- 1Nutrition Department, Federal University of Rio Grande do Norte, Natal, Brazil
- 2Graduate Program in Social Sciences, Center for Human Sciences, Letters and Arts, Federal University of Rio Grande do Norte, Natal, Brazil
- 3Nutrition Department, School of Public Health, University of São Paulo, São Paulo, Brazil
The assessment of food biodiversity has gained importance in nutrition due to the positive association between the diversity of foods consumed and the quality of diets. To date, however, we do not know systematically how food consumption studies address food biodiversity. Our objective with this paper was to characterize how food consumption studies address biodiverse foods, both in terms of (i) new methods capable of overcoming the limitations of existing methods, and (ii) indicators capable of measuring the contribution of biodiversity to nutrition. We conducted a systematic review based on the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA), using four databases: Web of Science, Medline/PubMed (via National Library of Medicine), Scopus, and Google Scholar. We selected papers focused on the consumption of biodiverse foods without time constraints. In addition, we assessed the methodological quality of the studies we selected. We reviewed a total of 22 studies, and summarized the methods and indicators most used. We found that some researchers used biodiversity mapping strategies based on ethnographic approaches before the dietary assessment. Regarding dietary assessment tools, retrospective direct methods were the most used by researchers. We list 23 indicators used by the authors, among them the Dietary Species Richness (DSR), used in 18% of the studies. Studies that used biodiversity mapping strategies based on ethnographic approaches before the dietary assessment portrayed the local availability of biodiverse foods more consistently, i.e., presented lists with local edible species satisfactorily identified. We believe researchers in the future can avoid many of the limitations of current methods by ensuring that teams are interprofessional. We emphasize that most of the indicators we summarized are not sensitive enough to biodiversity since they do not measure edible resources at the species level. In this sense, the DSR is promising, because it fills information gaps, especially in the case of wild or neglected species.
Introduction
Biodiversity is the biological diversity of animals, plants, fungi, algae, and other organisms, including diversity within species, among species, and within ecosystems (1). The term biodiverse foods refers to the subset of these resources that are edible and available in a food system. This definition encompasses cultivars and varieties of conventional foods (e.g., types of beans, local chicken breeds), as well as species considered as non-conventional or of limited cultural use, also referred to as wild, native, neglected, and spontaneous species (2, 3).
In recent years, the assessment of food biodiversity consumption has gained importance in nutrition due to the positive association between the diversity of foods consumed and the quality of diet. For example, Lachat et al. (4) analyzed the contribution of diversity within food consumption of women and children (n = 6,226) in rural areas of seven low- and middle-income countries. They found a positive association between dietary species richness, or the count of the number of different species consumed per day, and the nutritional adequacy of diets. Besides, it is worth mentioning that the benefits related to food biodiversity are not limited to human health outcomes. Nowadays, we have the opportunity to address several global challenges related to nutrition by diversifying diets. Some of these challenges include meeting high per capita demand for nutrient-rich foods (5), fostering resilience to climate change and to the emergence of new zoonotic outbreaks, and promoting stability in food supply within the food system (6). One of the most comprehensive reviews to date examining the role of biodiversity in sustainable development highlights that biological diversity can directly contribute to (i) increased food and nutrition security levels due to a rise in local food consumption; (ii) reduced poverty because local consumption generates a source of income for local farmers; (iii) improved health and wellbeing due to higher access to more nutritious foods; and (iv) increased ability to mitigate climate change by considering as food those species adapted to local ecosystems (1). Therefore, biodiversity not only contributes to the nutritional quality of diets, but it also fosters planetary health.
Despite the strategic role of biodiversity to promote human and environmental health, food consumption studies still have a narrow approach to the topic due to two main limitations: lack of food composition data and lack of appropriate food consumption assessment tools for mapping biodiverse foods in current food systems (3). First, regarding composition data, we know that many food composition tables do not present satisfactorily the edible biodiversity of their countries of origin, which leads to misinterpretations in the assessment of diets, which consequently can lead to inefficient nutrition policies, such as supplementation or fortification programs (7). Furthermore, by analyzing food at the taxonomic level below species, we know that the nutrient content and bioactive compounds can vary significantly among different varieties or cultivars of the same species (8). Burlingame et al. (9) present valuable examples of how high this variation can be considering species of conventional plants, such as rice, potatoes, mangoes, and bananas. For example, considering the content of carotenoids, they observe that some bananas may have up to 8,500 times more beta carotene when compared to other varieties. Furthermore, recent studies have highlighted the nutritional relevance of wild or native species, showing that even though the contribution of these neglected species to energy content of diets seems to be insignificant, they are a relevant source of several micronutrients of global nutritional interest, such as iron, zinc, vitamin A, and folate (10). Second, regarding dietary assessment methods, we know that many research protocols have limitations in addressing food diversity due to inadequate cultural adaptation of the survey tools (3). The lack of cultural adaptation of dietary assessment tools can led to two major mistakes: first, to over- or underestimate energy, macro- and micronutrients, bioactive compounds, and anti-nutritional factors in dietary assessments; second, to ignore under- or over-reporting of some food resources in dietary inquiries (11). This weakness of dietary assessment tools compromises our capacity to perceive the presence of local varieties in diets and thereby limits our ability to analyze the nutritional relevance of the species and their varieties to food and nutrition security (9).
In recent years, however, the assessment of local biodiversity has started to play a part in food consumption studies, especially those with a focus on species considered native, indigenous, wild, and traditional (12–15). However, to date, we do not know systematically how these assessments address food biodiversity, both in terms of (i) new methods capable of overcoming the limitations of existing methods, and (ii) indicators capable of measuring the contribution of biodiversity to nutrition. Therefore, with this systematic review, we seek to answer the following question: “What are the methods and indicators used in the assessment of biodiverse foods in food consumption studies?”
