- 1Department of Cardiology, China Academy of Chinese Medical Sciences, Xiyuan Hospital, Beijing, China
- 2National Clinical Research Center for Chinese Medicine Cardiology, Beijing, China
- 3Department of Graduate School, Beijing University of Chinese Medicine, Beijing, China
Background: Patients with metabolic syndrome (MetS) have increased cardiovascular risk. Capsaicin (CAP) has been shown to reduce lipids, but efficacy for patients with MetS is unknown.
Methods: A systematic review was performed according to PRISMA guidelines, to compare the effects of CAP against a placebo. Differences in the weight mean difference (WMD) with 95% confidence intervals (95% CI) were then pooled using a random effects model.
Results: Nine randomized controlled trials including 461 patients were identified in the overall analysis. CAP significantly decreased total cholesterol (TC) (WMD = −0.48, 95% CI: −0.63 to −0.34, I2= 0.00%) and low-density lipoprotein cholesterol (LDL-C) (WMD = −0.23, 95% CI: −0.45 to −0.02, I2 = 68.27%) among patients with MetS. No significant effects of CAP were found on triglycerides (TG) or high-density lipoprotein cholesterol (HDL-C) (WMD = −0.40, 95% CI: −1.50 to 0.71, I2 = 98.32%; WMD = −0.08, 95% CI: −0.21 to 0.04, I2 = 86.06%). Subgroup analyses indicated that sex and intervention period were sources of heterogeneity. The results revealed that CAP decreased TG levels in women (WMD = −0.59, 95% CI: −1.07 to −0.10) and intervention period <12 weeks (WMD = −0.65; 95% CI: −1.10 to −0.20). And there was no potential publication bias according to funnel plot, Begg' test and Egger regression test.
Conclusions: CAP supplementation is a promising approach to decreasing TC and LCL-C levels in patients with MetS. However, short-term (<12 weeks) use of CAP in women may also reduce TG levels.
Systematic Review Registration: Identifier: CRD42021228032.
Introduction
Metabolic syndrome (MetS) represents a cluster of metabolic risk factors, including dyslipidemia (raised triglycerides and lowered high-density lipoprotein cholesterol), obesity (especially abdominal obesity), elevated blood pressure, and dysglycemia (1). It is estimated that patients with MetS are twice as likely to develop cardiovascular disease (CVD) in the next 5 to 10 years compared to those without the syndrome, posing a huge burden on global health and the economy (1). The management of each MetS component has been proved to be effective in reducing the incidence of CVD and reducing the risk for major adverse cardiovascular events (MACE) (2).
Dyslipidemia is an important component of the contemporary consensus definition of MetS and also a major risk factor for CVD, which is a leading cause of death worldwide (3–7). Epidemiological data revealed that in addition to decreased levels of high-density lipoprotein cholesterol (HDL-C), elevated levels of low-density lipoprotein cholesterol (LDL-C), triglycerides (TG) and total cholesterol (TC) are also independent predictors of the risk of CVD (8–12). Therefore, lowering blood lipid levels is of great significance in the management of MetS.
Recently there has been rising attention to single dietary components or natural compounds due to the fact they are inexpensive, readily available, and have beneficial effects in the treatment of various diseases such as MetS. For example, studies have shown that daily intake of red yeast rice can reduce LDL-C levels from 15% to 25% (13). In addition, glucomannan (14, 15), probiotics (14, 16), garlic (17), berberine (18), omega-3 (ω′-3) and fatty acids (19), also have a positive effect on improving MetS.
Capsaicin (CAP) (trans-8-methyl-N-vanillyl-6-nonenamide) is the main component in red chili peppers that give chili peppers their spice, which belongs to the Solanaceae family (20, 21). Previous studies have demonstrated that CAP has antioxidant activity (22), analgesic activity (23, 24), and can aid in lowering rates of obesity (25). The lipid-lowering effects of the CAP remain controversial. Numerous studies have shown that CAP can reduce TC, TG, LDL-C and increase HDL-C levels (20, 26, 27). However, a study by Urbina et al. (28) showed that CAP supplementation does not affect serum TG, TC, LDL-C levels, but does decrease HDL-C levels in serum, which is not consistent with evidence reported in previous studies. There have been no systematic reviews or meta-analyses to summarize the available data. Therefore, we carry out a systematic review and meta-analysis to assess the effects of CAP on lipids in patients with MetS.
Methods
Data Sources and Searches
The meta-analysis protocol was registered on PROSPERO (CRD42021228032, https://www.crd.york.ac.uk/prospero/), and conducted following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (29). PubMed, EMBASE, MEDLINE, and the Cochrane Library were systematically searched from inceptions to February 1, 2021. MeSH terms and free words were used reasonably through the characteristics of literature databases. Detailed search strategies are listed in Supplementary Material 1.
Selection Criteria
The selected studies were screened using the PICOS (participants, interventions, comparisons, outcomes, and study design) criteria:
1. Population: patients with MetS were diagnosed according to recognized diagnostic criteria (IFD, WHO, or NCEP-ATP III) (1).
