- Department of General Surgery/Shanghai Clinical Nutrition Research Center, Zhongshan Hospital, Fudan University, Shanghai, China
Background: Skeletal muscle mass deterioration is common in gastric cancer (GC) patients and is linked to poor prognosis. However, information regarding the effect of skeletal muscle mass changes in the postoperative period is scarce. This study was to investigate the link between postoperative loss of skeletal muscle mass and survival following GC surgery.
Methods: Patients who underwent GC surgery between January 2015 and December 2016 were recruited into the study. Computed tomography at L3 vertebral level was used to examine skeletal muscle index prior to surgery and about 6 months after surgery. Skeletal muscle index changes were categorized as presence or absence of ≥5% loss. Overall survival (OS) and disease-free survival (DFS) were analyzed, and Cox proportional hazard models used to identify their predictors.
Results: The study comprised of 318 gastric cancer patients of which 63.5% were male. The group's mean age was 58.14 ± 10.77 years. Sixty-five patients experienced postoperative skeletal muscle index loss ≥5% and had poorer OS (P = 0.004) and DFS (P = 0.020). We find that postoperative skeletal muscle index loss ≥ 5% predicts OS [hazard ratio (HR): 2.769, 95% confidence interval (CI): 1.865–4.111; P < 0.001] and DFS (HR: 2.533, 95% CI: 1.753–3.659; P < 0.001).
Conclusions: Loss of skeletal muscle mass postoperatively is linked to poor survival following GC surgery. Further studies are needed to determine whether stabilizing or enhancing skeletal muscle mass after surgery improves survival.
Introduction
While its incidence rate continues to decrease in most parts of the world, gastric cancer (GC) accounts for the fifth common cancer and become the third leading cause of cancer-related death worldwide (1, 2). GC is mostly diagnosed after it has progressed to an advanced stage, and as such, has a low 5-year survival (3). Surgical resection is the most effective therapeutic intervention against GC (4). However, despite advances in operative techniques and perioperative care, GC prognosis after surgery remains poor (5). Numerous studies have shown that cancer prognosis is conditioned not only by non-modifiable tumor-specific factors such as histology and stage but also modifiable patient-individual factors such as performance status (i.e., patients' physical functioning associated with activities of daily life) and body composition (6–8). Thus, timely identification of these modifiable factors is needed for effective targeted interventions and improved prognosis.
Examination of body composition and its influence on cancer outcomes has drawn growing interest in surgical oncology. Notably, loss of skeletal muscle mass has cancer prognostic value (6–8). Preoperative reduction in skeletal muscle mass is related to poor prognosis after surgical treatment of various cancers, including GC (9–11). Identifying skeletal muscle mass preoperative loss is prognostic and may allow timely therapeutic intervention for better GC outcomes. However, GC patients are often malnourished before surgery, and their malnutrition is often worsened by various factors like postoperative chemotherapy and reduced stomach volume (12). Significant postoperative weight loss has been reported after GC surgery (13, 14), suggesting that muscle wasting might occur in the postoperative period. We have recently reported that after GC surgery, reduced skeletal muscle mass occurs in 3 months after hospital discharge (15). Postoperative skeletal muscle mass has also been reported to negatively impact survival after digestive tract cancer surgeries, including pancreatic, colorectal, and esophageal cancer (7, 16, 17). However, as far as we know, earlier studies mainly concentrated on the influence of preoperative skeletal muscle mass loss on postsurgical GC prognosis. Thus, it is unclear whether the loss of skeletal muscle postoperatively is a risk factor for poor GC prognosis after surgery. If this were the case, then serial assessment of skeletal muscle mass postoperatively may guide efficient interventions.
Here, we aimed to assess postoperative changes in skeletal muscle mass using computed tomography (CT) after GC surgery and to determine whether these changes affect overall and disease-free survival.
Materials and Methods
Study Population
Patients aged > 18 years, who underwent GC surgery between January 2015 and December 2016 at the Department of General Surgery/Shanghai Clinical Nutrition Research Center, Zhongshan Hospital, Fudan University, China, were recruited into the study from our prospective clinical database. Patients under palliative or emergency surgery were excluded from the study. Our institutional ethics committee provided ethical approval for the study, which was conducted based on the Declaration of Helsinki ethical standards.
Assessment of Skeletal Muscle Mass
We utilized routine patient abdominal CT scans to examine skeletal muscle mass, as we previously described (18). The CT images used were either contrast-enhanced or unenhanced multiphase acquisitions, 5 mm thick. Two adjacent CT images at L3 vertebral levels in the same series were chosen in the non-contrast phase. Next, total skeletal muscle area (SMA) was quantified using ImageJ2 software (The National Institutes of Health, Washington, MD, USA) between −29 to +150 Hounsfield units (HU) for skeletal muscle on both slices, and the average SMA reported. Skeletal muscle index (SMI) was computed using the formula: SMI = SMA/height2, expressed in cm2/m2. Anonymized CT images were analyzed by an experienced study evaluator who was not aware of the order of images. All included patients underwent abdominal CT scans within 7 days before surgery and about 6 months after surgery, and SMI changes were calculated. Because skeletal muscle losses ≥ 5% have previously been associated with poor clinical outcomes, including short survival in cancer treatment (19), we used this cutoff threshold to define the postoperative loss of skeletal muscle mass by grouping patients as SMI loss ≥ 5% or SMI loss < 5%.
