- 1Changsha Social Work College, Changsha, China
- 2Department of Orthopaedics, Xiangya Hospital, Central South University, Changsha, China
- 3National Clinical Research Center for Geriatric Disorders, Xiangya Hospital, Central South University, Changsha, China
Background: Epidemiological studies have investigated the association between dietary zinc intake and metabolic syndrome (MetS). However, their results are conflicting. This meta-analysis was therefore employed to investigate the associations further.
Methods: A comprehensive literature search was employed by using the electronic database of PubMed, Web of Science, and Embase up to November 2021. The pooled relative risk (RR) of MetS for the highest vs. lowest dietary zinc intake category, and the weighted mean difference (WMD) of dietary zinc intake for MetS vs. control subjects as well as their corresponding 95% confidence interval (CI) were calculated.
Results: A total of 13 observational studies (18,073 participants) were identified in this meta-analysis. The overall multi-variable adjusted RR demonstrated that the dietary zinc intake was inversely associated with MetS (RR = 0.75, 95%CI: 0.61 to 0.93; P = 0.009). The subgroup analysis confirmed such findings in cross-sectional (RR = 0.70, 95%CI: 0.55 to 0.87; P = 0.002), NCEP-ATP III (RR = 0.64, 95%CI: 0.48 to 0.84; P = 0.002), adult (RR = 0.77, 95%CI: 0.62 to 0.96; P = 0.02), dietary recall method (RR = 0.70, 95%CI: 0.55 to 0.87; P = 0.002), and >500 sample-sized study (RR = 0.79, 95%CI: 0.64 to 0.99; P = 0.002), respectively. On the other hand, the overall combined WMD showed that the dietary zinc intake in MetS was also lower than that in control subjects (WMD = −0.21, 95%CI: −0.42 to 0.00; P = 0.05).
Conclusions: Our results suggest that the dietary zinc intake is negatively associated with MetS. However, due to the limitation of available evidence. More well-designed prospective cohort studies are still needed.
Introduction
Metabolic syndrome (MetS) is defined as a cluster of elevated fasting blood glucose, triglycerides, blood pressure, waist circumference, and decreased high-density lipoprotein cholesterol (at least three of the above metabolic abnormalities) (1). Metabolic syndrome is closely associated with diabetes mellitus, stroke and coronary heart disorders (2–4). The global prevalence of MetS is between 11.6 and 62.5%, which is still progressively growing (5). The etiology of MetS is not well-understood yet. However, the dietary factors are deemed to be significantly involved in MetS (6–10).
As the second most common trace metal in the body, zinc is associated with DNA replication and transcriptions, protein synthesis, and cellular division and differentiation (11). Zinc is an important antioxidant, which stabilizes membrane, prevents cellular apoptosis, and is also important for endothelial integrity (12, 13). It is widely accepted that zinc improves chronic inflammation, oxidative stress, and insulin resistance (14, 15), which is closely associated with the pathogenesis of MetS. Moreover, epidemiological data have indicated a negative relationship between dietary zinc intake and MetS-related context (e.g., diabetes) (16). Therefore, the dietary zinc intake is speculated to be inversely associated with MetS.
As far as we know, a number of observational studies have explored the association between dietary zinc intake and MetS (17–29). However, their results are still conflicting. Thus, this meta-analysis of observational studies is employed to investigate the issue further. It is hypothesized that the dietary zinc intake is inversely associated with MetS.
Materials and Methods
Search Strategy
Our meta-analysis was employed according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines (30). Combine the keywords that related to MetS (“metabolic syndrome”) and zinc (“zinc,” “zn”), the electronic database of PubMed, Web of Science, and Embase were searched up to November 2021. No language restriction was set in the search strategy. The titles and abstracts of all articles were screened firstly, and the full articles were then read to identify the eligible studies.
Study Selection
The titles, abstracts and full texts of all retrieved studies were comprehensively reviewed by two researchers independently. Disagreements were resolved by discussions. The included studies were required to meet the following criteria: (1) the study design is observational study; (2) the association between dietary zinc intake and MetS; (3) the relative risk (RR), odds ratio (OR), or weighted mean difference (WMD) with 95% confidence interval (CI) were reported. The exclusion criteria were listed as follows: (1) duplicated or irrelevant articles; (2) randomized controlled trials; (3) reviews, letters, or case reports; (4) non-human studies.
Data Extraction
The effect estimates from each included studies were extracted by two researchers independently, and disagreements were resolved by discussion. The information about the first author, year of publication, location, age, gender, sample size, study design, adjustments, dietary zinc assessment, exposure, effect estimates, and diagnostic criteria of MetS, was collected. The corresponding effect estimates of MetS for the highest vs. lowest dietary zinc intake category that adjusted for the maximum number of confounding variables were extracted for analysis. Moreover, the dietary zinc intake in MetS vs. control was also extracted to calculate the WMD (mean ± SD).
Quality Assessment
We employed a quality assessment according to the Newcastle-Ottawa (NOS) criteria for non-randomized studies, which is based on three broad perspectives: the selection process of study cohorts, the comparability among different cohorts, and the identification of either the exposure or outcome of study cohorts. Disagreements were resolved by discussion.
