- 1Department of Pharmacy, Hubei Cancer Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
- 2Department of Nutrition, Hengyang Central Hospital, Hengyang, China
- 3Department of Clinical Nutrition, Hubei Cancer Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
- 4Department of Lymphoma Medicine (Breast Cancer and Soft Tissue Tumor Medicine), Hubei Cancer Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
Objective: Several studies have reported conflicting results regarding the association between sarcopenia and outcomes in patients with diffuse large B-cell lymphoma (DLBCL). This meta-analysis aimed to evaluate the prognostic value of sarcopenia in patients with DLBCL.
Methods: PubMed, Embase, and Cochrane Library databases were searched to identify trials exploring the association between sarcopenia and prognosis in patients with DLBCL treated with chemotherapy. A meta-analysis of overall survival (OS), progression-free survival (PFS), treatment completion, and rate of complete response (CR) was performed.
Results: Twelve studies that involved 2,324 patients with DLBCL were included. Sarcopenia was associated with poor OS and PFS in patients with DLBCL, even after adjusting for confounders. Patients with sarcopenia had lower rates of CR and treatment completion than patients without sarcopenia.
Conclusions: Sarcopenia is a negative predictor of prognosis in patients with DLBCL. Additional and prospective studies investigating the diagnostic criteria for sarcopenia are warranted.
Introduction
Lymphomas are solid tumors in the immune system. Non-Hodgkin lymphoma accounts for ~90% of all lymphomas (1). Diffuse large B-cell lymphoma (DLBCL) is the most common type of non-Hodgkin lymphoma in the United States and worldwide (2, 3). Compared to the chemotherapy regimen of cyclophosphamide, vincristine, doxorubicin, and prednisone (CHOP), the combination of immunotherapy with rituximab (R-CHOP) has been found to significantly improve outcomes. Although progression-free survival (PFS) and overall survival (OS) have improved as validated by many randomized controlled trials, ~40% of patients experience relapse or progression (4). Clinicians and researchers have found that the prognosis of DLBCL is not only related to age, disease stage, and extranodal involvement but also closely to the patients' nutritional status and skeletal muscle loss.
Sarcopenia is defined as a progressive and generalized skeletal muscle disorder associated with an increased likelihood of adverse outcomes, including falls, fractures, physical disability, and mortality (5). Sarcopenia is prevalent in patients with cancer: 15–50% had skeletal muscle loss (6), while 38–70% were diagnosed with sarcopenia (6). Sarcopenia can increase the risk of death (7), reduce chemotherapy tolerance (8), increase the risk of postoperative complications, and reduce the quality of life (9) and survival (8, 10). Furthermore, several meta-analyses have also verified the prognostic role of sarcopenia in patients with lung cancer, ovarian cancer, gastric cancer, hepatocellular carcinoma, and head and neck cancer (11–15).
Recent studies have explored sarcopenia as a prognostic factor for patients with DLBCL. However, the results were inconsistent and controversial. One meta-analysis reported that sarcopenia predicted OS in patients with malignant hematological diseases, while only four studies on patients with DLBCL were included in the meta-analysis (16). Importantly, several recently published studies, which were not included in the above meta-analysis, further explored the prognostic role of sarcopenia in patients with DLBCL. To fill this knowledge gap, we conducted a comprehensive systematic review and meta-analysis of these studies. The impact of sarcopenia on clinical outcomes in patients with DLBCL undergoing immunochemotherapy was evaluated.
Materials and Methods
Search Strategy and Selection Criteria
This meta-analysis was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement. We searched all the published articles in the PubMed, Embase, and Cochrane Library databases until July 2021 for all references using the keywords, MeSH terms “sarcopenia” and “diffuse large B-cell lymphoma,” and other related words. The complete search used for PubMed was {[sarcopenia (MeSH Term)] OR muscle OR cachexia OR body composition} AND {[diffuse large B-cell lymphoma (MeSH Term)] OR [non-Hodgkin lymphoma (MeSH Term)]}. Unpublished studies and original data were not included. To avoid oversights in the literature search, two independent researchers searched for the relevant trials twice.
Study Selection and Data Extraction
Studies were included if (1) the study was designed as a prospective cohort study or a retrospective study; (2) patients diagnosed with DLBCL were treated with chemotherapy; (3) skeletal muscle mass or function was measured before treatment; and (4) outcomes included OS, PFS, treatment completion, and rate of complete response (CR). Studies published as abstracts and case reports were excluded. Studies in which participants were not diagnosed with DLBCL and the diagnosis of sarcopenia was not clearly defined were also excluded.
Data from the included studies were extracted by two authors and checked by another author. The following data were collected: name of the first author, year of publication, characteristics of the study participants, number of participants, definition of sarcopenia, method to measure muscle, muscle measurement time, prevalence of sarcopenia, and anti-tumor therapy method for DLBCL and outcomes.
Quality Assessment
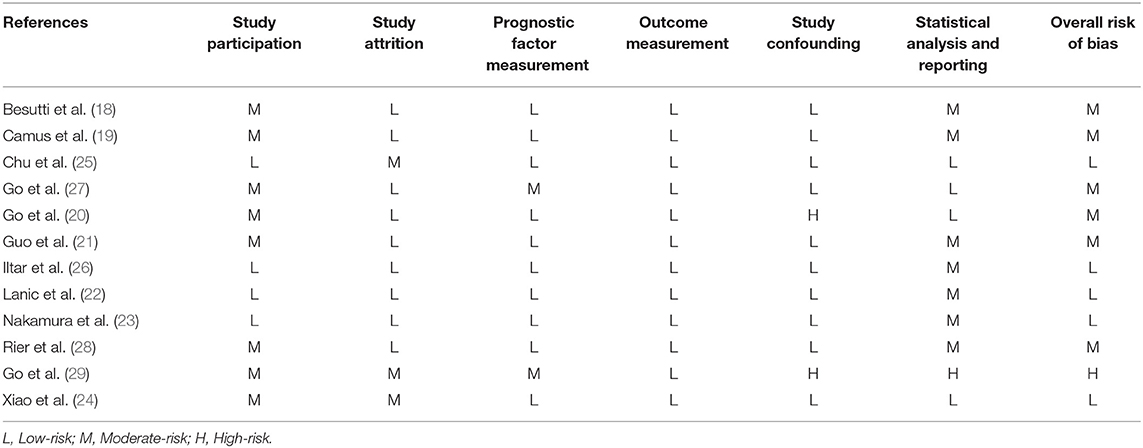
The quality of the included trials was evaluated using the Quality In Prognostic Studies (QUIPS) tool by two reviewers independently—study participation, study attrition, prognostic factor measurement, outcome measurement, study confounding, and statistical analysis and reporting were assessed. If more than four of these six criteria had a low risk of bias, the study was considered to have a low risk of bias, and if two or more criteria had a high risk of bias, the study was considered to have a high risk of bias. The remaining studies were classified as having a moderate risk of bias (17).
