
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Nutr. , 29 March 2022
Sec. Nutritional Epidemiology
Volume 9 - 2022 | https://doi.org/10.3389/fnut.2022.800996
This article is part of the Research Topic Mediterranean Diet: Health Benefits, Diversity, and Sustainability View all 7 articles
Background: The diet-center hypothesis has gained much support from the apparent protective effect of the Mediterranean diet on breast cancer. However, the evidence of the association between Mediterranean diet adherence and breast cancer molecular subtypes remains small, especially in non-Mediterranean populations.
Methods: The subjects from the Chinese Wuxi Exposure and Breast Cancer Study, a population-based case-control study, included 818 patients and 935 healthy controls. A validated food frequency questionnaire used for diet assessment and a modified version of the alternate Mediterranean Diet Score, which is called the alternate Chinese Diet Score, was developed to assess adherence to a migrated Chinese version of the Mediterranean diet, which we called the vegetable-fruit-soy dietary pattern. Soy foods, rapeseed oil, and coarse cereals replaced legumes, olive oil, and whole grains reflecting the cuisine of the region. We examined the association between the vegetable-fruit-soy diet adherence and breast cancer risk, stratified by menopause status (pre- or postmenopausal) and receptor status [estrogen-receptor (ER), progesterone-receptor (PR) status, and human epidermal growth factor 2 (HER2)] oncogene expression, followed by five specific combinations (ER+, ER–, ER+/PR+,ER–/PR–, and ER–/PR–/HER2–).
Results: The results suggest that the vegetable-fruit-soy dietary pattern was inversely associated with postmenopausal breast cancer risk [4th vs. 1st quartile, odds ratio (OR) = 0.57, 95%CI = 0.41, 0.80; P trend < 0.001] and that the inverse association was somewhat stronger to detect among ER- subtypes (OR = 0.63; 95%CI = 0.37, 0.94; P trend = 0.003) and ER–/PR–subtypes (OR = 0.64; 95%CI = 0.41, 0.93; P trend = 0.012). We did not observe any significant association between the vegetable-fruit-soy diet characteristics and ER+ subtype, as well as between PR+ and ER+/PR+ subtypes.
Conclusion: The favorable influence from the Mediterranean diet may also apply to Chinese women. The vegetable-fruit-soy dietary pattern may reduce the risk of postmenopausal breast cancer, particularly among ER- subtype, and ER–/PR–subtype.
Some extensive cohort studies reported the inverse association for the dietary pattern characterized by the traditional diet among populations in Mediterranean countries of breast cancer risk (1–8), which implies that the Mediterranean diet may be a unique dietary combination for breast cancer prevention. Review studies are reinforcing this hypothesis (9). The Mediterranean diet is a way of eating based on the traditional cuisines of Greece, Italy, and other countries that border the Mediterranean Sea. Plant-based foods, such as whole grains, vegetables, legumes, fruits, nuts, seeds, herbs, and spices, are the foundation of the diet.
Of particular interest were the low breast cancer incidences that have been reported in Asian countries (10). It has been noticed that the traditional Asian diet has much in common with the Mediterranean diet, as both of them are characterized by the high consumption of vegetables (11) and fruits, take more high-quality white meat rather than red meat (12, 13), and place emphasis on monounsaturated:saturated fat ratio (14). So, a hypothesis is proposed that the health benefits of the Mediterranean diet should be transmissible if the food components and their combinations in the Mediterranean diet play an etiologic role in breast cancer.
However, it is almost impossible to perform a long-term feeding trial in a large-scale population in achieving high compliance to follow the Mediterranean diet except with intensive intervention (15). Therefore, based on the original Cretan Mediterranean diet, we modified the diet according to Chinese diet habits; soy foods, rapeseed oil, and coarse cereals replaced legumes, olive oil, and whole grains, reflecting the cuisine of the region. In addition, due to the differences in etiology, it is important to distinguish between premenopausal and postmenopausal breast cancer and different breast tumor molecular subtypes. The recent meta-analysis summaries of six cohort studies that included 680,450 subjects suggested that the Mediterranean diet adherence (MD-adherence) is inversely associated with postmenopausal breast cancer and the inverse association is somewhat stronger among estrogen-receptor (ER)- tumors than ER+ tumors (16). If MD adherence is significantly associated with ER- tumors, which may have important implications for prevention, because of the poorer prognosis of ER- breast cancer.
This study aims to scrutinize the association between a migrated Chinese version of the Mediterranean diet, which we called the vegetable-fruit-soy dietary pattern, and breast cancer risk among Chinese women, stratified by the menopausal status and different breast tumor molecular subtypes.
The Chinese Wuxi Exposure and Breast Cancer Study (2013–2014) was a case-control study of the role of biology, diet, lifestyle, and environmental factors in the etiology of breast cancer in Asian women. Subjects were all adult women and were restricted to local residents who have lived in Wuxi for at least 5 years. All newly diagnosed (the diagnosis date was within 1 year before enrolment) women with breast cancer (ICD code: C50) among local residents identified by cancer registries were eligible to be included as cases. Secondary and recurrent cancers were excluded. Controls were derived from the local area as cases and were 1:1 individually matched with cases by age (±2 years) and residence. As personal information such as name, address, date of birth, and sex for all residents was available in the local demographic information database; eligible controls were randomly identified from this database. For each control chosen, two additional subjects were selected as a backup at the same time. When the first control could not be interviewed, an alternative was enrolled in the study. The selection procedure repeated until an eligible subject was interviewed. A total of 1,042 eligible breast cancer cases and 1,042 health controls were identified during the study period. Case and controls with major dietary habits changed over the past 10 years, which were excluded from this study. In analysis, 818 cases and 935 controls were included, with a frequency match (cases and controls have the same distributions over age and residence). All subjects gave voluntary written informed consent to participate in this research. The Institutional Review Boards of the Jiangsu Centers for Disease Control approved the study protocol.
