
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Nutr. , 29 April 2022
Sec. Nutrition and Food Science Technology
Volume 9 - 2022 | https://doi.org/10.3389/fnut.2022.825690
This article is part of the Research Topic Classification of Foods According their Processing Level View all 6 articles
With increasing advocacy for plant food consumption, the sub-Saharan Africa landscape is home to diverse plant-based food commodities. The need to leverage the advantages of unprocessed/minimally processed foods (PFs) over ultra-processed foods (UPFs) is a system that requires exploitation. Most of the crops produced in the continent are either classified as traditionally or moderately PFs. However, the rise in industrialization and formalization of markets is impacting and marginalizing traditional food processing (FP). Current FP classification frameworks are briefly discussed. The level of processing of cereals, grains, fruits, vegetables, roots, and tuber crops in the continent requires intervention from nutritionists, food scientists, and scientific and governmental bodies to gain a holistic view and tackle the issue of food insecurity in Africa. This study reviews the levels of processing of African foods, challenges, and future directions.
Human foods can be classified based on the food type (i.e., plant- or animal-based), food groups (e.g., cereals, fruits, vegetables, roots and tubers, and bakery products), and level of processing. Food processing (FP) encompasses the sequence of unit operations any raw food material is subjected to, such as cleaning, cutting, crushing, milling, freezing, heating, and packaging, thereby leading to the physical and chemical transformation of the food from its natural state (1, 2). Foods are processed for preservation, shelf-life extension, safety, quality improvement, and sensory attributes (2, 3). FP is important in dietary needs as it provides consumers with safe foods with no harmful pathogenic microorganisms, reduced antinutritional compounds, and high functional, nutritional, and sensory properties. Processed foods (PFs) also offer convenience, a diversified diet, reduced preparation time, and constant supply to the consumer market during the off-season and adverse climate conditions, thereby guaranteeing regular supply to remote regions (4).
These methods and processes are designed to preserve natural foods, to make them suitable for more extended storage, and still fit for human consumption. Some minimally PFs are prepared and cooked as dishes or meals in kitchens at home or in restaurants or canteens combined with some PFs (5, 6). They vary in energy density and their content and balance of fats, carbohydrates, proteins and their fractions, vitamins, minerals, and other bioactive compounds (4). PFs are mainly processed and consumed as part of meals or dishes or may be used together with ultra-processed products to replace food-based freshly prepared dishes and meals (7, 8). Types of foods that are included in this group are canned or bottled vegetables and legumes (pulses) preserved in brine; peeled or sliced fruits preserved in syrup; tinned whole or pieces of fish kept in oil; salted nuts; reconstituted processed meats such as ham, ham bacon, and smoked fish; and cheese (9). Ultra-processed foods (UPFs) are commonly known as “highly processed foods,” which are produced and are added to some food items such as salt, sweeteners, or fat to include artificial colors and flavors, and preservatives that promote shelf-life, preserve texture, and increase palatability (10).
The continent of Africa is endowed with lots of plant-based foods that have been transformed into PFs over the years. Some of these foods are present in other continents, while others are specific, only to the African continent (11). Transformation of these plant products into shelf-stable food products is achieved through FP, leading to sustainable food systems for the continent (12). This study reviews the current FP classification systems, which category some African foods fall into, challenges, and future directions.
Eight FP classification frameworks identified are (i) Food Standards Australian New Zealand (FSANZ), (ii) International Food Policy and Research Institute (IFPRI), (iii) International Agency for Research on Cancer and European Prospective Investigation into Cancer and Nutrition (IARC-EPIC), (iv) National Institute of Public Health (NIPH), (v) NOVA, (vi) International Food and Information Council (IFIC), (vii) Poti, and (viii) Siga (13). As reviewed by Reardon et al. the FP evolution over the past five decades has seen a shifting trend from processing foods at home (traditional) to buying PFs and then preparing them at home (early to mid-transitional) to now eating out frequently (modern) (7). The intention of these classification frameworks was all epidemiological, except for Siga, whose intent was based on the development of food products and portfolios and to provide proper guidance to consumers on the overview of the food to help them make better choices (13, 14). The lowest level for all frameworks was unprocessed, and the highest level was UPF or highly PFs. Despite the different approaches of classification used by the frameworks, they all ultimately grouped foods as processed or unprocessed in a similar yet distinct manner. Several studies have explained and reviewed the various food classification frameworks (13–16). For up-to-date, detailed information on the conceptualization and challenges with the existing classification frameworks, refer to the study by Sadler et al. (13).
The FSANZ method only classified foods as processed and unprocessed (Table 1), thus making the framework open to several interpretations. This was quite ambiguous (15, 17). The unprocessed class was not defined, while the processing was defined as treatments that caused significant changes to the food from its original state (15, 18). The IFPRI framework classified foods based on the degree of processing and was not elaborated. There were no definitions for the categories of unprocessed (e.g., fruits, nuts, fresh, and dried milk) and partially processed (e.g., lard, butter, and evaporated milk) foods. The PF category was defined as “foods that have undergone secondary processing into readily edible form, likely to contain high levels of added sugars, fats or salt.” Examples include patisserie and confectionaries. More emphasis was placed on industrial processing, while home processing was left out (13, 19). The NIPH framework encompasses unprocessed, locally made, non-industrialized vs. industrialized, and traditional vs. industrialized foods based on processing and temporality (19). The NOVA classification is a system of food classification based on the extent and purpose of their processing while considering the physical and chemical methods used for processing and the use of additives Monteiro et al. (8, 20, 21). This system places foods into four groups (13, 20, 22). NOVA group 1 is classified as unprocessed foods obtained directly from the plant or animal and have not been altered, which include grains, fresh fruits, and milk. The minimal level of processing of NOVA aims to preserve and extend the shelf-life of the food through washing, grating, freezing, crushing, and packaging (20). The foods in NOVA group 2 are processed culinary ingredients and derived from food group 1 through extraction, pressing, centrifugation, and mining processes. Examples include oils/fats, salt, and sugar. NOVA group 3 foods are called PFs and are created by combining food from groups 1 and 2 through industrial manufacturing processes such as canning, fermentation, and baking. Examples include unpackaged bread, cheese, and canned goods (vegetables/fruits/legumes). Finally, NOVA group 4 is called UPF. These are foods formulated by combining products from the other three groups through advanced industrial techniques such as extrusion. Examples include infant formula, reconstituted meat products, candies, and carbonated drinks (21).
The primary basis of the IARC-EPIC was the degree of processing at major comparison points such as raw vs. cooked, industrial vs. artisanal, and minimal vs. high processing. Minimal processing was not defined, but examples suggest that modest processing is “close to the natural process” (13, 23). The IFIC classification system defined FP as any intentional change to food outside its original derivation, based on the intricacies of processing with its accompanying physicochemical and organoleptic changes (24). This classification includes homemade foods at level 1 (minimally processed, e.g., homemade soup) or level 3 (mixtures of combined ingredients). The IFIC framework focuses on preserving the intrinsic properties of foods by comparing minimal processing vs. complex preparation and the level of value-added convenience (13).
The Poti classification system was developed by researchers at the University of North Carolina. It defined FP as any alteration of food from its natural state by the industry (13). The Poti framework has four classification levels based on the degree of industrial processing vs. convenience, the caloric content of each category in 12 years, and a comparison of the additives (e.g., sugar, fat, and sodium content) (25). They hypothesized that the nutritional quality of foods purchased in the supermarkets by American households might be due to a high correlation between the degree of FP and convenience. The system was developed by categorizing all bar-coded foods sold in supermarkets in the United States using product-specific ingredients and nutrients as markers (25, 26). Also, this study reveals that the United States market is dominated by highly processed and RTE foods with high sugar, fat, and sodium content over the periods assessed.
