- 1Department of Diagnostic Imaging, The Hospital for Sick Children, Toronto, ON, Canada
- 2Department of Psychology, University of Toronto, Toronto, ON, Canada
- 3Neuroscience and Mental Health Program, The Hospital for Sick Children Research Institute, Toronto, ON, Canada
- 4Department of Nutritional Sciences, University of Toronto, Toronto, ON, Canada
- 5Department of Translational Medicine, The Hospital for Sick Children Research Institute, Toronto, ON, Canada
- 6Department of Paediatrics, University of Toronto, Toronto, ON, Canada
- 7Department of Paediatrics, Sinai Health, Toronto, ON, Canada
- 8Division of Neonatology, The Hospital for Sick Children, Toronto, ON, Canada
Children born very low birth weight (VLBW, <1,500 g) are at high risk for cognitive and academic difficulties later in life. Although early nutrition (e.g., breastfeeding) is positively correlated with IQ in children born VLBW, the association between dietary intake in childhood and cognitive performance is unknown. Thus, our study is the first to investigate the relationship between diet quality, as measured by the Healthy Eating Index-2010 (HEI-2010) and cognitive performance in a Canadian cohort of 5-year-old children born VLBW (n = 158; 47% female). Diet quality was measured using two 24-h diet recalls obtained from parents and cognitive performance was assessed using the Wechsler Preschool and Primary Scale of Intelligence-IV (WPPSI-IV). To account for additional sociodemographic factors that could influence neurodevelopment, linear regression analyses were adjusted for sex, household income above/below the poverty line, maternal education, birth weight and breastfeeding duration. Mean ± SD HEI-2010 score was 58.2 ± 12.4, with most children (67%) having diets in “need of improvement” (scores 51–80). HEI-2010 scores were not significantly associated with IQ or any other WPPSI-IV composite score. Significant predictors of IQ in our model were birth weight, sex, and maternal education. Our findings emphasize the important role of maternal education and other sociodemographic factors on neurodevelopment in children born VLBW. Further, despite not finding any significant association between HEI-2010 scores and IQ, our results highlight the need to improve diet quality in young children born VLBW. Further research is needed to confirm the impact of diet quality on cognitive performance in this vulnerable population.
Introduction
Very low birth weight (VLBW, <1,500 g) infants are born early in the third trimester of pregnancy, a critical period of brain development, putting them at higher risk of cognitive impairments and academic underachievement later in life (1–3). Convergent findings support overall lower academic performance and IQ scores in preterm [<37 weeks gestational age (GA)] compared to full-term children, with one meta-analysis reporting almost a 12-point difference in IQ among school-aged children born preterm, independent of socioeconomic status [SES; (4)]. Several factors, as a consequence of preterm birth, increase the risk of sub-optimal neurodevelopment, including inadequate nutrient intake and morbidity during initial hospitalization [e.g., sepsis, necrotizing enterocolitis, chronic lung disease; (5–7)]. After hospital discharge, SES factors play a central role in the neurodevelopment of preterm infants, but other factors such as childhood nutrition have not yet been explored (8, 9).
While the role of post-discharge diet on neurodevelopment in infants born VLBW is unknown, evidence from studies of full-term infants suggests that a moderate association exists between diet and academic outcomes (8). In full-term children, different components of the diet (e.g., fruits and vegetables, breakfast consumption) are associated with cognition (8, 10, 11). In one large Canadian cohort (n = 5,200) of grade 5 students, higher diet quality scores, assessed by the Diet Quality Index-International and Healthy Eating Index (HEI), were associated with a reduced likelihood of failing school literacy assessments (12). More recently, a cross-sectional analysis in a Spanish cohort of children and adolescents (n = 1,371, 8–18 years) found better adherence to a Mediterranean diet, as measured by the KIDMED diet quality index, was associated with higher academic performance (13). A Mediterranean diet is typically rich in vegetables, fruit, nuts, fish and olive oil. Importantly, these findings remained significant after adjusting for various confounders including maternal education, sex, age, birth weight and GA. Infants born VLBW, however, were not included in these studies.
In preterm and VLBW children, the only studies investigating the relation between diet and cognition have focused on nutrient intake during early infancy. In one study, greater breastmilk intake during initial hospitalization in preterm infants (<37 weeks GA) was associated with higher verbal IQ in adolescence [n = 50, 13–19 years; (14)]. In boys, breastmilk intake was also linked with higher IQ scores, as well as total brain and white matter volume. Importantly, in the seminal meta-analytic paper by Anderson et al. (1), the authors found that low birth weight infants who were breastfed derived a greater cognitive benefit than normal birth weight infants, even after adjusting for covariates such as SES or maternal education. More recent systematic reviews and a cluster-randomized control trial also support the positive association between breastfeeding and neurodevelopment that persists after adjustment for maternal IQ in both full-term and preterm children (15, 16).
