- Jiangxi Province Key Laboratory of Animal Nutrition, Engineering Research Center of Feed Development, Jiangxi Agricultural University, Nanchang, China
To investigate the effect of Puerarin on intramuscular fat deposition in heat-stressed beef cattle and its underlying mechanism. Thirty-two healthy Jinjiang bulls were randomly divided into four groups and dietary with 0 (Control), 200 (Pue200), 400 (Pue400), and 800 (Pue800) mg/kg Puerarin in the feed concentrate. The results showed that Puerarin treatment enhanced the concentration of crude fat, fatty acid (C14:1 and C17:1), and the activity of fatty acid synthase in Longissimus thoracis (LT), but decreased the levels of blood leptin (P < 0.05). High-throughput sequencing of mRNA technology (RNA-Seq) was used and the analysis showed that 492 genes were down-regulated and 341 genes were up-regulated in LT, and these genes were significantly enriched to the pathways related to lipid metabolism. These results indicated that dietary supplemental with Puerarin enhanced intramuscular fat deposition by regulating lipid metabolism of heat-stressed beef cattle.
Introduction
Heat stress caused by high temperature is one of the most critical environmental stressors challenging in cattle production (1), which leads to endocrine disorder, abnormal nutrient metabolism and changes in body tissue composition (2, 3). Among the main components of the body, body fat is the most changeable. Many reports have shown that heat stress can inhibit the growth of beef cattle and reduce the deposition of body fat, especially intramuscular fat (4, 5).
Intramuscular fat is one of the main factors used to determine the beef quality grade in many countries due to its beneficial effect on the tenderness, aroma, juiciness, and palatability of beef (6). Intramuscular fat deposition is the result of comprehensive effects of animal growth, body fat distribution, fatty acid composition, key genes of fat metabolism and transcription regulators (7, 8).
Puerarin is the main active component of Pueraria lobata, and the latter is a traditional Chinese herbal medicine in China that play an important role in relieving muscle, alleviating pain and reducing fever (9, 10). Several reports have demonstrated that Puerarin has a protective effect on regulating lipid metabolism, anti-oxidative and anti-inflammation (11, 12). A previous study revealed that Puerarin, like estrogen, could affect the hormone secretion levels, thus improve the production performance of animals (13).
Moreover, our previous study has found that Puerarin enhanced the immune function and antioxidant capacity of beef cattle in summer, and improved the growth performance and meat quality of heat-stressed beef cattle (14).
However, little attention has been paid to the effect of Puerarin on intramuscular fat deposition. In light of the above considerations, the objective of this study was to evaluate the potential efficacy of the supplementation of Puerarin on intramuscular fat deposition and analyze its mechanism by RNA-Seq sequencing technology combined with bioinformatics.
Materials and Methods
Animal Ethics
All the experimental procedures applied in this study were reviewed and approved by the Committee for the Care and Use of Experimental Animals at Jiangxi Agricultural University (JXAULL-20190015). All procedures involving live animals handling, management, and health care followed the regulations of laboratory animals used for scientific purposes and were implemented within it.
Puerarin, Animals, and Experimental Design
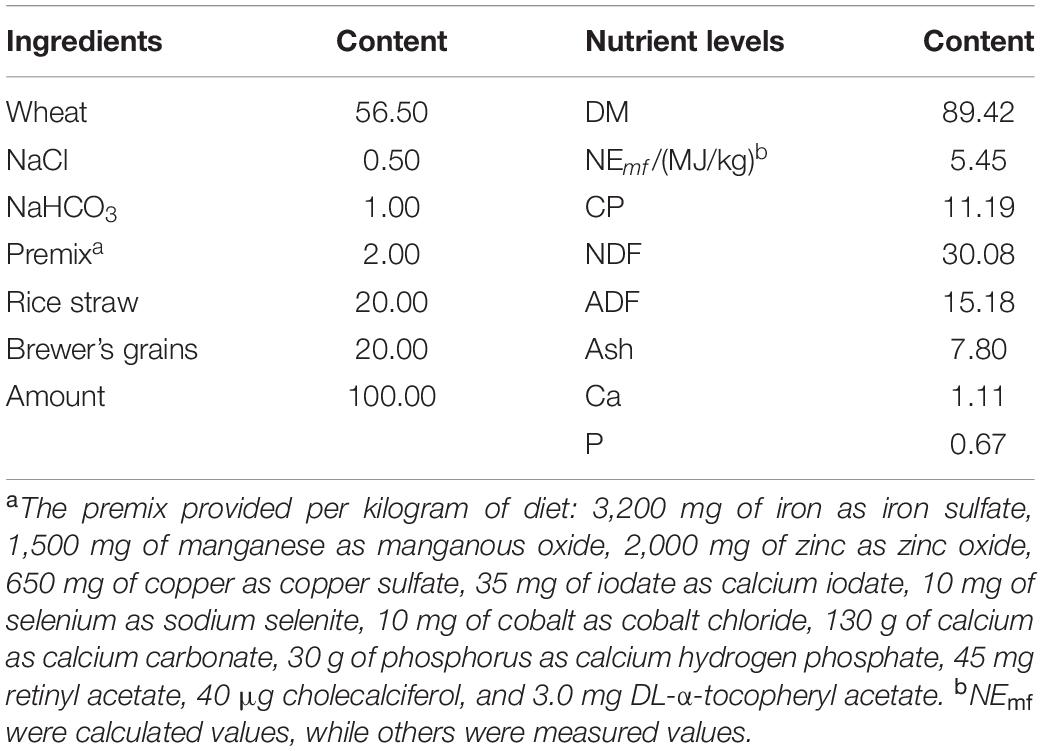
Puerarin was purchased from a company in Xi’an, whose content was 98.1% by analysis of liquid chromatography. The experimental cattle’s feeding and management have been described in detail in our previous study (15). In brief, thirty-two Jinjiang bulls at 15-month-old (291.65 ± 8.84 kg) were randomly divided into four groups (n = 8): control group, Pue200, Pue400, Pue800 group (200 mg/kg, 400 mg/kg, and 800 mg/kg Puerarin in the feed concentrate), respectively. The composition and nutrient levels of the basal diet were shown in Table 1. The feeding trial lasted for 70 days including a 10-day adaptation period and another 60-day experimental period (July 1 to September 8, and the temperature, relative humidity, and humidity index in cattle house were 30.68°C, 68.05%, and 81.81, respectively).
Serum Biochemical Indexes Analysis
On day 60, blood samples (15 mL each, n = 6) were collected at 14:00 from the jugular vein and then serum was prepared immediately. The concentrations of serum insulin (INS), triiodothyronine (T3), thyroxine (T4), cortisol (COR), adiponectin (ADPN), and leptin (LEP) were determined by using radioimmunoassay kits (Beijing Sinouk Institute of Biological Technology, China). The levels of total cholesterol (TC), triglyceride (TG), high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C) in serum were measured by using spectrophotometric kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China).
Muscle Sample Collection and Analysis
According to the results of blood samples, at the end of the experiment, four bulls with medium body weight were selected from control, Pue400 groups, Pue800 group, respectively. These bulls were transferred to the slaughterhouse and sacrificed at a commercial abattoir following the standard procedures. And then, approximately 200 g of the longissimus thoracis (LT) muscle samples from the right half-carcasses between the 12th and 13th ribs were quickly separated, 20 g samples were frozen immediately in liquid nitrogen and stored at −80°C until RNA isolation, and 50 g samples were stored at −20°C for the analysis of the fatty acid composition and the activity of the fatty metabolizing enzyme, other remaining samples were used to determine the contents of moisture, crude protein (CP), crude fat (CF), crude ash (CA), calcium(Ca), and total phosphorus (P) according to Association of Official Analytical Chemists (AOAC). In brief, the content of CA was obtained by incinerating the samples in a muffle furnace at 550°C for 3 h; CP was calculated by quantitative analysis of nitrogen using the Kjeldahl method with copper sulfate and potassium sulfate as catalysts; CF was extracted with diethyl ether using a Soxhlet extractor; Ca and P were determined colorimetrically.
Fatty Acid Composition and Fatty Metabolizing Enzymes in Muscle Analysis
Briefly, the crude fat in the LT muscle was extracted, and positive hexane, sodium methanol and methyl ester were added in crude fat in turn for fat esterification, and then ethyl acetate was added to obtain fatty acid methyl ester. Fatty acids were expressed as percentages of the total fatty acid methyl esters, which analyzed by a gas chromatograph (Shimadzu, Japan) and the method was referenced to Wang (16). The enzyme activity of fatty acid synthase (FAS), acetyl CoA carboxylase (ACC), hormone sensitive lipase (HSL), and lipoprotein lipase (LPL) in LT muscle of beef cattle were tested using ELISA kits (Delivery code number: ml077321; ml061000; ml061693; ml076623) purchased from Shanghai Enzyme-linked Biotechnology Co., Ltd., (Shanghai, China). Both the in-batch and interbatch coefficients of variation were less than 10%.
RNA-Seq Library Preparation and Data Analysis
The 60 g of longissimus thoracis samples were randomly selected from each of the control and Pue400 groups for RNA-Seq analysis. Total RNA was isolated from eight LT samples from control and Pue400 groups by Trizol reagent (Invitrogen, Waltham, MA, United States) according to the manufacturer’s instructions. The quantity and quality of total RNA were assessed using the Agilent 2100 Bioanalyzer (Agilent, CA, United States). Then, transcriptomic sequencing was performed by Shanghai Majorbio Biopharm Technology Co., (Shanghai, China).
Raw reads of all eight samples were pre-processed through the removal of containing adaptors-read with more than 17% unknown nucleotides. The valid reads of each samples were aligned to the Bos taurus genome assembly.1
To analyze gene expression, the number of unique-match reads was calculated and normalized to FPKM (Fragment Per Kilo base of exon model per Million mapped reads), which was used to indicate the condition of transcriptional expression. The amount of expression was calculated for each read of the eight sequenced samples by Cuffdiff (17).
To determine the functional categories of differentially expressed genes (DEGs), all DEGs were subjected to GO and KEGG pathway analyses. GO enrichment analysis was used to map all DEGs to GO terms in the GO database.2 The significance was calculated using a hypergeometric test by Yang (18).
