
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Nutr. , 07 June 2022
Sec. Nutrition and Microbes
Volume 9 - 2022 | https://doi.org/10.3389/fnut.2022.862498
 Yuying Li1,2
Yuying Li1,2 Xuetai Bao1,2
Xuetai Bao1,2 Fan Yang1,2
Fan Yang1,2 Junquan Tian1,2
Junquan Tian1,2 Wenxuan Su1,2
Wenxuan Su1,2 Jie Yin3
Jie Yin3 Kang Yao1*
Kang Yao1* Tiejun Li1,2
Tiejun Li1,2 Yulong Yin1,2
Yulong Yin1,2
Enterotoxigenic Escherichia coli (ETEC) is one of the main causes of diarrhea in weaned piglets, and ornithine α-ketoglutarate (OKG) as a food supplement has been shown to improve intestinal immune status in animals and humans. However, it remains unknown whether OKG alleviates inflammation through the regulation of gut microbiota and its metabolites on ETEC-infected piglets. This study was conducted to explore the impact of OKG on growth performance, immunity, and ileal mucosa microbiota and its metabolites in piglets infected with ETEC. On a total of 40 pigs, a 2 × 2 factor design was performed; the major factors were diet (basal diet or 1% OKG diet) and challenge (E. coli or LB Broth). The results showed that ETEC-infection inhibited growth performance, and OKG supplementation alleviated growth performance. Interestingly, ETEC-infection increased the serum TNF-α and IL-6, decreased the serum IL-10, downregulated the mRNA expression of IL-1β, IL-6, MyD88, and improved the mRNA expression of IL-8, IL-18, and TLR4. OKG inhibited serum IL-6, suppressed the phosphorylation of downstream signals of NF-κB/JNK in the ileum, and enhanced serum IL-10 and ileum SIgA in ETEC-challenged piglets. OKG supplementation enhanced the mRNA expression of IL-1β and IL-10 and reduced NF-κB and MyD88 in the ileum. Importantly, OKG reversed intestinal microbiota dysfunction, including the diversity of ileal microbiota, the relative abundances of Actinobacillus, Turicibacter, and [Acetivibrio]_ethanolgignens_group, which significantly affected arachidonic acid metabolism and primary bile acid biosynthesis. Collectively, our results suggest that OKG improves growth performance, regulates immunity, and ileal mucosa microbiota and its metabolites in ETEC-infected piglets.
Early weaned pigs generally exhibit hypoplasia of the immune system, disorder of the digestive system, and diarrhea after weaning (1). Neonatal and post-weaning piglets raise the opportunity for digestive pathogens, such as enterotoxigenic Escherichia coli (ETEC), to invade or colonize the gut. ETEC is a universal intestinal inhabitant for diarrhea in young animals, resulting in significant growth retardation and economic losses in pig production due to severe diarrhea, morbidity, mortality, and impaired growth during weaning transition (2, 3). ETEC K88 is often colonized in the small bowel and continuously secretes enterotoxins that damage the functions of the intestinal epithelium and triggers inflammation via increasing cell cation exchanges and reducing water absorption (4, 5). To solve the above problems, antibiotics are widely used to treat pathogen infections (6). In livestock, however, the overuse of antibiotics has ultimately led to serious problems, such as drug-resistant bacteria (7). Therefore, an effective nutritional feed ingredient is necessary to improve health status and inhibit diarrhea.
Ornithine α-ketoglutarate (OKG) is a nutritional compound that consists of one molecule of α-ketoglutarate and two molecules of ornithine, a precursor of glutamine, arginine, and proline, which benefit the immune response by altering inflammatory processes during times of challenge (8–12). More specifically, OKG improves glutamine in blood and has antitumor functions in combination with the glutaminase 1 enzyme pathway inhibitors (8). In recent years, OKG in stock farming has attracted growing attention. For instance, in ovo, 0.2 or 0.4% of OKG injection can promote early growth and pectoral muscles of chicks (8). Administration of 0.4 g/kg of OKG improves bone properties and amino acid synthesis in rapidly growing turkeys (13). Interestingly, our previous study found that 0.5 or 1% of OKG alters pig gut microbe, especially decreases the Proteobacteria (including Escherichia_coli), increases the serum of glutamate, proline, aspartate, threonine, and branch chain amino acid levels, and alleviates growth-suppression induced by D-galactose chronic oxidative stress (14). Dietary 0.75% OKG increases daily gain and feed intake in weaning pigs (15). Also, OKG (0.5, 1.5, and 4.5 g/kg/day) increases tissue glutamine concentration and N balance in endotoxemia rats (12). A total of 10 g/day OKG can cure pressure ulcers in elderly patients (11). The beneficial role of OKG in various physiological benefits has been validated, but the anti-inflammatory effect of OKG in intestinal inflammation was rarely revealed. Therefore, we aimed to explore the possible mechanism of the OKG in alleviating inflammation and regulating intestinal microbiota.
Forty healthy piglets (Duroc × Landrace × Yorkshire, half-castrated males and half females, average body weights of 8.25 kg) weaned at 28 days were used. After 3 days of adaption, all pigs were fed a standard diet. Then, the pigs were randomly assigned to either a basal (CON) or 1% OKG (n = 20/diet, NRC2012, Table 1). Each group of animals was randomly assigned to two sub-groups (ETEC and OKG ETEC; n = 10/treatment group). Pigs in the ETEC and OKG ETEC received orally 10 ml (2 × 109 CFU/ml) of the enterotoxic strain of E. coli K88, respectively, whereas pigs in the CON and OKG groups were given an equal volume of LB Broth on day 15. The piglets were housed individually and had free access to diets and water. Three days after the challenge, all animals were euthanized. Samples of serum, ileum, ileal mucosa, and feces were immediately frozen at −80°C for analysis. All animal procedures were approved by the Animal Care Committee of the Institute of Subtropical Agriculture, Chinese Academy of Sciences.
The body weight was recorded on days 0, 14, and 17, the feed intake was recorded daily to calculate average daily feed intake, and the feces score was recorded 0, 24, and 48 h after ETEC-infection.
The serum level of interferon-gamma (IFN-γ), tumor necrosis factor-alpha (TNF-α), interleukin-1 (IL-1), interleukin-10 (IL-10), interleukin-6 (IL-6), as well as the ileum level of secretory immunoglobulin A (IgA) were determined by a commercially available ELISA kit (Jiangsu Yutong Biological Technology Co., Ltd) according to the manufacturer’s instructions (16).
mRNA was isolated from frozen liquid nitrogen and ground ileum tissues with TRIzol reagent. The expression of β-actin (house-keeping gene) and intestinal immune-associated genes were determined by RT-PCR according to our previous study (17–19). Primers used were designed according to the sus scrofa sequence (Table 2). The relative expression of mRNA was calculated with ΔΔCt = (CtTarget–Ctβ –actin) treatment–(CtTarget–Ctβ –actin) control (20). Relative expression was normalized and expressed as a ratio to the expression in the CON group.
Sequencing procedures and data analyses were performed by a commercial company (Novogene Co., Ltd., Beijing, China). In brief, total genome DNA from ileal mucosa samples was extracted for amplification using the specific primer with the barcode (V3-V4 regions) (17, 21). Then, the sequencing libraries were generated, assessed, and sequenced on Illumina MiSeq Sequencer (18). The raw tags were paired, filtered, and then analyzed using the operational taxonomic unit (OTU) cluster (14, 22). Principal coordinate analysis (PCoA) to unweighted UniFrac distance metric matrices were applied to beta-diversity. Observed species, Shannon, Simpson, Chao1, ACE, Goods coverage, and PD whole tree were performed to determine alpha-diversity. Beta-diversity and alpha-diversity were used to evaluate the complexity of species diversity. The bacterial relative abundance at the phylum, order, and genus levels was further compared between the four groups; the top 10 most abundant families were defined as dominant genera flora and compared them, respectively. OTUs were then performed for the genome prediction of microbial communities by Phylogenetic Investigation of Communities by Reconstruction of Unobserved States (PICRUSt).
Ileal mucosa was separately pestle with liquid nitrogen and the homogenate was resuspended with pre-chilled 80% methanol through the vortex. The samples were incubated on ice for 5 min and subsequently centrifuged at 15,000 g at 4°C for 20 min. The supernatants were further transferred to a new Eppendorf tube, centrifuged at 15,000 g at 4°C for 20 min, and injected into the LC-MS/MS system analysis (Novogene Co., Ltd., Beijing, China) (23). Data were processed, analyzed, and the metabolite identified according to a previous study (24).
The detailed process of Western blot was described according to our previous study (18). The primary antibodies included NF-κB (bs-0465R, 1:1000, Bioss), p-NFκB (#3033, 1:1000, CST), p38 (ab170099, 1:1000, abcam), p-p38 (ab47363, 1:500, abcam), JNK (#9252, 1:1000, CST), p-JNK (#4668, 1:1000, CST), and β-actin (66009-1-Ig, 1:5000, Proteintech).
Data in pigs were evaluated using independent samples t-test or factorial ANOVA and p < 0.05 was taken to indicate statistical significance (IBM SPSS statistics 20 software). The statistical model contained the effects of infect (LB or ETEC), diet (basal or OKG), and their interaction. Data were expressed as mean ± standard error of the mean (SEM). The Pearson correlation analysis was used to measure the correlation between ileal mucosa microbiota and metabolites by using GraphPad Prism 7.
