
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Nutr. , 10 March 2022
Sec. Nutrition and Metabolism
Volume 9 - 2022 | https://doi.org/10.3389/fnut.2022.855573
This article is part of the Research Topic Obesity as a Systemic Disease View all 5 articles
Obesity as a multi-organ disease that affects the entire human organism. Notably, the skin is no exclusion from this postulate. Skin changes in obese patients have been widely studied with regards to mechanical friction, skin infections, and skin hypertrophic conditions, such as acanthosis nigricans and, most commonly, fibromas (skin tags). Almost 60–70% of obese patients present with a variety of skin changes. Herein, we discuss our own experience and review the complex skin changes in obesity. The role of metabolic syndrome and obesity are responsible for the epidemiological prevalence and are involved in the pathogenesis of chronic inflammatory skin diseases, such as psoriasis, atopic dermatitis, and skin malignancies. Here, we comment on the role of nutritional interventions in these patients as it has been proven that low-calorie diet and weight loss is related to improvement of inflammatory skin diseases. The readership of this paper will receive up-to-date overview on the connection between obesity and the skin that is of a practical importance to any clinician working in the field.
Skin, as the largest organ, accomplishes multiple defensive functions and contributes to the mammalian organism homeostasis. Skin physiology and pathology are influenced by the interplay of hormones, immune signaling molecules, and growth factors. Therefore, skin homeostasis reflects the inner state of the organism (1, 2). For centuries, the skin has been accepted as a “mirror,” in which the health status of internal organs is reflected. Classic examples of skin changes in systemic diseases include paraneoplastic skin syndromes, accumulation disorders (e.g., Fabry disease), and genetic inherited conditions with cutaneous involvement (3).
In the past decades, the concept of obesity, formerly accepted as an isolated condition, has evolved and is now disclosed as a systemic disease with multiple organ and system involvement (4). Numerous co-morbidities beyond the cardiovascular system have been reported, i.e., non-alcoholic fatty liver disease, autoimmune disorders, asthma, atopic dermatitis, and chronic inflammatory conditions such as psoriasis, rheumatoid arthritis, and cancer (5). Despite this, skin changes in obesity have been underestimated, with almost 50% of obese and overweight patients displaying skin comorbidities in relation to a metabolic disorder (6). In addition, a plethora of skin diseases, such as psoriasis, atopic dermatitis (AD), and melanoma, are influenced by comorbid obesity. Considering the increasing pandemic prevalence of obesity and the accessibility of the skin for inspection on the other, skin changes in obesity should be recognized by clinicians.
The aim of this paper is to summarize the current knowledge on the link between obesity and the skin. We describe skin physiology changes in obese and overweigh patients and specific skin manifestations of obesity are disclosed. A review on the affection of chronic skin diseases from comorbid obesity is presented together with the adverse skin reactions to anti-obesity medications.
The skin of an obese patient is characterized by increased subcutaneous fat, larger skin folds, and higher surface roughness (7–9). Early animal studies in obese-hyperglycemic mice revealed increased skin surface area, collagen abnormalities related to abundance of reducible cross-links, and glycosylated lysine in relation to decreased skin mechanical resilience (10). Reduced expression of certain ceramide types (structural components of epidermal lipids) was observed in obese females (9). This could be of clinical relevance for the development of stretch marks (striae distensae) and impaired wound healing in obesity.
Data on the permeability barrier function, measured by the assessment of the insensible transepidermal water loss (TEWL), is controversial. While increased basal TEWL was disclosed in the adult (11) and children population (12), suggesting increased skin barrier permeability, some patients can present contrasting values (7). This may be due to inconsistencies in the study related to acclimatization of subjects before measurement and the use of closed chamber device. Lower TEWL in patients who are overweight and with low grade obesity was noted in comparison to lean and excessively obese individuals (13). The authors attributed these discrepancies as an adaptation mechanism of the permeability water barrier to weight gain.
Skin dryness is a profound physiologic feature of obese patients’ skin, which has been confirmed by non-invasive measurements of skin electrical conductivity (9, 14). The objective changes correlated well with the self-perception of skin xerosis.
Skin erythema (redness) is increased in obese patients in relation to increased skin blood flow (11). However, several studies contradicted that erythema is, instead, due to decreased skin microcirculation and inadequate vascular reactivity of the skin capillaries that were maximally recruited at baseline (15, 16). This together with the decreased skin lymphatic drainage in obesity could explain the hindered wound healing and the development of stasis conditions and lymphedema.
Obesity and overweightness are correlated with specific skin changes. The association between monogenic obesity syndromes, such as Prader–Willy, McCune–Albright, and fragile X syndromes, and skin pathology is beyond the scope of this review. Hence, the readership is referred to the work of Millington for a detailed overview (17). For deductive purposes, we propose the following classification of specific skin changes related to obesity.
Analysis of 156 obese patients revealed the plantar “horseshoe-like” hyperkeratosis (Figure 1) as the most typical cutaneous stigma in this population (18). This reaction, together with the decrease of the plantar arch, is a defensive mechanism of the skin against mechanical friction and increased gravity load of the heels. The management of this condition requires topical keratolytic agents (most commonly based on urea or salicylic acid).
Striae distensae, also known as stretch marks, results from the mechanical stretching and tearing of dermal elastic fibers (Figure 2). They represent longitudinal atrophic marks most commonly on the breasts, abdomen, tights, and buttocks (8, 18, 19). While the newly appeared lesions are erythematous, with time, they become pearl-colored and atrophic. Such changes could be a manifestation of Cushing’s syndrome and elevated cortisol levels. Hence, patients with striae distensae should seek medical examination. Currently there is no effective treatment for this condition despite the multiple treatment modalities offered by aesthetic dermatologists.
