
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Nutr., 17 February 2022
Sec. Nutrition and Metabolism
Volume 9 - 2022 | https://doi.org/10.3389/fnut.2022.828824
This article is part of the Research TopicInsights in Nutrition and MetabolismView all 18 articles
 Hongna Mu1
Hongna Mu1 Ruiyue Yang1
Ruiyue Yang1 Siming Wang1
Siming Wang1 Wenduo Zhang2
Wenduo Zhang2 Xinyue Wang2
Xinyue Wang2 Hongxia Li1
Hongxia Li1 Jun Dong1
Jun Dong1 Wenxiang Chen3
Wenxiang Chen3 Xue Yu2*
Xue Yu2* Fusui Ji2*
Fusui Ji2*Ketone bodies, including β-hydroxybutyrate (BHB), acetoacetate (AA), and acetone, can substitute and alternate with glucose under conditions of fuel/food deficiency. Ketone-body metabolism is increased in a myriad of tissue-metabolism disorders. Perturbations in metabolism are major contributors to coronary artery disease (CAD). We investigated the association of BHB with CAD. A total of 2,970 people of Chinese Han ethnicity were enrolled. The Gensini score was calculated for all patients who had positive findings. The serum level of BHB and other laboratory parameters were measured. The association of serum levels of metabolites with traditionally risk factors and CAD severity was analyzed. The BHB was found to be associated with some traditional risk factors of CAD and CAD severity, as determined by the Gensini score or the number of diseased regions. Moreover, BHB was associated with the T3/T1 tertiles of the Gensini score after the adjustment for traditional risk factors by multivariable logistic regression analysis. The association of BHB with CAD severity was more obvious in women. Taken together, these data suggest that the circulating BHB level is independently associated with CAD severity, and that this association is more pronounced in women.
Coronary artery disease (CAD) caused by atherosclerosis, has been reported to be the leading cause of death worldwide. Although new medical therapies have emerged in recent years, the risk of death from CAD is still high (1). Therefore, there is a need for the development of more accurate diagnostic methods, identification of novel biomarkers, more efficacious drugs and identification of new therapeutic targets to reduce the risk of death from CAD (2, 3).
Metabolic dysfunction is a hallmark of CAD pathophysiology, reflecting not only the altered metabolism of the myocardium but also the overall contributions from peripheral tissues and organs (4, 5). These metabolic changes influence disease pathophysiology directly, and may serve as earlier and more sensitive markers of CAD (6, 7). Abnormal accumulation or deficiency of specific metabolites within the circulation can be powerful markers in the management of CAD, with regard to the detecting disease, evaluating disease progression, and assessing therapeutic efficacy (8, 9).
Ketone bodies [e.g., β-hydroxybutyrate (BHB), acetoacetate (AA), and acetone] are water-soluble molecules that contain the ketone groups produced from fatty acids by the liver (ketogenesis). Ketone bodies are derived from increased beta oxidation of free fatty acid, BHB is a major component of the ketone body (10). During perturbed tissue metabolism, an increase in BHB may help circumvent the metabolic malfunctions that directly contribute to disease pathophysiology (11). Recent studies have shown that BHB level was increased in a myriad of pathological conditions, such as type 2 diabetes mellitus (T2DM) (12), arrhythmogenic cardiomyopathy (13), and heart failure (14). In addition, it was reported that BHB was increased in patients with non-ST segment elevation acute coronary syndrome, which reflects the oxidative stress and hypoxic state that myocardial cells suffer. Hence, BHB could be used for the early diagnosis of acute coronary syndrome (15). Obokata and colleagues showed that an increased serum level of BHB was independently associated with cardiovascular events and all-cause death in patients undergoing hemodialysis (16). Those studies implied a potential association of BHB with CAD. However, studies on the associations between BHB and CAD in Chinese Han populations have not been carried out. Here, we evaluated the association of BHB with CAD, as well as the severity of CAD, and risk factors of CAD in a case–control study.
The protocol for this cross-sectional study was approved (2016BJYYEC-121-03) by the Ethics Committee of Beijing Hospital (Beijing, China). Written informed consent was provided by all participants.
The exclusion criteria were patients: (i) with severe congenital heart disease, severe cardiac insufficiency, primary pulmonary hypertension, hepatic/renal dysfunction, severe peripheral arterial disease, or related conditions which are contraindications to cardiac catheterization; (ii) receiving radiotherapy or chemotherapy; (iii) who were pregnant or nursing a baby; (iv) suffering from alcoholism or drug abuse; and (v) under treatment for mental illness.
Patients suspected of having CAD (or adjudicated to have a CAD history) and subjected to coronary angiography in Beijing Hospital between March 2017 and October 2020 were enrolled. The demographic characteristics (e.g., age and gender), medical history (e.g., DM, hypertension, and dyslipidemia), cigarette smoking, and body mass index (BMI) of the study cohort were recorded. Before coronary angiography, samples of venous blood were collected after an overnight fast. The serum was isolated, aliquoted, and stored at −80°C until analyses.
Coronary angiography was performed for CAD assessment by experienced cardiologists. All targeted coronary lesions of enrolled patients were analyzed by the built-in QCA software of the Allura Xper FD20 Angiography System (Philips Healthcare, Amsterdam, the Netherlands). Fifteen coronary segments were analyzed quantitatively based on the American Heart Association classification. According to current guidelines, “coronary stenosis” was defined as a reduction of >50% in the arterial diameter, and if this was not present, was diagnosed as non-CAD. In addition, individuals with negative findings on CT of the coronary arteries or stress myocardial perfusion imaging were categorized as the non-CAD group.
The Gensini score was used to assess the severity of damage to coronary arteries (17). The Gensini score was computed by assigning a severity score for each coronary stenosis depending on the degree of luminal narrowing and its importance based on location. Luminal stenoses of 25, 50, 75, 90, 99%, and total occlusion were given a Gensini score of 1, 2, 4, 8, 16, and 32, respectively. Then, these scores were multiplied by a factor according to location: 5, left main coronary artery; 2.5, proximal segment of the left anterior descending coronary artery and proximal segment of the circumflex artery; 1.5, mid-segment of the left anterior descending coronary artery; 1.0, right coronary artery, distal segment of the left anterior descending coronary artery, posterior descending artery, and obtuse marginal artery; and 0.5, other segments. Finally, the Gensini score was calculated by summation of the scores for individual coronary segments. The Gensini score was calculated in 2,047 patients who had not undergone percutaneous arterial intervention previously.