Statistical Analyses
The RR for MetS and WMD for dietary zinc intake were the outcome measures in the present study. The I2 statistic was employed to measure the heterogeneity by the percentage of total variation across studies (I2 > 50% was considered as heterogeneity). If significant heterogeneity was observed among the studies, the random-effects model was used; otherwise, the fixed effects model was accepted. Begg's test was employed to assess the publication bias (31). A p-value < 0.05 was considered as statistically significant. Moreover, a subgroup analysis was performed for study design, diagnostic criteria of MetS, population, exposure assessment, and sample size, respectively.
Results
Study Identification and Selection
Figure 1 presented the flow diagram of study identification and selection. Initially, a total of 711 articles (PubMed: 223, Embase: 298, and Web of Science: 190) were retrieved from the database during the literature search. After eliminating 309 duplicated articles, 402 articles were screened according to the titles and abstracts. Thereafter, 246 irrelevant studies were removed. Then, 81 reviews, case reports or letters, 51 non-human studies, 11 randomized controlled trials studies were excluded. Eventually, a total of 13 studies were identified for this meta-analysis.
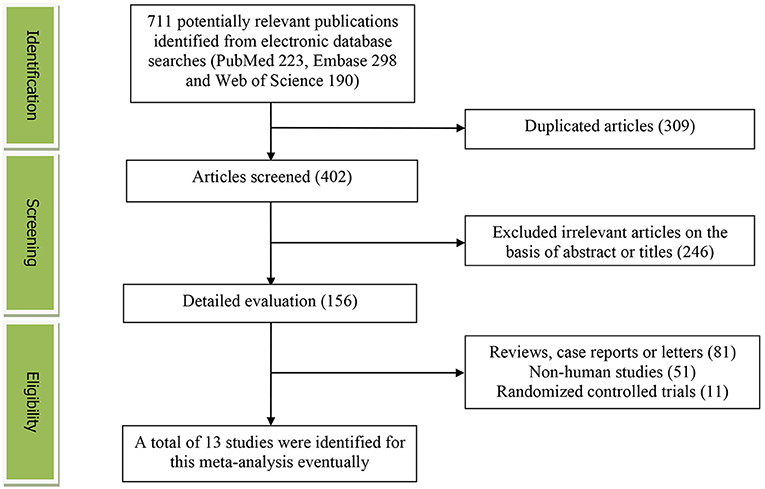
Figure 1. The detailed flow diagram of the study identification and selection in this meta-analysis.
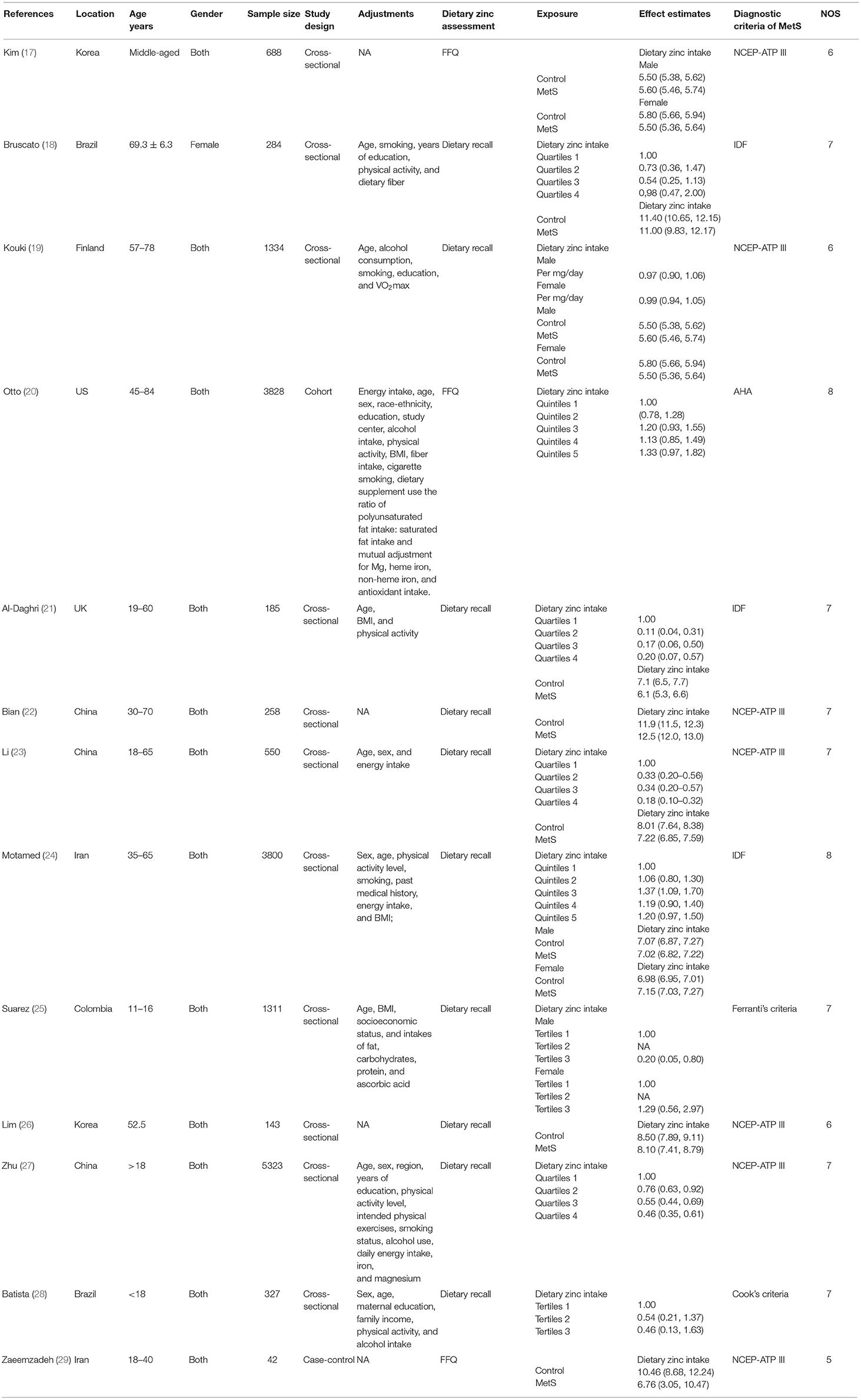
Study Characteristics
The main characteristics of the identified studies were presented in Table 1. These studies were published between 2008 and 2021. Seven of them were employed in Asian countries [China (22, 23, 27), Korea (17, 26), and Iran (24, 29)], and the other six ones were conducted in Brazil (18, 28), US (20), UK (21), Finland (19), and Columbia (25), respectively. Most studies