
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Nutr. , 06 January 2023
Sec. Clinical Nutrition
Volume 9 - 2022 | https://doi.org/10.3389/fnut.2022.1092059
This article is part of the Research Topic Nutritional Assessment Tools for Identification and Monitoring of Malnutrition in Patients with Chronic Disease, Volume II View all 14 articles
Background: Prognostic nutritional index (PNI) is an independent predictor of the prognosis of various diseases. However, the prognosis value of PNI in patients with decompensated liver cirrhosis (DLC) remains unknown. The study aimed to investigate the prognostic significance of PNI in patients with DLC.
Methods: A total of 214 eligible patients were enrolled in the study’s development cohort between January 2018 and March 2021. The clinical primary study endpoints were mortality at 3 and 6 months. Receiver operating characteristic (ROC) curve analysis was used to assess the PNI’s prediction accuracy, and Youden’s index was utilized to determine the PNI’s optimal cut-off value. Moreover, based on the optimal cut-off value, patients were categorized into high and low PNI groups. Multivariate logistic regression analysis was used to determine independent risk factors for mortality, while the relationship between PNI and the risk of death was identified and demonstrated using restricted cubic splines (RCS). A validation cohort of 139 patients was to verify the predictive power of the PNI.
Results: In the development cohort, the mortality rate at 3 and 6 months were 10.3% (22) and 14.0% (30), respectively. The PNI had comparable predictive power with the MELD score at all follow-up endpoints. Decreased PNI was an independent predictor of adverse prognosis at all follow-up endpoints. The RCS revealed a linear correlation between PNI and the risk of death. We confirmed that lower PNI was an independent predictor of poor prognosis in the validation cohort.
Conclusion: The findings showed that lower PNI is an independent factor of poor outcomes and might be utilized as a potentially promising prognostic predictor in patients with DLC.
Liver cirrhosis possesses a significant global morbidity and mortality rate, resulting in a million deaths annually, and is the 11th leading cause of death globally (1, 2). As compensated liver cirrhosis is difficult to identify, most patients are diagnosed with decompensated liver cirrhosis (DLC) in the hospital due to numerous complications (3). DLC has an unfavorable prognosis, with a median survival time of approximately 2 years, placing a heavy financial burden on healthcare (4). Despite the availability of several therapies, the mortality rate remains high for patients with DLC (5, 6). As a result, there is a need for a practical and simple predictor to evaluate the risk of death in patients with DLC in order to improve clinical management and subsequently reduce mortality.
Prognostic Nutritional Index (PNI) is a simple and objective index of inflammatory and nutrition status derived from serum albumin (ALB) and lymphocyte counts. Recently, PNI has been reported to be an independent prognostic predictor for patients with cancer, stroke, heart disease, chronic kidney disease, acute exacerbation of the chronic obstructive pulmonary disease, sepsis, COVID-19, and autoimmune disease (7–17). However, no research has been conducted to examine the relationship between PNI and the prognosis of patients with DLC. Several studies have shown that systemic inflammatory response and malnutrition are associated with poor prognosis in patients with liver cirrhosis (18–24). Hence, it seems reasonable to hypothesize that there may be a significant correlation between PNI and the risk of death in patients with DLC when PNI is used as an indicator of inflammatory and nutrition status. The current study aimed to evaluate the prognostic role of PNI in patients with DLC.
Between January 2018 and March 2021, we recruited patients with DLC who were admitted to the Department of Gastroenterology, Yijishan Hospital of Wannan Medical College as the development cohort of the study. We enrolled patients with DLC attending the Yijishan Hospital of Wannan Medical College between April 2021 and February 2022 as the validation cohort. DLC was defined as biochemical, clinical, endoscopic manifestations, imaging signs, and complications of ascites, gastrointestinal bleeding, hepatorenal syndrome, or hepatic encephalopathy (25). Reasons for exclusion were: (1) non-first admission, (2) malignant diseases, (3) cardio-cerebrovascular disease, (4) autoimmune diseases, (5) hyperpyrexia, (6) primary kidney disease, (7) incomplete data, and (8) loss to follow-up. The flow chart of the patient selection process is provided in Supplementary Figure 1.
On admission, variables including sex, age, cause of liver cirrhosis, modes of decompensation, and laboratory variables were collected (Table 1). PNI was calculated as serum albumin (ALB) concentration (g/L) + 5 × total lymphocyte count (109/L) (16). The Model for End-Stage Liver Disease (MELD) score was utilized to evaluate the severity and prognosis of liver disease (26). The mortality rate at 3 and 6 months was assessed using medical records or direct telephone conversations with patients or their relatives.
