
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Nutr., 05 April 2022
Sec. Nutrition and Sustainable Diets
Volume 9 - 2022 | https://doi.org/10.3389/fnut.2022.821096
This article is part of the Research TopicInnovation and Trends in the Global Food Systems, Dietary Patterns and Healthy Sustainable Lifestyle in the Digital AgeView all 16 articles
 Jana Jabbour1,2†
Jana Jabbour1,2† Yasmin Rihawi2†‡
Yasmin Rihawi2†‡ Assem M. Khamis3†‡
Assem M. Khamis3†‡ Layal Ghamlouche2,4
Layal Ghamlouche2,4 Bayan Tabban2
Bayan Tabban2 Gloria Safadi5†
Gloria Safadi5† Nour Hammad2,6†
Nour Hammad2,6† Ruba Hadla7†
Ruba Hadla7† Marwa Zeidan8
Marwa Zeidan8 Dana Andari8,9
Dana Andari8,9 Riwa Nour Azar2,10
Riwa Nour Azar2,10 Nadine Nasser2,11
Nadine Nasser2,11 Marlene Chakhtoura12*†
Marlene Chakhtoura12*†Background: Scientists have been investigating efficient interventions to prevent and manage obesity. This network meta-analysis (NMA) compared the effect of different diets [moderate macronutrients (MMs), low fat/high carbohydrate (LFHC), high fat/low carbohydrate (HFLC), and usual diet (UD)] on weight, body mass index (BMI), and waist circumference (WC) changes at ≥12 months.
Methods: We searched Medline, Embase, PubMed databases, and the Cochrane Library. We systematically assessed randomized controlled trials (RCTs) evaluating dietary interventions on adults (mean BMI ≥ 25 kg/m2) receiving active dietary counseling for ≥12 months. We pooled the data using a random-effect NMA. We assessed the quality of the included RCTs using the Cochrane risk of bias (ROB) tool.
Results: We included 36 trials, 14 of which compared HFLC with MM diets. Compared with UD, all diets were associated with a significant weight loss (WL) at ≥12 months, HFLC [mean difference in kg (95% CI): −5.5 (−7.6; −3.4)], LFHC [−5.0 (−7.1; −2.9)] and MM [−4.7 (−6.8; −2.7)]. HFLC, compared with MM diet, was associated with a slightly higher WL (of −0.77 kg) and drop in BMI (of −0.36 kg/m2), while no significant difference was detected in other dietary comparisons. WC was lower with all diets compared to UD, with no significant difference across specific diets. There was no significant interaction of the results with the pre-specified sub-groups. The ROB was moderate to high, mostly related to unclear allocation concealment, high dropout rate and unclear or lack of blinding of participants, providers, and outcome assessors.
Conclusion: Dietary interventions extending over ≥12 months are superior to UD in inducing weight, BMI and WC loss. HFLC might be associated with a slightly higher WL compared with MM diets.
Systematic Trial Registration: https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=103116, PROSPERO (CRD42018103116).
Obesity has almost increased three times in the last three decades to reach pandemic levels (1). Obesity is associated with a decreased lifetime expectancy of 5–20 years, depending on the severity and the presence of comorbidities (2–4). With more than half of the world population being overweight or obese, scientists are continuously exploring efficient interventions to prevent and manage this pandemic (5, 6).
Diet therapy remains one of the cornerstones of the multi-disciplinary approach to weight management. However, obesity treatment guidelines have variable recommendations regarding the most appropriate diet (Supplementary Table S1). While almost all agree on a reduced calorie meal plan, and a modification of macronutrient composition to enhance the dietary adherence and improve the metabolic profile, (7) (Supplementary Table S1), there is still no consensus yet on the most optimal macronutrient dietary pattern for weight management.
A network meta-analysis (NMA) is a meta-analysis (MA) technique that allows evaluation of at least three interventions in one analysis, using both direct and indirect comparisons (8). It is an “evidence synthesis method” gaining interest in the field of nutrition research (9). Two recent NMAs of randomized controlled trials (RCTs) assessed the short term (6–12 months) effect of different diets (10, 11). The first NMA included the following diet categories: lifestyle, exercise, attitudes, relationships, nutrition (LEARN), low carbohydrate, low fat, and moderate macronutrients (MMs) (10). The results (n = 7,286 participants) revealed that, compared with no diet, and as expected, any dietary intervention resulted in a significant weight loss (WL) at 6 months, of 5.1–8.7 kg (10). The WL response was attenuated at 12 months, with a weight reduction of 1–2 kg less compared with the 6-month follow up (10). The comparison of diets with different macronutrient composition between each other showed that a low carbohydrate diet was better than a MMs diet, at 6 and 12 months, with a small difference in mean WL of 1.9 and 1.5 kg, respectively, while none of the other comparisons reached significance (10). The more recent NMA (n = 21,942 participants) by Ge et al. included trials published until September 2018 and similarly showed that the low carbohydrate, low fat, and MM diets were superior to a usual diet (UD) at 6 months (WL of 4.6, 4.4, and 3.1 kg, respectively), with no significant difference comparing diets among each other's (11). WL decreased by 1.5 kg on average at 12 months compared with the 6-month assessment point (11). Several systematic reviews and meta-analyses (SR/MA) assessed the long term weight reducing effects of various diets beyond 12 months follow up (12–17). Two SR/MAs compared a low fat diet to any higher fat diet, including UD, and did not demonstrate any significant difference in the achieved weight at follow-up (15, 18). Two other SR/MAs compared a high protein/low or very low carbohydrate diet to other diets and showed a significant MD in WL of 0.4–0.9 kg, favoring the former diet (12, 14). One SR/MA compared Atkins, Weight Watchers diet, South Beach and Zone, and demonstrated a modest and comparable WL across all (13). The main limitations of the aforementioned SR/MAs assessing the long-term effects of dietary interventions stem from the inclusion of RCTs on patients with chronic diseases, such as diabetes mellitus (DM) and cancer, that might affect the WL response, the inclusion of RCTs with an active diet intervention extending over <1 year, or the lack of a systematic description and investigation of the effect of co-interventions, such as exercise and behavioral therapy.
Given the heavy burden of obesity, its chronic relapsing nature (19), and the lack of consensus on the most optimal diet composition, if any, for weight reduction, this SR/NMA aims at evaluating the association of long term dietary interventions, categorized using the Acceptable Macronutrient Distribution Ranges (AMDR), with changes in weight parameters. The AMDR, recommended by the Institute of Medicine (IOM), is widely used by clinicians and defines the ranges of macronutrient contribution to energy intake that have been linked to a lower risk of chronic diseases (20, 21).
The protocol for this SR/NMA followed the preferred reporting items for systematic reviews and meta-analyses (PRISMA), and was registered on PROSPERO (CRD42018103116) (22).
