
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Nutr., 07 December 2022
Sec. Food Chemistry
Volume 9 - 2022 | https://doi.org/10.3389/fnut.2022.1058639
 Zhengwei Liang1,2,3†
Zhengwei Liang1,2,3† Kunyi Liu2,3,4†
Kunyi Liu2,3,4† Ruoyu Li2,3,5†
Ruoyu Li2,3,5† Baiping Ma6
Baiping Ma6 Wei Zheng6
Wei Zheng6 Shengchao Yang1,2,3
Shengchao Yang1,2,3 Guanghui Zhang1,2,3
Guanghui Zhang1,2,3 Yinhe Zhao1
Yinhe Zhao1 Junwen Chen1,2,3*
Junwen Chen1,2,3* Ming Zhao2,3,5*
Ming Zhao2,3,5*Introduction: Radix Notoginseng, one of the most famous Chinese traditional medicines, is the dried root of Panax notoginseng (Araliaceae). Stems and leaves of P. notoginseng (SLPN) are rich in secondary metabolites and nutrients, and authorized as a food resource, however, its utilization needs further research.
Methods: A SLPN-instant beverage was manufactured from SLPN through optimization by response surface design with 21-fold of 48.50% ethanol for 39 h, and this extraction was repeated twice; the extraction solution was concentrated to 1/3 volume using a vacuum rotatory evaporator at 45°C, and then spray dried at 110°C. Nutritional components including 14 amino acids, ten mineral elements, 15 vitamins were detected in the SLPN-instant beverage; forty-three triterpenoid saponins, e.g., ginsenoside La, ginsenoside Rb3, notoginsenoside R1, and two flavonoid glycosides, as well as dencichine were identified by UPLC-MS.
Results: The extraction rate of SLPN-instant beverage was 37.89 ± 0.02%. The majority nutrients were Gly (2.10 ± 0.63 mg/g), His (1.23 ± 0.07 mg/g), α-VE (18.89 ± 1.87 μg/g), β-VE (17.53 ± 1.98 μg/g), potassium (49.26 ± 2.70 mg/g), calcium (6.73 ± 0.27 mg/g). The total saponin of the SLPN-instant beverage was 403.05 ± 34.98 mg/g, majority was notoginsenoside Fd and with contents of 227 ± 2.02 mg/g. In addition, catechin and γ-aminobutyric acid were detected with levels of 24.57 ± 0.21 mg/g and 7.50 ± 1.85 mg/g, respectively. The SLPN-instant beverage showed good antioxidant activities with half maximal inhibitory concentration (IC50) for scavenging hydroxyl (OH–) radicals, superoxide anion (O2–) radicals, 1,1-diphenyl-2-picrylhydrazyl (DPPH) radicals and 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonate) (ABTS+) radicals were 0.1954, 0.2314, 0.4083, and 0.3874 mg/mL, respectively.
Conclusion: We optimized an analytical method for in depth analysis of the newly authorized food resource SLPN. Together, an instant beverage with antioxidant activity, rich in nutrients and secondary metabolites, was manufactured from SLPN, which may improve the utilization of SLPN.
Panax notoginseng (Burk) F.H. Chen is a perennial medicinal plant and a member of the Araliaceae family. The dried root of P. notoginseng is defined as Radix Notoginseng, which is a famous Chinese medicinal material and has been used for thousands of years in China (1). This herb is extensively used in more than 594 Chinese medicines (2) and the total saponins in Radix Notoginseng are thought to be pharmacological active components (3). The cultivation area of P. notoginseng has reached 1.03 × 104 hm2 in China (4), and the total value of agricultural output has reached $ 0.87 billion (about ¥ 5.5 billion) in (5). Interestingly, total saponin including ginsenosides and notoginsenosides are also found in the stems and leaves of P. notoginseng (SLPN) (6), which is similar to that in roots of P. notoginseng. More than 20-million-kilogram SLPN are produced each year in China (7), but the utilization rate is <5%.
Stems and leaves of P. notoginseng contain secondary metabolites and nutrients, including polyphenols, flavonoids, volatile oil, dencichine, amino acids, vitamins, mineral elements etc. For example, SLPN were reported to contain vitamins (11.39 mg/100 g), crude fiber (8.33–20.45%), protein (9.63–15.18%), and fat (0.46–0.82%) (8). It mineral elements contents include zinc (Zn) (31.99–328.78 mg/kg), iron (Fe) (126.06–832.80 mg/kg), manganese (Mn) (102.58–431.18 mg/kg), calcium (Ca) (9.35–16.95 g/kg) and magnesium (Mg) (2.41–3.59 g/kg) (9). Besides, SLPN also contains abundant secondary metabolites and the total saponins in SLPN is 5–7% (10). To date, 945 ginsenosides have been identified in SLPN (11), such as notoginsenoside R1, ginsenoside Rb1, ginsenoside Rb2, notoginsenoside R2, notoginsenoside Fa, ginsenosides Rg1, ginsenosides Rg3, ginsenosides Rb3, ginsenosides Rc, ginsenosides Rd and the gypenoside family (12). The total sum content of notoginsenoside R1, ginsenosides Rb1, ginsenosides Rb2 amounts to 10.68 ± 0.97 mg/g in SLPN, and this is more than half of that in the roots (6). Two polyphenolic compounds, quercetin and gallic acid, are detected in SLPN with an average content of 0.16 and 0.02%, respectively (13). Therefore, SLPN are rich in nutritional components and secondary metabolites valued deeply for application.
Fortunately, the local food safety standard (DBS53/024 - 2017) for SLPN has been authenticated by the Health and Family Planning Commission of Yunnan province, China, and nowadays SLPN has been authorized as a food resource (14). It has been reported that vigorous ginsenoside transformation occurs in SLPN processed by sun-air drying and hot-air drying at 50°C, but not by shade-air drying, hot-air drying at 25°C and steaming prior to drying (15). Up to now, Radix Notoginseng saponins shows good antioxidant activity (3). Steamed Radix Notoginseng displayed a significant increase both in cell viability and oxygen radical absorption capacity values (16). Several foods containing ginsenosides which are extracted from SLPN have been on the market, e.g., ginsenoside Rb3 herbal teas (17), P. notoginseng stems and leaves extract candy (18), stem-leaves of P. notoginseng ginsenosides healthful-foods (6). Additionally, there is a Chinese medicine entitled by Qiyeshen’an tablets containing total saponin extracted from SLPN (19). The extraction methods of SLPN nutrition and secondary metabolites include hot water or high concentration hot ethanol extraction, ultrasonic extraction, enzyme-ultrasonic assisted extraction, enzyme extraction, and homogenate extraction (20). However, the products of SLPN or its extract are rarely, and utilization form are also need further research.
In this work, the environmentally friendly extraction method for nutritional and secondary metabolites from SLPN was optimized at room temperature, and the spray-drying process was used to manufacture an SLPN-instant beverage. The secondary metabolites, nutritional components, pesticides and heavy metal residues in SLPN and SLPN-instant beverage were analyzed by the vanillin-perchloric acid method, ultra-performance liquid chromatography coupled with electrospray time-of-flight mass spectrometry (UPLC-MS) and high performance liquid chromatography (HPLC). The antioxidant activity of the SLPN-instant beverage was determined in vitro. We provide a valuable process for the deep development and utilization of SLPN.
In this study, three-year cultivation fresh SLPN was collected from the Panax notoginseng planting base of Gaotian Co. Ltd (104.27 E, 23.35 N, 1631 meters above sea level, Wenshan, China) in September 2021. Previous research indicates that vigorous ginsenosides transformation occurs in SLPN processed by sun-air drying and hot-air drying at 50°C, but not shade-air drying at 25°C (15). Therefore, in our experiments, SLPN were shade-air dried at 25°C for further experiments, and all samples are expressed as dry weight (DW).
Ginsenoside Re (G-Re), ginsenoside Rc (G-Rc), ginsenoside Rb1 (G-Rb1), ginsenoside Rb2 (G-Rb2), ginsenoside Rb3 (G-Rb3), ginsenoside Rg1 (G-Rg1), ginsenoside Rd (G-Rd), ginsenoside Rh2 (G-Rh2), notoginsenoside R1 (NG-R1), notoginsenoside R2 (NG-R2), notoginsenoside Fa (NG-Fa), notoginsenoside Fc (NG-Fc), notoginsenoside Fe (NG-Fe), notoginsenoside Fd (NG-Fd) and ginsenoside F1 (G-F1) were dissolved to 1.00 mg/ml as a standard solution with 70% (v/v) methanol, respectively. Ten polyphenols including catechin (C), gallic acid (GA), epicatechin (EC), 1,4,6-tri-O-galloyl-β-D-glucose (GG), epigallocahechin-3-gallate (EGCG), epicatechin-3-gallate (ECG), gallocatechin (GC), catechin gallate (CG), quercetin (Qc) and delphinidin (Dp) were prepared (1.00 mg/ml) as a standard solution in methanol, respectively. All the above chemicals were obtained from Chengdu Must Bio-technology Co. Ltd. (Chengdu, China), as reference standards (≥ 99% purity). Seventeen amino acid mixed standard reference with 0.1 nmol/μl were provided by Agilent Inc, including alanine (Ala), L-arginine (Arg), aspartic acid (Asp), cysteine (Cys), glutamic acid (Glu), glycine (Gly), histidine (His), isoleucine (Ile), leucine (Leu), serine (Ser), threonine (Thr), tyrosine (Tyr), valine (Val), methionine (Met), phenylalanine (Phi) and proline (Pro). Additionally, γ-aminobutyric acid (GABA) and dencichine (Dc) were dissolved in deionized water (1.00 mg/ml) as a standard solution. The commercial kits for in vitro antioxidant activity analysis, including scavenging activities (SCs) of 1,1-diphenyl-2-picrylhydrazyl (DPPH), 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonate) (ABTS⋅+) radicals, hydroxyl (OH–) radicals and superoxide anion (O2–) radicals were purchased from Grace Biotechnology Co. Ltd. (Suzhou, China). Acetonitrile (ACN), methanol, and formic acid for HPLC analysis were purchased from MREDA (MREDA Technology Inc., Columbia, TN, USA). The deionized water was prepared using an ultrapure water on-line resistivity monitor (Shanghai, China).