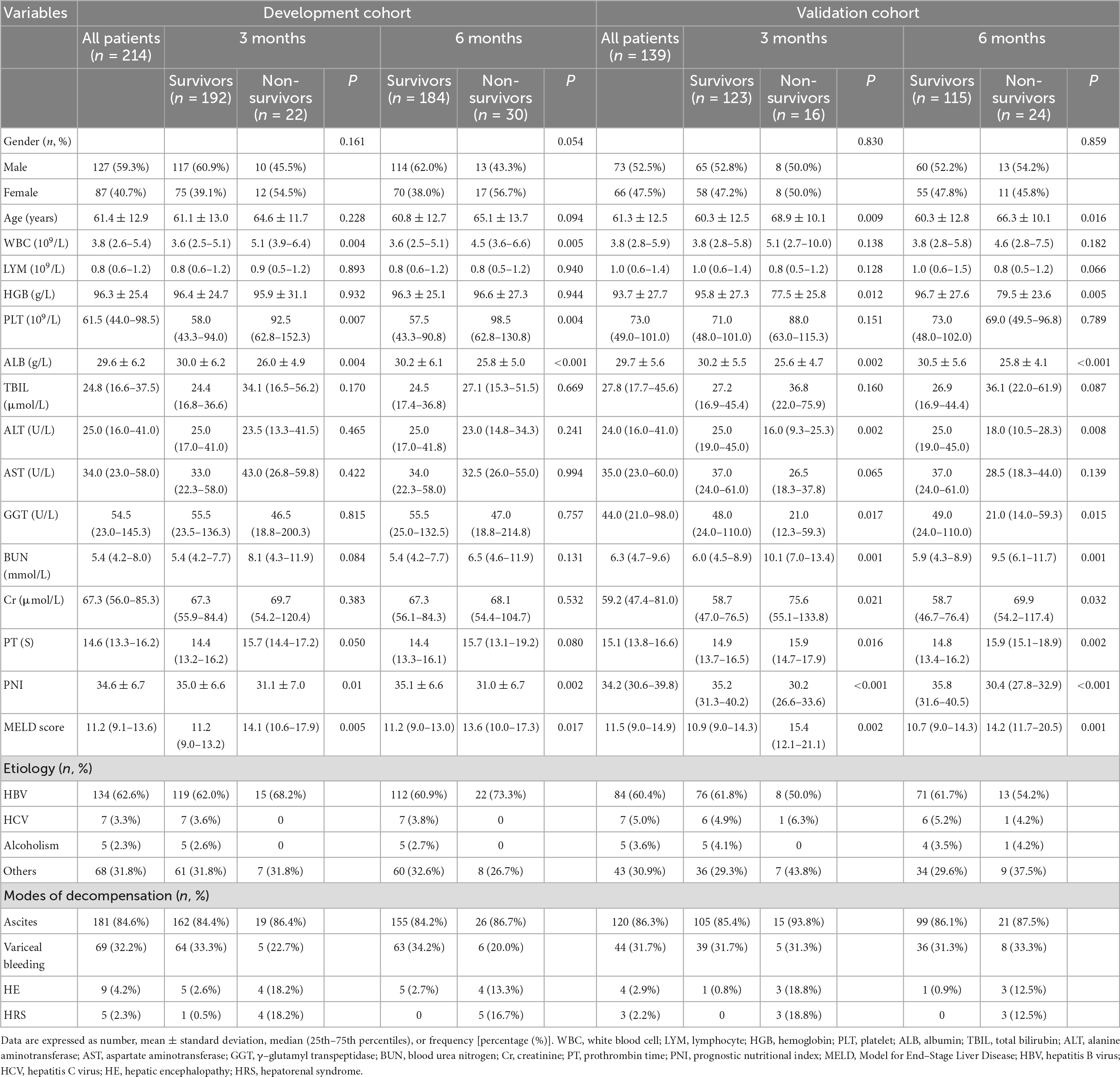
Table 1. Clinical and laboratory characteristics of the decompensated liver cirrhosis patients in the development and validation cohort at the 3-month, and 6-month follow-ups.
Categorical data were reported as frequency and percentage, while continuous variables were expressed as means ± standard deviation or medians (25th–75th percentiles). The independent sample t-test (normally distributed data) or the Mann-Whitney U-test (non-normally distributed data) were used to examine the differences in continuous variables. Categorical data were assessed by the chi-square test (27). Associations between MELD score and PNI were analyzed using Spearman’s analysis (28). Multivariate logistic regression analysis was used to determine independent predictors of mortality in patients with DLC. The degree of multicollinearity among the variables was measured by calculating the variance inflation factor (VIF) values, and VIF > 10 was considered to have multicollinearity (29). The diagnostic accuracy of PNI was assessed by analyzing the area under the receiver operating characteristic (ROC) curve (30), and the PNI’s optimal cutoff value was determined using Youden’s index (31). The values of the area under the ROC curve (AUC) were compared using the DeLong test (32). The patients were then categorized into high and low groups based on the optimal cut-off value. The Kaplan-Meier analysis with log-rank test was used to estimate survival between high and low groups. Furthermore, based on multivariate analysis, restricted cubic spline (RCS) was applied to assess the non-linear association between the PNI and the risk of death (33). The number of knots between three and five was chosen based on the minimum value for the Akaike information criterion to obtain the best fit and avoid overfitting in the main splines (34). Two-side P-value < 0.05 was considered statistically significant. The SPSS (version 25.0), R (version 4.0.2), and MedCalc (Version 15.2) were used to perform the statistical analyses in the study.
A total of 353 patients with DLC who met the inclusion criteria were recruited for the study. In the development cohort, ascites (84.6%) were found to be the most common type of decompensation, followed by variceal bleeding (32.2%), hepatic encephalopathy (4.2%), and hepatorenal syndrome (2.3%). Cirrhosis was caused by chronic hepatitis B virus in most cases, and the patients’ average age was 61.4 ± 12.9 years. The mortality rate at 3 and 6 months were 10.3 and 14.0%, respectively. The baseline characteristics of patients in the development and validation cohort are shown in Table 1 and Supplementary Figure 2.
In the development cohort, the PNI and ALB of the survivor were significantly higher than the non-survivor, while the white blood cell (WBC), platelet (PLT), and MELD scores of the survivor were considerably lower than the non-survivor at any phase of the follow-up (P < 0.05). At all follow-up endpoints, no significant differences were found between the two groups in lymphocyte (LYM), hemoglobin (HGB), total bilirubin (TBIL), alanine aminotransferase (ALT), aspartate aminotransferase (AST), γ–glutamyl transpeptidase (GGT), blood urea nitrogen (BUN), creatinine (Cr), and gender. The clinical and laboratory characteristics between non-survivors and survivors in the development and validation cohort are shown in Table 1.
In the development cohort, patients were categorized into two groups based on Youden’s index, with cut-off values of 35.47 at 3, and 6 months. Patients in the low PNI group were significantly associated with increased mortality, TBIL, PT, and MELD score, and decreased LYM, ALB, and HGB compared to those of the high PNI group at all follow-up endpoints (Table 2). Furthermore, a negative correlation was found between PNI and MELD score (r =–0.41, P < 0.001) (Figure 1). In addition, the clinical and laboratory characteristics of patients in the high and low PNI groups in the validation cohort are shown in Table 2.

Figure 1. Scatter graphs illustrating the association between the prognostic nutritional index and the MELD score.
