
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
BRIEF RESEARCH REPORT article
Front. Nutr., 30 March 2022
Sec. Nutritional Immunology
Volume 9 - 2022 | https://doi.org/10.3389/fnut.2022.843437
This article is part of the Research TopicDietary Management in Children with Immune-Related DiseasesView all 5 articles
 Emilia Vassilopoulou1
Emilia Vassilopoulou1 Gavriela Feketea2,3*
Gavriela Feketea2,3* George N. Konstantinou4
George N. Konstantinou4 Dimitris Zekakos Xypolias1
Dimitris Zekakos Xypolias1 Mina Valianatou5
Mina Valianatou5 Maria Petrodimopoulou1
Maria Petrodimopoulou1 Vasiliki Vourga1
Vasiliki Vourga1 Ioannis Tasios1
Ioannis Tasios1 Nikolaos G. Papadopoulos5
Nikolaos G. Papadopoulos5Background: The aim of the current investigation was to explore the association of food protein-induced allergic proctocolitis (FPIAP) with the maternal diet during pregnancy and breastfeeding in Greek infants.
Methods: A multicenter retrospective case-control study was conducted in 6 regions in Greece, with 96 mothers of infants with and 141 mothers of infants without a history of FPIAP. Maternal dietary habits during pregnancy and breastfeeding were evaluated with the following validated questionnaires: (a) The Mediterranean Diet Score and (b) The Mediterranean Oriented Culture-Specific Semi-Quantitative Food Frequency Questionnaire.
Results: FPIAP was associated with cow's milk (83.6%), egg (7.3%), wheat (6.4%), and beef (6.4%) in the maternal diet. Adherence to Mediterranean Diet was similar among the mothers. Mothers of FPIAP infants consumed more vegetables. Elastic net prediction models showed that, in this Mediterranean population, increased consumption during pregnancy and lactation of common allergens, whole grain products, homemade food, fish and shellfish, and fruits was associated with a decreased risk of FPIAP. Conversely, a high intake of vegetables, sugar and total fat, and non-stick/grilled cooking, were associated with increased risk of FPIAP, as was a high intake of salt and white flour during lactation only.
Conclusions: Components of a maternal Mediterranean Diet may protect against FPIAP when traditional cooking methods are adopted and fish, fruit, and whole wheat products are consumed frequently during pregnancy and breastfeeding.
Food protein-induced allergic proctocolitis, a major cause of colitis in infants, is a transient benign condition. Typically, food protein-induced allergic proctocolitis (FPIAP) presents in the first months of life, as a non-immunoglobulin E (non-IgE) mediated immune response to one or more foods, with inflammation in the distal colon (1), eosinophilic infiltration of the rectal mucosa, and blood in the feces (2).
Most cases of FPIAP are in breastfed infants (60%) and are triggered by food proteins consumed by the mother, mainly cow's milk, egg, soy, and corn (3). Elimination of the offending food from the maternal diet usually results in gradual alleviation of symptoms, without discontinuation of breastfeeding (4). In formula-fed infants, the triggers are mainly cow's milk and soy; hypoallergenic or extensively hydrolyzed formulas are rarely incriminated (4–10%) (5). The disease presents at a later stage in breastfed babies and with milder histopathological lesions (6).
The reported prevalence varies, possibly due to inaccurate diagnosis, ranging widely from 0.16% in healthy children to 18–64% among infants who show fecal blood, and is higher in areas with low rates of IgE-mediated food allergy (FA), such as Greece and Brazil (7). Coexistent eczema is reported in 22% and a family history of atopic disease in 25% of cases (5).
Pregnancy is a period of immunological, metabolic, and hormonal changes, which is needed to ensure normal fetal development, the timely onset of labor, and successful delivery. Recent evidence subverts the dogma that intrauterine life is sterile (8), and exposure of the fetus to bacteria/bacterial components might be altered by maternal food variety during pregnancy, affecting allergies in infancy and childhood (9).
In breastfed infants, lactation is a critical period for immune system modulation, and maternal food variety and nutritional adequacy are currently recommended for healthy infant growth, in contrast to earlier guidelines that proposed restricted diets (10). Regarding FA, the effects of maternal diet are unclear (11), but possible benefits of the Mediterranean diet (MedDiet) warrant investigation (12). The purpose of this study was to explore the association of maternal diet during pregnancy and breastfeeding in the occurrence of FPIAP in Greece.
For this retrospective, observational, multicenter, case-control study, conducted between May 2018 to November 2020, mothers of breastfed infants with a history of FPIAP were recruited from 6 regions in Greece including Athens, Thessaloniki, Amaliada, Volos, Kavala, and Kozani (FPIAP group). A control population was selected of mothers of breastfed infants with no history of FPIAP, matched for age, sex, and place of residence [healthy control (HC) group]. The study was approved by the Hospital Ethics and Scientific Committee of the Amaliada General Hospital of Ilia and the Medical Association of Thessaloniki on behalf of all study centers (Study IDs: 53/2021 and 4170/2018), and conducted in accordance with the code of Ethics of the World Medical Association (Declaration of Helsinki). All participants were fully informed of the scope and procedures of the study and provided written consent.
The diagnosis of FPIAP was based on the history of scant bright red rectal bleeding with mucus in an otherwise healthy infant, which disappeared after a two-week maternal elimination diet and reoccurred after reintroduction of the culprit food. Infections and other causes of rectal bleeding, such as intussusception, volvulus, Hirschsprung's disease, and necrotizing enterocolitis, were excluded. We recorded the symptoms, the infant's age at onset and development of tolerance, physical examination findings, stool examination results, allergenic food(s) implicated in FPIAP, other allergies, and family history of atopy or asthma.
