
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
PERSPECTIVE article
Front. Nutr., 09 March 2022
Sec. Clinical Nutrition
Volume 9 - 2022 | https://doi.org/10.3389/fnut.2022.847215
This article is part of the Research TopicExercise and Cancer: From Clinical Association to Mechanistic InsightsView all 9 articles
The 2019 coronavirus (COVID-19) epidemic, has caused unprecedented global social and economic impacts and many deaths. Many risk factors have been identified in the progression of COVID-19 to severe and critical stages, and it is shown that the coronavirus appears more severely in people with cancer. Pro-inflammatory status and weakened immune system due to cancer-related treatments can be determinants in the immune system’s response to the coronavirus in these patients. Higher physical activity levels are associated with lower hospitalization rates and mortality in COVID-19. Also, regular exercise training can improve immune system responses, modulate inflammatory responses, and improve psychological parameters in cancer patients. The interactive effects of nutritional supplements on immune responses and anti-inflammatory status have been shown in some studies. The purpose of this perspective article was to investigate the interaction between dietary supplementation and regular physical exercise in controlling risk factors associated with coronavirus in cancer patients. In addition to appropriate dietary habits, some nutritional supplements, especially vitamin D, have been shown to improve the immune system’s response against COVID-19 and cancer. Using lifestyle strategies such as regular physical activity and intake of functional compounds as supplements can be effective in treatment outcomes, quality of life, and overall survival in cancer patients. We proposed that combining dietary supplements and exercise training in cancer patients can boost immune responses against COVID-19 and probably improve vaccine responses. Angiotensin (ANG)-(1-7) Mas receptor axis can probably activate following exercise training and vitamin D combination. And can prevent pulmonary injury, hematological alterations, and hyperinflammatory state in COVID-19.
Humans are exposed to different viruses; and the human body is rapidly involved in an immune response to eradicate the virus, with a pattern of detection and the production of memory cells (1). Coronavirus (COVID-19) has been observed in China since 2019 and has become an epidemic (2). The virus is caused by acute respiratory syndrome (SARS) Coronavirus 2 (SARS-CoV-2). According to the statistics published as of September 21, 2021, the number of infected people worldwide was approximately 230 million; and the number of victims was about 4.7 million, which continues so far, and 14% of patients experiences acute and severe conditions (3). Coronavirus can cause uncontrolled releases of pro-inflammatory cytokines, which leads to cytokine release syndrome (CRS) or “cytokine storm” (4). Activation of CRS can worsen acute respiratory syndrome and lead to multiple organ dysfunction (4). Evidence suggests that patients with SARS-CoV-2, who previously suffered from rheumatological immune diseases and other inflammatory diseases are more fragile to an acute respiratory syndrome caused by CRS (5). Despite global vaccinations to tackle this pandemic, we can see new infections and the prevalence of new variants. Still observing social distancing and staying at home is one of the main ways to keep the COVID-19 under control (6, 7).
In particular, cancer patients appear to be a high-risk category to experience COVID-19 with more severe symptoms, especially due to damage to immune defenses and the consequences of anti-neoplastic treatments (8). Chronic cancer-related inflammation can create an immunosuppressive tumor microenvironment to help the tumor to escape immune monitoring (9). Also, cancer is associated with over-expression of immunosuppressive cytokines, decreased proinflammatory risk signals, and increased populations of functional immunosuppressive leukocytes, which weaken the immune responses and increase the risk of infectious complications (10, 11). Therefore, these patients cannot show effective immune responses when exposed to the virus.
In addition to regular cancer treatments, appropriate dietary habits and regular physical exercise have been considered in these patients. Regular physical exercise training can induce positive changes in anxiety, depression, immune and physiological responses in cancer (12). Moreover, some dietary supplements have always been considered to boost immune responses in cancer patients (13). Higher physical activity levels are associated with lower hospitalization rates and mortality in COVID-19 (14). Positive effects of acute or chronic physical exercise were observed on innate and acquired immune responses and modulation of inflammatory status (15). Also, the effects of regular physical exercise on angiotensin-converting enzyme 2 (ACE2) as an effective agent in the pathogenesis of COVID-19 are suggested in some studies (16). In addition, the effects of physical exercise on nitric oxide and oxidative stress have been proposed as possible effective mechanisms in the prevention and recovery of the COVID-19 (17). Impaired immune responses, inflammatory status, and oxidative stress have been observed in cancer patients (18). Also, some studies showed different expressions of ACE2 in cancer patients, which makes them more susceptible to SARS-CoV-2 (19). The modulatory effects of regular physical exercise on ACE2 and its receptors may be effective in preventing severe cases of COVID-19 in cancer patients.
In addition, the positive effects of various dietary supplements such as probiotics, omega-3 fatty acids, multivitamins, or vitamin D supplements in COVID-19 are suggested in some studies (20). Considering the observed effects of dietary supplements and physical exercise in cancer and COVID-19, synergic effects of combining regular physical exercise and supplementation can improve the immune system responses in cancer patients against coronavirus. The possible effects of combining regular physical exercise and dietary supplementation in cancer and COVID-19 were discussed following a systematic search in Scopus, Web of Science, ISC, and Pub-Med databases. And the results were divided into two main topics 1) Physical Exercise in Cancer and COVID-19, 2) Nutritional Supplements in Cancer and COVID-19.
Angiotensin-converting enzyme 2 (ACE2) is one of the causes of SARS-CoV-2 entry and infection (21). It has recently been found that ACE2 receptors are also more common in cancer patients (19). Once CoV-2 entry into the target cell, the host response is determinant of the severity of the ensuing pathogenesis (22). The immune system plays a critical role in COVID-19, and in addition to the genetic profile, environmental indices can be effective on the immune responses (23).
The first immune system responses are when the COVID-19 enters the body through the innate immune system. Inflammation is the body increases when a person becomes infected with COVID-19. As seen in patients with SARS-CoV, Viral infection and proliferation in airway epithelial cells can cause high levels of virus-associated pyroptosis associated with vascular leakage (24). During inflammation, type I interferons (IFNs) are activated by the innate immune system, and the presence of type I interferons can regulate myeloid cell activation and migration (25). Rapid and appropriate activation of type I IFNs can effectively limit virus replication and reduce immune pathological damage. It also limits the hyperactive inflammatory response (26). Also, in the innate immune system, natural killer cells (NK) play a vital role in infectious diseases. NK cells decreased in patients with COVID-19 (27). Activation of NK cells lead to cytotoxic degranulation and production of inflammatory cytokines and destroys target cells (28). In addition, the adaptive immune system would activate to help innate immunity for further controlling the infection. The three critical components of the adaptive immune system against SARS-CoV-2 are B cells, CD4+ T cells, and CD8+ T cells (29). CD4+ T cell responses are more prominent than CD8+ T cell responses and play a role in controlling primary infection earlier (30). It has also been shown that the function of B cells is very important in controlling viral infections. But the critical point is that COVID-19 can disrupt the phenotype of T and B cells and reduce their function (29).