Insulin resistance, a common finding in obese patients (20), has been shown to induce specific skin changes. Acanthosis nigricans (Figure 3), which presents with velvety pigmented and verrucous plaques on the skin folds (neck, axilla, inguinal, and sub-mammary), results from the stimulation of the proliferation of dermal fibroblasts and epidermal keratinocytes by insulin, insulin-like growth factor 1 (IGFR1), fibroblasts, and epidermal growth factors (8, 21). This condition should be distinguished from the obligatory paraneoplastic acanthosis nigricans (22). Weight reduction, topical retinoids, keratolytic agents, and agents targeted against insulin resistance have been shown to be effective in the treatment of this condition (23).
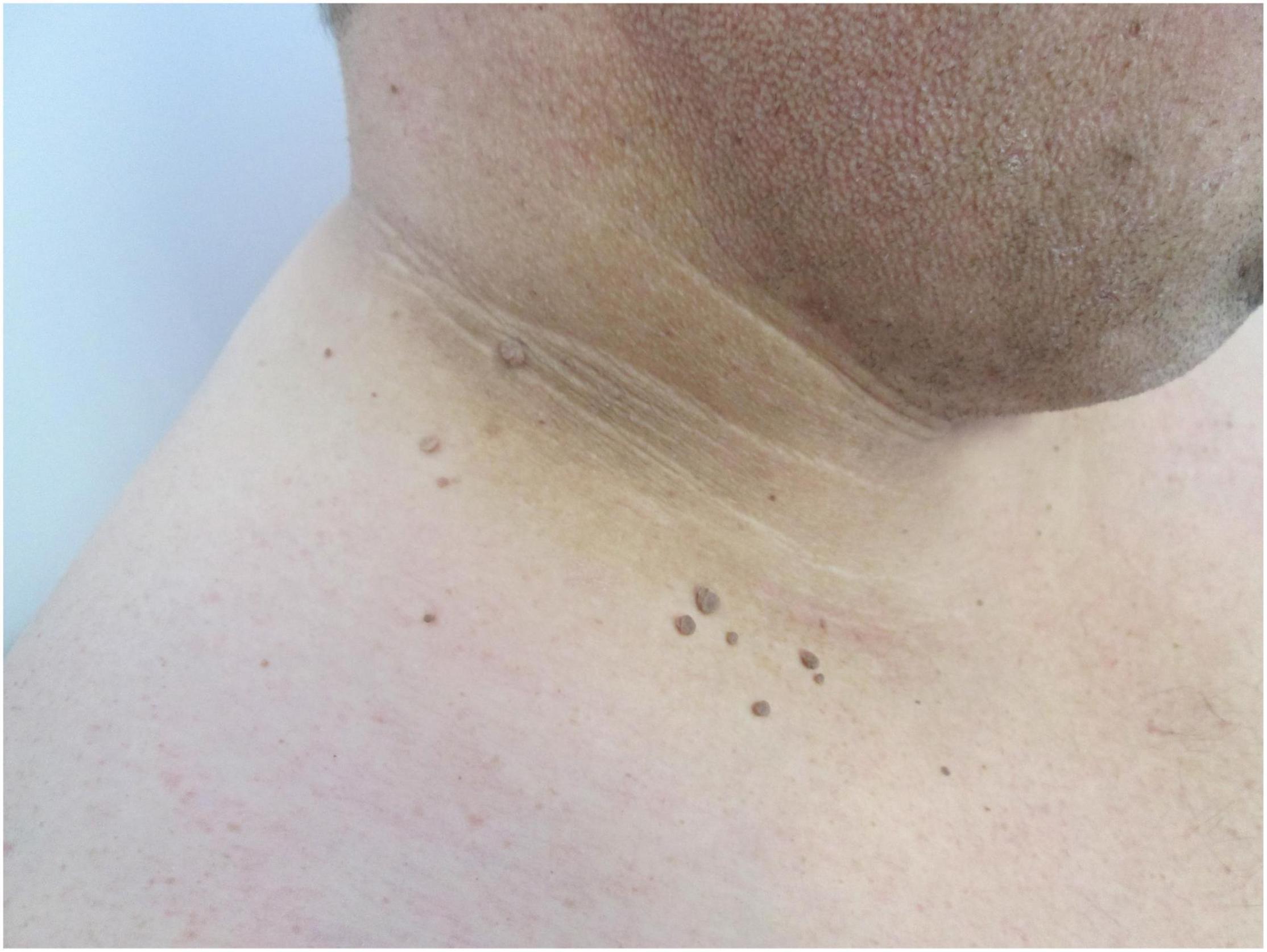
Figure 3. Acanthosis nigricans presented with velvety, brown plaques, and skin tags (fibroma pendulum) on the neck of the patient.
Fibroma pendulum (skin tags, acrochordons) represent pedunculated skin-colored papules, most commonly involving the predilection skin sites for acanthosis nigricans (skin folds) (Figure 3) (24). The number and the distribution of skin tags has been correlated to the degree of hyperinsulinemia (25). The management of fibromas require surgical excision and resides in the field of aesthetic medicine.
Keratosis pilaris, a widely prevalent condition, has been linked to increased body mass index (BMI) (26). The disorder is characterized by tiny keratotic follicular papules on the outer upper arms, buttocks, tights, and the cheeks. Topical moisturizers, keratolytic agents, and retinoids may be helpful in the management of keratosis pilaris (27).
Hyperandrogenism, resulting from adipose tissue or ovarian production of androgens, has been linked to virilism, manifested by hirsutism (most often facial), acne, androgenic alopecia, seborrhea, and several syndromes such as the hyperandrogenism, insulin resistance and acanthosis nigricans (HAIR-AN) and seborrhea, acne, hirsutism, alopecia (SAHA) (24, 28). Anti-androgen hormonal therapy is a treatment of choice in these conditions.
Skin infection complications are associated with obesity and overweightness. Staphylococcal and streptococcal infection of the skin and its appendages is typical (29, 30). In Figure 4A, we present a male obese patient with scrotal bacteria cellulitis (erysipelas) who presented with diffuse erythema and edema. In Figure 4B, the residual changes after repeated bacterial folliculitis can be observed. The pattern resembles a “moon crater” due to the excessive tension forces in the process of resolving Staphylococcal folliculitis.