Information on maternal diet during pregnancy and breastfeeding was collected by a personal interview with a qualified dietician, using the following questionnaires:
a) The Mediterranean Diet Score Questionnaire, which estimates adherence to the MedDiet, recording consumption of the 11 main components (non-refined cereals, fruit, vegetables, potatoes, legumes, olive oil, fish, red meat, poultry, full-fat dairy products, and alcohol). The resultant MedDiet score is categorized: 0–13: no adherence; 14–27: insufficient; 28–41: satisfactory; 42–55: very good adherence (13).
b) The Mediterranean Oriented Culture Specific Semi-Quantitative Food Frequency Questionnaire, which includes 221 foods, subdivided into 22 food sections: (1) white grain products, including rice and potato; (2) whole wheat grain products; (3) breads/pastries; (4) stews; (5) pulses; (6) raw and cooked vegetable salads; (7) fruit and homemade juices; (8) nuts; (9) milk/dairy products; (10) meat/traditional meat dishes; (11) red meat products; (12) white meat products; (13) eggs; (14) fish/seafood; (15) fats/spreads (including olive oil); (16) traditional dips/sauces/dressings; (17) sugar/sweet preserves/confectionary; (18) “ready-to-eat” foods; (19) chips/salty puffed snacks; (20) herbals/teas; (21) soft drinks/nonalcoholic beverages; (22) alcoholic drinks. Four additional questions concern (23) addition of extra salt, (24) use of non-stick casseroles/grilled food, and (25) traditionally cooked Mediterranean homemade food; (26) food supplements.
Food consumption frequency is scored as follows: (1) never or less than once per month, (2) 1–3 times per month, (3) once per week, (4) 2–4 times per week, (5) 5–6 times per week, (6) once per day, (7) 2–3 times per day, (8) 4–5 times per day, and (9) 6 or more times per day (14).
The data were shown as mean (±SD) or median and IQR. Statistical analysis included inferential analysis, statistical tests, and modeling, using R software (version 4.04) and R studio (version 1.4.1106) (Boston, USA). The normality of the distribution of continuous variables was checked using Shapiro-Wilk's test. Comparisons between the FPIAP and HC groups were made by t-test (normal distribution) and Wilcoxon test (non-normal distribution). P-values ≤ 0.05 were considered statistically significant. To investigate the FPIAP risk in relation to the maternal diet during pregnancy and breastfeeding, the elastic net regression model was applied, which combines Lasso and Ridge regression (15), using the glmnet package in R (version 4.1-1).
The study included 96 mothers and their breastfed infants with the diagnosis of FPIAP made by a pediatric allergist (FPIAP group), and 141 mothers with healthy breastfed infants (HC group). The mean age of the FPIAP infants (48 boys, 50%) was 18 ± 12.14 months and that of the HC infants (62 boys, 44%) 18.7 ± 12.8 months. The birth weight showed no significant difference between groups. The mean current height in HC infants (86.09 ± 12.31 cm) was lower than that in FPIAP infants (93.83 ± 9.5 cm) (p < 0.01) (Table A in Appendix).
The median age at diagnosis and resolution of FPIAP was 2 months (IQR 1–3 months) and 12 months (IQR 11–14 months), respectively. The symptoms were the following: blood in feces (80.1%) and mucus in feces (79%); 2.7% presented anemia and 0.9% presented hypoalbuminemia. The symptoms were induced by maternal consumption of cow's milk (83.6%), egg (7.3%), wheat (6.4%), and beef (6.4%), and by lamb, peanut, rice, pear, grape, and fish (1% each) (Table B in Appendix). After diagnosis, the mothers continued breastfeeding, following an elimination diet for the offending food until FPIAP was resolved.
Table 1 presents the allergy history of the study infants. Among the 96 infants with a history of FPIAP, at the age of data collection, 83 self-report reactions to foods including cow's milk at 59%, egg at 8%, wheat at 4%, peanut at 1%, soya at 1%, beef at 5.2%, lamb at 1%, apple at 1%, and the carrot at 1%. Wheezing was present in 8.3%, while the other 22% had eczema. In contrast, of the 141 infants in the HC group, only two had FA (one to milk and one to apple), one (0.7%) had asthma, and 2% had eczema.
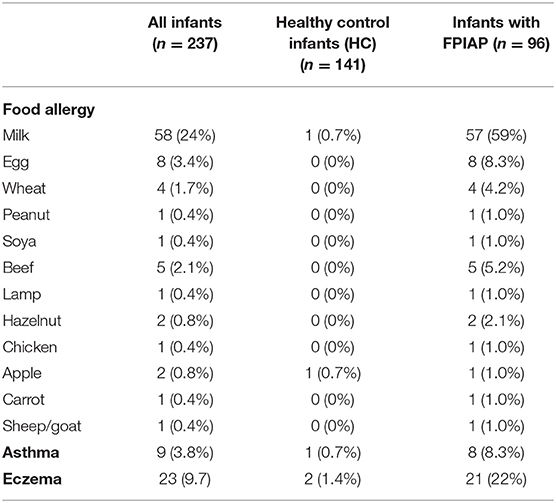
Table 1. Allergy history of study infants, other than food protein-induced allergic proctocolitis (FPIAP).
The family characteristics and family history of allergies of the study infants are presented in Figure 1. The FPIAP families were more frequently urban residents (85 vs. 79%). A higher parental educational level was significantly less common in the FPIAP group (mothers 27% and fathers 24.5%) than in the HC group (mothers 62% and fathers 59%). Maternal smoking was more frequent in the FPIAP group (14 vs. 7.8%), and 13% of fathers in both groups were smokers (Figure 1A).
Infants with FPIAP more often had a family history of allergy/atopy; 20% of FPIAP mothers had documented allergy/atopy (17% FA, 1% asthma, and 1% allergic rhinitis), compared with 2.8% of HC mothers who reported FA. FPIAP fathers also had a higher rate of reported allergy/atopy, specifically FA (25%), asthma (5.4%), and eczema (14.5), than HC fathers (FA in 2.7% and 0% asthma and eczema)) (Figure 1B).
