
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Nutr. , 22 March 2022
Sec. Nutrition and Metabolism
Volume 9 - 2022 | https://doi.org/10.3389/fnut.2022.860575
This article is part of the Research Topic The Model of Ramadan Diurnal Intermittent Fasting: Unraveling the Health Implications - Volume 1 View all 16 articles
Background: In recent years, intermittent fasting (IF) has gained popularity in the health and wellness in the world. There are numerous types of IF, all of which involve fasting periods that last longer than an overnight fast and involve limited meal time-windows, with or without calorie restriction. The objective of this review is to summarize the current evidence for the effects of Ramadan and non-Ramadan IF on gut microbiome.
Methods: We explored PubMed, Scopus, Web of Science, and Google Scholar according to the PRISMA criteria (Preferred Reporting Items for Systematic Reviews and Meta-Analysis). Animal and human studies were screened and reviewed separately by two researchers.
Results: Twenty-eight studies were selected after screening. Some of the studies were performed on animal models and some on humans. The results of these studies indicate a significant shift in the gut microbiota, especially an increase in the abundance of Lactobacillus and Bifidobacteria following fasting diets. The results of some studies also showed an increase in the bacterial diversity, decrease inflammation and increased production of some metabolites such as short-chain fatty acids (SCFAs) in individuals or samples under fasting diets. Moreover, Ramadan fasting, as a kind of IF, improves health parameters through positive effects on some bacterial strains such as Akkermansia muciniphila and Bacteroide. However, some studies have reported adverse effects of fasting diets on the structure of the microbiome.
Conclusion: In general, most studies have seen favorable results following adherence from the fasting diets on the intestinal microbiome. However, because more studies have been done on animal models, more human studies are needed to prove the results.
People can calorically restrict while feeling hungry, and this approach has already been demonstrated in various mammalian species to enhance life span, increase numerous physiological indicators, and lower metabolic parameters for chronic illness (1, 2). There are numerous types of intermittent fasting (IF), all of which involve fasting periods that last longer than an overnight fast and involve limited meal time-windows, with or without calorie restriction (3, 4).
The Islamic lunar calendar’s ninth month, Ramadan, is 11–12 days shorter than the Gregorian solar calendar. This indicates that the month of Ramadan revolves around the four seasons and the 12 months of the year. Fasting during Ramadan is an obligatory duty for all healthy adult Muslims, as stated in the Holy Quran where ALLAH says, “O you who believe, fasting is prescribed for you as it was prescribed for those before you, that you may develop God-consciousness” (Surat Al-Baqarah 2:183). Ramadan fasting is one of the most common types of fasting diets in which millions of Muslims around the world do not receive any food or drink for a daily time varies between 12 and 22 h (mean 12–14 h), depending on the geographical location and season during a special month for a month. Ramadan also spelled Ramazan, Ramzan, Ramadhan, or Ramathan, is the ninth month of the Islamic calendar, observed by Muslims worldwide as a month of fasting (sawm), prayer, reflection and community (5). According to Islamic law, during the days of Ramadan, healthy adults must fast at certain times of the day, while fasting is not required for premature children, the elderly, the sick, and pregnant and lactating women (6) #42; (7) #32.
In addition to Ramadan fasting diets, in recent years, there has been an increased interest in following modified fasting diets aimed at weight loss or the management of some chronic diseases among people in different countries (8). IF have greatly increased in recent decades as weight loss and some other metabolic benefits (9). The effectiveness of these diets in weight loss or management of metabolic parameters has varied depending on the type and duration of fasting diets (10).
The human gastrointestinal microbiome, which contains millions of organisms can be affected by various environmental factors such as diet. On the other hand, various studies have shown that adverse changes in the intestinal microbiome can be associated with the development of various chronic diseases (11). Some findings have revealed that fasting diets can also cause changes in the microbiome (12, 13).
The objective of this review is to summarize the current evidence for the effects of Ramadan and non-Ramadan IF on gut microbiome. We first review the evidence from pre-clinical studies to provide a background on the purported mechanisms by which fasting diets induces changes in gut microbiome and then focused on human studies.