Regular exercise throughout life is effective in cardiovascular fitness and self-reported mood, anxiety, and depressive symptoms. In addition to the physiological effects of regular physical exercise, it can improve immune responses. Moderate intensity training is directly associated with a lower upper respiratory tract infection (URTI) (31). Regular physical exercise can improve innate and acquired immune system responses (32). In particular, secondary antibody responses to some traditional vaccines approved after exercise training, especially in elderly people (33).
Moreover, it is shown that physical exercise has anti-inflammatory effects, especially in chronic diseases and obesity (34). Modulation of adipose tissue macrophages, the release of anti-inflammatory cytokines after acute physical exercise, and interaction of skeletal muscle and immune system can be effective in exercise-induced anti-inflammatory effects (34). The anti-inflammatory effects of exercise and changes in various indicators of the innate immune system following acute physical exercise can improve the innate immune system responses to infections. In addition, regular physical exercise training creates effective responses in the acquired immune system especially in elderly people (35). Physical exercise has been shown to reduce mortality from illnesses such as the flu, and higher levels of physical activity have reduced the incidence of severe cases, hospitalization rates, and mortality in COVID-19 (36, 37).
Cancer-related inflammation is a prominent feature of cancer (38). Cancer-related inflammation, which in different stages of tumorigenesis, contributes to genomic instability, epigenetic modification, induction of cancer cell proliferation, strengthening cancer anti-apoptosis pathways, stimulating angiogenesis, and ultimately spreading cancer (38). In the early stages of tumor growth, cytotoxic immune cells such as NK cells and T+CD8 cells detect and destroy cancer cells (39). In addition, the interaction between NK cells, effective T cells, and antitumor macrophages by secreting IFN-γ and tumor necrosis factor-alpha (TNF-α) at the tumor site increases the cytotoxic ability of NK cells (39). When tumors escape primary anti-tumor immunity, they undergo different strategies that shift the balance toward immune tolerance, as they reduce the effect of inherently compatible immune cells at different levels and through different mechanisms. Tumor cells escape immune attacks using two main strategies; avoid immune detection and stimulate an immunosuppressive environment. Firstly, cancer cells may lose expression of tumor antigens on the cell surface, thus preventing detection by cytotoxic T cells. In this sense, mutations and deletions may lead to low regulation of the antigen-providing device and possibly show resistance to effective T-cell molecules such as TNF-α and IFN-γ (40). Also, agents derived from cancer cells stimulate the expression of inhibitory inspection molecules such as programmed death-ligand 1 (PD-L1), CTLA β-4, and Tregs expression by tumor-derived chemokines of the immune-resistant environment by tumor-derived chemokines (18). Overall, these strategies lead to a complex and efficient system for safe escape.
Adjuvant therapies such as physical exercise that makes changes in different aspects of the immune system in cancer may improve the efficacy of current immunotherapy. The diagnosis of cancer and the treatments that cancer patients undergo have a significant impact on their mental and physical health. Some studies showed that regular physical exercise is effective in body weight and body mass index controlling, and improving patients’ quality of life and sleep quality (41). It also seems that regular exercise through myocyte secretion, decreased inflammatory factors within the tumor, decreased tumor angiogenesis, and increased expression of factors involved in apoptosis can slow tumor growth (42). Regular exercise and physical activity can prevent the disease from recurring by affecting sex steroid hormones, metabolic hormones, inflammatory markers, cytokines and adipokines, myokines, and stress hormones (43). There are also observed effects of regular and long-term exercise on immune responses to diseases such as cancer (44). Prolonged exercise promotes anti-inflammatory effects and improves immune responses in cancer patients. In addition, recent studies have shown the positive effects of physical exercise on antitumor immunity that can affect tumor growth (45). Tumor induces physiological changes in its environment such as changes in acidity and metabolism in order to suppress antitumor immunity. Physiological responses following physical exercise can increase the infiltration of macrophages, neutrophils, NK cells, and regulatory and cytosolic T lymphocytes to the tumor microenvironment, which can be effective in tumor suppression (46).
Unfortunately, cancer patients are among the groups most at risk for severe cases of COVID-19. The psychological, physiological, and especially immunological effects of physical exercise can help these patients respond better to infection and possibly even better immune responses to the vaccine. However, the effects of exercise training have always been considered along with nutritional factors. We discussed the effects of nutritional supplements on the immune system in cancer patients to prevent severe cases of COVID-19, we also discussed the combined effects of exercise and supplementation.
Many studies have approved that proper nutrition and some nutritional supplements can improve immune system function and reduce infection (47, 48). Long-term use of foods and supplements rich in antioxidants such as tart cherry juice, pomegranate juice, beetroot juice, creatine, omega-3 polyunsaturated fatty acids, and vitamin D3, watermelon juice has been associated with effective immune responses (49). In addition, the effect of ginger and some of its compounds against inflammation and improving immune responses have been reported in some studies (50–52).
Among vitamins, vitamin E is one of the most effective nutrients known to modulate the function of the immune system (53). There are reports of improved immune systems in human and animal specimens by taking this supplement. Improving immune function with vitamin E is clinically important because, in addition to allergic diseases such as asthma and the flu, it also affects infectious diseases such as respiratory infections (54). Also, evidence showed that vitamin E supplementation for 30 days increased Th1 immune responses, improved lung damage, and reduced influenza infection in mice (55).
In addition, Vitamin D is a fat-soluble steroid that plays an important role in regulating calcium and phosphorus levels. Vitamin D receptors are located on immune cells, and all leukocytes can synthesize the active metabolite of vitamin D. Moreover, vitamin D can act autonomously, boosting the innate immune response and inhibiting the acquired immune response (56). Low concentrations of vitamin D have been reported to be associated with upper respiratory tract infections and allergic asthma (57). Vitamin D enhances chemotaxis, antimicrobial peptides, and macrophage differentiation. It can also inhibit the maturation of DCs, the differentiation of Th1 and Th17, and boost the functions of regulatory T cells (57). Moreover, vitamin D has been reported to play an important role in influenza virus infection (58).