The serum concentration of BHB was quantified using an LC-MS/MS system comprising a 1,260 Infinity II Series high-performance liquid chromatography (HPLC) (Agilent, Santa Clara, CA, USA) coupled with a Sciex 5,500 QTRAP mass spectrometer (AB Sciex, Foster City, CA, USA). MS parameters were set as previously described (18). BHB standards, serum samples, and quality controls were, respectively, mixed with the isotopically labeled internal standard (BHB-D4) solution and proteins were precipitated with methanol containing 0.5% formic acid. After vortexing and centrifuging, the metabolites were separated by a Kinetex™ hydrophilic interaction LC column (2.6 μm, 150 mm × 2.1 mm, Phenomenex, Torrance, CA, USA) and eluted with a mobile phase of 75% methanol, containing 5 mmol/L ammonium formate, and 0.05% formic acid at a flow rate of 0.3 ml/min. BHB and internal standard were detected with positive electrospary ionization in multiple reaction monitor mode using characteristic precursor–product ion transitions of m/z 103.0 → 59.0 and m/z 107.0 → 59.0, respectively. The concentration of BHB was calculated using standard curves. The analytical coefficients of variation for BHB measurements were below 3%. Applied Biosystems Analyst version 1.6.2 software was used for system control, data collection, and processing.
In addition, the serum samples were tested for fasting blood glucose (FBG), total cholesterol (TC), triglycerides (TG), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), creatinine (Crea), and uric acid (UA) using the respective assay kits (Sekisui Medical Technologies, Osaka, Japan) on a chemistry analyzer (7,180 series; Hitachi, Tokyo, Japan). Two quality control materials, prepared by mixed fresh serum samples, were analyzed with patient samples in each run in LC-MS/MS and the laboratory assays to monitor the performance of the measurements.
The normal distribution of samples was tested. Parameters with normal distribution are expressed as mean ± SD, while parameters with a skewed distribution are presented as the median and percentile (25–75th). Count data are reported as frequencies and percentages. The one-way ANOVA was conducted to compute the differences for continuous variables, while the non-parametric Jonckheere-Terpstra test was used to determine the differences when the data did not have a normal distribution. For categorical variables, the Chi-square test was used. Spearman's correlation analysis was employed to examine the associations between BHB and traditional CAD risk factors. The multivariable logistic regression analysis was employed to determine the relationship between the BHB level and the Gensini score. Potential confounding variables (age, gender, smoking status, obesity or overweight, hypertension, dyslipidemia, DM, history of stroke, and family history of premature CAD) were controlled in the regression models. Results are presented as odds ratios (ORs) and 95% CIs. The value of p < 0.05 (two-tailed) was considered significant. Statistical analysis was performed using SPSS 25.0 (IBM, Armonk, NY, USA).
The study population comprised 2,970 patients (62.5% men). Patient characteristics at baseline according to the tertile of the BHB levels are summarized in Table 1. Individuals with a higher BHB level also had a higher systolic blood pressure (SBP), higher risk of a family history of premature CAD as well as a higher prevalence of DM and dyslipidemia. Furthermore, with the increase of BHB levels, FBG, TC, and LDL-C significantly increased (p < 0.001 for trend). People with a higher BHB level tended to be older (but not significantly so). Whereas, levels of BMI, diastolic blood pressure (DBP), TG, HDL-C, Crea, and UA were similar among the groups. In addition, associations were not found between the BHB level and overweight/obesity, hypertension, history of stroke, smoking status, or statins use.
A significant positive correlation was found between the serum BHB level and the level of FBG (r = 0.067, p < 0.001), TC (r = 0.070, p < 0.001), and LDL-C (r = 0.066, p = 0.001). The serum BHB level was significantly positively correlated with age (r = 0.041, p < 0.05) and SBP (r = 0.040, p < 0.05). However, an association between the BHB level and level of DBP, TG, HDL-C, Crea, UA, and BMI was not significant (Table 2).
To analyze the relationship between the BHB level and CAD severity, patients with positive findings upon coronary angiography were classified into subgroups for those with 1–3 and >3 stenosed regions. The BHB level was significantly increased (ptrend = 0.016) with an increase in the number of stenosed regions. Then, the patients with CAD were divided into subgroups with 1 stenosed or >1 stenosed vessels. As shown in Figure 1, no significant change was found for the BHB level among groups. The Gensini score is widely used to evaluate the severity of coronary atherosclerosis, hence the studied patients were divided into different subgroups according to tertile of the Gensini score: the BHB level was associated with the Gensini score (ptrend = 0.05).
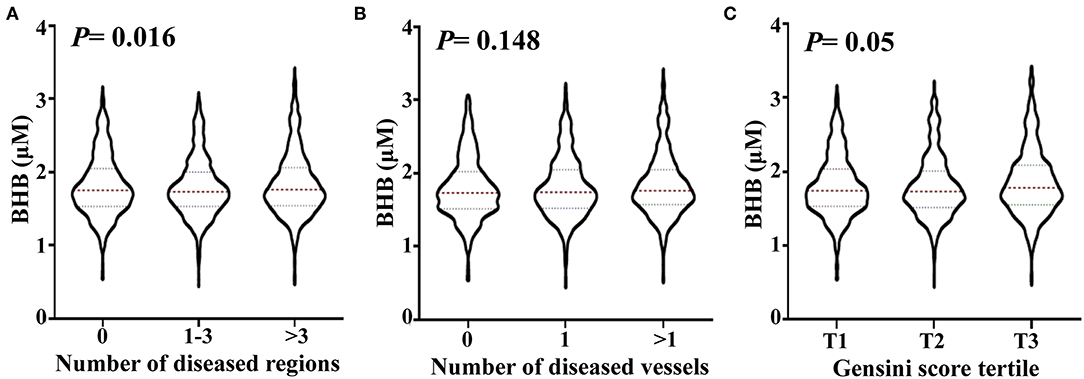
Figure 1. Association of the serum BHB level with the severity of coronary artery lesion. Violin plots of serum BHB concentrations at presentation with the number of stenosed regions (A), the number of stenosed vessels (B) and tertile of the Gensini scores (C), showing median (red dashed line) and interquartile ranges (blue dashed line) on a log 10 scale. p < 0.05 considered statistically significant.