Data Collection
The clinical data collected included demographics, preoperative characteristics [including BMI (body mass index), ECOG (eastern cooperative oncology group) performance status, serum hemoglobin and albumin level, and comorbidities], operative and pathologic features [including tumor location, type of resection, type of reconstruction, histology, and cancer stage based on the 8th AJCC (American joint committee on cancer) edition], postoperative characteristics (postoperative hospital stay, postoperative complications examined based on the Dindo and Clavien classification), number of patients needing chemotherapy, and chemotherapy tolerance (defined as chemotherapy modification including dose reduction, delay, or termination, and evaluated using a dichotomous scale of absent vs. present) (20). Data on overall survival (OS) and disease-free survival (DFS) were collected. In our prospective clinical database, the follow-up period for all patients was 1st-month post-surgery and after every 3 months, until June 2020.
Statistical Analysis
Statistical analysis was done on SPSS 23.0 software (SPSS Inc., Chicago, IL, USA). Continuous data are expressed as mean ± standard deviation (SD), whereas categorical data are shown as percentages and numbers. Independent-samples t-test or Mann-Whitney U test was employed to analyze continuous variables. χ2 test or the Fisher exact test was used to compare categorical data. Kaplan-Meier analyses were used to generate OS and DFS curves. Variations in survival were analyzed using the log-rank test. The impact of postoperative skeletal muscle mass loss on survival was investigated using Cox proportional hazard models. First, univariate analyses were performed respectively for all potential variables that were chosen based on clinical information. Multivariate analysis was then done using Cox proportional backward stepwise procedure, including all variables with P < 0.05 in the univariate analysis. P < 0.05 indicates statistical significance.
Results
Patient Characteristics
Of the 363 patients who consecutively underwent curative GC surgery from January 2015 to December 2016, 318 patients (63.5% male, mean age 58.14 years) met the inclusion criteria. 65 patients exhibited SMI losses ≥ 5%, while 253 had SMI losses < 5%. Participant characteristics are shown on Table 1. The groups with ≥ 5% SMI loss and the one with < 5% SMI loss were similar with regards to gender, diabetes, respiratory and cardiovascular comorbidity, serum albumin and hemoglobin, preoperative BMI, preoperative SMI, preoperative ECOG performance status, tumor location, type of resection, type of reconstruction, histology, and postoperative hospital stay (P > 0.05). However, ≥ 5% loss significantly correlated with advanced age (60.86 ± 10.73 vs. 57.45 ± 10.68 years, P = 0.022), higher incidence of postoperative complications (26.2 vs. 15.4%; P = 0.043), higher rates of postoperative chemotherapy (78.5 vs. 65.2%; P = 0.041), and chemotherapy modification including dose reduction, delay, or termination (35.4 vs. 19.8%; P = 0.008). Moreover, AJCC stage differed significantly between the two groups (P = 0.010).
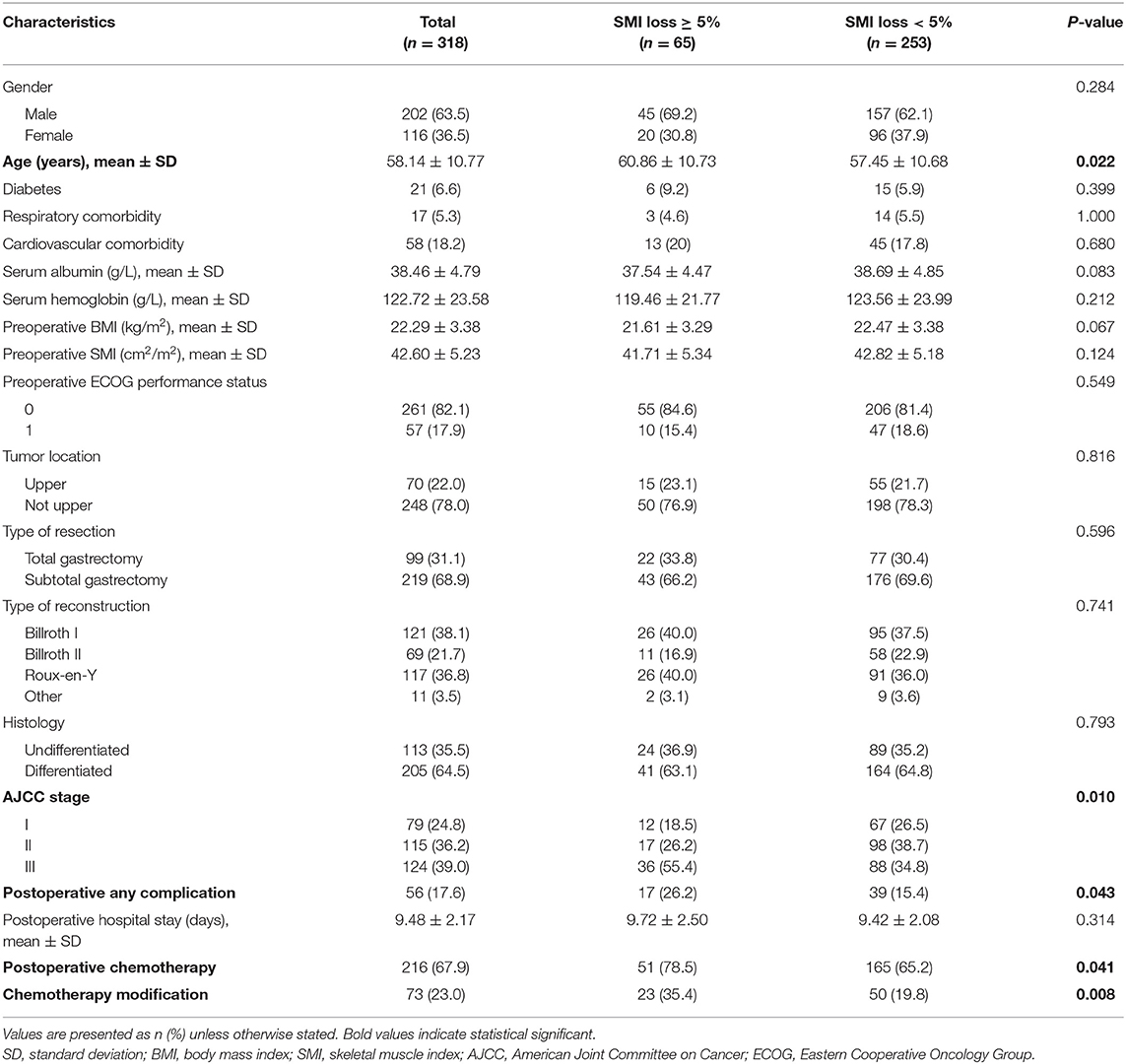
Table 1. Patient demographic and clinical characteristics according to postoperative skeletal muscle mass loss.