The American Cancer Research Institute (AICR) has always recommended a low-fat diet, high in fruits, vegetables, and whole-grain products for cancer survivors. It also considers adequate levels of major macronutrients and various vitamins and minerals necessary to maintain health (59). During cancer treatments with chemotherapy or radiotherapy, patients often experience nausea, vomiting, diarrhea, and loss of appetite, leading to fewer food combinations and weight loss (60). Supplementation with essential vitamins and minerals may seem desirable, but it may not always. Food interactions with treatment may affect the outcome of treatment. Of particular concern here are dietary supplements with antioxidant properties, but supplements without antioxidant properties may also affect the effectiveness of cancer treatment (61). Many non-oxidant supplements are widely consumed by cancer patients, although their effects on the effectiveness of chemotherapy treatments are controversial. Vitamin D is one of the non-oxidant vitamins that is probably useful in cancer treatment (62). It has been observed that vitamin D receptor (VDR) is expressed significantly in the immune system, raising the possibility that vitamin D and similar may have immunomodulating activity (63). Cell studies state that vitamin D modulates the activity of various defense and immune cells including blood monocytes, macrophages, antigen-providing cells, and activated CD4+ T cells or epithelial cells (64, 65). In general, vitamin D metabolites have significant anti-neoplastic activity in clinical models. The immune system is a suitable target for the anti-neoplastic effects of vitamin D. Vitamin D receptor (VDR) is expressed in different immune cells. Vitamin D can have inhibitory effects on chronic inflammation, resulting in the proliferation of immune cells. Also, it is suggested that some types of cancer may be more sensitive to the effects of vitamin D supplementation than others (66).
Another supplement that has been considered in cancer research is selenium (67). Selenium is an essential component in several major metabolic pathways, including the antioxidant defense system and the immune system. Selenium is incorporated into 30 different selenoproteins (68). Selenoproteins play an important role in antioxidant and DNA stability and may have anti-cancer effects. Also, selenium is involved in cell proliferation and apoptotic cell death in healthy and malignant cells. Low selenium levels are associated with a high prevalence of several different types of cancer and cancer mortality (69). Also, the effects of foods and supplements such as omega 3, vitamins E and C on the immune system responses in cancer have been specifically considered and confirmed. However, due to the stage and treatments related to cancer, the use of various supplements has always been cautious. Antioxidants are not always effective during cancer treatment, and also higher doses of some supplements may cause side effects. In the following, we discussed the possible effects of some supplements in combination with exercise training as a possible modulator and the possible effects in the COVID-19.
Limited studies have been performed on the combined effects of dietary supplementation and regular physical exercise on the prevention and treatment of COVID-19. It seems that regular physical exercise and dietary intake of functional compounds as two main parts of a proper lifestyle that can prevent severe cases of this disease. Also, it is shown that patients with chronic disease are susceptible to severe cases of COVID-19, and a combination of exercise and proper nutrition have always been effective in reducing some chronic diseases (70). So, one of the main considerations would be lifestyle changes, including increasing physical exercise and intake of functional compounds to improve immune function and induce antiviral effects. Cancer patients as one of the chronic diseases are involved in more severe cases of COVID-19 (71). It is probable that some dietary supplements especially in high doses with anti-inflammatory and antioxidant effects can interfere with cancer treatment. Combining exercise training with dietary supplements can be effective in modulating the effects of supplements in these patients and improve immune system’s response to infectious diseases, especially COVID-19.
In the athletes, nutritional supplements such as probiotics, glutamine, a variety of vitamins and minerals, selenium, etc., have always been considered to improve the immune system responses and prevent upper respiratory system infections (72). It has also been shown that the combined effects of exercise with a proper diet can be effective in the reduction of inflammation, leukocytes adhesion, and chemotaxis capacity, and creating anti-oxidative effects in metabolic syndrome and obesity. This has been especially true for the elderly due to immunosenescence (73). Regular physical exercise and dietary intake of functional compounds can induce immune-boosting and decrease the adverse effects of age-related immune dysfunction.
Cancer survivors are often highly motivated to seek about dietary choices, physical activity, and dietary supplements to improve their treatment outcomes, quality of life, and overall survival. Many dietary supplements contain levels of antioxidants that are significantly higher than the recommended dietary intake. Research has shown that taking high doses of vitamin D or selenium supplements can improve cancer and reduce tumor volume (74). On the other hand, it has been suggested that taking high doses of supplements with antioxidant activity during chemotherapy or radiation therapy may not be wise, as antioxidants can potentially reduce cellular oxidative damage to cancer cells, which contributes to the effectiveness of these therapies (75). Recent studies have suggested the possible role of exercise training in reducing the side effects of high doses of antioxidant supplementation in breast cancer tumors. The results of studying the use of high-dose vitamin D with exercise training in women with breast cancer have shown that a combination of exercise training and a high dose of vitamin D can modulate leukocytes’ cell survival-related gene expression in breast cancer survivors (76). It is suggested that response to vitamin D supplementation in cancer modulated via vitamin D receptor (77). The effects of physical exercise on the increase of vitamin D receptors in various tissues have been observed in some studies (78). Also, Lithgow et al. (79) showed that moderate aerobic exercise increases T-cell vitamin D receptor expression in vitamin D-deficient men (79). Changes in vitamin D receptors in various tissues, including the immune system following physical exercise, can be used as a mechanism to enhance the immune system’s response to vitamin D supplementation. However, more studies are needed in this area.
It has also been suggested that daily doses of 100–200 mcg of selenium consumption, inhibit genetic damage and cancer development in humans (80). About 400 mcg of selenium per day is considered the upper limit. Higher doses of RDA are needed to inhibit genetic damage and cancer. However, it is assumed that taking excessive selenium may cause oxidative damage and lead to genomic instability (81). A study in mice with cancer confirmed the effects of using selenium nanoparticles on cancer-induced cachexia (82). Concomitant use of exercise while improving the immune response, including T cells and antitumor immunity, prevented cachexia in animals (83). It seems that the modulating effects of exercise on selenium-induced responses in cancer can be effective in enhancing antitumor immunity.
