
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Nutr. , 12 May 2022
Sec. Nutrition and Metabolism
Volume 9 - 2022 | https://doi.org/10.3389/fnut.2022.846600
This article is part of the Research Topic The Model of Ramadan Diurnal Intermittent Fasting: Unraveling the Health Implications - Volume 1 View all 16 articles
 Alexander Kieu1,2*†
Alexander Kieu1,2*† Ashley Iles3†
Ashley Iles3† Moien AB Khan1†
Moien AB Khan1† Linda Östlundh1†
Linda Östlundh1† Duston Boyd4,5†
Duston Boyd4,5† MoezAlIslam Ezzat Faris6†
MoezAlIslam Ezzat Faris6†Background: Muslims with insulin-requiring type 2 diabetes are at high risk of hypo- and hyperglycemia while fasting during the month of Ramadan. Although a few reviews on diabetic management during Ramadan have been published, surveys reveal knowledge gaps remain among physicians.
Aim: This systematic review qualitatively analyzes what insulin dosing recommendations are likely to reduce hypoglycemic events and improve glycemic control during the Ramadan fasting for this high-risk group.
Methods: A comprehensive search in six databases and gray sources was performed from August 10, 2001, to August 10, 2021, for studies assessing which types of insulin and/or what dosing recommendations reduce hypoglycemic events and improve glycemic control during Ramadan. We excluded studies focusing mainly on oral antihyperglycemic medications, type 1 diabetes, persons with insulin pumps, and studies older than 20 years. Hypoglycemic event rates, pre-, and post-iftar blood glucose levels, overall average blood glucose, and hemoglobin A1c were analyzed, and a narrative synthesis was performed.
Results: Out of 1,101 collected articles, 14 eligible studies including 2,969 participants with an average age of 54.8 years, we found that insulin dose reduction may prevent hypoglycemia without causing subsequent hyperglycemia, and rapid-acting insulin analogs may improve post-iftar and overall blood glucose without incurring hypoglycemia.
Conclusions: Though initial findings are promising, more research is needed to confirm the benefits of insulin dose reduction, rapid-acting insulin analogs, and ultra-long-acting insulins.
Systematic Review Registration: https://www.crd.york.ac.uk/prospero/, identifier: CRD42021268943.
The burden of diabetes mellitus continues to rise globally across all regions of the world (1). Approximately 463 million adults are living with diabetes worldwide, and this figure is projected to increase by 51% in the next 25 years (2). Of all the people with diabetes globally, 150 million are estimated to be Muslim (3). One of the five pillars of Islam, central to the Muslim faith, is annual fasting during the holy month of Ramadan. During this month, all healthy Muslims who have reached puberty are required to fast from dawn to sunset, which includes refraining from eating, drinking, use of oral medications, and smoking (4). Exemptions are available for certain populations, such as Muslims who are elderly, traveling, expecting, or nursing mothers and Muslims with serious medical conditions including diabetes. However, many Muslims with these conditions still voluntarily choose to observe the practice of fasting during Ramadan (5). Epidemiologic studies have shown that Muslims with diabetes fast for an average of 27–28 days in the month of Ramadan (4, 6–8). Even 43.9% of Muslims with a “high” or “very high” risk classification of diabetes (according to the American Diabetes Association, ADA) fasted for an average of 28 days during Ramadan despite medical advice (9).
Hypoglycemia during Ramadan is a major concern, particularly for those who fast for up to 20 h consecutively (4). Recurrent hypoglycemia may increase the risk of cognitive impairment and mortality (10). This concern is heightened for patients with insulin-requiring diabetes, many of whom are categorized as very high risk by the ADA risk index (9). One study showed this very high-risk cohort has a 13.8% incidence of hypoglycemia compared to 4.2% for low-risk individuals (9). The same study showed that persons with type 2 diabetes taking only insulin during Ramadan had a greater incidence (16.8%) of hypoglycemia than those treated with only oral hypoglycemic agents (5.3%) (9).
In addition to hypoglycemia, hyperglycemia during Ramadan is also a major concern. One large epidemiologic study revealed a significantly increased risk of severe hyperglycemia or ketoacidosis during Ramadan (0.05 ± 0.35) compared to before Ramadan (0.01 ± 0.05) (4). A possible explanation for this may be because the meal that breaks the fast after sunset (iftar) is typically larger than average, and it has been shown that the risk of hyperglycemia is consequently higher (6). A more recent study that used flash-glucose monitoring on insulin-treated patients during Ramadan showed an increase in time in hyperglycemia and a reduced time in the target range (11). The long-term effects of elevated uncontrolled blood glucose are well-known including increased risk of cardiovascular disease, nephropathy, neuropathy, and ophthalmopathy.
Although the Diabetes and Ramadan International Alliance (DaR) and the International Diabetes Federation (IDF) collaboratively published practice guidelines (5, 12), healthcare providers continue to have knowledge gaps pertaining to diabetes management during Ramadan. Beshyah et al. conducted a survey on 260 physicians from 27 countries—almost all Muslim majority countries. Many physicians surveyed (54.1%) admitted to having a knowledge gap in the practical management of high-risk diabetic groups, and 49.2% believed there is limited data on high-risk patients. Additionally, respondents most desired knowledge on how to best organize healthcare before, during, and after Ramadan, and if newer pharmacological agents are better than older ones if used correctly. Most physicians agreed that the two most appropriate types of articles to disseminate knowledge about Ramadan fasting are original research (73.3% of respondents) and systematic reviews (64.3%) (13).