To better understand the biological function of DEGs, all DEGs were annotated to KEGG (Kyoto encyclopedias of genes and genomes) pathways.3
Quantitative RT-PCR Validation
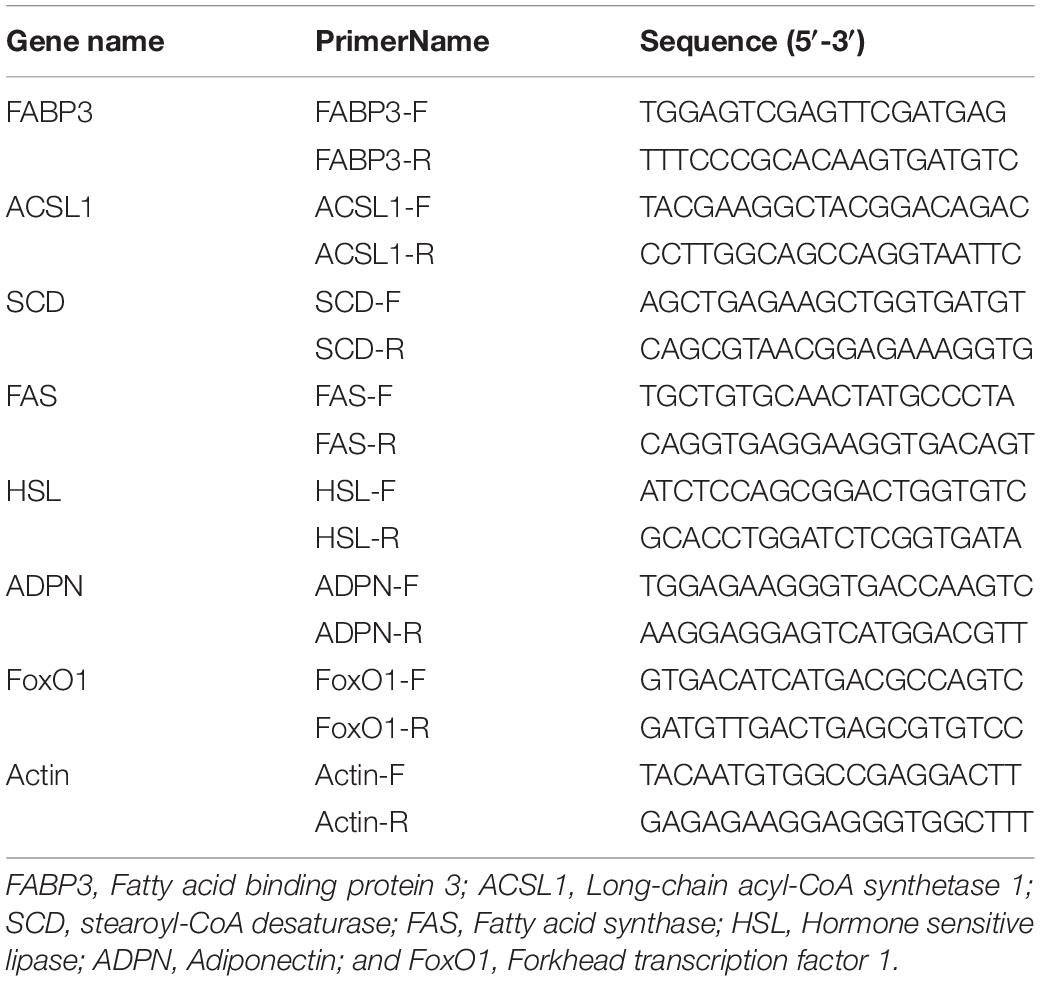
In order to verify the reproducibility and repeatability of gene expression data obtained by RNA-Seq, seven genes were selected for QRT PCR verification. In brief, cDNA was generated from total RNA using the PrimeScript II 1 st Strand cDNA Synthesis Kit (Takara, Dalian, China) following the manufacturer’s instruction. Quantitative RT-PCR analysis was carried out with the cDNA using SYBR green on a Roche LightCycler 96 real-time PCR machine (Roche, Basel, Switzerland). The b-Actin was used as a reference gene for the standardization of the results. The relative expression levels were calculated as described previously (15). Three biological repeats were measured for each sample. The primers used were shown in Table 2.
Statistical Analyses
The serum biochemistry and hormone indexes (n = 6) were statistically analyzed by one-way ANOVA with SPSS statistical software (Ver.20 for windows, SPSS), and Tukey–Kramer’s test was used to compare differences among the treatment groups. The muscle nutrients, fatty acid composition, and activity of the fatty metabolizing enzyme of muscle (n = 4) were statistically analyzed by T-test with SPSS statistical software (Ver.20 for windows, SPSS). All values were expressed as mean ± SE, P-value < 0.05 was considered to be significant and 0.05 ≤ P < 0.10 was considered as a tendency.
Results
Blood Biochemical Characteristics
As presented in Table 3, compared with the control group, dietary supplementation with Puerarin by 400 mg/kg and 800 mg/kg decreased the levels of LEP (P < 0.001), and the content of TC was reduced in the Pue200 group compared to control and Pue800 (P = 0.048). Moreover, the concentration of COR in the Pue400 group was decreased compared with the Pue200 group (P = 0.056). No difference was noticed on the contents of INS, T3, T4, ADPN, TG, HDL-C, and LDL-C among all groups.
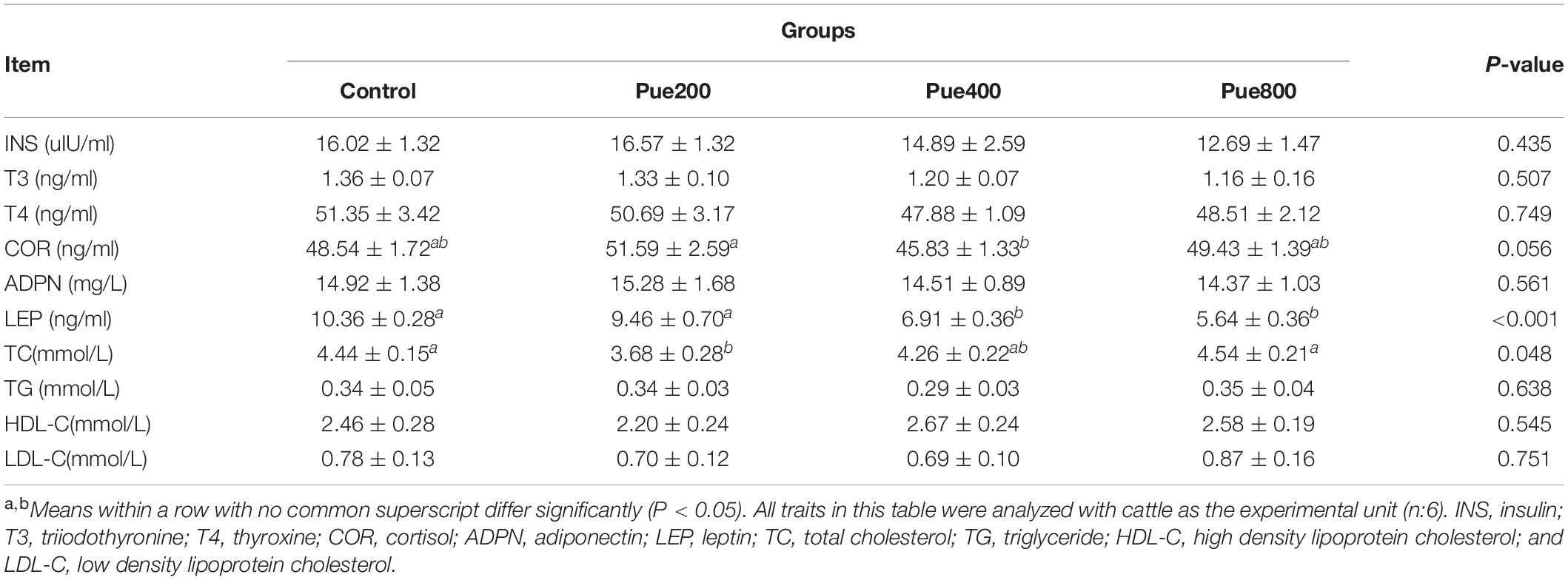
Table 3. Effects of puerarin on the blood biochemical characteristics in beef cattle under hot environment.
Nutritional Components and Fatty Acid Composition of Muscle
As presented in Table 4, compared with the control group, the concentration of CP and CF were increased in the Pue800 group (P = 0.039 and P = 0.025, respectively). No difference was noticed about the contents of moisture, CA, Ca, and P among all groups. However, dietary supplementation with Puerarin by 400 mg/kg enhanced the contents of tetradecenoic acid (C14:1) and heptadecenoic acid (C17:1) compared with the control group (P = 0.038 and P = 0.020, respectively). Moreover, the Pue400 treatment tended to increase the contents of hexadecenoic acid (C16:1) compared with the control group in Table 5 (P = 0.079).
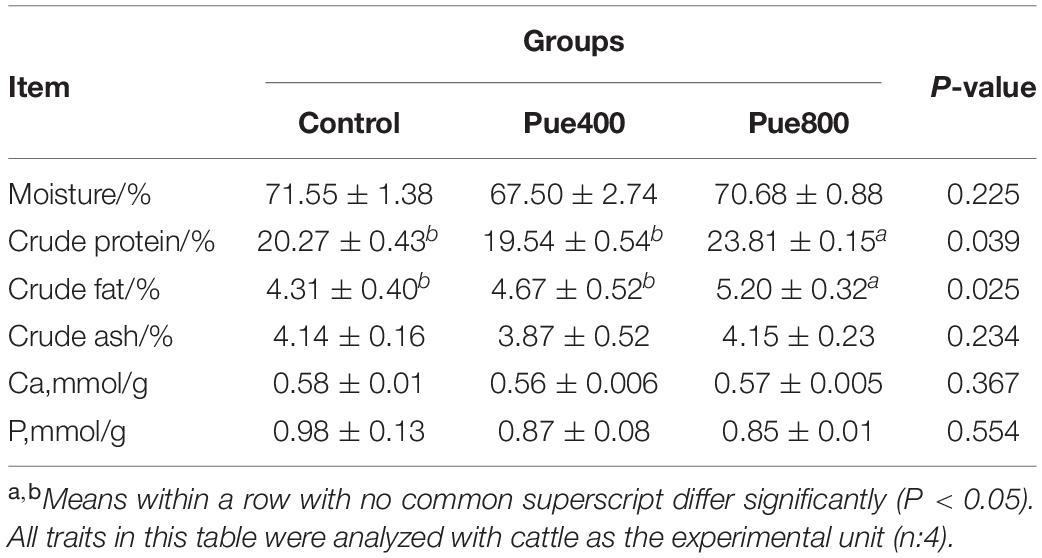
Table 4. Effect of Puerarin on the nutritional components of muscle in beef cattle under heat stress.
