- 1Student Research Committee, Faculty of Nutrition and Food Science, Tabriz University of Medical Sciences, Tabriz, Iran
- 2Student Research Committee, Ahvaz Jundishapour University of Medical Science, Ahvaz, Iran
- 3Department of Community Nutrition, School of Nutrition and Food Science, Shiraz University of Medical Sciences, Shiraz, Iran
- 4Students' Research Committee, Shiraz University of Medical Sciences, Shiraz, Iran
- 5Health Policy Research Center, Institute of Health, Shiraz University of Medical Sciences, Shiraz, Iran
- 6Department of Statistics and Epidemiology, School of Public Health, Tabriz University of Medical Sciences, Tabriz, Iran
- 7Department of Clinical Nutrition, School of Nutrition and Food Sciences, Shiraz University of Medical Sciences, Shiraz, Iran
- 8Nutrition Research Center, Department of Biochemistry and Diet Therapy, Faculty of Nutrition and Food Sciences, Tabriz University of Medical Sciences, Tabriz, Iran
Purpose: The relationship between the inflammatory and antioxidant potential of an athlete's diet and their oxidative biomarkers is an important area of investigation. Therefore, this study aimed to assess the excretion of 8-hydroxy-2-deoxyguanosine (8-OHdG) and F2alpha-isoprostane (F2a-IP) in the urine of male football players and healthy non-athlete controls. This study also aimed to examine the associations among the dietary inflammatory index (DII), the dietary total antioxidant capacity (DTAC), and the dietary phytochemical index (PI) with 8-OHdG and F2a-IP.
Methods: In this descriptive-analytical study, 45 male football players and 45 healthy non-athletes, who were individually matched based on age and body mass index (BMI), were recruited from Shiraz City, Iran. Fasted urine samples were analyzed for 8-OHdG and F2a-IP levels. Anthropometric measurements were performed, and body composition was assessed using a body composition analyzer. A valid food frequency questionnaire (FFQ) was used to calculate DII, DTAC, and PI scores. Data analysis was conducted using a generalized estimating equation (GEE) model.
Results: We found that 8-OHdG (β = −6.96), F2a-IP (β = −82.58), and DII (β = −2.06) were significantly lower, while DTAC (β = 2.37) and PI (β = 0.084) were significantly higher in the football player group compared with the non-athlete group (P < 0.001 for all variables). In all participants, dietary indices were significantly associated with oxidative biomarkers. DII was positively associated with 8-OHdG (β = 2.25; P < 0.001) and F2a-IP (β = 38.34; P < 0.001). Furthermore, negative associations between DTAC (β = −1.42; P < 0.001) and PI (β = −35.37; P < 0.001) with 8-OHdG were found. Moreover, DTAC (β = −17.34; P < 0.001) and PI (β = −428.11; P = 0.003) were negatively associated with F2a-IP.
Conclusion: The results of this study highlighted the importance of a healthy diet in reducing oxidative stress among football athletes. The levels of urinary biomarkers for DNA and lipid oxidation were found to be lower in football players compared to non-athletes. This suggests that following an anti-inflammatory and antioxidant-rich diet may help reduce oxidative stress in these individuals.
Introduction
Football, the world's most popular sport (1), is a team endurance-speed sport that involves jumps, sprints, changes of direction, and high-intensity running; it relies on both aerobic and anaerobic energy pathways. Consequently, these demanding physical efforts lead to the development of inflammation, muscle damage, and oxidative stress (OS) (2–5).
OS is defined as an unfavorable imbalance between the production of reactive oxygen species (ROS) and antioxidant defense that causes damage to cellular DNA, lipids, and proteins, which can result in reduced athletic performance and impaired recovery (6, 7). The relationship between exercise-induced ROS and cellular damage has been explained by the hormesis theory, which is a bell-shaped curve in which regular/moderate exercise leads to positive consequences. In contrast, high/strenuous exercise leads to negative consequences (8). Oxidative stress-derived DNA and lipid damages can be measured by two reliable biomarkers: urinary excretion rates of 8-hydroxy-2-deoxyguanosine (8-OHdG) (6) and F2alpha-isoprostane (F2a-IP) (9), respectively.
8-OHdG formed from hydroxyl radical attack at the C-8 position of deoxyguanosine (6) and F2a-IP generated from free radical-catalyzed peroxidation of arachidonic acid (9). Several studies have suggested an association between increased levels of urinary 8-OHdG and F2a-IP with some diseases such as cancer, diabetes, and atherosclerosis (10). Factors that have been associated with the levels of urinary 8-OHdG and F2a-IP are body mass index (BMI), age, gender, smoking, dietary factors, and exercise (6, 10). A meta-analysis study concluded that a significant increase in 8-OHdG levels remains between 2 h and one day after acute aerobic exercise but not within 5–28 days post-exercise (11). Another review study summarized that “acute exercise leads to a short-lived increase in plasma and skeletal muscle F2a-IP levels, but regular exercise reveals a trend for decreased urinary F2a-IP levels” (12).
Given the body of evidence that supports the relationship between inflammation status and 8-OHdG and F2a-IP concentrations (13), as well as the evidence for the role of various dietary factors and different food groups in the regulation of inflammatory processes (14, 15), it seems that assessment of the dietary inflammatory index (DII) as a validated tool for predicting the inflammatory potential of diet (16) is useful in expanding our knowledge in this field.
In addition, some previous studies demonstrated that urinary excretion rates of 8-OHdG and F2a-IP have been linked to dietary antioxidant nutrients and antioxidant-rich foods (10, 17). In order to assess the overall dietary and plasma status of antioxidants, dietary total antioxidant capacity (DTAC) is a useful, validated tool (18). In this line, a study has reported a negative correlation between DTAC and the levels of 8-isoprostaglandin F2alpha in athletes (19). Moreover, the dietary phytochemical index (PI) is a suitable tool to evaluate the received amount of phytochemicals (20). Phytochemicals, which are bioactive chemicals in antioxidant-rich foods, as anti-inflammatory agents and antioxidants, play beneficial roles in OS, immunity, and inflammation status (21).
To our knowledge, to date, no single study has been conducted regarding the association between urinary biomarkers of oxidative stress and dietary pro-inflammatory and antioxidant indices in football players. Given the limited data, the objective of the present study was to assess the urinary excretions of 8-OHdG and F2a-IP and also evaluate DII, DTAC, and PI scores, as well as investigate the relationships between the abovementioned dietary indices with 8-OHdG and F2a-IP in male football players and their healthy non-athlete controls.
Materials and methods
Study design and participants
The present study was a descriptive-analytical investigation that compared two groups of 90 men (45 football players and 45 healthy non-athlete controls). The two groups were matched based on age and BMI. The football players and non-athletes were recruited through cluster sampling among 36 football clubs in Shiraz City, Iran, and 10 schools at Shiraz University of Medical Sciences, respectively. Among the football clubs, five clubs were randomly selected, and nine football players who met the inclusion criteria were randomly included from each selected club. Likewise, five schools were randomly selected among Shiraz University's schools, and after that, nine eligible persons were randomly included from each selected school.
