
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Nutr., 23 May 2022
Sec. Clinical Nutrition
Volume 9 - 2022 | https://doi.org/10.3389/fnut.2022.844242
This article is part of the Research TopicInsights in Clinical NutritionView all 30 articles
Non-alcoholic fatty liver disease (NAFLD) has become prevalent in recent decades, especially in developed countries; yet the approaches for preventing and treating NAFLD are not clear. This study aimed to summarize meta-analyses of randomized controlled trials that examined the effects of probiotics on NAFLD. We systematically searched PubMed, Scopus, Embase, Web of Science, and Cochrane Central Library databases up to August 2021. All Meta-analysis studies assessing the effect of probiotics on liver function tests [alanine aminotransferase (ALT), aspartate aminotransferase (AST), and Gamma-glutamyl transferase (GGT)] were included. Meta-analysis was conducted using a random-effects model. Sensitivity and subgroup analyses were also performed. The umbrella study covered ten eligible studies involving 5,162 individuals. Beneficial effects of probiotics supplementation were revealed on ALT (ES = −10.54 IU/L; 95% CI: −12.70, −8.39; p < 0.001; I2 = 60.9%, p = 0.006), AST (ES = −10.19 IU/L, 95%CI: −13.08, −7.29, p < 0.001; I2 = 79.8%, p < 0.001), and GGT (ES = −5.88 IU/L, 95% CI: −7.09, −4.67, p = 0.009; I2 = 0.0%, p = 0.591) levels. Probiotics have ameliorating effects on ALT, AST, and GGT levels in patients with NAFLD. Overall, Probiotics could be recommended as an adjuvant therapeutic method for the management of NAFLD.
Non-alcoholic fatty liver disease (NAFLD) is characterized by lipid deposition in liver cells (1). On the other hand, untreated NAFLD can cause non-alcoholic hepatitis (NASH) as well as other liver-related severe sicknesses like cirrhosis, liver failure, and liver cancer (2). Considering the newest epidemiological studies, the estimated prevalence of NAFLD in Middle Eastern countries is 31.8%, the highest in comparison with other regions in the world (3). Also, studies display a higher prevalence of NAFLD in patients having obesity or hyperlipidemia (4). NAFLD has a strong relationship with metabolic syndromes like obesity, insulin resistance, type 2 diabetes, dyslipidemia, and hypertension (1, 5–8). Besides liver, this disease can progress and affect many other organs, such as systemic arteries, heart and kidneys, and causes irreversible problems and death (7). It has been suggested that lifestyle changes such as diet and exercise interventions, can help manage NAFLD and lead to a decrease in occurrence and progression of NAFLD (6, 7, 9). Therefore, studies showed that nutritional changes and exercise could lower liver lipid, improve liver enzyme functions and reduce plasma triglyceride (10, 11). However, earlier studies reported that natural compounds can reduce the complications caused by NAFLD (1, 12).
Some studies have shown that there is an association between the gut-liver axis and NAFLD. More than 10,000 microbiomes live in a synergetic relationship with the intestinal tract and have different effects on the host’s health and disease condition (13–15). Recently, microbiome-targeted treatments (MTTs) are a proposed way to influence the gut microbiome. In this regard, probiotics are used as a treatment strategy. Probiotics are explained as live microbial organisms used as dietary supplements that are beneficial for the human or animal host in terms of health. Moreover, when provided in an adequate dosage and for a capable duration, probiotics can improve intestinal microbial balance (16) and cause disease occurrence to delay by balancing intestinal flora, permeability, and inflammations (17). Improved production of short-chain fatty acids such as butyrate through microbial pathways impacts the metabolism of energy in the intestine and overall body (18, 19). In addition, probiotics can develop anti-microbial compounds and acidity of the intestinal lumen and cause a reduction in generation of pathogens (20). Finally, a variety of microorganism-based products can impact the immunity of the host positively (21, 22).
Although, many accumulative evidences have shown the beneficial effects of probiotics on liver enzymes by affecting specific biological processes; however, there are some contradictions in this regard (23–27). For more definite results, we aimed to investigate the effects of probiotics supplementation on serum levels of alanine transaminase (ALT), AST, and GGT in an umbrella meta-analysis study.
We followed standardized methods to carry out this umbrella review (a systematic review of multiple meta-analyses) to provide a clear understanding in terms of probiotics supplementation and serum levels of ALT, AST, and GGT. The scientific international databases including, PubMed, Scopus, EMBASE, Web of Science, and Cochrane Central Library databases were searched for relevant studies published up to August 2021. The pattern of search strategy is provided in Supplementary Table 1. To enhance the sensitivity of our search strategy, the wild-card term “*” was used. English-language articles were included in this study.
Meta-analysis studies examining the effects of probiotics supplementation on liver enzymes (ALT, AST, and GGT) which reported the effect sizes (ES) and corresponding confidence intervals (CI), were included in the umbrella meta-analysis. Additionally, we excluded the following studies: in vitro, in vivo, and ex-vivo studies, case reports, observational studies, quasi-experimental studies, and controlled clinical trials.
The methodological quality of meta-analyses was evaluated by two reviewers (VM and SSA) independently using the AMSTAR questionnaire (28). The AMSTAR questionnaire contains 11 items that asks reviewers to answer “Yes,” “No,” “Can’t answer” or “Not applicable.” The maximum score is 11. Articles with scores higher than seven are considered as high-quality studies.
Two independent reviewers (VM and SSA) screened the articles based on mentioned eligibility criteria. After checking and excluding irrelevant studies by the titles and abstracts, the full text of the relevant articles was evaluated to identify the study’s eligibility for the umbrella meta-analysis. Any disagreement was resolved through the consensus with the third author (PD).