2. Intervention: circulating CAP supplements, dietary CAP or CAP-related supplements.
3. Comparison: no use of CAP or CAP-related supplements categories of exposure.
4. Outcome: lipid parameters (TC, TG, HDL-C and LDL-C).
5. Study design: randomized controlled trials (RCTs).
Data Extraction
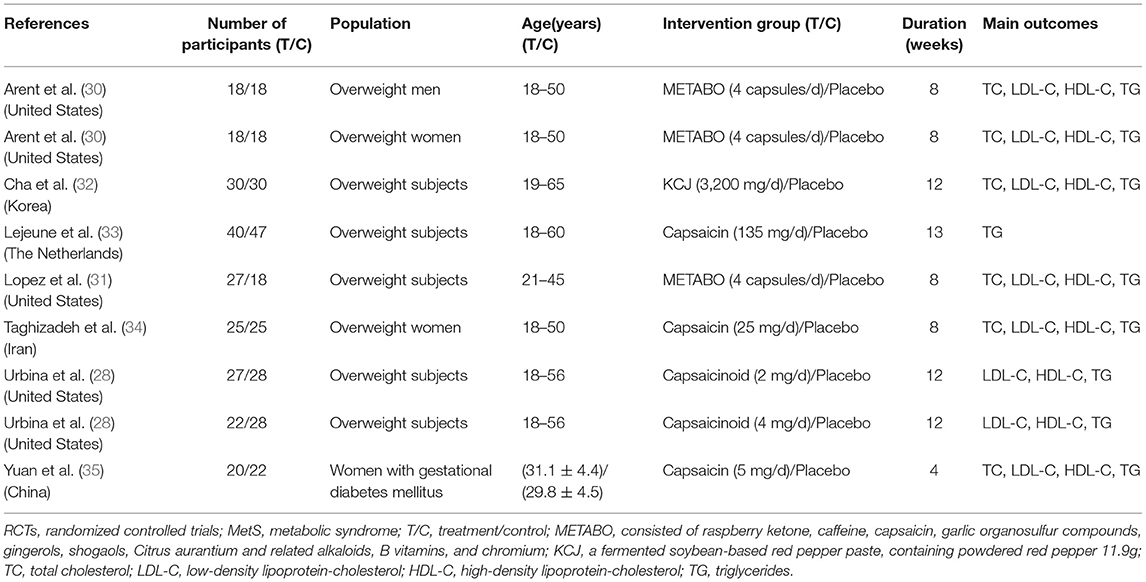
Two reviewers independently extracted data using standardized data extraction forms. Any disagreements would be resolved by consensus or consulting the third reviewer. If the information is incomplete or unclear, when necessary, the author was contacted. Data extraction included study design type and participant characteristics (age, sex, and country), intervention and placebo details (sample size, study duration, CAP dose, and controls group used). Outcomes included lipid levels (TC, TG, HDL-C, LDL-C).
Data Synthesis and Analysis
Data for the effect of continuous outcomes were extracted as the weight mean difference (WMD), which represents the mean difference between the intervention and control groups in standard deviation units, with 95% confidence intervals (CIs). Clinical heterogeneity was assessed by I2 and Chi-squared (χ2) test at α = 0.1. When Cochrane's test showed that I2 <50%, there was no statistical heterogeneity among the studies, and therefore a fixed-effect model (with inverse variance method) was used for meta-analysis. If I2 ≥ 50%, there was statistical heterogeneity among the studies, and as such the random effects model (DerSimonian and Laird method) was used to analyze the causes of the heterogeneity. Sensitivity analysis was performed by using the leave-one-out method and/ or a subgroup analysis according to that factor. Publication bias was examined using funnel plots, the Begg' test and the Egger regression test. Statistical analysis was conducted using Review Manager, Version 5.3 (Cochrane Collaboration, Oxford, UK) and Stata version 16.0 (Stata Corp., College Station, TX). The protocol for the present meta-analysis was registered on the international prospective register of systematic reviews (PROSPERO, CRD42021228032).
Quality Assessment
The recommendations of the Cochrane Intervention Systems Review Manual (updated September 2009) were used to evaluate the risk of bias. Studies were stratified as high risk, low risk, or unclear risk. The risk of bias included the following six evaluation criteria: generation of a random sequence, allocation concealment, use of blind method, integrity of result data, and selection of reporting outcome.
Results
Data Sources and Search Results
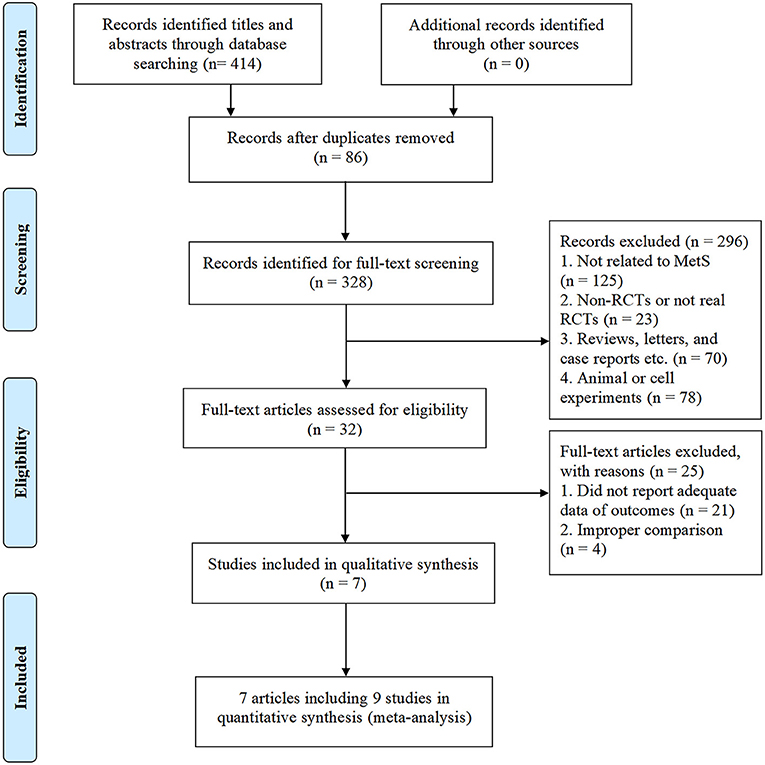
As shown in Figure 1, a total of 414 potentially relevant studies were identified in our initial literature search. We evaluated 328 potentially related articles for eligibility after removing the duplicates of 86 studies from different databases. After screening the titles and abstracts of these studies, we excluded 296 studies for the following reasons: subject was not related to MetS (n = 125); study not a RCTs (n = 23); reviews, letters, and case reports etc. (n = 70); animal or cell experiments (n = 78). Of the retrieved studies, a total of 32 met our inclusion criteria. However, 25 studies were excluded because they did not have sufficient data of outcomes (n = 21) or improper comparison (n = 4). Finally, seven studies entered into our meta-analysis, involving 461 patients (227 [49.2%] in the CAP group, 234 [50.8%] in the control group). Three studies (28, 30, 31) were conducted and published in full in the United States, one in Korea (32), one in the Netherlands (33), one in Iran (34), and one in China (35). The average sample size of the trials was 51 participants (ranging from 36 to 87 participants per trial). The course of treatment fluctuated between 4-weeks and 13-weeks. In one of the included trials (28), two different doses of CAP (2 and 4 mg) were administered, hence we considered it as two separate studies. In another trial (30), results were divided by sex despite the same intervention, so we also treated this study as two separate studies. The characteristics of the included trials are shown in Table 1.