The Siga FP classification was formed to improve the NOVA framework. The Siga index classifies foods using the cumulative effect of a few factors such as the quantity, nature, function and degree of processing, and risk assessment of additives (sugar, salt, and fat addition) based on the scientific opinions of health agencies such as the WHO AND EFSA and the effect of these additives on the nutrient thresholds of food (27, 28). The Siga framework considered the degree of transformation of the ingredients and the loss of the “matrix” effect to achieve an even more holistic and realistic classification. This framework adopts a holistic approach instead of other frameworks where FP classification is reduced to just a sum of certain nutrients. This is termed a “reductionist approach” (29). They further explained that a sum of all the nutrients in the food matrix might have a more synergistic effect than just capitalizing on a few essential nutrients. They defined the holistic paradigm as “an approach in food processing would lead technologists and food scientists to consider foods as systems that are not only a sum of their nutrients but rather a package of bioactive compounds included in a complex food structure.” The Siga system contains four holistic groups and four reductionist subgroups based on the impact of processing on the food on the food matrix (14).
Crino et al. compared six frameworks, namely, FSANZ, Poti, IFC, IFPRI, NOVA, and IARC-EPIC. The authors tested 135 food categories of Euromonitor by applying the frameworks to several food types including packaged foods, detailing industrial FP specifically, and categorizing foods based on the levels of processing. A fundamental dichotomy of processed vs. unprocessed foods was noted for all frameworks, with several layers/levels occurring in the PF section. Their findings showed some similarities. However, the frameworks did not precisely match the PF category. The NOVA framework had the highest agreement with the other five (15). Some authors have argued that the NOVA system is not holistic because the aspect of food safety and domestic processing was not taken into consideration (4). In measuring the strength of the UNC, NOVA, and IFIC FP classification systems, Bleiweiss-Sande et al. assessed 100 foods consumed by children by comparing the three frameworks based on nutrient quality, inter-reliability, and similarity of the systems. Although a significant relationship was observed between nutrient content and processing category, they alluded that current classification systems may not be able to distinguish common foods consumed by children in the United States satisfactorily. This is partly due to the small scope of the study in terms of the number of systems and food mass studied (26). Due to the ambiguity caused by the different purposes of the various FP classifications, Martinez-Perez et al. hypothesized that the NOVA, IFIC, IARC-EPIC, and Poti classification frameworks would result in varying degrees of association between UPFs and cardiometabolic biomarkers. Their study showed a need to standardize the FP classification due to distinct differences in cardiometabolic biomarkers. However, all the assessed frameworks showed that UPF consumption negatively impacted nutrient quality (16).
Although the application of the NOVA classification showed a direct correlation between consumption of UPFs and metabolic diseases (30), these foods have been labeled bad due to high fat, sugar, sodium, energy density, and low dietary fiber and essential nutrients (8). This may be entirely unacceptable to categorize all UPFs as bad foods (14). A case in point is a study where 50 foods classified as UPFs were analyzed using the NOVA framework and European Regulation (EC) No 1924/2006 Nutrition Claims. No statistically significant direct relationships were found between the number of ingredients and energy, saturated fat, total sugar, sodium, AOAC fiber, and protein. The majority of UPFs identified had 60–80% less sugar, salt, and saturated fat and had 60% fiber and 30% protein. The author concluded that not all foods classified as UPFs are unhealthy (17). In fact, some UPFs are not health-friendly. However, all UPFs should not be discarded and labeled as evil because some raw materials must undergo ultra-processing before becoming edible (14, 17). A point in case is that unprocessed raw cassava contains hydrogen cyanide—a toxic compound that minimal processing may not remove, rendering it inedible (31). Therefore, processes such as fermentation, starch extraction, and grinding are necessary to transform the tuber into value-added products without depletion of nutrients (32). Another example is fresh milk which is not shelf-stable for long without processing to extend the shelf life or into other dairy products such as cheese, butter, and yoghurt (12). Consumers may be at risk of food infection without necessary food safety checks at home while handling fresh milk. Therefore, FP is required to provide consumers with safe, shelf-stable, and nutritious food. It is paramount to include home processing as a factor in classifying FP frameworks. It has been noted that there is a higher risk of food contamination due to poor handling and processing practices in the home environment (33). For instance, FP at home may pose more health risks than industrial PFs because the consumers’ addition of sugar and salt to food is not regulated. There is also the risk of pathogenic cross-contamination between foods.
The above examples show that we are still far from a one-size-fits-all system that can classify PFs due to the discrepancies amongst the frameworks (34). This is due to socio-cultural differences, intent, and goal of classification. The purpose of current and new classification systems that may be proposed in the future should be clearly defined with explicit examples. In addition, a holistic approach should be applied while classifying. This encompasses the entire steps across the food chain, including the processing extent place of processing (home vs. industrial), while also considering food waste reduction, food storage, transportation, environmental and epidemiological impact, and nutrition (17).
Due to increased urbanization, there has been a sharp rise in the purchase of PFs in Africa over the last five decades (7). This has led to an increase in PFs in the form of ready-to-eat, ready-to-heat, and quick-cook foods requiring less preparation due to the “fast life” in urban regions compared with rural areas home-cooked meals that are still cherished (3). UPFs undergo several changes from their natural state. Raw agricultural commodities are introduced to different technological processes such as washing, cleaning, milling, cutting, chopping, heating, pasteurizing, blanching, cooking, canning, freezing, drying, dehydrating, mixing, packaging, or other procedures that alter the food from its natural state (38). Ingredients are also added, such as preservatives, storing, filtering, fermenting, extracting, concentrating, microwaving, and packaging (8). These products are characteristically ready-to-eat industrial formulations of cheap homogenized ingredients obtained from high-yield crops, notably sugars and syrups, refined starches, oils and fats, protein isolates, and sometimes from remnants of intensively reared animals. UPFs are produced to attract customers because they have a good appearance, smell, and better taste. The manufacturer uses sophisticated formulations of different food substances such as flavors, colors, emulsifiers, preservatives, sweeteners, thickeners, and other cosmetic additives (39).
According to the Siga framework, UPFs are either balanced (C01), greedy (C02), or processed to limit (C1, C2, C3). It is advised that the latter be avoided or reduced to occasional indulgence due to at-risk additives, which could be harmful to human health (28). However, some preservatives play a significant role in the promotion of food safety of food by preventing the growth of molds and bacteria. The most common preservatives used in the production of foods are ascorbic acid, sodium benzoate, potassium sorbate, tocopherols, and emulsifiers that prevent the separation of liquids and solids, e.g., soy lecithin monoglycerides. Examples of thickeners to add texture to foods are hydrocolloids such as xanthan gum, pectin, carrageenan, and guar gum (40, 41). Furthermore, food fortification is performed to produce nutritionally balanced UPFs where they were otherwise lacking. Fortified foods contain vitamins and minerals that are added after processing due to loss during processing, or they were added because they are lacking in the average diet (42). Mainly used fortificants are vitamin B (e.g., riboflavin, niacin, niacinamide, folate, or folic acid), beta carotene, iron (ferrous sulfate), vitamin C (ascorbic acid), vitamin D (42, 43), or amino acids to boost protein content (10).
Ultra-processing is characterized by different methods and ingredients to produce highly profitable branded products (44). UPFs are also available at low cost with a long shelf-life which is liable to displace the production and consumption of unprocessed or minimally PFs, PFs, and freshly prepared dishes and meals, or simply “real food” for short. UPFs are primarily formulated to increase human demands and cravings so that customers may enjoy eating them and can purchase more of such foods (44). Examples are sugary drinks, cookies, crackers, chips, breakfast cereals, frozen dinners, and luncheon meats, which minimally replace PFs in some consumers’ diets (21).
There are several disadvantages of consuming some UPFs that are related to human health, such as obesity, hypertension, cardiovascular diseases, dyslipidaemia, metabolic syndrome, gastrointestinal disorders, breast cancer, depression, and all-causes high death rate (14, 21, 28).