A few reviews have analyzed both glycemic control and adverse events during Ramadan for insulin-requiring diabetes, specifically. However, to our knowledge, the original research from which these reviews and guidelines derived their recommendations have not been critically appraised. Additionally, there is no systematic review dedicated exclusively to insulin management in type 2 diabetes during Ramadan. Given the growing body of literature on Ramadan and diabetes, this systematic review seeks to answer the question: what insulin dosing recommendations are likely to reduce hypoglycemia and improve glycemic control for persons with insulin-requiring type 2 diabetes who participate in the Ramadan fast?
The review is reported in accordance with the 2020 Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) statement and informed by the Cochrane Handbook for Systematic Reviews of Interventions (14, 15). A review protocol was prospectively registered online in PROSPERO (Prospective, International Register of Systematic Reviews) under the registration number: CRD42021268943.
A medical librarian specialized in systematic reviews (LÖ) conducted a comprehensive search for literature in six electronic databases: PubMed (NML), EMBASE (Elsevier), CINAHL (Ebscohost), Scopus (Elsevier), Web of Science (Clarivate), and Cochrane Library (Cochrane Collaboration). Gray literature sources were located via OAlster Gray Repository, World Health Organization Institutional Repository (WHO IRIS), ClinicalTrials.gov, BASE, and Open Gray. The search was performed in August 2021. Pre-searches in PubMed and PubMed's MeSH to identify relevant search term variations and to develop the search string was conducted by LÖ in May-July 2021 in close collaboration with subject specialists (AK and MK). The search strategy developed in PubMed was later systematically repeated in all selected information sources. A combination of the search fields title, abstract, keywords (Text Word, TOPIC, or similar), and “MeSH”/“thesaurus” (when available) was used to ensure that the best possible evidence was located. The search was conducted without any language or geographical restrictions. Because of the recent advances in diabetic management, studies older than 20 years were excluded. Search details, dates, keywords, results, and notes for all databases and gray sources are reported in Appendix A.
All records identified in the database search were uploaded to the systematic review software Covidence (Veritas Health Innovation, 2021) for automatic de-duplication and prepared for blinded screening and data extraction (LÖ) (16). The results from the gray sources were de-duplicated by hand. Cabell's Predatory Report (Cabell's Scholarly Analytics, 2021) was consulted to verify the scientific status of included studies published in open access journals (17). Two independent reviewers (AK and MK) screened the titles and abstracts of unique records against the pre-set inclusion and exclusion criteria, which is summarized in Table 1.
Because this review aims to guide insulin management for type 2 diabetes during Ramadan, a concerted effort was made to include studies focusing on insulin and exclude studies with an emphasis on oral hypoglycemic agents. If studies were able to control for oral hypoglycemic agents and isolate the effects of insulin on hypoglycemia and glycemic control during Ramadan fasting, they were included for review. Additionally, if studies evaluated a specific insulin dosing strategy and measured its effects on blood glucose levels, they were included for review. If a study included persons with insulin-requiring type 2 diabetes during Ramadan, but the primary objective of the study was to evaluate the effects of an oral hypoglycemic agent, this study was excluded. If it was difficult to ascertain the effects of insulin specifically on diabetic management during Ramadan because an oral hypoglycemic agent was an uncontrolled variable in the study, this study was excluded.
A third independent reviewer (AI) resolved the conflicts identified by the software. Full-text papers were sought and uploaded to Covidence for blinded screening (AK and AI) and conflict resolution (MK). A PRISMA flow diagram with details of the screening and selection process is illustrated in Figure 1.
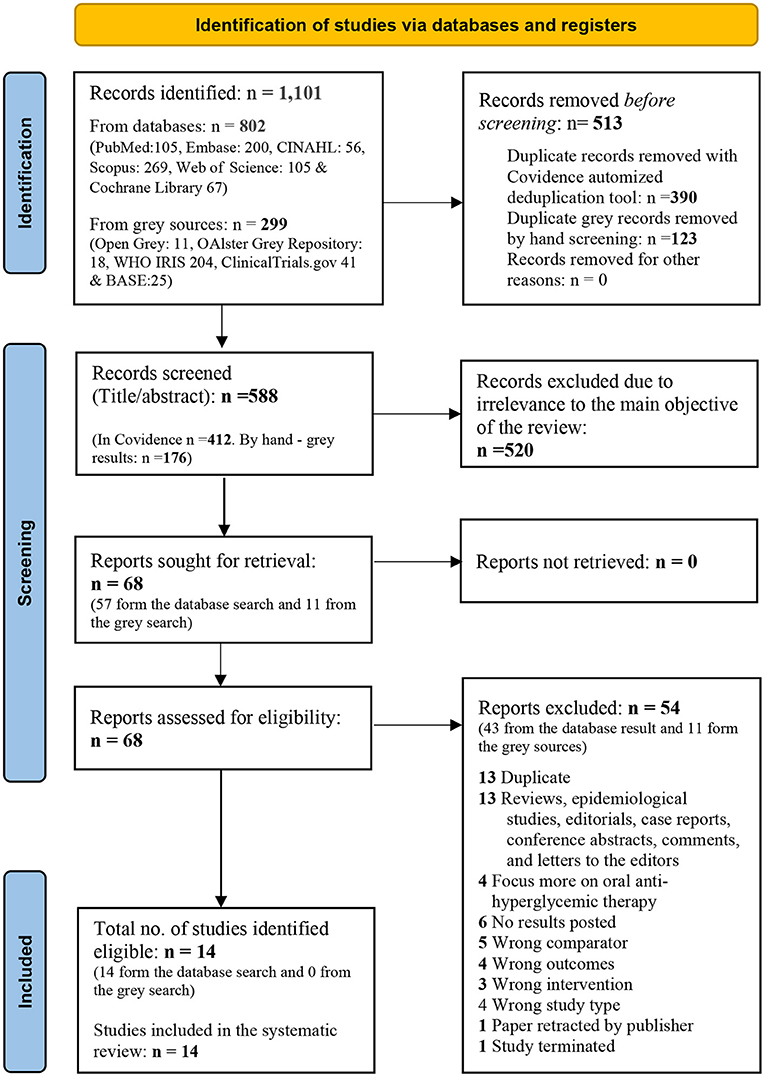
Figure 1. PRISMA 2020 flow diagram for new systematic reviews which included searches of databases and registers only. From: Page et al. (14). For more information, visit: http://www.prisma-statement.org/.