Quality of Included Studies
The assessment of the risk of bias for all included trials is shown in Figures 2A,B. All of the included studies reported randomly assigned participants, two (34, 35) of them described methodological operations for random sequence generation (using a computer-generated list of random numbers), and the remaining studies mentioned they were “random,” did not report it in detail. Two studies (28) reported the method of allocation concealment (by using numbered bottles). All trials were double-blind, of these, six studies (28, 30, 34, 35) described the specific blinding of participants and personnel, and detailed the blinding of the outcome assessment. Clinical trial registration and pre-published protocol were reported by the authors of eight studies (28, 30–32, 34, 35), but it was not feasible to effectively assess whether there was a risk of selective reporting bias.
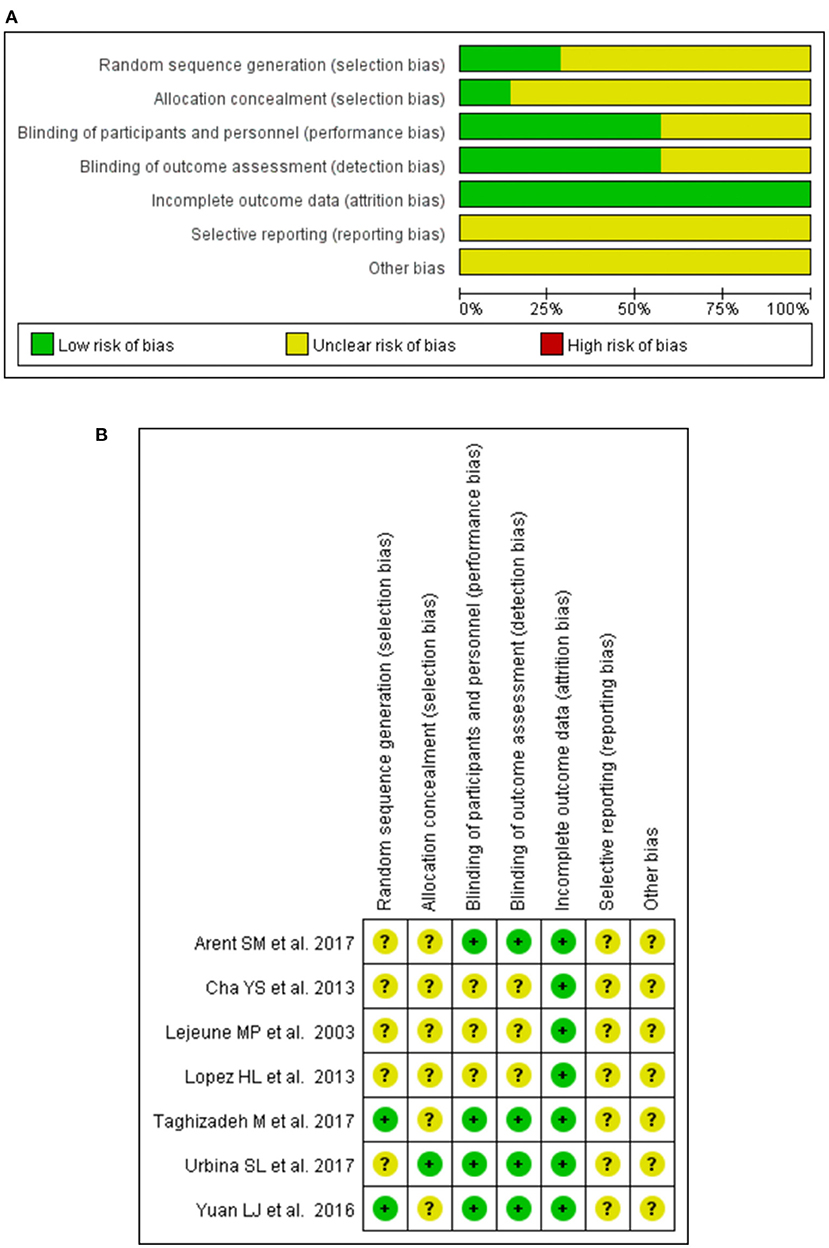
Figure 2. Graph of bias risk assessment for included studies by the Cochrane Collaboration's tool. (A) Risk of bias graph. (B) Risk of bias summary.
Effects of CAP on Lipid Levels
Total Cholesterol
Six studies (30–32, 34, 35) (138 patients in the CAP supplementation group vs. 131 patients in the placebo group) evaluated the effects of CAP supplementation on TC levels among patients with MetS. The overall results of the random-effect model exhibited a favorable effect on reducing TC levels following CAP supplementation (WMD = −0.48; 95% CI: −0.63 to −0.34; P = 0.00; I2 = 0.00%) (Figure 3A). The results of leave-one-out sensitivity analysis support the robustness of our findings (Figure 4A). Inter-group heterogeneity changed after subgroup analysis based on race, sex, dose and duration of CAP supplementation, but there was no significant difference in TC levels before and after the subgroup analysis (Figure 5).
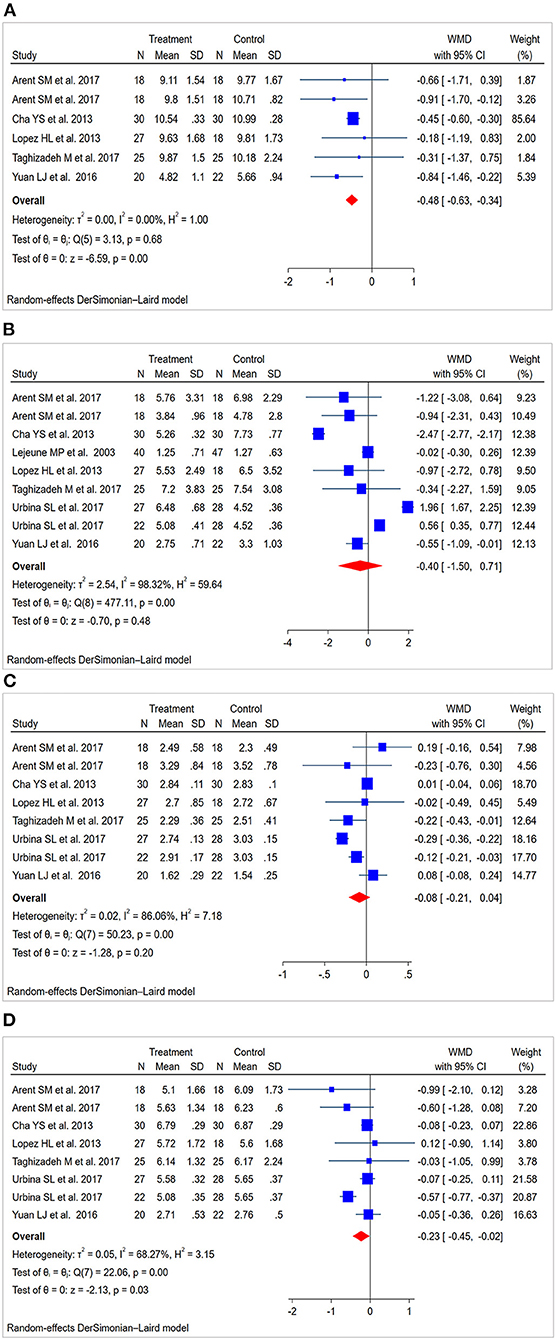
Figure 3. Forest plot for lipid levels: capsaicin vs. placebo (random-effect model). (A) Funnel plot for total cholesterol (TC). (B) Funnel plot for triglycerides (TG). (C) Funnel plot for high-density lipoprotein cholesterol (HDL-C). (D) Funnel plot for low-density lipoprotein cholesterol (LDL-C).