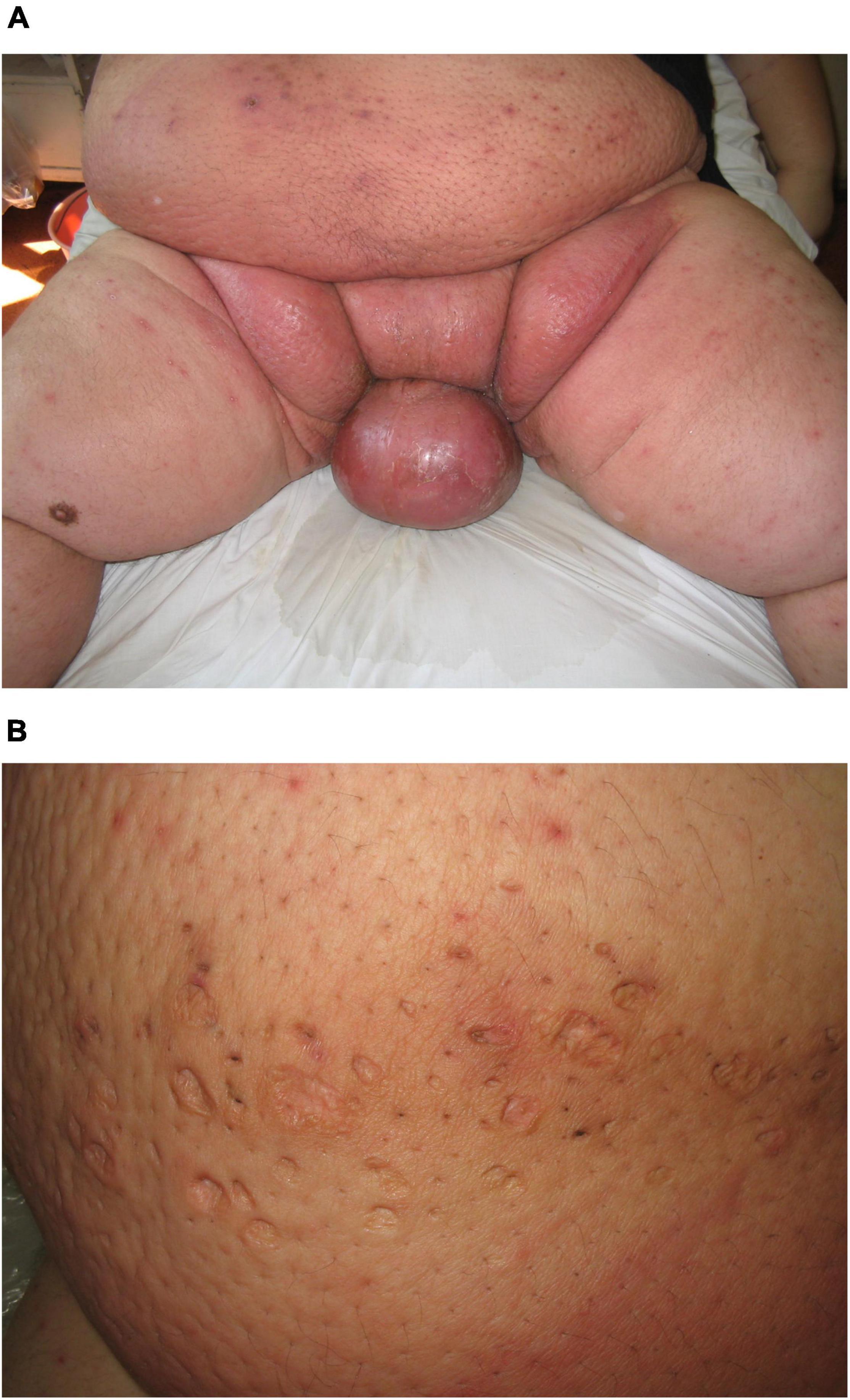
Figure 4. (A) Skin bacterial cellulitis of the scrotum and inguinal area of an obese male patient. (B) Atrophic round scars, secondary to the resolution of bacterial folliculitis, resembling “moon crater” pattern.
Intertrigo, a common skin fold (inguinal, axillar, sub-mammary) associated with obesity, is due to the increased friction, occlusion, and maceration in these sites and is most commonly associated with Candida or Gram positive bacteria overgrowth (31). This should not be confused with erythrasma – a corynebacterial skin fold infection also observed in obesity (8).
Onychomycosis, caused by different dermatophyte species, is commonly observed in obese patients. Patients with this condition are noted to present therapy resistance, particularly in obese and overweight patients (32).
The increased intra-abdominal pressure in obese patients results in chronic venous insufficiency (CVI) which manifests as varices, lower limb edema, leg ulcers, and lymphedema (33). In Figure 5, we present a rare case of elephantiasis nostras verrucosa, which resulted from CVI and secondary lymphedema of the lower limbs characterized by epidermal papillomatosis, in a 54-year-old patient with a BMI of 48.5.