The effects of physical exercise on cancer cells have been proven, including activation of invasion and metastasis, escaping growth inhibitors, reducing cell death, inhibiting cancer inflammatory cells, normalizing vessels, escaping immune damage, and reprogramming energy metabolism. Lactic acid is the final metabolite in the anaerobic glycolysis pathway. Increasing aerobic glycolysis in cancer cells can produce a large amount of lactic acid, which reduces pH in cancer cells (84). Proliferation, invasion, and metastasis of cancer cells are associated with angiogenesis, which is related to low pH levels in the tumor microenvironment. Also, lactic acid accumulation in cancer suppresses the immune responses, inhibits the T-cell response, and prevents lactic acid from leaving the T-cell, thereby disrupting the metabolic pathway. It has been stated that moderate-intensity exercise can reduce lactate levels in cells. Therefore, the metabolic process plays a role in the inhibition of anaerobic glycolysis in cancer metabolism (85). As a result, exercise can affect the reprogramming of cancer metabolism by improving the internal blood flow of cancer, angiogenesis, and cancer hypoxia. And rearranging in cancer cells metabolism and taking supplements that are effective in curing cancer, such as selenium and vitamin D, can show a synergistic effect on curing cancer and reducing tumor volume. Using a combination of selenium and other vitamins along with regular physical exercise also seems to be a promising approach to controlling genetic damage, and cancer development. Also, physical exercise can mitigate the side effects of high doses of antioxidants (86).
In addition, combining regular physical exercise and dietary supplementation can probably improve innate and acquired immune system responses (48, 87–89) and protect cancer patients against COVID-19. COVID-19 hyper-inflammatory responses and effects on the respiratory system are mediated by the ACE2, which ultimately leads to effects on other organs (90). Two receptors in the renin-angiotensin system by two opposite arms included: one classical composed by ACE/Angiotensin (Ang) II AT1 receptor (AT1R); and the alternative arm comprising ACE2/Ang-(1-7)/Mas receptor that have anti-inflammatory, vasodilatory, antiproliferative, cardioprotective, and renoprotective actions (91, 92). It is shown that COVID-19 is activated with renin-angiotensin system imbalance, which activates the classical arm (ACE/Ang II/AT1R) and leads to pulmonary injury, hematological alterations, and hyper-inflammatory state. Dysfunction of ACE2/Ang-(1-7)/Mas receptor has been observed in some cancers (91). Exercise training can activate the ACE2-Ang1-7-Mas receptor to induce an anti-inflammatory effect; and lead to inhibiting the ACE-Ang II-AT1 receptor pathway, inflammatory molecules, and oxidative stress in different tissues (93). Figure 1 summarizes the possible effects of exercise training in cancer and COVID-19.
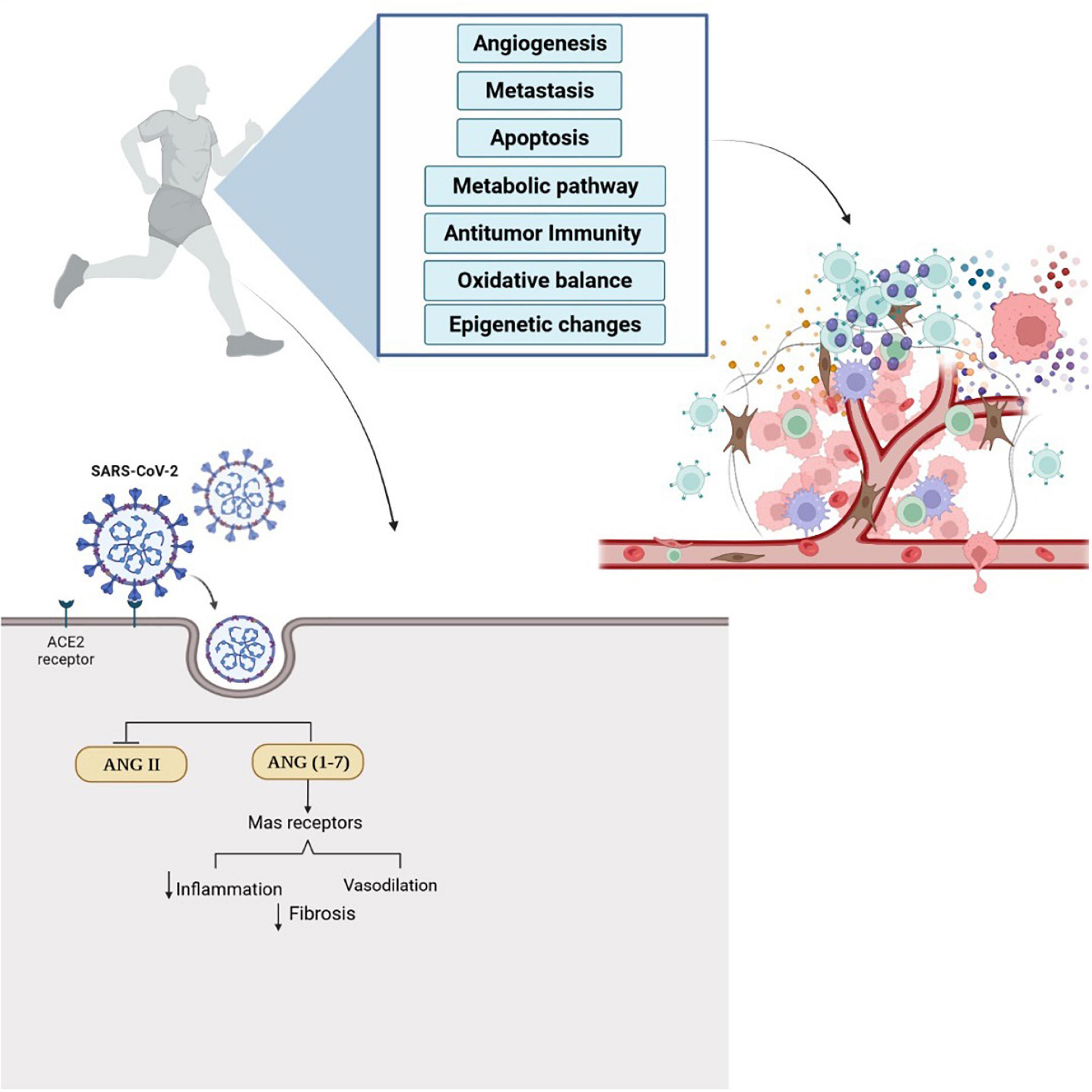
Figure 1. Regular physical exercise can affect tumor microenvironment and induce anti-tumor immunity. Also, exercise training augments the expression of ACE2/ANG-(1-7) Mas receptor and activate anti-inflammatory pathways. Physical exercise simultaneously inhibits the ACE-Ang II-AT1 receptor pathway, inflammatory molecules, and oxidative stress in different tissues and can decrease the possibility of infection with COVID-19. The effects of regular physical exercise on antitumor immunity and ACE2 receptors can probably help cancer patients in the prevention and treatment of COVID-19.