Given the high risk of academic underachievement and cognitive difficulties in children born VLBW, elucidating modifiable factors that impact cognitive outcomes in this population is an essential step for informing supports and strategies for families and educators (17, 18). Further, preschool age represents a critical time period to investigate the association between diet and cognitive development, as dietary preferences are established early in childhood (19), and cognitive ability at this age is associated with later academic achievement in full-term children (20, 21). Thus, our study estimated diet quality in children born VLBW using the HEI-2010 (22, 23) to evaluate associations with cognitive performance. In line with the full-term literature, we hypothesized that higher HEI-2010 scores (reflecting closer conformance with dietary guidelines) would correspond with higher IQ scores.
Methods
Participants
All surviving children and their families who consented to the original randomized trial were approached for the present follow-up study when the children were 5 years of age. The feeding intervention, study protocol and outcomes have been published (24–27). Briefly, VLBW infants were enrolled between October 2010 and December 2012, from four tertiary neonatal intensive care units in Ontario, Canada. Infants were eligible if their birth weight was <1,500 g, and if families consented within 4 days of birth. Exclusion criteria included chromosomal or congenital anomalies that could affect neurodevelopment. At the 5-year follow-up, written informed consent was obtained from parents and verbal assent from children.
Exposures: Diet Quality, Sociodemographic and Lifestyle Characteristics
A 24-h dietary recall was completed with parents during the study visit and parents were contacted 1 week later to complete a second recall by phone or email as described in detail previously (28). Briefly, the 24-h dietary recall asks parents to detail everything their child ate and drank in the previous day using the Automated Self-Administered 24-h (ASA24) Dietary Assessment tool, Canadian version (2016). The HEI-2010, a validated measure of diet quality in both children and adults (22, 23), was used to assess diet quality. Importantly, this tool considers diet quality distinctly from the quantity of food consumed (i.e., food groups are considered per 1000 kcal consumed).
The HEI-2010, created by the U.S. Department of Agriculture, consists of 12 components: nine adequacy components (that should be consumed in adequate amounts for optimal health) and three moderation components (that should be consumed in limited amounts for optimal health; see Supplementary Tables 1, 2 for the HEI-2010 Components and Standards for Scoring). Overall diet quality is reflected in the total index score, which is the sum of the 12 dietary components. HEI-2010 scores range from 0 to 100, with higher scores reflecting greater adherence to dietary requirements and better diet quality. Diets with HEI-2010 scores >80 are categorized as being “healthy”, with scores between 51 and 80 reflecting diets that “need improvement”, and scores <51 considered “poor” diets (22). HEI-2010 scores were calculated from the two non-consecutive 24-h dietary recalls using the multivariate Markov Chain Monte Carlo approach, which adjusts for weekday vs. weekend, season and recall sequence. A validated scoring algorithm was used to calculate individual HEI-2010 scores.
Outcome Measures: Cognitive Performance
The primary outcome measure for this study was cognitive performance assessed using Full-Scale IQ on the Wechsler Preschool and Primary Scale of Intelligence-IV [WPPSI-IV; (29)]; the version based on Canadian norms. The WPPSI-IV is a standardized measure of intelligence for children between 2 years and 6 months through to 7 years and 7 months of age. Secondary outcome measures included the following composite scores of the WPPSI-IV: verbal comprehension index (VCI), vocabulary acquisition index (VAI), visual spatial index (VSI), fluid reasoning index (FRI), processing speed index (PSI), and working memory index (WMI). All WPPSI-IV composite scores are calculated as standard scores with a mean of 100 and a standard deviation of 15, with “low average” scores defined as scores <90 (29). In line with other experts in the field, children who were unable to complete the WPPSI-IV assessment due to severe disability or who performed below the threshold of the test, were assigned a score of 49 (24, 30–32).
Demographic and Clinical Variables
Demographic and clinical factors including sex, birth weight, household income above/below the poverty line, maternal education, maternal ethnicity, and duration of breastfeeding were collected as described in detail previously (24). Briefly, sex and birth weight were collected from medical records and maternal education and ethnicity, as well as household income were reported from standardized parent questionnaires at birth. Maternal education was dichotomized as mothers having a university degree or not and household income above/below the poverty line was based on 2006 Statistics Canada family size-adjusted cut-off values (33). Duration of breastfeeding was calculated as the last recorded date that any breastmilk was provided, up to 18 months corrected age. During initial hospitalization, daily volumes of both parenteral and enteral nutrition were prospectively recorded in all infants. Enteral feed type during the intervention was characterized as receiving >50% of enteral feeds as either donor milk, preterm formula or mother's breastmilk.
Statistical Analyses
Normality of data were confirmed visually using histogram distributions and analytically using the Shapiro-Wilk test. Normally distributed variables were described as mean (standard deviation or 95% confidence interval [CI]) or median (interquartile range) when not normally distributed. Independent samples t-tests were used to compare differences between sexes for continuous outcome variables, and categorical variables were compared using chi-square tests.
Linear regression models were computed for primary and secondary outcomes. The association between HEI-2010 scores and WPPSI-IV composite scores was analyzed using linear regression models adjusted for sex, birth weight, breastfeeding duration, income above/below the poverty line, and maternal education (university degree or not). Interactions between exposure variables and sex were assessed in linear regression models to test whether the association between diet quality and IQ varied by sex. If the interaction was not statistically significant, it was removed from the model and the analyses were re-run. Multicollinearity was assessed using variance inflation factors with a cut-off value >10 considered to represent multicollinearity. Statistical analyses were performed in R 3.5.1 (34) using the rms statistical package. All hypothesis tests were two-sided and considered statistically significant if p-values were <0.05.
