- 1Community Nutrition Department, School of Nutritional Sciences and Dietetics, Tehran University of Medical Sciences (TUMS), Tehran, Iran
- 2Clinical Nutrition Department, School of Nutritional Sciences and Dietetics, Tehran University of Medical Sciences (TUMS), Tehran, Iran
Objective: We aimed to assess the potential association of dietary (DIS) and lifestyle inflammation score (LIS) and their joint association (DLIS) with cardiorespiratory fitness (CRF) in Tehranian adults.
Design: The present study was designed cross-sectional.
Participants: A total of 265 males and females aged 18–70 years (mean ± SD: 36.9 ± 13.3) were entered in the present cross-sectional study. Eligible participants were healthy men and women who were free of medications and had no acute or chronic infection or inflammatory disease.
Measures: The DIS was calculated by the use of data from 18 anti- and pro-inflammatory dietary components, and the LIS by three non-dietary components including physical activity, smoking status, and general adiposity, with higher scores indicating a more pro-inflammatory diet and lifestyle, respectively. The DLIS was calculated by summing the DIS and LIS. CRF was assessed by the Bruce protocol and VO2 max was measuredas the main variable of CRF. The odds ratio (OR) and 95% confidence interval (CI) of CRF across tertiles of the DIS, LIS, and DLIS were estimated by logistic regression analysis with considering age, gender, energy intake, marital and education status, and occupation as confounders.
Results: The DLIS ranged from −2.10 to 0.38 (mean ± SD: −1.25 ± 0.64). In the model that controlled for all variables, the ORs of CRF for the second and third tertiles of the DLIS as compared to the first tertile were 0.42 (95%CI: 0.20, 0.90) and 0.12 (95%CI: 0.05, 0.32), respectively (P-trend < 0.001). There was a strong inverse association between the LIS and CRF (ORthirdvs.firsttertile: 0.12, 95%CI: 0.05, 0.32). There was no association between DIS and CRF.
Conclusion: The present study examined the joint association of inflammation-related lifestyle behaviors with CRF and found a strong inverse association between a pro-inflammatory lifestyle with CRF. We did not find any association between dietary inflammatory properties with CRF. Future studies should address the relationship between the inflammatory potential of the diet and CRF.
Highlighting Key Findings
Strong inverse associated was found between inflammatory lifestyle and CRF. No association was found between dietary inflammatory index and CRF. No association was found between CRF and DLIS.
Introduction
Cardiorespiratory fitness (CRF) is applied to evaluate a person's ability to perform physical work, and its definition is maximum capacity of the cardiovascular and respiratory system to supply oxygen to the skeletal muscles through exercise (1). CRF represents the ability to transport inhaled oxygen to the mitochondria (2). A maximal cardiorespiratory exercise test is performed to measure CRF and accordingly, it is defined as the level of oxygen consumption at peak exercise performance (VO2 peak), or the maximal oxygen consumption (VO2 max), so VO2 peak or VO2 max is the main variable of CRF (2). CRF is an important health indicator because of its strong relationship with all-cause mortality (3). It is thought to be more important than other traditional risk factors such as smoking, hypertension, high serum cholesterol, and type 2 diabetes (2). CRF can be useful in predicting the risk of cardiovascular disease (CVD), systematic arterial hypertension, diabetes, metabolic syndrome, and cancer (4–9). It has been shown that CVD death rates in individuals with moderate to high levels of CRF were 71% lower than those who are unfit (10). CRF was inversely associated with circulating pro-inflammatory factors including C-reactive protein (CRP), Interleukin (IL)-6, and IL-18, and positively associated with the anti-inflammatory cytokine IL-10 (11). Recently, many studies have reported that high CRF is associated with lower levels of CRP (12, 13).
Although nearly 50% of the individual difference in CRF is thought to be determined by genetic factors, it can also be influenced by lifestyle-related factors such as physical activity, smoking, and body mass index (BMI) (14–16). A lifestyle that includes physical activity can improve the level of CRF especially in those who have a low level of CRF (16). The World Health Organization (WHO) has reported that the recommended physical activity (PA), defined as at least 30 min/d moderate PA, is not met by more than 60% of the world's population (17).
Meanwhile, cigarette smoking has unfavorable effects on CRF, in a way that, aerobic capacity and, thus, the ability of oxygen supply during exercise, declines substantially in smokers (8). Non-smokers had a higher level of VO2 max in comparison with current smokers and quit smokers (14). Increased BMI was also related to lower CRF (15). Poor dietary choices and a massive decrease in work-related PA in both gender and household women lead to the global prevalence of obesity during the past decades (18–20).
The relationship between CRF and dietary intake in young and older adults have been examined by some investigations. It has been documented that older adults who have higher CRF are more likely to follow the dietary recommendations than those who are unfit (21, 22). There is evidence that the diet-disease association may be mediated partly by the potential impact of dietary habits on CRF (23, 24).
Low CRF is an unfavorable health condition that is highly accompanied by low-grade systemic inflammation (25). Sedentary lifestyle (26), cigarette smoking (27), adiposity (28), and poor diet quality (29) are also important drivers of systemic inflammation in the human body. Therefore, the effects of dietary and lifestyle-related factors on CRF may be mediated, in part, by their unfavorable impacts on low-grade systemic inflammation. However, the combined effects of these factors on CRF has not been investigated.