Multivariate logistic regression analysis was done to examine the independent relationship between the BHB level and CAD severity. Here, we adopted tertiles of T3/T1 of the Gensini score to represent CAD severity. As shown in Table 3, the crude OR was increased with increasing BHB levels in Model 1 (p = 0.004). After adjustment for age and gender (Model 2), high BHB levels were significantly associated with CAD severity, with OR (95% CI) of 1.178 (1.045–1.327), p = 0.007. In Model 3, traditional risk factors of CAD [age, smoking (former and current smoking), hypertension, DM, and family history of premature CAD] were all positively associated with the Gensini score, whereas being female was a protective factor for CAD, with OR of 0.417 (0.308–0.564). An increased level of BHB continued to be independently associated with T3/T1 tertiles of the Gensini score (OR = 1.151, 95% CI: 1.012–1.309, p = 0.032), after adjustment for traditional risk factors, such as age, gender, smoking status, overweight/obesity, hypertension, dyslipidemia, DM, history of stroke, and family history of premature CAD (Table 3).
Stratified analysis of the association of the serum BHB level (per 1-SD increment) and the severity of coronary artery lesion was conducted in different groups of age, gender, BMI, smoking status, hypertension, dyslipidemia, DM, history of stroke, and family history of premature CAD. The interaction between the BHB level and these factors on CAD distribution was analyzed. As presented in Figure 2, there was a significant association between the BHB level and CAD in women, non-smokers, people with hypertension, DM, or a family history of premature CAD. Moreover, we observed that the association between the BHB level and CAD was modified by gender (Pinteraction = 0.015). The BHB level was associated with CAD in women (OR: 1.429, 95% CI: 1.138–1.793, p = 0.002), but not in men (OR: 1.028, 95% CI: 0.892–1.1840, p = 0.704), after adjustment for age, BMI, smoking status, hypertension, dyslipidemia, DM, history of stroke, and family history of premature CAD. However, an interaction was not observed between the BHB level and age, BMI, smoking status, hypertension, dyslipidemia, DM, history of stroke or family history of premature CAD (Figure 2). Then, we further analyzed the association of the BHB level with CAD severity in men and women, respectively, according to the Gensini score. Consistent with this, the BHB level showed a significant association with the Gensini score in women, but not in men (Figure 3).
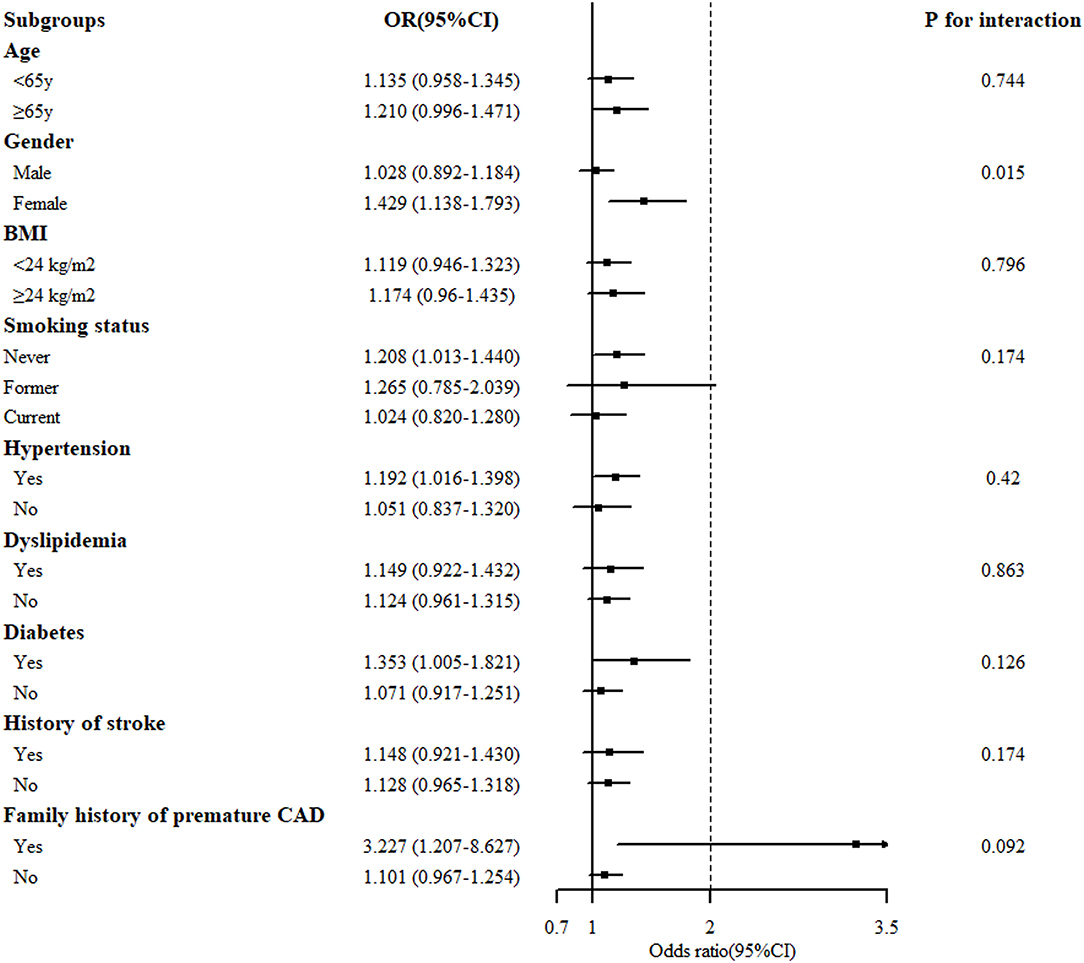
Figure 2. Stratified analysis of the association [odds ratio (OR) (95% CI)] between the circulating BHB level (per 1-SD increment) and the severity of coronary artery lesion. Values are adjusted for age, gender, smoking status, obesity or overweight, hypertension, dyslipidemia, diabetes, stroke, and family history of premature CAD, stratifying factors excepted. The p < 0.05 considered statistically significant.
