
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Nutr., 13 December 2022
Sec. Nutrition and Metabolism
Volume 9 - 2022 | https://doi.org/10.3389/fnut.2022.1092031
Background: The purpose of this meta-analysis was to explore the effects of low-carbohydrate ketogenic diets on cardiovascular risk factors in overweight or obese patients. However, there are limited literature data about effects of low-carbohydrate ketogenic diets on cardiovascular risk factors in obese or overweight patients.
Methods: We systematically searched PubMed, EMBASE, Web of Science, OVID, and Cochrane Library databases (last updated in September 2022) for randomized controlled trials (RCTs) which recruited overweight or obesity patients on ketogenic diets in order to control cardiovascular risk factors (blood glucose, weight, and lipids). The overall effect size for continuous variables was expressed as a weighted standardized mean difference (SMD) with a confidence interval of 95%. Considering type 2 diabetes mellitus (T2DM) status at baseline, subgroup analyses were performed when appropriate, based on T2DM comorbidity among patients. The effect model was selected according to heterogeneity.
Results: We finally selected 21 studies. Low carbohydrate ketogenic diets exerted a greater impact on cardiovascular risk factors in obese/ overweight patients with T2DM when compared with those on non-ketogenic diets, with lower fasting plasma glucose (FPG) (SMD, −0.75; P < 0.001) and hemoglobin A1c (HbA1c) (SMD, −0.53; P < 0.001) levels identified. Low-carbohydrate ketogenic diets significantly reduced body mass index (BMI) (SMD, −2.27; P = 0.032), weight (SMD, −6.72; P < 0.001), and waist circumference (SMD, −4.45; P = 0.003) in obese/ overweight patients with T2DM. Also, ketogenic diets improved lipid profiles in these patients; triglyceride (TG) (SMD, −0.32; P = 0.013) levels were lowered and high density lipoprotein (HDL) showed an upward trend with the P-value close to statistically significant level (SMD, −0.32; P = 0.052). In general, irrespective of diabetic status at baseline, ketogenic diets were more effective in reducing TG (SMD, −0.2; P = 0.02) and increasing HDL (SMD, 0.11; P = 0.03) levels when compared with non-ketogenic diets.
Conclusions: Low-carbohydrate ketogenic diets effectively improved cardiovascular risk factors (blood glucose, weight, and lipids) in obese/ overweight patients, especially those with T2DM when compared with non-ketogenic diets.
In both developing and developed countries, obesity levels continue to grow with ~38 and 20% of the world's adult population predicted to be overweight and obese by 2030 (1), respectively. On a global level, obesity is one of the most serious health issues (2, 3). Moreover, a recent study by Pi-Sunyer et al. (3) suggested that obesity was a major potential risk factor for chronic diseases, such as cardiovascular disease, diabetes mellitus (DM), cerebrovascular disease, metabolic syndrome, and cancer, thereby increasing social, medical, and economic burdens. Importantly, studies have also shown that normal weight reduces metabolic syndrome prevalence and all-cause and cardiovascular mortality when compared with obese or overweight individuals (4). The benefits of weight loss are not only related to blood sugar control, but also to several cardiovascular risk factors, such as blood pressure, high-density lipoprotein (HDL), total cholesterol (TC), triglyceride (TG), low-density lipoprotein (LDL), and uric acid (UA) levels (5). Dietary therapy for weight loss is commonly recommended in clinical practice (6).
Previous studies have proposed many different dietary approaches, including low-carbohydrate ketogenic diets which includes ~60% fat intake, 25% protein intake, and 15% carbohydrate intake, but without limiting caloric intake (7, 8). Ketogenic diets with low carbohydrate content may mimic starvation in the body and generate ketosis. Thus, the glucose-based energy model is replaced by the ketone body-based energy model, which requires fat to promote catabolism and reduce fat synthesis, while gluconeogenesis increases energy expenditure (9). To this end, insoluble TGs are converted to water-soluble ketone bodies. Therefore, ketone bodies can be eliminated by the body via urine excretion, taking away energy (10). In addition, increased ketone also bodies suppress appetite (11). Such diets are widely reported as being effective for weight loss and glycemic control (12). A recent review showed that low-carbohydrate diets reduced postprandial glucose levels and exerted cardioprotective effects (13). A meta-analysis of 14 studies showed that a ketogenic diet improved the metabolic parameters in overweight or obese patients when compared with a low-fat diet, it was particularly effective for metabolic parameters related to blood glucose, weight, and lipid control in patients with previous diabetes (14); however, Leow et al. (15) showed that low-carbohydrate diets potentially exacerbated lipid profiles in obese patients. The evidence supporting ketogenic diets is currently limited and the diet's potential risks are real (12). Therefore, appropriateness and potential risks of low-carbohydrate ketogenic diets in obese patients remain controversial. To address this issue, we investigated the clinical effects of ketogenic diets in overweight or obese patients when compared with non-ketogenic diets by assessing changes in cardiovascular risk factors related to glycemic, weight, UA, blood pressure, and lipid levels.
The following databases were systematically searched: Pubmed, EMBASE, OVID, Web of Science, and Cochrane Library (last updated September 2022). A combined Medical Subject Heading and free word search strategy was adopted. Key words included: ketogenic diet, obesity, overweight, abdominal obesity, type 2 diabetes, blood sugar, insulin resistance, hyperlipidemia, lipid metabolism, hyperuricemia, metabolic syndrome, cardiovascular risk factors, randomized controlled trials (RCTs), and clinical trials. Additionally, to avoid missing publications, bibliographies from previous reviews were also screened.
Inclusion criteria: (1) randomized clinical trials (RCTs) published up to September 2022 which investigated the effects of ketogenic diets on metabolic parameters in overweight or obese patients; (2) adequate original data which could be obtained from the original research, and (3) full original research texts.
Exclusion criteria: case-controls studies, cohort studies, cross-sectional studies, animal experiments, reviews, commentaries, editorials, and case reports. Study titles and summaries were independently assessed by two reviewers using the inclusion criteria. For potentially eligible studies, two reviewers assessed full texts. In case of disagreement, matters were settled through consultation and a consensus reached by the corresponding authors (XD and JZ).
Two reviewers independently extracted data from eligible articles into tables. Extracted data included: author's name, publication year, country, study population, mean age, gender, sample size, BMI, follow-up duration, diet type, and metabolic parameters of ketogenic and non-ketogenic diet groups (Table 1 and Supplementary Table S1).
Using the Cochrane Collaboration Risk of Bias Tool, RCT methodological quality was assessed by two reviewers (Supplementary Figure S2); areas were assessed with a low, medium, or high risk of deviation rather than as a range of scores. Differences were resolved by consultation and a consensus reached by corresponding authors (XD and ZJ).