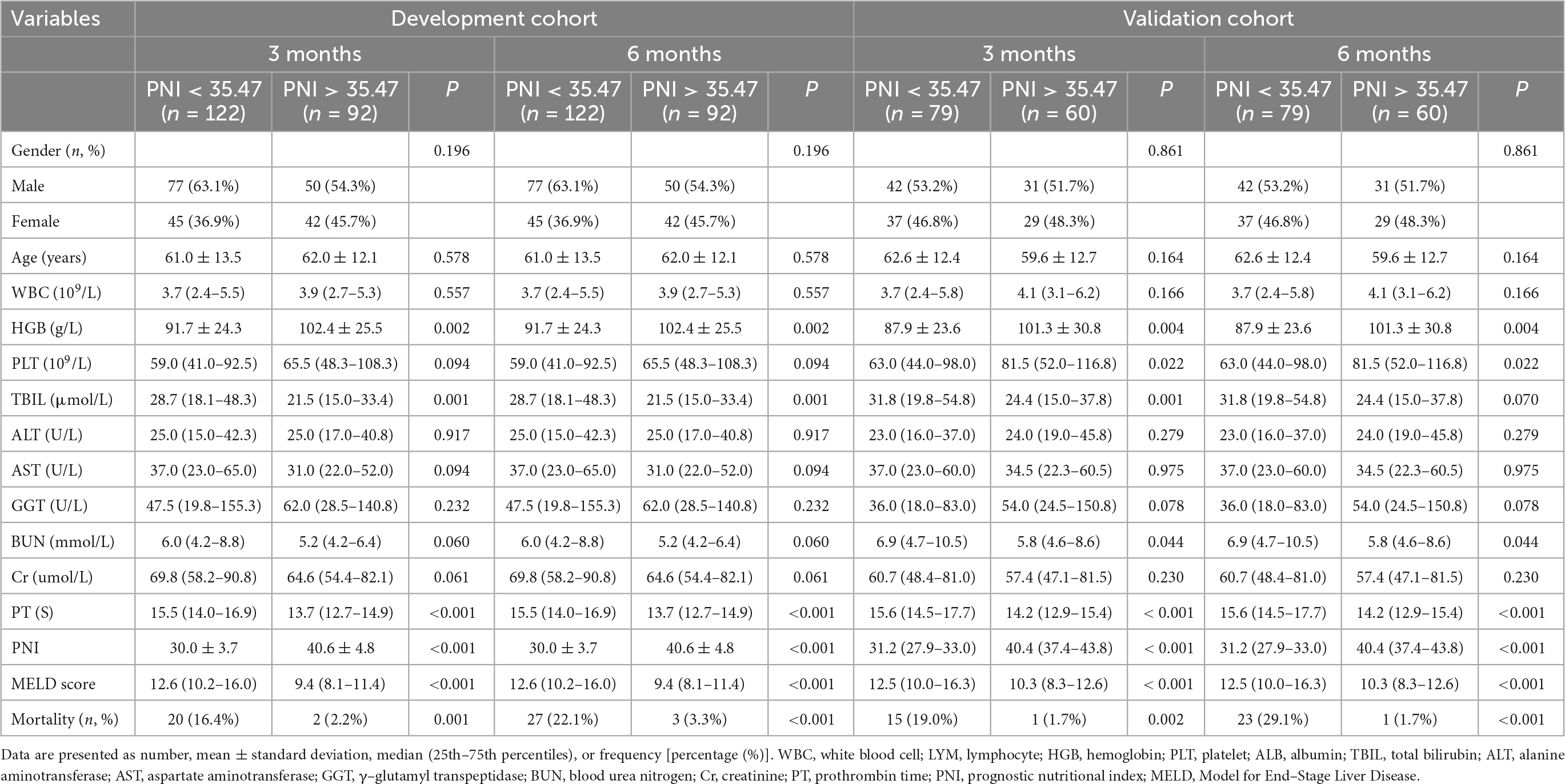
Table 2. Comparison of clinical and laboratory characteristics between low and high PNI groups in the development and validation cohort in patients with decompensated liver cirrhosis.
Kaplan-Meier analyses showed that mortality was significantly higher in patients with low PNI group than that of in patients with high PNI group (Supplementary Figure 3). In multivariate logistic regression analysis, lower PNI was identified as an independent predictor of adverse outcomes in patients with DLC in the development cohort after adjusting for the effect of confounders on mortality at 3, and 6 months, respectively (Table 3). The ROC analysis demonstrated that the AUC values of PNI and MELD scores were comparable at 3 months (0.684 vs. 0.683). The AUC values of PNI were higher than the MELD score (0.698 vs. 0.636) at 6 months, but the difference was not statistically significant (Delong test P-value > 0.05) (Figure 2).
