- 1Department of Soil, Plant and Food Sciences, University of Bari Aldo Moro, Bari, Italy
- 2Clinica Medica “A. Murri”, Department of Biomedical Sciences and Human Oncology, University of Bari Medical School, Bari, Italy
There is a growing need for gluten-free bakery products with an improved nutritional profile. Currently, gluten-free baked goods deliver low protein, fiber, and mineral content and elevated predicted glycaemic index (pGI). Olive cake (OC), a by-product from virgin olive oil extraction, is an excellent natural source of unsaturated fatty acids, dietary fiber and bioactive molecules, including polyphenols and tocopherols. In this framework, this study aimed at using two selected lactic acid bacteria and a yeast for increasing the antioxidant features and the phenol profile of the gluten-free breadsticks fortified with OC with the perspective of producing a functional food. Control (CTR) samples were prepared and compared with fermented ones (fCTR). Samples were added with either non-fermented OC (nfOC) or fermented for 12 and 20 h (fOC-12 and fOC-20). Our results showed that the predicted glycemic index (pGI) was influenced by both OC addition and sourdough fermentation. In fact, the lowest value of pGI was found in fOC-12, and hydrolysis index and pGI values of samples with OC (fOC-12 and nfOC) were statistically lower than fCTR. Both OC addition and fermentation improved the total phenol content and antioxidant activity of breadsticks. The most pronounced increase in hardness values was observed in the samples subjected to sourdough fermentation as evidenced both from texture profile analysis and sensory evaluation. Moreover, in most cases, the concentration of the detected volatile compounds was reduced by fermentation. Our work highlights the potential of OC to be upcycled in combination with fermentation to produce gluten-free breadsticks with improved nutritional profile, although additional trials are required to enhance textural and sensory profile.
Introduction
Celiac disease is a global health problem with ~1% of the world population being affected (1, 2). Moreover, gluten also causes other pathologies grouped under the term “gluten-related disorders,” namely, non-celiac gluten sensitivity, dermatitis herpetiformis, gluten ataxia and wheat allergy (3).
A gluten-free (GF) diet requires the complete exclusion of gluten, a protein complex present in food products from wheat, rye, barley, oats, spelt, kamut or their hybrids. It only comprises naturally GF food products (e.g., legumes, fruit and vegetables, unprocessed meat, fish, eggs, and dairy products) and/or substitutes of wheat-based foods, specially manufactured without gluten or having a gluten content lower than 20 mg/kg, according to the Commission Regulation (EC) No 41/2009 (4).
There are different challenges to face in the production of GF bakery products. First of all, gluten is responsible for several important technological features, and it is a big task to find ingredients that are able to mimic its properties and develop sensory acceptable GF baked goods. Moreover, the nutritional intake given by GF products must also be considered. Frequently, the celiac diet is unbalanced, with a high intake of saturated fatty acids and sugars and lacking in different nutrients, such as dietary fiber, iron, zinc, magnesium, calcium, B12 vitamin, and folate (5–7). Furthermore, GF products often present a high predicted glycaemic index (pGI) due to their starch-based composition. A high pGI represents a problem for subjects affected by metabolic disorders, such as obesity and diabetes (8).
The olive mill generates large amounts of different by-products, such as olive leaves, olive pomace, olive mill wastewaters and olive stones. These by-products have attracted attention for their possible exploitation in several industrial sectors (9, 10). The recently introduced multi-phase decanter technology for olive oil industrial extraction generates large quantities of olive cake (OC), a novel by-product made up of the partially defatted wet drupe pulp that contains very low traces of the stones. Recently, different authors reported that OC is an excellent natural source of unsaturated fatty acids, dietary fiber and biologically active substances, including polyphenols, triterpenic acids, tocochromanols and carotenoids (11, 12).
Different processing techniques may enhance the biological value of by-products by eliminating or reducing some technological drawbacks, such as unappealing taste, when used in baked goods. Sourdough fermentation, also in combination with enzymes, is one of the best tools for the above purposes (13). Bioconversion processes and fermentation strategies of agri-food by-products can increase digestibility, enhance nutritional value and decrease the levels of anti-nutritional factors in these substrates. Microbial fermentation of agri-food waste is used to produce ingredients with improved nutritional and health attributes, favoring the release of bioactives from the vegetable matrix, or through bioconversion of the compounds originally encountered in the by-products (14). For instance, fermentation of olive-mill wastewaters by Yarrowia lipolytica to produce citric and oleic acid has been reported (15). Specific compounds produced through microbial fermentation are largely dependent on the raw material characteristics and the specific microbial species used. Innovative valorization strategies include bioconversion of vegetables by-products using lactic acid bacteria (LAB) and other microorganisms to obtain enzymes, polysaccharides, nutraceuticals and beverages (16). Strategies based on sourdough fermentation to elaborate baked goods, which may ameliorate the symptoms of irritable bowel syndrome and with enhanced nutritional value, have been described (13, 17). The wide microbial biodiversity holds a great array of metabolic potential that is useful to design fermentation strategies and obtain added-value ingredients through vegetable residue fermentation, which would have the greatest environmental implication because of the large volume involved (14).
Based on those outcomes, our aim was to improve some nutritional features of GF breadsticks by upcycling OC and taking advantage of sourdough fermentation. Moreover, the effect on texture, nutritional, sensory and aromatic profile of breadsticks was evaluated.
Materials and Methods
Materials
Rice and cornflour, water, sunflower oil, salt and baking powder for the production of GF breadsticks were purchased from local retailers. Psyllium fiber and chicory inulin (COSURA groupe Warcoing S.A, Warcong, Belgium) were used in the formulation. OC, purchased from a local olive mill (Oleificio Ferrara, Trani, BAT), was lyophilized (Buchi LyovaporTM L-200, Switzerland) at −58°C, 0.8 mbar, until it reached 2–3% of moisture, then ground by a laboratory mill and stored in a powder form at −20°C until use.
Microbial Strains and Culture Conditions
Hanseniaspora uvarum CNG11, Kluyveromyces marxianus KL, Lactiplantibacillus plantarum V3-D0001, L. plantarum/Pediococcus pentosaceus CBD 100-D0001, Leuconostoc mesenteroides DDL1, Lc. mesenteroides KI6, Pichia kudriavzevii DCNa1, Saccharomyces cerevisiae DDNd10 and Saccharomyces subpellicelosus DFNb1 were used.
Lc. mesenteroides DDL1, P. kudriavzevii DCNa1, S. cerevisiae DDNd10, S. subpellicelosus DFNb1 (all isolated from date), Lc. mesenteroides KI6 (isolated from kiwi) and Hanseniaspora uvarum CNG11 (isolated from dogwood) strains belonging to the culture collection of the Department of Soil, Plant and Food Science of the University of Bari, Italy, were previously isolated and identified by partial sequencing of the 16S rRNA (18, 19). L. plantarum V3D0001, the mixed L. plantarum/P. pentosaceus CBD 100-D0001 and K. marxianus KL were commercial active dried strains (Sacco System s.r.l., Cadorago, Como, Italy) selected for their established role of vegetable starter cultures. Stock cultures were at −25°C, with 200 μl ml−1 glycerol as a cryoprotective agent. For the activation of the cultures, all strains were thawed at room temperature and then LAB strains were routinely cultivated at 1% (v/v) in De Man, Rogosa and Sharpe (MRS) (Oxoid, Basingstoke, Hampshire, England) broth at 30°C for 48 h. Yeasts were routinely propagated at 25°C on Yeast Extract Peptone Dextrose (YPD) broth, containing 10 g of glucose, 5 g of peptone, 3 g of yeast extract and 3 g of malt extract per liter (all ingredients were purchased by Oxoid).
Strains Selection for Olive-Cake Fermentation
The MRS or YPD broth with increasing OC concentration were used to evaluate the potential inhibitory effect of the OC extracts. OC was dissolved in saline (NaCl 9 g L−1) and added to MRS or YPD in a concentration series ranging from 1 to 20 g L−1, chosen based on both of the data reported in the literature and deriving from growth preliminary trials. In all cases, the OC solution was sterilized through membrane filtration using Millex® Syringe Filter Units (pore size, 0.22 μm; Merck, Millipore, Darmstadt, Germany) and added aseptically to the sterile MRS and YPD medium. An MRS and YPD broth without the addition of OC extract were used as control. Each LAB and yeast strain was cultivated two times in conventional MRS and YPD broth at 30 and 25°C until stationary growth phase. Cells were harvested by centrifugation at 10,000 × g for 10 min at 4°C, washed twice with 20 mM sterile potassium phosphate buffer (pH 7.0), and re-suspended in distilled water to a final cell density of 9 log colony forming unit (CFU) ml−1. Aliquots of each microbial suspension (100 μl) were singly used to inoculate 10 ml of the culture medium containing OC at a final cell density of ca. 7 log CFU ml−1. The incubation was at 30 and 25°C for LAB and yeast, respectively. After 24 h, cell density was estimated by pour-plating in MRS and YPD agar and incubated at 30 and 25°C for 48 h. The pH was estimated by a pH meter (Denver Instrument, USA). Three separate experiments were performed, and the average value and standard deviation were calculated. L. plantarum, L. plantarum/ P. pentosaceus and K. marxianus were the most growing and acidifying strains and were selected and used as mixed starters for dough fermentation.