Effects of Postoperative Skeletal Muscle Mass Loss on Overall Survival
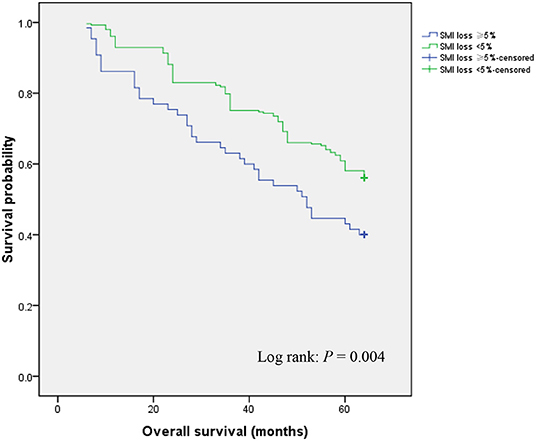
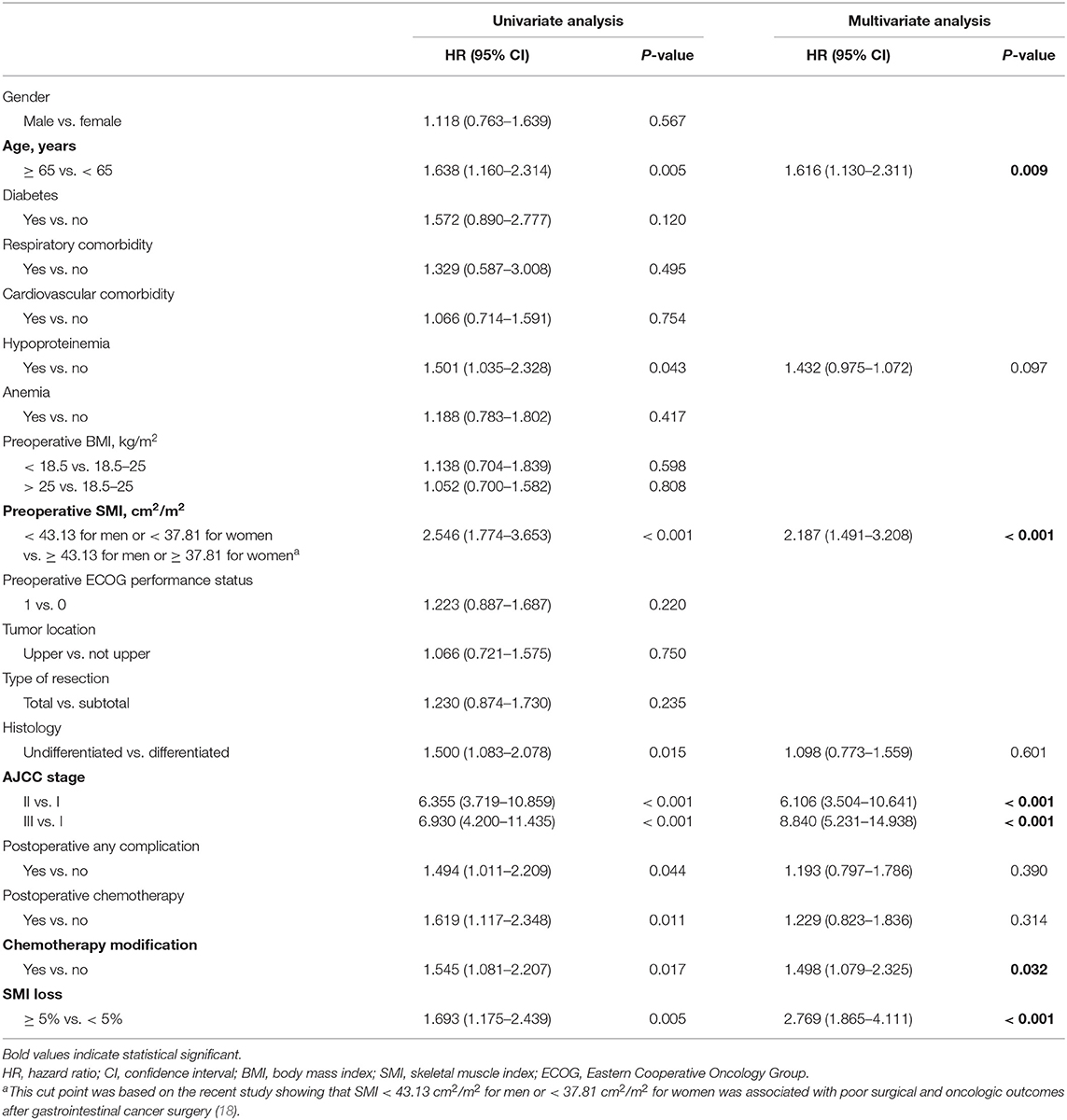
During follow-up, patients who exhibited ≥ 5% SMI loss showed significantly lower OS relative to those with < 5% SMI loss (40.0 vs. 56.1%; P = 0.004) (Figure 1). Univariate and multivariate analyses were used to identify factors influencing OS following GC curative surgery (Table 2). Univariate analysis revealed the following factor as significantly-associated with poor OS: age ≥ 65 years [hazard ratio (HR) = 1.638, 95% confidence interval (CI) = 1.160–2.314; P = 0.005], hypoproteinemia (HR = 1.501, 95% CI = 1.035–2.328; P = 0.043), preoperative SMI (HR = 2.546, 95% CI = 1.774–3.653; P < 0.001), histology (HR = 1.500, 95% CI = 1.083–2.078; P = 0.015), AJCC stage (II vs. I: HR = 6.355, 95% CI = 3.719–10.859; P < 0.001; III vs. I: HR = 6.930, 95% CI = 4.200–11.435; P < 0.001), postoperative any complication (HR = 1.494, 95% CI = 1.011–2.209; P = 0.044), postoperative chemotherapy (HR = 1.619, 95% CI = 1.117–2.348; P = 0.011), chemotherapy modification (HR = 1.545, 95% CI = 1.081–2.207; P = 0.017), and SMI loss ≥ 5% (HR = 1.693, 95% CI = 1.175–2.439; P = 0.005). Multivariate analysis identified the following factors as independently correlating with poor OS: age ≥65 years (HR = 1.616, 95% CI = 1.130–2.311; P = 0.009), preoperative SMI (HR = 2.187, 95% CI = 1.491–3.208; P < 0.001), AJCC stage (II vs. I: HR = 6.106, 95% CI = 3.504–10.641; P < 0.001; III vs. I: HR = 8.840, 95% CI = 5.231–14.938; P < 0.001), chemotherapy modification (HR = 1.498, 95% CI = 1.079–2.325; P = 0.032), and SMI loss ≥ 5% (HR = 2.769, 95% CI = 1.865–4.111; P < 0.001).
Effects of Postoperative Loss of Skeletal Muscle Mass on Disease-Free Survival
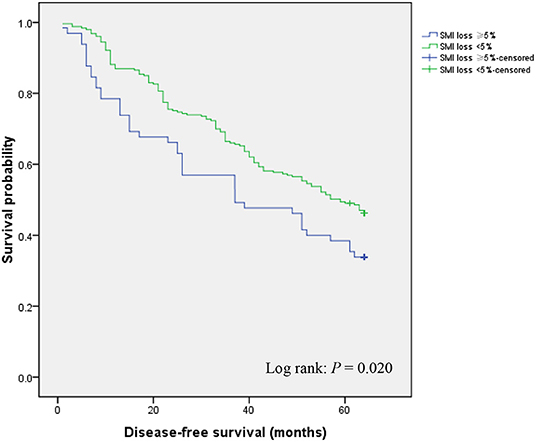
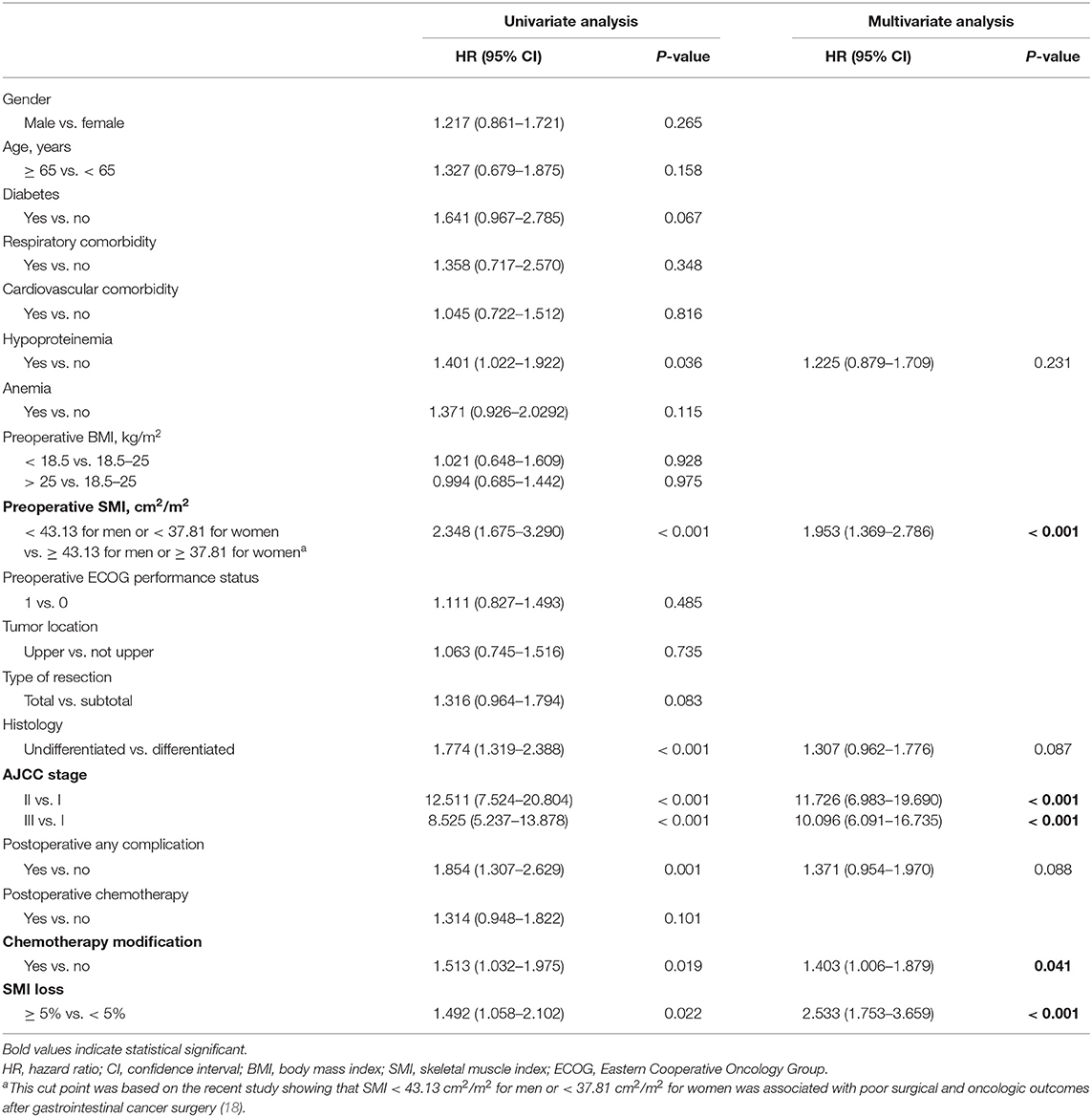
In the course of follow-up, patients who exhibited ≥ 5% SMI loss showed considerably lower DFS rates relative to those with < 5% SMI loss (33.8 vs. 46.2%; P = 0.020) (Figure 2). Univariate and multivariate analyses were used to identify factors influencing DFS following GC curative surgery (Table 3). Univariate analysis revealed the following factors as significantly correlating with poor DFS: hypoproteinemia (HR = 1.401, 95% CI = 1.022–1.922; P = 0.036), preoperative SMI (HR = 2.348, 95% CI = 1.675–3.290; P < 0.001), histology (HR = 1.774, 95% CI = 1.319–2.388; P < 0.001), AJCC stage (II vs. I: HR = 12.511, 95% CI = 7.524–20.804); P < 0.001; III vs. I: HR = 8.525, 95% CI = 5.237–13.878; P < 0.001), postoperative any complication (HR = 1.854, 95% CI = 1.307–2.629; P = 0.001), chemotherapy modification (HR = 1.513, 95% CI = 1.032–1.975; P = 0.019), and ≥ 5% SMI loss (HR = 1.492, 95% CI = 1.058–2.102; P = 0.022). Multivariate analysis identified preoperative SMI (HR = 1.953, 95% CI = 1.369–2.786; P < 0.001), AJCC stage (II vs. I: HR = 11.726, 95% CI = 6.983–19.690; P < 0.001; III vs. I: HR = 10.096, 95% CI = 6.091–16.735; P < 0.001), chemotherapy modification (HR = 1.403, 95% CI = 1.006–1.879; P = 0.041), and SMI loss ≥ 5% (HR = 2.533, 95% CI = 1.753–3.659; P < 0.001) as independently correlated with poor DFS.
Discussion
To our best knowledge, this study was the first report suggesting that postoperative loss of skeletal muscle mass negatively influences OS and DFS in patients following GC surgery. These findings may guide clinicians on the optimal use of prophylactic strategies to reduce postoperative skeletal muscle mass loss, aiming to improve GC outcomes after surgery.
Even though there has been a significant advancement in nutritional support therapy, surgical techniques, and increased recovery rates following surgery, GC surgery is still associated with high malnutrition risk as a result of gastrointestinal complications and reduced food intake. These problems are exacerbated by chronic comorbidities, unintentional weight loss prior to surgery, and postoperative chemotherapy (12). Poor nutrition is linked to poor clinical outcomes, which mainly include increased morbidity and mortality, as well as decreased survival (21–23). Thus, the management of malnutrition is critical for GC treatment and prognosis. Recently, loss of skeletal muscle mass emerged as a prognostic indicator in various cancers during surgery (6–11). However, studies conducted previously primarily focused on the effects of preoperative skeletal muscle mass loss after gastric cancer surgery, and it is unclear whether postoperative skeletal muscle mass loss affects post-GC surgery prognosis. Additionally, postoperative skeletal muscle mass loss negatively impacts survival after digestive tract surgery due to the pancreatic, colorectal, and esophagus cancers (7, 16, 17). Here, we sought to examine skeletal muscle mass postoperative changes after GC surgery and to determine whether these changes affect OS and DFS.