The growth performance, diarrhea, serum, and small intestinal immunity are shown in Figure 1. The ETEC-challenge significantly decreased average daily feed intake (ADFI) in pigs fed the basal or OKG diet. Compared with the basal diets, OKG increased body weight and ADFI after ETEC-infection (p < 0.05). Similarly, ETEC-challenge significantly increased the feces score in pigs fed with the basal or OKG diet. Compared with the CON group, in the OKG group, the feces score was decreased after ETEC-infection (p < 0.05). Meanwhile, ETEC-challenge markedly increased the serum TNF-α and IL-6 and decreased the serum IL-10, while OKG treatment markedly increased the serum IL-10 and ileum SIgA and decreased serum IL-6 (p < 0.05). We further measured the ileal gene expression of ILs, TNF-α, nuclear factor kappa B (NF-κB), myeloid differentiation primary response gene 88 (MyD88), and transmembrane Toll-like receptors (TLR2 and TLR4) by RT-PCR. ETEC-challenge markedly downregulated the gene expression of IL-1β, IL-6, and MyD88 and improved the mRNA expression of IL-8, IL-18, and TLR4, whereas OKG treatment markedly improved the gene expression of IL-1β and IL-10 and downregulated the mRNA expression of NF-κB and MyD88 (p < 0.05).
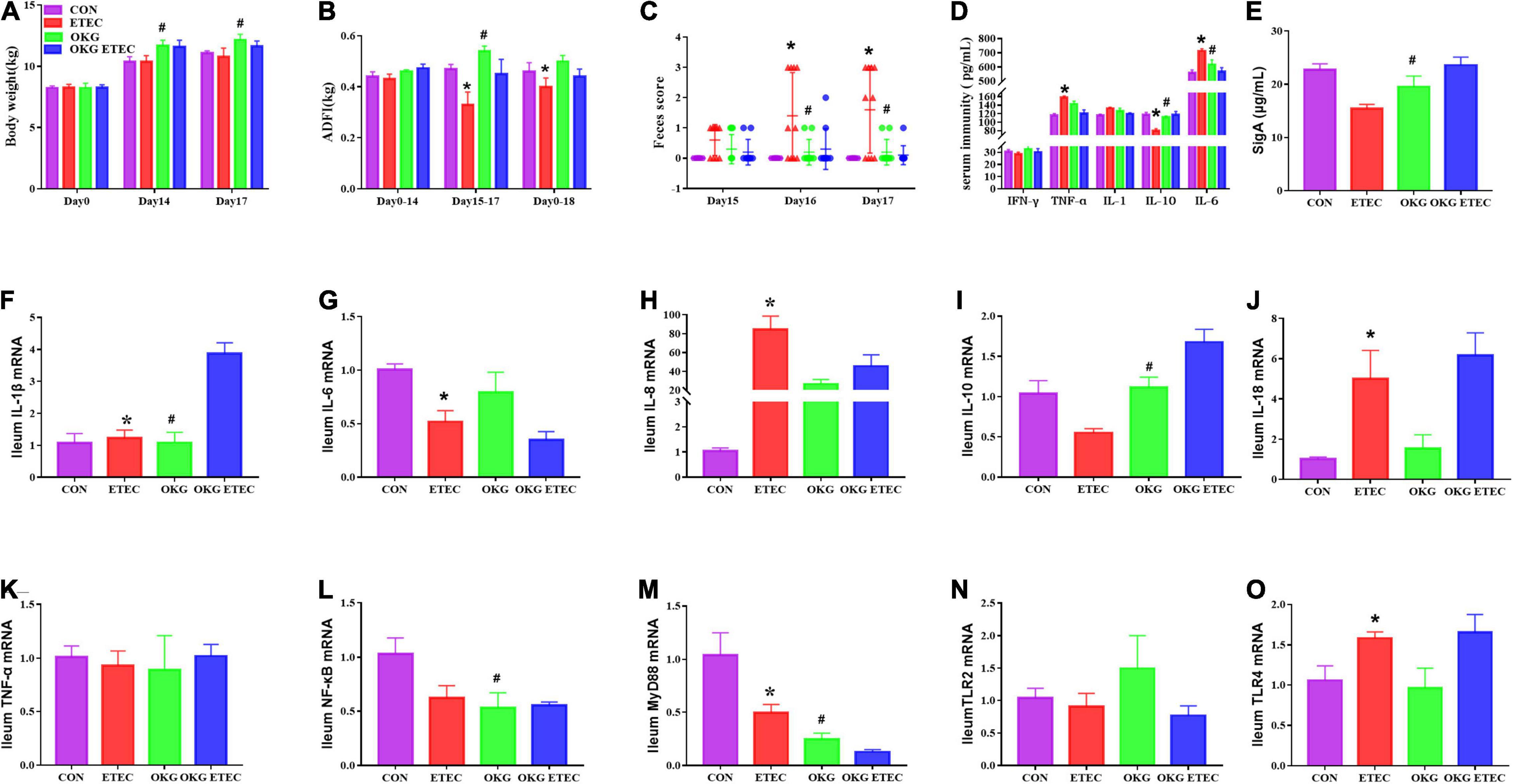
Figure 1. Effects of OKG supplementation on growth performance, feces score, and serum and small intestinal immunity in ETEC- infected pigs. (A) Body weight (kg), (B) average daily feed intake (kg), (C) feces score, (D) serum immunity (pg/mL), (E) ileum SIgA (μg/ml), (F–O) gene expressions of immunity in ileum. Values are presented as mean ± SEM. *Indicates a statistically significant difference for the challenge (ETEC or LB Broth) (p < 0.05). #Indicates a statistically significant difference for dietary treatment (basal or OKG diet) (p < 0.05).
The OKG treatment significantly decreased community richness, including observed species, Chao1, and AEC (Figure 2, p < 0.05). Dietary supplementation OKG treatment or not presented a clear separation clustering of microbial community composition in beta-diversity (Figure 2).
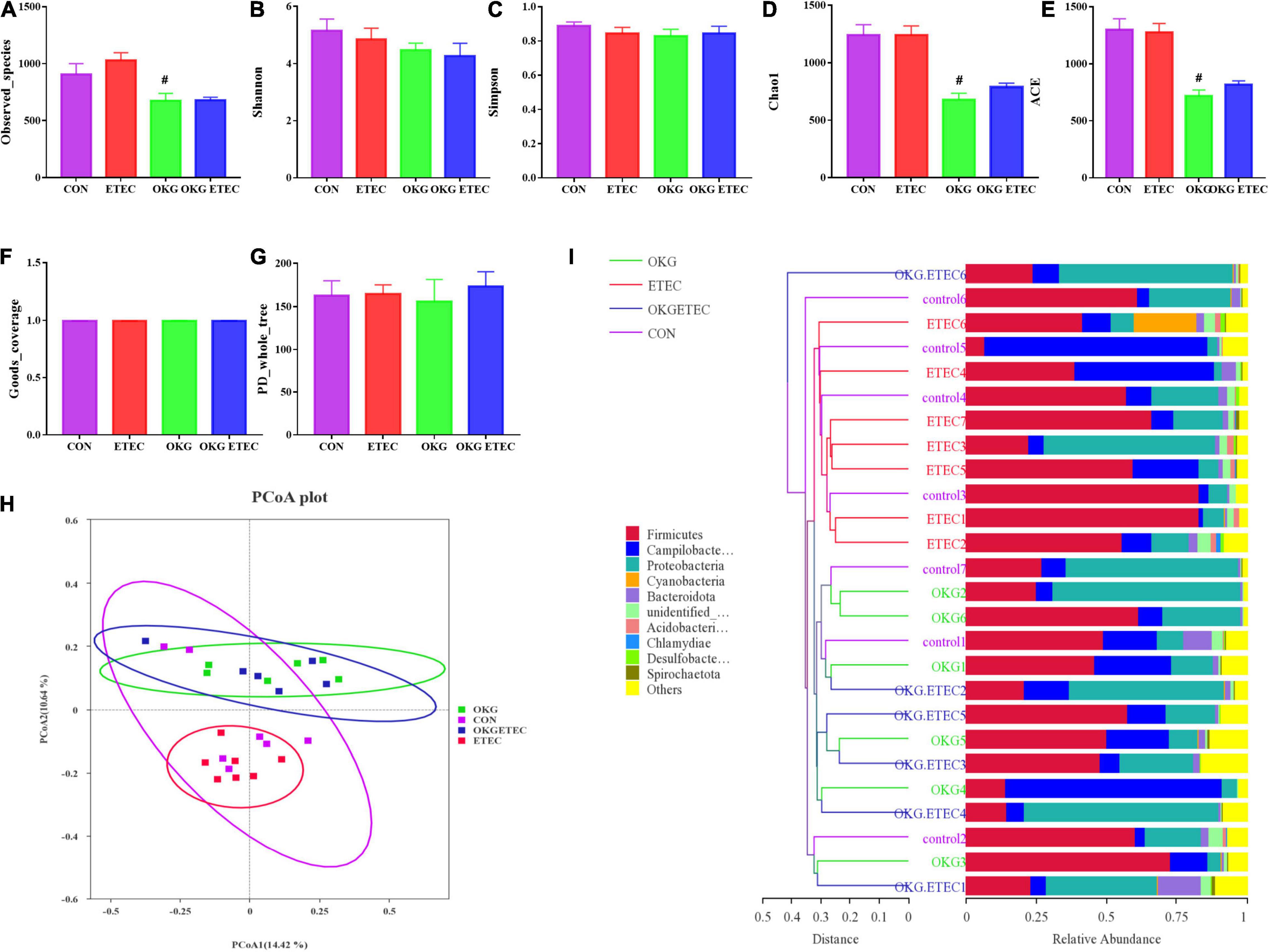
Figure 2. Effects of OKG supplementation on ileal mucosa microbiota diversity in ETEC- infected pigs. (A–G) α-diversity analysis, (H) PCoA plot analysis from each sample, (I) weighted UniFrac distance in each sample. Values are presented as mean ± SEM. *Indicates a statistically significant difference for the challenge (ETEC or LB Broth) (p < 0.05). #Indicates a statistically significant difference for dietary treatment (basal or OKG diet) (p < 0.05).