A global migration from indigenous and traditional food crops and agricultural production has changed the food scene in the last 50 years, especially in developing countries such as sub-Saharan Africa (Table 2). Statistical analysis of the world market showed higher consumption of UPFs in high-income countries such as the United States, Canada, the United Kingdom, and Australia. It indicated a rapid growth of UPFs in middle-income countries. Between 1998 and 2012, the market sales of sugary and salty snacks and soft drinks increased by 50% in upper-middle-income countries and more than 100% in lower-middle-income countries (21). Euromonitor statistical data showed that the per capita retail sales of three UPFs, namely, frozen products, snacks, and soft drinks in some low-middle income African countries (Cameroon, Egypt, Morocco, and Nigeria) increased by 180, 115, and 273%, respectively. In contrast, an increase of 129, 46, and 48% was reported in upper-middle African countries such as Algeria and South Africa (36, 45). This study concludes that the rise in the consumption of UPFs we see now is primarily influenced by the presence of industrial food manufacturing of UPFs, its retailing, and fast-food corporations (36). Most African countries, especially South Africa, have reported an increase in obesity in adults aged 18 years and above due to the consumption of UPFs (46–48). This is a direct result of the easy accessibility of UPFs on the shelves Hunter-Adams et al. (49). Most South African citizens eat more junk foods than indigenous minimal PFs (25, 43).
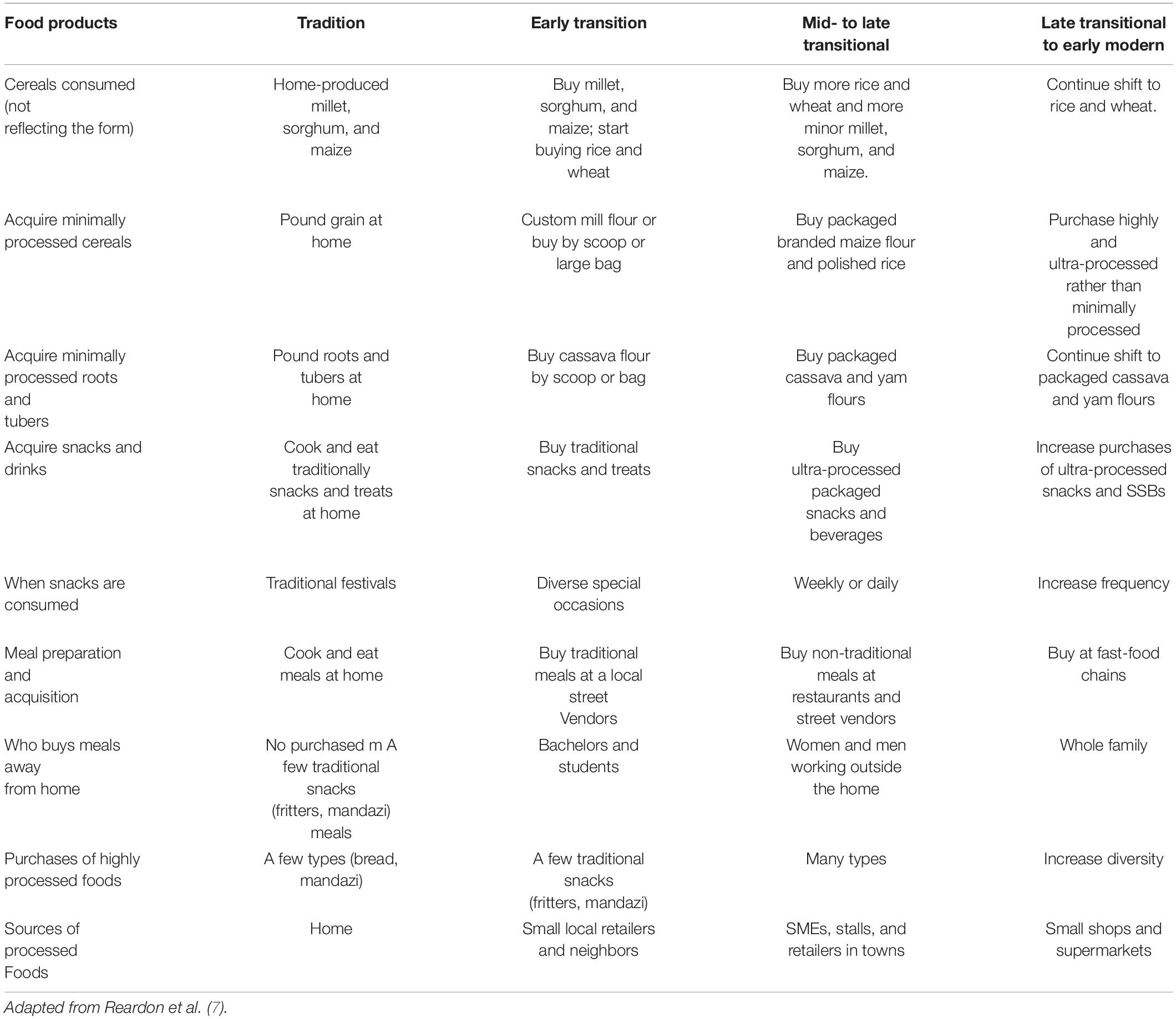
Table 2. Overview of 11 critical shifts in the 50-year evolution of processed food consumption in sub-Saharan Africa: who, what, when, where, and how.
Traditional or indigenous crops play an important role as a symbol of heritage, trademark, and culture, besides offering an essential opportunity to diversify the food base through different ethnic groups (11, 12). Therefore, it is necessary to preserve diverse food practices, especially food preparation and consumption elements, as this knowledge can easily be lost over a few generations (2, 50). There is a significant risk that the knowledge around indigenous foods and potentially crucial ways of living more sustainably has already vanished (51). The African communities lose their identity of preserving those products that are easy to prepare because they are usually minimally processed, leading to food insecurity and livelihood in sub-Saharan Africa. Researchers argue that indigenous food plants played an essential role in the diet of African communities, the industrialization of food, and formalization of markets in countries such as South Africa have resulted in a decrease in the utilization of established domesticated wild plants and foods that had been stable for decades (11). The African traditional foods have been marginalized due to a lack of information on their use and importance in rural economies/cultures and their economic value. Moreover, there are limited reliable methods for measuring their contribution to farm households and the rural economy; lack of world markets (except for a handful of products); irregularities in supply; quality standards; and storage and processing technology (52). Traditional foods that were consumed back then have more nutritional values because some of them were consumed without adding salt, sugar, oil, and other food additives, which are reported to cause some chronic diseases. Many modifications are done to the indigenous crops for converting them into value-added products that increase the availability of products in large quantities to reduce food security. The major problem with the value-added product is the increased harmful diseases caused by the substances added to the final product (11, 12, 52). This section reviewed some food groups (e.g., cereals and grains, fruits and vegetables, and roots and tuber crops) specific to the African continent and their reported level of processing.
Cereals and grains form the more significant percentage of food consumed worldwide as they are excellent energy sources. The various cereals grown in Africa include wheat, millet, maise, fonio, teff, sorghum, and rice (Figure 1). Grains require various levels of processing to transform them into edible products. Since it is the source of carbohydrate foods consumed by most people, several bakery products have been formulated from cereals (Table 3). Several research studies have been conducted on cereal grain processing (42, 53, 54). According to the Siga framework (Table 1), the level of processing has been assigned to the foods in Table 3.

Figure 1. Cereals and grains of Africa. (https://www.google.com/search?q=cereal+grains&sxsrf=AOaemvL2cmQ98ztkUbsjOzKZQi6eK6n95A:1638149496818&source=lnms&tbm=isch&sa=X&ved=2ahUKEwiryeK2trz0AhUcQUEAHf4CDsYQ_AUoAXoECAEQAw&biw=1366&bih=657&dpr=1.