A standard technique for measuring skeletal muscle mass is lacking. Different methods like dual-energy X-ray absorptiometry and CT scanning, are applied to quantitatively measure skeletal muscle mass in clinical practice and research (24). Of the widely used techniques, CT scan has emerged as a reliable method of skeletal muscle mass measurement (25–27). Cross-sectional areas of skeletal muscle tissue on single CT slices at L3 vertebral levels have been shown to strongly correlate with total body skeletal muscle tissue. CT images provide objective quantitative measures of skeletal muscle mass via SMI calculation (28–30). Thus, the assessment of skeletal muscle mass using CT scan at L3 vertebral levels combined with SMI calculation is increasingly used to examine the impact of preoperative skeletal muscle mass changes on clinical outcomes after digestive tract cancer surgery (18, 31, 32). Here, we used CT scan to measure skeletal muscle mass before and approximately 6 months after surgery and calculated SMI changes. We considered ≥ 5% skeletal muscle loss indicative of significant postoperative skeletal muscle mass loss, since it has been previously associated with poor clinical outcomes, including short survival with cancer treatment. Our data revealed 65 of 318 patients as having ≥ 5% SMI loss in the 6 months after GC surgery. ≥ 5% SMI loss significantly correlated with older age, higher incidence of postoperative complications, and higher postoperative chemotherapy and chemotherapy modification (like dose reduction, delay/termination). There was a significant difference in AJCC stage between the two groups. However, the ≥ 5% SMI loss group was comparable to the < 5% SMI loss group with regards to gender, diabetes, respiratory and cardiovascular comorbidity, serum albumin and hemoglobin, preoperative BMI, preoperative SMI, preoperative ECOG performance status, tumor location, type of resection, type of reconstruction, histology, and postoperative hospital stay. These indicate that the ≥ 5% SMI loss criteria used in this study represents significant postoperative skeletal muscle mass loss after GC surgery. Moreover, skeletal muscle mass measurement using abdominal CT scans can be employed to postoperatively evaluate patients, as abdominal CT scans are regularly used, inexpensive, and easy to execute during follow up after GC surgery.