The overall microbial composition at the phylum, order, and genus levels is presented in Figure 3. Firmicutes, Campylobacterales, and Escherichia-Shigella were the predominant flora in phylum, order, and genus level, respectively. ETEC-challenge markedly increased Acidobacteriota, while OKG treatment markedly reversed these alterations (p < 0.05). OKG ETEC groups had a higher proportion of Proteobacteria, but a lower proportion of Firmicutes (p < 0.05). At the order levels, Lactobacillales were decreased in relative abundance by ETEC-challenged pigs (p < 0.05). OKG treatment markedly increased the proportion of Enterobacterales but decreased the proportion of Peptostreptococcales-Tissierellales. Similar alterations were observed for Actinobacillus, [Acetivibrio]_cthanolgignens_group, and Turicibacter at the genus level (p < 0.05).
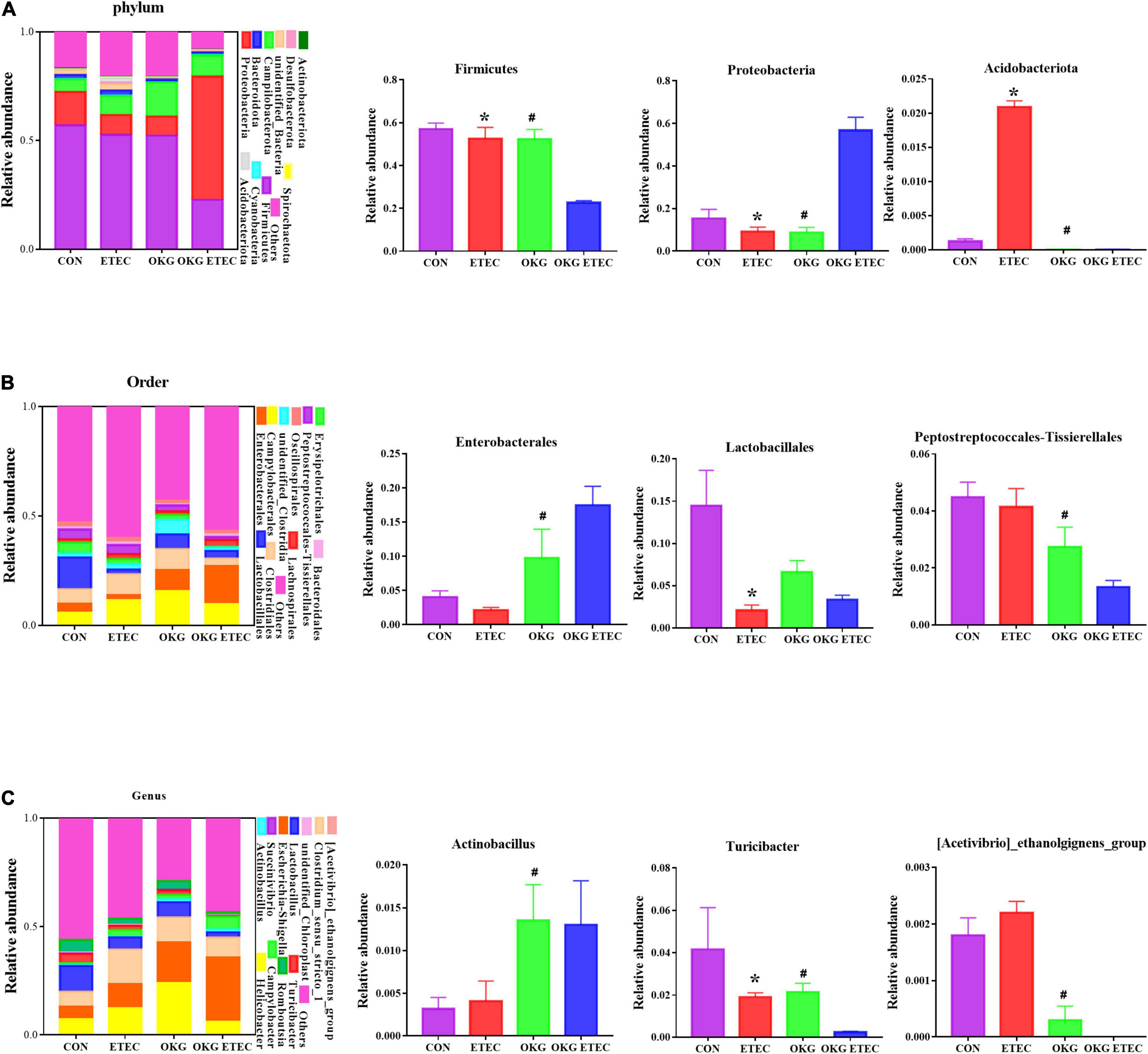
Figure 3. Effects of OKG supplementation on ileal mucosa microbiota compositions at phylum, order, and genus level in ETEC-infected pigs. (A) Microbiota compositions at the phylum level, (B) microbiota compositions at the order level, and (C) microbiota compositions at the genus level. Values are presented as mean ± SEM. *Indicates a statistically significant difference for the challenge (ETEC or LB Broth) (p < 0.05). #Indicates a statistically significant difference for dietary treatment (basal or OKG diet) (p < 0.05).
Phylogenetic Investigation of Communities by Reconstruction of Unobserved States analysis showed that ETEC treatment markedly increased ribosome and mitochondrial biogenesis (p < 0.05, Figure 4). OKG treatment significantly increased amino-acid related enzymes, amino sugar and nucleotide sugar metabolism, aminoacyl-tRNA biosynthesis, chromosome and associated proteins, cysteine and methionine metabolism, DNA repair and recombination proteins, DNA replication proteins, glycolysis/gluconeogenesis, homologous recombination, mismatch repair, peptidoglycan biosynthesis, and degradation proteins, purine metabolism, pyrimidine metabolism, ribosome, starch and sucrose metabolism, and transfer RNA biogenesis, but decreased bacterial motility proteins, quorum sensing, and the two-component system (p < 0.05).
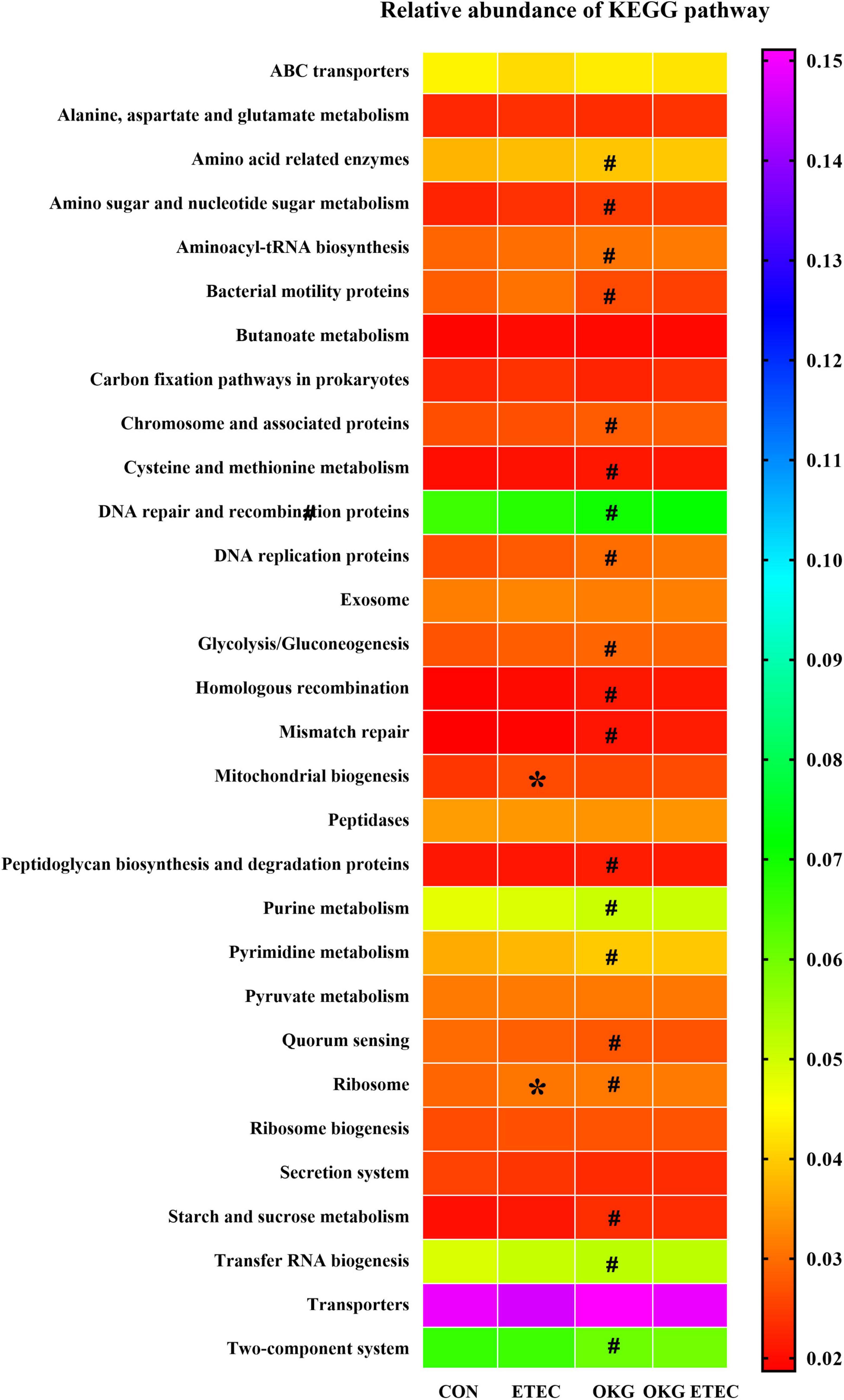
Figure 4. Predictive functional profiling of microbial communities by PICRUSt. KEGG pathway annotations in level 3. Values are presented as mean ± SEM. *Indicates a statistically significant difference for the challenge (ETEC or LB Broth) (p < 0.05). #Indicates a statistically significant difference for dietary treatment (basal or OKG diet) (p < 0.05).