Sensitivity Analyses
To ensure that imputation of scores did not impact study findings, sensitivity analyses were performed by excluding children who were assigned a score of 49. In addition, in-hospital enteral feed type (receiving >50% mother's breastmilk, donor milk, or preterm formula) was included as a covariate in multivariable models to test for the effect of supplemental milk provided in-hospital. We also tested the interaction between enteral feed type and maternal education (university degree or not) in linear regression models. If the interaction was not statistically significant, it was removed from the model.
Results
Participant Characteristics
One hundred and fifty-eight of 316 (50% follow-up rate) eligible children born VLBW participated in the 5-year follow-up [mean (SD) age: 5.7 (0.2) years]. Birth and parental baseline characteristics are summarized in Table 1, including sex, birth weight, birth GA, mother's ethnicity, mother's education, family income, enteral feed type, and breastfeeding duration. Mean (SD) birth weight and GA were 1013 (264) g and 28 (3) weeks, respectively. Fifty percent of mothers in our sample had an education level of university or above and 20% had incomes below the poverty line. As reported previously, except for maternal education, no statistically significant differences in baseline characteristics were found between children who participated in the 5-year follow-up and those who did not (28). Mothers of children who participated in the follow-up study were more educated than mothers of children who did not (p = 0.02).
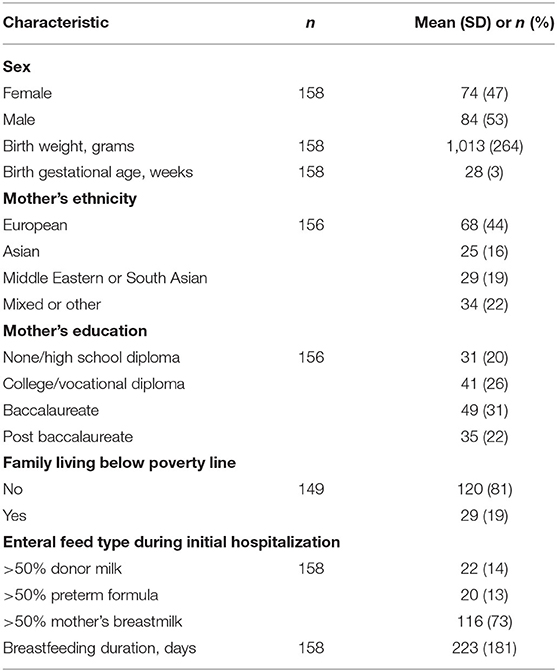
Table 1. Birth and parental baseline characteristics of children and families who participated in follow-up at 5 years.
Cognitive Assessments
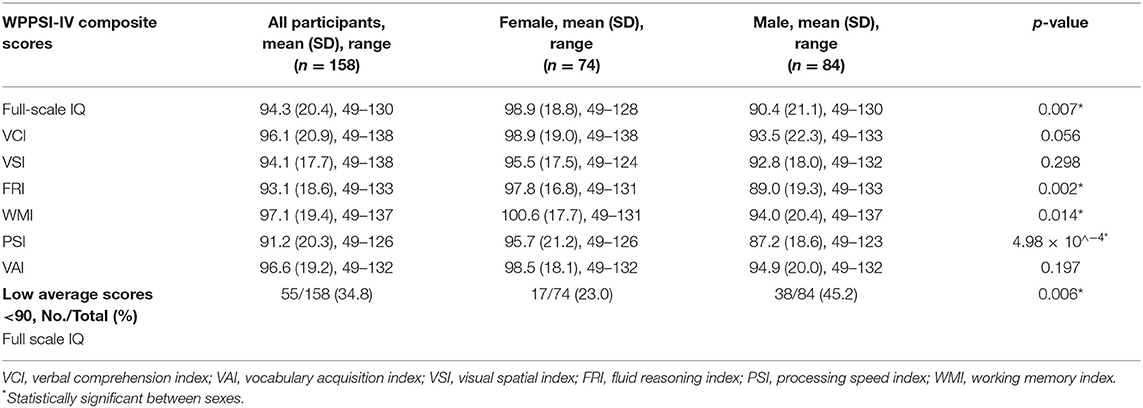
Complete WPPSI-IV data were obtained for 91.1% (144 of 158) of children born VLBW. Fourteen children who attended the follow-up visits were unable to complete the WPPSI-IV (see Figure 1 for participant flowchart). In these cases, an IQ score of 49 (the lower limit of IQ) was assigned. The mean (SD) of IQ was 94.3 (20.4), which is within the average range of standard IQ classification (90–109). The mean (SD) for all WPPSI-IV composite scores are summarized in Table 2. Males had significantly lower scores for IQ, FRI, WMI, and PSI. In addition, more males had an IQ score below 90, which according to the WPPSI-IV manual (29) is indicative of low average or borderline level cognitive ability (p = 0.006).