The usual diet was assessed by a validated, semiquantitative food frequency questionnaire (FFQ), which included 149 items along with the recipes commonly used in China; a detailed description is given in Zhao et al. (17). Nutrient and energy intake were calculated through the Chinese Food Composition Database (2018, 6th version).
Dietary intake assessment included whether the food was consumed, consumption frequency (times of per day/week/month/year), and the average amount of food consumption at each time. The 149 food items in the FFQ were classified into 18 predefined food groups based on similarities in nutrient profile and culinary usage.
A modified version on the alternate Mediterranean Diet Score (aMED) which we called the alternate Chinese Diet Score (aCHD) was developed to assess adherence to a migrated Chinese version of the Mediterranean diet, which we called the vegetable-fruit-soy dietary pattern. Soy foods, rapeseed oil, and coarse cereals replaced legumes, olive oil, and whole grains, reflecting the cuisine of the region. The aMED (18, 19) is an adapted version of the traditional Mediterranean Diet Score created by Trichopoulou et al. (20, 21). The aMED established by Fung et al. (18) included nine dietary components that are typical of the Mediterranean diet. For each of the presumed beneficial food items [vegetables, legumes, fruits, nuts, whole grains, fish, and the ratio of monounsaturated to saturated fatty acid intake (MUFA:SFA)], one point was given when the intake was at least the sex-specific median intake, and zero otherwise. For red and processed meat, 1 point was given (and 0 otherwise) when the intake was below the sex-specific median intake. In the full aMED, 1 additional point is normally given when alcohol intake is between 5 and 25 g/day, and 0 otherwise (19), with a 9-point sum score (minimal to maximal conformity). The aCHD also ranged from zero to nine points in the present analysis (Appendix).
To test the role of the vegetable-fruit-soy dietary pattern in the etiology of breast cancer, we design an interaction test between the aCHD and the duration of diet habits. The “duration” indicator was based on interviewing subjects about how long they have maintained their current diet habits, and the medians were calculated based on the distribution of the premenopausal controls and postmenopausal controls, respectively.
Demographic, lifestyle characteristics, menstrual and reproductive events, dietary intake, medical history, and physical activity-related data were obtained from a structured questionnaire through in-person interviews conducted by trained interviewers. Anthropometric measures were obtained by trained personnel following standardized protocols; the core elements of anthropometry are height, weight, body mass index (BMI), body circumferences (waist, hip, and limbs), and skinfold thickness. Physical activity was measured by referencing the Global Physical Activity Questionnaire (22). Women were considered to be postmenopausal at an absence of menstruation in the past 12 months.
The molecular subtypes of breast tumor based on ER status, progesterone-receptor (PR) status, and human epidermal growth factor 2 (HER2) oncogene expression, followed by five specific combinations (ER+, ER–, ER+/PR+, ER–/PR–, and ER–/PR–/HER2–).
The demographic characteristics and anthropometric measures of the subjects were presented as means± SD for normal distribution or median (Q1 and Q3) for non-normal distribution and frequency percentages. A Student’s t-test or the Wilcoxon rank-sum test and chi-squares tests were performed for continuous and categorical variables, respectively, to compare the differences between cases and controls.
To estimate the impact of the separate foods component of the vegetable-fruit-soy dietary pattern on breast cancer, we calculated adjusted odds ratios (ORs) for the intake ≥ median of the specific foods component compared to the intake < median (as reference), medians were calculated based on the distribution of the premenopausal controls and postmenopausal controls, respectively.
Associations between the aCHD score (divided into four quartiles, as a nominal predictor) and breast cancer were estimated in terms of adjusted ORs and respective 95%CIs by logistic regression models.
In the sensitivity analysis, soy foods were often considered to be an independent potential benefit for breast cancer prevention in Asian populations (23, 24). Thus, we examined the independent effect of soy foods on breast cancer, adjusting other foods components that were considered to be beneficial factors in the migrated vegetable-fruit-soy diet, namely, vegetables, fruit, nuts, cereals, fish, and monounsaturates. In addition, we designed a modified soy-free aCHD score with all soy foods in computation excluded for scrutinizing the joint effects of the vegetable-fruit-soy dietary pattern except for soy foods.
In analyses, we controlled potential confounders associated with breast cancer risk, included age at diagnosis for cases or enrollment for controls (by years), education (ordered as illiterate and primary, middle, and high school, university and above), tobacco smoking (no or yes: including smoking and second-hand smoking ≥ 3days/week), moderate physical activity (minutes/day), oral contraceptives use (no or yes: current use or ever use), hormone replacement therapy (no or yes: current use or ever use), family history of breast cancer (no or yes: in a first-degree relative), history of benign breast disease (no or yes: including lactation mastitis, plasma cell mastitis, cyclomastopathy, fibroadenoma of breast, and galactocele), age at menarche (by years), number of full term births (ordered as 0, 1, 2,or≥3), age at first full-term delivery (by years), breastfeeding (no or yes), height (by cm), BMI (in kg/m2), energy intake (kcal/day). In addition, postmenopausal stratification analysis was further adjusted for the menopausal age (by years). All subjects were included in the present analysis, with the missing covariates data imputed by using the fully conditional specification Multivariate Imputation by the Chained Equations method. Interactions were examined by the likelihood ratio tests (LRTs).
All analyses were performed with R version 4.0.2 (The R Project for Statistical Computing, China).1 Using CIs to measure effect size and to gauge precision, and precise P-values (not just whether P-values are above or below 0.05 or some other threshold), P-value near 0.05 taken by itself offers only weak evidence against the null hypothesis.
The demographic characteristics and anthropometric measures of the subjects stratified by menopausal status are presented in Table 1. The education level of the case group was lower than that of the control group, and the proportion of overweight rate, family history of breast cancer, and history of benign breast disease was higher than that of the control group.