Methods
We conducted a systematic review based on the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) Statement (16)—Supplementary File 1. Our protocol for this review was not previously registered because our research does not analyze directly any health-related outcomes.
Selection Criteria
We selected articles following these eligibility criteria: (i) original articles, published in English, Spanish, or Portuguese; (ii) papers focused on the assessment of food consumption of human populations; (iii) research presenting outcomes related to the consumption of biodiverse foods; and, finally, (iv) our investigation considered papers without time constraints. We excluded (i) repeated articles and (ii) review products.
Search Sources and Strategy
During March 2021, MFAM performed the search using four databases: Web of Science, Medline/PubMed (via National Library of Medicine), Scopus, and Google Scholar. The search consisted of applying the descriptors in each database. The entire search strategy is available in Supplementary File 2.
Study Selection
With the assistance of the tool Mendeley, MFAM organized all records and deleted duplicates. By applying the eligibility criteria previously outlined, two authors (MM and SS) selected the articles individually. Initially, titles and abstracts underwent a first screening, at which point we excluded those that did not meet the selection criteria. In cases of discrepancies or uncertainties about inclusion, we consulted a third author (MJ). Then, we proceeded to a full reading of potentially eligible texts.
Data Extraction
Three authors (MFAM, SGBS, and CDT) extracted data from the selected articles into a spreadsheet designed to assist us in answering the research question. Next, MCMJ verified the accuracy and scope. We gathered the following information: (i) article data (authors, year of publication, and journal), (ii) research setting and number of participants, (iii) research design, (iv) objective, (v) biodiverse foods assessed (e.g., wild plants, cultivated plants, bushmeat, mushrooms), (vi) methods applied to assess biodiversity, (vii) indicators, (viii) main results, (ix) main limitations reported, and (x) quality.
Quality Analysis
We evaluated the methodological quality of the studies by adapting a consolidated protocol to the objectives of our study (17). The consolidated protocol chosen, considering the design of the studies we reviewed, was the STROBE (Strengthening the Reporting of Observational Studies in Epidemiology Statement). STROBE consists of a checklist of 22 essential items applied to observational epidemiological studies. Considering the specificity of our analysis, and the absence of more specific protocols, we added three new items to the checklist in order to evaluate (i) whether the research team mapped foods species previously to the dietary assessment, (ii) if the authors report having checked the taxonomy of species, and (iii) whether the paper specifies environmental conditions when characterizing the setting (e.g., climate, soil, etc.) due to the importance of this information to biodiversity analysis. The adapted instrument contains 25 items.
After analyzing all the items, the studies received a point for each criterion fulfilled. Based on the grades received, we used three categories for quality assessment: strong—when the study met more than 80% of the criteria; moderate—from 50 to 80%; weak— <50% (18).
Summary of Results
We synthesized results by producing narrative summaries of each of the articles eligible for a full reading. During the reading process, we focused on detecting (i) methods used in the assessment of biodiverse foods and (ii) indicators employed to measure the contribution of biodiversity to nutrition reported in the manuscripts.
To present our results more clearly, we grouped the methods and indicators in the following ways. We grouped the methods into those applied (i) to map then classify known and consumed species and (ii) to assess food consumption. As for the indicators, we grouped them into four levels of analysis ranging from the landscape to the nutrient level: (i) production, collection, or supply: indicators that measure the number of species and varieties found within a given area, sample, farm, or local market; (ii) food consumption by population or household: indicators that refer to the number of food groups or food items consumed in a given population or household; (iii) food consumption by individuals: indicators that refer to the number of food groups or food items consumed by a person; and, finally, at the most specific level, (iv) dietary intake by individuals: indicators that measure individual food consumption at the nutrient level.
Results
Study Selection
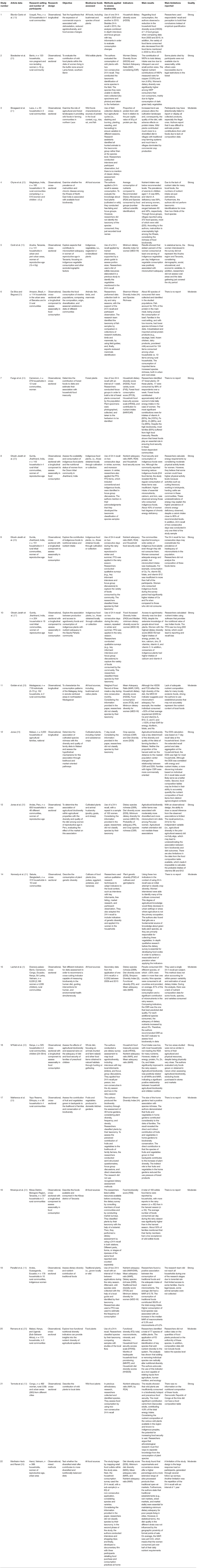
The search in the databases led to the recovery of 892 studies (138 in the Web of Science, 112 in Medline/PubMed, 401 in Scopus, and 241 in Google Scholar). After excluding 119 duplicates, we considered 773 articles as eligible for the next stage of selection. Based on titles and abstracts, we selected 91 papers for a full reading. At this stage, we excluded 69 articles that did not fit our inclusion criteria. Therefore, a total of 22 articles make up this review. Figure 1 shows the study selection process and the related flowchart.
Study Characteristics and Quality
Table 1 provides an overview of the main characteristics of the 22 studies included in this review.
The earliest study included in this review dates back to 2005 (31). Of the 22 papers, 20 were published in the last 10 years, with 73% from 2016 onwards. All studies have an observational design: 18 cross-sectional (six have a longitudinal component to assess seasonality in food consumption), three longitudinal, and one mixed methods (ethnographic and food consumption approaches).
