- 1Department of Gastroenterology, Dietetics and Internal Medicine, Poznan University of Medical Sciences, Poznan, Poland
- 2Doctoral School, Poznan University of Medical Sciences, Poznan, Poland
- 3Inflammatory Bowel Disease Unit, Department of Gastroenterology, S. Filippo Neri Hospital, Rome, Italy
Inflammatory bowel diseases (IBD) are chronic, progressive and relapsing inflammatory disorders of unknown etiology that may cause disability over time. Data from epidemiologic studies indicate that diet may play a role in the risk of developing and the course of IBD. It is known that the group of beneficial bacteria was reduced in the IBD and that the Mediterranean diet (MD)—which is defined as eating habits characterized by high consumption of plant foods, mainly cereals, vegetables, fruit as well as olive oil, and small portions of dairy products, sweets, sugar and meat products—affects gut microbiota, enriching beneficial bacteria, which support gut barrier function and reduce inflammation. Although several studies support different favorable effects of MD on IBD, adherence to MD by IBD patients is generally low, including patients from the Mediterranean Basin. Patients avoid many products which are elements of MD because there cause gastrointestinal symptoms. Patients should be encouraged to have a healthy and well-balanced diet according to individual tolerance of products. A good option seems to be good modified MD, changing hard-to-digest products to easy digest.
1. Introduction
The number of patients with inflammatory bowel diseases (IBD), including Crohn's disease (CD) and ulcerative colitis (UC), is rising globally (1). Many medications may modulate the course of the disease (2); however, looking for no pharmacological therapy for IBD seems to be a worth emphasizing element in improving patients' quality of life (QoL). One of these factors is diet. The study showed that the Western diet disturb gut microbiota and contains pro-inflammatory potential food. The opposite of the Western diet is the Mediterranean diet (MD), which basis are plant food, such us fruits, legumes, vegetables and whole grain food, as well as fish (2).
In this paper, we analyse the possible role of MD on IBD courses, offering a critical and comprehensive review of available evidences.
1.1. Inflammatory bowel diseases
IBD are disorders with chronic-relapsing course that may lead to disability. The etiology of IBD remains unknown: genetic and environmental factors, gut microbiota and immunological unbalance play a complex role in the pathogenesis (3–6).
IBD affect people in every age- from early childhood to late adulthood- even if, more than 80% of new cases are diagnosed in the second or third decade of life.
Nowadays an increasing prevalence of IBD is observed (7): in 1990, there were 3.7 million of people suffering from IBD around the world, and in 2017 they raised to 6.6 million (1). At a regional level, the highest age-standardized prevalence rate of IBD was observed in North America (422.0 cases per 100,000 population), while the lowest age-standardized prevalence rate was observed in the Caribbean (6.7 cases per 100,000 population). The incidence and prevalence of CD and UC appear to be lower in Asia and the Middle East; however, the frequency of IBD in some countries of Africa, Asia and South America, which were industrialized in last years, has also been rising (8). Furthermore, it seems to be a north-to-south gradient: higher incidence rates of CD and UC is observed in northern locations compared with southern latitudes (9). In fact, Jewish population presents higher frequency of IBD when compare with non-Jewish (10, 11), and the incidence is lower in Hispanic and Black populations than in White populations (10, 11). These, differences of geographical and ethnic are related to environmental and lifestyle factors as well as genetic factors. As an example, in a large Danish population-based study, the risk of IBD was lower in first-generation immigrants compared with individuals from Denmark (incidence rate ratio [IRR] 0.76, 95% CI 0.74–0.79) and the risk of IBD, however, was not significantly different for second-generation immigrants (11).
The most common symptoms of IBD patients, affecting QoL are abnormal bowel functions, rectal bleeding and abnominal pain.
About 50% of IBD patients have mild course of disease with low frequency of relapses and complications; on the other hand, the remaining patients may present a severe course with frequent relapses, need of hospitalisations, and evolutive behavior toward intestinal complications and need to surgical intervention (12, 13).
In UC, inflammation occurs in mucosa and submucosa of rectum and colon. The inflammation extends proximally and continuous. Proctitis (lesions are present only in the rectum) affects 30–35% of patients, left-sided colitis (lesions are present also in the left colon) affects 30–45% of patients and about 20–25% of patients suffer from extensive (also the right colon is involved by lesions). During disease course, the frequency of pancolitis may raise and affect ~50% of patients after 20 years of disease. During the course of the disease, in some patients, UC may be complicated by toxic megacolon, massive hemorrhage, colon perforation, colon-rectal cancer (12, 13).
In CD, inflammation is transmural and may affect various gastrointestinal tract sites. Ileocolonic disease is present in about 40% of CD patients at diagnosis, and both isolated ileal disease and isolate colonic disease in 30% for each localization. Upper gastrointestinal and perianal affected among 5–15% and 20–30% of patients, respectively (12, 13). Localization of disease is relatively constant, but the clinical manifestation is dynamic with many changes over time (14, 15). During follow-up, at least one-third of CD patients develop complications: intestinal strictures, internal or perianal fistulas or abscesses and are then classified as structuring or penetrating disease (15), and about ½ of CD patients have an aggressive disease course with more frequently exacerbation, hospitalizations and complications, which may lead often to surgery interventions (16).
The Montreal classification is commonly used to evaluate disease subtypes and their prognosis and to guide the challenge of choosing the most appropriate therapies for each disease subtype (17).
The goals of current treatment options (antibiotics, steroids, immunosuppressive drugs, biological therapies, small molecules) are to induce symptomatic remission, maintain steroid-free remission, enhance the QoL, prevent/treat complications of the disease avoiding short and long-term toxicity of therapy. Therapy should modify the course of the disease and prevent the disabling condition and irreversible tissue damage. Furthermore, therapy should be tailored according to patients' risk of developing disabling disease (18).