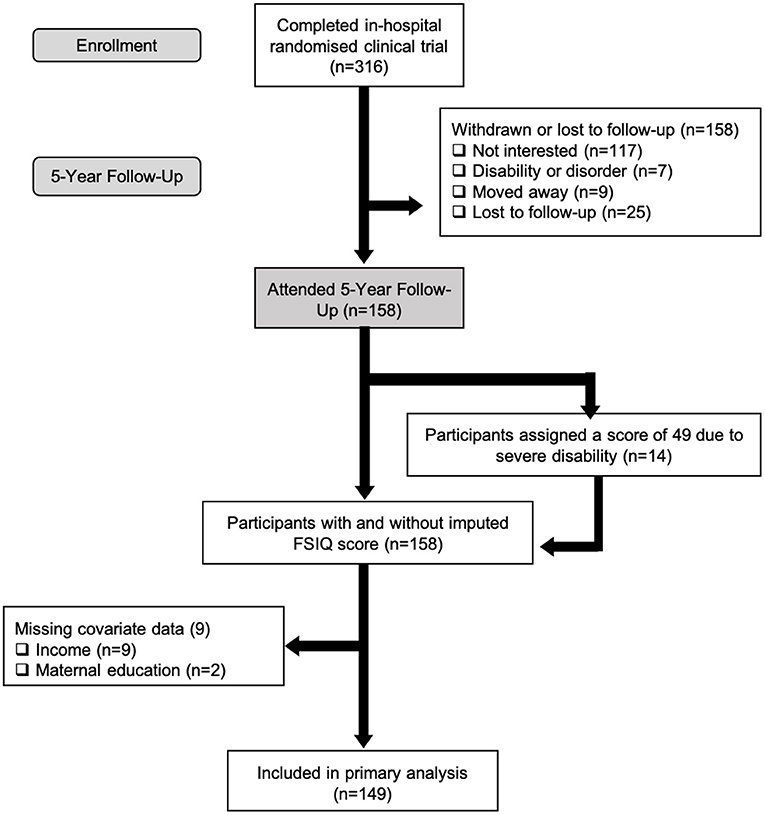
Figure 1. Flow of VLBW participants through the randomized clinical trial from initial enrollment to inclusion in primary analysis.
Diet Quality
HEI-2010 diet quality scores ranged from 19.2 to 87.1, with a mean (SD) of 58.2 (12.4). As previously reported (28), most children had diet scores that fell within the “poor” (43/158 children) or “needs improvement” (106/158 children) diet quality categories. Only 6% of our cohort had HEI-2010 scores that were above 80, indicative of a good quality diet meeting recommended age-specific guidelines. There were no sex differences in HEI-2010 total scores.
Association Between Exposure and Outcome Variables
Of the 158 children who were tested, 149 children were included in the primary analyses due to missing covariate data (Figure 1). HEI-2010 scores were not significantly associated with IQ scores (Table 3). However, statistically significant variables in our model were birth weight, sex, and maternal education. Every additional gram of birth weight was associated with a gain of 0.02 points in IQ (95% CI, 0.01–0.03). On average, males had IQ scores that were 7.7 points below females (95% CI, 1.5–13.8). The strongest predictor of IQ in our model was maternal education. Children whose mothers had a university degree were found to have an IQ of 9.1 points greater, or a 0.6 standard deviation difference, than children whose mothers were college-educated or had no post-secondary education (95% CI, 1.8–16.2). The interaction between HEI-2010 scores and sex were assessed in the adjusted linear regression model to test whether the association between diet quality and IQ varied by sex. This interaction term, however, was not significant (p = 0.83) and therefore removed from the model. Further, the associations between the different components of the HEI-2010 (i.e., total fruits, total vegetables, whole grains, etc.) with IQ were also investigated in adjusted linear regression models but revealed no significant associations (Supplementary Table 3).
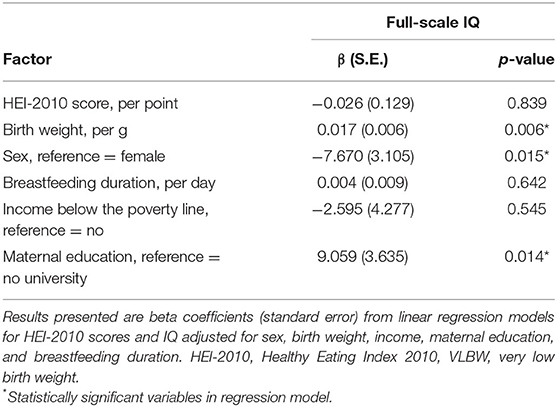
Table 3. Associations between diet quality, sociodemographic variables and IQ in children born VLBW (n = 149).
Secondary outcome variables (other WPPSI-IV composite scores) were regressed on HEI-2010 scores and revealed non-significant associations (p > 0.05). These findings remained unchanged in a sensitivity analysis that excluded children who were unable to complete the assessments and who would have otherwise been assigned a score of 49 (Supplementary Table 4). In sensitivity analyses, in-hospital enteral feed type (e.g., >50% mother's breastmilk, donor milk, or preterm formula) was included in the General linear model (GLM) as a covariate but was not statistically significant.
