
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Nutr., 08 April 2022
Sec. Nutrition and Metabolism
Volume 9 - 2022 | https://doi.org/10.3389/fnut.2022.867099
The nutritional state before and throughout pregnancy has a critical impact on the women's health and the baby's development and growth. The release of placental hormones during pregnancy induces/ increases maternal insulin resistance and promotes nutrition utilization by the fetus. Gestational Diabetes Mellitus (GDM) is the most common medical complication in pregnancy and is associated with significant maternal and fetal morbidity. Several studies have examined the effect of physical activity, healthy eating, and various food supplements on the risk of developing gestational diabetes (GDM) and related outcomes. Among those, Myo-Inositol supplementation has shown encouraging results in the prevention of GDM. Maternal vitamin D deficiency has been associated with an elevated risk of GDM, and supplementation can improve glucose haemostasis by lowering fasting blood glucose, HbA1c, and serum insulin concentration. Probiotics modulate the gut microbiota leading to an improved glucose and lipid metabolism, which is proposed to reduce the risk of GDM. We aim to review the strength and limitation of the current evidence for using some nutritional supplements either as single agents or in combinations on the risk of developing GDM and on glycaemic control.
Appropriate nutritional health before and during pregnancy is essential for favorable outcomes and the long-term health of the offspring. One or more nutritional deficits in mothers before and in early pregnancy are not uncommon and increase adverse pregnancy outcomes. Gestational diabetes mellitus (GDM) is a common pregnancy disorder associated with an increased risk of pregnancy complications and long-term metabolic complications for both mothers and offspring (1). Pre-eclamptic toxemia, preterm labor, large for gestational age, neonatal hypoglycaemia, and cesarean delivery are serious pregnancy complications associated with GDM. Besides, GDM increases the future risk of hypertension, type 2 diabetes (T2DM), fatty liver disease, and cardiovascular disease in women and offspring (1).
Dietary patterns before conception and during pregnancy are associated with a reduced risk of GDM. On the one hand, increasing the intake of vegetables, fruits, whole grains, nuts, legumes, and fish can lower the risk of GDM (2). While, high consumption of red meat, processed meat, and eggs can increase GDM risk (3). Dietary screening questionnaire tools can identify these nutritional habits in early pregnancy and implement tailored feedback interventions (4). Dietary counseling is the cornerstone in the treatment of GDM, and all women with GDM should be offered dietary advice by a trained clinical dietician (5).
Maternal obesity is the most critical and modifiable risk factor of GDM. While obesity is a synonym for overnutrition, obese people have higher micronutrient deficiencies than normal-weight individuals (6, 7). Most critical, some nutritional deficiencies might exacerbate insulin resistance in obese women leading to increased risk of GDM or worsening of glycaemic control in women with GDM. Hence, it is vital to understand the impacts caused either by deficiency or excess of some micronutrients on the risk of GDM.
In the last decade, there have been significant efforts to improve the outcome of pregnancy in women with GDM. Among these are various nutritional intervention strategies to reduce the risk of GDM. While considerable progress has been made, several challenges remain, including; non-compliance with dietary advice, reluctance to ingest tablets or use insulin injections, difficulty adhering to the treatment, and ongoing concerns over the long-term safety of oral agents on the mother and offspring.
Nutritional supplements are safe and generally well-tolerated and provide an alternative option for the treatment and prevention of GDM. However, studies examining the role of probiotics, omega 3 fatty acids, Myo-Inositol, vitamin D, selenium, zinc, and magnesium for the prevention of GDM are limited by the small sample size and the lack of reproducibility. The strength of the evidence for some commonly studied supplements is discussed in this narrative review focusing more on systematic reviews and meta-analysis. Besides, we will discuss the future directions for the prevention and treatment of GDM. A summary of the most recent systematic reviews and meta-analyses is included in Table 1.
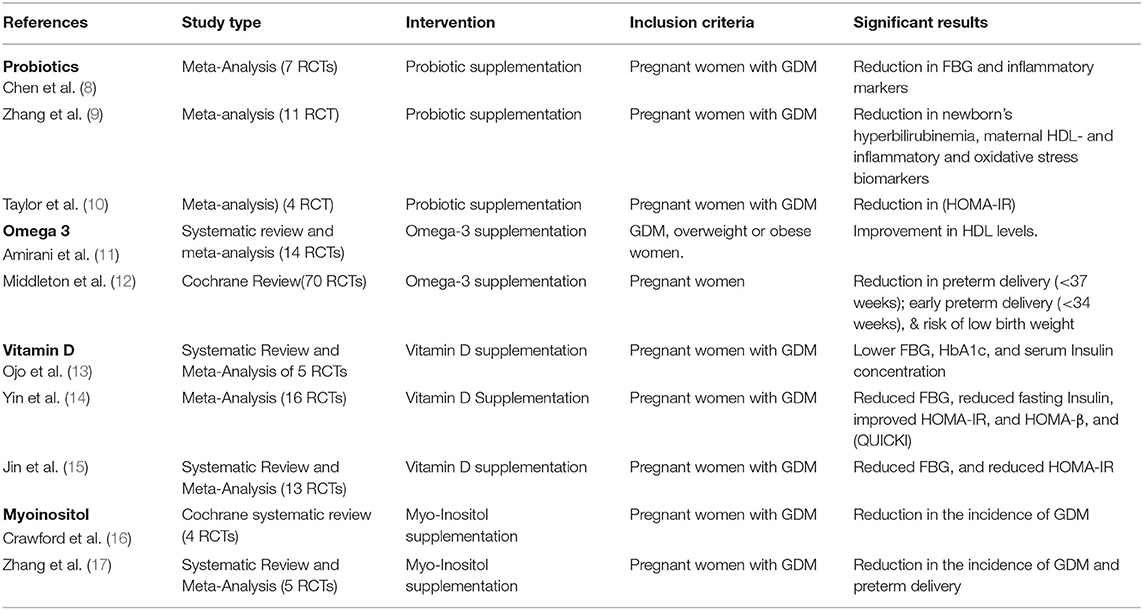
Table 1. Summary of systematic reviews and meta-analyses of RCTs on the effects of nutritional supplementation during pregnancy.
Microorganisms are found in human tissues and bodily fluids, including the skin, mouth, saliva, nose, vagina, seminal fluid, and mammary glands. Most of them dwell in the gut due to nutrient accessibility (18). The human gut microbiota is believed to exceed the total cells in the human body by a factor of ten, including roughly 100 trillion microorganisms representing 5,000 different species (19). The gut microbiota plays a fundamental role in many physiological processes such as digestion, immunity, neurological signaling, endocrine function, drug and toxins metabolism, and producing secondary compounds that influence the host physiology (20).