The overall effect size for continuous variables was expressed as a weighted standardized mean difference (SMD) with a confidence interval (CI) of 95%. Post-treatment metabolic parameters were compared between patients on ketogenic and non-ketogenic diets. Considering T2DM status at baseline and to minimize potential heterogeneity sources, subgroup analyses were performed when appropriate, based on T2DM comorbidity among patients. Heterogeneity was assessed using I2 and Cochran's Q tests, with I2 > 50% and p < 0.01 indicating significant heterogeneity. If significant, the random-effect model was adopted; otherwise, the fixed-effect model was applied. Two-tailed P < 0.05 values were considered statistically significant. All analyses were performed using STATA version 14.0. Sensitivity analyses were performed by omitting one study at a time. Egger's linear regression was used to test publication bias, which was evaluated by the trim-and-fill method.
This meta-analysis included 21 RCTs (9, 16–35) involving 1,074 subjects. The process and literature screening results are shown (Supplementary Figure S1). The basic characteristics and results from selected studies are shown (Table 1). Raw extracted data are shown (Supplementary Table S1).
Subgroup analysis of obese/overweight patients with T2DM showed that when compared with the non-ketogenic diet group, FPG (SMD, −0.75; 95% CI, −1.15 to −0.35; I2 = 0.00%, Z = 3.7; P < 0.001) and HbA1c levels (SMD, −0.53; 95% CI, −0.78 to −0.29; I2 = 0.00%, Z = 4.24; P < 0.001) were significantly decreased in the ketogenic diet group. Subgroup analysis of non-diabetic patients showed that the glycemic index was not significantly decreased. In general, ketogenic diets were more effective in reducing FPG (SMD, −0.23; 95% CI, −0.42 to −0.04, I2 = 90.6%, Z = 2.38, P = 0.017) and HbA1c (SMD, −0.22; 95% CI, −0.42 to −0.03, I2= 67.9%, Z = 2.24, P = 0.025) levels when compared with non-ketogenic diets (Figure 1).
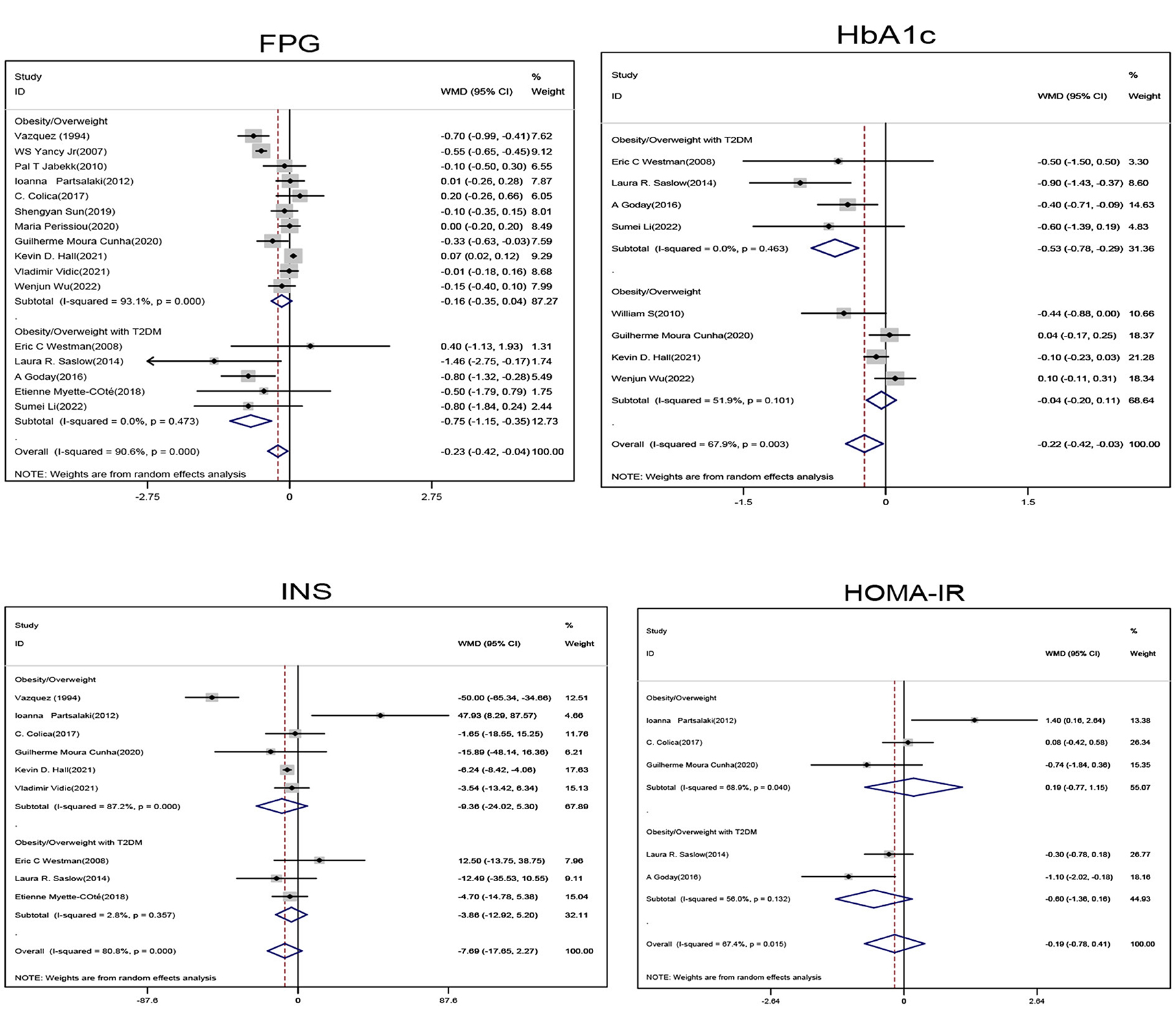
Figure 1. Forest plot showing weighted mean difference and 95% confidence intervals (CIs) for low carbohydrate ketogenic diet effects on glucose metabolism. FPG, Fasting blood glucose; HbA1c, Glycosylated hemoglobin; INS, Insulin; HOMA-IR, Homeostatic model assessment.
Subgroup analysis of obese/overweight patients with T2DM showed that when compared with the non-ketogenic diet group, TG levels (SMD, −0.32; 95% CI,− 0.57 to−0.07; I2 = 51%, Z = 2.48; P = 0.013) were significantly decreased in the ketogenic diet group, and HDL levels showed an upward trend and a P-value close to statistically significant level (SMD, 0.07; 95% CI, −0.00 to 0.14; I2 = 0.00%, Z = 1.95; P = 0.052). Subgroup analyses of obese/overweight patients without T2DM showed that when compared with the non-ketogenic diet, HDL levels were significantly higher in the ketogenic diet group (SMD, 0.13,95% CI, 0.04 to 0.21, I2 = 82.2%, Z = 2.85, P = 0.004), and TG levels showed a downward trend and a P-value close to statistically significant level (SMD, −0.15; 95% CI, −0.31 to 0.01; I2 = 81.1%, Z = 1.88; P = 0.06). In general, ketogenic diets were more effective in reducing TG (SMD, −0.2; 95% CI, −0.32 to −0.07, I2 = 75.7%, Z = 3.03, P = 0.02) and increasing HDL (SMD, 0.11; 95% CI, 0.04 to 0.18, I2 = 81.5%, Z = 2.98, P = 0.03) levels when compared with non-ketogenic diets. However, ketogenic diets had no significant effect on changes on TC and LDL levels (Figure 2).