considered both male and female participants, whereas Bruscato's study only recruited females (18). The sample size ranged from 42 to 5,323 for a total of 18,073. The dietary zinc intake was assessed by food-frequency questionnaire (FFQ) in three studies (17, 20, 29), and dietary recall method in 10 studies (18, 19, 21–28). The criteria for MetS were National Cholesterol Education Program-Adult Treatment Panel III (NCEP ATP III) (17, 19, 22, 23, 26, 27, 29), International Diabetes Federation (IDF) (18, 21, 24), and American Heart Association (AHA) (20) in 7, 3, and 1 studies, respectively. Moreover, the Ferranti's (32) and Cook's (33) criteria were employed for adolescent population (25, 28).
Relative Risk of MetS for the Highest vs. Lowest Dietary Zinc Intake Category
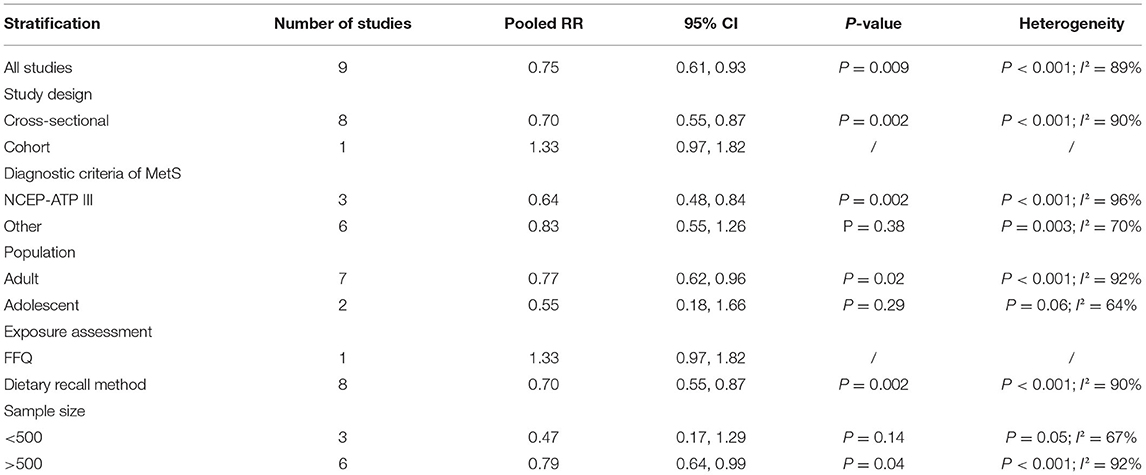
The overall multi-variable adjusted RR showed that the dietary zinc intake was inversely associated with MetS (RR = 0.75, 95%CI: 0.61 to 0.93; P = 0.009) (Figure 2). A substantial level of heterogeneity was observed among the various studies (P < 0.001, I2 = 89.4%). No evidence of publication bias was observed among the included studies according to Begg's rank-correlation test (P = 0.276). The results of subgroup analysis were presented in Table 2. Such findings were confirmed in cross-sectional (RR = 0.70, 95%CI: 0.55 to 0.87; P = 0.002), NCEP-ATP III (RR = 0.64, 95%CI: 0.48 to 0.84; P = 0.002), adult (RR = 0.77, 95%CI: 0.62 to 0.96; P = 0.02), dietary recall method (RR = 0.70, 95%CI: 0.55 to 0.87; P = 0.002), and >500 sample sized study (RR = 0.79, 95%CI: 0.64 to 0.99; P = 0.002), but not cohort (RR = 1.33, 95%CI: 0.97 to 1.82), other criteria of MetS (RR = 0.83, 95%CI: 0.55 to 1.26; P = 0.38), adolescent (RR = 0.55, 95%CI: 0.18 to 1.66; P = 0.29), FFQ (RR = 1.33, 95%CI: 0.97 to 1.82), and <500 sample sized (RR = 0.47, 95%CI: 0.17 to 1.29; P = 0.14) study.