To give a high extraction rate and saponin content of SLPN, single factors, including ethanol concentration (v/v) (20–70%), material liquid ratio (m:V) (1:5–1:25) and extraction time (24, 36, 48, 60 and 72 h) were optimized. Then, a three-factor, three-level Box-Behnken design model (BBD) (Khatib et al. (21)) of ethanol concentration: –1 (30%), 0 (40%), +1 (50%), material liquid ratio (m:V): –1 (1:15), 0 (1:20), +1 (1:25) and extraction time: –1 (24 h), 0 (36 h), +1 (48 h) were examined using Design Expert 12.0.3.0 (Stat-Ease, USA) (Table 1). The design comprised 17 randomized runs with five replicates as the center points. The total saponin contents of SLPN-instant beverage were detected by vanillin-perchloric acid method (21), the extraction rate of SLPN in single factor experiment was calculated as Eq. 1:
M0 is the mass of SLPN, unit (g); M1 is the mass of the evaporating dish, unit (g); M2 is the total mass of the evaporating dish and SLPN instant beverage, unit (g); ω is the water content of SLPN, unit (%), and the moisture (ω) of SLPN is 10.12 ± 0.17%.
A pilot-scale experiment was further carried out in a custom-designed tank reactor (50 L), and SLPN-instant beverage was manufactured as follows: one kilogram of SLPN was extracted by optimal methods, and the extraction solution was concentrated to 1/3 volume using a vacuum rotatory evaporator at 45°C, and then spray dried at 110°C. Then a powdered SLPN-instant beverage was obtained.
Seventeen amino acids were analyzed by HPLC which was reported in our previous paper (22) The method is given briefly below: 1 g SLPN powder was extracted with 50 ml distilled water for 2 h at 80°C and filtered through filter paper, the SLPN-instant beverage was dissolved in 50 ml distilled water. Then, 1 ml of SLPN extract and SLPN-instant beverage solution were mixed with 200 μl chloroform (CHCl3) and centrifuged at 12,000 g for 10 min at 4°C. Then 800 μl of each supernatant was filtered through a 0.2 μm nylon filter prior to the HPLC analysis.
The vitamins and mineral elements were determined by the Sanshu Co. Ltd. (Nanjing, China). The pesticide and heavy metal residues were evaluated by the Institute of Product Quality Supervision and Inspection, Yunnan, China. Fourteen pesticide residues, including hexachlorobenzene (BHC), dichloro-diphenyl-trichloroethane (DDT) were measured according to the People’s Republic of China national standard GB/T 5009.19; carbendazim and dimethomorph were measured according to the People’s Republic of China national standard GB/T 20769; procymidone, myclobutanil, propiconazole and oxadixyl were measured according to the People’s Republic of China national standard GB 23200; thiophanate-methyl was measured according to the People’s Republic of China national standard NY/T 1680; cyhalothrin was measured according to the People’s Republic of China national standard GB/T 5009.146. Heavy metal residues including As, Pb, Cd, and Hg were measured by Inductively Coupled Plasma Mass Spectrometer according to the People’s Republic of China national standards (GB 5009.11, GB 5009.12, GB 5009.15, GB 5009.17).
The amount of total saponin was determined by the vanillin-perchloric acid method, which is based on a color reaction of the acid-hydrolysis products of the saponin (i.e., sapogenins) with vanillin (21). One gram of SLPN powder was extracted with 50 ml of 70% (v/v) methanol for 1.5 h at 85°C. Subsequently, the extract solution was filtered to obtain the SLPN total saponin solution. One gram of SLPN-instant beverage was dissolved in 50 ml of 70% (v/v) methanol as a sample solution. Triple replicates of each SLPN and SLPN-instant beverage sample were extracted twice. A volume of 50 μl prepared sample solution was transferred into a colorimetric tube, then the solvent was volatilized at 60°C in a water bath. Freshly prepared 1.0% vanillin-perchloric acid solution reagent (0.5 ml) was added, mixed and incubated for 15 min at 60°C. Then, 5.0 ml 77% sulfuric acid (v/v) was added after immersion in an ice-water bath for 2 min. The absorbance was monitored using a spectrophotometer (Tu-190, Shanghai, China) at 535 nm using G-Re as a standard, while 70% (v/v) methanol and vanillin-perchloric acid was used as blank control. A standard curve of y=0.2229x− 0.0141 (R2 = 0.9979) with a linearity range of 0.5–8.5 mg/g was calculated, where y is the content of G-Re (mg) and x is the absorbance value. The results were expressed as mg/g-DW.
The principal secondary metabolites were subjected to qualitative analysis using UPLC-MS and HPLC system equipped with an evaporative light scattering detector (ELSD) (Waters, Milford, USA) at the Institute of Radiation Medicine, Academy of Military Medical Sciences (Beijing, China). The mobile phase flow rate was 0.35 ml/min with gradient elution of H2O + 0.1% formic acid (A) and acetonitrile (B) using the following gradient program: 0–3 min, 10–25% B; 3–8 min, 25–30% B; 8 - 13 min, 30–33% B; 13–15 min, 33–34% B; 15–17 min, 34–40% B; 17–19 min, 40–46% B; 19–21 min, 46–52% B; 21–23 min, 52–58% B; 23–25 min, 58–64% B; 25–26 min, 64–95% B; 26–27 min, 95% B; 27.5–29 min, 10% B. The column temperature and the detection wavelength were set 40°C and 203 nm, respectively. The MS conditions are below: Waters Vion IMS-Q-TOF system, ionization mode: electrospray ionization (ESI) ESI– and ESI+, source temperature: 110°C, Desolvation temperature: 450°C, Desolvation gas flow: 850 L/h, capillary voltage: 2.5 kV (ESI–), 3.0 kV (ESI+), collision energy: low energy: 6 eV (ESI–), 6 eV (ESI+), high energy: 30–50 eV (ESI–), 40–65 eV (ESI+), collision gas: argon. The identification method of SLPN-instant beverage main components is mainly based on the molecular weight and error fragments of the UNIFI database as well as the reference related analytical literature. Identification of ginsenoside and polyphenol compounds were dependent on the primary and secondary MS data, annotated against an in-house database, or the matches of both retention times and MS data to some standard compounds in the UNIFI database.
Monomer saponins were quantitative analyzed by high-performance liquid chromatography (HPLC-1290 identify II) system equipped with a diode array detector (DAD), quaternary pump, column compartment and autosampler. A Poroshell EC-C18 chromatographic column (4.6 × 150 mm, 4 μm) (Agilent, USA) was adopted for the analyses. A gradient elution system consisted of ultra-pure water (A) and acetonitrile (B) using the following gradient program: 0–20 min, 20% B; 20–55 min, 20–36% B; 55–70 min, 36–45% B; 70–79 min, 45–60% B; 79–80 min, 80% B; 80–81 min, 80% B; 81.5–83 min, 20% B. The flow rate was set at 0.5 ml/min and the sample volume was set at 10 μl. The column temperature and the detection wavelength were set 30°C and 203 nm, respectively.
Polyphenols was measured on an HPLC system (Agilent 1200) according to the method described in our previous paper (23). The HPLC was equipped with a variable wavelength detector (VWD) and Poroshell 120 EC-C18 chromatographic column (4.6 × 100 mm, 2.7 μm, Agilent, USA). SLPN polyphenol extract preparation was as follows: SLPN samples were ground to a fine powder and passed through a 40-mesh sieve. SLPN sample (1 g) was extracted with 44 ml MeOH-hydrochloric acid (40:4 v/v) in a flask equipped with a reflux condenser, the extraction was performed for 90 min in an 85°C water bath. Then, SLPN polyphenol extract and SLPN-instant beverage were diluted to 50 ml. The diluted solutions were then filtered through a 0.45 μm nylon filter and immediately analyzed by HPLC. The optimal elution conditions were as follows: A (ultra-pure water + 5% ACN + 0.261% ortho-phosphoric acid), B (80% MeOH); 0–16 min, 10–45% B; 16–22 min, 45–65% B; 22–25.9 min, 100% B; 25.9–29.9 min, held at 100% B. The flow rate was 0.8 ml/min, and each sample was detected in triplicate.