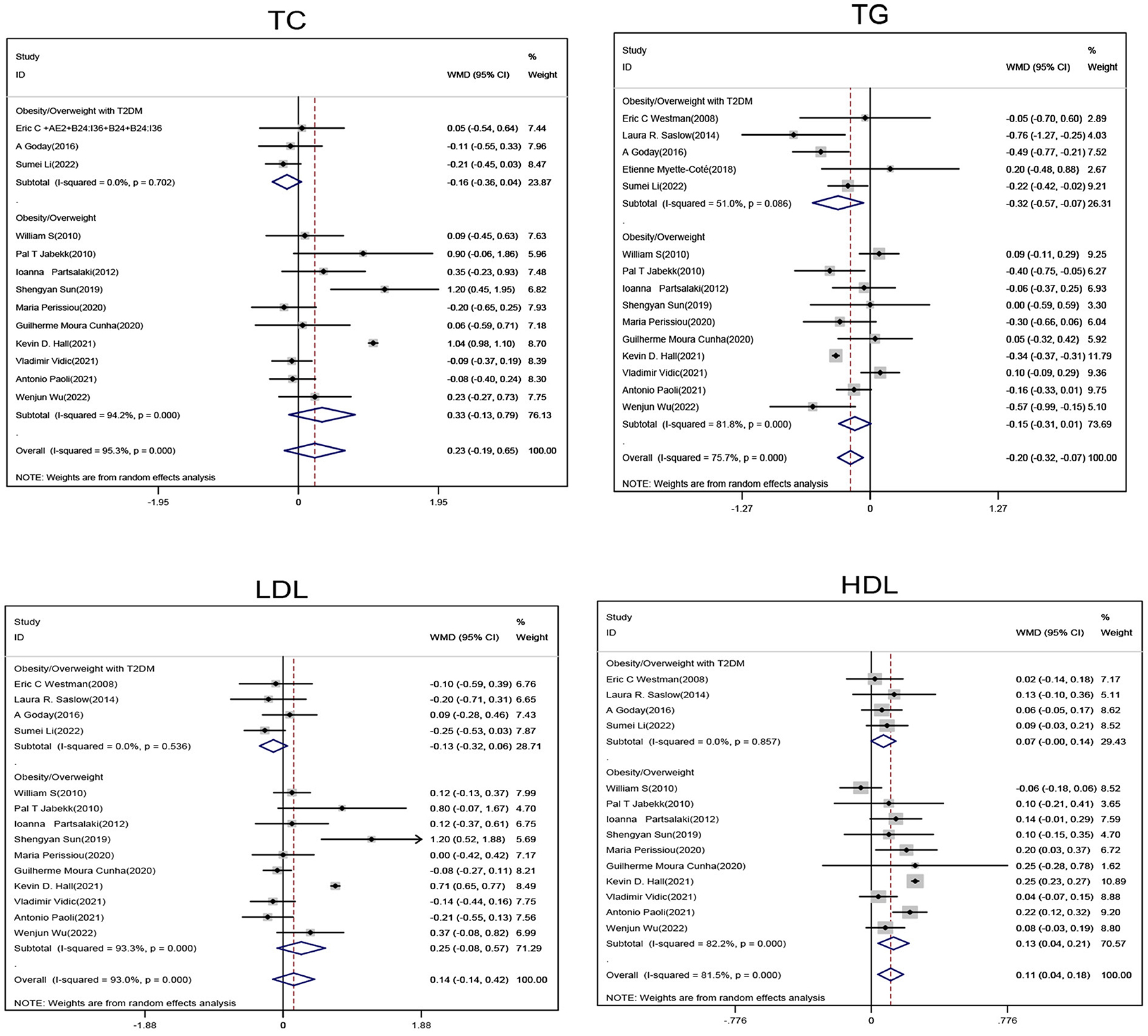
Figure 2. Forest plot showing weighted mean difference and 95% confidence intervals (CIs) for low carbohydrate ketogenic diet effects on lipid metabolism. TC, Total cholesterol; TG, Triglyceride; LDL, Low density lipoprotein; HDL, High density lipoprotein.
Subgroup analysis of obese/overweight patients with T2DM showed that when compared with the non-ketogenic diet group, subjects in the ketogenic diet group showed significant reductions in weight (SMD, −6.72; 95% CI, −10.42 to −3.02; I2 = 0.00%, Z = 3.56; P < 0.001), BMI (SMD, −2.27; 95% CI, −4.33 to −0.2; I2 = 0.00%, Z = 2.15; P = 0.032) and waistline circumference (SMD, −4.45; 95% CI, −7.34 to −1.56; I2 = 0.00%, Z = 3.01; P = 0.003). Subgroup analysis of obese/overweight patients without T2DM showed that when compared with non-ketogenic diet, subject's body fat mass (BFM) (SMD, −1.48; 95% CI, −2.55 to −0.4; I2 = 0.00%, Z = 2.69; P = 0.007) was significantly decreased in the ketogenic diet group. In general, weight (SMD, −2.52; 95% CI, −4.45 to −0.6; I2 = 11.7%, Z = 2.58; P = 0.01), BMI (SMD, −1.02; 95% CI, −1.79 to −0.24; I2 = 0.00%, Z = 2.57; P = 0.01), and waistline circumference (SMD, −1.92; 95% CI, −3.59 to −0.25; I2 = 41.6%, Z = 2.25; P = 0.025) were significantly decreased in the ketogenic diet group (Figure 3).
