
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Nutr. , 10 January 2023
Sec. Clinical Nutrition
Volume 9 - 2022 | https://doi.org/10.3389/fnut.2022.1084455
 Mohammad Zamani1
Mohammad Zamani1 Mahnaz Rezaei Kelishadi2
Mahnaz Rezaei Kelishadi2 Damoon Ashtary-Larky3
Damoon Ashtary-Larky3 Niusha Amirani4
Niusha Amirani4 Kian Goudarzi5
Kian Goudarzi5 Iman Attackpour Torki6
Iman Attackpour Torki6 Reza Bagheri7
Reza Bagheri7 Matin Ghanavati8*
Matin Ghanavati8* Omid Asbaghi9,10*
Omid Asbaghi9,10*Purpose: A bulk of observational studies have revealed the protective role of green tea supplementation in cardiovascular diseases. The current systematic review and meta-analysis study aimed to establish the effects of green tea supplementation on cardiovascular risk factors including lipid profile, blood pressure, glycemic control markers and CRP.
Methods: A systematic literature search of randomized clinical trials (RCTs) that investigated the effects of green tea supplementation and cardiovascular risk factors was undertaken in online databases including PubMed/Medline, Scopus, Web of Science, and Embase using a combination of green tea and cardiovascular risk factors search terms. Meta-analyses were carried out using a random-effects model. The I2 index was used to assess the heterogeneity of RCTs.
Results: Among the initial 11,286 studies that were identified from electronic databases search, 55 eligible RCTs with 63 effect sizes were eligible. Results from the random effects meta-analysis showed that GTE supplementation significantly reduced TC (WMD = −7.62; 95% CI: −10.51, −4.73; P = < 0.001), LDL-C (WMD = −5.80; 95% CI: −8.30, −3.30; P = < 0.001), FBS (WMD = −1.67; 95% CI: −2.58, −0.75; P = < 0.001), HbA1c (WMD = −0.15; 95% CI: −0.26, −0.04; P = 0.008), DBP (WMD = −0.87; 95% CI: −1.45, −0.29; P = 0.003), while increasing HDL-C (WMD = 1.85; 95% CI: 0.87, 2.84; P = 0.010). Subgroup analyses based on the duration of supplementation (≥ 12 vs. < 12 weeks), dose of green tea extract (GTE) (≥1,000 vs. < 1,000 mg/d), sex (male, female, and both), baseline serum levels of lipid profile, and glycemic control factors demonstrated different results for some risk factors.
Conclusion: The current study suggests improvements in the lipid and glycemic profiles following green tea supplementation. These findings support previous evidence showing the health benefits of green tea supplementation on cardiometabolic risk factors.
Many people have considered green tea as a drink with health-promotion properties ranging from weight management to cancer prevention (1). Green tea extract (GTE) is a dietary supplement derived from Camellia sinensis leaves (2). To stop the fermentation process which reduces the polyphenols content of tea, freshly green tea leaves are steamed immediately upon harvest (3). The fact that GTE contains a large number of concentrated components, including non-oxidized polyphenols, vitamins, and antioxidants, is the basis for their current rise in popularity. The major phenolic compounds found in green tea are flavonoids accounting for nearly 70% of its total polyphenols (4). Catechins and their derivatives especially epigallocatechin-3-gallate (EGCG) are the most abundant flavonoids in green tea which are responsible for potential preventive effects of green tea on oxidative stress-caused diseases such as cancer, cardiovascular and neurodegenerative diseases (5).
Globally, cardiovascular diseases (CVD) continue to be the leading cause of death (6). Observational studies have suggested the primary preventive role of green tea against CVD such as stroke, coronary heart disease, and coronary atherosclerosis (7–9). In this regard, results from a large cohort study showed that daily consumption of 2 cups of green tea was associated with a 22-33% reduction in CVD-cause mortality among the Japanese population (10). Accumulating evidence has examined the effects of green tea products on traditional and novel cardiovascular risk factors such as hypertension, lipid disorders, diabetes, oxidative stress, endothelial dysfunction, and inflammation (11). Among lifestyle modification strategies for controlling CVD risk factors, regular consumption of functional foods rich in antioxidants and polyphenols such as coffee (12), dark chocolate (13), nuts (14) and green tea (12) have been proposed to promote cardiometabolic risk factors.
Although many factors play a pathogenic role, increased oxidative stress is a common potential cause of various CVD (15). The bulk of evidence has shown that the cardio-protective activity of green tea is mainly attributed to the antioxidant properties of its catechins which act by inducing anti-oxidant enzymes, inhibiting pro-oxidant enzymes, and scavenging free radicals (16, 17). In line with animal studies where green tea catechins had lowering effects on cholesterol (18, 19), the administration of green tea catechins has been reported to reduce total cholesterol (TC) and low-density lipoprotein (LDL) in human clinical trial studies (20). Although the exact mechanism of action of green tea to reduce cholesterol is not fully understood, an increase in thermogenesis, enhance gene expression of enzymes involved in bile acid production and appetite suppression has been proposed as potential mechanisms (21). Also, the supplementation with GTE with a high amount of catechins exerted favorable effects on glycemic control (22) and blood pressure (23). However, inconsistency between the results of recent studies has been identified regarding the effects of green tea supplementation on some CVD risk factors. For instance, 3-week high doses of green tea polyphenols supplementation failed to improve CVD risk factors except for TC: high-density lipoprotein (HDL) ratio among healthy men (24). Likewise, Mousavi et al. (25) did not report a significant reduction in TC, triglyceride (TG), LDL, or glycemic control markers in diabetic patients following the 8-week drinking of four cups of green tea compared to the control group. Owing to this consistency across clinical trial studies, the main objective of this systematic review and meta-analysis was to summarize the effects of green tea supplementation on cardiovascular risk factors including glycemic control markers (fasting blood sugar (FBS), hemoglobin A1C (HbA1c), homeostatic model assessment for insulin resistance (HOMA-IR), fasting insulin), blood pressure (systolic blood pressure [SBP] and diastolic blood pressure [DBP]) lipid profile (TG, TC, LDL, HDL) and C-reactive protein (CRP).
Guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) were considered in the current review. Data were searched in PubMed/MEDLINE, Scopus, Web of Science, and Cochrane library from inception up to 27 August 2022 for all relevant published articles. The search was applied using the following MESH and non-MESH terms: (“green tea” OR “green tea extract” OR “catechin” OR “catechins” OR “Camellia sinensis” OR “Thea sinensis”) AND (Intervention OR “Intervention Study” OR “Intervention Studies” OR “controlled trial” OR randomized OR randomized OR random OR randomly OR placebo OR “clinical trial” OR Trial OR “randomized controlled trial” OR “randomized clinical trial” OR RCT OR blinded OR “double blind” OR “double blinded” OR trial OR “clinical trial” OR trials OR “Pragmatic Clinical Trial” OR “Cross-Over Studies” OR “Cross-Over” OR “Cross-Over Study” OR parallel OR “parallel study” OR “parallel trial”) (Supplementary File 1). No restriction was considered on time and language of publications. Reference lists of the related papers were also manually checked to prevent missing any pertinent papers. In addition, duplicate citations were removed after including all searched articles in the Endnote software.
The inclusion criteria for the present review are listed as follows: (a) randomized clinical trials (RCT) (either parallel or cross-over design), (b) investigations on adult population (age > 18y), (c) studies that administered any types of green tea supplement, (d) clinical trials with at least one week’s of the follow-up period, and (e) articles that provided sufficient information on the baseline and final levels of cardiovascular risk factors or represented required information for calculation of those effect sizes. In the case of more than one published article for one dataset, we included the most complete one. If there were clinical studies with an extra intervention group, we considered them as two separate investigations. The following criteria were also considered to exclude studies: (a) experimental, (b) those studies with a cohort, cross-sectional, and case-control design, (c) review articles, (d) ecological investigations, (e) clinical trials with no random allocation and no control group, and (f) investigations carried out on children and adolescents.
Data extraction including author’s name, publication year and the country where the study was performed, participants’ health condition, age, sex, body mass index (BMI), study design (parallel/cross-over), number of contributors in each study group, dose, and duration of prune administration, post-intervention mean and standard deviation (SD) of cardiovascular risk factors in both prune and control groups, post-intervention mean (SD) changes in cardiovascular risk factors in both study groups, and confounders adjusted in the analysis was completed by two researchers independently. If standard errors (SEs) or interquartile ranges were reported, we converted them to SDs. In addition, a chief researcher settled any controversies.
We systematically evaluated the bias in the included trials by using the Cochrane Collaboration’s tool risk of bias criteria (26). Two independent investigators assessed the quality details of the studies in seven domains including random sequence generation, allocation concealment, reporting bias, performance bias, detection bias, attrition bias, and other sources of bias based on the Cochrane Handbook for Systematic Reviews. To assess each domain, the terms “Low”, “High”, or “Unclear” was applied (Table 2).
