
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Nutr. , 23 March 2022
Sec. Food Chemistry
Volume 9 - 2022 | https://doi.org/10.3389/fnut.2022.862689
This article is part of the Research Topic Chemical and Biological Changes of Polyphenols Caused by Food Thermal Processing View all 7 articles
The aim of this study was to observe the effect of purple corn anthocyanin on the light-induced antioxidant activity, free radicals, volatile compounds, color parameters, and sensory properties of milk during storage. There were four groups: (1) negative control, no addition of anthocyanins + exposure to fluorescent light (NC); (2) positive control 1, no addition of anthocyanins + protected from fluorescent light (PC1); (3) positive control 2, the addition of 0.3% (w/v) anthocyanins + exposure to fluorescent light (PC2); and (4) the addition of 0.3% anthocyanins + protected from fluorescent light (AC). The results indicated that the concentration of antioxidant activity parameters in the NC group decreased during the entire storage period, whereas antioxidant activity parameters were unchanged except for the glutathione peroxidase (GSH-Px) in the AC group. Moreover, the NC group showed lower levels of 2,2-diphenyl-1-picrylhydrazyl (DPPH) scavenging activity and higher levels of superoxide anion and hydrogen peroxide than the other groups after 1 d of storage period. The extent of malondialdehyde accumulation and lipid peroxidation in the control groups were greater than that of the AC group. Twenty-two volatile compounds were determined in milk, which consisted of eight alcohols, three ketones, five aldehydes, two esters, and four hydrocarbons by headspace gas chromatography mass spectrometer analysis. Specifically, individual aldehydes, esters and hydrocarbons in the AC group remained at relatively stable values during storage relative to the other three groups. Stronger positive correlations were detected between several antioxidant activities (superoxide dismutase, GSH-Px) and DPPH scavenging activity as well as total ketones in milk. Adding of anthocyanin did not impact on the color values of L*, a* and b* in light-protected milk during the entire storage period. Some sensory evaluation parameters (flat, garlic/onion/weedy, oxidized-light, oxidized-metal, rancid) in AC group were significantly higher than that of the control group at the end of the period. In conclusion, the current study revealed that the addition of purple corn anthocyanin pigment to light-protected milk had the potential to prevent lipid oxidation, enhance antioxidant activity, maintain volatile compounds and increase the sensory scores.
Milk is a wholesome, natural food with high nutrition and easy absorption and is popular among people. The unsaturated fatty acids (UFAs) in milk could strengthen the human immune system, playing important roles in body health (1). However, milk is prone to oxidation influenced by promoting oxidation components (such as flavor, metal ions, and oxidative enzymes) during the storage period (2). Indeed, lipid oxidation has been a major contributor to milk flavor defects, and oxygen (O2), metal ions, heat, and light are the main factors that affect lipid oxidation in the milk system because they contribute to the formation of several lipid radicals, lipid hydroperoxides, and volatile compounds (3).
Specifically, volatile compounds play an important role in the acceptability and quality of milk. Studying volatile components would help to better understand the change of milk. The volatile compounds in cow milk was analyzed by Toso et al. (4), and forty-one volatile compounds were identified, including eight ketones, nine aldehydes, eight alcohols, six hydrocarbons, three sulfur compounds, four esters, and three terpenes. Many volatile compounds, such as alcohols, esters, and hydrocarbons, are produced by the oxidation of UFAs in milk (5). There were two pathways for oxidation in milk: (1) dependent upon the superoxide radical, which can be generated by enzymes such as xanthine oxidase and lactoperoxidase; and (2) dependent upon hydroxyl radical and added copper (6). Hence, the antioxidant activity of milk has practical value for the food industry, especially for food formulations. Milk antioxidants can function by removing the formation of radicals or scavenging FR to prevent milk oxidation, improving milk antioxidant activity (7). In addition, natural antioxidants are usually added to milk as FR scavengers. For example, Pihlanto (8) demonstrated that natural antioxidants could enhance antioxidant activity and prevent the oxidation reaction in milk.
The natural active polyphenol compounds are responsible for the total antioxidant potential of many fruits and other purple materials (9). Anthocyanins are a source of secondary metabolites of plants that are effective natural antioxidant and free radical (FR) scavengers, have various kinds of significant physiological functions for consumers, and have broad prospects for development and application (10). Therefore, anthocyanins can improve the activities of antioxidant enzymes, such as superoxide dismutase (SOD), glutathione peroxidase (GSH-Px), and catalase (CAT), to further inhibit FR in milk. Indeed, anthocyanins have been reported to show strong antioxidant potential to protect against lipid oxidation in milk during the storage period (11). Silva et al. (12) reported that anthocyanin-rich Isabel grapes positively influenced the quality characteristics of goat milk yogurt during refrigerated storage.
Milk was suggested to reduce oral bioavailability of anthocyanins, whereas it could be absorbed by humans to improve people's health. However, milk is susceptible to light-induced oxidation because of the photosensitizer riboflavin and lipids, and they are the primary targets for photooxidation. Light-induced oxidation results in lipids quickly producing FR or the very reactive singlet O2, affecting the sensorial product quality and degrading valuable nutrients into oxidation products (13). We hypothesized that the addition of purple corn anthocyanin pigment (PCP) could prevent lipid oxidation, improving the antioxidant activity of light-protected milk during the storage period. In this regard, to provide a deep understanding of anthocyanins as radical scavengers for milk, the current study investigates the effect of anthocyanin pigments of purple corn on antioxidant activity, oxidative stability, volatile compounds, color parameters and sensory evaluation in milk during storage and light prevention.