This section describes the Population, Intervention, Control, and Outcome (PICO) elements and details the eligibility criteria of this SR. We selected RCTs as we expected to have a complete summary of the evidence on the topic by gathering data from interventional studies. We included RCTs conducted in adults with overweight/obesity (mean body mass index (BMI) at baseline ≥ 25 kg/m2) (population), comparing an active dietary intervention of ≥12(±1) months (intervention), to another dietary regimen or UD (control), and reporting on one or more outcomes of interest, change in weight, BMI, or waist circumference (WC), at ≥12 months follow-up (outcome). We included only papers written in English. To assess the effect of dietary interventions in healthy individuals, we excluded trials where the majority of participants (>75%) were pregnant women, had chronic diseases (such as cancer, diabetes mellitus, advanced liver, or renal disease), or received medications inducing weight gain (such as anti-psychotic drugs), as these conditions are expected to affect the WL response. We excluded trials if the intensity of the intervention or the co-intervention differed between arms or in case a detailed description of the intervention (such as duration and macronutrient composition) was not provided in the trial or trial protocol publication. In addition, we excluded interventions consisting of a change in a single food item or that solely relied on the supplementation, as we aimed to assess the impact of comprehensive dietary changes. Exclusion criteria included RCTs assigning very-low-calorie diets (<800 Kcal/day) or meal replacement liquids (since such diets are not recommended as long term interventions), and RCTs where diets were assigned based on participants' genetic profiles (22).
We conducted a systematic search in the following electronic databases Medline, Embase, PubMed, and the Cochrane Library, without time restriction and until December 2020. We used Medical Subject Heading (MeSH) terms and keywords relevant to the dietary intervention and overweight or obesity (as shown in Supplementary Text S1). Moreover, we manually searched the references of SRs on the topic to identify any potentially relevant studies that may have been missed. We contacted experts in the field and searched ClinicalTrials.gov for potentially completed and non-published trials.
We conducted the screening of citations and full texts, and data abstraction in duplicate and independently, using standardized forms, prepared a priori. At each step, we conducted a calibration exercise until discrepancy rate between reviewers got to <5%. The calibration exercise involved training and cross training of the researchers on the eligibility criteria and data abstraction, to make sure that the process was standardized. We prepared screening sheets for each step based on our research question. At all stages, we resolved disagreement between reviewers by discussion and through intervention from content experts (MC and JJ).
We assessed the risk of bias (ROB) of the included RCTs in duplicate and independently using the Cochrane ROB assessment tool (23). We assessed the risk of publication bias by visually checking the symmetry of the funnel plot of the included studies in the traditional meta-analyses for comparisons including ≥10 RCTs. In the funnel plot, for each trial, we plotted the effect by the inverse of its SE.
We presented the characteristics of the included RCTs as counts (percentages) and means (ranges or SD) for categorical and continuous variables, respectively. We used complete case analysis in the quantitative analysis. We categorized the dietary interventions of the included RCTs using the AMDR as defined by the IOM (20). MM diets referred to diets where all macronutrients were within the AMDR ranges: carbohydrate (45–65% of energy), protein (10–35% of energy), and fat (20–35% of energy). High fat/low carbohydrate (HFLC) diets had a total fat percentage that exceeded the AMDR range (>35% of energy) and/or carbohydrate percentage below the lower AMDR limit (≤45% of energy). Low fat/high carbohydrate (LFHC) diets had a total fat percentage below the lower AMDR limit (≤20% of energy) and/or carbohydrates percentage exceeding the upper AMDR limit (>65% of energy). UD were control diets where participants were asked not to change their dietary intake from their usual lifestyle.
We used Bayesian random-effects model to assess the pooled direct and the NMA estimates. We derived the latter using Markov chain Monte Carlo simulation techniques (24, 25). The outcome measures of interest were the change in weight (kg), the change in BMI (kg/m2), and the change in WC (cm). When the change in the outcome measure was not reported, we used baseline and study end mean (SD) values of the outcome measure to calculate the mean difference (MD) of the change of this outcome. When the mean of the change was available but the SD was missing, we calculated the SD using the SE, 95% CI and/or p, when available, as suggested by the Cochrane Handbook (26). For the diets included in the NMA, we assessed the likelihood for every diet to be ranked first, second, etc. (27). We evaluated the statistical heterogeneity between studies for direct comparisons using I2 statistic, defining moderate heterogeneity for I2 of 40–70% and high for I2 > 70%.
For the traditional MA, we calculated the MD and 95% CI of continuous variables, when at least 2 RCTs were included in a given comparison, using a random-effects model. We used imputation methods when needed, as described above. We explored the reasons for moderate to high heterogeneity, by conducting sub-group analyses for the following variables, by outcome and by comparison, as applicable, when a given sub-group included at least 2 RCTs: gender (>75% of participants being men or women), baseline mean BMI (<30 vs. ≥ 30 kg/m2), age category (younger vs. older adults based on a cutoff point of 50 years, as a surrogate of menopausal status), intervention duration (12 months vs. 13–24 months), and the presence vs. the absence of concomitant exercise and/or behavioral prescription. We considered any explicit instruction on exercise, whether advised or supervised, as a physical activity (PA) co-intervention. Similarly, we considered any kind of behavioral support received, irrespective of the provider, as a behavioral co-intervention. We did not have data to explore the impact of compliance, dietary fiber content, and dietary restriction vs. ad libitum on the outcomes of interest. All the sub-group analyses were pre-specified in the protocol (22), with the exception of gender.
We used RStudio v1.4.1106 (Integrated Development for R. RStudio, PBC, Boston, MA) for the NMA and the league tables. We used Stata 17 to generate the network nodes (StataCorp. 2021. Stata Statistical Software: Release 17. College Station, TX: StataCorp LLC). We conducted the traditional meta-analysis, subgroup analyses, and funnel plots on Review Manager [(RevMan) (Computer program) Version 5.3. Copenhagen: The Nordic Cochrane Center, The Cochrane Collaboration, 2014].
The search strategy yielded 22,929 citations. After removal of duplicates, we screened 22,853 citations, 3,082 full text, and included 50 publications relevant to 36 trials (Figure 1). We identified 24 RCTs reporting on weight change (total n = 4,916 participants), 17 RCTs on BMI change (total n = 3,260 participants), and 15 RCTs on WC change (total n = 2,734 participants), and comparing diets with different macronutrient distribution between each other or to UD, at ≥12 months follow-up.