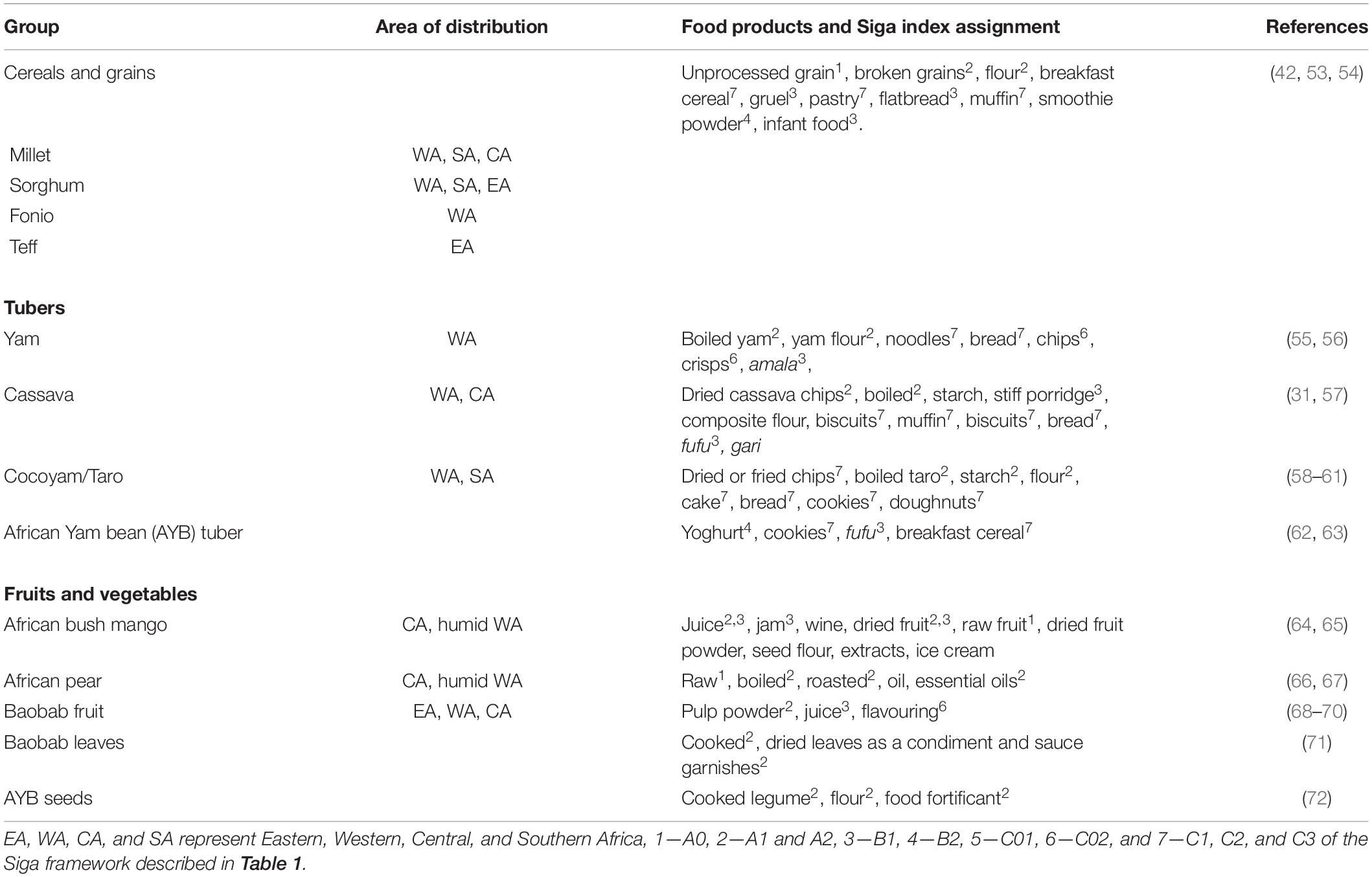
Table 3. Classification of African foods based on their level of processing based on the Siga food processing index.
Root and tuber crops supply energy and nutrition to over two billion people and are an essential income source for farmers in rural and marginalized communities. Root and tuber crops are economically versatile, providing cash, food security, and regular food crops. The waste products (peels) can be used as industrial raw materials and livestock feed (73). The tubers discussed in this section are grown and consumed on the African continent and their normal processing levels.
African yam bean (AYB) (Sphenostylis stenocarpa) plant is mainly grown and consumed in West, Central, and East Africa (Figure 2). It is a potential food security crop that is versatile due to its edible seed and roots George, Ajibola (48, 50, 72, 74). Although regarded as an orphan crop, this plant is suitable for potential food security in Africa for the following reasons: it survives in broad climatic conditions, contains essential nutrients, has a cultural link to Africa, and provides the potential for food-to-food fortification of staple foods Ojuederie, Teye (49, 51, 73, 75). AYB tuber has superior protein content (15%) compared with cassava and yam at < 2%, thus making it a valuable tuber for the development of protein-rich food products Ojuederie (49, 73). The starch of AYB tuber and seed showed superior thermal properties and were resistant to amylolytic enzymes indicating its potential use for the development of low Glycemic index (GI) foods Malumba (52, 76).

Figure 2. African tuber crops (https://www.google.com/search?q=tuber+crops+in+africa&sxsrf=AOaemvIjAfPFMI4V4BheAZXga3ORPC33wQ:1637923213131&source=lnms&tbm=isch&sa=X&ved=2ahUKEwjPlqa667~×~0AhWB87sIHcIoBp8Q_AUoAXoECAEQAw&biw=1366&bih=657&dpr=1).
The AYB seed is helpful for food fortification to compensate for nutrient loss in foods due to processing. Its seeds are primarily consumed in Nigeria, West Africa. This is because the proteins (20–30%), carbohydrates (50–64%), dietary fibers (3–10%), total minerals (2–5%), and amino acid contents compare favorably with other legumes such as soybean. AYB seed starch has been extracted and shown to possess superior functional properties (76). The seeds are usually boiled with salt, pepper, and palm oil and consumed as a bean soup. Recently, other products developed from AYB seed include a yoghurt-like product (77), biscuits (78), breakfast cereal (76, 79), noodles (80), and cassava enriched fufu (81).
The highest production of cassava (Manihot esculenta) in Africa is in Nigeria—accounting for 19% of the total world’s population, followed by other non-African countries such as Thailand (11%), Indonesia (9%), and Brazil (8%). This tuber crop offers a potential income stream for exportation. Still, due to problems such as weak trade, transport routes, market access, and quick deterioration of the tuber, most of the cassava produced on the continent is consumed in Africa (82). Cassava-based products are primarily consumed in traditional forms, such as fermented cassava granules (garri) and stiff porridge (fufu, eba), retaining their essential nutrients. More recently, cassava flour has been used alone and composited with other flours to produce biscuits (82), bread (83, 84), muffins (85), Dewi et al. (86), Ramírez et al. (87), and pasta (88). However, these ultra-processed products are not yet widespread or available on the shelves; hence, there is a need for more commercialisation of cassava flour on the continent and the exportation of these products within and outside of the African continent.
Cocoyam (Colocasia esculenta [L.] Schott) is a tropical root crop that originated in Asia and spread to the rest of the world. The global production statistics show that 69.42% is cultivated in Africa, with Nigeria ranking the highest at 27.14%. Surprisingly, none of the African countries cultivating taro make it to the top ten exporters (84). This is a saddening gap in economic turnover for cocoyam farmers in Africa. The two species extensively grown in Africa belong to the Araceae family, namely, Xanthosoma sagittifolium and C. esculenta. Despite its long-standing existence, it is marginalized in food production, export, and industrial usage. However, that narrative is changing as scientific investigations into its utilisation as a food fortificant (59, 60, 89, 90) and food ingredient (91–94) are currently underway. Nutritionally, taro is superior to cassava and potatoes in protein (11% dry weight) and carbohydrate (87% dry weight) contents. The corm of taro root has been consumed over the years because the starch granule of taro is small and easily digestible compared with other tubers. This makes it an ideal source of carbohydrates for people with digestive problems, especially the elderly (90, 95, 96).
Apart from the known commercial fruits in the market such as orange, mango, apple, pear, and grapes, numerous indigenous fruits have not been exploited for food security in Africa. Some of these fruits were adequately reviewed by Stadlmayr et al. (68). Examples of the indigenous fruits of Africa (Figure 3) are Adansonia digitata L., Balanites aegyptiaca (L.) Delile, Dacryodes edulis (G. Don) H. J. Lam, Irvingia gabonensis, Sclerocarya birrea, Syzygium guineense (Willd.) DC., Tamarindus indica L., Uapaca kirkiana Müll. Arg., Vitex doniana Sweet, Ziziphus mauritiana Lam., and Chrysophyllum albidum (68). The focus of this section is to bring to the limelight the underutilized African fruits and vegetables and their levels of processing.

Figure 3. African native fruits (https://www.google.com/search?q=fruits+in+africa&sxsrf=AOaemvID5L7IffILh1Ps_tXHgaANzaTqpA:1638116809389&source=lnms&tbm=isch&sa=X&ved=2ahUKEwjcipjUvLv0AhUMKewKHQs_CnYQ_AUoAXoECAEQAw).