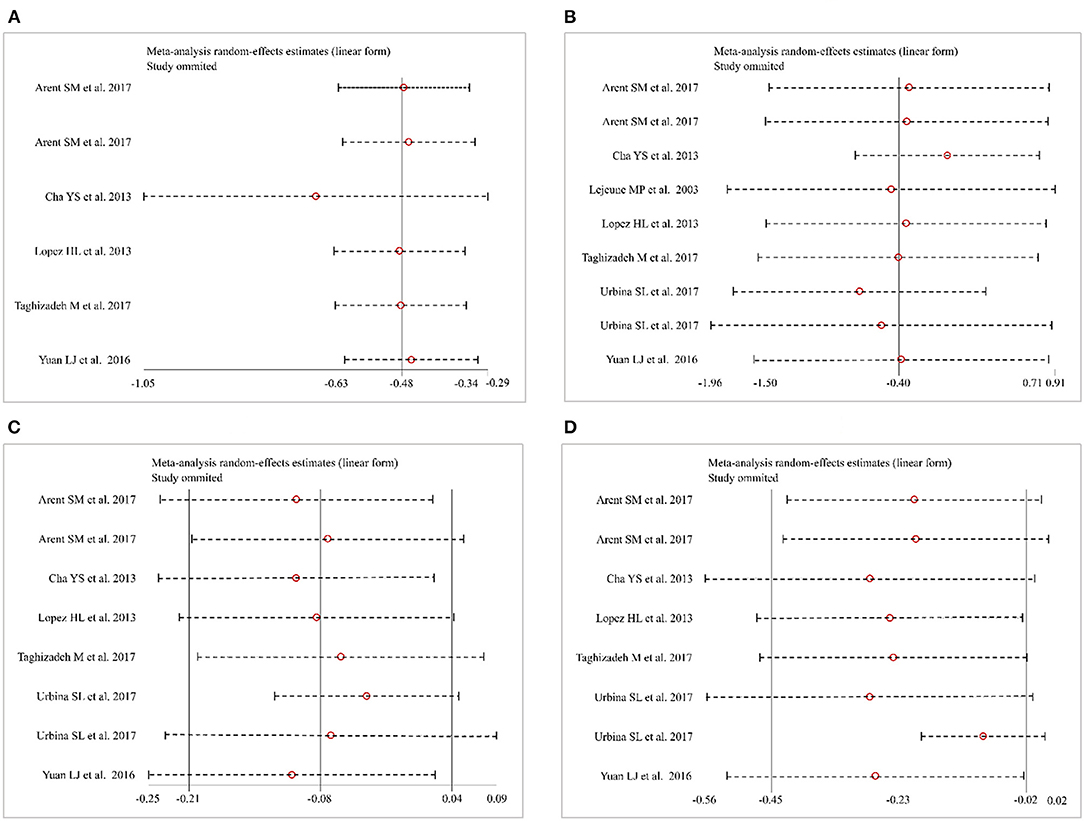
Figure 4. Sensitivity analysis. (A) Total cholesterol (TC). (B) Triglycerides (TG). (C) High-density lipoprotein cholesterol (HDL-C). (D) Low-density lipoprotein cholesterol (LDL-C).

Figure 5. Results of subgroup analysis. (A,E,I,M) Effect of capsaicin (CAP) on total cholesterol (TC), triglycerides (TG), high-density lipoprotein cholesterol (HDL-C), and low density lipoprotein cholesterol (LDL-C) in different races. (B,F,J,N) Effect of CAP on TC, TG, HDL-C and LDL-C in different genders. (C,G,K,O) Effect of dose of CAP on TC, TG, HDL-C and LDL-C. (D,H,L,P) Effect of duration of CAP intervention on TC, TG, HDL-C and LDL-C. WMD, weighted mean difference.
Triglycerides
Nine studies (28, 30–35) (227 patients in the CAP supplementation group vs. 234 patients in the placebo group) compared the effects of CAP and placebo on TG levels in patients with MetS. The results showed that CAP had no significant effect on TG levels compared with placebo (WMD = −0.40; 95% CI: −1.50 to 0.71; P = 0.48; I2 = 98.32%) (Figure 3B). Sensitivity analysis showed that the results did not change before and after sensitivity analysis (Figure 4B).
Figure 5 summarizes the subgroup analysis results of the effects of CAP on TG levels in patients with MetS. TG levels in women were significantly decreased after CAP supplementation (WMD = −0.59; 95% CI: −1.07 to −0.10). Furthermore, serum TG levels decreased after CAP supplementation for <12 weeks (WMD = −0.65; 95% CI: −1.10 to −0.20).
High-Density Lipoprotein Cholesterol
Eight studies (28, 30–32, 34, 35) (187 patients in the CAP supplementation group vs. 187 patients in the placebo group) reported the effects of CAP on serum HDL-C. The random effects model showed that the pooled mean effect size was not significant (WMD = −0.08; 95% CI: −0.21 to 0.04; P = 0.20), with significant heterogeneity (I2= 86.06%) (Figure 3C). The results of the sensitivity analysis were not altered after excluding the individual trials (Figure 4C). The heterogeneity changed with differences in race, gender, intervention time groups and dose, but there were no significant differences before and after subgroup analysis (Figure 5).