The PubMed, Web of Science, Scopus, and Google Scholar databases were searched from their inception until December 2021 according to the PRISMA criteria (Preferred Reporting Items for Systematic Reviews and Meta-Analysis). We used from the keywords included “gut microbiome” OR “Fecal microbiota” OR “Gut microbial profile” OR “Gut microbiota” OR “gut flora” OR “intestinal flora” OR “intestinal microbiota” in combination with Fasting OR “Intermittent fasting” OR “Ramadan Fasting” OR “Islamic fasting.” Additional items were added after examining the referenced articles (Figure 1). Two authors (MR and SM) independently assessed the abstract and full text of the articles, and animal and human studies, which evaluated the effect of different types of fasting on the microbiome, were screened (Table 1). Disagreements were resolved by consensus. Studies were included in this review article if the following conditions were met: (1) animal and human studies investigating the effect of fasting diets on the gut microbiota, (2) in order to evaluating the gut microbiota alterations in various fasting conditions and probable mechanisms in improving overall metabolic health, types of fasting regimens were classified into two main subgroups (time restricted fasting including Ramadan fasting and 8/16 h fasting program and calorie restricted fasting including alternate day fasting, water only fasting and the Buchinger program). Studies were excluded if the main text was not available or was not in the English language. Reviews, protocols, conference papers and case reports were also excluded. Therefore, only original researches with original data on animal models or human patients exploring any kind of fasting regimes on gut microbiota were included in the present study.
Twenty-eight articles were included in the qualitative synthesis. The characteristics of the evaluated studies and their results, including the results of animal studies and human studies, are listed in Tables 2, 3. The following are the results of animal studies and then the results of human studies.
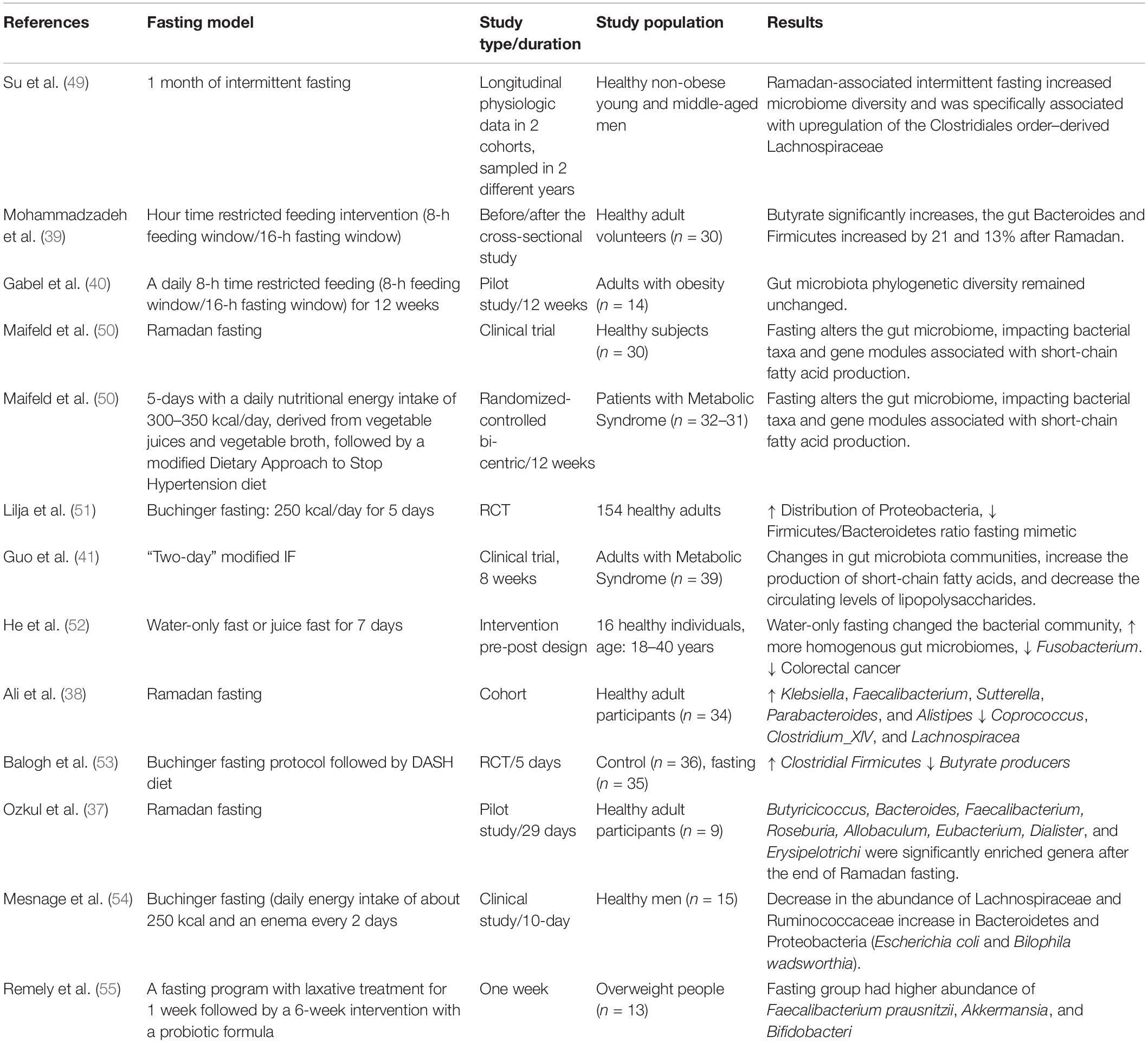
Table 3. Summary of the human studies investigating the effects of fasting on gut microbiota alterations.