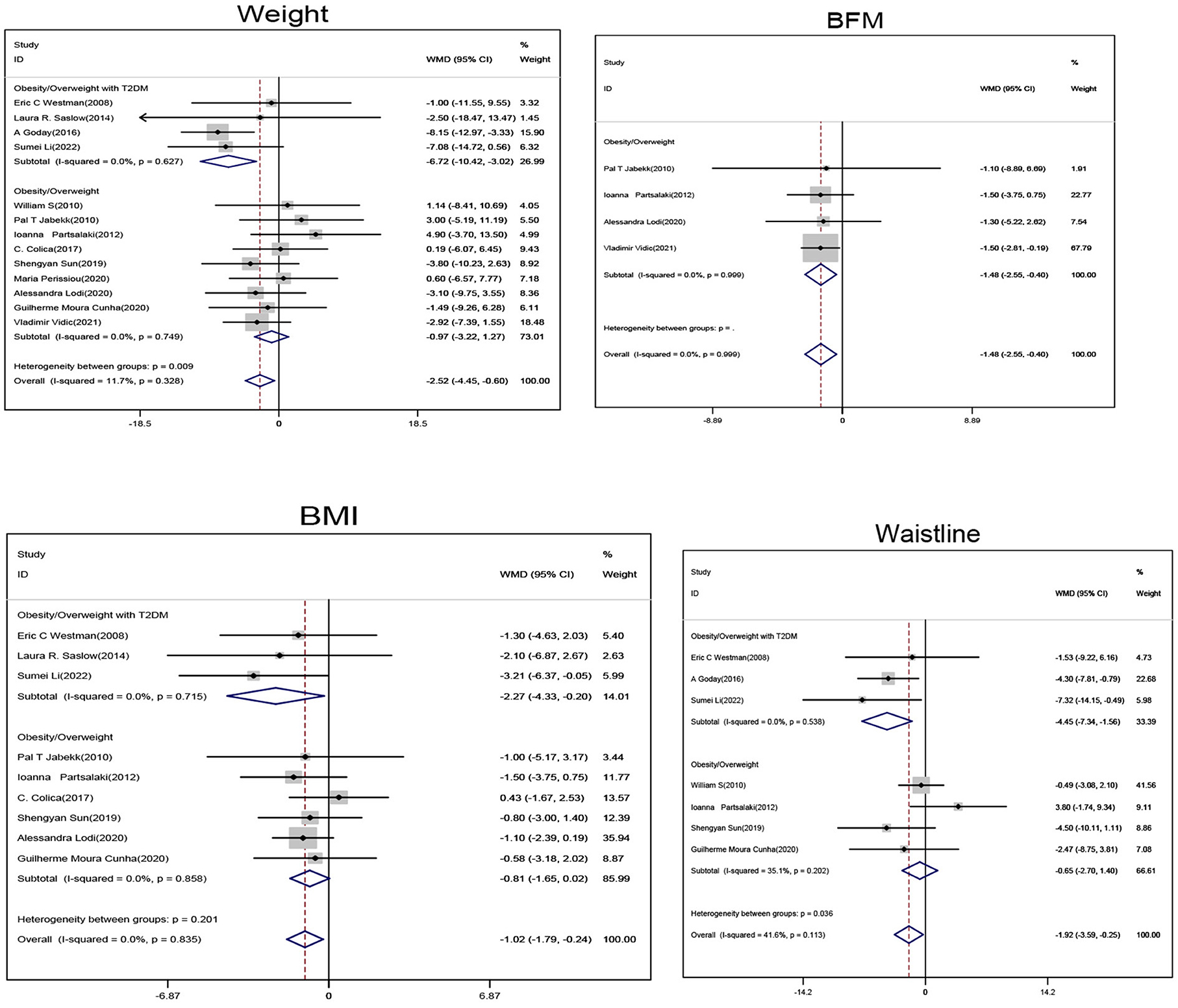
Figure 3. Forest plot showing weighted mean difference and 95% confidence intervals (CIs) for low carbohydrate ketogenic diet effects on weight control. BFM, Body fat volume; BMI, Body mass index.
Obese/overweight patients on ketogenic diet showed no statistically significant changes in Uric acid (UA), blood urea nitrogen (BUN), and creatinine levels when compared with those on non-ketogenic diet (Figure 4).
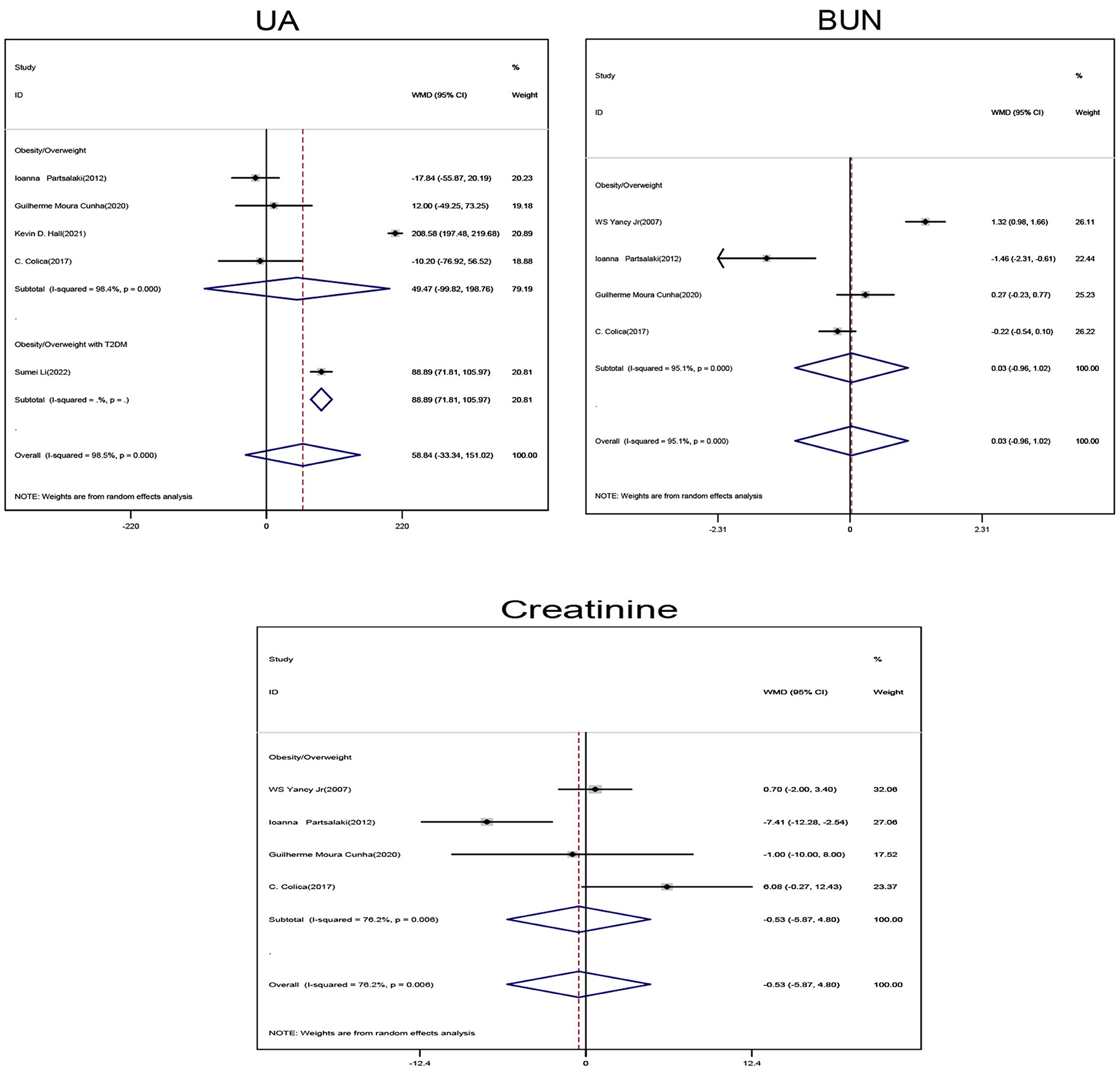
Figure 4. Forest plot showing weighted mean difference and 95% confidence intervals (CIs) for low carbohydrate ketogenic diet effects on kidney related functions. UA, Uric acid; BUN, Urea nitrogen.