The antioxidant activities of SLPN and SLPN-instant beverage were investigated in vitro, the methods were referenced by Wang et al reports (24). The antioxidant activity SLPN and SLPN-instant beverage were evaluated using the following assays: scavenging activities (SC) of DPPH, ABTS⋅+, OH–, and O2– radicals, Trolox solution was used as the positive control. These activities were determined using commercial kits (Grace Biotechnology Co. Ltd., Suzhou, China) according to the manufacturer’s instructions.
Data analysis was performed in SPSS 26.0 (IBM, New York, USA) and the results are expressed as mean ± standard deviation (SD). Single factors experiment and antioxidant activity results were analyzed and plotted with GraphPad prism 8.3.0 (GraphPad Prism, San Diego, California, USA). Response surface design and analysis of variance (ANOVA) were performed by Design Expert 12.0 software. The heat maps of the chemical components were plotted by TB-tools v 0.068 (25). Three replicates of each sample were extracted, and each extraction was analyzed twice. P < 0.05 was considered to be statistically significant.
Three-factor single experiments were used to optimize the extraction process. The extraction rates and total saponin contents of SLPN at 30% ethanol concentration (v/v) (Figure 1A), 1:20 material liquid ratios (m:V) (Figure 1B) and 36 h extraction time (Figure 1C) were significantly higher than the ones at other extract conditions, respectively (P < 0.05). The BBD model of ethanol concentrations A (30, 40, and 50%), material liquid ratio B (1:15, 1:20, 1:25) and extraction time C (24, 36 and 48 h) were further developed to optimize the extraction conditions, and consequently the extraction rate was increased from 31.49 to 41.60% (Table 1). According to the ANOVA analysis as shown in Table 2, alcohol concentration (A), secondary terms A2 and C2 showed highly significant effects on the extraction rate (P < 0.01), and the secondary term B2 also had a significant effect on the extraction rate (P < 0.05). In “lack of fit” analysis an F-value = 0.9043 and P-value = 0.18 indicates that the quadratic model data are suitable for representing the whole experimental data. The three-dimensional response surface plots and corresponding two-dimensional contour plots constructed by AB showed that the extraction rate of SLPN-instant beverage was increased when the ethanol concentration (A) increased from 20 to 48%, material liquid ratio (B) increased from 1:5 to 1:20. While the extraction rate decreased with the further increase in the two factors (Figures 2A1,A2). For the interaction between the ethanol concentration (A) and the extraction time (C), the extraction rate of SLPN-instant beverage was increased along with the extraction time increasing from 12 to 38 h. However, it decreased if the extraction time was continually extended (Figures 2B1,B2). Moreover, the extraction rate was highest when the material liquid ratio (B) approached 1:20 and the extraction time (C) was extended to 38 h (Figures 2C1,C2). These results indicated that the extraction rates were extremely affected by the ethanol concentration, material liquid ratio, and extraction time. Consequently, the regression equation of the extraction rate is fitted as below (Eq. 2).
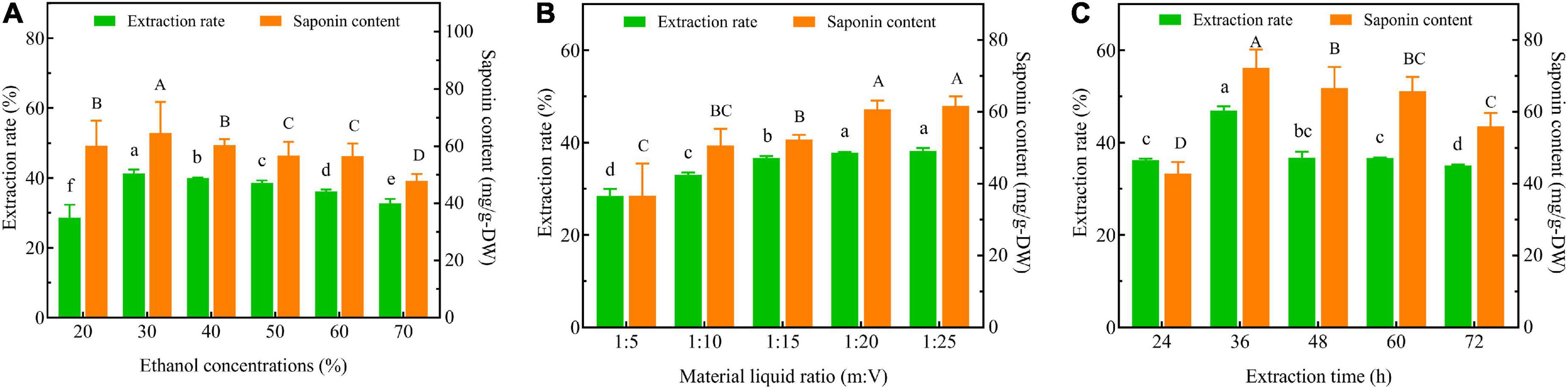
Figure 1. Extraction rate and total saponin content of SLPN-instant beverage optimized by single factor test. Extraction rate (%) as a function of (A) ethanol concentration (%), (B) material liquid ratio (m:V) and (C) extraction time (h) (Different letters indicate significant differences based on ANOVA post-hoc test at P < 0.05).
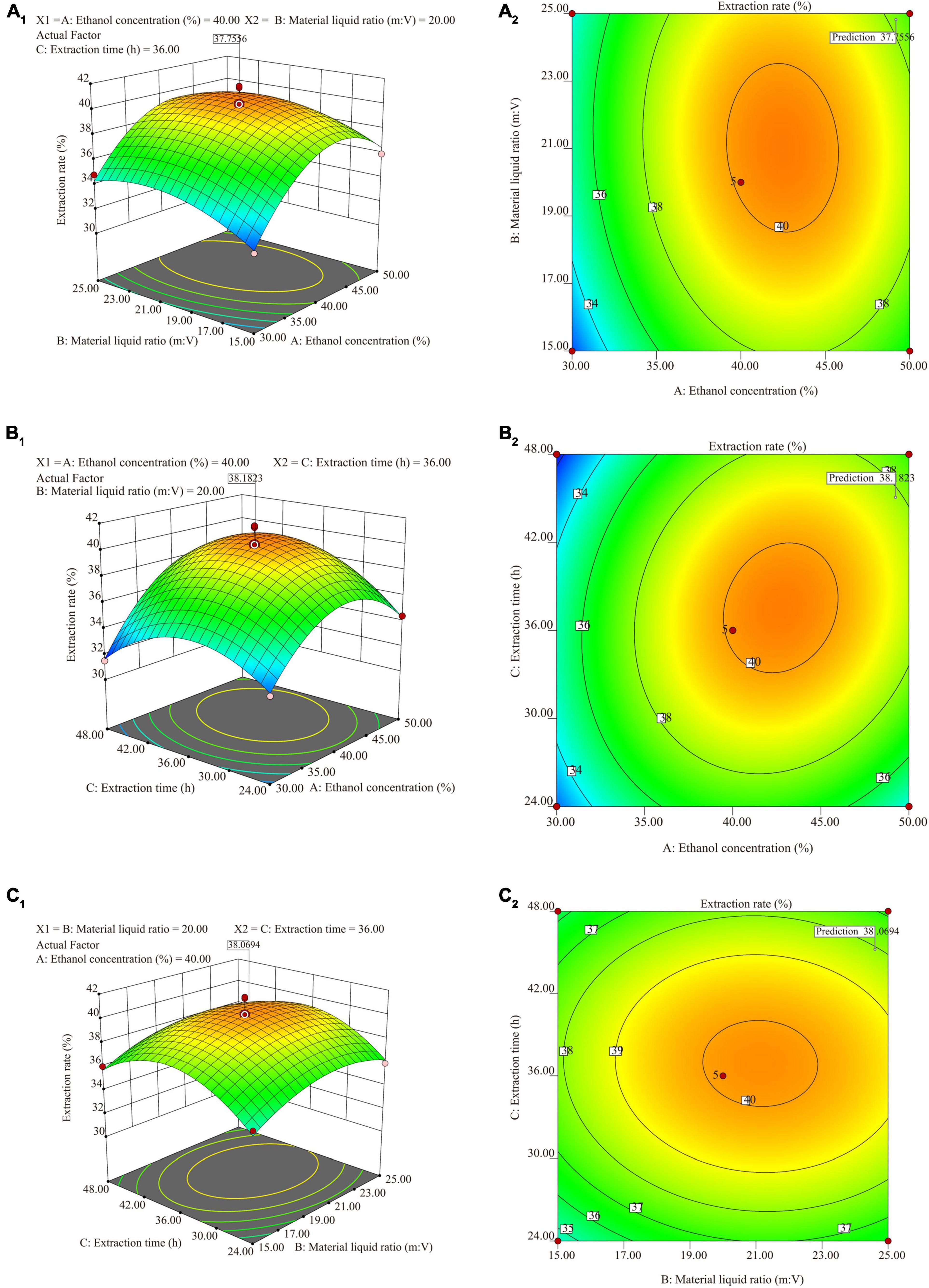
Figure 2. The three-dimensional curved surface graph and contour plots for ethanol concentration vs. material liquid ratio (m:V) (A1,A2); ethanol concentration vs. extraction time (B1,B2); material liquid ratio vs. extraction time (C1,C2).