The peaks extracted from all experimental samples and QC samples were analyzed by Principal component analysis (PCA; Figures 5A,B). The QC samples were clustered, which indicated that the data were reliable. Also, the partial least squares discriminant analysis (PLS–DA) score plots presented that ETEC and OKG led to significant metabolite changes (Figures 5C–J).
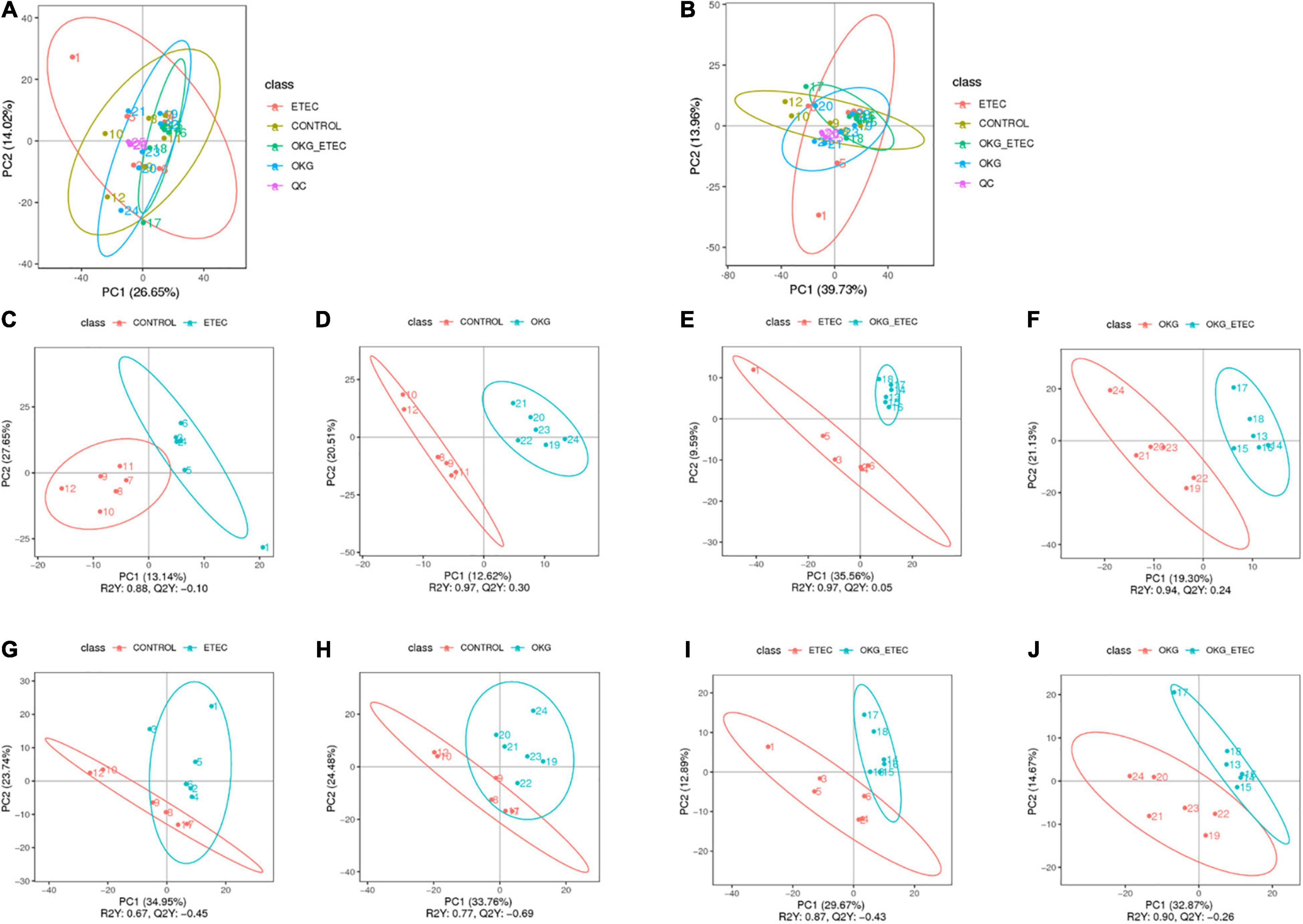
Figure 5. Score plots of principal component analysis (PCA) and Partial least squares discrimination analysis (PLS-DA) models. (A,B) PCA based on positive and negative ileal mucosa metabolite profiles. (C–J) PLS-DA based on positive and negative ileal mucosa metabolite profiles.
Metabolome analyzed a total of 156 positive ions and 39 negative ions with functional annotations. Among them, 21 (20 up and 1 down), 50 (13 up and 37 down), 53 (30 up and 234 down), and 71 (47 up and 24 down) metabolites with functional annotations were significantly different (p < 0.05 and VIP > 1) in the four pairwise comparisons (ETEC vs. CON, CON vs. OKG, ETEC vs. OKG ETEC, and OKG vs. OKG ETEC; Figure 6A). Their m/z, VIP values, and p-values are listed in Supplementary Table 1. In Figures 6B,C, the volcano plots showed the differentially expressed metabolites in the comparisons. Next, the Kyoto Encyclopedia of Genes and Genomes (KEGG) was used to analyze the pathways of the metabolites that differed between the two groups (Figure 7). Compared with the control group, the main metabolic pathways in the OKG group that were enriched are arachidonic acid metabolism, serotonergic synapse, and primary bile acid biosynthesis, and the main metabolic pathways in the ETEC group that were enriched are steroid biosynthesis (P < 0.05). Compared with the OKG ETEC group, the main metabolic pathways in the OKG group that were enriched are beta-alanine metabolism, bile secretion, glutathione metabolism, protein digestion and absorption, and arginine and proline metabolism, and the main metabolic pathways in the ETEC group that were enriched are taurine and hypotaurine metabolism (p < 0.05).
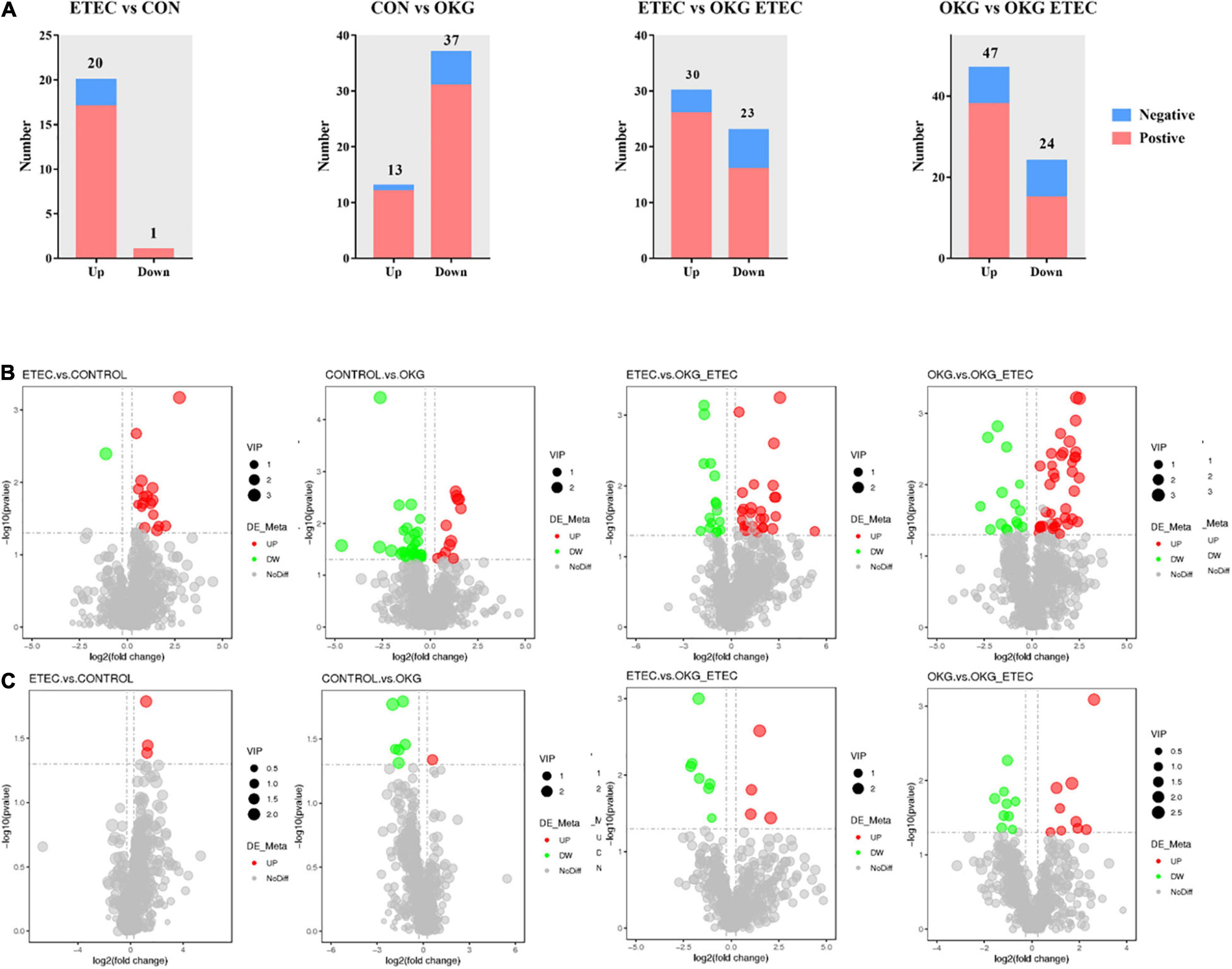
Figure 6. Effects of OKG supplementation on ileal mucosa metabolites in ETEC- infected pigs. (A) The number of differentially expressed metabolites with functional annotations (p < 0.05 and VIP > 1) in the four groups of piglets. (B,C) Volcano plots in differential metabolites, red or green spots indicate a statistically significant up-regulation or down-regulation in the four pairwise comparisons (ETEC vs. CON, CON vs. OKG, ETEC vs. OKG ETEC, and OKG vs. OKG ETEC).