Dough and Gluten-Free Breadstick Preparation
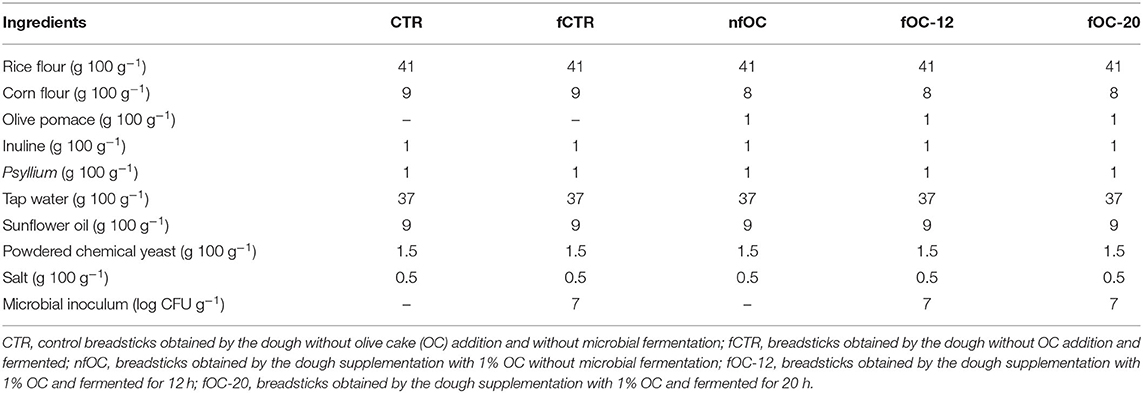
Gluten-free (GF) breadstick preparation was performed in a pilot plant belonging to the Department of Soil, Plant and Food Sciences of the University of Bari (Italy) on 3 consecutive days (a total of 3 batches for each breadsticks variant). Tap water (37 g 100 g−1 dough) at room temperature was mixed with oil (9 g 100 g−1 dough). Then, inulin (1 g 100 g−1 dough) and psyllium fiber (1 g 100 g−1 dough) were added and dissolved by gently stirring to obtain a gel. Rice flour (41 g 100 g−1 dough), cornflour (9 g 100 g−1 dough), salt (0.5 g 100 g−1 dough) and baking powder (1.5 g 100 g−1 dough) were added to the gel. The ingredients were kneaded for 10 min to obtain the dough. Figure 1 reports the flowchart of the sampling. Different types of GF breadsticks (Table 1) were produced as follows: control breadsticks (CTR) obtained by the dough without OC addition and without microbial fermentation; breadsticks obtained by the dough without OC addition and fermented by L. plantarum, L. plantarum/P. pentosaceus and K. marxianus (fCTR); breadsticks obtained by the dough supplemented with 1% OC without microbial fermentation (nfOC); and breadsticks obtained by the dough supplemented with 1% OC and fermented by L. plantarum, L. plantarum/P. pentosaceus and K. marxianus (fOC). To determine the best fermentation time, both fCTR and fOC doughs were inoculated by the selected mixed starter and fermented at 30°C for 24 h. The pH decrease was then monitored. The selection of fermentation parameters was based on the time to reach the 4.6 pH value. OC was added in substitution of an equal amount of cornflour; thus, the amount of dry ingredients and water was the same in the control and in the experimental doughs. For fermented doughs, overnight cultures of the selected strains (cell count of ~9.0 ± 0.2 log CFU ml−1) were harvested by centrifugation (10,000×g, 10 min, 4°C) and washed twice with sterile 20 mM potassium phosphate buffer, pH 7.0. Then, each cell pellets were re-suspended in 1 ml of tap water needed for making the dough and added at an initial cell density of ca. 7.0 and 5.0 log CFU g−1 for LAB and yeasts, respectively. The resulting doughs (immediately or at the end of fermentation) were manually shaped to form the breadsticks (9 × 1 cm), which were boiled in water for 3 min and then dried for 5 min on a clean cloth. The boiled breadsticks were baked in a forced-air convention oven at 200°C for 26 min. Baked samples were immediately subjected to the direct analyses and/or to the extraction process. At the beginning and at the end of fermentation, pH was recorded.
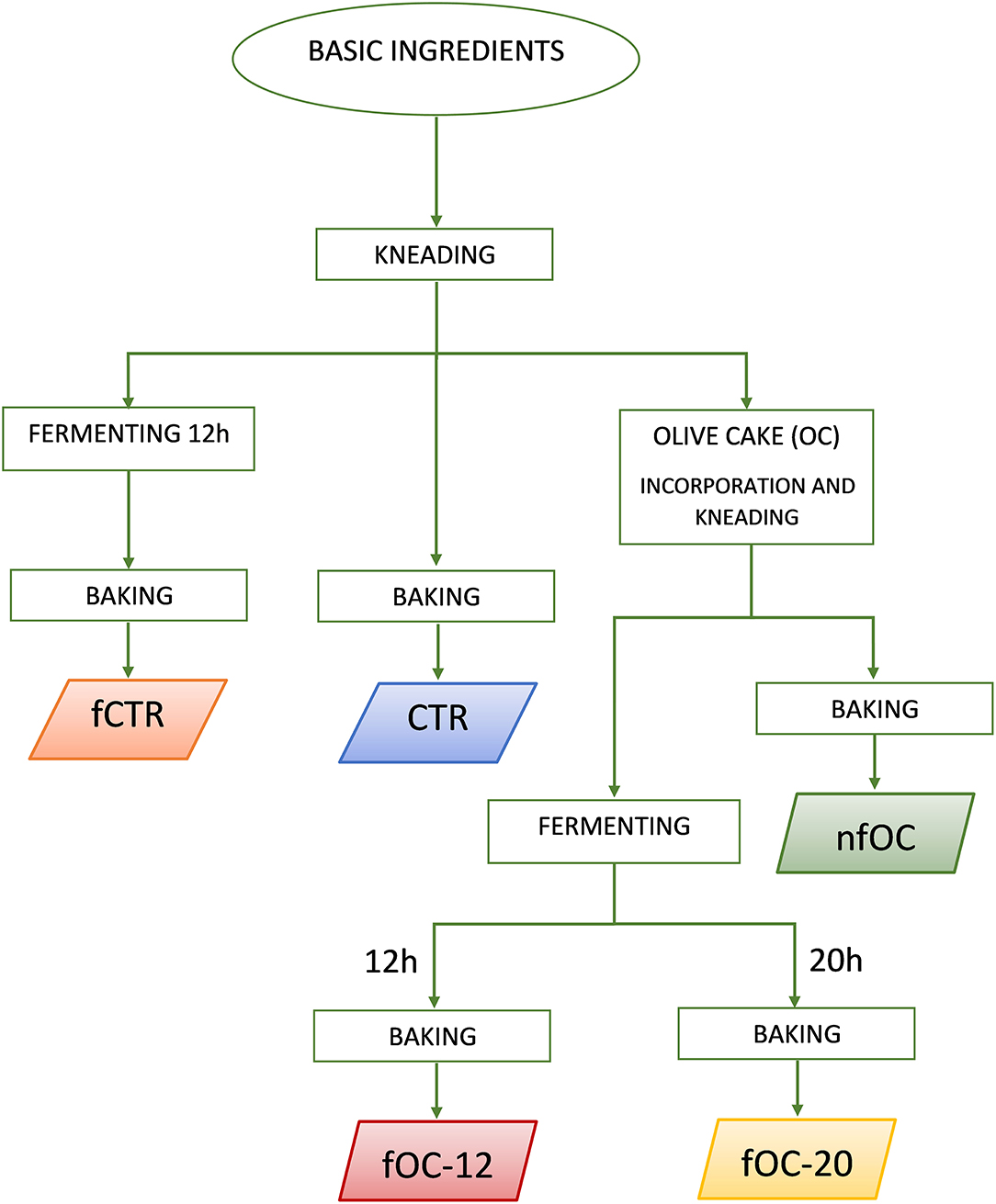
Figure 1. Flowchart of the breadstick sampling. CTR, control breadsticks obtained by the dough without olive cake (OC) addition and without microbial fermentation; fCTR, breadsticks obtained by the dough without OC addition and fermented; nfOC, breadsticks obtained by the dough supplementation with 1% OC without microbial fermentation; fOC-12, breadsticks obtained by the dough supplementation with 1% OC and fermented for 12 h; fOC-20, breadsticks obtained by the dough supplementation with 1% OC and fermented for 20 h.
Proximate Composition of Olive Cake and Breadsticks
The lipids, proteins (total nitrogen × 6.25), ash and total dietary fibers were determined according to Association of Official Analytical Collaboration (AOAC) methods 945.38F, 979.09, 923.03 and 991.43, respectively. Moisture content was determined by an automatic moisture analyser at 105°C (Mod. MAC 110/NP, Rodwang Wagi Elektroniczne, Poland). The carbohydrate content was determined as difference: 100 – (moisture + proteins + lipids + ash). The determinations were carried out in triplicate.
Enumeration of LAB and Yeasts Cell Densities
Lactic acid bacteria (LAB) and yeast cell densities were determined according to methods previously described (20, 21), using culture media and supplements purchased from Oxoid. For each sample, 10 g were diluted with 90 ml of 9 g L−1 NaCl solution, homogenized with a Stomacher Lab-Blender 400 (Seward Medical, London, United Kingdom) for 3 min, and serially diluted and plated in triplicate in selective media. LAB was enumerated using MRS agar plates with cycloheximide (0.1 g L−1) at 30°C for 48 h under anaerobiosis (AnaeroGen and AnaeroJar, Oxoid). The number of yeast cells was estimated at 25°C by using YPD agar supplemented with chloramphenicol (0.1 g L−1) for 48 h. The microbiological counts were confirmed by taking representative colonies for each medium, which were analyzed for morphology, motility, Gram staining reaction and catalase test.