To estimate the inflammatory potential of dietary and lifestyle behaviors, a new index has been developed to take important anti- and pro-inflammatory dietary and non-dietary components into account. The dietary and lifestyle inflammation index (DLIS) is a new-developed index (30) that combined the effects of anti- and pro-inflammatory dietary components, as well as four lifestyle-related components including PA, alcohol drinking, cigarette smoking, and BMI to present a broad picture of the effect of human lifestyle on inflammation. Thus, considering the vital role of CRF in health, the objective of this cross-sectional study was to determine the association of DLIS with CRF in adults.
Methods
Study Population
In this cross-sectional study, 270 adult men and women with an age range from 18 to 70 years who lived in Tehran were recruited. Eligible individuals were invited to attend the School of [double blinded] through advertisement in the local media. Eligible subjects were selected from volunteers according to pre-specified inclusion criteria by using a convenience sampling method. Eligible participants were healthy men and women, aged between 18 and 70 years, who were free of medications and had no acute or chronic infection or inflammatory disease. Subjects were excluded if they used any supplementation, or were lactating or pregnant at the time of the study. All participants received information concerning the background and procedures of the study. Written informed consent was obtained from each participant before data collection. The ethical committee [double blinded].
Demographic Factors
Trained interviewers recorded data about age, sex (male, female), education (Having or not having university education), marriage (single or married), smoking (never or former smoker, current smoker), and occupation (employee, housekeeper, retired, unemployed) by using pre-specified data extraction forms.
Physical Activity
The generally validated International Physical Activity Questionnaire (IPAQ) was applied to evaluate PA levels. PA levels were expressed as metabolic equivalent minutes per week (MET-min/week) (31), and accordingly, subjects were classified into two groups as follow: no or low PA (<600 MET-minute/week) and moderate and high low PA (>600 MET-minute/week).
Anthropometric Measurements
Anthropometric variables consisting of weight and height were measured by trained dieticians. Height was measured using a wall stadiometer (Seca, Germany) and recorded to the nearest 0.1 cm. Weight was measured by an adult's digital scales (808 Seca, Germany) with a sensitivity of 0.1 kg. Anthropometric measurements were performed barefoot and in light clothing. BMI was calculated as weight in kilograms divided by the square of height in meters.
Dietary Assessment
A reliable and validated food frequency questionnaire (FFQ) with 168 food items which was common in Iran (32), was applied to measure usual dietary intakes. Trained nutritionists through face-to-face interviews have asked the frequency (on a daily, weekly, monthly, and annual basis) and amount of consumption of each food item during the past year from the participant. Dietary intakes were then converted to g/d according to household measures (33). Finally, the daily intake of energy and nutrients was estimated using Nutritionist IV software based on the US Department of Agriculture food composition database modified for Iranian foods (34).
Calculating the Dietary and Lifestyle Inflammation Score
The method presented by Byrd et al. was used to calculate the DLIS in the present study (30) (Supplementary Table 1). The construction of this method was validated by a previous study. This score includes dietary inflammation score (DIS) and lifestyle inflammation score (LIS). Components of the diet include 19 variables and components of the lifestyle include four variables. The validation study reported that these dietary and non-dietary components have a significant effect on circulating concentrations of some pro-inflammatory biomarkers including IL-6, IL-8, and CRP or anti-inflammatory biomarkers such as IL-10. Then, the inflammatory potential of each component was scored according to whether it increased pro-inflammatory or declined anti-inflammatory markers, or it reduced pro-inflammatory or increased anti-inflammatory factors.
The DIS components include leafy greens and cruciferous vegetables, tomatoes, apples and berries, deep yellow or orange vegetables and fruit, other fruits and real fruit juices, other vegetables, legumes, fish, poultry, red and organ meats, processed meats, added sugars, high-fat dairy, low-fat dairy, coffee and tea, nuts, other fats, refined grains, starchy vegetables, and supplement score. All of these components were used other than supplement score due to the lack of information regarding supplement use in the study participants. The LIS components include smoking status (“former/never” or “current”), physical activity (“high or moderate” and “low or no physical activity”), and BMI (kg/m2) [“overweight (25–29.9)” and “obese (≥30)]” and alcohol intake. Alcohol intake was not included in the score because of the lack of information regarding the intake of alcohol in Iranian culture. In this study, the DLIS for each subject was calculated by summing the DIS and LIS. Higher DLIS (more positive) presents a more pro-inflammatory diet and lifestyle.
Assessment of Cardiorespiratory Fitness
The maximum rate of oxygen consumed (VO2 max) was measured using a treadmill and respiratory gas analyzer (Cortex Metabolizer 3B) as the main variable of CRF. The subjects warmed up for 5 min on the treadmill at a speed of 5 km/h and then, the Bruce test was used to determine the VO2 max using standard procedures (35). After completing the Bruce test, the subjects walked at a speed of 4 km/h to cool down for 3 min and were encouraged to perform 5 to 10 min of stretching. The conditions for test cessation were: the heart rate up to 90% of the maximum heart rate, a respiratory exchange ratio over 1.1, and having a plateau in oxygen intake, despite increases in exercise intensity. The CRF was expressed as VO2 max and those in the above-median category (>32 vs. <32) were considered to have CRF.
Statistical Analysis
The mean and standard deviation (SD) of continuous variables across tertiles of the DLIS were compared by one-way ANOVA test. The frequency of categorical variables across tertile of the DLIS was assessed by the chi-square test. The odds ratios (OR) and 95% confidence intervals (CI) of CRF (the above-median group as compared with the below-median group) across tertiles of the LIS, DIL, and DLIS and P-trend were determined through binary logistic regression analysis in the crude and adjusted models. In the first model, we adjusted for age, gender, and energy intake. Further adjustments were made for marital status, education, and occupation in the second model. Participants in the first quartile of the DLIS were considered as the reference group. The Statistical Package for the Social Sciences (SPSS version 26; SPSS Inc) was used for performing all statistical analyses. The statistical significance level was defined as p <0.05.