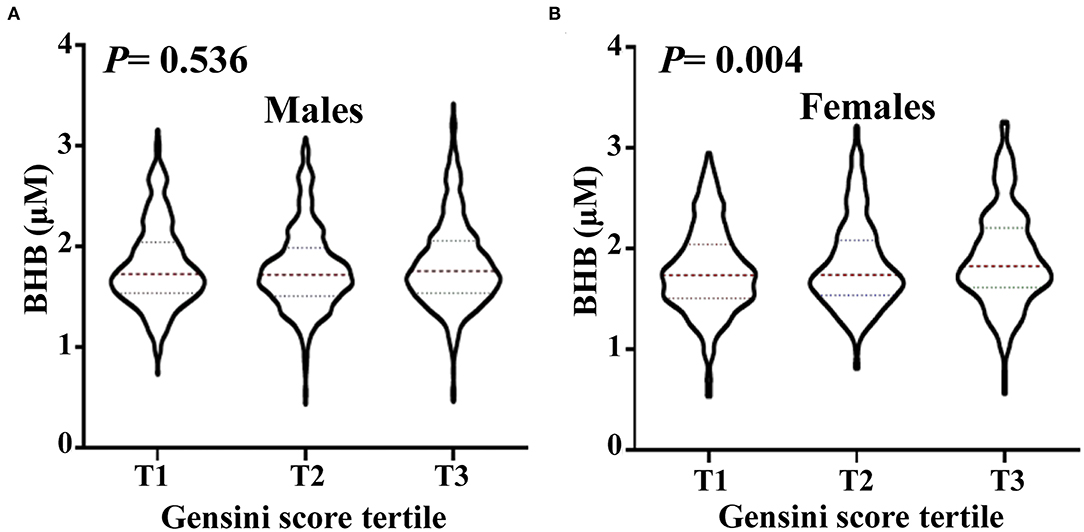
Figure 3. The level of circulating BHB in men and women, respectively, according to Gensini score tertile. Violin plots of serum BHB concentrations in men (A) and women (B) by Gensini score tertile, showing median (red dashed line) and interquartile ranges (blue dashed line) on a log10 scale. The p < 0.05 considered statistically significant.
In this study, we observed circulating BHB concentration was associated with the traditional risk factors and severity of CAD. An increased BHB level was associated with increased CAD severity, as defined by Gensini score or number of stenosed regions. This association of the BHB level with the Gensini score remained significant after adjustment for the conventional risk factors of CAD by multivariable logistic regression analysis. An increased level of BHB in patients with CAD indicated a more severe state of the disease and more severe systemic metabolic perturbations, especially in women (Figure 4). To the best of our knowledge, this study is the first to report that an increased BHB level to be associated with an increased risk of CAD in a Chinese population.
The heart is the most metabolically demanding organ in the body, so impaired metabolism may lead to CAD, thus, the identification and quantification of changes in levels of these metabolites is critical for the diagnosis and treatment of CAD (19). Ketone bodies, such as BHB, AA, and acetone, are “small fuel substrates,” derived from increased beta oxidation of free fatty acid. These substances can uniquely substitute and alternate with glucose under conditions of fuel/food deficiency (20). There is considerable evidence that the level of ketone bodies, in particular BHB, is increased in metabolic disorders. The most robust evidence is from a retrospective cohort study which demonstrates that the BHB level is independently and significantly associated with adverse cardiovascular events and therefore could serve as a novel biomarker predicting the survival and risk of cardiovascular events in patients undergoing hemodialysis (16). Furthermore, the circulating level of ketone bodies is increased in patients presenting with ST-segment elevation myocardial infarction and a higher level of ketone bodies after 24 h is associated with functional outcomes. Hence, the increased metabolism of ketones may play a part in the response to myocardial ischemia (21). Accumulating evidence has shown that the BHB level is increased in patients with heart failure (14, 22), obese and/or T2DM (12, 23). Of importance, with metabolic dysfunction as an inherent feature of the CAD pathophysiology, whether circulating BHB is increased in CAD remains poorly understood. We investigated if the BHB level is associated with CAD in a Chinese Han population.
First we analyzed the association between the circulating BHB concentrations with CAD traditional risk factors. The result revealed a strong association between the BHB level with the concentration of FBG, TC, and LDL-C, as well as a significant association between the BHB concentration and age and SBP. These results were not in line with some of the previous reports (13, 24), possibly on account of different populations studied. High TC and high LDL-C all play a role in CAD (25–28). We discovered a significant association between BHB with TC and LDL-C (Table 2). However, multivariable logistic regression analysis revealed dyslipidemia not to be associated with CAD severity. On account of the use of statins therapy, the relationship between the clinical diagnosis of dyslipidemia and CAD was not significant.
Next, we analyzed the relation of BHB with CAD and CAD severity. The study population was first divided into the non-CAD group and CAD group according to coronary angiography. People who developed CAD did not have a significantly higher BHB level in serum compared with that in healthy controls (data not shown). In a pilot study undertaken in Japan, Omori et al. found the BHB level to be significantly lower in the CAD group compared with that in the control subjects (in patients with DM), but the study cohorts were quite small (5). To analyze the association between the BHB level and CAD severity, the CAD group was divided into subgroups, based on the number of stenosed vessels or regions: the BHB level was associated with the severity of CAD according to the number of stenosed regions. Gensini score is widely used for evaluating the coronary atherosclerotic severity with the judgement of the position, number, and stenosis of blood vessels caused by coronary atherosclerosis (17). The CAD patients were divided into 3 groups according to the Gensini score tertile, and the BHB level showed a significant association with the Gensini score tertile (Figure 1). Multivariable logistic regression analysis revealed the BHB level to be independently associated with the T3/T1 tertiles of the Gensini score, even after adjustment for other well-known risk factors (Table 3).