1.2. Mediterranean diet
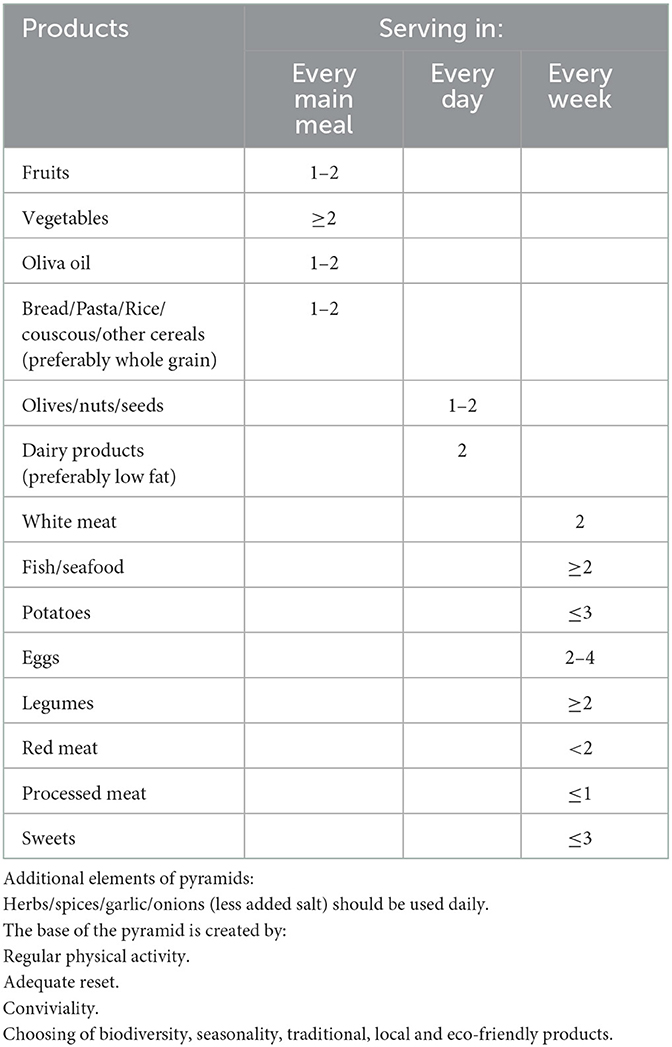
Ancel Benjamin Keys introduced the term “Mediterranean diet” (MD) for the first time in the 1960s. His milestone study showed that the populations living along the Mediterranean Basin had a lower mortality rate and incidence of cardiovascular disease and cancer than other populations. MD thus define eating habits characterized by high consumption of cereals, vegetables, fruit, olive oil, and small portions of dairy products, pastry, sugar and meat products at the same time. Additionally, the intake of fish and wine is moderate. Although the Pyramid of Mediterranean lifestyle did not define the type of wine—white, red or rose—it is mainly red wine, containing more resveratrol than white wine (19, 20).
The pyramid of the Mediterranean diet was presented in 2011 by Mediterranean Diet Foundation. The components and portions of this diet are presented in Table 1 (21).
Some diseases may be related to chronic inflammation and gut microbiota imbalance. According to meta-analysis, MD may reduce inflammation and positively affects gut microbiota (22). Additionally, systematic review reported that adherence of MD reduce biomarkers of inflammation and oxidative stress (23). Koloverou et al. (24) also reported that MD decreased systematic inflammation and increased total antioxidant capacity. Higher MD score was associated with decreased inflammatory markers—lower NF-κB; additionally, the adiponectin level was higher (25). Additionally, Mitjavila et al. (26) reported that MD protects lipids and DNA from oxidative damages in patients with the metabolic syndrome. Moreover, MD supplemented extra virgin olive oil and nuts affect serum nitric oxide (NO) concentration, endothelin-1 and endothelin-1 receptors, which influence on blood pressure and endothelial function (27). Mediterranean diet is especially important in cardiovascular disease prevention; however, many studies present a beneficial effect on other human body systems. According to a meta-analysis, Mediterranean diet adherence improved survival among people with cardiovascular disease (28). Moreover, MD reduce the risk of cardiovascular disease in patients with non-alcoholic fatty liver disease, metabolic syndrome and atrial fibrillation by lowering oxidative stress, improving antioxidant status, and decreasing insulin resistance (29). Patients with metabolic syndrome and higher adherence to MD presented lower alteration of anthropometric parameters and better oxidative and inflammatory status (30). However, MD does not decrease the risk of all-cause mortality among patients with a history of heart failure (31). It is vital to notice that also, interventions for MD improve endothelial function, which might prevent the atherosclerotic process (32). Urpi-Sarda et al. (33) reported that MD has an anti-inflammatory effect on the cardiovascular system. Moreover, MD reduces the risk of metabolic syndrome as well as components of this (waist circumference, high-density lipoprotein cholesterol level, triglycerides level, MD may also reduce the risk of cardiovascular disease, stroke, breast cancer (34) and lower systolic and diastolic blood pressure (35). Pintó et al. (36) reported that MD supplemented with extra virgin olive oil decrease risk of hepatic steatosis among elderly patients with high cardiovascular risk. Additionally, MD may decrease the risk of chronic kidney disease (37). The study showed that MD might also influence cognitive health. According to a meta-analysis, high adherence to the Mediterranean diet reduces the global cognitive decline risk in older adults without dementia (38). Moreover, Hill et al. (39) reported that MD has a small but significant effect on biomarkers of Alzheimer's disease. According to a systematic review, MD protects from Alzheimer's and Parkinson's disease (40). Based on the above evidence, MD is a dietary pattern that plays an important role in preventing many diseases, including inflammatory diseases and causes of mortality.
2. Mediterranean lifestyle and IBD
Data from epidemiologic studies suggest, also that dietary factors may play a role in the risk of developing IBD:
- Increased consumption of total fat, animal fat, and polyunsaturated fatty acids has been correlated with an increased incidence of IBD (41, 42);
- A high consumption of dietary fibers, particularly from fruit and cruciferous vegetables, has been linked with a decreased risk of CD (43);
- A high intake of omega-3 fatty acids as well as high intake of omega-6 fatty acids has been linked with a higher risk of developing CD (41);
- Intake of vitamin D is negatively associated with the risk of CD (whereas deficiency of vitamin D is common among IBD patients) (44–46).
Food antigens are thought to cause an immune system response leading to the development of intestinal inflammation; however, we do not know a specific pathogenic antigens, which are responsible for that (42). It is vital to notice that, the possible role of some dietary components, therapeutic enteral nutrition appears to be effective in CD, especially in children (47). In addition, smoking habits and some drugs (antibiotic, nonsteroidal anti-inflammatory, oral contraceptives, and hormone replacement) may increase the risk of developing IBD, but the magnitude of the risk appears small (5).