Subgroup analyses of obese/overweight patients with or without T2DM on ketogenic diet showed no statistically significant changes in systolic and diastolic blood pressure levels when compared with non-ketogenic diet (Figure 5).
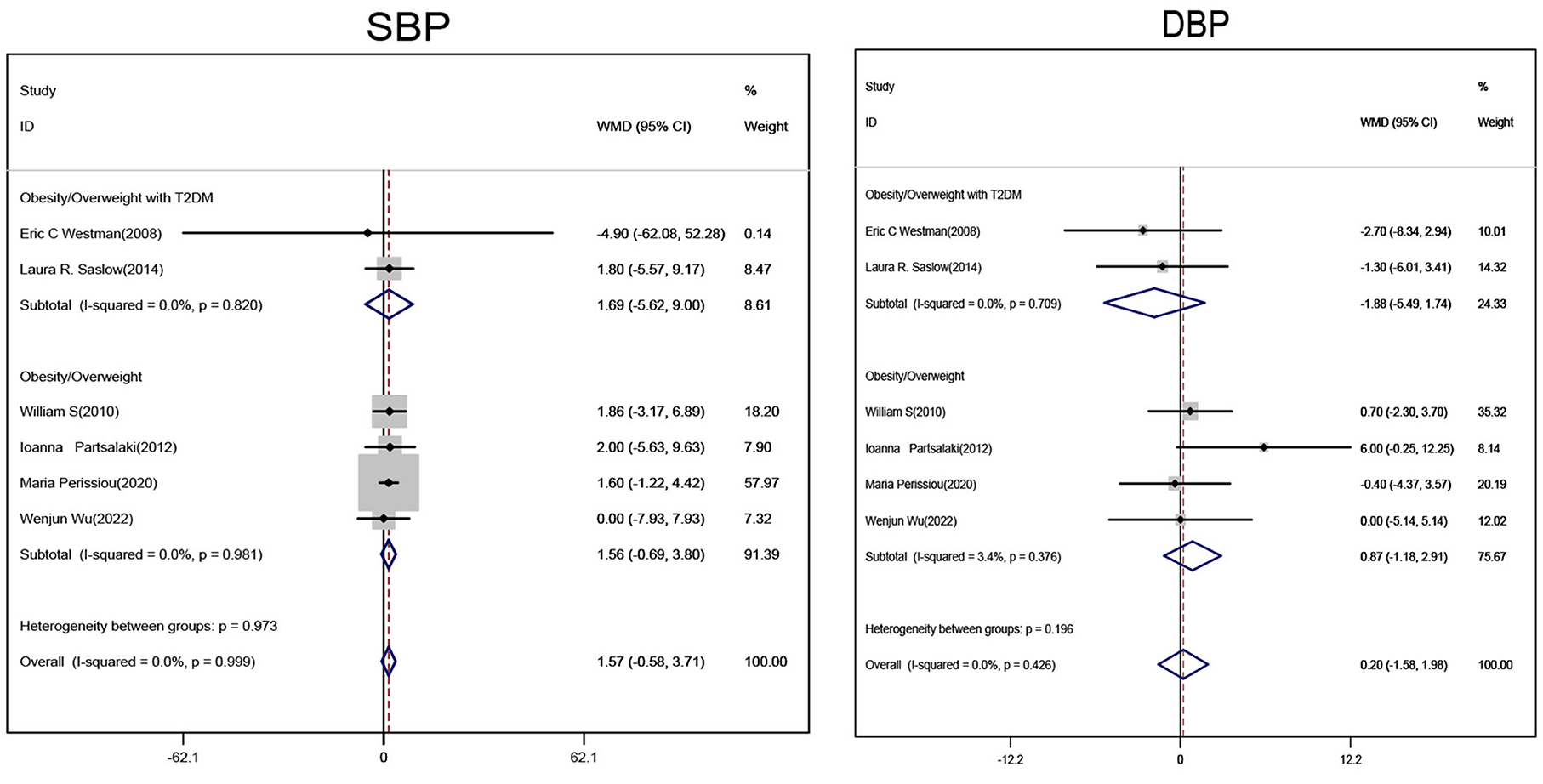
Figure 5. Forest plot showing weighted mean difference and 95% confidence intervals (CIs) for low carbohydrate ketogenic diet effects on blood pressure. SBP, Systolic pressure; DBP, Diastolic pressure.
In sensitivity analyses, suspected heterogeneity sources were identified and removed when data were pooled (Supplementary Figures S3–S9).
Egger linear regression analyses were used to test for publication bias. Ketogenic diets showed significant publication bias toward TC, LDL, HDL, and UA (Egger Test's P = 0.004, 0.006, 0.002, and 0.048, respectively) (Supplementary Tables S3–S6).
These results showed that publication bias had no effects on our observations, because the P-values of combined effect values before and after trim-and-fill analyses were not reversed. We observed no significant differences in heterogeneity and merging results before and after trim and fill analyses, suggesting our stable results (Supplementary Table S7).
In this meta-analysis of 21 RCTs which included 1,074 participants, we investigated the efficacy of ketogenic diets when compared with non-ketogenic diets toward cardiovascular risk factor control in overweight or obese patients. Our results showed: (1) In obese/overweight patients with T2DM, low-carbohydrate ketogenic diets significantly improved FPG and HbA1c levels and showed diets were more effective in controlling blood glucose. (2) In obese/overweight patients with T2DM and overall patients, low-carbohydrate ketogenic diets significantly decreased TG levels and increased HDL levels. (3) In obese/overweight patients with T2DM, after ketogenic diet intervention, weight, BMI, and waist circumference indices were significantly decreased, while BFM was also significantly decreased in non-diabetic patients. Our findings suggested that low-carbohydrate ketogenic diets were more beneficial dietary options for obese/overweight T2DM patients and improved cardiovascular risk factors related to glycemic, weight, and lipid control.
Concomitant with increased global obesity levels, cardiovascular disease levels have similarly increased (36). Obesity is also a major risk factor for insulin resistance and T2DM (37). An insulin resistance status is often associated with ectopic lipid accumulation, especially in the liver and skeletal muscle (38). Obesity and diabetes often co-exist in the same patient (37). Clear associations have been identified between obesity and cardiovascular disease incidence, including hypertension, coronary artery disease, heart failure, and sudden death. Many individuals with insulin resistance, the leading cause of T2DM, tend to have higher TG and lower HDL levels (39–41). Obesity, diabetes and dyslipidemia are all risk factors for cardiovascular disease (39–41).