The first authors’ name, year of publication, sample size, study location, dosage, and duration range of supplementation, ESs and CIs for ALT, AST, and GGT were extracted from the selected meta-analyses.
ESs and CIs were used to estimate the overall effect sizes. Heterogeneity was determined by I2 statistics and Cochrane’s Q-test. I2-value > 50% or p < 0.1 for the Q-test was considered as significant between-study heterogeneity. A random-effects model was applied to perform meta-analysis when the between-study heterogeneity was significant; otherwise, the fixed-effects model was employed. To detect probable sources of heterogeneity, subgroup analyses were performed according to the predefined variables, including type of effect size, study duration, mean age, sample size, and study location. Sensitivity analysis was used to identify the dependency of the overall effect size on a special study. The Egger’s and Begg’s tests was performed to detect a small-study effect. Publication bias was identified by visual inspection of the funnel plot. If there was any evidence of publication bias or small-study effect, trim and fill analysis was carried out accordingly. The meta-analysis was done using Stata, version 16 (Stata Corporation, College Station, TX, US). P-value < 0.05 was considered as significance level.
A total number of 85 articles were identified through a systematic search of electronic databases. Putting aside the 31 duplicate articles, 54 articles were screened carefully by titles and abstracts, among which 22 articles were selected for consideration by full-text evaluation. Considering the inclusion criteria, ten articles were included in the umbrella meta-analysis. Figure 1 presented the flow diagram of the study selection process. According to the studied variables, the distribution of identified articles was ten articles for ALT, nine for AST, and three for GGT. The included studies were conducted between 2013 and 2019, and the mean age of participants was 44 years. The average dose of probiotics in studies was between 2.6 × 109 and 5 × 1011 CFU. Studies were fulfilled in China (23, 25, 26, 29, 30), United States (31–33), India (34), and France (27). The duration of studies ranged between 8 and 20 weeks. Cochrane Risk of Bias Tool (35), adapted from Littell et al. (36), Physiotherapy Evidence Database (PEDro) scale tool (37) and Jadad scores (38) were used for quality assessment. Overall, almost 90% of meta-analyses included high quality RCTs. The quality of the RCTs included in the meta-analyses is summarized in Table 1.
AMSTAR checklist assessments for the methodological quality of the covered studies are summarized in Table 2. All meta-analyses included in the umbrella meta-analysis had a high-quality.
There was a significant reducing impact of probiotics on ALT (Figure 2A). Notable heterogeneity was observed among studies (I2 = 60.9%, p = 0.006). The high heterogeneity was reduced after subgroup analysis based on the type of effect size, sample size, study location, and duration of intervention. Reductions in ALT levels were more pronounced in the subgroups of sample size (<300) and an intervention duration ≥ 16 weeks when compared to their counterparts (Table 3). The sensitivity analysis revealed that the calculated overall effect sizes for ALT were not significantly changed after omitting each study. No significant small-study effects was found using Egger’s and Begg’s tests (p = 0.1 and p = 0.074, respectively). Moreover, visual inspection of the funnel plot indicated an asymmetric distribution of studies (Figure 2B). Thus, trim and fill analysis was conducted with ten studies (none imputed studies). The corrected effect size for publication bias didn’t show any change after trim and fill analysis.
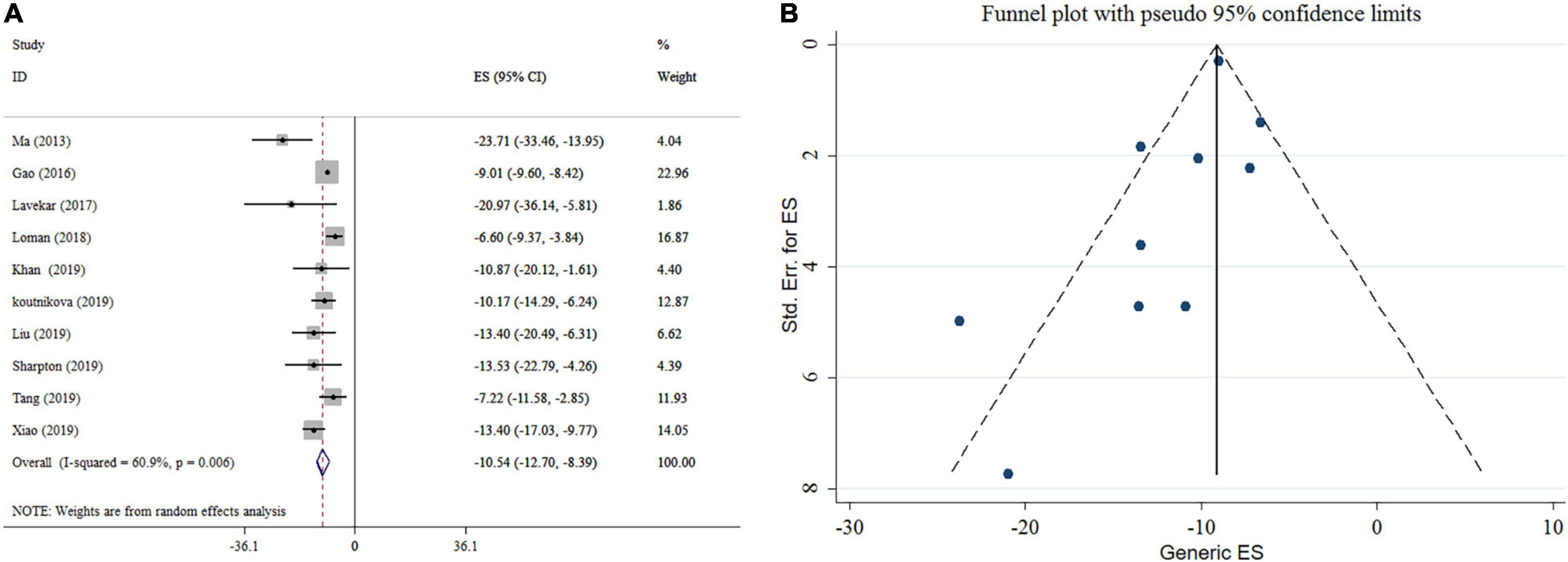
Figure 2. Forest plot (A) detailing mean difference and 95% confidence intervals (CIs) and funnel plot (B) displaying publication bias in the studies reporting, the effects of probiotics supplementation on ALT levels.