We found the quality of studies to be either moderate or strong. Characteristics that most contributed to a moderate rating were: not mapping edible species prior to dietary assessment; not reporting taxonomic classifications of species; lack of precision in reporting numbers of participants in each stage of the study; and lack of explanation concerning losses of participants at each stage. We did not classify any study as weak.
Geographical Coverage
Researchers conducted the studies with human participants on three continents: Africa, Asia, and South America (see Table 1). We observed that all studies prioritized areas marked by (i) nutritional deficiencies at the population level and (ii) consumption of foods hunted and gathered in the context of local food systems. The samples ranged from n = 33 households (21) to n = 6,226 individuals (4).
Considering rural-urban classification, 73% of the studies reported that data collection was conducted in rural areas exclusively. Of the remaining 27%, there were 9% in urban and rural areas, 9% in urban and peri-urban areas, and others that did not report geographic coverage considering urbanization levels.
Biodiversity Assessment in Food Consumption Studies
Authors analyzed a variety of biodiverse foods. Some categories mentioned overlap, such as wild foods and wild plants. Some may express the same type of food, but with different terminologies, such as traditional plants, indigenous vegetables, and indigenous food plants. Regardless, in the following synthesis, we preserved the terms used by the authors. The biodiverse foods assessed were: all food sources; whole diet, with a focus on animals (hunted and farm-raised) and other food items obtained in natural habitats through hunting or gathering; animals; food plants; forest plants; cultivated plants; traditional plants; wild plants; indigenous vegetables; indigenous food plants; wild foods; and finally, fruits and vegetables produced in home gardens.
In general, studies showed that dietary diversity is positively associated with the consumption of wild plants (20, 21, 34). Studies have also shown that the greater the dietary diversity, the greater the adequacy of micronutrients consumed by a given human group (4, 14, 15, 25–27). Agricultural biodiversity within farms and home gardens was also associated with the nutritional quality of diets (29, 30, 32, 35) and with the livelihood potential of families (33). However, despite the richness of local food biodiversity, nutrient intake levels in several cases in rural areas were below recommendations, and food insecurity was also recurrent (12, 22, 24, 28). Some authors suggest that the socio-economic context (e.g., poverty, lack of education) undermines the capacity of some communities to recognize and exploit natural resources properly (12). Concerning urban areas, some authors argue that traditional establishments (e.g., open-air markets, street food markets) are essential to maintaining diversity and, consequently, minimal dietary adequacy (36).
Methods Used in the Assessment of Biodiverse Foods in Food Consumption Studies
Table 2 summarizes the main methods used in food consumption studies to address food biodiversity.
As Table 2 demonstrates, some researchers mapped local biodiversity before conducting dietary survey fieldwork, by using various ethnographic approaches and by collecting samples of local foods to identify with the help of herbariums and animal collections at universities and research institutes. During the dietary survey in the field, researchers used the following methods to identify species: collecting samples of local foods to identify a posteriori; photographing samples; keeping diaries with lists of local edible species; running focus group discussions; conducting interviews with key informants among community members and with agricultural technicians; performing market research; counting with the support of a taxonomist; and using a field guide. After fieldwork, consulting literature, collections, and lists of plants commonly available in the region was the only method applied.
The technique most used to map local food biodiversity, in 37% of the studies, was the collection of samples before or during the dietary survey fieldwork to identify resources a posteriori. Tied in second place were pre-field qualitative research and post-field consultation (literature, collections, and lists), present in 27% of the studies. Next, focus group discussion and interviews with key local informants, both during the dietary survey stage, were present in 23% of the studies.
Concerning taxonomic classification, 45% of the studies did not mention having addressed it and did not justify its absence. Among them, one used secondary data previously classified (4), and another highlighted the lack of taxonomic classification as a weakness of the investigation (22).
Retrospective direct methods were the most used by researchers. For example, the 24-h recall appeared in 82% of the studies, followed by the Food Frequency Questionnaire (FFQ), present in 23% of the studies (see Table 2). Among prospective direct methods, one study used weighed food records (28).
Still concerning methods, we identified weaknesses before, during, and after fieldwork. In the pre-fieldwork, the absence of taxonomic classification was the major limitation, but it was not reported by the majority of studies. During fieldwork, we identified several crucial limitations. Broegaard et al. (21) noted that, with direct observation, participants would intentionally fail to report all food products consumed due to legal constraints in protected areas. Furthermore, the authors recognized that the lack of repeated dietary assessments (e.g., 24-h recall and FFQ) hampered the analysis of within-person variability and estimation of usual dietary intake (4). After the dietary survey, the most frequent limitation was the lack of nutritional data for particular species in food composition tables (4, 20–22, 26, 28, 30, 35). Faced with this gap, Ghosh-Jerath et al. (25) ran food composition analyses of species that did not have data available. Due to budget constraints, other researchers analyzed diets by considering data of similar species (i.e., food matching) (35).
Indicators Employed to Measure the Contribution of Biodiversity to Nutrition
In Table 3, we summarize the indicators that enabled the assessment of food biodiversity in the reviewed studies. In total, authors mentioned 23 indicators.