The inclusion criteria for football players consist of (1) the willingness of subjects to participate in this research and complete the consent form; (2) individuals aged 20–30 years with a BMI of 20–25 (kg/m2); (3) football experience at least for the last 2–3 years and following the training protocol of 3–4 days/week and 90–120 min/session; (4) a metabolic equivalent of task (MET) of more than 3,000 (min/week); (5) stability in eating behaviors and weight within the last 2 months; (6) not taking omega-3 and antioxidant supplements within the last month; (7) no alcohol or smoking; and (8) giving up caffeine within 24 h before urine sampling. The inclusion criteria for non-athletes included the willingness of subjects to participate and sign the consent form, individuals matching with football players (based on age and BMI), a MET between 600 and 3,000 (min/week), and items #5-8, the criteria mentioned above for the football player group.
The following were regarded as exclusion criteria for both groups: (1) the presence of infectious, inflammatory, cardiovascular, liver, kidney, and respiratory diseases, hypertension, high blood lipids, stroke, thyroid problems, and malignancies; (2) taking drugs that alter the metabolism of oxidants and antioxidants in the past month; (3) being a consumer of non-steroidal anti-inflammatory drugs (NSAIDs); (4) diseases that affect the oxidation of nucleic acids, such as diabetes, hemochromatosis, schizophrenia, and bipolar disorder; (5) hormone therapy; (6) smoking and drinking alcohol; and (7) those who answered < 90% of items on the food frequency questionnaire (FFQ).
Sample size
The sample size was calculated using G-power software based on the mean difference in the urinary level of 8-OHdG between athlete and non-athlete groups from Rahimi et al.'s study (22). Due to the existing correlation between matched athletes and non-athletes, the sample size was calculated based on paired-design studies by considering a 0.15 correlation between the urinary level of 8-OHdG in matched athletes and non-athletes, 80% power, and 95% confidence. The required sample size for each group was 43 participants. By considering about 5% of the sample drop, the final sample size for each group increased to 45 participants.
Measurements
The inclusion criteria were assessed on the first visit, and then anthropometric measurements, a dietary assessment, and urine samples were taken from eligible participants on the second visit. Interviewer-administered questionnaires were used to collect personal anthropometric, physical activity, medical history, general nutrition, and dietary intake information. To prevent measurement errors, all items were measured by the same person. Anthropometric measures and urine sampling were obtained in the morning.
The height and weight were measured using a wall-mounted Seca stadiometer and a digital Seca scale (with a sensitivity of 0.1 cm and 0.1 kg) (Seca, Germany), respectively, while the participant wore light clothes without shoes. BMI was considered to be weight (kg) divided by height squared (m2). Waist circumference (WC) was measured using an inelastic tape at the point midway between the lowest rib and the iliac crest at the end of a normal exhalation without imposing any pressure on the body surface to the nearest 0.1 cm. Hip circumference (HC) was measured at the maximum level of buttock extension using an inelastic tape measure (with a precision of 0.1 cm). Waist-to-hip ratio (WHR) was calculated by dividing WC (cm) by HC (cm).
Body composition, including fat mass (FM), fat-free mass (FFM), total body water (TBW), and total body fat (TBF), was estimated using the InBody 270 body composition analyzer. Participants were instructed to follow certain guidelines before the test: (1) avoiding heavy physical activity during the last 3 days; (2) adequate hydration during the last 2 h; (3) abstaining from caffeine and large meals during the last 24 and 12 h, respectively; (4) emptying their bladder. Physical activity was calculated using the validated short form of the international physical activity questionnaire (IPAQ) (23) and expressed as the MET hours per week (MET-h/week).
Laboratory assays
After a 12-h fasting period, morning urine samples were collected from 8–10 (a.m.) from all participants (for football players, 3 days after the last football training). Urinary levels of 8-OHdG and F2a-IP were determined by an enzyme-linked immunosorbent assay (ELISA) using commercial kits (the 8-OHdG kit, abx150312, Abbexa, United Kingdom, and the 8-epi PGF2a kit, abx150311, Abbexa, United Kingdom) according to the manufacturer's instructions. Values for 8-OHdG and F2a-IP were normalized by creatinine measured in urine (spectrophotometry creatinine test kit, Pars Azmoon, Tehran, Iran).
Assessment of dietary intake
Dietary intake over the previous year was obtained by applying a valid 168-item, semi-quantitative FFQ (24) via a face-to-face interview with a trained dietitian. This questionnaire reported the frequency of consumption of each food item on a daily, weekly, monthly, or yearly basis. Next, the reported frequency of each food intake was converted to a gram per day using household measures (25). Then, Nutritionist IV software (First Databank Division, The Hearst Corporation, San Bruno, CA, USA) modified for Iranian foods was used to calculate the energy and nutrient content of each food item.
The DII, DTAC, and PI were calculated according to the dietary data derived from the FFQ.
Calculation of DII
The DII score was calculated based on the method proposed in the study by Shivappa et al. (16). In the current study, we used 31 food parameters to calculate DII [energy, carbohydrate, protein, fat, vitamin B12, vitamin B9, vitamin B6, vitamin B3, vitamin B2, vitamin B1, beta-carotene, vitamin A, vitamin C, vitamin D, vitamin E, fiber, cholesterol, saturated fat, trans fat, monounsaturated fatty acids (MUFA), polyunsaturated fatty acids (PUFA), omega3, omega6, iron, zinc, selenium, magnesium, caffeine, garlic, onion, and tea].
Initially, energy-adjusted amounts of each nutrient were estimated using the residual method (26). Then, the z-score was calculated by subtracting the “standard global mean” from the actual food parameter value and dividing it by its “global standard deviation.” After that, the z-score was converted to a percentile and centered by multiplying by two and subtracting one score in order to minimize the effect of outliers or right-skewing, as earlier studies did. This value was then multiplied by the respective food inflammatory effect score to obtain the food parameter-specific DII score. Then, all of them were summed up to create the overall DII score for each participant. A more positive DII score indicates a greater pro-inflammatory potential of the participant's diet.
Calculation of DTAC
DTAC was calculated based on the ferric-reducing antioxidant power (FRAP) values from the study by Carlsen et al. (27). The FRAP assay measures the potential of dietary antioxidants to reduce Fe3+ to Fe2+ ions and is expressed as mmol per 100 g of food (mmol/100 g) (28). At first, the frequency of consumption of each food item (gr) was multiplied by its related antioxidant capacity value per 100 g and then summed to obtain the total dietary antioxidant capacity. For similar food items, for example, several types of bread, the overall mean value of DTAC was considered. For food items for which data were not available, the value of the nearest comparable food was assigned.
Calculation of PI
As developed by McCarty (20), the PI was calculated as the percentage of the daily dietary energy derived from phytochemical-rich foods (kcal) divided by the total daily energy intake (kcal) [PI = (phytochemical kcal/total kcal) * 100]. Phytochemical-rich foods considered in this study were as follows: whole grains, nuts, legumes, seeds, vegetables, fruits, and others (olive oil, tea, and coffee).