Supplementary Table S2 showcases the detailed study characteristics of each of the included RCTs. Table 1 presents a summary of the characteristics of the included RCTs. The comparison of MM to HFLC diets included the largest number of RCTs. The sample size of the RCTs ranged between 7 and 318 participants per arm and the duration of the active intervention spanned over 12–24 months. The range of mean age of participants was 22–67 years, and the range of mean BMI at baseline was 25.9–43.6 Kg/m2. The majority of RCTs included both genders, and women represented >50% of the population, with the exception of 3 RCTs (28–30). Most of the trials were conducted in North America (44%) and Europe (28%). Furthermore, seventeen RCTs included participants with cardio-metabolic co-morbidities (e.g., coronary heart disease, hypertension, dyslipidemia, and metabolic syndrome) in <40% of the population. There were few exceptions where the majority of participants had metabolic syndrome (65–100% of the population) (31–35), hypertension (81% of the population) (33), and hyperinsulinemia (100% of the population) (36). The delivery of the dietary intervention was achieved in most of the trials through face-to-face individual and/or group assessment and education. Few trials incorporated a follow-up over the phone and/or via email (29, 37–41). Behavioral therapy and PA were co-interventions in 39 and 36% of the RCTs, respectively, and 8 RCTs had both co-interventions administered concomitantly (Table 1). Nine RCTs administered diets that have a similar macronutrient composition falling within the same AMDR classification, and therefore were not included in our quantitative analysis (35, 36, 41–47).
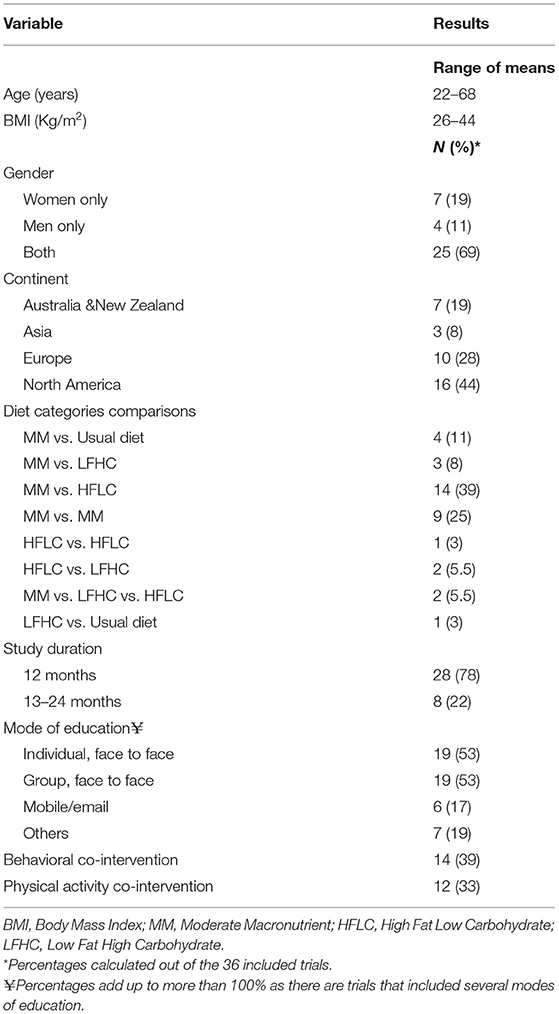
Table 1. Summary of the population and intervention characteristics of the included randomized controlled trials (RCTs).
The compliance to the dietary intervention was assessed in about 50% of the trials using a variety of methods, the most common being food records (32, 40, 43, 48–50), followed by food frequency questionnaires and dietary recalls (32, 35, 51), urine urea nitrogen (41, 52, 53), and educational sessions' attendance (30, 32, 33, 54). The dropout rate was reported in most studies. It was <20% in 20 RCTs, and larger reaching 30–60% in 14 RCTs. The highest dropout rates were observed in one RCT with young female participants (18–25 years) (55) and other trials with participants following HFLC diets (41, 49, 55, 56). When the funding was described, the source consisted of national or international scientific organizations, with the exception of one trial funded by a private company (55).
Supplementary Table S3 features the ROB assessment of the included RCTs. Most studies (72%) had a “High” overall ROB. The high ROB was related to a poor description or lack of appropriate allocation concealment and blinding of participants/personnel and outcome assessors', high dropout rate, and incomplete outcome reporting. Seventeen RCTs have their protocols posted online. Among the other risks of bias, the imbalance in baseline characteristics was the most common limitation. We were able to assess the risk of publication bias only for the comparison of HFLC to MM, as it included more than 10 RCTs (Supplementary Figures S1–S3). The funnel plots revealed asymmetry in the publications reporting weight changes. No asymmetry was noted for RCTs describing BMI and WC changes.
We conducted an NMA for each of the outcomes of interest (Figure 2). The largest number of direct comparisons was between MM and HFLC diets for all outcome measures (Figure 2). All estimates were fed by direct and indirect comparisons, with the exception of the one derived from UD vs. HFLC diet for the weight, BMI, and WC changes, and the one derived from UD vs. LFHC diet for the WC change, that were only based on indirect comparisons.
We identified 24 RCTs reporting on the weight changes (total n = 4,916 participants) at ≥ 12 months of follow-up (28, 29, 31–34, 37–40, 49–51, 53–63). The NMA revealed that, compared with UD, all diets were associated with a significant and comparable WL, at 12 months and beyond (Table 2), HFLC diets [MD (95% CI): −5.5 (−7.6; −3.4)] kg, LFHC diets [−5.0 (−7.1; −2.9)] kg and the MM diets [−4.7 (−6.8; −2.7)] kg (Table 2). When comparing dietary interventions between each other, the only significant difference was for the HFLC diets, associated with a higher WL compared with MM diets [MD (95% CI): −0.77 (−1.3; −0.19)] kg. The HFLC diet had the highest probability of being superior to all other diets (82%). The ROB of the included studies was moderate to high, mostly related to unclear allocation concealment and blinding, and high dropout rate of 20–60% (Supplementary Figure S1).
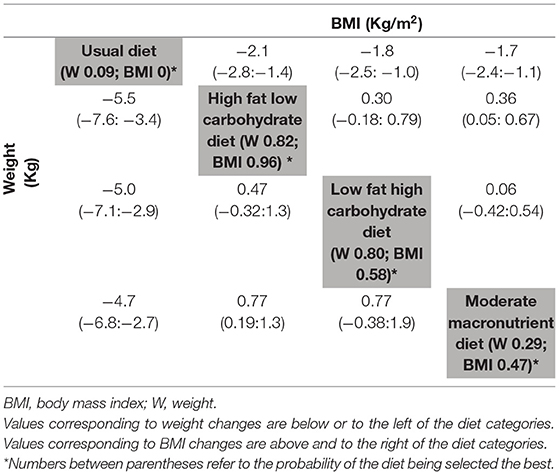
Table 2. League table of network meta-analysis results of weight changes (Kg) and BMI changes (Kg/m2) at study end (≥ 12 months).