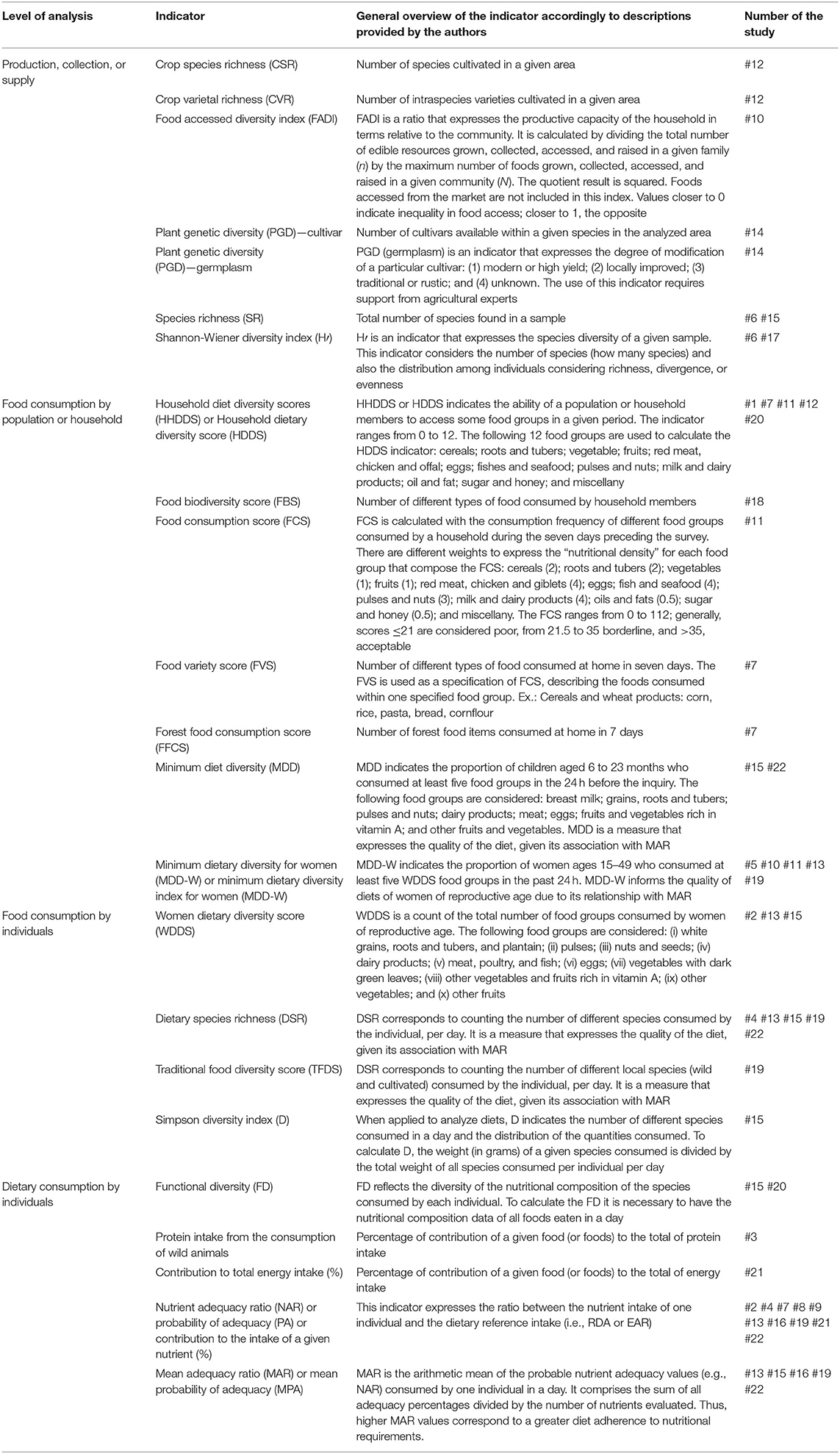
Table 3. Summary of indicators employed to measure the contribution of biodiversity to nutrition in food consumption studies.
At the first level of analysis, “Production, collection, or supply,” Species Richness (SR) and Shannon-Wiener Diversity Index (H′) were the indicators most used, comprising 9% of the studies. Regarding the second level, “Food Consumption by Population or Household,” we found that Household Diet Diversity Scores (HHDDS), also known as Household Dietary Diversity Score (HDDS), and Minimum Dietary Diversity for Women (MDD-W) were the most frequently used, one or both of which appeared in 22% of the studies. At the third level, “Food Consumption by Individuals,” Dietary Species Richness (DSR) was used in 18% of the studies. Finally, at the fourth level, “Dietary Consumption by Individuals,” Nutrient Adequacy Ratio (NAR) appeared in 45% of the studies, being the indicator most used within this category and among all indicators.
Even if we have described in Table 1 food security indicators, we do not present them in Table 3 or discuss them because they do not directly address food consumption of edible resources as a group or as species.
Discussion
Our main objective with this review was to identify methods and indicators most used in food consumption studies to date. Based on our analysis, we highlight the following aspects.
We found that some researchers used biodiversity mapping strategies based on ethnographic approaches before the dietary assessment (12, 25–27). The studies developed by these researchers portrayed the local availability of biodiverse foods more consistently, i.e., presenting lists with local edible species satisfactorily identified. A rapid ethnonutrition assessment before the dietary survey provides data about cultural variables that interfere when elaborating lists of local foods, e.g., food classifications, food processing, food perception, seasonality (11). During this pre-assessment stage, several data gathering techniques allow the researcher to build a broader perspective of the local diet, considering food at the system level. Some of the methods used in the studies we reviewed were: interviews, focus group discussions, local market surveys, and free listing. The guidelines to assess biodiverse foods in dietary surveys indicate these methods as pathways to map the edible resources and related cultural uses by a given population (3).