Additional stratification analysis revealed the BHB level to have a strong association with CAD in women (Figure 2). Furthermore, in accordance with this, Figure 3 revealed that the BHB level was associated with CAD severity in women not men, according to the Gensini score. These results indicated that BHB may be involved in the progress of atherosclerosis especially in women. Actually, cardiac metabolism and the burden of risk factors differ by gender (29, 30), gender may have an influence on both the development and progression of CAD and on the pattern of compositional plaque progression, and women have a higher prevalence of mortality from CAD than men (31). Thus, the role of BHB in the development and progression of CAD in women merits further investigation.
Traditionally, severe ketosis, or ketoacidosis, is known as a harmful event. Ketone bodies are synthesized in the liver from acetyl-coenzyme-A derived primarily from fatty acid β-oxidation and are transported to extrahepatic tissues for terminal oxidation as a source for adenosine triphosphate generation at the expense of glucose oxidation (32). Myocardial ischemia occurs when coronary blood flow is inadequate, and hence, the oxygen supply to the myocardium is not sufficient to meet the oxygen demand. Ischemia elicits disturbances in the balance between energy metabolism and cardiac function (33). It seems that hepatic ketogenesis is activated and cardiac utilization is not enhanced, the concentration of blood ketone bodies is highly increased in patients with ischemic heart disease (29, 33). In addition, people suffering from DM not only have increased production of ketones, their ketone clearance is also decreased significantly, because of low insulin levels, and decreased activities of succinyl-coenzyme-A: 3 oxoacid coenzyme-A-transferase and β-hydroxybutyrate dehydrogenase, which all contribute to an increased level of ketones (34). DM is a major risk factor for CAD, and many patients with DM have CAD, which is frequently associated with insulin resistance; a similar scenario has been noted for patients with CAD with an increased level of BHB (35, 36). A clinical study also supports this concept: patients with CAD have deficits in metabolic fuel extraction, with reduced extraction of fatty acid and ketone compared with that in controls (37). What's more, an increased level of BHB has an effect on upregulating expression of intercellular adhesion molecule-1 in endothelial cells (38), increasing the secretion of tumor necrosis factor-alpha in cultured U937 monocytes (39), and promoting lipid peroxidation levels in patients with DM (40). These phenomena would be implicated in the development of vascular disease and atherosclerosis (34).
However, recent evidence from experimental studies and clinical research has uncovered a protective role for ketones in cardiovascular disease. BHB infusion has been shown to specifically improve working memory performance in patients with T2DM in the absence of changes in global cognition (41). Even in healthy people, exogenous administration of ketones has been shown to improve the cardiac function (42). In addition, data from animal experiments have shown that ketones aid the prevention and treatment of heart failure by reducing inflammation (43), normalizing myocardial adenosine triphosphate production (44), and ameliorating pathologic cardiac remodeling and dysfunction (45). A hepatocyte-macrophage “AA shuttle” has been shown to coordinate the fibrogenic response to hepatic injury via mitochondrial metabolism in tissue macrophages after high fat diet-induced fibrosis (46). Furthermore, BHB has been found to protect the heart from ischemia/reperfusion injury (47) and attenuate atherosclerosis (48) in different mice models. Indeed, ketone bodies possess various capabilities and participate in multiple cellular processes (49), especially in cardiovascular disease, further studies to ascertain the action and specific mechanism of BHB in CAD are warranted.
During fast, DM and other pathological conditions, ketone bodies are all increased, the blood levels of BHB can be used to reflect ketotic state of a patient and American Diabetes Association prefers quantitative determination of blood BHB for the diagnosis and follow-up of ketoacidosis (50, 51). In ketone bodies, BHB is the most abundant in mammals. BHB has been found at a higher concentration than that of AA and acetone in subjects of DM and myocardial infarction. In cohort studies, BHB is the ketone body studied most often (21, 34, 52). Therefore, we focused only on BHB in this study, which is one limitation. The serum level of acetone is very low and the potential role of acetone in CAD is poorly known. AA is the central ketone body, and the other ketone bodies are derived from it. An increased level of AA has a role in oxidative modification of LDLs and then causes impaired cholesterol uptake and its deposition in the arterial wall and atherosclerosis in patients with DM (53). AA can also increase interleukin-6 secretion in U937 monocytes (54), upregulate expression of intercellular adhesion molecule-1 (55), facilitate the activity of NADPH oxidase (56), and promote glutathione depletion (57) in human umbilical vein endothelial cells. Those actions aggravate the inflammatory response, monocyte adhesion, and oxidative stress and contribute to the initiation and progression of CAD.
In this study, the control group already had high levels of cardiovascular risk factors and these people did not represent a true healthy control, which may have led to underestimation of the association between the metabolites and CAD and limited the power of this study. This is another limitation of the study.
Third, we did not adequately address that BHB as a biomarker for CAD can be an adaptive response that lack of enough evidence. Additionally, this study was a cross-sectional study, and we only addressed the association of BHB with CAD, this association does not inform causality. Therefore, further studies are necessary and the findings need to be confirmed in future prospective analysis. This is also a limitation of our study.
The circulating level of BHB was independently associated with CAD severity, and a gender-related difference in this association was documented. Further studies are needed to determine the exact role of BHB in CAD.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
The studies involving human participants were reviewed and approved by the Ethics Committee of Beijing Hospital. The patients/participants provided their written informed consent to participate in this study.
HM, FJ, and XY designed the research and drafted the article. HM, RY, and SW measured BHB in serum, performed the biochemical assays, and processed the data. WZ and XW performed coronary angiography and calculated the Gensini score. HL, JD, and WC calculated the number of stenosed vessels and regions. FJ and XY revised the manuscript. All authors contributed to the article and approved the submitted version.