The raw cow milk samples were collected from Guizhou University Farm (Guiyang, China). The milk was immediately mixed together, placed into a plastic bucket with an ice pack and transported to the laboratory. The addition of anthocyanin levels and methods were performed according to Güneşer (14) and Serafini et al. (15) with minor modifications. Briefly, all milk samples were prepared by the addition of PCP (0 and 0.3%, respectively) and blended vigorously with a blender (TMHL-200CL, Tianjin Taist Instrument Co., Ltd., Tianjin, China) for 30 s. In addition, high pasteurization (75°C for 15 s) could ensure milk hygienization while producing smaller losses in antioxidant potential (16) and could maintain the PCP concentration (17). Hence, milk samples were pasteurized at 75°C for 15 s using a minipasteurizer (Shandong Zolanbo Electrical Equipment Co., Ltd., Shandong, China). The four groups were as follows: (1) negative control, no addition of anthocyanins + exposure to fluorescent light (NC); (2) positive control 1, no addition of anthocyanins + protected from fluorescent light (PC1); (3) positive control 2, the addition of 0.3% (w/v) anthocyanins + exposure to fluorescent light (PC2); and (4) the addition of 0.3% anthocyanins + protected from fluorescent light (AC). The NC and PC1 groups were placed under fluorescent light bulbs (20 cm; 23 W, E27 crew-type, Philips, Amsterdam, the Netherlands), and the PC2 and AC groups were placed under the same conditions, but they were obtained by covering sample bottles with aluminum foil. Light exposure was regulated at an intensity of 1,100 to 1,300 lx throughout the experimental period (18). All samples were placed into 150-mL plastic tubes (Nanjing Metasequoia Technology Co., Ltd., Nanjing, China) within 2 h and kept at 4°C cooler for periods of 0, 1, 3, and 7 d, respectively. There were six replicates of each sampling time point. Milk has been properly stored by freezing at −80°C until the analysis of antioxidant activity, free radicals, volatile compounds, color measurement and sensory evaluations were conducted according to the reference of Smith et al. (19).
The PCP material was purchased from Nanjing Herd Source Biotechnology Co., Ltd., Nanjing, China. The anthocyanin composition of PCP was determined by High performance liquid chromatography-mass spectrometer (MS)/MS according to the method of Tian et al. (10). The PCP had 2,619 μg/g total anthocyanins.
Fresh milk was collected and mixed thoroughly, and the pH value of milk was detected immediately using a portable pH meter (pH 818, smart sensor, Guangdong, China). The pH meter was calibrated using standard pH solutions (pH values of 4.0, 6.8, and 9.18) before measurement. Dry matter (DM) was determined by the method of Güneşer (14). The milk compositions of protein, fat, lactose, total solids (TS), and solids-not-fat (SNF) were measured by a MilkoScan analyzer (MilkoScanTM FT2, FOSS, Hillerod, Denmark). The results were as followed: pH was 6.52, DM was 12.24%, protein was 3.21%, fat was 3.42%, lactose was 4.62%, TS was 12.74%, and SNF was 9.32%.
The milk sample was thawed and centrifuged at 10,000 × g for 30 min at 4°C (Gallop Technology Co., Ltd., Dongguan, Guangdong, China) and the supernatant was collected and mixed with 4% acetic acid before centrifugation at 10,000 × g for 30 min at 4°C. The supernatant was immediately transferred to a 1.5-mL tube and stored at −80°C until further analysis for antioxidant activity, free radical, and lipid peroxidation parameters (20). The antioxidant activity parameters of total antioxidant capacity (TAC), SOD, GSH-Px, oxidized glutathione (GSSG), reduced glutathione (GSH), and CAT; FR parameters of superoxide anion (), and hydrogen peroxide (H2O2); and lipid oxidation parameters of malondialdehyde (MDA), lipid peroxidation (LPO) were determined using commercially available kits from Nanjing Jiangcheng Bioengineering Institute (Nanjing, China; product codes were A015, A001-1, A005, A061-2, A006-1, A007-1, A003-1, A052, A018, A064-1, and A106-1, respectively) (20, 21). All measurement operation procedures strictly followed the manufacturers' protocol. Furthermore, 200 μL of final reaction solution was moved to a 96-well plate (TCP011096, JET-BIOFIL®, Beiden Biological Technology Co. Ltd., Nanjing, China) and was assayed via a microplate reader (Epoch, BioTek, Luzern, Switzerland).
The 2,2-diphenyl-1-picrylhydrazyl (DPPH; Pcode: 101845869, Sigma–Aldrich, St. Louis, MO, USA) scavenging activity was determined according to Tian et al. (22). Briefly, an aliquot of 0.35 mL of the milk sample was mixed with 1 mL of 0.1 mmol/L DPPH solution in a 1.5 mL tube. The mixture was centrifuged at 3,000 × g for 10 min at 4°C, and then 200 μL of supernatant was immediately transferred to a 96-well plate and incubated in the dark at room temperature for 30 min. The absorbance was analyzed at 517 nm via a microplate reader. The equation for calculation was as follows: DPPH scavenging activity (%) = (Ac – As) × 100/Ac, where Ac is the absorbance of the control and As is the absorbance of the milk sample.
The individual volatile compounds in milk were identified according to the method of Tian et al. (23) with a minor modification. Briefly, the gas chromatography (GC; Agilent Technologies, Santa Clara, CA, USA) conditions were: column was a fused silica capillary column (Agilent 19091S-436HP-5MS; 60 m ×250 μm ×0.25 μm); initial temperature was 40°C (kept for 2 min), warming up to 180°C (3.5°C/min), and to 310°C (10°C/min); carrier gas was He gas, and carrier gas flow rate was 1.0 mL/min; the total run time was 55 min. The MS (SCIEX-6500Qtrap; AB Allen-Bradley, Milwaukee, WI, USA) conditions were: the ion source was EI ionization, the ionization temperature was 230°C, the quadrupole temperature was 150°C, the ionization energy was 70 eV, the emission current was 34.6 μA, the multiplier voltage was 1,847 V, the interface temperature was 28°C, and the mass range was 29~500 Da. The volatile compounds were analyzed by GC/MS and run in a manual injector with 2 cm-50/30 μm DVB/CAR/PDMS StableFlex of the fiber tip. The qualitative identification of volatile compounds was noted by the standard mass spectra in NIST17 and Wiley275 libraries. The percentage of each volatile compound was obtained according to the peak area normalization method.
The color of milk sample was analyzed by using an Opto-Star equipment (Opto-Star, Matthäus, Nobitz-Klausa, Germany) according to Popov-Raljić et al. (24) with a minor modification. Briefly, a colorimeter was calibrated by a white standard plate, and then milk sample was placed in the glass cell (0.5 cm high and 2.5 cm diameter), and color values of L* (lightness), a* (red to green) and b* (blue to yellow) were read directly at different time intervals (0, 1, 3, and 7 d). The determination was repeated three times for each of sample.
The sensory evaluation of milk sample was carried out by the Institute of Animal Nutrition and Feed Science at Guizhou University. The relatively fixed full-time sensory evaluation personnel was used to form a sensory evaluation team to avoid errors caused by personnel changes in the current study. Thus, sensory evaluation of milk samples throughout product shelf life were developed by thirty panelists (including staffs, undergraduate and graduate students from Guizhou University) A total of 13 descriptive terms were selected for the major sensory attribute category as listed in Table 1 together with flavor defect and descriptions (25–27). The nine main points of the hedonic scale used are: (9) like extremely; (8) like very much; (7) like moderately; (6) like slightly; (5) neither like nor dislike; (4) dislike slightly; (3) dislike moderately; (2) dislike very much; and (1) dislike extremely. Samples were served in randomized order over panelists within each replicate. The panelists had a training session 2 d prior to evaluations to refresh their memory regarding the descriptors and the products. In these training sessions, examples of all samples that later would be evaluated were presented to the panelists.