Regarding the survival following cancer surgery, it always receives a significant concern for the prognostic gain following oncologic surgery. Studies have mainly evaluated the association between skeletal muscle mass loss and survival postoperatively. Here, we primarily assessed the effect of postoperative skeletal muscle mass loss on OS and DFS after GC surgery. Our data show that postoperative skeletal muscle mass loss significantly correlates with lower OS and DFS following GC surgery. Multivariate analyses reveal that it is an unfavorable prognostic indicator of disease-free survival. These findings are consistent with previous reports on surgical treatment of other gastrointestinal cancers (7, 16, 17, 33, 34), indicating independent relationship between skeletal muscle mass loss postoperatively and cancer endpoints. Although we did not examine the reasons underlying the strong link between postoperative skeletal muscle mass loss and survival, we speculate that it may be due to multiple factors, including poor tolerability of systemic chemotherapy. Previous studies, including our recent one on digestive cancer surgery, show that loss of skeletal muscle mass may reduce the ability to tolerate systemic chemotherapy. Thus, patients exhibiting low skeletal muscle mass are more likely to experience extreme treatment-associated toxicities, leading to fewer completed chemotherapy cycles (18, 26, 35). Here, postoperative skeletal muscle mass loss was related to more chemotherapy modifications, like dose reduction, delay/termination, and was identified as a risk factor for poor OS and DFS following GC surgery. This could lead to poorer disease control and low survival. Nevertheless, these findings highlight the importance of identifying skeletal muscle mass loss after surgery because it allows prophylactic strategies including the use of proper nutritional support therapy and physical exercise aiming to reduce postoperative skeletal muscle mass loss.
We acknowledge the following limitations in our study. First, our analysis did not examine nutritional intake and physical activity, which are linked to skeletal muscle mass and may affect survival (36). The inclusion of these data would more comprehensively highlight the causal link between skeletal muscle mass loss and poor survival. Secondly, being a single-center study, it may exaggerate the impact of postoperative skeletal muscle mass loss on survival. Thus, there is a need to conduct international multicenter studies to verify these findings. Thirdly, recent evidence indicates that both low skeletal muscle mass and decreased skeletal muscle function influence clinical outcomes (37, 38). However, our study did not capture data on skeletal muscle functions like grip strength/walking speed because of the retrospective design of the study cohort. Further research should evaluate both skeletal muscle mass and function, to evidently reveal the effect of skeletal muscle changes on cancer patients post-surgery. Finally, there were apparent differences in participant characteristics between the two groups, such as AJCC stage, which may affect OS and DFS. The Propensity Score Matching will be conducted to comprehensively answer the question regarding the impact of postoperative loss of skeletal muscle mass on survival after GC surgery. In addition, the univariate and multivariate analyses of risk factors affecting OS and DFS in our study are of great significance, and due to the confounding factors, the significance of OS and DFS in patients with different skeletal muscles is unclear and unreliable. Thus, we will include the related analysis in our future studies in this field of research.
In this study, our data show that postoperative skeletal muscle mass loss negatively affects survival and that it has a strong, independent, prognostic value after GC surgery. Identification of postoperative skeletal muscle mass loss by abdominal CT imaging after GC surgery and targeted approaches to reduce postoperative skeletal muscle mass loss may improve GC outcomes.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by Zhongshan Hospital Fudan University. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
GW supervised the entire project and ST designed the study. QZ, ZZ, SL, JX, JW, YZ, QX, QM, and YJ performed data collection. QZ and ZZ conducted data analyses. ST wrote and revised the manuscript. All authors critically reviewed and approved the final manuscript.
Funding
This study was sponsored by Clinical Research Special Fund of Zhongshan Hospital, Fudan University (2020ZSLC17) and Construction Program of Key but Weak Disciplines of Shanghai Health Commission-Clinical Nutrition (2019ZB0105).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
ST expresses his deepest love, sincerest gratitude and endless miss to his father, Mr. Weilong Tan, for his cultivating parenting and always encouraging during his life and work.