Regarding siblings, 69/96 FPIAP infants and 37/141 of the HC infants were the only children in the family. Among the infants with siblings, in the FPIAP group (27 infants with 41 siblings), a documented FA in a sibling was reported in 58.5% (12/41 milk, 7/41 egg, 3/41 fish, 4/41 shellfish, 2/41 wheat, 2/41 peanut, 2 hazelnuts, 2/41 walnut, 2/41 pepper allergy), and 15/41 had asthma, 12/41 eczema, and two (4.8%) had a history of FPIAP. Among the HC siblings (104 HC infants with 107 siblings), only 3.7% reported a FA (2/107 milk, 1/107 fish, 2/107 pepper allergy), 2/107 had a history of FPIAP, and one of eczema.
Overall, the study mothers showed satisfactory adherence to the MedDiet, similar in the two groups (MedDiet score: FPIAP 33.87 vs. HC 33.38, p = 0.38). No differences in the total MedDiet score were observed among women living in the different Greek regions. No significant dietary changes were found between pregnancy and breastfeeding. Significant differences in the consumption of vegetables and olive oil were recorded between the FPIAP and the HC mothers in both study periods: for vegetables 2.19 ± 0.91 vs. 1.85 ± 0.99 in pregnancy, p = 0.008, and during breastfeeding 2.1 ± 0.89 vs. 1.8 ± 0.95, p = scores of 0.018; for olive oil 4.78 ± 0.64 vs. 4.56 ± 0.79 in pregnancy, p = 0.023, and during breastfeeding 4.35 ± 1.09 vs. 4.74 ± 0.74, p = 0.003.
According to their responses on the Mediterranean Oriented Culture-Specific Semi-Quantitative Food Frequency Questionnaire, maternal consumption of certain food groups differed significantly between groups, as well as between pregnancy and lactation, as shown in Table 2.
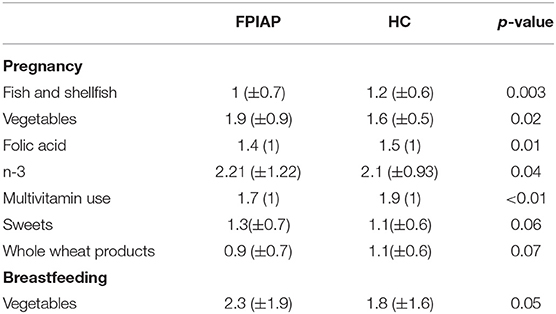
Table 2. Food consumption and food supplement use during pregnancy and breastfeeding of mothers of infants with FPIAP and healthy control subjects (HC).
During pregnancy, FPIAP mothers consumed less fish and shellfish (p = 0.003) and more vegetables (p = 0.02), than HC mothers, and they used less food supplements, specifically folic acid (p = 0.01), multivitamins (p < 0.01), and omega-3 (p = 0.04).
During breastfeeding the FPIAP mothers consumed significantly more vegetables than during pregnancy (p = 0.05), while vegetables' consumption was not altered by the HC mothers in the two study periods (p > 0.05).
Elastic net regression was used to construct multifactorial models to explore the effect of maternal dietary factors on FPIAP risk. The pregnancy model presented high statistical significance (p < 0.01) and good accuracy (accuracy = 0.83), with an area under the curve (AUC) of 0.87, confirming the ability of the model to distinguish maternal dietary factors affecting FPIAP. According to this model, pregnancy factors associated with FPIAP diagnosis were n-3 and vitamin C supplements, sugary products, vegetables, total fat, and the use of non-stick kitchenware and grills. Protective factors were traditionally cooked homemade food, dairy products, nuts, whole grain products, fish and shellfish, fruits, multivitamins, vitamin A, vitamin D, B-complex and folic acid, and dietary fiber (Figure 2A).
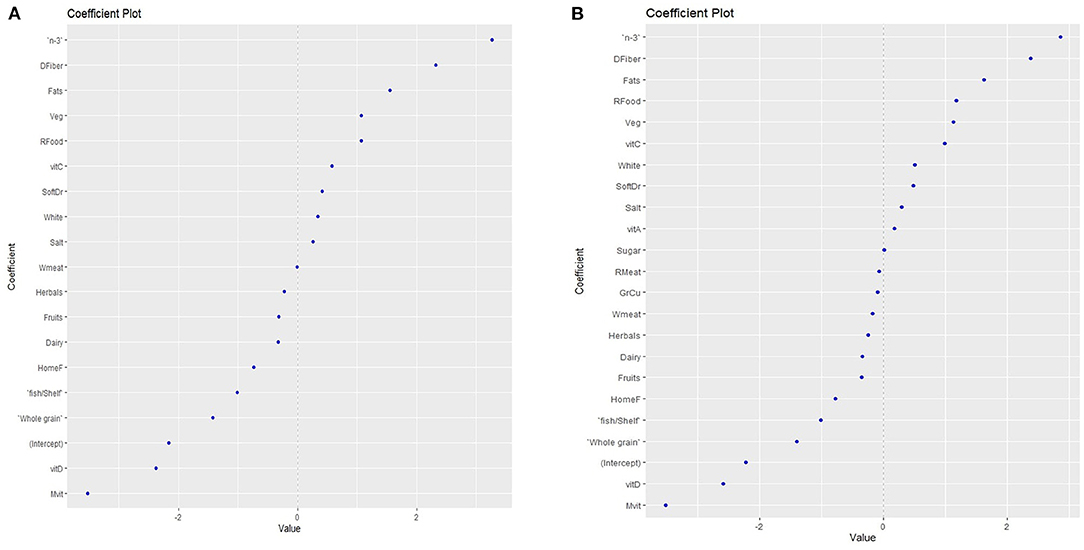
Figure 2. Maternal dietary factors that are associated with increased food protein-induced allergic proctocolitis (FPIAP) risk or protection, as predicted with the Elastic Net Regression model during pregnancy. Food products with values < 0 protect, while values > 0 increase the risk, (A) during pregnancy (B) during breastfeeding. n-3 = omega 3 polyunsaturated fatty acids; DFiber = dietary fiber; Veg = vegetables; RFood = ready made food; vitC = vitamin C; SoftDr = soft drinks; White = white grains; White meat = white meat/meat products homemade food; fish/Shelf = fish/ shellfish; Rmeat = red meat/meat products; Mvit = multivitamins.