Taxonomic identification of resources consumed locally is a critical step to build robust dietary surveys. The scientific identification of species will allow us to know precisely to which species people refer when using a given vernacular name. This step is crucial since popular names vary enormously among and even within different communities. For example, we know that traditional human populations from the Brazilian Caatinga use the popular name bredo for three edible species of three different botanical genera: Portulaca oleracea L., Amaranthus viridis L., and Talinum fruticosum (L.) Juss. Similarly, we can have different popular names to refer to the same species, as is the case of cumaru and amburana-açu, both referring to Amburana cearensis (Allemão) A.C.Sm (37). In the study developed by Chyne et al. (22), during data gathering, the researchers noticed a vegetable called jalynniar, with consumption reported in 15 different villages, consumed in ~100 g portions. As the researchers did not perform scientific identification of this plant, they also did not have the means to evaluate its nutritional properties. Omissions of this type can lead to results that do not express the accurate dietary profile, leading to wrong conclusions about the role of local biodiversity to foster good nutrition. We think that a possible explanation for this kind of weakness is the academic background of the research team. For example, research teams without specialists in biological and environmental sciences failed more frequently to provide taxonomic identification. We came to this conclusion after verifying the academic background of the authors of all selected studies. We identified that the absence of taxonomic identification was more common among manuscripts produced by disciplinary teams from social sciences and health sciences. On the other hand, a group exclusively composed of biological and environmental sciences scholars failed to choose a proper dietary assessment tool to gather consumption data (33). Multidisciplinary teams that included professionals from nutrition, natural sciences, and social sciences designed more robust approaches, choosing adequate methods and indicators to assess food biodiversity within diets.
A practical and efficient approach to address species identification in food consumption studies is to produce, before the dietary survey, a photographic guide containing all foods of interest to the assessment (3). None of the studies that we analyzed reported having used photo guides for this purpose. In two papers (12, 15), authors used photo guides to assess portion sizes and kitchen utensils, which are essential tools in well-designed food consumption studies, but they do not assess biodiversity information. The practice of using photo guides containing biodiverse foods is still incipient, and their absence can compromise the accuracy of the analyses due to poor species identification. Jacob et al. (37) recently developed a photo guide to assess biodiverse foods plants in the Brazilian Caatinga biome. The development of this guide involved six steps comprising (i) elaboration of the list of species, (ii) selection and production of pictures, (iii) assessment of the first version of the guide by local experts, (iv) adjustment of content and design, (v) assessment of the guide by botanists, and (vi) processing of the final version. In this paper, the authors also present the REA, which stands for Rapid Ethnonutrition Assessment method. This method allows prototyping dietary assessments with high efficiency, considering time and budget constraints. The article published by the authors explains REA step-by-step, giving clear examples of how this method benefits research teams by amplifying the perception and control of cultural variables that interfere with food consumption.
Another challenge to overcome when mapping biodiverse foods is related to wild animals. Some authors described difficulties with underreporting in dietary inquiries due to legal restrictions regarding the consumption of these animals (14, 20). In these cases, when food consumption conflicts with legal rules and ethical limits, building a good rapport and trust are research practices that allow the interviewees to feel more inclined to report consumption (38–40). Another challenge reported by the authors of the manuscripts we analyzed was obtaining samples of wild animals to develop composition analyses. Dialogue with local authorities can be a strategy for accessing samples since dead animals sometimes are apprehended and discarded by these authorities.
Seasonality is another topic to consider when mapping foods available or when conducting dietary surveys. Reproduction cycles of animals, plants, algae, and fungi vary in different seasons. This factor is especially relevant in traditional food systems in rural areas, where diets tend to change with the season (41). We can approach seasonality by mapping biodiversity in different seasons, by running systematic reviews, and by consulting official data capable of informing edible biodiversity. Furthermore, capturing seasonal variation by dietary surveys is essential to estimate usual dietary intake (42). We can approach this task by conducting a repeated 24-h recall, by applying food record methods, and by using FFQ.
Even after overcoming barriers in the data gathering stage, composition data of biodiverse foods are frequently insufficient to perform a dietary analysis. Data in food composition tables often are limited because they include analysis of food available in markets and do not consider local crop varieties that vary in their nutrition content due to individual characteristics, climate, soil, etc. For instance, a recent study analyzing the nutrition content of millets showed that some varieties could have three times more iron than others (43). Therefore, using these varieties could be strategic to reduce iron deficiency anemia with low-cost potential. This example is beneficial to illustrate that the lack of local foods in food composition tables can lead to over- or underestimating energy, macro- and micronutrients, bioactive compounds, and anti-nutritional factors in dietary assessments. The problem of analysis gets even more serious due to the fact that some countries do not even have their own food composition tables, e.g., the Democratic Republic of Congo (12). Other countries neglect to include in their composition tables species used by traditional communities. For example, 80% of the wild species consumed by people in the Brazilian Caatinga are absent from food composition tables of Brazil (18). The lack of composition data sometimes makes the effort to gather consumption details fruitless since researchers will not have the means to interpret these data. When composition tables do not provide the data, and there is no means to analyze the food directly, Food Matching (FM) is the strategy recommended by the Food and Agriculture Organization of the United Nations (44). In FM, the researcher chooses the best substitute for the missing data in composition tables from other countries, scientific articles, theses, gray literature, and food labels. When the data is not available from any of these sources, the recommendation is to choose three similar food items present in composition tables and calculate the average of nutrients. This strategy has severe limitations, such as over- or underestimating nutrients and bioactive compounds content. However, it is an analytical solution in the complete absence of data.