In addition, the effects of supplementation in COVID-19 can probably be discussed in more detail concerning the ACE2. Although there is conflicting information about the effects of this supplement on the prevention and treatment of COVID-19, some studies have suggested an active form of Vitamin D improved immune system response against COVID-19 (64). Also, it can probably induce ACE2/Ang-(1-7)/MasR axis activity and inhibits renin and the ACE/Ang II/AT1R axis (94). Vitamin D hydroxylate in the kidney yield 1,25(OH)2D3, and it binds to the VDR to activate vitamin D response elements within target genes (95). Considering the effects of exercise training on VDR, the combination of exercise training and vitamin D supplementation can also be effective in regulating the effects of ACE2 and preventing severe cases of COVID-19 in cancer patients (96). However, more studies are needed, especially in cancer patients with different cancers and at different stages of cancer.
Social distance and vaccination are still the best way to prevent severe cases of COVID-19. Despite the observed effects on immune responses to traditional vaccines, especially in older people (97), there is no information on the possible effects of physical exercise or dietary supplements on the COVID-19 vaccines. More studies with assessment of the combination of exercise training and dietary supplements on the potential efficacy of COVID-19 vaccines may be on the agenda of future studies.
COVID-19 is spreading with new variants, and cancer patients are prone to severe cases of COVID-19. However, using lifestyle strategies such as regular physical activity and intake of functional compounds as supplements can be effective in treatment outcomes, quality of life, and overall survival. In addition, we cannot ignore the role of regular physical exercise due to metabolic, physiological, and psychological effects. Exercise training and supplementation can improve immune system responses. Combining these two factors can be an important strategy for improving immune responses against COVID-19 and probably improving vaccine responses. However, more studies are needed on nutrition, exercise, and their combined effects on cancer and improving immune responses to infectious diseases in cancer.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
MM and KS conceived the review, drafted, and approved the final version of the manuscript. AH and AV made some additions to the text, revised the manuscript, and approved the final version. All authors contributed to the article and approved the submitted version.
This work was supported by the Tarbiat Modares Univeristy, Tehran, Iran.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
2. Hu L, Chen S, Fu Y, Gao Z, Long H, Ren H-W, et al. Risk factors associated with clinical outcomes in 323 coronavirus disease 2019 (COVID-19) hospitalized patients in Wuhan, China. Clin Infect Dis. (2020) 71:2089–98. doi: 10.1093/cid/ciaa539
3. Smallwood N, Harrex W, Rees M, Willis K, Bennett CM. COVID-19 infection and the broader impacts of the pandemic on healthcare workers. Respirology. (2022). 26:1–16. doi: 10.1111/resp.14208
4. Hirano T, Murakami M. COVID-19: a new virus, but a familiar receptor and cytokine release syndrome. Immunity. (2020) 52:731–3. doi: 10.1016/j.immuni.2020.04.003
5. Bakasis AD, Mavragani CP, Boki KA, Tzioufas AG, Vlachoyiannopoulos PG, Stergiou IE, et al. COVID-19 infection among autoimmune rheumatic disease patients: data from an observational study and literature review. J Autoimmun. (2021) 123:102687. doi: 10.1016/j.jaut.2021.102687
6. Sen-Crowe B, McKenney M, Elkbuli A. Social distancing during the COVID-19 pandemic: staying home save lives. Am J Emerg Med. (2020) 38:1519–20. doi: 10.1016/j.ajem.2020.03.063
7. Xiao H, Shu W, Li M, Li Z, Tao F, Wu X, et al. Social distancing among medical students during the 2019 coronavirus disease pandemic in China: disease awareness, anxiety disorder, depression, and behavioral activities. Int J Environ Res Public Health. (2020) 17:5047.
8. Chamilos G, Lionakis MS, Kontoyiannis DP. Are all patients with cancer at heightened risk for severe Coronavirus disease 2019 (COVID-19)? Clin Infect Dis. (2021) 72:351–6. doi: 10.1093/cid/ciaa1079
9. Nakamura K, Smyth MJ. Targeting cancer-related inflammation in the era of immunotherapy. Immunol Cell Biol. (2017) 95:325–32. doi: 10.1038/icb.2016.126
10. Li L, Yu R, Cai T, Chen Z, Lan M, Zou T, et al. Effects of immune cells and cytokines on inflammation and immunosuppression in the tumor microenvironment. Int Immunopharmacol. (2020) 88:106939. doi: 10.1016/j.intimp.2020.106939
11. Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. (2008) 454:436–44. doi: 10.1038/nature07205
12. Ramírez-Vélez R, Zambom-Ferraresi F, García-Hermoso A, Kievisiene J, Rauckiene-Michealsson A, Agostinis-Sobrinho C. Evidence-based exercise recommendations to improve mental wellbeing in women with breast cancer during active treatment: a systematic review and meta-analysis. Cancers. (2021) 13:264. doi: 10.3390/cancers13020264
13. Zitvogel L, Pietrocola F, Kroemer G. Nutrition, inflammation and cancer. Nat Immunol. (2017) 18:843–50.
14. Sallis R, Young DR, Tartof SY, Sallis JF, Sall J, Li Q, et al. Physical inactivity is associated with a higher risk for severe COVID-19 outcomes: a study in 48 440 adult patients. Br J Sports Med. (2021) 55:1099–105. doi: 10.1136/bjsports-2021-104080
15. Jee Y-S. Influences of acute and/or chronic exercise on human immunity: third series of scientific evidence. J Exerc Rehabil. (2020) 16:205. doi: 10.12965/jer.2040414.207
17. Fernández-Lázaro D, González-Bernal JJ, Sánchez-Serrano N, Navascués LJ, Ascaso-del-Río A, Mielgo-Ayuso J. Physical exercise as a multimodal tool for COVID-19: could it be used as a preventive strategy? Int J Environ Res Public Health. (2020) 17:8496. doi: 10.3390/ijerph17228496