This work was supported by the Beijing Natural Science Foundation (7214250), the National Key R&D Program of China (2020YFC2008304), the Non-profit Central Research Institute Fund of the Chinese Academy of Medical Sciences (2019TX310001), and the Beijing Hospital Special Fund Project (BJ-2019-184).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The authors thank Ranran Zhang and Ziyun Li for their excellent technical assistance.
BHB, β-hydroxybutyrate; BMI, body mass index; CAD, coronary artery disease; Crea, creatinine; DBP, diastolic blood pressure; DM, diabetes mellitus; FBG, fasting blood glucose; HDL-C, high density lipoprotein cholesterol; LDL-C, low density lipoprotein cholesterol; SBP, systolic blood pressure; TC, total cholesterol; TG, triglyceride; UA, uric acid.
1. Senthong V, Li XS, Hudec T, Coughlin J, Wu Y, Levison B, et al. Plasma trimethylamine N-Oxide, a gut microbe-generated phosphatidylcholine metabolite, is associated with atherosclerotic burden. J Am Coll Cardiol. (2016) 67:2620–8. doi: 10.1016/j.jacc.2016.03.546
2. Tunon J, Barbas C, Blanco-Colio L, Burillo E, Lorenzo O, Martin-Ventura JL, et al. Proteomics and metabolomics in biomarker discovery for cardiovascular diseases: progress and potential. Expert Rev Proteomics. (2016) 13:857–71. doi: 10.1080/14789450.2016.1217775
3. Kordalewska M, Markuszewski MJ. Metabolomics in cardiovascular diseases. J Pharm Biomed Anal. (2015) 113:121–36. doi: 10.1016/j.jpba.2015.04.021
4. Franssens BT, Van Der Graaf Y, Kappelle LJ, Westerink J, De Borst GJ, Cramer MJ, et al. Body weight, metabolic dysfunction, and risk of type 2 diabetes in patients at high risk for cardiovascular events or with manifest cardiovascular disease: a cohort study. Diabetes Care. (2015) 38:1945–51. doi: 10.2337/dc15-0684
5. Omori K, Katakami N, Yamamoto Y, Ninomiya H, Takahara M, Matsuoka TA, et al. Identification of metabolites associated with onset of CAD in diabetic patients using CE-MS analysis: a pilot study. J Atheroscler Thromb. (2019) 26:233–45. doi: 10.5551/jat.42945
6. Ussher JR, Elmariah S, Gerszten RE, Dyck JR. The emerging role of metabolomics in the diagnosis and prognosis of cardiovascular disease. J Am Coll Cardiol. (2016) 68:2850–70. doi: 10.1016/j.jacc.2016.09.972
7. Wang Z, Zhao Y. Gut microbiota derived metabolites in cardiovascular health and disease. Protein Cell. (2018) 9:416–31. doi: 10.1007/s13238-018-0549-0
8. Dona AC, Coffey S, Figtree G. Translational and emerging clinical applications of metabolomics in cardiovascular disease diagnosis and treatment. Eur J Prev Cardiol. (2016) 23:1578–89. doi: 10.1177/2047487316645469
9. Chen ZZ, Gerszten RE. Metabolomics and proteomics in type 2 diabetes. Circ Res. (2020) 126:1613–27. doi: 10.1161/CIRCRESAHA.120.315898
10. Moller N. Ketone body, 3-hydroxybutyrate: minor metabolite - major medical manifestations. J Clin Endocrinol Metab. (2020) 105:dgaa370. doi: 10.1210/clinem/dgaa370
11. Schugar RC, Moll AR, Andre D'avignon D, Weinheimer CJ, Kovacs A, Crawford PA. Cardiomyocyte-specific deficiency of ketone body metabolism promotes accelerated pathological remodeling. Mol Metab. (2014) 3:754–69. doi: 10.1016/j.molmet.2014.07.010
12. Vigili De Kreutzenberg S, Avogaro A. The role of point-of-care 3-hydroxybutyrate testing in patients with type 2 diabetes undergoing coronary angiography. J Endocrinol Invest. (2017) 40:627–34. doi: 10.1007/s40618-017-0615-0
13. Song JP, Chen L, Chen X, Ren J, Zhang NN, Tirasawasdichai T, et al. Elevated plasma beta-hydroxybutyrate predicts adverse outcomes and disease progression in patients with arrhythmogenic cardiomyopathy. Sci Transl Med. (2020) 12:1–13. doi: 10.1126/scitranslmed.aay8329
14. Flores-Guerrero JL, Westenbrink BD, Connelly MA, Otvos JD, Groothof D, Shalaurova I, et al. Association of beta-hydroxybutyrate with development of heart failure: sex differences in a dutch population cohort. Eur J Clin Invest. (2021) 51:e13468. doi: 10.1111/eci.13468
15. Laborde CM, Mourino-Alvarez L, Posada-Ayala M, Alvarez-Llamas G, Serranillos-Reus MG, Moreu J, et al. Plasma metabolomics reveals a potential panel of biomarkers for early diagnosis in acute coronary syndrome. Metabolomics. (2014) 10:414–24. doi: 10.1007/s11306-013-0595-9
16. Obokata M, Negishi K, Sunaga H, Ishida H, Ito K, Ogawa T, et al. Association between circulating ketone bodies and worse outcomes in hemodialysis patients. J Am Heart Assoc. (2017) 6:1–9. doi: 10.1161/JAHA.117.006885
17. Gensini GG. A more meaningful scoring system for determining the severity of coronary heart disease. Am J Cardiol. (1983) 51:606. doi: 10.1016/s0002-9149(83)80105-2
18. Tang Y, Wang S, Zhang W, Yang R, Yu X, Wang X, et al. A single-run, rapid polarity switching method for simultaneous quantification of cardiovascular disease-related metabolites using liquid chromatography–tandem mass spectrometry. Int J Mass Spec. (2021) 461:1–9. doi: 10.1016/j.ijms.2020.116500
19. Chen XF, Chen X, Tang X. Short-chain fatty acid, acylation and cardiovascular diseases. Clin Sci. (2020) 134:657–76. doi: 10.1042/CS20200128
20. Nielsen R, Moller N, Gormsen LC, Tolbod LP, Hansson NH, Sorensen J, et al. Cardiovascular effects of treatment with the ketone body 3-hydroxybutyrate in chronic heart failure patients. Circulation. (2019) 139:2129–41. doi: 10.1161/CIRCULATIONAHA.118.036459
