- 1School of Life Sciences, Henan University, Kaifeng, China
- 2Institute of Nursing and Health, Henan University, Kaifeng, China
- 3Department of Orthopedics, Henan Provincial People's Hospital, Zhengzhou, China
- 4Henan International Joint Laboratory for Nuclear Protein Regulation, Henan University, Kaifeng, China
In nutrition science, malnutrition is a state of imbalance between intake and the needs of the organism, leading to metabolic changes, impaired physiological functions, and weight loss. Regardless of the countless efforts being taken and researched for years, the burden of malnutrition is still alarming and considered a significant agent of mortality across the globe. Around 45% of 12 million children deaths (0–5 years old) annually are due to malnutrition, mostly from developing countries. Malnutrition develops associations with other infections and leads to substantial clinical outcomes, such as mortality, more visits to hospitals, poor quality of life and physical frailty, and socioeconomic issues. Here, in this review, we intend to provide an overview of the current burden, underlying risk factors, and co-existence of malnutrition and other infections, such as cancer. Following the rising concern of the vicious interplay of malnutrition and other medical illnesses, we believed that this narrative review would highlight the need to re-make and re-define the future strategies by giving comprehensive and sustainable programs to alleviate poverty and combat the rampant infectious diseases and those nutrition-related health problems. Furthermore, the study also raises the concern for hospitalized malnourished cancer patients as it is crucially important to knowledge the caregiver healthcare staff for early interventions of providing nutritional support to delay or prevent the onset of malnutrition.
Introduction
For in nutrition sciences, malnutrition is the state of the disproportion of food intake and energy requirement of an organism, which leads to impaired metabolism, body dysfunction, and diminishing of body mass (1, 2). Malnutrition is considered as a multifactorial and diverse ailment of all ages. It has been reported worldwide and has gained substantial significance as a leading health issue of the current time. Statistically, literature revealed that about 12 million children (aged 0–5years) die every year and the major portion of these deaths is malnutrition (3). Findings reveal that malnutrition is the major contributor to disease burden in developing countries where one out of every three preschool-age children were affected by malnutrition (4–6). Sub-Saharan Africa is experiencing the highest burden of malnutrition with the high level of undernutrition and growing burden of overweight/obesity and diet-related non-communicable diseases (7). It is estimated that around 1.9 billion adults are overweight while more than 462 million adults are underweight (8). According to a study supported by Bill and Melinda Gates Foundation, malnutrition is the predominant risk factor for death in children younger than 5 years of age in every state of India (9). The results of prevalence studies demonstrate the worse look of malnutrition in hospitals where 50% of patients were found malnourished at admission and more patients may be malnourished upon the hospital discharge (10, 11). In health-related outcomes, malnutrition affects individual physical growth, mobility, cognitive development, and extended length of hospital stay (12), institutionalization (11), poor quality of life (13), and physical frailty in hospitalized patients (14). Malnutrition develops a vicious association with other diseases, such as diarrhea, measles, cancer, and HIV, that lead to increased malnutrition-related death that further imposes both manpower and economic loss epically in poor countries (15–17).
Presently, malnutrition is considered as a significant agent of mortality across the globe, and it is urgently needed to design the strategies to cope with the challenges about the prevention of malnutrition: common nomenclature, screening methods, accurately reported data, and defined distinct silos to address the malnutrition by bridging the existing gaps (18–21). The current narrative review aimed to summarize the literature and provide up to date on underlying causes, characteristics, and the prevalence of malnutrition. With more emphasis, the review highlights the complex interactions and co-existence of malnutrition and other diseases, which govern a vicious cycle to end life especially in cancer-related malnutrition.
Method and Literature Mining Strategy
Relevant articles were selected against the specific keywords as per outlines of study by using the different search databases, PubMed, Google, Google Scholar, and Research Gate. In addition to the relevancy of the title and abstracts, for strong conceptual and logical understanding, more recent studies were selected for inclusion based on the year of publication, which was between 2016 and 2021. Though, little older publications and some news agencies, government data reports were also cited to strengthen the background of the subject. All selected articles have been cited accordingly.
Malnutrition: Now and Then
Nowadays, malnutrition is considered as a complex problem to solve and has been recognized as a world health crisis: a major risk factor for various medical illnesses. Thanks to the vision “Sustainable Development Goals (SDG) 2030, since 1990, there has been a 50% reduction in under-5 years” children deaths by malnutrition (22). To address the challenge of malnutrition, SDG aimed to reduce the under-5 mortality in all countries to at least as low as 25/1,000 live births by 2030 (23). WHO and United Nations International Children's Emergency Fund (UNICEF) integrated measurements are aimed to reduce the number of stunted children by 40% and wasting to <5% by 2025 (24). Although, we have made large-scale efforts on the lives of malnourished children in developing countries around the world, however, due to the exponentially raised population number, however, due to rising population, complexities in screening, and the association of malnutrition with other infections, current estimates predict that these measures are still insufficient to treat, control, and reduce the burden. More effective prevention and treatment of malnutrition are needed urgently.
Underlying Causes of Malnutrition
Any disease, whether chronic or acute, is a significant risk factor of malnutrition in third world countries and could cause or exaggerate malnutrition in some respects. As aforementioned, malnutrition is considered as a complex problem to solve due to the contribution of several driving factors in its development, so there is no way to list out the factors that may contribute to malnutrition in the population (21). With this complex manifestation across the life courses in different ways, it has been found that the risk factors and co-occurrence of malnutrition are not only related to nutrient intake but also linked with the levels of water sanitation and hygiene and the influence of infections on nutritional status (25–28).
In elderly persons, malnutrition is a serious threat that leads to disability, institutionalization, and more exposure to illnesses. In these patients, the underlying cause of being malnourished is anorexia of aging: an individual's food intake decreases with age (29). Clinically, metabolism-related malnutrition might be a result of reduced assimilation of nutrients and malabsorption, any infection in the digestive system, trauma, or any type of inflammation (30–32). Different studies have revealed that there are several mediators, such as cytokines (interleukin 1, interleukin 6, and tumor necrosis factors alpha), glucocorticoid, and lack of insulin growth factor 1, whose catabolic effects have altered food intake (33–35). Poor dentistry is also the result of a number of factors, especially in geriatric patients, such as dementia and immobilization (36–38). Other factors, such as loss of appetite, altered taste, and nausea, can reduce food intake and thus malnutrition (39, 40). Socioeconomic factors, such as isolation, poverty, and poor living conditions, are involved in the progression of malnutrition (17, 41). Malnutrition commonly occurs in elderly patients and is highly prevalent in patients with malignant/severe chronic liver disease, heart or kidney disease, HIV/AIDS, chronic obstructive pulmonary disease, impaired intestinal flora and cystic fibrosis, respiratory tuberculosis, and other diseases (42–51). Various studies have shown that patients tend to receive less nutritional care due to the inexperience of hospital staff (52). In addition, malnutrition is also associated with loss of appetite, poor diet, physical disability, depression, and loneliness (53, 54). Diseases are one of the major factors of malnutrition development and the risk increases with the severity of the diseases and it makes it exceedingly difficult to analyze the effect of malnutrition on prognosis alone. This can only be done in well-characterized settings, where analysis can be stratified according to the severity of the disease. A diagrammatic description of all direct or indirect factors that mediate in bringing the malnutrition has been shown in Figure 1.