The overall effect sizes were computed as mean differences and SDs of glycemic markers and CRP between prune and control groups. All data were inserted as means ± SD. It should be noted that in studies where findings have been reported as SEs and interquartile ranges, means ± SD was calculated by statistical computations. The effect sizes were indicated as standardized mean difference (SMD) and 95% confidence interval (CI). The random-effects model considering between-study variations was chosen to acquire the overall effect sizes. Heterogeneity between studies was evaluated by I-square (I2) index and I2 > 50% was assumed as considerable between-study heterogeneity (27). Subgroup analysis was performed to find any probable sources of heterogeneity based on the predefined variables including duration of supplementation (≥ 12 vs. < 12 weeks), the dose of GTE (≥ 1,000 vs. < 1,000 mg/d), sex (male, female, and both), baseline TG (≥ 150 vs. < 150 mg/dl), TC (≥ 200 vs. < 200 mg/dl), LDL (≥ 100 vs. < 100 mg/dl), HDL (≥ 50 vs. < 50 mg/dl), FBS (≥ 100 vs. < 100 mg/dl), and HbA1c (≥ 6.5 vs. < 6.5%), past medical history of type 2 diabetes mellitus (T2DM; Non-T2DM patients and T2DM patients), and baseline values of BMI (normal, overweight, and obese), DBP (≥ 80 vs. < 80 mmHg) and SBP (≥ 130 vs. < 130 mmHg). Fractional polynomial modeling was applied to detect the non-linear effects of green tea dosage (g/d) on each variable level. We performed a sensitivity analysis to identify the impact of one single study removal on the overall effect size. Publication bias evaluation was carried out through visual inspection of funnel plot for each variable and statistical tests including Begg’s adjusted rank correlation and Egger’s regression asymmetry tests (28, 29). All statistical analysis was accomplished by STATA® version 14.0 (StataCorp, College Station, Lakeway, TX, USA) and P-value less than 0.05 was assumed statistically significant.
We graded the overall certainty of evidence across the studies based on the guidelines of the GRADE (Grading of Recommendations Assessment, Development, and Evaluation) Working Group. According to the corresponding evaluation criteria, four categories of high, moderate, low, and very low represented the quality of evidence.
As disclosed in Figure 1, our initial search found a total of 11,286 relevant papers, of which 68 remained after duplicates removing (n = 3,529), and a wide range of screening of the titles and abstracts (n = 7,689). The entire of suitable articles were carefully checked and 13 irrelevant studies were excluded. Finally, 55 eligible clinical trials with 63 effect sizes were included in the present quantitative review based on the research topic.
Table 1 represented the characteristics of all included studies. Overall, clinical trials with a total of 4,874 participants were included (2,487 participants in the green tea group and 2,387 in the placebo group), participants’ mean age ranged between 18 and 68.7 years, and the period of intervention ranged between two to 48 weeks. Some of the studies enrolled only males or females and some of them included both genders. In addition, participants with various health conditions were enrolled in included clinical trials. Twenty-nine studies involved healthy participants (24, 30–58), 15 recruited diabetic patients (59–72), two enrolled patients with hypercholesterolemia (73, 74), two included polycystic ovarian syndrome (PCOS) patients (75, 76), two recruited obese patients with hypertension (77, 78), three recruited patients with liver disorders (79–81), one involved obese patients with metabolic syndrome (82), and another study investigated the effect of GTE supplementation on patients with chronic stable angina (83). In addition, mentioned clinical trials were executed in different countries including Australia, USA, Iran, Brazil, UK, China, Spain, Japan, Taiwan, Lithuania, Poland, Netherlands, Finland, Pakistan, and Mexico.
green tea supplementation had a non-significant effect on TG (WMD = −5.31; 95% CI: −12.32, 1.68; P = 0.137) based on our analysis of 40 arms of clinical trials (Figure 2A). Moreover, remarkable heterogeneity was observed between studies (P = < 0.001, I2 = 89.0%). Subgroup analysis was carried out according to the duration and dosage of supplementation, baseline values of BMI and TG, past medical history of T2DM, and sex (Table 3). The findings of subgroup analysis suggested that green tea supplementation contributed to a significant reduction in TG if both males and females were included and the duration of intervention was more than 12 weeks. However, there was no significant effect of green tea supplementation on TG after subgroup analysis by a dose of intervention, baseline values of BMI and TG, past medical history of T2DM, and sex.
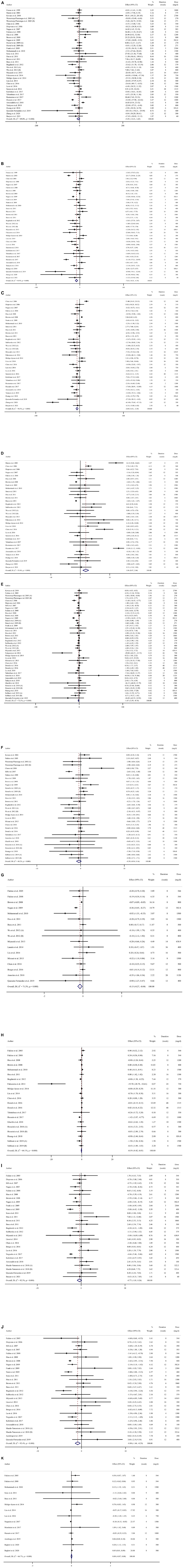
Figure 2. Forest plot detailing weighted mean difference and 95% confidence intervals (CIs) for the effects of green tea extract supplementation on (A) TG (mg/dL); (B) TC (mg/dL); (C) LDL (mg/dL); (D) HDL (mg/dL); (E) FBS (mg/dL); (F) fasting insulin (μlU/ml); (G) HbA1c (%); (H) HOMA-IR; (I) SBP (mmHg), (J) DBP (mmHg); (K) CRP (mg/dL).
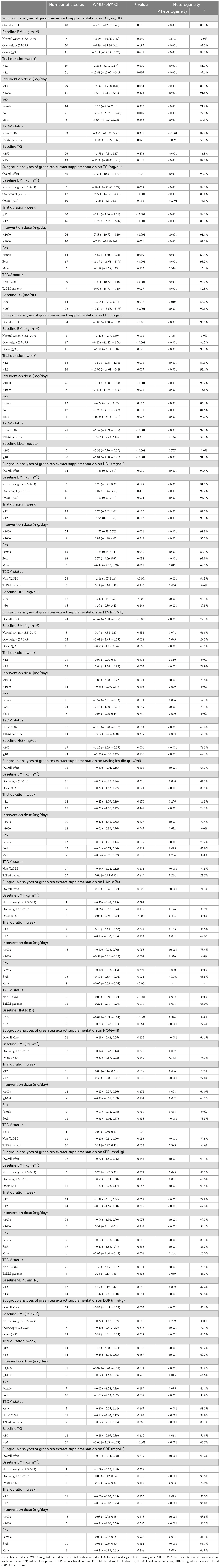
Table 3. Subgroup analyses of green tea extract supplementation on some cardiovascular risk factors in adults.
The effect of green tea supplementation on TC was examined in 36 arms of clinical trials. Pooled mean difference from the inverse variance method demonstrated a significant decrease in TC (WMD = −7.62; 95% CI: −10.51, −4.73; P = < 0.001) (Figure 2B). In addition, considerable between-study heterogeneity was disclosed (P = < 0.001, I2 = 90.9%). Subgroup analysis was accomplished based on duration and dosage of supplementation, baseline values of BMI and TC, past medical history of T2DM, and sex (Table 3). According to the results of subgroup analysis, green tea supplementation significantly decreased TC when females or both males and females were included, the dosage of supplementation was less than 1,000 mg/d, the baseline BMI was between 25-29.9 kg.m–2, and the baseline value of TC was more than 200 mg/dl.
The overall finding of our meta-analysis on 34 arms of clinical trials demonstrated that green tea supplementation had a significant decreasing effect on LDL (WMD = −5.80; 95% CI: −8.30, −3.30; P = < 0.001) (Figure 2C). In addition, considerable between-study heterogeneity was found (P < 0.001, I2 = 90.5%). Subgroup analysis was performed according to the duration and dosage of supplementation, baseline values of BMI and LDL, past medical history of T2DM, and sex (Table 3). The findings of subgroup analysis suggested that green tea supplementation contributed to a significant reduction in LDL if males or both males and females were included, the baseline BMI was between 25-29.9 kg.m–2 and participants were not affected by T2DM.
The overall finding of our meta-analysis on 34 arms of clinical trials exhibited that green tea supplementation significantly increased HDL (WMD = 1.85; 95% CI: 0.87, 2.84; P = 0.010) (Figure 2D). Also, there was heterogeneity among studies (P = < 0.001, I2 = 94.4%). Subgroup analysis was performed according to the duration and dosage of supplementation, baseline values of BMI and HDL, past medical history of T2DM, and sex (Table 3). The results of subgroup analysis revealed a significant elevation in HDL if females were included, the baseline BMI was lower more than 30 kg.m–2, there was no past medical history of T2DM, the duration of intervention was more than 12 weeks, the dosage of supplementation was less than 1000 mg/d, and baseline values of HDL were more than 50 mg/dl.
Combining effect sizes from 44 arms of clinical trials significantly decreased FBS after green tea supplementation (WMD = −1.67; 95% CI: −2.58, −0.75; P = < 0.001) (Figure 2E). In addition, considerable heterogeneity was found among studies (P = < 0.001, I2 = 72.2%). Subgroup analysis was conducted based on the duration and dosage of supplementation, baseline values of BMI and FBS, past medical history of T2DM, and sex (Table 3). The findings of subgroup analysis indicated a significant decrease in FBS when the baseline BMI of participants was between 25-29.9 kg.m–2, female or both male and female were included, the duration of intervention was more than 12 weeks, the dosage of supplementation was less than 1000 mg/d, and baseline values of FBS were less than 100 mg/dl.