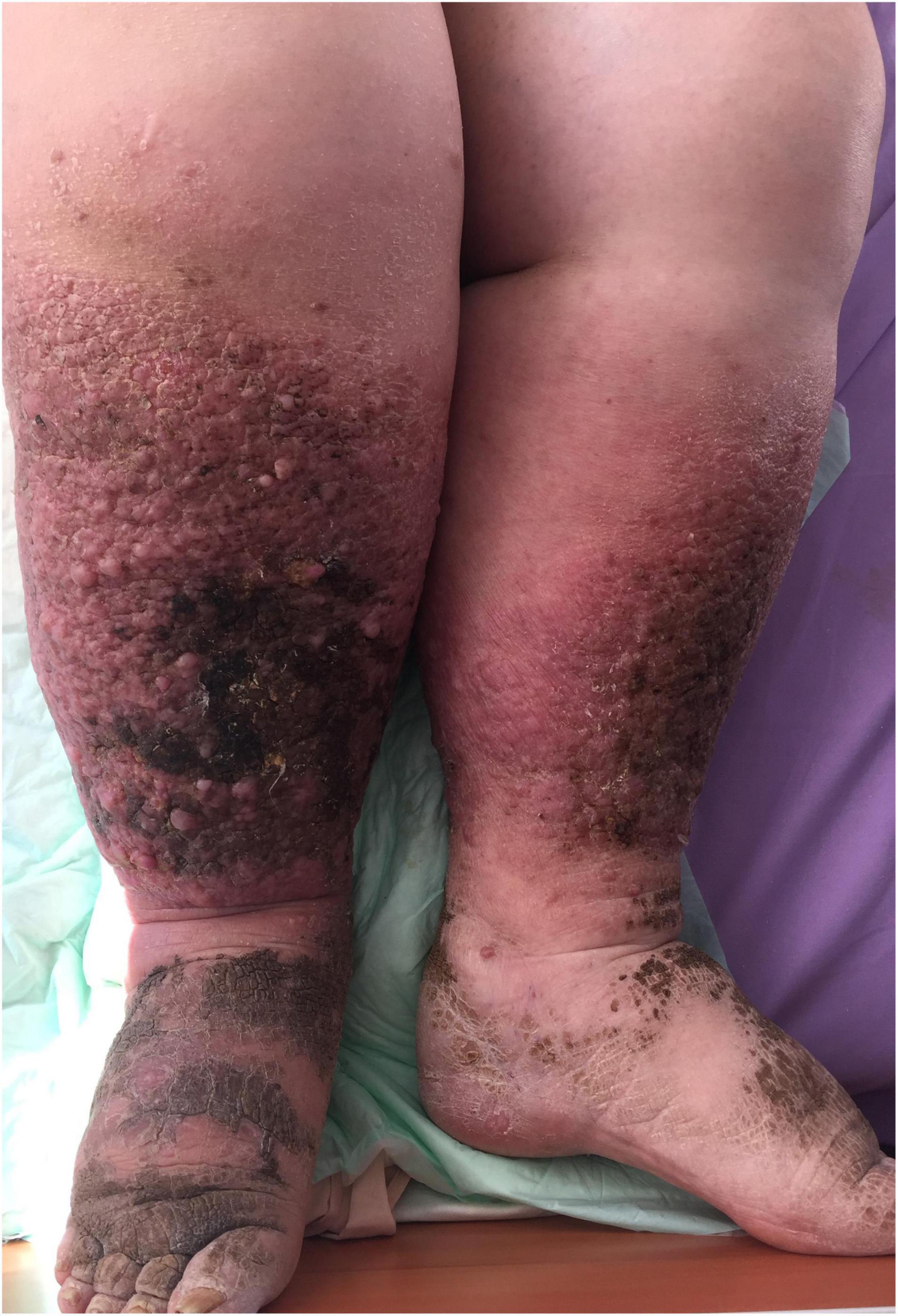
Figure 5. Elephantiasis nostras verrucosa with skin papillomatosis, pigmentation, and soft tissue overgrowth.
In the past decades, the role of obesity in chronic skin conditions such as psoriasis, hidradenitis suppurativa (HS), and atopic dermatitis has been reevaluated. Far from mechanistic association, common pathophysiology mechanisms have been disclosed in chronic inflammatory skin disorders and obesity (34).
Psoriasis as a chronic systemic inflammatory disease that shares pro-inflammatory mechanisms with obesity, including Th1 cytokines [interferon-γ, interleukin (IL)-2, IL-12, and tumor necrosis factor (TNF)-α] (35). More recently, the family of adipokines have been involved in the obesity-driven inflammation in psoriasis (36). Obesity is an independent risk factor associated with psoriasis severity and response to systemic therapy (i.e., methotrexate, cyclosporine, apremilast, and biologic agents) (37–39). Finally, dietary interventions and weight loss improve the disease course and severity of comorbid psoriasis (40, 41).
Hidradenitis suppurativa (HS) characterized by chronic inflammatory nodules, cysts, and abscesses, resolves with scar formation and engage the skin folds. Obesity is a risk factor for HS both in children and in adults (42, 43). Weight loss is associated with disease improvement (44), while bariatric surgery has been shown to worsen or induce de novo HS (45). A survey revealed that Western diet rich in high glycemic index and fat food exacerbates the course of HS, while fruit and vegetable-rich diets were reported to alleviate HS symptoms. Still, prospective cohort studies are required to evaluate the effect of diet on the disease prognosis.
The link between obesity has been disclosed and reviewed by several studies (46). The severity of atopic dermatitis (AD) correlated well with BMI (47). In addition, children whose mothers exhibited pre-pregnancy obesity and overweightness had a higher risk for AD development in early childhood (48). Plausible explanations for the increased incidence and comorbid state of AD and obesity is the sub-clinical systemic inflammation with BMI increase and the immune modulating properties of adipokines such as leptin, resistin, and ghrelin (49, 50). Weight reduction results in better AD treatment outcomes (47).
Increased BMI has been revealed as a risk factor for skin melanoma. However, the link is not as strong as in other malignancies (51, 52). In contrast, even a “obesity paradox” was disclosed for melanoma (51). Gut microbiome modulation by diet could be the explanation and, possibly, the link between obesity and melanoma (53). Still, the molecular mechanisms of this interaction remain to be elucidated.
Acne vulgaris is a common disorder with increasing prevalence. It has a certain link to obesity, insulin resistance, and altered peripheral skin receptor sensitivity to sex hormones (54, 55). High glycemic index and Western diet have been linked to acne worsening (56).