Probiotics supplementation significantly reduced AST level according to the meta-analysis of nine studies (Figure 3A). Heterogeneity was observed between studies (I2 = 79.8%, p < 0.001). For AST; pooled analysis, type of effect size, sample size, study location, and duration of intervention were possible sources of heterogeneity. Subgroup analysis indicated that probiotics supplementation with an intervention duration ≥ 16 weeks and a sample size of < 300 contributes to a more significant effect in lowering AST (Table 3). Sensitivity analysis revealed that no single study likely affected the pooled results. No significant small-study effect was shown using Egger’s and Begg’s tests (p = 0.318 and 0.466, respectively). Visual inspection of the funnel plot demonstrated a significant publication bias among included studies (Figure 3B). In the following trim and fill analysis, the effect size did not alter.
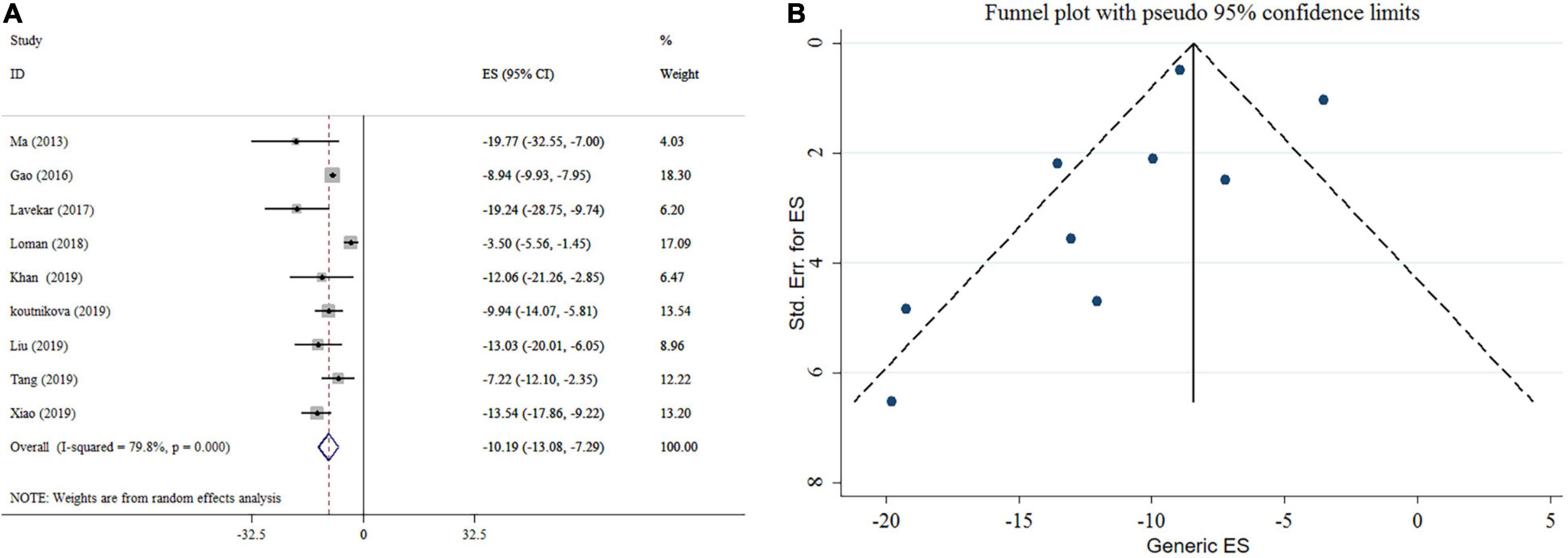
Figure 3. Forest plot (A) detailing mean difference and 95% confidence intervals (CIs) and funnel plot (B) displaying publication bias in the studies reporting, the effects of probiotics supplementation on AST levels.
The effect of probiotics on GGT level was reported in three studies (Figure 4). Our analysis revealed a significant reduction in GGT levels by probiotics intervention. No remarkable heterogeneity was observed between studies (I2 = 0.0%, p = 0.591). Subgroup analysis wasn’t conducted on studies. Sensitivity analysis provided no evidence of the impact of an individual study on the overall effect size. After performing the Begg’s tests, no small-study effects was detected (p = 0.296).