Our preliminary analysis on 17 arms of clinical trials proposed a significant decrease in HbA1c following green tea supplementation (WMD = −0.15; 95% CI: −0.26, −0.04; P = 0.008) (Figure 2G). Also, there was heterogeneity among included studies (P = < 0.001, I2 = 71.3%). Subgroup analysis was carried out based on the duration and dosage of intervention, baseline values of BMI and HbA1c, past medical history of T2DM, and sex (Table 3). A significant decrease in HbA1c was found if the duration of intervention was ≤ 12 weeks, the dosage of supplementation was ≥ 1,000 mg/d, baseline values of HbA1c were less than 6.5%, male or both genders were involved, and the baseline value of BMI was ≥ 30 kg.m–2.
Non-significant effect on fasting insulin was observed following green tea supplementation (WMD = −0.39; 95% CI: −0.94, 0.16; P = 0.165) according to our analysis of 32 arms of clinical trials (Figure 2F). Also, there was heterogeneity among studies (P = < 0.001, I2 = 68.2%). Subgroup analysis was done based on the duration and dosage of supplementation, baseline values of BMI, past medical history of T2DM, and sex (Table 3). The results of the subgroup analysis indicated that green tea supplementation had non-significant effects on fasting insulin after subgroup analysis by all aforementioned factors.
Non-significant effect on HOMA-IR was observed following green tea supplementation (WMD = −0.18; 95% CI: −0.42, 0.05; P = 0.122) according to our analysis of 21 arms of clinical trials (Figure 2H). Also, there was heterogeneity among studies (P = < 0.001, I2 = 64.1%). Subgroup analysis was carried out based on the duration and dosage of supplementation, baseline values of BMI, past medical history of T2DM, and sex (Table 3). The results of the subgroup analysis suggested that green tea supplementation had non-significant effects on HOMA-IR after subgroup analysis by all aforementioned factors.
The overall finding of our meta-analysis on 28 arms of clinical trials demonstrated that green tea supplementation had no significant effect on SBP (WMD = −0.77; 95% CI: −1.80, 0.26; P = 0.144) (Figure 2I). In addition, considerable heterogeneity was found among studies (P = < 0.001, I2 = 92.3%). Subgroup analysis was accomplished based on duration and dosage of supplementation, baseline values of BMI and SBP, past medical history of T2DM, and sex (Table 3). The results of subgroup analysis reported a significant decreasing effect of green tea supplementation on SBP if the male was only included and participants were not affected by T2DM. However, there was no significant effect of green tea on SBP after subgroup analysis by the dosage and duration of intervention and baseline values of BMI and SBP.
A significant decreasing effect on DBP was observed following green tea supplementation (WMD = −0.87; 95% CI: −1.45, −0.29; P = 0.003) according to our analysis of 28 arms of clinical trials (Figure 2J). Also, remarkable heterogeneity was observed between studies (P < 0.001, I2 = 92.4%). Subgroup analysis was performed based on duration and dosage of supplementation, baseline values of BMI and DBP, past medical history of T2DM, and sex (Table 3). A significant decrease in DBP was observed if the duration of intervention was ≤ 12 weeks, the dosage of supplementation was less than 1,000 mg/d, baseline values of DBP were more than 80 mmHg, and the baseline value of BMI was ≥ 30 kg.m–2.
Non-significant effect on CRP was found following green tea supplementation (WMD = −0.03; 95% CI: −0.14, 0.08; P = 0.619) according to our analysis of 16 arms of clinical trials (Figure 2K). Also, there was heterogeneity among included clinical trials (P = < 0.001, I2 = 90.2%). Subgroup analysis was done based on the duration and dosage of intervention, baseline values of BMI, and sex (Table 3). The results of the subgroup analysis disclosed non-significant effects of green tea supplementation on CRP after subgroup analysis by all aforementioned factors.
Visual inspection of the funnel plot (Supplementary Figure 1) and the results of Egger’s test did not find any publication bias in clinical trials investigating the effects of green tea supplementation on TG (Egger’s test, P = 0.131), fasting insulin (Egger’s test, P = 0.645), HbA1c (Egger’s test, P = 0.223), HOMA-IR (Egger’s test, P = 0.057), SBP (Egger’s test, P = 0.086), DBP (Egger’s test, P = 0.238), and CRP (Egger’s test, P = 0.902). However, there was publication bias for TC (Egger’s test, P = 0.021), LDL (Egger’s test, P = 0.024), HDL (Egger’s test, P = 0.001), and FBS (Egger’s test, P = 0.019).
To assess the potential association between alterations in TG, TC, LDL, HDL, FBS, fasting insulin, HbA1c, HOMA-IR, SBP, DBP, and CRP and dose and duration of green tea supplementation, meta-regression analysis using the random-effects model was applied (Supplementary Figures 2, 3, 4, 5). Based on the findings of meta-regression analysis, there was no linear association between absolute alterations in TC, LDL, HDL, FBS, fasting insulin, HbA1c, SBP, DBP, and CRP, and dose of intervention. However there is a significant linear relationship between absolute alterations in TG and dose (Coefficient: 7.60, P-value = 0.049).
Also, non-linear association between absolute changes in TC, LDL, HDL, FBS, fasting insulin, HbA1c, SBP and CRP, and duration of intervention was observed. However, there was a linear association between absolute changes in TG (Coefficient: −9.43, P-value = 0.017), HOMA-IR (Coefficient: −2.74, P-value < 0.001) and DBP (Coefficient: 0.90, P-value = 0.037) and the duration of the intervention. In addition, a linear association between absolute changes in TG (Coefficient: 15.88, P-value = 0.042) and HDL (Coefficient: −3.21, P-value = 0.044) and the dose of intervention was found.
To assess the certainty of the evidence, the GRADE protocol was applied (Table 4) and obtained findings revealed that TC, LDL, HDL, FBS, HbA1c, and DBP-related evidence had moderate quality due to the serious inconsistency reasons. Additionally, it was shown that evidence regarding TG, fasting insulin, SBP, and CRP had low quality due to serious imprecision and inconsistency reasons. The evidence relating to HOMA-IR was also downgraded to very low quality because of the serious inconsistency, imprecision, and publication bias.
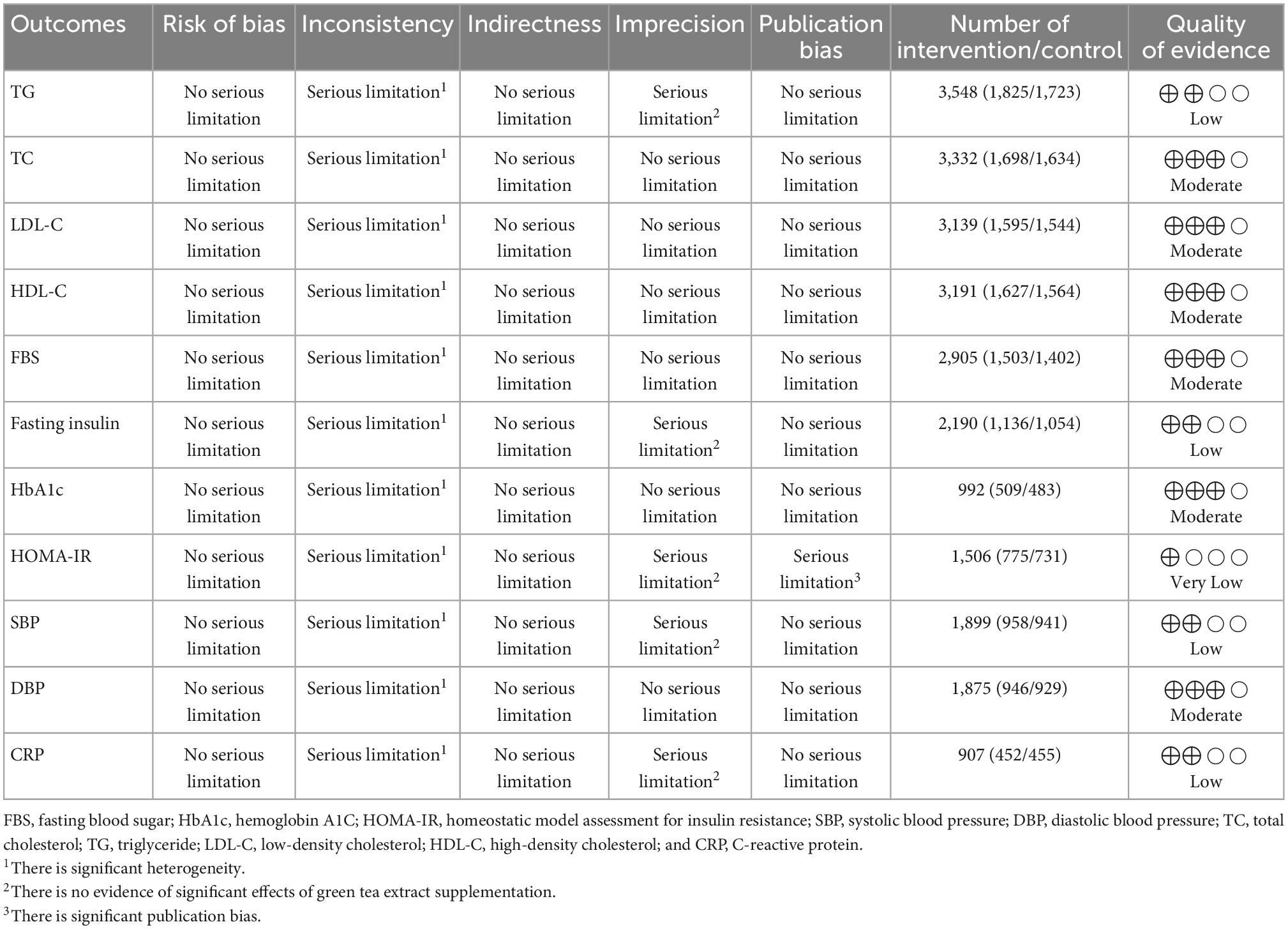
Table 4. GRADE profile of green tea extract supplementation for some cardiovascular risk factors in adults.
