- 1Fujian Provincial Key Laboratory of Environment Factors and Cancer, Department of Epidemiology and Health Statistics, School of Public Health, Fujian Medical University, Fuzhou, China
- 2Key Laboratory of Ministry of Education for Gastrointestinal Cancer, Fujian Key Laboratory of Tumor Microbiology, Fujian Medical University, Fujian, China
- 3Department of Oral and Maxillofacial Surgery, The First Affiliated Hospital of Fujian Medical University, Fujian, China
- 4Laboratory Center, School of Public Health, The Major Subject of Environment and Health of Fujian Key Universities, Fujian Medical University, Fujian, China
Objective: To investigate the association between dietary fatty acid (FA) patterns and the risk of oral cancer.
Method: A case-control study which included 446 patients with oral cancer and 448 controls subjects was conducted in Southeast China. A structured food frequency questionnaire was used to assess the dietary FA consumption before cancer diagnosis. FA patterns were identified using the principal component analysis, and the relationship between the dietary FA patterns and oral cancer was analyzed by logistic regression.
Results: General differences in FA intake were observed between the patient and control groups. The intakes of saturated FAs (SFAs) C14:0, C16:0, C18:0, and monounsaturated FA C18:1 were higher in the patient group than the control group (p < 0.001). Four FA patterns were derived by principal component analysis. The “SFA” pattern, “Polyunsaturated FA” pattern, “Monounsaturated FA” pattern, and “Medium- and long-chain FA” pattern, which could explain 75.7% of the variance of the dietary FA intake, were submitted to logistic regression analysis. A positive association was observed between the “SFA” pattern and oral cancer risk. Compared with the lowest quartile score, the OR of the highest quartile score was 3.71 (95%CI: 2.31, 5.94, Ptrend < 0.001) in the multivariate logistic regression model. No significant association was found among the other three patterns and oral cancer risk.
Conclusions: General differences in dietary FA intake were observed between patients with oral cancer and controls. A positive association between the “SFA” pattern and risk of oral cancer was observed after adjusting for potential confounders.
Introduction
Oral cancer is one of the foremost cancers in head and neck cancers with nearly 40,000 new cases recognized in China in 2015 (1). According to GLOBOCAN 2018, the incidence and mortality of oral cancer in China were 2.0/100,000 and 0.97/100,000, respectively, in 2018 (2). The recognized etiologic factors of oral cancer consist of smoking, drinking, oral hygiene, human papillomavirus (HPV), and betel quid consumption (3–9). In addition to the above-mentioned traditional risk factors, diet is also involved in the etiology of oral cancer (10–12). Additionally, the potential role of fatty acids (FAs) in tumorigenesis has got increased interest.
Fatty acids, including saturated FA (SFA), n-3 and n-6 polyunsaturated FA (PUFA), and trans fatty acid (TFA), have been reported to be associated with the risk of varied types of cancer such as prostate cancer (13, 14), pancreatic cancer (15, 16), colorectal cancer (17, 18), and lung cancer (19). However, reports about the association between FA and head and neck tumors, especially oral cancer, are rare.
Most of the previous studies have taken individual FAs as separate exposures. However, individual FAs were consumed together and tended to be correlated with each other and to be interactive or synergistic, partially due to shared food sources and metabolic pathways (20, 21). Because of the complexity of diet and the highly interrelated nature of dietary exposures, FA pattern analysis could instead offer a more comprehensive view of separate FAs and shed light on the biological interactions between different FAs and their relation with disease risk (22–24).
Due to the limited evidence of the role of FA in oral cancer, we performed a case-control study to explore the potential FA intake patterns in oral cancer and their role in the development of oral cancer.
Materials and Methods
Study Design and Population
In this case-control study from September, 2016, to July, 2020, 446 newly diagnosed patients with oral cancer and 448 control participants were recruited from the First Affiliated Hospital of Fujian Medical University in Fujian province, China. Cancers of the lip, oral cavities, and parotid corresponded to codes C00 to C07 according to the 10th revision of the International Classification of Diseases (ICD-10) (25) were referred to as oral cancer in this study. The inclusion criteria of the patients were as follows: (1) histologically confirmed primary oral cancer; (2) Chinese Han population and residence in Fujian Province; (3) age above 18 years old. Patients with second primary, recurrent, or metastasized cancer, and previous radiotherapy or chemotherapy were excluded. Control participants were recruited from the health examination center of the same hospital during the same period. Those with a history of cancer were excluded. Additionally, we excluded those with extreme daily caloric intake (>4,200 or <700 kcal/day for men; >3,500 or <500 kcal/day for women).
All participants provided signed informed consent. The study protocol was approved by the Institutional Review Board of Fujian Medical University (Approval number: 2011053; Approval date: March 10, 2011) and conducted following the ethical standards described in the Declaration of Helsinki.