Two independent reviewers (AK, AI) used the Covidence software to extract study characteristics and outcomes. The primary outcome assessed was the difference in hypoglycemic incidence or event rate between the insulin types and/or insulin dosing recommendations. Hypoglycemia was defined in most studies as a serum blood glucose level < 70 mg/dL (3.9 mmol/L) except in two studies it was <63 mg/dL (3.5 mmol/L), and one study defined hypoglycemia as <60 mg/dL (3.3 mmol/L). The secondary outcomes obtained reflected glycemic control which was measured by the changes in pre-and post-iftar blood glucose (mg/dL, mmol/L), overall blood glucose (mg/dL, mmol/L), and HbA1c (mmol/mol) between the insulin subtypes and/or dosing recommendations. Additionally, insulin dosing strategies were examined and compared between studies. Type 2 diabetes-associated adverse events, such as DKA or HHS was also extracted when available in addition to each study's funding sources. A third reviewer (MK) was available to resolve any conflicts if necessary.
Two independent reviewers (AK, MK) used the Newcastle-Ottawa Scale (NOS) and the National Heart, Lung, and Blood Institute's (NHLBI) quality assessment tools to assess the quality and risk of bias of each observational cohort study and controlled intervention study, respectively (18, 19). A third reviewer (DB) resolved conflicts. The Covidence software enabled a systematic and blinded approach.
The characteristics and outcomes of each study were summarized in a comprehensive table, allowing us to group and compare similar findings relevant to our primary and secondary outcomes. Our primary outcome, the hypoglycemic incidence or event rate, was expressed as a difference in the percentage between different insulin types and/or dosing recommendations, and the p-values from each study were noted. The CREED epidemiologic study reported a hypoglycemia incidence rate of 13.8% for insulin-requiring type 2 diabetes during Ramadan, so this was the benchmark value that we used in our analysis. Our secondary outcomes concerning glycemic control were measured as the mean difference in blood glucose levels between different insulin types and/or dosing recommendations while considering the p-value of each respective measurement. If a study found that a specific insulin subtype or dosing recommendation decreased the hypoglycemia incidence rate to <13.8% without significantly increasing post-iftar blood glucose, overall blood glucose, or severe hyperglycemia (blood glucose > 300 mg/dL, 16.7 mmol/L), this would be an acceptable finding. A narrative synthesis was done to analyze which insulin types and dosing recommendations are superior for improving glycemic control and reducing hypoglycemic events during Ramadan. Due to the high heterogeneity of study designs, interventions, and outcomes, we did not perform a meta-analysis.
Of the 1,101 records located in the literature search, 588 unique studies remained for the title and abstract screening after de-duplication. All studies were found to be in the English language. Sixty-eight papers were selected for full-text screening, of which fourteen were identified as eligible to be included in the systematic review. A PRISMA 2020 flow diagram detailing the search, de-duplication, screening, and selection process is summarized in Figure 1.
The total number of study participants was 2,969 between all fourteen studies reviewed. These studies were conducted across four continents in 25 different countries; there were six in Africa: Algeria (n = 1), Egypt (n = 3), Libya (n = 1), Morocco (n = 2), South Africa (n = 2), and Tunisia (n = 1), seventeen in Asia: Bangladesh (n = 1), China (n = 1), India (n = 5), Indonesia (n = 1), Iraq (n = 1), Israel (n = 1), Jordan (n = 4), Kuwait (n = 1), Lebanon (n = 3), Malaysia (n = 3), Oman (n = 1), Pakistan (n = 3), Qatar (n = 2), Saudi Arabia (n = 3), Singapore (n = 1), Turkey (n = 1), the United Arab Emirates (n = 1), one in Europe: the United Kingdom (n = 1), and one in North America: Canada (n = 1). Among all fourteen studies, the average age is 54.8 years, the average duration of diabetes is 11.1 years, 52.3% were female, and 47.7% male.
Five studies were RCTs, and the remaining nine studies were observational cohort studies. A comprehensive summary of all included studies is organized in Table 2 with RCTs in the top five rows. Six studies analyzed how insulin dosage adjustment affects glycemic control and hypoglycemia during Ramadan, three examined newer ultra-long-acting insulins, three compared insulin analogs (synthetic insulin designed to mimic the body's natural insulin release pattern) to regular human insulin, and two studied specific medical regimens for the Ramadan fast. We have grouped these studies according to the previously mentioned categories concerning hypoglycemia and glycemic control. These findings are summarized in Tables 3, 4.