Lichen planus, a chronic itchy inflammatory skin disease characterized by flat round and polygonal papules, has been linked to abdominal obesity and other features of the metabolic syndrome (57).
Systemic lupus erythematosus, as an autoimmune disorder, is worsened with the increase in BMI. In addition, physical activity has been shown to ameliorate the pro-inflammatory effect of the higher body mass (58). The link between obesity and autoimmunity has been disclosed in mice. Hence, low-fiber diet could be the milestone in the complex disease interactions (59).
The pandemic spread of obesity has led to the development of multiple medications and interventions for its treatment. Orlistat has been reported to induce leukocytoclastic vasculitis (60, 61) and lichenoid skin eruption (62, 63). Dexfenfluramine and phentermine have been linked to the development of rash (2%), alopecia, sweating, urticaria, and pruritus (64). Sibutramine could provoke rash (3.8%), sweating (2.5%), herpes simplex (1.3%), acne (1.0%), and, though rarely, bullous drug eruptions (65). Novel players, such as liraglutide, was shown to induce injection site reactions such as erythema, pruritus, and rash. A single report also disclosed vesiculopustular dermatosis caused by this agent (66, 67). Beyond injection site reactions, semaglutide has been suspected to cause hair loss. However, this was not confirmed by other studies (67, 68). We refer the reader to a former work of our group revealing the adverse skin reactions to metformin, commonly used off-label for the treatment of obesity (69). Rarely, reactions to anti-obesity food supplements have been documented, as in the case of lichen planus pemphigoides (70).
Bariatric surgery induces specific skin manifestations that could be divided into (1) diseases due to metabolic and nutritional disorders, and (2) those derived from cutaneous structural changes after major weight reduction (71). The most specific adverse skin reactions to this intervention include bowel-associated dermatosis and arthritis syndrome, erythematous macular eruption with a neutrophilic infiltrate, non-pruritic papular eruption with IgG and C3 deposition, and erythema nodosum (71, 72). In addition, a new subset of HS associated with malabsorption and deficiency in micronutrients has been described (73). Zinc deficiency resulting from malabsorption in bariatric surgery has been linked to the development of the rare condition known as necrotic migratory erythema (74). Despite the clinical improvement of acanthosis nigricans, the development of alopecia has been described in a cohort of post bariatric surgery in relation to malabsorption (75). In post-bariatric abdominoplasty, delayed wound healing, skin infections, and umbilical necrosis have been shown to be significantly correlated with certain predictors such as initial BMI, type 2 diabetes, tobacco smoking, and the amount and timing of abdominoplasty (76).
The variety of skin comorbidities in obesity is rich, and their understanding is of relevance both for dermatologists and physicians involved in obesity management. The most common specific stigmata of obesity on the skin include the “horseshoe-like” plantar hyperkeratosis, skin tags (fibroma pendulum), and acanthosis nigricans, with the latter specific for the insulin resistance in obesity. Therefore, these signs are considered relevant to be screened in obesity and overweight patients.
On the other hand, the link between certain chronic skin conditions is a subject of a growing body of evidence. Therefore, patients with chronic inflammatory skin disorders such as psoriasis, AD, hidradenitis suppurativa, and systemic lupus erythematosus should be monitored for obesity with the primary goal of losing weight. Evidence suggests that weight loss could improve the course and severity of chronic skin comorbidities such as AD and psoriasis (47, 77). Missing in the literature is the correlation of the specific types of obese patients (e.g., morbid obesity, abdominal obesity) and specific skin diseases. It is an open question whether there is a threshold on the amount of weight loss to improve skin lesions.
Finally, the safety profile of anti-obese drugs and procedures with regard to the skin and its appendages is enriched by multiple case reports, and real-world post-marketing data will broaden our understanding on the diverse nature of obesity.
All authors listed have made a substantial, direct, and intellectual contribution to the work, and approved it for publication.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Sampaio AL, Bressan AL, Vasconcelos BN, Gripp AC. Skin manifestations associated with systemic diseases – part I. An Bras Dermatol. (2021) 96:655–71. doi: 10.1016/j.abd.2021.02.008
2. Tsankov N, Kazandjieva J, Darlenski R. The skin as a target organ in multisystemic diseases II. Clin Dermatol. (2015) 33:509–11. doi: 10.1016/j.clindermatol.2015.05.011
3. Manuelyan KL, Bogdanov IA, Darlenski RB. Skin signs of systemic infections and neoplastic diseases. G Ital Dermatol Venereol. (2017) 152:489–99. doi: 10.23736/S0392-0488.17.05719-4
4. Fruhbeck G, Busetto L, Dicker D, Yumuk V, Goossens GH, Hebebrand J, et al. The ABCD of obesity: an EASO position statement on a diagnostic term with clinical and scientific implications. Obes Facts. (2019) 12:131–6. doi: 10.1159/000497124
5. Schelbert KB. Comorbidities of obesity. Prim Care. (2009) 36:271–85. doi: 10.1016/j.pop.2009.01.009
6. Lecerf JM, Reitz C, de Chasteigner A. Evaluation of discomfort and complications in a population of 18,102 patients overweight or obese patients. Presse Med. (2003) 32:689–95.