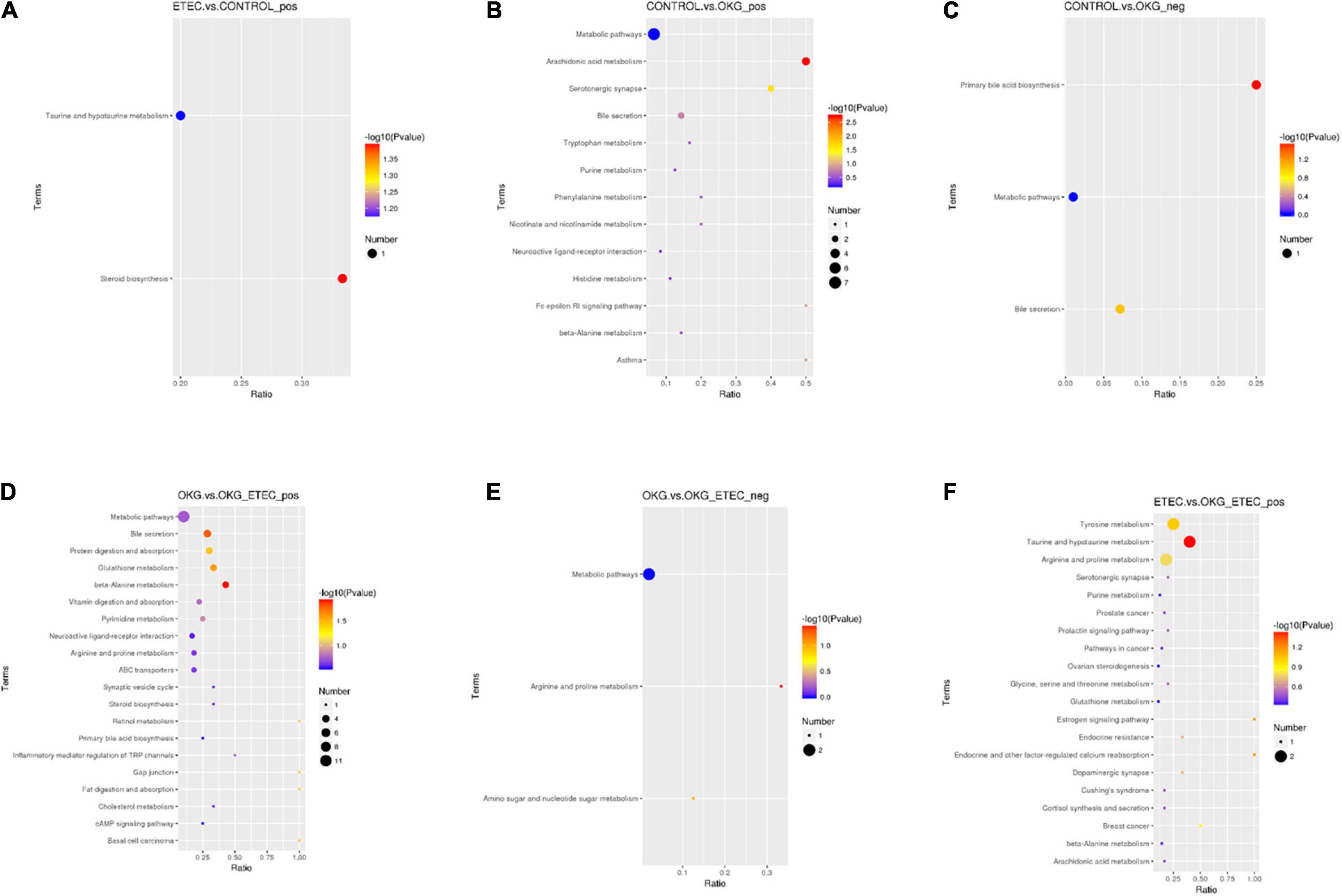
Figure 7. Effects of OKG supplementation on KEGG pathway enrichment in ETEC- infected pigs. (A) ETEC vs. CON on positive mode. (B–C) CON vs. OKG on positive and negative mode. (D–E) OKG vs. OKG ETEC on positive and negative mode. (F) ETEC vs. OKG ETEC on positive mode.
Based on the comparison of the data with KEGG, 14 identified endogenous metabolites were characterized. These metabolites were ergocalciferol, leukotriene C4, 16(R)-HETE, thromboxane B2, chenodeoxycholic acid, sulfoacetic acid, hypotaurine, spermidine, tyramine, spermine, cholesterol, L-histidine, cadaverine, serotonin, and D-proline. Among these metabolites, ergocalciferol was increased significantly in the ETEC pigs, and leukotriene C4, 16(R)-HETE, thromboxane B2, chenodeoxycholic acid were increased clearly in the OKG pigs compared to the controls (p < 0.05). Compared with the OKG ETEC group, sulfoacetic acid and hypotaurine were clearly increased in the ETEC pigs, and spermidine, tyramine, cholesterol, L-histidine, cadaverine, serotonin were significantly increased, while spermine and D-proline were markedly decreased in the OKG group (p < 0.05).
The results of Pearson correlation (r) between metabolites and microbiota are shown in Figure 8. We found different levels of correlation in two groups of correlation analyses (p < 0.05): cadaverine vs. Actinobacillus (R2 = 0.2387; p = 0.0154; Figure 8A) and L-histidine vs. Turicibacter (R2 = 0.4068; p = 0.0033; Figure 8B).
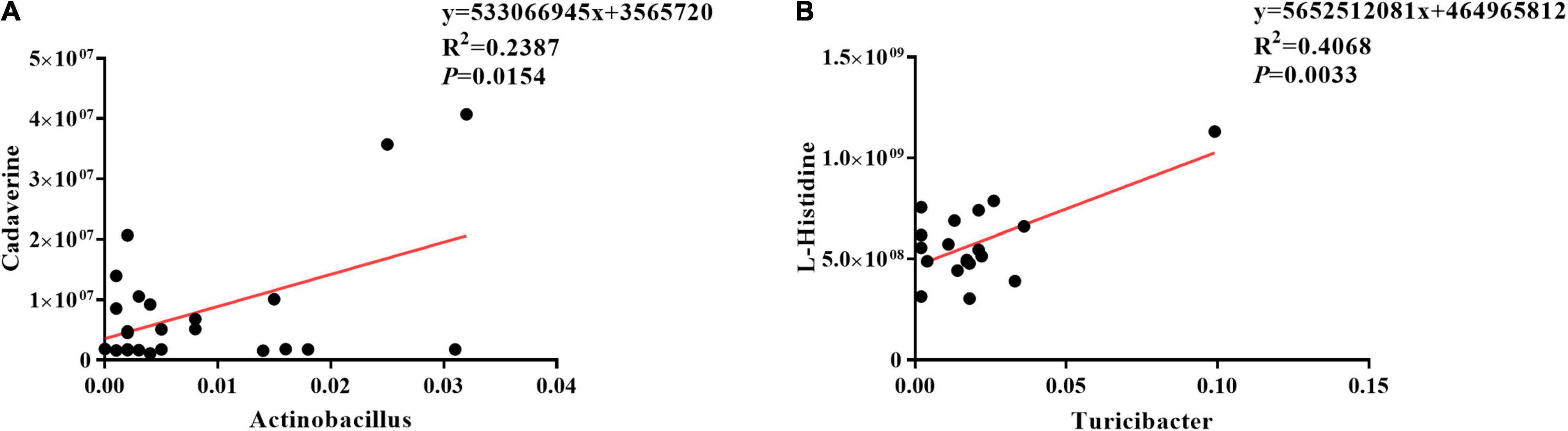
Figure 8. Correlation analyses between ileal mucosa microbiota and metabolites. (A) Cadaverine vs. Actinobacillus, (B) L-histidine vs. Turicibacter.
Ornithine α-ketoglutarate treatment failed to affect NF-κB expression in ETEC-challenged pigs. We further determined several downstream signals of NF-κB (i.e., JNK and p38). The results indicated that OKG treatment suppressed the phosphorylation of JNK and p38 (p < 0.05, Figure 9).