The bacterial phyla Bacteroidetes and Firmicutes phyla dominate the gut microbiota in healthy adults (21). The gut microbiota also plays a central role in producing short-chain fatty acids (SCFAs), such as acetate, butyrate, and propionate, via fermentation of non-digestible dietary fibers (22). SCFAs can activate G-coupled receptors and stimulate the secretion of Glucagon-like peptide 1 (GLP-1) and Peptide YY from the L-cells. It increases leptin secretion from adipocytes increases both insulin secretion and insulin sensitivity and satiety (23). There is a growing body of evidence regarding the association between gut microbiota and metabolic disorders, including obesity, T2DM, and GDM. Dysregulation of the gut microbiota, known as dysbiosis, reduces the release of SCFA and other antimicrobial molecules (20). These changes can lead to dysregulation in insulin secretion, insulin sensitivity, and appetite regulation. Most critical is the increase in gut wall permeability, allowing endotoxins' systemic entrance, resulting in low-grade inflammation. Women with GDM have altered gut microbiota compared to normal glucose tolerance (24).
Probiotics are defined by the World Health Organization (WHO) as “live microorganisms which when administered in adequate amounts confer a health benefit on the host” (21). They are reported to improve microbial balance in the gastrointestinal tract, increase the colonic microbial diversity, improve intestinal barrier function (25, 26), attenuate inflammation and regulate insulin production (27). Additionally, probiotic supplementation during pregnancy modulates gut microbiota composition and improves glucose and lipid metabolism (28). Experimental and clinical evidence support that this effect could be beneficial in preventing GDM (29). Furthermore, combination probiotics (Lactobacillus and Bifidobacterium strains) during pregnancy reduces the risk of inflammatory events and preeclampsia and improves maternal glucose metabolism (30, 31).
A meta-analysis by Chen et al. (8), which included seven studies, showed that probiotic supplementation reduced fasting glucose (FBG) (WMD:−3.19 mg/dl, 95% CI:−5.55 to−0.82, P = 0.008) in pregnant women with GDM. This association was more significant in patients with a baseline FBG ≥ 92 mg/dl, a duration of probiotic treatment ≤ 6 weeks and a dose <6 × 109 colony-forming unit (CFU). In addition, the supplementation was effective in reducing some of the biomarkers of inflammation and oxidative stress like high-sensitivity CRP and malonaldehyde. Similarly, a meta-analysis of 11 randomized controlled trials assessed the effects of probiotic supplementation for 4 to 8 weeks showed that probiotic supplementation in women with GDM reduced the incidence of a newborn's hyperbilirubinemia and improved maternal HDL-cholesterol and markers of inflammation and oxidative stress (9).
Conversely, other studies did not support these findings and showed that probiotics did not prevent GDM in overweight and obese pregnant women (32, 33). Another meta-analysis showed that 6–8 weeks of probiotic supplementation significantly reduces the homeostasis model of assessment-estimated insulin resistance (HOMA-IR) but did not affect the FBG or LDL-cholesterol (10).
One explanation of the above disparity could be that probiotics differ in their ability to resist gastric acid and bile acids colonizing the intestinal tract (34). Therefore, not all probiotics exert similar clinical benefits; whether used as a single or combination of species (35), this could explain the un-unifying evidence about their impact on GDM prevention and maternal glucose metabolism. Besides, the individual response to the treatment may play a role. Hence, personalized and precise supplementations should be tested, considering interaction with diet and the host gut microbiota composition during pregnancy.
In summary, gut microbiota dysbiosis associated with inflammation, adiposity, and glucose intolerance, was observed in women with GDM, which resembles the gut microbiota profile of adults with other metabolic disorders such as T2DM. Microbiota-targeted interventions such as probiotics/prebiotic or combination symbiotic supplementation have already shown promising results and could enhance health outcomes in women with GDM.
A combination of lifestyle changes like exercise and diet, microbiota targeted, and novel approaches should be considered and tested, considering the side effects of such intervention. Thus, long-term studies using multi-strain probiotics/postbiotic or engineered microbes are warranted involving large GDM cohorts from different ethnicities.
Fish are rich in long-chain polyunsaturated fatty acids. The relationship between fish intake and metabolic disorders is controversial (36). While white and oily fish was associated with a reduced risk of T2DM, fried and shellfish were associated with a higher risk of T2DM (37). Besides, the relationship between T2DM and fish intake varies by gender and geographical location (38). In pregnancy, there is more focus on the mercury contents of fish, and there are no uniform recommendations on fish intake (39). However, fish are not the only source of long-chain polyunsaturated fatty acids. Omega-3 polyunsaturated fatty acids (O-3-PUFA) [Eicosapentaenoic acid (EPA) and Docosahexaenoic acid (D.H.)] are also derived from plants, such as leafy greens, seeds, and nuts (40). O-3-PUFA modulates inflammatory pathways and exert anti-inflammatory and anti-coagulant effects (40). Furthermore, O-3-PUFA has several potential benefits, including antilipidemic, anti-hypertensive effects; modulation of gut microbiota; regulation of satiety; and enhancement of insulin sensitivity (40–42). In the growing fetus, 0-3-PUFA are critical for the structural and the functional development of many organs, particularly the brain and the eyes (43).
The antilipidemic effects of O-3-PUFA are of particular interest in pregnancy with or without GDM. Pregnancy impairs lipid metabolism and alters the serum concentration of free fatty acids (FFA), triglycerides (T.G.), total cholesterol (T.C.), High-density lipoprotein (HDL), and Low-density lipoprotein (LDL) (44). Indeed, there is a steady rise in T.G. and lipoproteins from the eighth week of gestation (44). GDM further aggravates lipids metabolism. Compared to women with normal glucose tolerance, women with GDM had higher levels of T.G., dense LDL particles, T.C., similar levels of LDL, but lower HDL levels (45). There is a shred of growing evidence that, in GDM, maternal T.G. levels positively correlate with neonatal weight and the risk for LGA and macrosomia (46–48). Furthermore, higher T.G. levels were found to worsen GDM- associated endothelial dysfunction in the umbilical vein (49).
Outside pregnancy, treatment with Omega-3 (EPA and D.H.) at a dose of 4 g/day were shown to be “an effective and safe option for reducing triglycerides as monotherapy” (50). Lower doses were not shown to be effective in reducing T.G. levels. Recently, a purified form of EPA, Icosapent ethyl (at a dose of 4 g/day), was shown to reduce the composite of cardiovascular death, non-fatal myocardial infarction, or non-fatal stroke in patients with established cardiovascular disease and elevated T.G. levels (51).