Low-Density Lipoprotein Cholesterol
Eight studies (28, 30–32, 34, 35) indicated beneficial results from taking CAP supplementation (n = 187), as seen by a reduction in serum LDL-C levels, compared to that with placebo group (n = 187) (WMD = −0.23; 95% CI: −0.45 to −0.02, P = 0.03; I2 = 68.27%) using the random-effect model (Figure 3D). Sensitivity analysis showed no significant change in the overall estimate of effect size after the elimination of individual trials (Figure 4D). The subgroup analysis of the LDL-C levels showed no significant differences within subgroups based on the dose of CAP, duration of CAP use, race or gender (Figure 5).
Publication Bias
A funnel plot, Begg' test and Egger regression test were used to evaluate the effects of CAP on TC levels, and no publication bias was found (Egger regression test, coefficient, −0.42; 95% CI, −1.72 to 0.89; P = 0.43). For the effects of CAP on TG and HDL-C levels, there was no evidence of publication bias according to the results of a funnel plot, Begg' test and Egger regression test (coefficient, −2.15; 95% CI, −13.08 to 8.79; P = 0.66; coefficient, −0.07; 95% CI, −4.22 to 4.08; P = 0.97). Lastly, no potential publication bias for LDL-C was identified according to funnel plot, Begg' test and Egger regression test (coefficient, −0.59; 95% CI, −3.39 to 2.21; P = 0.62) (Figure 6).
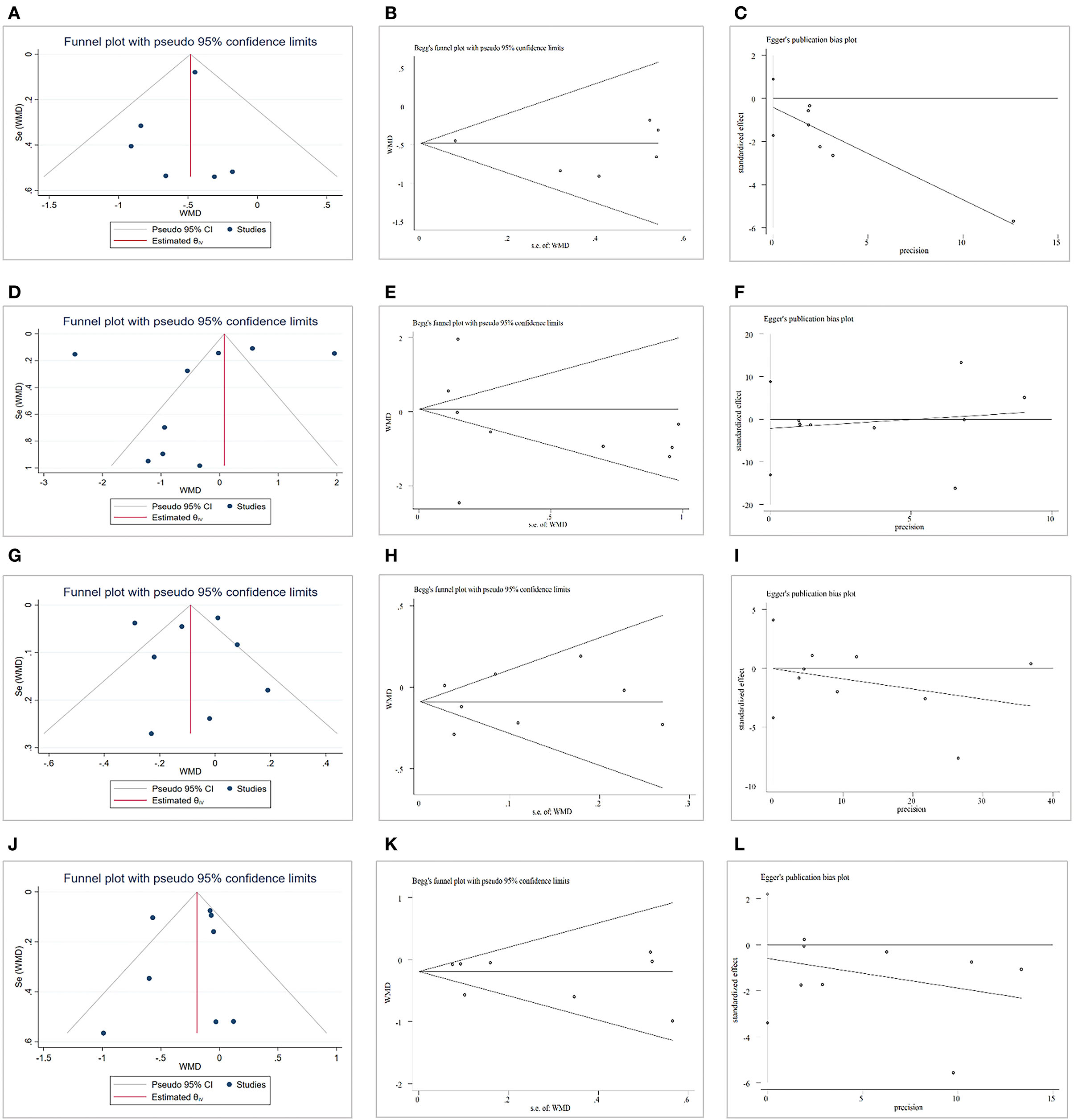
Figure 6. Publication bias. (A) Funnel plot for total cholesterol (TC). (B) Begg' test for TC. (C) Egger test for TC. (D) Funnel plot for triglycerides (TG). (E) Begg' test for TG. (F) Egger test for TG. (G) Funnel plot for high-density lipoprotein cholesterol (HDL-C). (H) Begg' test for HDL-C. (I) Egger test for HDL-C. (J) Funnel plot for low-density lipoprotein cholesterol (LDL-C). (K) Begg' test for LDL-C. (L) Egger test for LDL-C
Discussion
To the best of our knowledge, this is the first systematic review and meta-analyses of RCTs evaluating the effects of CAP supplementation on lipid levels among patients with MetS. A total of nine studies (involving 461 patients) were included in this meta-analysis. The main finding of our meta-analysis was that CAP supplementation may have beneficial therapeutic effects in reducing TC and LDL-C levels. No significant effects of CAP were found regarding TG and HDL-C levels. However, subgroup analyses revealed that CAP reduced TG levels in women and at <12 weeks of the intervention.