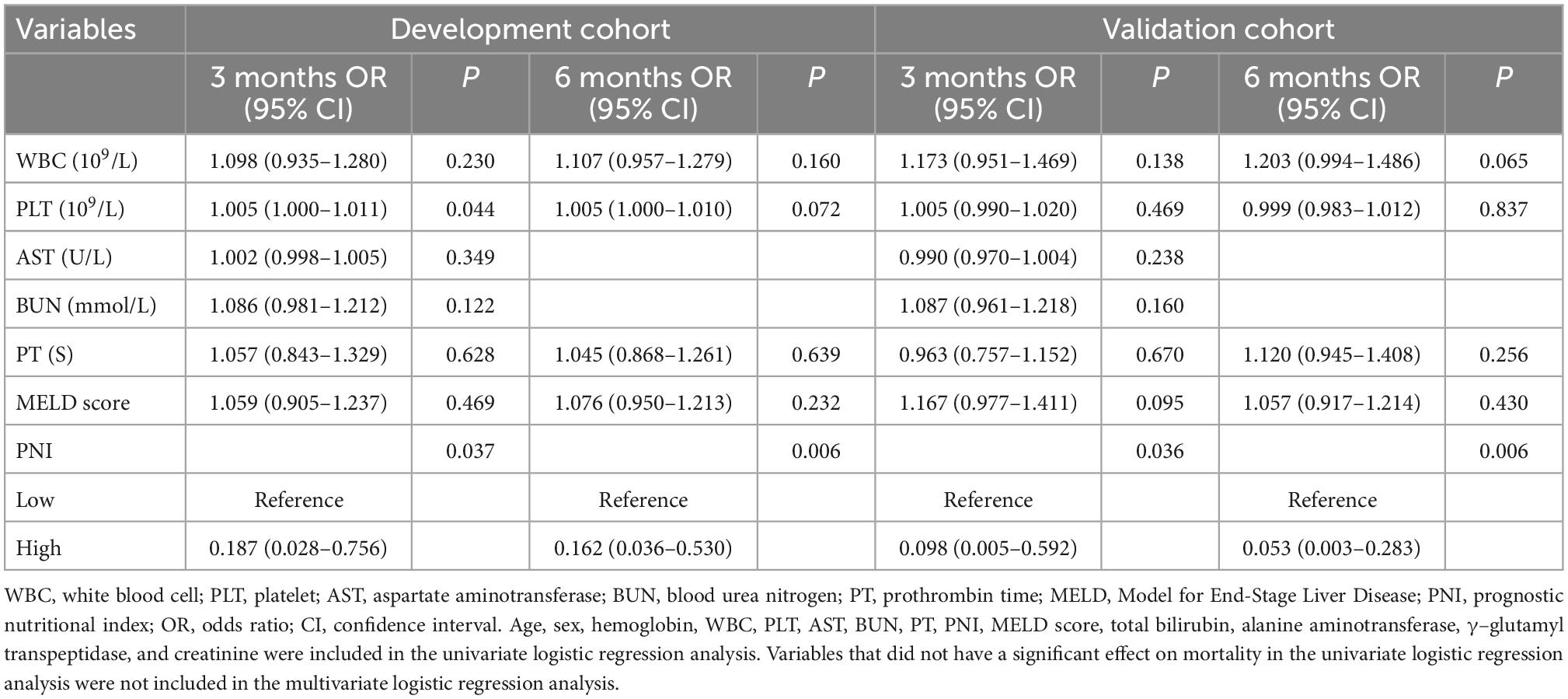
Table 3. Factors correlated with 3-month, and 6-month mortality in multivariate analyses in patients with decompensated liver cirrhosis in the development cohort and validation cohort.
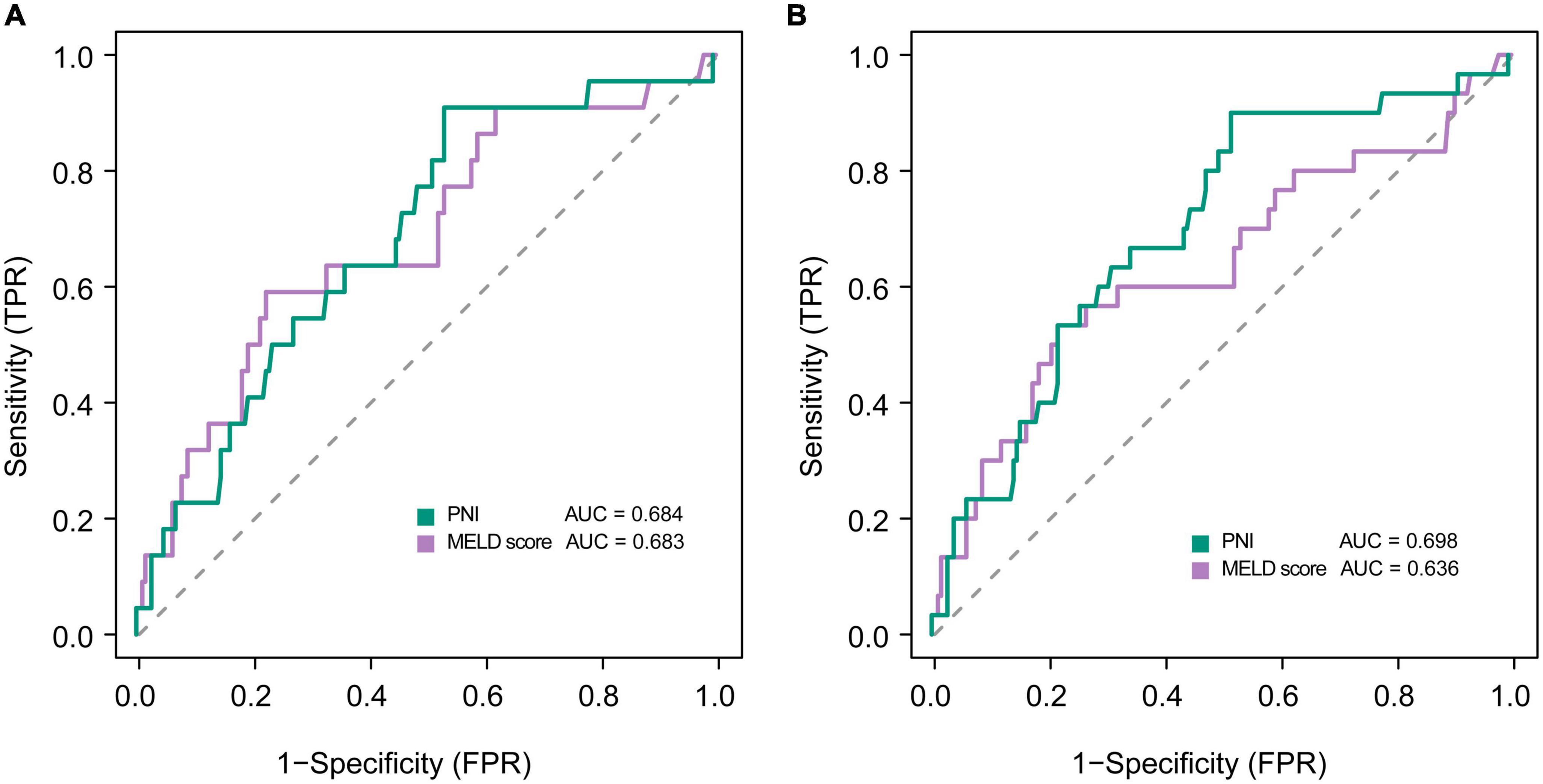
Figure 2. Receiver operating curves showing predictive accuracy of the MELD score and the PNI for mortality at (A) 3, and (B) 6 months in patients with decompensated liver cirrhosis.