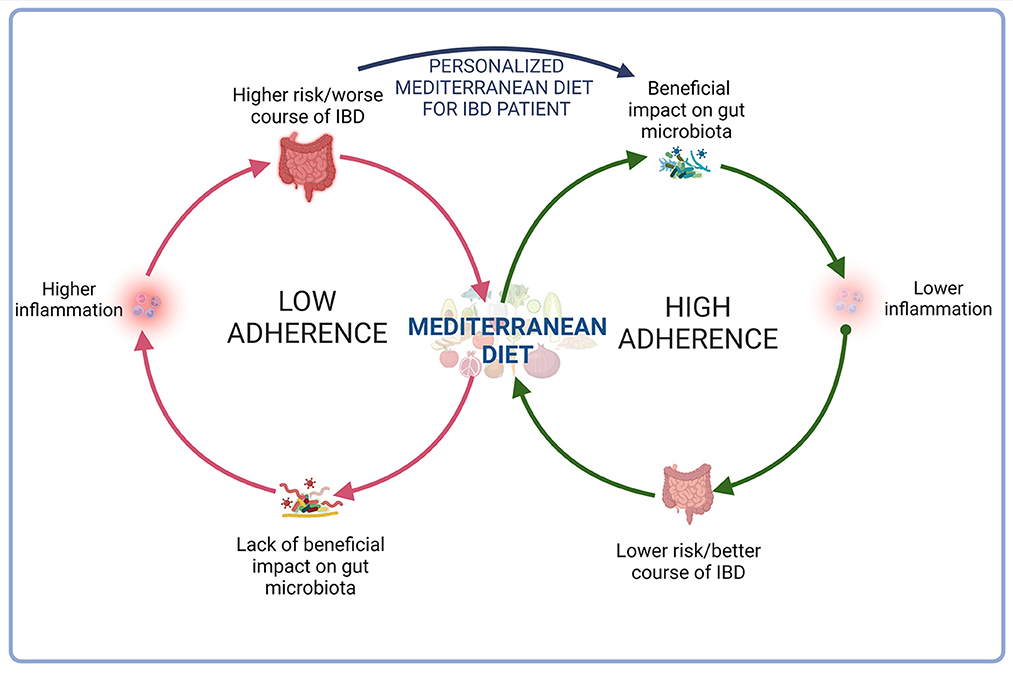
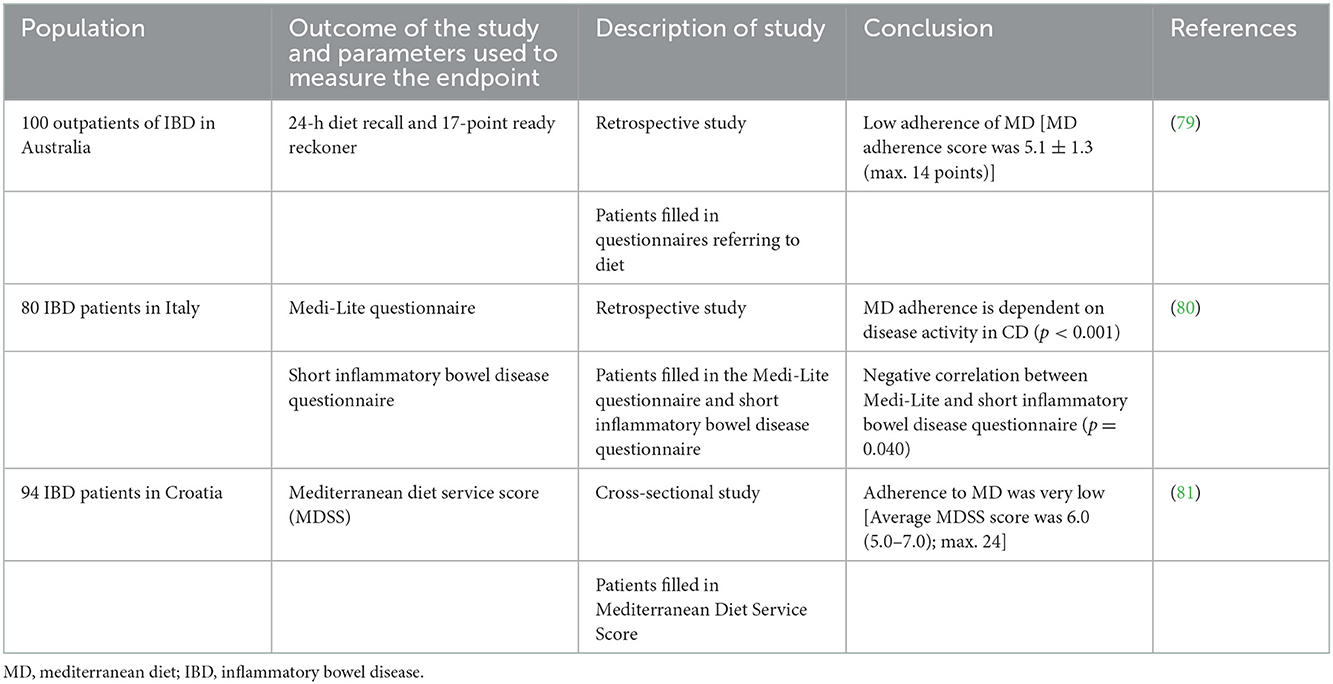
It is known that the group of beneficial bacteria was reduced in the IBD and that MD affects gut microbiota, enriching beneficial bacteria, which support gut barrier function and reduce inflammation (48). MD stimulates Bifidobacteria, Lactobacilli, Eubacteria, Bacteroides, and Prevotella (49). Among healthy adults, subjects with high MD adherence presented lower Escherichia coli counts and higher bifidobacteria/E. coli ratio (50). The data suggest, MD affect gut microbiota positively, decreasing risk of IBD (51). Mediterranean-like dietary pattern reduces intestinal inflammation among healthy first-degree relatives of Crohn's disease patients (52) and higher adherence to MD is associated with a lower risk of later-onset Crohn's disease (53). However, the study showed that only 1/4 of males and 1/5 of females with CD met the criteria of fruit consumption in the Mediterranean diet. Additionally, 21% of men and 32% of women consumed fish and/or shellfish three or more times per week. According to MD, over 70% of patients limited butter, beverages with added sugar, and red and processed meat (54). Although several studies support different favorable effects of MD on IBD (Table 2), adherence of MD by IBD patients is generally low, including patients from the Mediterranean Basin (Table 3).
MD also contains many bioactive compounds, such as polyphenols (55, 56), which present antioxidant activity (57). Moreover, consuming polyphenols improve epithelium cells' function and present an antioxidant effect (58). It is vital to notice that polyphenols modulate gut microbiota and promote the growth of lactobacilli and bifidobacterial. Gut microbiota changes may decrease gut inflammation (59). Sterniolo and Moreno reported that resveratrol, occurring mainly in red wine, may protect against colorectal cancer by inhibiting eicosanoids growth and Caco-2 growth (60).