In pregnancy, most studies did not show a benefit of Omega-3 supplementations, likely due to (i) low dose of Omega-3 used in the trials, (ii) short duration of exposure to the treatment, and (iii) the small sample size. A meta-analysis of 14 RCT showed favorable effects of Omega-3 treatment on HDL but not on T.G., HOMA-IR, and fasting glucose (11). Of the 14 studies, only one used a dose of 4 grams; most studies lasted for <20 weeks and enrolled <100 subjects (11). Some studies have shown limited benefits from Omega-3 in women with GDM. Supplementation of Omega-3 fatty acid (at a dose of 1 g per day) in 52 women with GDM had some beneficial effects on insulin resistance but did not impact plasma glucose (HOMA-B), QUICKI, and lipids profile (52). Co-supplementation of omega-3 fatty acids (at a dose of 2 g per day) and vitamin D for 6 weeks in 140 women with GDM improves fasting glucose, insulin levels, serum triglycerides, and VLDL cholesterol (49). An RCT of 40 women with GDM showed that supplementation of omega-3 fatty acids (at a dose of 2 g per day) for 6 weeks significantly improved gene expression of peroxisome proliferator-activated receptor-gamma (PPAR-γ), interleukin-1 (IL-1), and tumor necrosis factor-alpha (TNF-α) (53). Co-supplementation of omega-3 fatty acids (at a dose of 1 g per day) and vitamin E for 6 weeks in 60 women with GDM did not improve insulin resistance or lipids but improved plasma total antioxidant capacity plasma malondialdehyde, and nitric oxide (54). A study of the long-term influence of GDM and the effect of omega-3 polyunsaturated fatty acids on the pancreas of the offspring found that supplementation lowered the pancreatic oxidative stress and inflammation, as evident by pancreatic fatty infiltration (55).
A recent Cochrane review has shown other potential benefits of Omega-3 supplementation in pregnancy (12). The Cochrane review included 70 RCTs involving 19,927 pregnant women showed a high risk of attrition bias in most studies. The use of Omega-3 supplementation showed a reduction in preterm delivery (<37 week's gestation), early preterm delivery (<34 week's gestation), and the risk of low birth weight.
In summary, the current evidence is insufficient to recommend for or against the routine supplementing of pregnant women with Omega-3. However, there are multiple potential benefits in pregnant women with or without GDM that should be examined in RCT. We recommend that future trials start at an early gestational age, be sufficiently powered, and use 4 g of Omega-3.
Vitamin D (Vit D) deficiency is not uncommon amongst pregnant women and has been linked with some pregnancy complications such as GDM, preeclampsia, preterm birth, and small for gestational age (56). The true prevalence of Vit D deficiency during pregnancy is ill-defined as different cut-off levels of 25-hydroxyvitamin D level (25 OH D) was used in studies. A study from the Middle East and North Africa (MENA) region found that the prevalence of Vit D deficiency amongst pregnant women [defined as 25 (OH) D <50 nmol/l] ranged between was 54 and 90% (57). Saraf et al. (58) meta-analysis found that the global prevalence of Vit D ranged between 46 and 87% for cut off levels of 25(OH) D <50 nmol/; and between 9 and 79% for cut off levels <25 nmol/l (59).
Many studies have shown an association between Vit D deficiency and increased risk of GDM (59–66), while others did not show similar results (67). Furthermore, high serum 25(OH) D levels (>81 nmol/l) were found to be protective against GDM when compared to moderate-high Vit D levels (63–81 nmol/L, RR: 0.47; 95% CI: 0.23, 0.96) (68).
Vit D supplementation can improve glucose metabolism in women with GDM by lowering FBG, HbA1c, and serum insulin concentration (13). A meta-analysis showed that Vit D supplementation significantly reduced FBG (SMD =-1.87, 95% CI-3.39-0.35) and the incidence of GDM (OR = 0.42, 95% CI 0.30–0.60) in pregnant women. It also showed that Vit D supplementation significantly reduced FBG insulin levels, improved the HOMA-IR and HOMA-β, and increased the quantitative insulin sensitivity check index (QUICKI) in women with GDM (14). However, a multicentre European study (The DALI vitamin D randomized controlled trial for GDM prevention) showed no significant benefit from Vit D intervention besides improving vitamin D status (69). In this trial, pregnant women were randomized into eight groups; healthy eating (HE), physical activity (PA), HE & PA, HE& PA + Vit D3 1600 IU/day, HE& PA + placebo, Vit D3 1600 IU/day, and placebo. While 98% of the subjects enrolled on Vit D arms achieved a serum 25(OH)D ≥ 50 nmol/l, there was no reduction in the risk of GDM despite a small but significantly lower FPG (-0.14 mmol/l; CI95 −0.28, −0.00) compared to placebo (69).
Supplementation of Vit D in combination with other supplements have also been studied during pregnancy. Aside from improving insulin resistance markers in women with GDM, Vit D and evening primrose oil significantly reduced serum triglycerides, VLDL, total cholesterol, and LDL concentrations (70). Vitamin D-magnesium-zinc-calcium co-supplementation to women with GDM for 6 weeks was shown to reduce inflammation and oxidative stress biomarkers (CRP and plasma malondialdehyde concentrations) and to increase total antioxidant capacity levels compared to placebo. In addition, this study also found a decreasing trend in birth weight and the rate of macrosomia (3.3 vs. 16.7%, P = 0.08) (71).
A recent meta-analysis illustrated that vit D supplementation significantly reduced FBG and regulated HOMA-IR and that vit D supplementation was superior to omega-3 (-3.64 mg/dL, 95% CI:-5.77 to-1.51), zinc (-5.71 mg/dL, 95% CI: -10.19 to -1.23), probiotics (-6.76 mg/dL, 95% CI:-10.02 to -3.50), and placebo (-12.13 mg/dL, 95% CI:-14.55 to -9.70) for improving FBG (15).
Personalized variations in response to different treatments among different study populations are expected due to genetics and lifestyle factors, a concept that has been increasingly supported in this era of precision medicine (72). Given the evidence presented above, Vit D supplementation may be beneficial in preventing and treating GDM. Hence, assessing vitamin D status in early pregnancy may be clinically helpful for risk assessment and developing effective and personalized interventions for the prevention and treatment of GDM.
Myo-Inositol is the most abundant isomer of Inositol, a naturally occurring polyol sugar commonly found in cereals, beans, nuts, meat, legumes, and fresh citrus fruits. Myo-Inositol and Chiro-Inositol are inositol precursors, known as inositol phosphoglycans (IPGs) (73).
Myo-Inositol is a component of the membranes of all living cells and can be produced by the human body from D-glucose (74). It also plays a role in synthesizing lipids and occurs in its free form as a component of phospholipids or as phytic acid. It is one of the intracellular mediators of the insulin-signaling pathway and correlates with insulin sensitivity in T2DM (74). It is an insulin-sensitizing mediator, which is reported to reduce plasma glucose levels improve insulin sensitivity and ovulatory function in young women with PCOS (75). Myo-Inositol is needed for both production and activation of PI3 Kinase, essential for normal cell glucose metabolism (76). In patients with PCOS, Myo-Inositol restored glucose uptake, similar to Metformin, through an AMPK activation leading to an increase in GLUT-4 levels and glucose uptake by human endometrial cells (77). It has also been reported that conditions associated with insulin resistance are characterized by a high level of urinary inositol metabolites (78).