A linear association was observed between the PNI and the risk of death at all follow-up time points (all P for non-linearity > 0.05) (Figure 3). PNI was found to be negatively associated with the risk of death, indicating that the risk of death increased with the decrease in PNI.
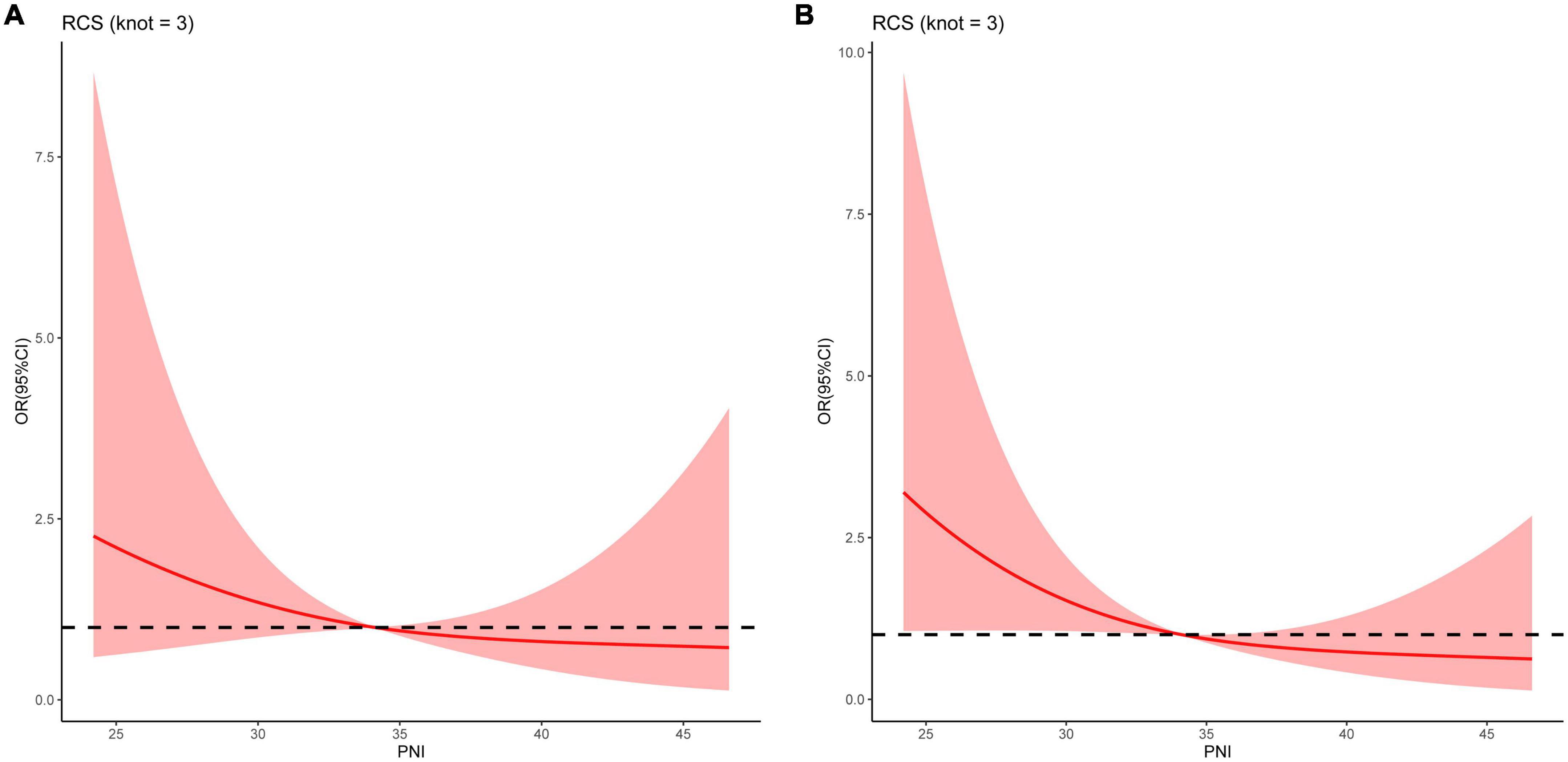
Figure 3. Restricted cubic spline curves for the relationships between the prognostic nutritional index and the risk of death at (A) 3, and (B) 6 months in patients with decompensated liver cirrhosis.
In the validation cohort, we confirmed that the PNI was an independent predictor of 3- and 6-month mortality in patients with DLC (Table 3). In addition, the ROC analysis demonstrated that the PNI had a comparable predictive ability with the MELD score (All Delong test P-value > 0.05) (Supplementary Figure 4).
The findings demonstrated that lower PNI was an independent predictor for adverse outcomes at all follow-up endpoints and PNI had a potential predictive value for mortality in patients with DLC. Furthermore, a linear correlation between PNI and the risk of death was observed, indicating that mortality increased with the decrease in PNI. Currently, the MELD score is the most extensively used scoring system for stratifying disease severity and predicting mortality in advanced liver disease but requires complicated calculations that are inconvenient for clinical practice (26). In contrast, PNI is a straightforward, effective, and simple index that uses serum albumin level and total lymphocyte count (8). Our findings revealed a significant negative correlation between PNI and MELD scores, and indicated that PNI had comparable predictive power to MELD score at all follow-up endpoints. Previous research found some inflammatory and nutrition indicators, such as the albumin-bilirubin scores, the international normalized ratio-to-albumin ratio, the neutrophil-to-albumin ratio, and the neutrophil-to-lymphocyte ratio, were independent predictors of mortality in patients with liver cirrhosis (35–38). To our knowledge, this is the first study which identified that PNI could be used as an independent predictor for mortality in patients with DLC.