The changes of antioxidant activity, FR, lipid peroxidation, volatile compound, and color parameters were assessed using multiple comparisons with the Duncan's test, using SAS System Version 9.1.3 (SAS Institute Inc., Cary, NC, USA). The sensory score data were analyzed using the comparison difference analysis according to Larmond (28). Each plastic tube was an experimental unit. The results were presented as the mean ± standard deviation. Pearson correlation coefficients (r) were calculated to detect the relationship between antioxidant activity and FR, lipid peroxidation, and volatile compounds in milk (29). Differences were set statistically significant at a P-value < 0.05.
The temperature and pH factors that affect anthocyanin degradation could be considered to be negligible in the current research. As a result, light became the only factor impacting anthocyanin degradation in milk. Moreover, photooxidation is the main inducement of milk fat oxidation because pigments can absorb visible or ultraviolet light, resulting in photooxidation of milk lipids. Walsh et al. (30) demonstrated that photooxidation can promote the rancidity of dairy products, which was related to the wavelength, intensity and duration of light. The three components of milk can be selected as the most practical photochemically important components: (1) the proteins are related to the activated flavor of light-exposed milk; (2) the lipids are related to the other oxidized flavor; and (3) various antioxidants in milk are photoehemically changed, damaging nutritional quality (31). Reddy et al. (32) indicated that the addition of anthocyanin-rich black tea to milk could prevent oxidative damage. Hence, we found that the level of TAC in the light-protected groups was unchanged (P > 0.05; Table 2), suggesting that anthocyanins add antioxidant activity to milk, which could protect milk from light oxidation. This protection might be the result of single anthocyanin action and might also be related to anthocyanin synergism with other antioxidants to strengthen milk antioxidant activity (33).
Milk has several kinds of enzymatic and non-enzymatic antioxidant components (SOD, GSH-Px, CAT, polyphenols, vitamin E), showing high levels of antioxidant potential and DPPH scavenging activity (34). Arreola et al. (35) did show that natural antioxidants in milk had high levels of antiproliferative activity and scavenging activity. However, light-exposed milk is also an exceptionally good breeding ground for bacteria, and its nutrition is destroyed during the storage period, especially decreasing antioxidative factors and natural antioxidants (36). Thus, we found that antioxidant activity in light-exposed groups tended to decrease (P < 0.05; Table 2) during the storage period, possibly because anthocyanins were sensitive to light, thereby decreasing milk antioxidant activity (37). This result was consistent with Gutierrez et al. (38), who indicated that TAC was significantly decreased in light-induced milk during the storage period.
Oksuz et al. (39) showed that cherry anthocyanin pigments had a relatively low level of stability in dairy food. However, the casein in milk could react with anthocyanins, leading to anthocyanins becoming stable in neutral food (40). In this study, the mean pH value was 6.52, nearly the same as that in aqueous solution, which potentially provides a condition for anthocyanin reactions with milk protein to stabilize anthocyanins in milk. Consequently, complex compounds may form between anthocyanins and proteins in milk, increasing the stability of anthocyanins to provide a necessary condition for preventing milk oxidation. Van Aardt et al. (18) showed that supplementation with 0.025% α-tocopherol in light-induced milk could prevent lipid and protein oxidation. Moreover, antioxidant enzymes can reduce reactive oxygen species and maintain the oxygen balance, thus enhancing the antioxidant activity (41, 42). In the present study, the additional anthocyanin groups showed higher (P < 0.05; Table 2) levels of SOD, GSH-Px, and CAT than the no additional anthocyanin groups, perhaps because PCP provided a high concentration of exogenous natural antioxidant, resulting in a greater antioxidant activity (43). These results were in accordance with Gutierrez et al. (38), who revealed that the addition of exogenous antioxidants in milk could enhance antioxidant activity. In addition, four sources of structure changed the color of different anthocyanin compositions, among which colorless colors of carbinol pseudobase and chalcone were unstable relative to the red color of flavylium cation, thereby more easily degrading to other products (44). Clifford (45) demonstrated that the structure of chalcone might degrade by oxidation reactions with high molecular weights. Light-exposed milk might be the result of changes in the four structures of anthocyanins, and they decreased in response to increasing storage days (10). Hence, antioxidant activity parameters tended to decline (P < 0.05; Table 2) for all groups as the storage time extended.
UFAs oxidize lipid radicals, oxidize peroxyl radicals with O2, and then change FA hydroperoxide with hydrogen, thus negatively affecting milk antioxidant enzymes (46). Živković et al. (47) revealed that anthocyanins might react in the water and lipid phases, which could be seen as a source of radical scavengers in milk. Moreover, Vinson et al. (48) reported that the antioxidant properties of polyphenols in milk could provide a health benefit for humans. In the current work, the AC group showed lower (P < 0.05; Table 3) concentrations of and H2O2 compared to control groups. There could be several reasons as follows: (1) the light-protected environment provided conditions for preventing milk oxidation; (2) the AC group contained high levels of anthocyanins; and (3) the H atom in anthocyanins may react with FR, leading to its stability and improved antioxidant enzymes. As expected, the AC group showed higher (P < 0.05) levels of DPPH scavenging activity after 1 d of storage period, providing further proof that anthocyanins commonly have reducing power and inhibition ability on oxidation of the liposome system in milk. A similar conclusion was found by Denise et al. (49), who demonstrated that adding anthocyanin-rich grape extract to milk can improve antioxidant activity by improving the DPPH radical scavenging ability.
The chemical properties of UFAs are very unstable and might appear to increase susceptibility to oxidation, producing cytotoxic lipid peroxides in milk (50). Additionally, the high reactivity of FRs have an impact on antioxidant enzymes in milk, resulting in lipid peroxidation (51). In this study, the concentrations of MDA and LPO in the control groups were increased (P < 0.05; Figure 1) during the storage period compared to the AC group, perhaps because light was one of the important impacting factors that increased milk lipid oxidation. This result was consistent with a previous report by Gutierrez (52), who indicated that light treatment could increase milk oxidized flavor and lipid peroxidation parameters during a refrigerated storage period. As expected, the AC group displayed the lowest (P < 0.05; Figure 1) MDA and LPO contents in milk at the end of the period, suggesting that PCP could inhibit lipid peroxidation and enhance antioxidative effects in light-protected milk. The reason may be that the anthocyanin-casein complex could promote autoxidation of iron, inhibiting lipid peroxidation (53). Similar to our current findings, Gualdrón et al. (54) indicated that the addition of anthocyanin-rich grape pigment could protect against oxidation of lipids and protein in yogurt during the 21-day storage period.