Statistical analysis
Statistical Package for Social Sciences (SPSS) version 25.0 was applied to perform statistical data analysis. The normality of the data distribution was evaluated by the Kolmogorov and Smirnov tests. The characteristics of all participants are expressed as mean ± standard deviation (SD) for normally distributed variables. By considering matching design in data analysis, a generalized estimating equation (GEE) model with an identity link function and an exchangeable correlation structure was performed for all data analysis. For comparing the mean values of baseline characteristics, DII items, and PI components in study groups, a suited GEE model was employed. In order to assess the differences of 8-OHdG, F2a-IP, DII, DTAC, and PI in the football player group in comparison to the non-athlete group, a suited GEE approach that adjusted for potential confounders (FM, FFM, and WHR) was undertaken. The association of DII, DTAC, and PI with 8-OHdG and F2a-IP in all participants was analyzed by linear regression analysis with the GEE. P < 0.05 were considered statistically significant.
Results
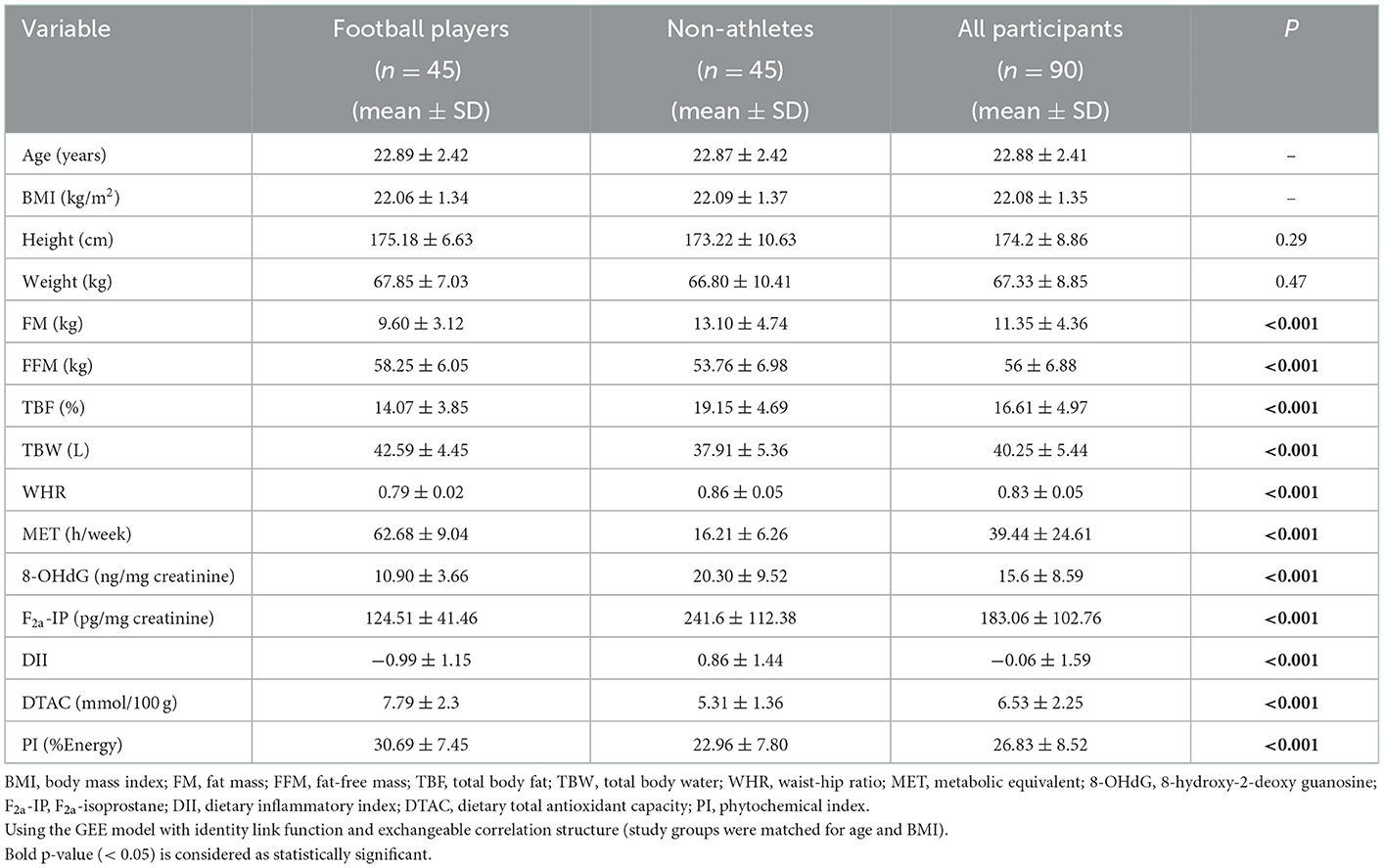
The participant's characteristics are summarized in Table 1. The mean age of participants was 22.88 years (SD = 2.41), and their BMI was 22.08 kg/m2 (SD = 1.35). The mean values of FFM (kg), TBW (L), MET (h/week), DTAC (mmol/100 g), and PI (%Energy) were significantly higher in the football player group, while FM (kg), TBF (%), WHR, DII, and urinary levels of 8-OHdG (ng/mg creatinine) and F2a-IP (pg/mg creatinine) were significantly lower in the football player group compared with the non-athlete group (P < 0.001 for all values).