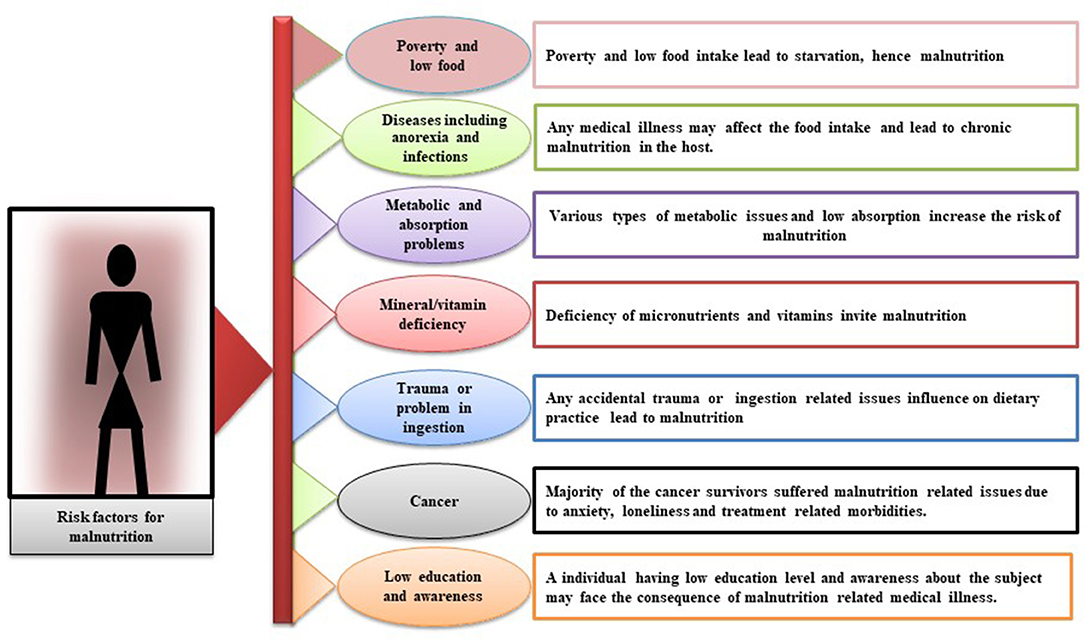
Figure 1. Diagrammatic description of risk factors that contribute directly or indirectly to the development of malnutrition. Leading facts are poverty and low food, diseases, such as anorexia and infections, metabolic and absorption problems, mineral/vitamin deficiency, trauma or problem in ingestion, cancer, and low education and awareness.
Protein Energy Malnutrition and Infection
Protein energy malnutrition is a range of pathological conditions arising out of coincident of lack of protein and energy in varying proportions, most frequently seen in infants, and young children and usually associated with infections (55). It is understandable that the body needs extra energy to activate and replenish the body's immune system in response to an infection. In nutrition sciences, this association of energy expenditure during infection can be expressed in the form of PEM, which provides an outlook to understand the relationship between malnutrition and infection (56). WHO has identified PEM as the most lethal form of malnutrition as it affects the most vulnerable segment of the population: children under the age of 5 (57). Through the years of research on the association of malnutrition and infection, it has been well-established that, in malnourished individuals, the immune capacity of energy expenditure is further impaired, and therefore the host becomes susceptible to infection and determines the outcome of infection (58).
Although, deficiencies in non-nutrients, such as vitamins, amino acids, iron, and even micronutrients, can affect the normal functioning of the body, however, PEM is considered to be associated with delayed recovery from disease, poorer quality of life, and increased risk of morbidity and mortality (59). It makes the body vulnerable to major human infectious diseases, especially in children in low-income countries (22). Overviewing the published data, it has been found that around half of the 10.8 million annual deaths of children under the age of 5 by malnutrition in less developed countries are under the deadly co-existence of PEM and infections (60). Hence, PEM determines the susceptibility of infection in the body and energy of the individual, which further leads to a loss of work efficiency at the community level and catalyzes the alarming spiral of infection, disease, and blatant poverty in society. Figure 2 demonstrates impose-impact of malnutrition on an individuals' life, such as impaired growth, economic loss, hard to recover from other medical illnesses, low immunity, and prone to infections.
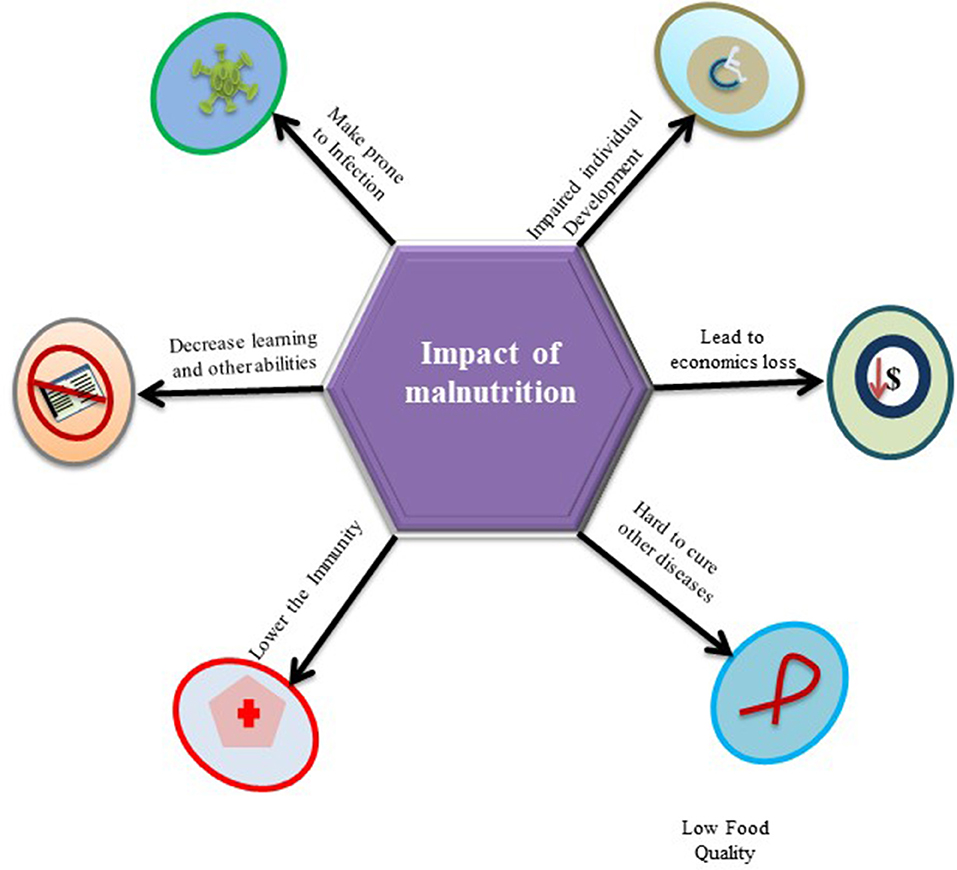
Figure 2. The figurative presentation of impose-impact in the form of low immunity and prone to infections with other related outcomes by malnutrition on an individuals' life.
Malnutrition Increase Risk of Infection
A vicious relationship between malnutrition and infectious diseases has been long established. Malnutrition causes an exponential decrease in the chances of survival per exposure to any fatal infectious disease while non-fatal and subclinical infections may impair the process of growth (61). Published research revealed that malnourished children have a clear excess risk of infectious morbidity and mortality (62). Concurrently, the latest estimates show that about 50% of 10.6 million under 5 child death per year globally has been ascribed to the five infectious diseases (pneumonia, diarrhea, malaria, measles, and AIDS) (25, 63). Malnutrition impaired the integrity of the gastrointestinal mucosa of patients leading to reduced gastric acid secretion and increased susceptibility to some pathogens (64, 65). Besides the direct organ-specific effects of infection (e.g., intestinal loss of nutrients during diarrhea), there are other illnesses, such as metabolic and immune systems, which are the result of this association (66). It has been well-established that HIV infection and malnutrition are inter-linked by the complex interplay of different etiological factors, which contribute to the progression of the disease and increase the risk of mortality. Hence, addressing the nutrition right from the time of HIV diagnosis is a good strategy. This reduction of immune cells, known as nutritionally acquired immunodeficiency syndrome, plays a key role in the body's susceptibility to any infection (67). Severely malnourished patients have lacked a strong immunological response and show weak acquired immunity and innate host defense mechanisms. These patients are more susceptible to infection factors than the patients with HIV/AIDS and their opportunistic pathogens (68). A remarkable example of malnutrition and infection was seen after the Second World War when thousands of patients with AIDS and malnourished children were repeatedly diagnosed with opportunistic fungal pneumonia with other illnesses (69). Similarly, Noma is another deadly opportunistic infection. Noma is a result of complex interactions of different factors, such as poverty, malnutrition, compromised immune system, and poor oral hygiene, typically occurring in sub-Saharan Africa in children under 4 years of age (70). Severe malnutrition, PEM, promotes acute and chronic infections, further reduces food intake, increases metabolic requirements, and reduces anabolic nutrient losses by impairing linear growth in affected children. Additionally, the acute phase of PEM disrupts bone remodeling required for longitudinal growth (71). It has been pointed out that the association between malnutrition and growth retardation helps researchers to assess the nutritional status of individuals. Numerous studies have demonstrated a significant correlation between acute malnutrition and acute infections. It has been well-established by the years of research that one cannot separate infection and its risk factors as determinants of the whole malnutrition burden. These determinants of malnutrition and infections are significantly present in the under-5 years of age children in developing countries and adversely impact children's health and development (25, 72, 73). Overviewing the published literature, it has been concluded that the relationship between malnutrition and infection is considered bidirectional (74). Evidence of the inverse relationship between malnutrition and infection is also found where an infection alters the nutrients intake, absorption, secretion, catabolism, and consumption. Such a vicious cycle of infection and malnutrition appears commonly in nature (25). Figure 3 represents the bidirectional associations between malnutrition and infections.