Based on the desirability of reducing the sample consumption and reagents in the experiment, the optimal factors were obtained according to the BBD model while taking economic development and environmental protection requirements into consideration. The BBD model suggests that the maximum value of the extraction rate might reach 39.28% when the ethanol concentration (A) is 48.42% (w/v), material liquid ratio (B) is 1:20.64 (m:V) and extraction time (C) is 38.58 h, respectively. Triplicate experiments were used to verify the model’s reliability and accuracy, and consequently the extraction rate was 37.89 ± 0.02%. To further investigate the practical availability in industrial application, one kilogram of SLPN was extracted by the above-suggested method, and consequently 361.48 ± 4.57 g of SLPN-instant beverage was obtained, and the extraction yield was 36.15 ± 0.46%, being in good agreement with the predicted values. Organoleptic evaluation results showed that the SLPN-instant beverage was a fine powder with a yellow-green color (Figures 3A–C), and it showed good solubility in both cold and hot water (Figure 3D), and the taste of the solution was slightly bitter with a sweet aftertaste.

Figure 3. The manufacturing process of SLPN-instant beverage. (A) Fresh SLPN material; (B) SLPN material dried at 25°C shade-air; (C) SLPN-instant beverage; (D) infusion of SLPN-instant beverage.
We analyzed the pesticide and heavy metals residues in SLPN and SLPN-instant beverage, the results showed that the content of lead (Pb), cadmium (Cd), carbendazim, procymidone, and dimethomorph were 2.20 mg/kg, 0.94 mg/kg, 140 mg/kg, 4.48 mg/kg and 42.8 mg/kg in SLPN, respectively, were higher than the DBS 53 024–2017 standard limits (26). Interestingly, these contaminants were 0.11, 0.08 mg/kg, 0.47 mg/kg, 0.03 mg/kg and 0.19 mg/kg in the SLPN-instant beverage, respectively (Table 3), all being below the DBS 53 024–2017 limits. These results showed that our extraction method could reduce the pesticide and heavy metal residues in the instant beverage.
The nutritional components in SLPN and SLPN-instant beverage including amino acids, vitamins, and mineral elements were also analyzed, respectively. Fourteen amino acids were detected in SLPN and SLPN-instant beverage (Figure 4A). In SLPN, the contents of γ-aminobutyric acid were 6.5 ± 0.38 mg/g-DW, Asp 2.40 ± 0.10 mg/g-DW, Glu 2.74 ± 0.17 mg/g-DW and Gly 0.35 ± 0.06 mg/g-DW, whereas in SLPN-instant beverage, the contents of γ-aminobutyric acid were 7.50 ± 1.85 mg/g-DW, Gly 2.10 ± 0.63 mg/g-DW and His 1.23 ± 0.07 mg/g-DW, respectively. Additionally, ten mineral elements were determined, and potassium 49.26 ± 2.70 mg/g-DW, magnesium 6.12 ± 0.38 mg/g-DW and sodium 0.83 ± 0.02 mg/g-DW were enriched in SLPN-instant beverage, but calcium 6.73 ± 0.27 mg/g-DW and phosphorus 2.42 ± 0.07 mg/g-DW were relatively decreased (Figure 4B). Fifteen vitamins, including vitamin B, vitamin C, vitamin D, vitamin E were detected in SLPN. The contents of different monomer vitamins ranged from 0.01 μg/g-DW to 355.45 μg/g-DW, and the main components were α-VE 355.45 ± 25.75 μg/g-DW, γ-VE 148.28 ± 10.61 μg/g-DW, δ-VE 72.66 ± 5.73 μg/g-DW and VK 24.14 ± 0.41 μg/g-DW. Meanwhile, the contents of different monomer vitamins ranged from 0 to 18.89 μg/g-DW in SLPN-instant beverage and the main components were α-VE 18.89 ± 1.87 μg/g-DW, γ-VE 11.66 ± 1.24 μg/g-DW, δ-VE 17.53 ± 1.98 μg/g-DW, and VB5 10.19 ± 005 μg/g-DW, respectively (Figure 4C).
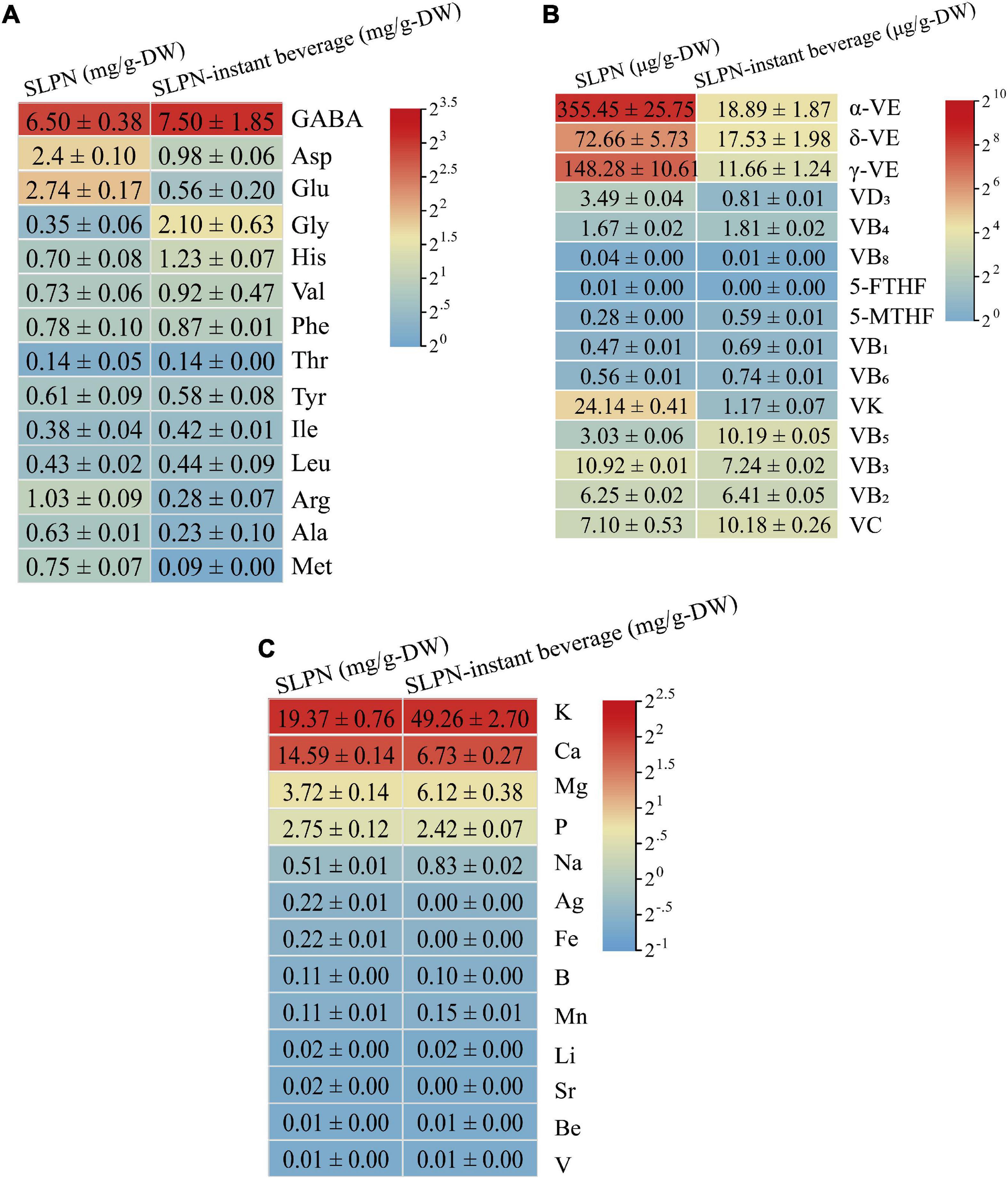
Figure 4. The contents of (A) fourteen amino acids (B) fifteen vitamins and (C) thirteen mineral elements in SLPN and SLPN-instant beverage.
The result of UV spectrophotometry showed that the total saponin in SLPN was 100.19 ± 10.08 mg/g-DW, while it reached 403.05 ± 34.98 mg/g-DW in SLPN-instant beverage. The content of total saponin in SLPN-instant beverage was increased about quadruple than that of SLPN raw material. Forty-three triterpenoid ginsenosides (e.g., ginsenoside La, G-Rb3, notoginsenoside D, NG-R1), and two flavonoid glycoside including quercetin-3-O-β-D-galactose (2→1) glucoside and kaempferol-3-O-β-D-galactose (2→1) glucoside, as well as dencichine were identified in SLPN by UPLC-MS, as showed in Supplementary Table 1 and Supplementary Figure 2. Additionally, the secondary metabolites of SLPN-instant beverage were tentatively identified by HPLC-ELSD, the results were shown in Table 4 and Supplementary Figure 2B. In our experiments, fourteen monomer ginsenosides were quantitatively analyzed by HPLC, the content of each monomer ginsenoside was between 0 and 63.3 mg/g-DW in SLPN, and the main ginsenosides were G-F1 63.30 ± 2.24 mg/g-DW, G-Rb3 22.08 ± 11.71 mg/g-DW, G-Rb2 16.9 ± 11.04 mg/g-DW, and G-Rb1 8.83 ± 4.57 mg/g-DW, respectively. The more abundant ginsenosides in the SLPN-instant beverage were NG-Fd 227.45 ± 2.02 mg/g-DW, NG-Fe 51.80 ± 2.33 mg/g-DW, G-Rb3 41.04 ± 2.48 mg/g-DW, and G-Rh2 1.64 ± 0.14 mg/g-DW (Figure 5A).