Concerning the geographical coverage of assessments, we highlight that food consumption studies conducted in urban contexts present particular challenges to the approach of biodiversity. Some of these challenges are the higher degree of complexity of urban and peri-urban food systems and the higher contributions of processed foods to diets in these settings (4). It is urgent to find ways to overcome these limitations in order to better analyze the role of biodiversity in diets in these contexts since these settings concentrate several challenges, and several opportunities, in the implementation of the agenda of the sustainable development goal. So far, considering the available evidence, some authors show that consumption of diversified foods is more significant in urban areas than in rural and peri-urban areas due to a better supply and access to food (15, 23). However, we need to evaluate this information carefully. In some cases, a large number of species does not necessarily reflect a higher quality of diets. For example, the frequency of consumption of ultra-processed and industrialized foods in urban areas when compared to rural areas tends to be high (23). Conti et al. (15) also highlight that households in peri-urban regions tend to have more land available to establish home gardens when compared to those in urban areas, which can increase the consumption of in natura and minimally processed food by those households. Considering food selling points, Wertheim-Heck and Raneri (36), who conducted research in two low-income urban districts in Hanoi, Vietnam, reported that one of the biggest challenges in urban contexts is understanding the impact of types of food retailers on dietary diversity. They formulated a research experiment to assess this problem. To measure the consumption of biodiverse foods, they tried to use the Dietary Species Richness (DSR) indicator, but they could not apply it properly due to issues in the taxonomic identification of species. The complexities of biodiverse food consumption in urban and peri-urban contexts need to be better framed by researchers in order to guide policy-making that affects people living in these settings.
We believe that the DSR is the most promising indicator to evaluate the impact of biodiversity in diets because it provides a proxy to assess simultaneously diets and biodiversity conservation. Lachat et al. (4) highlight that, given the conflicts in reconciling environmental and nutrition policies, the DSR is a valuable tool that integrates biodiversity, food, and health. In addition, DSR provides more specific details at the species level than classic indicators that focus on food groups. This characteristic allows a more accurate analysis at the nutrient level. We also highlight the value of using agrobiodiversity indicators in food consumption studies because they can inform pathways to promote sustainable diets at the food systems level. We can use these indicators to test whether agricultural biodiversity is protective of nutrition. For example, using the Crop Species Richness (CSR) indicator, Jones (29) demonstrated a relationship between crop production and dietary diversity. Finally, to consider the genetic diversity of edible resources below the species level and its relationship with nutritional profile, the Plant Genetic Diversity (PGD) indicator provides a reference useful for understanding, protecting, and promoting genetic diversity (31).
This study has one limitation: the lack of specific protocols for assessing the overall quality of the studies. To address this limitation, we adapted a consolidated protocol.
Conclusion
Our study summarizes methods and indicators that researchers use to address biodiversity in food consumption studies. We believe researchers in the future can avoid many of the limitations of current methods by ensuring that teams are interprofessional. In our opinion, one professional trained in food consumption studies, a person with a background in ethnographic methods, and a taxonomist are fundamental actors on a team. We emphasize that most of the indicators we summarized are not sensitive enough to biodiversity since they do not measure edible resources at the species level. This limitation creates information gaps, especially in the case of wild or neglected species, which hampers our ability to estimate the actual contribution of these foods within diets. In this sense, the DSR is promising, even if applying this indicator can be a real challenge due to the necessity to prospect the species considering their taxonomic classification. Finally, to correctly evaluate the role of biodiverse foods in diets, we need good composition data for these species or the resources to conduct composition analyses. Securing resources is a challenge due to the limited funding available. Many biodiversity hotspots are underdeveloped countries with limited resources to fund science and lack of scientific experts who are well-trained to conduct the well-designed studies systematically. By considering the strategic role of food biodiversity in transforming global food systems, especially in light of the environmental crisis we are currently facing worldwide, we suggest that international research agencies could apply more resources to scholars in the Global South working within the food biodiversity agenda.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Author Contributions
MM, SS, and MJ analyzed and interpreted the data and were major contributors in writing the manuscript and responsible for all components of the research and manuscript. CT assisted in analyses, data interpretation, and in writing the first drafting and final version of the paper. SL and DM had substantially revised the first drafting and final version of the paper. All authors have made important contributions in the research conception and by reading and approving the final manuscript.
Funding
This study was funded by the Federal University of Rio Grande do Norte through a Scientific Initiation research scholarship to MM (UFRN call 01/2019). This funding source had no role in the design of this study nor in its execution, interpretation of data, analyses, or decision to submit results. The Brazilian Coordenação de Aperfeiçoamento de Pessoal de Nível Superior financed the fee to publish this article (Finance Code 001).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2022.832288/full#supplementary-material
References
1. Blicharska M, Smithers RJ, Mikusiński G, Rönnbäck P, Harrison PA, Nilsson M, et al. Biodiversity's contributions to sustainable development. Nat Sustain. (2019) 2:1083–93. doi: 10.1038/s41893-019-0417-9
3. FAO. Guidelines on Assessing Biodiverse Foods in Dietary Intake Surveys. Rome: Food and Agriculture Organization of the United Nations (2017).