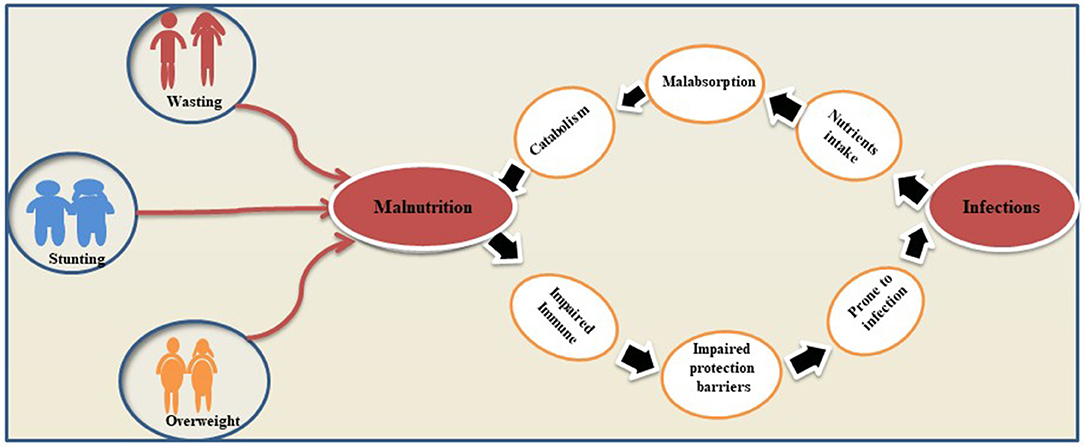
Figure 3. Bidirectional associations between malnutrition and infections. The figure gives an explanation on how malnutrition and infections reinforce each other's and lead to maximum health damage of host.
Malnutrition and Chronic Diseases
It is a need of time to add important nuance to establish facts and understandings about the complex associations of chronic diseases, such as diarrhea, respiratory infection, HIV, malaria, measles, and the most hateful disease cancer, with malnutrition for the specific therapeutic approaches to break these associations and treat the diseases.
The vicious cycle between malnutrition and diarrhea is being explored for decades of years. According to a statistic report, around 1.5 million children die every year of diarrhea (75). It has been understood that, as compared to better nourished children with diarrhea, malnourished children with diarrhea have a far higher risk of death (76, 77). Diarrhea is the most common morbidity in severe acute malnutrition children and needs to be addressed. Effective adherence to the management protocol of dehydration and prompt modification of therapeutic feed can bring satisfactory health outcomes.
Followed by diarrhea, respiratory infections are the leading causes of death in children under the age of 5 years (78). It is plausible to assess the relationship between respiratory infection and malnutrition as poor growth. The low immune response of the host and susceptibility to pathogens lead to mortality and morbidity among the children across the globe (79). Going through the literature, it has been found that the published articles that concluded that malnourished children are very susceptible to several respiratory infections as compared to the others (80–82). Malnutrition especially in children is the risk factor for many bacterial and viral infections. A study on hospitalized children with the severe respiratory syncytial virus (RSV) demonstrates that poor infant growth increases the risk for severe RSV infection and leads to prolonging hospitalized stay (83). Similarly, there are also findings about the frequency of co-morbidities related to measles, pneumonia, tuberculosis, and malaria in the children presenting with severe acute malnutrition (84, 85). In an observational study, it has been known that 22% of 104 severe acute malnourished children were diagnosed with tuberculosis while 3.8% were diagnosed with malaria and measles (86). The research published in the American Journal of Clinical Nutrition, with the objective to find whether undernutrition is an underlying cause of children deaths associated with diarrhea, pneumonia, malaria, and measles, reveals that overall 52.5% of all deaths in young children were attributable to undernutrition varying from 44.8% for death by measles and 60.7% for death because of diarrhea (87).
Due to the desperate consequences of malnutrition, more than 1 million children die each year with the co-occurrence of HIV (88). The comorbidity rate of malnutrition and HIV makes HIV-infected children three times more likely to die than non-infected children (88, 89). Sub-Saharan Africa is the most affected region by the deadly association of malnutrition and HIV where 31.2% of stunting, 7.4% of wastage, and 5.2% of overweight children are reported (90). HIV infection in malnourished individuals is the result of several physiological and socioeconomic factors (91, 92). Muenchhoff confirmed strong associations with other comorbidities, such as diversification of the gut microbiota, epithelial malformations, chronic intestinal inflammation, and immune activation in malnourished HIV-infected children, which further lead to advances in disease pathogenesis (88). According to a recent study, it has been observed that micronutrient deficiency, such as selenium and vitamin C, determines the mycobacterial progression in HIV-positive patients, thus an imbalance of these micronutrients can be considered as an important risk factor (93). Furthermore, it has been found that in HIV-positive cases, metabolic problems, such as high lipids, can lead to increased long-term risk of atherosclerosis and cardiovascular abnormalities in malnourished patients (94). Figure 4 represents the vicious cycle of malnutrition and HIV.
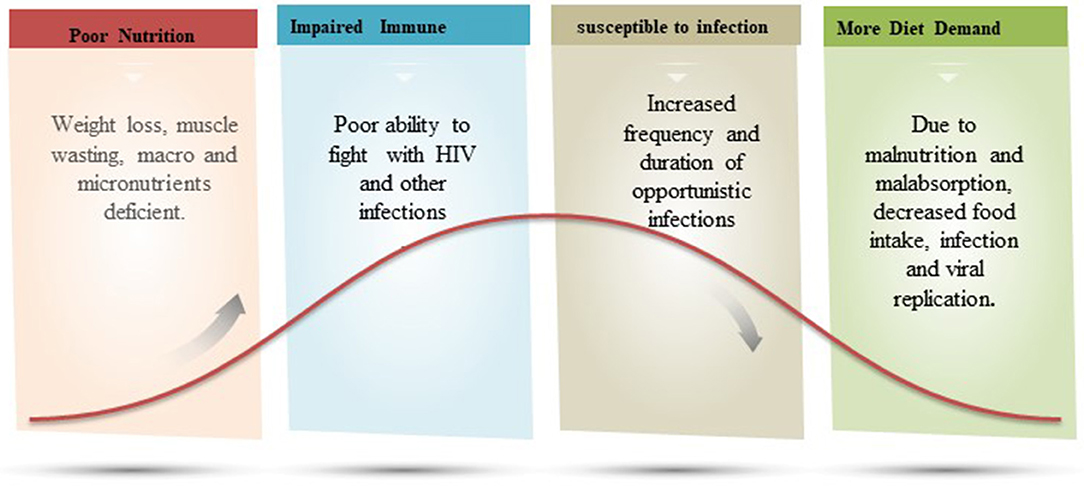
Figure 4. A figurative explanation on the relation of malnutrition and immune fighting ability against HIV infection. In a vicious cycle of malnutrition-HIV, the patient has poor immunity and increased frequency and duration of opportunistic infection.
Despite the immense volume of laboratory and field research, there is still an adequate gap in knowledge about the subject and in defining the relationship between malnutrition and chronic diseases. To deal with this worldwide health crisis, diverse minds of researchers are continuously exploring the deadly associations of malnutrition and chronic diseases for the vision of a “world free of malnutrition.”
Here, we are illuminating the current understanding of the interactions between malnutrition and cancer. As secondary data, this is an objective study to summarizing the literature and providing an update on the association of malnutrition and said disease.
Malnutrition and Cancer
Malnutrition is exceedingly common in cancer patients. Cancer-associated malnutrition is a multifactorial syndrome characterized by persistent skeletal muscle loss due to different combinations of reduced dietary intake and metabolic changes (42). This association might express at the time of diagnosis or develop later and as the treatment and disease progressed, the situation became worse. The nutritional status of cancer patients who underwent treatment plays a vital role in promoting multimodality cancer care (95).