Data Collection
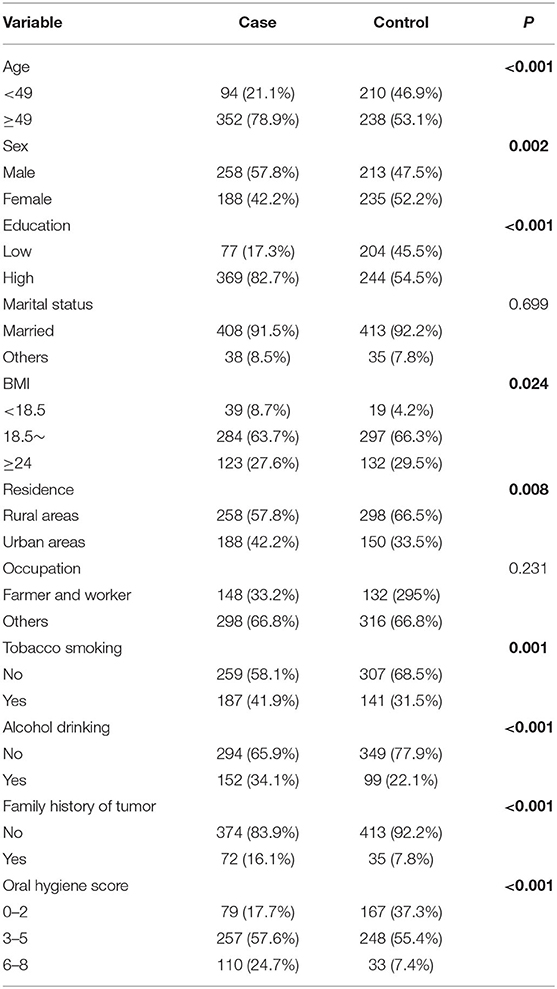
A structured questionnaire was used to collect information through face-to-face interviews conducted by well-trained interviewers. The questionnaire included socio-demographic characteristics (age, sex, education, marital status, residence, occupation, and family history of cancers) and lifestyle indicators (tobacco smoking, alcohol drinking, and oral hygiene). Subjects who had smoked at least 100 cigarettes during their lifetime were considered tobacco smokers. Alcohol drinker was defined as consuming at least one drink per week and lasting for more than 6 months continuously (26). A complete description of the oral hygiene score is available in our previous study (3). Oral hygiene score = teeth brushing + the number of missing teeth + wearing dentures + regular dental visits + recurrent dental ulceration. The range of oral hygiene score was 0–8, and a higher score indicated worse oral hygiene. Detailed coding information of variables included in the analysis was as follows: age (<49 years/≥49 years, based on the median of controls), sex (male/female), marital status (married/others), residence (rural areas/urban areas), occupation (farmer and worker/others), tobacco smoking (no/yes), alcohol drinking (no/yes), oral hygiene (0–2/3–5/6–8), and family history of cancer (no/yes). Educational was defined as low (lower vocational training or primary school), or high (secondary school and above) level groups. Height and weight were measured by the nurse of the hospital. The body mass index (BMI) was calculated as weight (in kilograms) divided by the square of the height (in meters) and was classified into three categories (<18.5/18.5–23.9/≥24).
A validated food frequency questionnaire (FFQ) (27) was utilized to collect the habitual dietary intake from each participant. The dietary intake of the year before the interview was collected. The dietary items were grouped into 8 broad categories (grains; beans and soy products; vegetables; fruits; animal food; algal fungi and nuts; beverages and soup; fried foods and pickled foods) and 17 sub-categories (grains; beans and soy products; dark vegetables; light color vegetables; purple vegetables; fresh beans; fruits; livestock; poultry; fish; processed meat; red meat; eggs; dairy; algal fungi and nuts; fried foods; pickled foods). For each food item or food group, participants were asked how frequently (daily, weekly, monthly, yearly, or never) they consumed the food or food group, which was followed by a question on the amount consumed in lians per unit of time. Lian is a unit of weight in China (1 lian = 50 g). The Chinese Food Composition Tables (28) were used to estimate the intake levels of macronutrients and FAs for participants.
Statistical Analysis
The intakes of energy and nutrients were log transformed and then FA intakes were adjusted for total energy intake using the residuals method (29). The quantitative data were presented as median with inter-quartile range, while the qualitative variables were presented as frequency (numbers and percentages). The chi-square test was used to compare the main characteristics between patients and controls. The Wilcoxon rank-sum test was used to analyze the distribution of dietary FAs. The Pearson correlation coefficients were calculated, and the hierarchical cluster tree and heatmap were generated to visualize the correlation between FAs (30). Hierarchical cluster analysis was performed using the Ward's method on correlation coefficient using the pheatmap package in R software.
Fatty acid patterns were derived by principal component analysis (PCA) using the intake of 32 FAs and PCs identified were referred to as FA patterns. The correlation pattern matrix from PCA was then used to calculate the scores of each pattern which were then categorized into quartiles, and the lowest quartiles were used as reference. The FA pattern score was evaluated categorically in the logistic regression model, and the ORs and their 95% CIs were calculated. Associations between FA pattern and intakes of 17 food groups and macronutrients were assessed by the Spearman correlation analysis. In addition, the restricted cubic spline (RCS) was used to plot and investigate the possible non-linear association between FA pattern and oral cancer risk.
All analyses were performed using the R software (version 4.0.3), with 2-tailed p-values <0.05 considered statistically significant.
Results
Characteristics of the Study Population
The distributions of the demographical characteristics and lifestyle factors are shown in Table 1. Compared with the patient group, the case group was characterized by a higher proportion of subjects with tobacco abuse, alcohol consumption, tumor history, and worse oral hygiene. In addition, the distribution of gender, education levels, BMI, and residence was significantly different between the patient and control groups (p < 0.05). General differences in FA intake were observed between the patient and control groups. The intake of saturated FAs C14:0, C16:0, C18:0, and monounsaturated FA C18:1 were higher in the patient group than the control group (p < 0.001). The distribution of dietary FAs between the case and control groups are shown in Supplementary Figure 1.