The association between the vegetable-fruit-soy diet adherence and breast cancer is shown in Table 2. We found a significant trend of postmenopausal breast cancer risk reductions with a high aCHD score (P-value for trend < 0.001) and women with the highest quartile aCHD score had a significantly lower risk compared with women with the lowest quartile aCHD score (OR = 0.57; 95%CI = 0.41, 0.80), while no significant association was found for premenopausal breast cancer (Figure 1). Furthermore, we found a significant interaction between the vegetable-fruit-soy diet adherence and the duration of diet habits on postmenopausal breast cancer (P interaction = 0.013). Long-term (≥median) vegetable-fruit-soy diet adherence (highest quartile of aCHD) significantly reduced postmenopausal breast cancer risk compared with short-term (<median) adherence and showed lowest quartile of aCHD (OR = 0.42, 95%CI = 0.26–0.69), Table 3.
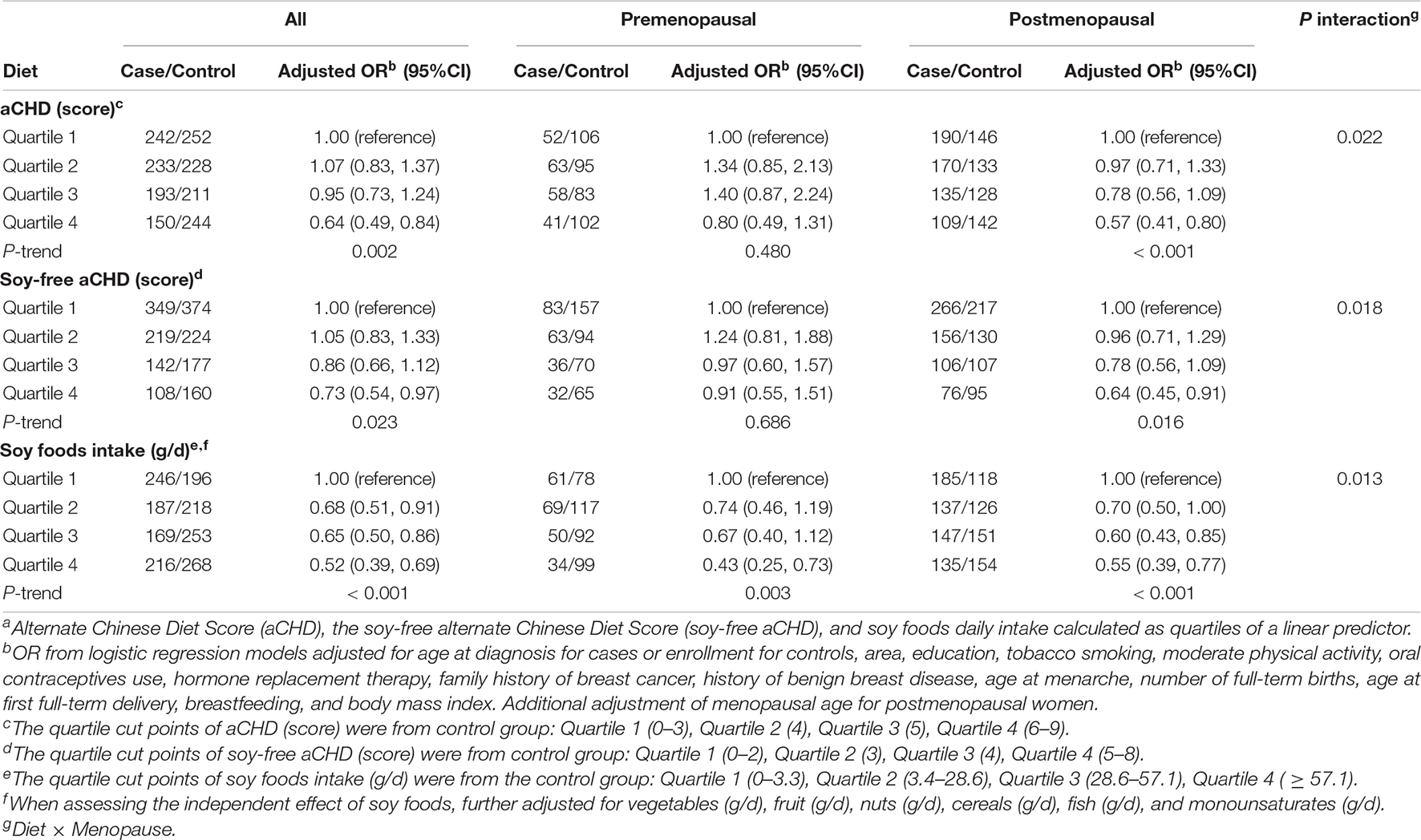
Table 2. Associations (OR and 95%CIs) between the alternate Chinese Diet Score (aCHD), the soy-free alternate Chinese Diet Score (soy-free aCHD), and soy foods daily intake and breast cancer, stratified by menopausal status.a

Table 3. Interaction between the vegetable-fruit-soy diet adherence and the duration on breast cancer risk, stratified by menopausal status.
Breast cancer risk in association with the quartile of soy foods intake and the modified soy-free aCHD score is also shown in Table 2. After controlling other types of beneficial foods components in the vegetable-fruit-soy dietary pattern, the consumption of soy foods still showed a stable inverse association with breast cancer among postmenopausal women (4th vs. 1st quartile, OR was 0.55; 95%CI = 0.39, 0.77; P-value for trend < 0.001). When all soy foods were excluded from the aCHD score calculation, a high soy-free aCHD score was also found to be inversely associated with postmenopausal breast cancer (OR was 0.64; 95%CI = 0.45, 0.91; P-value for trend = 0.016).
For the impact of the separate food components of the vegetable-fruit-soy dietary pattern on breast cancer, we found alcohol consumption to be a significant risk factor for postmenopausal breast cancer (≥median intake vs. < median intake, OR = 3.29; 95%CI = 1.25, 10.3), and a high intake of soy foods was inversely associated with breast cancer (≥median intake vs. <median intake, OR = 0.53; 95%CI = 0.41, 0.70). No significant association was observed between separate food components intake and premenopausal breast cancer risk (Table 4). The average intake of separate foods components of the vegetable-fruit-soy dietary pattern of cases and controls are presented in supplementary material (Supplementary Table 1).