Various animal studies have evaluated changes in the gut microbiome following a variety of fasting diets. Most animal studies on this interaction have been conducted in the past 5 years. Liu et al. in an experimental study compared the effect of intermediate fasting (IF) with melatonin administration on clinical variables and changes in the intestinal microbiome. They found that IF compared to the control group led to a significant increase in the abundance of Lactobacillus, Ruminococcus, and Akkermansia strains. Also, they found a significant reduction in the abundance of Helicobacter, Prevotella, and Parasutterella in the IF group (14). In another study on farmed mink (Neovision vision), the gut microbiota load and diversity showed no change after 3 days of fasting. Firmicutes were as the major phylum in the gut of these animals, however the Proteobacteria and Fusobacteria also were seen in another study (15). The rapid movement of food through the gastrointestinal tract may not allow enough time for bacterial metabolism to provide an environment that is suitable for growth of anaerobes (16).
Beli et al., evaluated the effects of long-term IF on gut microbiome, retinopathy and prolongs survival in db/db mice. The animals were fed ad libitum (AL) before the IF was initiated at 4 months of age. The db/db mice in the intervention group were then exposed to IF daily for up to 7 months. Microbiome analysis revealed increased levels of Firmicutes and decreased levels of Bacteroidetes and Verrucomicrobia in the IF group than control. Compared to the db/db mice on AL feeding, microbiome changes in the fasting group were associated with an increase in the gut mucin, goblet cell number, villi length, and reductions in plasma peptidoglycan (12). It has been reported in the previous studies that higher Firmicutes to Bacteroidetes ratio is associated with obesity (16, 17), as well as improve energy harvesting capacity (18). In this study, researchers used measurement of plasma peptidoglycan levels as an indicator of damage to the blood-brain barrier, and the results showed that IF regimen reduced plasma peptidoglycan levels and improved blood-brain barrier integrity. It has also been shown that a decrease in peptidoglycan concentration following IF is consistent with a reduction in endotoxemia (12). Therefore, fasting diets effect on weight loss through changes in the gut microbiota diversity and number, as well as peptidoglycan production. Gut microbiota involves major energy metabolic processes (19). Some studies have found a significant association between intestinal dysbiosis and energy dysmetabolism-induced chronic diseases such as diabetes, metabolic syndrome, and obesity (20). The positive results of the IF regimen on animal models with hypertension have also been shown in some studies (21).
Another part of animal studies has evaluated the effect of fasting on intestinal microbiome in animal models of neurodegenerative diseases. Cignarella et al. evaluated the effects of IF on gut microbiome and clinical symptoms of animal models of multiple sclerosis (MS), which named experimental autoimmune encephalomyelitis (EAE). They found that IF led to a significant improvement in the gut bacteria richness, enrichment of the Lactobacillaceae, Bacteroidaceae, and Prevotellaceae families and enhanced antioxidative microbial metabolic pathways. The results of this study also showed that the IF reduced the differentiation of native T cells into T17 cells, which secrete proinflammatory cytokines, and, conversely, increased the differentiation into regulatory T cells. Interestingly, the results of this study showed that fecal microbiome transplantation from mice under the fasting diet to mice with EAE ameliorated the symptoms, which could indicate the positive effect of the fasting diet (22).