7. Guida B, Nino M, Perrino NR, Laccetti R, Trio R, Labella S, et al. The impact of obesity on skin disease and epidermal permeability barrier status. J Eur Acad Dermatol Venereol. (2010) 24:191–5. doi: 10.1111/j.1468-3083.2009.03503.x
8. Hirt PA, Castillo DE, Yosipovitch G, Keri JE. Skin changes in the obese patient. J Am Acad Dermatol. (2019) 81:1037–57. doi: 10.1016/j.jaad.2018.12.070
9. Mori S, Shiraishi A, Epplen K, Butcher D, Murase D, Yasuda Y, et al. Characterization of skin function associated with obesity and specific correlation to local/systemic parameters in American women. Lipids Health Dis. (2017) 16:214. doi: 10.1186/s12944-017-0608-1
10. Enser M, Avery NC. Mechanical and chemical properties of the skin and its collagen from lean and obese-hyperglycaemic (ob/ob) mice. Diabetologia. (1984) 27:44–9. doi: 10.1007/BF00253500
11. Loffler H, Aramaki JU, Effendy I. The influence of body mass index on skin susceptibility to sodium lauryl sulphate. Skin Res Technol. (2002) 8:19–22. doi: 10.1046/j.0909-752x
12. Nino M, Franzese A, Ruggiero Perrino N, Balato N. The effect of obesity on skin disease and epidermal permeability barrier status in children. Pediatr Dermatol. (2012) 29:567–70. doi: 10.1111/j.1525-1470.2012.01738.x
13. Monteiro Rodrigues LM, Palma L, Santos O, Almeida MA, Bujan J, Tavares L. Excessive weight favours skin physiology – up to a point: another expression of the obesity paradox. Skin Pharmacol Physiol. (2017) 30:94–101. doi: 10.1159/000464338
14. de Farias Pires T, Azambuja AP, Horimoto AR, Nakamura MS, de Oliveira Alvim R, Krieger JE, et al. A population-based study of the stratum corneum moisture. Clin Cosmet Investig Dermatol. (2016) 9:79–87. doi: 10.2147/CCID.S88485
15. Andreieva IO, Riznyk OI, Myrnyi SP, Surmylo NN. State of cutaneous microcirculation in patients with obesity. Wiad Lek. (2021) 74:2039–43.
16. Francischetti EA, Tibirica E, da Silva EG, Rodrigues E, Celoria BM, de Abreu VG. Skin capillary density and microvascular reactivity in obese subjects with and without metabolic syndrome. Microvasc Res. (2011) 81:325–30. doi: 10.1016/j.mvr.2011.01.002
17. Millington GW. Obesity, genetics and the skin. Clin Exp Dermatol. (2013) 38:50–6; quiz 6. doi: 10.1111/ced.12024
18. Garcia-Hidalgo L, Orozco-Topete R, Gonzalez-Barranco J, Villa AR, Dalman JJ, Ortiz-Pedroza G. Dermatoses in 156 obese adults. Obes Res. (1999) 7:299–302. doi: 10.1002/j.1550-8528.1999.tb00410.x
19. Garcia Hidalgo L. Dermatological complications of obesity. Am J Clin Dermatol. (2002) 3:497–506. doi: 10.2165/00128071-200203070-00006
20. Klimontov VV, Semenova JF. Glucose variability in subjects with normal glucose tolerance: relations with body composition, insulin secretion and sensitivity. Diabetes Metab Syndr. (2022) 16:102387. doi: 10.1016/j.dsx.2022.102387
21. Zhang L, Li G, Su L, Du L, Zhou D, Cheng X, et al. [Correlation between total testosterone levels and insulin resistance in patients with acanthosis nigricans and non-acanthosis nigrican]. Nan Fang Yi Ke Da Xue Xue Bao. (2021) 41:1780–6. doi: 10.12122/j.issn.1673-4254.2021.12.04
22. Fonia A, Baran R. Cutaneous paraneoplastic syndromes with nail involvement. Dermatol Clin. (2021) 39:175–82. doi: 10.1016/j.det.2020.12.003
23. Unluhizarci K, Karaca Z, Kelestimur F. Role of insulin and insulin resistance in androgen excess disorders. World J Diab. (2021) 12:616–29. doi: 10.4239/wjd.v12.i5.616
24. Uzuncakmak TK, Akdeniz N, Karadag AS. Cutaneous manifestations of obesity and themetabolic syndrome. Clin Dermatol. (2018) 36:81–8. doi: 10.1016/j.clindermatol.2017.09.014
25. Shaheen MA, Abdel Fattah NS, Sayed YA, Saad AA. Assessment of serum leptin, insulin resistance and metabolic syndrome in patients with skin tags. J Eur Acad Dermatol Venereol. (2012) 26:1552–7. doi: 10.1111/j.1468-3083.2011.04401.x
26. Yosipovitch G, Mevorah B, Mashiach J, Chan YH, David M. High body mass index, dry scaly leg skin and atopic conditions are highly associated with keratosis pilaris. Dermatology. (2000) 201:34–6. doi: 10.1159/000018425
27. Reddy S, Brahmbhatt H. A narrative review on the role of acids, steroids, and kinase inhibitors in the treatment of keratosis pilaris. Cureus. (2021) 13:e18917. doi: 10.7759/cureus.18917
28. Yosipovitch G, DeVore A, Dawn A. Obesity and the skin: skin physiology and skin manifestations of obesity. J Am Acad Dermatol. (2007) 56:901–16; quiz 17–20. doi: 10.1016/j.jaad.2006.12.004
29. Cranendonk DR, Lavrijsen APM, Prins JM, Wiersinga WJ. Cellulitis: current insights into pathophysiology and clinical management. Neth J Med. (2017) 75:366–78.
30. Hahler B. An overview of dermatological conditions commonly associated with the obese patient. Ostomy Wound Manage. (2006) 52:34–6, 8, 40 passim.
31. Mathur AN, Goebel L. Skin findings associated with obesity. Adolesc Med State Art Rev. (2011) 22:146–56, ix.
32. Rosen T. Evaluation of gender as a clinically relevant outcome variable in the treatment of onychomycosis with efinaconazole topical solution 10. Cutis. (2015) 96:197–201.
33. Schneider C, Stratman S, Kirsner RS. Lower extremity ulcers. Med Clin North Am. (2021) 105:663–79. doi: 10.1016/j.mcna.2021.04.006
34. Grozdev I, Korman N, Tsankov N. Psoriasis as a systemic disease. Clin Dermatol. (2014) 32:343–50. doi: 10.1016/j.clindermatol.2013.11.001
35. Onsun N, Akaslan TC, Sallahoglu K, Gulcan AS, Bulut H, Yabaci A. Effects of TNF inhibitors and an IL12/23 inhibitor on changes in body weight and adipokine levels in psoriasis patients: a 48-week comparative study. J Dermatolog Treat. (2021) 1–16. doi: 10.1080/09546634.2021.1901845 [Epub ahead of print].