Irvingia gabonensis has a morphology like that of mango, hence the name “African bush mango.” Other names include bush mango, dika nut, odika, ogbono, manguier sauvage, or chocolatier (65). It is indigenous to the humid forest zone of some African countries such as Congo, Uganda, Nigeria, Angola, and the Ivory Coast. It has an edible fruit pulp and an oil-rich kernel enclosed in a hard stony nut. It is widely used as food and medicine. The fruit pulp and seed (Figure 3) have gained prominence in pharmaceutical weight loss supplements in the United States (97). There are two species common in West Africa. The sweet, yellow-fleshed edible fruit (Irvingia gabonensis) and the one that is processed for cooking are characterized by a bitter and non-edible mesocarp (Irvingia wobolu). The vitamin C (51–76 mg/100 g) content of I. gabonensis fruit is higher than the common mango (40 mg/100 g). The ripe and unripe bush mango fruit has a shelf-life of 2 and 10 days, respectively. Therefore, processing is highly needed to prevent postharvest losses. The level of processing of the fruit is minimal to average ranging from drying, grinding, pressing, and fermentation. These have yielded processed products such as juice, jam, wine, dried fruits, and dried fruit powder (64, 65, 98).
Dacryodes edulis, also known as butter fruit, bush pear, and African plum, is a fruit tree native to West African countries. Its local name differs in different countries. It matures into a pink olive-like fruit and turns dark purple upon ripening. It is called ube in Nigeria, atanga in Gabon, and safou in Cameroon (67). D. edulis is a highly underutilized tropical crop. The fruit is usually eaten raw as a snack, and it can also be roasted or boiled as side dishes. The fruit pulp has a rich lipid content accounting for 72.6% of the whole fruit, 44% of the pulp, and 27.3% of the seed and fatty acids up to 60% oleic and palmitic acid (66). The protein content (34% in the seed and 26% in pulp) of D. edulis is superior to soybean (14%). Essential oils such as α-pinene α-phellandrene, limonene, and β-pinene have been extracted from the resin, fruit, and seed of D. edulis for medicinal use (99). The fruit is high in calcium, potassium, and ascorbic acid. The array of nutrients in D. edulis makes it a “superfood” and a potential food security crop to combat malnutrition in the malnourished population of Africa. Cold-pressed D. edulis oil made in Africa is available on market shelves (Figure 4).

Figure 4. Some African processed foods (https://www.google.com/search?q=african+processed+foods&rlz=1C1CHBF_enZA990ZA990&sxsrf=APq-WBtQ l6wnr_h-2NX_pG9IAOcEX6L9_g:1647524662762&source=lnms&tbm=isch&sa=X&ved=2ahUKEwj09qDSo832AhUBgFwKHeIeCDEQ_AUoAXoECAEQAw&biw= 1366&bih=657&dpr=1).
Adansonia digitata L., known as baobab in English, is naturally distributed in Eastern, Western, and Southern Africa (68). Baobab is used as food, medicine, and animal feed. Every part of the baobab plant, i.e., bark, leaves, flower, root, fruit pulp, and seeds, is edible (100). African names for baobab are isimuhu (South Africa), kouka (Nigeria), sira (Mali), mwambo (Kenya), and mlonje (Malawi) (101). The fruit pulp is said to have antioxidant, anti-inflammatory, anti-microbial, and analgesic properties. The powder is derived from the fruit pulp and used in various ways such as adding to maize gruel, mixing with water as juice, and using in fermentation (69, 70). Due to high levels of anti-nutrient compounds such as tannins and phytic acid, the processing of baobab fruit into a value-added product is necessary. Even though the baobab is classified as the fruit of Africa, the leaves are often overlooked. Baobab leaves can be cooked just like any green vegetable in season and dried, ground into powder, and used as food condiments during off-seasons. The leaves are rich in iron, vitamin A, and protein (71).
There are increasing global efforts, substantial interest, and scientific research on plant foods from sub-Saharan Africa. A lot of research still needs to be performed on the entire value chain of farms to consumers. Further research is required for all aspects of the plant crop to optimize its benefits and valorization. Most plant-based foods on the African continent are still minimally prepared in their natural state, therefore, offering the benefits of whole foods—supporting the holism paradigm. However, the challenges that plague the low-income countries of Africa, such as unreliable road networks, poor storage infrastructures, and electricity issues, most foods are prone to spoilage. Therefore, the level of processing that ensures nutritional balance and fewer additives and promotes less wastage with a minimal or no negative environmental impact is encouraged. Future directions range from the engagement of policymakers to advancing scientific understanding using the various technologies of the fourth industrial revolution, including green technologies enabling maximum utilization of the different underutilized crops in addressing global food security and nutrition. Intensifying research on plant foods of Africa brings these crops at par with socioeconomic and scientific knowledge as crops from other parts of the world. The following areas, among others, are recommended for a balanced FP in Africa: (a) scoring foods in a hierarchy where a holistic index is first applied, followed by a compositional index, avoiding excessive valorization of UPFs, (b) understanding plant starch chemistry involving composition, isolation, physicochemical properties, and starch modification methods in search of novel properties and the application of modified starch in food systems, (c) modification of plant proteins for improved functionality highlights that although, through minimal physical, mechanical, and biological techniques are widely being adapted to produce a functional ingredient such as texturized vegetable proteins, hydrolyzed vegetable protein, clean label protein concentrates, de-flavored protein isolates, protein flour, and grits, and (d) promotion of holistic approach in line with the Siga framework.
AIOJ conceptualized the manuscript. OOO crafted the outline and led the manuscript writing. SER, OOO, and AIOJ wrote different portions of the manuscript. OOO formatted the final version of the manuscript. AIOJ reviewed the manuscript. All authors approved the submitted version of the manuscript.
This study was funded by the Directorate of Research and Innovation, University of Venda South Africa.
AIOJ is currently engaged with VicFame Pty Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Carretero C, Clotet R, Colomer Y, Fernando GG, De Frías J, Guamis B, et al. Food Classification Report: The Concept “Ultra-Processed”. (2020). Available online at: http://www.triptolemos.org/en/portfolio_page/food-clasification-report-ultraprocessed-concept/ (accessed November 19, 2021).
2. USDA – United States Department of Agriculture. Processed Foods are Excluded from COOL Requirements. How is a Processed Food Defined?. (2016). Available online at: https://www.ams.usda.gov/sites/default/files/media/COOL%20FAQs%20Final.pdf (accessed November 16, 2021).