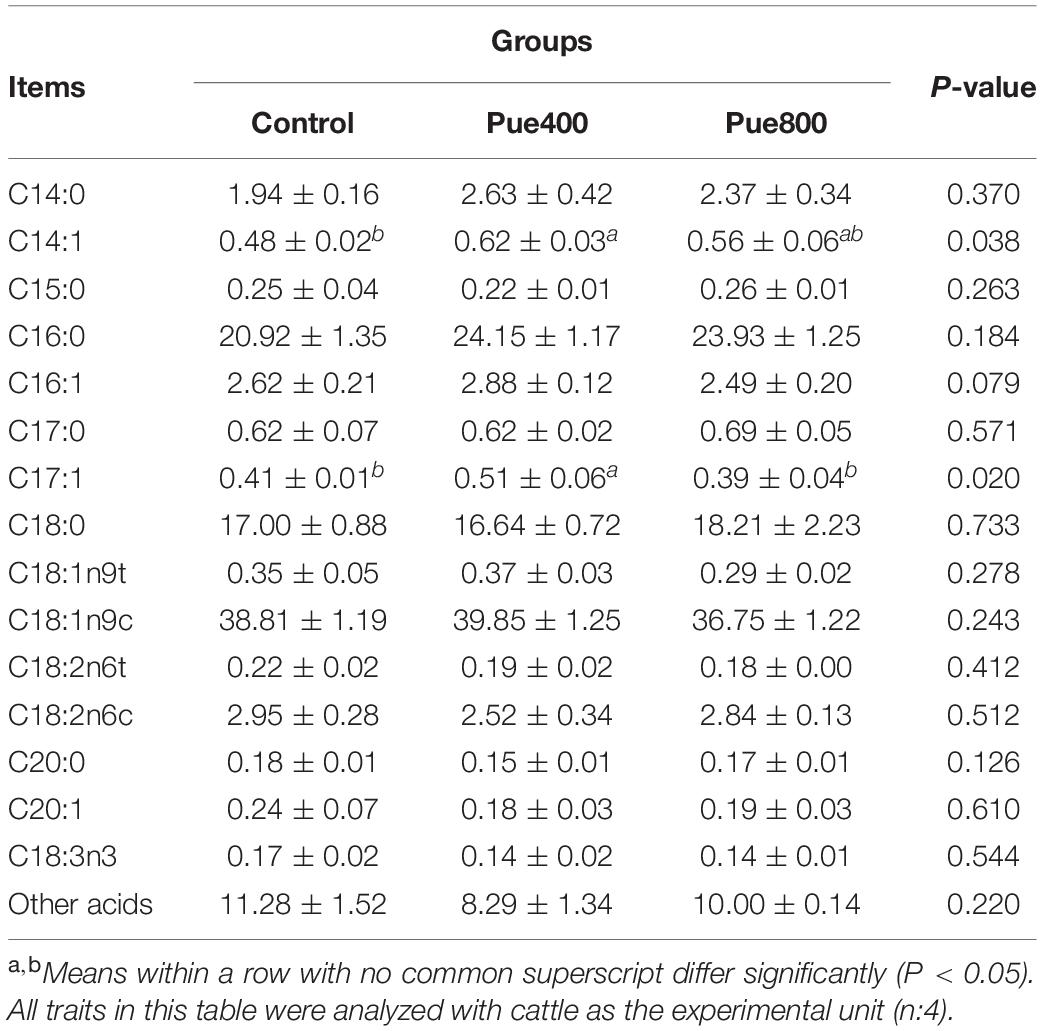
Table 5. Effects of Puerarin on fatty acid composition of longissimus thoracis muscle beef under heat stress (%).
The Activity of the Fatty Metabolizing Enzyme
The results in Figure 1 showed that diet supplemented with 400 mg/kg Puerarin improved the activity of FAS in LT (D) (P = 0.044), but the activity of HSL in the Pue800 was decreased compared with the control group (A) (P = 0.006). No difference was noticed on the activity of LPL and ACC among the three groups.
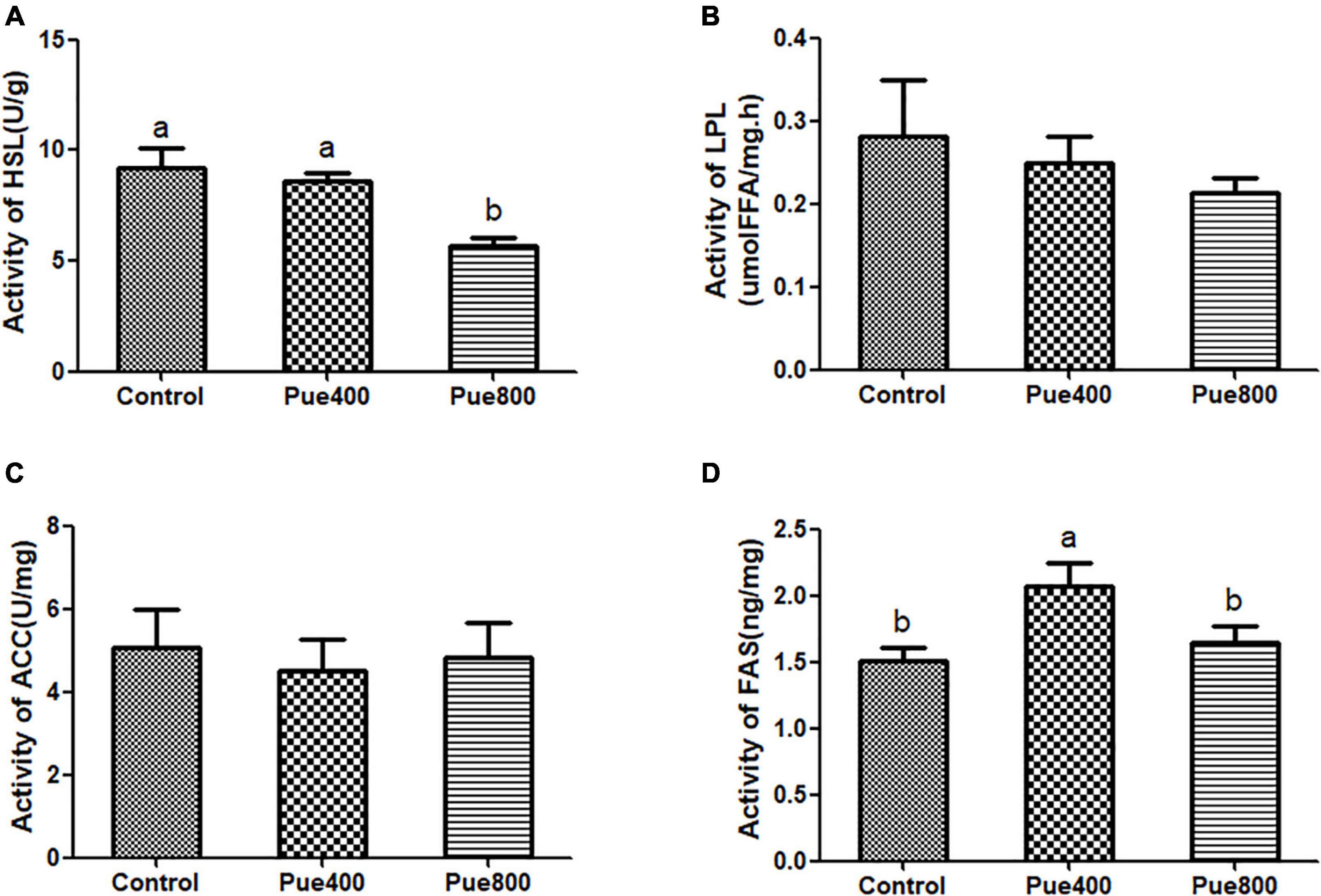
Figure 1. Effect of Puerarin on the activity of fat metabolizing enzymes in heat stressed beef cattle (n = 4). Control: dietary supplementation with 0 mg/kg Puerarin in the feed concentrate; Pue400: dietary supplementation with 400 mg/kg Puerarin in the feed concentrate; Pue800: dietary supplementation with 800 mg/kg Puerarin in the feed concentrate. HSL, hormone sensitive lipase (A); LPL, lipoprotein lipase (B); ACC, acetyl CoA carboxylase (C); FAS, fatty acid synthase (D). a,bMeans within a row with no common superscript differ significantly (P < 0.05).
Overall Assessment for Mapping Statistics
The overall assessment for mapping statistics is shown in Table 6. The RNA-Seq of eight LT samples yielded around 4 billion raw reads. After quality filtering, the high-quality sequence data in each muscle sample was approximately 5.03 gigabases (Gb), ranging from 4.48 to 5.87 Gb. The correlation analysis based on the gene expression profiles found that the correlations between biological replicates were greater than 0.952 (Figure 2), the high reproductivity between samples indicated that the sequencing data could be used for further analyses.
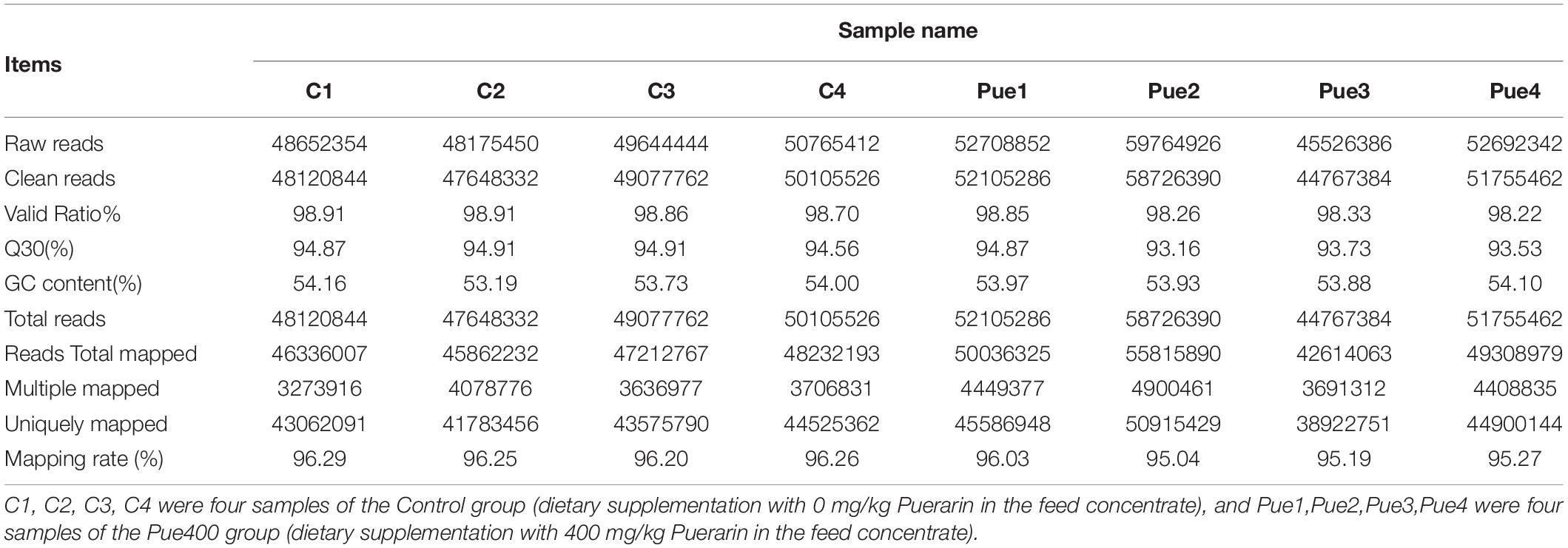
Table 6. Summary statistics for sequence quality and alignment information of eight longissimus thoracis muscle samples in two groups.