The breastfeeding model did not reveal statistical significance (p = 0.08). However, there was a trend towards a non significant association of FPIAP cases with increased consumption of dietary fiber, total fat, vegetables, sugar/sugary products and soft drinks, white flour products, and salt, whereas no significant protective factors included increased intake of fruits, fish and shellfish products, whole grain products, homemade food, vitamin D, and multivitamin supplementation (Figure 2B).
This retrospective, observational, multicenter case-control study, the first to explore the risk of FPIAP in relation to maternal dietary habits during pregnancy and lactation, provided evidence that the traditional MedDiet can be protective against FPIAP, when whole wheat products, fish, and fruit are consumed frequently by the mother, using traditional cooking methods. A high intake of fat, sugar, and vegetables increases the risk of FPIAP, together with the use of grills and non-stick kitchenware. As in other allergies, the inclusion of common food allergens in the maternal diet appears to protect infants from FPIAP.
Current evidence does not support maternal dietary restrictions during pregnancy or lactation for prevention of allergic disease in the offspring (10, 16); consumption of allergenic foods in pregnancy (17) and during lactation is reported to prevent allergic sensitization to these foods (17, 18), and in line with this, frequent consumption of common allergenic foods (dairy products, nuts, whole-grain foods, and fish/shellfish) as part of a balanced diet, was associated with a reduction in the risk of FPIAP (17, 19, 20).
Earlier studies suggest that the MedDiet is associated with reduced allergic outcomes (21), and a systematic review confirmed the beneficial effect of the MedDiet during pregnancy and lactation (12). In the current study, both groups of mothers recorded similar satisfactory adherence to the MedDiet, thus allowing the study of the impact of specific foods within this dietary pattern. Home-cooked food, exemplifying Mediterranean dietary habits, appeared to be associated with a protective effect. The apparent beneficial effect of specific components of MedDiet and/or traditional cooking methods in the protection against allergies has generated interest in a proposal for a large prospective randomized controlled trial (RCT) during pregnancy to assess its effect on infant allergy prevention (22, 23).
Conversely, a high-fat diet and the use of non-stick kitchenware and grills were associated with FPIAP. The consumption of olive oil did not appear as a significant individual factor as it did not differ between our study groups, but the total fat intake was a risk factor for FPIAP. Results from a mouse model suggest that a high-fat diet increases the risk of FA due to microbiome alterations (24). Grilling food, mainly meat, at home may affect the fetal immune system, and probably aggravate the risk of food allergies in neonates and children, via the carcinogenic compounds produced at high temperatures (25).
Sugary products and soft drinks were associated with an increase in the risk of FPIAP in our population, in agreement with a recent metanalysis of high sugar consumption during pregnancy and lactation and allergies in the offspring (26). Moreover, high salt intake during lactation appeared to increase the risk of FPIAP. Documentation is lacking about the effect of salt intake during pregnancy and lactation on infant allergy risk.
Whole grain products were associated with a reduced risk of FPIAP in both periods, but white flour consumption during breastfeeding was associated with an increase in the risk of FPIAP. Dietary fiber supplementation during pregnancy appeared to exert a beneficial effect. Dietary fiber acts as a prebiotic, ensuring gut microbial biodiversity during pregnancy. The microbiome is transferred from mother to offspring. Loss of microbial communities or species occurs in the mother-infant dyad when the diet is rich in highly processed foods (27). Breast milk benefits the infant gut microbiome diversity, and formula-fed infants lack dominant microbes; formula enriched with symbiotics have not increased gut biodiversity or provided protection in high-risk infants (28–30). Increased insoluble dietary fiber intake during pregnancy is associated specifically with reduced risk of wheeze and increased risk of eczema in the infant (31). Conversely, maternal fiber supplementation during lactation was associated with an increase in the risk of FPIAP.
An unexpected finding was the link of vegetable intake with FPIAP. Other studies associated maternal vegetable intake with allergy prevention (25, 32), although offspring of women with a higher intake of total vegetables, folate-rich, and green and yellow vegetables were reported to have a higher risk of allergies, including FA (33). In countries where the MedDiet is widespread, vegetables are frequently incriminated in FA (34–36). In Greece, pregnant and lactating women are encouraged to consume washed and peeled fruits without restrictions, but they are often advised to consume only specific vegetables during the following events: a) during pregnancy, to eat only cooked or boiled vegetables, for prevention of toxoplasmosis, transmitted through vegetables grown in soil contaminated with cat feces (37); b) during lactation, to prefer green leafy vegetables, but to avoid vegetables that may cause colic in the baby, such as broccoli (38). Cooking methods affect the antioxidant vitamins, with loss in vitamins C, E, β-carotene, and K (39), dietary fiber, and slight increases in insoluble dietary fiber (40).
A high intake of seasonal fruits during pregnancy and lactation, both fresh and dried, was associated with a reduced risk of FPIAP. The seasonal availability of a wide variety of Mediterranean fruits rich in antioxidants protects against oxidative exposures, abating allergy risk during pregnancy, lactation (12) infancy (41) and childhood (42, 43). The high fruit content of soluble dietary fibers enhances the development of a healthy intestinal microbiota (23), and fruit antioxidants, including polyphenols, carotenoids, and vitamins A and C, are anti-inflammatory (44). Vitamin C supplements increased the risk of FPIAP, and relevant data are conflicting, indicating low to no protection against allergies (21).
Several researchers report a protective effect of high fish and seafood intake in pregnancy and infancy against allergic diseases in childhood (45–47), and our study confirmed a reduced risk of FPIAP. In our population, n-3 long-chain polyunsaturated fatty acid (LC-PUFA) supplementation was a risk factor for FPIAP, and although n-3 PUFA supplementation has been suggested for allergy prevention (48), especially in lower socioeconomic populations (49) its benefits are obscure (50–52).