The importance of a proper balanced diet can-not be underestimated when managing and preventing chronic diseases. The American Diabetes Association recommends a combination of physical activity, diet management, and medication intake to control blood glucose, weight, and other abnormal metabolic factors. Ketogenic diets have many health benefits for obese/overweight patients with T2DM (11, 42). These diets provide energy via fat oxidation, and when the body experiences extreme hunger or limited carbohydrate levels, ketone bodies are produced and released into the circulation via fatty acid conversion in the liver (43). Nutritional ketosis is distinct to severe pathological diabetic ketosis; blood ketone bodies are maintained in the 0.5–3.0 mmol/l range, blood glucose is decreased, and no nutritional ketosis symptoms are present (44).
Our meta-analysis confirmed that ketogenic diets significantly reduced FPG and HbA1c levels in obese/overweight patients with T2DM. A possible mechanism for this health benefit could be extreme dietary sugar restriction which reduces intestinal monosaccharide absorption, leading to lower blood glucose levels and reduced glucose fluctuations. Considerable evidence now suggests the effectiveness of ketogenic diets in regulating glucose metabolism (14, 45). HbA1c effectively reflects blood glucose control in the previous 2–3 months in patients with diabetes. When HbA1c levels drop, the risks of myocardial infarction and microvascular complications are reportedly decreased (21, 46).
Ketogenic diets not only improve glucose metabolism, but also improve lipid metabolism (in particular for obese/overweight patients with T2DM), and decrease TG and increase HDL levels so as, to improve abnormal blood lipid levels. Dyslipidemia is lipotoxic to cells and causes and/or exacerbates insulin resistance. The condition is characterized by elevated TG and free fatty acids (FFA) levels. Increased FFA is an independent risk factor for insulin resistance and may increase cardiovascular disease risks (47, 48). Also, in considering that low HDL and high TG levels are independent risk factors for insulin resistance and cardiovascular disease (49), our analyses suggest a cardioprotective effect for ketogenic diets in patients with diabetes; ketogenic diets appear to significantly affect TG and HDL levels in these individuals. Therefore, improved dyslipidemia is not only beneficial for regulating insulin sensitivity, but also protects against cardiovascular disease (49, 50).
In obese/overweight individuals with T2DM, ketogenic diets significantly reduced weight, BMI, and waist circumference. Hall et al. suggested that diets high in carbohydrates were prone to generating obesity as they tended to increase insulin production (51). Insulin-guided energy distribution is stored in adipose tissue in the form of fat, rather than being oxidized by metabolically active tissue, and is believed to result in a perceived state of hunger within cells (51). In response, hunger and appetite increase and metabolism is suppressed, thereby promoting a positive energy balance associated with obesity development (51). Ketogenic diets trigger gluconeogenesis and ketogenesis in the liver, producing ketone bodies from glucose-substituting fatty acids. Ketone body production usually occurs when endogenous glucose production is depleted and lowers insulin levels in the blood, which further limits fat and glucose storage in the body (50, 52). A clear association exists between obesity and cardiovascular disease incidence; however, with improved weight control, cardiovascular disease incidence and mortality is also decreased (53).
Our RCT meta-analysis had the following advantages: firstly, as an RCT meta-analysis, we investigated the effects of ketogenic diets on cardiovascular risk factors (glycemic control, weight loss, and lipid control) in various non-ketogenic diets. Accordingly, obese/overweight patients with T2DM were more likely to benefit from ketogenic diets in terms of weight loss, improved blood glucose levels, and lipid control. Secondly, the meta-analysis included a large number of participants (n = 1,074). Thirdly, comprehensive sensitivity analyses, publication bias, and trim and fill evaluations indicated the reliability of our results. Fourthly, all selected studies were RCTs, which further increased the reliability of our conclusions.
However, our meta-analysis had the following limitations: firstly, we combined RCT ketogenic diets with different control diets. Secondly, cardiovascular risk factors were used to explore the metabolic effects of ketogenic diets, including blood glucose levels, weight, and lipid composition, rather than to assess cardiovascular disease incidence and mortality in patients. However, the effects of ketogenic diets on cardiovascular risk factors could help validate the effects of low-carbohydrate ketogenic diets on clinical cardiovascular event endpoints. Finally, heterogeneity was evident in our data and was possibly due to differences in control diets and follow-up durations across the 21 studies. However, our comprehensive sensitivity analysis, publication bias, and trim and fill evaluation indicated the reliability of our observations.
In conclusion, this is a large, comprehensive and RCTs meta-analysis designed to explore the effect of low-carbohydrate ketogenic diets on cardiovascular risk factors in obese/overweight patients. Our results showed that low-carbohydrate ketogenic diets were more effective than control diets in improving cardiovascular risk factors (body weight, blood glucose, and lipid levels) in obese/overweight patients especially those with T2DM. But there remains a requirement for further prospective studies, to determine the long-term effects of low-carbohydrate ketogenic diets on cardiovascular risk markers in obese/overweight populations, and to determine their impact on cardiovascular event endpoints in these populations.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
WL and JZ had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: WL, DX, and JZ. Acquisition of data: YZ and QY. Drafting of the manuscript: WL. Statistical analysis: WL, ZQ, and JZ. Study supervision: DX and JZ. Analysis and interpretation of data and critical revision of the manuscript for important intellectual content: All authors.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2022.1092031/full#supplementary-material
1. Sahoo K, Sahoo B, Choudhury AK, Sofi NY, Kumar R, Bhadoria AS. Childhood obesity: causes and consequences. J Family Med Prim Care. (2015) 4:187–92. doi: 10.4103/2249-4863.154628
2. Rubini A, Bosco G, Lodi A, Cenci L, Parmagnani A, Grimaldi K, et al. Effects of twenty days of the ketogenic diet on metabolic and respiratory parameters in healthy subjects. Lung. (2015) 193:939–45. doi: 10.1007/s00408-015-9806-7
3. Pi-Sunyer X. The medical risks of obesity. Postgrad Med. (2009) 121:21–33. doi: 10.3810/pgm.2009.11.2074
4. Katzmarzyk PT, Church TS, Janssen I, Ross R, Blair SN. Metabolic syndrome, obesity, and mortality: impact of cardiorespiratory fitness. Diabetes Care. (2005) 28:391–7. doi: 10.2337/diacare.28.2.391
5. Yu Z, Nan F, Wang LY, Jiang H, Chen W, Jiang Y. Effects of high-protein diet on glycemic control, insulin resistance and blood pressure in type 2 diabetes: a systematic review and meta-analysis of randomized controlled trials. Clin Nutr. (2020) 39:1724–34. doi: 10.1016/j.clnu.2019.08.008
6. Hussain TA, Mathew TC, Dashti AA, Asfar S, Dashti HM. Effect of low-calorie versus low-carbohydrate ketogenic diet in type 2 diabetes. Nutrition. (2012) 28:1016–21. doi: 10.1016/j.nut.2012.01.016
7. Masood W, Annamaraju P, Uppaluri KR. Ketogenic Diet. Statpearls. Treasure Island, FL: StatPearls Publishing Copyright 2022, StatPearls Publishing LLC (2022).