Three studies (two RCTs) out of four demonstrated that insulin dosage reduction of between 25 and 40% TDD decreases rates of hypoglycemia for some participants (23, 24, 28). The average hypoglycemic incidence rate from these two RCTs was 22.5% [21.4% in Shehadeh et al. (23), 23% in Zaghlol et al. (24)] in the control group and 4.5% [4.8% from Shehadeh et al. (23), 4.2% in Zaghlol et al. (24)] in the intervention group. Another study compared premixed twice-daily dosing to short-acting insulin at iftar with intermediate at suhur (the meal consumed early in the morning before dawn and before fasting commences) and found no difference in hypoglycemia (26). Ahmedani et al.'s strategy of flexible glycemic targets (100–200 mg/dl during fasting hours and 100–180 mg/dl during non-fasting hours) reported only a 0.6% rate of hypoglycemia (25).
Ultra-long-acting insulins (IDegAsp twice daily) demonstrated a 62% reduction in overall hypoglycemia compared to BIAsp 30 twice daily (p = 0.007) in one RCT (21) and reported no severe hypoglycemic episodes in another cohort study (32). Utilization of Gla-300 resulted in no severe hypoglycemic episodes during or post-Ramadan (30). Participants taking basal + Non-SU-OHA reported zero episodes of hypoglycemia (27). The use of nighttime Levemir with reduced total daily insulin dosing resulted in a lower adverse event rate (0.04 vs. 0.07, p = 0.010) and a lower hypoglycemic event rate (4.8 vs. 21.4%, p < 0.001) compared to usual care (23). Salti et al. found that using insulin glargine with glimepiride resulted in minimal severe hypoglycemic episodes and that keeping fasting blood glucose > 120 mg/dL (6.7 mmol/L) had a protective effect on hypoglycemia (33).
Although three studies (2 RCTs) found that rapid-acting insulin analogs can improve glycemic control, each study comparing insulin lispro/protamine to human mixed insulin found no statistically significant difference in hypoglycemic event rate between the two arms (20, 22, 31). Hajjaji et al. reported three minor hypoglycemic episodes in both the intervention (rapid-acting insulin analog) and control (human insulin) groups (20), Mattoo et al. recorded a similar number of hypoglycemic episodes (0.4 episodes per participant) for both groups (22), and Hui et al. did not record a statistically significant difference in hypoglycemic events between the group using a rapid-acting insulin analog (0.04 events per participant reduction) vs. the group using human insulin (0.15 events per participant increase) (31).
Two RCTs and four observational cohort studies analyzed how insulin dosage adjustment affects glycemic control. Three of the studies reduced the total insulin daily dose by 25% in the experimental groups, and the result was no difference in hyperglycemia compared to regular dosing (24), no episodes of DKA or NKHS (no comparator) (28), and no difference in A1c or average blood glucose compared to regular dosing (29). Shehadeh et al. reduced TDD by 40% in their intervention group, giving 60% of the reduced dose as biphasic insulin 70 for iftar and 40% as Levemir at suhur, and their result showed no difference in A1c compared to usual care. In fact, although the insulin TDD was reduced, there was a decreased event rate of >300 mg/dL (16.7 mmol/L) blood glucose in the intervention group (p = 0.026) (23). Altemimi compared human premixed (NPH/regular) insulin dosed as 2/3 TDD pre-iftar and 1/3 TDD pre-suhur vs. human regular insulin and NPH dosed as TDD pre-iftar and TDD pre-suhur, respectively, and the result was no statistical difference in average A1c or hyperglycemic events (26). Ahmedani et al. allowed for flexible targets: 100–200 mg/dl during fasting hours and 100–180 mg/dl during non-fasting hours. This resulted in an A1c reduction of 0.88 (p < 0.0001) without any DKA or HHS (25).
Five studies examined long and ultra-long-acting insulins and their effects on glycemic control. IDegAsp was found to significantly lower pre-iftar glucose by −8 mg/dL (−0.54 mmol/L, p = 0.0247) compared to BIAsp 30 (21). Similarly, Gla-300 decreased A1c (−0.4%) and fasting plasma glucose (−13.5 mg/dl) during Ramadan (30). Another small study found that 5 out of 6 participants who switched from premixed or NPH to IDeg or IDegAsp resulted in 12–25% insulin dose reduction (32). For long-acting insulin, one study used Levemir and a reduced total daily dosing strategy, which resulted in a significantly reduced > 300 mg/dL (16.7 mmol/L) event rate (23). Salti et al. found that insulin glargine with glimepiride helped improve FBG (176–124 mg/dL, 9.8–6.9 mmol/L, p < 0.0001) and A1c (8.6–7.7%, p < 0.0001) in non-insulin naïve participants (33).
Rapid-acting insulin analog mixes, such as lispro/protamine, improved glycemic control in two RCTs and one cohort study when compared to human mixed insulin. During Ramadan, lispro/protamine improved pre-iftar blood glucose 6 mg/dL (0.4 mmol/L, p = 0.034) (22), 2-h post iftar blood glucose 20 mg/dL (1.1 mmol/L, p = 0.0001) (20), mean postprandial blood glucose 21.1 mg/dL (1.2 mmol/L, p < 0.001) (20), mean A1c 0.4% (p = 0.01) (31), and overall blood glucose 9 mg/dL (0.6 mmol/L, p = 0.004) compared to human insulin 30/70 (22). Another cohort study demonstrated improved A1c by 0.48% (p = 0.0001) when using lispro/protamine mix 50 at iftar as opposed to an increase in A1c by 0.28% (p = 0.007) in the comparator group using human insulin mix 30 at iftar (31).