Results
General and Body Composition Characteristics
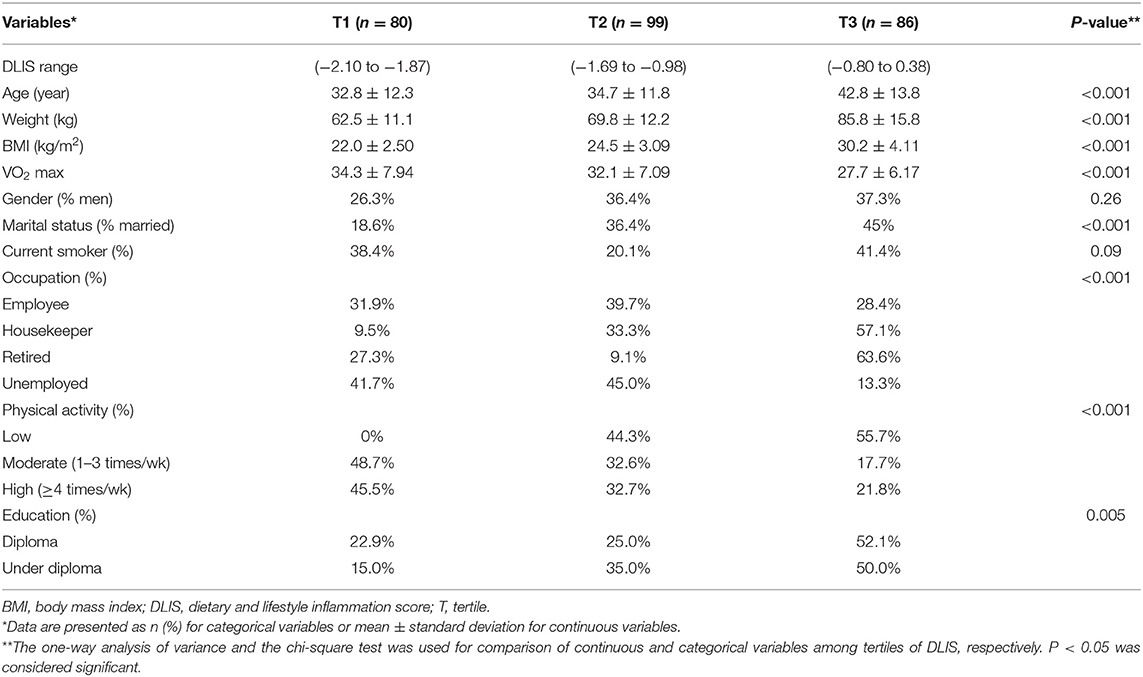
Subject characteristics within each tertile of the DLIS are shown in Table 1. The mean age of participants was 36.9 ± 13.3 years. Those in the top tertile were older and had heavier weight as compared to the first tertile. The mean BMI was 25.7 ± 4.7 kg/m2 that increased proportionally from the first to the third tertile (p < 0.001). The ranges of the DLIS and VO2 max in the study participants were from −2.10 to 0.38 (mean ± SD: −1.25 ± 0.64) and 17 to 54 (mean ± SD: 31.40 ± 7.51), respectively. The mean of VO2 max significantly declined across tertiles of the DLIS (p < 0.001). The DLIS was significantly related to marital status, occupation, PA (P for all < 0.001), and education status (P = 0.005), but was not related to current smoking (P = 0.09).
Dietary Characteristics
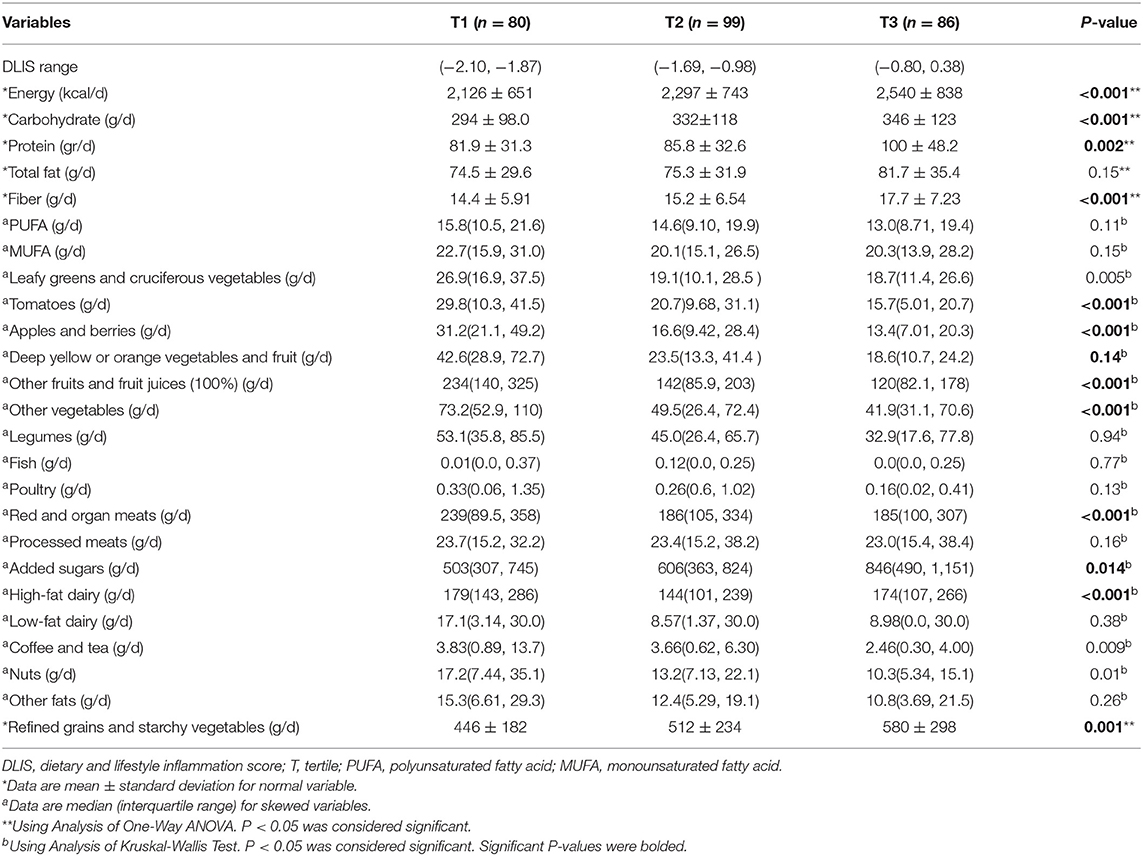
Dietary intakes of the study participants among tertiles of the DLIS are reported in Table 2. Subjects in the highest tertile of the DLIS showed a higher intake of energy, carbohydrate, protein, fiber, leafy greens and cruiferous vegtables, apples and berries, tomatoes, other fruits and juices, other vegtables, reg and organ meat, high-far dairy, coffe and teanuts and refined grains and starchy vegtables (P <0.001). Participants in the highest tertile of the DLIS had higher consumption of added sugars. The intake of other dietary variables did not differ significantly across tertiles of the DLIS.