Recently, studies demonstrate that many European hospitals have 30–60% of cancer patients at risk of malnutrition who receive nutrition through other means, such as oral supplements, enteral nutrition, or parenteral nutrition (96, 97). Another European study showed that 40% of cancer-related severe malnutrition was misclassified by doctors, resulting in patients losing nutritional interventions (43). It has been also noted that damage of cancer-related malnutrition went uncontrolled when patients and their relatives underestimate it after the doctor's diagnosis (98–100).
The increasing frequency of malnutrition has become an emerging health problem of cancer survivors as it decreases the efficacy of homeopathy treatments and prolongs hospital stay especially in the citizen of the United States of America (101, 102). According to a follow-up study, it has been obtained that malnourished patients with cancer are at about 2- to 5-fold greater risk of death (103). The development of cancer-related malnutrition is the result of several factors, which are considerably different from simple starvation, such as mental health issues, poor food intake, dysfunction of the gastrointestinal tract, increase in the energy need, low physical activity, and altered metabolism (42, 43, 104). Tumor-derived cytokines also mediate the disruption of metabolism, suppress the appetite, enhance muscle wasting, depression, fatigue, and subsequently, lead to impaired physical activity and anorexia (43, 105). Mentioned conditions may worsen the prognosis and lead to poor quality of life, prolonged hospital stay, tolerance to treatment, and therapeutic efficacy that lead to the end of life (106, 107). Additionally, cancer patients often report poor appetite along with reduced food enjoyment putting them at risk for malnutrition. This reduction in food intake may increase due to the site of the tumor. For example, oral or esophagus cancer may halt the eating and support the malnutrition onset (107, 108). On the other hand, the use of steroid drugs and hormone therapy has been considered as a standard of care for a different advanced type of cancer, however, there are findings that reveal that these steroid or hormone intake may contribute to the development of malnutrition (109, 110). Furthermore, unlikely other disease-related malnutrition, cancer-related malnutrition, are thought to be different from those that lack nutrients in the absence of underlying diseases (i.e., hunger and anorexia nervosa). It has been observed that metabolic changes in cancer patients, such as inflammation, increased catabolic rate, and ineffective cyclic anabolic resistance, achieved through a tumor or cancer treatment may invite malnutrition in the patient (111, 112). Apart from other clinical aspects in cancer-related malnutrition, the lack of adequate knowledge of nutritional intervention for cancer patients in caregivers is also a significant contribution in cancer-related malnutrition loss. A recent study demonstrates that almost half of the hospital oncologists involved in the survey report ascribe the underestimation of nutritional treatment to the insufficient training of healthcare professionals (95). In conclusion, to end up this deadly link of malnutrition and cancer, medical science is an urgent need to introduce advanced, state-of-the-art diagnostics, and care mechanisms and emphasizes the concern institutes to spread and raised the prevention and awareness literature to the public. Figure 5 gives the multi ways associations and outcomes of the relation between malnutrition and cancer.
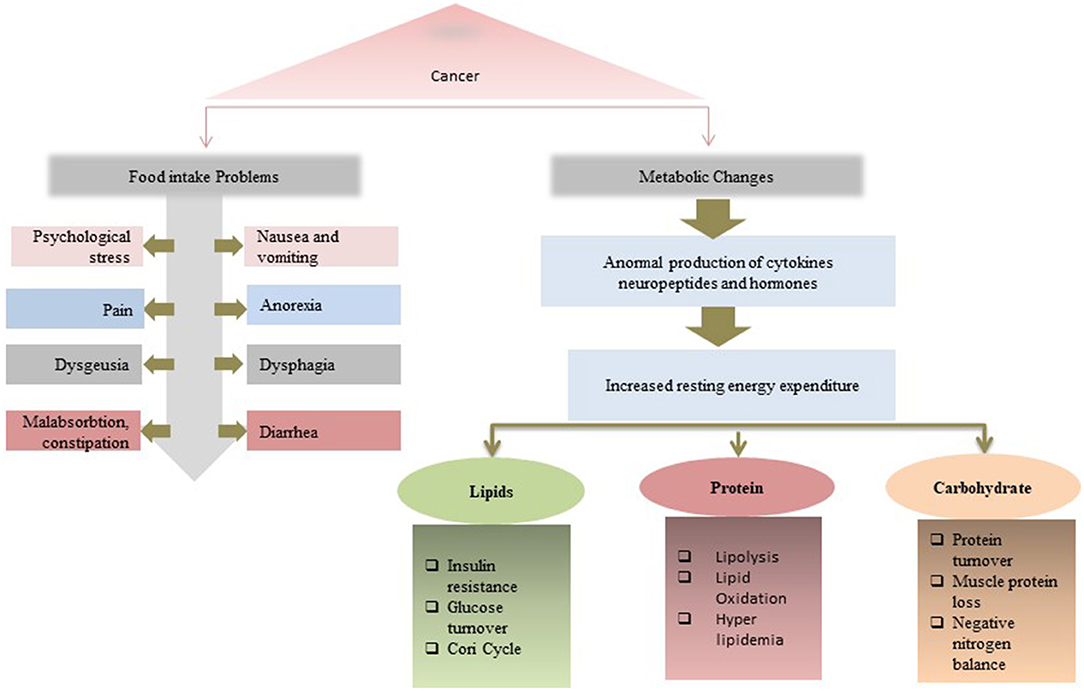
Figure 5. Multi ways associations and outcomes of the relation between malnutrition and cancer. A malnutrition-cancer patient faces issues both in food intake and metabolic changes that lead to the reduction in treatment efficiency.
Prevention and Awareness of Malnutrition: Public Health Nursing
Exceedingly damage by malnutrition-related co-morbidities demands the development of the specific protocol and practical recommendations rather than official meetings for the prevention and awareness about this global health crisis.
Multidisciplinary approaches are needed for the long-term nutritional support and benefits of geriatric malnutrition prevention, detection, and treatment. These approaches must explain acceptable different screening/assessment models and involve healthcare professionals (113). To examine and prevent the PEM especially in growing children from developing countries, there is a critical need of employing electrical health records and other mobiles systems. Adaptation of health information technology is a single advanced way forward of achieving better health outcomes across the globe (114). Malnutrition manifests its worse outcome in hospitalized malnutrition form and needs effective strategies to promote the awareness and overcome the burden. The nutrition committee of the French pediatric society (ePINUT) has endorsed an awareness strategy to lessen the burden and released its recommendations about the prevention and promotion of malnutrition education awareness in patient relatives, healthcare staff, and other caregivers (115). The vicious association of malnutrition cancer imposes significant health and economic loss. Cancer-related malnutrition is a globally rising health concern and appeals to add novel nuance to break this association. According to a current survey report, there is a lack of collaboration between oncologists and clinical nutritionists, which is the first obstacle to overcome. We are in urgent need to start internationally accepted educational intersociety initiatives, aimed to strengthen oncologist knowledge to improve nutritional support management and identify and remove the barriers to delivering optimal nutrition care in cancer survivors (116, 117). To lessen the burden and to the way of the world free of malnutrition, current understandings of knowledge about the subject deduced the following key steps to start a war against malnutrition. (1) Awareness about all forms of malnutrition must be raised: frontline staff who provide care must understand the importance of nutrition to basic patient care and consequences to patients if they neglected; (2) there is a critical need to develop worldwide accepted state of the art screening and assessments methods; (3) malnourished individual or those at risk must be on the right care pathway and received the right treatments; (4) frontline staff in all care settings must receive appropriate training on the importance of good nutritional care to speed up the recovery; and (5) world organizations must development management structure in place to ensure best practice especially in high prevalence parts of the world: Sub-Saharan Africa.
Conclusion
The literature revealed the need for important implications of nutritional intervention drives for elderly and child survival programs for developing countries to ensure the access of children who fight against chronic diseases. In conclusion, to fill the gaps in prioritizing standardized measurements and reporting of malnutrition status worldwide, it is imperative to enhance an in-depth understanding of the development causes and co-existence of malnutrition with other illnesses. To overcome malnutrition, it also demands to re-make and re-define the future by giving comprehensive but achievable and sustainable programs to alleviate poverty and combat the rampant infectious diseases and other nutrition-related health problems. To improve health outcomes especially in hospitalized cancer patients, it is crucially important to knowledge the caregiver healthcare staff for early interventions of supplying nutritional support to delay or prevent the onset of malnutrition.