On the other hand, some studies have shown that IF cause weight loss, reduce lipid peroxidation, and hepatic steatosis on obese mice through changes in microbial profile. Also, it has been reported in this study that IF led to a significant increase in the intestinal flora community diversity [Firmicutes to Bacteroidetes (F/B ratio) and relative increase in the Allobaculum abundance] (23). Increasing the abundance of Firmicutes following fasting diets can increase the production of short-chain fatty acids (SCFAs). SCFAs have the ability to increase the integrity of gut barrier, strengthen the immune system, reduce weight and insulin resistance (24). Moreover, fasting diets effect on the α-diversity (richness) and β-diversity (variety) of gut microbiota (12). Some pre-clinical studies have shown that IF could increase β-diversity, but the results on the effect of fasting diets on α-diversity are contradictory (12, 22, 25). Seven months IF on mice gut microbiota increased β-diversity compared in animals (12). Furthermore, weight loss introduced as the important and effective factor on α-diversity of gut microbiota (22), however it varies greatly during the day and dependents to dietary content (26, 27).
On the other hand, some studies have evaluated the effect of fasting diets on gut microbiota changes. Li et al. evaluated the effect of 12, 16, or 20-h fasting diets on the gut microbiome for 1 month. The results of this study showed that the composition of the gut microbiome changed in all types of fasting diets. At genus level, 16 h fasting led to increased level of Akkermansia and decreased level of Alistipes, but these effects disappeared after the cessation of fasting. No taxonomic differences were identified in the other two groups (28). In some previous findings, an increase in Akkermansia strains has been associated with metabolic benefits such as a reduction in the severity of fatty liver and intestinal inflammation (29). Increased levels of Alistipes can also exacerbate gut inflammation (30, 31).
Given that different metabolites are produced by the gastrointestinal microbiome, some other studies have evaluated these metabolites produced by microbiota following fasting diets. It has been reported an increased plasma levels of some metabolites such as tryptophan, serotonin, tryptophan, various bile acids, propionate, and acetate following the administration of fasting diets in animal samples (25, 32, 33). These results have also been confirmed in some human studies (34). Changes in the production of some metabolites can affect processes such as inflammation in the body. For example, some preclinical studies have shown that fasting diets exert inhibitory effects on the biosynthesis pathways of lipopolysaccharides by altering the intestinal microbiome. Lipopolysaccharides are among the major constituents of the outer membrane of Gram-negative bacteria, and studies have shown that increased production of lipopolysaccharides can induce toll like receptor-4 (TLR-4). TLR4 represents a key receptor on which both infectious and non-infectious stimuli converge to induce a proinflammatory response (35).
According to the positive results of pre-clinical studies, in recent years, various human studies have evaluated the association between intestinal microbiome and fasting. In some human studies, fasting diet of Ramadan type on intestinal microbiome has been evaluated (13, 36–39). The duration of fasting time was 12–18 h per day in these studies. The results of these studies mainly showed changes in the intestinal microbiome following adherence to Ramadan fasting, some of which are mentioned below. In a clinical study in 2021, Mohammadzadeh et al. evaluated the effect of Ramadan fasting on serum levels of butyrate, intestinal microbiome and lipid profile. The results of this study showed that the serum level of butyrate in the fasting group increased significantly after 1 month. There was also a significant increase in the bacteroides and filminus strains in the intervention group (39). In another study, which conducted on Pakistani and Chinese participants, researchers evaluated the effect of a 29-day Ramadan fasting on alpha and beta diversity. The results of this study showed that the population of some bacterial strains such as Bacteroidetes and Firmicutes increased in the Pakistani population following fasting, however no noticeable changes were observed in the Chinese population. In addition, it has been reported that fasting in both populations affects beta diversity. Moreover, lower levels of genus Coprococcus observed after Ramadan fasting suggesting that fasting could have implications on health. On the other hand, fasting could also have harmful effects on health (38). A study of two cohort data showed that following a Ramadan-associated IF increased microbiome diversity and was specifically associated with upregulation of the Clostridiales order–derived Lachnospiraceae. In fact, the fasting diet in this study increased the expression of butyric acid-producing Lachnospiraceae. These alternations were independent of living area, body weight and diet composition and disappeared again when fasting was stopped (13). Various studies have shown that changes in the intestinal microbiome cause changes in physiological functions and reduce energy intake. Thus, human microbiome can be an effector for physiologic effects of IF (13). In another preliminary study, it was found that following the Islamic fasting diet caused significant changes in the intestinal microbiome, so that the number of A. muciniphila and Bacteroides fragilis group members increased, however, Lactobacillus spp. and Bifidobacterium spp. remained relatively unchanged perhaps due to low fiber intake (36).