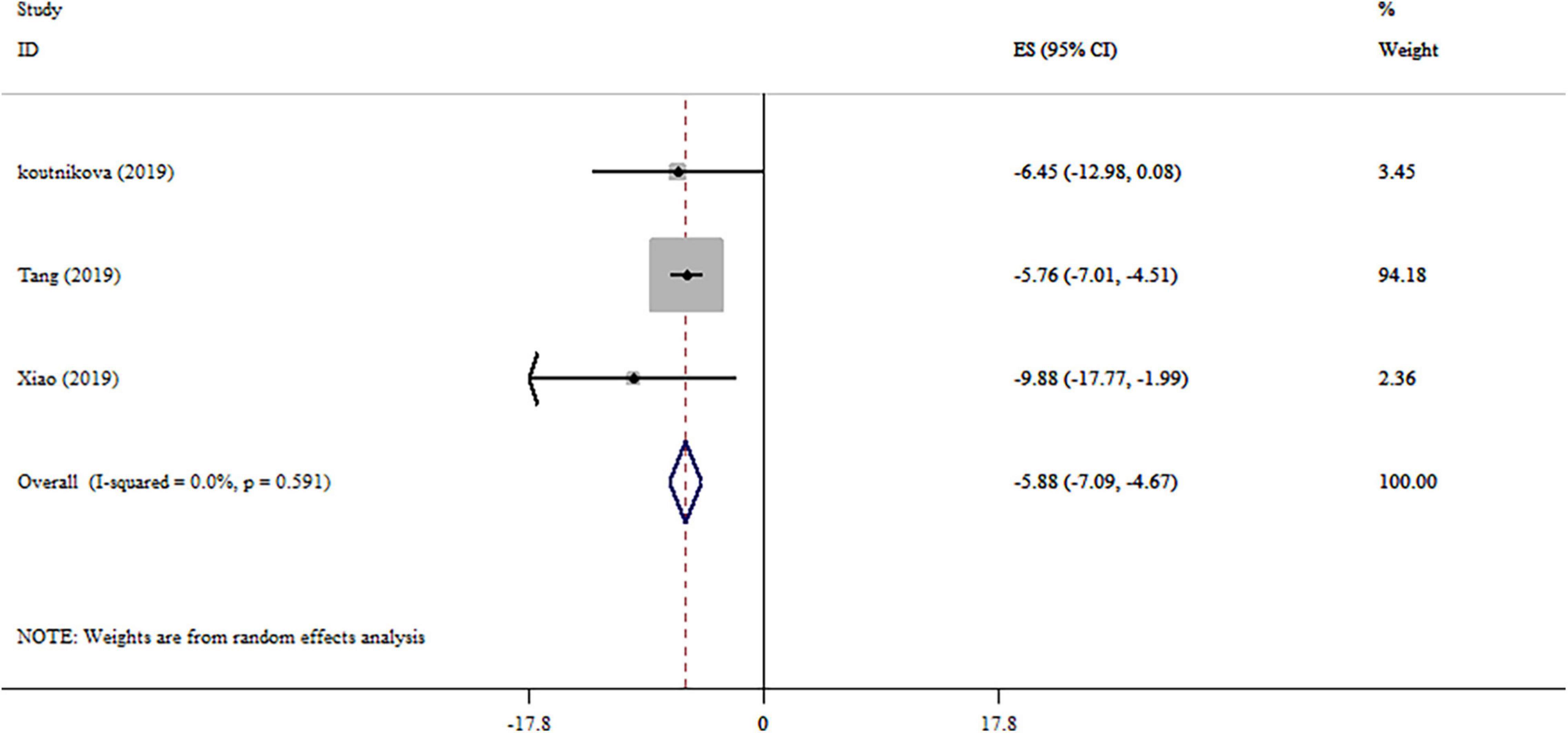
Figure 4. Forest plot detailing mean difference and 95% confidence intervals (CIs), the effects of probiotics supplementation on GGT levels.
Exposure to probiotics has been the subject of numerous meta-analyses in the realm of health as well as a diverse range of disorders. We conducted this umbrella review to summarize the available evidence and draw conclusions for the effects of probiotics in NAFLD treatment. We identified 10 meta-analyses of RCTs which had high quality based on the AMSTAR checklist. There seem to be beneficial associations between probiotic consumption and liver function in terms of serum ALT, AST, and GGT levels. All-inclusive, probiotic therapy generally appears to be safe without any evidence of adverse effects.
Even though the findings suggest that probiotics supplementation may be efficacious for controlling NAFLD, it must be stated that, the effects of probiotics on liver enzymes (ALT & AST) were heterogeneous. Differences in treatment dosage, sample size, gender, and duration of intervention, mean age of participants, study location, and population may explain this heterogeneity. Also, the evidence from this study implies that probiotic supplementation in studies with sample size < 300 and supplement duration ≥ 16 weeks can meaningfully decrease ALT. In line with ALT reduction, probiotic supplementation with an intervention duration ≥ 16 weeks and a sample size of < 300 contributes to a more significant effect in lowering AST. Also, probiotic consumption had beneficial effect on reducing GGT level without any significant heterogeneity (39, 40).
NAFLD is a chronic liver disease with a worldwide prevalence of 20–30%. It can be attributed to numerous roots, including environmental parameters, metabolic, genetic, and gut microbial factors (41). In the past decade, considerable evidence has been accumulated regarding the critical role of gut microbiota unbalance and various metabolic disorders, including NAFLD. The causal role of gut microbiota dysbiosis (an imbalance in microbial homeostasis) in NAFLD genesis and development has been reported (42, 43). Host physiology, age, drugs like antibiotics, diet, and environmental factors can influence the gut microbial ecosystem (44).
The gut dysbiosis may influence the development of NAFLD via various signaling pathways including (45, 46):
• Impact on intestinal hormone production affecting glucose control.
• Effect on short-chain production affecting glucose and lipid metabolism.
• Increasing liver toxicity and cardiovascular risk.
• Disturbance of bile acid homeostasis.
• Contribution in metabolic endotoxemia via bacterial lipopolysaccharide (LPS) which subsequently triggers intestinal and hepatic inflammation.