36. Ruiyang B, Panayi A, Ruifang W, Peng Z, Siqi F. Adiponectin in psoriasis and its comorbidities: a review. Lipids Health Dis. (2021) 20:87. doi: 10.1186/s12944-021-01510-z
37. Zachariae C, Skov L. Obesity as a risk factor for psoriasis. J Eur Acad Dermatol Venereol. (2020) 34:915–6. doi: 10.1111/jdv.16434
38. Enos CW, O’Connell KA, Harrison RW, McLean RR, Dube B, Van Voorhees AS. Psoriasis severity, comorbidities, and treatment response differ among geographic regions in the United States. JID Innov. (2021) 1:100025. doi: 10.1016/j.xjidi.2021.100025
39. Graier T, Weger W, Sator PG, Salmhofer W, Gruber B, Jonak C, et al. Effectiveness and clinical predictors of drug survival in psoriasis patients receiving apremilast: a registry analysis. JAAD Int. (2021) 2:62–75. doi: 10.1016/j.jdin.2020.10.012
40. Castaldo G, Pagano I, Grimaldi M, Marino C, Molettieri P, Santoro A, et al. Effect of very-low-calorie ketogenic diet on psoriasis patients: a nuclear magnetic resonance-based metabolomic study. J Proteome Res. (2021) 20:1509–21. doi: 10.1021/acs.jproteome.0c00646
41. Klingberg E, Bjorkman S, Eliasson B, Larsson I, Bilberg A. Weight loss is associated with sustained improvement of disease activity and cardiovascular risk factors in patients with psoriatic arthritis and obesity: a prospective intervention study with two years of follow-up. Arthritis Res Ther. (2020) 22:254. doi: 10.1186/s13075-020-02350-5
42. Garcovich S, Fania L, Caposiena D, Giovanardi G, Chiricozzi A, De Simone C, et al. Pediatric hidradenitis suppurativa: a cross-sectional study on clinical features and treatment approaches. J Cutan Med Surg. (2021) 12034754211039993. doi: 10.1177/12034754211039993 [Epub ahead of print].
43. Fabbrocini G, Ruina G, Giovanardi G, Dini V, Raone B, Venturini M, et al. Hidradenitis suppurativa in a large cohort of Italian patients: evaluation of the burden of disease. Dermatology. (2021) 1–11. doi: 10.1159/000517412 [Epub ahead of print].
44. Kromann CB, Deckers IE, Esmann S, Boer J, Prens EP, Jemec GB. Risk factors, clinical course and long-term prognosis in hidradenitis suppurativa: a cross-sectional study. Br J Dermatol. (2014) 171:819–24. doi: 10.1111/bjd.13090
45. Choi F, Lehmer L, Ekelem C, Mesinkovska NA. Dietary and metabolic factors in the pathogenesis of hidradenitis suppurativa: a systematic review. Int J Dermatol. (2020) 59:143–53. doi: 10.1111/ijd.14691
46. Zhang A, Silverberg JI. Association of atopic dermatitis with being overweight and obese: a systematic review and metaanalysis. J Am Acad Dermatol. (2015) 72:606–16 e4. doi: 10.1016/j.jaad.2014.12.013
47. Jung MJ, Kim HR, Kang SY, Kim HO, Chung BY, Park CW. Effect of weight reduction on treatment outcomes for patients with atopic dermatitis. Ann Dermatol. (2020) 32:319–26. doi: 10.5021/ad.2020.32.4.319
48. Wei X, Huang P, Gao C, Shen S, Tu S, Guo Y, et al. Associations of maternal weight status with the risk of offspring atopic dermatitis and wheezing by 1 year of age. Pediatr Allergy Immunol. (2022) 33:e13703. doi: 10.1111/pai.13703
49. Jimenez-Cortegana C, Ortiz-Garcia G, Serrano A, Moreno-Ramirez D, Sanchez-Margalet V. Possible role of leptin in atopic dermatitis: a literature review. Biomolecules. (2021) 11:1642. doi: 10.3390/biom11111642
50. Jaworek AK, Szepietowski JC, Szafraniec K, Jaworek M, Halubiec P, Wojas-Pelc A, et al. Adipokines as biomarkers of atopic dermatitis in adults. J Clin Med. (2020) 9:2858. doi: 10.3390/jcm9092858
51. Pellegrini M, D’Eusebio C, Ponzo V, Tonella L, Finocchiaro C, Fierro MT, et al. Nutritional interventions for patients with melanoma: from prevention to therapy-an update. Nutrients. (2021) 13:4018. doi: 10.3390/nu13114018
52. Saginala K, Barsouk A, Aluru JS, Rawla P, Barsouk A. Epidemiology of Melanoma. Med Sci (Basel). (2021) 9:63. doi: 10.3390/medsci9040063
53. Warner AB, McQuade JL. Modifiable host factors in melanoma: emerging evidence for obesity, diet, exercise, and the microbiome. Curr Oncol Rep. (2019) 21:72. doi: 10.1007/s11912-019-0814-2
54. Alshammrie FF, Alshammari R, Alharbi RM, Khan FH, Alshammari SK. Epidemiology of acne vulgaris and its association with lifestyle among adolescents and young adults in Hail, kingdom of Saudi Arabia: a community-based study. Cureus. (2020) 12:e9277. doi: 10.7759/cureus.9277
55. Sas K, Reich A. High body mass index is a risk factor for acne severity in adolescents: a preliminary report. Acta Dermatovenerol Croat. (2019) 27:81–5.