Based on the sensitivity analysis findings, for all considered cardiovascular risk factors including lipid profiles, glycemic indices, SBP and DBP, and CRP, there was no significant difference in results with removing one single study.
In the present meta-analysis, we weighed the effects of green tea supplementation on cardiovascular risk factors, including lipid (TG, TC, HDL, and LDL) and glycemic profiles (FBS, fasting insulin, HbA1c, and HOMA-IR), BP (SBP and DBP), and CRP as the marker of systemic inflammation. According to the findings, green tea supplementation was associated with small but significant improvements in the lipid profile by decreasing TC and LDL. Interestingly, green tea supplementation resulted in increases in HDL. In terms of TG, subgroup analyses showed that green tea supplementation had significantly favorable effects on TG in long-term interventions. green tea also showed favorable effects on the glycemic profile by decreasing FBS and HbA1c without any changes in fasting insulin and HOMA-IR. Moreover, our results demonstrated a small decline in DBP, highlighting the possible hypotensive effects of green tea supplementation. However, green tea had no significant effects on CRP.
Primary observations from in vitro and animal studies indicate that green tea supplementation inhibits CVD processes, which suggested the possible protective role of green tea against this disease (16). Moreover, previous epidemiological studies showed the significance of drinking green tea in the prevention of CVD (84, 85). For example, Kuriyama et al. reported that green tea consumption is associated with reduced mortality due to CVD in a population-based, prospective cohort study initiated among 40,530 Japanese adults aged 40 to 79 years (10). Furthermore, in another cohort of 165,000 adult men, Liu et al. showed that regular green tea consumption is associated with a significantly reduced risk of death from all-cause, and CVD among Chinese adults (86).
Our finding on the possible favorable effects of green tea supplementation on lipid profile is similar to the previous meta-analysis. A meta-analysis by Onakpoya et al. revealed a significant reduction in TC and LDL without any changes in HDL and TG (87). These results were repeated in the more recent systematic review and meta-analysis studies (88–90). However, our findings underlined that green tea also can have positive effects on lipid profile by increasing HDL which was not seen in the previous meta-analyses. Moreover, we showed that green tea supplementation can decrease TG if intervention lasts more than 12 weeks. The possible mechanisms underlying the positive effects of green tea on lipid profile. The hypolipidemic effects of GTE can be attributed to the high content of flavonoids, especially catechins, which are potent antioxidants (91). One of these catechins high in green tea is epigallocatechin (57). It is well-known that dietary supplements with antioxidant properties may have hypolipidemic effects (92–95). In terms of green tea antioxidants, previous in vitro studies showed that epigallocatechin can inhibit lipoprotein oxidation, namely, against LDL oxidation (96). The GTE can improve lipid profile by reducing micellar solubility and intestinal absorption of cholesterol, and reducing hepatic cholesterol concentration (97, 98). It should be noted that another possible mechanism involved in the favorable effects of green tea consumption on lipid profile is its anti-obesity property. Previous studies indicated that weight reduction can improve lipid profiles (99, 100). Previous studies reported anti-obesity effects of green tea by a small but significant effect of green tea on body mass (101).
Large population cohort studies reported that regular green tea intake may decrease the risk of T2DM. For example, a cohort study of 0.5 million adults aged 30–79 years suggested that daily green tea consumption was associated with a lower risk of incident T2DM and a lower risk of all-cause mortality in patients with diabetes (102). A more recent prospective cohort study among the 27 841 rural community residents in Deqing County revealed that drinking green tea may reduce the risk of T2DM among the adult population in rural China (103). Regarding the hypoglycemic of green tea and its effects on glycemic profile, although most systematic reviews and meta-analyses underlined the favorable effects of green tea, there are some inconsistencies between them. For example, Xu et al. reported that GTE supplementation significantly reduced FBS without any changes in other glycemic indices (104). These findings were repeated in a more recent meta-analysis (89). In contrast, the results of our previous systematic review and meta-analysis indicated that the green tea supplementation had no significant effect on FBS, fasting insulin, HbA1c, and HOMA-IR in patients with T2DM (105). However, our findings revealed that green tea supplementation has favorable effects on the glycemic profile by decreasing both FBS and HbA1c. Although the antioxidant content and anti-obesity of green tea (which is discussed above) are involved in the favorable effects of GTE on glycemic profile, (99, 106–108) in our study, some other possible mechanisms can contribute. It has been shown that green tea can increase circulating adiponectin (91). It is well-documented that adiponectin is the most abundant peptide secreted by adipocytes, whose increases are considered a therapeutic target in obesity-related diseases, including insulin resistance and T2DM (109, 110). Therefore, the adiponectin-increasing effects of green tea can be a possible mechanism for its hypoglycemic effects.
Hypertension is one of the chief risk factors for CVD (111). There are meta-analyses studies conducted to evaluate the effects of green tea consumption on BP. Increasingly, these studies reported inconsistent findings. For example, a primary meta-analysis published in 2014 showed that GTE supplementation resulted in significant reductions in SBP but not DBP (87). Xu et al. showed that even short-term GTE supplementation significantly reduced SBP and DBP (112) which is consistent with Igho-Osagie et al. study which revealed that short-term tea and green tea consumption is not associated with a reduction in blood pressure (113). In another systematic review and meta-analysis, Mahdavi-Roshan et al. suggested the positive effects of regular green tea consumption on BP in participants with elevated BP or hypertension by decreasing both SBP and DBP (114). Recently, an umbrella review and meta meta-analysis study showed that regular consumption of green tea significantly decreases SBP and DBP (89). However, our analysis demonstrated a small decline in DBP without any changes in SBP. It should be noted that the hypotensive effects of green tea were small and may not reach clinical importance. The small hypotensive effects of green tea may be the cause of its antioxidant contents. Previous studies underlined the significant role of antioxidant agents as a hypotensive treatment (115–117). Furthermore, green tea catechin cleanses reactive oxygen and nitrogen species; it also enhances antioxidant enzymes such as catalase and superoxide dismutase, thereby protecting endothelial cells from oxidative damage and regulating BP (118, 119). Moreover, green tea has been shown to increase circulating adiponectin. Evidence suggests that adiponectin has a potent role in regulating blood pressure. Adiponectin reduces blood presure through anti-atherogenic and insulin sensitivity effects and reversed salt-induced hypertenstion (120).
A large volume of clinical data indicates that the detection of CRP is of predictive value in CVD (121, 122). It has been hypothesized that green tea has CRP-lowering effects through inhibition of the Nuclear factor kappa-light-chain-enhancer of activated B cells (NF-kB) pathway and stimulation of nitric oxide (NO) production (123–125). This hypothesis underlined in some previous studies. For example, in our previous systematic review and meta-analysis study, we indicated that GTE supplementation significantly reduced CRP in patients with T2DM (126). However, these favorable effects were not seen in other systematic reviews and meta-analyses (127, 128). Our finding also underlined that green tea had no significant effects on CRP.
This meta-analysis contains some strengths and limitations. The main strength of this study is the relatively acceptable number of studies and high sample size. Moreover, we analyzed a wide range of biomarkers that are linked to CVD. Another advantage is performing a dose-response meta-regression analysis to evaluate the association between pooled effect size, dosage, and duration of green tea supplementation. Another strength of this meta-analysis relates to the inclusion of several long-term studies, which certainly has the advantage of documenting the long-term effects of GTE on CVD markers and allowing comparisons to shorter-duration designs (e.g., TG was shown to decrease to a greater extent in studies of longer duration). Finally, we graded the overall certainty of evidence across the studies according to the GRADE guidelines. Regarding limitations, statistical heterogeneity is apparent in our analysis. This may be attributed to methodological diversity (different study designs) and/or differences in treatment regimens (doses/durations) or the intervention type (different types of green tea which is mentioned in Table 1). In addition, there was publication bias for some biomarkers is another limitation of our study.
green tea supplementation was associated with a small but significant improvement in the lipid profile by decreasing TC and LDL while increasing HDL. Moreover, green tea supplementation had significantly favorable effects on TG in long-term interventions. green tea also showed favorable effects on the glycemic profile by decreasing FBS and HbA1c without any changes in fasting insulin and HOMA-IR. Moreover, our results demonstrated a small but significant decline in DBP. Moreover, green tea had no significant effects on CRP.