Author Contributions
YF, YL, TJ, and QY reviewed the literature and prepared the manuscript draft and the figure. EJ and JZ made the final editing and offered his expert suggestions and insights in preparing this work. All authors have read and agreed to the published version of the manuscript.
Funding
This review was supported by the National Natural Science Foundation of China (No. 81900375) and the Henan Provincial Science and Technology Research Project (No. 212102310147).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. da Silva Fink J, Marcadenti A, Rabito EI, Silva FM. The new european society for clinical nutrition and metabolism definition of malnutrition: application for nutrition assessment and prediction of morbimortality in an emergency service. JPEN J Parenter Enteral Nutr. (2018) 42:550–6. doi: 10.1177/0148607117695248
2. Ooi PH, Thompson-Hodgetts S, Pritchard-Wiart L, Gilmour SM, Mager DR. Pediatric sarcopenia: a paradigm in the overall definition of malnutrition in children? JPEN J Parenter Enteral Nutr. (2020) 44:407–18. doi: 10.1002/jpen.1681
3. Shrivastava SR, Shrivastava P. Necessity to urgently respond to the challenge of malnutrition: world health organization. Cukurova Med J. (2019) 44:303–4. doi: 10.17826/cumj.483943
4. Pelletier DL, Frongillo EA. Changes in child survival are strongly associated with changes in malnutrition in developing countries. J Nutr. (2003) 133:107–19. doi: 10.1093/jn/133.1.107
5. Jensen GL, Cederholm T. Global leadership initiative on malnutrition: progress report from aspen clinical nutrition week 2017. JPEN J Parenter Enteral Nutr. (2018) 42:266–7. doi: 10.1177/0148607117707761
6. Clark H, Coll-Seck AM, Banerjee A, Peterson S, Dalglish SL, Ameratunga S, et al. A future for the world's children? a WHO-UNICEF-lancet commission. Lancet. (2020) 395:605–58. doi: 10.1016/S0140-6736(19)32540-1
7. Onyango AW, Jean-Baptiste J, Samburu B, Mahlangu TLM. Regional overview on the double burden of malnutrition and examples of program and policy responses: african region. Ann Nutr Metab. (2019) 75:127–30. doi: 10.1159/000503671
8. World Health Organization. Malnutrition (2021). Available online at: https://www.who.int/news-room/fact-sheets/detail/malnutrition (accessed June 9, 2021).
9. Swaminathan S, Hemalatha R, Pandey A, Kassebaum NJ, Laxmaiah A, Longvah T, et al. The burden of child and maternal malnutrition and trends in its indicators in the states of India: the global burden of disease study 1990–2017. The Lancet Child. (2019) 3:855–70. doi: 10.1016/S2352-4642(19)30273-1
10. Pineda JCC, Garcia AG, Velasco N, Graf JIDP, Adames AM, de la Torre AM. Nutritional assessment of hospitalized patients in Latin America: association with prognostic variables. The ENHOLA study. Nutr Hosp. (2016) 33:275. doi: 10.20960/nh.275
11. Correia MI, Hegazi RA, Diaz-Pizarro Graf JI, Gomez-Morales G, Fuentes Gutiérrez C, Goldin MF, et al. Addressing disease-related malnutrition in healthcare: a Latin American perspective. JPEN J Parenter Enteral Nutr. (2016) 40:319–25. doi: 10.1177/0148607115581373
12. Bwakura-Dangarembizi M, Amadi B, Bourke CD, Robertson RC, Mwapenya B, Chandwe K, et al. Health outcomes, pathogenesis and epidemiology of severe acute malnutrition (HOPE-SAM): rationale and methods of a longitudinal observational study. BMJ Open. (2019) 9:e023077. doi: 10.1136/bmjopen-2018-023077
13. Rasheed S, Woods RT. Malnutrition and quality of life in older people: a systematic review and meta-analysis. Ageing Res Rev. (2013) 12:561–6. doi: 10.1016/j.arr.2012.11.003
14. Lorenzo-Lopez L, Maseda A, de Labra C, Regueiro-Folgueira L, Rodriguez-Villamil JL, Millan-Calenti JC. Nutritional determinants of frailty in older adults: a systematic review. BMC Geriatr. (2017) 17:1–13. doi: 10.1186/s12877-017-0496-2
15. Bagriansky J, Champa N, Pak K, Whitney S, Laillou A. The economic consequences of malnutrition in Cambodia, more than 400 million US dollar lost annually. Asia Pac J Clin Nutr. (2014) 23:524–31. doi: 10.6133/apjcn.2014.23.4.08
16. Besora-Moreno M, Llaurado E, Tarro L, Sola R. Social and economic factors and malnutrition or the risk of malnutrition in the elderly: a systematic review and meta-analysis of observational studies. Nutrients. (2020) 12:737. doi: 10.3390/nu12030737
17. Adebisi YA, Ibrahim K, Lucero-Prisno DE, Ekpenyong A, Micheal AI, Chinemelum IG, et al. Prevalence and socio-economic impacts of malnutrition among children in Uganda. Nutr Metab Insights. (2019) 12. doi: 10.1177/1178638819887398
18. Elia M. Defining, recognizing, and reporting malnutrition. Int J Low Extrem Wounds. (2017) 16:230–7. doi: 10.1177/1534734617733902
19. Harris PS, Payne L, Morrison L, Green SM, Ghio D, Hallett C, et al. Barriers and facilitators to screening and treating malnutrition in older adults living in the community: a mixed-methods synthesis. BMC Fam Pract. (2019) 20:100. doi: 10.1186/s12875-019-0983-y
20. Craven DL, Lovell GP, Pelly FE, Isenring E. Community-living older adults' perceptions of body weight, signs of malnutrition and sources of information: a descriptive analysis of survey data. J Nutr Health Aging. (2018) 22:393–9. doi: 10.1007/s12603-017-0942-z
21. Wells JC, Sawaya AL, Wibaek R, Mwangome M, Poullas MS, Yajnik CS, et al. The double burden of malnutrition: aetiological pathways and consequences for health. Lancet. (2020) 395:75–88. doi: 10.1016/S0140-6736(19)32472-9
22. Adepoju AA, Allen S. Malnutrition in developing countries: nutrition disorders, a leading cause of ill health in the world today. J Paediatr Child Health. (2019) 29:394–400. doi: 10.1016/j.paed.2019.06.005
23. Sabbahi M, Li J, Davis C, Downs SM. The role of the sustainable development goals to reduce the global burden of malnutrition. Advances in food security and sustainability. Elsevier. (2018) 3:277–333. doi: 10.1016/bs.af2s.2018.09.007
24. World Health Organization. Levels and trends in child malnutrition: UNICEF/WHO/The World Bank Group joint child malnutrition estimates: key findings of the 2020 edition. (2020). Available online at: https://www.who.int/publications/i/item/jme-2020-edition (accessed March 31, 2020).
25. De Vita MV, Scolfaro C, Santini B, Lezo A, Gobbi F, Buonfrate D, et al. Malnutrition, morbidity and infection in the informal settlements of Nairobi, Kenya: an epidemiological study. Ital J Pediatr. (2019) 45:12. doi: 10.1186/s13052-019-0607-0
26. Wang XF, Gao P, Liu YF, Li HF, Lu F. Predicting thermophilic proteins by machine learning. Curr Bioinform. (2020) 15:493–502. doi: 10.2174/1574893615666200207094357
27. Niu MT, Lin Y, Zou Q. sgRNACNN: identifying sgRNA on-target activity in four crops using ensembles of convolutional neural networks. Plant Mol Biol. (2021) 105:483–95. doi: 10.1007/s11103-020-01102-y
28. Sun SW, Xu L, Zou Q, Wang GH. BP4RNAseq: a babysitter package for retrospective and newly generated RNA-seq data analyses using both alignment-based and alignment-free quantification method. Bioinformatics. (2021) 37:1319–21. doi: 10.1093/bioinformatics/btaa832
29. Di Francesco V, Pellizzari L, Corrà L, Fontana G. The anorexia of aging: impact on health and quality of life. Geriatr Care. (2018) 4:7324. doi: 10.4081/gc.2018.7324
30. Wu T-T. Malabsorption and malnutrition disorders. In: Surgical Pathology of Non-Neoplastic Gastrointestinal Diseases, eds Zhang L, Chandan VS, Wu T-T, (Cham: Springer Nature Switzerland AG) (2019). p. 191–238.