Discussion
Our findings demonstrate that diet quality at 5 years of age was not correlated with IQ or any other WPPSI-IV composite measures in children born VLBW. There are no comparable data in the preterm or VLBW populations, but our findings are inconsistent with the few studies who report a positive association between diet quality and cognition in full-term children, even after adjusting for a range of confounding factors (12, 35, 36). Unlike our findings, Khan et al. (36) in a cross-sectional study from the United States assessing diet quality using the HEI-2005 version, found positive associations with executive function in full-term children (n = 65, 7–9 years). Similarly, Haapala et al. (35) in a large Finnish cohort of full-term children (n = 428, aged 6–8 years), found that poor diet quality assessed using the Baltic Sea Diet Score, was associated in a dose-dependent manner with lower cognitive scores on the Raven's Colored Progressive Matrices; this relation was strongest for the boys. Similar to the HEI-2010, the Baltic Sea diet score assesses diet quality but is specific to Nordic dietary recommendations. Both full-term studies, however, did not directly measure IQ and were performed in older cohorts of children, which may explain why we did not find significant associations between diet quality and cognitive performance in our study (35, 36). Another reason for this discrepancy is the index of diet quality used in these studies, which makes comparison difficult. Further, with the exception of the previously mentioned studies (35, 36), few other studies have used validated measures of diet quality, such as the HEI-2010 in children. Thus, future studies should focus on investigating diet quality using validated tools to better understand its impact on cognition in young children.
In this cohort, the average HEI-2010 score was 58.2 out of 100, with the majority of children (94%) having diets in the “needs improvement” or “poor quality” range. Although diet quality scores were low in our sample of children born VLBW, they are similar to those reported in Khan et al.'s cohort of 7–9-year-old full-term children (mean HEI-2005 score: 66.4) and Florence et al.'s cohort of 10–11-year-old full-term children (mean DQI-I score: 62.4), both of which found significant associations between diet and cognitive performance. However, the lack of variability in our diet scores may be one reason why we did not find significant associations between diet quality and IQ. It is also possible that the association between diet and cognition, may become more apparent with increasing age as IQ scores begin to stabilize over childhood (12, 36). Our results may also indicate that other factors are more critical in supporting neurodevelopment in children born VLBW that were not assessed in this study, such as early growth (9, 37–39). More specifically, it is important to consider the confounding role of SES factors related to neurodevelopment and diet quality in children such as maternal education.
In this cohort, maternal education was the strongest predictor of IQ, with children whose mothers had a university degree, compared to no university degree, having a 9-point gain in IQ. SES indicators such as maternal education have been reported to be predisposing risk factors of preterm birth and have also been linked with adverse cognitive and academic outcomes in both preterm and full-term populations (40, 41). Our findings are similar to those of Voss et al. (41), who reported that 10–13-year-old children born preterm (n = 148, mean: birth weight = 798 g, GA = 27.1 weeks) from mothers with low educational background (high school or less) were almost 22 times more likely to have an IQ score ≤ 85 compared to those from more highly-educated mothers (college or higher). The reasons for this association may be due to many factors, including parenting style and quality of cognitive stimulation (41). Given the current and previous findings that maternal education is predictive of cognitive performance in preterm children (42, 43), early interventions focused on supporting families with low maternal education may help attenuate cognitive impairment related to preterm birth.
Despite previous findings that children born preterm are consistently below full-term children in neurocognitive outcomes, encouragingly mean IQ scores were within the average range in our cohort. Males, however, had significantly lower IQ, FRI, WMI and PSI scores compared to females and consistently underperformed in all other cognitive domains. This is in line with previous developmental studies, showing females have a slight IQ advantage in early to mid-childhood due to earlier maturational processes (44, 45). Consistent with this, Lean et al. (46) found males born very preterm were at higher risk of poor intellectual outcomes compared to very preterm-born females at 5 years of age. Thus, future studies and interventions should consider the heightened “triple-risk” of being born male, preterm and to socioeconomically disadvantaged mothers, to mitigate later cognitive risk.
Our study has many strengths, including using the HEI-2010, a measure of diet quality that has been validated in young children (22). In addition, dietary intake was assessed using two 24-h recalls that were adjusted for usual intake using the NCI method. To our knowledge, this is the first study to assess the association between diet quality and cognitive performance in young children born VLBW. Further, our study adjusted for sociodemographic variables such as maternal education and household income, which many other studies fail to take into account. However, despite the many strengths of our study, there are some limitations to consider. Only 50% of the survivors from the original trial participated in this 5-year follow-up. Great efforts were made to contact all 316 families who participated in the initial trial, however, due to various reasons indicated in Figure 1, many families were unable or not interested in participating in this follow-up study. While sample retention is a common challenge faced in prospective cohort studies, we acknowledge that our sample may not be representative of all children born VLBW. However, our sample represented diversity in educational attainment and household income, as well as similar incidence of major morbidity to national data of VLBW infants (47). Another limitation is the lack of a matched control group in our study. Although this study was performed cross-sectionally (i.e., association between diet quality and cognitive performance at 5 years), children were part of a prospective cohort study, from birth, in which they were recruited from birth. Follow-up studies such as ours are costly and time-intensive, making it difficult to include a control group. We did, however, find that average diet quality scores in full-term children were similar to our results (12, 36). In addition, we also acknowledge the possibility of other unmeasured confounding variables, such as the amount and quality of preschool education, that may influence the relations between diet and cognition. We did, however, collect and control for various other sociodemographic factors that could influence neurodevelopment such as sex, household income above/below the poverty line, maternal education, birth weight and breastfeeding duration. Further research is also necessary to assess the impact of specific micronutrients (e.g., iron) and its association with neurodevelopment, which was not assessed in the current study.