Data Synthesis and Statistical Analysis
Stata software (version 15.0, StataCorp., College Station, TX, USA) was used for statistical analyses. A heterogeneity test was performed for each analysis, and I2 > 50% indicated heterogeneity. When heterogeneity across studies was identified (I2 > 50%), the random-effects model was used to calculate the pooled hazard ratio (HR) and corresponding 95% confidence intervals (CIs). If studies were homogeneous, a fixed-effects model was used for the analysis. Most of the HR values were extracted from the univariate and multivariate Cox regression analyses, and a few were calculated using the Kaplan–Meier curves. If several methods were used to diagnose sarcopenia, such as skeletal muscle index (SMI) and skeletal muscle density (SMD), SMI was used in the meta-analysis for OS and PFS. A predefined subgroup analysis based on the anti-tumor treatment, skeletal muscle measurement method, and rate of sarcopenia was performed to identify the potential sources of heterogeneity and further explore the prognostic role of sarcopenia. Publication bias was assessed using. The funnel plots and Egger's regression intercept analysis.
Results
Study Selection and Characteristics
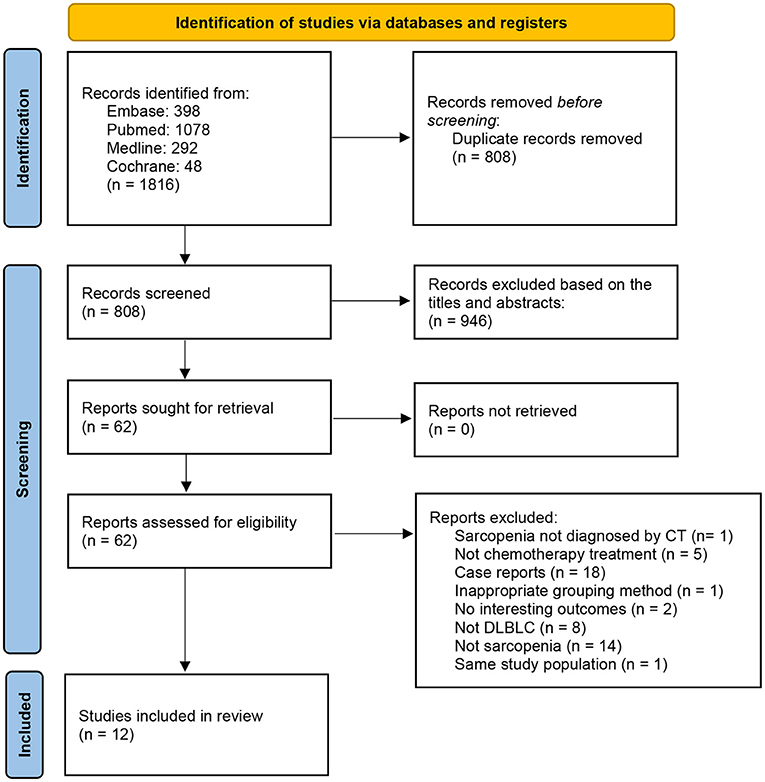
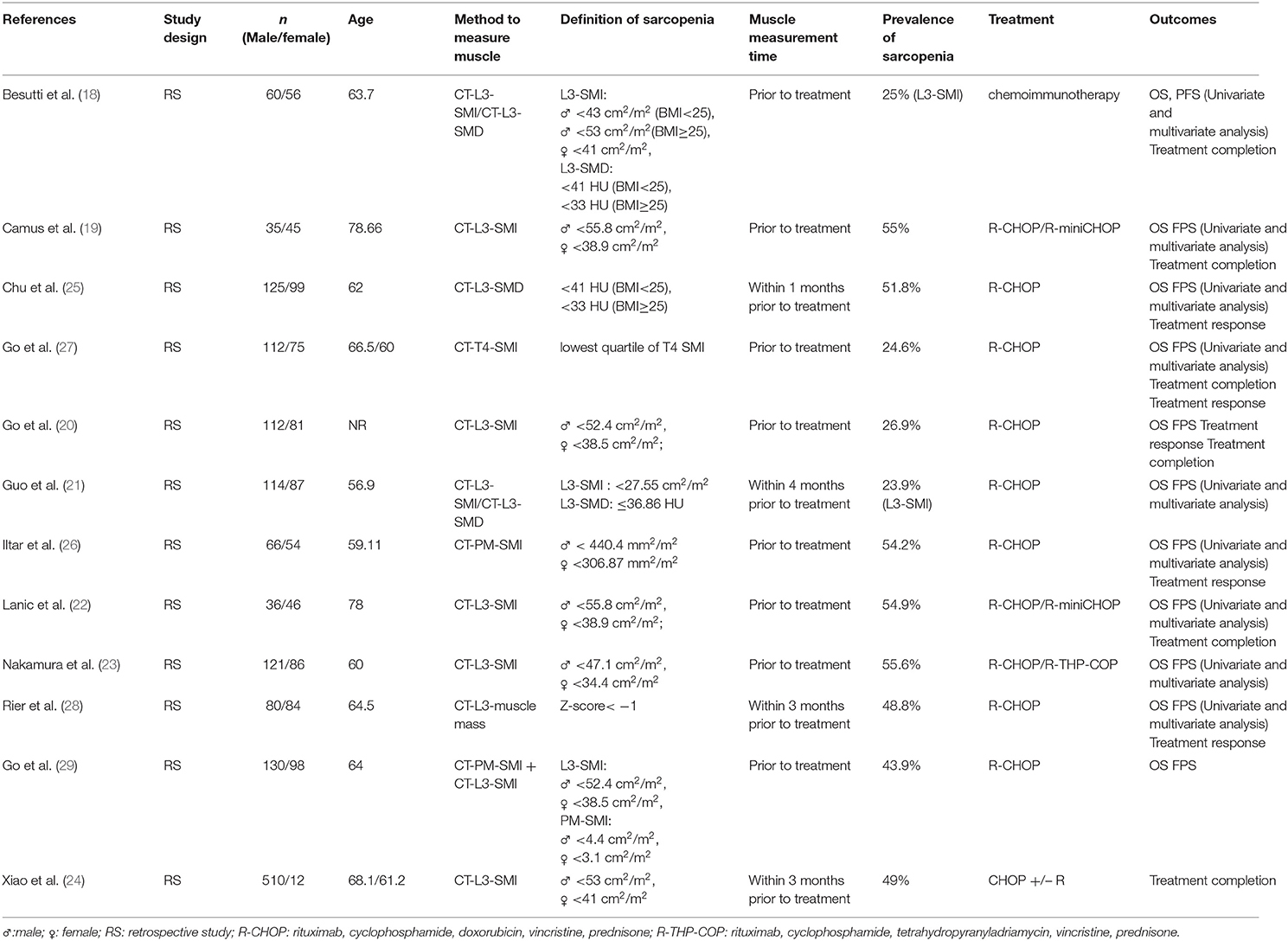
A total of 659 related studies were extracted from the above-mentioned databases; 12 met the eligibility criteria and were thus included in the meta-analysis (Figure 1). The sample size ranged from 80 to 522 in the 12 studies. All included studies were retrospective cohort studies. The 12 included studies included 2,324 participants, among whom 996 were diagnosed with sarcopenia (18, 19). Seven studies used SMI at the third lumbar vertebra level (L3) on computed tomography (CT) (CT-L3-SMI) for the measurement of skeletal muscle mass (18–24). In the other three studies, SMD at the L3 level on CT imaging (CT-L3-SMD) was used for the measurement of skeletal muscle mass (18, 21, 25). In the two studies, skeletal muscle mass measured using SMI at the psoas level (CT-PM-SMI) (26) and fourth thoracic levels (CT-T4-SMI) (27) were used as the representative skeletal muscle mass of the whole body. One study used muscle mass at the L3 level on CT (CT-L3-muscle mass) as a diagnostic criterion for sarcopenia (28), while another study used a combination of CT-PM-SMI and CT-L3-SMI to diagnose sarcopenia (29). Eleven studies reported the OS and FPS. Five studies evaluated the impact of sarcopenia on treatment completion, and five studies assessed the rate of CR of anti-tumor therapy. The characteristics of the trials included in this meta-analysis are presented in Table 1.