Moreover, 17 RCTs presented BMI changes (total n = 3,260 participants) at ≥ 12 months (29, 31, 34, 37–39, 49, 50, 54, 57–59, 61, 62, 64–66). The NMA on BMI change showed results similar to WL. There was a significant drop in BMI across all diets compared with UD, HFLC [−2.1 (−2.8; −1.4)] kg/m2, LFHC [−1.8 (−2.5; −1.0)] kg/m2, and the MM diets [−1.7 (−2.4; −1.1)] kg/m2 (Table 2). When comparing dietary interventions between each other, the only significant difference was for the HFLC diets, associated with a significantly lower BMI compared with MM diets [MD (95% CI): −0.36 (−0.67; −0.05) kg/m2]. The HFLC diets had the highest probability of being superior to all other diets (96%). The ROB of the included studies was moderate to high, mostly related to unclear sequence generation, allocation concealment and blinding, and incomplete data reporting, with a high dropout rate of 20–60% and other bias (Supplementary Figure S2).
We identified 15 RCTs reporting on WC change (total n = 2,734 participants) at ≥12 months (29, 31, 34, 37, 38, 49–51, 53–55, 57–59, 65). The results on WC changes echoed those of weight and BMI changes, with the HFLC associated with the highest changes [MD (95% CI): −5.6 cm (−8.6; −2.6)], followed by LFHC and MM diets, compared with UD. Yet, there was no significant difference in WC when diets were compared with each other (Table 3). The ROB of the included studies was moderate to high mostly related to unclear allocation concealment and blinding, and high dropout (Supplementary Figure S3).
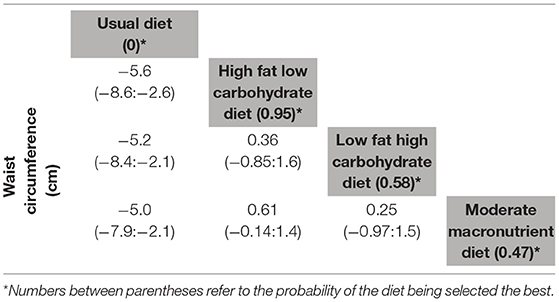
Table 3. League table of network meta-analysis results of waist circumference changes (cm) at study end (≥ 12 months).
We conducted subgroup analyses for the MM vs. LFHC comparison, as it had a moderate to high heterogeneity in the traditional MA for weight change (I2 56%), BMI change (I2 77%), and WC change (I2 40%) (Supplementary Table S4). There was a larger drop in BMI with a longer intervention duration of 12 months or more [MD −0.73 (−1.23; −0.27) kg/m2], compared with a duration of 12 months only [MD −0.19 (−0.36; −0.02) kg/m2], of borderline significance with a p-subgroup analysis 0.05. However, this finding was not reproduced for other outcome measures. Sub-group analysis by the type of co-intervention showed a trend for a larger difference in the mean change in WC in trials that administered both co-interventions (PA and behavioral therapy), p-subgroup analysis 0.05. We did not find any interaction by gender. We detected a consistent trend for a slightly larger effect in younger (age <50 years), compared with older (age ≥50 years) individuals, across various outcome measures. We could not assess the impact of baseline BMI given the narrow range of mean BMI (of 30–40 kg/m2) in most studies.
The adverse events were reported only in 6/36 trials (28, 33, 35, 52, 53, 57). Most adverse events were not related to the dietary interventions. Some diet related adverse events included increased urine micro-albumin to creatinine ratio in patients following high-protein diets (52) and hypoglycemia following an oral glucose tolerance test (57).
This NMA exclusively analyzes the long-term (≥ 12 months) effect of dietary interventions with active counseling, using the IOM macronutrient categorization brackets for diets categorization. The conduct of such an NMA is important given the wide utilization and reference of the AMDR (20). All diet categories (MM, LFHC, and HFLC), compared with UD, were associated with a significant WL of about 5 kg, a significant drop in BMI of 2 kg/m2, and a significant drop in WC of 5 cm, at 12–24 months follow-up. Diets did not differ among each other, with the exception of the HFLC diet that was slightly better than MM diet, with a larger WL (of 0.8 kg) and BMI loss (0.4 kg/m2). Since these differences in weight and BMI have a minimal clinical significance, our findings confirm that all diets have the same efficacy on weight management, and provide the evidence for obesity guidelines recommendations, not favoring a specific diet beyond the one that would optimize patient adherence and the adoption of healthy eating patterns (7, 67). Our conclusion contrasts with what diet advertisements claim to the public about the superiority of certain diets over others (68–70). Popular diets may be helpful as a jumpstart but do not affect long-term weight and WC changes (71).
Results of this NMA are aligned with those of two previous NMAs that assessed the effect of dietary interventions at 6–12 months follow-up (10, 11). Both NMAs showed that the different diet categories were superior to UD at 12 months, with a drop in weight (of 5–7 kg) and BMI of around 2 kg/m2, similar to our findings. When comparing diets among each other, both NMAs showed a slightly higher effect of a low carbohydrate diet compared with the MM diet (10, 11), as demonstrated in our findings. Ge et al. showed a higher effect of a low-fat diet compared with an MM diet (11). While our NMA assessed the effect of dietary interventions of at least 12 months, in patients without chronic diseases potentially affecting WL, it confirmed the result of previous SR/MA on the topic, and hence showed comparable results, suggesting weight maintenance at a certain plateau with continuous dietary efforts beyond 12 months (72).
Our significant findings in one comparison only may be related to a higher power to detect significance given the larger number of included trials comparing HFLC to MM diets. The potential superiority of HFLC over an MM on weight and BMI changes may be explained by several reasons. A low-carbohydrate diet implies a higher protein intake and a reduced sugar consumption, and therefore more satiety (73). A HFLC diet may be associated with an higher secretion of the anorexigenic peptide YY hormone (74, 75), and a higher energy expenditure, compared with other diets (76). Furthermore, the carbohydrate-insulin model of obesity favors a low carbohydrate and a low glycemic index diet, allowing less fat deposition and higher energy expenditure, compared to a traditional low fat diet (77). However, this model has been criticized for being “too simplistic” (78), and further research is needed to explore the implication of this model in specific patient populations.
Aging is associated with a decrease in the total energy expenditure, and a change in various hormones that affect body composition and appetite (79). However, we did not detect a significant interaction by age, but a trend for a larger effect in younger individuals. Moreover, our subgroup analysis revealed a trend for better results in WC among recipients of PA and behavioral therapy interventions, compared with either one. Such findings highlight the importance of a multidisciplinary approach in weight management, such as diet, exercise, and psychologic support (80). Noteworthy, there was a wide heterogeneity in the intensity of delivery of the physical activity, consisting of education about healthy habits or supervised exercise sessions (29, 30, 34, 37, 44, 49, 51, 54, 55, 57, 59, 62). Similarly, the behavioral support consisted of counseling by behavioral therapists as well as support sessions provided by healthcare providers non-specialized in the behavioral field (34, 37, 39, 43, 44, 46, 50, 51, 53, 57, 59–62).