Chemical Characterization and Antioxidant Activity
The chemical composition and the antioxidant profile of olive cake (antioxidant activity, total phenols and tocopherols) were performed as reported in Difonzo et al. (12). The determination of total phenols was carried out according to Caponio et al. (22) with some modifications. Two g of ground breadsticks were added to 2 ml of hexane, to remove the oil phase, and 10 ml of methanol:water (80:20 v/v). The samples were stirred with a vortex at 2,000 rpm for 15 min, then sonicated (Ultrasonic cleaner CP104, EIA) for 15 min and centrifugated at 10.000 g min−1 for 10 min at 24°C (Thermo Fisher Scientific, Osterodeam Harz, Germany). The hydroalcoholic extract was collected, while the pellet was subjected to a second extraction phase following the same procedure. The hydroalcoholic extracts were filtered through a nylon filter (pore size 0.45 mm, Sigma, Ireland). Total phenols were determined according to the Folin-Ciocalteu assay. Twenty microliters of extract and 100 μl of Folin-Ciocalteu reagent were added to 980 μl of Milli-Q water. After 3 min, 800 μl of Na2CO3 were added and then incubated for 1 h. The absorbance was read at 720 nm and the results were expressed as mg of gallic acid equivalents per g of breadsticks. Antioxidant activity was determined by 2,2-diphenyl-1-picrylhydrazyl (DPPH) assay as reported by Difonzo et al. (23). Specifically, 50 μl of each extract was added to 950 μl of DPPH solution and incubated for 30 min in the dark. The absorbance was read at 517 nm and the results expressed as μmol Trolox equivalent (TE) g−1 dry weight. The determinations were carried out in triplicate.
In vitro Starch Hydrolysis
In vitro starch hydrolysis was determined as previously described (24). The procedure mimicked the in vivo digestion of starch. Aliquots of doughs, containing 1 g of starch (determined in dough), were subjected to enzymatic process (pancreatic amylase and pepsin-HCl), and the released glucose content was measured with D-Fructose/D-Glucose Assay Kit (Megazyme, Wicklow, Ireland). Simulated digests were dialyzed (cut-off of the membrane: 12,400 Da) for 180 min. Aliquots of dialysate, containing free glucose and partially hydrolysed starch, were sampled every 30 min and further treated with amyloglucosidase. Then, free glucose was determined using the above-mentioned enzyme-based kit and finally converted into hydrolysed (digested) starch in the dough. Control white wheat bread was used as the control to estimate the hydrolysis index (HI = 100). The pGI was calculated using the equation: pGI = 0.549 × HI + 39.71 (25). Each sample was analyzed in triplicate.
Color Determination
The breadsticks color was determined by Chromameter CM-600d (Konica Minolta Sensing, Osaka, Japan). The color indices analyzed for breadsticks were lightness (L*), redness (a*), and yellowness (b*). The analyses were carried out in triplicate.
Textural Properties
Textural properties of GF breadsticks were determined through a three-point bending test. A Z1.0 TN (Zwick/Roell, Ulm, Germany) was used, equipped with 1 KN load cell. The distance between the support bars was set at 60 mm, and the breadsticks were placed centrally with the probe speed set at 3 mm s−1. The maximum force required to break the breadstick (N) and the distance before the breadstick broke (mm) were measured, which represent the hardness and brittleness, respectively. The analyses were carried out in triplicate.
Sensory Analysis
The sensory analysis was performed by a semi-trained panel of seven panelists that included women and men. Ethical guidelines of the laboratory of Food Science and Technology of the Department of Soil, Plant, and Food Science-University of Bari (Italy) were followed. Panelists were usual consumers of the food category under the study who underwent 1 h training on the definition of the attributes via physical references; information about study aims were given, and individually written informed consent was obtained from each participant. The five GF breadsticks were randomly coded with a three-digit number and analyzed on the same day of production. Nine descriptors were chosen: 2 for visual analysis (color intensity, surface homogeneity), 2 for retro nasal analysis (malty and olive), 2 for texture attributes (hardness, crispness) and 3 for taste attributes (bitterness, astringency and oily). The perception intensity of the descriptors was assessed on a scale ranging from 0 (no perception) to 10 (maximum intensity).
Volatile Profile
Volatile compounds of GF breadsticks were analyzed by a headspace solid phase micro-extraction (HS-SPME) coupled with gas-chromatography/mass spectrometry (GC-MS) following the procedure reported by Difonzo et al. (26). In particular, 0.5 g of each sample were added with 4 ml of a saturated aqueous solution of NaCl and 150 μl of 1-propanol (internal standard). Vials were homogenized using a laboratory vortex for 2 min. The extraction of volatile compounds was performed by exposing a 75 μm Carboxen/polydimethylsiloxane (CAR/PDMS) SPME fiber (Supelco, Bellefonte, PA, USA) in the headspace of the vials containing the samples at 50°C for 40 min. The fiber was desorbed for 6 min in the injection port of the gas-chromatography operating a split-less mode at 230°C for 3.5 min. The determination was carried out using an Agilent 6580 gas-chromatography equipped with an Agilent 5975 mass-spectrometer (Agilent Technologies Inc., Santa Clara, CA, USA). Volatile compounds were separated with a capillary column HP-Innowax (60 m length × 0.25 mm i.d. × 0.25 μm film thickness) under the following conditions: injector temperature of 250°C, oven temperature was held at 35°C for 5 min, then increased to 210°C at 5.5°C min−1. This temperature was kept constant for 5 min. Mass detector was set at the following conditions: interface temperature of 230°C, source temperature of 230°C, ionization energy of 70 eV, scan range of 33–260 amu. Helium was used as a carrier with a pressure 30 kPa and a flow of 1 ml min−1. Volatile compounds were identified comparing the peak areas of compounds of interest with the area of internal standard. The analyses were carried out in triplicate.
Statistical Analysis
Significant differences between the values of all parameters were determined at p < 0.05, according to the analysis of variance (ANOVA) followed by the Tukey's Honestly Significant Difference (HSD) test for multiple comparisons. The statistical analysis was performed by the Minitab Statistical Software (Minitab Inc., State College, PA, USA).
Results and Discussion
Olive Cake Characterization
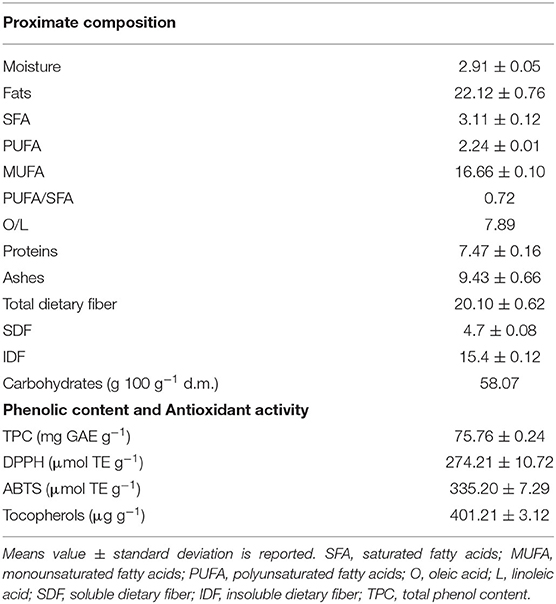
The proximate composition of the freeze-dried OC, as well as the antioxidant activity in terms of phenolic compounds and tocopherols is detailed in Table 2. The final moisture of the OC was about 3 g 100 g−1, with a protein content of 7.47 g 100 g−1 and ashes of 9.43 g 100 g−1. The lipid fraction is a mixture of saturated (SFA), monounsaturated (MUFA) and polyunsaturated fatty acids (PUFA); MUFA represented the main fraction with a content of 16.66 g 100 g−1 of dried cake. In fact, the fatty acid composition resulted that the oleic acid was the most abundant, representing 75% of the total fatty acids followed by palmitic, linoleic and stearic acids. The other fatty acids were found in small quantities (data not shown). These results agree with the literature for olive products and by-products (27, 28). The PUFA/SFA and oleic/linoleic acid (O/L) ratios were 0.72 and 7.89, respectively, in line with those of extra virgin olive oil. The PUFA/SFA ratio is one of the main parameters used to assess the nutritional quality of the lipid fraction of foods. Nutritional guidelines recommend a PUFA/SFA ratio higher than 0.4 (28). In addition, values of O/L equal or higher than 7 indicate a good oxidative stability.
Olive cake (OC) is also a good source of functional molecules, such as dietary fiber, polyphenols, tocopherols and phytosterols, as shown in Table 2. Moreover, OC showed an interesting, though expected, antioxidant activity, especially due to the presence of polyphenols and tocopherols (12, 29).
Strains Selection for Olive-Cake Fermentation
Due to the increasing need for GF bakery products, food processing methods that improve the nutritional profile and enhance the bioactive compounds are a crucial research topic. As already reported (30–32), it is possible to improve the nutritional properties of OC through fermentation carried out by microorganisms. Sourdough fermentation by selected microorganisms enhances bioactive capacity and improves the technological traits of baked goods (33). To select a suitable microbial strain for OC sourdough fermentation, in the present study, several LAB and yeast strains were screened based on their acidification and growing capacity in OC-added medium after 24 h of fermentation. The OC concentrations that were used in the formulations of the cultivation media (1, 2, 5, 10 and 20 g L−1) were chosen considering some preliminary experiments (data not shown) and literature data. Nanditha and Prabhasankar (34) used the synthetic antioxidant butylated hydroxy anisole (BHA) in a range of concentrations between 250 and 2500 ppm, obtaining the inhibition of bacterial and yeast growth (34). Overall, the tested strains, previously recognized as vegetables microbial starter culture or isolated from vegetable source, were able to grow and acidify on an OC-added medium although with different extent (Supplementary Table S1). Maximal growth occurred in broth containing 1 g L−1 OC with average increases in cell density of 2.12 log units and an average decrease of pH of 1.60 delta pH units, starting from a pH value of 6.2. No microbial growth was observed when 20 g L−1 of OC was added. The highest OC concentration, which allows microbial growth in the tested range, was 10 g L−1. In this condition, after 24 h of incubation, the cell density increase was between 0 and 1.59 log units and the pH decrease was between 0.14 and 1.74 pH units (average values). Among the tested strains, the best tolerance was shown by commercial L. plantarum, L. plantarum/P. pentosaceus and K. marxianus cultures, which showed that the higher cell density increased (1.50 ± 0.01, 1.55 ± 0.05 and 1.59 ± 0.11 log units, respectively) and pH decreased (1.74 ± 0.02, 1.65 ± 0.04 and 0.79 ± 0.11 pH units, respectively) (Supplementary Table S1). The reduced growth, compared to positive control media, can be attributed to the antimicrobial activities of OC extracts related to their content of phenols which have effective antimicrobial activities against a wide array of microorganisms (35), as pointed out by the relevant antiradical activity observed in the extracts (36). OC extracts were rich in phenolic compounds; therefore, phenol tolerance is an important selection criterion for a microorganism to be used as a starter. LAB, mainly belonging to Lactiplantibacillus, Leuconostoc and Pediococcus genera, has to be adapted to the characteristics of the plant raw materials where phenolic compounds are abundant. In addition, L. plantarum is the commercial starter most frequently used in the fermentation of food products of plant origin (21, 22, 37). K. marxianus has been shown to be able to grow in olive pomace by solid-state fermentation and improve its nutritive value by simultaneous production of gallic acid (38). In a recent work (39), olive mill wastewater and olive paste were used to improve the chemical quality of bread and pasta. The fermented olive paste was also used to increase the functionality of Italian bakery products taralli (11).