Additionally, resveratrol metabolites may control apoptosis and cell cycle (61). In vivo, resveratrol also inhibits epithelial-mesenchymal transition (62). Another product rich in bioactive compounds is extra virgin olive oil—phenolic alcohols in them present chemoprotective activity. Additionally, oil polyphenols decrease inflammation and proliferation (63). Therefore, polyphenols may positively affect the course of IBD.
2.1. Mediterranean diet and course of IBD
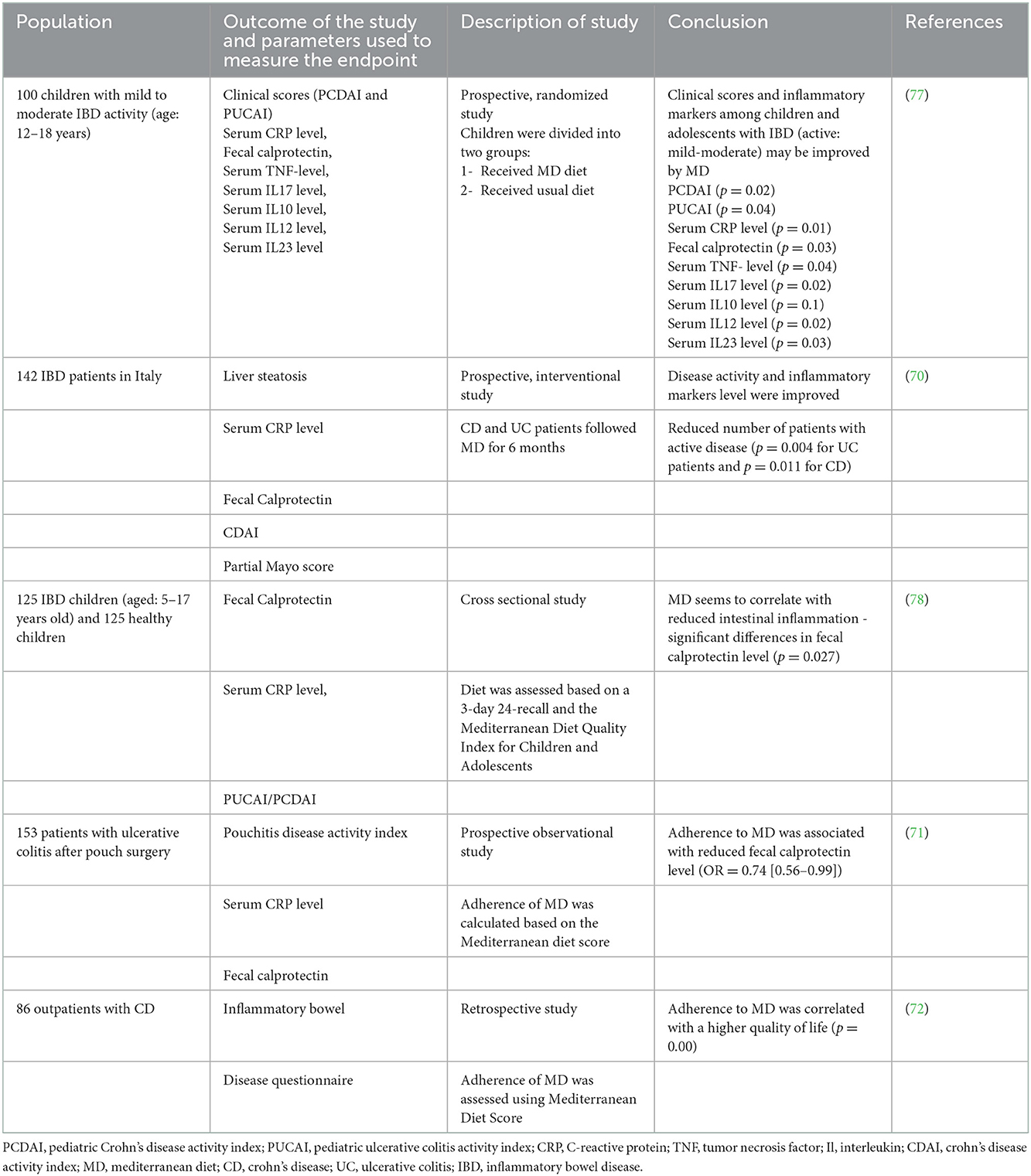
Although a number of evidences, mainly coming from population-based observational studies, support a protective effect of MD against cardiovascular disease, stroke, metabolic disorders, several types of cancer, allergic diseases and Parkinson's and Alzheimer's disease (64–67), as state above, the impact of MD on IBD course remain unclear. Several experimental studies on epigenetic and transcriptomics analysis demonstrated that adherence to MD has a role in modulating the expression of inflammation-related genes and suggested a potential favorable anti-inflammatory effect (68, 69). More recently, the different studies from Italy, Greece and Israel, all of them conducted in the Mediterranean area, have linked the adherence to MD to an improvement of clinical and laboratory disease activity index in both CD and UC patients, suggesting a role of MD in reducing intestinal inflammation again (70–72).
However, to date, there is no evidence that MD, similarly to other alimentary regimens, can modify by itself disease course in IBD patients by reducing strong outcomes such as disease flares, hospitalisations, and need for additional treatments and surgery. Accordingly, ESPEN guidelines do not recommend to date any diet as a unique strategy for treating IBD patients (73).
3. Recommendations for the use of the Mediterranean diet in patients with IBD
The Mediterranean diet is high in vegetables, fruits, whole grains and nuts, and low in red and processed meat. However, data about the impact of MD on IBD are limited (74). However, MD is rich in fiber. Therefore, adherence to MD by IBD patients may be difficult, especially for patients with active disease. On the other hand, ESPEN does not recommend a specific diet as well as a high fiber diet during remission of IBD (75). Therefore, patients suffering from IBD can introduce MD in remission. However, they should observe gastrointestinal symptoms and eliminate products which worsen symptoms. Additionally, the Mediterranean diet meets the recommendation of the International Organization for the Study of Inflammatory Bowel Diseases (76). They recommend reducing red meat and myristic acid intake and increasing consumption of omega-3 fatty acids. According to these guidelines, CD patients should reduce saturated fatty acids consumption and increase their intake of fruits and vegetables. Finally, CD and UC patients should reduce the intake of emulsifiers, thickeners, maltodextrin, artificial sweeteners, and processed food containing titanium dioxide and sulphites. Certain food additives may increase intestinal permeability and higher inflammatory markers in gastrointestinal tissues (76) Avoiding trans-fatty acids and unpasteurised dairy products is recommended. Unpasteurised dairy products may cause infections, and trans-fatty acids increase lacking and inflammation (76). Patients should be encouraged to have a healthy and balanced diet according to individual tolerance of products. We want to propose some modifications to the Mediterranean diet for IBD patients:
1. IBD patients should choose easy-to-digest fruits and vegetables. They can peel them and cook lightly.
2. Patients must not choose whole grain bread, pasta, rice, couscous and other cereals. However, if they tolerated them well, choose it.