PNI is a simple index developed by Onodera et al., reflecting immune, inflammatory, and nutritional status (39). According to recent studies, PNI significantly correlates with adverse outcomes in various diseases (7–17). Zheng et al. reported that a lower PNI is an independent risk factor for higher mortality in patients with respectable esophageal squamous cell carcinoma (7). According to Chen et al., a lower PNI is independently correlated with increased cardiovascular disease death and overall mortality in patients with heart failure (11). Bodolea et al. found that a lower PNI is an independent predictor of higher mortality in patients with severe COVID-19 (17). Similarly, our results showed that a lower PNI is an independent predictor of poor prognosis in patients with DLC.
There are specific explanations as to why PNI can predict the prognosis in patients with DLC. Firstly, albumin can reflect systemic inflammation and nutritional status (40, 41). Serum albumin had a negative relationship with the intensity of the systemic inflammatory response (37, 38). It has been recognized to have a significant role in the pathogenesis of end-stage liver cirrhosis and is related to poor outcome (21–24). A recent study has also demonstrated that low serum albumin may be caused by a combination of hepatic reorganization of protein synthesis in the body and redistribution of albumin in and out of blood vessels under high inflammatory conditions (41). In patients with liver cirrhosis, although albumin levels are primarily influenced by hepatic synthetic function, it is also influenced by other factors such as decreased protein intake, increase of the catabolic state, increased vascular permeability, systemic inflammatory response, protein-losing enteropathy secondary to portal hypertension, and impaired immunity (38, 40–48). Additionally, Topan et al. reported that low albumin levels were associated with malnutrition in patients with liver cirrhosis (49). Secondly, a previous study has shown that activated and differentiated CD4 + T lymphocytes are recruited to the inflamed liver and cause liver inflammation (50). Lymphopenia has been identified to be a marker of malnutrition and impaired immune response in patients with chronic liver disease (51, 52). Low lymphocytes were found to be associated with mortality in patients with advanced liver cirrhosis who were waiting for liver transplantation (53). Hence, the combination of lymphocytes and albumin primarily reflects inflammatory and immune status and partially reflects malnutrition that may help in predicting the prognosis of patients with DLC.
There are certain drawbacks in the current study. Firstly, it is a single-centered, retrospective, observational study, and selection bias cannot be avoided. Secondly, the predominant etiology of the patients in this study was hepatitis B virus infection, requiring caution to extrapolate these findings to other populations, especially in western cirrhosis populations where alcohol and NASH predominate. Third, this study did not compare the predictive ability of PNI with other nutritional indicators, such as skeletal muscle index or phase angle markers, for the prognosis of patients with DLC. Hence, prospective multicentered research with large sample numbers is required to further evaluate the clinical relevance of the PNI in patients with DLC.
Prognostic nutritional index may be a potential and promising predictor of prognosis in patients with DLC. It is readily available and could be used to identify patients at high risk of death. This finding may be used to improve the prognosis in patients with DLC by adjusting the treatment strategies in clinical practice.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by the Scientific Research and New Technology Institutional Review Board of Yijishan Hospital of Wannan Medical College. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
WW designed the research. YX and CH collected the data. YX and WW analyzed the data and wrote the main manuscript text. YX prepared all tables and figures. WW and CH revised the manuscript. All authors have read and approved the final manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2022.1092059/full#supplementary-material
Supplementary Figure 1 | The flow chart of the patient selection process.
Supplementary Figure 2 | The graphical presentation of the variables on admission. The units of variables were as follows: age, years; white blood cell (WBC), 109/L; lymphocyte (LYM), 109/L; hemoglobin (HGB), g/L; platelet (PLT), 109/L; albumin (ALB), g/L; total bilirubin (TBIL), μmol/L; alanine aminotransferase (ALT), U/L; aspartate aminotransferase (AST), U/L; γ–glutamyl transpeptidase (GGT), U/L; blood urea nitrogen (BUN), mmol/L; creatinine (Cr), μmol/L; prothrombin time (PT), s.
Supplementary Figure 3 | Kaplan-Meier analysis curves for survival according to prognostic nutritional index levels in the development and validation cohort.
Supplementary Figure 4 | Receiver operating curves of the MELD score, and the prognostic nutritional index for prediction of mortality in patients with decompensated liver cirrhosis in the validation cohort at (A) 3, and (B) 6 months.
1. Ginès P, Krag A, Abraldes J, Solà E, Fabrellas N, Kamath P. Liver cirrhosis. Lancet. (2021) 398:1359–76. doi: 10.1016/s0140-673601374-x
2. Ge P, Runyon B. Treatment of patients with cirrhosis. N Engl J Med. (2016) 375:767–77. doi: 10.1056/NEJMra1504367
3. D’Amico G, Garcia-Tsao G, Pagliaro L. Natural history and prognostic indicators of survival in cirrhosis: a systematic review of 118 studies. J Hepatol. (2006) 44:217–31. doi: 10.1016/j.jhep.2005.10.013
4. Stepanova M, De Avila L, Afendy M, Younossi I, Pham H, Cable R, et al. Direct and indirect economic burden of chronic liver disease in the united states. Clin Gastroenterol Hepatol. (2017) 15:759–66.e5. doi: 10.1016/j.cgh.2016.07.020
5. Wang S, Wang J, Chen J, Giri R, Chen M. Natural history of liver cirrhosis in south china based on a large cohort study in one center: a follow-up study for up to 5 years in 920 patients. Chin Med J. (2012) 125:2157–62.