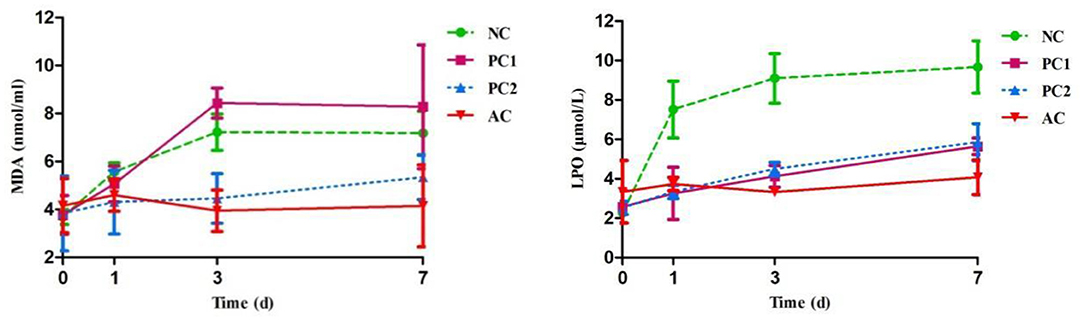
Figure 1. Effect of PCP on lipid peroxidation parameters in milk during storage period. NC, negative control of no addition anthocyanins + exposure to fluorescent light; PC1, positive control 1 of no addition anthocyanins + protected from fluorescent light; PC2, positive control 2 of the addition of 0.3% (w/v) anthocyanins + exposure to fluorescent light; AC, the addition of 0.3% (w/v) anthocyanins + protected from fluorescent light; MDA, malondialdehyde; LPO, lipid peroxidation.
Twenty-two volatile compounds were determined in milk, which consisted of eight alcohols (ethanol, 1-pentanol, 1-hexanol, 1-heptanol, 1-octen-3-ol, 2-ethyl-1-hexanol, (Z)-2-octen-1-ol, and 1-octanol), three ketones (2,3-butanedione, 2-pentanone, 2,3-octanedione), five aldehydes (hexanal, heptanal, octanal, nonanal, decanal), two esters (ethyl acetate, ethyl hexanoate), and four hydrocarbons (octane, 1-nitro-hexane, dodecane, tetradecane) by GC/MS analysis (Table 4). Milk volatile compounds are produced mainly from amino acid degradation, the Maillard reaction and the thermal oxidation of lipids (55). Scanlan et al. (56) who showed that the heat processing could increase lipid oxidation in milk resulted in formation of flavor substances. Moreover, the high pasteurization could maintain milk anthocyanin level and improve the antioxidant activity (17). Anthocyanins are hydrogen donors of lipid FRs in the process of lipid oxidation and can be transformed into more stable FRs because anthocyanins can effectively intercept peroxy radicals, block chain propagation and inhibit the formation of peroxide (57). As a consequence, some volatile compounds, such as ethanol, 1-heptanol tended to increase without anthocyanin groups after heat processing compared to the anthocyanin groups. Lipid oxidation is an important cause of undesirable flavors of non-enzymatic origin in milk because O2 is linked to the methylene adjacent to a double bond in UFAs, thereby forming allylic hydroperoxides. Alternatively, molecular O2 is reduced to superoxide radicals, and it is converted into singlet O2 and peroxide anions. Anthocyanis can provide H atoms to peroxy radicals, thus inhibiting the oxidation of FA by chain radical termination and decreasing milk alcohol concentrations (57). As mentioned previously, light was the main factor affecting lipid oxidation, and anthocyanins can increase antioxidant activity and decrease lipid peroxidation parameters in milk. Thus, aldehydes, esters and hydrocarbons, all individual volatile compounds in the AC group remained at relatively stable (P > 0.05; Table 4) values during storage relative to the other three groups. Of interest, various ketones are associated with fruity and floral notes, so the presence of these volatile compounds can be considered to positively influence the flavor of milk (58). In the present research, the level of ketones increased rapidly in anthocyanin groups during storage and had higher (P < 0.05; Table 4) relative contents of ketones compared to the other groups, perhaps because anthocyanins could inhibit lipid oxidation, delaying the decline of milk flavor quality, and enriching the types of flavor substances (59). Conservatively, anthocyanins play a positive role in the aroma of light-protected milk, which not only enriches the taste of fruity and floral notes but also retains the nutritive value of milk, so this kind of milk might be popular with consumers.
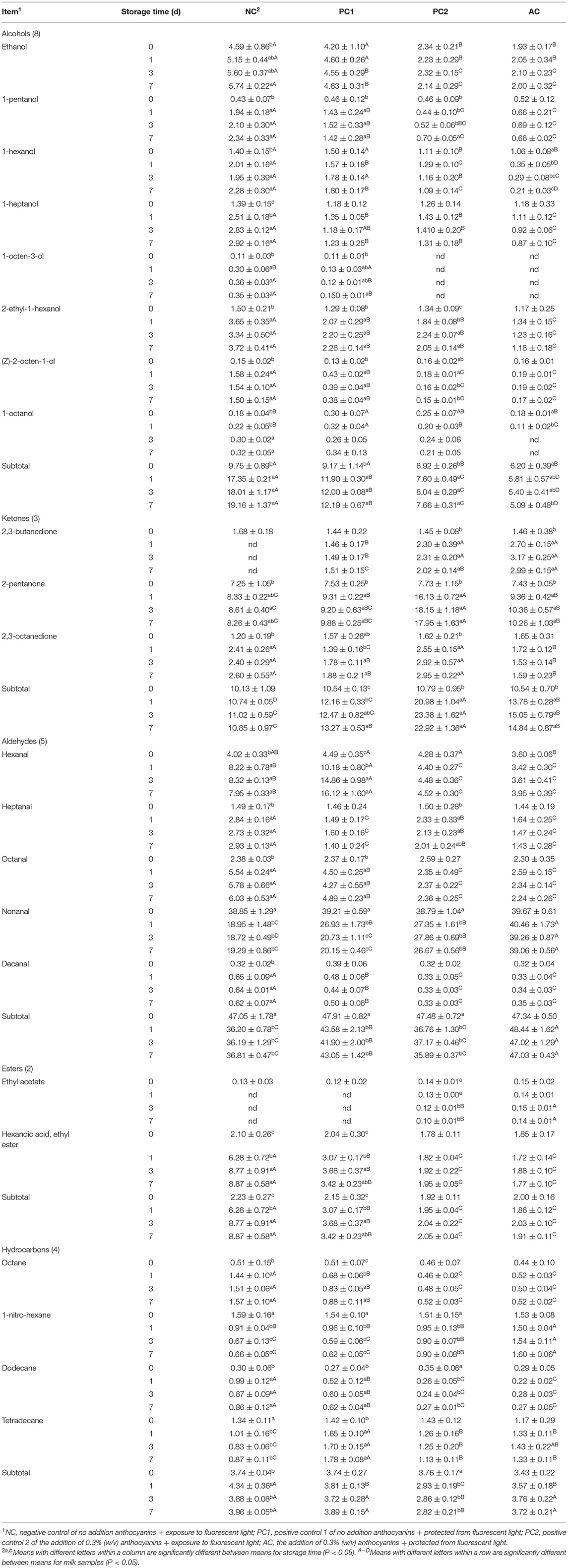
Table 4. Effect of PCP on relative contents of volatile compounds in milk during storage period (%).