56. Clatici VG, Voicu C, Barinova E, Lupu M, Tatu AL. Butterfly effect and acne-the role of diet. Dermatol Ther. (2020) 33:e13832. doi: 10.1111/dth.13832
57. Arias-Santiago S, Buendia-Eisman A, Aneiros-Fernandez J, Giron-Prieto MS, Gutierrez-Salmeron MT, Mellado VG, et al. Cardiovascular risk factors in patients with lichen planus. Am J Med. (2011) 124:543–8. doi: 10.1016/j.amjmed.2010.12.025
58. Sola-Rodriguez S, Vargas-Hitos JA, Gavilan-Carrera B, Rosales-Castillo A, Rios-Fernandez R, Sabio JM, et al. Physical fitness attenuates the impact of higher body mass and adiposity on inflammation in women with systemic lupus erythematosus. Front Immunol. (2021) 12:729672. doi: 10.3389/fimmu.2021.729672
59. Schafer AL, Eichhorst A, Hentze C, Kraemer AN, Amend A, Sprenger DTL, et al. Low dietary fiber intake links development of obesity and lupus pathogenesis. Front Immunol. (2021) 12:696810. doi: 10.3389/fimmu.2021.696810
60. Lazic T, Fonder M, Robinson-Bostom L, Wilkel CS, Della Torre L. Orlistat-induced bullous leukocytoclastic vasculitis. Cutis. (2013) 91:148–9.
61. Gonzalez-Gay MA, Garcia-Porrua C, Lueiro M, Fernandez ML. Orlistat-induced cutaneous leukocytoclastic vasculitis. Arthritis Rheum. (2002) 47:567. doi: 10.1002/art.10670
62. Koca Kalkan I, Kalpaklioglu AF, Atasoy P, Karabulut AA. Orlistat and obesity: be aware of lichenoid drug eruption. Eur J Dermatol. (2011) 21:456–7. doi: 10.1684/ejd.2011.1401
63. Sergeant A, Milne G, Shaffrali F. Lichenoid eruption associated with orlistat. Br J Dermatol. (2006) 154:1020–1. doi: 10.1111/j.1365-2133.2006.07220.x
65. Goh BK, Ng PP, Giam YC. Severe bullous drug eruption due to sibutramine (reductil). Br J Dermatol. (2003) 149:215–6. doi: 10.1046/j.1365-2133.2003.05413.x
66. Besemer F, Verschoor AJ, Diamant M, Hoogma RP. Vesiculopustular dermatosis: an uncommon side-effect of liraglutide? J Diabetes Complications. (2012) 26:458–9. doi: 10.1016/j.jdiacomp.2012.05.018
67. Vosoughi K, Atieh J, Khanna L, Khoshbin K, Prokop LJ, Davitkov P, et al. Association of glucagon-like peptide 1 analogs and agonists administered for obesity with weight loss and adverse events: a systematic review and network meta-analysis. EClinicalMedicine. (2021) 42:101213. doi: 10.1016/j.eclinm.2021.101213
68. Srivastava G, Kumar RB. Once-weekly semaglutide in adults with overweight or obesity. N Engl J Med. (2021) 385:e4. doi: 10.1056/NEJMc2106918
69. Yaneva M, Demerdjieva Z, Darlenski R. Adverse skin reactions to metformin: a case report and mini review. EMJ Dermatol. (2020) 8:50–3.
70. Rosmaninho A, Sanches M, Oliveira A, Alves R, Selores M. Lichen planus pemphigoides induced by a weight reduction drug. Cutan Ocul Toxicol. (2011) 30):306–8. doi: 10.3109/15569527.2011.566234
71. Manzoni AP, Weber MB. Skin changes after bariatric surgery. An Bras Dermatol. (2015) 90:157–66. doi: 10.1590/abd1806-4841.20153139
72. Rosen J, Darwin E, Tuchayi SM, Garibyan L, Yosipovitch G. Skin changes and manifestations associated with the treatment of obesity. J Am Acad Dermatol. (2019) 81:1059–69. doi: 10.1016/j.jaad.2018.10.081
73. Garcovich S, De Simone C, Giovanardi G, Robustelli E, Marzano AV, Peris K. Post-bariatric surgery hidradenitis suppurativa: a new patient subset associated with malabsorption and micronutritional deficiencies. Clin Exp Dermatol. (2019) 44:283–9. doi: 10.1111/ced.13732
74. Salaheldin Y, El Ansari W, Aljaloudi E, Elhag W. Third reported case of rare necrolytic migratory erythema associated with bacteraemia due to severe zinc deficiency after revisional Roux-En-Y gastric bypass: case report and literature review. Eat Weight Disord. (2021). doi: 10.1007/s40519-021-01154-z [Epub ahead of print].
75. Itthipanichpong Y, Damkerngsuntorn W, Tangkijngamvong N, Udomsawaengsup S, Boonchayaanant P, Kumtornrut C, et al. Skin manifestations after bariatric surgery. BMC Dermatol. (2020) 20:21. doi: 10.1186/s12895-020-00120-z
76. De Paep K, Van Campenhout I, Van Cauwenberge S, Dillemans B. Post-bariatric abdominoplasty: identification of risk factors for complications. Obes Surg. (2021) 31:3203–9. doi: 10.1007/s11695-021-05383-0
Keywords: acne, lichen planus, psoriasis, melanoma, anti-obesity drugs, overweight, bariatric surgery
Citation: Darlenski R, Mihaylova V and Handjieva-Darlenska T (2022) The Link Between Obesity and the Skin. Front. Nutr. 9:855573. doi: 10.3389/fnut.2022.855573
Received: 15 January 2022; Accepted: 11 February 2022;
Published: 10 March 2022.
Edited by:
Luca Busetto, Università degli Studi di Padova, ItalyReviewed by:
Themistoklis Tzotzas, St. Luke’s Hospital, GreeceCopyright © 2022 Darlenski, Mihaylova and Handjieva-Darlenska. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Razvigor Darlenski, ZGFybGVuc2tpQGdtYWlsLmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.