3. Jones JM. Food processing: criteria for dietary guidance and public health? Proc Nutr Soc. (2019) 78:4–18. doi: 10.1017/S0029665118002513
4. Petrus RR, do Amaral Sobral PJ, Tadini CC, Gonçalves CB. The NOVA classification system: a critical perspective in food science. Trend Food Sci Technol. (2021) 16:603–8. doi: 10.1016/j.tifs.2021.08.010
5. Bansal V, Siddiqui MW, Rahman MS. Minimally processed foods: overview. In: Siddiqui M, Rahman M, editors. Minimally Processed Foods. Food Engineering Series. Cham: Springer (2015). doi: 10.1007/978-3-319-10677-9_1
6. Nasreddine L, Tamim H, Itani L, Nasrallah MP, Isma’eel H, Na Nakhoul NF, et al. A minimally processed dietary pattern is associated with lower odds of metabolic syndrome among Lebanese adults. Public Health Nutr. (2018) 21:160–71. doi: 10.1017/S1368980017002130
7. Reardon T, Tschirley D, Liverpool-Tasie LSOL, Awokuse T, Fanzo J, Minten B, et al. The processed food revolution in African food systems and the double burden of malnutrition. Glob Food Secur. (2021) 28:100466. doi: 10.1016/j.gfs.2020.100466
8. Monteiro CA, Cannon G, Moubarac JC, Levy RB, Louzada MLC, Jaime PC. The UN decade of nutrition, the NOVA food classification and the trouble with ultra-processing. Public Health Nutr. (2018) 21:5–17. doi: 10.1017/S1368980017000234
9. Fardet A, Rock E. Ultra-processed foods and food system sustainability: what are the links? Review. Sustainability. (2020) 12:6280. doi: 10.3390/su12156280
10. Gibney MJ. Ultra-processed foods: definitions and policy. Curr Dev Nutr. (2019) 3:nzy077. doi: 10.1093/cdn/nzy077
11. Adeyeye SAO. The role of food processing and appropriate storage technologies in ensuring food security and food availability in Africa. Nutr Food Sci. (2017) 47:122–39. doi: 10.1108/nfs-03-2016-0037
12. Kuyu CG, Bereka TY. Review on the contribution of indigenous food preparation and preservation techniques to the attainment of food security in Ethiopian. Food Sci Nutr. (2020) 8:3–15. doi: 10.1002/fsn3.1274
13. Sadler CR, Grassby T, Hart K, Raats M, Sokolović M, Timotijevic L. Processed food classification: conceptualisation and challenges. Trend Food Sci Technol. (2021) 112:149–62. doi: 10.1016/j.tifs.2021.02.059
14. Fardet A, Rock E. Ultra-processed foods: a new holistic paradigm? Trend Food Sci Technol. (2019) 93:174–84. doi: 10.1016/j.tifs.2019.09.016
15. Crino M, Barakat T, Trevena H, Neal B. Systematic review and comparison of classification frameworks describing the degree of food processing. Nutr Food Technol. (2017) 3:138. doi: 10.16966/2470-6086.138
16. Martinez-Perez C, San-Cristobal R, Guallar-Castillon P, Martínez-González MÁ, Salas-Salvadó J, Corella D, et al. Use of different food classification systems to assess the association between ultra-processed food consumption and cardiometabolic health in an elderly population with metabolic syndrome (PREDIMED-Plus Cohort). Nutrients. (2021) 13:2471. doi: 10.3390/nu13072471
17. Derbyshire E. Are all ultra-processed foods nutritional demons? A commentary and nutritional profiling analysis. Trend Food Sci Technol. (2019) 94:98–104. doi: 10.1016/j.tifs.2019.08.023
18. Food Standards Australia New Zealand. Australia New Zealand Food Standards Code – Standard 3.2.2 – Food Safety Practices and General Requirements. Canberra, ACT: Food Standards Australia New Zealand (2014).
19. Moubarac JC, Parra DC, Cannon G, Monteiro CA. Food classification systems based on food processing: significance and implications for policies and actions: a systematic literature review and assessment. Curr Obes Rep. (2014) 3:256–72. doi: 10.1007/s13679-014-0092-0
20. Monteiro CA, Levy RB, Claro RM, Castro IRRD, Cannon G. A new classification of foods based on the extent and purpose of their processing. Cadernos de Saude Publica. (2010) 26:2039–49. doi: 10.1590/s0102-311x2010001100005
21. Monteiro CA, Cannon G, Levy RB, Moubarac JC, Louzada ML, Rauber F, et al. Ultra-processed foods: what they are and how to identify them. Public Health Nutr. (2019) 22:936–41. doi: 10.1017/S1368980018003762
22. Sarfo J, Pawelzik E, Keding GB. Dietary patterns as characterized by food processing levels and their association with the health outcomes of rural women in East Africa. Nutrients. (2021) 13:2866. doi: 10.3390/nu13082866
23. Slimani N, Deharveng G, Southgate DAT, Biessy C, Chajes V, Van Bakel MME, et al. Contribution of highly industrially processed foods to the nutrient intakes and patterns of middle-aged populations in the European prospective investigation into cancer and nutrition study. Eur J Clin Nutr. (2009) 63:S206–25. doi: 10.1038/ejcn.2009.82
24. Eicher-Miller HA, Fulgoni VLI, Keast DR. Energy and nutrient intakes from processed foods differ by sex, income status, and race/ethnicity of US adults. J Acad Nutr Diet. (2015) 115:907–18.e6. doi: 10.1016/j.jand.2014.11.004
25. Poti JM, Bianca Braga MA, Bo Qin B. Ultra-processed food intake and obesity: what really matters for health – processing or nutrient content? Curr Obes Rep. (2017) 6:420–31. doi: 10.1007/s13679-017-0285-4
26. Bleiweiss-Sande R, Chui K, Evans EW, Goldberg J, Amin S, Sacheck J. Robustness of food processing classification systems. Nutrients. (2019) 11:1344. doi: 10.3390/nu11061344
27. SIGA. Siga, Une d;Emarche Scientifique Pour am’Eliorer et Promouvoir la Qualit’e des Aliments [Siga, a Scientific Approach to Improve and Promote the Quality of Food]. (2019). Available online at: https://siga.care/ (accessed March 9, 2022).
28. Davidou S, Christodoulou A, Frank K, Fardet A. A study of ultra-processing marker profiles in 22,028 packaged ultra-processed foods using the Siga classification. J Food Comp Anal. (2021) 99:103848. doi: 10.1016/j.jfca.2021.103848
29. Fardet A, Rock E. From a reductionist to a holistic approach in preventive nutrition to define new and more ethical paradigms. Healthcare. (2015) 3:1054–63. doi: 10.3390/healthcare3041054
30. Dicken SJ, Batterham RL. The role of diet quality in mediating the association between ultra-processed food intake, obesity and health-related outcomes: a review of prospective cohort studies. Nutrients. (2021) 14:23. doi: 10.3390/nu14010023
31. Udoro EO, Anyasi TA, Jideani AIO. Process-induced modifications on quality attributes of cassava (Manihot esculenta Crantz) flour. Process. (2021) 9:1891. doi: 10.3390/pr9111891
32. Udoro EO, Kehinde AT, Olasunkanmi SG, Charles TA. Studies on the physicochemical, functional and sensory properties of gari processed from dried cassava chips. J Food Process Technol. (2014) 5:293. doi: 10.4172/2157-7110.1000293
33. Langiano E, Ferrara M, Lanni L, Viscardi V, Abbatecola AM, De Vito E. Food safety at home: knowledge and practices of consumers. J Public Health. (2012) 20:47–57. doi: 10.1007/s10389-011-0437-z
34. de Araújo TP, de Moraes MM, Afonso C, Santos C, Rodrigues SS. Food processing: comparison of different food classification systems. Nutrients. (2022) 14:729. doi: 10.3390/nu14040729
35. Chajès V, Biessy C, Byrnes G, Deharveng G, Saadatian-Elahi M, Jenab M, et al. Ecological-level associations between highly processed food intakes and plasma phospholipid elaidic acid concentrations: results from a cross-sectional study within the European prospective investigation into cancer and nutrition (EPIC). Nutr Cancer. (2011) 63:1235–50. doi: 10.1080/01635581.2011.617530
36. Monteiro CA, Moubarac JC, Cannon G, Ng SW, Popkin B. Ultra-processed products are becoming dominant in the global food system. Obes Rev. (2013) 14:21–8. doi: 10.1111/obr.12107
37. Monteiro CA, Cannon G, Levy RB, Moubarac JC, Jaime P, Martins AP, et al. NOVA. The star shines bright. World Nutr. (2016) 7:28–38.