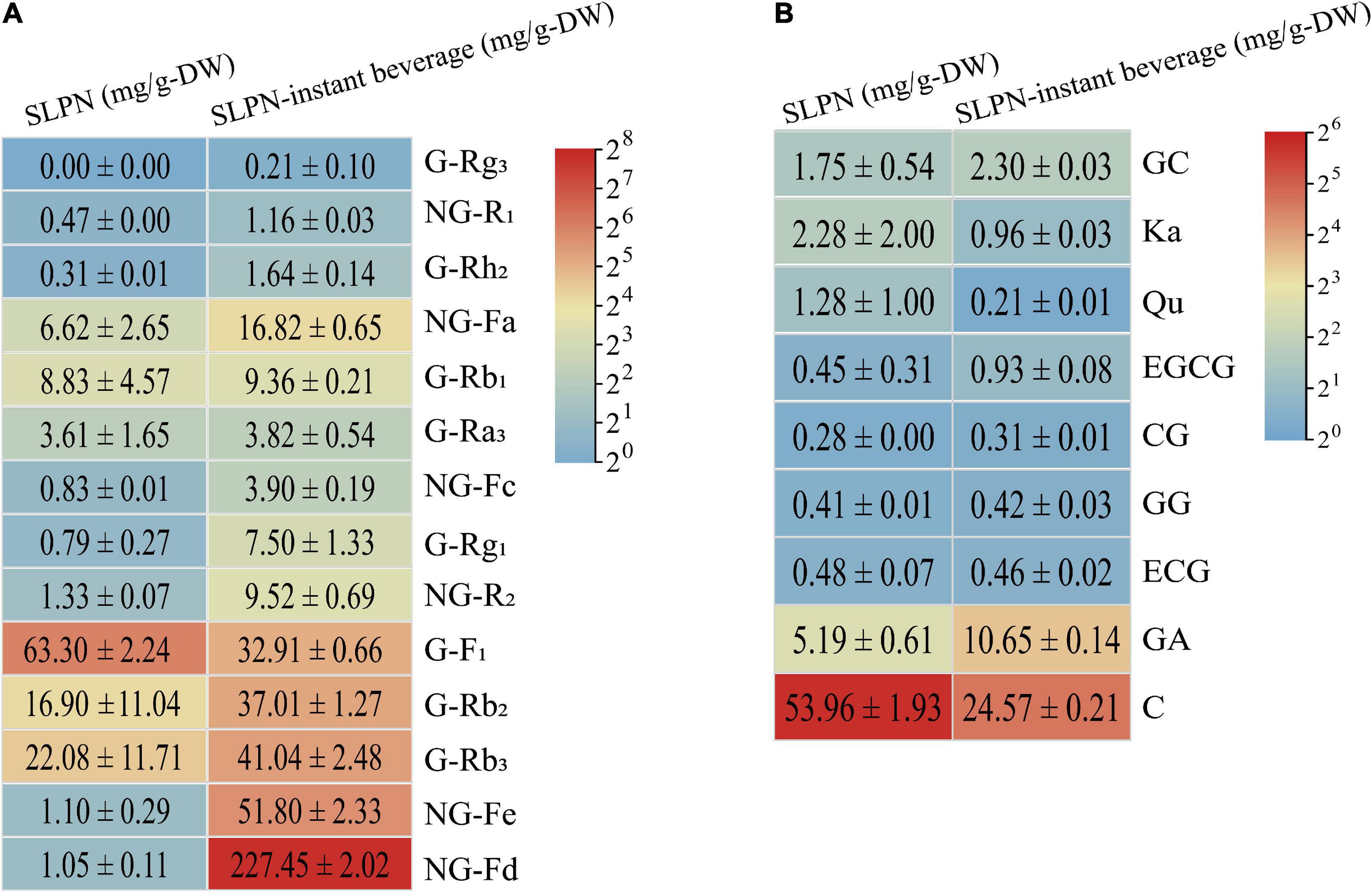
Figure 5. The contents of (A) fourteen saponin and (B) nine polyphenols in SLPN and SLPN-instant beverage.
Additionally, nine phenolic compounds, including catechin, GA, GC, CG etc., were detected in SLPN and its beverage (Figure 5B). The content of total polyphenol did not show a significant difference between SLPN and SLPN-instant beverage. In SLPN, the main phenolic compounds were catechin 53.96 ± 1.93 mg/g-DW, GA 5.19 ± 0.61 mg/g-DW, Ka 2.28 ± 2.00 mg/g-DW, GC 1.75 ± 0.54 mg/g-DW, and Qu 1.28 ± 1.00 mg/g-DW. After extraction, the contents of catechin 24.57 ± 0.21 mg/g-DW, ECG 0.46 ± 0.02 mg/g-DW, Qu 0.21 ± 0.01 mg/g-DW and Ka 0.96 ± 0.03 mg/g-DW were decreased in SLPN-instant beverage. While the level of GA 10.65 ± 0.14 mg/g-DW was increased in the SLPN-instant beverage.
According to the optimized extraction method, nutrients and secondary metabolites of SLPN are enriched in this experiment. SLPN-instant beverage is rich in nutrients and secondary metabolites, including saponins (G-Rb1, G-Rb3, NG-Fd), polyphenols (C, GA, GC), amino acids (Asp, Glu, Gly), and multivitamin etc. In vitro assays, SLPN-instant beverage showed good scavenging activities (SCs) for hydroxyl radicals (Figure 6A), superoxide anion radicals (Figure 6B), DPPH (Figure 6C), and ABTS⋅+ (Figure 6D) with dose-dependent behavior. The IC50 for SCs of OH– radicals, O2– radicals, DPPH radicals and ABTS+ radicals were 0.5826, 0.4966, 0.8331, and 0.6368 mg/ml, respectively (Figure 6E). While it was 0.1954, 0.2314, 0.4083, and 0.3874 mg/ml for SLPN-instant beverage to scavenge these radicals (Figure 6E). Previous reports indicated that Gypenoside IX (NG-Fd) isolated from SLPN significantly suppressed the nitric oxide production and inflammatory cytokines including tumor necrosis factor-α, interleukin 10, interferon-inducible protein 10 and interleukin-1β, shows good antioxidant stress and anti-inflammatory effects (27), ginsenoside Rb3 could decrease oxidative stress and protect endothelial function in hypertension (28).
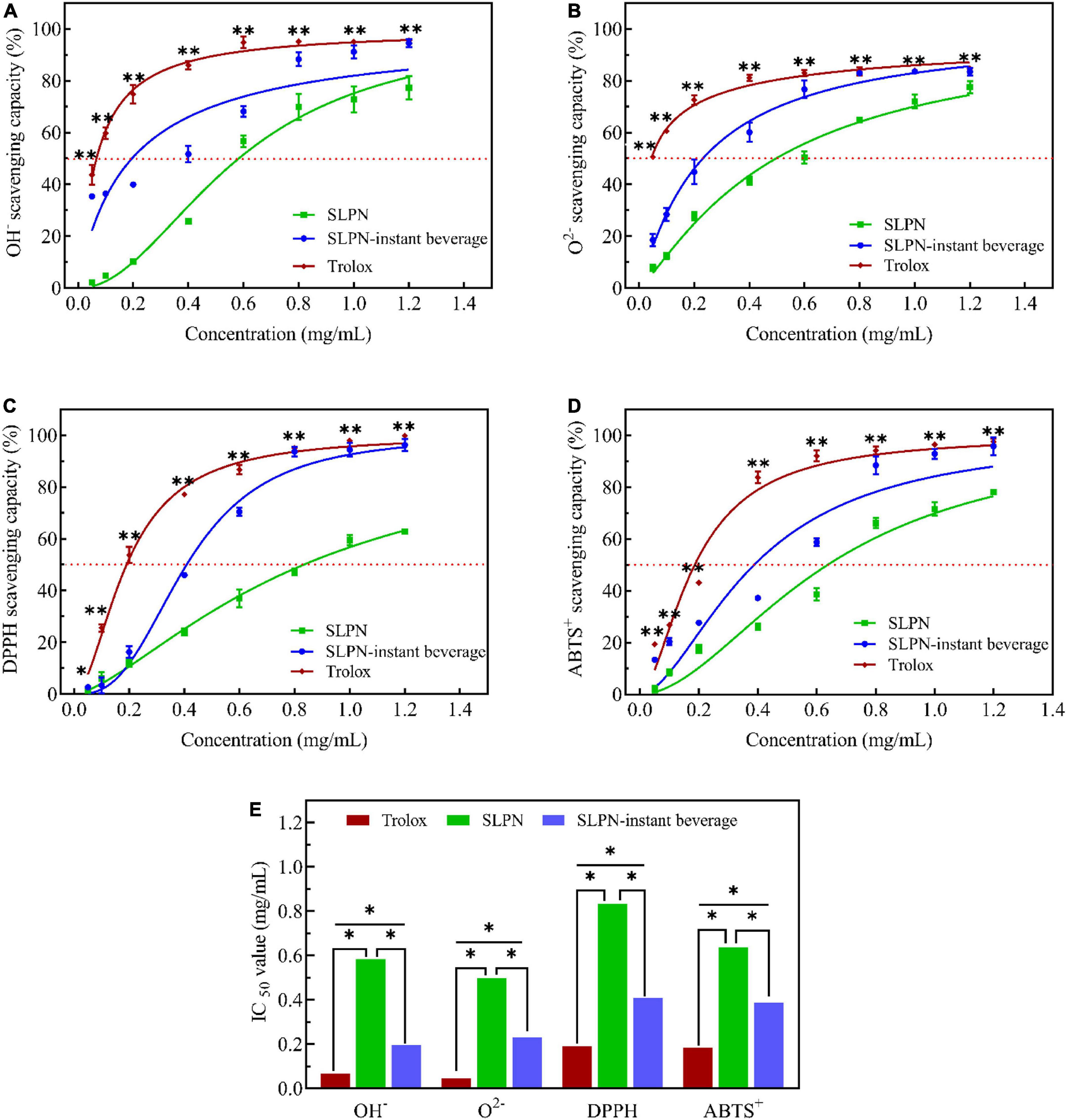
Figure 6. Scavenging activities of OH– radicals (A), O2– radicals (B), DPPH radicals (C), ABTS+ radicals (D), and IC50 (E) in SLPN and SLPN-instant beverage, Trolox as positive control. *Indicates a significant difference, **Indicates a very significant difference based on ANOVA post-hoc test (including t-test) at P < 0.05.