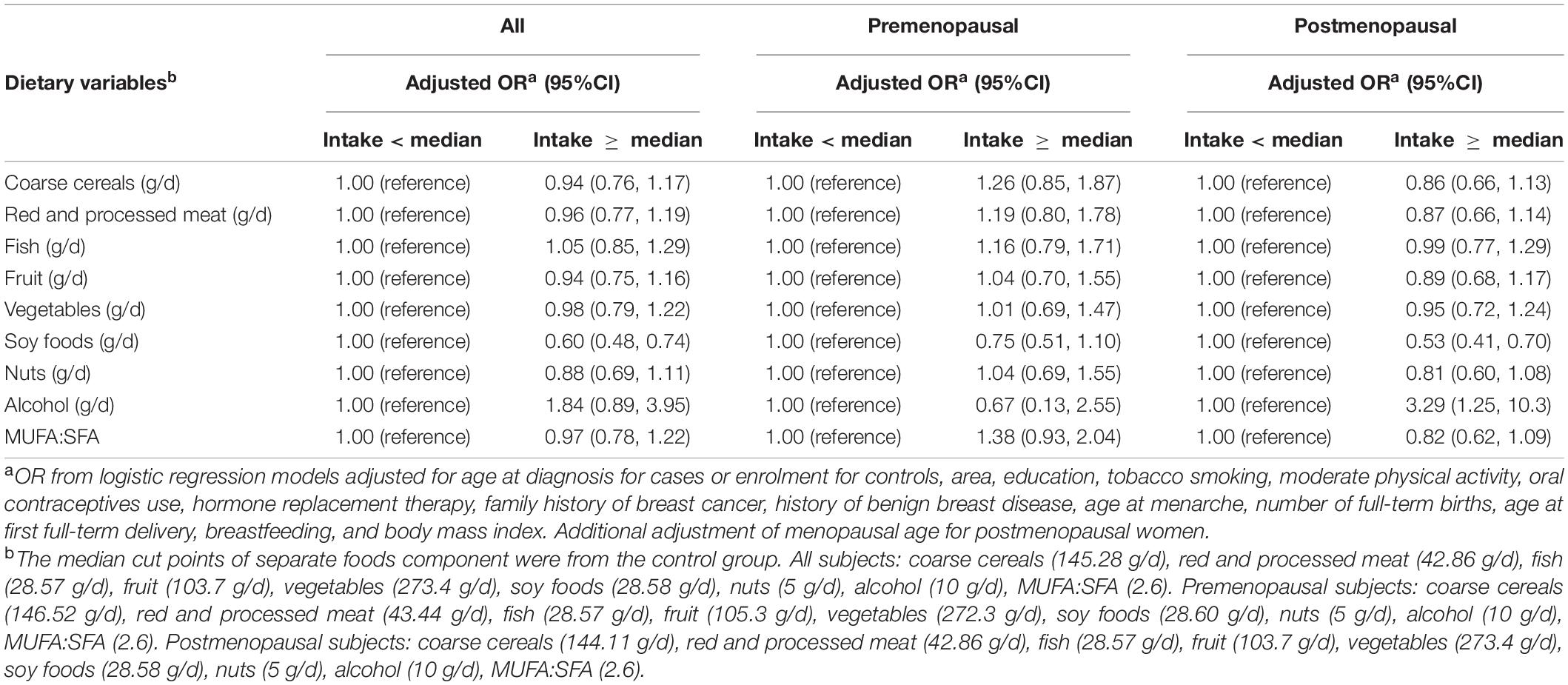
Table 4. Associations (OR and 95%CIs) between separate foods component of the vegetable-fruit-soy dietary pattern and breast cancer, stratification by menopausal status.
Since the significant inverse association with the vegetable-fruit-soy diet adherence was only found in postmenopausal breast cancer, further analyses for different breast tumor molecular subtypes were restricted only to the postmenopausal participants. We observed the vegetable-fruit-soy diet adherence was significantly inversely associated with of ER- subtype (4th vs. 1st quartile, OR = 0.63; 95%CI = 0.37, 0.94; P trend = 0.003); results were similar for ER-/PR- subtype (4th vs. 1st quartile, OR = 0.64; 95%CI = 0.41, 0.93; P trend = 0.012) (Table 5). We did not observe any significant associations between the vegetable-fruit-soy diet adherence and ER+ subtype (4th vs. 1st quartile, OR = 0.84; 95%CI = 0.41, 1.51; P trend = 0.371). A test for heterogeneity examines the null hypothesis that the beneficial effect of vegetable-fruit-soy diet adherence on the ER- subtype is the same as that of the ER+ subtype. However, the test of heterogeneity yields a P-value of 0.013, conventionally interpreted as being non-significant. Results for ER+/PR+ and ER–/PR– breast cancers were similar to those for ER+ and ER– breast tumors, respectively; results for ER–/PR– /HER2– were not significant.