The NOS was used for the risk of bias and quality assessment for the nine included observational cohort studies. Five out of nine cohort studies were rated as Good according to the NOS scale, and the remaining four cohort studies were rated as Poor. Most cohort studies rated as Poor were rated as such because the analytic design of the study did not control for confounders. The NHLBI quality assessment was used for the five RCTs with two studies rated as Fair, and three studies rated as Good. Tables 5, 6 summarize our ratings for the nine observational cohort studies and the five RCTs, respectively.
Despite the risks involved, up to 43.9% of Muslims with high-risk diabetes choose to fast during Ramadan (9, 10). Although large epidemiologic studies have demonstrated increased hypo- and hyperglycemia during the fasting month (6–9), most physicians acknowledge inexperience with managing diabetes during Ramadan (13). It is incumbent upon all physicians to identify safe and effective insulin dosing recommendations during Ramadan for Muslims, a cohort claiming almost one-third of all persons with diabetes worldwide (3). In this review, we found that insulin dosing adjustment and long and ultra-long acting insulins can reduce hypoglycemic events, and rapid-acting insulin analogs can improve post-iftar and overall blood glucose during Ramadan.
Reducing the pre-Ramadan TDD of insulin by 25–40% during the fasting month appears to effectively decrease the rate of hypoglycemia (23, 24, 28) from a combined average incidence rate of 22.5–4.5% (23, 24, 28). By comparison, the CREED epidemiologic study recorded a hypoglycemia incidence rate of 13.8% in a similar demographic during Ramadan (7). Theoretically, lowering the insulin dose may consequently increase rates of hyperglycemia, however, studies have shown that decreasing the pre-Ramadan TDD of insulin does not subsequently increase the rate of hyperglycemia (23, 24), DKA (28), or A1c (23, 29). In fact, the large epidemiologic EPIDIAR study reported a severe hyperglycemic event rate of 4% during Ramadan compared to a 3% event rate in the intervention group in Shehadeh et al.'s study (23). This finding can be explained by the strategy of administering the long-acting insulin at suhur prior to the daytime fast, thus preventing hypoglycemia, and giving a higher insulin dose with the larger meal (iftar), which helps mitigate a large glucose load.
Previous reviews have similar recommendations: the IDF/DAR guidelines (5), the South Asian Health Foundation (UK) guidelines (34), Ibrahim et al. applied principles of the ADA/EASD consensus guidelines in 2020 (35), and Sadikot et al. (36) all recommend reducing the basal dose by 15–30% and the suhur dose by 25–50%. After a comprehensive systematic review and critical appraisal of the original research, we have also found that reducing the TDD of insulin is a dosing recommendation that may help reduce hypoglycemic events during Ramadan. However, it must be acknowledged that we found and reviewed only four studies relating to insulin dose adjustment, limiting our confidence in this conclusion.
Novel insulin dosing strategies may lead to improved outcomes for persons with diabetes during Ramadan. The IDF/DAR guidelines in 2021 relied on standard glycemic targets through the suhur, pre-iftar, and post-iftar periods (90–130 mg/dL) (5). However, the study done by Ahmedani and colleagues opens the door for more research regarding the possible superiority of flexible glycemic targets up to 200 mg/dL during fasting hours and tightened glycemic targets to <180 mg/dL during non-fasting hours (25). Their result of only a 0.6% hypoglycemia incidence rate appears very promising compared to the 9.2% incidence rate in a similar demographic in the CREED study (7). Future studies will need to examine the long-term impact of annually recurring permissive hyperglycemia as the study by Ahmedani et al. was only 2 months in duration.
Long and ultra-long-acting insulins, when given at sunrise, may reduce the risk of hypoglycemia during Ramadan (27, 30, 33), particularly when compared to intermediate-acting insulins (21, 23, 30). Long-acting insulins have a more attenuated peak and can accommodate fasting hours lasting as long as 20 h (37), thus resulting in fewer episodes of hypoglycemia. However, regarding hyperglycemia, each study recorded a beneficial, but different, outcome [lower pre-iftar glucose (21), lower A1c (30), insulin dose reduction (32), and reduced hyperglycemia > 300 mg/dL, 16.7 mmol/L (23)]. Because we cannot compare these outcomes to each other, we are unable to conclude long and ultra-long-acting insulins and their effects on hyperglycemia. Additionally, three of the six studies examining long and ultra-long-acting insulins had insufficient quality per the NOS, further limiting our confidence (27, 30, 32). More high-quality RCTs need to confirm whether long and ultra-long-acting insulins reduce hypoglycemic events and improve glycemic control during Ramadan.