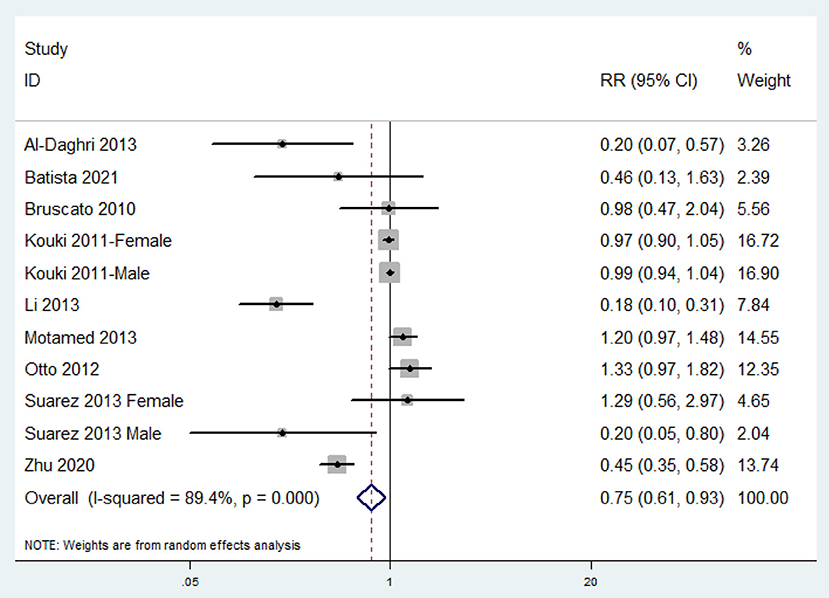
Figure 2. Forest plot of meta-analysis: Overall multi-variable adjusted RR of MetS for the highest vs. lowest dietary zinc intake category.
Weighted Mean Difference of the Dietary Zinc Intake for MetS vs. Control Subjects
The combined WMD demonstrated that the dietary zinc intake in MetS was lower than that in control subjects (WMD = −0.21, 95%CI: −0.42 to 0.00; P = 0.05) (Figure 3). A substantial level of heterogeneity was observed among the various studies (P = 0.001, I2 = 65.1%). No evidence of publication bias was observed according to Begg's rank-correlation test (P = 0.304).
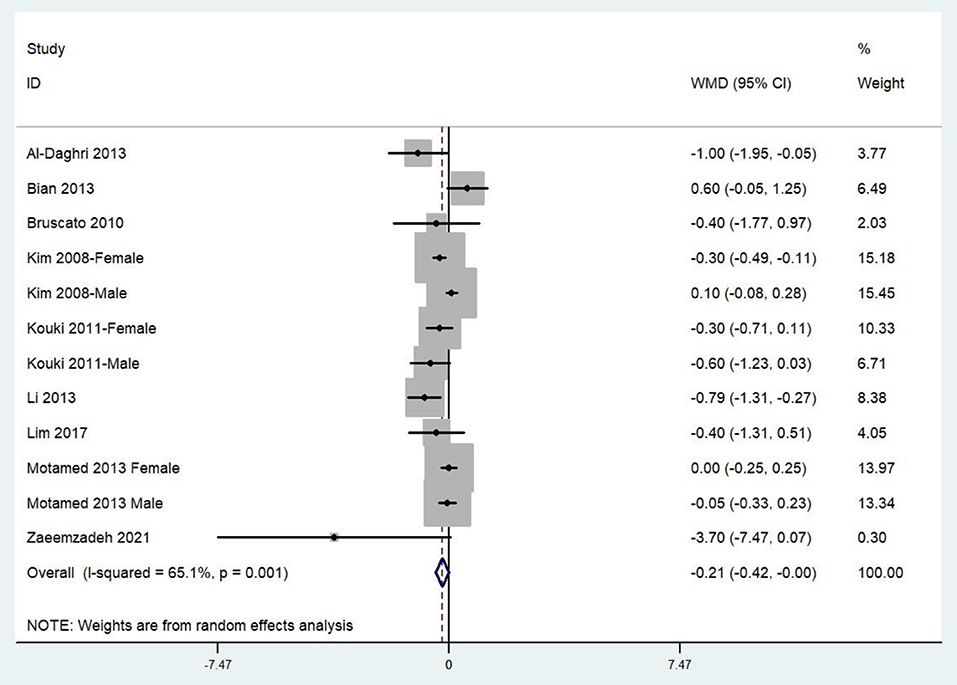
Figure 3. Forest plot of meta-analysis: Weighted mean difference of dietary zinc intake for MetS vs. control subjects.
Discussion
In this study, a total of 13 observational studies are identified for meta-analysis. The results show that the dietary zinc intake is inversely associated with MetS. Moreover, the dietary zinc intake in MetS is lower than that in control either.