3. Nuts and seeds will be tolerated better after grinding.
4. Patients ought to avoid full fat as well as skimmed dairy products. Full fatty contains many saturated fatty acids, and skimmed are poor in fat-soluble vitamins and low-caloric. Semi-fatty will be the best option.
5. Hard-boiled, scrambled eggs or fried eggs may be hard to digest. Scrambled and fried eggs contain many fats, which make them harder to digest and cause long-time persist in stomach. Patients should choose soft-boiled eggs or poached eggs.
6. Patients should be careful during consuming legumes. Most of them are hard to digest, because are rich in fibers. The best option for IBD patients will be lentils, tempeh or tofu.
4. Summary
Mediterranean diet may be beneficial element, next to pharmacotherapy, influencing course of disease and lifestyle quality in IBD patient. In fact, MD reduces inflammation, and decreases also risk of other diseases, e.g., cardiovascular disease. Nevertheless, some products appearing in MD may be not tolerated by patients with gastrointestinal disorders, including inflammatory bowel diseases. For this reason, introducing MD to CD or UC patients, it should be paid attention on tolerance of this diet. MD should be personalized for each patients with the aim to reduce malaise and improving disease course.
Author contributions
Conceptualization and supervision: IK-K. Writing—original draft preparation: AER, SF, AA, and CP. Writing—review and editing: AER, SF, AA, CP, AD, and IK-K. All authors have read and agreed to the published version of the manuscript.
Funding
AER is participants of STER Internationalization of Doctoral Schools Programme from NAWA Polish National Agency for Academic Exchange No. PPI/STE/2020/1/00014/DEC/02.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Alatab S, Sepanlou SG, Ikuta K, Vahedi H, Bisignano C, Safiri S, et al. The global, regional, and national burden of inflammatory bowel disease in 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol. (2020) 5:17–30. doi: 10.1016/S2468-1253(19)30333-4
2. Rizzello F, Spisni E, Giovanardi E, Imbesi V, Salice M, Alvisi P, et al. Implications of the westernized diet in the onset and progression of IBD. Nutrients. (2019) 11:33. doi: 10.3390/nu11051033
3. Schirbel A, Fiocchi C. Inflammatory bowel disease: established and evolving considerations on its etiopathogenesis and therapy. J Dig Dis. (2010) 11:266–76. doi: 10.1111/j.1751-2980.2010.00449.x
4. Cho JH. The genetics and immunopathogenesis of inflammatory bowel disease. Nat Rev Immunol. (2008) 8:458–66. doi: 10.1038/nri2340
5. Zhang Y-Z, Li Y-Y. Inflammatory bowel disease: pathogenesis. World J Gastroenterol. (2014) 20:91–9. doi: 10.3748/wjg.v20.i1.91
6. Chassaing B, Darfeuille–Michaud A. The commensal microbiota and enteropathogens in the pathogenesis of inflammatory bowel diseases. Gastroenterology. (2011) 140:1720-1728.e3. doi: 10.1053/j.gastro.2011.01.054
7. Shivashankar R, Tremaine WJ, Harmsen WS, Loftus EV. Incidence and prevalence of Crohn's disease and ulcerative colitis in Olmsted county, Minnesota from 1970 through 2010. Clin Gastroenterol Hepatol. (2017) 15:857–63. doi: 10.1016/j.cgh.2016.10.039
8. Ng SC, Shi HY, Hamidi N, Underwood FE, Tang W, Benchimol EI, et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet. (2018) 390:2769–78. doi: 10.1016/S0140-6736(17)32448-0
9. Khalili H, Huang ES, Ananthakrishnan AN, Higuchi L, Richter JM, Fuchs CS, et al. Geographical variation and incidence of inflammatory bowel disease among US women. Gut. (2012) 61:1686–92. doi: 10.1136/gutjnl-2011-301574
10. Calkins BM, Lilienfeld AM, Garland CF, Mendeloff AI. Trends in incidence rates of ulcerative colitis and Crohn's disease. Dig Dis Sci. (1984) 29:913–20. doi: 10.1007/BF01312480
11. Agrawal M, Corn G, Shrestha S, Nielsen NM, Frisch M, Colombel J-F, et al. Inflammatory bowel diseases among first-generation and second-generation immigrants in Denmark: a population-based cohort study. Gut. (2021) 70:1037–43. doi: 10.1136/gutjnl-2020-321798
12. Vatn MH. Natural history and complications of IBD. Curr Gastroenterol Rep. (2009) 11:481–7. doi: 10.1007/s11894-009-0073-8
13. Cosnes J, Gower-Rousseau C, Seksik P, Cortot A. Epidemiology and natural history of inflammatory bowel diseases. Gastroenterology. (2011) 140:1785–94. doi: 10.1053/j.gastro.2011.01.055
14. Louis E, Collard A, Oger AF, Degroote E, Aboul Nasr El Yafi FA, Belaiche J. Behaviour of Crohn's disease according to the Vienna classification: changing pattern over the course of the disease. Gut. (2001) 49:777–82. doi: 10.1136/gut.49.6.777
15. Cosnes J, Cattan S, Blain A, Beaugerie L, Carbonnel F, Parc R, et al. Long-term evolution of disease behavior of Crohn's disease. Inflamm Bowel Dis. (2002) 8:244–50. doi: 10.1097/00054725-200207000-00002
16. Solberg IC, Vatn MH, Høie O, Stray N, Sauar J, Jahnsen J, et al. IBSEN Study Group. Clinical course in Crohn's disease: results of a Norwegian population-based 10-year follow-up study. Clin Gastroenterol Hepatol. (2007) 5:1430–8. doi: 10.1016/j.cgh.2007.09.002
17. Satsangi J, Silverberg MS, Vermeire S, Colombel J-F. The Montreal classification of inflammatory bowel disease: controversies, consensus, and implications. Gut. (2006) 55:749–53. doi: 10.1136/gut.2005.082909
18. Lamb CA, Kennedy NA, Raine T, Hendy PA, Smith PJ, Limdi JK, et al. British society of gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut. (2019) 68:s1–s106. doi: 10.1136/gutjnl-2019-318484