The strains of L. plantarum, L. plantarum/P. pentosaceus and K. marxianus, which demonstrated to well adapt to OC matrix that was rich in polyphenols, were used as a mixed starter to ferment GF dough. In agreement with traditional sourdoughs, which showed that the ratio of cell counts of yeasts to lactobacilli ranges from 1:10 to 1:100 (40), in this work, we used the ratio of 1:100. The acidification curve of the mixed starter during preliminary dough fermentation at 30°C shows that the 4.6 pH value was reached after 20 and 12 h of fermentation with and without the addition of 10 g L−1 of OC, respectively. Therefore, these fermentation times were adopted in subsequent fCTR (20 h) and fOC (12 and 20 h, referred to as fOC-12 and fOC-20) experiments. Fermented GF breadstick doughs were thus prepared under these conditions and compared to unfermented ones with and without OC addition (CTR and nfOC). Changes in LAB and yeast cell densities and acidification were monitored in all doughs before and after driven fermentation (Figure 2). LAB and yeasts profiles changed as a consequence of OC addition and/or microbial fermentation. Before fermentation, all fermented doughs were characterized for ca. 7 and 5 log CFU g−1 for LAB and yeast microbial densities, respectively, corresponding to the initial inoculum of the starter, while cell densities were <1 log CFU g−1 in non-inoculated doughs. In fOC samples, the final cell count of presumptive LAB after 12 and 20 h of fermentation at 30°C was ~8.41 ± 0.1 and 8.73 ± 0.12 log CFU g−1. Yeast number increased up to 1 log cycle in fOC-20 dough. As already reported, sourdough originates from the starter culture-driven or spontaneous fermentation of a blend of flour and water, and in the first step, redox potential decreases favoring the growth of LAB (13). The pH values of doughs varied between 6.48 (CTR) and 6.00 (in dough with OC before fermentation). In fermented doughs, after 20 h, the lower pH was observed in fOC-20 (4.54). Previous studies already reported the ability of LAB, especially L. plantarum, to grow in the presence of relatively high polyphenol concentrations. Particularly, L. plantarum was able to colonize olives and its derivates, as shown by Blana et al. (41), in agreement with a previous work performed in our laboratory on bacterial microbiota on the surface of table olives fermented with the same commercial L. plantarum strain (21). Not only Lactiplantibacillus strains, but also Pediococcus strains should be adapted to the environment of the fermentation of olive products. As reported by Bonatsou et al. (42), Pediococcus strains are able to ferment olive products and decrease the pH value through the production of specific organic acids in the presence of phenolic compounds.
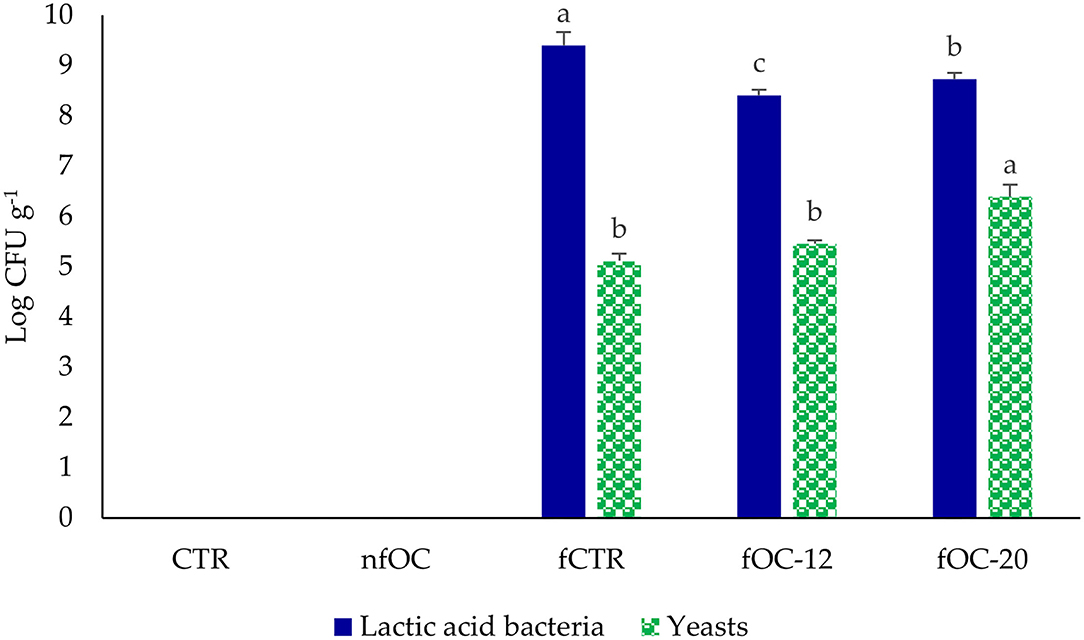
Figure 2. Cell density (log CFU g−1) of presumptive lactic acid bacteria and yeasts in gluten-free breadstick doughs. CTR, control breadsticks obtained by the dough without OC addition and without microbial fermentation; fCTR, breadsticks obtained by the dough without OC addition and fermented; nfOC, breadsticks obtained by the dough supplementation with 1% OC without microbial fermentation; fOC-12, breadsticks obtained by the dough supplementation with 1% OC and fermented for 12 h; fOC-20, breadsticks obtained by the dough supplementation with 1% OC and fermented for 20 h. The values represent means of triplicates; different letters indicate significant differences (p < 0.05) according to Tukey's Honestly Significant Difference (HSD) test.
Chemical and Nutritional Characterization
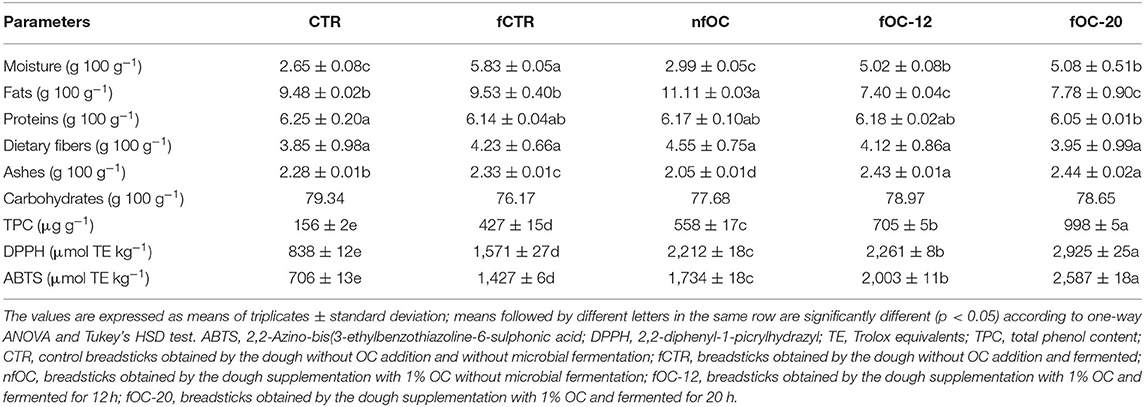
Gluten-free (GF) breadsticks were characterized by proximate composition, showing that the final moisture in the samples was significantly higher in the samples subjected to the fermentation process, reaching the highest value in fCTR (5.83 g 100 g−1). The highest fat content was found in nfOC due to the contribution of lipids from OC. However, in the fermented samples added with OC, the fat content was lower than the other samples, probably due to a loss of lipids that was observed during cooking. In fact, fermentation can affect the molecular structure of starch with subsequent modifications in dough rheology (43). Slight differences were found between the samples for proteins. The dietary fiber content was higher than 3 g 100 g−1, allowing to label the product with the claim “rich in fiber,” due the use of ingredients, such as inulin, psyllium and OC even if the samples added with OC did not show a significantly higher content in fiber than the CTR samples (Table 3).
As reported in Table 3, OC is a source of different bioactive compounds, such as phenolic compounds and tocopherols. Antioxidant activities of two different fermented GF breadstick doughs fortified with OC were investigated and compared to a non-inoculated GF breadstick dough added with OC and to two control GF breadstick doughs that were without OC-fermented and not fermented, respectively. The total phenol content (TPC) and the antioxidant activity of the GF breadsticks are reported in Table 3. Both OC addition and fermentation improved TPC and antioxidant activity of breadsticks. Fermentations may lead to significant changes to the health-promoting features of plant foods. Cereals per se contain classes of phytochemicals, but processing conditions markedly influence their levels and bioavailability (44). Among processing conditions, the sourdough fermentation has the most pronounced impact on the levels and bioavailability of phytochemicals. Those can be attributed to the capacity of sourdough fermentation to increase the levels of extractable phenolic compounds (13) which, in turn, were responsible for the enhancement of the antioxidant capacity, as measured by 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) and DPPH assays. Difonzo et al. (26) showed how olive leaves polyphenols increased the antioxidant activity of baked salty snacks. Moreover, despite the loss of phenolic compounds during kneading and cooking phases ranging from 20 to 47%, different authors found that olive phenolics are able to reduce the oxidation degree in baked products and bring health-related benefits by lowering the postprandial oxidized low-density lipoproteins (45, 46). The incorporation of extracts from olive leaves and wastewater also resulted in improved nutritional and functional properties of GF breadsticks, as evidenced by the changes observed in the insoluble/soluble polyphenol ratio in favor of the soluble fraction, the enhanced bioavailability of polyphenols, and the higher antioxidant activity (47).