6. Harrison P. Management of patients with decompensated cirrhosis. Clin Med. (2015) 15:201–3. doi: 10.7861/clinmedicine.15-2-201
7. Zheng Z, Zhu H, Cai H. Preoperative prognostic nutritional index predict survival in patients with resectable esophageal squamous cell carcinoma. Front Nutr. (2022) 9:824839. doi: 10.3389/fnut.2022.824839
8. Wu H, Zhou C, Kong W, Zhang Y, Pan D. Prognostic nutrition index is associated with the all-cause mortality in sepsis patients: a retrospective cohort study. J Clin Lab Anal. (2022) 36:e24297. doi: 10.1002/jcla.24297
9. Correa-Rodriguez M, Pocovi-Gerardino G, Callejas-Rubio J, Fernandez R, Martin-Amada M, Cruz-Caparros M, et al. The prognostic nutritional index and nutritional risk index are associated with disease activity in patients with systemic lupus erythematosus. Nutrients. (2019) 11:638. doi: 10.3390/nu11030638
10. Wang D, Hu X, Xiao L, Long G, Yao L, Wang Z, et al. Prognostic nutritional index and systemic immune-inflammation index predict the prognosis of patients with HCC. J Gastrointest Surg. (2021) 25:421–7. doi: 10.1007/s11605-019-04492-7
11. Cheng Y, Sung S, Cheng H, Hsu P, Guo C, Yu W, et al. Prognostic nutritional index and the risk of mortality in patients with acute heart failure. J Am Heart Assoc. (2017) 6:4876. doi: 10.1161/JAHA.116.004876
12. Peng J, Nie F, Li Y, Xu Q, Xing S, Gao Y. Prognostic nutritional index as a predictor of 30-day mortality among patients admitted to intensive care unit with acute exacerbation of chronic obstructive pulmonary disease: a single-center retrospective cohort study. Med Sci Monit. (2022) 28:e934687. doi: 10.12659/MSM.934687
13. Xie H, Wei L, Yuan G, Liu M, Tang S, Gan J. Prognostic value of prognostic nutritional index in patients with colorectal cancer undergoing surgical treatment. Front Nutr. (2022) 9:794489. doi: 10.3389/fnut.2022.794489
14. Keskin M, Hayiroglu M, Keskin T, Kaya A, Tatlisu M, Altay S, et al. A novel and useful predictive indicator of prognosis in st-segment elevation myocardial infarction, the prognostic nutritional index. Nutr Metab Cardiovasc Dis. (2017) 27:438–46. doi: 10.1016/j.numecd.2017.01.005
15. Zhang H, Tao Y, Wang Z, Lu J. Evaluation of nutritional status and prognostic impact assessed by the prognostic nutritional index in children with chronic kidney disease. Medicine. (2019) 98:e16713. doi: 10.1097/MD.0000000000016713
16. Liu Y, Yang X, Kadasah S, Peng C. Clinical value of the prognostic nutrition index in the assessment of prognosis in critically ill patients with stroke: a retrospective analysis. Comput Math Methods Med. (2022) 2022:4889920. doi: 10.1155/2022/4889920
17. Bodolea C, Nemes A, Avram L, Craciun R, Coman M, Ene-Cocis M, et al. Nutritional risk assessment scores effectively predict mortality in critically Ill patients with severe covid-19. Nutrients. (2022) 14:105. doi: 10.3390/nu14102105
18. Shiraki M, Nishiguchi S, Saito M, Fukuzawa Y, Mizuta T, Kaibori M, et al. Nutritional status and quality of life in current patients with liver cirrhosis as assessed in 2007-2011. Hepatol Res. (2013) 43:106–12. doi: 10.1111/hepr.12004
19. Maharshi S, Sharma B, Srivastava S. Malnutrition in cirrhosis increases morbidity and mortality. J Gastroenterol Hepatol. (2015) 30:1507–13. doi: 10.1111/jgh.12999
20. Alberino F, Gatta A, Amodio P, Merkel C, Di Pascoli L, Boffo G, et al. Nutrition and survival in patients with liver cirrhosis. Nutrition. (2001) 17:445–50. doi: 10.1016/s0899-900700521-4
21. Bajaj J, Heuman D, Hylemon P, Sanyal A, White M, Monteith P, et al. Altered profile of human gut microbiome is associated with cirrhosis and its complications. J Hepatol. (2014) 60:940–7. doi: 10.1016/j.jhep.2013.12.019
22. Arroyo V, Angeli P, Moreau R, Jalan R, Claria J, Trebicka J, et al. The systemic inflammation hypothesis: towards a new paradigm of acute decompensation and multiorgan failure in cirrhosis. J Hepatol. (2021) 74:670–85. doi: 10.1016/j.jhep.2020.11.048
23. Cazzaniga M, Dionigi E, Gobbo G, Fioretti A, Monti V, Salerno F. The systemic inflammatory response syndrome in cirrhotic patients: relationship with their in-hospital outcome. J Hepatol. (2009) 51:475–82. doi: 10.1016/j.jhep.2009.04.017
24. Abdel-Khalek E, El-Fakhry A, Helaly M, Hamed M, Elbaz O. Systemic inflammatory response syndrome in patients with liver cirrhosis. Arab J Gastroenterol. (2011) 12:173–7. doi: 10.1016/j.ajg.2011.11.006
25. Angeli P, Bernardi M, Villanueva C, Francoz C, Mookerjee R, Trebicka J, et al. Easl clinical practice guidelines for the management of patients with decompensated cirrhosis. J Hepatol. (2018) 69:406–60. doi: 10.1016/j.jhep.2018.03.024
26. Freeman R Jr., Wiesner R, Harper A, McDiarmid S, Lake J, Edwards E, et al. The new liver allocation system: moving toward evidence-based transplantation policy. Liver Transpl. (2002) 8:851–8. doi: 10.1053/jlts.2002.35927
27. du Prel J, Rohrig B, Hommel G, Blettner M. Choosing statistical tests: part 12 of a series on evaluation of scientific publications. Dtsch Arztebl Int. (2010) 107:343–8. doi: 10.3238/arztebl.2010.0343
28. Spearman C. The proof and measurement of association between two things. Int J Epidemiol. (2010) 39:1137–50. doi: 10.1093/ije/dyq191
29. Liao Y, Yin G, Fan X. The positive lymph node ratio predicts survival in T1-4n1-3m0 non-small cell lung cancer: a nomogram using the seer database. Front Oncol. (2020) 10:1356. doi: 10.3389/fonc.2020.01356
30. Walter S. The partial area under the summary roc curve. Stat Med. (2005) 24:2025–40. doi: 10.1002/sim.2103
31. Fluss R, Faraggi D, Reiser B. Estimation of the youden index and its associated cutoff point. Biom J. (2005) 47:458–72. doi: 10.1002/bimj.200410135