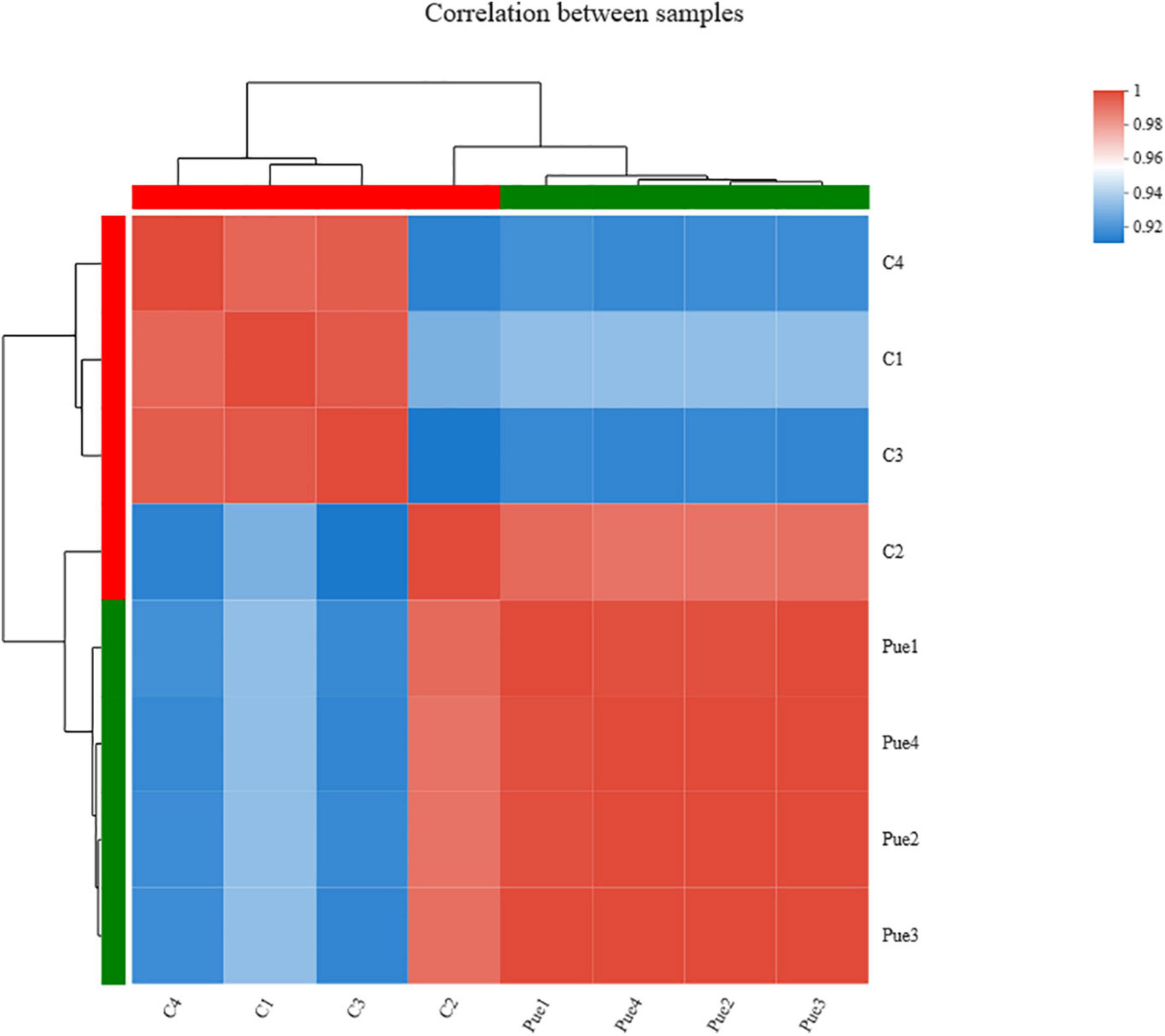
Figure 2. Correlations of eight samples (n = 4). C1, C2, C3, C4 were four samples of the Control group (dietary supplementation with 0 mg/kg Puerarin in the feed concentrate), and Pue1, Pue2, Pue3, Pue4 were four samples of the Pue400 group (dietary supplementation with 400 mg/kg Puerarin in the feed concentrate). In the figure, the right and lower sides are the sample names, the left and upper sides are the sample clustering, and the different color squares represent the correlation of the two samples.
Gene Differential Expression Analysis
As shown in Figure 3, there were 833 differentially expressed genes (DEGs) were found in LT muscles between the control and Puerarin groups, these DEGs were categorized into three gene ontology categories: molecular function, biological process, and cellular component (Figure 4). The top five cellular component categories of DEGs between the control and Puerarin groups included “binding,” “catalytic activity,” “molecular function regulator,” “transport activity” and “molecular transducer activity.” The top five biological processes of DEGs included “cellular process,” “biological regulation,” “metabolic process,” “response stimulus” and “developmental process.” The top five cellular components of DEGs included “cell part,” “organelle,” “membrane part,” “membrane” and “organelle part.” Among the total 833 DEGs, 341 DEGs upregulated and 492 DEGs downregulated were identified in the Puerarin group compared with the control group.
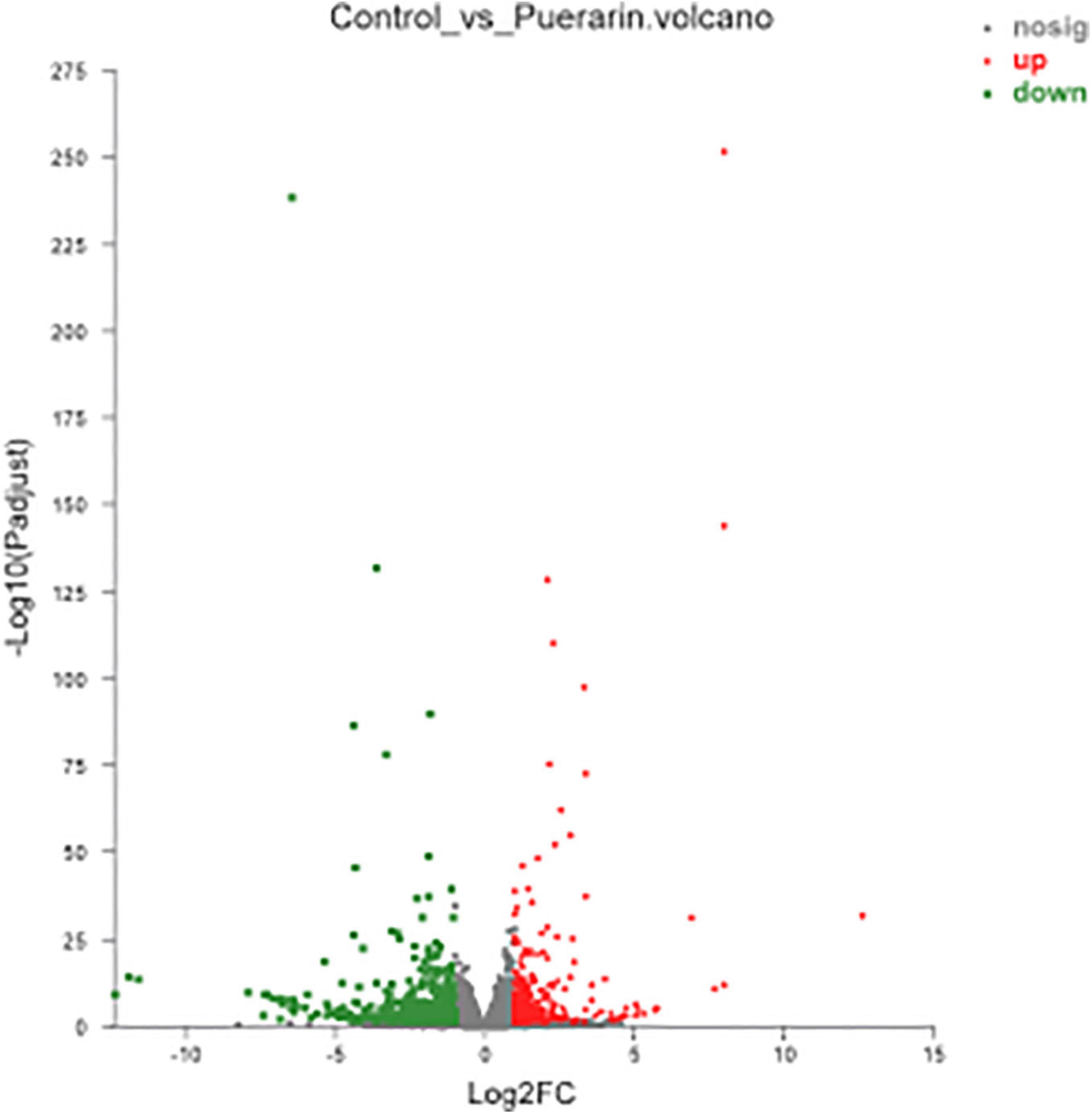
Figure 3. Volcano plot of the differentially expressed genes between control and Puerarin groups of longissimus thoracis muscles (n = 4). Control: dietary supplementation with 0 mg/kg Puerarin in the feed concentrate; Puerarin: dietary supplementation with 400 mg/kg Puerarin in the feed concentrate. The red dots represent the up-regulated DEGs, the green dots represent the down-regulated DEGs and the blue dots represent non-DEGs.