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2018) 68:394–424. doi: 10.3322/caac.21492
2. Arnold M, Park JY, Camargo MC, Lunet N, Forman D, Soerjomataram I. Is gastric cancer becoming a rare disease? a global assessment of predicted incidence trends to 2035. Gut. (2020) 69:823–9. doi: 10.1136/gutjnl-2019-320234
3. Thrift AP, El-Serag HB. Burden of gastric cancer. Clin Gastroenterol Hepatol. (2020) 18:534–42. doi: 10.1016/j.cgh.2019.07.045
4. Yu J, Huang C, Sun Y, Su X, Cao H, Hu J, et al. Effect of laparoscopic vs open distal gastrectomy on 3-year disease-free survival in patients with locally advanced gastric cancer: the CLASS-01 randomized clinical trial. JAMA. (2019) 321:1983–92. doi: 10.1001/jama.2019.5359
5. Petrillo A, Pompella L, Tirino G, Pappalardo A, Laterza MM, Caterino M, et al. Perioperative Treatment in Resectable Gastric Cancer: Current Perspectives and Future Directions. Cancers. (2019) 11:399. doi: 10.3390/cancers11030399
6. Dolan RD, Almasaudi AS, Dieu LB, Horgan PG, McSorley ST, McMillan DC. The relationship between computed tomography-derived body composition, systemic inflammatory response, and survival in patients undergoing surgery for colorectal cancer. J Cachexia Sarcopenia Muscle. (2019) 10:111–22. doi: 10.1002/jcsm.12357
7. Basile D, Parnofiello A, Vitale MG, Cortiula F, Gerratana L, Fanotto V, et al. The IMPACT study: early loss of skeletal muscle mass in advanced pancreatic cancer patients. J Cachexia Sarcopenia Muscle. (2019) 10:368–77. doi: 10.1002/jcsm.12368
8. Huang CY, Yang YC, Chen TC, Chen JR, Chen YJ, Wu MH, et al. Muscle loss during primary debulking surgery and chemotherapy predicts poor survival in advanced-stage ovarian cancer. J Cachexia Sarcopenia Muscle. (2020) 11:534–46. doi: 10.1002/jcsm.12524
9. Choi MH, Yoon SB, Lee K, Song M, Lee IS, Lee MA, et al. Preoperative sarcopenia and post-operative accelerated muscle loss negatively impact survival after resection of pancreatic cancer. J Cachexia Sarcopenia Muscle. (2018) 9:326–34. doi: 10.1002/jcsm.12274
10. Lu J, Zheng ZF Li P, Xie JW, Wang JB, Lin JX, et al. A novel preoperative skeletal muscle measure as a predictor of postoperative complications, long-term survival and tumor recurrence for patients with gastric cancer after radical gastrectomy. Ann Surg Oncol. (2018) 25:439–48. doi: 10.1245/s10434-017-6269-5
11. Shichinohe T, Uemura S, Hirano S, Hosokawa M. Impact of preoperative skeletal muscle mass and nutritional status on short-and long-term outcomes after esophagectomy for esophageal cancer: a retrospective observational study: impact of psoas muscle mass and body mass on esophagectomy. Ann Surg Oncol. (2019) 26:1301–10. doi: 10.1245/s10434-019-07188-z
12. Oh SE, Choi MG, Seo JM, An JY, Lee JH, Sohn TS, et al. Prognostic significance of perioperative nutritional parameters in patients with gastric cancer. Clin Nutr. (2019) 38:870–6. doi: 10.1016/j.clnu.2018.02.015
13. Hatao F, Chen KY, Wu JM, Wang MY, Aikou S, Onoyama H, et al. Randomized controlled clinical trial assessing the effects of oral nutritional supplements in postoperative gastric cancer patients. Langenbecks Arch Surg. (2017) 402:203–11. doi: 10.1007/s00423-016-1527-8
14. Kong SH, Lee HJ, Na JR, Kim WG, Han DS, Park SH, et al. Effect of perioperative oral nutritional supplementation in malnourished patients who undergo gastrectomy: a prospective randomized trial. Surgery. (2018) 164:1263–70. doi: 10.1016/j.surg.2018.05.017
15. Meng Q, Tan S, Jiang Y, Han J, Xi Q, Zhuang Q, et al. Post-discharge oral nutritional supplements with dietary advice in patients at nutritional risk after surgery for gastric cancer: a randomized clinical trial. Clin Nutr. (2020) 40:40–6. doi: 10.1016/j.clnu.2020.04.043
16. Brown JC, Caan BJ, Meyerhardt JA, Weltzien E, Xiao J, Cespedes Feliciano EM, et al. The deterioration of muscle mass and radiodensity is prognostic of poor survival in stage I-III colorectal cancer: a population-based cohort study (C-SCANS). J Cachexia Sarcopenia Muscle. (2018) 9:664–72. doi: 10.1002/jcsm.12305
17. Takahashi K, Watanabe M, Kozuki R, Toihata T, Okamura A, Imamura Y, et al. Prognostic significance of skeletal muscle loss during early postoperative period in elderly patients with esophageal cancer. Ann Surg Oncol. (2019) 26:3727–35. doi: 10.1245/s10434-019-07616-0
18. Zhang S, Tan S, Jiang Y, Xi Q, Meng Q, Zhuang Q, et al. Sarcopenia as a predictor of poor surgical and oncologic outcomes after abdominal surgery for digestive tract cancer: A prospective cohort study. Clin Nutr. (2019) 38:2881–8. doi: 10.1016/j.clnu.2018.12.025
19. Lee J, Lin JB, Wu MH, Jan YT, Chang CL, Huang CY, et al. Muscle radiodensity loss during cancer therapy is predictive for poor survival in advanced endometrial cancer. J Cachexia Sarcopenia Muscle. (2019) 10:814–26. doi: 10.1002/jcsm.12440
20. Blauwhoff-Buskermolen S, Versteeg KS, de van der Schueren MA, den Braver NR, Berkhof J, Langius JA, et al. Loss of Muscle Mass During Chemotherapy Is Predictive for Poor Survival of Patients With Metastatic Colorectal Cancer. J Clin Oncol. (2016) 34:1339–44. doi: 10.1200/JCO.2015.63.6043