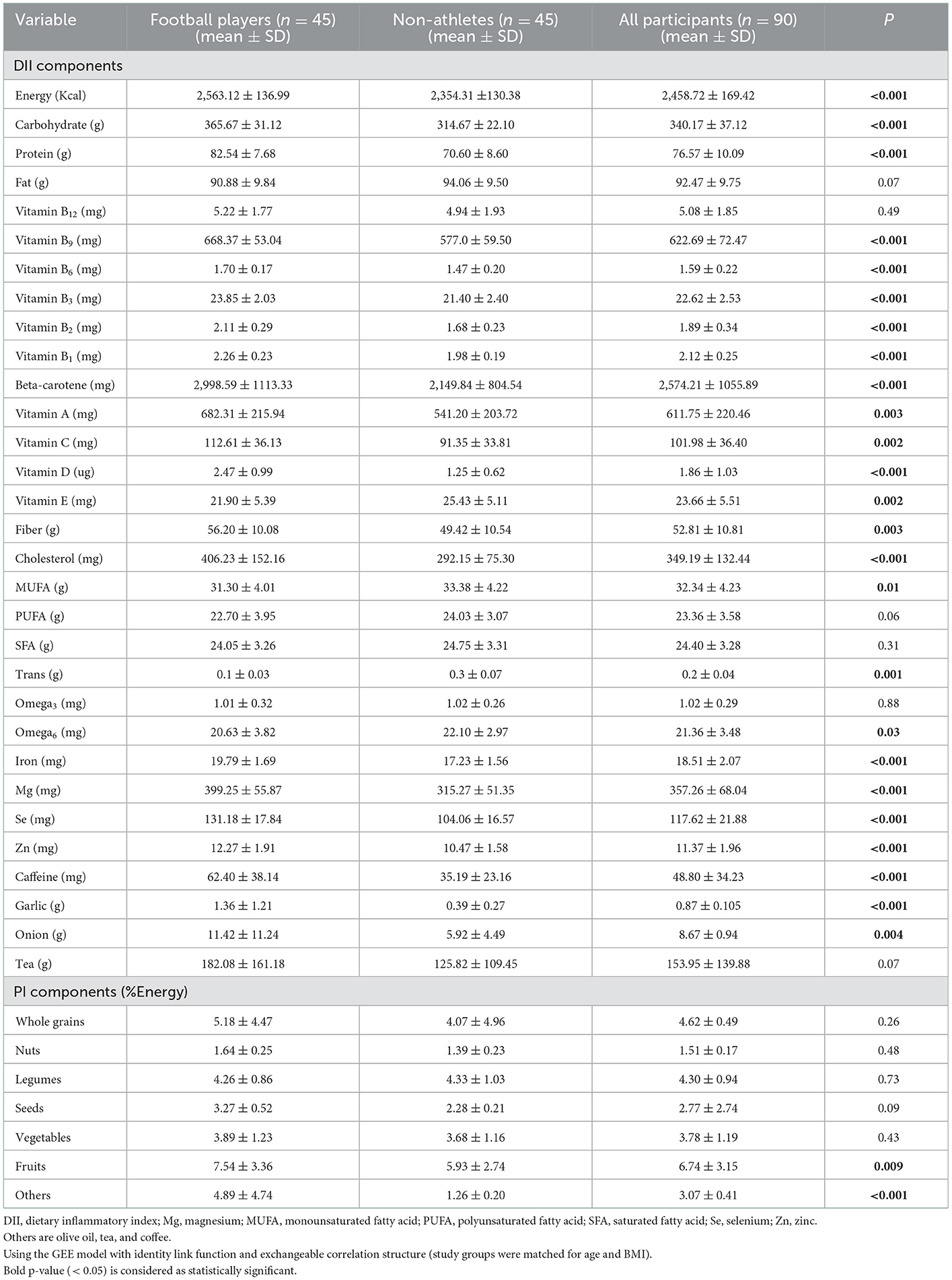
The DII and PI components of the subject groups are shown in Table 2. Significant differences were detected among some components. Among the average daily intakes of various DII components; energy (Kcal), carbohydrate (g), protein (g), vitamin B9 (mg), vitamin B6 (mg), vitamin B3 (mg), vitamin B2 (mg), vitamin B1 (mg), beta-carotene (mg), vitamin A (mg), vitamin C (mg), vitamin D (ug), fiber (g), cholesterol (mg), iron (mg), Mg (mg), Se (mg), Zn (mg), caffeine (mg), garlic (g), onion (g), and also among PI components; fruits (%Energy) and others (olive oil, tea, and coffee) (%Energy) were higher in the football player group [P < 0.001 for all values except vitamin A (P = 0.003), vitamin C (P = 0.002), fiber (P = 0.003), onion (P = 0.004), fruits (P = 0.009)], while vitamin E (mg) (P = 0.002), MUFA (g) (P = 0.01), trans fat (g) (P = 0.001), and omega6 (mg) (P = 0.03) were higher in the non-athlete group.
The comparison of urinary parameters and questionnaire indices revealed differences between the two groups after considering the adjustment for confounders (FM, FFM, and WHR) are shown in Table 3. These results indicated that the football player group was significantly lower in 8-OHdG (ng/mg creatinine) (β = −6.96), F2a-IP (pg/mg creatinine) (β = −82.58), and DII (β = −2.06), and significantly higher in DTAC (mmol/100 g) (β = 2.37) and PI (%Energy) (β = 0.084) than the non-athlete group (P < 0.001 for all variables).
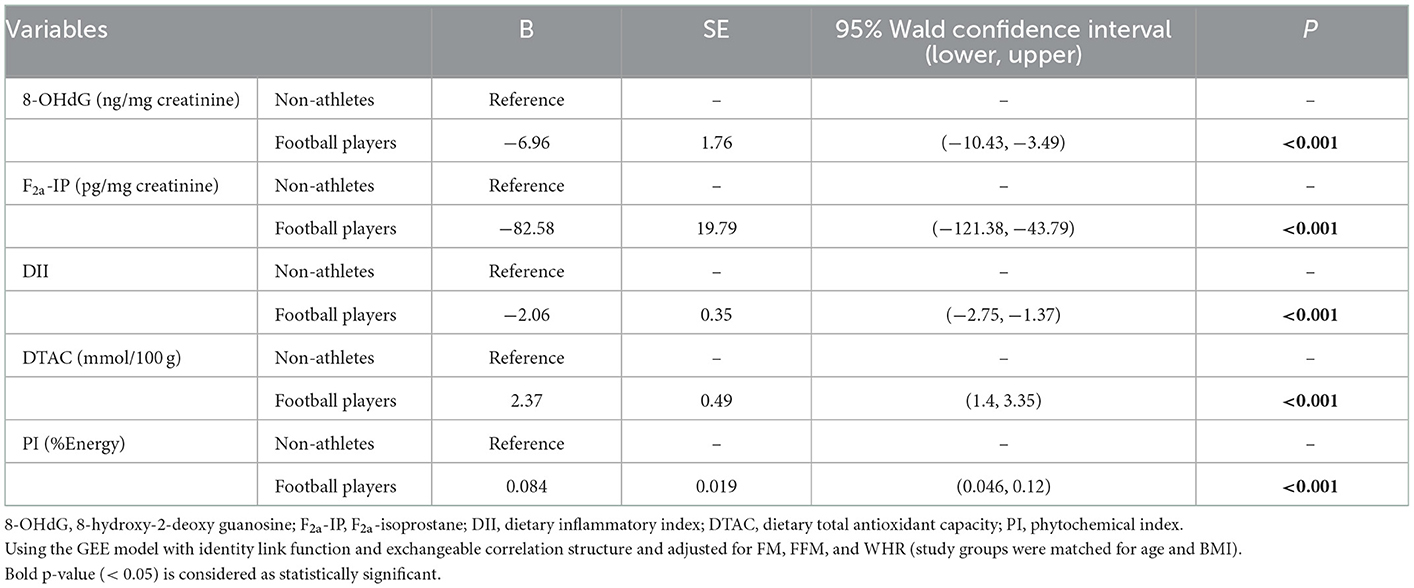
Table 3. GEE results for comparing oxidative biomarkers and dietary indices between football player and non-athlete groups.
The results of the linear regression analysis for the associations among DII, DTAC, and PI with 8-OHdG in all participants are presented in Table 4. Significant associations were detected. The results revealed that DII was positively associated with 8-OhdG; as DII increased by one unit, 8-OHdG (ng/mg creatinine) increased by 2.25 (P < 0.001). Moreover, negative associations were shown between DTAC and PI with 8-OHdG; as DTAC (mmol/100 g) and PI (%Energy) increased by one unit, 8-OHdG (ng/mg creatinine) decreased by 1.42 (P < 0.001) and 35.37 (P < 0.001), respectively.