Association Between DLIS With CRF
The ORs and 95%CIs of CRF among the tertiles of the DLIS, DIS, and LIS are shown in Table 3. The results showed a strong inverse association between higher DLIS and odds of CRF. In the crude model, there was no significant association between the second tertile of the DLIS and odds of CRF, but the third tertile was significantly and strongly associated with lower odds (OR: 0.28, 95%CI: 0.12, 0.44; P for trend < 0.001). The associations became much stronger when we controlled for confounding variables including age, sex, energy intake, marital status, education status, and occupation. In the fully adjusted model, the OR and 95%CI of the CRF for the second and third tertiles of the DLIS were 0.42 (95%CI: 0.20, 0.90) and 0.12 (95%CI: 0.04, 0.32), respectively (P for trend < 0.001).
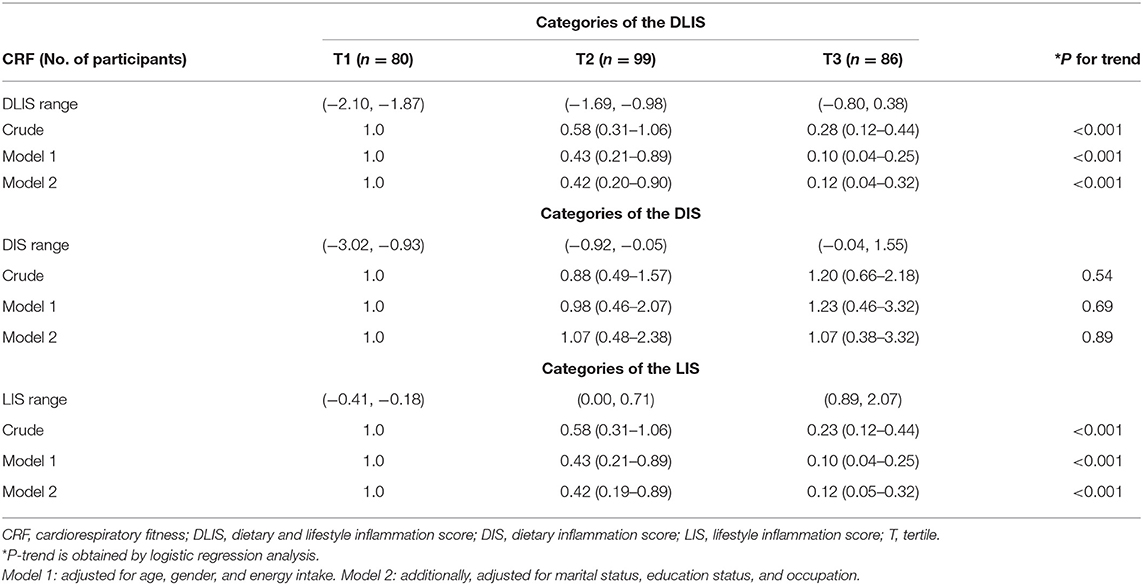
Table 3. The association between the DLIS and cardiorespiratory fitness in adults (odds ratio and 95% confidence interval).
The OR of CRF among the tertiles of DIS showed that there was no significant association between DIS and CRF. This result remained stable after controlling for confounders (OR: 1.07, 95%CI: 0.37, 3.05, P for trend 0.89). Table 3 presents a strong association between LIS and CRF, in a way that odds of having CRF decreased proportionally from the first to the third tertile of LIS in the crude model. Adjustment for confounders made this result stronger (ORthirdvs.firsttertile: 0.12, 95%CI: 0.05, 0.32, P for trend < 0.001).