21. Fujiya K, Kawamura T, Omae K, Makuuchi R, Irino T, Tokunaga M, et al. Impact of malnutrition after gastrectomy for gastric cancer on long-term survival. Ann Surg Oncol. (2018) 25:974–83. doi: 10.1245/s10434-018-6342-8
22. Li YF, Nie RC, Wu T, Li SM, Chen S, Wang W, et al. Prognostic value of the nutritional risk screening 2002 scale in metastatic gastric cancer: a large-scale cohort study. J Cancer. (2019) 10:112–9. doi: 10.7150/jca.27729
23. Yang Y, Gao P, Song Y, Sun J, Chen X, Zhao J, et al. The prognostic nutritional index is a predictive indicator of prognosis and postoperative complications in gastric cancer: A meta-analysis. Eur J Surg Oncol. (2016) 42:1176–82. doi: 10.1016/j.ejso.2016.05.029
24. Buckinx F, Landi F, Cesari M, Fielding RA, Visser M, Engelke K, et al. Pitfalls in the measurement of muscle mass: a need for a reference standard. J Cachexia Sarcopenia Muscle. (2018) 9:269–78. doi: 10.1002/jcsm.12268
25. Poltronieri TS, de Paula NS, Chaves GV. Assessing skeletal muscle radiodensity by computed tomography: an integrative review of the applied methodologies. Clin Physiol Funct Imaging. (2020) 40:207–23. doi: 10.1111/cpf.12629
26. Duan K, Gao X, Zhu D. The clinical relevance and mechanism of skeletal muscle wasting. Clin Nutr. (2020) 40:27–37. doi: 10.1016/j.clnu.2020.07.029
27. Borggreve AS, den Boer RB, van Boxel GI, de Jong PA, Veldhuis WB, Steenhagen E, et al. The predictive value of low muscle mass as measured on ct scans for postoperative complications and mortality in gastric cancer patients: a systematic review and meta-analysis. J Clin Med. (2020) 9:199. doi: 10.3390/jcm9010199
28. Viertel M, Bock C, Reich M, Löser S, Plauth M. Performance of CT-based low skeletal muscle index, low mean muscle attenuation, and bioelectric impedance derived low phase angle in the detection of an increased risk of nutrition related mortality. Clin Nutr. (2019) 38:2375–80. doi: 10.1016/j.clnu.2018.10.018
29. Nakashima Y, Saeki H, Nakanishi R, Sugiyama M, Kurashige J, Oki E, et al. Assessment of sarcopenia as a predictor of poor outcomes after esophagectomy in elderly patients with esophageal cancer. Ann Surg. (2018) 267:1100–4. doi: 10.1097/SLA.0000000000002252
30. Zwart AT, van der Hoorn A, van Ooijen PMA, Steenbakkers R, de Bock GH, Halmos GB. CT-measured skeletal muscle mass used to assess frailty in patients with head and neck cancer. J Cachexia Sarcopenia Muscle. (2019) 10:1060–9. doi: 10.1002/jcsm.12443
31. Rinninella E, Cintoni M, Raoul P, Pozzo C, Strippoli A, Bria E, et al. Muscle mass, assessed at diagnosis by L3-CT scan as a prognostic marker of clinical outcomes in patients with gastric cancer: a systematic review and meta-analysis. Clin Nutr. (2020) 39:2045–54. doi: 10.1016/j.clnu.2019.10.021
32. Tan S, Meng Q, Jiang Y, Zhuang Q, Xi Q, Xu J, et al. Impact of oral nutritional supplements in post-discharge patients at nutritional risk following colorectal cancer surgery: A randomised clinical trial. Clin Nutr. (2020) 40:47–53. doi: 10.1016/j.clnu.2020.05.038
33. Kudou K, Saeki H, Nakashima Y, Kimura K, Ando K, Oki E, et al. Postoperative skeletal muscle loss predicts poor prognosis of adenocarcinoma of upper stomach and esophagogastric junction. World J Surg. (2019) 43:1068–75. doi: 10.1007/s00268-018-4873-6
34. Kudou K, Saeki H, Nakashima Y, Sasaki S, Jogo T, Hirose K, et al. Postoperative development of sarcopenia is a strong predictor of a poor prognosis in patients with adenocarcinoma of the esophagogastric junction and upper gastric cancer. Am J Surg. (2019) 217:757–63. doi: 10.1016/j.amjsurg.2018.07.003
35. Sealy MJ, Dechaphunkul T, van der Schans CP, Krijnen WP, Roodenburg JLN, Walker J, et al. Low muscle mass is associated with early termination of chemotherapy related to toxicity in patients with head and neck cancer. Clin Nutr. (2020) 39:501–9. doi: 10.1016/j.clnu.2019.02.029
36. Abiri B, Vafa M. Nutrition and sarcopenia: a review of the evidence of nutritional influences. Crit Rev Food Sci Nutr. (2019) 59:1456–66. doi: 10.1080/10408398.2017.1412940
37. Chen L-K, Woo J, Assantachai P, Auyeung T-W, Chou M-Y, Iijima K, et al. Asian working group for sarcopenia: 2019 consensus update on sarcopenia diagnosis and treatment. J Am Med Dir Assoc. (2020) 21:300–7.e2. doi: 10.1016/j.jamda.2019.12.012
Keywords: muscle loss, gastric cancer, surgery, survival, prognosis
Citation: Tan S, Zhuang Q, Zhang Z, Li S, Xu J, Wang J, Zhang Y, Xi Q, Meng Q, Jiang Y and Wu G (2022) Postoperative Loss of Skeletal Muscle Mass Predicts Poor Survival After Gastric Cancer Surgery. Front. Nutr. 9:794576. doi: 10.3389/fnut.2022.794576
Received: 13 October 2021; Accepted: 10 January 2022;
Published: 01 February 2022.
Edited by:
Kalliopi-Anna Poulia, Agricultural University of Athens, GreeceReviewed by:
Gerasimos Terzis, National and Kapodistrian University of Athens, GreeceXinying Wang, Medical School of Nanjing University, China
Copyright © 2022 Tan, Zhuang, Zhang, Li, Xu, Wang, Zhang, Xi, Meng, Jiang and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guohao Wu, prowugh@163.com
†These authors have contributed equally to this work
 Shanjun Tan
Shanjun Tan Qiulin Zhuang†
Qiulin Zhuang† Guohao Wu
Guohao Wu