Risk of Bias of Individual Studies
Table 2 presents an assessment of the risk of bias in the trials. According to the QUIPS checklist, five included studies had an overall low risk of bias, six trials had an overall moderate risk of bias, and one study had an overall high risk of bias.
Association Between Pretreatment Sarcopenia and OS
In total, 11 studies reported OS as an outcome (Figure 2A). A fixed-effect model indicated moderate heterogeneity between studies (I2 = 47.7%), in which patients with sarcopenia tended to have a shorter OS than those without sarcopenia (HR = 2.25; 95% CI = 1.90–2.67, P < 0.01). According to the multivariate analysis of eight trials, the association between pretreatment sarcopenia and poor OS was significant (HR = 1.90; 95% CI = 1.52–2.37, P < 0.01; I2 = 38.4%) (Figure 2B).
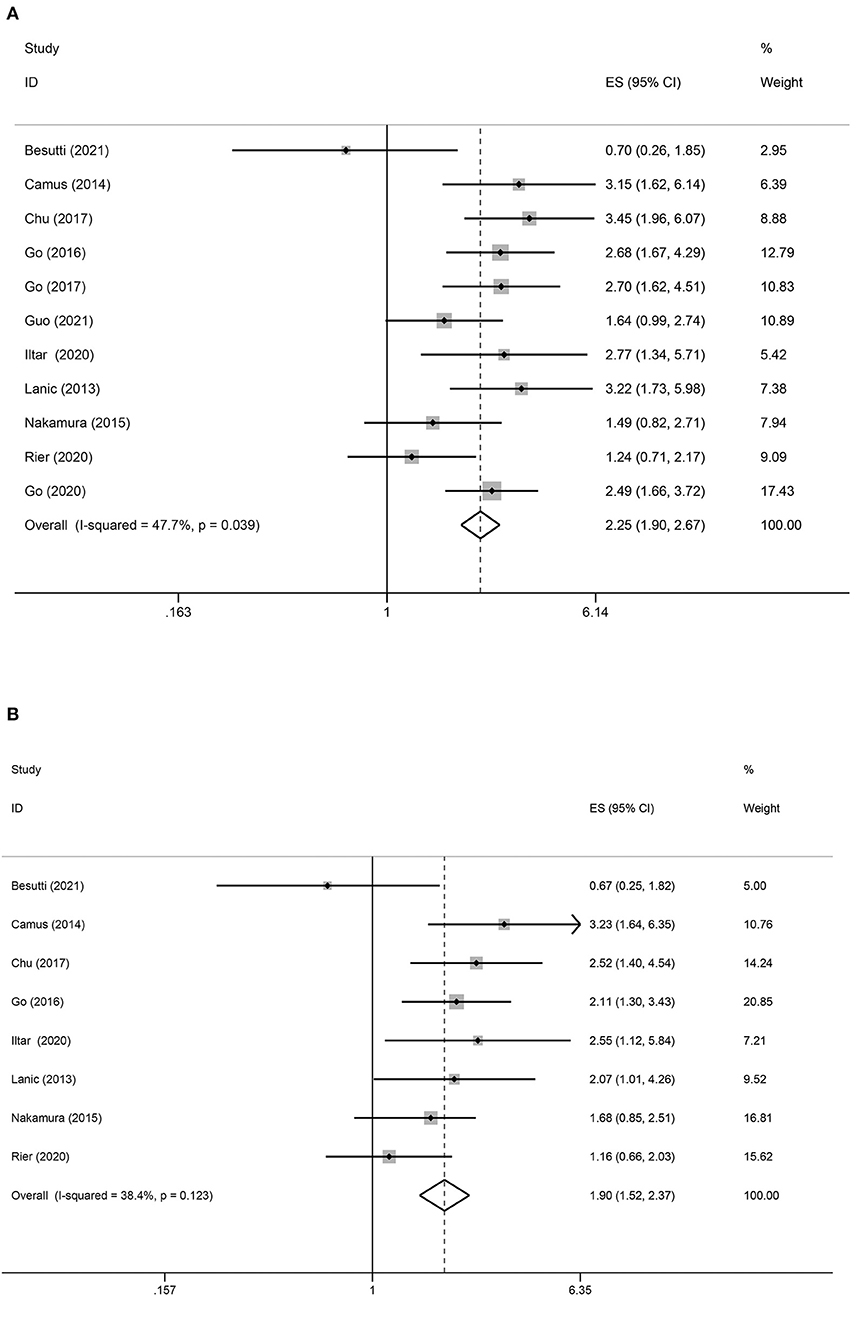
Figure 2. Forest plots of the association between sarcopenia and OS in patients with DLBCL undergoing chemotherapy. (A) Univariate analysis (B) Multivariate analysis. (A) Univariate analysis of pooled results of the association between sarcopenia and OS. The pooled HR was 2.25 (95% CI = 1.90–2.67, p < 0.01, I2 = 47.7%). (B) Multivariate analysis of pooled results of the association between sarcopenia and OS from. The pooled adjusted HR was 1.90 (95% CI = 1.52–2.37, p < 0.01; I2 = 38.4%).
To comprehensively evaluate the association between sarcopenia and OS in DLBCL, subgroup analysis was performed based on the anti-tumor treatment, skeletal muscle measurement, and rate of sarcopenia. In most subgroup meta-analyses, the pooled data indicated an association between sarcopenia and shorter OS in patients with DLBCL. Subgroup analysis also showed that sarcopenia had no significant impact on OS of patients with DLBCL treated with multiple treatment methods (HR = 1.21; 95% CI = 0.73–2.02, P > 0.05; I2 = 39.8%; Table 3).