Many small studies have reported that CAP might decrease lipid levels among patients with MetS. This effect has also been proposed CAP as one of the agents treating dyslipidemia. In most instances, however, the studies regarding CAP and lipid levels had methodological limitations (mainly owing to small numbers of patients included), leaving this hypothesis unproven. Our meta-analysis pooled all the RCTs regarding the effects of CAP on lipid levels, and the results showed that CAP supplementation may be beneficial in reducing TC, and LDL-C. Overall, CAP may be a complementary approach in the patients with dyslipidemia who cannot be treated with statins or other LDL-C-lowering therapies.
It is essential to actively manage risk factors for MetS, such as dyslipidemia. Studies have shown that lowering atherogenic cholesterol levels can effectively reduce morbidity and mortality of CVD (36–38). The ATP-III guidelines emphasized that LDL-C reduction is the primary target of lipid management in MetS, and low HDL-C and TG are secondary targets (39). Large LDL-C reductions, such as 2 to 3 mmol/L (77.4–116.1 mg/dL), can reduce the relative risk of CVD by 40–50% (37). The availability and use of lipid-lowering medication, such as statin therapy and ezetimibe, or proprotein convertase subtilisin-kexin type 9 (PCSK9) inhibitors, significantly reduces lipid levels. In turn, reducing the number of patients with hyperlipidemia and therefore the risk of an acute cardiovascular event (40, 41). Statin therapies also generally reduced the risk of an acute cardiovascular event by 25 to 45%, which was noted over 5 years of follow-ups (6). However, despite the existence of effective treatments and well-established treatment guidelines, lipid abnormalities are still very common in adults, with an estimated 53% (105.3 M) of U.S. adults having at least one lipid abnormality, 27% (53.5 million) having high LDL-C, 23% (46.4 million) having low HDL, and 30% (58.9 million) with high TG (42). A clinical guideline for the management of dyslipidemia conducted by Downs and O'Malley showed that 10 to 20% of patients using statins experienced muscle-related symptoms (43). Hereby, our meta-analysis showed that CAP can improve dyslipidemia and has the advantages of a lower price and easy availability. For these reasons, CAP supplementation as an adjunct nutritional therapy for the treatment of dyslipidemia in MetS patients is easy to implement and may lead to better compliance in patients with MetS.
There are several mechanisms which could potentially explain the effects of CAP on lipid levels. It has been shown that CAP plays a role in countering the detrimental effects of a high-fat diet, such as glucose intolerance and/or hypercholesterolemia. It does this primarily by increasing the expression of metabolically important thermogenic genes, including uncoupling protein 1 (UCP-1), bone morphogenetic protein 8b (BMP 8b), Sirtuin1 (SIRT-1), peroxisome proliferator-activated receptor-γ co-activator-1α (PGC-1α), and positively regulated domain containing zinc finger protein 16 (PRDM-16) (44). CAP activates its receptor transient receptor vanilloid subtype 1 (TRPV1), which can activate sympathetically-mediated brown adipose tissue (BAT) thermogenesis and reduce body fat (45). In addition, CAP inhibits the expression of peroxisome proliferator-activated receptor-γ (PPARγ), CCAAT-enhancer-binding protein-α (C/EBP-α) and leptin; but induces up-regulation of adiponectin at the protein level. Therefore, it can effectively induce apoptosis of 3T3-L1 pre-adipocytes and adipocytes; and inhibit adipogenesis in vitro (46).
The meta-analysis has a number of limitations, of which heterogeneity across the included studies is the most important. We conducted a sensitivity and subgroup analysis to determine the factors (race, gender, dose, and duration, etc.) that might cause large heterogeneity and thus to explore the source of heterogeneity. Heterogeneity changed after analysis, but the overall results were stable and reliable. In clinical practice, disparate formulations and delivery routes of CAP may affect the results, which should be noted in future research. Second, this meta-analysis was limited by the small number of studies and the small size of existing RCTs. Therefore, additional studies are needed to confirm our findings and to expand our understanding of CAP.
In conclusion, the findings of this meta-analysis demonstrated that CAP supplementation is effective in improving lipid levels and should be considered in the prevention and treatment of MetS. Large-scale, high-quality, and precise RCTs are needed to further demonstrate the effects of CAP on lipid levels, and we will follow up on this study.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Materials, further inquiries can be directed to the corresponding author/s.
Author Contributions
ZJ and HQ were involved in the conception and design. ZJ, HQ, GL, and ZG were involved in literature retrieval, data collection, extraction and analysis. KC and DS were involved in systematic review and meta-analysis. KC and ZG are responsible for the final approval of the version to be published. All authors revised and approved the final version of the manuscript.