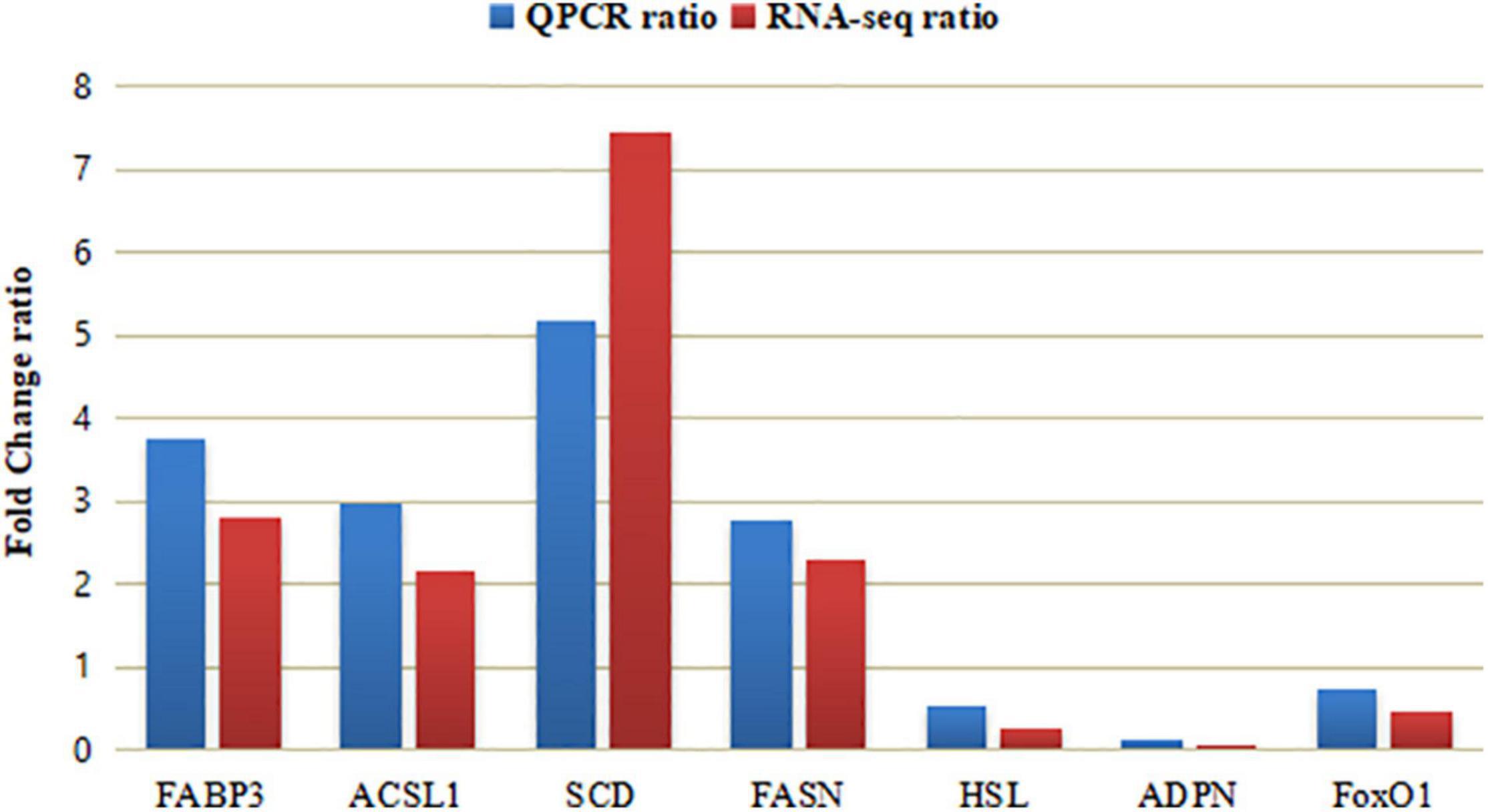
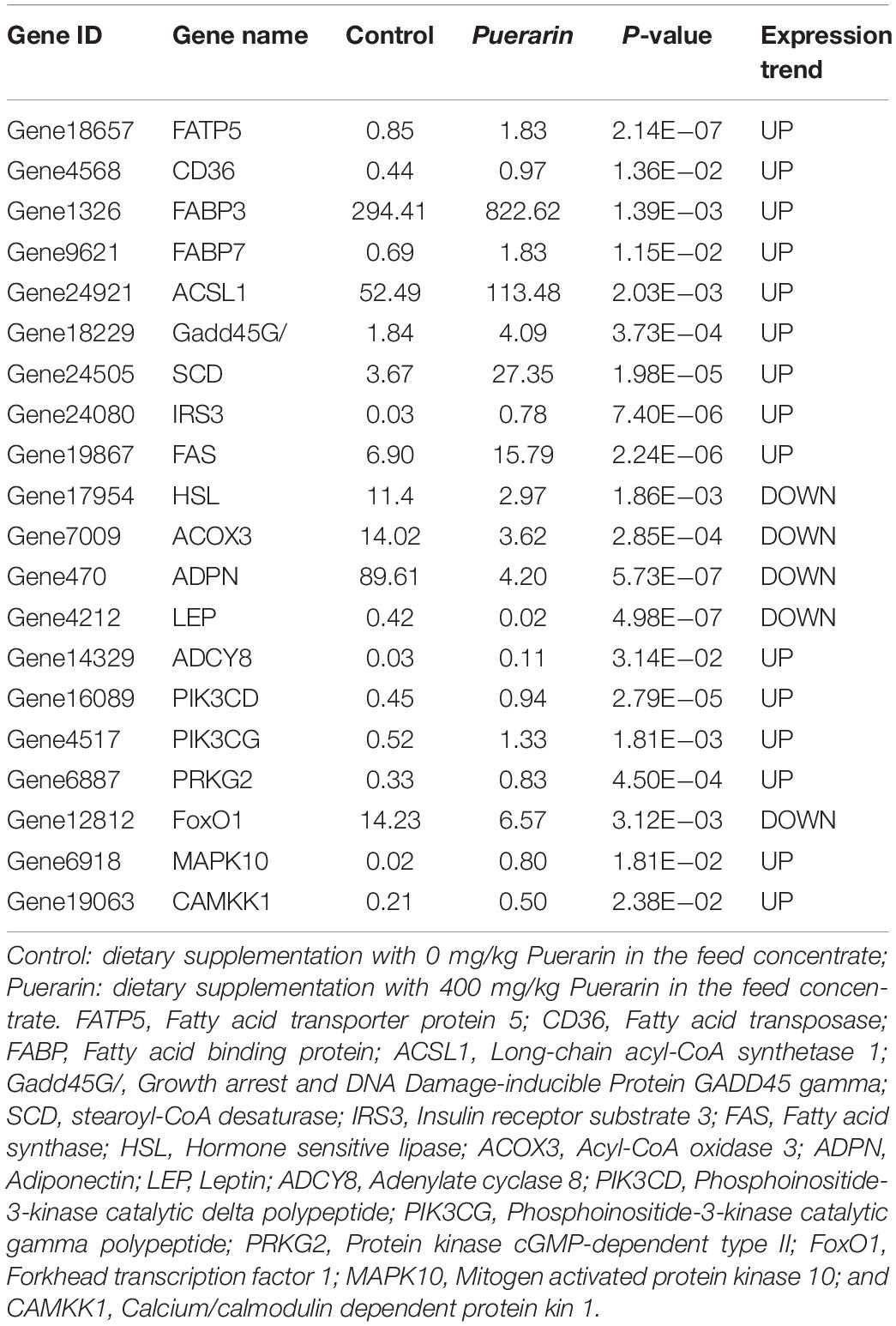
There were 20 DEGs related to lipid metabolism, and Puerarin treatment enhanced the expression of 15 genes including FATP5, CD36, FABP3, FABP7, ACSL1, Gadd45G, SCD, IRS3, FAS (Table 7). To validate the reliability of the transcriptomic sequencing analyses, 7 differentially expressed genes were randomly selected for qRT-PCR verification (Table 2). As shown in Figure 5, the results from both methods were largely consistent, suggesting that the RNA-Seq results were credible.
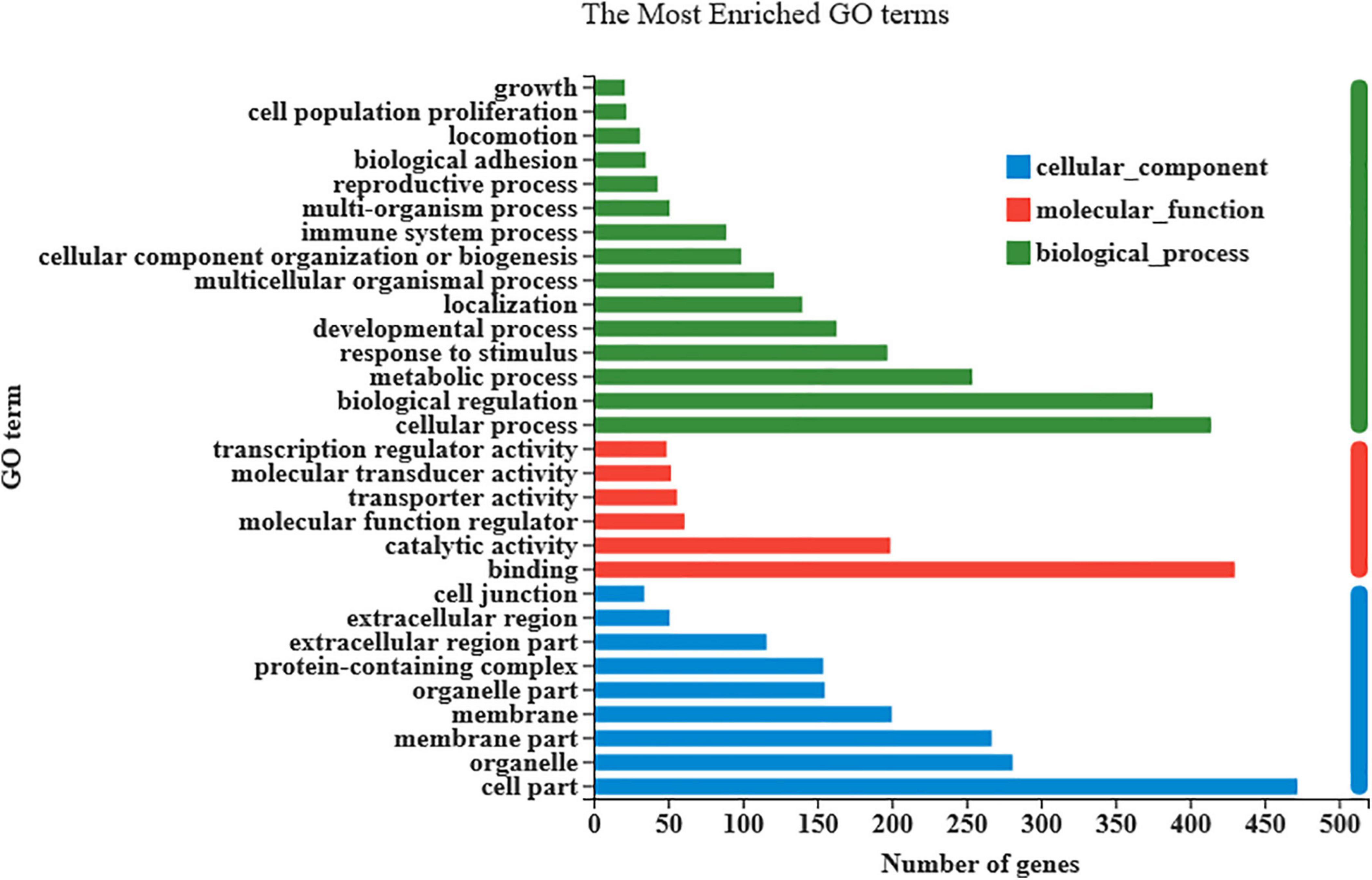
Figure 5. Comparing analysis of relative gene expression in Control and Puerarin group (n = 4). Control: dietary supplementation with 0 mg/kg Puerarin in the feed concentrate; Puerarin: dietary supplementation with 400 mg/kg Puerarin in the feed concentrate. FABP3, Fatty acid binding protein 3; ACSL1, Long-chain acyl-CoA synthetase 1; SCD, stearoyl-CoA desaturase; FAS, Fatty acid synthase; HSL, Hormone sensitive lipase; ADPN, Adiponectin; and FoxO1, Forkhead transcription factor 1.