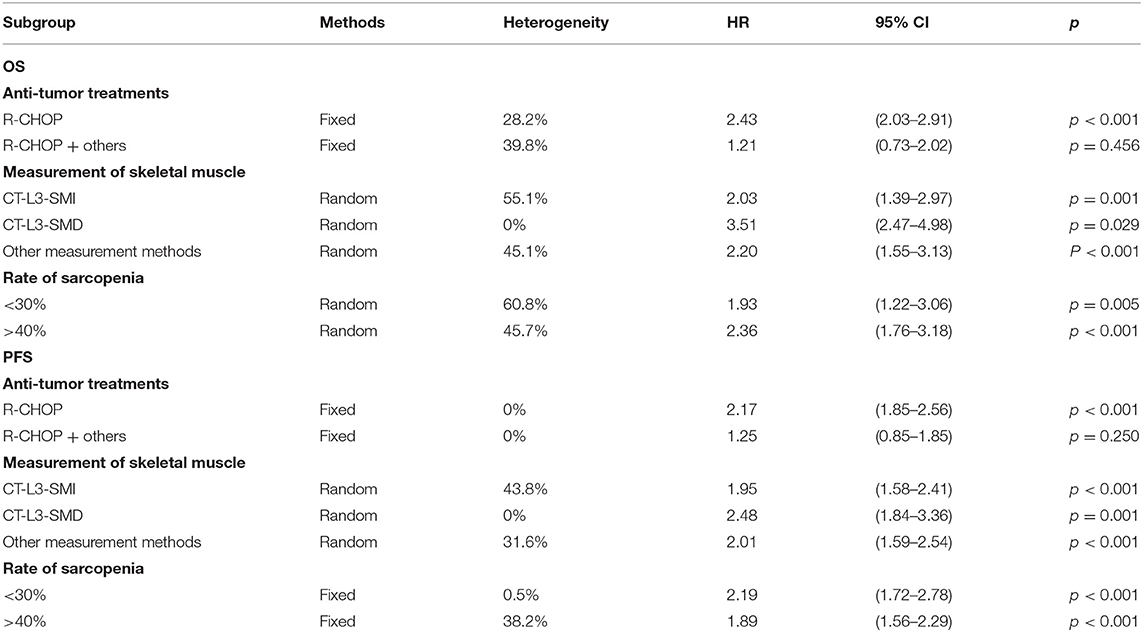
Table 3. Subgroup analysis of the association between sarcopenia and survival of patients with DLBCL treated with chemotherapy.
Association Between Pretreatment Sarcopenia and PFS
Eleven studies reported an association between pretreatment sarcopenia and PFS in patients with DLBCL. The crude pooled HR of skeletal muscle mass loss for PFS was 2.00 (95% CI = 1.72–2.32, P < 0.01), while low, non-significant heterogeneity was detected (I2 = 26.4%; P = 0.08). The adjusted summary HR from eight selected trials was 1.64 (95% CI = 1.32–2.03, P < 0.01), in which low, non-significant heterogeneity was detected (I2 = 0.0%) (Figure 3).
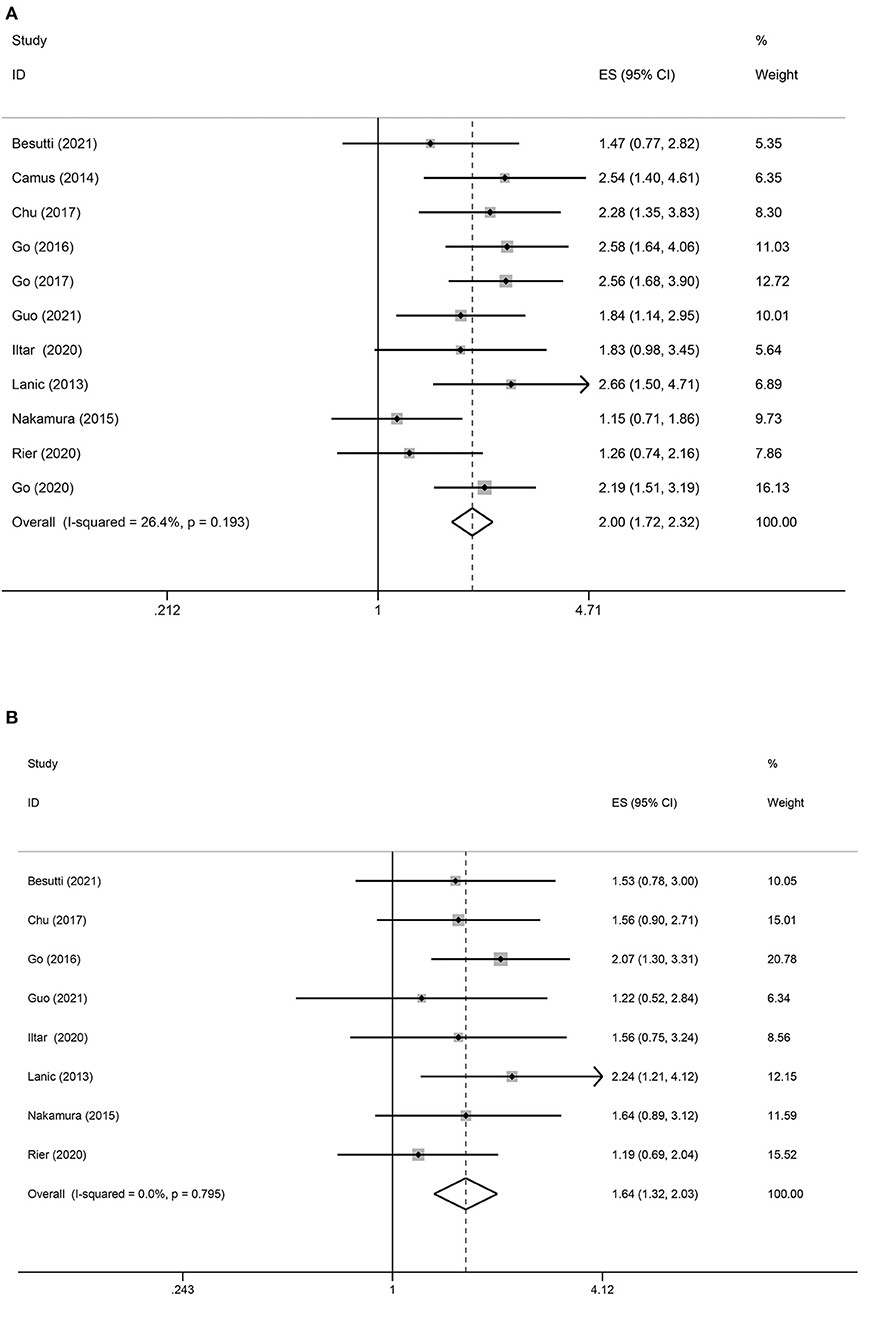
Figure 3. Forest plots of the association between sarcopenia and PFS in patients with DLBCL undergoing chemotherapy. (A) Univariate analysis (B) Multivariate analysis. (A) Univariate analysis of pooled results of the association between sarcopenia and PFS. The pooled HR was 2.00 (95% CI = 1.72–2.32, p < 0.01, I2 = 26.4%). (B) Multivariate analysis of pooled results of the association between sarcopenia and OS. The pooled adjusted HR in total was 1.64 (95% CI = 1.32–2.03, p < 0.01; I2 = 0.0%).
Subgroups analysis based on the anti-tumor treatments suggested that sarcopenia predicted negative PFS in patients with DLBCL treated with R-CHOP (HR = 2.17; 95% CI = 1.85–2.56, P < 0.01; I2 = 0%) but not in those treated with multiple treatment methods (HR = 1.25; 95% CI = 0.85–1.85, P = 0.250; I2 = 0%). The other subgroup analyses showed an association between sarcopenia and poor PFS (Table 3).
Sarcopenia and Treatment Completion
Five studies assessed the association between sarcopenia and treatment completion. The pooled results from the fixed model indicated that sarcopenia decreased the rate of treatment completion [odds ratio (OR) = 0.50; 95% CI = 0.37–0.65, P < 0.01]. Heterogeneity between studies was low (I2 = 21.1%) (Figure 4).