A prospective RCT included pregnant women with a parent who had T2DM but not those who were obese or had a history of PCOS, GDM, or pre-gestational diabetes. From the end of the first trimester, patients were randomly assigned to either 2 g Myo-Inositol and 200 mcg folic acid twice a day or 200 mcg folic acid twice a day. Myo-Inositol supplementation reduced the incidence of GDM (6 vs. 15.3 %, P = 0.04) and the incidence of macrosomia (79). When given at the end of the first trimester, Myo-Inositol also reduced the incidence of GDM (14 vs. 33.6%) in obese women (80). A Cochrane systematic review of four randomized controlled trials of 567 Italian women; most studies had small sample sizes, and two were open-label. The Cochrane review showed that Myo-Inositol was associated with a reduction in the incidence of GDM [risk ratio (RR) 0.43, 95% confidence interval (CI) 0.29 to 0.64]. However, there was no consensus on the neonatal outcomes (16). A more recent systematic review and meta-analysis of 5 RCTs showed that Myo-Inositol was associated with a significant reduction in the incidence of GDM and preterm delivery. However, Myo-Inositol supplementation had no impact on 2-h glucose of oral glucose tolerance test, gestational age at birth, birth weight, or macrosomia (17).
In conclusion, Myo-Inositol's potential benefit in improving insulin sensitivity suggests that it may help prevent GDM in obese, overweight women, women with PCOS or a family history of T2DM. However, large RCTs in different ethnic groups and more comprehensive risk factor profiles are required before Myo-Inositol could be recommended to prevent GDM.
Magnesium supplementation improves glucose metabolism in people with diabetes and improves insulin sensitivity parameters in those at high risk of diabetes (81). In addition, Magnesium supplementation for 6 weeks in women with GDM has been shown to have beneficial effects on the expression of inflammatory markers and genes related to insulin and lipid metabolism (82, 83). Ultimately, this might decrease metabolic complications in women with GDM. A recent meta-analysis demonstrated that omega-3, magnesium, Vit D, zinc, and probiotics improved FBG, serum insulin, and HOMA-IR compared to placebo. Magnesium supplementation was superior to other supplements in decreasing serum insulin (15).
Zinc and selenium are trace elements required for the activity of glutathione peroxidase and other antioxidant functions (84). The level of the two supplements is reported to be low during pregnancy (85). A meta-analysis showed that serum selenium concentration is significantly lower in women with GDM compared to normoglycemic women (86). On the other hand, there was no difference in the zinc status between women with and without GDM (87). Overall, there is insufficient evidence that zinc supplementation during pregnancy improves maternal or neonatal outcomes (88).
There is no evidence to support an association between iron supplementation and the risk of GDM. On the contrary, pregnant women with iron deficiency anemia are less likely to develop GDM, as suggested by a meta-analysis of six studies including over 15,000 women (OR 0.61; 95% CI 0.47–0.80; PA = 0.0003) (89). A meta-analysis by Kataria et al. (90) including over 30 studies investigating the association of iron biomarkers and dietary iron exposure with GDM, demonstrated high iron biomarkers associated with GDM. Due to the high heterogeneity of the analyses, these findings should be interpreted with caution. Overall, there is no conclusive evidence linking routine iron supplementation in non-anemic women to an increased risk of GDM.
Finally, because the studies were conducted in different populations with different ethnicities and socioeconomic backgrounds, it is vital to approach all evidence of these supplements' association with GDM with caution. Well-designed prospective studies are required to understand the dynamic relationship between these minerals and GDM risk.
The use of vitamin D, Myo-Inositol, Probiotics and Omega 3 fatty acids in pregnancy is associated with a reduction in systemic inflammation and lipolysis, improvement in Insulin resistance and Insulin signaling through direct or indirect mechanisms. Thus, the use of these supplements appears to improve glucose haemostasis in women with or without GDM, which is summarized in Figure 1. Indeed, a recent meta-analysis published in 2020 found that omega-3, magnesium, Vit D, zinc, and probiotics were more effective than placebo in improving FPG, serum insulin, and HOMA-IR. FPG was significantly reduced, and HOMA-IR was regulated by vitamin D supplementation. Magnesium supplementation was more effective than other nutrient supplements in lowering serum insulin levels (15).
A recent randomized controlled, double-blind trial investigated the effect of Myo-Inositol, probiotics, and multiple micronutrients on gestational normoglycemia and preterm birth during the preconception and antenatal period (91). There were no differences between the intervention and control groups in any glucose measurements during the OGTT, nor in the incidence of GDM, birth weight, or gestational age at birth. This trial found a reduction in the incidence of major postpartum hemorrhage independent of cesarean section rates, parity, or birth size, as well as a potential benefit of Myo-Inositol-containing supplements in reducing preterm birth (91). Multi-ethnic women from three continents participated in this study. However, certain ethnicities were underrepresented, with less than half of the participants being overweight or obese. It did not analyze data separately for each ethnicity and relied on sachet counts to assess adherence to Myo-Inositol supplementation.
The summary of the evidence for the different interventions comes from a very recent Cochrane database of systematic reviews (92). It demonstrated that there was unknown benefit or harm of the following interventions on the risk of GDM: dietary advice vs. standard care, a low glycaemic index diet vs. a moderate-high glycaemic index diet, probiotic with dietary intervention vs. placebo with dietary intervention, Vit D, and calcium supplementation vs. placebo, and exercise interventions vs. standard antenatal care. On the other hand, there was a possible benefit of combined diet and exercise interventions during pregnancy vs. routine care (RR 0.85, 95% CI 0.71 to 1.01). There was also possible evidence of benefit for Myo-Inositol and Vit D supplementation during pregnancy vs. control in reducing the risk of GDM (RR 0.43, 95% CI 0.29 to 0.64 and RR 0.51, 95% CI 0.27 to 0.97, respectively) (91). However, there was clear evidence of no effect for omega-3 fatty acid supplementation on GDM risk.
The high prevalence of GDM and the associated maternal and perinatal morbidity significantly strain current and future healthcare resource utilization. More importantly, GDM remains an important surrogate marker for developing T2DM in the future. As a result, pregnancy provides a unique and critical window of opportunity for interventions to reduce the long-term burden of diabetes. Antenatal supplementation with Myo-Inositol, vitamin D, and probiotics to prevent GDM is a relatively new and novel intervention. These readily available supplements as single agents or in combination are approved food supplements. Although the limited emerging evidence indicates that their use may be beneficial in reducing the incidence of GDM, further studies from different ethnic contexts and with differing risk factors are needed to assess its effects on maternal and neonatal outcomes. Given their availability as dietary supplements and their relatively low cost compared to traditional interventions for preventing GDM, exploring its potential role in reducing GDM is a much needed and timely study for high-risk populations. In the same context, a double-blind, randomized controlled trial is currently underway at Sidra Medicine, Doha, Qatar, to examine the effect of antenatal dietary Myo-Inositol supplementation on the incidence of gestational diabetes mellitus and fetal outcome (93). We believe the results from this study will contribute to the generalizable knowledge and direction of future research on GDM.
Before drawing meaningful conclusions about the effects of dietary supplements on maternal glycemia, we need to look at different subgroup populations, different nutritional supplement dose regimens, and the appropriate time for starting such supplements.
The assessment of longitudinal changes in Myo-Inositol levels and other nutritional supplements may help define potential pathways of effect and should be considered in the future when designing more definitive trials.
All authors have contributed to the literature review, writing the manuscript, and agreed to the published version.