Identification of FA Patterns
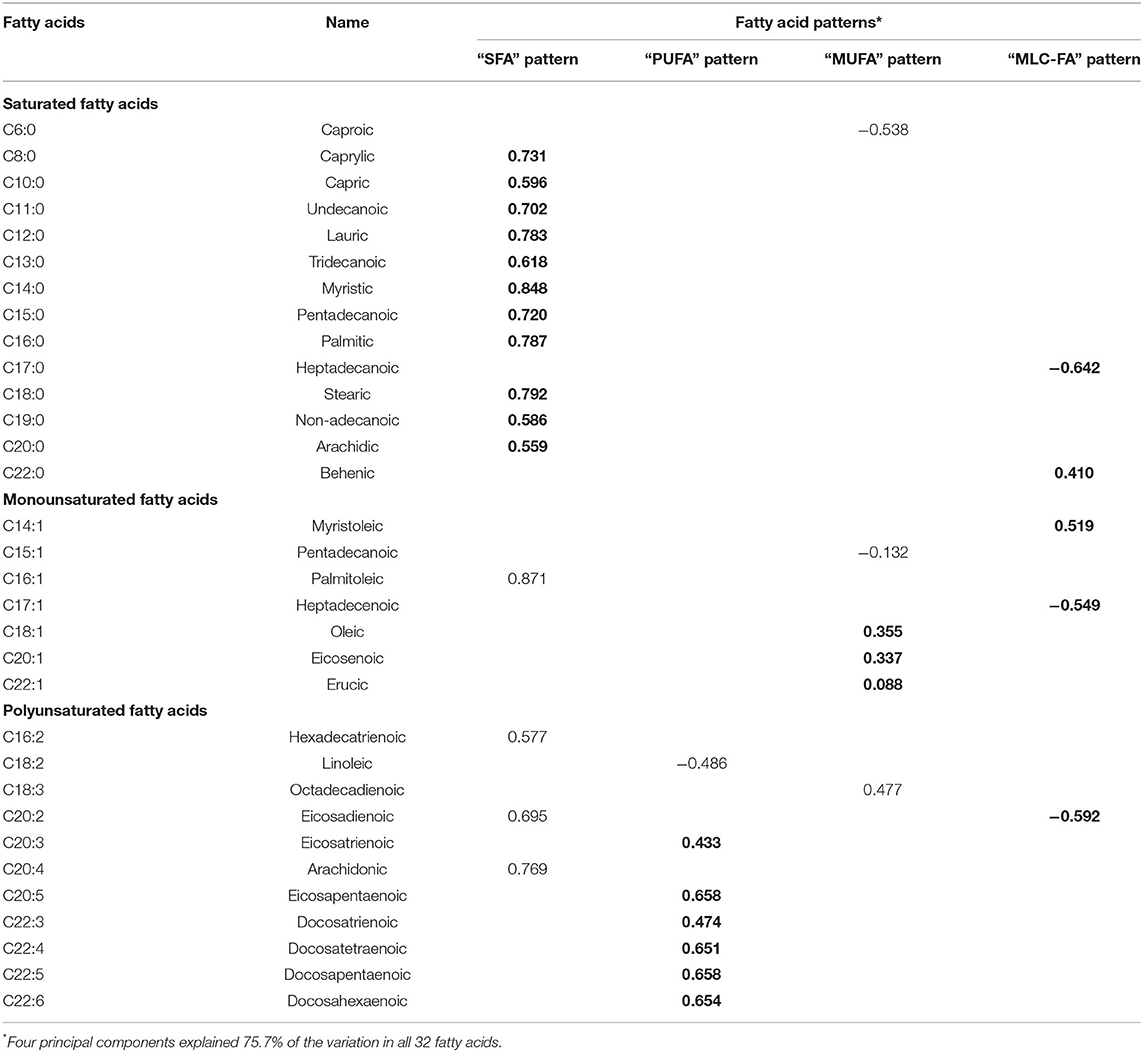
Four FA patterns were identified by applying PCA which could explain 75.7% of the variance of the dietary FA consumption, as the scree plot shown in Figure 1. Pattern 1 was characterized with saturated FA (the “SFA” pattern), which mainly included octanoic acid (C8:0), undecanoic acid (C11:0), lauric acid (C12:0), myristic acid (C14:0), and pentadecanoic acid (C15:0). Pattern 2 (the “PUFA” pattern) had high factor loading of eicosatrienoic acid (C20:3), eicosapentaenoic acid (C20:5), docosatrienoic acid (C22:3), docosatetraenoic acid (C22:4), docosapentaenoic acid (C22:5), and docosahexaenoic acid (C22:6). Pattern 3 (the “MUFA” pattern) was characterized with oleic acid (C18:1), eicosenoic acid (C20:1), and erucic acid (C22:1). Pattern 4 [the “medium- and long-chain FA (MLC-FA)” pattern] was dominated by heptadecanoic acid (C17:0), behenic acid (C20:0), myristoleic acid (C14:1), heptadecenoic acid (C17:1), and eicosadicnoic acid (C20:2). The factor loadings of individual FAs in the four FA patterns are shown in Table 2. Additionally, a correlation analysis among individual FAs was performed, and a heatmap was derived using correlation coefficients among individual FAs. A similar pattern was identified in the cluster analysis, as FAs adjacent in the tree had similar loading values (Figure 1).
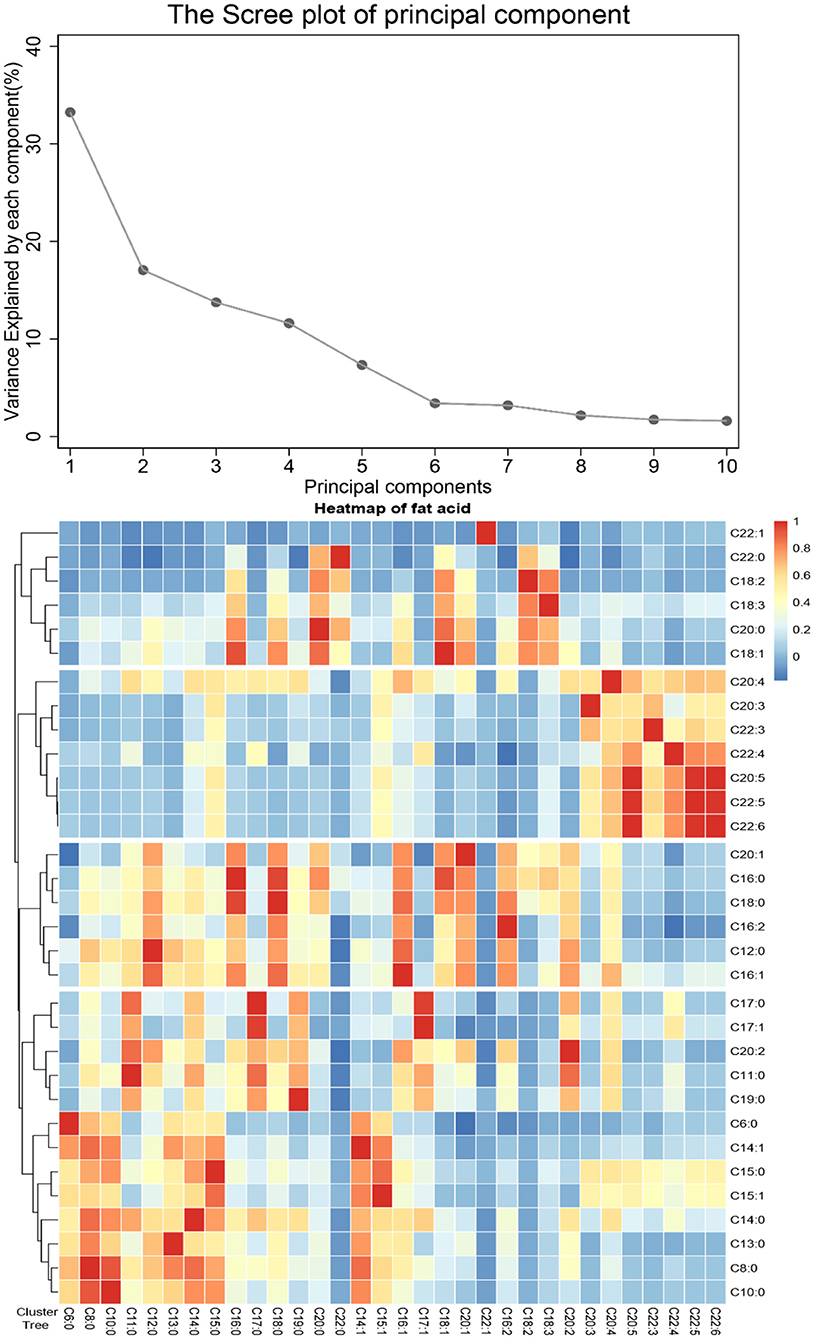
Figure 1. Principal components and clusters of 32 fatty acids. (A) The proportion of total variance of 32 fatty acids explained by each principal component. (B) Association among 32 fatty acids, the hierarchical cluster tree on the left, and the heatmap of fatty acid on the right.