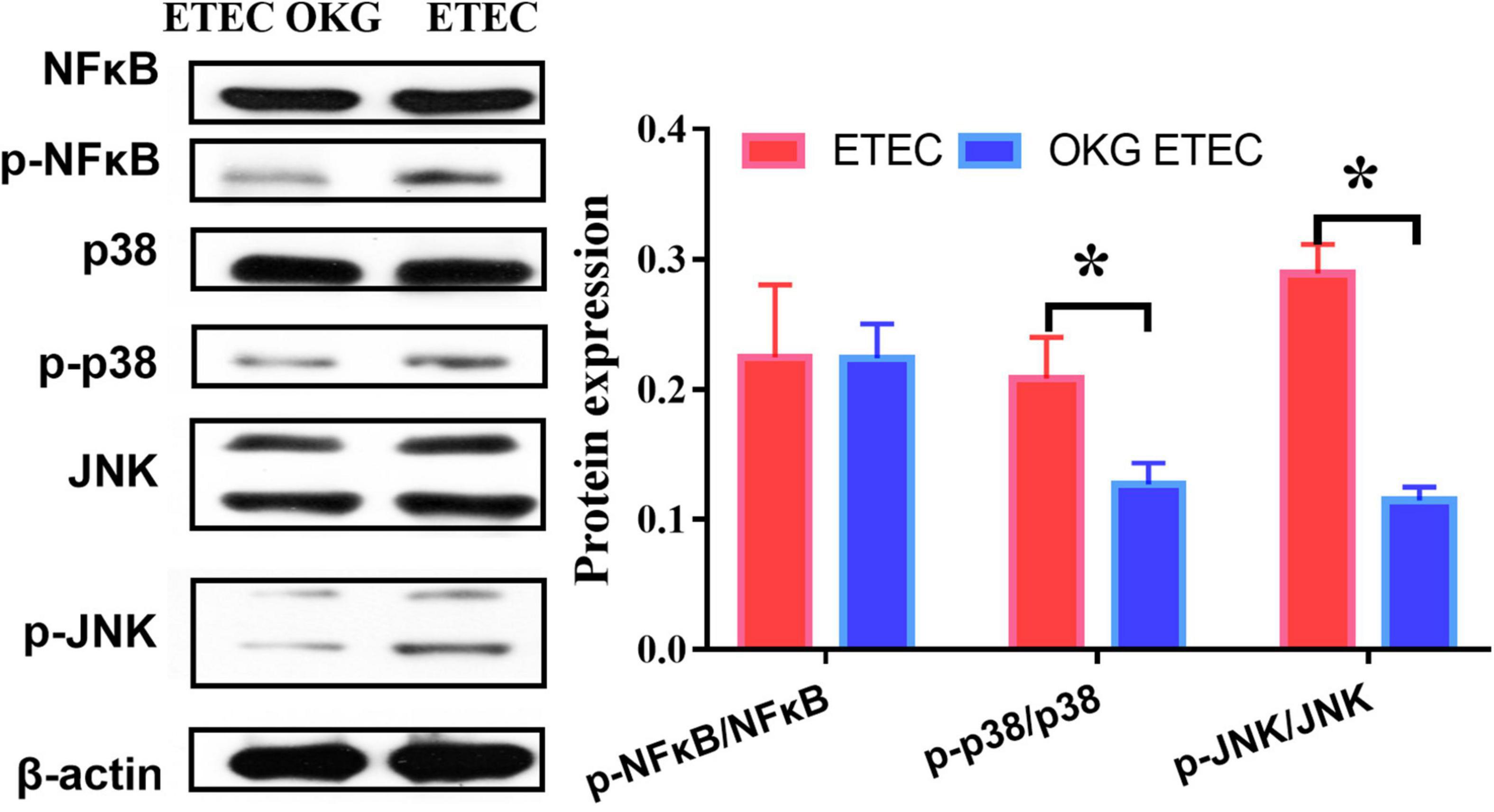
Figure 9. Effects of OKG supplementation on NF-κB/JNK inflammatory pathways in ETEC infected pigs. Values are presented as mean ± SEM. *Indicates a statistically significant difference for the challenge (ETEC or LB Broth) (p < 0.05).
Enterotoxigenic Escherichia coli is considered to be a potential pathogen threatening human and animal diarrhea, resulting in huge economic losses (25). ETEC triggers inflammation and intestinal dysfunction, ultimately leading to diarrhea (26). To induce pig diarrhea at least 109 to 1010 ETEC is required that is generally obtained 1–5 days after ETEC infection (27). The nutritional supplement can reduce ETEC infection (20). In detail, arginine or glutamine supplementation suppresses ETEC colonization in the ETEC-challenged mouse (28). Our group and others have previously demonstrated that OKG changes or reverses growth performance, intestinal microbes, and immunity in animal models (14, 29). OKG is a nutrient compound obtained from ornithine and α-ketoglutarate (30), which is the precursor of glutamine, arginine, proline, and polyamines (12). However, there is no direct evidence that OKG mediated gut microbiota and immunity in ETEC-infected pig models based on previous reports. Thus, in the current study, we focused on OKG to prevent ETEC-infection diarrhea via regulation of immunity, alterations of ileal mucosa microbiota and metabolites. Notably, growth performance was inhibited in ETEC infection, and OKG supplementation improved growth performance, which is similar to the effect of OKG on pigs induced with D-galactose chronic oxidative stress and OKG on tumor-bearing rats (14, 29). In ETEC K88-infection pigs, the day immediately after inoculation, a clear peak of diarrhea could also be observed in infected animals, and Chito-oligosaccharide reduced the incidence of diarrhea (1). Similarly, our study found that OKG supplementation markedly reduced the feces score after ETEC infection, which is in accordance with previous reports.
Pro-inflammatory cytokines such as IFN-γ, TNF-α, IL-1, and IL-6 are measured as biomarkers for gastrointestinal disorders, including ETEC-infection diarrhea (31). An NF-κB signaling pathway is associated with inflammation (26). Intermittent fasting can inhibit the NF-κB/JNK inflammatory pathways and thus reduce inflammation and alleviate type-2 diabetes symptoms (32). Our results also demonstrate that ETEC-infection increases the serum TNF-α, and IL-6, while OKG can significantly decrease the serum IL-6 and suppress the phosphorylation of downstream signals of NF-κB/JNK. Thus, the anti-inflammatory effect of OKG may be associated with suppressing the NF-κB pathway. IL-10 can inhibit the production of other inflammatory factors by blocking the activation of NF-κB and is an important regulator of the immune response (33, 34). SIgA is the main part of the intestinal immunological barrier and the most abundant immunoglobulin in the body, which also can clear pathogenic microorganisms (6, 35, 36). Nucleotides supplementation promotes the development of small intestinal villus and secretory IgA in neonatal piglets, prevents diarrhea, and increases the weaning weight of piglets (19). Meantime, we found similar results that OKG supplementation can increase the ileum SIgA and serum IL-10 secretion in piglets.
Chitosan supplementation enhances the gene expression of IL-1β and IL-6 in the intestine compared with ETEC, alleviates intestinal inflammation, and enhances the cell-mediated immune response (1). ETEC-infection can suppress the gene expression of pro-inflammatory cytokines, including IL-1β, IL-6, IL-8, TNF-α, and IL-17A, indicating ETEC infection inhibits the inflammatory reaction (37). In the neonatal piglets, nucleotides upregulate the gene expression of IL-17, IL-8, IL-6, IL-1β, IL-10, and TNF-α in the intestine, prevent diarrhea, and increase the weaning weight of piglets (19). Meanwhile, pathogen-associated molecular patterns adaptors include MyD88 (38). Asp supplementation suppresses TLRs and MyD88 expression in LPS-induced weaned pigs (39). Similarly, our experimental results indicate that ETEC-infection downregulates the mRNA expression of IL-1β, IL-6, and MyD88 and improves the mRNA expression of IL-8, IL-18, and TLR4, while OKG supplementation increases the gene expression of IL-1β and IL-10 and reduces the NF-κB and MyD88 in the ileum. However, OKG supplementation fails to affect NF-κB expression, and it only suppresses the phosphorylation of downstream signals of NF-κB/JNK. mRNA–protein relation is generally linear; however, mRNA to protein in vivo involves transcription, translation, and the turnover of proteins, and thus the determinants of protein abundance (40, 41). These observations suggested that OKG may alleviate intestinal inflammation and prevent diarrhea when ETEC infection represses the intestinal inflammatory response
The gastrointestinal microbes act as the first line against harmful endogenous and exogenous substances entering the body (42–45). The homeostasis between the host and gastrointestinal microbiota is disturbed so that it results in the incidence of diarrhea (26). OKG administration can prevent bacterial dissemination so that endotoxemia is reduced (46). In ETEC infection-induced diarrhea piglet model, the diversity, structure, and function of the gut microbial community are decreased and ETEC could induce diarrhea via intestinal microbiota in piglets (47). However, OKG groups change the diversity, structure, and function of the gut microbial community, including Observed species, Chao1 and ACE, decreased and difference in beta-diversity presented difference. The reason may be that the substantially higher relative abundance of Enterobacterales, which is consistent with OKG, suppressed alpha diversity in our previous study (14). At the genus level, OKG increased Actinobacillus, but decreased Turicibacter and [Acetivibrio]_ethanolgignens_group. Intestinal fora [Acetivibrio]_ethanolgignens_group and Turicibacter are involved in immune function (47, 48). [Acetivibrio]_ethanolgignens_group can induce metabolic disorder and liver inflammation (48). Turicibacter has adverse effects on intestinal health and lipid metabolism of the host (49, 50). Additionally, increased Actinobacillus can impair barrier function (51). However, OKG supplementation failed to reduce the Actinobacillus abundance. The reason may be that OKG decreased other pro-inflammatory bacteria, including Turicibacter and [Acetivibrio]_ethanolgignens_group, thus improving growth performance and regulating immunity. Predictive functional profiling of microbial communities further confirms that altered microbiota mainly involves genes related to the ribosome and mitochondrial biogenesis, amino acid-related enzymes, amino sugar and nucleotide sugar metabolism, aminoacyl-tRNA biosynthesis, chromosome and associated proteins, cysteine and methionine metabolism, DNA repair and recombination proteins, DNA replication proteins, glycolysis/gluconeogenesis, homologous recombination, mismatch repair, peptidoglycan biosynthesis and degradation proteins, purine metabolism, pyrimidine metabolism, ribosome, starch and sucrose metabolism, and transfer RNA biogenesis, but decreased bacterial motility proteins, quorum sensing, and two-component system. These suggested that OKG prevents ETEC-infection diarrhea via alterations of the ileal mucosa microbiota.