Multivitamin supplements, folic acid, vitamins A, D, and B-complex during pregnancy and vitamin D and multivitamin supplements during lactation were associated with a reduced risk of FPIAP, and other researchers have reported vitamin protection against allergy outcomes in the offspring, mainly in undernourished mothers, especially from vitamin A and folic acid, and also iron (53, 54).
Vitamin D supplementation, in both pregnancy and lactation, appeared to reduce the risk of FPIAP, and other researchers report that it protects against asthma and FA in the offspring (55, 56). Inadequate levels of vitamin D in infants have been associated with clinical manifestations of cow's milk allergy, including non-IgE forms (57), and the role of vitamin D in the prevention of FA (58) and other atopic disease is documented (55, 56, 59).
Food protein-induced allergic proctocolitis (FPIAP) is frequently, but not exclusively, caused by cow's milk protein, even in exclusively breastfed infants (3, 60, 61). In 83.6% of the cases proved by the challenge in this study, cow's milk was incriminated in FPIAP.
A striking finding in our study was that approximately 25% of our FPIAP infants had parents with higher education compared with 60% of HC infants. In the Mediterranean area, parental tertiary education has been found associated with high consumption of fruits and vegetables, and low childhood asthma prevalence (62).
In line with other reports (63), the FPIAP infants more often had a family history of allergy and atopy than the HC infants. Infants with FPIAP are reported to develop IgE-mediated FA (64).
Children who are pre- and postnatally exposed to tobacco smoke have a significantly higher risk of FA (65), and in our study maternal smoking was associated with FPIAP. Paternal smoking was equivalent in the two groups and had less effect, probably because fathers spend less time in close contact with the infants (66).
Our results support the need for increased counseling on maternal diet during pregnancy and breastfeeding, especially when there is a family history of allergy, in the context of the prevailing Mediterranean diet, as certain foods, nutrients, and cooking methods appear to be associated with FPIAP.
A limitation of the current investigation was the retrospective design, data based on self-reported questionnaires, and recall bias when they report their past eating habits during pregnancy and lactation. There is a need for a prospective, intervention study to investigate the apparent benefits of the specific components of the Mediterranean diet model for infant allergy prevention during pregnancy and lactation.
In this first cross-sectional, multicenter study on the association of maternal diet during pregnancy and breastfeeding with the development of FPIAP in the offspring, we found that frequent consumption of common allergens, food home-cooked according to the traditional Mediterranean customs, wholewheat grains, fruits, fish and shellfish, and folic acid, vitamins D, A, B-complex, and multivitamin supplementation appeared to protect against FPIAP in infancy. Conversely, a diet with high content of fat, sugary products, salt, vegetables and dietary fiber, vitamin C and n-3 PUFA supplementation, and use of non-stick kitchenware/grills appeared to increase the risk of FPIAP. A family history of allergy was a strong predisposing factor. These results show that elements of the MedDiet may be protective. This evidence should be further evaluated in prospective intervention studies and used to develop dietary guidelines for mothers during pregnancy and lactation, for the prevention of FPIAP.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by Hospital Ethics and Scientific Committee of the Amaliada General Hospital of Ilia (53/03-09-2021) and the Medical Association of Thessaloniki (4170/18-4-2018). Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin.
EV and NP: conceptualization. EV, GF, GK, MV, MP, and IT: data collection. EV and GF: drafting the article. EV and DZ: data analysis and interpretation. All authors: critical revision and approval.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We would like to express our gratitude to F. Tsiogka, I. Papamichali, D. Kalafati, E. Mpazouki, and L. Doulgeri who participated in patient recruitment procedures, and all mothers who participated in the study.
MedDiet, Mediterranean diet; FPIAP, Food Protein-Induced Allergic Proctocolitis; FA, Food Allergy; HC, Healthy Control.
1. Martin VM, Virkud YV, Seay H, Hickey A, Ndahayo R, Rosow R, et al. Prospective assessment of pediatrician-diagnosed food protein-induced allergic proctocolitis by gross or occult blood. J Allergy Clin Immunol Pract. (2020) 8:1692–9.e1. doi: 10.1016/j.jaip.2019.12.029
2. Arvola T, Ruuska T, Keranen J, Hyoty H, Salminen S, Isolauri E. Rectal bleeding in infancy: clinical, allergological, microbiological examination. Pediatrics. (2006) 117:e760–8. doi: 10.1542/peds.2005-1069
3. Caubet JC, Szajewska H, Shamir R, Nowak-Wegrzyn A. Non-IgE-mediated gastrointestinal food allergies in children. Pediatr Allergy Immunol. (2017) 28:6–17. doi: 10.1111/pai.12659