38. Jones JM, Clemens RA. Introductory brain teaser for the cereal chemist—how do we categorize processed and ultraprocessed foods? AACCI. (2017) 62:120–3. doi: 10.1094/cfw-62-3-0120
39. Crimarco A, Landry MJ, Gardner CD. Ultra-processed foods, weight gain, and co-morbidity risk. Curr Obes Rep. (2021) 10: 1–13. doi: 10.1007/s13679-021-00460-y
40. Franco F, Navarro G, Martínez-Pinilla E. Antioxidants versus food antioxidant additives and food preservatives. Review. Antioxidants. (2019) 8:542. doi: 10.3390/antiox8110542
41. Silva MMN, Albuquerque TL, Pereira KS, Coelho MAZ. Food additives used in non-alcoholic water-based beverages– a review. J Nutr Health Food Eng. (2019) 9:109–121. doi: 10.15406/jnhfe.2019.09.00335
42. Ramashia SE, Anyasi TA, Gwata ET, Meddows-Taylor S, Jideani AIO. Processing, nutritional composition and health benefits of finger millet in sub-Saharan Africa. Food Sci Technol. (2019) 39:253–66. doi: 10.1590/fst.25017
43. Steyn NP, Wolmarans P, Nel JH, Bourne LT. National fortification of staple foods can make a significant contribution to the micronutrient intake of South African adults. Public Health Nutr. (2007) 11:307–13. doi: 10.1017/S136898000700033X
44. Igumbor EU, Sanders D, Puoane TR, Tsolekile L, Schwarz C, Purdy C, et al. “Big food,” the consumer food environment, health, and the policy response in South Africa. PLoS Med. (2012) 9:e1001253. doi: 10.1371/journal.pmed.1001253
45. Baker P, Machado P, Santos T, Sievert K, Backholer K, Hadjikakou M, et al. Ultra-processed foods and the nutrition transition: global regional and national trends food systems transformations and political economy drivers. Obes Rev. (2020) 21:e13126. doi: 10.1111/obr.13126
46. Otitoola O, Oldewage-Theron W, Egal A. Prevalence of overweight and obesity among selected schoolchildren and adolescents in Cofimvaba South Africa. South Afri J Clin Nutr. (2021) 34:97–102. doi: 10.1080/16070658.2020.1733305
47. Letswalo BP, Schmid-Zalaudek K, Brix B, Matjuda EN, Klosz F, Obernhumer N, et al. Cardiometabolic risk factors and early indicators of vascular dysfunction: a cross-sectional cohort study in South African adolescents. BMJ Open. (2021) 11:e042955. doi: 10.1136/bmjopen-2020-042955
48. Bosire EN, Cohen E, Erzse A, Goldstein SJ, Hofman KJ, Norris SA. ‘I’d say I’m fat I’m not obese’: obesity normalisation in urban-poor South Africa. Public Health Nutr. (2020) 23:1515–26. doi: 10.1017/S1368980019004440
49. Hunter-Adams J, Battersby J, Oni T. Food insecurity in relation to obesity in peri-urban Cape Town South Africa: implications for diet-related non-communicable disease. Appetite. (2019) 137:244–9. doi: 10.1016/j.appet.2019.03.012
50. Raji MNA, Karim SAB, Ishak FAC, Arshad MM. Past and present practices of the Malay food heritage and culture in Malaysia. J Ethn Food. (2017) 4:122–231. doi: 10.1016/j.jef.2017.11.001
51. Masekoameng MR, Molotja MC. The role of indigenous foods and indigenous knowledge systems for rural households’ food security in Sekhukhune district Limpopo province South Africa. J Consum Sci. (2019) 4:34–48. Available online at: https://www.ajol.info/index.php/jfecs/article/view/191563
52. Akinola R, Pereira LMT, Mabhaudhi T, de Bruin FM, Rusch L. A review of indigenous food crops in Africa and the implications for more sustainable and healthy food systems. Sustainability. (2020) 12:3493. doi: 10.3390/su12083493
53. Wolter A, Hager AS, Zannini E, Arendt EK. In vitro starch digestibility and predicted glycaemic indexes of buckwheat oat quinoa Sorghum teff and commercial gluten-free bread. J Cereal Sci. (2013) 58:431–6. doi: 10.1016/j.jcs.2013.09.003
54. Zhu F. Fonio grains: physicochemical properties nutritional potential and food applications. Compr Rev Food Sci Food Saf. (2020) 19:3365–89. doi: 10.1111/1541-4337.12608
55. Abulude FO, Ojediran VA. Development and quality evaluation of fortified “amala”. Acta Sci Polonor Technol Aliment. (2006) 5:127–33.
56. Ojokoh AO, Gabriel RA. A comparative study on the storage of yam chips (gbodo) and yam flour (elubo). Afr J Biotechnol. (2010) 9:3175–7.
57. Rachman A, Brennan MA, Morton J, Brennan CS. Gluten-free pasta production from banana and cassava flours with egg white protein and soy protein addition. Int J Food Sci Technol. (2020) 55:3053–60. doi: 10.1111/ijfs.14608
58. Sanful RE, Darko S. Production of cocoyam cassava and wheat flour composite rock cake. Pak J Nutr. (2010) 9:810–4. doi: 10.3923/pjn.2010.810.814
59. Mongi RJ, Ndabikunze BK, Chove BE, Mamiro P, Ruhembe CC, Ntwenya JG. Proximate composition bread characteristics and sensory evaluation of cocoyam-wheat composite breads. Afr J Food Agric Nutr Dev. (2011) 11:5586–99. doi: 10.18697/ajfand.48.11315
60. Orhevba BA, Ndanaimi Y. Proximate and sensory properties of wheat-cocoyam (Colocasia esculenta) composite bread. Eur J Agric Food Sci. (2021) 3:86–90. doi: 10.24018/ejfood.2021.3.3.297
61. Akonor PT, Tortoe C, Buckman ES. Evaluation of cocoyam-wheat composite flour in pastry products based on proximate composition physicochemical functional and sensory properties. J Culin Sci Technol. (2018) 16:52–65. doi: 10.1080/15428052.2017.1333937
62. Malumba P, Bungu MD, Katanga KJ, Doran L, Danthine S, Béra F. Structural and physicochemical characterization of Sphenostylis stenocarpa (Hochst. ex A. Rich.) harms tuber starch. Food Chem. (2016) 212:305–12. doi: 10.1016/j.foodchem.2016.05.181
63. Sotunde AJ, Awofadeju OFJ, Olapade AA. Production and characterisation of extruded African yam bean based ready-to-eat breakfast product. J Res For Wildl Environ. (2021) 13:172–87.
64. Aworh OC. Promoting food security and enhancing Nigeria’s small farmers’ income through value-added processing of lesser-known and under-utilized indigenous fruits and vegetables. Food Res Int. (2015) 76:986–91. doi: 10.1016/j.foodres.2015.06.003
65. Mateus-Reguengo L, Barbosa-Pereira L, Rembangouet W, Bertolino M, Giordano M, Rojo-Poveda O, et al. Food applications of Irvingia gabonensis (Aubry-Lecomte ex. O’Rorke) Baill. the ‘bush mango’: a review. Crit Rev Food Sci Nutr. (2020) 60:2446–59. doi: 10.1080/10408398.2019.1646704
66. Tee LH, Yang B, Nagendra KP, Ramanan RN, Sun J, Chan ES, et al. Nutritional compositions and bioactivities of Dacryodes species: a review. Food Chem. (2014) 165:247–55. doi: 10.1016/j.foodchem.2014.05.084
67. Onuegbu NC. Dacryodes edulis: composition and physico-chemical properties. In: A Mariod editor. Wild Fruits: Composition, Nutritional Value and Products. Cham: Springer (2019). doi: 10.1007/978-3-030-31885-7_20
68. Stadlmayr B, Charrondiere UR, Eisenwagen S, Jamnadass R, Kehlenbeck K. Nutrient composition of selected indigenous fruits from Sub-Saharan Africa. J Sci Food Agric. (2013) 93:2627–36. doi: 10.1002/jsfa.6196
69. Tembo DT, Holmes MJ, Marshall LJ. Effect of thermal treatment and storage on bioactive compounds organic acids and antioxidant activity of baobab fruit (Adansonia digitata) pulp from Malawi. J Food Compos Anal. (2017) 58:40–51. doi: 10.1016/j.jfca.2017.01.002
70. Ismail BB, Liu D, Pu Y, He Q, Guo M. High-intensity ultrasound processing of baobab fruit pulp: effect on quality bioactive compounds and inhibitory potential on the activity of α-amylase and α-glucosidase. Food Chem. (2021) 361:130144. doi: 10.1016/j.foodchem.2021.130144
71. Rashford J. The use of baobab leaves (Adansonia digitata L.) for food in Africa: a review. Econ Bot. (2018) 72:478–95. doi: 10.1007/s12231-018-9438-y
72. George TT, Obilana AO, Oyeyinka SA. The prospects of African yam bean: past and future importance. Heliyon. (2020) 6:e05458. doi: 10.1016/j.heliyon.2020.e05458
73. Ojuederie OB, Balogun MO. African yam bean (Sphenostylis stenocarpa) tubers for nutritional security. J Underutil Legum. (2019) 1:56–68.