Discussion
To the best of our knowledge, this is the first study that assessed the association of the inflammatory potential of the diet and lifestyle combined with CRF in young adults. The main finding of our study was that higher adherence to a pro-inflammatory diet and lifestyle, reflected by high DLIS, had a strong inverse association with odds of having CRF. There was also a strong inverse association between LIS and CRF. However, our results indicated no association between the inflammatory potential of the diet, as assessed by DIS, with CRF.
In this study, participants were more likely to have a lower level of CRF with a pro-inflammatory lifestyle. The association between single components of the DLIS and levels of CRF has been investigated in previous research. Recent investigations from the Aerobics Center Longitudinal Study in the US presented that adopting a more anti-inflammatory lifestyle including having higher PA, being at a normal weight, and not smoking were related to higher levels of CRF in both men and women (36). Existing evidence suggests an inverse association between cigarette smoking and levels of CRF (37). De Borba et al. reported that non-smokers showed a higher level of VO2 max compared to active smokers and passive smokers. However, the VO2 max of passive smokers did not differ from active smokers (14). An experimental investigation indicated that even a short-time passive smoker exposure in non-smoking adults can exert substantial adverse effects on the cardiorespiratory and immune response to PA (38).
A randomized controlled trial demonstrated evidence for a dose-response association between PA and progress in CRF. The study mentioned that promoting either quality or quantity of PA seems to have extra influence on CRF (39). Chatrath et al. showed that overweight children are more likely to perform poorly physical tests than their non-obese peers (40); however, such a result was not found in boys (15).
There is also evidence for an association between dietary factors and CRF. Carbone et al. reported that carbohydrate intake, especially in the form of sugars, was inversely, and higher consumption of mono- and polyunsaturated fats were positive, associated with CRF (41). A cross-sectional evaluation within the CARDIA study presented that there is a positive association between diet quality, as assessed by a pre-defined diet quality index, and CRF. The study also showed a negative association between a data-driven meat pattern, rich in red and processed meat, and CRF, and in contrast, a positive association for fruit and vegetable patterns (42). Borney et al. demonstrated that lower consumption of carbohydrates, fiber, folate, calcium, vitamin A, vitamin B-6, and vitamin C; and higher consumption of total, saturated and monounsaturated fats and cholesterol were associated with low physical fitness in both genders (21). However, similar to our findings, some investigations have reported no association between dietary habits and CRF (43–46). The present study is the first try to examine the association of inflammatory potential of the diet with CRF and this highlights the need for further research to fully investigate the association between DIS and CRF.
Recent investigations presented that there is an association between diet and lifestyle with inflammatory factors (47). Montonen et al. documented that there was a relationship between high consumption of whole-grain bread and lower levels of high-sensitive C-reactive protein, whereas a high intake of red meat was related to higher levels (48). Dietary patterns characterized by high intakes of refined starches, sugar, and saturated and trans-fatty acids and low intakes of natural antioxidants, nuts, fiber (from fruits and vegetables), and whole grains may lead to a stimulation of the innate immune system, most likely by an extreme creation of pro-inflammatory cytokines related to a declined production of anti-inflammatory cytokines. Choosing healthy sources of carbohydrate, fat, and protein, in association with regular PA and not smoking have a vital role against inflammatory biomarkers (49–51). Lifestyle factors such as smoking cessation, augmenting PA, and weight loss are associated with a reduction in CRP concentration (52, 53). Therefore, CRF may be associated with inflammatory factors (54, 55), and as a result, the strong inverse association between the DLIS and levels of CRF found in the present study may be mediated partly by inflammatory pathways.
Strengths of this study that are worth to be mentioned are the careful measurements of dietary intakes of each participant which were recorded by skilled nutritionists and valid and reliable questionnaires. Also, we applied a valid tool to calculate the DLIS. Potential limitations should also be considered. The results of our investigation ought to be taken with caution due to its cross-sectional design. Hence, prospective studies are needed to confirm the long-term influence of the DLIS on CRF. Moreover, in this study two items were not included for calculating the DLIS. Missing items include supplementation score and alcohol intake. Although we adjusted several confounding variables in our study, the results may have been affected by residual confounding. Finally, we used FFQ for dietary assessment that has been shown to have some limitations in evaluating dietary information (56). Also, we have to mentioned some of variables were skewed.
Conclusions and Implications
In conclusion, the present study examined the joint association of inflammation-related lifestyle behaviors including cigarette smoking, sedentary lifestyle, and being overweight/obese with CRF and found a strong inverse association between a pro-inflammatory lifestyle with CRF. We did not find any association between dietary inflammatory properties with CRF. Future studies should address the relationship between the inflammatory potential of the diet and CRF.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by the Ethical Committee of the Tehran University of Medical Sciences approved the study protocol (Ethical Approval ID: IR.TUMS.VCR.REC.1397.472). The patients/participants provided their written informed consent to participate in this study.
Author Contributions
SS-B and KD contributed to the conception/design of the research. ZN and NJ contributed to the acquisition of data. MF and ZN participated in the analysis and interpretation of the data. MF drafted the manuscript. AJ critically revised the manuscript. SS-B agrees to be fully accountable for ensuring the integrity and accuracy of the work. All authors read and approved the final manuscript.