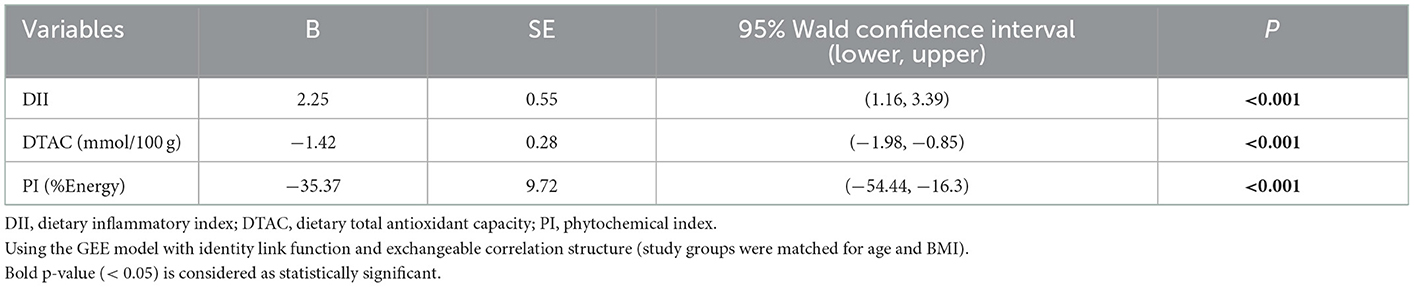
Table 4. Linear regression model using the GEE method for assessing the association of DII, DTAC, and PI with 8-OHdG in all participants.
Table 5 shows the results of the linear regression analysis for the association of DII, DTAC, and PI with F2a-IP in all participants. Significant associations were found. Results indicate that DII was positively associated with F2a-IP; as DII increased by one unit, F2a-IP (pg/mg creatinine) increased by 28.34 (P < 0.001). In addition, negative associations were observed between DTAC and PI with F2a-IP; as DTAC (mmol/100 g) and PI (%Energy) increased by one unit, F2a-IP (pg/mg creatinine) decreased by 17.34 (P < 0.001), and 428.11 (P = 0.003), respectively.
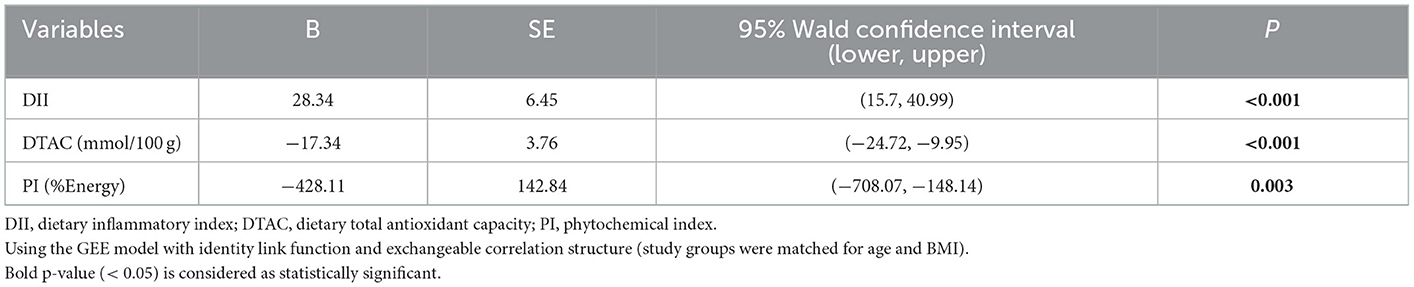
Table 5. Linear regression model using the GEE method for assessing the association of DII, DTAC, and PI with F2a-IP in all participants.
Discussion
In the present study, urinary oxidative biomarkers and their association with the inflammatory and antioxidant potential of diet in male football players and healthy non-athlete controls were investigated. Overall, our findings demonstrated that oxidative stress was significantly lower in football players, and there were also significant relationships between dietary pro-inflammatory and antioxidant indices and oxidative stress.
Long-term and exhaustive exercises are associated with a significant accumulation of oxidative radicals (29). Due to the high energy requirement during exercise, the absorption of oxygen (O2) from the blood increases to help muscle contraction. The exercise increases O2 consumption in the muscles up to 200 times compared to the resting state (30). It has been concluded that contracting skeletal muscles are an important tissue for ROS production during exercise, and mounting evidence indicates that nicotinamide adenine dinucleotide phosphate (NADPH) is likely to be a major ROS-generating source in contracting skeletal muscle (8, 31). As mentioned previously, the interaction of ROS with the DNA strand and lipids leads to the formation of 8-OHdG and F2a-IP, respectively (9, 32).
In the present study, it was indicated that the pro-inflammatory capacity of the diet (assessed by the DII) was significantly lower in athletes compared to non-athletes. Furthermore, the results showed that the DII score could significantly increase 8-OHdG and F2a-IP levels, which is consistent with previous similar studies. A study conducted by Moradi et al. reported a positive and negative relationship between DII and malondialdehyde (an OS biomarker) and total antioxidant capacity in healthy people, respectively (33). In addition, it has been demonstrated that a pro-inflammatory diet can induce oxidative processes in the body and that an oxidant-antioxidant imbalance with high levels of nitrogen and ROS can increase DNA damage (34). A study performed by Shahinfar et al. proved that higher DII scores were related to reduced muscle endurance and strength in adults (35). Ramezani et al. also demonstrated that a lower DII is correlated with a higher aerobic capacity (36). Therefore, in football, which relies more on the aerobic pathway (37), a lower DII score may be useful in improving the performance of football players.
The findings showed that the DTAC score was significantly higher in football players compared to non-athletes. Moreover, a negative and significant association was observed among DTAC, 8-OHdG, and F2a-IP levels. These findings indicated that DTAC had a significant impact on antioxidant defense against OS caused by exercise. A study conducted on ultra-endurance athletes proved that there was a significant negative correlation between changes in levels of 8-isoprostaglandin F2a and dietary antioxidant intake (FRAP) comparing post-exercise and pre-exercise (19). In addition, Pérez et al. demonstrated an inverse correlation between dietary antioxidants and 8-OHdG (38). Furthermore, a study conducted by Anderson et al. on premenopausal women revealed that there were significant inverse associations between F2a-IP and physical activity and dietary antioxidant nutrients (17). In contrast, a study indicated that the composite dietary antioxidant index and dietary antioxidant quality score were not significantly associated with OS (39).
In the current study, the mean dietary antioxidant content calculated with the FRAP assay was 7.79 mmol/day in football players. In the study by Koivisto et al., the FRAP score was considered to be 21.2 mmol/day for an antioxidant-rich diet and 2.8 mmol/day for a low-antioxidant diet in elite endurance athletes (40). Comparing the results of the current study with the findings of the above-mentioned research, it seems the total intake of dietary antioxidants by football players was moderate.