It is well known that both oxidative stress and inflammation plays significant role in the pathophysiology of MetS (34), and the antioxidant and anti-inflammatory property of zinc may mainly account for the negative relationship between dietary zinc intake and MetS. Consistently, several randomized controlled trials have revealed that zinc supplementation improves insulin resistance, oxidative stress, and inflammation in MetS subjects (35, 36). Moreover, zinc supplementation also leads to a higher level of TNF-α bound monocytes, which may benefit the immune response system (37). On the other hand, some fundamental experimental evidence indicates that long term zinc supplementation directly improves MetS in animal model (38), and decreases several metabolic disorder makers, lipid accumulation, and toxicity (39–41). Above all, the existing clinical and experimental data are strongly consistent with our results.
Interestingly, the inverse relationship between dietary zinc intake and MetS is only obtained in cross-sectional studies. Nevertheless, the number of cohort studies is rather small (only one), which may inevitably reduce the reliability. Moreover, the inconsistent result with regard to diagnostic criteria of MetS, exposure assessment and sample size is also acquired. It is speculated that NCEP ATP III criteria, dietary recall method, and lager sample size (>500) are more precise and suitable for this analysis. On the other hand, our findings only exist in adult, but not adolescent population. Indeed, the adolescent is a less concerned population for MetS (MetS is a chronic disorder, and only two studies are identified for adolescent). Our results preliminarily suggest a potential effect of age on the relationship between dietary zinc intake and MetS. Taken together, more well-designed prospective cohort study with the specification of population age (adult/adolescent) is still needed.
Several similar meta-analysis studies should also be noted. Capdor et al. find that zinc supplementation reduces glucose concentrations and HbA1c, which may contribute to the management of hyperglycemia in individuals with MetS (42). Moreover, Khazdouz et al. further indicates that zinc supplementation has beneficial effects on glycemic indices and lipid profile, which contributes to a reduction in risk of atherosclerosis (43). In addition, Karamali et al. demonstrates that 30 mg/day zinc supplementation for 6 weeks has beneficial effects on metabolic profiles in gestational diabetes subjects (44). These evidences strongly suggest a potential beneficial effect of zinc supplementation on MetS, which is a significant supplement for our results.
The relationship between serum zinc level and MetS has been deeply discussed in our previous work (45). It demonstrates that the serum zinc level in MetS is slightly higher than that in control, and an increased serum zinc level might be associated with a higher risk of MetS. However, these results seem to be limited by available evidence. More importantly, the development of MetS is associated with the chronic inflammation and oxidative stress (46–48), which lead to a lower serum zinc level. In turn, zinc can also reduce inflammatory cytokine production and oxidative stress (14, 45). As a consequence, the level of serum zinc might be dynamic in MetS condition. Alternatively, the dietary zinc intake is also served as a valid and reliable indicator for zinc status (49–53). Interestingly, a negative relationship between dietary zinc intake and MetS was obtained in our present study, which may encourage to build a collaboration between physicians and nutritionists to reinforce the dietary education in MetS subjects. Nevertheless, the toxicity of excess zinc intake should not be ignored neither. Excess zinc intake leads to the aggravation of renal function and an increase in systemic blood pressure predominantly through the oxidative stress (54). Moreover, excess dietary zinc intake may have negative impacts on epithelial signaling pathways, barrier function, and luminal ecology in the intestine, which may have long-term consequences on intestinal health (55). Therefore, a careful clinical validation is still needed before its application.
Our study has several strengths. First, this is the first meta-analysis of observational studies on the association between dietary zinc intake and MetS. Second, the included studies are analyzed based on the adjusted results and large samples. Third, our results may be beneficial for the nutritional management in MetS. The limitations of the present study should also be acknowledged. First, the reliability of our results might be influenced by the substantial level of heterogeneity. Second, due to the limitation in the relevant literature, only one prospective cohort study is identified (precludes causal relationships). Third, the classification of exposure varies greatly among individuals. Fourth, the selection of adjusted factors and definition of MetS are not uniform. Finally, only two studies have considered the adolescent population. These limitations may weaken the significance of this study.
Conclusions
Our results suggest that the dietary zinc intake is negatively associated with MetS. However, due to the limited evidence, more well-designed prospective cohort study with the specification of population age is still needed to elaborate the issues examined in this study.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary materials, further inquiries can be directed to the corresponding author.