MB was employed by Hamad Medical Corporation.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Damm P, Houshmand-Oeregaard A, Kelstrup L, Lauenborg J, Mathiesen ER, Clausen TD. Gestational diabetes mellitus and long-term consequences for mother and offspring: a view from Denmark. Diabetologia. (2016) 59:1396–9. doi: 10.1007/s00125-016-3985-5
2. Raghavan R, Dreibelbis C, Kingshipp BL, Wong YP, Abrams B, Gernand AD, et al. Dietary patterns before and during pregnancy and maternal outcomes: a systematic review. Am J Clin Nutr. (2019) 109:705S−28. doi: 10.1093/ajcn/nqy216
3. Asadi M, Shahzeidi M, Nadjarzadeh A, Hashemi Yusefabad H, Mansoori A. The relationship between pre-pregnancy dietary patterns adherence and risk of gestational diabetes mellitus in Iran: a case–control study. Nutr Diet. (2019) 76:597–603. doi: 10.1111/1747-0080.12514
4. Hrolfsdottir L, Gunnarsdottir I, Birgisdottir BE, Hreidarsdottir IT, Smarason AK, Hardardottir H, et al. Can a simple dietary screening in early pregnancy identify dietary habits associated with gestational diabetes? Nutrients. (2019) 11:1868. doi: 10.3390/nu11081868
5. Rasmussen L, Poulsen CW, Kampmann U, Smedegaard SB, Ovesen PG, Fuglsang J. Diet and healthy lifestyle in the management of gestational diabetes mellitus. Nutrients. (2020) 12:1–24. doi: 10.3390/nu12103050
6. Kimmons JE, Blanck HM, Tohill BC, Zhang J, Khan LK. Associations between body mass index and the prevalence of low micronutrient levels among US adults. MedGenMed. (2006) 8:59.
7. Kaidar-Person O, Person B, Szomstein S, Rosenthal RJ. Nutritional deficiencies in morbidly obese patients: a new form of malnutrition? Part B Miner Obes Surg. (2008) 18:1028–34. doi: 10.1007/s11695-007-9350-5
8. Chen Y, Yue R, Zhang B, Li Z, Shui J, Huang X. Effects of probiotics on blood glucose, biomarkers of inflammation and oxidative stress in pregnant women with gestational diabetes mellitus: a meta-analysis of randomized controlled trials. Med Clin. (2020) 154:199–206. doi: 10.1016/j.medcli.2019.05.041
9. Zhang J, Ma S, Wu S, Guo C, Long S, Tan H. Effects of probiotic supplement in pregnant women with gestational diabetes mellitus: a systematic review and meta-analysis of randomized controlled trials. J Diabetes Res. (2019) 2019. doi: 10.1155/2019/5364730
10. Taylor BL, Woodfall GE, Sheedy KE, O'Riley ML, Rainbow KA, Bramwell EL, et al. Effect of probiotics on metabolic outcomes in pregnant women with gestational diabetes: a systematic review and meta-analysis of randomized controlled trials. Nutrients. (2017) 9:461. doi: 10.3390/nu9050461
11. Amirani E, Asemi Z, Asbaghi O, Milajerdi A, Reiner Ž, Mansournia MA, et al. The effects of omega-3 fatty acids supplementation on metabolic status in pregnant women: a systematic review and meta-analysis of randomized controlled trials. J Diabetes Metab Disord. (2020) 19:1685–99. doi: 10.1007/s40200-020-00558-5
12. Middleton P, Gomersall J, Gould J, Shepherd E, Olsen S, Makrides M. Omega-3 fatty acid addition during pregnancy. Cochrane database Syst Rev. (2018) 11:1–421. doi: 10.1002/14651858.CD003402.pub3
13. Ojo O, Weldon SM, Thompson T, Vargo EJ. The effect of vitamin d supplementation on glycaemic control in women with gestational diabetes mellitus: a systematic review and meta-analysis of randomized controlled trials. Int J Environ Res Public Health. (2019) 16:1716. doi: 10.3390/ijerph16101716
14. Yin W, Jin D, Yao M, Yu W, Zhu P. [Effect of vitamin D supplementation on gestational diabetes mellitus:a meta-analysis]. Wei Sheng Yan Jiu. (2019) 48:811–21.
15. Jin S, Sha L, Dong J, Yi J, Liu Y, Guo Z, et al. Effects of nutritional strategies on glucose homeostasis in gestational diabetes mellitus: a systematic review and network meta-analysis. J Diabetes Res. (2020) 2020:6062478. doi: 10.1155/2020/6062478
16. Crawford TJ, Crowther CA, Alsweiler J, Brown J. Antenatal dietary supplementation with myo-inositol in women during pregnancy for preventing gestational diabetes. Cochrane Database Syst Rev. (2015) 2015:CD011507. doi: 10.1002/14651858.CD011507.pub2
17. Zhang H, Lv Y, Li Z, Sun L, Guo W. The efficacy of myo-inositol supplementation to prevent gestational diabetes onset: a meta-analysis of randomized controlled trials. J Matern Fetal Neonatal Med. (2019) 32:2249–55. doi: 10.1080/14767058.2018.1428303
18. Huttenhower C, Gevers D, Knight R, Abubucker S, Badger JH, Chinwalla AT, et al. Structure, function and diversity of the healthy human microbiome. Nature. (2012) 486:207–14. doi: 10.1038/nature11234
19. Gill SR, Pop M, DeBoy RT, Eckburg PB, Turnbaugh PJ, Samuel BS, et al. Metagenomic analysis of the human distal gut microbiome. Science. (2006) 312:1355–9. doi: 10.1126/science.1124234
20. Lynch S V, Pedersen O. The human intestinal microbiome in health and disease. N Engl J Med. (2016) 375:2369–79. doi: 10.1056/NEJMra1600266
21. Lloyd-Price J, Abu-Ali G, Huttenhower C. The healthy human microbiome. Genome Med. (2016) 8:51. doi: 10.1186/s13073-016-0307-y
22. Bäckhed F, Ley RE, Sonnenburg JL, Peterson DA, Gordon JI. Host-bacterial mutualism in the human intestine. Science. (2005) 307:1915–20. doi: 10.1126/science.1104816
23. Peterson J, Garges S, Giovanni M, McInnes P, Wang L, Schloss JA, et al. The NIH human microbiome project. Genome Res. (2009) 19:2317–23. doi: 10.1101/gr.096651.109
24. Medici Dualib P, Ogassavara J, Mattar R, Mariko Koga da Silva E, Atala Dib S, de Almeida Pititto B. Gut microbiota and gestational diabetes mellitus: a systematic review. Diabetes Res Clin Pract. (2021) 180:109078. doi: 10.1016/j.diabres.2021.109078
25. Seth A, Yan F, Polk D, Rao R. Probiotics ameliorate the hydrogen peroxide-induced epithelial barrier disruption by a PKC- and MAP kinase-dependent mechanism. Am J Physiol Gastrointest Liver Physiol. (2008) 294:G1060–9. doi: 10.1152/ajpgi.00202.2007
26. Jones S, Versalovic J. Probiotic Lactobacillus reuteri biofilms produce antimicrobial and anti-inflammatory factors. BMC Microbiol. (2009) 9:35. doi: 10.1186/1471-2180-9-35