Association Between FA Patterns and Oral Cancer Risk
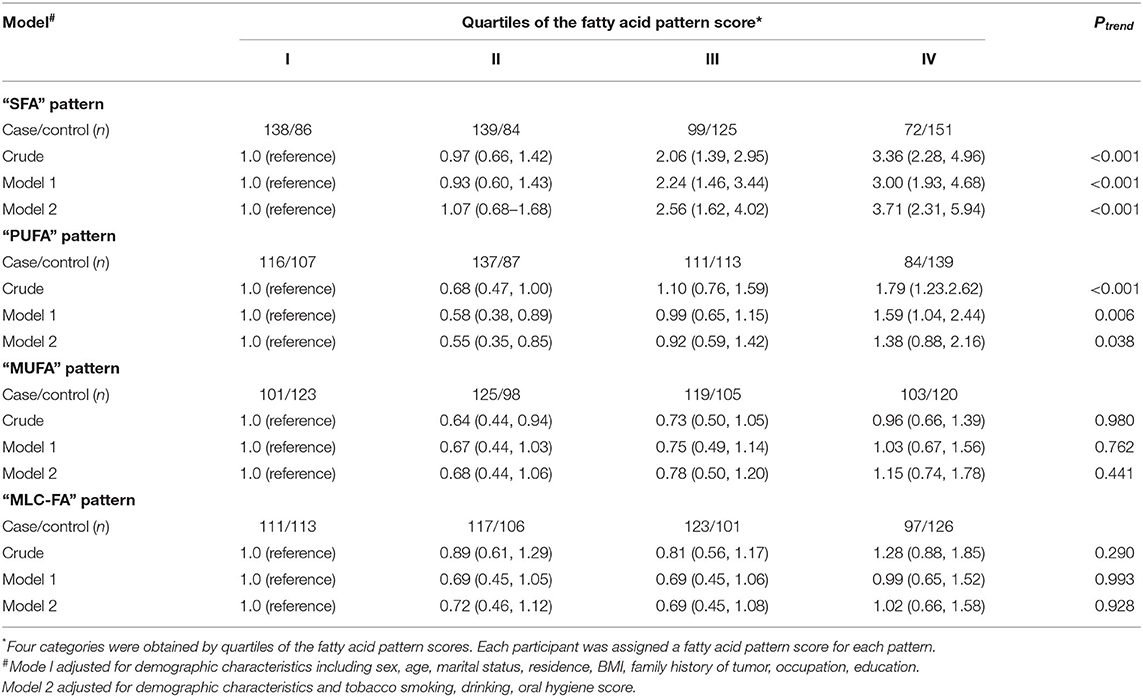
Crude and multivariable-adjusted OR and 95% CI for oral cancer across quartile categories of dietary FA pattern scores are shown in Table 3. A positive association between the “SFA” pattern and the risk of oral cancer was observed. In the crude model, those in the highest quartile of the “SFA” pattern had an increased risk of oral cancer compared with the lowest quartile, with a statistically significant linear trend (OR = 3.36; 95% CI: 2.28–4.96; Ptrend < 0.001). In model 1, after adjusting for sex, age, marital status, education levels, residence, BMI, occupation, and family history of tumor, the individuals in the highest quartile of the “SFA” pattern tended to have higher oral cancer risk (OR = 3.00; 95%CI: 1.93–4.68; Ptrend < 0.001) compared with those in the lowest quartile. In model 2, the result remained statistically significant after further adjustment for lifestyle factors, including tobacco smoking, alcohol drinking, and oral hygiene score (OR = 3.71; 95% CI: 2.31–5.94; Ptrend < 0.001). Compared with the lowest quartile, the ORs of the second quartile of the “PUFA” pattern were 0.58 (95% CI: 0.38–0.89) and 0.55 (95% CI: 0.35–0.85) in the crude model and model 2. Additionally, the ORs of the highest quartile of the “PUFA” pattern were 1.79 (95% CI: 1.23–2.62) and 1.59 (95% CI: 1.04–2.44) compared to the lowest quartile in the crude model and model 1. However, the result showed no statistical significance after further adjustment in model 3 (OR = 1.38; 95% CI: 0.88–2.16). Neither the “MUFA” nor the “MLC-FA” pattern was observed to be associated with oral cancer in all the three models (P > 0.05).
Additionally, we evaluated the correlations between the “SFA” pattern with intakes of macronutrients and food groups, the result of which is shown in Supplement Table 1. The “SFA” pattern was positively associated with the intake of protein, total fat (r = 0.207, 0.368, respectively, all p < 0.001), but negatively related to fiber (r = −0.185, P < 0.001). As for food groups, the “SFA” pattern was positively correlated with the intakes of fish, eggs, dairy, and red meat (r = 0.372, 0.320, 0.283, 0.282, respectively, all p < 0.05), but negatively correlated with grain and vegetables (r = −0.403, −0.100, respectively, all p < 0.05).