18. Loose D, Van de Wiele C. The immune system and cancer. Cancer Biother Radiopharm. (2009) 24:369–76.
19. Gottschalk G, Knox K, Roy A. ACE2: at the crossroad of COVID-19 and lung cancer. Gene Rep. (2021) 23:101077. doi: 10.1016/j.genrep.2021.101077
20. Lordan R, Rando HM, Consortium C-R, Greene CS. Dietary supplements and nutraceuticals under investigation for COVID-19 prevention and treatment. ArXiv [Preprint]. (2021). arXiv:2102.02250v02251. doi: 10.1128/mSystems.00122-21
21. Vaduganathan M, Vardeny O, Michel T, McMurray JJV, Pfeffer MA, Solomon SD. Renin-angiotensin-aldosterone system inhibitors in patients with Covid-19. N Engl J Med. (2020) 382:1653–9. doi: 10.1056/NEJMsr2005760
22. Dalan R, Bornstein SR, El-Armouche A, Rodionov RN, Markov A, Wielockx B, et al. The ACE-2 in COVID-19: foe or friend? Horm Metab Res. (2020) 52:257–63. doi: 10.1055/a-1155-0501
23. Onder G, Rezza G, Brusaferro S. Case-Fatality rate and characteristics of patients dying in relation to COVID-19 in Italy. JAMA. (2020) 323:1775–6. doi: 10.1001/jama.2020.4683
24. Zhang H, Zhou P, Wei Y, Yue H, Wang Y, Hu M, et al. Histopathologic changes and SARS-CoV-2 immunostaining in the lung of a patient with COVID-19. Ann Intern Med. (2020) 172:629–32.
25. Goritzka M, Makris S, Kausar F, Durant LR, Pereira C, Kumagai Y, et al. Alveolar macrophage–derived type I interferons orchestrate innate immunity to RSV through recruitment of antiviral monocytes. J Exp Med. (2015) 212:699–714. doi: 10.1084/jem.20140825
26. Palermo E, Di Carlo D, Sgarbanti M, Hiscott J. Type I interferons in COVID-19 pathogenesis. Biology. (2021) 10:829.
27. Wang F, Nie J, Wang H, Zhao Q, Xiong Y, Deng L, et al. Characteristics of peripheral lymphocyte subset alteration in COVID-19 pneumonia. J Infect Dis. (2020) 221:1762–9. doi: 10.1093/infdis/jiaa150
29. Sette A, Crotty S. Adaptive immunity to SARS-CoV-2 and COVID-19. Cell. (2021) 184:861–80. doi: 10.1016/j.cell.2021.01.007
30. Moderbacher CR, Ramirez SI, Dan JM, Grifoni A, Hastie KM, Weiskopf D, et al. Antigen-specific adaptive immunity to SARS-CoV-2 in acute COVID-19 and associations with age and disease severity. Cell. (2020) 183:996.–1012. doi: 10.1016/j.cell.2020.09.038
31. Nieman DC, Wentz LM. The compelling link between physical activity and the body’s defense system. J Sport Health Sci. (2019) 8:201–17. doi: 10.1016/j.jshs.2018.09.009
32. Suzuki K. Chronic inflammation as an immunological abnormality and effectiveness of exercise. Biomolecules. (2019) 9:223. doi: 10.3390/biom9060223
33. Furtado GE, Letieri RV, Caldo-Silva A, Sardão VA, Teixeira AM, de Barros MP, et al. Sustaining efficient immune functions with regular physical exercise in the COVID-19 era and beyond. Eur J Clin Invest. (2021) 51:e13485. doi: 10.1111/eci.13485
34. Metsios GS, Moe RH, Kitas GD. Exercise and inflammation. Best Pract Res Clin Rheumatol. (2020) 34:101504. doi: 10.1016/j.berh.2020.101504
35. Improta-Caria AC, Soci ÚPR, Pinho CS, Aras Júnior R, De Sousa RAL, Bessa TCB. Physical exercise and immune system: perspectives on the COVID-19 pandemic. Rev Associação Méd Bras. (2021) 67:102–7. doi: 10.1590/1806-9282.67.Suppl1.20200673
36. Hekmatikar AHA, Shamsi MM, Ashkazari ZSZ, Suzuki K. Exercise in an overweight patient with COVID-19: a case study. Int J Environ Res Public Health. (2021) 18:5882. doi: 10.3390/ijerph18115882
37. Khoramipour K, Basereh A, Hekmatikar AA, Castell L, Ruhee RT, Suzuki K. Physical activity and nutrition guidelines to help with the fight against COVID-19. J Sports Sci. (2021) 39:101–7. doi: 10.1080/02640414.2020.1807089
38. Colotta F, Allavena P, Sica A, Garlanda C, Mantovani A. Cancer-related inflammation, the seventh hallmark of cancer: links to genetic instability. Carcinogenesis. (2009) 30:1073–81. doi: 10.1093/carcin/bgp127
39. Shimasaki N, Jain A, Campana D. NK cells for cancer immunotherapy. Nat Rev Drug Discov. (2020) 19:200–18.
40. O’Sullivan D, Sanin DE, Pearce EJ, Pearce EL. Metabolic interventions in the immune response to cancer. Nat Rev Immunol. (2019) 19:324–35. doi: 10.1038/s41577-019-0140-9
41. Hojman P, Gehl J, Christensen JF, Pedersen BK. Molecular mechanisms linking exercise to cancer prevention and treatment. Cell Metab. (2018) 27:10–21. doi: 10.1016/j.cmet.2017.09.015
42. Zhang X, Ashcraft KA, Betof Warner A, Nair SK, Dewhirst MW. Can exercise-induced modulation of the tumor physiologic microenvironment improve antitumor immunity? Cancer Res. (2019) 79:2447–56. doi: 10.1158/0008-5472.CAN-18-2468
43. Khosravi N, Hanson E, Farajivafa V, Agha-Alinejad H, Haghighat S, Molanouri Shamsi M, et al. Changes in monocyte populations following acute aerobic exercise in breast cancer survivors. Iran Q J Breast Dis. (2018) 11:7–16.
44. Koelwyn GJ, Wennerberg E, Demaria S, Jones LW. Exercise in regulation of inflammation-immune axis function in cancer initiation and progression. Oncology. (2015) 29:214800.
45. Molanouri Shamsi M, Mohammad Hassan M. Chapter 42: exercise, selenium and cancer cells. In: Preedy V, Vinood B, editors. Cancer: Oxidative Stress and Dietary Antioxidants. 2nd ed. Cambridge, MA: Academic Press (2020). doi: 10.1016/B978-0-12-819547-5.00042-0
46. Kruijsen-Jaarsma M, Révész D, Bierings MB, Buffart LM, Takken T. Effects of exercise on immune function in patients with cancer: a systematic review. Exerc Immunol Rev. (2013) 19:120–43.