In addition, gut dysbiosis cause the intestinal more permeable which leading to an elevation in fatty acid absorption, migration of the bacteria via the gut epithelial barrier, release of toxic bacterial products and pro- inflammatory cytokines that can initiate an inflammatory cascade. Interestingly, recent studies indicated that gut dysbiosis is related to higher fecal concentrations of some metabolites (2-butanone and 4-methyl-2-pentanone) which lead to hepatocellular toxicity (47).
Probiotics are believed to be important for liver health via specific biological processes as follows: (1) increment in insulin sensitivity; (2) decreasing the absorption of glucose and LDL cholesterol; (3) modification of gut dysbiosis and inducing the production of short-chain fatty acids; (4) decrement in endotoxin concentrations; (5) reducing the oxidative stress status and inflammatory markers; (6) diminishing total cholesterol levels and (7) trapping of bile acids’ substances (48–51). Interestingly, probiotics have been introduced as an effective agent in inhibiting pathogens. The fermented products of many probiotic bacteria, containing lactic and acetic acids, are significant causes of probiotics’ antimicrobial features. Furthermore, bacteriocins (small antimicrobial proteins secreted by some probiotics) are another cause for this trait (52).
The overall evidence indicates that supplementation with probiotics led to a reduced level of liver enzymes (53). ALT, AST, and GGT are often used to indicate the quality of liver function. The correlation of the entitled enzymes with NAFLD has been shown in some previous studies (54, 55). In recent prospective studies, GGT is considered as a sensitive indicator of liver damage and a novel marker for oxidative stress as well as for inflammation (56, 57). In addition, there is an association between liver aminotransferase enzymes’ concentration (AST and ALT) and the quality of liver function (58, 59). Based on previous reports, increased ALT levels in patients with NAFLD may be related to insulin resistance and intrahepatic fat content (60). Lower AST and ALT levels were perceptible in various meta-analyses following probiotics supplementation (25–27, 29–34). Beyond that, microbial therapies reduced circulating GGT according to other evidence-based meta-analyses (25, 27, 31). Several probiotic strains have particular abilities to improve liver function through the modulation of the gastrointestinal tract. These improvements were mostly observed with Bifidobacterium, Lactobacillus, and Streptococcus or multispecies probiotic therapy (23, 27). Studies specifically analyzing the gut microbiome composition revealed that the imbalanced gut–liver axis can be a major factor in NAFLD development and progression (61). In general, the potential therapeutic effects of the gut microbiome manipulation by probiotics and changes in the relative abundance of selective bacteria can be considered as an alternative therapy in NAFLD patients.
This umbrella review used systematic methods with robust search strategies of various databases and independent study selection and extraction methods, which systematically summarized the current evidence regarding the effects of probiotics supplementation on serum levels of ALT, AST, and GGT. Moreover, the quality of covered systematic reviews and meta-analyses were evaluated using the AMSTAR questioner. However, the use of existing meta-analyses is the main limitation of the umbrella review. The results may depend on what assessments to choose from each preliminary study and how to report them in the meta-analysis. Finally, the most critical limitation of this study is to consider that an impressive body of pioneering studies has supported the concept that in the absence of aminotransferase levels abnormalities, the NAFLD may pretend (62–64). So in fact these criteria may not be reliable for the diagnosis of NAFLD.
The beneficial impacts of probiotics as new promising therapeutic agents for patients with NAFLD have been summarized in this studies. Convincing evidence was obtained regarding associations between probiotic intake and liver function improvement reflecting as the serum levels of ALT, AST, and GGT.
However, due to the high heterogeneity of the results and the small number of studies included in each subgroup, the outcomes of the present study should be interpreted with caution.
Even though most studies showed hepatic enzymes as biomarkers for liver function, there is still no comprehensive agreement on counting on the enzyme levels in order to indicate liver function. Adding other non-invasive assessments in line with enzyme levels in RCTs seems to be a necessity. Conclusively, further studies need to be conducted for a definite presumption.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s. All the materials used in this systematic review and meta-analysis have been fully referenced.
VM and SA wrote the original manuscript and contributed to the conception of the article. VM and NR contributed to data collection and analyze and provided advice and consultation. PD contributed to the final revision of the manuscript. All authors read and approved the final manuscript.
The research protocol was approved and supported by the Student Research Committee, Tabriz University of Medical Sciences (Registration code: 69606).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2022.844242/full#supplementary-material
Supplementary Table 1 | Electronic search strategy.
NAFLD, Non-alcoholic fatty liver disease; ALT, Alanine aminotransferase; AST, Aspartate aminotransferase; GGT, Gamma-glutamyl transferase; NASH, Non-alcoholic hepatitis; MTTs, microbiome-targeted treatments; ES, effect sizes; CI, corresponding confidence intervals.