OA contributed to the conception and design of the study, data analysis, and supervised the study. DA-L and MK contributed to the data extraction. MZ and MK screened article for inclusion criteria. MG and DA-L contributed to the manuscript drafting. RB, KG, NA, and IT revised the manuscript. All authors approved the final version of the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2022.1084455/full#supplementary-material
1. Abe S, Inoue M. Green tea and cancer and cardiometabolic diseases: a review of the current epidemiological evidence. Eur J Clin Nutr. (2021) 75:865–76. doi: 10.1038/s41430-020-00710-7
2. Khan N, Mukhtar H. Tea polyphenols for health promotion. Life Sci. (2007) 81:519–33. doi: 10.1016/j.lfs.2007.06.011
3. Chacko S, Thambi P, Kuttan R, Nishigaki I. Beneficial effects of green tea: a literature review. Chin Med. (2010) 5:13. doi: 10.1186/1749-8546-5-13
4. Lorenzo J, Munekata P. Phenolic compounds of green tea: health benefits and technological application in food. Asian Pac J Trop Biomed. (2016) 6:709–19. doi: 10.1016/j.foodres.2017.11.047
5. Musial C, Kuban-Jankowska A, Gorska-Ponikowska M. Beneficial properties of green tea catechins. Int J Mol Sci. (2020) 21:1744. doi: 10.3390/ijms21051744
6. Roth Gregory A, Mensah George A, Johnson Catherine O, Addolorato G, Ammirati E, Baddour Larry M, et al. Global burden of cardiovascular diseases and risk factors, 1990–2019. J Am Coll Cardiol. (2020) 76:2982–3021.
7. Kokubo Y, Iso H, Saito I, Yamagishi K, Yatsuya H, Ishihara J, et al. The impact of green tea and coffee consumption on the reduced risk of stroke incidence in Japanese population. Stroke. (2013) 44:1369–74. doi: 10.1161/STROKEAHA.111.677500
8. Tian C, Huang Q, Yang L, Légaré S, Angileri F, Yang H, et al. Green tea consumption is associated with reduced incident CHD and improved CHD-related biomarkers in the Dongfeng-Tongji cohort. Sci Rep. (2016) 6:24353. doi: 10.1038/srep24353
9. Kishimoto Y, Saita E, Taguchi C, Aoyama M, Ikegami Y, Ohmori R, et al. Associations between green tea consumption and coffee consumption and the prevalence of coronary artery disease. J Nutr Sci Vitaminol. (2020) 66:237–45. doi: 10.3177/jnsv.66.237
10. Kuriyama S, Shimazu T, Ohmori K, Kikuchi N, Nakaya N, Nishino Y, et al. Green tea consumption and mortality due to cardiovascular disease, cancer, and all causes in Japan: the Ohsaki study. JAMA. (2006) 296:1255–65. doi: 10.1001/jama.296.10.1255
11. Alexopoulos N, Vlachopoulos C, Stefanadis C. Role of green tea in reduction of cardiovascular risk factors. Nutr Diet Suppl. (2010) 2:85–95. doi: 10.2147/NDS.S6025
12. Ding F, Ma B, Nazary-Vannani A, Kord-Varkaneh H, Fatahi S, Papageorgiou M, et al. The effects of green coffee bean extract supplementation on lipid profile in humans: a systematic review and meta-analysis of randomized controlled trials. Nutr Metab Cardiovasc Dis. (2020) 30:1–10. doi: 10.1016/j.numecd.2019.10.002
13. Tokede O, Gaziano J, Djoussé L. Effects of cocoa products/dark chocolate on serum lipids: a meta-analysis. Eur J Clin Nutr. (2011) 65:879–86. doi: 10.1038/ejcn.2011.64
14. Ghanavati M, Rahmani J, Clark C, Hosseinabadi S, Rahimlou M. Pistachios and cardiometabolic risk factors: a systematic review and meta-analysis of randomized controlled clinical trials. Complement Ther Med. (2020) 52:102513. doi: 10.1016/j.ctim.2020.102513
15. Dubois-Deruy E, Peugnet V, Turkieh A, Pinet F. Oxidative stress in cardiovascular diseases. Antioxidants. (2020) 9:864. doi: 10.3390/antiox9090864
16. Frei B, Higdon J. Antioxidant activity of tea polyphenols in vivo: evidence from animal studies. J Nutr. (2003) 133:3275s–84s. doi: 10.1093/jn/133.10.3275S
17. Zheng J, Lee H, Bin Sattar M, Huang Y, Bian J. Cardioprotective effects of epigallocatechin-3-gallate against doxorubicin-induced cardiomyocyte injury. Eur J Pharmacol. (2011) 652:82–8. doi: 10.1016/j.ejphar.2010.10.082
18. Alkhafaji N, Latif A. Effects of green tea extract on prevention and treatment of Dyslipidemia in cholesterol-fed male rabbits. Kufa Med J. (2012) 15:175–82.
19. Muramatsu K, Fukuyo M, Hara Y. Effect of green tea catechins on plasma cholesterol level in cholesterol-fed rats. J Nutr Sci Vitaminol. (1986) 32:613–22. doi: 10.3177/jnsv.32.613
20. Kim A, Chiu A, Barone M, Avino D, Wang F, Coleman C, et al. Green tea catechins decrease total and low-density lipoprotein cholesterol: a systematic review and meta-analysis. J Am Diet Assoc. (2011) 111:1720–9. doi: 10.1016/j.jada.2011.08.009
21. Rains T, Agarwal S, Maki K. Antiobesity effects of green tea catechins: a mechanistic review. J Nutr Biochem. (2011) 22:1–7. doi: 10.1016/j.jnutbio.2010.06.006
22. Liu K, Zhou R, Wang B, Chen K, Shi L, Zhu J, et al. Effect of green tea on glucose control and insulin sensitivity: a meta-analysis of 17 randomized controlled trials. Am J Clin Nutr. (2013) 98:340–8.
23. Li G, Zhang Y, Thabane L, Mbuagbaw L, Liu A, Levine M, et al. Effect of green tea supplementation on blood pressure among overweight and obese adults: a systematic review and meta-analysis. J Hypertens. (2015) 33:243–54. doi: 10.1097/HJH.0000000000000426
24. Frank J, George T, Lodge J, Rodriguez-Mateos A, Spencer J, Minihane A, et al. Daily consumption of an aqueous green tea extract supplement does not impair liver function or alter cardiovascular disease risk biomarkers in healthy men. J Nutr. (2009) 139:58–62. doi: 10.3945/jn.108.096412
25. Mousavi A, Vafa M, Neyestani T, Khamseh M, Hoseini F. The effects of green tea consumption on metabolic and anthropometric indices in patients with type 2 diabetes. J Res Med Sci. (2013) 18:1080–6.
26. Higgins J, Altman D, Gotzsche P, Juni P, Moher D, Oxman A, et al. The cochrane collaboration’s tool for assessing risk of bias in randomised trials. BMJ. (2011) 343:d5928. doi: 10.1136/bmj.d5928
27. Akl E, Altman D, Aluko P, Askie L, Beaton D, Berlin J, et al. Cochrane Handbook for Systematic Reviews of Interventions. New York, NY: John Wiley & Sons (2019).
28. Begg C, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. (1994) 50:1088–101. doi: 10.2307/2533446
29. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. (1997) 315:629–34. doi: 10.1136/bmj.315.7109.629
30. Brown A, Lane J, Coverly J, Stocks J, Jackson S, Stephen A, et al. Effects of dietary supplementation with the green tea polyphenol epigallocatechin-3-gallate on insulin resistance and associated metabolic risk factors: randomized controlled trial. Br J Nutr. (2009) 101:886–94. doi: 10.1017/S0007114508047727
31. Brown A, Lane J, Holyoak C, Nicol B, Mayes A, Dadd T. Health effects of green tea catechins in overweight and obese men: a randomised controlled cross-over trial. Br J Nutr. (2011) 106:1880–9. doi: 10.1017/S0007114511002376
32. Chen I, Liu C, Chiu J, Hsu C. Therapeutic effect of high-dose green tea extract on weight reduction: a randomized, double-blind, placebo-controlled clinical trial. Clin Nutr. (2016) 35:592–9. doi: 10.1016/j.clnu.2015.05.003
33. Diepvens K, Kovacs E, Vogels N, Westerterp-Plantenga M. Metabolic effects of green tea and of phases of weight loss. Physiol Behav. (2006) 87:185–91. doi: 10.1016/j.physbeh.2005.09.013
34. Hsu C, Tsai T, Kao Y, Hwang K, Tseng T, Chou P. Effect of green tea extract on obese women: a randomized, double-blind, placebo-controlled clinical trial. Clin Nutr. (2008) 27:363–70. doi: 10.1016/j.clnu.2008.03.007
35. Hill A, Coates A, Buckley J, Ross R, Thielecke F, Howe P. Can EGCG reduce abdominal fat in obese subjects? J Am Coll Nutr. (2007) 26:396s–402s.
36. Kovacs E, Lejeune M, Nijs I, Westerterp-Plantenga M. Effects of green tea on weight maintenance after body-weight loss. Br J Nutr. (2004) 91:431–7.
37. Lu P, Hsu C. Does supplementation with green tea extract improve acne in post-adolescent women? A randomized, double-blind, and placebo-controlled clinical trial. Complement Ther Med. (2016) 25:159–63. doi: 10.1016/j.ctim.2016.03.004
38. Mielgo-Ayuso J, Barrenechea L, Alcorta P, Larrarte E, Margareto J, Labayen I. Effects of dietary supplementation with epigallocatechin-3-gallate on weight loss, energy homeostasis, cardiometabolic risk factors and liver function in obese women: randomised, double-blind, placebo-controlled clinical trial. Br J Nutr. (2014) 111:1263–71. doi: 10.1017/S0007114513003784
39. Miyazaki R, Kotani K, Ayabe M, Tsuzaki K, Shimada J, Sakane N, et al. Minor effects of green tea catechin supplementation on cardiovascular risk markers in active older people: a randomized controlled trial. Geriatr Gerontol Int. (2013) 13:622–9. doi: 10.1111/j.1447-0594.2012.00952.x
40. Nagao T, Hase T, Tokimitsu I. A green tea extract high in catechins reduces body fat and cardiovascular risks in humans. Obesity. (2007) 15:1473–83. doi: 10.1038/oby.2007.176
41. Suliburska J, Bogdanski P, Szulinska M, Stepien M, Pupek-Musialik D, Jablecka A. Effects of green tea supplementation on elements, total antioxidants, lipids, and glucose values in the serum of obese patients. Biol Trace Elem Res. (2012) 149:315–22. doi: 10.1007/s12011-012-9448-z
42. Tadayon M, Movahedi S, Abedi P, Syahpoosh A. Impact of green tea extract on serum lipid of postmenopausal women: a randomized controlled trial. J Tradit Complement Med. (2018) 8:391–5.