In vitro Starch Hydrolysis
As shown in Figure 3, after 12 h of fermentation (fOC-12), pGI and HI changed significantly (p < 0.05) compared to CTR. In fact, the lowest value of pGI was found in fOC-12 at approximately 64.11 ± 0.42. CTR has a statistically higher pGI value (77.94) than other samples. The HI and pGI values of samples with OC (fOC-12 and nfOC) were statistically lower than samples without OC (fCTR). However, samples fermented without OC also showed statistically lower pGI values than CTR.
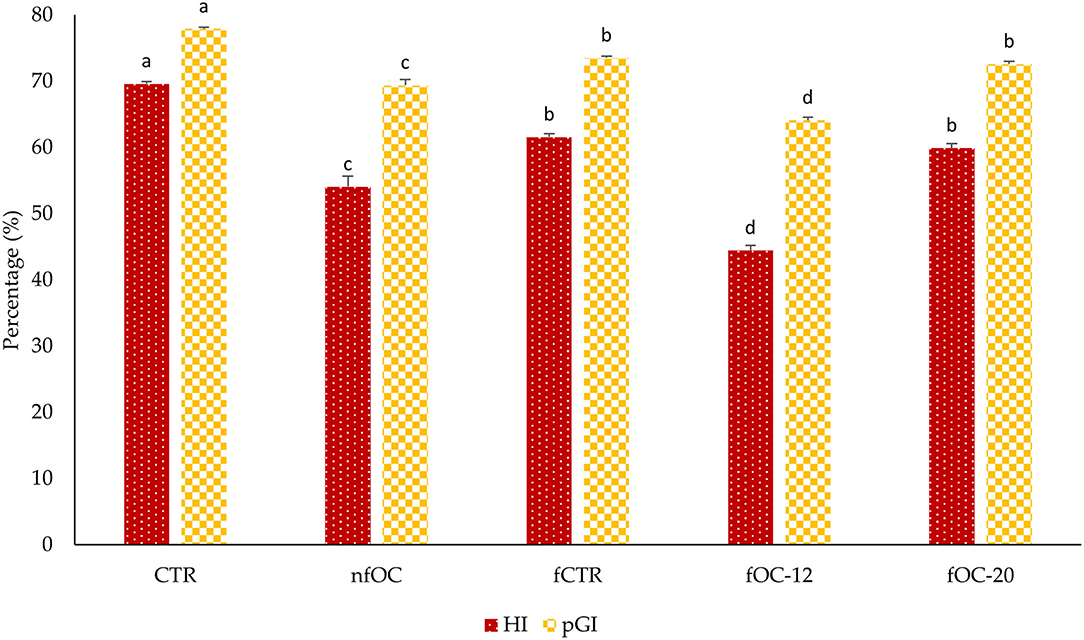
Figure 3. Results of hydrolysis index (HI) and predicted glycemic index (pGI). CTR, control breadsticks obtained by the dough without OC addition and without microbial fermentation; fCTR, breadsticks obtained by the dough without OC addition and fermented; nfOC, breadsticks obtained by the dough supplementation with 1% OC without microbial fermentation; fOC-12, breadsticks obtained by the dough supplementation with 1% OC and fermented for 12 h; fOC-20, breadsticks obtained by the dough supplementation with 1% OC and fermented for 20 h. The values represent means of triplicates; different letters indicate significant differences (p < 0.05) according to Tukey's HSD test.
It is well recognized that fermentation reduced pGI. Taking an example of bread, consumption of sourdough bread reduced postprandial blood glucose and insulin response. This mechanism could explain why organic acids, such as lactic and acetic acids, produced in sourdough lower its estimated pGI. Acetic acid appears to be associated with a delay in gastric emptying, whereas lactic acid induces interactions between starch and gluten during dough baking and reduces starch availability (48). These results were in accordance with Curiel et al. (49). In fact, the biological acidification of microorganisms is one of the main factors that decrease starch hydrolysis rate and HI. Moreover, wheat bread fortified with sourdough-fermented hemp flour showed a lower pGI compared to bread fermented with baker's yeast (50).
Colorimetric and Textural Evaluation
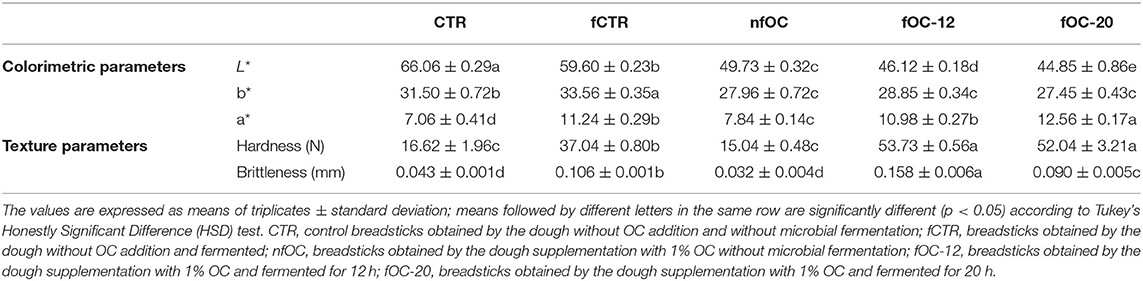
As shown in Table 4, the addition of OC led to an L* decrease due to the darkening of breadsticks; this effect is due to the dark color of OC and could be related to the presence of sugars. In fact, Kocadagli et al. (51) found that the concentration of reducing sugars in cookies increased significantly during baking and contributed to browning through caramelization, suggesting that reducing sugars from OC may contribute to browning. The same trend was found for the yellowness index b* that was the highest in fCTR, whereas the redness index a* increased with OC addition.
The texture is one of the most critical quality attributes in breadsticks because consumers appreciate a crisp and crunchy texture (52). From a textural point of view, baked snacks like breadsticks are characterized by a rigid, stiff structure with a little tendency to deform before fracture when subjected to small forces (47). In the present study, the different mechanical behavior of the experimental breadsticks was measured on the day of baking to assess their quality in terms of hardness and brittleness. As reported in Table 4, both CTR and nfOC significantly (p < 0.05) lowered the maximum force needed to break the experimental breadsticks. In particular, while the control sample and the samples added with OC showed the lowest values of force at break, the most pronounced increase in hardness values was observed in the samples subjected to dough fermentation. Brittleness, which is a textural parameter describing the distance crossed by the blade through the sample before its breaking and thus, how far a sample can be deformed before fracture, showed the lowest values in CTR and nfOC according to the results from hardness. In fact, the higher the brittleness value, the less friable is the product. Texture profile analysis had been created as an imitative test that resembles what goes on in the human mouth and is a parameter to determine the human perception of the texture of the product and how it behaved when handled and eaten. Furthermore, it incorporates all the attributes (mechanical, geometric and surface) of the food. In fact, physical texture is also an important quality to determine the porous structure of baked products where the texture is related to the geometric and mechanical properties of the product, which heavily depends on its cellular structure, such as cell wall thickness, cell size and uniformity (53). A possible explanation for this hardening effect could be related to the loss of lipids during cooking of fOC-12 and fOC-20. Therefore, the use of leavening agent, as sourdough, may drive changes in product structure, altering water-holding capacity of the products which can significantly affect oil uptake capacity of the products, as observed by Marquez et al. (54). This evaluates the possibility to obtain taralli biscuits with limited water absorption during storage and consequently, prolonged hardness and crispness characteristics. In addition, during the baking stage in the oven, the water vapor carries fats/oils emulsified with it toward the external layer. While the vapor leaves the structure, the oil stays on the surface. Moreover, sourdough fermentation is known to cause transformations of lipids and macromolecules (40, 55). Oxidation of components, such as lipids and proteins (including protein carbonylation), as well as the interactions between raw material components (e.g., protein-lipid or protein-protein interactions), take place during fermentation (56). Based on this consideration, differences between breadsticks obtained by baking powder only or sourdough technology on fat loss may be influenced by different structural components, such as changes in the pH value of the dough system which, in turn, affects this phenomenon.
Sensory Evaluation and Aromatic Profile
Some of the selected sensory attributes were influenced by the OC addition and fermentation (p < 0.05) as reported in Figure 4. In novel food product formulation, maintaining the sensory characteristics of the product is of great importance. The fermentation led to a worsening of textural attributes. In fact, all the fermented samples had the higher scores in hardness and the lowest in crispness according to the results obtained from the texture profile analysis (Table 4). However, CTR and the samples added with OC without dough fermentation led to the highest scores in crispness and overall acceptability. The addition of OC did not induce an astringency and bitterness perception, except for fOC-12 that was perceived as the more bitter. The results of overall acceptability were completely in line with the results of hardness and crispness, highlighting that these parameters affected the whole liking of the breadsticks.