Furthermore, we visualized the association between the “SFA” pattern score and the risk of oral cancer using restricted cubic splines. Generally, the risk of oral cancer increased with the increase of the “SFA” pattern score and there is no evidence of non-linear association between the score and oral cancer risk (Pnon−linearity = 0.097). However, the risk of oral cancer was relatively flat until around −0.68 of the “SFA” pattern scores and then started to increase rapidly afterward (Figure 2).
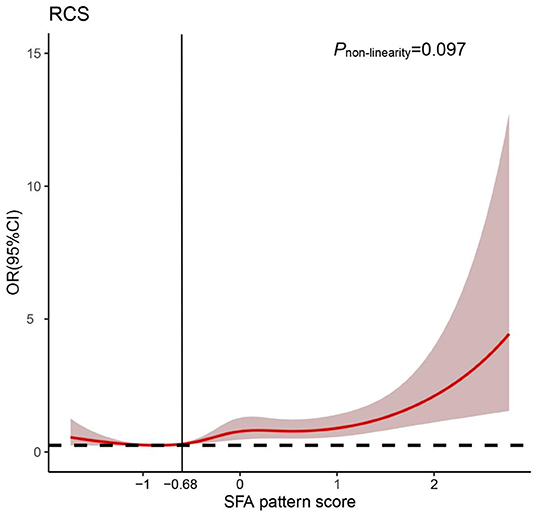
Figure 2. Odds ratio (OR) based on saturated fatty acid “(SFA) pattern” score and restricted cubic spline (Pnon−linearity = 0.097). OR was adjusted for the same variates as in model 2.
Association Between “SFA” Pattern and Oral Cancer Risk by Stratification Analysis
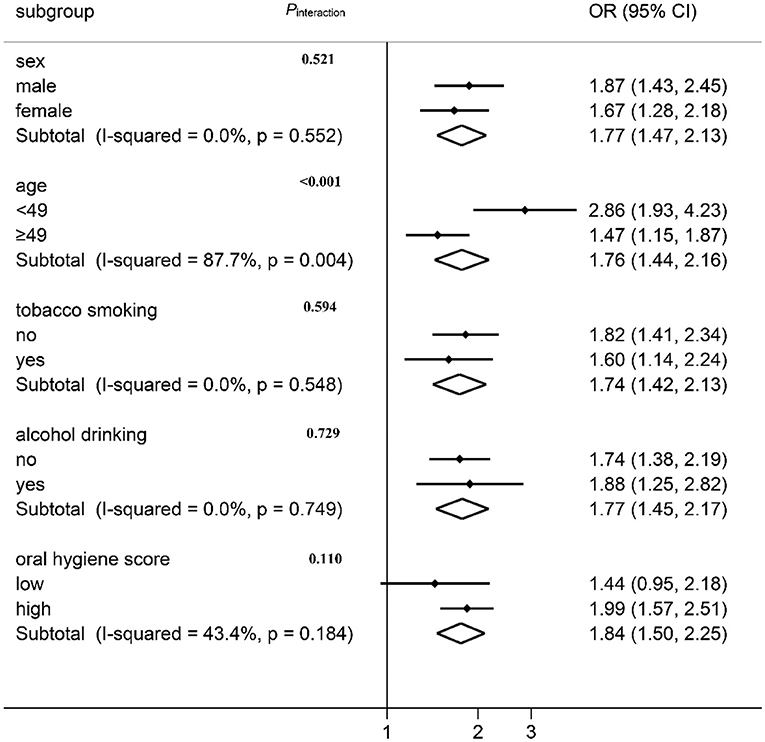
The association between the “SFA” pattern and oral cancer risk was stratified by the demographic characteristics and lifestyle factors, the result of which is shown in Figure 3. A positive association between oral cancer risk and the “SFA” pattern was observed in all subgroups except for the lower oral hygiene score group. No effect modification was observed by sex, tobacco smoking, alcohol drinking, or oral hygiene score (Pheterogeneity > 0.05). The association varied across different age groups (Figure 3; I2 = 87.8%, Pheterogeneity = 0.004). The interaction was further tested by multiplying the variates of “SFA” pattern score with age in the logistic regression model, and a multiplicative interaction was observed (Pinteraction < 0.001).
Discussion
In this case-control study conducted in Southeast China, we observed that the intake of FAs varied between patients with oral cancer and healthy controls. Four FA patterns, the “SFA” pattern, “PUFA” pattern, “MUFA” pattern, and “MLC-FA” pattern, were derived by PCA. The “SFA” pattern was found to be positively associated with oral cancer risk while no statistically significant association was found between the other three patterns and disease risk.
Dietary FAs, especially saturated FAs, have been hypothesized to increase cancer risk. Kim et al. performed a cross-sectional study, in which the results showed that the risk of colorectal cancer increased with higher SFA intake in Korean adults (31). Several epidemiological studies discovered that increased consumption of SFA correlated with increased odds of prostate cancer and may also be directly associated with the risk of biochemical recurrence and cancer progression (30, 32, 33). However, there is also evidence supporting that dietary SFA is not associated with cancer risk or even negatively associated with cancer risk. Cao et al. performed a meta-analysis of prospective cohort studies, in which the results showed that the highest vs. lowest levels of dietary SFA were not associated with the risk of breast cancer (34). A meta-analysis of prospective cohort research shows a null association between the SFA intake and colon cancer risk (35). No associations were observed in the Nurses' Health Study cohort of dietary SFAs and epithelial ovarian cancer risk (36). In the European Prospective Investigation into Cancer and Nutrition (EPIC), Aglago et al. (17) found an inverse association between dietary total SFA and colorectal cancer. To the best of our knowledge, reports of the association between dietary SFA and oral cancer are rare. A FA pattern characterized by SFA was identified in our study and was found to be positively associated with oral cancer risk. The inconsistent findings across studies may be partly due to differences in the type of cancer, study design and population, sample size, and varied measuring of dietary intake and confounding elimination.