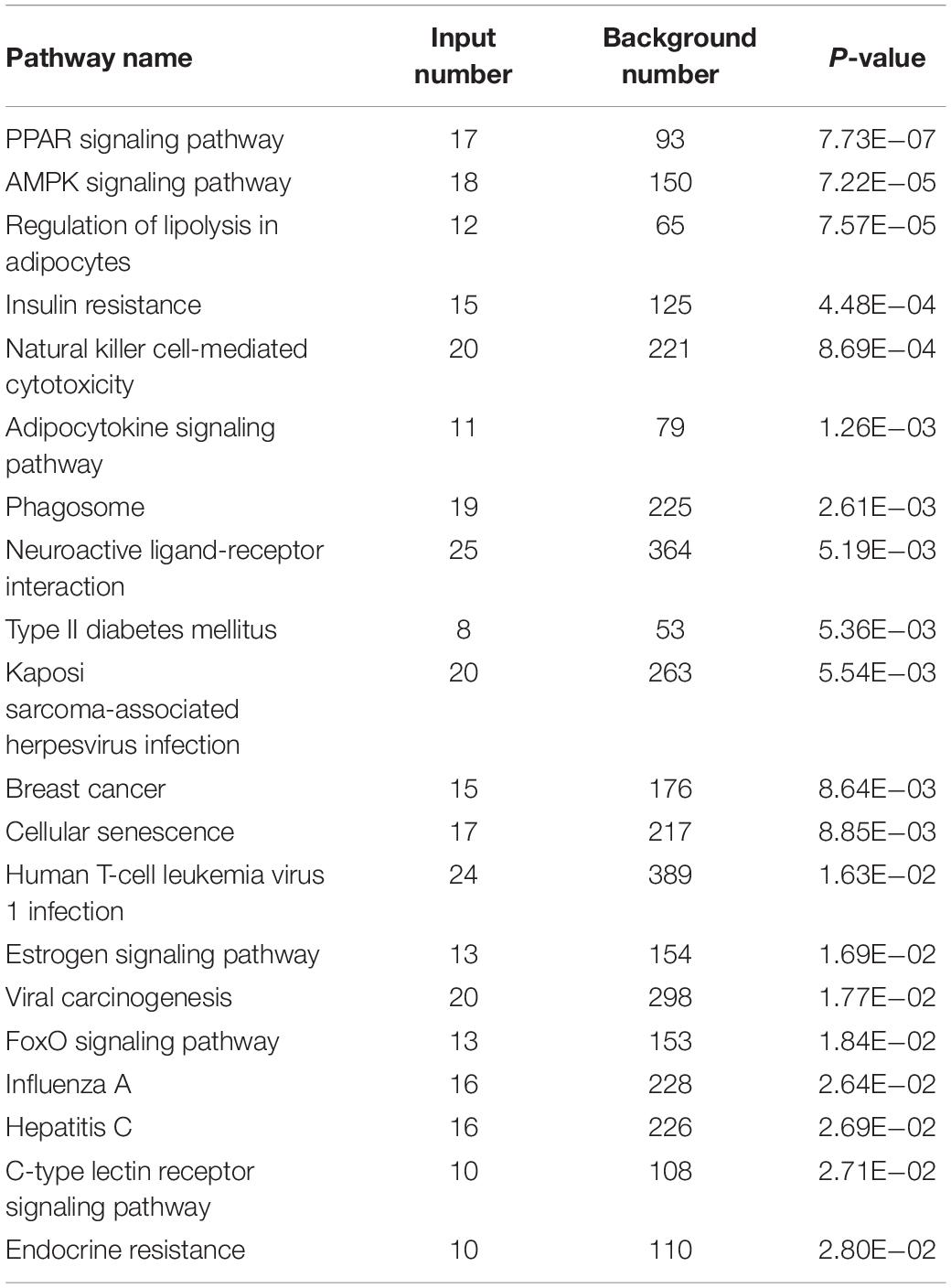
Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analysis on the downregulated genes demonstrated. There were 36 significantly enriched signaling pathways (P < 0.05). As shown in Table 8 in the top 20 significantly enriched signaling pathways, peroxisome proliferator-activated receptor (PPAR) signaling pathway (P = 7.73E−07), adenylate activated protein kinase (AMPK) signaling pathway (P = 7.22E−05) and forkhead transcription factor (FoxO) signaling pathway (P = 1.84E−02) were closely related to lipid metabolism and meat quality in animals.
Discussion
The present study was performed under high temperature and humidity during the summer months (the average THI = 81.81), which indicated that the experimental beef cattle were in a state of heat stress according to the report of Armstrong (19). Studies have shown that heat-stressed can activate the hypothalamic-pituitary-adrenal (HPA) axis, causing a series of complex physiological and metabolic changes, such as elevated level of corticosterone hormone and leptin, which can indirectly reflect the impact of heat-stressed on animals (20). Leptin, an adipokines secreted by adipose, plays an effective role in energy homeostasis, neuroendocrine function and metabolism (21). A study has shown that the concentration of leptin in the blood would increase under heat stress (22). The current results showed that Puerarin treatments declined the levels of leptin significantly compared with the control group, which indicates dietary supplementation with Puerarin may relieve the disordered endocrine function of beef cattle due to heat stress. Puerarin could block the increased levels of the adrenocortico-tropic hormone in the serum, which is induced by single prolonged stress (SPS) (23). Therefore, we can conclude that Puerarin can relieve the response of heat stress.
Puerarin has been shown to have a direct effect on lipid metabolism in our study. The concentrations of triglyceride (TG) and total cholesterol (TC) in serum can be used as an important index of lipid metabolism, and leptin can inhibit the expression of fatty acid synthase, which is negatively correlated with fat deposition. In this experiment, Puerarin treatment with 200 mg/kg decreased the levels of TC, and the levels of leptin in Pue400 and Pue800 groups were significantly lower than those in the control group, which confirmed that Puerarin had a direct role in promoting lipid metabolism. Some studies have mentioned that Puerarin has a negative effect on animal fat deposition (24, 25). While, other studies have shown that the addition of Puerarin enhanced preadipocyte differentiation as well as lipid accumulation (26, 27). The reason for different result may be the treatment concentration of puerarin and the species of laboratory animal.
The content and composition of fatty acids in muscle are closely related to muscle quality (28). Yang found that the content of monounsaturated fatty acids (such as oleic acid and linolenic acid) was correlated with flavor positively, which can help prevent diseases and is beneficial to human health when absorbed unsaturated fatty acids appropriately (29). Tan found that adding isoflavones to the diet can reduce the level of saturated fatty acids in the muscle of the goat, increase the level of monounsaturated fatty acids, and increase the ratio of n-6 to n-3 fatty acids (30). Study has shown that grazing mutton has a better flavor than barn-fed sheep meat, another study found that C14:1 in grazing sheep meat was significantly higher than that in barn-fed sheep meat (31, 32). In this experiment, adding 400 mg/kg Puerarin significantly increased the content of C14:1 and C17:1 in muscle, which indicated that Puerarin can improve the flavor of postmortem beef.
Fatty acid synthase play an important catalytic role in the synthesis of long-chain fatty acids (33), and HSL is the key enzyme of regulating the rate of lipolysis. In this experiment, the activities of FAS in LT were significantly increased in the Pue400 group, while the activities of HSL were decreased in the Pue800 group, which suggested that Puerarin could regulate fat metabolism and promote fat synthesis. These results agree well with the findings of Zhao, which indicated that adding Daidzein, a similar structure with Puerarin, can affect the lipid metabolism and promote intramuscular fat deposition of Xiangzhong black cattle (34).
Further, to reveal the molecular mechanism of Puerarin promoting intramuscular fat deposition, high-throughput sequencing of mRNA technology (RNA-Seq) was performed. The current results showed that Puerarin treatment with 400 mg/kg up-regulated 341 DEGs and down-regulated 492 DEGs in LT muscle, and these DEGs are mainly enriched in the PPAR signaling pathway, AMPK signaling pathway, and FoxO signaling pathway. Among them, the PPAR signaling pathway and AMPK signaling pathway are correlated with lipid metabolism and meat quality, and the FoxO signaling pathway is associated with cell-lipid differentiation. The FoxO signaling pathway plays an important role in regulating preadipocyte differentiation (35). After differentiation of preadipocytes, the synthesis and deposition of triglycerides in adipose cells were accelerated, which increased the volume of fat cells. Lu found that FoxO could enhance glucose synthesis and lipolysis (36). Sakamoto suggested that the expression of the Gadd45 gene could be regulated directly by the FoxO signaling pathway, which could promote the differentiation of preadipocytes by participating in DNA methylation in cells (37). Chen found that activated FOXO1 binds to the PPARγ promoter and inhibits the transcriptional activity of PPARγ by competitively suppressing the formation of functional PPARγ/RXR/DNA complex, thereby inhibiting lipogenesis and adipocyte differentiation (38). Our results showed that the expression of FoxO1 genes in the FoxO signaling pathway in the Puerarin group was down-regulated significantly and the expression of the Gadd45 gene was up-regulating, which indicated Puerarin could promote the differentiation of fat cells in the heat-stressed beef cattle muscles.