Anthocyanins have the ability to scavenge oxygen FR in milk and are the main contributors to antioxidant potential in milk (60). Thus, stronger correlations (P < 0.05; Table 5) were noted between some antioxidant activities and DPPH scavenging activity as well as total ketones. These results were in agreement with Mann et al. (61), who demonstrated that there was a stronger correlation between TAC and DPPH scavenging activity in milk. Moreover, numerous FRs and lipid peroxidations are produced in the process of lipid oxidation (62, 63). Polyphenols could protect milk oxidation by removing the concentrations of FRs and reducing lipid peroxidation, leading to the maintenance of volatile compounds in milk (64). Therefore, the correlation analysis showed negative (P < 0.05; Table 5) correlations between antioxidant activity and , H2O2, MDA, LPO, alcohols, and esters in milk. Consistent with our observations, Stapelfeldt et al. (65) indicated that there were negative correlations between FR concentrations and antioxidant levels in milk.

Table 5. Pearson correlation coefficients between antioxidant activity, free radical, lipid peroxidation, and volatile compounds in milk.
Anthocyanins have been associated with the color change of milk added with purple corn extract; and the color values were impacted on the anthocyanin concentration, storage temperature and storage duration (66). All of milk samples were pasteurized and placed in tubes, and kept at 4°C during the 7 d period in the present research. A previous study has been showed that adding of anthocyanin in milk can be stored at 4°C temperature condition, and the degrade rate of anthocyanins should be extended (67). Therefore, adding of anthocyanin did not impact (P > 0.05; Table 6) on the color values of L* and b* in milk among all groups during the entire storage period. However, light is one of the major environmental factors that affecting anthocyanin degradation (68). As a result, anthocyanin decreased significantly in response to increasing storage time (11). More specifically, secondary complexes may also form between polyphenols and proteins, which can reduce the color rendering ability of the anthocyanins. These previous reports lead us to presume that PCP could keep the stability of anthocyanin concentration in light-protected milk. Accordingly, the red color of milk began to decrease (P < 0.05) in PC2 group at 1 d storage, but it did not differ (P > 0.05) in the light-protected group during the whole storage period. In summary, our results suggest that adding of PCP in light-protected milk can maintain their color value at a low temperature condition. Consistent with our result, Sawale et al. (69) who found that addition of anthocyanin-rich Pueraria tuberosa extract to milk can decrease lightness value, whereas it can increase yellowness and redness values.
In consideration of consumer acceptance, enrichment of pasteurized milk with natural antioxidants without compromising the sensory attributes of milk is very essential (70). Furthermore, sensory evaluation is necessary during the development of the dairy products. The attributes of acid, bitter, feed, fermented/fruity, flat, foreign/atypical, garlic/onion/weedy, lacks freshness (stale), malty, oxidized-light (light-induced), oxidized-metal (metal-induced), rancid and salty for the milk samples were evaluated as being in the range of 5 to 9, suggesting that adding of anthocyanin in milk had no negative impact on the sensory evaluation of consumers. Of interest, adding of anthocyanins in milk may have bitter taste because attributed to the interaction of polyphenol-salivary protein (69). However, the lipid oxidation can decrease nutritive value and sensory quality in milk (59). Thus, adding of 0.3% PCP had the potential to improve the acceptability of consumers. These results showed that no attributes changed if adding of PCP in the pasteurized milk. A previous study has shown that purple corn anthocyanins can remain stable at 80°C (71). It was safe to assume that adding of PCP in pasteurization of fresh milk could maintain the active constituent anthocyanin content in dark condition and improve consumer health. In the present study, most of sensory evaluation parameters in anthocyanin groups showed relatively (Table 7) higher values compared to without anthocyanin groups, perhaps due to milk supplementation of PCP and light prevention during storage period. Current experiment results are in agreement with the finding of Ma et al. (67), who observed adding of anthocyanins in milk had no negative effect on the sensory quality parameters. Similarly, Sawale et al. (69) who demonstrated that no adverse effect on sensory attributes of milk with 0.4% anthocyanin-rich Pueraria tuberosa extract. Overall, these results indicated that the process of anthocyanin and light prevention did not influence the sensory characteristics of cow milk; most importantly, adding of PCP might not affect the consumer acceptance of products containing pasteurized milk as a major ingredient. Therefore, from the view of practical application, adding of anthocyanin in pasteurized milk was a feasible and effective method.
The present results indicated that the addition of PCP had the potential to prevent milk oxidation because: (1) it could enhance antioxidant activity and reduce the extent of FRs and lipid peroxidation parameters, (2) it could maintain volatile compounds and enrich the taste of fruity and floral notes, and (3) it could increase the sensory scores to improve the acceptability of consumers in light-protected milk within the 7-day storage period. Further studies are needed to elucidate the mechanism by which anthocyanins protect against milk oxidation.
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
The animal study was reviewed and approved by the Rules of the College of Animal Science, Guizhou University.
X-ZT conducted all the experiments, data analyzing, and writing—original draft preparation and project administration. XW, CB, Q-YL, and J-XL contributed by the investigation, data curation. QL conducted methodology, supervising data, and project administration. All authors contributed to the article and approved the submitted version.