31. Blanar V, Hödl M, Lohrmann C, Amir Y, Eglseer D. Dysphagia and factors associated with malnutrition risk: a 5-year multicentre study. J Adv Nurs. (2019) 75:3566–76. doi: 10.1111/jan.14188
32. Dijkink S, Meier K, Krijnen P, Yeh DD, Velmahos GC, Schipper IB. Malnutrition and its effects in severely injured trauma patients. Eur J Trauma Emerg Surg. (2020) 46:993–1004. doi: 10.1007/s00068-020-01304-5
33. Roberts BL, Zhu M, Zhao H, Dillon C, Appleyard SM. High glucose increases action potential firing of catecholamine neurons in the nucleus of the solitary tract by increasing spontaneous glutamate inputs. Am J Physiol Regul Integr Comp Physiol. (2017) 313:R229–39. doi: 10.1152/ajpregu.00413.2016
34. Rachakonda V, Borhani AA, Dunn MA, Andrzejewski M, Martin K, Behari J. Serum leptin is a biomarker of malnutrition in decompensated cirrhosis. PLoS ONE. (2016) 11:e0159142. doi: 10.1371/journal.pone.0159142
35. DeBoer MD, Scharf RJ, Leite AM, Ferrer A, Havt A, Pinkerton R, et al. Systemic inflammation, growth factors, and linear growth in the setting of infection and malnutrition. Nutrition. (2017) 33:248–53. doi: 10.1016/j.nut.2016.06.013
36. Jacobsen EL, Brovold T, Bergland A, Bye A. Prevalence of factors associated with malnutrition among acute geriatric patients in Norway: a cross-sectional study. BMJ Open. (2016) 6:e011512. doi: 10.1136/bmjopen-2016-011512
37. Soysal P, Dokuzlar O, Erken N, Gunay FSD, Isik AT. The relationship between dementia subtypes and nutritional parameters in older adults. J Am Med Dir Assoc. (2020) 21:1430–5. doi: 10.1016/j.jamda.2020.06.051
38. Pourhassan M, Rommersbach N, Lueg G, Klimek C, Schnatmann M, Liermann D, et al. The impact of malnutrition on acute muscle wasting in frail older hospitalized patients. Nutrients. (2020) 12:1387. doi: 10.3390/nu12051387
39. Lau S, Pek K, Chew J, Lim JP, Ismail NH, Ding YY, et al. The simplified nutritional appetite questionnaire (SNAQ) as a screening tool for risk of malnutrition: optimal cutoff, factor structure, and validation in healthy community-dwelling older adults. Nutrients. (2020) 12:2885. doi: 10.3390/nu12092885
40. Chen CC, Schilling LS, Lyder CH. A concept analysis of malnutrition in the elderly. J Adv Nurs. (2001) 36:131–42. doi: 10.1046/j.1365-2648.2001.01950.x
41. Islam MR, Rahman MS, Rahman MM, Nomura S, de Silva A, Lanerolle P, et al. Reducing childhood malnutrition in Bangladesh: the importance of addressing socio-economic inequalities. Public Health Nutr. (2020) 23:72–82. doi: 10.1017/S136898001900140X
42. Baracos VE. Cancer-associated malnutrition. Eur J Clin Nutr. (2018) 72:1255–9. doi: 10.1038/s41430-018-0245-4
43. Arends J, Baracos V, Bertz H, Bozzetti F, Calder PC, Deutz NEP, et al. ESPEN expert group recommendations for action against cancer-related malnutrition. Clin Nutr. (2017) 36:1187–96. doi: 10.1016/j.clnu.2017.06.017
44. Widodo AD, Soelaeman EJ, Dwinanda N, Narendraswari PP, Purnomo B. Chronic liver disease is a risk factor for malnutrition and growth retardation in children. Asia Pac J Clin Nutr. (2017) 26:S57–60. doi: 10.6133/apjcn.062017.s10
45. Tabib A, Aryafar M, Ghadrdoost B. Prevalence of malnutrition in children with congenital heart disease. J Compr Pediatr. (2019) 10:e84274. doi: 10.5812/compreped.84274
46. Noce A, Vidiri M, Marrone G, Moriconi E, Bocedi A, Capria A, et al. Is low-protein diet a possible risk factor of malnutrition in chronic kidney disease patients? Cell Death Discov. (2016) 2:16026. doi: 10.1038/cddiscovery.2016.26
47. Mukhopadhyay DK, Biswas R, Chakraborty M, Sadhukhan SK, and Banik KK. Anthropometric failure, a new approach to measure undernutrition: an experience from a rural community of West Bengal, India. J Indian Med Assoc. (2009) 107:211–4.
48. Sehgal IS, Dhooria S, Agarwal R. Chronic obstructive pulmonary disease and malnutrition in developing countries. Curr Opin Pulm Med. (2017) 23:139–48. doi: 10.1097/MCP.0000000000000356
49. Attia S, Feenstra M, Swain N, Cuesta M, Bandsma RHJ. Starved guts: morphologic and functional intestinal changes in malnutrition. J Pediatr Gastroenterol Nutr. (2017) 65:491–5. doi: 10.1097/MPG.0000000000001629
50. Slae M, Wilschanski M. Prevention of malnutrition in cystic fibrosis. Curr Opin Pulm Med. (2019) 25:674–9. doi: 10.1097/MCP.0000000000000629
51. Tellez-Navarrete NA, Ramon-Luing LA, Munoz-Torrico M, Osuna-Padilla IA, Chavez-Galan L. Malnutrition and tuberculosis: the gap between basic research and clinical trials. J Infect Dev Countr. (2021) 15:310–9. doi: 10.3855/jidc.12821
52. Eglseer D, Halfens RJG, Lohrmann C. Use of an electronic malnutrition screening tool in a hospital setting: effects on knowledge, attitudes and perceived practices of healthcare staff. Brit J Nutr. (2018) 120:150–7. doi: 10.1017/S0007114518001447
53. Schorr AV, Yehuda I, Tamir S. Ethnic differences in loneliness, depression, and malnutrition among older adults during COVID-19 quarantine. J Nutr Health Aging. (2021) 25:311–7. doi: 10.1007/s12603-020-1540-z
54. Ramic E, Pranjic N, Batic-Mujanovic O, Karic E, Alibasic E, Alic A. The effect of loneliness on malnutrition in elderly population. Med Arh. (2011) 65:92–5. Available online at: https://www.ncbi.nlm.nih.gov/pubmed/21585182
55. Jee KO. Protein energy malnutrition: an overview. International Journal of Homoeopathic Sciences. (2021) 5:368–73. doi: 10.33545/26164485.2021.v5.i1f.341
56. Batool R, Butt MS, Sultan MT, Saeed F, Naz R. Protein–energy malnutrition: a risk factor for various ailments. Crit Rev Food Sci Nutr. (2015) 55:242–53. doi: 10.1080/10408398.2011.651543
57. Satapathy A, Satapathy A, Rout DS, Prusty AK, Rout S. Prevalence of protein energy malnutrition among under-five children in Odisha: a review. J Phytopharm. (2021) 10:272–6. doi: 10.31254/phyto.2021.10410
58. Bartelt LA, Bolick DT, Mayneris-Perxachs J, Kolling GL, Medlock GL, Zaenker EI, et al. Cross-modulation of pathogen-specific pathways enhances malnutrition during enteric co-infection with Giardia lamblia and enteroaggregative Escherichia coli. PLoS Pathog. (2017) 13:e1006471. doi: 10.1371/journal.ppat.1006471
59. Cederholm T, Barazzoni R, Austin P, Ballmer P, Biolo G, Bischoff SC, et al. ESPEN guidelines on definitions and terminology of clinical nutrition. Clin Nutr. (2017) 36:49–64. doi: 10.1016/j.clnu.2016.09.004
60. Echendu ST, Okeke KN, Ebenebe JC, Ugochukwu EF, Onubogu CU, Umeadi EN, et al. Prevalence of protein energy malnutrition in HIV-infected under five children and the effects of highly active antiretroviral therapy on their nutritional status in Nigeria. MSARR. (2021) 1:53–61. doi: 10.30574/msarr.2021.1.3.0024
61. Jones KD, Thitiri J, Ngari M, Berkley JA. Childhood malnutrition: toward an understanding of infections, inflammation, and antimicrobials. Food Nutr Bull. (2014) 35(2 Suppl):S64–70. doi: 10.1177/15648265140352S110
62. Prendergast AJ. Malnutrition and vaccination in developing countries. Philos Trans R Soc Lond B Biol Sci. (2015) 370:20140141. doi: 10.1098/rstb.2014.0141
63. UNICEF. The State of the World's Children. (2019). Available online at: https://www.unicef.org/reports/state-of-worlds-children-2019 (accessed October 31, 2019).