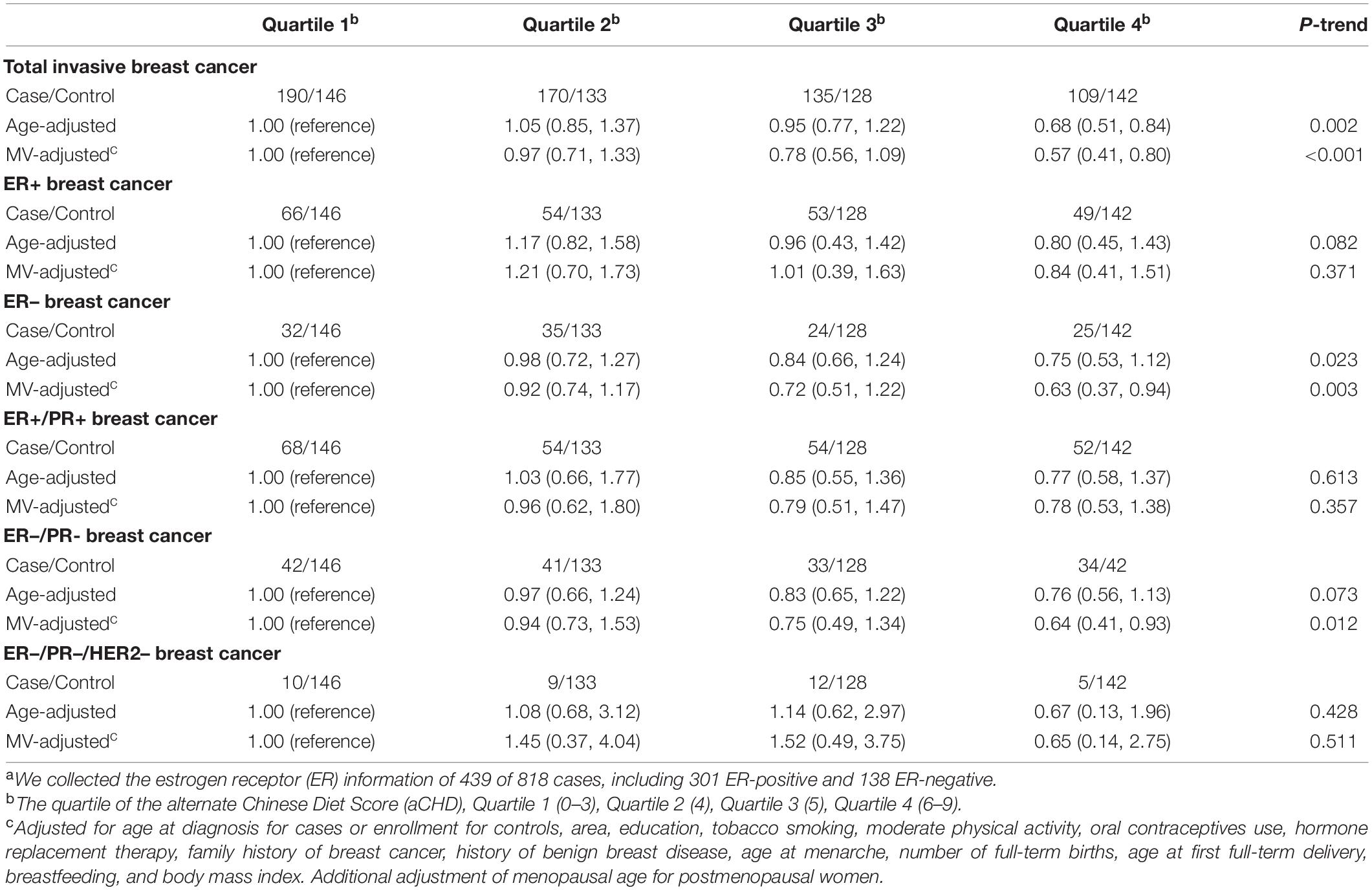
Table 5. Associations (OR and 95%CIs) between the vegetable-fruit-soy diet adherence and different breast tumor molecular subtypes in postmenopausal women.a
We found that the vegetable-fruit-soy diet adherence was inversely associated with postmenopausal breast cancer risk, especially with ER- and ER-/PR-subtypes. This favorable influence of the vegetable-fruit-soy diet adherence on postmenopausal breast cancer risk cannot be explained by the single effect of soy foods since only a very slight attenuation in estimates was noted after excluding the effect of soy foods. Our finding supports the hypothesis that food components and their combinations in the vegetable-fruit-soy dietary pattern play an etiologic role in breast cancer.
The abundant vegetables and moderate fruits intake are the common features of the Mediterranean and Chinese traditional diets (11). Both diets recommend taking more high-quality white meat rather than red meat (12, 13). However, there are also obvious differences between the two; the main differences are the preference of the two diets in the choice of legumes and oils. Legumes in the traditional Mediterranean diet which includes lentils, peas, beans, chickpeas, and the like; chickpeas (also known as garbanzo beans) and fava beans are two of the most common beans in Mediterranean cooking, but other varieties, such as black beans and kidney beans, can also be seen. On the other side, beans are a typical component in the traditional Chinese diet, which included soybeans, broad beans, peas, mung beans, black beans, and many other varieties, and soybeans and related products are the most common. Another major difference is the choice of oils; the Mediterranean region mainly consumes olive oil while the Chinese use other unsaturated fat cooking oils other than olive oil, such as rapeseed, soybean, flaxseed, peanut, corn, and sunflower oils, and rapeseed oils and soybean oils are the most common. Therefore, the migrated vegetable-fruit-soy diet is not a traditional Mediterranean diet because soybeans and rapeseed oils were not traditional Mediterranean foods. However, both of them could be associated with healthy fat characteristics of the traditional Mediterranean diet, and beans provide fiber, protein, carbohydrate, B vitamins, iron, copper, magnesium, manganese, zinc, and phosphorous. They are naturally low in fat and are practically free of saturated fat, and because they are plant foods, they are cholesterol-free as well. Olives oils provide potential breast cancer benefits, which may because they have a high monounsaturated/saturated fat ratio, while rapeseed oils also have a high polyunsaturated/saturated fat ratio with a similar protective effect to that of monounsaturated oil in reducing breast cancer risk. In terms of grain intake, the Mediterranean inhabitants included whole grains as the main carbohydrate source years ago, as was also used in China before the 1990s (25). The distinction is that the former diet included non-refined barley and wheat, the latter mainly consumes coarse cereals. Although differences in dietary composition between the two regions exist, the main aim of the study is to conclude the main commonalities that are major protective factors of breast cancer.