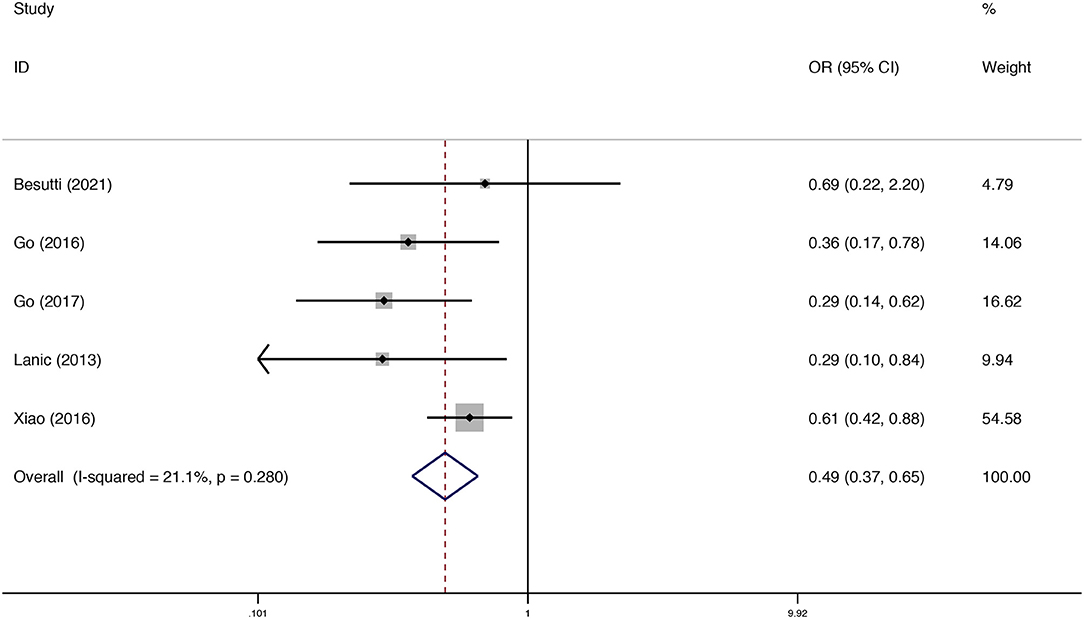
Figure 4. Forest plots of the meta-analysis of the effect of sarcopenia on treatment completion in patients with DLBCL undergoing chemotherapy. Pooled results of the OR of the rate of treatment completion in patients with DLBCL. The pooled OR of patients with DLBCL was 0.49 (95% CI = 0.37–0.65, I2 = 21.1%).
Sarcopenia and Rate of CR
Five studies analyzed the relationship between sarcopenia and the rate of CR in DLBCL. As shown in Figure 5, sarcopenia predicted a low rate of CR (OR = 0.47; 95% CI = 0.24–0.93, P < 0.01). Howev er, there was significant heterogeneity between studies (I2 = 72.3%).
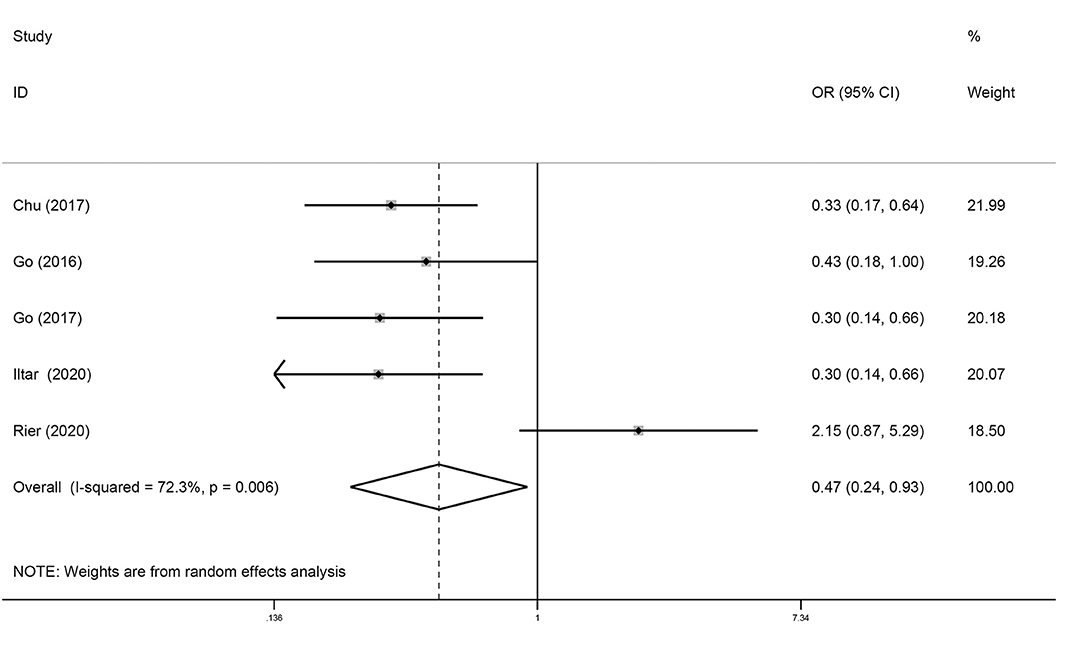
Figure 5. Forest plots of the meta-analysis of the effect of sarcopenia on the rate of CR in patients with DLBCL undergoing chemotherapy. Pooled results of OR of rate of CR in patients with DLBCL. The pooled OR of patients with DLBCL was 0.47 (95% CI = 0.24–0.93, I2 = 72.3%).
Publication Bias
The Begg's funnel plots and Egger's publication bias plots were used to assess the potential publication bias for OS in the univariate analysis. No publication bias was detected using the Egger's test (P = 0.344) (Figure 6).
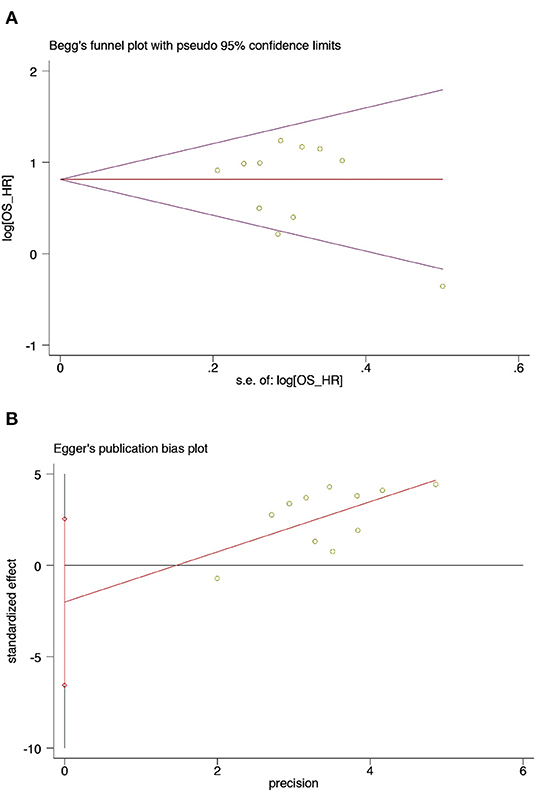
Figure 6. Begg's test and Egger's test of sarcopenia and OS from univariate analysis in patients with DLBCL undergoing chemotherapy (A) Begg's test (B) Egger's test. (A) Begg's funnel plots of publication bias for the association between sarcopenia and OS from univariate analysis (p = 0.755). (B) Egger's test of publication bias for the association between sarcopenia and OS from univariate analysis (p = 0.344).