This work was funded by the Science and Technology Project of Guizhou Province (Qiankehe foundation-ZK[2021] General 164), Youth Science and Technology Talent Development Project of Guizhou Province (Qianjiaohe KY [2022]150), the Cultivating Project of Guizhou University (2019-33), and the Start-up Funds of Guizhou University (2016-76; 2019-26), respectively.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Ruxton CHS, Calder PC, Reed SC, Simpson MJA. The impact of long-chain n-3 polyunsaturated fatty acids on human health. Nutr Res Rev. (2005) 18:113–29. doi: 10.1079/NRR200497
2. Hanna N, Ahmed K, Anwar M, Petrova A, Hiatt M, Hegyi T. Effect of storage on breast milk antioxidant activity. Arch Dis Child Fetal. (2004) 89:518–20. doi: 10.1136/adc.2004.049247
3. Correddu F, Nudda A, Manca MG, Pulina G, Dalsgaard TK. Light-induced lipid oxidation in sheep milk: effects of dietary grape seed and linseed, alone or in combination, on milk oxidative stability. J Agr Food Chem. (2015) 63:3980–6. doi: 10.1021/acs.jafc.5b01614
4. Toso B, Procida G, Stefanon B. Determination of volatile compounds in cows' milk using headspace GC-MS. J Dairy Res. (2002) 69:569–77. doi: 10.1017/S0022029902005782
5. Forss DA. Mechanisms of formation of aroma compounds in milk and milk products. J Dairy Res. (1979) 46:691–706. doi: 10.1017/S0022029900020768
6. Hill RD, Van Leeuwen V, Wilkinson RA. Some factors influencing the autoxidation of milks rich in linoleic acid. New Zeal J Dairy Sci Tech. (1977) 12:69–77.
7. Taylor MJ, Richardson T. Antioxidant activity of skim milk: effect of heat and resultant sulfhydryl groups. J Dairy Sci. (1980) 63:1783–95. doi: 10.3168/jds.S0022-0302(80)83140-7
8. Pihlanto A. Antioxidative peptides derived from milk proteins. Int. Dairy J. (2006) 16:1306–1314. doi: 10.1016/j.idairyj.2006.06.005
9. Zhao Y, Zhang X, Zhang N, Zhou Q, Fan D, Wang M. Lipophilized apigenin derivatives produced during the frying process as novel antioxidants. Food Chem. (2022) 379:32178. doi: 10.1016/j.foodchem.2022.132178
10. Tian XZ, Li JX, Luo QY, Zhou D, Long QM, Wang X, et al. Effects of purple corn anthocyanin on blood biochemical indexes, ruminal fluid fermentation, and rumen microbiota in goats. Front Vet Sci. (2021) 8:715710. doi: 10.3389/fvets.2021.715710
11. Tian XZ, Lu Q, Paengkoum P, Paengkoum S. Effect of purple corn pigment on change of anthocyanin composition and unsaturated fatty acids during milk storage. J Dairy Sci. (2020) 103:7808–12. doi: 10.3168/jds.2020-18409
12. Silva FA, de Oliveira MEG, de Figueirêdo RMF, Sampaio KB, de Souza EL, de Oliveira CEV, et al. The effect of Isabel grape addition on the physicochemical, microbiological and sensory characteristics of probiotic goat milk yogurt. Food Funct. (2017) 8:2121–32. doi: 10.1039/C6FO01795A
13. Mestdagh F, Kerkaert B, Cucu T, Meulenaer BD. Interaction between whey proteins and lipids during light-induced oxidation. Food Chem. (2011) 126:1190–7. doi: 10.1016/j.foodchem.2010.11.170
14. Güneşer O. Pigment and color stability of beetroot betalains in cow milk during thermal treatment. Food Chem. (2016) 196:220–7. doi: 10.1016/j.foodchem.2015.09.033
15. Serafini M, Testa MF, Villaño D, Pecorari M, Van Wieren K, Azzini E, et al. Antioxidant activity of blueberry fruit is impaired by association with milk. Free Radical Bio Med. (2009) 46:769–74. doi: 10.1016/j.freeradbiomed.2008.11.023
16. Silvestre D, Miranda M, Muriach M, Almansa I, Jareño E, Romero FJ. Antioxidant capacity of human milk: effect of thermal conditions for the pasteurization. Acta Pdiatrica. (2009) 97:1070–4. doi: 10.1111/j.1651-2227.2008.00870.x
17. Muche BM, Speers RA, Rupasinghe HPV. Storage temperature impacts on anthocyanins degradation, color changes and haze development in juice of “Merlot” and “Ruby” grapes (Vitis vinifera). Front Nutr. (2018) 5:100. doi: 10.3389/fnut.2018.00100
18. Van Aardt M, Duncan SE, Marcy JE, Long TE, O'Keefe SF, Nielsen-Sims SR. Effect of antioxidant (α-tocopherol and ascorbic acid) fortification on light-induced flavor of milk. J Dairy Sci. (2005) 88:872–80. doi: 10.3168/jds.S0022-0302(05)72753-3
19. Smith TJ, Campbell RE, Jo Y, Drake MA. Flavor and stability of milk proteins. J. Dairy Sci. (2016) 99:4325–46. doi: 10.3168/jds.2016-10847
20. Zhao X, Wang J, Yang Y, Bu D, Cui H, Sun Y, et al. Effects of different fat mixtures on milk fatty acid composition and oxidative stability of milk fat. Anim Feed Sci Tech. (2013) 185:35–42. doi: 10.1016/j.anifeedsci.2013.06.009
21. Hu YJ, Gao KG, Zheng CT, Wu ZJ, Yang XF, Wang L, et al. Effect of dietary supplementation with glycitein during late pregnancy and lactation on antioxidative indices and performance of primiparous sows. J Anim Sci. (2015) 93:2246–54. doi: 10.2527/jas.2014-7767
22. Tian XZ, Paengkoum P, Paengkoum S, Thongpea S, Ban C. Comparison of forage yield, silage fermentative quality, anthocyanin stability, antioxidant activity, and in vitro rumen fermentation of anthocyanin-rich purple corn (Zea mays L.) stover and sticky corn stover. J Integr Agr. (2018) 17:2082–95. doi: 10.1016/S2095-3119(18)61970-7
23. Tian XZ, Lu Q, Zhao SG, Li JX, Luo QY, Wang X, et al. Purple corn anthocyanin affects lipid mechanism, flavor compound profiles, and related gene expression of longissimus thoracis et lumborum muscle in goats. Animals. (2021) 11:2407. doi: 10.3390/ani11082407
24. Popov-Raljić JV, Lakić NS, Laličić-Petronijević JG, Barać MB, Sikimić VM. Color changes of UHT milk during storage. Sensors Basel. (2008) 8:5961–74. doi: 10.3390/s8095961
25. Hedegaard RV, Kristensen D, Nielsen JH, Frøst MB, Østdal H, Hermansen JE, et al. Comparison of descriptive sensory analysis and chemical analysis for oxidative changes in milk. J Dairy Sci. (2006) 89:495–504. doi: 10.3168/jds.S0022-0302(06)72112-9
26. Clark S, Costello M, Drake M, Bodyfelt F. The Sensory Evaluation of Dairy Products. 2nd ed. New York, NY: Springer (2009). doi: 10.1007/978-0-387-77408-4
27. Schiano AN, Harwood WS, Drake M A. A 100-Year review: sensory analysis of milk. J Dairy Sci. (2017) 100:9966–86. doi: 10.3168/jds.2017-13031