Mammalian body contains four important fat depots, namely, visceral, subcutaneous, intermuscular, and intramuscular (IM) fat. But among them, the IM fat is considered one of the most important factors that determines carcass quality traits (39). PUFAs was decreased with the increasing IMF%, which may account for the reduce of PUFAs content in the Puerarin group (40). From the view of molecular, the deposition of adipose tissue is essentially the result of spatiotemporal specific expression regulation of many adipose synthesis genes (41). Studies have shown that the PPAR signaling pathway plays a leading role in the process of lipid synthesis, which can directly regulate the expression of SCD, FAS, FABP, GLUT4 and other genes (42, 43), and promote glucose absorption and fat synthesis of adipocytes when it was activated (44).
The PPAR signaling pathway regulates cellular differentiation, energy balance, and lipid metabolism (45). PPAR has three exists isoforms, α, β and γ (46). Furthermore, it was reported that activation of PPARγ is to be essential for deposition of intramuscular fat (47). PPARγ can increase lipid deposition in adipocytes by regulating the levels of expression of HSL, LEP, ADPN and other cytokines produced by adipose tissue (48), and regulating the transcription of a variety of genes involved in fat synthesis, such as FATP, FABP, CD36 (49, 50). After the activation of PPARγ, the levels of expression of FABP3/FABP7, ACSL1, CD36, IRS3, SCD, FAS and other genes in the pathway were significantly up-regulated, and the levels of expression of HSL, LEP, ADPN and other genes were significantly down-regulated. Among the up-regulated genes, FABP is a member of the fatty acid-binding protein family and plays a very important role in the uptake of long-chain fatty acids. ACSL1 can prolong the long-chain fatty acids in cells (51), IRS3 is the receptor of short-chain fatty acids on cell membrane (52), SCD is the main enzyme for de novo synthesis of monounsaturated fatty acids (53), FAS is the key enzyme in the process of fatty acid synthesis. Among the down-regulated genes, HSL, LEP and ADPN are the key factors affecting lipolysis. ACSL1 is elevated by PPARγ agonists in the adipose tissue, and ACSL1 overexpression can promote triglyceride accumulation in adipocytes (54, 55). A previous study showed that the higher ACSL1 expression in the F line than the C line coincided with the greater IMF deposition found in the former (56), which was in line with our result. In bovine mammary glands, mRNA abundance at 60 days postpartum of FABP3 and ACSL1 were 80- and 7-fold greater relative to 15 days antenatal, respectively, which are significantly associated with milk fat synthesis (57). Kae found that isoflavone daidzein and its metabolite equol enhance adipocyte differentiation through activating PPARγ (58). Genistein, a main soy isoflavone, can directly bind to and activate peroxisome proliferators-activated receptor a (PPARa) or PPARc (59). Therefore, the activation of PPAR and the expression of its downstream regulatory genes are the most fundamental reason for promoting fat deposition, especially intramuscular fat deposition.
Conclusion
In a word, Puerarin can activate the PPARγ signaling pathway, up-regulate the levels of expression of genes related to fat synthesis, and down-regulated genes expression promoting muscle fatty acid oxidation, so as to regulate lipid metabolism, improve the beef flavor of Jinjiang cattle and enhance intramuscular fat deposition in LT muscle of heat-stressed beef cattle.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/supplementary material.
Ethics Statement
The animal study was reviewed and approved by the Committee for the Care and Use of Experimental Animals at Jiangxi Agricultural University (JXAULL-20190015).
Author Contributions
XSo, HC, and TP designed the overall study. HS, XSh, XZ, and MQ performed the animal feeding experiment and sample analysis. XSo and HC wrote the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This study was financially supported by the National Natural Science Foundation of China (31660672 and 32060768), and the China Agriculture Research System of MOF and MARA (CARS-37).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors appreciated all the help from our colleagues and collaborators, and the technical assistance provided by Gao’an Yufeng Agricultural and Livestock Co., Ltd., in Jiangxi province of China.
Footnotes
- ^ ftp://ftp.ensembl.org/pub/release-75/fasta/bos_taurus/dna/
- ^ http://geneontology.org
- ^ https://www.genome.jp/kegg/pathway.html
References
1. Silanikove N. Effects of heat stress on the welfare of extensively managed domestic ruminants. Lives Sci. (2000) 67:1–18. doi: 10.1016/s0301-6226(00)00162-7
2. Herbut P, Angrecka S, Dorota G, Hoffmann G. The physiological and productivity effetcts of heat stress in cattle – a review. Ann Anim Sci. (2019) 19:579–94. doi: 10.2478/aoas-2019-0011
3. Mishra SR. Thermoregulatory responses in riverine buffaloes against heat stress: an updated review. J Therm Biol. (2021) 96:102844. doi: 10.1016/j.jtherbio.2021.102844
4. Zhang M, Dunshea FR, Warner RD, Digiacomo K, Chauhan SS. Impacts of heat stress on meat quality and strategies for amelioration: a review. Int J Biometeorol. (2020) 64:1613–28. doi: 10.1007/s00484-020-01929-6
5. Summer A, Lora I, Formaggioni P, Gottardo F. Impact of heat stress on milk and meat production. Anim Front. (2019) 9:39–46. doi: 10.1093/af/vfy026
6. Hausman GJ, Dodson MV, Ajuwon K, Azain M, Barnes KM, Guan LL, et al. Board-invited review: the biology and regulation of preadipocytes and adipocytes in meat animals. J Anim Sci. (2009) 87:1218–46. doi: 10.2527/jas.2008-1427
7. Liu S, Huang J, Wang X, Ma Y. Transcription factors regulate adipocyte differentiation in beef cattle. Anim Genet. (2020) 51:351–7. doi: 10.1111/age.12931
8. Park SJ, Beak SH, Da J, Sang YK, Jeong IH, Piao MY, et al. Genetic, management, and nutritional factors affecting intramuscular fat deposition in beef cattle–a review. Asian Austral J Anim. (2018) 31:1043–61. doi: 10.5713/ajas.18.0310
9. Yao XJ, Yin JA, Xia YF, Wei ZF, Luo YB, Liu M, et al. Puerarin exerts antipyretic effect on lipopolysaccharide-induced fever in rats involving inhibition of pyrogen production from macrophages. J Ethnopharmacol. (2012) 141:322–30. doi: 10.1016/j.jep.2012.02.038
10. Chen X, Huang C, Sun H, Hong H, Jin J, Bei C, et al. Puerarin suppresses inflammation and ECM degradation through Nrf2/HO-1 axis in chondrocytes and alleviates pain symptom in osteoarthritic mice. Food Funct. (2021) 12:2075–89. doi: 10.1039/D0FO03076G
11. Jiang M, Yun Q, Niu G, Gao Y, Shi F, Yu S. Puerarin prevents inflammation and apoptosis in the neurocytes of a murine Parkinson’s disease model. Genet Mol Res. (2016) 15:1–9. doi: 10.4238/gmr.15047501
12. Yun J, Yu Y, Zhou G, Luo X, Jin H, Zhao Y, et al. Effects of puerarin on the AKT signaling pathway in bovine preadipocyte differentiation. Asian Austral J Anim Sci. (2020) 33:4–11. doi: 10.5713/ajas.19.0004
13. Shen Y, Yang S, Hu X, Zhang M, Ma X, Wang Z, et al. Natural product puerarin activates AKT and ameliorates glucose and lipid metabolism dysfunction in hepatic cells. J Funct Foods. (2019) 55:296–304. doi: 10.1016/j.jff.2019.02.035
14. Peng T, Shang HL, Yang MR, Li YJ, Luo JR, Qu MR, et al. Puerarin improved growth performance and postmortem meat quality by regulating lipid metabolism of cattle under hot environment. Anim Sci J. (2021) 92:e13543. doi: 10.1111/asj.13543
15. Li YJ, Shang HL, Zhao XH, Qu MR, Peng T, Guo BB, et al. Radix puerarin extract (puerarin) could improve meat quality of heat-stressed beef cattle through changing muscle antioxidant ability and fiber characteristics. Front Vet Sci. (2020) 7:615086. doi: 10.3389/fvets.2020.615086
16. Wang BH, Yang L, Luo YL, Su RN, Su L, Zhao LH, et al. Effects of feeding regimens on meat quality, fatty acid composition and metabolism as related to gene expression in Chinese Sunit sheep. Small Ruminant Res. (2018) 169(Suppl. 4):127–33. doi: 10.1016/j.smallrumres.2018.08.006
17. Trapnell C, Roberts A, Goff L, Pertea G, Kim D, Kelley DR, et al. Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nat Protoc. (2012) 7:562–78. doi: 10.1038/nprot.2012.016
18. Yang Z, Zhao X, Xiong X, Bao L, Pan K, Zhou S, et al. Uncovering the mechanism whereby dietary nicotinic acid increases the intramuscular fat content in finishing steers by RNA sequencing analysis. Anim Prod Sci. (2019) 59:1620. doi: 10.1071/an18205
19. Armstrong DV. Heat stress interaction with shade and cooling. J Dairy Sci. (1994) 77:2044–50. doi: 10.3168/jds.s0022-0302(94)77149-6
20. He XF, Lu Z, Ma BB, Zhang L, Li JL, Jiang Y, et al. Chronic heat stress alters hypothalamus integrity, the serum indexes and attenuates expressions of hypothalamic appetite genes in broilers. J Therm Biol. (2019) 81:110–7. doi: 10.1016/j.jtherbio.2019.02.025
21. Li S, Li X. Leptin in normal physiology and leptin resistance. Sci Bull. (2016) 61:1480–8. doi: 10.1007/s11434-015-0951-4