The present study also determined ileal mucosal microbe-derived metabolites consistent with changes in the ileal mucosa microbiome. OKG increased leukotriene C4, 16(R)-HETE, thromboxane B2, and chenodeoxycholic acid. Leukotriene C4, 16(R)-HETE, and thromboxane B2, a product of arachidonic acid, can improve anti-inflammation, enhance intestinal health, and cure disease (52–55). For instance, 16(R)-HETE can suppress the activation of polymorphonuclear leukocytes and reduce intracranial pressure (53). Chenodeoxycholic acid, which is involved in primary bile acid biosynthesis, inhibits pro-inflammatory cytokines (56). These suggested that OKG regulates immune status and alleviates the suppression in piglets infected by ETEC via stimulating the ileal mucosa microbe and metabolites. Our data indicate that the cadaverine level was positively correlated with Actinobacillus and L-histidine level was positively correlated with Turicibacter abundance.
In conclusion, dietary supplementation with 1% OKG regulates serum levels of TNF-α, IL-6, and IL-10, alters the genes expression related to ileal immunity, and alleviates the growth-suppression of piglets infected by ETEC, which may be associated with positive alterations in the composition of gut microbiota and modulation in the gut metabolites, especially for Actinobacillus, [Acetivibrio]_ethanolgignens_group, and Turicibacter. Further studies with the use of OKG in healthy animals and clinical application are anticipated.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: NCBI, PRJNA808781.
The animal study was reviewed and approved by Committee on Animal Care of the Institute of Subtropical Agriculture, Chinese Academy of Sciences (Changsha, CAS20190409). Written informed consent was obtained from the owners for the participation of their animals in this study.
YL and KY conceived the study. YL wrote the original draft of the manuscript. All authors have contributed to the development of the methodology, design of the study, reviewed, and edited the manuscript.
This research was supported by the Key Programs of Frontier Scientific Research of the Chinese Academy of Sciences (QYZDY-SSW-SMC008), TaiShan Industrial Experts Program (tscy20190121).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We would like to thank members of the laboratory of YY for helpful discussions. We also thank Baolikang Biological Feed Co., Ltd. and Yichun Dahaigui Life Science Co., Ltd. for the animal feeding support. We are grateful to the Public Service Technology Center, Institute of Subtropical Agriculture, Chinese Academy of Sciences for technical support.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2022.862498/full#supplementary-material
Supplementary Data Sheet 1 | Effects of OKG supplementation on ileal mucosa differential metabolites in ETEC- infected pigs.
1. Xiao D, Wang Y, Liu G, He J, Qiu W, Hu X, et al. Effects of chitosan on intestinal inflammation in weaned pigs challenged by enterotoxigenic Escherichia coli. PLoS One. (2014) 9:e104192. doi: 10.1371/journal.pone.0104192
2. Hansen LHB, Nielsen B, Boll EJ, Skjot-Rasmussen L, Wellejus A, Jorgensen L, et al. Functional in vitro screening of probiotic strains for inoculation of piglets as a prophylactic measure towards enterotoxigenic Escherichia coli infection. J Microbiol Methods. (2021) 180:106126. doi: 10.1016/j.mimet.2020.106126
3. Dubreuil JD. Enterotoxigenic Escherichia coli and probiotics in swine: what the bleep do we know? Biosci Microbiota Food Health. (2017) 36:75–90. doi: 10.12938/bmfh.16-030
4. Qiao J, Sun Z, Liang D, Li H. Lactobacillus salivarius alleviates inflammation via NF-kappaB signaling in ETEC K88-induced IPEC-J2 cells. J Anim Sci Biotechnol. (2020) 11:76. doi: 10.1186/s40104-020-00488-5
5. Massacci FR, Tofani S, Forte C, Bertocchi M, Lovito C, Orsini S, et al. Host genotype and amoxicillin administration affect the incidence of diarrhoea and faecal microbiota of weaned piglets during a natural multiresistant ETEC infection. J Anim Breed Genet. (2020) 137:60–72. doi: 10.1111/jbg.12432
6. Zhao Y, Wang J, Wang H, Huang Y, Qi M, Liao S, et al. Effects of GABA supplementation on intestinal SIgA secretion and gut microbiota in the healthy and ETEC-infected weanling piglets. Mediators Inflamm. (2020) 2020:7368483. doi: 10.1155/2020/7368483
7. Jimenez MJ, Berrios R, Stelzhammer S, Bracarense A. Ingestion of organic acids and cinnamaldehyde improves tissue homeostasis of piglets exposed to enterotoxic Escherichia coli (ETEC). J Anim Sci. (2020) 98:skaa012. doi: 10.1093/jas/skaa012
8. Mirabile A, Rivoltini L, Daveri E, Vernieri C, Mele R, Porcu L, et al. Metabolism and Immune Modulation in patients with solid tumors: systematic review of preclinical and clinical evidence. Cancers (Basel). (2020) 12:E1153. doi: 10.3390/cancers12051153
9. Albaugh VL, Mukherjee K, Barbul A. Proline precursors and collagen synthesis: biochemical challenges of nutrient supplementation and wound healing. J Nutr. (2017) 147:2011–7. doi: 10.3945/jn.117.256404
10. Goncalves ES, Rabelo CM, Prado Neto AX, Garcia JH, Guimaraes SB, Vasconcelos PR. Effect of short-term ornithine alpha-ketoglutarate pretreatment on intestinal ischemia-reperfusion in rats. Acta Cir Bras. (2011) 26(Suppl. 1):2–7. doi: 10.1590/s0102-86502011000700002
11. Meaume S, Kerihuel JC, Constans T, Teot L, Lerebours E, Kern J, et al. Efficacy and safety of ornithine alpha-ketoglutarate in heel pressure ulcers in elderly patients: results of a randomized controlled trial. J Nutr Health Aging. (2009) 13:623–30. doi: 10.1007/s12603-009-0173-z
12. Pernet P, Coudray-Lucas C, Schneid C, Jardel A, Cynober L. Dose dependency of the effect of ornithine α-ketoglutarate on tissue glutamine concentrations and hypercatabolic response in endotoxaemic rats. Br J Nutr. (2007) 92:627. doi: 10.1079/bjn20041254
13. Tatara MR, Sliwa E, Krupski W, Brodzki A, Pasternak K. Ornithine alpha-ketoglutarate increases mineralization and mechanical properties of tibia in turkeys. Bone. (2006) 39:100–5. doi: 10.1016/j.bone.2005.12.016
14. Li Y, Wang P, Yin J, Jin S, Su W, Tian J, et al. Effects of ornithine α-ketoglutarate on growth performance and gut microbiota in a chronic oxidative stress pig model induced by D-galactose. Food Funct. (2020) 11:472–82. doi: 10.1039/c9fo02043h
15. Guzik AC, Southern LL, Matthews JO, Bidner TD, Ladner JP. Ornithine alpha-ketoglutarate and creatine effects on growth and plasma metabolites of nursery pigs. J Anim Sci. (2000) 78:1022–8. doi: 10.2527/2000.7841022x
16. Xu M, Zhang X, Ren F, Yan T, Wu B, Bi K, et al. Essential oil of Schisandra chinensis ameliorates cognitive decline in mice by alleviating inflammation. Food Funct. (2019) 10:5827–42. doi: 10.1039/c9fo00058e
17. Li Y, Han H, Yin J, He X, Tang Z, Li T, et al. D- and L-Aspartate regulates growth performance, inflammation and intestinal microbial community in young pigs. Food Funct. (2019) 10:1028–37. doi: 10.1039/c8fo01410h
18. Yin J, Li YY, Han H, Chen S, Gao J, Liu G, et al. Melatonin reprogramming of gut microbiota improves lipid dysmetabolism in high-fat diet-fed mice. J Pineal Res. (2018) 65:e12524. doi: 10.1111/jpi.12524
19. Gao L, Xie C, Liang X, Li Z, Li B, Wu X, et al. Yeast-based nucleotide supplementation in mother sows modifies the intestinal barrier function and immune response of neonatal pigs. Anim Nutr. (2021) 7:84–93. doi: 10.1016/j.aninu.2020.06.009
20. Chen S, Wu X, Xia Y, Wang M, Liao S, Li F, et al. Effects of dietary gamma-aminobutyric acid supplementation on amino acid profile, intestinal immunity, and microbiota in ETEC-challenged piglets. Food Funct. (2020) 11:9067–74. doi: 10.1039/d0fo01729a
21. Yin J, Li Y, Han H, Liu ZJ, Zeng XF, Li T, et al. Long-term effects of lysine concentration on growth performance, intestinal microbiome, and metabolic profiles in a pig model. Food Funct. (2018) 9:4153–63. doi: 10.1039/c8fo00973b
22. Manuel M. A new semi-subterranean diving beetle of the Hydroporus normandi-complex from south-eastern France, with notes on other taxa of the complex (Coleoptera: Dytiscidae). Zootaxa. (2013) 3652:453–74. doi: 10.11646/zootaxa.3652.4.4
23. Want EJ, Masson P, Michopoulos F, Wilson ID, Theodoridis G, Plumb RS, et al. Global metabolic profiling of animal and human tissues via UPLC-MS. Nat Protoc. (2013) 8:17–32. doi: 10.1038/nprot.2012.135
24. Wen B, Mei Z, Zeng C, Liu SJBB. metaX: a flexible and comprehensive software for processing metabolomics data. BMC Bioinformatics. (2017) 18:183. doi: 10.1186/s12859-017-1579-y