In addition to Ramadan fasting, some studies have examined the effect of restricted feeding in a form of IF on the intestinal microbiome. One of the major problems seen in these studies is the low sample size. Therefore, it is difficult to generalize the results of these studies to large populations. Gabei et al. in a pilot study evaluated the effect of fasting in a form of IF on the intestinal microbiome in adults with obesity. They found that IF led to a significant weight loss. Baseline evaluation of fecal microbiome by 16 S rRNA (ribosomal ribonucleic acid) gene sequencing showed that the predominant strains included Firmicutes and Bacteroidetes. However, at the end of 12 weeks of fasting diet, no significant change was observed in the abundance and distribution of dominant bacterial strains (40). However, the results of some other studies were inconsistent with this study. Guo et al. in a RCT study were evaluated the effects of 8 weeks of “2-day” modified IF in patients with metabolic syndrome. The results of this study revealed that 8 weeks of “2-day” modified IF led to a significant reduction in fat mass, oxidative stress, inflammatory cytokines, and improved vasodilatory parameters. On the other hand, the results of this study showed that following the 8 weeks of “2-day” modified IF caused a significant change in the composition of the intestinal microbiome, increased the production of SCFA and decreased lipopolysaccharide levels (41).
In this review study, we evaluated the effects of Ramadan and non-Ramadan IF on gut microbiome. The results of most animal and human studies indicate the positive effects of fasting on the composition and structure of the gut microbiome. In addition to the positive role of fasting on the composition and abundance of intestinal microbiome, in some studies, other positive results have been observed following the observance of fasting regimes. Positive alterations in gut microbiota, such as overexpression of A. muciniphila, B. fragilis, Bacteroides, and butyric acid-producing Lachnospiraceae, were found to be associated with improved health indicators and decreasing disease development during Ramadan fasting. However, factors such as the duration of fasting diets, the presence of chronic diseases and obesity can affect the results. Considering the role of intestinal microbiome changes in the management of various diseases, future studies, especially clinical studies, should evaluate the impact of fasting regimes, especially Ramadan, on the management of various diseases through changes in the intestinal microbiome.
MR and ER: conception and design, and systematic search. SM and JH: screening and data extraction. MR and RT: manuscript writing. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Klempel MC, Kroeger CM, Varady KA. Alternate day fasting (ADF) with a high–fat diet produces similar weight loss and cardio–protection as ADF with a low–fat diet. Metabolism. (2013) 62:137–43. doi: 10.1016/j.metabol.2012.07.002
2. Gabel K, Kroeger CM, Trepanowski JF, Hoddy KK, Cienfuegos S, Kalam F, et al. Differential effects of alternate–day fasting versus daily calorie restriction on insulin resistance. Obesity. (2019) 27:1443–50. doi: 10.1002/oby.22564
3. Varady K. Intermittent versus daily calorie restriction: which diet regimen is more effective for weight loss? Obes Rev. (2011) 12:e593–601. doi: 10.1111/j.1467-789X.2011.00873.x
4. Chow LS, Manoogian EN, Alvear A, Fleischer JG, Thor H, Dietsche K, et al. Time–restricted eating effects on body composition and metabolic measures in humans who are overweight: a feasibility study. Obesity. (2020) 28:860–9. doi: 10.1002/oby.22756
6. Bazzano AN, Potts KS, Mulugeta A. How do pregnant and lactating women, and young children, experience religious food restriction at the community level? A qualitative study of fasting traditions and feeding behaviors in four regions of Ethiopia. PLoS One. (2018) 13:e0208408. doi: 10.1371/journal.pone.0208408
7. Abdelrahim D, Faris ME, Hassanein M, Shakir AZ, Yusuf AM, Almeneessier AS, et al. Impact of Ramadan diurnal intermittent fasting on hypoglycemic events in patients with type 2 diabetes: a systematic review of randomized controlled trials and observational studies. Front Endocrinol. (2021) 12:624423. doi: 10.3389/fendo.2021.624423
8. Bagudu KA, Noreen S, Rizwan B, Bashir S, Khan M, Chishti K, et al. Intermittent fasting effect on weight loss: a systematic review. Biosci Res. (2021) 18:622–31.