8. Lee HS, Lee J. Effects of combined exercise and low carbohydrate ketogenic diet interventions on waist circumference and triglycerides in overweight and obese individuals: a systematic review and meta-analysis. Int J Environ Res Public Health. (2021) 18:828. doi: 10.3390/ijerph18020828
9. Li S, Lin G, Chen J, Chen Z, Xu F, Zhu F, et al. The Effect of periodic ketogenic diet on newly diagnosed overweight or obese patients with type 2 diabetes. BMC Endocr Disord. (2022) 22:34. doi: 10.1186/s12902-022-00947-2
10. Urbain P, Bertz H. Monitoring for compliance with a ketogenic diet: what is the best time of day to test for urinary ketosis? Nutr Metab. (2016) 13:77. doi: 10.1186/s12986-016-0136-4
11. Nymo S, Coutinho SR, Jørgensen J, Rehfeld JF, Truby H, Kulseng B, et al. Timeline of changes in appetite during weight loss with a ketogenic diet. Int J Obes. (2017) 41:1224–31. doi: 10.1038/ijo.2017.96
12. Joshi S, Ostfeld RJ, McMacken M. The ketogenic diet for obesity and diabetes-enthusiasm outpaces evidence. JAMA Intern Med. (2019) 179:1163–4. doi: 10.1001/jamainternmed.2019.2633
13. Hamdy O, Tasabehji MW, Elseaidy T, Tomah S, Ashrafzadeh S, Mottalib A. Fat vs. carbohydrate-based energy-restricted diets for weight loss in patients with type 2 diabetes. Curr Diab Rep. (2018) 18:128. doi: 10.1007/s11892-018-1103-4
14. Choi YJ, Jeon SM, Shin S. Impact of a ketogenic diet on metabolic parameters in patients with obesity or overweight and with or without type 2 diabetes: a meta-analysis of randomized controlled trials. Nutrients. (2020) 12:2005. doi: 10.3390/nu12072005
15. Leow ZZX, Guelfi KJ, Davis EA, Jones TW, Fournier PA. The glycaemic benefits of a very-low-carbohydrate ketogenic diet in adults with type 1 diabetes mellitus may be opposed by increased hypoglycaemia risk and dyslipidaemia. Diabet Med. (2018) 3:13663. doi: 10.1111/dme.13663
16. Vazquez JA, Kazi U. Lipolysis and gluconeogenesis from glycerol during weight reduction with very-low-calorie diets. Metabolism. (1994) 43:1293–9. doi: 10.1016/0026-0495(94)90225-9
17. Yancy WS, Olsen MK, Guyton JR, Bakst RP, Westman EC, A. low-carbohydrate, ketogenic diet versus a low-fat diet to treat obesity and hyperlipidemia: a randomized, controlled trial. Ann Intern Med. (2004) 140:769–77. doi: 10.7326/0003-4819-140-10-200405180-00006
18. Westman EC, Yancy WS, Olsen MK. Effect of a low-carbohydrate, ketogenic diet program compared to a low-fat diet on fasting lipoprotein subclasses. Int J Cardiol. (2006) 110:212–6. doi: 10.1016/j.ijcard.2005.08.034
19. Yancy WS, Olsen MK, Dudley T, Westman EC. Acid-base analysis of individuals following two weight loss diets. Eur J Clin Nutr. (2007) 61:1416–22. doi: 10.1038/sj.ejcn.1602661
20. Westman EC, Yancy WS, Mavropoulos JC, Marquart M, McDuffie JR. The effect of a low-carbohydrate, ketogenic diet versus a low-glycemic index diet on glycemic control in type 2 diabetes mellitus. Nutr Metab. (2008) 5:36. doi: 10.1186/1743-7075-5-36
21. Yancy WS, Westman EC, McDuffie JR, Grambow SC, Jeffreys AS. Bolton, J, et al. A randomized trial of a low-carbohydrate diet vs orlistat plus a low-fat diet for weight loss. Arch Intern Med. (2010) 170:136–45. doi: 10.1001/archinternmed.2009.492
22. Jabekk PT, Moe IA, Meen HD, Tomten SE, Høstmark AT. Resistance training in overweight women on a ketogenic diet conserved lean body mass while reducing body fat. Nutr Metab. (2010) 7:17. doi: 10.1186/1743-7075-7-17
23. Partsalaki I, Karvela A, Spiliotis BE. Metabolic impact of a ketogenic diet compared to a hypocaloric diet in obese children and adolescents. J Pediatr Endocrinol Metab. (2012) 25:697–704. doi: 10.1515/jpem-2012-0131
24. Saslow LR, Kim S, Daubenmier JJ, Moskowitz JT, Phinney SD, Goldman V, et al. A randomized pilot trial of a moderate carbohydrate diet compared to a very low carbohydrate diet in overweight or obese individuals with type 2 diabetes mellitus or prediabetes. PLoS ONE. (2014) 9:e91027. doi: 10.1371/journal.pone.0091027
25. Goday A, Bellido D, Sajoux I, Crujeiras AB, Burguera B, García-Luna PP, et al. Short-term safety, tolerability and efficacy of a very low-calorie-ketogenic diet interventional weight loss program versus hypocaloric diet in patients with type 2 diabetes mellitus. Nutr Diabetes. (2016) 6:e230. doi: 10.1038/nutd.2016.36
26. Colica C, Merra G, Gasbarrini A, De Lorenzo A, Cioccoloni G, Gualtieri P, et al. Efficacy and safety of very-low-calorie ketogenic diet: a double blind randomized crossover study. Eur Rev Med Pharmacol Sci. (2017) 21:2274–89.