Discussion
Our meta-analysis showed that sarcopenia was associated with poor survival, even after adjustment for confounders. Furthermore, the meta-analysis outcomes showed a low rate of CR in patients with sarcopenia after R-CHOP therapy. In addition, patients with sarcopenia tended to fail to complete the treatment plan compared to patients without sarcopenia. These results are consistent with a recently published meta-analysis study, which demonstrated that overall mortality of hematopoietic cancer was significantly associated with sarcopenia. However, only two studies that involved patients with DLBCL were included, and several recently updated researches were not (30).
In our study, the incidence of sarcopenia ranged from 23.9 to 55.6%, which was consistent with the previous study involving United States veterans with DLBCL, which was >30% based on CT diagnosis before chemotherapy (24). The significant differences in the incidence of sarcopenia among patients with DLBCL were due to the lack of uniform diagnostic criteria and cut-off values. Additionally, patients with DLBCL also experience further muscle loss during chemotherapy, with skeletal muscle area decreasing by ~2.8% after treatment (24).
The mechanism of sarcopenia is complex. Aging is a crucial risk factor for sarcopenia. The aging process breaks the balance between muscle protein synthesis and catabolism, eventually leading to gradual loss of skeletal muscle loss (31). The mechanism of aging related sarcopenia also involves negative protein turnover characterized by reduction of myofibrillar and mitochondrial protein synthesis and increased proteolysis via the ubiquitin proteasome and calcium-dependent activation of proteases (32, 33). The decreased number of type II fiber satellite cells and the intramuscular and intermuscular fat infiltration (myosteatosis) caused by aging contribute to sarcopenia at the cellular level (31, 34). Systemic inflammation is also crucial for the pathogenesis of muscle loss in later life. The chronic pro-inflammatory state caused by increased production of pro-inflammatory cytokines is a possible underlying cause of muscle loss (35). The high incidence of sarcopenia in patients with DLBCL can be explained by the average age of the study cohort included in this meta-analysis, which ranged from 60 to 70 years. These patients are susceptible to sarcopenia. Moreover, various pro-inflammatory cytokines, including prostaglandins, interleukin-6, interleukin-1, tumor necrosis factor, interferon gamma, and leukemia inhibitory factor, secreted by the tumors also elicit catabolism in the skeletal muscles and accelerate muscle loss (36, 37). In addition, the abnormal metabolism of proteins and amino acids caused by the tumor exacerbates muscle loss. Malnutrition due to the tumor and anti-tumor therapy also affects muscle mass in patients with DLBCL (38). Furthermore, physical activity is also greatly limited in patients with cancer.
Our findings suggested an overall negative effect of sarcopenia in the rate of CR. However, this result was inconsistent with the previous studies. In the study by Rier, when low muscle mass (LMM) was used as the diagnostic criteria for sarcopenia, there was no difference in the rate of CR between the sarcopenia and non-sarcopenia groups (28). Another study showed that there was no difference in the rate of CR between the groups in patients whose chemotherapy treatment was uninterrupted (29). Upon reviewing the literature, we found the decreased clearance of the anti-tumor drugs in the tissues of patients with muscle reduction may improve the therapeutic effect while accompanying side effects, including increased toxicity (39). The study by Guo confirmed that grade 3–4 toxicity during or after immunochemotherapy was associated with poor body composition. For every 5 cm2/m2 decrease in SMI, the risk of any grade 3–4 toxicity increased by 34% (21). Another study also confirmed that the sarcopenia group had more frequent grade 3 anemia, grade 3–4 and 4 thrombocytopenia, and grade 4–5 non-hematologic toxicity (21). Increased toxicity may lead to early discontinuation of treatment, which may be a reason for the reduction in the rate of CR. The pooled data from this meta-analysis also indicated that sarcopenia was associated with the inability to complete the standard number of treatment cycles due to toxicity. Moreover, the association between lower SMG, SMD, SMI, and lean body mass (LBM) and the increasing risk of dose delay/reduction was also demonstrated in the study by Guo (21).
Most importantly, the meta-analysis provided convincing evidence that sarcopenia was associated with low OS and PFS. The association between sarcopenia and prognosis was independent of other prognostic factors, such as age, sex, prognostic index, and hypoalbuminemia. The pathogenesis of poor survival in patients with cancer and sarcopenia is unclear. A commonly postulated mechanism is that greater drug toxicity results in decreased treatment tolerance and reduction in effective drug doses (15, 40). Another potential explanation is that decreased levels of insulin-like growth factor-1 (IGF-1) are secreted by the smaller skeletal muscle and that both insulin-like growth factor-1 receptor (IGF-1R) density and signaling are impaired in the aged skeletal muscle (41). Recent studies have suggested that the IGF-1/IGF-1R signaling pathway may contribute to the progression of DLBCL and other cancers (42–44). In addition, muscle loss is a manifestation of malnutrition. Malnutrition encountered in patients with DLBCL also leads to increased incidence of treatment-related toxicity, which influences the occurrence of poor survival outcomes (38). Sarcopenia is also a hallmark of cancer cachexia. Poor OS and PFS may be a consequence of cancer cachexia rather than sarcopenia. Patients with cancer cachexia tend to have progressive functional impairment and worse clinical outcomes due to the profound systemic inflammation associated with cancer.
Although sarcopenia was associated with poor survival in patients with DLBCL, the prognostic value of low body mass index (BMI) in the included studies remains controversial. Two studies showed that low BMI was associated with poor OS and PFS (19, 22). However, in the four studies, underweight was not significantly associated with poor survival (20, 26, 27, 29). In Besutti's study, no significant difference was observed between different the BMI groups (<25, 25–30, ≥30) in terms of OS and PFS. However, sarcopenia in patients with obesity have the worst survival after further stratifying patients into sarcopenia and obesity group (18). As a result, the prognostic value of sarcopenia in DLBCL cancer patients with obesity, normal weight, and underweight should be further studied.
Our study has some limitations. All included trials were retrospective studies. There was considerable heterogeneity in the meta-analysis with respect to patient cohorts as a result of using different diagnostic criteria for sarcopenia. Although the muscle mass in all included studies was measured on CT, the scan level of CT used in each study was different, and the cut-off values varied. In addition, the various treatment strategies used in the studies were also likely to contribute to heterogeneity. Another limitation of our meta-analysis is the different definitions of OS and PFS in the studies. Further, the lack of measurement of relevant indicators of muscle function is a limitation. The included studies were retrospective and evaluated muscle mass only on CT, but not by muscle function. The definition of sarcopenia includes not only the reduction of muscle mass but also the degeneration of muscle function. Moreover, gene mutations also play a key role in determining the prognosis of patients with DLBCL. However, none of the included articles investigated the effect of sarcopenia on prognosis in patients with DLBCL with different gene mutations. Therefore, further studies using rigorous design are warranted to verify the strength of prognostic role of sarcopenia in patients with DLBCL.