The extraction process could be optimized for maximizing the contents of functional components from ginger using a single factor test and a response surface BBD design (29). In a single factor test experiment, when the ethanol concentration was increased from 20 to 30%, the extraction rate was increased from 28.65 ± 3.77% to 41.26 ± 1.13% (Figure 1A). According to the principle of liquid-liquid mass transfer (30), the soluble components separate out from the extraction process, but it might be adsorbed on the SLPN interface with the increase in ethanol concentration that reduces the total mass transfer coefficient and the extraction rate of SLPN-instant beverage. The extraction rate of SLPN-instant beverage increases with the increase of the material liquid ratio (m:V). When the material liquid ratio was increased from 1:20 to 1:25, the extraction rate was increased from 37.82% ± 0.15% to 38.23% ± 0.64% (Figure 1B), indicating that the material liquid ratio 1:20 is suitable for the extraction process. In our study, when the extraction time exceeded 36 h, the extraction rate decreased rapidly. The solvent-terminated dispersive liquid-liquid microextraction model (31) has explained that with the prolonged extraction time, the ethanol extractant either floats or sinks depending on the density of aqueous SLPN as has been observed in our study (Figure 1C). Therefore, the bioextraction technology is a complex process, a single factor cannot completely investigate the best extraction process of SLPN-instant beverages, since it involves interacting multiple extraction factors (32).
In the response surface design experiment, when the ethanol concentration (A) was 30–50%, material liquid ratio (B) 1:15–1:20, extraction time (C) 36 h, the highest point of the extraction rate in 3D hook face reached 37.76% (Figures 2A1,A2). As shown in Table 2, low P-values (P < 0.01 and P < 0.5) of BBD model associated with high F-values (10.24 and 17.19) implied the significance of the model (33). The extraction condition of the present study is similar to that of the other study that polyphenols are extracted from P. quinquefolius L., and the extraction rate reached 48% (34). The interaction between (A) and (C) showed that when the ethanol concentration is 30–40%, material liquid ratio 1:20, extracted time 24–36 h, the highest extraction rate reaches 38.18% (Figures 2B1,B2). When the material liquid ratio was 1:15–1:20, extracted time 24–36 h, with 40% of ethanol concentration, the highest extraction rate reached 38.18% (Figures 2C1,C2). Consequently, the BBD model recommends that the optimal condition is 48.42% ethanol concentration, material liquid ratio 1:20.64, and extraction time 38.58 h. To facilitate the experimental operation, we have made a minor adjustment, that is, 48.50% of ethanol concentration, material to liquid 1:21, extracted time 39 h, and repeated twice. The extraction rate under the interaction of the three factors can reach 37.89 ± 0.02%. In our previous work, the extraction yield of P. notoginseng flowers is 46.63 ± 0.81%, being higher than the extract yield of SLPN-instant beverage based on the adjusted method. Furthermore, a pilot-scale experiment was further carried out in a custom-designed tank reactor (50 L), one kilogram of SLPN was extracted by the adjusted method, and consequently 361.48 ± 4.57 g-DW of SLPN-instant beverage was obtained, and the extraction yield was 36.15 ± 0.46% (unpublished data). Thus, our experiment optimized a stable extraction method from a response surface model to manufacture SLPN-instant beverage (Figures 3A–D).
It has been reported that SLPN contain amino acids, vitamins, polysaccharides, and other nutrients (9, 35). The total content of eighteen free amino acids in SLPN is 0.344% (36). In our experiment, fourteen key amino acids in SLPN and SLPN-instant beverage were detected by HPLC, and the main amino acids in SLPN-instant beverage were γ-aminobutyric acid, Gly, Asp, Val and His (Figure 4A). In many plant-derived foods, γ-aminobutyric acid acts as a main inhibitory neurotransmitter in the central nervous system (37). Thus, SLPN and SLPN-instant beverage might improve anti-fatigue and extend the sleeping function (38). Vitamins are one of the important parts in food nutrition, and in our study fifteen vitamins were detected in SLPN and SLPN-instant beverage. Unfortunately, the main components in SLPN-instant beverage such as α-VE, γ-VE, δ-VE, and VB had not been enriched in our experiments (Figure 4B). Ten mineral elements including potassium, magnesium and sodium were enriched in the SLPN-instant beverage (Figure 4C). We found that the content of pesticides and heavy metal residues in the SLPN-instant beverage conformed to the limit of the food standard DBS 53 024–2017. Therefore, SLPN-instant beverage is safe and edible, and it can be used as one of the supplementary of nutrition sources for human body.
Saponin is the most important secondary metabolites in plants of the genus Panax (39). In our experiment, the total saponin of SLPN was 100.19 ± 10.08 mg/g-DW, within the reported scope of saponin content ranging from 60 to 100 mg/g-DW (40, 41). While the total saponin content of SLPN-instant beverage was 403.05 ± 34.98 mg/g-DW, it increased nearly four times as compared with that of SLPN. Total saponin of SLPN show multiple pharmacological activities, e.g., cardioprotective (42), hepatoprotective (43), anti-inflammatory (8) and inhibition of depression effects (44). The total saponin of SLPN could activate the PI3K/Akt/mTOR signaling pathway to attenuate excessive autophagy and apoptosis in myocardial cells in heart tissue induced by sleep deprivation (42). Furthermore, fermented P. notoginseng leaves could increase the content of secondary metabolites, and show a good antifatigue effect in vivo and in vitro (45), and the ethanol-induced metabolic perturbations are restored when treated by SLPN saponin (42). In our experiment, forty-three triterpenoid ginsenosides were identified in SLPN by UPLC-MS (Supplementary Table 1, Supplementary Figure 2A). G-Rb3, G-Rc, G-Rb2, G-Rb1, and NG-Fd have been regarded as the most important ginsenoside compounds in P. notoginseng leaves (46). While, in the SLPN-instant beverage, the principal components were NG-Fa, notoginsenoside-Fc/FP2, malonylfloralginsenosides Rc1/2/3/4, and gypenoside IX (GP-IX)/notoginsenoside Fe/notoginsenoside Ft1 (Table 4, Supplementary Figure 2B).
The most abundant secondary metabolite was NG-Fd at 1.05 ± 0.11 mg/g-DW, 227.45 ± 2.02 mg/g-DW in SLPN and SLPN-instant beverage, respectively (Figure 5A). Recent research has shown that 30 mg of refined SLPN extract could produce 8.4 mg of NG-Fd with a purity of 87.3% (47). NG-Fd might suppress reactive astrogliosis to treat neuroinflammatory disorders (48) and show a moderate anti-inflammatory effect (8). Therefore, NG-Fd could be served as an important pharmacological active saponin in the SLPN. Although the content of NG-Fe was 1.10 ± 0.29 mg/g-DW in SLPN, it was 51.80 ± 2.33 mg/g-DW in the SLPN-instant beverage (Figure 5A). NG-Fe could decrease food intake and body weight, protect liver structure integrity and normal function, as well as promote a resting metabolic rate in C57BL/6 mice (49). Also, NG-Fe is one of the key components to improve chronic obstructive pulmonary disease (50). G-Rb3 is one of the most important components in the SLPN and its content is 6.66–29.85 mg/g (51). In our experiment, 22.08 ± 11.71 mg/g-DW and 41.04 ± 2.48 mg/g-DW of G-Rb3 was detected in SLPN and the SLPN-instant beverage, respectively (Figure 5A). G-Rb3 might inhibit pro-inflammatory cytokines (52), suppress myocardial fibrosis (53), provide protective effects against cisplatin-induced nephrotoxicity (54), decrease oxidative stress and protect endothelial function in hypertension (28) and alleviate smoke-induced lung injury (55). Furthermore, in our study the content of minor ginsenoside Rh2 in the SLPN-instant beverage was 1.64 ± 0.14 mg/g-DW, being five times higher than that in SLPN. G-Rh2 could inhibit colorectal cancer cell growth (56), suppress the proliferation of A549 cells (57), breast cancer cell growth (58), and moreover the combination of G-Rh2 could induce lung cancer (59). Up to date, G-Rh2 is being developed as a new antitumor drug. Overall, SLPN-instant beverage might qualify a potential application in functional foods as it is rich in total saponin and polyphenols.