To our knowledge, this is the most comprehensive review on the long-term effect (≥ 12 months) of diets, assessing the WL response, in the general population with overweight/obesity. We have used a rigorous approach in the identification, data abstraction, and analysis of relevant RCTs. Our findings are based on RCTs mostly derived from Western populations and therefore might not be generalizable to non-Western countries. In addition, with the exception of few trials conducted exclusively in men, women constituted the majority of the participants, and therefore, we were not able to explore the gender effect on the response to dietary interventions. Our limitations stem from challenges identified in the included RCTs, most of which were of low quality, with a high ROB. We could not assess the impact of the quality of RCTs on the results given the paucity of high-quality trials. Although the International Committee of Medical Journal Editors (ICMJE) required, as of 2005, trials protocol registration for publication, and same did the World Health Organization as of 2006, we identified several studies published after 2007, without an available published protocol. While blinding is an essential component in RCTs to reduce the risk of performance and detection bias, blinding of participants and dieticians to dietary interventions is very difficult, as previously recognized (81–83). There was a wide heterogeneity in the intensity and delivery mode of the dietary interventions, and this could have affected the WL response. Furthermore, compliance was assessed qualitatively in the majority of studies, implying the lack of accurate assessment of participants' adherence to dietary intervention. Few trials used quantitative methods and these included 24-h recall, self-reported food records, and food frequency questionnaires, and fewer ones chose urine urea nitrogen, urinary ketone levels, and respiratory quotient; the latter are preferred methods as they are subjected to less recall and social desirability biases. Due to the difference in the assessment methodology, there were no means of evaluating the relationship between adherence rate and changes in weight parameters. Finally, we noted an under-reporting of adverse events, as described previously (10, 11). Although various diet therapies are safe, limited data are available on their long-term effects (84).
Compared with the usual diet, all dietary interventions allow a sustained modest WL during the follow-up of 12 months and beyond. A HFLC diet seems to be slightly better than a MM diet, while all other comparisons between diets yield similar results. A major limitation of the findings stems from the lack of compliance/adherence data, the wide variability of the delivery of dietary interventions, and the low quality of RCTs. A formal and standardized delivery of diet therapy, a qualitative assessment of participants' adherence to diets, efforts to improve on blinding of participants and researchers, and to reduce the participants' attrition are essential in future trials. While our findings apply to the general population of patients with overweight/obesity, the long-term impact of dietary approaches on patients with chronic diseases is worth investigation in a separate systematic review of the literature.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
MC and JJ conceived, designed the study, coordinated data screening and abstraction, and wrote and reviewed the manuscript. MC, JJ, and AK analyzed and interpreted the data. DA, RA, RH, LG, NH, NN, GS, BT, YR, and MZ performed data screening. DA, RH, LG, NH, GS, BT, YR, and MZ performed data abstraction. YR and NH managed the data. All authors revised the article critically for important intellectual content, gave final approval of the version to be published, and agreed to be accountable for all aspects of the work. All authors contributed to the article and approved the submitted version.
LG is employed by Qualisus Consulting.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The authors would like to thank professionals who have supported this research project. Ms. Aida Farha and Ms. Layal Hneiny for their support in designing the search strategy and extracting the articles. Dr. Maria Armache for developing the search strategy. Ms. Noor Al Rubaye, Mr. Karim El Mokaddem, Ms. Nour El Ghawi, Ms. Sally El Sammak, Ms. Lara Haidous, Ms. Anna Maria Markarian, Ms. Nadine Omran, and Mr. Bassel Tfayli for their support in the abstract screening phase and data abstraction.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2022.821096/full#supplementary-material
1. Blüher M. Obesity: global epidemiology and pathogenesis. Nat Rev Endocrinol. (2019) 15:288–98. doi: 10.1038/s41574-019-0176-8
2. Collaboration PS. Body-mass index and cause-specific mortality in 900 000 adults: collaborative analyses of 57 prospective studies. Lancet. (2009) 373:1083–96. doi: 10.1016/S0140-6736(09)60318-4
3. Fontaine KR, Redden DT, Wang C, Westfall AO, Allison DB. Years of life lost due to obesity. JAMA. (2003) 289:187–93. doi: 10.1001/jama.289.2.187
4. Berrington de Gonzalez A, Hartge P, Cerhan JR, Flint AJ, Hannan L, MacInnis RJ, et al. Body-mass index and mortality among 146 million white adults New England. J Med. (2010) 363:2211–9. doi: 10.1056/NEJMoa1000367
5. WHO. Obesity and overweight 2021 [updated June 9, 2021]. Available online at: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed August 10, 2021).
6. Meldrum DR, Morris MA, Gambone JC. Obesity pandemic: causes, consequences, and solutions—but do we have the will? Fertil Steril. (2017) 107:833–9. doi: 10.1016/j.fertnstert.2017.02.104
7. Garber AJ, Abrahamson MJ, Barzilay JI, Blonde L, Bloomgarden ZT, Bush MA, et al. Consensus statement by the american association of clinical endocrinologists and american college of endocrinology on the comprehensive type 2 diabetes management algorithm – 2016 executive summary. Endocr Pract. (2016) 22:84–113. doi: 10.4158/EP151126.CS
8. Chaimani A, Caldwell DM, Li T, Higgins JP, Salanti G. Undertaking network meta-analyses. Cochrane Handbook for Systematic Reviews of Interventions. Bristol: The Cochrane Collaboration (2019). p. 285–320.
9. Schwingshackl L, Buyken A, Chaimani A. Network meta-analysis reaches nutrition research. Eur. J. Nutr. Springer (2019). 58:1–3.
10. Johnston BC, Kanters S, Bandayrel K, Wu P, Naji F, Siemieniuk RA, et al. Comparison of weight loss among named diet programs in overweight and obese adults: a meta-analysis. JAMA. (2014) 312:923–33. doi: 10.1001/jama.2014.10397
11. Ge L, Sadeghirad B, Ball GD, da Costa BR, Hitchcock CL, Svendrovski A, et al. Comparison of dietary macronutrient patterns of 14 popular named dietary programmes for weight and cardiovascular risk factor reduction in adults: systematic review and network meta-analysis of randomised trials. BMJ. (2020) 369:m696. doi: 10.1136/bmj.m696
12. Bueno NB, de Melo ISV, de Oliveira SL, da Rocha Ataide T. Very-low-carbohydrate ketogenic diet v. low-fat diet for long-term weight loss: a meta-analysis of randomised controlled trials. Br J Nutr. (2013) 110:1178–87. doi: 10.1017/S0007114513000548
13. Atallah R, Filion KB, Wakil SM, Genest J, Joseph L, Poirier P, et al. Long-term effects of 4 popular diets on weight loss and cardiovascular risk factors: a systematic review of randomized controlled trials. Circ Cardiovasc Qual Outcomes. (2014) 7:815–27. doi: 10.1161/CIRCOUTCOMES.113.000723
14. Clifton P, Condo D, Keogh J. Long term weight maintenance after advice to consume low carbohydrate, higher protein diets–a systematic review and meta analysis. Nutr Metabol Cardiovasc Dis. (2014) 24:224–35. doi: 10.1016/j.numecd.2013.11.006
15. Tobias DK, Chen M, Manson JE, Ludwig DS, Willett W, Hu FB. Effect of low-fat diet interventions versus other diet interventions on long-term weight change in adults: a systematic review and meta-analysis. Lancet Diabetes Endocrinol. (2015) 3:968–79. doi: 10.1016/S2213-8587(15)00367-8
16. Mancini JG, Filion KB, Atallah R, Eisenberg MJ. Systematic review of the Mediterranean diet for long-term weight loss. Am J Med. (2016) 129:407–15. e4. doi: 10.1016/j.amjmed.2015.11.028
17. Anton SD, Hida A, Heekin K, Sowalsky K, Karabetian C, Mutchie H, et al. Effects of popular diets without specific calorie targets on weight loss outcomes: systematic review of findings from clinical trials. Nutrients. (2017) 9:822. doi: 10.3390/nu9080822
18. Lu M, Wan Y, Yang B, Huggins CE, Li D. Effects of low-fat compared with high-fat diet on cardiometabolic indicators in people with overweight and obesity without overt metabolic disturbance: a systematic review and meta-analysis of randomised controlled trials. Br J Nutr. (2018) 119:96–108. doi: 10.1017/S0007114517002902
19. Bray G, Kim K, Wilding J, Federation WO. Obesity: a chronic relapsing progressive disease process. A position statement of the World Obesity Federation. Obes Rev. (2017) 18:715–23. doi: 10.1111/obr.12551
20. Medicine Io. Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids. Washington, DC: The National Academies Press (2005). p. 1358.