Between two RCTs and one cohort study, rapid-acting insulin analogs significantly improved post-iftar blood glucose (20, 31) and overall blood glucose compared to regular human insulin without the risk of increased hypoglycemia (20, 22, 31). In 2015, Lessan et al. demonstrated that persons with insulin-treated diabetes (with/without oral antidiabetic drugs) recorded a significant difference in the mean amplitude of glycemic excursion during Ramadan (176 mg/dL, 9.8 mmol/L) compared to persons without diabetes (44 mg/dL, 2.4 mmol/L) (38). Other groups on only oral antidiabetic drugs did not show a significant difference (38). Physiologically, rapid-acting insulin analogs can better counteract these post-iftar excursions than regular-acting ones. Our findings are consistent with the previous reviews (3, 34–36), but because our review included only three relevant studies, two of which funded by industry, further research is necessary to confirm that rapid-acting insulin analogs improve glycemic control without increasing hypoglycemic events during Ramadan.
The strength of this review is that it is, to our knowledge, the only systematic review exclusively analyzing insulin subtypes and dosing strategies for insulin-treated type 2 diabetes during Ramadan. A comprehensive search of relevant literature was conducted, including gray literature, and a critical appraisal of the original research was performed. We reviewed recent literature which includes an investigation of second-generation basal insulin and the use of flexible glycemic targets. Acknowledged limitations include heterogeneity of study designs and study outcomes which precluded rigorous meta-analysis. We were able to group similar interventions and comparators between studies but given the variety of insulin types and dosing recommendations, our conclusions are limited. Additionally, six of the fourteen included studies were funded by industry which may introduce bias.
This review did not examine how combining insulin with other non-insulin antidiabetic medications affects hypoglycemic events and glycemic control. Abdelrahim et al. performed a large comprehensive review in 2021 and found that certain oral hypoglycemic agents combined with insulin are preferable in preventing hypoglycemic events and improving glycemic control during Ramadan, namely non-sulfonylureas such as incretin mimetics (39). In this review, we have shed light on which types of insulin and dosing strategies are beneficial during Ramadan. However, practically, many regimens include both insulin and oral hypoglycemic agents, so further research on the superiority of insulin subtypes and dosing strategies in combination with oral hypoglycemic agents during Ramadan would help close the knowledge gap which physicians have expressed (14).
The research and body of literature pertaining to insulin-requiring diabetes and Ramadan remain sparse. Many reviews and guidelines have been published, but the original research had not been critically appraised, which we have done here. Insulin dose reduction may prevent hypoglycemic events, and rapid-acting insulin analogs may improve glycemic control without incurring subsequent hypoglycemia during Ramadan. However, more randomized controlled trials need to be performed before conclusions can be made. Though initial findings are promising, more research is needed to confirm the benefits of ultra-long-acting insulins as well as the use of flexible glycemic targets. While certain types of insulin and particular dosing strategies demonstrate some advantages, these recommendations should be tailored to the context of each person with diabetes to make the appropriate regimen adjustments in preparation for intensive fasting practices during the month of Ramadan.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
AK conceived of the presented idea. AK, AI, MK, and LÖ designed the review. LÖ conducted the literature search and prepared the articles for screening. AK and MK conducted the title/abstract screening while AI resolved conflicts. AK and AI did the full-text screen with MK resolving conflicts. AK and AI extracted data. AK and MK performed the quality assessment with DB resolving conflicts. AK, AI, and LÖ wrote the manuscript. DB and MF designed tables. All authors edited the manuscript, discussed the results, analyzed the data, and contributed to the final manuscript. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We thank Ms. Mariam Al Ahbabi for her strategic support in locating all full-text articles and for consulting Cabell's Predatory Reports to verify the academic status of open access papers.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2022.846600/full#supplementary-material
1. Khan MAB, Hashim MJ, King JK, Govender RD, Mustafa H, Al Kaabi J. Epidemiology of type 2 diabetes-global burden of disease and forecasted trends. J Epidemiol Glob Health. (2020) 10:107–11. doi: 10.2991/jegh.k.191028.001
2. Diabetes Facts & Figures. International Diabetes Federation (2020). Available online at: https://www.idf.org/aboutdiabetes/what-is-diabetes/facts-figures.html (accessed November 25, 2021).
3. Diabetes and Ramadan. International Diabetes Federation (2020). Available online at: https://www.idf.org/our-activities/education/diabetes-and-ramadan.html (accessed November 27, 2021).
4. Salti I, Benard E, Detournay B, Bianchi-Biscay M, Le Brigand C, Voinet C, et al. A population-based study of diabetes and its characteristics during the fasting month of Ramadan in 13 countries: results of the epidemiology of diabetes and Ramadan 1422/2001 (EPIDIAR) study. Diabetes Care. (2004) 27:2306–11. doi: 10.2337/diacare.27.10.2306
5. Diabetes and Ramadan: Practical Guidelines. International Diabetes Federation and the DAR International Alliance (2021). Available online at: https://www.daralliance.org/daralliance/idf-dar-practical-guidelines-2021/ (accessed November 27, 2021).