9. Collier R. Intermittent fasting: the next big weight loss fad. Can Med Assoc. (2013) 185:E321–2. doi: 10.1503/cmaj.109-4437
10. Correia JM, Santos I, Pezarat-Correia P, Silva AM, Mendonca GV. Effects of ramadan and non-ramadan intermittent fasting on body composition: a systematic review and meta-analysis. Front Nutr. (2021) 7:625240. doi: 10.3389/fnut.2020.625240
11. Asnicar F, Berry SE, Valdes AM, Nguyen LH, Piccinno G, Drew DA, et al. Microbiome connections with host metabolism and habitual diet from 1,098 deeply phenotyped individuals. Nat Med. (2021) 27:321–32. doi: 10.1038/s41591-020-01183-8
12. Beli E, Yan Y, Moldovan L, Vieira CP, Gao R, Duan Y, et al. Restructuring of the gut microbiome by intermittent fasting prevents retinopathy and prolongs survival in db/db mice. Diabetes. (2018) 67:1867–79. doi: 10.2337/db18-0158
13. Su J, Wang Y, Zhang X, Ma M, Xie Z, Pan Q, et al. Remodeling of the gut microbiome during Ramadan–associated intermittent fasting. Am J Clin Nutr. (2021) 113:1332–42. doi: 10.1093/ajcn/nqaa388
14. Liu J, Zhong Y, Luo XM, Ma Y, Liu J, Wang H. Intermittent fasting reshapes the gut microbiota and metabolome and reduces weight gain more effectively than melatonin in mice. Front Nutr. (2021) 8:784681. doi: 10.3389/fnut.2021.784681
15. Bahl MI, Hammer AS, Clausen T, Jakobsen A, Skov S, Andresen L. The gastrointestinal tract of farmed mink (Neovison vison) maintains a diverse mucosa-associated microbiota following a 3-day fasting period. Microbiologyopen. (2017) 6:e00434. doi: 10.1002/mbo3.434
16. Ley RE, Bäckhed F, Turnbaugh P, Lozupone CA, Knight RD, Gordon JI. Obesity alters gut microbial ecology. Proc Natl Acad Sci. (2005) 102:11070–5. doi: 10.1073/pnas.0504978102
17. Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Human gut microbes associated with obesity. Nature. (2006) 444:1022–3.
18. Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity–associated gut microbiome with increased capacity for energy harvest. Nature. (2006) 444:1027–31. doi: 10.1038/nature05414
19. Rinninella E, Cintoni M, Raoul P, Ianiro G, Laterza L, Lopetuso LR, et al. Gut microbiota during dietary restrictions: new insights in non–communicable diseases. Microorganisms. (2020) 8:1–23. doi: 10.3390/microorganisms8081140
20. Rong B, Wu Q, Saeed M, Sun C. Gut microbiota–a positive contributor in the process of intermittent fasting–mediated obesity control. Anim Nutr. (2021) 7:1283–95. doi: 10.1016/j.aninu.2021.09.009
21. Shi H, Zhang B, Abo-Hamzy T, Nelson JW, Ambati CSR, Petrosino JF, et al. Restructuring the gut microbiota by intermittent fasting lowers blood pressure. Circ Res. (2021) 128:1240–54.
22. Cignarella F, Cantoni C, Ghezzi L, Salter A, Dorsett Y, Chen L, et al. Intermittent fasting confers protection in CNS autoimmunity by altering the gut microbiota. Cell Metab. (2018) 27: 1222-1235.e6. doi: 10.1016/j.cmet.2018.05.006
23. Deng Y, Liu W, Wang J, Yu J, Yang LQ. Intermittent fasting improves lipid metabolism through changes in gut microbiota in diet–induced obese mice. Med Sci Monit. (2020) 26:e926789. doi: 10.12659/MSM.926789
24. Canfora EE, Jocken JW, Blaak EE. Short–chain fatty acids in control of body weight and insulin sensitivity. Nat Rev Endocrinol. (2015) 11:577–91. doi: 10.1038/nrendo.2015.128
25. Li G, Xie C, Lu S, Nichols RG, Tian Y, Li L, et al. Intermittent fasting promotes white adipose browning and decreases obesity by shaping the gut microbiota. Cell Metab. (2017) 26:672–685.e4.