43. Wu A, Spicer D, Stanczyk F, Tseng C, Yang C, Pike M. Effect of 2-month controlled green tea intervention on lipoprotein cholesterol, glucose, and hormone levels in healthy postmenopausal women. Cancer Prev Res. (2012) 5:393–402. doi: 10.1158/1940-6207.CAPR-11-0407
44. Sone T, Kuriyama S, Nakaya N, Hozawa A, Shimazu T, Nomura K, et al. Randomized controlled trial for an effect of catechin-enriched green tea consumption on adiponectin and cardiovascular disease risk factors. Food Nutr Res. (2011) 55:55–64. doi: 10.3402/fnr.v55i0.8326
45. Dostal A, Samavat H, Espejo L, Arikawa A, Stendell-Hollis N, Kurzer M. Green tea extract and catechol-O-methyltransferase genotype modify fasting serum insulin and plasma adiponectin concentrations in a randomized controlled trial of overweight and obese postmenopausal women. J Nutr. (2016) 146:38–45. doi: 10.3945/jn.115.222414
46. Dostal A, Arikawa A, Espejo L, Kurzer M. Long-term supplementation of green tea extract does not modify adiposity or bone mineral density in a randomized trial of overweight and obese postmenopausal women. J Nutr. (2016) 146:256–64.
47. Kafeshani M, Entezari M, Karimian J, Pourmasoumi M, Maracy M, Amini M, et al. A comparative study of the effect of green tea and sour tea on blood pressure and lipid profile in healthy adult men. ARYA Atheroscler. (2017) 13:109–16.
48. Maeda-Yamamoto M, Nishimura M, Kitaichi N, Nesumi A, Monobe M, Nomura S, et al. Randomized, placebo-controlled study on the safety and efficacy of daily ingestion of green tea (Camellia sinensis L.) cv. “Yabukita” and “Sunrouge” on eyestrain and blood pressure in healthy adults. Nutrients. (2018) 10:569. doi: 10.3390/nu10050569
49. Nantz M, Rowe C, Bukowski J, Percival S. Standardized capsule of Camellia sinensis lowers cardiovascular risk factors in a randomized, double-blind, placebo-controlled study. Nutrition. (2009) 25:147–54. doi: 10.1016/j.nut.2008.07.018
50. Hursel R, Westerterp-Plantenga M. Green tea catechin plus caffeine supplementation to a high-protein diet has no additional effect on body weight maintenance after weight loss. Am J Clin Nutr. (2009) 89:822–30. doi: 10.3945/ajcn.2008.27043
51. Westerterp-Plantenga M, Lejeune M, Kovacs E. Body weight loss and weight maintenance in relation to habitual caffeine intake and green tea supplementation. Obes Res. (2005) 13:1195–204. doi: 10.1038/oby.2005.142
52. Freese R, Basu S, Hietanen E, Nair J, Nakachi K, Bartsch H, et al. Green tea extract decreases plasma malondialdehyde concentration but does not affect other indicators of oxidative stress, nitric oxide production, or hemostatic factors during a high-linoleic acid diet in healthy females. Eur J Nutr. (1999) 38:149–57.
53. Samavat H, Newman A, Wang R, Yuan J, Wu A, Kurzer M. Effects of green tea catechin extract on serum lipids in postmenopausal women: a randomized, placebo-controlled clinical trial. Am J Clin Nutr. (2016) 104:1671–82.
54. Amozadeh H, Shabani R, Nazari M. The effect of aerobic training and green tea supplementation on cardio metabolic risk factors in overweight and obese females: a randomized trial. Int J Endocrinol Metab. (2018) 16:e60738. doi: 10.5812/ijem.60738
55. Rostamian M, Bijeh N. The Effect of short-term aerobic exercise and green tea consumption on MFO, ıFatmax, body composition and lipid profile in sedentary postmenopausal ııwomen. Int J Appl Exerc Physiol. (2017) 6:21–31.
56. Zhang T, Li N, Chen S, Hou Z, Saito A. Effects of green tea extract combined with brisk walking on lipid profiles and the liver function in overweight and obese men: a randomized, double-blinded, placebo-control trial. An Acad Bras Cienc. (2020) 92:e20191594. doi: 10.1590/0001-3765202020191594
57. Bagheri R, Rashidlamir A, Ashtary-Larky D, Wong A, Grubbs B, Motevalli M, et al. Effects of green tea extract supplementation and endurance training on irisin, pro-inflammatory cytokines, and adiponectin concentrations in overweight middle-aged men. Eur J Appl Physiol. (2020) 120:915–23. doi: 10.1007/s00421-020-04332-6
58. Bagheri R, Rashidlamir A, Ashtary-Larky D, Wong A, Alipour M, Motevalli M, et al. Does green tea extract enhance the anti-inflammatory effects of exercise on fat loss? Br J Clin Pharmacol. (2020) 86:753–62. doi: 10.1111/bcp.14176
59. Fukino Y, Shimbo M, Aoki N, Okubo T, Iso H. Randomized controlled trial for an effect of green tea consumption on insulin resistance and inflammation markers. J Nutr Sci Vitaminol. (2005) 51:335–42.
60. Fukino Y, Ikeda A, Maruyama K, Aoki N, Okubo T, Iso H. Randomized controlled trial for an effect of green tea-extract powder supplementation on glucose abnormalities. Eur J Clin Nutr. (2008) 62:953–60. doi: 10.1038/sj.ejcn.1602806
61. Nagao T, Meguro S, Hase T, Otsuka K, Komikado M, Tokimitsu I, et al. A catechin-rich beverage improves obesity and blood glucose control in patients with type 2 diabetes. Obesity. (2009) 17:310–7. doi: 10.1038/oby.2008.505
62. Mohammadi S, Hasseinzadeh Attar M, Karimi M, Hossainnezhad A, Eshraghian M, Hosseini S, et al. The effects of green tea extract on serum adiponectin concentration and insulin resistance in patients with type 2 diabetes mellitus. J Adv Med Biomed Res. (2010) 18:44–57.
63. Hsu C, Liao Y, Lin S, Tsai T, Huang C, Chou P. Does supplementation with green tea extract improve insulin resistance in obese type 2 diabetics? A randomized, double-blind, and placebo-controlled clinical trial. Altern Med Rev. (2011) 16:157–63.
64. Lasaite L, Spadiene A, Savickiene N, Skesters A, Silova A. The effect of Ginkgo biloba and Camellia sinensis extracts on psychological state and glycemic control in patients with type 2 diabetes mellitus. Nat Prod Commun. (2014) 9:1345–50.
65. Liu C, Huang C, Huang L, Chen I, Chiu J, Hsu C. Effects of green tea extract on insulin resistance and glucagon-like peptide 1 in patients with type 2 diabetes and lipid abnormalities: a randomized, double-blinded, and placebo-controlled trial. PLoS One. (2014) 9:e91163. doi: 10.1371/journal.pone.0091163
66. Borges C, Papadimitriou A, Duarte D, Lopes de Faria J, Lopes de Faria J. The use of green tea polyphenols for treating residual albuminuria in diabetic nephropathy: a double-blind randomised clinical trial. Sci Rep. (2016) 6:28282. doi: 10.1038/srep28282
67. Zandi Dareh Gharibi Z, Faramarzi M, Banitalebi E. The effect of rhythmic aerobic exercise and green tea supplementation on visfatin levels and metabolic risk factors in obese diabetic women. JMPIR. (2018) 17:145–56.
68. Sobhani F, Haghshenas R, Rahimi M. Effect of eight weeks aerobic training and supplementation of green tea on apelin plasma levels and insulin resistance in elderly women with type 2 diabetes. J Mazand Univ Med Sci. (2019) 28:84–93.
69. Quezada-Fernández P, Trujillo-Quiros J, Pascoe-González S, Trujillo-Rangel W, Cardona-Müller D, Ramos-Becerra C, et al. Effect of green tea extract on arterial stiffness, lipid profile and sRAGE in patients with type 2 diabetes mellitus: a randomised, double-blind, placebo-controlled trial. Int J Food Sci Nutr. (2019) 70:977–85. doi: 10.1080/09637486.2019.1589430
70. de Amorim L, Vaz S, Cesário G, Coelho A, Botelho P. Effect of green tea extract on bone mass and body composition in individuals with diabetes. J Funct Foods. (2018) 40:589–94.
71. Hosseini S, Alipour M, Zakerkish M, Cheraghian B, Ghandil P. The gene-treatment interaction of FTO-RS9939609 gene polymorphism and epigallocatechin-gallate intervention on anthropometric indices, fasting blood sugar and insulin resistance/sensitivity in patients with type 2 diabetes mellitus. Iran Red Crescent Med J. (2018) 20:12–8.