The mechanism concerning dietary SFA and risk of cancer had also been widely discussed. It was reported that SFA intake influenced the risk of oral cancer through several mechanisms including chronic inflammation, insulin resistance, and fatty acylation, which were all related to carcinogenesis. Firstly, dietary SFA, particularly lauric acid and palmitic acid, were capable of stimulating inflammatory response through the toll-like receptors 4 (TLR4) (37), which could be exacerbated by the production of reactive oxygen species (ROS) in vivo (38). Inflammation was a key cause of the development and progression of many chronic diseases, including cancer (39). Moreover, inflammatory cytokines such as tumor necrosis factor (TNF)-a, induced by SFA, may influence insulin sensitivity (40), which favored the establishment of a pro-tumorigenic environment (41). Fatty acylation was another potential carcinogenic mechanism of SFA. It was shown that an SFA-rich diet could lead to an increase of myristoylated Src kinase and Src-mediated oncogenic signaling which accelerated tumor progression (42).
Dietary intake of SFAs consists of both animal and plant origins. The association between dietary FAs and cancer risk may depend on types and food sources of FAs (43, 44). The “SFA” pattern identified in this study was verified by performing a correlation analysis between the “SFA” pattern score and intakes of nutrients and food groups. It was found that the intake of red meat and dairy products was significantly higher in individuals with higher “SFA” pattern scores, which was consistent with previous studies about relation between varied food components and oral cancer. A study from Italy suggested that animal-derived foods such as dairy products and red meat could increase the risk of oral cancer (45). Epidemiological evidence from Greece also indicated that meat products were positively associated with the risk of oral cancer (46). However, we did not observe significant food components of plant origin that were related to SFA intake. So, it was unclear whether the association between the “SFA” pattern and oral cancer was partially attributed to the origin of SFA intake. This remains unclear for now and warrants investigation.
Additionally, in stratification analysis, we found that the association between the “SFA” pattern and oral cancer risk varied with age. The “SFA” pattern was positively associated with oral cancer risk in both age groups, but the association was more significant in the age group younger than 49 years. Compared with MUFA and PUFA, SFA is more likely to come from red meat, processed meat, and dairy products. Red meat is a primary source of total SFA, which has been identified as a dietary risk factor closely associated with various cancers (47, 48). In addition to red meat, excessive intake of dairy products could also contribute to cancer risks (49). Therefore, the origin of SFAs may modulate the effect of SFAs on oral cancer risk. Actually, in this study, we found that the “SFA” pattern was more strongly associated with dairy products in the younger-age group than the older-age group (Supplement Table 2). The results indicate that younger-age groups may consume more saturated FAs from dairy products, such as cakes, cheese, and ice cream bars, which may be positively associated with the risk of oral cancer.
There were several limitations in this study. Firstly, the selection of controls was not well-matched with the case, which resulted in distribution differences between the case and control groups in characteristics such as sex, age, and education. This could imply a selection bias, even when these variables were adjusted in the models. Secondly, recall bias and measurement error in dietary assessment using FFQ could be hardly avoided in a case-control study. Lastly, this was a single-center study and the sample size was limited. A prospective study with a large-scale sample size is needed to verify the current findings
Conclusion
In conclusion, the study provides support for a possible positive relationship between the “SFA” pattern and the risk of oral cancer. In addition, potential interactions were found between “SFA” pattern and age in oral cancer risk. Our findings support previous findings that there is suggestive evidence of a link between dietary patterns with head and neck cancer, but go beyond this by highlighting the role of specific FA patterns in oral cancer susceptibility.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Materials, further inquiries can be directed to the corresponding author/s.
Ethics Statement
The studies involving human participants were reviewed and approved by the Institutional Review Board (IRB) of Fujian Medical University. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
YF, JW, QC, and FL conceptualized the original idea for the study and have been involved in data collection, data analysis, and manuscript drafting. YQ, LL, LP, and BS were involved in data and blood samples collection. SW, YWa, YL, YWe, and JQ carried out the initial analysis. FC and BH assisted with revisions. All authors have made substantial contributions to the conception and design of the study, read and approved the final manuscript, and agreed to be accountable for all aspects of the work.
Funding
This work was supported by the Fujian Natural Science Foundation Program (grant number: 2020J01639), Technology Development Fund from the Department of Education of Fujian Province (grant number: 2019L3006), and Fujian Provincial Health Technology Project (No. 2018-1-57).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors appreciate the patients with oral cancer and control participants who contributed to this study.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2022.864098/full#supplementary-material