There is evidence suggesting that an antioxidant-rich diet provides several bioactive dietary components called phytochemicals that help to remove ROS and prevent DNA damage by inducing the body's antioxidant defenses (19). It was found that the PI score was higher in football athletes than in non-athletes. Diets containing whole grains, nuts, fresh fruits and vegetables, legumes, and plant foods such as olive oil are rich in phytochemicals, fiber, and antioxidants (41), and the PI score is a simple way to assess phytochemical intake (42). In terms of PI components, there was a significant difference between the two groups of football players and the non-athletes in the intake of fruits, olive oil, tea, and coffee. Furthermore, in the present study, an inverse and significant relationship was observed between the PI score and the 8-OHdG and F2a-IP levels. What is more, the findings showed that the PI, compared to DTAC, exerted a greater effect on reducing 8-OHdG and F2a-IP. Furthermore, a study showed that a diet rich in phytochemicals can reduce OS (42).
It has been demonstrated that phytochemicals such as flavonoids, carotenoids, and bioflavonoids provide antioxidant support (43). De Carvalho et al. reported that the intake of fruits was inversely related to 8-OHdG, but this relationship was not observed for the consumption of vegetables (44). As mentioned earlier, in this study, there was a significant difference between the two groups in terms of receiving fruits, but no statistically significant difference was observed in terms of vegetable intake. In total, it seems the diet rich in phytochemicals with antioxidant impacts was effective in reducing 8-OHdG and F2a-IP levels.
Our results, taken together with those from previous studies, postulated that adherence to a balanced diet rich in natural antioxidants and phytochemicals is the best recommendation regarding exercise and antioxidants in athletes and physically active persons. Regular consumption of various fresh fruits and vegetables, legumes and beans, whole grains, sprouts, and seeds is a safe and effective approach to meeting all antioxidant requirements (45).
The evidence indicates that a diet rich in antioxidants is positively associated with post-exercise blood lactate removal and can increase the running time to exhaustion (19). Therefore, the consumption of a diet rich in antioxidants by football players who are running for a long time can both help to relieve their fatigue faster after exercise and to increase the duration of time they can run without causing fatigue.
In the current study, it was shown that urinary levels of 8-OHdG and F2a-IP were significantly lower in football players compared to non-athletes. There is a lot of evidence that supports the hypothesis that physical exercise can increase the production of free radicals and cause OS in the body (46). But as mentioned earlier, regular/moderate exercise can lead to positive consequences such as improving muscle endurance and strength (3, 8). Furthermore, regular exercise can induce adaptive anti-oxidative effects and an improvement of antioxidant capacity that results in a decrease in oxidative markers (22, 47). It is noteworthy that the response of the antioxidant defense to physical effort varies based on exercise type, intensity, duration, and volume (3).
To the best of our knowledge, there is no study investigating the urinary excretion of 8-OHdG in football players, and only one study has evaluated the urinary level of F2a-IP in football players, which reported an elevated value of 8-isoprostaglandin F2a soon after the match and returned to baseline within two days' post-match, which is consistent with our results (48). Furthermore, in line with our findings, a meta-analysis study by Tryfidou et al. revealed a trend for decreased DNA damage (8-OHdG) within 5–28 days of post-acute aerobic exercise (11), and also a review study by Nikolaidis et al. reported that regular exercise could lead to decrease in lipid peroxidation (urinary F2a-IP) (12). Research shows that supplemental or dietary interventions ameliorate OS (49). The findings of the present study showed the low score of the DII and the high scores of the DTAC and PI in football players. Consequently, lower levels of oxidative biomarkers can be attributed to these dietary indicators and also to adherence to regular football training.
Strengths and limitations
This study's strengths lie in its novelty, as it is the first study to evaluate both urinary 8-OHdG and F2a-IP levels and simultaneously the relationships among DII, DTAC, and PI with 8-OHdG and F2a-IP in football players. Moreover, dietary intake was assessed using the valid and reliable FFQ. Moreover, controlling confounding factors such as FM, FFM, and WHR was another strength of the study. Although matching design in participant recruitment was considered and confounding factors such as FM, FFM, and WHR have been adjusted, the impact of better physical fitness on OS levels should also be considered. The FFQ is still the most appropriate tool for collecting subjects' usual intake in the long term, but reporting relied on respondents' memories, which may cause recall bias; nevertheless, we tried to solve these problems by using a trained dietitian to collect data face-to-face. Another limitation is that this study did not assess the level of inflammatory biomarkers due to financial limitations. This suggests that future studies evaluating inflammatory biomarkers should be conducted. Additionally, due to the cross-sectional nature of the study, it is not possible to discuss the mechanisms underlying the results of the present study.
Conclusion
The present study revealed that urinary excretion rates of 8-OHdG and F2a-IP were lower in football athletes compared to non-athletes. In addition, the DII score was lower, and the DTAC and PI scores were higher in football players. The findings showed that diet-related inflammation was correlated with OS levels. As such, dietary factors such as dietary antioxidants and fruit intake had an inverse relationship with OS levels. Furthermore, results indicated that dietary phytochemical intake exerted the greatest effect on decreasing OS biomarkers. Altogether, adherence to an anti-inflammatory and antioxidant-rich diet is likely to be effective for football players due to decreased OS.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving human participants were reviewed and approved by Ethics Committee of Tabriz University of Medical Sciences (Ethical Code: IR.TBZMED.REC.1399.1009). The patients/participants provided their written informed consent to participate in this study.
Author contributions
MZ conducted research, data analysis, interpretation of the data, and preparation of a draft manuscript. ZS contributed to writing the manuscript. MN contributed to the interpretation of data and the drafting of the manuscript. PS contributed to the data analysis. ME contributed to the study conception and design. BP contributed to the study idea, design, and revision of the manuscript. All the authors read and approved the final manuscript.
Funding
The current study was financially supported by the Vice-Chancellor for Research at Tabriz University of Medical Sciences and the Student Research Committee at Tabriz University of Medical Sciences, Tabriz, Iran. The results of this article were extracted from the MSc thesis of MZ (Pazhoohan: 66826).
Acknowledgments
The authors sincerely appreciate all the participants of this study. They would like to thank the Tabriz University of Medical Sciences, Tabriz, Iran, for their assistance in their research.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
BMI, body mass index; DII, dietary inflammatory index; DTAC, dietary total antioxidant capacity; F2a-IP, F2alpha-isoprostane; FFM, fat-free mass; FFQ, food frequency questionnaire; FM, fat mass; FRAP, ferric reducing antioxidant power; GEE, generalized estimating equation; 8-OHdG, 8-hydroxy-2-deoxyguanosine; MET, metabolic equivalent of task; OS, oxidative stress; PI, phytochemical index; ROS, reactive oxygen species; TBF, total body fat; TBW, total body water; WHR, waist-hip ratio.