4. Lachat C, Raneri JE, Smith KW, Kolsteren P, Damme PV, Verzelen K, et al. Dietary species richness as a measure of food biodiversity and nutritional quality of diets. Proc Natl Acad Sci USA. (2018) 115:127–32. doi: 10.1073/pnas.1709194115
5. Spiker ML, Knoblock-Hahn A, Brown K, Giddens J, Hege AS, Sauer K, et al. Cultivating sustainable, resilient, and healthy food and water systems: a nutrition-focused framework for action. J Acad Nutr Diet. (2020) 120:1057–67. doi: 10.1016/j.jand.2020.02.018
6. Jacob M, Chaves V, Rocha C. Biodiversity towards sustainable food systems: four arguments. In: Jacob M, Albuquerque U, editors. Local Food Plants of Brazil. Cham: Springer (2021). doi: 10.1007/978-3-030-69139-4_1
7. Michaelsen KF, Neufeld LM, Prentice AM editors. Global Landscape of Nutrition Challenges in Infants Children. Nestlé Nutrition Institution Workshop Series. Basel: Nestlé Nutrition Institute, Switzerland (2020). p. 39–50. doi: 10.1159/isbn.978-3-318-06649-4
8. Toledo A, Burlingame B. Biodiversity and nutrition: a common path toward global food security and sustainable development. J Food Compos Anal. (2006) 19:477–83. doi: 10.1016/j.jfca.2006.05.001
9. Burlingame B, Charrondiere UR, Mouille B. Food composition is fundamental to the cross-cutting initiative on biodiversity for food and nutrition. J Food Compos Anal. (2009) 22:361–5. doi: 10.1016/j.jfca.2009.05.003
10. Powell B, Ickowitz A, Mcmullin S, Jamnadass R, Padoch C, Pinedo-Vasquez, et al. The role of forests, trees and wild biodiversity for nutrition-sensitive food systems and landscapes. In: Expert Background Paper for the International Conference on Nutrition. Rome: FAO (2013)
11. Jacob MCM, Feitosa IS, de Araujo JYM, Batista NA do N, Silva TLL da, Motta VW de L, et al. Rapid ethnonutrition assessment method is useful to prototype dietary assessments with a focus on local biodiverse food plants. Ecol Food Nutr. (2020) 60:334–50. doi: 10.1080/03670244.2020.1852227
12. Termote C, Bwama Meyi M, Dhed'a Djailo B, Huybregts L, Lachat C, Kolsteren P, et al. A biodiverse rich environment does not contribute to a better diet: a case study from DR Congo. PLoS ONE. (2012) 7:e30533. doi: 10.1371/journal.pone.0030533
13. Powell B, Maundu P, Kuhnlein HV, Johns T. Wild foods from farm and forest in the east Usambara Mountains, Tanzania. Ecol Food Nutr. (2013) 52:451–78. doi: 10.1080/03670244.2013.768122
14. Penafiel D, Cevallos-Valdiviezo H, Espinel R, Van Damme P. Local traditional foods contribute to diversity and species richness of rural women's diet in Ecuador. Public Health Nutr. (2019) 22:2962–71. doi: 10.1017/S136898001900226X
15. Conti MV, De Giuseppe R, Monti MC, Mkindi AG, Mshanga NH, Ceppi S, et al. Indigenous vegetables: a sustainable approach to improve micronutrient adequacy in women of childbearing age. Eur J Clin Nutr. (2021) 75:1475–82. doi: 10.1038/s41430-021-00865-x
16. Moher D, Liberati A, Tetzlaff J, Altman DG, The The PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. (2009) 6:e1000097. doi: 10.1371/journal.pmed.1000097
17. Von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JB. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Int J Surg. (2014) 12:1495–9. doi: 10.1016/j.ijsu.2014.07.013
18. Jacob MCM, Medeiros MFA, Albuquerque UP. Biodiverse food plants in the semiarid region of Brazil have unknown potential: a systematic review. PLoS ONE. (2020) 15:e0230936. doi: 10.1371/journal.pone.0230936
19. Blundo-Canto G, Cruz-Garcia GS, Talsma EF, Francesconi W, Labarta R, Sanchez-Choy J, et al. Changes in food access by mestizo communities associated with deforestation and agrobiodiversity loss in Ucayali, Peruvian Amazon. Food Sec. (2020) 12:637–58. doi: 10.1007/s12571-020-01022-1
20. Boedecker J, Termote C, Assogbadjo AE, Van Damme P, Lachat C. Dietary contribution of Wild Edible Plants to women's diets in the buffer zone around the Lama forest, Benin – an underutilized potential. Food Secur. (2014) 6:833–49. doi: 10.1007/s12571-014-0396-7
21. Broegaard RB, Rasmussen LV, Dawson N, Mertz O, Vongvisouk T, Grogan K. Wild food collection and nutrition under commercial agriculture expansion in agriculture-forest landscapes. Forest Policy Econ. (2017) 84:92–101. doi: 10.1016/j.forpol.2016.12.012
22. Chyne DAL, Meshram II, Rajendran A, Kodali V, Getti N, Roy P, et al. Nutritional status, food insecurity, and biodiversity among the Khasi in Meghalaya, North-East India. Matern Child Nutr. (2017) 13:e12557. doi: 10.1111/mcn.12557
23. Da Silva AL, Begossi A. Biodiversity, food consumption and ecological niche dimension: a study case of the riverine populations from the Rio Negro, Amazonia, Brazil. Environ Dev Sustain. (2009) 11:489–507. doi: 10.1007/s10668-007-9126-z
24. Fungo R, Muyonga J, Kabahenda M, Kaaya A, Okia CA, Donn P, et al. Contribution of forest foods to dietary intake and their association with household food insecurity: a cross-sectional study in women from rural Cameroon. Public Health Nutr. (2016) 19:3185–96. doi: 10.1017/S1368980016001324
25. Ghosh-Jerath S, Singh A, Lyngdoh T, Magsumbol MS, Kamboj P, Goldberg G. Estimates of indigenous food consumption and their contribution to nutrient intake in Oraon Tribal Women of Jharkhand, India. Food Nutr Bull. (2018) 39:581–94. doi: 10.1177/0379572118805652