64. Martinsen TC, Bergh K, Waldum HL. Gastric juice: a barrier against infectious diseases. Basic Clin Pharmacol Toxicol. (2005) 96:94–102. doi: 10.1111/j.1742-7843.2005.pto960202.x
65. Peterson LW, Artis D. Intestinal epithelial cells: regulators of barrier function and immune homeostasis. Nat Rev Immunol. (2014) 14:141–53. doi: 10.1038/nri3608
66. Benson MJ, Pino-Lagos K, Rosemblatt M, Noelle RJ. All-trans retinoic acid mediates enhanced T reg cell growth, differentiation, and gut homing in the face of high levels of co-stimulation. J Exp Med. (2007) 204:1765–74. doi: 10.1084/jem.20070719
67. Bourke CD, Berkley JA, Prendergast AJ. Immune dysfunction as a cause and consequence of malnutrition. Trends Immunol. (2016) 37:386–98. doi: 10.1016/j.it.2016.04.003
68. Weger-Lucarelli J, Auerswald H, Vignuzzi M, Dussart P, Karlsson EA. Taking a bite out of nutrition and arbovirus infection. PLoS Negl Trop Dis. (2018) 12:e0006247. doi: 10.1371/journal.pntd.0006247
69. Evans HM, Bryant GL, Garvya BA. The life cycle stages of pneumocystis murina have opposing effects on the immune response to this opportunistic fungal pathogen. Infect Immun. (2016) 84:3195–205. doi: 10.1128/IAI.00519-16
70. Brattström-Stolt L, Funk T, Sié A, Ndiaye C, Alfvén T. Noma—knowledge and practice competence among primary healthcare workers: a cross-sectional study in Burkina Faso. Int Health. (2019) 11:290–6. doi: 10.1093/inthealth/ihy088
71. Dolan E, Varley I, Ackerman KE, Pereira RMR, Elliott-Sale KJ, Sale C. The bone metabolic response to exercise and nutrition. Exerc Sport Sci Rev. (2020) 48:49–58. doi: 10.1249/JES.0000000000000215
72. Ibrahim MK, Zambruni M, Melby CL, Melby PC. Impact of childhood malnutrition on host defense and infection. Clin Microbiol Rev. (2017) 30:919–71. doi: 10.1128/CMR.00119-16
73. Walson JL, Berkley JA. The impact of malnutrition on childhood infections. Curr Opin Infect Dis. (2018) 31:231–6. doi: 10.1097/QCO.0000000000000448
74. Schlaudecker EP, Steinhoff MC, Moore SR. Interactions of diarrhea, pneumonia, and malnutrition in childhood: recent evidence from developing countries. Curr Opin Infect Dis. (2011) 24:496–502. doi: 10.1097/QCO.0b013e328349287d
75. Black RE, Morris SS, Bryce J. Where and why are 10 million children dying every year? Lancet. (2003) 361:2226–34. doi: 10.1016/S0140-6736(03)13779-8
76. Troeger C, Blacker BF, Khalil IA, Rao PC, Cao SJ, Zimsen SRM, et al. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of diarrhoea in 195 countries: a systematic analysis for the global burden of disease study 2016. Lancet Infect Dis. (2018) 18:1211–28. doi: 10.1016/S1473-3099(18)30362-1
77. Olofin I, McDonald CM, Ezzati M, Flaxman S, Black RE, Fawzi WW, et al. Associations of suboptimal growth with all-cause and cause-specific mortality in children under five years: a pooled analysis of ten prospective studies. PLoS ONE. (2013) 8:e64636. doi: 10.1371/journal.pone.0064636
78. Cunha AL. Relationship between acute respiratory infection and malnutrition in children under 5 years of age. Acta Paediatrica. (2000) 89:608–9. doi: 10.1111/j.1651-2227.2000.tb00347.x
79. Farhadi S, Ovchinnikov RS. The relationship between nutrition and infectious diseases: a review. Biomed Biotech Res J. (2018) 2:168–72. doi: 10.4103/bbrj.bbrj_69_18
80. Bhat RY, Manjunath N. Correlates of acute lower respiratory tract infections in children under 5 years of age in India. Int J Tuberc Lung Dis. (2013) 17:418–22. doi: 10.5588/ijtld.12.0117
81. Mukhopadhyay DK, Biswas R, Chakraborty M, Sadhukhan SK, Banik KK. Anthropometric failure, a new approach to measure undernutrition: an experience from a rural community of West Bengal, India. J Indian Med Assoc. (2009) 107:211–4, 236. Available online at: https://www.ncbi.nlm.nih.gov/pubmed/19810363
82. Brennhofer S, Reifsnider E, Bruening M. Malnutrition coupled with diarrheal and respiratory infections among children in Asia: a systematic review. Public Health Nursing. (2017) 34:401–9. doi: 10.1111/phn.12273
83. Paynter S, Ware RS, Lucero MG, Tallo V, Nohynek H, Weinstein P, et al. Malnutrition: a risk factor for severe respiratory syncytial virus infection and hospitalization. Pediatr Infect Dis J. (2014) 33:267–71. doi: 10.1097/INF.0000000000000096
84. Page AL, de Rekeneire N, Sayadi S, Aberrane S, Janssens AC, Rieux C, et al. Infections in children admitted with complicated severe acute malnutrition in Niger. PLoS ONE. (2013) 8:e68699. doi: 10.1371/journal.pone.0068699
85. Fatima S, Haider M, Hameed A, Saleem SG, Karim S. Comorbidities and their outcomes in children with severe acute malnutrition visiting pediatric emergency department at a tertiary care hospital in urban slums of Karachi, Pakistan. Ann Pediatr. (2021) 4:1068.