4. Maloney JA. Nowak-Wegrzyn Educational clinical case series for pediatric allergy and immunology: allergic proctocolitis, food protein-induced enterocolitis syndrome and allergic eosinophilic gastroenteritis with protein-losing gastroenteropathy as manifestations of non-IgE-mediated cow's milk allergy. Pediatr Allergy Immunol. (2007) 18:360–7. doi: 10.1111/j.1399-3038.2007.00561.x
5. Nowak-Wegrzyn. Food protein-induced enterocolitis syndrome and allergic proctocolitis. Allergy Asthma Proc. (2015) 36:172–84. doi: 10.2500/aap.2015.36.3811
6. Labrosse R, Graham F, Caubet J-C. Non-IgE-Mediated Gastrointestinal Food Allergies in Children: An Update. Nutrients. (2020) 12:2086. doi: 10.3390/nu12072086
7. Mennini M, Fiocchi AG, Cafarotti A, Montesano M, Mauro A, Villa MP, et al. Food protein-induced allergic proctocolitis in infants: literature review and proposal of a management protocol World. Allergy Org J. (2020) 13:100471. doi: 10.1016/j.waojou.2020.100471
8. Stinson LF, Boyce MC, Payne MS, Keelan AJ. The not-so-sterile womb: evidence that the human fetus is exposed to bacteria prior to birth. Front Microbiol. (2019) 10:1124. doi: 10.3389/fmicb.2019.01124
9. Nyangahu DD, Jaspan BH. Influence of maternal microbiota during pregnancy on infant immunity. Clin Exp Immunol. (2019) 198:47–56. doi: 10.1111/cei.13331
10. Greer FR, Sicherer SH, Burks WA. The effects of early nutritional interventions on the development of atopic disease in infants and children: the role of maternal dietary restriction, breastfeeding, hydrolyzed formulas, and timing of introduction of allergenic complementary foods. Pediatrics. (2019) 143:e20190281. doi: 10.1542/peds.2019-0281
11. Perkin MR, Togias A, Koplin J, Sicherer S. Food allergy prevention: more than peanut. J Allergy Clin Immunol Pract. (2020) 8:1–13. doi: 10.1016/j.jaip.2019.11.002
12. Netting MJ, Middleton PF, Makrides M. Does maternal diet during pregnancy and lactation affect outcomes in offspring? A systematic review of food-based approaches. Nutrition. (2014) 30:1225–41. doi: 10.1016/j.nut.2014.02.015
13. Panagiotakos DB, Pitsavos C, Stefanadis C. Dietary patterns: a Mediterranean diet score and its relation to clinical and biological markers of cardiovascular disease risk. Nutr Metab Cardiovasc Dis. (2006) 16:559–68. doi: 10.1016/j.numecd.2005.08.006
14. Athanasiadou E, Kyrkou C, Fotiou M, Tsakoumaki F, Dimitropoulou A, Polychroniadou E, et al. Development and validation of a mediterranean oriented culture-specific semi-quantitative food frequency questionnaire. Nutrients. (2016) 8:522. doi: 10.3390/nu8090522
15. Chase EC, Boonstra SP. Accounting for established predictors with the multistep elastic net. Stat Med. (2019) 38:4534–44. doi: 10.1002/sim.8313
16. Fleischer DM, Spergel JM. Assa'ad AH, Pongracic AJ. Primary prevention of allergic disease through nutritional interventions. J Allergy Clin Immunol Pract. (2013) 1:29–36. doi: 10.1016/j.jaip.2012.09.003
17. Fujimura T, Lum SZC, Nagata Y, Kawamoto S, Oyoshi KM. Influences of maternal factors over offspring allergies and the application for food allergy. Front Immunol. (2019) 10:1933. doi: 10.3389/fimmu.2019.01933
18. Du Toit G, Roberts G, Sayre PH, Bahnson HT, Radulovic S, Santos AF, et al. Randomized trial of peanut consumption in infants at risk for peanut allergy. N Engl J Med. (2015) 372:803–13. doi: 10.1056/NEJMoa1414850
19. Fleischer DM. Primary Prevention of Allergic Disease: Maternal Diet in Pregnancy and Lactation. Netherlands (Global); Philadelphia, PA: Wolters Kluwer.
20. Stråvik M, Barman M, Hesselmar B, Sandin A, Wold AE, Sandberg SA. Maternal intake of cow's milk during lactation is associated with lower prevalence of food allergy in offspring. Nutrients. (2020) 12:680. doi: 10.3390/nu12123680
21. Venter C, Agostoni C, Arshad SH, Ben-Abdallah M, Du Toit G, Fleischer DM, et al. Dietary factors during pregnancy and atopic outcomes in childhood: a systematic review from the European Academy of Allergy and Clinical Immunology. Pediatr Allergy Immunol. (2020) 31:889–912. doi: 10.1111/pai.13303
22. Sewell DA, Hammersley VS, Robertson A, Devereux G, Stoddart A, Weir CJ, et al. Sheikh. A pilot randomised controlled trial investigating a Mediterranean diet intervention in pregnant women for the primary prevention of allergic diseases in infants. J Hum Nutr Diet. (2017) 30:604–14. doi: 10.1111/jhn.12469
23. Skypala IJ, McKenzie R. Nutritional issues in food allergy. Clin Rev Allergy Immunol. (2019) 57:166–78. doi: 10.1007/s12016-018-8688-x
24. Hussain M, Bonilla-Rosso G, Kwong Chung CKC, Bäriswyl L, Rodriguez MP, Kim BS, et al. High dietary fat intake induces a microbiota signature that promotes food allergy. J Allergy Clin Immunol. (2019) 144:157–70.e8. doi: 10.1016/j.jaci.2019.01.043
25. Baïz N, Just J, Chastang J, Forhan A, de Lauzon-Guillain B, Magnier A-M, et al. Maternal diet before and during pregnancy and risk of asthma and allergic rhinitis in children. Allergy Asthma Clin Immunol. (2019) 15:40. doi: 10.1186/s13223-019-0353-2
26. Gupta A, Singh A, Fernando RL, Dharmage SC, Lodge CJ, Waidyatillake TN. The association between sugar intake during pregnancy and allergies in offspring: a systematic review and a meta-analysis of cohort studies. Nutr Rev. (2021) nuab052. doi: 10.1093/nutrit/nuab052