27. Amabebe E, Anumba DOC. Female gut and genital tract microbiota-induced crosstalk and differential effects of short-chain fatty acids on immune sequelae. Front Immunol. (2020) 11:2184. doi: 10.3389/fimmu.2020.02184
28. Amabebe E, Robert FO, Agbalalah T, Orubu ESF. Microbial dysbiosis-induced obesity: role of gut microbiota in homoeostasis of energy metabolism. Br J Nutr. (2020) 123:1127–37. doi: 10.1017/S0007114520000380
29. Homayouni A, Bagheri N, Mohammad-Alizadeh-Charandabi S, Kashani N, Mobaraki-Asl N, Mirghafurvand M, et al. Prevention of gestational diabetes mellitus (GDM) and probiotics: mechanism of action: a review. Curr Diabetes Rev. (2019) 16:538–45. doi: 10.2174/1573399815666190712193828
30. VandeVusse L, Hanson L, Safdar N. Perinatal outcomes of prenatal probiotic and prebiotic administration: an integrative review. J Perinat Neonatal Nurs. (2013) 27:288–301. doi: 10.1097/JPN.0b013e3182a1e15d
31. Dallanora S, Medeiros de Souza Y, Deon R, Tracey C, Freitas-Vilela A, Wurdig Roesch L, et al. Do probiotics effectively ameliorate glycemic control during gestational diabetes? a systematic review. Arch Gynecol Obstet. (2018) 298:477–85. doi: 10.1007/s00404-018-4809-2
32. Callaway LK, McIntyre HD, Barrett HL, Foxcroft K, Tremellen A, Lingwood BE, et al. Probiotics for the prevention of gestational diabetes mellitus in overweight and obese women: findings from the SPRING double-blind randomized controlled trial. Diabetes Care. (2019) 42:364–71. doi: 10.2337/dc18-2248
33. Pellonperä O, Mokkala K, Houttu N, Vahlberg T, Koivuniemi E, Tertti K, et al. Efficacy of fish oil and/or probiotic intervention on the incidence of gestational diabetes mellitus in an at-risk group of overweight and obese women: a randomized, placebo-controlled, double-blind clinical trial. Diabetes Care. (2019) 42:1009–17. doi: 10.2337/dc18-2591
34. Yan F, Cao H, Cover T, Whitehead R, Washington M, Polk D. Soluble proteins produced by probiotic bacteria regulate intestinal epithelial cell survival and growth. Gastroenterology. (2007) 132:562–75. doi: 10.1053/j.gastro.2006.11.022
35. Preidis GA, Weizman AV, Kashyap PC, Morgan RL. AGA technical review on the role of probiotics in the management of gastrointestinal disorders. Gastroenterology. (2020) 159:708–38. doi: 10.1053/j.gastro.2020.05.060
36. Yanai H, Hamasaki H, Katsuyama H, Adachi H, Moriyama S, Sako A. Effects of intake of fish or fish oils on the development of diabetes. J Clin Med Res. (2015) 7:8–12. doi: 10.14740/jocmr1964w
37. Patel PS, Sharp SJ, Luben RN, Khaw KT, Bingham SA, Wareham NJ, et al. Association between type of dietary fish and seafood intake and the risk of incident type 2 diabetes. Diabetes Care. (2009) 32:1857–63. doi: 10.2337/dc09-0116
38. Pastorino S, Bishop T, Sharp SJ, Pearce M, Akbaraly T, Barbieri NB, et al. Heterogeneity of associations between total and types of fish intake and the incidence of type 2 diabetes: federated meta-analysis of 28 prospective studies including 956,122 participants. Nutrients. (2021) 13:1223. doi: 10.3390/nu13041223
39. Taylor CM, Emmett PM, Emond AM, Golding J. A review of guidance on fish consumption in pregnancy: is it fit for purpose? Public Health Nutr. (2018) 21:2149–59. doi: 10.1017/S1368980018000599
40. Itsiopoulos C, Marx W, Mayr HL, Tatucu-Babet OA, Dash SR, George ES, et al. The role of omega-3 polyunsaturated fatty acid supplementation in the management of type 2 diabetes mellitus: a narrative review. J Nutr Intermed Metab. (2018) 14:42–51. doi: 10.1016/j.jnim.2018.02.002
41. Cooper JA, Stevenson JL, Paton CM. Hunger and satiety responses to saturated fat-rich meals before and after a high PUFA diet. FASEB J. (2016) 30:405.7. doi: 10.1016/j.nut.2017.03.008
42. Fedor D, Kelley DS. Prevention of insulin resistance by n-3 polyunsaturated fatty acids. Curr Opin Clin Nutr Metab Care. (2009) 12:138–46. doi: 10.1097/MCO.0b013e3283218299
43. Herrera E, Ortega-Senovilla H. Implications of lipids in neonatal body weight and fat mass in gestational diabetic mothers and non-diabetic controls. Curr Diab Rep. (2018) 18:7. doi: 10.1007/s11892-018-0978-4
44. Cibickova L, Schovanek J, Karasek D. Changes in serum lipid levels during pregnancy in women with gestational diabetes. a narrative review. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. (2021) 165:8–12. doi: 10.5507/bp.2021.009
45. Toescu V, Nuttall SL, Martin U, Nightingale P, Kendall MJ, Brydon P, et al. Changes in plasma lipids and markers of oxidative stress in normal pregnancy and pregnancies complicated by diabetes. Clin Sci. (2004) 106:93–8. doi: 10.1042/CS20030175
46. Schaefer-Graf UM, Graf K, Kulbacka I, Kjos SL, Dudenhausen J, Vetter K, et al. Maternal lipids as strong determinants of fetal environment and growth in pregnancies with gestational diabetes mellitus. Diabetes Care. (2008) 31:1858–63. doi: 10.2337/dc08-0039
47. Simeonova-Krstevska S, Krstevska B, Velkoska-Nakova V, Hadji Lega M, Samardjiski I, Serafimoski V, et al. Effect of lipid parameters on foetal growth in gestational diabetes mellitus pregnancies. Pril. (2014) 35:131–6. doi: 10.2478/prilozi-2014-0017
48. Son GH, Kwon JY, Kim YH, Park YW. Maternal serum triglycerides as predictive factors for large-for-gestational age newborns in women with gestational diabetes mellitus. Acta Obstet Gynecol Scand. (2010) 89:700–4. doi: 10.3109/00016341003605677
49. Jamilian M, Samimi M, Ebrahimi FA, Hashemi T, Taghizadeh M, Razavi M, et al. The effects of vitamin D and omega-3 fatty acid co-supplementation on glycemic control and lipid concentrations in patients with gestational diabetes. J Clin Lipidol. (2017) 11:459–68. doi: 10.1016/j.jacl.2017.01.011
50. Contreras-Duarte S, Carvajal L, Garchitorena MJ, Subiabre M, Fuenzalida B, Cantin C, et al. Gestational diabetes mellitus treatment schemes modify maternal plasma cholesterol levels dependent to women's weight: possible impact on feto-placental vascular function. Nutrients. (2020) 12:506. doi: 10.3390/nu12020506