Funding
This manuscript has been granted by Tehran University of Medical Sciences (Grant No: 33887). The funder had no role in the design, analysis or writing of this article.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors thank all those who participated in this study.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2022.730841/full#supplementary-material
References
1. Myers J, Kokkinos P, Nyelin E. Physical activity, cardiorespiratory fitness, and the metabolic syndrome. Nutrients. (2019) 11:1652. doi: 10.3390/nu11071652
2. Ross R, Blair SN, Arena R, Church TS, Després J-P, Franklin BA, et al. Importance of assessing cardiorespiratory fitness in clinical practice: a case for fitness as a clinical vital sign: a scientific statement from the American Heart Association. Circulation. (2016) 134:e653–e99. doi: 10.1161/CIR.0000000000000461
3. Lee D-c, Artero EG, Sui X, Blair SN. Mortality trends in the general population: the importance of cardiorespiratory fitness. J Psychopharmacol. (2010) 24:27–35. doi: 10.1177/1359786810382057
4. Barlow CE, LaMonte MJ, FitzGerald SJ, Kampert JB, Perrin JL, Blair SN. Cardiorespiratory fitness is an independent predictor of hypertension incidence among initially normotensive healthy women. Am J Epidemiol. (2006) 163:142–50. doi: 10.1093/aje/kwj019
5. LaMonte MJ, Barlow CE, Jurca R, Kampert JB, Church TS, Blair SN. Cardiorespiratory fitness is inversely associated with the incidence of metabolic syndrome: a prospective study of men and women. Circulation. (2005) 112:505–12. doi: 10.1161/CIRCULATIONAHA.104.503805
6. Lee C-D, Sui X, Hooker SP, Hébert JR, Blair SN. Combined impact of lifestyle factors on cancer mortality in men. Ann Epidemiol. (2011) 21:749–754. doi: 10.1016/j.annepidem.2011.04.010
7. Sawada SS, Lee I-M, Naito H, Noguchi J, Tsukamoto K, Muto T, et al. Long-term trends in cardiorespiratory fitness and the incidence of type 2 diabetes. Diabetes care. (2010) 33:1353–7. doi: 10.2337/dc09-1654
8. Turnovska TH, Mandadzhieva SK, Marinov BI, Kostianev SS. Respiratory and cardiovascular functions among smoking and nonsmoking girls from two regions with different air pollution degree. Int J Hyg Environ Health. (2007) 210:61–8. doi: 10.1016/j.ijheh.2006.08.002
9. Vanhecke TE, Franklin BA, Miller WM, Dejong AT, Coleman CJ, McCullough PA. Cardiorespiratory fitness and sedentary lifestyle in the morbidly obese. Clin Cardiol. (2009) 32:121–4. doi: 10.1002/clc.20458
10. Barlow CE, Kohl HW 3rd, Gibbons L, Blair S. Physical fitness, mortality and obesity. Int J Obes Relat Metab Disord. (1995) 19:S41–4.
11. Wedell-Neergaard A-S, Krogh-Madsen R, Petersen GL, Hansen ÅM, Pedersen BK, Lund R, et al. Cardiorespiratory fitness and the metabolic syndrome: Roles of inflammation and abdominal obesity. PloS ONE. (2018) 13:e0194991. doi: 10.1371/journal.pone.0194991
12. Church TS, Barlow CE, Earnest CP, Kampert JB, Priest EL, Blair SN. Associations between cardiorespiratory fitness and C-reactive protein in men. Arterioscler Thromb Vasc Biol. (2002) 22:1869–76. doi: 10.1161/01.ATV.0000036611.77940.F8
13. Lavie CJ, Church TS, Milani RV, Earnest CP. Impact of physical activity, cardiorespiratory fitness, and exercise training on markers of inflammation. J Cardiopulm Rehabil Prev. (2011) 31:137–45. doi: 10.1097/HCR.0b013e3182122827
14. de Borba AT, Jost RT, Gass R, Nedel R, Cardoso FB, Pohl DM, et al. The influence of active and passive smoking on the cardiorespiratory fitness of adults. Multidiscip Respir Med. (2014) 9:34. doi: 10.1186/2049-6958-9-34
15. Mota J, Flores L, Ribeiro JC, Santos MP. Relationship of single measures of cardiorespiratory fitness and obesity in young schoolchildren. Am J Hum Biol. (2006) 18:335–41. doi: 10.1002/ajhb.20513
16. Oliveira VH, Perazzo JD, Josephson RA, Deminice R, Webel AR. Association between the 6-minute walk test distance and peak cardiorespiratory fitness among people living with HIV varies by fitness level. J Assoc Nurses AIDS Care. (2018) 29:775. doi: 10.1016/j.jana.2018.05.005
17. World Health Organization. The Atlas of Heart Disease and Stroke/Judith Mackay and George Mensah with Shanthi Mendis and Kurt Greenland. World Health Organization (2004). Available online at: https://apps.who.int/iris/handle/10665/43007
18. Archer E, Shook RP, Thomas DM, Church TS, Katzmarzyk PT, Hébert JR, et al. 45-Year trends in women's use of time and household management energy expenditure. PloS ONE. (2013) 8:e56620. doi: 10.1371/journal.pone.0056620