26. Zarrinpar A, Chaix A, Yooseph S, Panda S. Diet and feeding pattern affect the diurnal dynamics of the gut microbiome. Cell Metab. (2014) 20:1006–17. doi: 10.1016/j.cmet.2014.11.008
27. Zeb F, Wu X, Chen L, Fatima S, Haq I-U, Chen A, et al. Effect of time-restricted feeding on metabolic risk and circadian rhythm associated with gut microbiome in healthy males. Br J Nutr. (2020) 123:1216–26. doi: 10.1017/S0007114519003428
28. Li L, Su Y, Li F, Wang Y, Ma Z, Li Z, et al. The effects of daily fasting hours on shaping gut microbiota in mice. BMC Microbiol. (2020) 20:65. doi: 10.1186/s12866-020-01754-2
29. Anhê FF, Roy D, Pilon G, Dudonné S, Matamoros S, Varin TV, et al. A polyphenol–rich cranberry extract protects from diet–induced obesity, insulin resistance and intestinal inflammation in association with increased Akkermansia spp. population in the gut microbiota of mice. Gut. (2015) 64:872–83. doi: 10.1136/gutjnl-2014-307142
30. Saulnier DM, Riehle K, Mistretta TA, Diaz MA, Mandal D, Raza S, et al. Gastrointestinal microbiome signatures of pediatric patients with irritable bowel syndrome. Gastroenterology. (2011) 141:1782–91. doi: 10.1053/j.gastro.2011.06.072
31. Naseribafrouei A, Hestad K, Avershina E, Sekelja M, Linløkken A, Wilson R, et al. Correlation between the human fecal microbiota and depression. Neurogastroenterol Motil. (2014) 26:1155–62. doi: 10.1111/nmo.12378
32. Martin AM, Yabut JM, Choo JM, Page AJ, Sun EW, Jessup CF, et al. The gut microbiome regulates host glucose homeostasis via peripheral serotonin. Proc Natl Acad Sci USA.. (2019) 116:19802–4. doi: 10.1073/pnas.1909311116
33. Zhou ZL, Jia XB, Sun MF, Zhu YL, Qiao CM, Zhang BP, et al. Neuroprotection of fasting mimicking diet on MPTP-induced Parkinson’s disease mice via gut microbiota and metabolites. Neurotherapeutics. (2019) 16:741–60. doi: 10.1007/s13311-019-00719-2
34. Liu Z, Wei Z-Y, Chen J, Chen K, Mao X, Liu Q, et al. Acute sleep–wake cycle shift results in community alteration of human gut microbiome. mSphere. (2020) 5:e914–9. doi: 10.1128/mSphere.00914-19
35. D’Hennezel E, Abubucker S, Murphy LO, Cullen TW. Total lipopolysaccharide from the human gut microbiome silences toll–like receptor signaling. mSystems. (2017) 2:e46–17. doi: 10.1128/mSystems.00046-17
36. Özkul C, Yalınay M, Karakan T. Islamic fasting leads to an increased abundance of Akkermansia muciniphila and Bacteroides fragilis group: a preliminary study on intermittent fasting. Turk J Gastroenterol. (2019) 30:1030–5. doi: 10.5152/tjg.2019.19185
37. Ozkul C, Yalinay M, Karakan T. Structural changes in gut microbiome after Ramadan fasting: a pilot study. Benef Microbes. (2020) 11:227–33. doi: 10.3920/BM2019.0039
38. Ali I, Liu K, Long D, Faisal S, Hilal MG, Ali I, et al. Ramadan fasting leads to shifts in human gut microbiota structured by dietary composition. Front Microbiol. (2021) 12:642999. doi: 10.3389/fmicb.2021.642999
39. Mohammadzadeh A, Roshanravan N, Mesri Alamdari N, Safaiyan A, Mosharkesh E, Hadi A, et al. The interplay between fasting, gut microbiota, and lipid profile. Int J Clin Pract. (2021) 75:e14591. doi: 10.1111/ijcp.14591
40. Gabel K, Marcell J, Cares K, Kalam F, Cienfuegos S, Ezpeleta M, et al. Effect of time restricted feeding on the gut microbiome in adults with obesity: a pilot study. Nutr Health. (2020) 26:79–85. doi: 10.1177/0260106020910907
41. Guo Y, Luo S, Ye Y, Yin S, Fan J, Xia M. Intermittent fasting improves cardiometabolic risk factors and alters gut microbiota in metabolic syndrome patients. J Clin Endocrinol Metab. (2021) 106:64–79. doi: 10.1210/clinem/dgaa644