Many have studied the relationship between individual dietary factors and breast cancer. Alcohol consumption appears to be a convincing risk factor (26, 27), whereas fresh fruit and vegetables appear to be protective (28, 29). However, there is still little evidence for the role of most individual foods or nutrients on breast cancer risk. In this study, alcohol intake was also positively associated with breast cancer, while soy foods consumption seems to be the only food ingredient that was negatively correlated with breast cancer. Soy foods are an important potential beneficial factor in traditional Chinese diet practice and our findings are consistent with previous studies (23, 24). The biological mechanisms for soy isoflavones to reduce the risk of breast cancer may be related to preferential binding to ER-b relative to ER-a (30). Soy isoflavones may also act via estrogen-independent mechanisms, such as inhibiting the nuclear transcription factor jB DNA binding activity and the Akt signaling pathway (31), both of which are vital to maintaining a steady-state balance between survival and apoptosis of the cell. Although soy foods seem to be the only food ingredient that may reduce the risk of breast cancer in the present analysis; the favorable influence of the dietary pattern cannot only be explained by the independent effect of separate food ingredients. This is because, when we control the favorable influence of soy foods, the beneficial effects of the vegetable-fruit-soy dietary pattern were still significant.
The possible beneficial effects of the vegetable-fruit-soy dietary pattern on breast cancer can borrow from the physiological mechanisms of the traditional Mediterranean diet. The Mediterranean diet is characterized by a diversity of various polyphenols, especially from nuts, fruits, and legumes, and suggests a potential approach in population to increase polyphenols intake (32). Epidemiological evidence indicates that long-term consumption of a diet rich in plant polyphenols could have a negative impact on the initiation and proliferation of breast cancer, especially with the anti-inflammatory and antioxidant effects that can neutralize free radicals and prevent DNA damage (5, 33–36). Phytoestrogens may have a tumor-inhibiting role as they act like estrogen. The favorable fatty acid profile of the Mediterranean diet may influence breast cancer risk by reducing hyperinsulinemia in cells (3, 16). As fiber intake increases, circulating estrogen and androstenedione levels may decrease, which affects the carcinogenesis of breast cancer (37, 38). The heterogeneity of menopausal status on breast cancer may be due to the greater influence of genetic factors and early-life events of premenopausal breast cancer, which result in the possible protective effect of the vegetable-fruit-soy dietary pattern being more difficult to observe in premenopausal breast cancer (5, 16).
Several prospective studies examined relations between the Mediterranean diet and breast cancer risk, with most (2–6, 8) studies demonstrating an inverse correlation and especially among hormone receptor-negative, but not all (39, 40). The etiology of different breast tumor molecular subtypes may be different, but the evidence remains unclear, especially in different racial populations (40–42). Our finding suggests that the vegetable-fruit-soy dietary pattern is inversely associated with postmenopausal breast cancer and that the inverse association was greater and easier to detect among ER- tumors. As has been suggested before (2, 5), any potential influence of dietary factors may be difficult to detect in ER+ tumors given the strong influence of hormonal factors. In ER- tumors, other risk factors, including diet, may exert a relatively larger influence and may be more easily detectable (2, 16). Findings between different breast tumor molecular subtypes and the vegetable-fruit-soy diet adherence may be particularly important because ER- tumors have a poor prognosis and lower survival rates than ER+ tumors (8, 16, 43).
Several limitations of this study should be noted. First, the data were collected from a case-control study, which might be partially influenced by the selection and recall biases inherent in the design. However, we only included newly diagnosed patients with breast cancer to reduce the recall bias. In addition, the dietary preference was collected based on composite measures, which were less likely to cause selective bias on specific foods/food groups. Second, the vegetable-fruit-soy dietary pattern is not a traditional Mediterranean diet, but the components of the alternative foods of the vegetable-fruit-soy diet that were related to the health characteristics of the traditional Mediterranean diet. The results demonstrated that the foods components and their combinations in the vegetable-fruit-soy dietary pattern may supply benefits in breast cancer prevention and are more suitable for the Chinese population. Finally, the sample size of molecular subtypes of breast tumor are small; we collected the ER information of 439 of 818 cases, including 301 ER-positive and 138 ER-negative. Although there is a suggestion that the Mediterranean diet is inversely associated with ER-/PR-/HER2- (“triple-negative” tumors) (16), our results may lack enough statistical power to find it.
The strengths of our study were the use of a validated food-frequency questionnaire and a modified questionnaire surrounding the Mediterranean dietary pattern and individual dietary factors to examine their association with breast cancer risk in the Chinese female population. Since the diet is a modifiable risk factor, identifying the characterization of the population most susceptible to harmful and beneficial dietary habits is critical for breast cancer prevention policy design. Our findings support vegetable-fruit-soy diet compliance associated with reduced postmenopausal breast cancer risk, especially with ER- or ER-/PR- subtypes.
The favorable influence from the Mediterranean diet pattern may also apply to Chinese women, and the vegetable-fruit-soy dietary pattern reduced the risk of postmenopausal breast cancer, particularly among ER- subtype, and ER–/PR–subtype.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
The studies involving human participants were reviewed and approved by the Review Boards of the Jiangsu Centers for Disease Control. The patients/participants provided their written informed consent to participate in this study.
MW, SC, and PW designed and conducted the study. QZ, ZZ, and JZ developed diet indices and data collection. SC and LL performed the statistical analyses and drafted the manuscript. PW and MW interpreted the data, critically revised the manuscript, and had full responsibility for the analyses and interpretation of the data. SC has full access to all study data. All authors contributed to the preparation of the manuscript and read and approved the final manuscript.
This study was supported by World Cancer Research Fund (2011/RFA/473).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We are grateful to all study participants for their contributions. We thank the entire data collection team. Breast cancer cases and healthy controls in this study were collected by the Wuxi Center for Disease Control and Prevention, Jiangsu Provincial Center for Disease Control and Prevention.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2022.800996/full#supplementary-material
1. Sieri S, Krogh V, Pala V, Muti P, Micheli A, Evangelista A, et al. Dietary patterns and risk of breast cancer in the ORDET cohort. Cancer Epidemiol Biomarkers Prev. (2004) 13:567–72.