47. Mrityunjaya M, Pavithra V, Neelam R, Janhavi P, Halami PM, Ravindra PV. Immune-boosting, antioxidant and anti-inflammatory food supplements targeting pathogenesis of COVID-19. Front Immunol. (2020) 11:2337. doi: 10.3389/fimmu.2020.570122
48. Wu D, Lewis ED, Pae M, Meydani SN. Nutritional modulation of immune function: analysis of evidence, mechanisms, and clinical relevance. Front Immunol. (2019) 9:3160. doi: 10.3389/fimmu.2018.03160
49. Harty PS, Cottet ML, Malloy JK, Kerksick CM. Nutritional and supplementation strategies to prevent and attenuate exercise-induced muscle damage: a brief review. Sports Med Open. (2019) 5:1–1. doi: 10.1186/s40798-018-0176-6
50. Aggarwal BB, Shishodia S. Suppression of the nuclear factor-kB activation pathway by spice-derived phytochemicals. Ann NY Acad Sci. (2004) 1030:434–41. doi: 10.1196/annals.1329.054
51. Grzanna R, Lindmark L, Frondoza CG. Ginger—an herbal medicinal product with broad anti-inflammatory actions. J Med Food. (2005) 8:125–32. doi: 10.1089/jmf.2005.8.125
52. Talpur AD, Ikhwanuddin M, Bolong A-MA. Nutritional effects of ginger (Zingiber officinale Roscoe) on immune response of Asian sea bass, Lates calcarifer (Bloch) and disease resistance against Vibrio harveyi. Aquaculture. (2013) 400:46–52.
53. Meydani SN, Lewis ED, Wu D. Perspective: should vitamin E recommendations for older adults be increased? Adv Nutr. (2018) 9:533–43. doi: 10.1093/advances/nmy035
54. Meydani SN, Han SN. Nutrient regulation of the immune response: the case of vitamin E. 8th ed. In: B Bowman, R Russell Present Knowledge in Nutrition. Washington, DC: (2001). p. 449–63.
55. Han S, Wu D, Ha W, Beharka A, Smith D, Bender B, et al. Vitamin E supplementation increases T helper 1 cytokine production in old mice infected with influenza virus. Immunology. (2000) 100:487–93. doi: 10.1046/j.1365-2567.2000.00070.x
56. Lagishetty V, Misharin AV, Liu NQ, Lisse TS, Chun RF, Ouyang Y, et al. Vitamin D deficiency in mice impairs colonic antibacterial activity and predisposes to colitis. Endocrinology. (2010) 151:2423–32. doi: 10.1210/en.2010-0089
57. Iruretagoyena M, Hirigoyen D, Naves R, Burgos PI. Immune response modulation by vitamin D: role in systemic lupus erythematosus. Front Immunol. (2015) 6:513. doi: 10.3389/fimmu.2015.00513
58. Martineau AR, Jolliffe DA, Hooper RL, Greenberg L, Aloia JF, Bergman P, et al. Vitamin D supplementation to prevent acute respiratory tract infections: systematic review and meta-analysis of individual participant data. BMJ. (2017) 356:i6583. doi: 10.1136/bmj.i6583
59. Wiseman M. The second world cancer research fund/American institute for cancer research expert report. Food, nutrition, physical activity, and the prevention of cancer: a global perspective: nutrition society and BAPEN medical symposium on ‘nutrition support in cancer therapy’. Proc Nutr Soc. (2008) 67:253–6. doi: 10.1017/S002966510800712X
60. Dewys WD, Begg C, Lavin PT, Band PR, Bennett JM, Bertino JR, et al. Prognostic effect of weight loss prior tochemotherapy in cancer patients. Am J Med. (1980) 69:491–7.
61. Allowances RD. Recommended Dietary Allowances. Washington, DC: National Research Council-National Academy Press (1989).
62. Mäkitie A, Tuokkola I, Laurell G, Mäkitie O, Olsen K, Takes RP, et al. Vitamin D in head and neck cancer: a systematic review. Curr Oncol Rep. (2021) 23:1–8.
63. Batra U, Sharma M. Association of vitamin D with cancer–catch me if you can! Cancer Res Stat Treat. (2020) 3:78.
64. Bae M, Kim H. The role of vitamin C, vitamin D, and selenium in immune system against COVID-19. Molecules. (2020) 25:5346.
65. Koivisto O, Hanel A, Carlberg C. Key vitamin D target genes with functions in the immune system. Nutrients. (2020) 12:1140. doi: 10.3390/nu12041140
66. Lazzeroni M, Serrano D, Pilz S, Gandini S. Vitamin D supplementation and cancer: review of randomized controlled trials. Anti Cancer Agents Med Chem. (2013) 13:118–25.
67. Zarei S, Hosseiniara SM. Selenium as a mineral with anti-cancer properties. Novelty Clin Med. (2022) 1:4–25.
68. Chekachak S, Molanouri SM, Soudi S. Assessment of aerobic training with selenium nanoparticles supplementation effects on cytokines levels of liver tissue in 4T1 breast cancer mice. Iran J Endocrinol Metab. (2017) 19:116–25.
69. Chekachak S, Molanouri Shamsi M, Soudi S. Investigating the effect of aerobic interval training with selenium nanoparticles on the content of IL-6, TNF-α and IL-4 cytokines in spleen tissue of mice with breast cancer. J Fasa Univ Med Sci. (2018) 8:608–17.
70. Cancello R, Lucchetti E, Gobbi M, Brunani A. Nutrition and Exercise 4. Rehabilitation Interventions in the Patient with Obesity. London: Springer (2020). 51 p.
71. Burki TK. Cancer guidelines during the COVID-19 pandemic. Lancet Oncol. (2020) 21:629–30. doi: 10.1016/S1470-2045(20)30217-5
72. Costagliola G, Spada E, Comberiati P, Peroni DG. Could nutritional supplements act as therapeutic adjuvants in COVID-19? Ital J Pediatr. (2021) 47:1–5. doi: 10.1186/s13052-021-00990-0
73. Calder PC. Nutrition, immunity and COVID-19. BMJ Nutr Prev Health. (2020) 3:74. doi: 10.1136/bmjnph-2020-000085
74. Akutsu T, Kitamura H, Himeiwa S, Kitada S, Akasu T, Urashima M. Vitamin D and cancer survival: does vitamin D supplementation improve the survival of patients with cancer? Curr Oncol Rep. (2020) 22:1–9. doi: 10.1007/s11912-020-00929-4
75. Myung SK, Kim Y, Ju W, Choi HJ, Bae WK. Effects of antioxidant supplements on cancer prevention: meta-analysis of randomized controlled trials. Ann Oncol. (2010) 21:166–79. doi: 10.1093/annonc/mdp286
76. Khedmati Zare V, Javadi M, Amani-shalamzari S, Kaviani M. The high dose of vitamin D supplementation combined with yoga training improve the leukocytes cell survival-related gene expression in breast cancer survivors. Nutr Metab. (2021) 18:80. doi: 10.1186/s12986-021-00607-7
77. Carlberg C, Velleuer E. Vitamin D and the risk for cancer: a molecular analysis. Biochem Pharmacol. (2021) 196:114735.