Author Contributions
YZ was the guarantor of the overall content, conceived the idea, and assessed each study. JD and YZ drafted this study. ZL and QL performed the statistical analysis. HG and JL selected and retrieved relevant papers. All authors revised and approved the final manuscript. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by National Natural Science Foundation of China (82102581), National Postdoctoral Science Foundation of China (2021M693562), Provincial Outstanding Postdoctoral Innovative Talents Program of Hunan (2021RC2020), Provincial Natural Science Foundation of Hunan (2019JJ40517), Young Investigator Grant of Xiangya Hospital, Central South University (2020Q14), and FuQing Postdoc Program of Xiangya Hospital, Central South University (176).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Maury E, Brichard SM. Adipokine dysregulation, adipose tissue inflammation and metabolic syndrome. Mol Cell Endocrinol. (2010) 314:1–16. doi: 10.1016/j.mce.2009.07.031
2. Gurka MJ, Guo Y, Filipp SL, DeBoer MD. Metabolic syndrome severity is significantly associated with future coronary heart disease in type 2 diabetes. Cardiovasc Diabetol. (2018) 17:17. doi: 10.1186/s12933-017-0647-y
3. DeBoer MD, Gurka MJ, Golden SH, Musani SK, Sims M, Vishnu A, et al. Independent associations between metabolic syndrome severity and future coronary heart disease by sex and race. J Am Coll Cardiol. (2017) 69:1204–5. doi: 10.1016/j.jacc.2016.10.088
4. Decker JJ, Norby FL, Rooney MR, Soliman EZ, Lutsey PL, Pankow JS, et al. Metabolic syndrome and risk of ischemic stroke in atrial fibrillation: ARIC study. Stroke. (2019) 50:3045–50. doi: 10.1161/STROKEAHA.119.025376
5. Ranasinghe P, Mathangasinghe Y, Jayawardena R, Hills AP, Misra A. Prevalence and trends of metabolic syndrome among adults in the asia-pacific region: a systematic review. BMC Public Health. (2017) 17:101. doi: 10.1186/s12889-017-4041-1
6. Marventano S, Salomone F, Godos J, Pluchinotta F, Del Rio D, Mistretta A, et al. Coffee and tea consumption in relation with non-alcoholic fatty liver and metabolic syndrome: a systematic review and meta-analysis of observational studies. Clin Nutr. (2016) 35:1269–81. doi: 10.1016/j.clnu.2016.03.012
7. Lee M, Lim M, Kim J. Fruit and vegetable consumption and the metabolic syndrome: a systematic review and dose-response meta-analysis. Br J Nutr. (2019) 122:723–33. doi: 10.1017/S000711451900165X
8. Hidayat K, Zhu WZ, Peng SM, Ren JJ, Lu ML, Wang HP, et al. The association between meat consumption and the metabolic syndrome: a cross-sectional study and meta-analysis. Br J Nutr. (2021). 2021:1–15. doi: 10.1017/S0007114521002452
9. Karimi G, Heidari Z, Firouzi S, Haghighatdoost F. A systematic review and meta-analysis of the association between fish consumption and risk of metabolic syndrome. Nutr Metab Cardiovasc Dis. (2020) 30:717–29. doi: 10.1016/j.numecd.2020.02.001
10. Sarrafzadegan N, Khosravi-Boroujeni H, Lotfizadeh M, Pourmogaddas A, Salehi-Abargouei A. Magnesium status and the metabolic syndrome: a systematic review and meta-analysis. Nutrition. (2016) 32:409–17. doi: 10.1016/j.nut.2015.09.014
11. Frederickson CJ. Neurobiology of zinc and zinc-containing neurons. Int Rev Neurobiol. (1989) 31:145–238. doi: 10.1016/S0074-7742(08)60279-2
12. Powell SR. The antioxidant properties of zinc. J Nutr. (2000) 130:1447S−54S. doi: 10.1093/jn/130.5.1447S
13. Hennig B, Wang Y, Ramasamy S, McClain CJ. Zinc deficiency alters barrier function of cultured porcine endothelial cells. J Nutr. (1992). 122:1242–7. doi: 10.1093/jn/122.6.1242
14. Seo JA, Song SW, Han K, Lee KJ, Kim HN. The associations between serum zinc levels and metabolic syndrome in the Korean population: findings from the 2010 Korean national health and nutrition examination survey. PLoS ONE. (2014) 9:e105990. doi: 10.1371/journal.pone.0105990
15. Ahn BI, Kim MJ, Koo HS, Seo N, Joo NS, Kim YS. Serum zinc concentration is inversely associated with insulin resistance but not related with metabolic syndrome in nondiabetic Korean adults. Biol Trace Elem Res. (2014) 160:169–75. doi: 10.1007/s12011-014-0045-1
16. Fernández-Cao J, Warthon-Medina M, Moran V, Arija V, Doepking C, Serra-Majem L, et al. Zinc intake and status and risk of type 2 diabetes mellitus: a systematic review and meta-analysis. Nutrients. (2019) 11:1027. doi: 10.3390/nu11051027
17. Kim WY, Kim JE, Choi YJ, Huh HB. Nutritional risk and metabolic syndrome in Korean type 2 diabetes mellitus. Asia Pac J Clin Nutr. (2008) 17(Suppl 1):47–51.