Conclusion
The prevalence of sarcopenia is higher in patients with DLBCL than in the general population. Loss of skeletal mass is associated with poor survival in patients with DLBCL. Sarcopenia also negatively affects the rate of treatment completion and CR to immunochemotherapy. Identification of consensus diagnostic criteria for sarcopenia and design of prospective studies that incorporate measurements of muscle strength and physical function are the areas for further research.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author Contributions
XJ: conceptualization, methodology, software, investigation, and writing—original draft. X-TX: methodology, software, and investigation. M-XT: resources, data curation, and investigation. D-LH and H-JW: writing—review and editing. All authors revised and approved the final manuscript.
Funding
This study was supported by the Provincial Natural Science Foundation of Hunan (2021JJ30066).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We would like to thank Jing Li, Yu Qian, Wen-Zhen Jiang, and Ren-Chong Hu for their help.
References
1. Shankland KR, Armitage JO, Hancock BW. Non-hodgkin lymphoma. Lancet. (2012) 380:848–57. doi: 10.1016/S0140-6736(12)60605-9
2. Teras LR, DeSantis CE, Cerhan JR, Morton LM, Jemal A, Flowers CR. 2016 US lymphoid malignancy statistics by world health organization subtypes. CA Cancer J Clin. (2016) 66:443–59. doi: 10.3322/caac.21357
3. McGuire S. World cancer report 2014. Geneva, Switzerland: world health organization, international agency for research on cancer, WHO press, 2015. Adv Nutr. (2016) 7:418–9. doi: 10.3945/an.116.012211
4. Chiappella A, Castellino A, Nicolosi M, Santambrogio E, Vitolo U. Diffuse large B-cell lymphoma in the elderly: standard treatment and new perspectives. Expert Rev Hematol. (2017) 10:289–97. doi: 10.1080/17474086.2017.1305264
5. Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyere O, Cederholm T, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. (2019) 48:16–31. doi: 10.1093/ageing/afy169
6. Peterson SJ, Mozer M. Differentiating sarcopenia and cachexia among patients with cancer. Nutr Clin Pract. (2017) 32:30–9. doi: 10.1177/0884533616680354
7. Feliciano EMC, Kroenke CH, Meyerhardt JA, Prado CM, Bradshaw PT, Kwan ML, et al. Association of systemic inflammation and sarcopenia with survival in nonmetastatic colorectal cancer: results from the C SCANS study. JAMA Oncol. (2017) 3:e172319. doi: 10.1001/jamaoncol.2017.2319
8. Blauwhoff-Buskermolen S, Versteeg KS., de van der Schueren MA, den Braver NR, Berkhof J, Langius JA, et al. Loss of muscle mass during chemotherapy is predictive for poor survival of patients with metastatic colorectal cancer. J Clin Oncol. (2016) 34:1339–44. doi: 10.1200/JCO.2015.63.6043
9. Wheelwright S, Darlington AS, Hopkinson JB, Fitzsimmons D, White A, Johnson CD, et al. Systematic review of health-related quality of life instruments in patients with cancer cachexia. Support Care Cancer. (2013) 21:2625–36. doi: 10.1007/s00520-013-1881-9
10. Ryan AM, Prado CM, Sullivan ES, Power DG, Daly LE. Effects of weight loss and sarcopenia on response to chemotherapy, quality of life, and survival. Nutrition. (2019) 67–68:110539. doi: 10.1016/j.nut.2019.06.020
11. Yang M, Shen Y, Tan L, Li W. Prognostic value of sarcopenia in lung cancer: a systematic review and meta-analysis. Chest. (2019) 156:101–11. doi: 10.1016/j.chest.2019.04.115
12. Ubachs J, Ziemons J, Minis-Rutten IJG, Kruitwagen R, Kleijnen J, Lambrechts S, et al. Sarcopenia and ovarian cancer survival: a systematic review and meta-analysis. J Cachexia Sarcopenia Muscle. (2019) 10:1165–74. doi: 10.1002/jcsm.12468
13. Yang Z, Zhou X, Ma B, Xing Y, Jiang X, Wang Z. Predictive value of preoperative sarcopenia in patients with gastric cancer: a meta-analysis and systematic review. J Gastrointest Surg. (2018) 22:1890–902. doi: 10.1007/s11605-018-3856-0
14. Chang KV, Chen JD, Wu WT, Huang KC, Hsu CT, Han DS. Association between loss of skeletal muscle mass and mortality and tumor recurrence in hepatocellular carcinoma: a systematic review and meta-analysis. Liver Cancer. (2018) 7:90–103. doi: 10.1159/000484950
15. Wong A, Zhu D, Kraus D, Tham T. Radiologically defined sarcopenia affects survival in head and neck cancer: a meta-analysis. Laryngoscope. (2021) 131:333–41. doi: 10.1002/lary.28616
16. Surov A, Wienke A. Sarcopenia predicts overall survival in patients with malignant hematological diseases: a meta-analysis. Clin Nutr. (2021) 40:1155–60. doi: 10.1016/j.clnu.2020.07.023
17. Foroutan F, Guyatt GH, O'Brien K, Bain E, Stein M, Bhagra S, et al. Prognosis after surgical replacement with a bioprosthetic aortic valve in patients with severe symptomatic aortic stenosis: systematic review of observational studies. BMJ. (2016) 354:i5065. doi: 10.1136/bmj.i5065
18. Besutti G, Massaro F, Bonelli E, Braglia L, Casali M, Versari A, et al. Prognostic impact of muscle quantity and quality and fat distribution in diffuse large B-cell lymphoma patients. Front Nutr. (2021) 8:620696. doi: 10.3389/fnut.2021.620696
19. Camus V, Lanic H, Kraut J, Modzelewski R, Clatot F, Picquenot JM, et al. Prognostic impact of fat tissue loss and cachexia assessed by computed tomography scan in elderly patients with diffuse large B-cell lymphoma treated with immunochemotherapy. Eur J Haematol. (2014) 93:9–18. doi: 10.1111/ejh.12285
20. Go SI, Park MJ, Song HN, Kim HG, Kang MH, Kang JH, et al. A comparison of pectoralis versus lumbar skeletal muscle indices for defining sarcopenia in diffuse large B-cell lymphoma - two are better than one. Oncotarget. (2017) 8:47007–19. doi: 10.18632/oncotarget.16552
21. Guo J, Cai P, Li P, Cao C, Zhou J, Dong L, et al. Body composition as a predictor of toxicity and prognosis in patients with diffuse large B-cell lymphoma receiving R-CHOP immunochemotherapy. Curr Oncol. (2021) 28:1325–37. doi: 10.3390/curroncol28020126