28. Larmond E. Methods for Sensory Evaluation of Food. Ottawa, ON: Department of Agriculture Publication (1977).
29. Kaps M, Lamberson W. R. Biostatistics for Animal Science. Cambridge, MA: CABI Publishing (2004). doi: 10.1079/9780851998206.0000
30. Walsh AM, Duncan SE, Potts H, Gallagher DL. Comparing quality and emotional responses as related to acceptability of light-induced oxidation flavor in milk. Food Res Int. (2015) 76:293–300. doi: 10.1016/j.foodres.2015.02.027
31. Wishner LA. Light-induced oxidations in milk. J Dairy Sci. (1964) 47:216–21. doi: 10.3168/jds.S0022-0302(64)88624-0
32. Reddy VC, Sagar GV, Sreeramulu D, Venu L, Raghunath M. Addition of milk does not alter the antioxidant activity of black tea. Ann Nutr Metab. (2005) 49:189–95. doi: 10.1159/000087071
33. Bolling BW, Chen YY, Chen CYO. Contributions of phenolics and added vitamin C to the antioxidant capacity of pomegranate and grape juices: synergism and antagonism among constituents. Int J Food Sci Tech. (2013) 48:2650–8. doi: 10.1111/ijfs.12261
34. Power O, Jakeman P, FitzGerald RJ. Antioxidative peptides: enzymatic production, in vitro and in vivo antioxidant activity and potential applications of milk-derived antioxidative peptides. Amino Acids. (2013) 44:797–820. doi: 10.1007/s00726-012-1393-9
35. Arreola AR, Lacroix M, Pacheco JRS, Morales EG, Padilla JAG, Castellanos EA, et al. Assessment of the biological activity in human milk power treated with different processes for their conservation. J Food Nutr Res. (2018) 6:329–34. doi: 10.12691/jfnr-6-5-8
36. Virtanen T, Pihlanto A, Akkanen S, Korhonen H. Development of antioxidant activity in milk whey during fermentation with lactic acid bacteria. J Appl Microbiol. (2007)102:106–15. doi: 10.1111/j.1365-2672.2006.03072.x
37. Braidot E, Petrussa E, Peresson C, Patui S, Bertolini A, Tubaro F, et al. Low-intensity light cycles improve the quality of lamb's lettuce (Valerianella olitoria [L.] Pollich) during storage at low temperature. Postharvest Biol Tec. (2014) 90:15–23. doi: 10.1016/j.postharvbio.2013.12.003
38. Gutierrez AM, Boylston TD, Clark S. Effects of pro-oxidants and antioxidants on the total antioxidant capacity and lipid oxidation products of milk during refrigerated storage. J Food Sci. (2018) 83:275–83. doi: 10.1111/1750-3841.14016
39. Oksuz T, Tacer-Caba Z, Nilufer-Erdil D, Boyacioglu D. Changes in bioavailability of sour cherry (Prunus cerasus L.) phenolics and anthocyanins when consumed with dairy food matrices. J Food Sci Tech. (2019) 56:4177–88. doi: 10.1007/s13197-019-03888-2
40. Jing P, Giusti MM. Characterization of anthocyanin-rich waste from purple corncobs (Zea mays L.) and its application to color milk. J Agr Food Chem. (2005) 53:8775–81. doi: 10.1021/jf051247o
41. Yang K, Tian X, Ma Z, Wu W. Feeding a negative dietary cation–anion difference to female goats is feasible, as indicated by the non-deleterious effect on rumen fermentation and rumen microbial population and increased plasma calcium level. Animals. (2021) 11:664. doi: 10.3390/ani11030664
42. Yang K, Wu W, Tian X, Han E, Sun L. Evaluation of sorghum hull serving as feed alternative on growth performance, nutrients digestibility and plasma metabolites for growing goats. Agroforest Syst. (2020) 94:1307–14. doi: 10.1007/s10457-018-0318-3
43. Tian XZ, Xin HL, Paengkoum P, Paengkoum S, Ban C, Sorasak T. Effects of anthocyanin-rich purple corn (Zea mays L.) stover silage on nutrient utilization, rumen fermentation, plasma antioxidant capacity, and mammary gland gene expression in dairy goats. J Anim Sci. (2019) 97:1384–97. doi: 10.1093/jas/sky477
44. Brouillard R. Chemical Structure of Anthocyanins. New York, NY: Academic Press (1982). doi: 10.1016/B978-0-12-472550-8.50005-6
45. Clifford MN. Anthocyanins–nature, occurrence and dietary burden. J Sci Food Agric. (2000) 80:1063–72.3. doi: 10.1002/(SICI)1097-0010(20000515)80:7<1063::AID-JSFA605>3.0.CO;2-Q
46. Lindmark-Månsson H, Åkesson B. Antioxidative factors in milk. Br J Nutr. (2000) 84:103–10. doi: 10.1017/S0007114500002324
47. Živković J, Sunarić S, Trutić N, Denić M, Kocić G, Jovanović T. Antioxidants and antioxidant capacity of human milk. Acta Fac Med Nai. (2015) 32:115–25. doi: 10.1515/afmnai-2015-0012
48. Vinson JA, Proch J, Zubik L. Phenol antioxidant quantity and quality in foods: cocoa, dark chocolate, and milk chocolate. J Agric Food Chem. (1999) 47:4821–4. doi: 10.1021/jf990312p
49. Denise F, Matumoto-Pintro PT, Bazinet L, Couillard C, Britten M. Effect of commercial grape extracts on the cheese-making properties of milk. J Dairy Sci. (2015) 98:1552–62. doi: 10.3168/jds.2014-8796
50. Timm-Heinrich M, Nielsen NS, Xu X, Jacobsen C. Oxidative stability of structured lipids containing C18:0, C18:1, C18:2, C18:3 or CLA in sn2-position–as bulk lipids and in milk drinks. Innov Food Sci Emerg. (2004) 5:249–61. doi: 10.1016/j.ifset.2003.08.006
51. Turoli D, Testolin G, Zanini R, Bellù R. Determination of oxidative status in breast and formula milk. Acta Paediatr. (2005) 93:1569–74. doi: 10.1111/j.1651-2227.2004.tb00845.x
52. Gutierrez AM. Effects of lipid oxidation initiators and antioxidants on the total antioxidant capacity of milk and oxidation products during storage (dissertation/master's thesis). Lowa: Lowa State University (2014).