19. De Luca I, Di Cristo F, Valentino A, Peluso G, Di Salle A, Calarco A. Food-derived bioactive molecules from mediterranean diet: nanotechnological approaches and waste valorization as strategies to improve human wellness. Polymers. (2022) 14:1726. doi: 10.3390/polym14091726
20. Menotti A, Puddu PE, Catasta G. Dietary habits, cardiovascular and other causes of death in a practically extinct cohort of middle-aged men followed-up for 61 years. Nutr Metabol Cardiovasc Dis. (2022) 32:1819–29. doi: 10.1016/j.numecd.2022.04.010
21. Bach-Faig A, Berry EM, Lairon D, Reguant J, Trichopoulou A, Dernini S, et al. Mediterranean diet pyramid today. Science and cultural updates Public Health. Nutrition. (2011) 14:2274–84. doi: 10.1017/S1368980011002515
22. Koelman L, Egea Rodrigues C, Aleksandrova K. Effects of dietary patterns on biomarkers of inflammation and immune responses: a systematic review and meta-analysis of randomized controlled trials. Adv Nutr. (2022) 13:101–15. doi: 10.1093/advances/nmab086
23. Aleksandrova K, Koelman L, Rodrigues CE. Dietary patterns and biomarkers of oxidative stress and inflammation: a systematic review of observational and intervention studies. Redox Biol. (2021) 42:101869. doi: 10.1016/j.redox.2021.101869
24. Koloverou E, Panagiotakos DB, Pitsavos C, Chrysohoou C, Georgousopoulou EN, Grekas A, et al. Adherence to Mediterranean diet and 10-year incidence (2002–2012) of diabetes: correlations with inflammatory and oxidative stress biomarkers in the ATTICA cohort study. Diabet Metabol Res Rev. (2016) 32:73–81. doi: 10.1002/dmrr.2672
25. Sood S, Feehan J, Itsiopoulos C, Wilson K, Plebanski M, Scott D, et al. Higher adherence to a Mediterranean diet is associated with improved insulin sensitivity and selected markers of inflammation in individuals who are overweight and obese without diabetes. Nutrients. (2022) 14:4437. doi: 10.3390/nu14204437
26. Mitjavila MT, Fandos M, Salas-Salvadó J, Covas M-I, Borrego S, Estruch R, et al. The Mediterranean diet improves the systemic lipid and DNA oxidative damage in metabolic syndrome individuals. A randomized, controlled, trial. Clin Nutr. (2013) 32:172–8. doi: 10.1016/j.clnu.2012.08.002
27. Storniolo CE, Casillas R, Bulló M, Castañer O, Ros E, Sáez GT, et al. A Mediterranean diet supplemented with extra virgin olive oil or nuts improves endothelial markers involved in blood pressure control in hypertensive women. Eur J Nutr. (2017) 56:89–97. doi: 10.1007/s00394-015-1060-5
28. Tang C, Wang X, Qin L-Q, Dong J-Y. Mediterranean diet and mortality in people with cardiovascular disease: a meta-analysis of prospective cohort studies. Nutrients. (2021) 13:2623. doi: 10.3390/nu13082623
29. Biccirè FG, Bucci T, Menichelli D, Cammisotto V, Pignatelli P, Carnevale R, et al. Mediterranean diet: a tool to break the relationship of atrial fibrillation with the metabolic syndrome and non-alcoholic fatty liver disease. Nutrients. (2022) 14:1260. doi: 10.3390/nu14061260
30. Quetglas-Llabrés MM, Monserrat-Mesquida M, Bouzas C, Gómez C, Mateos D, Ripoll-Vera T, et al. Inflammatory and oxidative stress markers related to adherence to the Mediterranean diet in patients with metabolic syndrome. Antioxidants. (2022) 11:901. doi: 10.3390/antiox11050901
31. Chang C-Y, Lee C-L, Liu W-J, Wang J-S. Association of adherence to the Mediterranean diet with all-cause mortality in subjects with heart failure. Nutrients. (2022) 14:842. doi: 10.3390/nu14040842
32. Shannon OM, Mendes I, Köchl C, Mazidi M, Ashor AW, Rubele S, et al. Mediterranean diet increases endothelial function in adults: a systematic review and meta-analysis of randomized controlled trials. J Nutr. (2020) 150:1151–9. doi: 10.1093/jn/nxaa002
33. Urpi-Sarda M, Casas R, Chiva-Blanch G, Romero-Mamani ES, Valderas-Martínez P, Arranz S, et al. Virgin olive oil and nuts as key foods of the Mediterranean diet effects on inflammatory biomakers related to atherosclerosis. Pharmacol Res. (2012) 65:577–83. doi: 10.1016/j.phrs.2012.03.006
34. Papadaki A, Nolen-Doerr E, Mantzoros CS. The effect of the Mediterranean diet on metabolic health: a systematic review and meta-analysis of controlled trials in adults. Nutrients. (2020) 12:3342. doi: 10.3390/nu12113342
35. Ndanuko RN, Tapsell LC, Charlton KE, Neale EP, Batterham MJ. Dietary patterns and blood pressure in adults: a systematic review and meta-analysis of randomized controlled trials. Adv Nutr. (2016) 7:76–89. doi: 10.3945/an.115.009753
36. Pintó X, Fanlo-Maresma M, Corbella E, Corbella X, Mitjavila MT, Moreno JJ, et al. A Mediterranean diet rich in extra-virgin olive oil is associated with a reduced prevalence of nonalcoholic fatty liver disease in older individuals at high cardiovascular risk. J Nutr. (2019) 149:1920–9. doi: 10.1093/jn/nxz147
37. Hansrivijit P, Oli S, Khanal R, Ghahramani N, Thongprayoon C, Cheungpasitporn W. Mediterranean diet and the risk of chronic kidney disease: a systematic review and meta-analysis. Nephrology. (2020) 25:913–8. doi: 10.1111/nep.13778
38. Coelho-Júnior HJ, Trichopoulou A, Panza F. Cross-sectional and longitudinal associations between adherence to Mediterranean diet with physical performance and cognitive function in older adults: a systematic review and meta-analysis. Ageing Res Rev. (2021) 70:101395. doi: 10.1016/j.arr.2021.101395