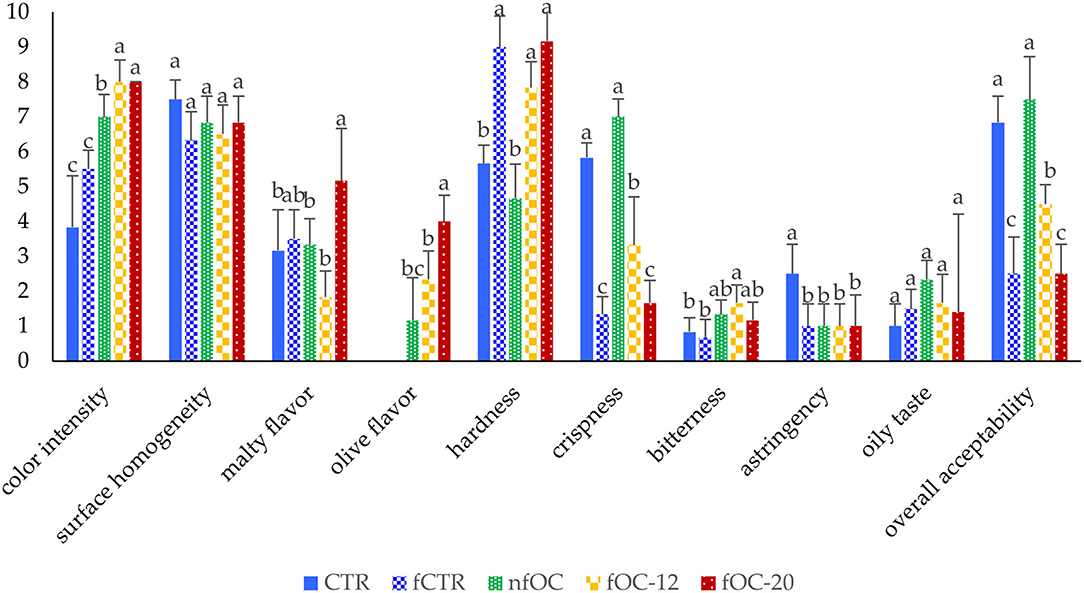
Figure 4. Sensory evaluation of the gluten-free breadsticks. Different letters indicate significantly different (p < 0.05) according to one-way ANOVA and Tukey's HSD test. CTR, control breadsticks obtained by the dough without OC addition and without microbial fermentation; fCTR, breadsticks obtained by the dough without OC addition and fermented; nfOC, breadsticks obtained by the dough supplementation with 1% OC without microbial fermentation; fOC-12, breadsticks obtained by the dough supplementation with 1% OC and fermented for 12 h; fOC-20, breadsticks obtained by the dough supplementation with 1% OC and fermented for 20 h.
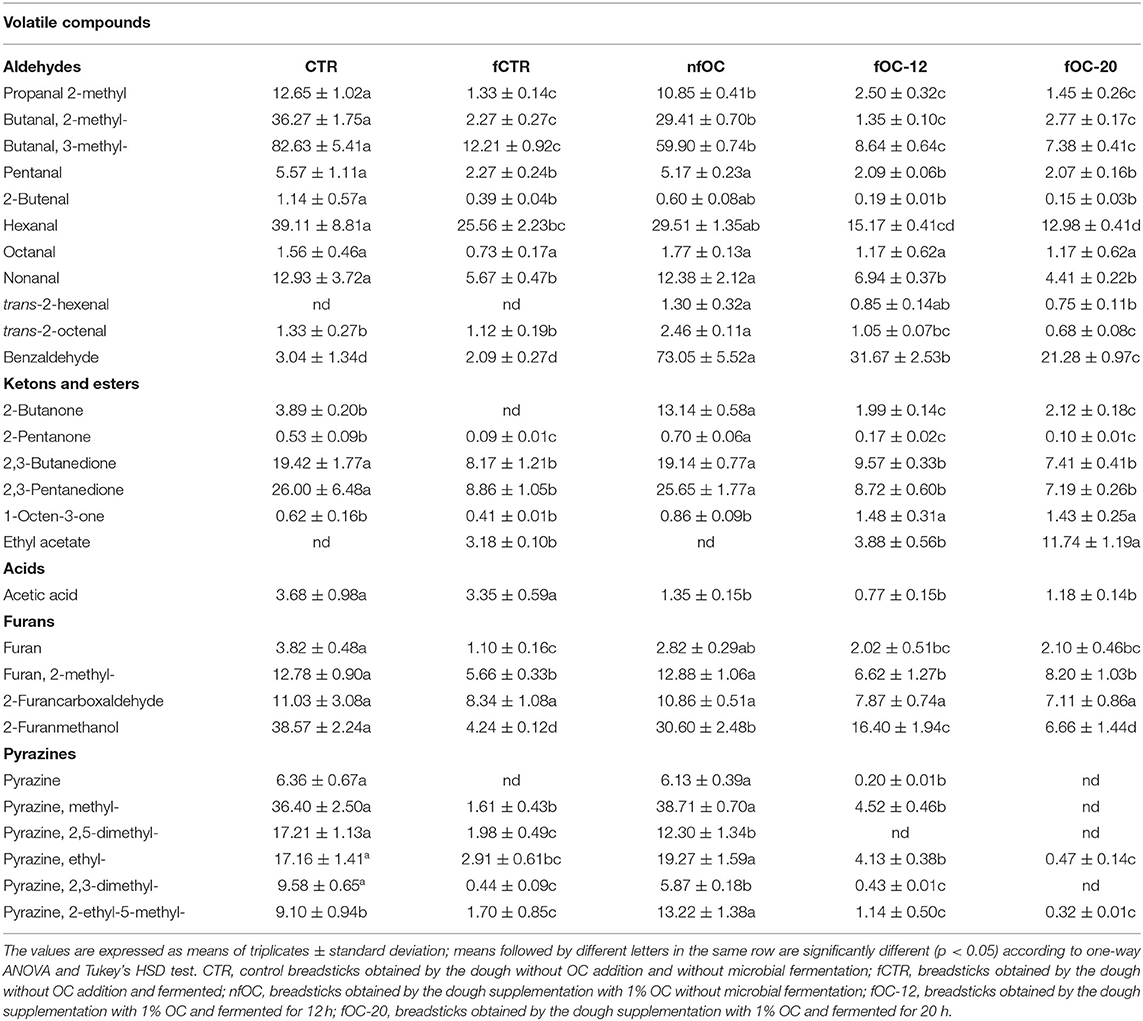
Fermentation is an important biotechnology strategy for maintaining and/or improving safety, nutritional, sensory and shelf-life properties of foods (33). Composition and concentration of volatile flavor-aroma compounds have been of great interest because of their important influence on sensory properties and consumer acceptance. Although, most of the volatile compounds are naturally present in the raw materials, it does not neglect that they might be synthesized as secondary metabolites by microbes (57). Considering the potential unpleasant aromatic properties of OC, lactic acid fermentation through selected strains may be considered as an interesting option to change the flavor profile of processed GF foods added to this by-product and ensure a better control of flavor changes during processing. In this framework, some authors have evaluated how different formulations and processing may affect the volatile and lipidic fraction of taralli (58–60). Table 5 reports the main volatile compounds detected in the samples. Several pyrazine derivatives, such as dimethyl-pyrazines or ethyl-methyl-pyrazines, whose origin could be linked to the Maillard reaction. Besides nitrogen-containing compounds, the Maillard reaction also arise several furans that were also detected in the experimental samples. Pico et al. (61, 62), in studying the aromatic profile of several GF breads obtained from flours and starches of different origins, selected a list of the main volatiles identified in all GF samples, including compounds deriving from fermentation process, lipid oxidation and Strecker degradation. In this framework, 2-methylbutanal and 3-methylbutanal, Strecker aldehydes deriving from Maillard reaction, were more present in the volatile pattern of control breadsticks followed by the samples added with OC. A similar trend was found for all the aldehydes, including the aldehydes from lipid oxidation with the lowest concentration in the fermented samples, irrespective of the OC addition. On the contrary, the amount of benzaldehyde was the highest in the samples added with OC since it is a compound generally founded in Drupaceae (63). 2-Butanone was already detected in baked goods in previous researches and its origin was attributed to β-chetoacids, which arose from thermal treatment of tryglicerides via decarboxylation (64). The furan compounds were mostly represented by furfural, a caramel-like odorant derived by Maillard reaction, which is significantly more abundant in acorn-added biscuits than in control. Furfural is typically present in biscuits as reported in several studies (65–68). Pyrazines, produced by Maillard reaction, were also detected. In most cases, the concentration of all these volatile compounds was reduced by dough fermentation. This behavior could be associated to the variation of the matrix composition during kneading and baking of the fermented samples, leading to modifications of the structure, viscosity and of physicochemical interactions established between volatile and non-volatile compounds (69). Moreover, lipid fraction also influences the release of volatile compounds in food matrices (70).
Conclusion
This study aimed to use two selected lactic acid bacteria and a yeast for increasing the antioxidant features and the phenol profile of GF breadsticks fortified with OC with the perspective of producing a functional food. Our results showed that both OC addition and fermentation improved TPC and antioxidant activity of breadsticks. Furthermore, the pGI was influenced by OC addition and dough fermentation. In fact, the lowest value of pGI was found in fOC-12, and HI and pGI values of samples with OC (fOC-12 and nfOC) were statistically lower than samples without OC (fCTR). The samples subjected to dough fermentation showed the highest hardness values, as evidenced from both texture profile analysis and sensory evaluation. Moreover, in most cases, the concentration of the detected volatile compounds was reduced by fermentation. Additional trials are necessary to improve the textural and sensorial features of the GF breadsticks.
In conclusion, the fermentation of agri-food residues by LAB, alone or in combination with other microorganisms, opens an avenue of opportunities for a sustainable circular economy. In fact, our work highlights the potential of OC to be exploited by fermentation to produce GF-baked goods with an improved nutritional profile.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author Contributions
GD and MDA dealt with conceptualization. GC, GD, GdG, and MC performed analysis, data elaboration, and the first drafting of the article. GC, GD, MC, and AP supervised the final revision of the manuscript. All authors approved the final version of the manuscript to be submitted for publication.