26. Ghosh-Jerath S, Singh A, Magsumbol MS, Lyngdoh T, Kamboj P, Goldberg G. Contribution of indigenous foods towards nutrient intakes and nutritional status of women in the Santhal tribal community of Jharkhand, India. Public Health Nutr. Aug. (2016) 19:2256–67. doi: 10.1017/S1368980016000318
27. Ghosh-Jerath S, Kapoor R, Singh A, Nilima, Downs S, Goldberg G, et al. Agroforestry diversity, indigenous food consumption and nutritional outcomes in Sauria Paharia tribal women of Jharkhand, India. Matern Child Nutr. (2021) 17:e13052. doi: 10.1111/mcn.13052
28. Golden CD, Vaitla B, Ravaoliny L, Vonona MA, Anjaranirina EG, Randriamady HJ, et al. Seasonal trends of nutrient intake in rainforest communities of north-eastern Madagascar. Public Health Nutr. (2019) 22:2200–9. doi: 10.1017/S1368980019001083
29. Jones AD. On-Farm crop species richness is associated with household diet diversity and quality in subsistence- and market-oriented farming households in Malawi. J Nutr. (2017) 147:86–96. doi: 10.3945/jn.116.235879
30. Jones AD, Creed-Kanashiro H, Zimmerer KS, de Haan S, Carrasco M, Meza K, et al. Farm-Level agricultural biodiversity in the Peruvian Andes is associated with greater odds of women achieving a minimally diverse and micronutrient adequate diet. J Nutr. (2018) 148:1625–37. doi: 10.1093/jn/nxy166
31. Kennedy G, Islam O, Eyzaguirre P, Kennedy S. Field testing of plant genetic diversity indicators for nutrition surveys: rice-based diet of rural Bangladesh as a model. J Food Compos Anal. (2005) 18:255–68. doi: 10.1016/j.jfca.2004.10.002
32. M'Kaibi FK, Steyn NP, Ochola S, Du Plessis L. Effects of agricultural biodiversity and seasonal rain on dietary adequacy and household food security in rural areas of Kenya. BMC Public Health. (2015) 15:422. doi: 10.1186/s12889-015-1755-9
33. Mathewos M, Hundera K, Biber-Freudenberger L. Planting fruits and vegetables in homegarden as a way to improve livelihoods and conserve plant biodiversity. Agriculture. (2018) 8:1–17. doi: 10.3390/agriculture8120190
34. Ntwenya JE, Kinabo J, Msuya J, Mamiro P, Mamiro D, Njoghomi E, et al. Rich food biodiversity amid low consumption of food items in Kilosa district, Tanzania. Food Nutr Bull. (2017) 38:501–11. doi: 10.1177/0379572117708647
35. Remans R, Flynn DFB, DeClerck F, Diru W, Fanzo J, Gaynor K, et al. Assessing nutritional diversity of cropping systems in African villages. PLoS ONE. (2011) 6:e21235. doi: 10.1371/journal.pone.0021235
36. Wertheim-Heck SCO, Raneri JE. A cross-disciplinary mixed-method approach to understand how food retail environment transformations influence food choice and intake among the urban poor: experiences from Vietnam. Appetite. (2019) 142:104370. doi: 10.1016/j.appet.2019.104370
37. Jacob M, Araújo N, Benedito A, Borges M, Jorge TP, Albuquerque U. Guia Fotográfico de Alimentos Biodiversos Consumidos na Caatinga. Manaus: Ed Polimatia (2020)
38. Albuquerque UP, Cruz da Cunha LVF, Lucena RFP, Alves RRN. Methods and Techniques in Ethnobiology and Ethnoecology. Cham: Humana Press (2014). doi: 10.1007/978-1-4614-8636-7
39. Senko J, Nichols WJ, Ross JP, Willcox AS. To eat or not to eat an endangered species: views of local residents and physicians on the safety of sea turtle consumption in northwestern Mexico. EcoHealth. (2009) 6:584–95. doi: 10.1007/s10393-010-0280-7
40. Chaves LS, Alves RRN, Albuquerque UP. Hunters' preferences and perceptions as hunting predictors in a semiarid ecosystem. Sci Total Environ. (2020) 726:138494. doi: 10.1016/j.scitotenv.2020.138494
42. FAO. Dietary Assessment: A Resource Guide to Method Selection and Application in Low Resource Settings. Rome: Food and Agriculture Organization of the United Nations (2018).
43. Anitha S, Kane-Potaka J, Botha R, Givens DI, Sulaiman NLB, Upadhyay S, et al. Millets can have a major impact on improving iron status, hemoglobin level, and in reducing iron deficiency anemia–a systematic review and meta-analysis. Front Nutr. (2020) 8:725529. doi: 10.3389/fnut.2021.725529
Keywords: food biodiversity, food consumption, food security, sustainable development goals, ethnonutrition
Citation: Medeiros MFAd, Silva SGB, Teixeira CD, Lima SCVC, Marchioni DM and Jacob MCM (2022) Assessment of Biodiversity in Food Consumption Studies: A Systematic Review. Front. Nutr. 9:832288. doi: 10.3389/fnut.2022.832288
Received: 09 December 2021; Accepted: 10 May 2022;
Published: 14 June 2022.
Edited by:
Hattie Wright, University of the Sunshine Coast, AustraliaReviewed by:
Meghit Boumediene Khaled, University of Sidi-Bel-Abbès, AlgeriaSeetha Anitha, International Crops Research Institute for the Semi-Arid Tropics (ICRISAT), India
Copyright © 2022 Medeiros, Silva, Teixeira, Lima, Marchioni and Jacob. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Michelle Cristine Medeiros Jacob, bWljaGVsbGUuamFjb2JAdWZybi5icg==
†ORCID: Maria Fernanda Araújo de Medeiros orcid.org/0000-0001-7674-9307
Stephanie Gomes Bezerra Silva orcid.org/0000-0003-2163-1146
Carla Djaine Teixeira orcid.org/0000-0002-3138-1323
Severina Carla Vieira Cunha Lima orcid.org/0000-0001-8268-1986
Dirce Maria Marchioni orcid.org/0000-0002-6810-5779
Michelle Cristine Medeiros Jacob orcid.org/0000-0002-4881-7285
 Maria Fernanda Araújo de Medeiros
Maria Fernanda Araújo de Medeiros Stephanie Gomes Bezerra Silva1†
Stephanie Gomes Bezerra Silva1† Carla Djaine Teixeira
Carla Djaine Teixeira Dirce Maria Marchioni
Dirce Maria Marchioni Michelle Cristine Medeiros Jacob
Michelle Cristine Medeiros Jacob