2. Fung TT, Hu FB, McCullough ML, Newby P, Willett WC, Holmes MD. Diet quality is associated with the risk of estrogen receptor–negative breast cancer in postmenopausal women. J Nutr. (2006) 136:466–72. doi: 10.1093/jn/136.2.466
3. Trichopoulou A, Bamia C, Lagiou P, Trichopoulos D. Conformity to traditional Mediterranean diet and breast cancer risk in the Greek EPIC (European prospective investigation into cancer and nutrition) cohort. Am J Clin Nutr. (2010) 92:620–5. doi: 10.3945/ajcn.2010.29619
4. Cade J, Taylor E, Burley V, Greenwood D. Does the Mediterranean dietary pattern or the healthy diet index influence the risk of breast cancer in a large British cohort of women? Eur J Clin Nutr. (2011) 65:920–8. doi: 10.1038/ejcn.2011.69
5. Buckland G, Travier N, Cottet V, Gonzalez C, Luján−Barroso L, Agudo A, et al. Adherence to the mediterranean diet and risk of breast cancer in the European prospective investigation into cancer and nutrition cohort study. Int J Cancer. (2013) 132:2918–27. doi: 10.1002/ijc.27958
6. Toledo E, Salas-Salvadó J, Donat-Vargas C, Buil-Cosiales P, Estruch R, Ros E, et al. Mediterranean diet and invasive breast cancer risk among women at high cardiovascular risk in the PREDIMED trial: a randomized clinical trial. JAMA Int Med. (2015) 175:1752–60. doi: 10.1001/jamainternmed.2015.4838
7. Masala G, Bendinelli B, Assedi M, Occhini D, Zanna I, Sieri S, et al. Up to one-third of breast cáncer cases in post-menopausal Mediterranean women might be avoided by modifying lifestyle habits: the EPIC Italy study. Breast Cancer Res Treat. (2017) 161:311–20. doi: 10.1007/s10549-016-4047-x
8. van den Brandt PA, Schulpen M. Mediterranean diet adherence and risk of postmenopausal breast cancer: results of a cohort study and meta−analysis. Int J Cancer. (2017) 140:2220–31. doi: 10.1002/ijc.30654
9. Bloomfield HE, Koeller E, Greer N, MacDonald R, Kane R, Wilt TJ. Effects on health outcomes of a Mediterranean diet with no restriction on fat intake: a systematic review and meta-analysis. Ann Intern Med. (2016) 165:491–500.
10. Torre LA, Siegel RL, Ward EM, Jemal A. Global cancer incidence and mortality rates and trends—an update. Cancer Epidemiol Biomark Prev. (2016) 25:16–27. doi: 10.1158/1055-9965.EPI-15-0578
11. Drewnowski A. New metrics of affordable nutrition: which vegetables provide most nutrients for least cost? J Acad Nutr Diet. (2013) 113:1182–7. doi: 10.1016/j.jand.2013.03.015
12. Pan A, Sun Q, Bernstein AM, Schulze MB, Manson JE, Willett WC, et al. Red meat consumption and risk of type 2 diabetes: 3 cohorts of US adults and an updated meta-analysis. Am J Clin Nutr. (2011) 94:1088–96. doi: 10.3945/ajcn.111.018978
13. He Y, Li Y, Yang X, Hemler EC, Fang Y, Zhao L, et al. The dietary transition and its association with cardiometabolic mortality among Chinese adults, 1982–2012: a cross-sectional population-based study. Lancet Diabetes Endocrinol. (2019) 7:540–8. doi: 10.1016/S2213-8587(19)30152-4
14. Woo J, Woo KS, Leung SS, Chook P, Liu B, Ip R, et al. The Mediterranean score of dietary habits in Chinese populations in four different geographical areas. Eur J Clin Nutr. (2001) 55:215–20. doi: 10.1038/sj.ejcn.1601150
15. Murphy KJ, Parletta N. Implementing a Mediterranean-style diet outside the Mediterranean region. Curr Atheroscler Rep. (2018) 20:28.
16. Coughlin SS, Stewart J, Williams LB. A review of adherence to the Mediterranean diet and breast cancer risk according to estrogen- and progesterone-receptor status and HER2 oncogene expression. Ann Epidemiol Public Health. (2018) 1:1002. doi: 10.33582/2639-4391/1002
17. Zhao W, Hasegawa K, Chen J. The use of food-frequency questionnaires for various purposes in China. Public Health Nutr. (2002) 5:829–33. doi: 10.1079/phn2002374
18. Fung TT, McCullough ML, Newby P, Manson JE, Meigs JB, Rifai N, et al. Diet-quality scores and plasma concentrations of markers of inflammation and endothelial dysfunction. Am J Clin Nutr. (2005) 82:163–73. doi: 10.1093/ajcn.82.1.163
19. Mitrou PN, Kipnis V, Thiébaut AC, Reedy J, Subar AF, Wirfält E, et al. Mediterranean dietary pattern and prediction of all-cause mortality in a US population: results from the NIH-AARP Diet and Health Study. Arch Intern Med. (2007) 167:2461–8. doi: 10.1001/archinte.167.22.2461
20. Trichopoulou A, Kouris-Blazos A, Wahlqvist ML, Gnardellis C, Lagiou P, Polychronopoulos E, et al. Diet and overall survival in elderly people. BMJ. (1995) 311:1457–60. doi: 10.1136/bmj.311.7018.1457
21. Trichopoulou A, Costacou T, Bamia C, Trichopoulos D. Adherence to a Mediterranean diet and survival in a Greek population. N Engl J Med. (2003) 348:2599–608. doi: 10.1056/nejmoa025039
22. Armstrong T, Bull F. Development of the world health organization global physical activity questionnaire (GPAQ). J Public Health. (2006) 14:66–70. doi: 10.1007/s10389-006-0024-x
23. Wu AH, Yu MC, Tseng CC, Pike MC. Epidemiology of soy exposures and breast cancer risk. Br J Cancer. (2008) 98:9–14. doi: 10.1038/sj.bjc.6604145