51. Bhatt DL, Steg PG, Miller M, Brinton EA, Jacobson TA, Ketchum SB, et al. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N Engl J Med. (2019) 380:11–22. doi: 10.1056/NEJMoa1812792
52. Samimi M, Jamilian M, Asemi Z, Esmaillzadeh A. Effects of omega-3 fatty acid supplementation on insulin metabolism and lipid profiles in gestational diabetes: Randomized, double-blind, placebo-controlled trial. Clin Nutr. (2015) 34:388–93. doi: 10.1016/j.clnu.2014.06.005
53. Jamilian M, Samimi M, Mirhosseini N, Ebrahimi FA, Aghadavod E, Taghizadeh M, et al. A randomized double-blinded, placebo-controlled trial investigating the effect of fish oil supplementation on gene expression related to insulin action, blood lipids, and inflammation in gestational diabetes mellitus-fish oil supplementation and gestation. Nutrients. (2018) 10:e163. doi: 10.3390/nu10020163
54. Jamilian M, Hashemi Dizaji S, Bahmani F, Taghizadeh M, Memarzadeh MR, Karamali M, et al. A randomized controlled clinical trial investigating the effects of omega-3 fatty acids and vitamin E co-supplementation on biomarkers of oxidative stress, inflammation and pregnancy outcomes in gestational diabetes. Can J Diabetes. (2017) 41:143–9. doi: 10.1016/j.jcjd.2016.09.004
55. Gao J, Huang T, Li J, Guo X, Xiao H, Gu J, et al. Beneficial effects of n-3 polyunsaturated fatty acids on offspring's pancreas of gestational diabetes rats. J Agric Food Chem. (2019) 67:13269–81. doi: 10.1021/acs.jafc.9b05739
56. Wei SQ, Qi HP, Zhong Cheng L, William D F. Maternal vitamin D status and adverse pregnancy outcomes: a systematic review and meta-analysis. J Matern Neonatal Med. (2013) 26:889–99. doi: 10.3109/14767058.2013.765849
57. Chakhtoura M, Rahme M, Chamoun N, El-Hajj Fuleihan G. Vitamin D in the Middle East and North Africa. Bone Reports. (2018) 8:135–46. doi: 10.1016/j.bonr.2018.03.004
58. Saraf R, Morton SMB, Camargo CA, Grant CC. Global summary of maternal and newborn vitamin D status – a systematic review. Matern Child Nutr. (2016) 12:647–68. doi: 10.1111/mcn.12210
59. Xia J, Song Y, Rawal S, Wu J, Hinkle SN, Tsai MY, et al. Vitamin D status during pregnancy and the risk of gestational diabetes mellitus: a longitudinal study in a multiracial cohort. Diabetes, Obes Metab. (2019) 21:1895–905. doi: 10.1111/dom.13748
60. Zhang M-X, Pan G-T, Guo J-F, Li B-Y, Qin L-Q, Zhang Z-L. Vitamin D deficiency increases the risk of gestational diabetes mellitus: a meta-analysis of observational studies. Nutrients. (2015) 7:8366–75. doi: 10.3390/nu7105398
61. Hu L, Zhang Y, Wang X, You L, Xu P, Cui X, et al. Maternal vitamin D status and risk of gestational diabetes: a meta-analysis. Cell Physiol Biochem. (2018) 45:291–300. doi: 10.1159/000486810
62. Zhang Y, Gong Y, Xue H, Xiong J, Cheng G. Vitamin D and gestational diabetes mellitus: a systematic review based on data free of Hawthorne effect. BJOG Int J Obstetr Gynaecol. (2018) 125:784–93. doi: 10.1111/1471-0528.15060
63. Lu M, Xu Y, Lv L, Zhang M. Association between vitamin D status and the risk of gestational diabetes mellitus: a meta-analysis. Arch Gynecol Obstetr. (2016) 293:959–66. doi: 10.1007/s00404-016-4010-4
64. Al-Ajlan A, Al-Musharaf S, Fouda MA, Krishnaswamy S, Wani K, Aljohani NJ, et al. Lower vitamin D levels in Saudi pregnant women are associated with higher risk of developing GDM. BMC Preg Childbirth. (2018) 18:86. doi: 10.1186/s12884-018-1723-3
65. Arnold DL, Enquobahrie DA, Qiu C, Huang J, Grote N, VanderStoep A, et al. Early pregnancy maternal vitamin d concentrations and risk of gestational diabetes mellitus. Paediatr Perinat Epidemiol. (2015) 29:200–10.doi: 10.1111/ppe.12182
66. Lacroix M, Battista M-C, Doyon M, Houde G, Ménard J, Ardilouze J-L, et al. Lower vitamin D levels at first trimester are associated with higher risk of developing gestational diabetes mellitus. Acta Diabetol. (2014) 51:609–16. doi: 10.1007/s00592-014-0564-4
67. Park S, Yoon HK, Ryu HM, Han YJ, Lee SW, Park BK, et al. Maternal vitamin D deficiency in early pregnancy is not associated with gestational diabetes mellitus development or pregnancy outcomes in Korean pregnant women in a prospective study. J Nutr Sci Vitaminol. (2014) 60:269–75. doi: 10.3177/jnsv.60.269
68. Wilson RL, Leviton AJ, Leemaqz SY, Anderson PH, Grieger JA, Grzeskowiak LE, et al. Vitamin D levels in an Australian and New Zealand cohort and the association with pregnancy outcome. BMC Preg Childbirth. (2018) 18:251. doi: 10.1186/s12884-018-1887-x
69. Corcoy R, Mendoza LC, Simmons D, Desoye G, Adelantado JM, Chico A, et al. The DALI vitamin D randomized controlled trial for gestational diabetes mellitus prevention: no major benefit shown besides vitamin D sufficiency. Clin Nutr. (2020) 39:976–84. doi: 10.1016/j.clnu.2019.04.006
70. Jamilian M, Karamali M, Taghizadeh M, Sharifi N, Jafari Z, Memarzadeh MR, et al. Vitamin D and evening primrose oil administration improve glycemia and lipid profiles in women with gestational diabetes. Lipids. (2016) 51:349–56. doi: 10.1007/s11745-016-4123-3
71. Jamilian M, Mirhosseini N, Eslahi M, Bahmani F, Shokrpour M, Chamani M, et al. The effects of magnesium-zinc-calcium-vitamin D co-supplementation on biomarkers of inflammation, oxidative stress and pregnancy outcomes in gestational diabetes. BMC Preg Childbirth. (2019) 19:107. doi: 10.1186/s12884-019-2258-y
72. Xie F, Chan J, Ma R. Precision medicine in diabetes prevention, classification and management. J Diabetes Investig. (2018) 9:998–1015. doi: 10.1111/jdi.12830
73. Caputo M, Bona E, Leone I, Samà MT, Nuzzo A, Ferrero A, et al. Inositols and metabolic disorders: from farm to bedside. J Tradit Complement Med. (2020) 10:252–9. doi: 10.1016/j.jtcme.2020.03.005
74. Larner J, Brautigan DL, Thorner MO. D-chiro-inositol glycans in insulin signaling and insulin resistance. Mol Med. (2010) 16:543–51. doi: 10.2119/molmed.2010.00107
75. Bizzarri M, Carlomagno G. Inositol: history of an effective therapy for polycystic ovary syndrome. Eur Rev Med Pharmacol Sci. (2014) 18:1896–903.