21. Lee E, Choi J, Ahn A, Oh E, Kweon H, Cho D. Acceptable macronutrient distribution ranges and hypertension. Clin Exp Hypertens. (2015) 37:463–7. doi: 10.3109/10641963.2015.1013116
22. Chakhtoura M, Jabbour J, Armache A, Khamis A, Halperin F, Rihawi Y. Effect of different diets on weight and other outcomes in long term trials. (2021). Available online at: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42018103116 (accessed November 1, 2021).
23. Higgins JPT, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. (2011) 343:d5928. doi: 10.1136/bmj.d5928
24. Lumley T. Network meta-analysis for indirect treatment comparisons. Stat Med. (2002) 21:2313–24. doi: 10.1002/sim.1201
25. Ades A, Sculpher M, Sutton A, Abrams K, Cooper N, Welton N, et al. Bayesian methods for evidence synthesis in cost-effectiveness analysis. Pharmacoeconomics. (2006) 24:1–19. doi: 10.2165/00019053-200624010-00001
26. Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. Cochrane Handbook for Systematic Reviews of Interventions. Chichester, UK: John Wiley & Sons (2019).
27. Veroniki AA, Straus SE, Fyraridis A, Tricco AC. The rank-heat plot is a novel way to present the results from a network meta-analysis including multiple outcomes. J Clin Epidemiol. (2016) 76:193–9. doi: 10.1016/j.jclinepi.2016.02.016
28. Stern L, Iqbal N, Seshadri P, Chicano KL, Daily DA, McGrory J, et al. The effects of low-carbohydrate versus conventional weight loss diets in severely obese adults: one-year follow-up of a randomized trial. Ann Intern Med. (2004) 140:778–85. doi: 10.7326/0003-4819-140-10-200405180-00007
29. Shai I, Schwarzfuchs D, Henkin Y, Shahar DR, Witkow S, Greenberg I, et al. Weight loss with a low-carbohydrate, Mediterranean, or low-fat diet. N Engl J Med. (2008) 359:229–41. doi: 10.1056/NEJMoa0708681
30. Gepner Y, Shelef I, Schwarzfuchs D, Cohen N, Bril N, Rein M, et al. Intramyocellular triacylglycerol accumulation across weight loss strategies; Sub-study of the CENTRAL trial. PLoS ONE. (2017) 12:e0188431. doi: 10.1371/journal.pone.0188431
31. Rusu E, Jinga M, Enache G, Rusu F, Dragomir AD, Ancuta I, et al. Effects of lifestyle changes including specific dietary intervention and physical activity in the management of patients with chronic hepatitis C-a randomized trial. Nutr J. (2013) 12:1–12. doi: 10.1186/1475-2891-12-119
32. Klemsdal TO, Holme I, Nerland H, Pedersen TR, Tonstad S. Effects of a low glycemic load diet versus a low-fat diet in subjects with and without the metabolic syndrome. Nutr Metabol Cardiovasc Dis. (2010) 20:195–201. doi: 10.1016/j.numecd.2009.03.010
33. Salas-Salvadó J, Fernández-Ballart J, Ros E, Martinez-Gonzalez M-A, Fitó M, Estruch R, et al. Effect of a Mediterranean diet supplemented with nuts on metabolic syndrome status: one-year results of the PREDIMED randomized trial. Arch Intern Med. (2008) 168:2449–58. doi: 10.1001/archinte.168.22.2449
34. Pavić E, HadŽiabdić MO, Mucalo I, Martinis I, Romić Ž, BoŽikov V, et al. Effect of the Mediterranean diet in combination with exercise on metabolic syndrome parameters: 1-year randomized controlled trial. Int J Vitamin Nutr Res. (2019) 89:132–43. doi: 10.1024/0300-9831/a000462
35. Ma Y, Olendzki BC, Wang J, Persuitte GM, Li W, Fang H, et al. A randomized trial of single-versus multi-component dietary goals for metabolic syndrome. Ann Intern Med. (2015) 162:248. doi: 10.7326/M14-0611
36. Keogh JB, Brinkworth GD, Clifton PM. Effects of weight loss on a low-carbohydrate diet on flow-mediated dilatation, adhesion molecules and adiponectin. Br J Nutr. (2007) 98:852–9. doi: 10.1017/S0007114507747815
37. Dale KS, McAuley KA, Taylor RW, Williams SM, Farmer VL, Hansen P, et al. Determining optimal approaches for weight maintenance: a randomized controlled trial. CMAJ. (2009) 180:E39–46. doi: 10.1503/cmaj.080974
38. Frisch S, Zittermann A, Berthold HK, Götting C, Kuhn J, Kleesiek K, et al. A randomized controlled trial on the efficacy of carbohydrate-reduced or fat-reduced diets in patients attending a telemedically guided weight loss program. Cardiovasc Diabetol. (2009) 8:1–10. doi: 10.1186/1475-2840-8-36
39. Gardner CD, Kiazand A, Alhassan S, Kim S, Stafford RS, Balise RR, et al. Comparison of the Atkins, Zone, Ornish, and LEARN diets for change in weight and related risk factors among overweight premenopausal women: the A TO Z Weight Loss Study: a randomized trial. JAMA. (2007) 297:969–77. doi: 10.1001/jama.297.9.969
40. Brinkworth GD, Wycherley TP, Noakes M, Buckley JD, Clifton PM. Long-term effects of a very-low-carbohydrate weight-loss diet and an isocaloric low-fat diet on bone health in obese adults. Nutrition. (2016) 32:1033–6. doi: 10.1016/j.nut.2016.03.003
41. Jesudason D, Nordin BC, Keogh J, Clifton P. Comparison of 2 weight-loss diets of different protein content on bone health: a randomized trial. Am J Clin Nutr. (2013) 98:1343–52. doi: 10.3945/ajcn.113.058586
42. Marniemi J, Seppänen A, Hakala P. Long-term effects on lipid metabolism of weight reduction on lactovegetarian and mixed diet. Int J Obes. (1990) 14:113–25.