22. Lanic H, Kraut-Tauzia J, Modzelewski R, Clatot F, Mareschal S, Picquenot JM, et al. Sarcopenia is an independent prognostic factor in elderly patients with diffuse large B-cell lymphoma treated with immunochemotherapy. Leuk Lymphoma. (2014) 55:817–23. doi: 10.3109/10428194.2013.816421
23. Nakamura N, Hara T, Shibata Y, Matsumoto T, Nakamura H, Ninomiya S, et al. Sarcopenia is an independent prognostic factor in male patients with diffuse large B-cell lymphoma. Ann Hematol. (2015) 94:2043–53. doi: 10.1007/s00277-015-2499-4
24. Xiao DY, Luo S, O'Brian K, Ganti A, Riedell P, Sanfilippo KM, et al. Impact of sarcopenia on treatment tolerance in United States veterans with diffuse large B-cell lymphoma treated with CHOP-based chemotherapy. Am J Hematol. (2016) 91:1002–7. doi: 10.1002/ajh.24465
25. Chu MP, Lieffers J, Ghosh S, Belch A, Chua NS, Fontaine A, et al. Skeletal muscle density is an independent predictor of diffuse large B-cell lymphoma outcomes treated with rituximab-based chemoimmunotherapy. J Cachexia Sarcopenia Muscle. (2017) 8:298–304. doi: 10.1002/jcsm.12161
26. Iltar U, Sozel H, Sozel YK, Atas U, Yucel OK, Salim O, et al. Prognostic impact of the psoas muscle index, a parameter of sarcopenia, in patients with diffuse large B-cell lymphoma treated with rituximab-based chemoimmunotherapy. Leuk Lymphoma. (2021) 62:1098–106. doi: 10.1080/10428194.2020.1856833
27. Go SI, Park MJ, Song HN, Kim HG, Kang MH, Lee HR, et al. Prognostic impact of sarcopenia in patients with diffuse large B-cell lymphoma treated with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone. J Cachexia Sarcopenia Muscle. (2016) 7:567–76. doi: 10.1002/jcsm.12115
28. Rier HN, Kharagjitsing H, van Rosmalen J, van Vugt J, Westerweel PE, de Jongh E, et al. Prognostic impact of low muscle mass and low muscle density in patients with diffuse large B-cell lymphoma. Leuk Lymphoma. (2020) 61:1618–26. doi: 10.1080/10428194.2020.1737686
29. Go SI, Kim HG, Kang MH, Park S, Lee GW. Prognostic model based on the geriatric nutritional risk index and sarcopenia in patients with diffuse large B-cell lymphoma. BMC Cancer. (2020) 20:439. doi: 10.1186/s12885-020-06921-2
30. Au PC Li HL, Lee GK Li GH, Chan M, Cheung BM, et al. Sarcopenia and mortality in cancer: a meta-analysis. Osteoporos Sarcopenia. (2021) 7:S28–33. doi: 10.1016/j.afos.2021.03.002
31. Cruz-Jentoft AJ, Sayer AA. Sarcopenia. Lancet. (2019) 393:2636–46. doi: 10.1016/S0140-6736(19)31138-9
32. Brioche T, Pagano AF, Py G, Chopard A. Muscle wasting and aging: experimental models, fatty infiltrations, and prevention. Mol Aspects Med. (2016) 50:56–87. doi: 10.1016/j.mam.2016.04.006
33. Dennison EM, Sayer AA, Cooper C. Epidemiology of sarcopenia and insight into possible therapeutic targets. Nat Rev Rheumatol. (2017) 13:340–7. doi: 10.1038/nrrheum.2017.60
34. Ciciliot S, Rossi AC, Dyar KA, Blaauw B, Schiaffino S. Muscle type and fiber type specificity in muscle wasting. Int J Biochem Cell Biol. (2013) 45:2191–9. doi: 10.1016/j.biocel.2013.05.016
35. De Martinis M, Franceschi C, Monti D, Ginaldi L. Inflamm-ageing and lifelong antigenic load as major determinants of ageing rate and longevity. FEBS Lett. (2005) 579:2035–9. doi: 10.1016/j.febslet.2005.02.055
36. Fearon KC, Glass DJ, Guttridge DC. Cancer cachexia: mediators, signaling, and metabolic pathways. Cell Metab. (2012) 16:153–66. doi: 10.1016/j.cmet.2012.06.011
37. Baracos VE, Martin L, Korc M, Guttridge DC, Fearon KCH. Cancer-associated cachexia. Nat Rev Dis Primers. (2018) 4:17105. doi: 10.1038/nrdp.2017.105
38. Park S, Han B, Cho JW, Woo SY, Kim S, Kim SJ, et al. Effect of nutritional status on survival outcome of diffuse large B-cell lymphoma patients treated with rituximab-CHOP. Nutr Cancer. (2014) 66:225–33. doi: 10.1080/01635581.2014.867065
39. Prado CM, Lima IS, Baracos VE, Bies RR, McCargar LJ, Reiman T, et al. An exploratory study of body composition as a determinant of epirubicin pharmacokinetics and toxicity. Cancer Chemother Pharmacol. (2011) 67:93–101. doi: 10.1007/s00280-010-1288-y
40. Prado CM, Baracos VE, McCargar LJ, Reiman T, Mourtzakis M, Tonkin K, et al. Sarcopenia as a determinant of chemotherapy toxicity and time to tumor progression in metastatic breast cancer patients receiving capecitabine treatment. Clin Cancer Res. (2009) 15:2920–6. doi: 10.1158/1078-0432.CCR-08-2242
41. Hameed M, Harridge SD, Goldspink G. Sarcopenia and hypertrophy: a role for insulin-like growth factor-1 in aged muscle? Exerc Sport Sci Rev. (2002) 30:15–9. doi: 10.1097/00003677-200201000-00004
42. Rigiracciolo DC, Nohata N, Lappano R, Cirillo F, Talia M, Scordamaglia D, et al. IGF-1/IGF-1R/FAK/YAP transduction signaling prompts growth effects in triple-negative breast cancer (TNBC) cells. Cells. (2020) 9(4). doi: 10.3390/cells9041010
43. Zhou X, Chen N, Xu H, Zhou X, Wang J, Fang X, et al. Regulation of Hippo-YAP signaling by insulin-like growth factor-1 receptor in the tumorigenesis of diffuse large B-cell lymphoma. J Hematol Oncol. (2020) 13:77. doi: 10.1186/s13045-020-00906-1
Keywords: sarcopenia, diffuse large B-cell lymphoma, overall survival, complete response, meta-analysis
Citation: Xu X-T, He D-L, Tian M-X, Wu H-J and Jin X (2022) Prognostic Value of Sarcopenia in Patients With Diffuse Large B-Cell Lymphoma Treated With R-CHOP: A Systematic Review and Meta-Analysis. Front. Nutr. 9:816883. doi: 10.3389/fnut.2022.816883
Received: 17 November 2021; Accepted: 01 February 2022;
Published: 25 February 2022.
Edited by:
Paula Ravasco, Santa Maria Hospital, PortugalReviewed by:
Parmanand Malvi, Yale University, United StatesShufang Xu, Wuhan Central Hospital, China
Liu Xiangliang, Jilin University, China
Copyright © 2022 Xu, He, Tian, Wu and Jin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xin Jin, amlueGlucmRAYWx1bW5pLmh1c3QuZWR1LmNu
†These authors have contributed equally to this work and share first authorship
 Xin-Tian Xu1†
Xin-Tian Xu1† Dong-Liang He
Dong-Liang He Hui-Jing Wu
Hui-Jing Wu Xin Jin
Xin Jin