53. Cervato G, Cazzola R, Cestaro B. Studies on the antioxidant activity of milk caseins. Int J Food Sci Nutr. (1999) 50:291–6. doi: 10.1080/096374899101175
54. Gualdrón TRJ, Alzate Ceballos JA, Rojano BA. Oxidative stability of a dairy product pike yogurt, with anthocyanin extracts of corozo (Bactris guineensis) and grape (Vitis vinifera). J Berry Res. (2019) 9:141–53. doi: 10.3233/JBR-180327
55. Zou Y, Kang D, Rui L, Qi J, Zhang W. Effects of ultrasonic assisted cooking on the chemical profiles of taste and flavor of spiced beef. Ultrason Sonochem. (2018) 46:36–45. doi: 10.1016/j.ultsonch.2018.04.005
56. Scanlan RA, Lindsay RC, Libbey LM, Day EA. Heat-induced volatile compounds in milk. J Dairy Sci. (1968) 51:1001–7. doi: 10.3168/jds.S0022-0302(68)87113-9
57. Narayan MS, Naidu KA, Ravishankar GA, Srinivas L, Venkataraman LV. Antioxidant effect of anthocyanin on enzymatic and non-enzymatic lipid peroxidation. Prostag Leukotr Ess. (1999) 60:1–4. doi: 10.1054/plef.1998.0001
58. Wang W, Chen H, Ke D, Chen W, Yun YH. Effect of sterilization and storage on volatile compounds, sensory properties and physicochemical properties of coconut milk. Microchem J. (2019) 153:104532. doi: 10.1016/j.microc.2019.104532
59. Yi J, Qiu M, Liu N, Tan L, Zhu X, Decker EA, et al. Inhibition of lipid and protein oxidation in whey protein-stabilized emulsions using a natural antioxidant: black rice anthocyanins. J Agric Food Chem. (2020) 68:10149–56. doi: 10.1021/acs.jafc.0c03978
60. Tian XZ, Paengkoum P, Paengkoum S, Chumpawadee S, Ban C, Thongpea S. Purple corn (Zea mays L.) stover silage with abundant anthocyanins transferring anthocyanin composition to the milk and increasing antioxidant status of lactating dairy goats. J Dairy Sci. (2019) 102:413–8. doi: 10.3168/jds.2018-15423
61. Mann S, Shandilya UK, Sodhi M, Kumar P, Mukesh M. Determination of antioxidant capacity and free radical scavenging activity of milk from native cows (Bos indicus), exotic cows (Bos taurus), and riverine buffaloes (Bubalus bubalis) across different lactation stages. Int J Dairy Sci Process. (2016) 3:66–70. doi: 10.19070/2379-1578-1600013
62. Zhao Y, Kong H, Zhang X, Hu X, Wang M. The effect of Perilla (Perilla frutescens) leaf extracts on the quality of surimi fish balls. Food Sci Nutr. (2019) 7:2083–90. doi: 10.1002/fsn3.1049
63. Tian XZ, Li JX, Luo QY, Wang X, Xiao MM, Zhou D, et al. Effect of Supplementation with selenium-yeast on muscle antioxidant activity, meat quality, fatty acids and amino acids in goats. Front Vet Sci. (2022) 8:813672. doi: 10.3389/fvets.2021.813672
64. Grazyna C, Hanna C, Adam A, Magdalena BM. Natural antioxidants in milk and dairy products. Int J Dairy Technol. (2017) 70:165–78. doi: 10.1111/1471-0307.12359
65. Stapelfeldt H, Nielsen KN, Jensen SK, Skibsted LH. Free radical formation in freeze-dried raw milk in relation to its α-tocopherol level. J Dairy Res. (1999) 66:461–6. doi: 10.1017/S0022029999003568
66. Wegrzyn TF, Farr JM, Hunter DC, Au J, Wohlers MW, Skinner MA, et al. Stability of antioxidants in an apple polyphenol-milk model system. Food Chem. (2008) 109:310–8. doi: 10.1016/j.foodchem.2007.12.034
67. Ma Y, Li S, Ji T, Wu W, Liu Y. Development and optimization of dynamic gelatin/chitosan nanoparticles incorporated with blueberry anthocyanins for milk freshness monitoring. Carbohyd Polym. (2020) 247:116738. doi: 10.1016/j.carbpol.2020.116738
68. Zhang P, Lu S, Liu Z, Zheng T, Dong T, Jin H, et al. Transcriptomic and metabolomic profiling reveals the effect of LED light quality on fruit ripening and anthocyanin accumulation in cabernet sauvignon grape. Front Nutr. (2021) 8:790697. doi: 10.3389/fnut.2021.790697
69. Sawale PD, Singh RRB, Arora S. Stability and quality of herb (Pueraria Tuberosa)-milk model system. J. Food Sci. Technol. (2015) 52:1089–1095. doi: 10.1007/s13197-013-1067-y
70. Mahato DK, Keast R, Liem DG, Russell CG, Gamlath S. Sugar reduction in dairy food: an overview with flavoured milk as an example. Foods. (2020) 9:1400. doi: 10.3390/foods9101400
Keywords: anthocyanin, antioxidant activity, volatile compound, color measurement, sensory evaluation
Citation: Tian X-Z, Wang X, Ban C, Luo Q-Y, Li J-X and Lu Q (2022) Effect of Purple Corn Anthocyanin on Antioxidant Activity, Volatile Compound and Sensory Property in Milk During Storage and Light Prevention. Front. Nutr. 9:862689. doi: 10.3389/fnut.2022.862689
Received: 26 January 2022; Accepted: 03 March 2022;
Published: 23 March 2022.
Edited by:
Yueliang Zhao, Shanghai Ocean University, ChinaReviewed by:
Yangdong Zhang, Institute of Animal Sciences (CAAS), ChinaCopyright © 2022 Tian, Wang, Ban, Luo, Li and Lu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qi Lu, bHVxaTI1NTY3MjhAMTYzLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.