Funding
This work was supported by POR Puglia 2014/2020-Asse X-Azione 10.4 Research for Innovation-REFIN Code No. E65BAEEE.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2022.830932/full#supplementary-material
References
1. Fasano A, Catassi C. Celiac disease. N Engl J Med. (2012) 367:2419–26. doi: 10.1056/NEJMcp1113994
2. Leonard MM, Sapone A, Catassi C, Fasano A. Celiac disease and nonceliac gluten sensitivity: a review. JAMA. (2017) 318:647–56. doi: 10.1001/jama.2017.9730
3. Foschia M, Horstmann S, Arendt EK, Zannini E. Nutritional therapy–Facing the gap between coeliac disease and gluten-free food. Int J Food Microbiol. (2016) 239:113–24. doi: 10.1016/j.ijfoodmicro.2016.06.014
4. Henkey VJ. Concerning the Composition and Labelling of Foodstuffs Suitable for People Intolerant to Gluten. Commission Regulation (EC) No. 41/2009 (2009)
5. Jnawali P, Kumar V, Tanwar B. Celiac disease: overview and considerations for development of gluten-free foods. Food Sci Hum Wellness. (2016) 5:169–76. doi: 10.1016/j.fshw.2016.09.003
6. Naqash F, Gani A, Gani A, Masoodi FA. Gluten-free baking: combating the challenges—a review. Trends Food Sci Technol. (2017) 66:98–107. doi: 10.1016/j.tifs.2017.06.004
7. Vici G, Belli L, Biondi M. Polzonetti V. Gluten free diet and nutrient deficiencies: a review. Clin Nutr. (2016) 35:1236–41. doi: 10.1016/j.clnu.2016.05.002
8. Di Cairano M, Galgano F, Tolve R, Caruso MC, Condelli N. Focus on gluten free biscuits: ingredients and issues. Trends Food Sci Technol. (2018) 81:203–12. doi: 10.1016/j.tifs.2018.09.006
9. Difonzo G, Troilo M, Squeo G, Pasqualone A, Caponio F. Functional compounds from olive pomace to obtain high-added value foods—a review. J Sci Food Agric. (2021) 101:15–26. doi: 10.1002/jsfa.10478
10. Gullon P, Gullon B, Astray G, Carpena M, Fraga-Corral M, Prieto MA, et al. Valorization of by-products from olive oil industry and added-value applications for innovative functional foods. Food Res Int. (2020) 137:109683. doi: 10.1016/j.foodres.2020.109683
11. Durante M, Bleve G, Selvaggini R, Veneziani G, Servili M, Mita G. Bioactive compounds and stability of a typical Italian bakery products “Taralli” enriched with fermented olive paste. Molecules. (2019) 24:3258. doi: 10.3390/molecules24183258
12. Difonzo G, Aresta A, Cotugno P, Ragni R, Squeo G, Summo C, et al. Supercritical CO2 extraction of phytocompounds from olive pomace subjected to different drying methods. Molecules. (2021) 26:598. doi: 10.3390/molecules26030598
13. Gobbetti M, De Angelis M, Di Cagno R, Calasso M, Archetti G, Rizzello CG. Novel insights on the functional/nutritional features of the sourdough fermentation. Int J Food Microbiol. (2019) 302:103–13. doi: 10.1016/j.ijfoodmicro.2018.05.018
14. Sabater C, Ruiz L, Delgado S, Ruas-Madiedo P, Margolles A. Valorization of vegetable food waste and by-products through fermentation processes. Front Microbiol. (2020) 11:2604. doi: 10.3389/fmicb.2020.581997
15. Sarris D, Rapti A, Papafotis N, Koutinas AA, Papanikolaou S. Production of added-value chemical compounds through bioconversions of olive-mill wastewaters blended with crude glycerol by a Yarrowia lipolytica strain. Molecules. (2019) 24:222. doi: 10.3390/molecules24020222
16. Vázquez J, Grilles K, Daum G, Mas A, Beltran G, Torija MJ. The role of the membrane lipid composition in the oxidative stress tolerance of different wine yeasts. Food Microbiol. (2019) 78:143–54. doi: 10.1016/j.fm.2018.10.001
17. Dingeo C, Difonzo G, Paradiso VM, Rizzello CG, Pontonio E. Teff type-I sourdough to produce gluten-free muffin. Microorganisms. (2020) 8:1149. doi: 10.3390/microorganisms8081149
18. Filannino P, Gobbetti M, De Angelis M, Di Cagno R. Hydroxycinnamic acids used as external acceptors of electrons: an energetic advantage for strictly heterofermentative lactic acid bacteria. Appl Environ Microbiol. (2014) 80:7574–82. doi: 10.1128/AEM.02413-14
19. Di Cagno R, Filannino P, Cantatore V, Polo A, Celano G, Martinovic A, et al. Design of potential probiotic yeast starters tailored for making a cornelian cherry (Cornus mas L.) functional beverage. Int J Food Microbiol. (2020) 323:108591. doi: 10.1016/j.ijfoodmicro.2020.108591
20. Rizzello CG, Nionelli L, Coda R, De Angelis M, Gobbetti M. Effect of sourdough fermentation on stabilisation, and chemical and nutritional characteristics of wheat germ. Food Chem. (2010) 119:1079–89. doi: 10.1016/j.foodchem.2009.08.016
21. De Angelis M, Campanella D, Cosmai L, Summo C, Rizzello CG, Caponio F. Microbiota and metabolome of un-started and started Greek-type fermentation of Bella di Cerignola table olives. Food Microbiol. (2015) 52:18–30. doi: 10.1016/j.fm.2015.06.002
22. Caponio F, Difonzo G, Calasso M, Cosmai L, De Angelis M. Effects of olive leaf extract addition on fermentative and oxidative processes of table olives and their nutritional properties. Int Food Res J. (2019) 116:1306–17. doi: 10.1016/j.foodres.2018.10.020
23. Difonzo G, Russo A, Trani A, Paradiso VM, Ranieri M, Pasqualone A, et al. Green extracts from Coratina olive cultivar leaves: antioxidant characterization and biological activity. J Funct Foods. (2017) 31:63–70. doi: 10.1016/j.jff.2017.01.039
24. Liljeberg H, Åkerberg A, Björck I. Resistant starch formation in bread as influenced by choice of ingredients or baking conditions. Food Chem. (1996) 56:389–94. doi: 10.1016/0308-8146(95)00199-9
25. Goñi I, Garcia-Alonso A, Saura-Calixto F. A starch hydrolysis procedure to estimate glycemic index. Nutr Res. (1997) 17:427–37. doi: 10.1016/S0271-5317(97)00010-9
26. Difonzo G, Pasqualone A, Silletti R, Cosmai L, Summo C, Paradiso VM, et al. Use of olive leaf extract to reduce lipid oxidation of baked snacks. Int Food Res J. (2018) 108:48–56. doi: 10.1016/j.foodres.2018.03.034
27. Orsavova J, Misurcova L, Ambrozova JV, Vicha R, Mlcek J. Fatty acids composition of vegetable oils and its contribution to dietary energy intake and dependence of cardiovascular mortality on dietary intake of fatty acids. Int J Mol Sci. (2015) 16:12871–90. doi: 10.3390/ijms160612871
28. Nunes MA, Costa AS, Bessada S, Santos J, Puga H, Alves RC, et al. Olive pomace as a valuable source of bioactive compounds: a study regarding its lipid-and water-soluble components. Sci Total Environ. (2018) 644:229–36. doi: 10.1016/j.scitotenv.2018.06.350
29. Tufariello M, Durante M, Veneziani G, Taticchi A, Servili M, Bleve G, et al. Patè olive cake: possible exploitation of a by-product for food applications. Front Nutr. (2019) 6:3. doi: 10.3389/fnut.2019.00003
30. Haddadin MS, Abdulrahim SM, Al-Khawaldeh GY, Robinson RK. Solid state fermentation of waste pomace from olive processing. J Chem Technol Biotechnol. (1999) 74:613–8. doi: 10.1002/(SICI)1097-4660(199907)74:7<613::AID-JCTB80>3.0.CO
31. Neifar M, Jaouani A, Ayari A, Abid O, Ben H, Boudabous A, et al. Improving the nutritive value of olive cake by solid state cultivation of the medicinal mushroom Fomes fomentarius. Chemosphere. (2013) 91:110–4. doi: 10.1016/j.chemosphere.2012.12.015
32. Medouni-Haroune L, Zaidi F, Medouni-Adrar S, Kecha M. Olive pomace: From an olive mill waste to a resource, an overview of the new treatments. J Crit Rev. (2018) 5:1–6. doi: 10.22159/jcr.2018v5i5.28840
33. Gobbetti M, Di Cagno R, Calasso M, Neviani E, Fox PF, De Angelis M. Drivers that establish and assembly the lactic acid bacteria biota in cheeses. Trends Food Sci Technol. (2018) 78:244–54. doi: 10.1016/j.tifs.2018.06.010
34. Nanditha B, Prabhasankar P. Antioxidants in bakery products: a review. Crit Rev Food Sci Nut. (2008) 49:1–27. doi: 10.1080/10408390701764104
35. Obied HK, Bedgood DR Jr, Prenzler PD, Robards K. Bioscreening of Australian olive mill waste extracts: biophenol content, antioxidant, antimicrobial and molluscicidal activities. Food Chem Toxicol. (2007) 45:1238–48. doi: 10.1016/j.fct.2007.01.004
36. Difonzo G, Squeo G, Calasso M, Pasqualone A, Caponio F. Physico-chemical, microbiological and sensory evaluation of ready-to-use vegetable pâté added with olive leaf extract. Foods. (2019) 8:138. doi: 10.3390/foods8040138
37. Rodríguez H, Curiel JA, Landete JM., de las Rivas B, de Felipe FL, Gómez-Cordovés C, et al. Food phenolics and lactic acid bacteria. Int J Food Microbiol. (2009) 132:79–90. doi: 10.1016/j.ijfoodmicro.2009.03.025
38. Fathy SA, Mahmoud AE, Rashad MM, Ezz MK, Mohammed AT. Improving the nutritive value of olive pomace by solid state fermentation of Kluyveromyces marxianus with simultaneous production of gallic acid. Int J Recycl Org Waste Agric. (2018) 7:135–41. doi: 10.1007/s40093-018-0199-5