43. Due A, Toubro S, Skov A, Astrup A. Effect of normal-fat diets, either medium or high in protein, on body weight in overweight subjects: a randomised 1-year trial. Int J Obes. (2004) 28:1283–90. doi: 10.1038/sj.ijo.0802767
44. Ebbeling CB, Leidig MM, Sinclair KB, Seger-Shippee LG, Feldman HA, Ludwig DS. Effects of an ad libitum low-glycemic load diet on cardiovascular disease risk factors in obese young adults. Am J Clin Nutr. (2005) 81:976–82. doi: 10.1093/ajcn/81.5.976
45. Tanumihardjo SA, Valentine AR, Zhang Z, Whigham LD, Lai HJ, Atkinson RL. Strategies to increase vegetable or reduce energy and fat intake induce weight loss in adults. Exp Biol Med. (2009) 234:542–52. doi: 10.3181/0810-RM-293
46. Sukumar D, Ambia-Sobhan H, Zurfluh R, Schlussel Y, Stahl TJ, Gordon CL, et al. Areal and volumetric bone mineral density and geometry at two levels of protein intake during caloric restriction: a randomized, controlled trial. J Bone Mineral Res. (2011) 26:1339–48. doi: 10.1002/jbmr.318
47. Venn BJ, Perry T, Green TJ, Skeaff CM, Aitken W, Moore NJ, et al. The effect of increasing consumption of pulses and wholegrains in obese people: a randomized controlled trial. J Am Coll Nutr. (2010) 29:365–72. doi: 10.1080/07315724.2010.10719853
48. Azadbakht L, Mirmiran P, Esmaillzadeh A, Azizi F. Better dietary adherence and weight maintenance achieved by a long-term moderate-fat diet. Br J Nutr. (2007) 97:399–404. doi: 10.1017/S0007114507328602
49. Dansinger ML, Gleason JA, Griffith JL, Selker HP, Schaefer EJ. Comparison of the Atkins, Ornish, Weight Watchers, and Zone diets for weight loss and heart disease risk reduction: a randomized trial. JAMA. (2005) 293:43–53. doi: 10.1001/jama.293.1.43
50. Mellberg C, Sandberg S, Ryberg M, Eriksson M, Brage S, Larsson C, et al. Long-term effects of a Palaeolithic-type diet in obese postmenopausal women: a 2-year randomized trial. Eur J Clin Nutr. (2014) 68:350–7. doi: 10.1038/ejcn.2013.290
51. Foster-Schubert KE, Alfano CM, Duggan CR, Xiao L, Campbell KL, Kong A, et al. Effect of diet and exercise, alone or combined, on weight and body composition in overweight-to-obese postmenopausal women. Obesity. (2012) 20:1628–38. doi: 10.1038/oby.2011.76
52. Sacks FM, Bray GA, Carey VJ, Smith SR, Ryan DH, Anton SD, et al. Comparison of weight-loss diets with different compositions of fat, protein, and carbohydrates. N Engl J Med. (2009) 360:859–73. doi: 10.1056/NEJMoa0804748
53. Bazzano LA, Hu T, Reynolds K, Yao L, Bunol C, Liu Y, et al. Effects of low-carbohydrate and low-fat diets: a randomized trial. Ann Intern Med. (2014) 161:309–18. doi: 10.7326/M14-0180
54. Santanasto A, Newman A, Strotmeyer E, Boudreau R, Goodpaster B, Glynn NW. Effects of changes in regional body composition on physical function in older adults: a pilot randomized controlled trial. J Nutr Health Aging. (2015) 19:913–21. doi: 10.1007/s12603-015-0523-y
55. Griffin H, Cheng H, O'Connor H, Rooney K, Petocz P, Steinbeck K. Higher protein diet for weight management in young overweight women: a 12-month randomized controlled trial. Diabetes Obes Metabol. (2013) 15:572–5. doi: 10.1111/dom.12056
56. Wycherley T, Brinkworth G, Clifton P, Noakes M. Comparison of the effects of 52 weeks weight loss with either a high-protein or high-carbohydrate diet on body composition and cardiometabolic risk factors in overweight and obese males. Nutr Diabetes. (2012) 2:e40-e. doi: 10.1038/nutd.2012.11
57. Gardner CD, Trepanowski JF, Del Gobbo LC, Hauser ME, Rigdon J, Ioannidis JP, et al. Effect of low-fat vs low-carbohydrate diet on 12-month weight loss in overweight adults and the association with genotype pattern or insulin secretion: the DIETFITS randomized clinical trial. JAMA. (2018) 319:667–79. doi: 10.1001/jama.2018.0245
58. Fernández AC, Casariego AV, Rodríguez IC. One-year effectiveness of two hypocaloric diets with different protein/carbohydrate ratios in weight loss and insulin resistance. Nutr Hosp. (2012) 27:2093–101. doi: 10.3305/nh.2012.27.6.6133
59. De Jonge L, Bray GA, Smith SR, Ryan DH, De Souza RJ, Loria CM, et al. Effect of diet composition and weight loss on resting energy expenditure in the POUNDS LOST study. Obesity. (2012) 20:2384–9. doi: 10.1038/oby.2012.127
60. Knopp RH, Walden CE, Retzlaff BM, McCann BS, Dowdy AA, Albers JJ, et al. Long-term cholesterol-lowering effects of 4 fat-restricted diets in hypercholesterolemic and combined hyperlipidemic men: the Dietary Alternatives Study. JAMA. (1997) 278:1509–15. doi: 10.1001/jama.1997.03550180059038
61. Pritchard JE, Nowson CA, Wark JD. A worksite program for overweight middle-aged men achieves lesser weight loss with exercise than with dietary change. J Am Diet Assoc. (1997) 97:37–42. doi: 10.1016/S0002-8223(97)00015-1
62. Wing RR, Venditti E, Jakicic JM, Polley BA, Lang W. Lifestyle intervention in overweight individuals with a family history of diabetes. Diabetes Care. (1998) 21:350–9. doi: 10.2337/diacare.21.3.350
63. Pereira MA, Swain J, Goldfine AB, Rifai N, Ludwig DS. Effects of a low–glycemic load diet on resting energy expenditure and heart disease risk factors during weight loss. JAMA. (2004) 292:2482–90. doi: 10.1001/jama.292.20.2482
64. Williams PT, Krauss RM, Stefanick ML, Vranizan KM, Wood PD. Effects of low-fat diet, calorie restriction, and running on lipoprotein subfraction concentrations in moderately overweight men. Metabolism. (1994) 43:655–63. doi: 10.1016/0026-0495(94)90210-0
65. Álvarez-Pérez J, Sánchez-Villegas A, Díaz-Benítez EM, Ruano-Rodríguez C, Corella D, Martínez-González MÁ, et al. Influence of a Mediterranean dietary pattern on body fat distribution: results of the PREDIMED–Canarias Intervention Randomized Trial. J Am Coll Nutr. (2016) 35:568–80. doi: 10.1080/07315724.2015.1102102
66. Brinkworth GD, Noakes M, Buckley JD, Keogh JB, Clifton PM. Long-term effects of a very-low-carbohydrate weight loss diet compared with an isocaloric low-fat diet after 12 mo. Am J Clin Nutr. (2009) 90:23–32. doi: 10.3945/ajcn.2008.27326
67. Garvey WT, Mechanick JI, Brett EM, Garber AJ, Hurley DL, Jastreboff AM, et al. American Association of Clinical Endocrinologists and American College of Endocrinology Comprehensive Clinical Practice Guidelines Formedical Care of Patients with Obesity. Endocr Pract. (2016) 22:1–203. doi: 10.4158/EP161365.GL