72. Mirzaei K, Hossein-Nezhad A, Karimi M, Hosseinzadeh-Attar M, Jafari N, Najmafshar A, et al. Effect of green tea extract on bone turnover markers in type 2 diabetic patients; a double- blind, placebo-controlled clinical trial study. DARU J Pharm Sci. (2015) 2015:7.
73. Huang L, Liu C, Wang L, Huang C, Hsu C. Effects of green tea extract on overweight and obese women with high levels of low density-lipoprotein-cholesterol (LDL): a randomised, double-blind, and cross-over placebo-controlled clinical trial. BMC Complement Altern Med. (2018) 18:294. doi: 10.1186/s12906-018-2355-x
74. Maron D, Lu G, Cai N, Wu Z, Li Y, Chen H, et al. Cholesterol-lowering effect of a theaflavin-enriched green tea extract: a randomized controlled trial. Arch Intern Med. (2003) 163:1448–53. doi: 10.1001/archinte.163.12.1448
75. Chan C, Koo M, Ng E, Tang O, Yeung W, Ho P. Effects of Chinese green tea on weight, and hormonal and biochemical profiles in obese patients with polycystic ovary syndrome–a randomized placebo-controlled trial. J Socr Gynecol Invest. (2006) 13:63–8. doi: 10.1016/j.jsgi.2005.10.006
76. Mombaini E, Jafarirad S, Husain D, Haghighizadeh M, Padfar P. The impact of green tea supplementation on anthropometric indices and inflammatory cytokines in women with polycystic ovary syndrome. Phytother Res. (2017) 31:747–54. doi: 10.1002/ptr.5795
77. Bogdanski P, Suliburska J, Szulinska M, Stepien M, Pupek-Musialik D, Jablecka A. Green tea extract reduces blood pressure, inflammatory biomarkers, and oxidative stress and improves parameters associated with insulin resistance in obese, hypertensive patients. Nutr Res. (2012) 32:421–7. doi: 10.1016/j.nutres.2012.05.007
78. Nogueira L, Nogueira Neto J, Klein M, Sanjuliani A. Short-term effects of green tea on blood pressure, endothelial function, and metabolic profile in obese prehypertensive women: a crossover randomized clinical trial. J Am Coll Nutr. (2017) 36:108–15. doi: 10.1080/07315724.2016.1194236
79. Fukuzawa Y, Kapoor M, Yamasaki K, Okubo T, Hotta Y, Juneja L. Effects of green tea catechins on nonalcoholic steatohepatitis (NASH) patients. J Funct Food. (2014) 9:48–59.
80. Tabatabaee S, Alavian S, Ghalichi L, Miryounesi M, Mousavizadeh K, Jazayeri S, et al. Green tea in non-alcoholic fatty liver disease; a double blind randomized clinical trial. Hepat Mon. (2017) 17:e14993.
81. Hussain M, Habib Ur R, Akhtar L. Therapeutic benefits of green tea extract on various parameters in non-alcoholic fatty liver disease patients. Pak J Med Sci. (2017) 33:931–6. doi: 10.12669/pjms.334.12571
82. Basu A, Du M, Sanchez K, Leyva M, Betts N, Blevins S, et al. Green tea minimally affects biomarkers of inflammation in obese subjects with metabolic syndrome. Nutrition. (2011) 27:206–13. doi: 10.1016/j.nut.2010.01.015
83. Lee T, Charng M, Tseng C, Lai L. A double-blind, randomized, placebo-controlled study to evaluate the efficacy and safety of STA-2 (green tea polyphenols) in patients with chronic stable angina. Acta Cardiol Sin. (2016) 32:439–49. doi: 10.6515/acs20150708d
84. Kuriyama S. The relation between green tea consumption and cardiovascular disease as evidenced by epidemiological studies. J Nutr. (2008) 138:1548S–53S. doi: 10.1093/jn/138.8.1548S
85. Nakachi K, Matsuyama S, Miyake S, Suganuma M, Imai K. Preventive effects of drinking green tea on cancer and cardiovascular disease: epidemiological evidence for multiple targeting prevention. Biofactors. (2000) 13:49–54. doi: 10.1002/biof.5520130109
86. Liu J, Liu S, Zhou H, Hanson T, Yang L, Chen Z, et al. Association of green tea consumption with mortality from all-cause, cardiovascular disease and cancer in a Chinese cohort of 165,000 adult men. Eur J Epidemiol. (2016) 31:853–65. doi: 10.1007/s10654-016-0173-3
87. Onakpoya I, Spencer E, Heneghan C, Thompson M. The effect of green tea on blood pressure and lipid profile: a systematic review and meta-analysis of randomized clinical trials. Nutr Metab Cardiovasc Dis. (2014) 24:823–36.
88. Asbaghi O, Fouladvand F, Moradi S, Ashtary-Larky D, Choghakhori R, Abbasnezhad A, et al. Effect of green tea extract on lipid profile in patients with type 2 diabetes mellitus: a systematic review and meta-analysis. Diabetes Metab Syndr. (2020) 14:293–301.
89. Neyestani T, Nikooyeh B. A comprehensive overview on the effects of green tea on anthropometric measures, blood pressure, glycemic and lipidemic status: an umbrella review and meta meta-analysis study. Nutr Metab Cardiovasc Dis. (2022) 32:2026–40. doi: 10.1016/j.numecd.2022.05.021
90. Xu R, Yang K, Li S, Dai M, Chen G. Effect of green tea consumption on blood lipids: a systematic review and meta-analysis of randomized controlled trials. Nutr J. (2020) 19:1–15.
91. Asbaghi O, Fouladvand F, Ashtary-Larky D, Bagheri R, Choghakhori R, Wong A, et al. Effects of green tea supplementation on serum concentrations of adiponectin in patients with type 2 diabetes mellitus: a systematic review and meta-analysis. Arch Physiol Biochem. (2020):1–8. doi: 10.1080/13813455.2020.1846202
92. Asbaghi O, Ashtary-Larky D, Bagheri R, Nazarian B, Pourmirzaei Olyaei H, Rezaei Kelishadi M, et al. Beneficial effects of folic acid supplementation on lipid markers in adults: a GRADE-assessed systematic review and dose-response meta-analysis of data from 21,787 participants in 34 randomized controlled trials. Crit Rev Food Sci Nutr. (2021) 62:8435–53. doi: 10.1080/10408398.2021.1928598
93. Asbaghi O, Naeini F, Ashtary-Larky D, Moradi S, Zakeri N, Eslampour E, et al. Effects of chromium supplementation on lipid profile in patients with type 2 diabetes: a systematic review and dose-response meta-analysis of randomized controlled trials. J Trace Elem Med Biol. (2021) 66:126741. doi: 10.1016/j.jtemb.2021.126741
94. Kelishadi M, Ashtary-Larky D, Davoodi S, Clark C, Asbaghi O. The effects of selenium supplementation on blood lipids and blood pressure in adults: a systematic review and dose-response meta-analysis of randomized control trials. J Trace Elem Med Biol. (2022) 74:127046. doi: 10.1016/j.jtemb.2022.127046
95. Namkhah Z, Ashtary-Larky D, Naeini F, Clark C, Asbaghi O. Does vitamin C supplementation exert profitable effects on serum lipid profile in patients with type 2 diabetes? A systematic review and dose-response meta-analysis. Pharmacol Res. (2021) 169:105665.
96. Coimbra S, Santos-Silva A, Rocha-Pereira P, Rocha S, Castro E. Green tea consumption improves plasma lipid profiles in adults. Nutr Res. (2006) 26:604–7.
97. Raederstorff D, Schlachter M, Elste V, Weber P. Effect of EGCG on lipid absorption and plasma lipid levels in rats. J Nutr Biochem. (2003) 14:326–32.
98. Haidari F, Shahi M, Zarei M, Rafiei H, Omidian K. Effect of green tea extract on body weight, serum glucose and lipid profile in streptozotocin-induced diabetic rats. A dose response study. Saudi Med J. (2012) 33:128–33.
99. Ashtary-Larky D, Ghanavati M, Lamuchi-Deli N, Payami S, Alavi-Rad S, Boustaninejad M, et al. Rapid weight loss vs. Slow weight loss: which is more effective on body composition and metabolic risk factors? Int J Endocrinol Metab. (2017) 15:e13249. doi: 10.5812/ijem.13249
100. Aucott L, Gray D, Rothnie H, Thapa M, Waweru C. Effects of lifestyle interventions and long-term weight loss on lipid outcomes–a systematic review. Obes Rev. (2011) 12:e412–25. doi: 10.1111/j.1467-789X.2010.00819.x
101. Asbaghi O, Fouladvand F, Gonzalez M, Aghamohammadi V, Choghakhori R, Abbasnezhad A. Effect of green tea on anthropometric indices and body composition in patients with type 2 diabetes mellitus: a systematic review and meta-analysis. Complement Med Res. (2021) 28:244–51. doi: 10.1159/000511665
102. Nie J, Yu C, Guo Y, Pei P, Chen L, Pang Y, et al. Tea consumption and long-term risk of type 2 diabetes and diabetic complications: a cohort study of 0.5 million Chinese adults. Am J Clin Nutr. (2021) 114:194–202. doi: 10.1093/ajcn/nqab006
103. Zhu B, Dong X, Zhu J, Wang N, Chen Y, Jiang Q, et al. Association between tea drinking and the risk of type 2 diabetes mellitus among rural adults in Deqing County: a prospective cohort study. Wei Sheng Yan Jiu. (2022) 51:12–7. doi: 10.19813/j.cnki.weishengyanjiu.2022.01.003
104. Xu R, Bai Y, Yang K, Chen G. Effects of green tea consumption on glycemic control: a systematic review and meta-analysis of randomized controlled trials. Nutr Metab. (2020) 17:56.