1. Parker HM, Johnson NA, Burdon CA, Cohn JS, O’Connor HT, George J. Omega-3 supplementation and non-alcoholic fatty liver disease: a systematic review and meta-analysis. J Hepatol. (2012) 56:944–51. doi: 10.1016/j.jhep.2011.08.018
2. Jennings J, Faselis C, Yao MD. NAFLD-NASH: an under-recognized epidemic. Curr Vasc Pharmacol. (2018) 16:209–13. doi: 10.2174/1570161115666170622074007
3. Perdomo CM, Frühbeck G, Escalada J. Impact of nutritional changes on nonalcoholic fatty liver disease. Nutrients. (2019) 11:677. doi: 10.3390/nu11030677
5. Guo X-F, Yang B, Tang J, Li D. Fatty acid and non-alcoholic fatty liver disease: meta-analyses of case-control and randomized controlled trials. Clin Nutr. (2018) 37:113–22. doi: 10.1016/j.clnu.2017.01.003
6. Le Yu MY, Wang L. The effect of omega-3 unsaturated fatty acids on non-alcoholic fatty liver disease: a systematic review and meta-analysis of RCTs. Pak J Med Sci. (2017) 33:1022. doi: 10.12669/pjms.334.12315
7. Lee C-H, Fu Y, Yang S-J, Chi C-C. Effects of omega-3 polyunsaturated fatty acid supplementation on non-alcoholic fatty liver: a systematic review and meta-analysis. Nutrients. (2020) 12:2769. doi: 10.3390/nu12092769
8. Zheng HJ, Guo J, Jia Q, Huang YS, Huang WJ, Zhang W, et al. The effect of probiotic and synbiotic supplementation on biomarkers of inflammation and oxidative stress in diabetic patients: a systematic review and meta-analysis of randomized controlled trials. Pharmacol Res. (2019) 142:303–13. doi: 10.1016/j.phrs.2019.02.016
9. Musa-Veloso K, Venditti C, Lee HY, Darch M, Floyd S, West S, et al. Systematic review and meta-analysis of controlled intervention studies on the effectiveness of long-chain omega-3 fatty acids in patients with nonalcoholic fatty liver disease. Nutr Rev. (2018) 76:581–602. doi: 10.1093/nutrit/nuy022
10. Hannah WN, Harrison SA. Lifestyle and dietary interventions in the management of nonalcoholic fatty liver disease. Digest Dis Sci. (2016) 61:1365–74. doi: 10.1007/s10620-016-4153-y
11. Thoma C, Day CP, Trenell MI. Lifestyle interventions for the treatment of non-alcoholic fatty liver disease in adults: a systematic review. J Hepatol. (2012) 56:255–66. doi: 10.1016/j.jhep.2011.06.010
12. Araya J, Rodrigo R, Videla LA, Thielemann L, Orellana M, Pettinelli P, et al. Increase in long-chain polyunsaturated fatty acid n− 6/n− 3 ratio in relation to hepatic steatosis in patients with non-alcoholic fatty liver disease. Clin Sci. (2004) 106:635–43. doi: 10.1042/CS20030326
13. Marra F, Svegliati-Baroni G. Lipotoxicity and the gut-liver axis in NASH pathogenesis. J Hepatol. (2018) 68:280–95. doi: 10.1016/j.jhep.2017.11.014
14. Zarezadeh M, Musazadeh V, Faghfouri AH, Roshanravan N, Dehghan P. Probiotics act as a potent intervention in improving lipid profile: an umbrella systematic review and meta-analysis. Crit Rev Food Sci Nutr. (2021):1–14. doi: 10.1080/10408398.2021.2004578
15. Yu JS, Youn GS, Choi J, Kim CH, Kim BY, Yang SJ, et al. Lactobacillus lactis and Pediococcus pentosaceus-driven reprogramming of gut microbiome and metabolome ameliorates the progression of non-alcoholic fatty liver disease. Clin Transl Med. (2021) 11:e634. doi: 10.1002/ctm2.634
16. Lynch SV, Pedersen O. The human intestinal microbiome in health and disease. N Engl J Med. (2016) 375:2369–79.
17. Buss C, Valle-Tovo C, Miozzo S, de Mattos AA. Probiotics and synbiotics may improve liver aminotransferases levels in non-alcoholic fatty liver disease patients. Ann Hepatol. (2014) 13:482–8. doi: 10.1016/s1665-2681(19)31246-3
18. Donohoe DR, Garge N, Zhang X, Sun W, O’Connell TM, Bunger MK, et al. The microbiome and butyrate regulate energy metabolism and autophagy in the mammalian colon. Cell Metab. (2011) 13:517–26. doi: 10.1016/j.cmet.2011.02.018
19. Den Besten G, Van Eunen K, Groen AK, Venema K, Reijngoud D-J, Bakker BM. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J Lipid Res. (2013) 54:2325–40. doi: 10.1194/jlr.R036012
20. Buffie CG, Pamer EG. Microbiota-mediated colonization resistance against intestinal pathogens. Nat Rev Immunol. (2013) 13:790–801. doi: 10.1038/nri3535
21. Rooks MG, Garrett WS. Gut microbiota, metabolites and host immunity. Nat Rev Immunol. (2016) 16:341–52. doi: 10.1038/nri.2016.42
22. Musazadeh V, Zarezadeh M, Faghfouri AH, Keramati M, Jamilian P, Jamilian P, et al. Probiotics as an effective therapeutic approach in alleviating depression symptoms: an umbrella meta-analysis. Crit Rev Food Sci Nutr. (2022) 62:1–9. doi: 10.1080/10408398.2022.2051164
23. Gao X, Zhu Y, Wen Y, Liu G, Wan C. Efficacy of probiotics in non-alcoholic fatty liver disease in adult and children: a meta-analysis of randomized controlled trials. Hepatol Res. (2016) 46:1226–33. doi: 10.1111/hepr.12671
24. Khalesi S, Johnson DW, Campbell K, Williams S, Fenning A, Saluja S, et al. Effect of probiotics and synbiotics consumption on serum concentrations of liver function test enzymes: a systematic review and meta-analysis. Eur J Nutr. (2018) 57:2037–53. doi: 10.1007/s00394-017-1568-y
25. Xiao M-W, Lin S-X, Shen Z-H, Luo W-W, Wang X-Y. Systematic review with meta-analysis: the effects of probiotics in nonalcoholic fatty liver disease. Gastroenterol Res Pract. (2019) 2019:1484598.
26. Tang Y, Huang J, Zhang WY, Qin S, Yang YX, Ren H, et al. Effects of probiotics on nonalcoholic fatty liver disease: a systematic review and meta-analysis. Ther Adv Gastroenterol. (2019) 12:1756284819878046.