27. Çavdar G, Papich T, Ryan PE. Microbiome, breastfeeding and public health policy in the United States: the case for dietary fiber. Nutr Metab Insights. (2019) 12:1178638819869597. doi: 10.1177/1178638819869597
28. Järvinen KM, Martin H, Oyoshi KM. Immunomodulatory effects of breast milk on food allergy. Ann Allergy Asthma Immunol. (2019) 123:133–43. doi: 10.1016/j.anai.2019.04.022
29. Fox A, Bird JA, Fiocchi A, Knol J, Meyer R, Salminen S, et al. Papadopoulos. The potential for pre-, pro- and synbiotics in the management of infants at risk of cow's milk allergy or with cow's milk allergy: an exploration of the rationale, available evidence and remaining questions. World Allergy Org J. (2019) 12:100034. doi: 10.1016/j.waojou.2019.100034
30. Sestito S, D'Auria E, Baldassarre ME, Salvatore S, Tallarico V, Stefanelli E, et al. The role of prebiotics and probiotics in prevention of allergic diseases in infants. Front Pediatr. (2020) 8:583946. doi: 10.3389/fped.2020.583946
31. Pretorius RA, Bodinier M, Prescott SL, Palmer JD. Maternal fiber dietary intakes during pregnancy and infant allergic disease. Nutrients. (2019) 11:1767. doi: 10.3390/nu11081767
32. Venter C, Palumbo MP, Glueck DH, Sauder KA, O'Mahony L, Fleischer DM, et al. The maternal diet index in pregnancy is associated with offspring allergic diseases: the Healthy Start study. Allergy. (2022) 77:162–72. doi: 10.1111/all.14949
33. Ogawa K, Pak K, Yamamoto-Hanada K, Ishitsuka K, Sasaki H, Mezawa H, et al. Children's Study Association between maternal vegetable intake during pregnancy and allergy in offspring: Japan Environment and Children's Study. PLoS ONE. (2021) 16:e0245782. doi: 10.1371/journal.pone.0245782
34. Plaza-Martin M. Food allergies in paediatrics: current concepts. Pediatric. (2016) 85:50.e1–5. doi: 10.1016/j.anpede.2016.01.007
35. Plaza-Martín AM, Jiménez-Feijoo R, Andaluz C, Giner-Muñoz MT, Martin-Mateos MA, Piquer-Gibert M, et al. Polysensitization to aeroallergens and food in eosinophilic esophagitis in a pediatric population. Allergol Immunopathol. (2007) 35:35–7. doi: 10.1016/S0301-0546(07)70227-6
36. Muñoz-García E, Luengo-Sánchez O, Moreno-Pérez N, Cuesta-Herranz J, Pastor-Vargas C, Cardona Lettuce V. Allergy is a lipid transfer syndrome-related food allergy with a high risk of severe reactions. J Investig Allergol Clin Immunol. (2017) 27:98–103. doi: 10.18176/jiaci.0110
37. Ahmed M, Sood A, Gupta J. Toxoplasmosis in pregnancy. Eur J Obstet Gynecol Reprod Biol. (2020) 255:44–50. doi: 10.1016/j.ejogrb.2020.10.003
38. Karcz K, Lehman I, Królak-Olejnik B. Foods to avoid while breastfeeding? Experiences and opinions of polish mothers and healthcare providers. Nutrients. (2020) 12:1644. doi: 10.3390/nu12061644
39. Lee S, Choi Y, Jeong HS, Lee J, Sung J. Effect of different cooking methods on the content of vitamins and true retention in selected vegetables. Food Sci Biotechnol. (2018) 27:333–42. doi: 10.1007/s10068-017-0281-1
40. Mattheé V, Appledorf H. Effect of cooking on vegetable fiber. J Food Sci. (1978) 43:1344–5. doi: 10.1111/j.1365-2621.1978.tb15310.x
41. Pali-Schöll I, Renz H, Jensen-Jarolim E. Update on allergies in pregnancy, lactation, early childhood. J Allergy Clin Immunol. (2009) 123:1012–21. doi: 10.1016/j.jaci.2009.01.045
42. Chatzi L, Apostolaki G, Bibakis I, Skypala I, Bibaki-Liakou V, Tzanakis N, et al. Cullinan. Protective effect of fruits, vegetables and the Mediterranean diet on asthma and allergies among children in Crete. Thorax. (2007) 62:677–83. doi: 10.1136/thx.2006.069419
43. Vassilopoulou E, Konstantinou GN, Dimitriou A, Manios Y, Koumbi L, Papadopoulos GN. The impact of food histamine intake on asthma activity: a pilot study. Nutrients. (2020) 12:3402. doi: 10.3390/nu12113402
44. Cui J, Lian Y, Zhao C, Du H, Han Y, Gao W, et al. Dietary fibers from fruits and vegetables and their health benefits via modulation of gut microbiota. Compreh Rev Food Sci Food Safety. (2019) 18:1514–32. doi: 10.1111/1541-4337.12489
45. Koletzko B, Boey CC, Campoy C, Carlson SE, Chang N, Guillermo-Tuazon MA, et al. Current information and Asian perspectives on long-chain polyunsaturated fatty acids in pregnancy, lactation, and infancy: systematic review and practice recommendations from an early nutrition academy workshop. Ann Nutr Metab. (2014) 65:49–80. doi: 10.1159/000365767
46. Zhang G-Q, Liu B, Li J, Luo C-Q, Zhang Q, Chen J-L, et al. Fish intake during pregnancy or infancy and allergic outcomes in children: a systematic review and meta-analysis. Pediatr Allergy Immunol. (2017) 28:152–61. doi: 10.1111/pai.12648
47. Loo EXL, Ong L, Goh A, Chia AR, Teoh OH, Colega MT, et al. Effect of maternal dietary patterns during pregnancy on self-reported allergic diseases in the first 3 years of life: results from the GUSTO study. Int Arch Allergy Immunol. (2017) 173:105–13. doi: 10.1159/000475497
48. Garcia-Larsen V, Ierodiakonou D, Jarrold K, Cunha S, Chivinge J, Robinson Z, et al. Diet during pregnancy and infancy and risk of allergic or autoimmune disease: a systematic review and meta-analysis. PLoS Med. (2018) 15:e1002507. doi: 10.1371/journal.pmed.1002507
49. Nordgren TM, Lyden E, Anderson-Berry A, Hanson C. Omega-3 fatty acid intake of pregnant women and women of childbearing age in the United States: potential for Deficiency? Nutrients. (2017) 9:197. doi: 10.3390/nu9030197