105. Asbaghi O, Fouladvand F, Gonzalez M, Ashtary-Larky D, Choghakhori R, Abbasnezhad A, et al. Effect of green tea on glycemic control in patients with type 2 diabetes mellitus: a systematic review and meta-analysis. Diabetes Metab Syndr. (2021) 15:23–31.
106. Hosseini S, Ghaedi E, Zakerkish M, Ghadiri A, Ashtary-larky D, Safari M, et al. Effects of ginseng extract on chemerin, apelin and glycemic biomarkers in type 2 diabetic patients. Indian J Physiol Pharmacol. (2017) 61:152–8.
107. Asbaghi O, Fatemeh N, Mahnaz R, Ehsan G, Elham E, Behzad N, et al. Effects of chromium supplementation on glycemic control in patients with type 2 diabetes: a systematic review and meta-analysis of randomized controlled trials. Pharmacol Res. (2020) 161:105098.
108. Asbaghi O, Ashtary-Larky D, Bagheri R, Moosavian S, Olyaei H, Nazarian B, et al. Folic acid supplementation improves glycemic control for diabetes prevention and management: a systematic review and dose-response meta-analysis of randomized controlled trials. Nutrients. (2021) 13:2355. doi: 10.3390/nu13072355
109. Achari A, Jain S. Adiponectin, a therapeutic target for obesity, diabetes, and endothelial dysfunction. Int J Mol Sci. (2017) 18:1321. doi: 10.3390/ijms18061321
110. Fisman E, Tenenbaum A. Adiponectin: a manifold therapeutic target for metabolic syndrome, diabetes, and coronary disease? Cardiovasc Diabetol. (2014) 13:103. doi: 10.1186/1475-2840-13-103
111. Ashtary-Larky D, Rezaei Kelishadi M, Bagheri R, Moosavian S, Wong A, Davoodi S, et al. The effects of nano-curcumin supplementation on risk factors for cardiovascular disease: a GRADE-assessed systematic review and meta-analysis of clinical trials. Antioxidants. (2021) 10:1015. doi: 10.3390/antiox10071015
112. Xu R, Yang K, Ding J, Chen G. Effect of green tea supplementation on blood pressure: a systematic review and meta-analysis of randomized controlled trials. Medicine. (2020) 99:e19047. doi: 10.1097/MD.0000000000019047
113. Igho-Osagie E, Cara K, Wang D, Yao Q, Penkert L, Cassidy A, et al. Short-term tea consumption is not associated with a reduction in blood lipids or pressure: a systematic review and meta-analysis of randomized controlled trials. J Nutr. (2020) 150:3269–79. doi: 10.1093/jn/nxaa295
114. Mahdavi-Roshan M, Salari A, Ghorbani Z, Ashouri A. The effects of regular consumption of green or black tea beverage on blood pressure in those with elevated blood pressure or hypertension: a systematic review and meta-analysis. Complement Ther Med. (2020) 51:102430. doi: 10.1016/j.ctim.2020.102430
115. Asbaghi O, Naeini F, Ashtary-Larky D, Kaviani M, Kelishadi M, Eslampour E, et al. Effects of chromium supplementation on blood pressure, body mass index, liver function enzymes and malondialdehyde in patients with type 2 diabetes: a systematic review and dose-response meta-analysis of randomized controlled trials. Complement Ther Med. (2021) 60:102755. doi: 10.1016/j.ctim.2021.102755
116. Asbaghi O, Nazarian B, Naeini F, Kaviani M, Moradi S, Askari G, et al. Lycopene supplementation and blood pressure: systematic review and meta-analyses of randomized trials. J Herb Med. (2022) 31:100521. doi: 10.1016/j.hermed.2021.100521
117. Asbaghi O, Salehpour S, Rezaei Kelishadi M, Bagheri R, Ashtary-Larky D, Nazarian B, et al. Folic acid supplementation and blood pressure: a GRADE-assessed systematic review and dose-response meta-analysis of 41,633 participants. Crit Rev Food Sci Nutr. (2021):1–16. doi: 10.1080/10408398.2021.1968787
118. Babu P, Sabitha K, Shyamaladevi C. Therapeutic effect of green tea extract on oxidative stress in aorta and heart of streptozotocin diabetic rats. Chem Biol Interact. (2006) 162:114–20. doi: 10.1016/j.cbi.2006.04.009
119. Simos Y, Verginadis I, Toliopoulos I, Velalopoulou A, Karagounis I, Karkabounas S. Effects of catechin and epicatechin on superoxide dismutase and glutathione peroxidase activity, in vivo. Redox Rep. (2012) 17:181–6. doi: 10.1179/1351000212Y.0000000020
120. Yiannikouris F, Gupte M, Putnam K, Cassis L. Adipokines and blood pressure control. Curr Opin Nephrol Hypertens. (2010) 19:195–200. doi: 10.1097/MNH.0b013e3283366cd0
121. Tian R, Tian M, Wang L, Qian H, Zhang S, Pang H, et al. C-reactive protein for predicting cardiovascular and all-cause mortality in type 2 diabetic patients: a meta-analysis. Cytokine. (2019) 117:59–64.
122. Hermans M, Ahn S, Rousseau M. Increased CRP: an extended biomarker of microvascular risk in men with type 2 diabetes, complications I. Increased CRP: an extended biomarker of microvascular risk in men with type 2 diabetes. J Diabetes Complications. (2019) 33:107413. doi: 10.1016/j.jdiacomp.2019.107413
123. Wahyudi S, Sargowo D. Green tea polyphenols inhibit oxidized LDL-induced NF-KB activation in human umbilical vein endothelial cells. Acta Med Indones. (2007) 39:66–70.
124. Yang F, De Villiers W, McClain C, Varilek G. Green tea polyphenols block endotoxin-induced tumor necrosis factor-production and lethality in a murine model. J Nutr. (1998) 128:2334–40. doi: 10.1093/jn/128.12.2334
125. Chan M, Fong D, Ho C, Huang H. Inhibition of inducible nitric oxide synthase gene expression and enzyme activity by epigallocatechin gallate, a natural product from green tea. Biochem Pharmacol. (1997) 54:1281–6. doi: 10.1016/S0006-2952(97)00504-2
126. Asbaghi O, Fouladvand F, Gonzalez M, Aghamohammadi V, Choghakhori R, Abbasnezhad A. The effect of green tea on C-reactive protein and biomarkers of oxidative stress in patients with type 2 diabetes mellitus: a systematic review and meta-analysis. Complement Ther Med. (2019) 46:210–6. doi: 10.1016/j.ctim.2019.08.019
127. Haghighatdoost F, Hariri M. The effect of green tea on inflammatory mediators: a systematic review and meta-analysis of randomized clinical trials. Phytother Res. (2019) 33:2274–87. doi: 10.1002/ptr.6432
128. Serban C, Sahebkar A, Antal D, Ursoniu S, Banach M. Effects of supplementation with green tea catechins on plasma C-reactive protein concentrations: a systematic review and meta-analysis of randomized controlled trials. Nutrition. (2015) 31:1061–71. doi: 10.1016/j.nut.2015.02.004
129. Azizbeigi K, Stannard SR, Atashak S. Green tea supplementation during resistance training minimally affects systemic inflammation and oxidative stress indices in obese men. Jundishapur J Nat Pharm Prod. (2019) 14:e61419. doi: 10.5812/jjnpp.61419
130. Bazyar H, Hosseini SA, Saradar S, Mombaini D, Allivand M, Labibzadeh M, et al. Effects of epigallocatechin-3-gallate of Camellia sinensis leaves on blood pressure, lipid profile, atherogenic index of plasma and some inflammatory and antioxidant markers in type 2 diabetes mellitus patients: a clinical trial. J Complement Integr Med. (2021) 18:405–11.
Keywords: green tea supplementation, cardiovascular risk factors, systematic review, meta-analysis, lipid profile, glycemic control, blood pressure
Citation: Zamani M, Kelishadi MR, Ashtary-Larky D, Amirani N, Goudarzi K, Torki IA, Bagheri R, Ghanavati M and Asbaghi O (2023) The effects of green tea supplementation on cardiovascular risk factors: A systematic review and meta-analysis. Front. Nutr. 9:1084455. doi: 10.3389/fnut.2022.1084455
Received: 30 October 2022; Accepted: 16 December 2022;
Published: 10 January 2023.
Edited by:
Lilia Castillo-Martinez, Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán (INCMNSZ), MexicoReviewed by:
Mehran Rahimlou, Zanjan University of Medical Sciences, IranCopyright © 2023 Zamani, Kelishadi, Ashtary-Larky, Amirani, Goudarzi, Torki, Bagheri, Ghanavati and Asbaghi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Omid Asbaghi,  b21pZC5hc2JhZ2hpQGdtYWlsLmNvbQ==; Matin Ghanavati,
b21pZC5hc2JhZ2hpQGdtYWlsLmNvbQ==; Matin Ghanavati,  bWF0aW5naGFuYXZhdGlAc2JtdS5hYy5pcg==
bWF0aW5naGFuYXZhdGlAc2JtdS5hYy5pcg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.