74. Ajibola CF, Malomo SA, Fagbemi TN, Aluko RE. Polypeptide composition and functional properties of African yam bean seed (Sphenostylis stenocarpa) albumin globulin and protein concentrate. Food Hydrocoll. (2016) 56:189–200. doi: 10.1016/j.foodhyd.2015.12.013
75. Teye E, Deha CI, Dadzie R, MacArthur RL. Delivering the nutritional needs by food-to-food fortification of staples using underutilized plant species in Africa. Int J Food Sci. (2020) 8826693. doi: 10.1155/2020/8826693
76. Malumba P, Doran L, Zanmenou W, Odjo S, Katanga J, Blecker C, et al. Morphological structural and functional properties of starch granules extracted from the tubers and seeds of Sphenostylis stenocarpa. Carbohydr Polym. (2017) 178:286–94. doi: 10.1016/j.carbpol.2017.09.013
77. Amakoromo E, Innocent-Adiele H, Njoku H. Microbiological quality of a yoghurt-like product from African yam bean. Nat Sci. (2012) 10:6–9.
78. Igbabul BD, Iorliam BM, Umana EN. Physicochemical and sensory properties of cookies produced from composite flours of wheat cocoyam and African yam beans. J Food Res. (2015) 4:150. doi: 10.5539/jfr.v4n2p150
79. Okafor GI, Usman GO. Production and evaluation of breakfast cereals from blends of African yam bean (Sphenostylis stenocarpa) maize (Zea mays) and defatted coconut (Cocus nucifera). J Food Process Preserv. (2014) 38:1037–43. doi: 10.1111/jfpp.12060
80. Ajibola GO, Olapade AA. Chemical composition anti-nutritional factors and pasting properties of cassava-African yam bean flour blends for noodle preparation. Int J Food Stud. (2021) 10:SI1–13. doi: 10.7455/ijfs/10.SI.2021.a1
81. Aniedu C, Aniedu OC. Fortification of cassava fufu flour with African yam bean flour: implications for improved nutrition in Nigeria. Asian J Plant Sci Res. (2014) 4:63–6.
82. Lu H, Guo L, Zhang L, Xie C, Li W, Gu B, et al. Study on quality characteristics of cassava flour and cassava flour short biscuits. Food Sci Nutr. (2020) 8:521–33. doi: 10.1002/fsn3.1334
83. Jensen S, Skibsted LH, Kidmose U, Thybo AK. Addition of cassava flours in bread-making: sensory and textural evaluation. LWT Food Sci Technol. (2015) 60:292–9. doi: 10.1016/j.lwt.2014.08.037
84. Monthe OC, Grosmaire L, Nguimbou RM, Dahdouh L, Ricci J, Tran T, et al. Rheological and textural properties of gluten-free doughs and breads based on fermented cassava sweet potato and sorghum mixed flours. LWT Food Sci Technol. (2019) 101:575–82. doi: 10.1016/j.lwt.2018.11.051
85. Rodriguez-Sandoval E, Prasca-Sierra I, Hernandez V. Effect of modified cassava starch as a fat replacer on the texture and quality characteristics of muffins. J Food Meas Charact. (2017) 11:1630–9. doi: 10.1007/s11694-017-9543-0
86. Dewi PS, Ulandari D, Susanto NS. Effect of glucomannan addition on physical and sensory characteristic of gluten-free muffin from modified cassava flour and maize flour. In: Proceedings of the IOP Conference Series: Earth and Environmental Science. (Vol. 33), Bristol: IOP Publishing (2021). p. 012085. doi: 10.1088/1755-1315/733/1/012085
87. Ramírez M, Tenorio MJ, Ramirez C, Jaques A, Nuñez H, Simpson R, et al. Optimization of hot-air drying conditions for cassava flour for its application in gluten-free pasta formulation. Food Sci Technol Int. (2019) 25:414–28. doi: 10.1177/1082013219828269
88. Odey GN, Lee WY. Evaluation of the quality characteristics of flour and pasta from fermented cassava roots. Int J Food Sci Technol (2020) 55:813–22. doi: 10.1111/ijfs.14364
89. Otekunrin OA, Sawicka B, Adeyonu AG, Otekunrin OA, Rachoń L. Cocoyam [Colocasia esculenta (L.) Schott]: exploring the production health and trade potentials in Sub-Saharan Africa. Sustainability. (2021) 13:4483. doi: 10.3390/su13084483
90. Mokhele TM. The Development of Amadumbe (Colocasia esculenta (l.) Schott)-Soya Composite Biscuits With Improved Nutritional and Sensory Properties. Masters dissertation. Johannesburg: University of South Africa Press. (2018). Available online at: http://hdl.handle.net/10500/25106 (accessed February 02, 2022).
91. Naidoo K, Amonsou EO, Oyeyinka SA. In vitro digestibility and some physicochemical properties of starch from wild and cultivated amadumbe corms. Carbohydr Polym. (2015) 125:9–15. doi: 10.1016/j.carbpol.2015.02.066
92. Mukurumbira A, Mariano M, Dufresne A, Mellem JJ, Amonsou EO. Microstructure thermal properties and crystallinity of amadumbe starch nanocrystals. Int J Biol Macromol. (2017) 102:241–7. doi: 10.1016/j.ijbiomac.2017.04.030
93. Manhivi VE, Amonsou EO, Kudanga T. Transglutaminase and tyrosinase as potential cross-linking tools for the improvement of rheological properties of gluten-free amadumbe dough. Int J Food Sci Technol. (2020) 55:2399–407. doi: 10.1111/ijfs.14489
94. Oyeyinka SA, Amonsou EO. Composition pasting and thermal properties of flour and starch derived from amadumbe with different corm sizes. J Food Sci Technol. (2020) 57:3688–95. doi: 10.1007/s13197-020-04401-w
95. Ubalua AO. Cocoyam (taro and tannia): staples with untapped enormous potentials-a review. Plant Knowl J. (2016) 5:27–35. doi: 10.21475/pkj.05.01.16.p7058
96. Boakye AA, Wireko-Manu FD, Oduro I, Ellis WO, Gudjónsdóttir M, Chronakis IS. Utilizing cocoyam (Xanthosoma sagittifolium) for food and nutrition security: a review. Food Sci Nutr. (2018) 6:703–13. doi: 10.1002/fsn3.602
97. Sun J, Chen P. Ultra-high-performance liquid chromatography with high-resolution mass spectrometry analysis of African mango (Irvingia gabonensis) seeds extract and related dietary supplements. J Agric Food Chem. (2012) 60:8703–9. doi: 10.1021/jf302703u
98. Mbaeyi IE, Anyanwu LN. Production and evaluation of yoghurt flavoured with solar-dried bush mango (Irvingia gabonensis) pulp. Agro Sci. (2010) 9:137–46. doi: 10.4314/as.v9i2.64808
99. Jirovetz L, Buchbauer G, Ngassoum MB, Parmentier M. Chemical composition and olfactory characterization of essential oils of fruits and seeds of African pear (Dacryodes edulis (G. Don) HJ Lam) from Cameroon. Flav Fragr J. (2005) 20:215–8. doi: 10.1002/ffj.1324
100. Kaboré D, Sawadogo-Lingani H, Diawara B, Compaoré CS, Dicko MH, Jakobsen M. A review of baobab (Adansonia digitata) products: effect of processing techniques medicinal properties and uses. Afr J Food Sci. (2011) 5:833–44.
Keywords: exotic fruits, ultra-processing, cereals, tuber, minimal processing
Citation: Jideani AIO, Onipe OO and Ramashia SE (2022) Classification of African Native Plant Foods Based on Their Processing Levels. Front. Nutr. 9:825690. doi: 10.3389/fnut.2022.825690
Received: 30 November 2021; Accepted: 22 March 2022;
Published: 29 April 2022.
Edited by:
Marco Dalla Rosa, University of Bologna, ItalyReviewed by:
Gustavo F. Gutiérrez-López, Instituto Politécnico Nacional (IPN), MexicoCopyright © 2022 Jideani, Onipe and Ramashia. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Afam I. O. Jideani, aWppZGVhbmlAZ21haWwuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.