50. Gunaratne AW, Makrides M, Collins TC. Maternal prenatal and/or postnatal n-3 long chain polyunsaturated fatty acids (LCPUFA) supplementation for preventing allergies in early childhood. Cochrane Database Syst Rev. (2015) 7:CD010085. doi: 10.1002/14651858.CD010085.pub2
51. Best KP, Gold M, Kennedy D, Martin J, Makrides M. Omega-3 long-chain PUFA intake during pregnancy and allergic disease outcomes in the offspring: a systematic review and meta-analysis of observational studies and randomized controlled trials. Am J Clin Nutr. (2016) 103:128–43. doi: 10.3945/ajcn.115.111104
52. Best KP, Sullivan T, Palmer D, Gold M, Kennedy DJ, Martin J, et al. Prenatal fish oil supplementation and allergy: 6-year follow-up of a randomized controlled trial. Pediatrics. (2016) 137:43. doi: 10.1542/peds.2015-4443
53. Devakumar D, Stocks J, Ayres JG, Kirkby J, Yadav SK, Saville NM, et al. Effects of antenatal multiple micronutrient supplementation on lung function in mid-childhood: follow-up of a double-blind randomised controlled trial in Nepal. Eur Respir J. (2015) 45:1566–75. doi: 10.1183/09031936.00188914
54. Checkley W, West KP, Wise RA, Baldwin MR, Wu L, LeClerq SC, et al. Maternal vitamin A supplementation and lung function in offspring. N Engl J Med. (2010) 362:1784–94. doi: 10.1056/NEJMoa0907441
55. Finkel J, Cira C, Mazzella L, Bartyzel J, Ramanna A, Strimel K, et al. Adequate vitamin D(3) supplementation during pregnancy: decreasing the prevalence of asthma and food allergies. Maternal Pediatr Nutr. (2015) 2:105. doi: 10.4172/2472-1182.1000105
56. Bountouvi E, Douros K, Papadopoulou A. Can getting enough vitamin D during pregnancy reduce the risk of getting asthma in childhood? Front Pediatr. (2017) 5:87. doi: 10.3389/fped.2017.00087
57. Silva CM, da Silva S, d Antunes MMC, d Silva GAP, Sarinho ESC, Brandt GK. Do infants with cow's milk protein allergy have inadequate levels of vitamin D? J Pediatr. (2017) 93:632–8. doi: 10.1016/j.jped.2017.01.006
58. Giannetti A, Bernardini L, Cangemi J, Gallucci M, Masetti R, Ricci G. Role of vitamin D in prevention of food allergy in infants. Front Pediatr. (2020) 8:447. doi: 10.3389/fped.2020.00447
59. Best CM, Xu J, Patchen BK, Cassano AP. Vitamin D supplementation in pregnant or breastfeeding women or young children for preventing asthma. Cochrane Database Syst Rev. (2019) 8:CD013396. doi: 10.1002/14651858.CD013396
60. Erdem SB, Nacaroglu HT, Karaman S, Erdur CB, Karkiner CU, Can D. Tolerance development in food protein-induced allergic proctocolitis: single centre experience. Allergol Immunopathol. (2017) 45:212–9. doi: 10.1016/j.aller.2016.10.005
61. Arik Yilmaz E, Soyer O, Cavkaytar O, Karaatmaca B, Buyuktiryaki B, Sahiner UM, et al. Characteristics of children with food protein-induced enterocolitis and allergic proctocolitis. Allergy Asthma Proc. (2017) 38:54–62. doi: 10.2500/aap.2017.38.4023
62. Antonogeorgos G, Priftis KN, Panagiotakos DB, Ellwood P, García-Marcos L, Liakou E, et al. Parental education and the association between fruit and vegetable consumption and asthma in adolescents: the Greek global asthma network (GAN) study. Children. (2021) 8:304. doi: 10.3390/children8040304
63. Nowak-Wegrzyn A, Katz Y, Mehr SS, Koletzko S. Non-IgE-mediated gastrointestinal food allergy. J Allergy Clin Immunol. (2015) 135:1114–24. doi: 10.1016/j.jaci.2015.03.025
64. Cetinkaya PG, Ocak M, Sahiner UM, Sekerel BE, Soyer O. Food protein-induced allergic proctocolitis may have distinct phenotypes. Ann Allergy Asthma Immunol. (2021) 126:75–82. doi: 10.1016/j.anai.2020.08.021
65. Kulig M, Luck W, Lau S, Niggemann B, Bergmann R, Klettke U, et al. Effect of pre- and postnatal tobacco smoke exposure on specific sensitization to food and inhalant allergens during the first 3 years of life Multicenter Allergy Study Group. Germany Allergy. (1999) 54:220–8. doi: 10.1034/j.1398-9995.1999.00753.x
66. Vassilopoulou E, Vardaka E, Efthymiou D, Pitsios C. Early life triggers for food allergy that in turn impacts dietary habits in childhood. Allergol Immunopathol. (2021) 49:146–52. doi: 10.15586/aei.v49i3.181
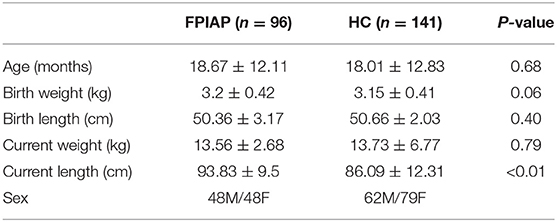
Table A. Characteristics of infants with food protein-induced allergic proctocolitis (FPIAP) and healthy control subjects (HC) included in the study.
Keywords: Mediterranean Diet, fish, food protein induced allergic proctocolitis, fruit, lactation, pregnancy, cooking methods, whole wheat
Citation: Vassilopoulou E, Feketea G, Konstantinou GN, Zekakos Xypolias D, Valianatou M, Petrodimopoulou M, Vourga V, Tasios I and Papadopoulos NG (2022) Food Protein-Induced Allergic Proctocolitis: The Effect of Maternal Diet During Pregnancy and Breastfeeding in a Mediterranean Population. Front. Nutr. 9:843437. doi: 10.3389/fnut.2022.843437
Received: 25 December 2021; Accepted: 11 February 2022;
Published: 30 March 2022.
Edited by:
Francisco José Pérez-Cano, University of Barcelona, SpainReviewed by:
Karine Adel-Patient, INRAE Centre Jouy-en-Josas, FranceCopyright © 2022 Vassilopoulou, Feketea, Konstantinou, Zekakos Xypolias, Valianatou, Petrodimopoulou, Vourga, Tasios and Papadopoulos. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gavriela Feketea, Z2FieWNocmlAb3RlbmV0Lmdy
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.