86. Kumar R, Singh J, Joshi K, Singh HP, Bijesh S. Co-morbidities in hospitalized children with severe acute malnutrition. Indian Pediatr. (2014) 51:125–7. doi: 10.1007/s13312-014-0343-x
87. Caulfield LE, de Onis M, Blossner M, Black RE. Undernutrition as an underlying cause of child deaths associated with diarrhea, pneumonia, malaria, and measles. Am J Clin Nutr. (2004) 80:193–8. doi: 10.1093/ajcn/80.1.193
88. Muenchhoff M, Healy M, Singh R, Roider J, Groll A, Kindra C, et al. Malnutrition in HIV-Infected children is an indicator of severe disease with an impaired response to antiretroviral therapy. AIDS Res Hum Retroviruses. (2018) 34:46–55. doi: 10.1089/aid.2016.0261
89. Lazzari TK, Forte GC, Silva DR. Nutrition status among HIV-positive and HIV-negative inpatients with pulmonary tuberculosis. Nutr Clin Pract. (2018) 33:858–64. doi: 10.1002/ncp.10006
90. Penda CI, Moukoko ECE, Nolla NP, Evindi NOA, Ndombo PK. Malnutrition among HIV infected children under 5 years of age at the Laquintinie hospital Douala, Cameroon. Pan Afr Med J. (2018) 30:91. doi: 10.11604/pamj.2018.30.91.15832
91. Takarinda KC, Mutasa-Apollo T, Madzima B, Nkomo B, Chigumira A, Banda M, et al. Malnutrition status and associated factors among HIV-positive patients enrolled in ART clinics in Zimbabwe. BMC Nutrition. (2017) 3:1–11. doi: 10.1186/s40795-017-0132-8
92. Jesson J, Leroy V. Challenges of malnutrition care among HIV-infected children on antiretroviral treatment in Africa. Med Mal Infect. (2015) 45:149–56. doi: 10.1016/j.medmal.2015.03.002
93. Rautiainen S, Manson JE, Lichtenstein AH, Sesso HD. Dietary supplements and disease prevention—a global overview. Nat Rev Endocrinol. (2016) 12:407–20. doi: 10.1038/nrendo.2016.54
94. Jesson J, Dahourou DL, Folquet MA, Malateste K, Yonaba C, N'Gbeche MS, et al. Malnutrition, growth response and metabolic changes within the first 24 months after ART Initiation in HIV-infected children treated before the age of 2 Years in West Africa. Pediatr Infect Dis J. (2018) 37:781–7. doi: 10.1097/INF.0000000000001932
95. Muscaritoli M, Corsaro E, Molfino A. Awareness of cancer-related malnutrition and its management: analysis of the results from a survey conducted among medical oncologists. Front Oncol. (2021) 11:682999. doi: 10.3389/fonc.2021.682999
96. Planas M, Alvarez-Hernandez J, Leon-Sanz M, Celaya-Perez S, Araujo K, Garcia de Lorenzo A, et al. Prevalence of hospital malnutrition in cancer patients: a sub-analysis of the PREDyCES(R) study. Support Care Cancer. (2016) 24:429–35. doi: 10.1007/s00520-015-2813-7
97. Hebuterne X, Lemarie E, Michallet M, de Montreuil CB, Schneider SM, Goldwasser F. Prevalence of malnutrition and current use of nutrition support in patients with cancer. JPEN J Parenter Enteral Nutr. (2014) 38:196–204. doi: 10.1177/0148607113502674
98. Caccialanza R, Lobascio F, Cereda E, Aprile G, Farina G, Traclò F, et al. Cancer-related malnutrition management: a survey among italian oncology units and patients' associations. Curr Probl Cancer. (2020) 44:100554. doi: 10.1016/j.currproblcancer.2020.100554
99. Wang S, Zhao Y, Li J, Lai H, Qiu C, Pan N, et al. Neurostructural correlates of hope: dispositional hope mediates the impact of the SMA gray matter volume on subjective well-being in late adolescence. Soc Cogn Affect Neurosci. (2020) 15:395–404. doi: 10.1093/scan/nsaa046
100. Zou Q, Xing P, Wei L, Liu B. Gene2vec: gene subsequence embedding for prediction of mammalian N6-methyladenosine sites from mRNA. RNA. (2019) 25:205–18. doi: 10.1261/rna.069112.118
101. Mays LC, Drummonds JW, Powers S, Buys DR, Watts PI. Identifying Geriatric Patients at Risk for Malnutrition: A Quality Improvement Project. J Nutr Gerontol Geriatr. (2019) 38:115–29. doi: 10.1080/21551197.2019.1604464
102. Zhang XT, Tang TL, Pang LD, Sharma SV, Li RS, Nyitray AG, et al. Malnutrition and overall survival in older adults with cancer: a systematic review and meta-analysis. J Geriatr Oncol. (2019) 10:874–83. doi: 10.1016/j.jgo.2019.03.002
103. Karami K, Pourmahmoudi A, Toori MA, Imani H, Hosseinikia M, Jonghani MN, et al. Malnutrition risk and related factors in cancer patients undergoing chemotherapy: a cross-sectional study. World Cancer Res J. (2021) 8:e1925. doi: 10.32113/wcrj_20213_1925
104. Muscaritoli M, Lucia S, Farcomeni A, Lorusso V, Saracino V, Barone C, et al. Prevalence of malnutrition in patients at first medical oncology visit: the PreMiO study. Oncotarget. (2017) 8:79884–96. doi: 10.18632/oncotarget.20168
105. Zhu CJ, Wang BQ, Gao Y, Ma XL. Prevalence and relationship of malnutrition and distress in patients with Cancer using questionnaires. BMC Cancer. (2018) 18:1–6. doi: 10.1186/s12885-018-5176-x
106. Sanz EA, Siles MG, Fernandez LR, Roldan RV, Dominguez AR, Abiles J. Nutritional risk and malnutrition rates at diagnosis of cancer in patients treated in outpatient settings: early intervention protocol. Nutrition. (2019) 57:148–53. doi: 10.1016/j.nut.2018.05.021
107. de Pinho NB, Martucci RB, Rodrigues VD, D'Almeida CA, Thuler LCS, Saunders C, et al. Malnutrition associated with nutrition impact symptoms and localization of the disease: results of a multicentric research on oncological nutrition. Clin Nutr. (2019) 38:1274–9. doi: 10.1016/j.clnu.2018.05.010
108. Galaniha LT, McClements DJ, Nolden A. Opportunities to improve oral nutritional supplements for managing malnutrition in cancer patients: a food design approach. Trends Food Sci Technol. (2020) 102:254–60. doi: 10.1016/j.tifs.2020.03.020
109. Sydes MR, Spears MR, Mason MD, Clarke NW, Dearnaley DP, de Bono JS, et al. Adding abiraterone or docetaxel to long-term hormone therapy for prostate cancer: directly randomised data from the STAMPEDE multi-arm, multi-stage platform protocol. Ann Oncol. (2018) 29:1235–48. doi: 10.1093/annonc/mdy072
110. Beirer A. Malnutrition and cancer, diagnosis and treatment. Memo-Mag Eur Med Oncol. (2021) 14:168–73. doi: 10.1007/s12254-020-00672-3
111. Verlande A, Chun SK, Goodson MO, Fortin BM, Bae H, Jang C, et al. Glucagon regulates the stability of REV-ERBα to modulate hepatic glucose production in a model of lung cancer–associated cachexia. Sci Adv. (2021) 7:eabf3885. doi: 10.1126/sciadv.abf3885
112. de van der Schueren MAE, Laviano A, Blanchard H, Jourdan M, Arends J, Baracos VE. Systematic review and meta-analysis of the evidence for oral nutritional intervention on nutritional and clinical outcomes during chemo(radio)therapy: current evidence and guidance for design of future trials. Ann Oncol. (2018) 29:1141–53. doi: 10.1093/annonc/mdy114
113. Keller H, Slaughter S, Gramlich L, Namasivayam-MacDonald A, Bell JJ. Multidisciplinary nutrition care: Benefitting patients with malnutrition across healthcare sectors. In: Interdisciplinary Nutritional Management and Care for Older Adults, eds Ólöf GG, Jack JB (Cham: Springer) (2021). p. 177–88.
114. Onaleye F. Protein energy malnutrition in children: Prevention system. In: Optimizing Health Monitoring Systems With Wireless Technology, ed Wickramasinghe N, (Hershey: IGI Global) (2021). p. 248–57. doi: 10.4018/978-1-5225-6067-8.ch017
115. De Luca A, Patel M, Mantha O, Peretti N, Hankard R. Promoting the awareness of hospital malnutrition in children: ePINUT 10th anniversary in 2020. Nutrition Clinique et Métabolisme. (2021) 35:85–92. doi: 10.1016/j.nupar.2021.01.001
116. Kiss N, Bauer J, Boltong A, Brown T, Isenring L, Loeliger J, et al. Awareness, perceptions and practices regarding cancer-related malnutrition and sarcopenia: a survey of cancer clinicians. Support Care Cancer. (2020) 28:5263–70. doi: 10.1007/s00520-020-05371-7
Keywords: malnutrition, infections, children under five, cancer, developing countries, future strategies
Citation: Fan Y, Yao Q, Liu Y, Jia T, Zhang J and Jiang E (2022) Underlying Causes and Co-existence of Malnutrition and Infections: An Exceedingly Common Death Risk in Cancer. Front. Nutr. 9:814095. doi: 10.3389/fnut.2022.814095
Received: 16 November 2021; Accepted: 24 January 2022;
Published: 23 February 2022.
Edited by:
Francesco Sofi, Università degli Studi di Firenze, ItalyReviewed by:
Sorush Niknamian, Liberty University, United StatesDewi Marhaeni Diah Herawati, Universitas Padjadjaran, Indonesia
Victor Musiime, Makerere University, Uganda
Copyright © 2022 Fan, Yao, Liu, Jia, Zhang and Jiang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Enshe Jiang, ZXNqaWFuZ0BnbWFpbC5jb20=; Junjuan Zhang, MzgzNTgzNDk2QHFxLmNvbQ==
†These authors have contributed equally to this work
 Yuanyuan Fan
Yuanyuan Fan Qianqian Yao2†
Qianqian Yao2† Enshe Jiang
Enshe Jiang