References
1. Giulianotti R, Robertson R. The globalization of football: a study in the globalization of the 'serious life'. Br J Sociol. (2004) 55:545–68. doi: 10.1111/j.1468-4446.2004.00037.x
2. de Oliveira DC, Rosa FT, Simões-Ambrósio L, Jordao AA, Deminice RJN. Antioxidant vitamin supplementation prevents oxidative stress but does not enhance performance in young football athletes. Nutrition. (2019) 63:29–35. doi: 10.1016/j.nut.2019.01.007
3. Sadowska-Krepa E, Bańkowski S, Kargul A, Iskra J. Changes in blood antioxidant status in American football players and soccer players over a training macrocycle. J Exerc Sci Fitness. (2021) 19:229–33. doi: 10.1016/j.jesf.2021.08.001
4. Luti S, Modesti A, Modesti PA. Inflammation, peripheral signals and redox homeostasis in athletes who practice different sports. Antioxidants. (2020) 9:1065. doi: 10.3390/antiox9111065
5. Le Moal E, Groussard C, Paillard T, Chaory K, Le Bris R, Plantet K, et al. Redox status of professional soccer players is influenced by training load throughout a season. Int J Sports Med. (2016) 37:680–6. doi: 10.1055/s-0035-1565199
6. Graille M, Wild P, Sauvain JJ, Hemmendinger M, Guseva Canu I, Hopf NB. Urinary 8-OHdG as a biomarker for oxidative stress: a systematic literature review and meta-analysis. International journal of molecular sciences. (2020) 21:3743. doi: 10.3390/ijms21113743
7. Powers SK, Talbert EE, Adhihetty PJ. Reactive oxygen and nitrogen species as intracellular signals in skeletal muscle. J Physiol. (2011) 589(Pt 9):2129–38. doi: 10.1113/jphysiol.2010.201327
8. Powers SK, Deminice R, Ozdemir M, Yoshihara T, Bomkamp MP, Hyatt HJ. Exercise-induced oxidative stress: friend or foe? J Sport Health Sci. (2020) 9:415–25. doi: 10.1016/j.jshs.2020.04.001
9. Córdova A, Sureda A, Albina ML, Linares V, Bellés M, Sánchez DJ. Oxidative stress markers after a race in professional cyclists. Int J Sport Nutr Exerc Metab. (2015) 25:171–8. doi: 10.1123/ijsnem.2014-0090
10. Narukawa T, Anzai Y, Murakami T, Isogai R, Nakagawa S, Yamada HJ. Effects of Body Mass Index (BMI), dietary intake and serum antioxidant vitamin concentration on urinary 8-hydroxydeoxyguanosine and F2-isoprostane excretions. Anti-Aging Med. (2011) 8:1–6. doi: 10.3793/jaam.8.1
11. Tryfidou DV, McClean C, Nikolaidis MG, Davison GWJ. DNA damage following acute aerobic exercise: a systematic review and meta-analysis. Sports Med. (2020) 50:103–27. doi: 10.1007/s40279-019-01181-y
12. Nikolaidis MG, Kyparos A, Vrabas IS. F2-isoprostane formation, measurement and interpretation: the role of exercise. Prog Lipid Res. (2011) 50:89–103. doi: 10.1016/j.plipres.2010.10.002
13. Black CN, Bot M, Revesz D, Scheffer PG, Penninx B. The association between three major physiological stress systems and oxidative DNA and lipid damage. Psychoneuroendocrinology. (2017) 80:56–66. doi: 10.1016/j.psyneuen.2017.03.003
14. Edwards MK, Shivappa N, Mann JR, Hebert JR, Wirth MD, Loprinzi PD. The association between physical activity and dietary inflammatory index on mortality risk in US adults. Phys Sportsmed. (2018) 46:249–54. doi: 10.1080/00913847.2018.1443665
15. Bawaked RA, Schröder H, Ribas-Barba L, Izquierdo-Pulido M, Pérez-Rodrigo C, Fíto M, et al. Association of diet quality with dietary inflammatory potential in youth. Food Nutr Res. (2017) 61:1328961. doi: 10.1080/16546628.2017.1328961
16. Shivappa N, Steck SE, Hurley TG, Hussey JR, Hébert JR. Designing and developing a literature-derived, population-based dietary inflammatory index. Public Health Nutr. (2014) 17:1689–96. doi: 10.1017/S1368980013002115
17. Anderson C, Milne GL, Sandler DP, Nichols HB. Oxidative stress in relation to diet and physical activity among premenopausal women. Br J Nutr. (2016) 116:1416–24. doi: 10.1017/S0007114516003226
18. Wang Y, Yang M, Lee SG, Davis CG, Koo SI, Chun OK. Dietary total antioxidant capacity is associated with diet and plasma antioxidant status in healthy young adults. J Acad Nutr Diet. (2012) 112:1626–35. doi: 10.1016/j.jand.2012.06.007
19. Devrim-Lanpir A, Bilgic P, Kocahan T, Deliceoglu G, Rosemann T, Knechtle B. Total dietary antioxidant intake including polyphenol content: is it capable to fight against increased oxidants within the body of ultra-endurance athletes? Nutrients. (2020) 12:1877. doi: 10.3390/nu12061877
20. McCarty MF. Proposal for a dietary “phytochemical index”. Med Hypoth. (2004) 63:813–7. doi: 10.1016/j.mehy.2002.11.004
21. Kim C, Park KJA. Association between phytochemical index and inflammation in Korean adults. Antioxidants. (2022) 11:348. doi: 10.3390/antiox11020348
22. Rahimi R, Sharafi H. The effect of a bout of resistance exercise on 8-Hydroxy-2′-Deoxyguanosine in athletes and non-athletes. Knowl Health. (2012) 7:1–7.
23. Moghaddam MB, Aghdam FB, Jafarabadi MA, Allahverdipour H, Nikookheslat SD, Safarpour S. The Iranian version of international physical activity questionnaire (IPAQ) in Iran: content and construct validity, factor structure, internal consistency and stability. World Appl Sci J. (2012) 18:1073–80.
24. Mirmiran P, Esfahani FH, Mehrabi Y, Hedayati M, Azizi F. Reliability and relative validity of an FFQ for nutrients in the Tehran lipid and glucose study. Public Health Nutr. (2010) 13:654–62. doi: 10.1017/S1368980009991698
25. Ghaffarpour M, Houshiar-Rad A, Kianfar H. The Manual for Household Measures, Cooking Yields Factors and Edible Portion of Foods. Tehran: Nashre Olume Keshavarzy (1999). p. 42–58.