6. Babineaux SM, Toaima D, Boye KS, Zagar A, Tahbaz A, Jabbar A, et al. Multi-country retrospective observational study of the management and outcomes of patients with Type 2 diabetes during Ramadan in 2010 (CREED). Diabet Med. (2015) 32:819–28. doi: 10.1111/dme.12685
7. Jabbar A, Mohamed WM, Spaepen E, Reed V, Tayeb K, Khalil SH, et al. Fasting experience of patients with type 2 diabetes mellitus on insulin therapy during Ramadan: VISION Ramadan substudy. Diabetes Res Clin Pract. (2019) 151:285–89. doi: 10.1016/j.diabres.2019.02.021
8. Hassanein M, Al Awadi FF, El Hadidy KES, Ali SS, Echtay A, Djaballah K, et al. The characteristics and pattern of care for the type 2 diabetes mellitus population in the MENA region during Ramadan: an international prospective study (DAR-MENA T2DM). Diabetes Res Clin Pract. (2019) 151:275–84. doi: 10.1016/j.diabres.2019.02.020
9. Jabbar A, Hassanein M, Beshyah SA, Boye KS, Yu M, Babineaux SM. CREED study: Hypoglycemia during Ramadan in individuals with type 2 diabetes mellitus from three continents. Diabetes Res Clin Pract. (2017) 132:19–26. doi: 10.1016/j.diabres.2017.07.014
10. Morales J, Schneider D. Hypoglycemia. Am J Med. (2014) 127:S17–24. doi: 10.1016/j.amjmed.2014.07.004
11. Saadane I, Ali T, El-Laboudi A, Lessan N. Ramadan fasting in insulin-treated patients is associated with potentially unfavourable changes in glucose metrics: a flash glucose monitoring (FGM) study. Diabetes Res Clin Pract. (2021) 172:108592. doi: 10.1016/j.diabres.2020.108592
12. Hassanein M, Afandi B, Ahmedani MY, Alamoudi RM, Alawadi F, Bajaj H, et al. Diabetes and Ramadan: practical guidelines 2021. Diabetes Res Clin Pract. (2022) 8:109185. doi: 10.1016/j.diabres.2021.109185
13. Beshyah SA, Ali KF, Hajjaji IM, Hafidh K, Raza SA, Ghour N, et al. Knowledge gaps and perceptions of future research directions on management of diabetes during Ramadan fasting: an online survey of physicians. Diabetes Res Clin Pract. (2021) 177:108923. doi: 10.1016/j.diabres.2021.108923
14. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Syst Rev. (2021) 10:89. doi: 10.1186/s13643-021-01626-4
15. Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ. Cochrane Handbook for Systematic Reviews of Interventions. (2019). Version 6.0. Available online at: www.training.cochrane.org/handbook (accessed December 1, 2021).
16. Veritas Health Innovation. (2021). Covidence Systematic Review Software. Veritas Health Innovation. Available online at: https://www.covidence.org (accessed December 31, 2021).
17. Cabell's Scholarly Analytics (2021). Cabell's Predatory Report. Cabell's Scholarly Analytics. Available online at: https://www2.cabells.com/about-predatory (accessed December 31, 2021).
18. Wells GA, Shea B, O'Connell D, Peterson J, Welch V, Losos M. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Non-randomised Studies in Meta-analyses. (2009). Available online at: http://wwwohrica/programs/clinical_epidemiology/oxford.htm (accessed February 1, 2009).
19. Study Quality Assessment Tools. National Heart, Lung and Blood Institute. Available online at: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools (accessed December 14, 2021).
20. Hajjaji IM, Eshwihdi N, Barrowman N. Comparison of analog insulin mix 50:50 with human insulin mix 30:70 in persons with type 2 diabetes during Ramadan. Int J Clin Pract. (2019) 73:e13348. doi: 10.1111/ijcp.13348
21. Hassanein M, Echtay AS, Malek R. Original paper: efficacy and safety analysis of insulin degludec/insulin aspart compared with biphasic insulin aspart 30: a phase 3, multicenter, international, open-label, randomised, treat-to-target trial in patients with Type 2 diabetes fasting during Ramadan. Diabetes Res Clin Pract. (2018) 135:218–26. doi: 10.1016/j.diabres.2017.11.027
22. Mattoo V, Milicevic Z, Malone JK, Schwarzenhofer M, Ekangaki A, Levitt LK, et al. A comparison of insulin lispro Mix25 and human insulin 30/70 in the treatment of Type 2 diabetes during Ramadan. Diabetes Res Clin Pract. (2003) 59:137–43. doi: 10.1016/S0168-8227(02)00202-4
23. Shehadeh N, Maor Y. Effect of a new insulin treatment regimen on glycemic control and quality of life of Muslim patients with Type 2 diabetes mellitus during Ramadan fast - an open-label, controlled, multicentre, cluster randomised study. Int J Clin Pract. (2015) 69:1281–8. doi: 10.1111/ijcp.12695
24. Zaghlol LY, Beirat AF, Amarin JZ, Hassoun Al Najar AM, Hasan YY, Qtaishat A, et al. Effect of dosage reduction of hypoglycemic multidrug regimens on the incidences of acute glycemic complications in people with type 2 diabetes who fast during Ramaḍān: a randomized controlled trial. Front Endocrinol. (2021) 12:613826. doi: 10.3389/fendo.2021.613826
25. Ahmedani MY, Ghafoor E. Achieving safer Ramadan fasting by keeping flexible glycemic targets during the day and tighter targets during the night in insulin-treated people with Type 2 diabetes. Diabetes Res Clin Pract. (2020) 165:108234. doi: 10.1016/j.diabres.2020.108234