39. Hill E, Goodwill AM, Gorelik A, Szoeke C. Diet and biomarkers of Alzheimer's disease: a systematic review and meta-analysis. Neurobiol Aging. (2019) 76:45–52. doi: 10.1016/j.neurobiolaging.2018.12.008
40. Solch RJ, Aigbogun JO, Voyiadjis AG, Talkington GM, Darensbourg RM, O'Connell S, et al. Mediterranean diet adherence, gut microbiota, and Alzheimer's or Parkinson's disease risk: a systematic review. J Neurol Sci. (2022) 434:166. doi: 10.1016/j.jns.2022.120166
41. Hou JK, Abraham B, El-Serag H. Dietary intake and risk of developing inflammatory bowel disease: a systematic review of the literature. Am J Gastroenterol. (2011) 106:563–73. doi: 10.1038/ajg.2011.44
42. Chapman-Kiddell CA, Davies PSW, Gillen L, Radford-Smith GL. Role of diet in the development of inflammatory bowel disease. Inflamm Bowel Dis. (2010) 16:137–51. doi: 10.1002/ibd.20968
43. Ananthakrishnan AN, Khalili H, Konijeti GG, Higuchi LM, de Silva P, Korzenik JR, et al. A prospective study of long-term intake of dietary fiber and risk of Crohn's disease and ulcerative colitis. Gastroenterology. (2013) 145:970–7. doi: 10.1053/j.gastro.2013.07.050
44. Abegunde AT, Muhammad BH, Bhatti O, Ali T. Environmental risk factors for inflammatory bowel diseases: Evidence based literature review. World J Gastroenterol. (2016) 22:6296–317. doi: 10.3748/wjg.v22.i27.6296
45. Ananthakrishnan AN, Khalili H, Higuchi LM, Bao Y, Korzenik JR, Giovannucci EL, et al. Higher predicted vitamin D status is associated with reduced risk of Crohn's disease. Gastroenterology. (2012) 142:482–9. doi: 10.1053/j.gastro.2011.11.040
46. Del Pinto R, Pietropaoli D, Chandar AK, Ferri C, Cominelli F. Association between inflammatory bowel disease and vitamin d deficiency: a systematic review and meta-analysis. Inflamm Bowel Dis. (2015) 21:2708–17. doi: 10.1097/MIB.0000000000000546
47. Yamamoto T, Nakahigashi M, Umegae S, Matsumoto K. Enteral nutrition for the maintenance of remission in Crohn's disease: a systematic review. Eur J Gastroenterol Hepatol. (2010) 22:1–8. doi: 10.1097/MEG.0b013e32832c788c
48. Illescas O, Rodríguez-Sosa M, Gariboldi M. Mediterranean diet to prevent the development of colon diseases: a meta-analysis of gut microbiota studies. Nutrients. (2021) 13:2234. doi: 10.3390/nu13072234
49. Singh RK, Chang H-W, Yan D, Lee KM, Ucmak D, Wong K, et al. Influence of diet on the gut microbiome and implications for human health. J Transl Med. (2017) 15:1–17. doi: 10.1186/s12967-017-1175-y
50. Mitsou EK, Kakali A, Antonopoulou S, Mountzouris KC, Yannakoulia M, Panagiotakos DB, et al. Adherence to the Mediterranean diet is associated with the gut microbiota pattern and gastrointestinal characteristics in an adult population. Br J Nutr. (2017) 117:1645–55. doi: 10.1017/S0007114517001593
51. Yan J, Wang L, Gu Y, Hou H, Liu T, Ding Y, et al. Dietary patterns and gut microbiota changes in inflammatory bowel disease: current insights and future challenges. Nutrients. (2022) 14:4003. doi: 10.3390/nu14194003
52. Turpin W, Dong M, Sasson G, Raygoza Garay JA, Espin-Garcia O, Lee S-H, et al. Mediterranean-like dietary pattern associations with gut microbiome composition and sub-clinical gastrointestinal inflammation. Gastroenterology. (2022) 163:685–95. doi: 10.1053/j.gastro.2022.05.037
53. Khalili H, Håkansson N, Chan SS, Chen Y, Lochhead P, Ludvigsson JF, et al. Adherence to a Mediterranean diet is associated with a lower risk of later-onset Crohn's disease: results from two large prospective cohort studies. Gut. (2020) 69:1637–44. doi: 10.1136/gutjnl-2019-319505
54. Taylor L, Almutairdi A, Shommu N, Fedorak R, Ghosh S, Reimer RA, et al. Cross-sectional analysis of overall dietary intake and Mediterranean dietary pattern in patients with Crohn's disease. Nutrients. (2018) 10:1761. doi: 10.3390/nu10111761
55. Stefaniak O, Dobrzyńska M, Drzymała-Czyz S, Przysławski J. Diet in the prevention of Alzheimer's disease: current knowledge and future research requirements. Nutrients. (2022) 14:4564. doi: 10.3390/nu14214564
56. Memmola R, Petrillo A, Di Lorenzo S, Altuna SC, Habeeb BS, Soggiu A, et al. Correlation between olive oil intake and gut microbiota in colorectal cancer prevention. Nutrients. (2022) 14:3749. doi: 10.3390/nu14183749
57. Tresserra-Rimbau A, Lamuela-Raventos RM, Moreno JJ. Polyphenols, food and pharma. Current knowledge and directions for future research. Biochem Pharmacol. (2018) 156:186–95. doi: 10.1016/j.bcp.2018.07.050
58. Biegańska-Hensoldt S, Rosołowska-Huszcz D. Polyphenols in preventing endothelial dysfunction. Postepy Hig Med Dosw. (2017) 71:227–35. doi: 10.5604/01.3001.0010.3808
59. Tomás-Barberán FA, Selma MV, Espín JC. Interactions of gut microbiota with dietary polyphenols and consequences to human health. Curr Opin Clin Nutr Metab Care. (2016) 19:471–6. doi: 10.1097/MCO.0000000000000314
60. Storniolo CE, Moreno JJ. Resveratrol analogs with antioxidant activity inhibit intestinal epithelial cancer caco-2 cell growth by modulating arachidonic acid cascade. J Agric Food Chem. (2019) 67:819–28. doi: 10.1021/acs.jafc.8b05982
61. Storniolo CE, Moreno JJ. Resveratrol metabolites have an antiproliferative effect on intestinal epithelial cancer cells. Food Chem. (2012) 134:1385–91. doi: 10.1016/j.foodchem.2012.03.036
62. Song Y, Chen Y, Li Y, Lyu X, Cui J, Cheng Y, et al. Resveratrol suppresses epithelial-mesenchymal transition in GBM by regulating smad-dependent signaling. BioMed Res Int. (2019) 2019:e1321973. doi: 10.1155/2019/1321973