78. Makanae Y, Ogasawara R, Sato K, Takamura Y, Matsutani K, Kido K, et al. Acute bout of resistance exercise increases vitamin D receptor protein expression in rat skeletal muscle. Exp Physiol. (2015) 100:1168–76. doi: 10.1113/ep085207
79. Lithgow H, Florida-James G, Ross M, Duncan G, Leggate M. Exercise acutely increases vitamin D receptor expression in T lymphocytes in vitamin D-deficient men, independent of age. Exp Physiol. (2021) 106:1460–9. doi: 10.1113/EP089480
80. Combs G Jr., Clark L, Turnbull B. An analysis of cancer prevention by selenium. Biofactors. (2001) 14:153–9.
81. Carlisle AE, Lee N, Matthew-Onabanjo AN, Spears ME, Park SJ, Youkana D, et al. Selenium detoxification is required for cancer-cell survival. Nat Metab. (2020) 2:603–11. doi: 10.1038/s42255-020-0224-7
82. Molanouri Shamsi M, Chekachak S, Soudi S, Quinn LS, Ranjbar K, Chenari J, et al. Combined effect of aerobic interval training and selenium nanoparticles on expression of IL-15 and IL-10/TNF-α ratio in skeletal muscle of 4T1 breast cancer mice with cachexia. Cytokine. (2017) 90:100–8. doi: 10.1016/j.cyto.2016.11.005
83. Molanouri Shamsi M, Chekachak S, Soudi S, Gharakhanlou R, Quinn LS, Ranjbar K, et al. Effects of exercise training and supplementation with selenium nanoparticle on T-helper 1 and 2 and cytokine levels in tumor tissue of mice bearing the 4 T1 mammary carcinoma. Nutrition. (2019) 57:141–7. doi: 10.1016/j.nut.2018.05.022
84. D’Ascenzi F, Anselmi F, Fiorentini C, Mannucci R, Bonifazi M, Mondillo S. The benefits of exercise in cancer patients and the criteria for exercise prescription in cardio-oncology. Eur J Prev Cardiol. (2021) 28:725–35.
85. Grande AJ, Silva V, Neto LS, Basmage JPT, Peccin MS, Maddocks M. Exercise for cancer cachexia in adults. Cochrane Datab Syst Rev. (2021) 3:1–26.
86. Thomas S, Alias M, Venkateswaramurthy N. Exercise in cancer and its benefits in cancer survivors. World J Pharm Sci. (2021) 9:14–21.
87. Molanouri Shamsi M, Hassan ZM, Quinn LS, Gharakhanlou R, Baghersad L, Mahdavi M. Time course of IL-15 expression after acute resistance exercise in trained rats: effect of diabetes and skeletal muscle phenotype. Endocrine. (2015) 49:396–403. doi: 10.1007/s12020-014-0501-x
88. Rezaei S, Shamsi MM, Mahdavi M, Jamali A, Prestes J, Tibana RA, et al. Endurance exercise training decreased serum levels of surfactant protein D and improved aerobic fitness of obese women with type-2 diabetes. Diabetol Metab Syndr. (2017) 9:74–74. doi: 10.1186/s13098-017-0273-6
89. Shao T, Verma HK, Pande B, Costanzo V, Ye W, Cai Y, et al. Physical activity and nutritional influence on immune function: an important strategy to improve immunity and health status. Front Physiol. (2021) 12:751374. doi: 10.3389/fphys.2021.751374
90. Zaim S, Chong JH, Sankaranarayanan V, Harky A. COVID-19 and multiorgan response. Curr Prob Cardiol. (2020) 45:100618. doi: 10.1016/j.cpcardiol.2020.100618
91. Lanza K, Perez LG, Costa LB, Cordeiro TM, Palmeira VA, Ribeiro VT, et al. Covid-19: the renin–angiotensin system imbalance hypothesis. Clin Sci. (2020) 134:1259–64. doi: 10.1042/CS20200492
92. Touyz RM, Li H, Delles C. ACE2 the Janus-faced protein–from cardiovascular protection to severe acute respiratory syndrome-coronavirus and COVID-19. Clin Sci. (2020) 134:747–50. doi: 10.1042/CS20200363
93. Heffernan KS, Jae SY. Exercise as medicine for COVID-19: an ACE in the hole? Med Hypotheses. (2020) 142:109835. doi: 10.1016/j.mehy.2020.109835
94. Hamming I, Timens W, Bulthuis M, Lely A, Navis GV, van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. (2004) 203:631–7. doi: 10.1002/path.1570
95. Bikle DD. Vitamin D metabolism, mechanism of action, and clinical applications. Chem Biol. (2014) 21:319–29. doi: 10.1016/j.chembiol.2013.12.016
96. Caballero-García A, Pérez-Valdecantos D, Guallar P, Caballero-Castillo A, Roche E, Noriega DC, et al. Effect of vitamin D supplementation on muscle status in old patients recovering from COVID-19 infection. Medicina (Kaunas). (2021) 57:1079. doi: 10.3390/medicina57101079
Keywords: aerobic exercise training, cancer, immune response, vitamin D, coronavirus
Citation: Molanouri Shamsi M, Vahed A, Hekmatikar AA and Suzuki K (2022) Combined Effects of Exercise Training and Nutritional Supplementation in Cancer Patients in the Context of the COVID-19: A Perspective Study. Front. Nutr. 9:847215. doi: 10.3389/fnut.2022.847215
Received: 01 January 2022; Accepted: 14 February 2022;
Published: 09 March 2022.
Edited by:
Yao Lin, Fujian University of Traditional Chinese Medicine, ChinaReviewed by:
Diego Fernández Lázaro, University of Valladolid, SpainCopyright © 2022 Molanouri Shamsi, Vahed, Hekmatikar and Suzuki. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mahdieh Molanouri Shamsi, bW9sYW5vdXJpQG1vZGFyZXMuYWMuaXI=; Katsuhiko Suzuki, a2F0c3Uuc3V6dUB3YXNlZGEuanA=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.