42. Zhang Z, Chen X, Loh YJ, Yang X, Zhang C. The effect of calorie intake, fasting, and dietary composition on metabolic health and gut microbiota in mice. BMC Biol. (2021) 19:51. doi: 10.1186/s12915-021-00987-5
43. Park S, Zhang T, Wu X, Yi Qiu J. Ketone production by ketogenic diet and by intermittent fasting has different effects on the gut microbiota and disease progression in an Alzheimer’s disease rat model. J Clin Biochem Nutr. (2020) 67:188–98. doi: 10.3164/jcbn.19-87
44. Kim JN, Song J, Kim EJ, Chang J, Kim C-H, Seo S, et al. Effects of short–term fasting on in vivo rumen microbiota and in vitro rumen fermentation characteristics. Asian Australas J Anim Sci. (2019) 32:776. doi: 10.5713/ajas.18.0489
45. Catterson JH, Khericha M, Dyson MC, Vincent AJ, Callard R, Haveron SM, et al. Short–term, intermittent fasting induces long–lasting gut health and TOR-independent lifespan extension. Curr Biol. (2018) 28: 1714-1724.e4. doi: 10.1016/j.cub.2018.04.015
46. Wei S, Han R, Zhao J, Wang S, Huang M, Wang Y, et al. Intermittent administration of a fasting–mimicking diet intervenes in diabetes progression, restores β cells and reconstructs gut microbiota in mice. Nutr Metab. (2018) 15:1–12. doi: 10.1186/s12986-018-0318-3
47. McCue MD, Passement CA, Meyerholz DK. Maintenance of distal intestinal structure in the face of prolonged fasting: a comparative examination of species from five vertebrate classes. Anat Rec. (2017) 300:2208–19. doi: 10.1002/ar.23691
48. Sonoyama K, Fujiwara R, Takemura N, Ogasawara T, Watanabe J, Ito H, et al. Response of gut microbiota to fasting and hibernation in Syrian hamsters. Appl Environ Microbiol. (2009) 75:6451–6. doi: 10.1128/AEM.00692-09
49. Su J, Braat H, Peppelenbosch MP. Gut microbiota–derived propionate production may explain beneficial effects of intermittent fasting in experimental colitis. J Crohns Colitis. (2021) 15:1081–2. doi: 10.1093/ecco-jcc/jjaa248
50. Maifeld A, Bartolomaeus H, Löber U, Avery EG, Steckhan N, Markó L, et al. Fasting alters the gut microbiome reducing blood pressure and body weight in metabolic syndrome patients. Nat Commun. (2021) 12:1970. doi: 10.1038/s41467-021-22097-0
51. Lilja S, Bäck H, Duszka K, Hippe B, Suarez L, Höfinger I, et al. Fasting and fasting mimetic supplementation address sirtuin expression, miRNA and microbiota composition. Funct Foods Health Dis. (2020) 10:439–55. doi: 10.31989/ffhd.v10i10.752
52. He Y, Yin J, Lei J, Liu F, Zheng H, Wang S, et al. Fasting challenges human gut microbiome resilience and reduces Fusobacterium. Med Microecol. (2019) 1:100003.
53. Balogh A, Bartolomaeus H, Löber U, Avery EG, Steckhan N, Marko L, et al. Fasting alters the gut microbiome with sustained blood pressure and body weight reduction in metabolic syndrome patients. medRxiv [Preprint]. (2020): doi: 10.1101/2020.02.23.20027029
54. Mesnage R, Grundler F, Schwiertz A, Le Maho Y, Wilhelmi De Toledo F. Changes in human gut microbiota composition are linked to the energy metabolic switch during 10 d of Buchinger fasting. J Nutr Sci. (2019) 8:e36. doi: 10.1017/jns.2019.33
Keywords: fasting, intermediate fasting, Ramadan, gut microbiome, review
Citation: Mousavi SN, Rayyani E, Heshmati J, Tavasolian R and Rahimlou M (2022) Effects of Ramadan and Non-ramadan Intermittent Fasting on Gut Microbiome. Front. Nutr. 9:860575. doi: 10.3389/fnut.2022.860575
Received: 23 January 2022; Accepted: 23 February 2022;
Published: 22 March 2022.
Edited by:
Hassane Zouhal, University of Rennes 2 – Upper Brittany, FranceReviewed by:
Sergio Perez-Burillo, University of Granada, SpainCopyright © 2022 Mousavi, Rayyani, Heshmati, Tavasolian and Rahimlou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mehran Rahimlou, cmFoaW1sdW1AZ21haWwuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.