Diet quality, assessed using the HEI-2010, was not associated with IQ in 5-year-old children born VLBW. Although this is inconsistent with previous studies reporting a positive association between diet quality and cognition in older cohorts of full-term children, our study is the first to assess this association in 5-year-old children born VLBW. While it is unclear which factors contribute to these discrepant findings, the age range of our cohort, the measure of diet quality used, or the lack of variability in the diet quality scores (most within the “needs improvement” range), all may contribute to these differences. In addition, previous studies did not directly assess the association between diet quality and IQ, which may explain differences with our study findings. Maternal education, however, was independently associated with cognitive performance, and was the strongest predictor of IQ in our model. Maternal education is an important predictor of neurodevelopment in children born VLBW and should be considered in future interventions to target those at risk for cognitive difficulties. Finally, most children in our study had diets that were “in need of improvement”, which has important implications for the health and developmental outcomes in this vulnerable population. Future studies are needed to confirm these findings and assess the impact of diet quality on cognitive performance in young children born VLBW.
Data Availability Statement
The datasets presented in this article are not readily available because deidentified individual participant data will not be made available in order to protect the privacy and confidentiality of our participants. We do not have consent from participating families to share their anonymized data, nor do we have permission from the research ethics boards of our participating hospitals. Requests to access the datasets should be directed to ZGVib3JhaC5vY29ubm9yQHV0b3JvbnRvLmNh.
Ethics Statement
The Hospital for Sick Children's Research Ethics Board (REB# 1000053053) approved the study protocol. Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin.
Author Contributions
JS, MM, SU, and DO'C contributed to conception and design of the study. JS, MM, and NB were involved in data collection. JS and MM performed the statistical analysis. JS wrote the first draft of the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
Funding
This study was funded by the Canadian Institutes of Health Research (FHG 129919) and the SickKids Research Institute Restracomp Scholarship to JS.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors thank the study families for their participation and ongoing support of this work. The authors also thank Aisling Conway for assisting with study visits, and Michael Jory for setting up and managing our study database (The Hospital for Sick Children).
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2022.874118/full#supplementary-material
References
1. Anderson PJ. Neuropsychological outcomes of children born very preterm. Semin Fetal Neonatal Med. (2014) 19:90–6. doi: 10.1016/j.siny.2013.11.012
2. Bhutta AT, Cleves MA, Casey PH, Cradock MM, Anand KJ. Cognitive and behavioral outcomes of school-aged children who were born preterm: a meta-analysis. JAMA. (2002) 288:728–37. doi: 10.1001/jama.288.6.728
3. Joseph RM, O'Shea TM, Allred EN, Heeren T, Hirtz D, Jara H, et al. Neurocognitive and academic outcomes at age 10 years of extremely preterm newborns. Pediatrics. (2016) 137:e20154343. doi: 10.1542/peds.2015-4343
4. Kerr-Wilson CO, Mackay DF, Smith GC, Pell JP. Meta-analysis of the association between preterm delivery and intelligence. J Public Health. (2012) 34:209–16. doi: 10.1093/pubmed/fdr024
5. Cormack BE, Harding JE, Miller SP, Bloomfield FH. The influence of early nutrition on brain growth and neurodevelopment in extremely preterm babies: a narrative review. Nutrients. (2019) 11:2029. doi: 10.3390/nu11092029
6. Rees CM, Pierro A, Eaton S. Neurodevelopmental outcomes of neonates with medically and surgically treated necrotizing enterocolitis. Arch Dis Child Fetal Neonatal Ed. (2007) 92:F193–8. doi: 10.1136/adc.2006.099929
7. Schlapbach LJ, Aebischer M, Adams M, Natalucci G, Bonhoeffer J, Latzin P, et al. Impact of sepsis on neurodevelopmental outcome in a Swiss National Cohort of extremely premature infants. Pediatrics. (2011) 128:e348–57. doi: 10.1542/peds.2010-3338
8. Burrows T, Goldman S, Pursey K, Lim R. Is there an association between dietary intake and academic achievement: a systematic review. J Hum Nutr Diet. (2017) 30:117–40. doi: 10.1111/jhn.12407