Medicinal plants P. notoginseng and SLPN are rich in secondary metabolites, especially saponins and polyphenols. In our experimental results, the SLPN-instant beverage is rich in G-Rb1, G-Rb3, NG-Fd, catechin, gallic acid, gallocatechin, multiple amino acids (Asp, Glu, Gly) and vitamins. SLPN flavonoid extract reveals a good antioxidant potential in vitro assay, the IC50 for O2– at 2.89 mg/ml, DPPH at 7.21 mg/ml and ABTS+ at 2.95 mg/ml (60). In our study, the IC50 for SLPN to OH– radicals was at 0.5826 mg/mL, O2– radicals at 0.4966 mg/mL, DPPH radicals at 0.8331 mg/mL and ABTS+ radicals at 0.6368 mg/mL, respectively. While the IC50 for SLPN-instant beverage to scavenge these radicals was 0.1954 mg/mL, 0.2314 mg/mL, 0.4083 mg/mL, 0.3874 mg/mL (Figure 6E). Thus, the antioxidant activity of the SLPN-instant beverage is better than that of SLPN aqueous extract. Saponins and polyphenols have been largely used in various pharmaceutical and food industries (61). It has been demonstrated that 5 μg/mL of P. notoginseng leaves total saponin could reduce cell death induced by H2O2 (62). Therefore, the SLPN-instant beverage manufactured from SLPN is rich in total saponins, polyphenols, ginsenosides and nutrition, shows a good antioxidation and might be neuroprotective in neurological disorders. Previous reports indicated that fermented SLPN increased liver glycogen and serum lactate dehydrogenase activity, decreased blood urea nitrogen, lactate acid, and malondialdehyde in mice, showed a good antifatigue effect and in vivo (45). SLPN saponins could inhibit abnormal autophagy and produce cardioprotective effects in mice (42). Thus, in future studies, we will take the main function of the SLPN-instant beverage as a research goal, and deeply explore the key target in vivo.
A SLPN-instant beverage has been manufactured from the food resources SLPN according to a single factor test and a response surface design, and the extraction rate reach 37.89 ± 0.02%. The total saponin in the SLPN-instant beverage is four times greater than that in SLPN. The SLPN-instant beverage is rich in ginsenosides, polyphenols, amino acids, vitamins, and mineral elements, and it shows a good antioxidant activity in vitro. Our experiments provide a simple, reliable, low cost and environmentally friendly technology to improve the utilization rate of the medicinal crops of P. notoginseng.
The original contributions presented in this study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
JC and MZ: conceiving and designing the project. ZL and KL: analyzing the data and writing the manuscript. BM: identification of chemical composition. GZ: funding acquisition. SY and YZ: providing laboratory platform. All authors contributed to the article and approved the submitted version.
This work was supported by the National Key Research and Development Plan of China (2021YFD1601003), National Natural Science Foundation of China (32160248 and 81860676), the Science and Technology Planning Project of Yunnan Science and Technology Department of China (202002AA1000051), and the Major Special Science and Technology Project of Yunnan Province (202102AA310048). Innovative Research Team of Science and Technology in Yunnan Province (202105AE160016) and the Independent Research Fund of Yunnan Characteristic Plant Extraction Laboratory (2022YKZY001).
We are grateful to all the staff, free detection service provided by Institute of Radiation Medicine, Academy of Military Medical Sciences, Beijing, and Yang Shi contributed to the photographs in this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2022.1058639/full#supplementary-material
1. Wang T, Guo R, Zhou G, Zhou X, Kou Z, Sui F, et al. Traditional uses, botany, phytochemistry, pharmacology and toxicology of Panax notoginseng (Burk.) F.H. Chen: a review. J Ethnopharmacol. (2016) 188:234–58. doi: 10.1016/j.jep.2016.05.005
2. China National Drug Administration. 17th G20 Summit and Delivers an Important Speech. Beijing: China National Drug Administration (2022).
3. Hu Y, Cui X, Zhang Z, Chen L, Zhang Y, Wang C, et al. Optimisation of ethanol-reflux extraction of saponins from steamed Panax notoginseng by response surface methodology and evaluation of hematopoiesis effect. Molecules. (2018) 23:1206. doi: 10.3390/molecules23051206
4. Li CY, Zhang J, Zhang P, Xue YF, Li Y, Chen C. Study on area of Panax notoginseng in Wenshan prefecture of Yunnan province based on sentinel-2. J Yunnan Univ. (2022) 44:89–97. doi: 10.7540/j.ynu.20200432
5. Weiheng A The Industry Data Analysis Report of China’s Panax notoginseng In 2020. (2022). Available online at: https://www.weihengag.com/home/data/productdetail/id/122/doc_id/7347.html
6. Zhang F, Tang S, Zhao L, Yang X, Yao Y, Hou Z, et al. Stem-leaves of Panax as a rich and sustainable source of less-polar ginsenosides: comparison of ginsenosides from Panax ginseng, American ginseng and Panax notoginseng prepared by heating and acid treatment. J Ginseng Res. (2021) 45:163–75. doi: 10.1016/j.jgr.2020.01.003
7. Cai S, Lin J. Forgotten treasure -Panax notoginseng stems and leaves. Pharm Inform. (2018) 7:141–3. doi: 10.12677/PI.2018.76023
8. Li J, Wang RF, Zhou Y, Hu HJ, Yang YB, Yang L, et al. Dammarane-type triterpene oligoglycosides from the leaves and stems of Panax notoginseng and their anti-inflammatory activities. J Ginseng Res. (2019) 43:377–84. doi: 10.1016/j.jgr.2017.11.008
9. Qu Y, Liu Y, Huang L, Guo L, Cui X. Analysis and evaluation of nutritive elements in aerial part of Panax notoginseng. Zhongguo Zhong Yao Za Zhi. (2014) 39:601–5. doi: 10.4268/cjcmm20140408
10. Liu F, Ma N, He C, Hu Y, Li P, Chen M, et al. Qualitative and quantitative analysis of the saponins in Panax notoginseng leaves using ultra-performance liquid chromatography coupled with time-of-flight tandem mass spectrometry and high performance liquid chromatography coupled with UV detector. J Ginseng Res. (2018) 42:149–57. doi: 10.1016/j.jgr.2017.01.007
11. Cao JL, Ma LJ, Wang SP, Deng Y, Wang YT, Li P, et al. Comprehensively qualitative and quantitative analysis of ginsenosides in Panax notoginseng leaves by online two-dimensional liquid chromatography coupled to hybrid linear ion trap orbitrap mass spectrometry with deeply optimized dilution and modulation system. Anal Chim Acta. (2019) 1079:237–51. doi: 10.1016/j.aca.2019.06.040
12. Wang DM, Yu HS, Song JG, Xu YF, Liu CY, Jin FX. A novel ginsenosidase from an Aspergillus strain hydrolyzing 6-o-multi-glycosides of Protopanaxatriol-type ginsenosides, named ginsenosidase type iv. J Microbiol Biotechn. (2011) 21:1057–63. doi: 10.4014/jmb.1101.01044
13. Liu Y Research and Development of the Aerial Part of Panax Notoginseng as New Resource Food. Kunming: Kunming University of Science and Technology (2015).
14. Yang G, Cui XM, Chen M, Qu Y, Huang LQ. Research on using stems and leaves of Panax notoginseng and flowers of Panax notoginseng as new food ingredients. J Chin Pharm Sci. (2017) 52:543–7. doi: 10.11669/cpj.2017.07.00
15. Ma LJ, Ma N, Cao JL, Wan JB. Characterizing the influence of different drying methods on chemical components of Panax notoginseng leaves by heart-cutting two-dimensional liquid chromatography coupled to orbitrap high-resolution mass spectrometry. Food Chem. (2022) 369:130965. doi: 10.1016/j.foodchem.2021.130965
16. Ma D, Wang J, Yin G, Wang L, Jin Y, Huang Y, et al. The study of steaming durations and temperatures on the chemical characterization, neuroprotective, and antioxidant activities of Panax notoginseng. Evid Based Complement Alternat Med. (2022) 2022:3698518. doi: 10.1155/2022/3698518
17. Cai SX, Zou JL, Xu YY, Wang DQ. Optimization of enzymatic hydrolysis process of Panax notoginseng stem-leaf tea by response surface methodology. Sichuan Food Ferment. (2021) 57:108–14. doi: 10.3969/j.issn.1674-506X.2021.02-016
18. Wang HY, Guo DM, Yu WP, Zhu Z, Kong QL, You Y. Development of pressed candy by stems and leaves of Panax notoginseng. J Xi Hua Univ. (2019) 38:69–72.