22. Min L, Cheng JB, Shi BL, Yang HJ, Zheng N, Wang JQ, et al. Effects of heat stress on serum insulin, adipokines, AMP-activated protein kinase, and heat shock signal molecules in dairy cows. J Zhejiang Univ Sci B. (2015) 6:541–8. doi: 10.1631/jzus.B1400341
23. Su AS, Zhang JW, Zou J. The anxiolytic-like effects of puerarin on an animal model of PTSD. Biomed Pharmacother. (2019) 115:108978. doi: 10.1016/j.biopha.2019.108978
24. Zheng G, Lin L, Zhong S, Zhang Q, Li D, Zane A. Effects of puerarin on lipid accumulation and metabolism in high-fat diet-fed mice. PLoS One. (2015) 10:e0122925. doi: 10.1371/journal.pone.0122925
25. Jung H, Kang A, Kang S, Park YK, Song MY. The root extract of Pueraria lobata and its main compound, puerarin, prevent obesity by increasing the energy metabolism in skeletal muscle. Nutrients. (2017) 9:33. doi: 10.3390/nu9010033
26. Prasain JK, Peng N, Rajbhandari R, Wyss JM. The Chinese Pueraria root extract (Pueraria lobata) ameliorates impaired glucose and lipid metabolism in obese mice. Phytomedicine. (2012) 20:17–23. doi: 10.1016/j.phymed.2012.09.017
27. Lee OH, Seo DH, Park CS, Kim YC. Puerarin enhances adipocyte differentiation, adiponectin expression, and antioxidant response in 3T3-L1 cells. Biofactors. (2010) 36:459–67. doi: 10.1002/biof.119
28. Enser M, Hallett KG, Hewett B, Fursey GAJ, Wood JD, Harrington G. Fatty acid content and composition of UK beef and lamb muscle in relation to production system and implications for human nutrition. Meat Sci. (1998) 49:329–41. doi: 10.1016/s0309-1740(97)00144-7
29. Yang A, Lanari MC, Brewster M, Tume RK. Lipid stability and meat colour of beef from pasture- and grain-fed cattle with or without vitamin E supplement. Meat Sci. (2002) 60:41–50. doi: 10.1016/s0309-1740(01)00103-6
30. Tan CY, Zhong RZ, Tan ZL, Han XF, Tang SX, Xiao WJ, et al. Dietary inclusion of tea catechins changes fatty acid composition of muscle in goats. Lipids. (2011) 46:239–47. doi: 10.1007/s11745-010-3477-1
31. Ling J, Zhang WJ, Wang B. Research progress on influence factors of mutton quality. China Anim Husb Vet Med. (2016) 43:1250–4. doi: 10.16431/j.cnki.1671-7236.2016.05.020
32. Liu MJ, Guo J, Yan XL, Sun HZ. Fatty acid profiles of meat from pasturing and barn-fed sheep in Inner Mongolia. Meat Res. (2020) 34:38–44. doi: 10.7506/rlyj1001-8123-20200429-110
33. Haemmerle G, Zimmermann R, Zechner R. Letting lipids go: hormone-sensitive lipase. Curr Opin Lipidol. (2003) 14:289–97. doi: 10.1097/00041433-200306000-00009
34. Zhao XH, Yang ZQ, Bao LB, Wang CY, Shan Z, Gong JM, et al. Daidzein enhances intramuscular fat deposition and improves meat quality in finishing steers. Exp Biol Med. (2014) 240:1152–7. doi: 10.1177/1535370214564755
35. Song YF, Gao Y, Hogstrand C, Li DD, Pan YX, Luo Z. Upstream regulators of apoptosis mediates methionine-induced changes of lipid metabolism. Cell Signal. (2018) 51:176–90. doi: 10.1016/j.cellsig.2018.08.005
36. Lu ZY, Meng Z, Wen MY, Kang XL, Zhang Y, Liu QS, et al. Overexpression of BmFoxO inhibited larval growth and promoted glucose synthesis and lipolysis in silkworm. Mol Genet Genomics. (2019) 294:1375–83. doi: 10.1007/s00438-019-01550-2
37. Sakamoto Y, Inoue K, Takahashi M, Taketa Y, Kodama Y, Nemoto K, et al. Different pathways of constitutive androstane receptor-mediated liver hypertrophy and hepatocarcinogenesis in mice treated with piperonyl butoxide or decabromodiphenyl ether. Toxicol Pathol. (2013) 41:1078–92. doi: 10.1177/0192623313482055
38. Chen J, Lu Y, Tian M, Huang Q. Molecular mechanisms of FOXO1 in adipocyte differentiation. J Mol Endocrinol. (2019) 62:R239–53. doi: 10.1530/JME-18-0178
39. Guo H, Khan R, Raza S, Suhail SM, Zan L. RNA-seq reveals function of Bta-miR-149-5p in the regulation of bovine adipocyte differentiation. Animals. (2021) 11:1207. doi: 10.3390/ani11051207
40. Realini CE, Pavan E, Purchas RW, Agnew M, Johnson PL, Bermingham EN, et al. Relationships between intramuscular fat percentage and fatty acid composition in m. longissimus lumborum of pasture-finished lambs in New Zealand. Meat Sci. (2021) 181:108618. doi: 10.1016/j.meatsci.2021.108618
41. De Jager N, Hudson NJ, Reverter A, Barnard R, Cafe LM, Greenwood PL, et al. Gene expression phenotypes for lipid metabolism and intramuscular fat in skeletal muscle of cattle. J Anim Sci. (2013) 91:1112–28. doi: 10.2527/jas.2012-5409
42. Brown JD, Plutzky J. Peroxisome proliferator activated receptors as transcriptional nodal points and therapeutic targets. Circulation. (2007) 115:518–33. doi: 10.1161/CIRCULATIONAHA.104.475673
43. Shamina MR, Mitchell AL. Peroxisome proliferator-activated receptor γ in diabetes and metabolism. Trends Pharmacol Sci. (2004) 25:331–6. doi: 10.1016/j.tips.2004.03.012
44. Lamartiniere CA, Wang J, Smith-Johnson M, Eltoum IE. Daidzein: bioavailability, potential for reproductive toxicity, and breast cancer chemoprevention in female rats. Toxicol Sci. (2002) 65:228–38. doi: 10.1093/toxsci/65.2.228
45. Gross B, Pawlak M, Lefebvre P, Staels B. PPARs in obesity-induced T2DM, dyslipidaemia and NAFLD. Nat Rev Endocrinol. (2017) 13:36–49. doi: 10.1038/nrendo.2016.135
46. Wu Z, Rosen ED, Brun R, Hauser S, Spiegelman BM. Cross-regulation of C/EBPα and PPARγ controls the transcriptional pathway of adipogenesis and insulin sensitivity. Mol Cell. (1999) 3:151–8. doi: 10.1016/s1097-2765(00)80306-8
47. Hausman GJ, Bergen WG, Etherton TD, Smith SB. The history of adipocyte and adipose tissue research in meat animal. J Anim Sci. (2018) 96:473–86. doi: 10.1093/jas/skx050
48. Yaacov B, Michael CN, Estelita SO, Ying ZJ, Pilar RL, Kenneth RC, et al. PPARγ is required for placental, cardiac, and adipose tissue development. Mol Cell. (1999) 4:585–95. doi: 10.1016/s1097-2765(00)80209-9
49. Moldes M, Zuo Y, Morrison RF, Silva D, Park BH, Liu JJ, et al. Peroxisome-proliferator-activated receptor γ suppresses Wnt/β-catenin signalling during adipogenesis. Biochem J. (2003) 376:607–13. doi: 10.1042/BJ20030426
50. Gandhi GR, Jothi G, Antony PJ, Balakrishna KP, Michael G, Ignacimuthu S, et al. Gallic acid attenuates high-fat diet fed-streptozotocin-induced insulin resistance via partial agonism of PPARγ in experimental type 2 diabetic rats and enhances glucose uptake through translocation and activation of GLUT4 in PI3K/p-Akt signaling pathway. Eur J Pharmacol. (2014) 745:201–16. doi: 10.1016/j.ejphar.2014.10.044
51. Harkewicz R, Du H, Tong Z, Alkuraya H, Bedell M, Sun W, et al. Essential role of ELOVL4 protein in very long chain fatty acid synthesis and retinal function. J Biol Chem. (2012) 287:11469–80. doi: 10.1074/jbc.M111.256073
52. Tazoe H, Otomo Y, Kaji I, Tanaka R, Kuwahara A. Roles of short-chain fatty acids receptors, gpr41 and gpr43 on colonic functions. J Physiol Pharmacol. (2008) 59:251–62. doi: 10.2170/physiolsci.RP006108
53. Elshorbagy AK, Valdivia-Garcia M, Mattocks DAL, Plummer JD, Smith AD, Drevon CA, et al. Cysteine supplementation reverses methionine restriction effects on rat adiposity: significance of stearoyl-coenzyme A desaturase. J Lipid Res. (2011) 52:104–12. doi: 10.1194/jlr.M010215
54. Martin G, Schoonjans K, Lefebvre AM, Staels B, Auwerx J. Coordinate regulation of the expression of the fatty acid transport protein and acyl-CoA synthetase genes by PPARalpha and PPARgamma activators. J Biol Chem. (1997) 272:28210–7. doi: 10.1074/jbc.272.45.28210
55. Souza SC, Muliro KV, Liscum L, Lien P, Yamamoto MT, Schaffer JE, et al. Modulation of hormone-sensitive lipase and protein kinase A-mediated lipolysis by perilipin A in an adenoviral reconstituted system. J Biol Chem. (2002) 277:8267–72. doi: 10.1074/jbc.M108329200
56. Liu L, Cui HX, Xing SY, Zhao GP, Wen J. Effect of divergent selection for intramuscular fat content on muscle lipid metabolism in chickens. Animals. (2019) 10:4. doi: 10.3390/ani10010004
57. Bionaz M, Loor JJ. ACSL1, AGPAT6, FABP3, LPIN1, and SLC27A6 are the most abundant isoforms in bovine mammary tissue and their expression in affected by stage of lactation. J Nutr. (2008) 138:1019–24. doi: 10.1093/jn/138.6.1019
58. Cho KW, Lee OH, Banz WJ, Moustaid-Moussa N, Shay NF, Kim YC. Daidzein and the daidzein metabolite, equol, enhance adipocyte differentiation and PPARγ transcriptional activity. J Nutr Biochem. (2010) 21:841–7. doi: 10.1016/j.jnutbio.2009.06.012
Keywords: puerarin, beef cattle, heat stress, intramuscular fat deposition, lipid metabolism, RNA-seq
Citation: Chen H, Peng T, Shang H, Shang X, Zhao X, Qu M and Song X (2022) RNA-Seq Analysis Reveals the Potential Molecular Mechanisms of Puerarin on Intramuscular Fat Deposition in Heat-Stressed Beef Cattle. Front. Nutr. 9:817557. doi: 10.3389/fnut.2022.817557
Received: 18 November 2021; Accepted: 10 February 2022;
Published: 21 March 2022.
Edited by:
Julio J. Ochoa, University of Granada, SpainReviewed by:
Susan Kay Duckett, Clemson University, United StatesZhigang Song, Shandong Agricultural University, China
Copyright © 2022 Chen, Peng, Shang, Shang, Zhao, Qu and Song. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaozhen Song, c29uZ3hpYW96aGVuMTExNkAxNjMuY29t
†These authors have contributed equally to this work and share first authorship
 Huan Chen
Huan Chen Tao Peng
Tao Peng Hanle Shang
Hanle Shang Xianghui Zhao
Xianghui Zhao