Funding
This work was financially supported by the National Science and Technology Major Project of the Ministry of Science and Technology of China (2019ZX09201005-002-006), Beijing Traditional Chinese Medicine Science and Technology Development Fund Project (JJ-2020-79), and the Capital Health Research and Development of Special (2018–1–4171).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2022.812294/full#supplementary-material
References
1. Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. (2009) 120:1640–5. doi: 10.1161/CIRCULATIONAHA.109.192644
2. Park S, Han K, Kim DK. Altered risk for cardiovascular events with changes in the metabolic syndrome status. Ann Intern Med. (2020) 172:707–8. doi: 10.7326/L20-0076
3. Rader DJ, Daugherty A. Translating molecular discoveries into new therapies for atherosclerosis. Nature. (2008) 451:904–13. doi: 10.1038/nature06796
4. Grundy SM, Brewer HB Jr, Cleeman JI, Smith SC Jr, Lenfant C. American Heart Association, et al. Definition of metabolic syndrome: report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation. (2004) 109:433–8. doi: 10.1161/01.CIR.0000111245.75752.C6
5. Grundy SM. Pre-diabetes, metabolic syndrome, and cardiovascular risk. J Am Coll Cardiol. (2012) 59:635–43. doi: 10.1016/j.jacc.2011.08.080
6. Toth PP. Treatment of dyslipidemia in elderly patients with coronary heart disease: there are miles to go before we sleep. J Am Coll Cardiol. (2015) 66:1873–5. doi: 10.1016/j.jacc.2015.06.1356
7. Kopin L, Lowenstein C. Dyslipidemia. Ann Intern Med. (2017) 167:ITC81–96. doi: 10.7326/AITC201712050
8. Rader DJ. New therapeutic approaches to the treatment of dyslipidemia. Cell Metab. (2016) 23:405–12. doi: 10.1016/j.cmet.2016.01.005
9. Jellinger PS, Handelsman Y, Rosenblit PD, Bloomgarden ZT, Fonseca VA, Garber AJ, et al. American association of clinical endocrinologists and American college of endocrinology guidelines for management of dyslipidemia and prevention of cardiovascular disease. Endocr Pract. (2017) 23:1–87. doi: 10.4158/EP171764.GL
10. Ganda OP, Bhatt DL, Mason RP, Miller M, Boden WE. Unmet need for adjunctive dyslipidemia therapy in hypertriglyceridemia management. J Am Coll Cardiol. (2018) 72:330–43. doi: 10.1016/j.jacc.2018.04.061
11. Sarwar N, Danesh J, Eiriksdottir G, Sigurdsson G, Wareham N, Bingham S, et al. Triglycerides and the risk of coronary heart disease: 10,158 incident cases among 262,525 participants in 29 Western prospective studies. Circulation. (2007) 115:450–8. doi: 10.1161/CIRCULATIONAHA.106.637793
12. Watts GF, Ooi EM, Chan DC. Demystifying the management of hypertriglyceridemia. Nat Rev Cardiol. (2013) 10:648–61. doi: 10.1038/nrcardio.2013.140
13. Cicero AFG, Fogacci F, Zambon A. Red yeast rice for hypercholesterolemia: JACC focus seminar. J Am Coll Cardiol. (2021) 77:620–8. doi: 10.1016/j.jacc.2020.11.056
14. Cicero AFG, Fogacci F, Stoian AP, Vrablik M, Al Rasadi K, Banach M, et al. Nutraceuticals in the management of dyslipidemia: which, when, and for whom? Could nutraceuticals help low-risk individuals with non-optimal lipid levels? Curr Atheroscler Rep. (2021) 23:57. doi: 10.1007/s11883-021-00955-y
15. Ho HVT, Jovanovski E, Zurbau A, Blanco Mejia S, Sievenpiper JL, Au-Yeung F, et al. A systematic review and meta-analysis of randomized controlled trials of the effect of konjac glucomannan, a viscous soluble fiber, on LDL cholesterol and the new lipid targets non-HDL cholesterol and apolipoprotein B. Am J Clin Nutr. (2017) 105:1239–47. doi: 10.3945/ajcn.116.142158
16. Companys J, Pla-Pagà L, Calderón-Pérez L, Llauradó E, Solà R, Pedret A, et al. Fermented dairy products, probiotic supplementation, and cardiometabolic diseases: a systematic review and meta-analysis. Adv Nutr. (2020) 11:834–63. doi: 10.1093/advances/nmaa030
17. Anaeigoudari A, Safari H, Khazdair MR. Effects of Nigella sativa, Camellia sinensis, and Allium sativum as food additives on metabolic disorders, a literature review. Front Pharmacol. (2021) 12:762182. doi: 10.3389/fphar.2021.762182
18. Banach M, Patti AM, Giglio RV, Cicero AFG, Atanasov AG, Bajraktari G, et al. The role of nutraceuticals in statin intolerant patients. J Am Coll Cardiol. (2018) 72:96–118. doi: 10.1016/j.jacc.2018.04.040
19. Shahidi F, Ambigaipalan P. Omega-3 polyunsaturated fatty acids and their health benefits. Annu Rev Food Sci Technol. (2018) 9:345–81. doi: 10.1146/annurev-food-111317-095850
20. Panchal SK, Bliss E, Brown L. Capsaicin in metabolic syndrome. Nutrients. (2018) 10:630. doi: 10.3390/nu10050630
21. Sanati S, Razavi BM, Hosseinzadeh H. A review of the effects of Capsicum annuum L. and its constituent, capsaicin, in metabolic syndrome. Iran J Basic Med Sci. (2018) 21:439–48. doi: 10.22038/IJBMS.2018.25200.6238
22. Yudkin JS, Stehouwer CD, Emeis JJ, Coppack SW. C-reactive protein in healthy subjects: associations with obesity, insulin resistance, and endothelial dysfunction: a potential role for cytokines originating from adipose tissue? Arterioscler Thromb Vasc Biol. (1999) 19:972–8. doi: 10.1161/01.ATV.19.4.972
23. Bastard JP, Jardel C, Bruckert E, Blondy P, Capeau J, Laville M, et al. Elevated levels of interleukin 6 are reduced in serum and subcutaneous adipose tissue of obese women after weight loss. J Clin Endocrinol Metab. (2000) 85:3338–42. doi: 10.1210/jcem.85.9.6839
24. Natali A, Toschi E, Baldeweg S, Ciociaro D, Favilla S, Saccà L, et al. Clustering of insulin resistance with vascular dysfunction and low-grade inflammation in type 2 diabetes. Diabetes. (2006) 55:1133–40. doi: 10.2337/diabetes.55.04.06.db05-1076
25. Phillips CM, Perry IJ. Does inflammation determine metabolic health status in obese and nonobese adults? J Clin Endocrinol Metab. (2013) 98:E1610–1619. doi: 10.1210/jc.2013-2038