24. Butler LM, Wu AH, Wang R, Koh W-P, Yuan J-M, Yu MC. A vegetable-fruit-soy dietary pattern protects against breast cancer among postmenopausal Singapore Chinese women. Am J Clin Nutr. (2010) 91:1013–9. doi: 10.3945/ajcn.2009.28572
25. Gong Q, Zhang P, Wang J, Ma J, An Y, Chen Y, et al. Morbidity and mortality after lifestyle intervention for people with impaired glucose tolerance: 30-year results of the Da Qing diabetes prevention outcome study. Lancet Diabetes Endocrinol. (2019) 7:452–61. doi: 10.1016/S2213-8587(19)30093-2
26. Jung S, Wang M, Anderson K, Baglietto L, Bergkvist L, Bernstein L, et al. Alcohol consumption and breast cancer risk by estrogen receptor status: in a pooled analysis of 20 studies. Int J Epidemiol. (2016) 45:916–28. doi: 10.1093/ije/dyv156
27. Choi YJ, Myung SK, Lee JH. Light alcohol drinking and risk of cancer: a meta-analysis of cohort studies. Cancer Res Treat. (2018) 50:474–87. doi: 10.4143/crt.2017.094
28. Jung S, Spiegelman D, Baglietto L, Bernstein L, Boggs DA, van den Brandt PA, et al. Fruit and vegetable intake and risk of breast cancer by hormone receptor status. J Natl Cancer Inst. (2013) 105:219–36. doi: 10.1093/jnci/djs635
29. Emaus MJ, Peeters PH, Bakker MF, Overvad K, Tjønneland A, Olsen A, et al. Vegetable and fruit consumption and the risk of hormone receptor–defined breast cancer in the EPIC cohort. Am J Clin Nutr. (2016) 103:168–77. doi: 10.3945/ajcn.114.101436
30. Ström A, Hartman J, Foster JS, Kietz S, Wimalasena J, Gustafsson J-Å. Estrogen receptor β inhibits 17β-estradiol-stimulated proliferation of the breast cancer cell line T47D. Proc Natl Acad Sci USA. (2004) 101:1566–71. doi: 10.1073/pnas.0308319100
31. Gong L, Li Y, Nedeljkovic-Kurepa A, Sarkar FH. Inactivation of NF-κ B by genistein is mediated via Akt signaling pathway in breast cancer cells. Oncogene. (2003) 22:4702–9. doi: 10.1038/sj.onc.1206583
32. Braakhuis AJ, Campion P, Bishop KS. Reducing breast cancer recurrence: the role of dietary polyphenolics. Nutrients. (2016) 8:547. doi: 10.3390/nu8090547
33. Krinsky NI. Actions of carotenoids in biological systems. Annu Rev Nutr. (1993) 13:561–87. doi: 10.1146/annurev.nu.13.070193.003021
34. Paiva SA, Russell RM. β-carotene and other carotenoids as antioxidants. J Am Coll Nutr. (1999) 18:426–33. doi: 10.1080/07315724.1999.10718880
35. Eliassen AH, Hendrickson SJ, Brinton LA, Buring JE, Campos H, Dai Q, et al. Circulating carotenoids and risk of breast cancer: pooled analysis of eight prospective studies. J Natl Cancer Inst. (2012) 104:1905–16. doi: 10.1093/jnci/djs461
36. Castello A, Pollán M, Buijsse B, Ruiz A, Casas A, Baena-Cañada J, et al. Spanish Mediterranean diet and other dietary patterns and breast cancer risk: case–control EpiGEICAM study. Br J Cancer. (2014) 111:1454–62. doi: 10.1038/bjc.2014.434
37. Rose DP, Goldman M, Connolly JM, Strong LE. High-fiber diet reduces serum estrogen concentrations in premenopausal women. Am J Clin Nutr. (1991) 54:520–5. doi: 10.1093/ajcn/54.3.520
38. Aune D, Chan DS, Greenwood DC, Vieira AR, Rosenblatt DA, Vieira R, et al. Dietary fiber and breast cancer risk: a systematic review and meta-analysis of prospective studies. Ann Oncol. (2012) 23:1394–402. doi: 10.1093/annonc/mdr589
39. Hirko KA, Willett WC, Hankinson SE, Rosner BA, Beck AH, Tamimi RM, et al. Healthy dietary patterns and risk of breast cancer by molecular subtype. Breast Cancer Res Treat. (2016) 155:579–88. doi: 10.1007/s10549-016-3706-2
40. Russnes HG, Lingjaerde OC, Borresen-Dale AL, Caldas C. Breast cancer molecular stratification: from intrinsic subtypes to integrative clusters. Am J Pathol. (2017) 187:2152–62. doi: 10.1016/j.ajpath.2017.04.022
41. Althuis MD, Fergenbaum JH, Garcia-Closas M, Brinton LA, Madigan MP, Sherman ME. Etiology of hormone receptor–defined breast cancer: a systematic review of the literature. Cancer Epidemiol Biomarkers Prev. (2004) 13:1558–68.
42. Anderson KN, Schwab RB, Martinez ME. Reproductive risk factors and breast cancer subtypes: a review of the literature. Breast Cancer Res Treat. (2014) 144:1–10. doi: 10.1007/s10549-014-2852-7
43. Petimar J, Park Y-MM, Smith-Warner SA, Fung TT, Sandler DP. Dietary index scores and invasive breast cancer risk among women with a family history of breast cancer. Am J Clin Nutr. (2019) 109:1393–401. doi: 10.1093/ajcn/nqy392
Keywords: breast cancer, Mediterranean diet, cancer prevention, molecular subtype, vegetable-fruit-soy diet
Citation: Cao S, Liu L, Zhu Q, Zhu Z, Zhou J, Wei P and Wu M (2022) Adherence to the Vegetable-Fruit-Soy Dietary Pattern, a Reference From Mediterranean Diet, Protects Against Postmenopausal Breast Cancer Among Chinese Women. Front. Nutr. 9:800996. doi: 10.3389/fnut.2022.800996
Received: 24 October 2021; Accepted: 25 February 2022;
Published: 29 March 2022.
Edited by:
Maria Hassapidou, International Hellenic University, GreeceReviewed by:
Tommaso Filippini, University of Modena and Reggio Emilia, ItalyCopyright © 2022 Cao, Liu, Zhu, Zhu, Zhou, Wei and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ming Wu, bWluZ3d1QHNldS5lZHUuY24=
†These authors share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.