19. Church TS, Thomas DM, Tudor-Locke C, Katzmarzyk PT, Earnest CP, Rodarte RQ, et al. Trends over 5 decades in US occupation-related physical activity and their associations with obesity. PLoS ONE. (2011) 6:e19657. doi: 10.1371/journal.pone.0019657
20. Swinburn B, Sacks G, Ravussin E. Increased food energy supply is more than sufficient to explain the US epidemic of obesity. Am J Clin Nutr. (2009) 90:1453–6. doi: 10.3945/ajcn.2009.28595
21. Brodney S, McPherson RS, Carpenter R, Welten D, Blair SN. Nutrient intake of physically fit and unfit men and women. Med Sci Sports Exerc. (2001) 33:459–67. doi: 10.1097/00005768-200103000-00020
22. Haraldsdóttir J, Andersen LB. Dietary factors related to fitness in young men and women. Prev Med. (1994) 23:490–7. doi: 10.1006/pmed.1994.1067
23. Héroux M, Janssen I, Lam M, Lee D-c, Hebert JR, Sui X, et al. Dietary patterns and the risk of mortality: impact of cardiorespiratory fitness. Int J Epidemiol. (2010) 39:197–209. doi: 10.1093/ije/dyp191
24. Johansson-Persson A, Ulmius M, Cloetens L, Karhu T, Herzig K-H, Onning Gl. A high intake of dietary fiber influences C-reactive protein and fibrinogen, but not glucose and lipid metabolism, in mildly hypercholesterolemic subjects. Eur J Nutr. (2014) 53:39–48. doi: 10.1007/s00394-013-0496-8
25. Madssen E, Skaug E-A, Wisløff U, Ellingsen Ø, Videm V. Inflammation is strongly associated with cardiorespiratory fitness, sex, BMI, and the metabolic syndrome in a self-reported healthy population: HUNT3 fitness study. Mayo Clin Proc. (2019) 94:803–10. doi: 10.1016/j.mayocp.2018.08.040
26. Bruunsgaard H. Physical activity and modulation of systemic low-level inflammation. J Leukoc Biol. (2005) 78:819–35. doi: 10.1189/jlb.0505247
27. Lee J, Taneja V, Vassallo R. Cigarette smoking and inflammation: cellular and molecular mechanisms. J Dent Res. (2012) 91:142–9. doi: 10.1177/0022034511421200
28. Lyon CJ, Law RE, Hsueh WA. Minireview: adiposity, inflammation, and atherogenesis. Endocrinology. (2003) 144:2195–200. doi: 10.1210/en.2003-0285
29. Galland L. Diet and inflammation. Nutr Clin Pract. (2010) 25:634–40. doi: 10.1177/0884533610385703
30. Byrd DA, Judd SE, Flanders WD, Hartman TJ, Fedirko V, Bostick RM. Development and validation of novel dietary and lifestyle inflammation scores. J Nutr. (2019) 149:2206–18. doi: 10.1093/jn/nxz165
31. Craig CL, Marshall AL, Sjöström M, Bauman AE, Booth ML, Ainsworth BE, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. (2003) 35:1381–95. doi: 10.1249/01.MSS.0000078924.61453.FB
32. Mirmiran P, Esfahani FH, Mehrabi Y, Hedayati M, Azizi F. Reliability and relative validity of an FFQ for nutrients in the Tehran lipid and glucose study. Public Health Nutr. (2010) 13:654–62. doi: 10.1017/S1368980009991698
33. Ghaffarpour M, Houshiar-Rad A, Kianfar H. The manual for household measures, cooking yields factors and edible portion of foods. Tehran: Nashre Olume Keshavarzy. (1999) 7:213.
34. Haytowitz D, Lemar L, Pehrsson P, Exler J, Patterson K, Thomas R, et al. USDA National Nutrient Database For Standard Reference, Release 24. Washington, DC, USA: US Department of Agriculture (2011).
35. Bruce RA, Kusumi F, Hosmer D. Maximal oxygen intake and nomographic assessment of functional aerobic impairment in cardiovascular disease. Am Heart J. (1973) 85:546–62. doi: 10.1016/0002-8703(73)90502-4
36. Jackson AS, Sui X, Hebert JR, Church TS, Blair SN. Role of lifestyle and aging on the longitudinal change in cardiorespiratory fitness. Arch Intern Med. (2009) 169:1781–7. doi: 10.1001/archinternmed.2009.312
37. Su FY, Wang SH, Lu HH, Lin GM. Association of tobacco smoking with physical fitness of military males in taiwan: the CHIEF study. Can Respir J. (2020) 2020:5968189. doi: 10.1155/2020/5968189
38. Flouris AD, Metsios GS, Jamurtas AZ, Koutedakis Y. Cardiorespiratory and immune response to physical activity following exposure to a typical smoking environment. Heart. (2010) 96:860–4. doi: 10.1136/hrt.2009.190744
39. Church TS, Earnest CP, Skinner JS, Blair SN. Effects of different doses of physical activity on cardiorespiratory fitness among sedentary, overweight or obese postmenopausal women with elevated blood pressure: a randomized controlled trial. JAMA. (2007) 297:2081–91. doi: 10.1001/jama.297.19.2081
40. Chatrath R, Shenoy R, Serratto M, Thoele D. Physical fitness of urban American children. Pediatr Cardiol. (2002) 23:608–12. doi: 10.1007/s00246-001-0074-3
41. Carbone S, Buckley LF, Trankle CR, Billingsley HE, Dixon DL, Mauro AG, et al. Dietary fat, sugar consumption, and cardiorespiratory fitness in patients with heart failure with preserved ejection fraction. JACC Basic Transl Sci. (2017) 2:513–25. doi: 10.1016/j.jacbts.2017.06.009