27. Koutnikova H, Genser B, Monteiro-Sepulveda M, Faurie J-M, Rizkalla S, Schrezenmeir J, et al. Impact of bacterial probiotics on obesity, diabetes and non-alcoholic fatty liver disease related variables: a systematic review and meta-analysis of randomised controlled trials. BMJ Open. (2019) 9:e017995. doi: 10.1136/bmjopen-2017-017995
28. Higgins JP, Lane PW, Anagnostelis B, Anzures-Cabrera J, Baker NF, Cappelleri JC, et al. A tool to assess the quality of a meta-analysis. Res Synthesis Methods. (2013) 4:351–66. doi: 10.1002/jrsm.1092
29. Liu L, Li P, Liu Y, Zhang Y. Efficacy of probiotics and synbiotics in patients with nonalcoholic fatty liver disease: a meta-analysis. Digest Dis Sci. (2019) 64:3402–12. doi: 10.1007/s10620-019-05699-z
30. Ma Y-Y, Li L, Yu C-H, Shen Z, Chen L-H, Li Y-M. Effects of probiotics on nonalcoholic fatty liver disease: a meta-analysis. World J Gastroenterol WJG. (2013) 19:6911. doi: 10.3748/wjg.v19.i40.6911
31. Loman BR, Hernández-Saavedra D, An R, Rector RS. Prebiotic and probiotic treatment of nonalcoholic fatty liver disease: a systematic review and meta-analysis. Nutr Rev. (2018) 76:822–39. doi: 10.1093/nutrit/nuy031
32. Khan MY, Mihali AB, Rawala MS, Aslam A, Siddiqui WJ. The promising role of probiotic and synbiotic therapy in aminotransferase levels and inflammatory markers in patients with nonalcoholic fatty liver disease – a systematic review and meta-analysis. Eur J Gastroenterol Hepatol. (2019) 31:703–15. doi: 10.1097/MEG.0000000000001371
33. Sharpton SR, Maraj B, Harding-Theobald E, Vittinghoff E, Terrault NA. Gut microbiome-targeted therapies in nonalcoholic fatty liver disease: a systematic review, meta-analysis, and meta-regression. Am J Clin Nutr. (2019) 110:139–49. doi: 10.1093/ajcn/nqz042
34. Lavekar AS, Raje DV, Manohar T, Lavekar AA. Role of probiotics in the treatment of nonalcoholic fatty liver disease: a meta-analysis. Euroasian J Hepato Gastroenterol. (2017) 7:130. doi: 10.5005/jp-journals-10018-1233
35. Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The cochrane collaboration’s tool for assessing risk of bias in randomised trials. BMJ. (2011) 343:d5928. doi: 10.1136/bmj.d5928
36. Littell JH, Corcoran J, Pillai V. Systematic Reviews and Meta-Analysis. Oxford: Oxford University Press (2008).
37. Maher CG, Sherrington C, Herbert RD, Moseley AM, Elkins M. Reliability of the PEDro scale for rating quality of randomized controlled trials. Phys Ther. (2003) 83:713–21. doi: 10.1093/ptj/83.8.713
38. Clark HD, Wells GA, Huët C, McAlister FA, Salmi LR, Fergusson D, et al. Assessing the quality of randomized trials: reliability of the Jadad scale. Control Clin Trials. (1999) 20:448–52. doi: 10.1016/s0197-2456(99)00026-4
39. Vallianou N, Christodoulatos GS, Karampela I, Tsilingiris D, Magkos F, Stratigou T, et al. Understanding the role of the gut microbiome and microbial metabolites in non-alcoholic fatty liver disease: current evidence and perspectives. Biomolecules. (2021) 12:56. doi: 10.3390/biom12010056
40. Jadhav K, Cohen TS. Can you trust your gut? Implicating a disrupted intestinal microbiome in the progression of NAFLD/NASH. Front Endocrinol. (2020) 11:592157. doi: 10.3389/fendo.2020.592157
41. Singal AG, Manjunath H, Yopp AC, Beg MS, Marrero JA, Gopal P, et al. The effect of PNPLA3 on fibrosis progression and development of hepatocellular carcinoma: a meta-analysis. Am J Gastroenterol. (2014) 109:325–34. doi: 10.1038/ajg.2013.476
42. Le Roy T, Llopis M, Lepage P, Bruneau A, Rabot S, Bevilacqua C, et al. Intestinal microbiota determines development of non-alcoholic fatty liver disease in mice. Gut. (2013) 62:1787–94. doi: 10.1136/gutjnl-2012-303816
43. Tarantino G, Finelli C. Systematic review on intervention with prebiotics/probiotics in patients with obesity-related nonalcoholic fatty liver disease. Future Microbiol. (2015) 10:889–902. doi: 10.2217/fmb.15.13
44. Aron-Wisnewsky J, Vigliotti C, Witjes J, Le P, Holleboom AG, Verheij J, et al. Gut microbiota and human NAFLD: disentangling microbial signatures from metabolic disorders. Nat Rev Gastroenterol Hepatol. (2020) 17:279–97. doi: 10.1038/s41575-020-0269-9
45. Scorletti E, Afolabi PR, Miles EA, Smith DE, Almehmadi A, Alshathry A, et al. Design and rationale of the INSYTE study: a randomised, placebo controlled study to test the efficacy of a synbiotic on liver fat, disease biomarkers and intestinal microbiota in non-alcoholic fatty liver disease. Contemp Clin Trials. (2018) 71:113–23. doi: 10.1016/j.cct.2018.05.010
46. Loscalzo J. Gut microbiota, the genome, and diet in atherogenesis. Mass Med Soc. (2013) 368:1647–9. doi: 10.1056/NEJMe1302154