21. De Koning MLY, Westenbrink BD, Assa S, Garcia E, Connelly MA, Van Veldhuisen DJ, et al. Association of circulating ketone bodies with functional outcomes after ST-segment elevation myocardial infarction. J Am Coll Cardiol. (2021) 78:1421–32. doi: 10.1016/j.jacc.2021.07.054
22. Du Z, Shen A, Huang Y, Su L, Lai W, Wang P, et al. 1H-NMR-based metabolic analysis of human serum reveals novel markers of myocardial energy expenditure in heart failure patients. PLoS ONE. (2014) 9:e88102. doi: 10.1371/journal.pone.0088102
23. Mahendran Y, Vangipurapu J, Cederberg H, Stancakova A, Pihlajamaki J, Soininen P, et al. Association of ketone body levels with hyperglycemia and type 2 diabetes in 9,398 Finnish men. Diabetes. (2013) 62:3618–26. doi: 10.2337/db12-1363
24. Stryeck S, Gastrager M, Degoricija V, Trbusic M, Potocnjak I, Radulovic B, et al. Serum concentrations of citrate, tyrosine, 2- and 3- hydroxybutyrate are associated with increased 3-month mortality in acute heart failure patients. Sci Rep. (2019) 9:6743. doi: 10.1038/s41598-019-42937-w
25. Miller M, Seidler A, Kwiterovich PO, Pearson TA. Long-term predictors of subsequent cardiovascular events with coronary artery disease and 'desirable' levels of plasma total cholesterol. Circulation. (1992) 86:1165–70. doi: 10.1161/01.cir.86.4.1165
26. Mostaza JM, Gomez MV, Gallardo F, Salazar ML, Martin-Jadraque R, Plaza-Celemin L, et al. Cholesterol reduction improves myocardial perfusion abnormalities in patients with coronary artery disease and average cholesterol levels. J Am Coll Cardiol. (2000) 35:76–82. doi: 10.1016/s0735-1097(99)00529-x
27. Maher VM, Brown BG, Marcovina SM, Hillger LA, Zhao XQ, Albers JJ. Effects of lowering elevated LDL cholesterol on the cardiovascular risk of lipoprotein(a). JAMA. (1995) 274:1771–4.
28. O'donoghue ML, Morrow DA, Tsimikas S, Sloan S, Ren AF, Hoffman EB, et al. Lipoprotein(a) for risk assessment in patients with established coronary artery disease. J Am Coll Cardiol. (2014) 63:520–7. doi: 10.1016/j.jacc.2013.09.042
29. Arima Y, Izumiya Y, Ishida T, Takashio S, Ishii M, Sueta D, et al. Myocardial ischemia suppresses ketone body utilization. J Am Coll Cardiol. (2019) 73:246–7. doi: 10.1016/j.jacc.2018.10.040
30. Hemal K, Pagidipati NJ, Coles A, Dolor RJ, Mark DB, Pellikka PA, et al. Sex differences in demographics, risk factors, presentation, and noninvasive testing in stable outpatients with suspected coronary artery disease: insights from the PROMISE trial. JACC Cardiovasc Imag. (2016) 9:337–46. doi: 10.1016/j.jcmg.2016.02.001
31. Lee SE, Sung JM, Andreini D, Al-Mallah MH, Budoff MJ, Cademartiri F, et al. Sex differences in compositional plaque volume progression in patients with coronary artery disease. JACC Cardiovasc Imag. (2020) 13:2386–96. doi: 10.1016/j.jcmg.2020.06.034
32. Cotter DG, Schugar RC, Crawford PA. Ketone body metabolism and cardiovascular disease. Am J Physiol Heart Circ Physiol. (2013) 304:H1060–76. doi: 10.1152/ajpheart.00646.2012
33. Lopaschuk GD, Ussher JR, Folmes CD, Jaswal JS, Stanley WC. Myocardial fatty acid metabolism in health and disease. Physiol Rev. (2010) 90:207–58. doi: 10.1152/physrev.00015.2009
34. Kanikarla-Marie P, Jain SK. Hyperketonemia and ketosis increase the risk of complications in type 1 diabetes. Free Radic Biol Med. (2016) 95:268–77. doi: 10.1016/j.freeradbiomed.2016.03.020
35. Naito R, Miyauchi K. Coronary artery disease and type 2 diabetes mellitus. Int Heart J. (2017) 58:475–80. doi: 10.1536/ihj.17-191
36. Rutter MK, Meigs JB, Sullivan LM, D'agostino RB Sr., Wilson PW. Insulin resistance, the metabolic syndrome, and incident cardiovascular events in the framingham offspring study. Diabetes. (2005) 54:3252–7. doi: 10.2337/diabetes.54.11.3252
37. Turer AT, Stevens RD, Bain JR, Muehlbauer MJ, Van Der Westhuizen J, Mathew JP, et al. Metabolomic profiling reveals distinct patterns of myocardial substrate use in humans with coronary artery disease or left ventricular dysfunction during surgical ischemia/reperfusion. Circulation. (2009) 119:1736–46. doi: 10.1161/CIRCULATIONAHA.108.816116
38. Rains JL, Jain SK. Hyperketonemia increases monocyte adhesion to endothelial cells and is mediated by LFA-1 expression in monocytes and ICAM-1 expression in endothelial cells. Am J Physiol Endocrinol Metab. (2011) 301:E298–306. doi: 10.1152/ajpendo.00038.2011
39. Jain SK, Kannan K, Lim G, Mcvie R, Bocchini Jr JA. Hyperketonemia increases tumor necrosis factor-alpha secretion in cultured U937 monocytes and Type 1 diabetic patients and is apparently mediated by oxidative stress and cAMP deficiency. Diabetes. (2002) 51:2287–93. doi: 10.2337/diabetes.51.7.2287
40. Jain SK, Mcvie R, Jackson R, Levine SN, Lim G. Effect of hyperketonemia on plasma lipid peroxidation levels in diabetic patients. Diabetes Care. (1999) 22:1171–5. doi: 10.2337/diacare.22.7.1171
41. Jensen NJ, Nilsson M, Ingerslev JS, Olsen DA, Fenger M, Svart M, et al. Effects of beta-hydroxybutyrate on cognition in patients with type 2 diabetes. Eur J Endocrinol. (2020) 182:233–42. doi: 10.1530/EJE-19-0710
42. Selvaraj S, Hu R, Vidula MK, Dugyala S, Tierney A, Ky B, et al. Acute echocardiographic effects of exogenous ketone administration in healthy participants. J Am Soc Echocardiogr. (2021). doi: 10.1016/j.echo.2021.10.017. [Epub ahead of print].