42. Shikany JM, Jacobs DR Jr, Lewis CE, Steffen LM, Sternfeld B, Carnethon MR, et al. Associations between food groups, dietary patterns, and cardiorespiratory fitness in the Coronary Artery Risk Development in Young Adults study. Am J Clin Nutr. (2013) 98:1402–9. doi: 10.3945/ajcn.113.058826
43. Butterworth DE, Nieman DC, Underwood BC, Lindsied KD. The relationship between cardiorespiratory fitness, physical activity, and dietary quality. Int J Sport Nutr Exerc Metab. (1994) 4:289–98. doi: 10.1123/ijsn.4.3.289
44. Chan R, Yau F, Yu B, Woo J. The role of dietary patterns in the contribution of cardiorespiratory fitness in community-dwelling older chinese adults in Hong Kong. J Am Med Dir Assoc. (2019) 20:558–63. doi: 10.1016/j.jamda.2018.12.009
45. Estrada-Reyes C, Tlatempa-Sotelo P, Valdés-Ramos R, Cabañas-Armesilla M, Manjarrez-Montes-de-Oca R. Dietary patterns and fitness level in mexican teenagers. J Nutr Metab. (2018). doi: 10.1155/2018/7159216
46. Saeedi P, Black KE, Haszard JJ, Skeaff S, Stoner L, Davidson B, et al. Dietary patterns, cardiorespiratory and muscular fitness in 9–11-year-old children from Dunedin, New Zealand. Nutrients. (2018) 10:887. doi: 10.3390/nu10070887
47. Norde MM, Fisberg RM, Marchioni DM, Rogero MM. Systemic low-grade inflammation–associated lifestyle, diet, and genetic factors: a population-based cross-sectional study. Nutrition. (2020) 70:110596. doi: 10.1016/j.nut.2019.110596
48. Montonen J, Boeing H, Fritsche A, Schleicher E, Joost H-G, Schulze MB, et al. Consumption of red meat and whole-grain bread in relation to biomarkers of obesity, inflammation, glucose metabolism and oxidative stress. Eur J Nutr. (2013) 52:337–45. doi: 10.1007/s00394-012-0340-6
49. Casas-Agustench P, López-Uriarte P, Bull ó M, Ros E, Cabré-Vila J, Salas-Salvadó J. Effects of one serving of mixed nuts on serum lipids, insulin resistance and inflammatory markers in patients with the metabolic syndrome. Nutr Metab Cardiovasc Dis. (2011) 21:126–35. doi: 10.1016/j.numecd.2009.08.005
50. Giugliano D, Ceriello A, Esposito K. The effects of diet on inflammation: emphasis on the metabolic syndrome. J Am Coll Cardiol. (2006) 48:677–85. doi: 10.1016/j.jacc.2006.03.052
51. Palafox-Carlos H, Ayala-Zavala JF, González-Aguilar GA. The role of dietary fiber in the bioaccessibility and bioavailability of fruit and vegetable antioxidants. J Food Sci. (2011) 76:R6–R15. doi: 10.1111/j.1750-3841.2010.01957.x
52. Kelishadi R, Mirghaffari N, Poursafa P, Gidding SS. Lifestyle and environmental factors associated with inflammation, oxidative stress and insulin resistance in children. Atherosclerosis. (2009) 203:311–9. doi: 10.1016/j.atherosclerosis.2008.06.022
53. van't Klooster C, van der Graaf Y, Ridker P, Westerink J, Hjortnaes J, Sluijs I, et al. The relation between healthy lifestyle changes and decrease in systemic inflammation in patients with stable cardiovascular disease. Atherosclerosis. (2020) 301:37–43. doi: 10.1016/j.atherosclerosis.2020.03.022
54. Jae SY, Heffernan KS, Lee M-K, Fernhall B, Park WH. Relation of cardiorespiratory fitness to inflammatory markers, fibrinolytic factors, and lipoprotein (a) in patients with type 2 diabetes mellitus. The American journal of cardiology. (2008) 102:700–3. doi: 10.1016/j.amjcard.2008.05.012
55. Martinez-Gomez D, Eisenmann J, Wärnberg J, Gomez-Martinez S, Veses A, Veiga O, et al. Associations of physical activity, cardiorespiratory fitness and fatness with low-grade inflammation in adolescents: the AFINOS Study. Int J Obes. (2010) 34:1501–7. doi: 10.1038/ijo.2010.114
Keywords: cardiorespiratory fitness, dietary and lifestyle inflammation score, diet, lifestyle, inflammation
Citation: Farazi M, Jayedi A, Noruzi Z, Janbozorgi N, Djafarian K and Shab-Bidar S (2022) Association of Dietary and Lifestyle Inflammation Score With Cardiorespiratory Fitness. Front. Nutr. 9:730841. doi: 10.3389/fnut.2022.730841
Received: 25 June 2021; Accepted: 28 February 2022;
Published: 30 March 2022.
Edited by:
Miguel Luiz Batista Júnior, Boston Medical Center, United StatesReviewed by:
Marcelo Santos, Universidade de São Paulo, BrazilEmmanouella Magriplis, Agricultural University of Athens, Greece
Copyright © 2022 Farazi, Jayedi, Noruzi, Janbozorgi, Djafarian and Shab-Bidar. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sakineh Shab-Bidar, c19zaGFiYmlkYXImI3gwMDA0MDt0dW1zLmFjLmly
 Mena Farazi
Mena Farazi Ahmad Jayedi
Ahmad Jayedi Zahra Noruzi
Zahra Noruzi Nasim Janbozorgi1
Nasim Janbozorgi1 Kurosh Djafarian
Kurosh Djafarian