References
1. Chen W, Zheng R, Baade P, Zhang S, Zeng H, Bray F, et al. Cancer statistics in China, 2015. CA Cancer J Clin. (2016) 66:115–32. doi: 10.3322/caac.21338
2. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2018) 68:394–424. doi: 10.3322/caac.21492
3. Chen F, He B, Yan L, Qiu Y, Lin L, Cai L. Influence of oral hygiene and its interaction with standard of education on the risk of oral cancer in women who neither smoked nor drank alcohol: a hospital-based, case-control study. Br J Oral Maxillofac Surg. (2017) 55:260–5. doi: 10.1016/j.bjoms.2016.11.316
4. Gormley M, Dudding T, Sanderson E, Martin R, Thomas S, Tyrrell J, et al. A multivariable Mendelian randomization analysis investigating smoking and alcohol consumption in oral and oropharyngeal cancer. Nat Commun. (2020) 11:6071–80. doi: 10.1038/s41467-020-19822-6
5. Hung L, Kung P, Lung C, Tsai M, Liu S, Chiu L. Assessment of the risk of oral cancer incidence in a high-risk population and establishment of a predictive model for oral cancer incidence using a population-based cohort in Taiwan. Int J Environ Res Public Health. (2020) 17:665–79. doi: 10.3390/ijerph17020665
6. Kadashetti V, Chaudhary M, Patil S, Gawande M, Shivakumar K, Patil S. Analysis of various risk factors affecting potentially malignant disorders and oral cancer patients of Central India. J Caner Res Ther. (2015) 11:280–6. doi: 10.4103/0973-1482.151417
7. Nair U, Bartsch H, Nair J. Alert for an epidemic of oral cancer due to use of the betel quid substitutes gutkha and pan masala: a review of agents and causative mechanisms. Mutagenesis. (2004) 19:251–62. doi: 10.1093/mutage/geh036
8. Radoi L, Paget-Bailly S, Cyr D, Papadopoulos A, Guida F, Schmaus A, et al. Tobacco smoking, alcohol drinking and risk of oral cavity cancer by subsite: results of a French population-based case-control study, the ICARE study. Eur J Cancer Prev. (2013) 22:268–76. doi: 10.1097/CEJ.0b013e3283592cce
9. Rietbergen M, Leemans C, Bloemena E, Heideman D, Braakhuis B, Hesselink A, et al. Increasing prevalence rates of HPV attributable oropharyngeal squamous cell carcinomas in the Netherlands as assessed by a validated test algorithm. Int J Cancer. (2013) 132:1565–71. doi: 10.1002/ijc.27821
10. Chen F, Yan L, Lin L, Liu F, Qiu Y, Wang J, et al. Dietary score and the risk of oral cancer: a case-control study in southeast China. Oncotarget. (2017)c 8:34610–16. doi: 10.18632/oncotarget.16659
11. Dalmartello M, Decarli A, Ferraroni M, Bravi F, Serraino D, Garavello W, et al. Dietary patterns and oral and pharyngeal cancer using latent class analysis. Int J Cancer. (2020) 147:719–27. doi: 10.1002/ijc.32769
12. Shivappa N, Hébert J, Rosato V, Garavello W, Serraino D, La Vecchia C. Inflammatory potential of diet and risk of oral and pharyngeal cancer in a large case-control study from Italy. Int J Cancer. (2017) 141:471–9. doi: 10.1002/ijc.30711
13. Dahm C, Gorst-Rasmussen A, Crowe F, Roswall N, Tjønneland A, Drogan D, et al. Fatty acid patterns and risk of prostate cancer in a case-control study nested within the European Prospective Investigation into Cancer and Nutrition. Am J Clin Nutr. (2012) 96:1354–61. doi: 10.3945/ajcn.112.034157
14. Perez-Cornago A, Huybrechts I, Appleby P, Schmidt J, Crowe F, Overvad K, et al. Intake of individual fatty acids and risk of prostate cancer in the European prospective investigation into cancer and nutrition. Int J Cancer. (2020) 146:44–57. doi: 10.1002/ijc.32233
15. Ghamarzad Shishavan N, Mohamadkhani A, Ghajarieh Sepanlou S, Masoudi S, Sharafkhah M, Poustchi H, et al. Circulating plasma fatty acids and risk of pancreatic cancer: results from the Golestan Cohort Study. Clin Nutr. (2021) 40:1897–904. doi: 10.1016/j.clnu.2020.09.002
16. Matejcic M, Lesueur F, Biessy C, Renault A, Mebirouk N, Yammine S, et al. Circulating plasma phospholipid fatty acids and risk of pancreatic cancer in a large European cohort. Int J Cancer. (2018) 143:2437–48. doi: 10.1002/ijc.31797
17. Aglago E, Murphy N, Huybrechts I, Nicolas G, Casagrande C, Fedirko V, et al. Dietary intake and plasma phospholipid concentrations of saturated, monounsaturated and trans fatty acids and colorectal cancer risk in the EPIC cohort. Int J Cancer. (2021) 149:865–82. doi: 10.1002/ijc.33615
18. Nguyen S, Li H, Yu D, Cai H, Gao J, Gao Y, et al. Dietary fatty acids and colorectal cancer risk in men: a report from the Shanghai Men's Health Study and a meta-analysis. Int J Cancer. (2021) 148:77–89. doi: 10.1002/ijc.33196
19. Luu H, Cai H, Murff H, Xiang Y, Cai Q, Li H, et al. A prospective study of dietary polyunsaturated fatty acids intake and lung cancer risk. Int J Cancer. (2018) 143:2225–37. doi: 10.1002/ijc.31608
20. Council N. Diet and Health: Implications for Reducing Chronic Disease Risk. Washington, DC: The National Academies Press (1989).
21. Hu F. Dietary pattern analysis: a new direction in nutritional epidemiology. Curr Opin Lipidol. (2002) 13:3–9. doi: 10.1097/00041433-200202000-00002
22. Edefonti V, Bravi F, La Vecchia C, Randi G, Ferraroni M, Garavello W, et al. Nutrient-based dietary patterns and the risk of oral and pharyngeal cancer. Oral Oncol. (2010) 46:343–8. doi: 10.1016/j.oraloncology.2009.11.017
23. Jacques P, Tucker K. Are dietary patterns useful for understanding the role of diet in chronic disease? Am J Clin Nutr. (2001) 73:1–2. doi: 10.1093/ajcn/73.1.1
24. Schulze M, Hoffmann K. Methodological approaches to study dietary patterns in relation to risk of coronary heart disease and stroke. Brit J Nutr. (2006) 95:860–9. doi: 10.1079/BJN20061731
25. World Health Organization. ICD-10: International Statistical Classification of Diseases and Related Health Problems: Tenth Revision. World Health Organization (2004).