63. Gorzynik-Debicka M, Przychodzen P, Cappello F, Kuban-Jankowska A, Marino Gammazza A, Knap N, et al. Potential health benefits of olive oil and plant polyphenols. Int J Mol Sci. (2018) 19:686. doi: 10.3390/ijms19030686
64. Tosti V, Bertozzi B, Fontana L. Health Benefits of the Mediterranean diet: metabolic and molecular mechanisms. J Gerontol A Biol Sci Med Sci. (2018) 73:318–26. doi: 10.1093/gerona/glx227
65. Seethaler B, Nguyen NK, Basrai M, Kiechle M, Walter J, Delzenne NM, et al. Short-chain fatty acids are key mediators of the favorable effects of the Mediterranean diet on intestinal barrier integrity: data from the randomized controlled LIBRE trial. Am J Clin Nutr. (2022) 116:928–42. doi: 10.1093/ajcn/nqac175
66. Delgado-Lista J, Alcala-Diaz JF, Torres-Peña JD, Quintana-Navarro GM, Fuentes F, Garcia-Rios A, et al. Long-term secondary prevention of cardiovascular disease with a Mediterranean diet and a low-fat diet (CORDIOPREV): a randomised controlled trial. Lancet. (2022) 399:1876–85. doi: 10.1016/S0140-6736(22)00122-2
67. Paknahad Z, Sheklabadi E, Derakhshan Y, Bagherniya M, Chitsaz A. The effect of the Mediterranean diet on cognitive function in patients with Parkinson's disease: a randomized clinical controlled trial. Compl Ther Med. (2020) 50:102366. doi: 10.1016/j.ctim.2020.102366
68. Marlow G, Ellett S, Ferguson IR, Zhu S, Karunasinghe N, Jesuthasan AC, et al. Transcriptomics to study the effect of a Mediterranean-inspired diet on inflammation in Crohn's disease patients. Hum Genom. (2013) 7:24. doi: 10.1186/1479-7364-7-24
69. Arpón A, Riezu-Boj JI, Milagro FI, Marti A, Razquin C, Martínez-González MA, et al. Adherence to Mediterranean diet is associated with methylation changes in inflammation-related genes in peripheral blood cells. J Physiol Biochem. (2016) 73:445–55. doi: 10.1007/s13105-017-0552-6
70. Chicco F, Magrì S, Cingolani A, Paduano D, Pesenti M, Zara F, et al. Multidimensional impact of Mediterranean diet on IBD patients. Inflamm Bowel Dis. (2021) 27:1–9. doi: 10.1093/ibd/izaa097
71. Godny L, Reshef L, Pfeffer-Gik T, Goren I, Yanai H, Tulchinsky H, et al. Adherence to the Mediterranean diet is associated with decreased fecal calprotectin in patients with ulcerative colitis after pouch surgery. Eur J Nutr. (2020) 59:3183–90. doi: 10.1007/s00394-019-02158-3
72. Papada E, Amerikanou C, Forbes A, Kaliora AC. Adherence to Mediterranean diet in Crohn's disease. Eur J Nutr. (2020) 59:1115–21. doi: 10.1007/s00394-019-01972-z
73. Forbes A, Escher J, Hébuterne X, Kłek S, Krznaric Z, Schneider S, et al. ESPEN guideline: clinical nutrition in inflammatory bowel disease. Clin Nutr. (2017) 36:321–47. doi: 10.1016/j.clnu.2016.12.027
74. Jiang Y, Jarr K, Layton C, Gardner CD, Ashouri JF, Abreu MT, et al. Therapeutic implications of diet in inflammatory bowel disease and related immune-mediated inflammatory diseases. Nutrients. (2021) 13:890. doi: 10.3390/nu13030890
75. Bischoff SC, Escher J, Hébuterne X, Kłek S, Krznaric Z, Schneider S, et al. ESPEN practical guideline: clinical nutrition in inflammatory bowel disease. Clin Nutr. (2020) 39:632–53. doi: 10.1016/j.clnu.2019.11.002
76. Levine A, Rhodes JM, Lindsay JO, Abreu MT, Kamm MA, Gibson PR, et al. Dietary guidance from the international organization for the study of inflammatory bowel diseases. Clin Gastroenterol Hepatol. (2020) 18:1381–92. doi: 10.1016/j.cgh.2020.01.046
77. El Amrousy D, Elashry H, Salamah A, Maher S, Abd-Elsalam SM, Hasan S. Adherence to the Mediterranean diet improved clinical scores and inflammatory markers in children with active inflammatory bowel disease: a randomized trial. J Inflamm Res. (2022) 15:2075–86. doi: 10.2147/JIR.S349502
78. Strisciuglio C, Cenni S, Serra MR, Dolce P, Martinelli M, Staiano A, et al. Effectiveness of Mediterranean diet's adherence in children with inflammatory bowel diseases. Nutrients. (2020) 12:E3206. doi: 10.3390/nu12103206
79. Marsh A, Radford-Smith G, Banks M, Lord A, Chachay V. Dietary intake of patients with inflammatory bowel disease aligns poorly with traditional Mediterranean diet principles. Nutr Diet. (2022) 79:229–37. doi: 10.1111/1747-0080.12715
80. Fiorindi C, Dinu M, Gavazzi E, Scaringi S, Ficari F, Nannoni A, et al. Adherence to Mediterranean diet in patients with inflammatory bowel disease. Clin Nutr ESPEN. (2021) 46:416–23. doi: 10.1016/j.clnesp.2021.09.726
Keywords: Mediterranean diet, inflammatory bowel diseases, gut microbiota, Crohn's disease, ulcerative colitis
Citation: Ratajczak AE, Festa S, Aratari A, Papi C, Dobrowolska A and Krela-Kaźmierczak I (2023) Should the Mediterranean diet be recommended for inflammatory bowel diseases patients? A narrative review. Front. Nutr. 9:1088693. doi: 10.3389/fnut.2022.1088693
Received: 03 November 2022; Accepted: 07 December 2022;
Published: 10 January 2023.
Edited by:
Rui Curi, Cruzeiro Do Sul University São Paulo, BrazilReviewed by:
Enzo Spisni, University of Bologna, ItalyJuan José Moreno, University of Barcelona, Spain
Copyright © 2023 Ratajczak, Festa, Aratari, Papi, Dobrowolska and Krela-Kaźmierczak. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Alicja Ewa Ratajczak,  YWxpY2phZXdhcmF0YWpjemFrJiN4MDAwNDA7Z21haWwuY29t
YWxpY2phZXdhcmF0YWpjemFrJiN4MDAwNDA7Z21haWwuY29t
 Alicja Ewa Ratajczak
Alicja Ewa Ratajczak Stefano Festa
Stefano Festa Annalisa Aratari
Annalisa Aratari Claudio Papi3
Claudio Papi3 Iwona Krela-Kaźmierczak
Iwona Krela-Kaźmierczak