32. DeLong E, DeLong D, Clarke-Pearson D. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. (1988) 44:837–45.
33. Harrell F Jr., Lee K, Pollock B. Regression models in clinical studies: determining relationships between predictors and response. J Natl Cancer Inst. (1988) 80:1198–202. doi: 10.1093/jnci/80.15.1198
34. Johannesen C, Langsted A, Mortensen M, Nordestgaard B. Association between low density lipoprotein and all cause and cause specific mortality in denmark: prospective cohort study. BMJ. (2020) 371:m4266. doi: 10.1136/bmj.m4266
35. Chen R, Cai Y, Wu J, Wang X, Song M, Wang Y, et al. Usefulness of albumin-bilirubin grade for evaluation of long-term prognosis for hepatitis b-related cirrhosis. J Viral Hepat. (2017) 24:238–45. doi: 10.1111/jvh.12638
36. Rice J, Dodge J, Bambha K, Bajaj J, Reddy K, Gralla J, et al. Neutrophil-to-lymphocyte ratio associates independently with mortality in hospitalized patients with cirrhosis. Clin Gastroenterol Hepatol. (2018) 16:1786–91.e1. doi: 10.1016/j.cgh.2018.04.045
37. Cai M, Han Z, He X, Zhang J. Usefulness of international normalized ratio to albumin ratio for evaluation of mortality in hepatitis b virus-associated decompensated cirrhosis. Biomed Res Int. (2021) 2021:6664574. doi: 10.1155/2021/6664574
38. Han Z, He X, Peng S. Neutrophil count to albumin ratio as a prognostic indicator for hbv-associated decompensated cirrhosis. J Clin Laborat Analy. (2021) 35:23730. doi: 10.1002/jcla.23730
39. Onodera T, Goseki N, Kosaki G. [Prognostic nutritional index in gastrointestinal surgery of malnourished cancer patients]. Nihon Geka Gakkai Zasshi. (1984) 85:1001–5.
40. Soeters P, Wolfe R, Shenkin A. Hypoalbuminemia: pathogenesis and clinical significance. JPEN J Parenter Enteral Nutr. (2019) 43:181–93. doi: 10.1002/jpen.1451
41. Evans D, Corkins M, Malone A, Miller S, Mogensen K, Guenter P, et al. The use of visceral proteins as nutrition markers: an aspen position paper. Nutr Clin Pract. (2021) 36:22–8. doi: 10.1002/ncp.10588
42. Gabay C, Kushner I. Acute-phase proteins and other systemic responses to inflammation. N Engl J Med. (1999) 340:448–54. doi: 10.1056/nejm199902113400607
43. Levitt D, Levitt M. Protein losing enteropathy: comprehensive review of the mechanistic association with clinical and subclinical disease states. Clin Exp Gastroenterol. (2017) 10:147–68. doi: 10.2147/ceg.S136803
44. Dahlqvist G, Jamar F, Zech F, Geubel A. In-111 transferrin scintigraphy in cirrhosis with hypoalbuminemia: evidence for protein-losing enteropathy in a small group of selected cases. Scand J Gastroenterol. (2012) 47:1247–52. doi: 10.3109/00365521.2012.696682
45. Takada A, Kobayashi K, Takeuchi J. Gastroenteric clearance of albumin in liver icrrhosis; relative protein-losing gastroenteropathy. Digestion. (1970) 3:154–64. doi: 10.1159/000197026
46. Lee S, Lee J, Lee S, Kim E, Chang J, Kim D, et al. Prediction of postoperative pulmonary complications using preoperative controlling nutritional status (conut) score in patients with resectable non-small cell lung cancer. Sci Rep. (2020) 10:12385. doi: 10.1038/s41598-020-68929-9
47. Roxburgh C, McMillan D. Role of systemic inflammatory response in predicting survival in patients with primary operable cancer. Fut Oncol. (2010) 6:149–63. doi: 10.2217/fon.09.136
48. Traub J, Reiss L, Aliwa B, Stadlbauer V. Malnutrition in patients with liver cirrhosis. Nutrients. (2021) 13:540. doi: 10.3390/nu13020540
49. Topan M, Sporea I, Dãnilã M, Popescu A, Ghiuchici A, Lupuşoru R, et al. Comparison of different nutritional assessment tools in detecting malnutrition and sarcopenia among cirrhotic patients. Diagnostics. (2022) 12:893. doi: 10.3390/diagnostics12040893
50. Wu W, Yan H, Zhao H, Sun W, Yang Q, Sheng J, et al. Characteristics of systemic inflammation in hepatitis B-precipitated aclf: differentiate it from no-aclf. Liver Int. (2018) 38:248–57. doi: 10.1111/liv.13504
51. Okeefe S. Malnutrition and immuno-incompetence in patients with liver disease. Lancet. (1980) 316:615–7. doi: 10.1016/s0140-673690284-6
52. Fernandez-Ruiz M, Lopez-Medrano F, Romo E, Allende L, Meneu J, Fundora-Suarez Y, et al. Pretransplant lymphocyte count predicts the incidence of infection during the first two years after liver transplantation. Liver Transpl. (2009) 15:1209–16. doi: 10.1002/lt.21833
Keywords: decompensated liver cirrhosis, prognostic nutritional index, prognostic factor, mortality, independent predictor, validation
Citation: Xie Y, He C and Wang W (2023) Prognostic nutritional index: A potential biomarker for predicting the prognosis of decompensated liver cirrhosis. Front. Nutr. 9:1092059. doi: 10.3389/fnut.2022.1092059
Received: 07 November 2022; Accepted: 19 December 2022;
Published: 06 January 2023.
Edited by:
Eloisa Colin-Ramirez, Universidad Anáhuac México Norte, MexicoReviewed by:
Inés Osvely Méndez Guerrero, Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán (INCMNSZ), MexicoCopyright © 2023 Xie, He and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wei Wang,  d3d3eUB3bm1jLmVkdS5jbg==
d3d3eUB3bm1jLmVkdS5jbg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.