26. Kim Y, Park YJ, Yang SO, Kim SH, Hyun SH, Cho S, et al. Hypoxanthine levels in human urine serve as a screening indicator for the plasma total cholesterol and low-density lipoprotein modulation activities of fermented red pepper paste. Nutr Res. (2010) 30:455–61. doi: 10.1016/j.nutres.2010.06.014
27. Lim JH, Jung ES, Choi EK, Jeong DY, Jo SW, Jin JH, et al. Supplementation with Aspergillus oryzae-fermented kochujang lowers serum cholesterol in subjects with hyperlipidemia. Clin Nutr. (2015) 34:383–7. doi: 10.1016/j.clnu.2014.05.013
28. Urbina SL, Roberts MD, Kephart WC, Villa KB, Santos EN, Olivencia AM, et al. Effects of twelve weeks of capsaicinoid supplementation on body composition, appetite and self-reported caloric intake in overweight individuals. Appetite. (2017) 113:264–73. doi: 10.1016/j.appet.2017.02.025
29. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372: n71. doi: 10.1136/bmj.n71
30. Arent SM, Walker AJ, Pellegrino JK, Sanders DJ, McFadden BA, Ziegenfuss TN, et al. The combined effects of exercise, diet, and a multi-ingredient dietary supplement on body composition and adipokine changes in overweight adults. J Am Coll Nutr. (2018) 37:111–20. doi: 10.1080/07315724.2017.1368039
31. Lopez HL, Ziegenfuss TN, Hofheins JE, Habowski SM, Arent SM, Weir JP, et al. Eight weeks of supplementation with a multi-ingredient weight loss product enhances body composition, reduces hip and waist girth, and increases energy levels in overweight men and women. J Int Soc Sports Nutr. (2013) 10:22. doi: 10.1186/1550-2783-10-22
32. Cha YS, Kim SR, Yang JA, Back HI, Kim MG, Jung SJ, et al. Kochujang, fermented soybean-based red pepper paste, decreases visceral fat and improves blood lipid profiles in overweight adults. Nutr Metab (Lond). (2013) 10:24. doi: 10.1186/1743-7075-10-24
33. Lejeune MP, Kovacs EM, Westerterp-Plantenga MS. Effect of capsaicin on substrate oxidation and weight maintenance after modest body-weight loss in human subjects. Br J Nutr. (2003) 90:651–9. doi: 10.1079/BJN2003938
34. Taghizadeh M, Farzin N, Taheri S, Mahlouji M, Akbari H, Karamali F, et al. The effect of dietary supplements containing green tea, capsaicin and ginger extracts on weight loss and metabolic profiles in overweight women: a randomized double-blind placebo-controlled clinical trial. Ann Nutr Metab. (2017) 70:277–85. doi: 10.1159/000471889
35. Yuan LJ, Qin Y, Wang L, Zeng Y, Chang H, Wang J, et al. Capsaicin-containing chili improved postprandial hyperglycemia, hyperinsulinemia, and fasting lipid disorders in women with gestational diabetes mellitus and lowered the incidence of large-for-gestational-age newborns. Clin Nutr. (2016) 35:388–93. doi: 10.1016/j.clnu.2015.02.011
36. Srikanth S, Deedwania P. Management of dyslipidemia in patients with hypertension, diabetes, and metabolic syndrome. Curr Hypertens Rep. (2016) 18:76. doi: 10.1007/s11906-016-0683-0
37. Cholesterol Treatment Trialists' (CTT) Collaboration, Baigent C, Blackwell L, Emberson J, Holland LE, Reith C, et al. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. (2010) 376:1670–81. doi: 10.1016/S0140-6736(10)61350-5
38. Cholesterol Treatment Trialists' (CTT) Collaborators, Mihaylova B, Emberson J, Blackwell L, Keech A, Simes J, et al. The effects of lowering LDL cholesterol with statin therapy in people at low risk of vascular disease: meta-analysis of individual data from 27 randomised trials. Lancet. (2012) 380:581–90. doi: 10.1016/S0140-6736(12)60367-5
39. Grundy SM, Cleeman JI, Merz CN, Brewer HB Jr, Clark LT, Hunninghake DB, et al. Implications of recent clinical trials for the National Cholesterol Education Program adult treatment panel III guidelines. Circulation. (2004) 110:227–39. doi: 10.1161/01.CIR.0000133317.49796.0E
40. Jang AY, Lim S, Jo SH, Han SH, Koh KK. New trends in dyslipidemia treatment. Circ J. (2021) 85:759–68. doi: 10.1253/circj.CJ-20-1037
41. Virani SS, Smith SC Jr, Stone NJ, Grundy SM. Secondary prevention for atherosclerotic cardiovascular disease: comparing recent US and European guidelines on dyslipidemia. Circulation. (2020) 141:1121–3. doi: 10.1161/CIRCULATIONAHA.119.044282
42. Tóth PP, Potter D, Ming EE. Prevalence of lipid abnormalities in the United States: the national health and nutrition examination survey 2003-2006. J Clin Lipidol. (2012) 6:325–30. doi: 10.1016/j.jacl.2012.05.002
43. Downs JR, O'Malley PG. Management of dyslipidemia for cardiovascular disease risk reduction: synopsis of the 2014 U.S. Department of Veterans Affairs and US Department of Defense clinical practice guideline. Ann Intern Med. (2015) 163:291–7. doi: 10.7326/M15-0840
44. Baskaran P, Krishnan V, Fettel K, Gao P, Zhu Z, Ren J, et al. TRPV1 activation counters diet-induced obesity through sirtuin-1 activation and PRDM-16 deacetylation in brown adipose tissue. Int J Obes (Lond). (2017) 41:739–49. doi: 10.1038/ijo.2017.16
45. Joo JI, Kim DH, Choi JW, Yun JW. Proteomic analysis for antiobesity potential of capsaicin on white adipose tissue in rats fed with a high fat diet. J Proteome Res. (2010) 9:2977–87. doi: 10.1021/pr901175w
Keywords: capsaicin, lipid levels, metabolic syndrome, randomized controlled trials, meta-analysis
Citation: Jiang Z, Qu H, Lin G, Shi D, Chen K and Gao Z (2022) Lipid-Lowering Efficacy of the Capsaicin in Patients With Metabolic Syndrome: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Front. Nutr. 9:812294. doi: 10.3389/fnut.2022.812294
Received: 12 November 2021; Accepted: 31 January 2022;
Published: 01 March 2022.
Edited by:
Ellen E. Blaak, Maastricht University, NetherlandsReviewed by:
Alessandro Cavarape, University of Udine, ItalyFederica Fogacci, University of Bologna, Italy
Copyright © 2022 Jiang, Qu, Lin, Shi, Chen and Gao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Keji Chen, a2pjaGVudmlwQDE2My5jb20=; Zhuye Gao, emh1eWVnYW8yMDIxQDE2My5jb20=
†These authors have contributed equally to this work
 Zhonghui Jiang
Zhonghui Jiang Hua Qu1,2†
Hua Qu1,2† Gongyu Lin
Gongyu Lin Dazhuo Shi
Dazhuo Shi Keji Chen
Keji Chen