18. Bruscato N, Vieira J, Nascimento N, Canto M, Stobbe J, Gottlieb M, et al. Dietary intake is not associated to the metabolic syndrome in elderly women. N Am J Med Sci. (2010) 2:182–8. doi: 10.4297/najms.2010.2182
19. Kouki R, Schwab U, Hassinen M, Komulainen P, Heikkilä H, Lakka T, et al. Food consumption, nutrient intake and the risk of having metabolic syndrome: the DR's EXTRA study. Eur J Clin Nutr. (2011) 65:368–77. doi: 10.1038/ejcn.2010.262
20. Otto M, Alonso A, Lee DH, Delclos GL, Bertoni AG, Rui Jiang R, et al. Dietary intakes of zinc and heme iron from red meat, but not from other sources, are associated with greater risk of metabolic syndrome and cardiovascular disease. J Nutr. (2012) 142:526–33. doi: 10.3945/jn.111.149781
21. Al-Daghri N, Khan N, Alkharfy KM, Al-Attas OS, Alokail MS, Alfawaz HA, et al. Selected dietary nutrients and the prevalence of metabolic syndrome in adult males and females in Saudi Arabia: a pilot study. Nutrients. (2013) 5:4587–604. doi: 10.3390/nu5114587
22. Bian S, Gao Y, Zhang M, Wang X, Liu W, Zhang D, et al. Dietary nutrient intake and metabolic syndrome risk in Chinese adults: a case-control study. Nutr J. (2013) 12:106. doi: 10.1186/1475-2891-12-106
23. Li Y, Guo H, Wu M, Liu M. Serum and dietary antioxidant status is associated with lower prevalence of the metabolic syndrome in a study in Shanghai, China. Asia Pac J Clin Nutr. (2013). 22:60–8. doi: 10.6133/apjcn.2013.22.1.06
24. Motamed S, Ebrahimi M, Safarian M, Mobarhan M, Mouhebati M, Azarpazhouh M, et al. Micronutrient intake and the presence of the metabolic syndrome. N Am J Med Sci. (2013) 5:377–85. doi: 10.4103/1947-2714.114171
25. Suarez-Ortegón MF, Ordoñez-Betancourth JE, Plata CA. Dietary zinc intake is inversely associated to metabolic syndrome in male but not in female urban adolescents. Am J Hum Biol. (2013) 25:550–4. doi: 10.1002/ajhb.22408
26. Lim HS, Shin EJ, Yeom JW, Park YH, Kim SK. Association between nutrient intake and metabolic syndrome in patients with colorectal cancer. Clin Nutr Res. (2017) 6:38–46. doi: 10.7762/cnr.2017.6.1.38
27. Zhu Z, He Y, Wu F, Zhao L, Wu C, Lu Y, et al. The associations of dietary iron, zinc and magnesium with metabolic syndrome in China's mega cities. Nutrients. (2020) 12:659. doi: 10.3390/nu12030659
28. Batista CC, Nascimento LM, Lustosa L, Rodrigues B, Campelo V, Frota K. Metabolic syndrome in adolescents and antioxidant nutrient intake: a cross-sectional study. Rev Assoc Med Bras. (2021) 67:918–25. doi: 10.1590/1806-9282.20200733
29. Zaeemzadeh N, Sadatmahalleh SJ, Ziaei S, Kazemnejad A, Movahedinejad M, Mottaghi A, et al. Comparison of dietary micronutrient intake in PCOS patients with and without metabolic syndrome. J Ovarian Res. (2021) 14:10. doi: 10.1186/s13048-020-00746-0
30. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. (2009) 339:b2700. doi: 10.1136/bmj.b2700
31. Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. (1994) 50:1088–101. doi: 10.2307/2533446
32. de Ferranti SD, Gauvreau K, Ludwig DS, Neufeld EJ, Newburger JW, Rifai N. Prevalence of the metabolic syndrome in American adolescents: findings from the Third National Health and Nutrition Examination Survey. Circulation. (2004) 110:2494–7. doi: 10.1161/01.CIR.0000145117.40114.C7
33. Cook S, Weitzman M, Auinger P, Nguyen M, Dietz WH. Prevalence of metabolic syndrome phenotype in adolescents: findings from the third National Health and Nutrition Examination Survey, 1988–1994. Arch Pediatr Adolesc Med. (2003) 157:821–27. doi: 10.1001/archpedi.157.8.821
34. Wong SK, Chin KY, Ima-Nirwana S. Vitamin C: a review on its role in the management of metabolic syndrome. Int J Med Sci. (2020) 17:1625–38. doi: 10.7150/ijms.47103
35. Kelishadi R, Hashemipour M, Adeli K, Tavakoli N, Movahedian-Attar A, Shapouri J, et al. Effect of zinc supplementation on markers of insulin resistance, oxidative stress, and inflammation among prepubescent children with metabolic syndrome. Metab Syndr Relat Disord. (2010) 8:505–10. doi: 10.1089/met.2010.0020
36. Hashemipour M, Kelishadi R, Shapouri J, Sarrafzadegan N, Amini M, Tavakoli N, et al. Effect of zinc supplementation on insulin resistance and components of the metabolic syndrome in prepubertal obese children. Hormones (Athens). (2009) 8:279–85. doi: 10.14310/horm.2002.1244
37. Meksawan K, Sermsri U, Chanvorachote P. Zinc supplementation improves anticancer activity of monocytes in type-2 diabetic patients with metabolic syndrome. Anticancer Res. (2014) 34:295–9.
38. Taneja S, Mandal R, Girhotra S. Long term excessive Zn-supplementation promotes metabolic syndrome-X in Wistar rats fed sucrose and fat rich semisynthetic diet. Indian J Exp Biol. (2006) 44:705–18.