27. Myette-Côté É, Durrer C, Neudorf H, Bammert TD, Botezelli JD, Johnson JD, et al. The effect of a short-term low-carbohydrate, high-fat diet with or without postmeal walks on glycemic control and inflammation in type 2 diabetes: a randomized trial. Am J Physiol Regul Integr Comp Physiol. (2018) 315:R1210–9. doi: 10.1152/ajpregu.00240.2018
28. Sun S, Kong Z, Shi Q, Hu M, Zhang H. Zhang, D, et al. Non-energy-restricted low-carbohydrate diet combined with exercise intervention improved cardiometabolic health in overweight. Chin Females Nutri. (2019) 11:3051. doi: 10.3390/nu11123051
29. Perissiou M, Borkoles E, Kobayashi K. Polman, R. The effect of an 8 week prescribed exercise and low-carbohydrate diet on cardiorespiratory fitness, body composition and cardiometabolic risk factors in obese individuals: a randomized controlled trial. Nutrients. (2020) 12:0482. doi: 10.3390/nu12020482
30. Lodi A, Zarantonello L, Bisiacchi PS, Cenci L, Paoli A. Ketonemia and glycemia affect appetite levels and executive functions in overweight females during two ketogenic diets. Obesity. (2020) 28:1868–77. doi: 10.1002/oby.22934
31. Cunha GM, Guzman G, Correa De Mello LL, Trein B, Spina L, Bussade I, et al. Efficacy of a 2-month very low-calorie ketogenic diet (VLCKD) compared to a standard low-calorie diet in reducing visceral and liver fat accumulation in patients with obesity. Front Endocrinol. (2020) 11:607. doi: 10.3389/fendo.2020.00607
32. Hall KD, Guo J, Courville AB, Boring J, Brychta R, Chen KY, et al. Effect of a plant-based, low-fat diet versus an animal-based, ketogenic diet on ad libitum energy intake. Nat Med. (2021) 27:344–53. doi: 10.1038/s41591-020-01209-1
33. Vidic V, Ilic V, Toskic L, Janković N, Ugarković D. Effects of calorie restricted low carbohydrate high fat ketogenic vs. non-ketogenic diet on strength, body-composition, hormonal and lipid profile in trained middle-aged men. Clin Nutr. (2021) 40:1495–502. doi: 10.1016/j.clnu.2021.02.028
34. Paoli A, Cenci L, Pompei P, Sahin N, Bianco A, Neri M, et al. Effects of 2 months of very low carbohydrate ketogenic diet on body composition, muscle strength, muscle area, and blood parameters in competitive natural body builders. Nutrients. (2021) 13:0374. doi: 10.3390/nu13020374
35. Wu W, Zhou Q, Yuan P, Qiao D, Deng S, Cheng H, et al. A novel multiphase modified ketogenic diet: an effective and safe tool for weight loss in Chinese obese patients. Diab Metabol Synd Obes Targets Therapy. (2022) 15:2521–34. doi: 10.2147/DMSO.S365192
36. Ng M, Fleming T, Robinson M, Thomson B, Graetz N, Margono C, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980-2013: a systematic analysis for the global burden of disease study 2013. Lancet. (2014) 384:766–81. doi: 10.1016/S0140-6736(14)60460-8
37. Koh-Banerjee P, Wang Y, Hu FB, Spiegelman D, Willett WC, Rimm EB. Changes in body weight and body fat distribution as risk factors for clinical diabetes in US men. Am J Epidemiol. (2004) 159:1150–9. doi: 10.1093/aje/kwh167
38. Fabbrini E, Sullivan S, Klein S. Obesity and non-alcoholic fatty liver disease: biochemical, metabolic, and clinical implications. Hepatology. (2010) 51:679–89. doi: 10.1002/hep.23280
39. Klop B, Elte JW, Cabezas MC. Dyslipidemia in obesity: mechanisms and potential targets. Nutrients. (2013) 5:1218–40. doi: 10.3390/nu5041218
40. McLaughlin T, Abbasi F, Cheal K, Chu J, Lamendola C, Reaven G. Use of metabolic markers to identify overweight individuals who are insulin resistant. Ann Intern Med. (2003) 139:802–9. doi: 10.7326/0003-4819-139-10-200311180-00007
41. Bitzur R, Cohen H, Kamari Y, Shaish A, Harats D. Triglycerides and Hdl cholesterol: stars or second leads in diabetes? Diabetes Care. (2009) 32 Suppl 2:S373–377. doi: 10.2337/dc09-S343
42. McKenzie AL, Hallberg SJ, Creighton BC, Volk BM, Link TM, Abner MK, et al. A novel intervention including individualized nutritional recommendations reduces hemoglobin A1c level, medication use, and weight in type 2 diabetes. JMIR Diabetes. (2017) 2:e5. doi: 10.2196/diabetes.6981
43. Athinarayanan SJ, Adams RN, Hallberg SJ, McKenzie AL, Bhanpuri NH, Campbell WW, et al. Long-term effects of a novel continuous remote care intervention including nutritional ketosis for the management of type 2 diabetes: a 2-year non-randomized clinical trial. Front Endocrinol. (2019) 10:348. doi: 10.3389/fendo.2019.00348
44. Gershuni VM, Yan SL, Medici V. Nutritional ketosis for weight management and reversal of metabolic syndrome. Curr Nutr Rep. (2018) 7:97–106. doi: 10.1007/s13668-018-0235-0
45. Bolla AM, Caretto A, Laurenzi A, Scavini M, Piemonti L. Low-carb and ketogenic diets in type 1 and type 2 diabetes. Nutrients. (2019) 11:0962. doi: 10.3390/nu11050962
46. Stratton IM, Adler AI, Neil HA, Matthews DR, Manley SE, Cull CA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. (2000) 321:405–12. doi: 10.1136/bmj.321.7258.405
47. Zhu B, Li MY, Lin Q, Liang Z, Xin Q, Wang M, et al. Lipid oversupply induces CD36 sarcolemmal translocation via dual modulation of PKCζ and TBC1D1: an early event prior to insulin resistance. Theranostics. (2020) 10:1332–54. doi: 10.7150/thno.40021
48. Akhtar DH, Iqbal U, Vazquez-Montesino, LM, Dennis BB, Ahmed A. Pathogenesis of insulin resistance and atherogenic dyslipidemia in nonalcoholic fatty liver disease. J Clin Transl Hepatol. (2019) 7:362–70. doi: 10.14218/JCTH.2019.00028
49. Accurso A, Bernstein RK, Dahlqvist A, Draznin B, Feinman RD, Fine EJ, et al. Dietary carbohydrate restriction in type 2 diabetes mellitus and metabolic syndrome: time for a critical appraisal. Nutr Metab. (2008) 5:9. doi: 10.1186/1743-7075-5-9
50. Yuan X, Wang J, Yang S, Gao M, Cao L, Li X, et al. Effect of the ketogenic diet on glycemic control, insulin resistance, and lipid metabolism in patients with T2dm: a systematic review and meta-analysis. Nutr Diabetes. (2020) 10:38. doi: 10.1038/s41387-020-00142-z
51. Hall KD. A review of the carbohydrate-insulin model of obesity. Eur J Clin Nutr. (2017) 71:679. doi: 10.1038/ejcn.2017.21
52. Brouns F. Overweight and diabetes prevention: is a low-carbohydrate-high-fat diet recommendable? Eur J Nutr. (2018) 57:1301–12. doi: 10.1007/s00394-018-1636-y
Keywords: low-carbohydrate ketogenic diet, overweight, obesity, T2DM, cardiovascular risk
Citation: Luo W, Zhang J, Xu D, Zhou Y, Qu Z, Yang Q and Lv Q (2022) Low carbohydrate ketogenic diets reduce cardiovascular risk factor levels in obese or overweight patients with T2DM: A meta-analysis of randomized controlled trials. Front. Nutr. 9:1092031. doi: 10.3389/fnut.2022.1092031
Received: 10 November 2022; Accepted: 29 November 2022;
Published: 13 December 2022.
Edited by:
Marija Takic, University of Belgrade, SerbiaReviewed by:
Nikoleta Lugonja, University of Belgrade, SerbiaCopyright © 2022 Luo, Zhang, Xu, Zhou, Qu, Yang and Lv. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jin Zhang, emhhbmdqaW4xMjNzbWlsZUAxMjYuY29t; Dan Xu, eHVkYW4xMjNzbWlsZUAxMjYuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.