26. Chen F, He B, Yan L, Liu F, Huang J, Hu Z, et al. Tea consumption and its interactions with tobacco smoking and alcohol drinking on oral cancer in southeast China. Eur J Clin Nutr. (2017) 71:481–5. doi: 10.1038/ejcn.2016.208
27. Villegas R, Yang G, Liu D, Xiang Y, Cai H, Zheng W, et al. Validity and reproducibility of the food-frequency questionnaire used in the Shanghai men's health study. Br J Nutr. (2007) 97:993–1000. doi: 10.1017/S0007114507669189
28. Jiang H, Zhang J, Du W, Su C, Zhang B, Wang H. Energy intake and energy contributions of macronutrients and major food sources among Chinese adults: CHNS 2015 and CNTCS 2015. Eur J Clin Nutr. (2021) 75:314–24. doi: 10.1038/s41430-020-0698-0
29. Willett W, Stampfer M. Total energy intake: implications for epidemiologic analyses. Am J Epidemiol. (1986) 124:17–27. doi: 10.1093/oxfordjournals.aje.a114366
30. Bassett J, Severi G, Hodge A, MacInnis R, Gibson R, Hopper J, et al. Plasma phospholipid fatty acids, dietary fatty acids and prostate cancer risk. Int J Cancer. (2013) 133:1882–91. doi: 10.1002/ijc.28203
31. Kim J, Oh S, Kim Y, Kwon H, Joh H, Lee J, et al. Association between dietary fat intake and colorectal adenoma in korean adults: a cross-sectional study. Medicine. (2017) 96:e5759–65. doi: 10.1097/MD.0000000000005759
32. Brasky T, Darke A, Song X, Tangen C, Goodman P, Thompson I, et al. Plasma phospholipid fatty acids and prostate cancer risk in the SELECT trial. J Natl Cancer Inst. (2013) 105:1132–41. doi: 10.1093/jnci/djt174
33. Liss M, Al-Bayati O, Gelfond J, Goros M, Ullevig S, DiGiovanni J. Higher baseline dietary fat and fatty acid intake is associated with increased risk of incident prostate cancer in the SABOR study. Prostate Cancer Prostatic Dis. (2019) 22:244–51. doi: 10.1038/s41391-018-0105-2
34. Cao Y, Hou L, Wang W. Dietary total fat and fatty acids intake, serum fatty acids and risk of breast cancer: a meta-analysis of prospective cohort studies. Int J Cancer. (2016) 138:1894–904. doi: 10.1002/ijc.29938
35. Kim M, Park KJN. Dietary fat intake and risk of colorectal cancer: a systematic review and meta-analysis of prospective studies. Int J Cancer. (2018) 10:1963–73. doi: 10.3390/nu10121963
36. Bertone ER, Rosner BA, Hunter DJ, Stampfer MJ, Speizer FE, Colditz GA, et al. Dietary fat intake and ovarian cancer in a cohort of US women. Am J Epidemiol. (2002) 156:22–31. doi: 10.1093/aje/kwf008
37. Hwang D, Kim J, Lee J. Mechanisms for the activation of Toll-like receptor 2/4 by saturated fatty acids and inhibition by docosahexaenoic acid. Eur J Pharmacol. (2016) 785:24–35. doi: 10.1016/j.ejphar.2016.04.024
38. Huang S, Rutkowsky J, Snodgrass R, Ono-Moore K, Schneider D, Newman J, et al. Saturated fatty acids activate TLR-mediated proinflammatory signaling pathways. J Lipid Res. (2012) 53:2002–13. doi: 10.1194/jlr.D029546
39. Aggarwal B, Shishodia S, Sandur S, Pandey M, Sethi G. Inflammation and cancer: how hot is the link? Biochem Pharmacol. (2006) 72:1605–21. doi: 10.1016/j.bcp.2006.06.029
40. Calder P. Functional roles of fatty acids and their effects on human health. JPEN J Parenter Enteral Nutr. (2015) 39:18–32S. doi: 10.1177/0148607115595980
41. Chiefari E, Mirabelli M, La Vignera S, Tanyolac S, Foti DP, Aversa A, et al. Insulin resistance and cancer: in search for a causal link. Int J Mol Sci. (2021) 22:11137–66. doi: 10.3390/ijms222011137
42. Kim S, Yang X, Li Q, Wu M, Costyn L, Beharry Z, et al. Myristoylation of Src kinase mediates Src-induced and high-fat diet-accelerated prostate tumor progression in mice. J Biol Chem. (2017) 292:18422–33. doi: 10.1074/jbc.M117.798827
43. Gerber M. Background review paper on total fat, fatty acid intake and cancers. Ann Nutr Metab. (2009) 55:140–61. doi: 10.1159/000229000
44. Thiebaut AC, Chajes V, Gerber M, Boutron-Ruault MC, Joulin V, Lenoir G, et al. Dietary intakes of omega-6 and omega-3 polyunsaturated fatty acids and the risk of breast cancer. Int J Cancer. (2009) 124:924–31. doi: 10.1002/ijc.23980
45. Barasch A, Litaker M. Nutrition and the risk of oral and pharyngeal cancer: the evidence for any association remains weak and clinical significance remains limited. J Evid Based Dent Pract. (2012) 12:263–4. doi: 10.1016/S1532-3382(12)70050-7
46. Petridou E, Zavras AI, Lefatzis D, Dessypris N, Laskaris G, Dokianakis G, et al. The role of diet and specific micronutrients in the etiology of oral carcinoma. Cancer. (2002) 94:2981–8. doi: 10.1002/cncr.10560
47. de Lorgeril M, Salen P. New insights into the health effects of dietary saturated and omega-6 and omega-3 polyunsaturated fatty acids. BMC Med. (2012) 10:50–53. doi: 10.1186/1741-7015-10-50
48. Pascual JV, Rafecas M, Canela MA, Boatella J, Bou R, Baucells MD, et al. Effect of increasing amounts of a linoleic-rich dietary fat on the fat composition of four pig breeds. Part I: backfat fatty acid evolution. Food Chem. (2006) 96:538–48. doi: 10.1016/j.foodchem.2005.02.042
Keywords: fatty acid pattern, saturated fatty acids, oral cancer, principal component analysis, case-control study
Citation: Fan Y, Qiu Y, Wang J, Chen Q, Wang S, Wang Y, Li Y, Weng Y, Qian J, Chen F, Wang J, Shi B, Pan L, Lin L, He B and Liu F (2022) Association Between Dietary Fatty Acid Pattern and Risk of Oral Cancer. Front. Nutr. 9:864098. doi: 10.3389/fnut.2022.864098
Received: 28 January 2022; Accepted: 30 March 2022;
Published: 16 May 2022.
Edited by:
Rafaela Rosário, University of Minho, PortugalReviewed by:
José María Huerta, Instituto de Salud Carlos III (ISCIII), SpainJavier Carballo, University of Vigo, Spain
Copyright © 2022 Fan, Qiu, Wang, Chen, Wang, Wang, Li, Weng, Qian, Chen, Wang, Shi, Pan, Lin, He and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Baochang He, aGJjNTE3JiN4MDAwNDA7MTYzLmNvbQ==; Fengqiong Liu, bGZxJiN4MDAwNDA7ZmptdS5lZHUuY24=
†These authors have contributed equally to this work
 Yi Fan1,2†
Yi Fan1,2† Yu Qiu
Yu Qiu Fa Chen
Fa Chen Bin Shi
Bin Shi Lizhen Pan
Lizhen Pan Lisong Lin
Lisong Lin Baochang He
Baochang He Fengqiong Liu
Fengqiong Liu