76. Croze ML, Soulage CO. Potential role and therapeutic interests of myo-inositol in metabolic diseases. Biochimie. (2013) 95:1811–27. doi: 10.1016/j.biochi.2013.05.011
77. Cabrera-Cruz H, Oróstica L, Plaza-Parrochia F, Torres-Pinto I, Romero C, Vega M. The insulin-sensitizing mechanism of myo-inositol is associated with AMPK activation and GLUT-4 expression in human endometrial cells exposed to a PCOS environment. Am J Physiol Endocrinol Metab. (2020) 318:E237–48. doi: 10.1152/ajpendo.00162.2019
78. Farren M, Daly N, McKeating A, Kinsley B, Turner MJ, Daly S. The prevention of gestational diabetes mellitus with antenatal oral inositol supplementation: a randomized controlled trial. Diabetes Care. (2017) 40:759–63. doi: 10.2337/dc16-2449
79. D'anna R, Scilipoti A, Giordano D, Caruso C, Cannata ML, Lieta Interdonato M, et al. myo-inositol supplementation and onset of gestational diabetes mellitus in pregnant women with a family history of type 2 diabetes a prospective, randomized, placebo-controlled study. Diabetes Care. (2013) 36:854–7. doi: 10.2337/dc12-1371
80. D'Anna R, Di Benedetto A, Scilipoti A, Santamaria A, Interdonato ML, Petrella E, et al. Myo-inositol supplementation for prevention of gestational diabetes in obese pregnant women: a randomized controlled trial. Obstet Gynecol. (2015) 126:310–5. doi: 10.1097/AOG.0000000000000958
81. Veronese N, Watutantrige-Fernando S, Luchini C, Solmi M, Sartore G, Sergi G, et al. Effect of magnesium supplementation on glucose metabolism in people with or at risk of diabetes: a systematic review and meta-analysis of double-blind randomized controlled trials. Eur J Clin Nutr. (2016) 70:1354–9. doi: 10.1038/ejcn.2016.154
82. Ahmadi S, Naderifar M, Samimi M, Mirhosseini N, Amirani E, Aghadavod E, et al. The effects of magnesium supplementation on gene expression related to inflammatory markers, vascular endothelial growth factor, and pregnancy outcomes in patients with gestational diabetes. Magnes Res. (2018) 31:131–42.doi: 10.1684/mrh.2019.0446
83. Jamilian M, Samimi M, Faraneh A, Aghadavod E, Hashemi Dizaji S, Chamani M, et al. Magnesium supplementation affects gene expression related to Insulin and lipid in patients with gestational diabetes. Magnes Res. (2017) 30:71–9. doi: 10.1684/mrh.2017.0425
84. Bo S, Lezo A, Menato G, Gallo M, Bardelli C, Signorile A, et al. Gestational hyperglycemia, zinc, selenium, and antioxidant vitamins. Nutrition. (2005) 21:186–91. doi: 10.1016/j.nut.2004.05.022
85. Iqbal S, Ali I, Rust P, Kundi M, Ekmekcioglu C. Selenium, zinc, and manganese status in pregnant women and its relation to maternal and child complications. Nutrients. (2020) 12:725. doi: 10.3390/nu12030725
86. Askari G, Iraj B, Salehi-Abargouei A, Fallah AA, Jafari T. The association between serum selenium and gestational diabetes mellitus: a systematic review and meta-analysis. J Trace Elem Med Biol. (2015) 29:195–201. doi: 10.1016/j.jtemb.2014.09.006
87. Wilson R, Grieger J, Bianco-Miotto T, Roberts C. Association between maternal zinc status, dietary zinc intake and pregnancy complications: a systematic review. Nutrients. (2016) 8:641. doi: 10.3390/nu8100641
88. Carducci B, Keats E, Bhutta Z. Zinc supplementation for improving pregnancy and infant outcome. Cochrane database Syst Rev. (2021) 3:CD000230. doi: 10.1002/14651858.CD000230.pub6
89. Tiongco RE, Arceo E, Clemente B, Pineda-Cortel MR. Association of maternal iron deficiency anemia with the risk of gestational diabetes mellitus: a meta-analysis. Arch Gynecol Obstet. (2019) 299:89–95. doi: 10.1007/s00404-018-4932-0
90. Kataria Y, Wu Y, Horskjær P de H, Mandrup-Poulsen T, Ellervik C. Iron status and gestational diabetes—a meta-analysis. Nutrients. (2018) 10:621. doi: 10.3390/nu10050621
91. Godfrey KM, Barton SJ, El-Heis S, Kenealy T, Nield H, Baker PN, et al. Myo-inositol, probiotics, and micronutrient supplementation from preconception for glycemia in pregnancy: nipper international multicenter double-blind randomized controlled trial. Diabetes Care. (2021) 44:1091–9. doi: 10.2337/figshare.13874705
92. Griffith RJ, Alsweiler J, Moore AE, Brown S, Middleton P, Shepherd E, et al. Interventions to prevent women from developing gestational diabetes mellitus: an overview of cochrane reviews. Cochrane Database Syst Rev. (2020) 2020:CD012394. doi: 10.1002/14651858.CD012394.pub3
93. Ibrahim I, Abdullahi H, Fagier Y, Ortashi O, Terrangera A, Okunoye G. Effect of antenatal dietary myo-inositol supplementation on the incidence of gestational diabetes mellitus and fetal outcome: protocol for a double-blind randomized controlled trial. BMJ Open. (2022) 12:e055314. doi: 10.1136/bmjopen-2021-055314
Keywords: pregnancy, gestational diabetes, Myo-Inositol, probiotics, vitamin D, fish oils, omega 3
Citation: Ibrahim I, Bashir M, Singh P, Al Khodor S and Abdullahi H (2022) The Impact of Nutritional Supplementation During Pregnancy on the Incidence of Gestational Diabetes and Glycaemia Control. Front. Nutr. 9:867099. doi: 10.3389/fnut.2022.867099
Received: 03 February 2022; Accepted: 18 March 2022;
Published: 08 April 2022.
Edited by:
Stefania Triunfo, University of Milan, ItalyReviewed by:
Annunziata Lapolla, University of Padua, ItalyCopyright © 2022 Ibrahim, Bashir, Singh, Al Khodor and Abdullahi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ibrahim Ibrahim, aWlicmFoaW0xQHNpZHJhLm9yZw==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.