39. Nour S, Taha A, Fahmy M, Mansour M. Effects of oral zinc supplementation in early neonatal live on development of obesity and metabolic syndrome in albino rats. Int J Pharmaceut Sci Res. (2021) 12:1433–41. doi: 10.13040/IJPSR.0975-8232.12(3).1433-41
40. Mustafa Z, Ali R, Ali D, Abdulkarimi R, Abdulkareem N, Akbari A. The combination of ginger powder and zinc supplement improves the fructose-induced metabolic syndrome in rats by modulating the hepatic expression of NF-κB, mTORC1, PPAR-α SREBP-1c, and Nrf2. J Food Biochem. (2021) 45:e13546. doi: 10.1111/jfbc.13546
41. Naito Y, Yoshikawa Y, Yoshizawa K, Takenouchi A, Yasui H. Beneficial effect of bis(hinokitiolato)Zn complex on high-fat diet-induced lipid accumulation in mouse liver and kidney. In Vivo. (2017) 31:1145–51. doi: 10.21873/invivo.11181
42. Capdor J, Foster M, Petocz P, Samman S. Zinc and glycemic control: a meta-analysis of randomized placebo controlled supplementation trials in humans. J Trace Elem Med Biol. (2013) 27:137–42. doi: 10.1016/j.jtemb.2012.08.001
43. Khazdouz M, Djalalinia S, Zadeh SS, Hasani M, Shidfar F, Ataie-Jafari A, et al. Effects of zinc supplementation on cardiometabolic risk factors: a systematic review and meta-analysis of randomized controlled trials. Biol Trace Elem Res. (2020) 195:373–98. doi: 10.1007/s12011-019-01870-9
44. Karamali M, Heidarzadeh Z, Seifati SM, Samimi M, Tabassi Z, Hajijafari M, et al. Zinc supplementation and the effects on metabolic status in gestational diabetes: a randomized, double-blind, placebo-controlled trial. J Diabetes Complications. (2015) 29:1314–9. doi: 10.1016/j.jdiacomp.2015.07.001
45. Zhang Y, Zhang D-Z. Relationship between serum zinc level and metabolic syndrome: a meta-analysis of observational studies. J Am Coll Nutr. (2018) 37:708–15. doi: 10.1080/07315724.2018.1463876
46. Roberts CK, Sindhu KK. Oxidative stress and metabolic syndrome. Life Sci. (2009) 84:705–12. doi: 10.1016/j.lfs.2009.02.026
47. Sutherland J, McKinley B, Eckel RH. The metabolic syndrome and inflammation. Metab Syndr Relat Disord. (2004) 2:82–104. doi: 10.1089/met.2004.2.82
48. Balaşoiu M, Balaşoiu AT, Stepan AE, Dinescu SN, Avrămescu CS, Dumitrescu D, et al. Proatherogenic adipocytokines levels in metabolic syndrome. Rom J Morphol Embryol. (2014) 55:29–33.
49. Gao JW, Zhang SL, Hao QY, Huang FF, Liu ZY, Zhang HF, et al. Association of dietary zinc intake with coronary artery calcium progression: the Multi-Ethnic Study of Atherosclerosis (MESA). Eur J Nutr. (2021) 60:2759–67. doi: 10.1007/s00394-020-02452-5
50. Joo YS, Kim HW, Lee S, Nam KH, Yun HR, Jhee JH, et al. Dietary zinc intake and incident chronic kidney disease. Clin Nutr. (2021) 40:1039–45. doi: 10.1016/j.clnu.2020.07.005
51. Hajianfar H, Mollaghasemi N, Tavakoly R, Campbell MS, Mohtashamrad M, Arab A. The association between dietary zinc intake and health status, including mental health and sleep quality, among Iranian female students. Biol Trace Elem Res. (2021) 199:1754–61. doi: 10.1007/s12011-020-02316-3
52. Vasseur P, Dugelay E, Benamouzig R, Savoye G, Hercberg S, Touvier M, et al. Dietary zinc intake and inflammatory bowel disease in the french NutriNet-Santé cohort. Am J Gastroenterol. (2020) 115:1293–7. doi: 10.14309/ajg.0000000000000688
53. Dharamdasani DH, Mitchell P, Russell J, Burlutsky G, Liew G, Gopinath B. Dietary zinc intake is associated with macular fluid in neovascular age-related macular degeneration. Clin Exp Ophthalmol. (2020) 48:61–8. doi: 10.1111/ceo.13644
54. Yanagisawa H, Miyazaki T, Nodera M, Miyajima Y, Suzuki T, Kido T, et al. Zinc-excess intake causes the deterioration of renal function accompanied by an elevation in systemic blood pressure primarily through superoxide radical-induced oxidative stress. Int J Toxicol. (2014) 33:288–96. doi: 10.1177/1091581814532958
Keywords: dietary zinc intake, metabolic syndrome, meta-analysis, observational studies, clinical nutrition
Citation: Ding J, Liu Q, Liu Z, Guo H, Liang J and Zhang Y (2022) Association Between Dietary Zinc Intake and Metabolic Syndrome. A Meta-Analysis of Observational Studies. Front. Nutr. 9:825913. doi: 10.3389/fnut.2022.825913
Received: 30 November 2021; Accepted: 07 January 2022;
Published: 03 February 2022.
Edited by:
Surasak Saokaew, University of Phayao, ThailandReviewed by:
Reza Rastmanesh, American Physical Society, United StatesSukrit Kanchanasurakit, University of Phayao, Thailand
Copyright © 2022 Ding, Liu, Liu, Guo, Liang and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yi Zhang, emhhbmd5aTAyMDVAY3N1LmVkdS5jbg==
 Jun Ding1
Jun Ding1 Qi Liu
Qi Liu Jieyu Liang
Jieyu Liang Yi Zhang
Yi Zhang