47. Nair S, Cope K, Terence RH, Diehl AM. Obesity and female gender increase breath ethanol concentration: potential implications for the pathogenesis of nonalcoholic steatohepatitis. Am J Gastroenterol. (2001) 96:1200–4. doi: 10.1111/j.1572-0241.2001.03702.x
48. Ghavami A, Roshanravan N, Alipour S, Barati M, Mansoori B, Ghalichi F, et al. Assessing the effect of high performance inulin supplementation via KLF5 mRNA expression in adults with type 2 diabetes: a randomized placebo controlled clinical trail. Adv Pharm Bull. (2018) 8:39–47. doi: 10.15171/apb.2018.005
49. Malaguarnera M, Vacante M, Antic T, Giordano M, Chisari G, Acquaviva R, et al. Bifidobacterium longum with fructo-oligosaccharides in patients with non alcoholic steatohepatitis. Dig Dis Sci. (2012) 57:545–53. doi: 10.1007/s10620-011-1887-4
50. Manzhalii E, Virchenko O, Falalyeyeva T, Beregova T, Stremmel W. Treatment efficacy of a probiotic preparation for non-alcoholic steatohepatitis: a pilot trial. J Digest Dis. (2017) 18:698–703. doi: 10.1111/1751-2980.12561
51. Wong VW, Won GL, Chim AM, Chu WC, Yeung DK, Li KC, et al. Treatment of nonalcoholic steatohepatitis with probiotics. A proof-of-concept study. Ann Hepatol. (2013) 12:256–62. doi: 10.1016/s1665-2681(19)31364-x
52. Shoaf K, Mulvey GL, Armstrong GD, Hutkins RW. Prebiotic galactooligosaccharides reduce adherence of enteropathogenic Escherichia coli to tissue culture cells. Infect Immun. (2006) 74:6920–8. doi: 10.1128/IAI.01030-06
53. Huang Z, Mu C, Chen Y, Zhu Z, Chen C, Lan L, et al. Effects of dietary probiotic supplementation on LXRα and CYP7α1 gene expression, liver enzyme activities and fat metabolism in ducks. Br Poultry Sci. (2015) 56:218–24. doi: 10.1080/00071668.2014.1000821
54. Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease—meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. (2016) 64:73–84. doi: 10.1002/hep.28431
55. Oh HJ, Kim TH, Sohn YW, Kim YS, Oh YR, Cho EY, et al. Association of serum alanine aminotransferase and γ-glutamyltransferase levels within the reference range with metabolic syndrome and nonalcoholic fatty liver disease. Korean J Hepatol. (2011) 17:27. doi: 10.3350/kjhep.2011.17.1.27
56. Lee DH, Blomhoff R, Jacobs DR Jr. Is serum gamma glutamyltransferase a marker of oxidative stress? Free Radic Res. (2004) 38:535–9. doi: 10.1080/10715760410001694026
57. Lee DH, Jacobs DR Jr. Serum gamma-glutamyltransferase: new insights about an old enzyme. J Epidemiol Commun Health. (2009) 63:884–6. doi: 10.1136/jech.2008.083592
58. Askarpour M, Djafarian K, Ghaedi E, Sadeghi O, Sheikhi A, Shab-Bidar S. Effect of L-carnitine supplementation on liver enzymes: a systematic review and meta-analysis of randomized controlled trials. Arch Med Res. (2020) 51:82–94. doi: 10.1016/j.arcmed.2019.12.005
59. Sanyal D, Mukherjee P, Raychaudhuri M, Ghosh S, Mukherjee S, Chowdhury S. Profile of liver enzymes in non-alcoholic fatty liver disease in patients with impaired glucose tolerance and newly detected untreated type 2 diabetes. Indian J Endocrinol Metab. (2015) 19:597–601. doi: 10.4103/2230-8210.163172
60. Maximos M, Bril F, Portillo Sanchez P, Lomonaco R, Orsak B, Biernacki D, et al. The role of liver fat and insulin resistance as determinants of plasma aminotransferase elevation in nonalcoholic fatty liver disease. Hepatology. (2015) 61:153–60. doi: 10.1002/hep.27395
61. Dai X, Hou H, Zhang W, Liu T, Li Y, Wang S, et al. Microbial metabolites: critical regulators in NAFLD. Front Microbiol. (2020) 11:567654. doi: 10.3389/fmicb.2020.567654
62. Manco M, Alisi A, Nobili V. Risk of severe liver disease in NAFLD with normal ALT levels: a pediatric report. Hepatology. (2008) 48:2087–8. doi: 10.1002/hep.22631
63. Kohli R. There’s more under the nonalcoholic fatty liver disease umbrella than an elevated alanine aminotransferase level. J Pediatr. (2014) 164:684–6. doi: 10.1016/j.jpeds.2013.12.026
Keywords: non-alcoholic fatty liver disease, probiotics, liver enzyme, umbrella meta-analysis, systematic review
Citation: Musazadeh V, Roshanravan N, Dehghan P and Ahrabi SS (2022) Effect of Probiotics on Liver Enzymes in Patients With Non-alcoholic Fatty Liver Disease: An Umbrella of Systematic Review and Meta-Analysis. Front. Nutr. 9:844242. doi: 10.3389/fnut.2022.844242
Received: 27 December 2021; Accepted: 02 May 2022;
Published: 23 May 2022.
Edited by:
Maurizio Muscaritoli, Sapienza Università di Roma, ItalyReviewed by:
Pietro Vajro, University of Salerno, ItalyCopyright © 2022 Musazadeh, Roshanravan, Dehghan and Ahrabi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Parvin Dehghan, ZGVoZ2hhbi5udXRAZ21haWwuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.