25. Yang D, Mai K, Zhu Y, Liu Y, Luo C, Zhou Q, et al. Research Progress on new vaccines against swine ETEC in both domestic and overseas. China Anim Husb & Vet Med. (2019) 46:2119–26. doi: 10.16431/j.cnki.1671-7236.2019.07.029
26. Yue Y, He Z, Zhou Y, Ross RP, Stanton C, Zhao J, et al. Lactobacillus plantarum relieves diarrhea caused by enterotoxin-producing Escherichia coli through inflammation modulation and gut microbiota regulation. Food Funct. (2020) 11:10362–74. doi: 10.1039/d0fo02670k
27. Sun Y, Kim SW. Intestinal challenge with enterotoxigenic Escherichia coli in pigs, and nutritional intervention to prevent postweaning diarrhea. Anim Nutr. (2017) 3:322–30. doi: 10.1016/j.aninu.2017.10.001
28. Liu G, Ren W, Fang J, Hu CA, Guan G, Al-Dhabi NA, et al. L-glutamine and L-arginine protect against enterotoxigenic Escherichia coli infection via intestinal innate immunity in mice. Amino Acids. (2017) 49:1945–54. doi: 10.1007/s00726-017-2410-9
29. Le Bricon T, Cynober L, Baracos VJM. Ornithine α-ketoglutarate limits muscle protein breakdown without stimulating tumor growth in rats bearing Yoshida ascites hepatoma. Metabolism. (1994) 43:899–905. doi: 10.1016/0026-0495(94)90274-7
30. Allouchi H, Ceolin R, Berthon L, Tombret F, Rietveld IB. Characterization of molecular associations involving L-ornithine and alpha-ketoglutaric acid: crystal structure of L-ornithinium alpha-ketoglutarate. Ann Pharm Fr. (2014) 72:238–43. doi: 10.1016/j.pharma.2014.04.005
31. Lopez-Colom P, Yu K, Barba-Vidal E, Saco Y, Martin-Orue SM, Castillejos L, et al. I-FABP, Pig-MAP and TNF-alpha as biomarkers for monitoring gut-wall integrity in front of Salmonella Typhimurium and ETEC K88 infection in a weaned piglet model. Res Vet Sci. (2019) 124:426–32. doi: 10.1016/j.rvsc.2019.05.004
32. Liu Z, Dai X, Zhang H, Shi R, Hui Y, Jin X, et al. Gut microbiota mediates intermittent-fasting alleviation of diabetes-induced cognitive impairment. Nat Commun. (2020) 11:855. doi: 10.1038/s41467-020-14676-4
33. Vallabhapurapu S, Karin M. Regulation and function of NF-kappa B transcription factors in the immune system. Ann Rev Immunol. (2009) 27:693–733. doi: 10.1146/annurev.immunol.021908.132641
34. Zheng L, Kelly CJ, Battista KD, Schaefer R, Lanis JM, Alexeev EE, et al. Microbial-derived butyrate promotes epithelial barrier function through IL-10 receptor–dependent repression of claudin-2. J Immunol. (2017) 199:2976–84. doi: 10.4049/jimmunol.1700105
35. Xiao D, Tang Z, Yin Y, Zhang B, Hu X, Feng Z, et al. Effects of dietary administering chitosan on growth performance, jejunal morphology, jejunal mucosal sIgA, occludin, claudin-1 and TLR4 expression in weaned piglets challenged by enterotoxigenic Escherichia coli. Int Immunopharmacol. (2013) 17:670–6. doi: 10.1016/j.intimp.2013.07.023
36. Song B, Zheng C, Zha C, Hu S, Yang X, Wang L, et al. Dietary leucine supplementation improves intestinal health of mice through intestinal SIgA secretion. J Appl Microbiol. (2020) 128:574–83. doi: 10.1111/jam.14464
37. Yang X, Xiao Z, Liu F, Chen S, Tang W, Zhang D, et al. Enterotoxigenic Escherichia coli infection alters intestinal immunity in mice. Mol Med Rep. (2016) 14:825–30. doi: 10.3892/mmr.2016.5302
38. Koliada A, Syzenko G, Moseiko V, Budovska L, Puchkov K, Perederiy V, et al. Association between body mass index and Firmicutes/Bacteroidetesratio in an adult Ukrainian population. BMC Microbiol. (2017) 17:120. doi: 10.1186/s12866-017-1027-1
39. Wang H, Liu Y, Shi H, Wang X, Zhu H, Pi D, et al. Aspartate attenuates intestinal injury and inhibits TLR4 and NODs/NF-kappaB and p38 signaling in weaned pigs after LPS challenge. Eur J Nutr. (2017) 56:1433–43. doi: 10.1007/s00394-016-1189-x
40. Buccitelli C, Selbach M. mRNAs, proteins and the emerging principles of gene expression control. Nat Rev Genet. (2020) 21:630–44. doi: 10.1038/s41576-020-0258-4
41. Guimaraes JC, Miguel R, Arkin AP. Transcript level and sequence determinants of protein abundance and noise in Escherichia coli. Nucleic Acids Res. (2014) 42:4791–9. doi: 10.1093/nar/gku126
42. Li HH, Li YP, Zhu Q, Qiao JY, Wang WJ. Wang, dietary supplementation with Clostridium butyricum helps to improve the intestinal barrier function of weaned piglets challenged with enterotoxigenic Escherichia coli K88. J Appl Microbiol. (2018) 125:964–75. doi: 10.1111/jam.13936
43. Yin LY, Han J, Chen H, Gao S, Liu J, Wu G, et al. Melatonin reprogramming of gut microbiota improves lipid dysmetabolism in high-fat diet-fed mice. J Pineal Res. (2018) 65:e12524.
44. Juan Z, Zhao-Ling S, Ming-Hua Z, Chun W, Hai-Xia W, Meng-Yun L, et al. Oral administration of Clostridium butyricum CGMCC0313-1 reduces ovalbumin-induced allergic airway inflammation in mice. Respirology. (2017) 22:898–904. doi: 10.1111/resp.12985
45. Goudarzi M, Khodayar MJ, Smt HT, Ghaznavi H, Fatemi I, Mehrzadi S. Mehrzadi, pretreatment with melatonin protects against cyclophosphamide-induced oxidative stress and renal damage in mice. Fundam Clin Pharmacol. (2017) 31:625–35. doi: 10.1111/fcp.12303
46. Schlegel L, Coudray-Lucas C, Barbut F, Le Boucher J, Jardel A, Zarrabian S, et al. Bacterial dissemination and metabolic changes in rats induced by endotoxemia following intestinal E. coli overgrowth are reduced by ornithine α-ketoglutarate administration. J Nutr. (2000) 130:2897–902. doi: 10.1093/jn/130.12.2897
47. Ma D, Wang AC, Parikh I, Green SJ, Hoffman JD, Chlipala G, et al. Ketogenic diet enhances neurovascular function with altered gut microbiome in young healthy mice. Sci Rep. (2018) 8:6670. doi: 10.1038/s41598-018-25190-5
48. Yuan GH, Zhang Z, Gao XS, Zhu J, Guo WH, Wang L, et al. Gut microbiota-mediated tributyltin-induced metabolic disorder in rats. RSC Adv. (2020) 10:43619–28. doi: 10.1039/d0ra07502g
49. Wan X, Li T, Liu D, Chen Y, Liu Y, Liu B, et al. Effect of Marine Microalga Chlorella pyrenoidosa ethanol extract on lipid metabolism and gut microbiota composition in high-fat diet-fed rats. Mar Drugs. (2018) 16:498. doi: 10.3390/md16120498
50. Fak F, Nyman M, Zhong Y. Modulation of gut microbiota in rats fed high-fat diets by processing whole-grain barley to barley malt. Mol Nutr Food Res. (2015) 59:2066–76. doi: 10.1002/mnfr.201500187
51. Liao J, Li Q, Lei C, Yu W, Deng J, Guo J, et al. Toxic effects of copper on the jejunum and colon of pigs: mechanisms related to gut barrier dysfunction and inflammation influenced by the gut microbiota. Food Funct. (2021) 12:9642–57. doi: 10.1039/d1fo01286j
52. Bednar MM, Gross CE, Balazy MK, Belosludtsev Y, Colella DT, Falck JR, et al. 16(R)-hydroxy-5,8,11,14-eicosatetraenoic acid, a new arachidonate metabolite in human polymorphonuclear leukocytes. Biochem Pharmacol. (2000) 60:447–55. doi: 10.1016/s0006-2952(00)00345-2
53. Bednar MM, Gross CE, Russell SR, Fuller SP, Ahern TP, Howard DB, et al. 16(R)-hydroxyeicosatetraenoic acid, a novel cytochrome P450 product of arachidonic acid, suppresses activation of human polymorphonuclear leukocytes and reduces intracranial pressure in a rabbit model of thromboembolic stroke. Neurosurgery. (2000) 47:1410–8. doi: 10.1093/neurosurgery/47.6.1410
54. Wang D, Li RS, Wei SZ, Gao SJ, Xu Z, Liu HH, et al. Metabolomics combined with network pharmacology exploration reveals the modulatory properties of Astragali Radix extract in the treatment of liver fibrosis. Chin Med. (2019) 14:30. doi: 10.1186/s13020-019-0251-z
55. Li X, Yu H, Gong YJ, Wu PJ, Feng QS, Liu C. Fuzheng Xiaozheng prescription relieves rat hepatocellular carcinoma through improving anti-inflammation capacity and regulating lipid related metabolisms. J Ethnopharmacol. (2022) 284:114801. doi: 10.1016/j.jep.2021.114801
Keywords: ornithine α-ketoglutarate, enterotoxigenic Escherichia coli, ileal mucosa microbiota, ileal mucosa metabolites, immune status, inflammation
Citation: Li Y, Bao X, Yang F, Tian J, Su W, Yin J, Yao K, Li T and Yin Y (2022) Ornithine α-Ketoglutarate Alleviates Inflammation via Regulating Ileal Mucosa Microbiota and Metabolites in Enterotoxigenic Escherichia coli-Infected Pigs. Front. Nutr. 9:862498. doi: 10.3389/fnut.2022.862498
Received: 26 January 2022; Accepted: 07 March 2022;
Published: 07 June 2022.
Edited by:
Kai Wang, Chinese Academy of Agricultural Sciences (CAAS), ChinaReviewed by:
Ashwinkumar Subramenium Ganapathy, The Pennsylvania State University, United StatesCopyright © 2022 Li, Bao, Yang, Tian, Su, Yin, Yao, Li and Yin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kang Yao, eWFva2FuZ0Bpc2EuYWMuY24=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.