43. Byrne NJ, Soni S, Takahara S, Ferdaoussi M, Al Batran R, Darwesh AM, et al. Chronically elevating circulating ketones can reduce cardiac inflammation and blunt the development of heart failure. Circ Heart Fail. (2020) 13:e006573. doi: 10.1161/CIRCHEARTFAILURE.119.006573
44. Yurista SR, Matsuura TR, Sillje HHW, Nijholt KT, Mcdaid KS, Shewale SV, et al. Ketone ester treatment improves cardiac function and reduces pathologic remodeling in preclinical models of heart failure. Circ Heart Fail. (2021) 14:e007684. doi: 10.1161/CIRCHEARTFAILURE.120.007684
45. Horton JL, Davidson MT, Kurishima C, Vega RB, Powers JC, Matsuura TR, et al. The failing heart utilizes 3-hydroxybutyrate as a metabolic stress defense. JCI Insight. (2019)4. doi: 10.1172/jci.insight.124079
46. Puchalska P, Martin SE, Huang X, Lengfeld JE, Daniel B, Graham MJ, et al. Hepatocyte-macrophage acetoacetate shuttle protects against tissue fibrosis. Cell Metab. (2019) 29:383–98 e387. doi: 10.1016/j.cmet.2018.10.015
47. Yu Y, Yu Y, Zhang Y, Zhang Z, An W, Zhao X. Treatment with D-beta-hydroxybutyrate protects heart from ischemia/reperfusion injury in mice. Eur J Pharmacol. (2018) 829:121–8. doi: 10.1016/j.ejphar.2018.04.019
48. Zhang SJ, Li ZH, Zhang YD, Chen J, Li Y, Wu FQ, et al. Ketone body 3-hydroxybutyrate ameliorates atherosclerosis via receptor Gpr109a-mediated calcium influx. Adv Sci. (2021) 8:2003410. doi: 10.1002/advs.202003410
49. Yurista SR, Chong CR, Badimon JJ, Kelly DP, De Boer RA, Westenbrink BD. Therapeutic potential of ketone bodies for patients with cardiovascular disease: JACC state-of-the-art review. J Am Coll Cardiol. (2021) 77:1660–9. doi: 10.1016/j.jacc.2020.12.065
50. Goldstein DE, Little RR, Lorenz RA, Malone JI, Nathan DM, Peterson CM, et al. Tests of glycemia in diabetes. Diabetes Care. (2004) 27(Suppl_1):S91–3. doi: 10.2337/diacare.27.2007.s91
51. Taboulet P, Deconinck N, Thurel A, Haas L, Manamani J, Porcher R, et al. Correlation between urine ketones (acetoacetate) and capillary blood ketones (3-beta-hydroxybutyrate) in hyperglycaemic patients. Diabetes Metab. (2007) 33:135–9. doi: 10.1016/j.diabet.2006.11.006
52. Stephens JM, Sulway MJ, Watkins PJ. Relationship of blood acetoacetate and 3-hydroxybutyrate in diabetes. Diabetes. (1971) 20:485–9. doi: 10.2337/diab.20.7.485
53. Jain SK, Mcvie R, Jaramillo JJ, Chen Y. Hyperketonemia (acetoacetate) increases the oxidizability of LDL + VLDL in Type-I diabetic patients. Free Radic Biol Med. (1998) 24:175–81. doi: 10.1016/s0891-5849(97)00213-x
54. Jain SK, Kannan K, Lim G, Matthews-Greer J, Mcvie R, Bocchini Jr JA. Elevated blood interleukin-6 levels in hyperketonemic type 1 diabetic patients and secretion by acetoacetate-treated cultured U937 monocytes. Diabetes Care. (2003) 26:2139–43. doi: 10.2337/diacare.26.7.2139
55. Rains JL, Jain SK. Effect of hyperketonemia (Acetoacetate) on nuclear factor-kappaB and p38 mitogen-activated protein kinase activation mediated intercellular adhesion molecule 1 upregulation in endothelial cells. Metab Syndr Relat Disord. (2015) 13:71–7. doi: 10.1089/met.2014.0101
56. Kanikarla-Marie P, Jain SK. Hyperketonemia (acetoacetate) upregulates NADPH oxidase 4 and elevates oxidative stress, ICAM-1, and monocyte adhesivity in endothelial cells. Cell Physiol Biochem. (2015) 35:364–73. doi: 10.1159/000369702
Keywords: coronary artery disease, ketone bodies, β-hydroxybutyrate, Gensini score, metabolic dysfunction
Citation: Mu H, Yang R, Wang S, Zhang W, Wang X, Li H, Dong J, Chen W, Yu X and Ji F (2022) Association of Serum β-Hydroxybutyrate and Coronary Artery Disease in an Urban Chinese Population. Front. Nutr. 9:828824. doi: 10.3389/fnut.2022.828824
Received: 04 December 2021; Accepted: 17 January 2022;
Published: 17 February 2022.
Edited by:
Ellen E. Blaak, Maastricht University, NetherlandsReviewed by:
Sushil K. Jain, Louisiana State University in Shreveport, United StatesCopyright © 2022 Mu, Yang, Wang, Zhang, Wang, Li, Dong, Chen, Yu and Ji. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fusui Ji, amlfZnVzdWlAMTYzLmNvbQ==; Xue Yu, eXV4dWVtZEBhbGl5dW4uY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.