26. Altemimi MT, Odhaib SA, Imran HJ, Alhamza A, Almomin A, Mansour AA. Comparison of human premixed and basal plus short-acting insulin regimens for individuals with type 2 diabetes during Ramadan fasting. Cureus. (2020) 12:e11976. doi: 10.7759/cureus.11976
27. Ba-Essa EM, Hassanein M, Abdulrhman S, Alkhalifa M, Alsafar Z. Attitude and safety of patients with diabetes observing the Ramadan fast. Diabetes Res Clin Pract. (2019) 152:177–82. doi: 10.1016/j.diabres.2019.03.031
28. Beano AM, Zmaili MA, Gheith ZH, Naser AM, Momani MS, Yousef AMF, et al. Predetermined anti-diabetic drug regimen adjustments during Ramadan fasting: an observational study of safety. Endocrinol Metab. (2017) 32:265–73. doi: 10.3803/EnM.2017.32.2.265
29. Elhadd T, Bashir M, Baager KA, Ali HA, Almohannadi DHS, Dabbous Z, et al. PROFAST Ramadan Study Group. Mitigation of hypoglycemia during Ramadan using the flash glucose monitoring system following dose adjustment of insulin and sulphonylurea in patients taking multiple glucose-lowering therapies (The PROFAST-IT Study). Diabetes Res Clin Pract. (2021) 172:108589. doi: 10.1016/j.diabres.2020.108589
30. Hassanein M, Buyukbese MA, Malek R, Pilorget V, Naqvi M, Berthou B, et al. Real-world safety and effectiveness of insulin glargine 300 U/ml in participants with Type 2 diabetes who fast during Ramadan: the observational ORION study. Diabetes Res Clin Pract. (2020) 166:108189. doi: 10.1016/j.diabres.2020.108189
31. Hui E, Bravis V, Salih S. Comparison of Humalog Mix 50 with human insulin Mix 30 in Type 2 diabetes patients during Ramadan. Int J Clin Pract. (2010) 64:1095–9. doi: 10.1111/j.1742-1241.2010.02347.x
32. Kalra S. Insulin degludec and insulin degludec/insulin aspart in Ramadan: a single center experience. Indian J Endocrinol Metab. (2016) 20:564–7. doi: 10.4103/2230-8210.180644
33. Salti I Diabetes and Ramadan Study Group. Efficacy and safety of insulin glargine and glimepiride in subjects with Type 2 diabetes before, during and after the period of fasting in Ramadan. Diabet Med. (2009) 26:1255–61. doi: 10.1111/j.1464-5491.2009.02836.x
34. Hanif W, Patel V, Ali SN, Karamat A, Saeed M, Hassanein M, et al. The South Asian Health Foundation (UK) guidelines for managing diabetes during Ramadan. Diabetes Res Clin Pract. (2020) 164:108145. doi: 10.1016/j.diabres.2020.108145
35. Ibrahim M, Davies MJ, Ahmad E, Annabi FA, Eckel RH, Ba-Essa EM, et al. Recommendations for management of diabetes during Ramadan: update 2020, applying the principles of the ADA/EASD consensus. BMJ Open Diabetes Res Care. (2020) 8:e001248. doi: 10.1136/bmjdrc-2020-001248
36. Sadikot S, Jothydev K, Zargar AH, Ahmad J, Arvind SR, Saboo B. Clinical practice points for diabetes management during RAMADAN fast. Diabetes Metab Syndr. (2017) 11 (Suppl. 2):S811–9. doi: 10.1016/j.dsx.2017.06.003
37. Types of Insulin. Diabetes Education Online: Diabetes Teaching Center at the University of California, San Francisco (2020). Available online at: https://dtc.ucsf.edu/types-of-diabetes/type2/treatment-of-type-2-diabetes/medications-and-therapies/type-2-insulin-rx/types-of-insulin/ (accessed August 31, 2020).
38. Lessan N, Hannoun Z, Hasan H. Glucose excursions and glycemic control during Ramadan fasting in diabetic patients: insights from continuous glucose monitoring (CGM). Diabetes Metab. (2015) 41:28–36. doi: 10.1016/j.diabet.2014.11.004
39. Abdelrahim D, Faris ME, Hassanein M, Shakir AZ, Yusuf AM, Almeneessier AS, et al. Impact of Ramadan diurnal intermittent fasting on hypoglycemic events in patients with type 2 diabetes: a systematic review of randomized controlled trials and observational studies. Front Endocrinol. (2021) 12:624423. doi: 10.3389/fendo.2021.624423
Keywords: type 2 diabetes, Islam, insulin, hypoglycemia, hyperglycemia
Citation: Kieu A, Iles A, Khan MAB, Östlundh L, Boyd D and Faris ME (2022) A Systematic Review of Insulin Management Recommendations to Improve Glycemic Control and Reduce Hypoglycemic Events During Ramadan Fasting in Patients With Insulin-Requiring Type 2 Diabetes. Front. Nutr. 9:846600. doi: 10.3389/fnut.2022.846600
Received: 31 December 2021; Accepted: 25 March 2022;
Published: 12 May 2022.
Edited by:
Clare Marie Reynolds, University College Dublin, IrelandReviewed by:
Abdurezak Ahmed Abdela, Addis Ababa University, EthiopiaCopyright © 2022 Kieu, Iles, Khan, Östlundh, Boyd and Faris. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Alexander Kieu, YWxleGFuZGVyLmtpZXVAZ21haWwuY29t
†ORCID: Alexander Kieu orcid.org/0000-0002-5434-9705
Ashley Iles orcid.org/0000-0002-4990-8701
Moien AB Khan orcid.org/0000-0003-4970-4618
Linda Ostlundh orcid.org/0000-0001-5091-604X
Duston Boyd orcid.org/0000-0002-5998-437X
MoezAlIslam Ezzat Faris orcid.org/0000-0002-7970-2616
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.