39. Cedola A, Cardinali A, D'Antuono I, Conte A, Del Nobile MA. Cereal foods fortified with by-products from the olive oil industry. Food Biosci. (2020) 33:100490. doi: 10.1016/j.fbio.2019.100490
40. Gänzle MG, Ehmann M, Hammes WP. Modeling of growth of Lactobacillus sanfranciscensis and Candida milleri in response to process parameters of sourdough fermentation. Appl Environ Microbiol. (1998) 64:2616–23. doi: 10.1128/AEM.64.7.2616-2623.1998
41. Blana VA, Grounta A, Tassou CC, Nychas GJE, Panagou EZ. Inoculated fermentation of green olives with potential probiotic Lactobacillus pentosus and Lactobacillus plantarum starter cultures isolated from industrially fermented olives. Food Microbiol. (2014) 38:208–18. doi: 10.1016/j.fm.2013.09.007
42. Bonatsou S, Tassou CC, Panagou EZ, Nychas GJE. Table olive fermentation using starter cultures with multifunctional potential. Microorganisms. (2017) 5:30. doi: 10.3390/microorganisms5020030
43. Sun L, Sun X, Du Y, Fang Y, Yang W, Hu Q, et al. Effect of the starch structure fermented by Lactobacillus plantarum LB-1 and yeast on rheological and thermomechanical characteristics of dough. Food Chem. (2022) 369:130877. doi: 10.1016/j.foodchem.2021.130877
44. Hassani A, Procopio S, Becker T. Influence of malting and lactic acid fermentation on functional bioactive components in cereal-based raw materials: a review paper. Int J Food Sci. (2016) 51:14–22. doi: 10.1111/ijfs.12965
45. Difonzo G, Squeo G, Pasqualone A, Summo C, Paradiso VM, Caponio F. The challenge of exploiting polyphenols from olive leaves: addition to foods to improve their shelf-life and nutritional value. J Sci Food Agric. (2021) 101:3099–116. doi: 10.1002/jsfa.10986
46. Mateos R, Martínez-López S, Arévalo GB, Amigo-Benavent M, Sarriá B, Bravo-Clemente L. Hydroxytyrosol in functional hydroxytyrosol-enriched biscuits is highly bioavailable and decreases oxidised low density lipoprotein levels in humans. Food Chem. (2016) 205:248–56. doi: 10.1016/j.foodchem.2016.03.011
47. Conte P, Pulina S, Del Caro A, Fadda C, Urgeghe PP, De Bruno A, et al. Gluten-free breadsticks fortified with phenolic-rich extracts from olive leaves and olive mill wastewater. Foods. (2021) 10:923. doi: 10.3390/foods10050923
48. Demirkesen-Bicak H, Arici M, Yaman M, Karasu S, Sagdic O. Effect of different fermentation condition on estimated glycemic index, in vitro starch digestibility, and textural and sensory properties of sourdough bread. Foods. (2021) 10:514. doi: 10.3390/foods10030514
49. Curiel JA, Coda R, Centomani I, Summo C, Gobbetti M, Rizzello CG. Exploitation of the nutritional and functional characteristics of traditional Italian legumes: the potential of sourdough fermentation. Int J Food Microbiol. (2015) 196:51–61. doi: 10.1016/j.ijfoodmicro.2014.11.032
50. Nionelli L, Montemurro M, Pontonio E, Verni M, Gobbetti M, Rizzello CG. Pro-technological and functional characterization of lactic acid bacteria to be used as starters for hemp (Cannabis sativa L) sourdough fermentation and wheat bread fortification. Int J Food Microbiol. (2018) 279:14–25. doi: 10.1016/j.ijfoodmicro.2018.04.036
51. Kocadagli T, Žilić S, Taş NG, Vančetović J, Dodig D, Gökmen V. Formation of α-dicarbonyl compounds in cookies made from wheat, hull-less barley and colored corn and its relation with phenolic compounds, free amino acids and sugars. Eur Food Res Technol. (2016) 242:51–60. doi: 10.1007/s00217-015-2517-8
52. Rainero G, Bianchi F, Rizzi C, Cervini M, Giuberti G, Simonato B. Breadsticks fortification with red grape pomace: effect on nutritional, technological, and sensory properties. J Sci Food Agric. (2021). doi: 10.1002/jsfa.11596
53. Rathnayake HA, Navaratne SB, Navaratne CM. Porous crumb structure of leavened baked products. Int J Food Sci. (2018) 2018:1–15. doi: 10.1155/2018/8187318
54. Marquez GR, Di Pierro P, Esposito M, Mariniello L, Porta R. Application of transglutaminase-crosslinked whey protein/pectin films as water barrier coatings in fried and baked foods. Food Bioproc Tech. (2014) 7:447–55. doi: 10.1007/s11947-012-1045-9
55. Angioloni A, Romani S, Pinnavaia GG, Dalla Rosa M. Characteristics of bread making doughs: influence of sourdough fermentation on the fundamental rheological properties. Eur Food Res Technol. (2006) 222:54–7. doi: 10.1007/s00217-005-0087-x
56. Wójciak KM, Dolatowski ZJ. Oxidative stability of fermented meat products. Acta Sci Pol Technol Aliment. (2012) 11:99–109.
57. Di Cagno R, Surico RF, Paradiso A, De Angelis M, Salmon JC, Buchin S, et al. Effect of autochthonous lactic acid bacteria starters on health-promoting and sensory properties of tomato juices. Int J Food Microbiol. (2009) 128:473–83. doi: 10.1016/j.ijfoodmicro.2008.10.017
58. Caponio F, Summo C, Pasqualone A, Paradiso VM, Gomes T. Influence of processing and storage time on the lipidic fraction of taralli. J Food Sci. (2009) 74:C701–6. doi: 10.1111/j.1750-3841.2009.01357.x
59. Giarnetti M, Caponio F, Paradiso VM, Summo C, Gomes T. Effect of the type of oil on the evolution of volatile compounds of taralli during storage. J Food Sci. (2012) 77:326–31. doi: 10.1111/j.1750-3841.2011.02613.x
60. Caponio F, Cosmai L, Giarnetti M, Gomes T, Summo C, Paradiso VM, et al. comparative study on oxidative and hydrolytic stability of monovarietal extra virgin olive oil in bakery products. Food Res Int. (2013) 54:1995–2000. doi: 10.1016/j.foodres.2013.06.022
61. Pico J, Bernal JL, Gómez M. Influence of different flours and starches on gluten-free bread aroma. J Food Sci Technol. (2017) 54:1433–41. doi: 10.1007/s13197-017-2562-3
62. Pico J, Hansen ÅS, Petersen MA. Comparison of the volatile profiles of the crumb of gluten-free breads by DHE-GC/MS. J Cereal Sci. (2017) 76:280–8. doi: 10.1016/j.jcs.2017.07.004
63. Cherfaoui M, Cecchi T, Keciri S, Boudriche L. Volatile compounds of Algerian extra-virgin olive oils: effects of cultivar and ripening stage. Int J Food Prop. (2018) 21:36–49. doi: 10.1080/10942912.2018.1437627
64. Pasqualone A, Makhlouf FZ, Barkat M, Difonzo G, Summo C, Squeo G, et al. Effect of acorn flour on the physico-chemical and sensory properties of biscuits. Heliyon. (2019) 5:e02242. doi: 10.1016/j.heliyon.2019.e02242
65. Giarnetti M, Paradiso VM, Caponio F, Summo C, Pasqualone A. Fat replacement in shortbread cookies using an emulsion filled gel based on inulin and extra virgin olive oil. LWT-Food Sci Technol. (2015) 63:339–45. doi: 10.1016/j.lwt.2015.03.063
66. Mohsen SM, Fadel HH, Bekhit MA, Edris AE, Ahmed MY. Effect of substitution of soy protein isolate on aroma volatiles, chemical composition and sensory quality of wheat cookies. Int J Food Sci. (2009) 44:1705–12. doi: 10.1111/j.1365-2621.2009.01978.x
67. Pasqualone A, Bianco AM, Paradiso VM, Summo C, Gambacorta G, Caponio F. Physico-chemical, sensory and volatile profiles of biscuits enriched with grape marc extract. Int Food Res J. (2014) 65:385–93. doi: 10.1016/j.foodres.2014.07.014
68. Pasqualone A, Bianco AM, Paradiso VM, Summo C, Gambacorta G, Caponio F, et al. Production and characterization of functional biscuits obtained from purple wheat. Food Chem. (2015) 180:64–70. doi: 10.1016/j.foodchem.2015.02.025
69. Seuvre AM, Philippe E, Rochard S, Voilley A. Retention of aroma compounds in food matrices of similar rheological behaviour and different compositions. Food Chem. (2006) 96:104–14. doi: 10.1016/j.foodchem.2005.02.014
Keywords: gluten-free, olive cake, glycemic index, texture, upcycling, Lactiplantibacillus plantarum, Leuconostoc mesenteroides
Citation: Caponio GR, Difonzo G, de Gennaro G, Calasso M, De Angelis M and Pasqualone A (2022) Nutritional Improvement of Gluten-Free Breadsticks by Olive Cake Addition and Sourdough Fermentation: How Texture, Sensory, and Aromatic Profile Were Affected? Front. Nutr. 9:830932. doi: 10.3389/fnut.2022.830932
Received: 07 December 2021; Accepted: 11 January 2022;
Published: 10 February 2022.
Edited by:
Kolawole Banwo, University of Ibadan, NigeriaReviewed by:
Pablo Ribotta, Universidad Nacional de Córdoba, ArgentinaTomás García-Cayuela, Monterrey Institute of Technology and Higher Education (ITESM), Mexico
Copyright © 2022 Caponio, Difonzo, de Gennaro, Calasso, De Angelis and Pasqualone. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Graziana Difonzo, Z3JhemlhbmEuZGlmb256b0B1bmliYS5pdA==
 Giusy Rita Caponio1,2
Giusy Rita Caponio1,2 Graziana Difonzo
Graziana Difonzo Giuditta de Gennaro
Giuditta de Gennaro Maria Calasso
Maria Calasso Maria De Angelis
Maria De Angelis Antonella Pasqualone
Antonella Pasqualone