68. Eugene A. Sugar Detox: Defeat Cravings and Restore Your Health. Summertown, TN: Book Publishing Company (2016).
69. Grout P. Jumpstart Your Metabolism: How to Lose Weight by Changing the Way You Breathe. New York, NY: Simon and Schuster (2010).
70. Jauho M, Pääkkönen J, Isotalo V, Pöyry E, Laaksonen S-M. How do trendy diets emerge? An exploratory social media study on the low-carbohydrate diet in Finland. Food Cult Soc. (2021) 1–26. doi: 10.1080/15528014.2021.1971436
71. Freire R. Scientific evidence of diets for weight loss: different macronutrient composition, intermittent fasting, and popular diets. Nutrition. (2020) 69:110549. doi: 10.1016/j.nut.2019.07.001
72. Hall KD, Kahan S. Maintenance of lost weight and long-term management of obesity. Med Clin. (2018) 102:183–97. doi: 10.1016/j.mcna.2017.08.012
73. Seid H, Rosenbaum M. Low carbohydrate and low-fat diets: What we don't know and why we should know it. Nutrients. (2019) 11:2749. doi: 10.3390/nu11112749
74. Wolever TM, Chiasson JL, Josse RG, Leiter LA, Maheux P, Rabasa-Lhoret R, et al. Effects of changing the amount and source of dietary carbohydrates on symptoms and dietary satisfaction over a 1-year period in subjects with type 2 diabetes: Canadian Trial of Carbohydrates in Diabetes (CCD). Can J Diabetes. (2017) 41:164–76. doi: 10.1016/j.jcjd.2016.08.223
75. Hu T, Yao L, Reynolds K, Niu T, Li S, Whelton P, et al. The effects of a low-carbohydrate diet on appetite: A randomized controlled trial. Nutr Metabol Cardiovasc Diseases. (2016) 26:476–88. doi: 10.1016/j.numecd.2015.11.011
76. Ebbeling CB, Swain JF, Feldman HA, Wong WW, Hachey DL, Garcia-Lago E, et al. Effects of dietary composition on energy expenditure during weight-loss maintenance. JAMA. (2012) 307:2627–34. doi: 10.1001/jama.2012.6607
77. Ludwig DS, Aronne LJ, Astrup A, de Cabo R, Cantley LC, Friedman MI et al. The carbohydrate-insulin model: a physiological perspective on the obesity pandemic. Am J Clin Nutr. (2021) 114:1873–1885. doi: 10.1093/ajcn/nqab270
78. Hall D. A review of the carbohydrate–insulin model of obesity. Eur J Clin Nutr. (2018) 72:183. doi: 10.1038/ejcn.2017.156
79. Villareal DT, Apovian CM, Kushner RF, Klein S. Obesity in older adults: technical review and position statement of the American Society for Nutrition and NAASO, The Obesity Society. Am J Clin Nutr. (2005) 82:923–34. doi: 10.1093/ajcn/82.5.923
80. Seo D-C, Sa J. A meta-analysis of psycho-behavioral obesity interventions among US multiethnic and minority adults. Preven Med. (2008) 47:573–82. doi: 10.1016/j.ypmed.2007.12.010
81. Staudacher HM, Irving PM, Lomer MC, Whelan K. The challenges of control groups, placebos and blinding in clinical trials of dietary interventions. Proc Nutr Soc. (2017) 76:203–12. doi: 10.1017/S0029665117000350
82. Mirmiran P, Bahadoran Z, Gaeini Z. Common Limitations and Challenges of Dietary Clinical Trials for Translation into Clinical Practices. Int J Endocrinol Metab. (2021) 19:e108170. doi: 10.5812/ijem.108170
83. Crichton GE, Howe PRC, Buckley JD, Coates AM, Murphy KJ, Bryan J. Long-term dietary intervention trials: critical issues and challenges. Trials. (2012) 13:111. doi: 10.1186/1745-6215-13-111
Keywords: diet, obesity, Acceptable Macronutrient Distribution Ranges (AMDR), weight loss, waist circumference, body mass index (BMI)
Citation: Jabbour J, Rihawi Y, Khamis AM, Ghamlouche L, Tabban B, Safadi G, Hammad N, Hadla R, Zeidan M, Andari D, Azar RN, Nasser N and Chakhtoura M (2022) Long Term Weight Loss Diets and Obesity Indices: Results of a Network Meta-Analysis. Front. Nutr. 9:821096. doi: 10.3389/fnut.2022.821096
Received: 23 November 2021; Accepted: 24 January 2022;
Published: 05 April 2022.
Edited by:
Reema Fayez Tayyem, Qatar University, QatarReviewed by:
Naomi Kakoschke, Commonwealth Scientific and Industrial Research Organisation (CSIRO), AustraliaCopyright © 2022 Jabbour, Rihawi, Khamis, Ghamlouche, Tabban, Safadi, Hammad, Hadla, Zeidan, Andari, Azar, Nasser and Chakhtoura. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Marlene Chakhtoura, bWMzOUBhdWIuZWR1Lmxi
†ORCID: Jana Jabbour orcid.org/0000-0002-0576-1031
Yasmin Rihawi orcid.org/0000-0002-4026-1868
Assem M. Khamis orcid.org/0000-0002-5567-7065
Gloria Safadi orcid.org/0000-0001-5110-5871
Nour Hammad orcid.org/0000-0001-9578-7261
Ruba Hadla orcid.org/0000-0002-3613-7848
Marlene Chakhtoura orcid.org/0000-0003-0012-986X
‡These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.