26. Willett WC, Howe GR, Kushi LH. Adjustment for total energy intake in epidemiologic studies. Am J Clin Nutr. (1997) 65:1220S−8S. doi: 10.1093/ajcn/65.4.1220S
27. Carlsen MH, Halvorsen BL, Holte K, Bøhn SK, Dragland S, Sampson L, et al. The total antioxidant content of more than 3100 foods, beverages, spices, herbs and supplements used worldwide. Nutr J. (2010) 9:1–11. doi: 10.1186/1475-2891-9-3
28. Milajerdi A, Keshteli AH, Afshar H, Esmaillzadeh A, Adibi P. Dietary total antioxidant capacity in relation to depression and anxiety in Iranian adults. Nutrition. (2019) 65:85–90. doi: 10.1016/j.nut.2018.11.017
29. Mastaloudis A, Leonard SW, Traber MG. Oxidative stress in athletes during extreme endurance exercise. Free Rad Biol Med. (2001) 31:911–22. doi: 10.1016/S0891-5849(01)00667-0
31. Sakellariou GK, Jackson MJ, Vasilaki A. Redefining the major contributors to superoxide production in contracting skeletal muscle. The role of NAD(P)H oxidases. Free Rad Res. (2014) 48:12–29. doi: 10.3109/10715762.2013.830718
32. Valavanidis A, Vlachogianni T., Fiotakis C. 8-hydroxy-2′-deoxyguanosine (8-OHdG): a critical biomarker of oxidative stress and carcinogenesis. J Environ Sci Health Part C. (2009) 27:120–39. doi: 10.1080/10590500902885684
33. Moradi F, Heidari Z, Teimori A, Ghazvini M, Imani ZF, Naeini AA. The association between the dietary inflammatory index (DII) and some serum oxidative stress markers in non-alcoholic fatty liver disease: case-control. Int J Prev Med. (2022) 13:93. doi: 10.4103/ijpvm.IJPVM_411_20
34. Czarny P, Wigner P, Galecki P, Sliwinski T. The interplay between inflammation, oxidative stress, DNA damage, DNA repair and mitochondrial dysfunction in depression. Prog Neuro-Psychopharmacol Biol Psychiatry. (2018) 80:309–21. doi: 10.1016/j.pnpbp.2017.06.036
35. Shahinfar H, Shahavandi M, Tijani AJ, Jafari A, Davarzani S, Djafarian K, et al. The association between dietary inflammatory index, muscle strength, muscle endurance, and body composition in Iranian adults. Eat Weight Disord Stud Anorex Bulimia Obes. (2022) 27:463–72. doi: 10.1007/s40519-020-01096-y
36. Ramezani A, Parastouei K, Delkhosh M, Rostami H. The relationship between dietary inflammatory index, physical performance and anthropometric indices in marines. Res Sq. (2021). doi: 10.21203/rs.3.rs-596924/v1
37. Bangsbo J, Mohr M, Krustrup P. Physical and metabolic demands of training and match-play in the elite football player. J Sports Sci. (2006) 24:665–74. doi: 10.1080/02640410500482529
38. Pérez DD, Strobel P, Foncea R, Díez MS, Vásquez L, Urquiaga I, et al. Wine, diet, antioxidant defenses, and oxidative damage. Ann N Y Acad Sci. (2002) 957:136–45. doi: 10.1111/j.1749-6632.2002.tb02912.x
39. LuuHung N, Wen W, Li H, Dai Q, Yang G, Cai Q, et al. Are dietary antioxidant intake indices correlated to oxidative stress and inflammatory marker levels? Antioxid Redox Signal. (2015) 22:951–59. doi: 10.1089/ars.2014.6212
40. Koivisto AE, Olsen T, Paur I, Paulsen G, Bastani NE, Garthe I, et al. Effects of antioxidant-rich foods on altitude-induced oxidative stress and inflammation in elite endurance athletes: a randomized controlled trial. PLoS ONE. (2019) 14:e0217895. doi: 10.1371/journal.pone.0217895
41. Rajaram S. The effect of vegetarian diet, plant foods, and phytochemicals on hemostasis and thrombosis. Am J Clin Nutr. (2003) 78:552S−8S. doi: 10.1093/ajcn/78.3.552S
42. Vincent HK, Bourguignon CM, Taylor AG. Relationship of the dietary phytochemical index to weight gain, oxidative stress and inflammation in overweight young adults. J Human Nutr Dietet. (2010) 23:20–9. doi: 10.1111/j.1365-277X.2009.00987.x
43. Schmidt MC, Askew E, Roberts DE, Prior RL, Ensign Jr W, Hesslink Jr RE. Oxidative stress in humans training in a cold, moderate altitude environment and their response to a phytochemical antioxidant supplement. Wilderness Environ Med. (2002) 13:94–105. doi: 10.1580/1080-6032(2002)013[0094:OSIHTI]2.0.CO;2
44. de Carvalho AM, Carioca AAF, Fisberg RM Qi L, Marchioni DM. Joint association of fruit, vegetable, and heterocyclic amine intake with DNA damage levels in a general population. Nutrition. (2016) 32:260–4. doi: 10.1016/j.nut.2015.08.018
45. Yavari A, Javadi M, Mirmiran P, Bahadoran Z. Exercise-induced oxidative stress and dietary antioxidants. Asian J Sports Med. (2015) 6:e24898. doi: 10.5812/asjsm.24898
46. Tsakiris S, Parthimos T, Parthimos N, Tsakiris T, Schulpis KH. The beneficial effect of L-cysteine supplementation on DNA oxidation induced by forced training. Pharmacol Res. (2006) 53:386–90. doi: 10.1016/j.phrs.2006.01.008
47. Larsen EL, Poulsen HE, Michaelsen C, Kjær LK, Lyngbæk M, Andersen ES, et al. Differential time responses in inflammatory and oxidative stress markers after a marathon: an observational study. J Sports Sci. (2020) 38:2080–91. doi: 10.1080/02640414.2020.1770918
48. Papapanagiotou A, Gissis I, Papadopoulos C, Souglis A, Bogdanis G, Giosos I, et al. Changes in homocysteine and 8-iso-PGF2a levels in football and hockey players after a match. Res Sports Med. (2011) 19:118–28. doi: 10.1080/15438627.2011.556532
Keywords: oxidative stress, 8-hydroxy-2-deoxyguanosine, F2alpha-isoprostane, dietary inflammatory index, dietary antioxidant capacity, dietary phytochemical index, football
Citation: Zare M, Shateri Z, Nouri M, Sarbakhsh P, Eftekhari MH and Pourghassem Gargari B (2023) Association between urinary levels of 8-hydroxy-2-deoxyguanosine and F2a-isoprostane in male football players and healthy non-athlete controls with dietary inflammatory and antioxidant indices. Front. Nutr. 9:1101532. doi: 10.3389/fnut.2022.1101532
Received: 17 November 2022; Accepted: 28 December 2022;
Published: 24 January 2023.
Edited by:
Felipe J. Aidar, Federal University of Sergipe, BrazilReviewed by:
Nikos Margaritelis, Aristotle University of Thessaloniki, GreeceNaheed Aryaeian, Iran University of Medical Sciences, Iran
Copyright © 2023 Zare, Shateri, Nouri, Sarbakhsh, Eftekhari and Pourghassem Gargari. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bahram Pourghassem Gargari,  YmFocmFtcGdAeWFob28uY29t;
YmFocmFtcGdAeWFob28uY29t;  cG91cmdoYXNzZW1iQHRiem1lZC5hYy5pcg==
cG91cmdoYXNzZW1iQHRiem1lZC5hYy5pcg==
†ORCID: Mahsa Zare orcid.org/0000-0002-6173-9904
Zainab Shateri orcid.org/0000-0003-3725-6686
Mehran Nouri orcid.org/0000-0002-7031-3542
Parvin Sarbakhsh orcid.org/0000-0002-4213-5152
Mohammad Hasan Eftekhari orcid.org/0000-0001-5428-1491
Bahram Pourghassem Gargari orcid.org/0000-0001-7667-099X
 Mahsa Zare
Mahsa Zare Zainab Shateri
Zainab Shateri Mehran Nouri
Mehran Nouri Parvin Sarbakhsh6†
Parvin Sarbakhsh6†