9. Wong HS, Edwards P. Nature or nurture: a systematic review of the effect of socio-economic status on the developmental and cognitive outcomes of children born preterm. Matern Child Health J. (2013) 17:1689–700. doi: 10.1007/s10995-012-1183-8
10. Hoyland A, Dye L, Lawton CL. A systematic review of the effect of breakfast on the cognitive performance of children and adolescents. Nutr Res Rev. (2009) 22:220–43. doi: 10.1017/S0954422409990175
11. Nyaradi A, Li J, Hickling S, Foster J, Oddy WH. The role of nutrition in children's neurocognitive development, from pregnancy through childhood. Front Hum Neurosci. (2013) 7:97. doi: 10.3389/fnhum.2013.00097
12. Florence MD, Asbridge M, Veugelers PJ. Diet quality and academic performance. J Sch Health. (2008) 78:209–41. doi: 10.1111/j.1746-1561.2008.00288.x
13. Esteban-Cornejo I, Izquierdo-Gomez R, Gómez-Martínez S, Padilla-Moledo C, Castro-Piñero J, Marcos A, et al. Adherence to the Mediterranean diet and academic performance in youth: the UPandDOWN study. Eur J Nutr. (2016) 55:1133–40. doi: 10.1007/s00394-015-0927-9
14. Isaacs EB, Fischl BR, Quinn BT, Chong WK, Gadian DG, Lucas A. Impact of breast milk on intelligence quotient, brain size, and white matter development. Pediatr Res. (2010) 67:357–62. doi: 10.1203/PDR.0b013e3181d026da
15. Horta BL, Loret de Mola C, Victora CG. Breastfeeding and intelligence: a systematic review and meta-analysis. Acta Paediatr. (2015) 104:14–9. doi: 10.1111/apa.13139
16. Kramer MS, Aboud F, Mironova E, Vanilovich I, Platt RW, Matush L, et al. Breastfeeding and child cognitive development: new evidence from a large randomized trial. Arch Gen Psychiatry. (2008) 65:578–84. doi: 10.1001/archpsyc.65.5.578
17. Beck S, Wojdyla D, Say L, Betran AP, Merialdi M, Requejo JH, et al. The worldwide incidence of preterm birth: a systematic review of maternal mortality and morbidity. Bull World Health Organ. (2010) 88:31–8. doi: 10.2471/BLT.08.062554
18. Huddy CL, Johnson A, Hope PL. Educational and behavioural problems in babies of 32-35 weeks gestation. Arch Dis Child Fetal Neonatal Ed. (2001) 85:F23–8. doi: 10.1136/fn.85.1.F23
19. McGuire S. Institute of Medicine (IOM) early childhood obesity prevention policies. Adv Nutr. (2012) 3:56–7. doi: 10.3945/an.111.001347
20. Clark CA, Woodward LJ. Neonatal cerebral abnormalities and later verbal and visuospatial working memory abilities of children born very preterm. Dev Neuropsychol. (2010) 35:622–42. doi: 10.1080/87565641.2010.508669
21. Tramontana MG, Hooper SR, Selzer SC. Research on the preschool prediction of later academic achievement: a review. Dev Rev. (1988) 8:89–146. doi: 10.1016/0273-2297(88)90001-9
22. Guenther PM, Kirkpatrick SI, Reedy J, Krebs-Smith SM, Buckman DW, Dodd KW, et al. The Healthy Eating Index-2010 is a valid and reliable measure of diet quality according to the 2010 Dietary Guidelines for Americans. J Nutr. (2014) 144:399–407. doi: 10.3945/jn.113.183079
23. Guenther PM, Casavale KO, Reedy J, Kirkpatrick SI, Hiza HA, Kuczynski KJ, et al. Update of the healthy eating index: HEI-2010. J Acad Nutr Diet. (2013) 113:569–80. doi: 10.1016/j.jand.2012.12.016
24. O'Connor DL, Gibbins S, Kiss A, Bando N, Brennan-Donnan J, Ng E, et al. Effect of supplemental donor human milk compared with preterm formula on neurodevelopment of very low-birth-weight infants at 18 months: a randomized clinical trial. JAMA. (2016) 316:1897–905. doi: 10.1001/jama.2016.16144
25. Unger S, Gibbins S, Zupancic J, O'Connor DL. DoMINO: donor milk for improved neurodevelopmental outcomes. BMC Pediatr. (2014) 14:123. doi: 10.1186/1471-2431-14-123
26. Ng D, Unger S, Asbury M, Kiss A, Bishara R, Bando N, et al. Neonatal morbidity count is associated with a reduced likelihood of achieving recommendations for protein, lipid, and energy in very low birth weight infants: a prospective cohort study. J Parenter Enteral Nutr. (2018) 42:623–32. doi: 10.1177/0148607117710441
27. Asbury MR, Unger S, Kiss A, Ng D, Luk Y, Bando N, et al. Optimizing the growth of very-low-birth-weight infants requires targeting both nutritional and nonnutritional modifiable factors specific to stage of hospitalization. Am J Clin Nutr. (2019) 110:1384–94. doi: 10.1093/ajcn/nqz227
28. McGee M, Unger S, Hamilton J, Birken CS, Pausova Z, Kiss A, et al. Associations between diet quality and body composition in young children born with very low body weight. J Nutr. (2020) 150:2961–8. doi: 10.1093/jn/nxaa281
29. Wechsler D. Wechsler Preschool and Primary Scale of Intelligence. Canadian Manual (WPPSI®–IV CDN). 4th ed. San Antonio, TX: Pearson (2012).
30. Belfort MB, Anderson PJ, Nowak VA, Lee KJ, Molesworth C, Thompson DK, et al. Breast milk feeding, brain development, and neurocognitive outcomes: a 7-year longitudinal study in infants born at less than 30 weeks' gestation. J Pediatr. (2016) 177:133–9.e1. doi: 10.1016/j.jpeds.2016.06.045
31. Stoll BJ, Hansen NI, Adams-Chapman I, Fanaroff AA, Hintz SR, Vohr B, et al. Neurodevelopmental and growth impairment among extremely low-birth-weight infants with neonatal infection. JAMA. (2004) 292:2357–65. doi: 10.1001/jama.292.19.2357
32. Wadhawan R, Oh W, Perritt RL, McDonald SA, Das A, Poole WK, et al. Twin gestation and neurodevelopmental outcome in extremely low birth weight infants. Pediatrics. (2009) 123:e220–7. doi: 10.1542/peds.2008-1126
33. Statistics Canada. Income Research Paper Series, low income cut-offs for 2006 and low income measures for 2005. Report No.: 75F0002MIE.
34. R Core Team. R: a Language Environment for Statistical Computing. Vienna, Austria (2020). Available online at: https://www.R-project.org/
35. Haapala EA, Eloranta AM, Venäläinen T, Schwab U, Lindi V, Lakka TA. Associations of diet quality with cognition in children - the Physical Activity and Nutrition in Children Study. Br J Nutr. (2015) 114:1080–7. doi: 10.1017/S0007114515001634
36. Khan NA, Raine LB, Drollette ES, Scudder MR, Kramer AF, Hillman CH. Dietary fiber is positively associated with cognitive control among prepubertal children. J Nutr. (2015) 145:143–9. doi: 10.3945/jn.114.198457
37. Cooke RW, Foulder-Hughes L. Growth impairment in the very preterm and cognitive and motor performance at 7 years. Arch Dis Child. (2003) 88:482–7. doi: 10.1136/adc.88.6.482
38. Der G, Batty GD, Deary IJ. Effect of breast feeding on intelligence in children: prospective study, sibling pairs analysis, and meta-analysis. BMJ. (2006) 333:945. doi: 10.1136/bmj.38978.699583.55
39. Ehrenkranz RA, Dusick AM, Vohr BR, Wright LL, Wrage LA, Poole WK. Growth in the neonatal intensive care unit influences neurodevelopmental and growth outcomes of extremely low birth weight infants. Pediatrics. (2006) 117:1253–61. doi: 10.1542/peds.2005-1368
40. Sirin SR. Socioeconomic status and academic achievement: a meta-analytic review of research. Rev Educ Res. (2005) 75:417–53. doi: 10.3102/00346543075003417
41. Voss W, Jungmann T, Wachtendorf M, Neubauer AP. Long-term cognitive outcomes of extremely low-birth-weight infants: the influence of the maternal educational background. Acta Paediatr. (2012) 101:569–73. doi: 10.1111/j.1651-2227.2012.02601.x
42. Patra K, Greene MM, Patel AL, Meier P. Maternal education level predicts cognitive, language, and motor outcome in preterm infants in the second year of life. Am J Perinatol. (2016) 33:738–44. doi: 10.1055/s-0036-1572532
43. ElHassan NO, Kaiser JR. Parenteral nutrition in the neonatal intensive care unit. Neoreviews. (2011) 12:e130–40. doi: 10.1542/neo.12-3-e130
44. Lynn R, Kanazawa S. A longitudinal study of sex differences in intelligence at ages 7, 11 and 16 years. Pers Individ Dif. (2011) 51:321–4. doi: 10.1016/j.paid.2011.02.028
45. Irwing P, Lynn R. Sex differences in means and variability on the progressive matrices in university students: a meta-analysis. Br J Psychol. (2005) 96(Pt 4):505–24. doi: 10.1348/000712605X53542
46. Lean RE, Paul RA, Smyser CD, Rogers CE. Maternal intelligence quotient (IQ) predicts IQ and language in very preterm children at age 5 years. J Child Psychol Psychiatry. (2018) 59:150–9. doi: 10.1111/jcpp.12810
47. The Canadian Neonatal Network. CNN annual reports 2010Ȕ2015. Available online at: http://www.canadianneonatalnetwork.org/portal/ (accessed May 01, 2019).
Keywords: preterm, very low birth weight, diet quality, cognition, children
Citation: Sato J, McGee M, Bando N, Law N, Unger S and O'Connor DL (2022) Diet Quality and Cognitive Performance in Children Born Very Low Birth Weight. Front. Nutr. 9:874118. doi: 10.3389/fnut.2022.874118
Received: 11 February 2022; Accepted: 16 June 2022;
Published: 19 July 2022.
Edited by:
Maria G. Grammatikopoulou, International Hellenic University, GreeceReviewed by:
Rosaura Leis, University of Santiago de Compostela, SpainAlan Emond, University of Bristol, United Kingdom
Copyright © 2022 Sato, McGee, Bando, Law, Unger and O'Connor. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Julie Sato, anVsaWUuc2F0b0BzaWNra2lkcy5jYQ==
 Julie Sato
Julie Sato Meghan McGee4,5
Meghan McGee4,5 Nicole Bando
Nicole Bando Sharon Unger
Sharon Unger Deborah L. O'Connor
Deborah L. O'Connor