19. Lin LC, Lin XZ, Zhou XJ. Rapid determination of ginsenoside Rb3 in Qiyeshen’an tablets by HPLC. Chem Anal Meterage. (2019) 28:66–9. doi: 10.3969/j.issn.1008-6145.2019.06.015
20. Tian H, Xie X, Wu X, Shi P, Liu X, Pan J, et al. Effects of differentextraction methods on the physicochemical properties and anti-inflammation activity of polysaccharides of Panax notoginseng leaves. Proceedings of the Second Natural Materials Research and Application Seminar in 2019. (2019). doi: 10.26914/c.cnkihy.2019.049236
21. Khatib I, Chow M, Ruan J, Cipolla D, Chan HK. Modeling of a spray drying method to produce ciprofloxacin nanocrystals inside the liposomes utilizing a response surface methodology: Box-Behnken experimental design. Int J Pharmaceut. (2021) 597:120277. doi: 10.1016/j.ijpharm.2021.120277
22. Zhao M, Fan J, Liu Q, Luo H, Tang Q, Li C, et al. Phytochemical profiles of edible flowers of medicinal plants of Dendrobium officinale and Dendrobium devonianum. Food Sci Nutr. (2021) 9:6575–86. doi: 10.1002/fsn3.2602
23. Nian B, Chen L, Yi C, Shi X, Jiang B, Jiao W, et al. A high performance liquid chromatography method for simultaneous detection of 20 bioactive components in tea extracts. Electrophoresis. (2019) 40:2837–44. doi: 10.1002/elps.201900154
24. Wang Z, Hwang SH, Guillen QY, Gonzales AP, Lim SS. Investigation of the antioxidant and aldose reductase inhibitory activities of extracts from Peruvian tea plant infusions. Food Chem. (2017) 231:222–30. doi: 10.1016/j.foodchem.2017.03.107
25. Chen CJ, Chen H, Zhang Y, Thomas HR, Frank MH, He YH, et al. TBtools: an integrative toolkit developed for interactive analyses of big biological data. Mol Plant. (2020) 13:1194–202. doi: 10.1016/j.molp.2020.06.009
26. Yunnan Provincial Health and Commission. Local Food Safety Standard – Dried Stems and Leaves of Panax Notoginseng. Kunming: Yunnan Provincial Health and Commission (2017). p. 1–7.
27. Li J, Ru-Feng W, Yue Z, Hai-Jun H, Ying-Bo Y, Li Y, et al. Dammarane-type triterpene oligoglycosides from the leaves and stems of Panax notoginseng and their antiinflammatory activities. J Ginseng Res. (2019) 43:377–84.
28. Wang Y, Dong J, Liu P, Lau CW, Gao Z, Zhou D, et al. Ginsenoside Rb3 attenuates oxidative stress and preserves endothelial function in renal arteries from hypertensive rats. Br J Pharmacol. (2014) 171:3171–81. doi: 10.1111/bph.12660
29. Cha J, Kim CT, Cho YJ. Optimizing extraction conditions for functional compounds from ginger (Zingiber officinale roscoe) using response surface methodology. Food Sci Biotechnol. (2020) 29:379–85. doi: 10.1007/s10068-019-00667-9
30. Pursell MR, Mendes-Tatsis MA, Stuckey DC. Effect of fermentation broth and biosurfactants on mass transfer during liquid-liquid extraction. Biotechnol Bioeng. (2004) 85:155–65. doi: 10.1002/bit.10840
31. Mansour FR, Danielson ND. Solvent-terminated dispersive liquid-liquid microextraction: a tutorial. Anal Chim Acta. (2018) 1016:1–11. doi: 10.1016/j.aca.2018.02.005
32. Liu Q, Sun B, Huo Y, Liu M, Shi J, Jiang T, et al. Nutrient bioextraction and microalgae growth inhibition using submerged macrophyte Myriophyllum spicatum in a low salinity area of east China sea. Mar Pollut Bull. (2018) 127:67–72. doi: 10.1016/j.marpolbul.2017.11.031
33. Wang Z, Zhang Y, Yan H. In situ net fishing of α-glucosidase inhibitors from evening primrose (Oenothera biennis) defatted seeds by combination of LC-MS/MS, molecular networking, affinity-based ultrafiltration, and molecular docking. Food Funct. (2022) 13:2545–58. doi: 10.1039/d1fo03975j
34. Zhu S. Optimization of extraction of polyphenol from Panax quinquefolius L. Using central composite design/response surface methodology. Starch Stärke. (2021) 73:2100020. doi: 10.1002/star.202100020
35. Liang ZW, Pan JQ, Chen JW, Ma BP, Ma Y, Zhang GH, et al. Extraction process optimization and analysis of active components from Panax notoginseng flowers. Food Sci Tech Brazil. (2021) 46:184–91.
36. Yang F, Chen B, Jiang M, Wang H, Hu Y, Wang H, et al. Integrating enhanced profiling and chemometrics to unveil the potential markers for differentiating among the leaves of Panax ginseng, P. quinquefolius, and P. notoginseng by ultra-high performance liquid chromatography/ion mobility-quadrupole time-of-flight mass spectrometry. Molecules. (2022) 27:5549. doi: 10.3390/molecules27175549
37. Gao M, Cao X, Wei S, Huang X, Ouyang H, Chang Y, et al. Quantitative comparison and chemical profile of different botanical parts of Panax notoginseng from different regions. Front Nutr. (2022) 9:841541. doi: 10.3389/fnut.2022.841541
38. Ngo DH, Vo TS. An updated review on pharmaceutical properties of gamma-aminobutyric acid. Molecules. (2019) 24:2678. doi: 10.3390/molecules24152678
39. Yang W, Qiao X, Li K, Fan J, Bo T, Guo DA, et al. Identification and differentiation of Panax ginseng, Panax quinquefolium, and Panax notoginseng by monitoring multiple diagnostic chemical markers. Acta Pharm Sin B. (2016) 6:568–75. doi: 10.1016/j.apsb.2016.05.005
40. Jae L, Bo-Ram C, Young-Chang K, Doo C, Young-Seob L, Geum-Soog K, et al. Comprehensive profiling and quantification of ginsenosides in the root, stem, leaf, and berry of Panax ginseng by UPLC-QTOF/MS. Molecules. (2017) 22:2147. doi: 10.3390/molecules22122147
41. Wan JB, Yang FQ, Li SP, Wang YT, Cui XM. Chemical characteristics for different parts of Panax notoginseng using pressurized liquid extraction and HPLC-ELSD. J Pharmaceut Biomed. (2006) 41:1596–601. doi: 10.1016/j.jpba.2006.01.058
42. Cao Y, Li QL, Yang YB, Ke ZJ, Chen SQ, Li MR, et al. Cardioprotective effect of stem-leaf saponins from Panax notoginseng on mice with sleep derivation by inhibiting abnormal autophagy through PI3K/AKT/MTOR pathway. Front Cardiovasc Med. (2021) 8:694219. doi: 10.3389/fcvm.2021.694219
43. Liu F, Wang M, Wang Y, Cao YW, Sun ZL, Chen MC, et al. Metabonomics study on the hepatoprotective effect of Panax notoginseng leaf saponins using UPLC/Q-TOF-MS analysis. Am J Chin Med. (2019) 47:559–75. doi: 10.1142/S0192415X19500290
44. Zhang H, Chen Z, Zhong Z, Gong W, Li J. Total saponins from the leaves of Panax notoginseng inhibit depression on mouse chronic unpredictable mild stress model by regulating circRNA expression. Brain Behav. (2018) 8:e1127. doi: 10.1002/brb3.1127
45. Yang M, Tao L, Zhao CC, Wang ZL, Yu ZJ, Zhou W, et al. Antifatigue effect of Panax notoginseng leaves fermented with microorganisms: in-vitro and in-vivo evaluation. Front Nutr. (2022) 9:824525. doi: 10.3389/fnut.2022.824525
46. Kim SA, Jeong EB, Oh DK. Complete bioconversion of protopanaxadiol-type ginsenosides to compound K by extracellular enzymes from the isolated strain Aspergillus tubingensis. J Agr Food Chem. (2021) 69:315–24. doi: 10.1021/acs.jafc.0c07424
47. Sun H, Ma LJ, Wan JB, Tong S. Preparative separation of Gypenoside XVII, ginsenoside Rd2, and notoginsenosides Fe and Fd from Panax notoginseng leaves by countercurrent chromatography and orthogonality evaluation for their separation. J Sep Sci. (2021) 44:2996–3003. doi: 10.1002/jssc.202100078
48. Wang X, Yang L, Yang L, Xing F, Yang H, Qin L, et al. Gypenoside ix suppresses P38 MAPK/AKT/NF-κB signaling pathway activation and inflammatory responses in astrocytes stimulated by proinflammatory mediators. Inflammation. (2017) 40:2137–50. doi: 10.1007/s10753-017-0654-x
49. Li H, Liu Y, Liu C, Luo L, Yao Y, Li F, et al. Notoginsenoside Fe suppresses diet induced obesity and activates paraventricular hypothalamic neurons. Rsc Adv. (2019) 9:1290–8. doi: 10.1039/c8ra07842d
50. Lin H, Wang C, Yu H, Liu Y, Tan L, He S, et al. Protective effect of total saponins from American ginseng against cigarette smoke-induced COPD in mice based on integrated metabolomics and network pharmacology. Biomed Pharmacother. (2022) 149:112823. doi: 10.1016/j.biopha.2022.112823
51. Li W, Yang X, Chen B, Zhao D, Wang H, Sun M, et al. Ultra-high performance liquid chromatography/ion mobility time-of-flight mass spectrometry-based untargeted metabolomics combined with quantitative assay unveiled the metabolic difference among the root, leaf, and flower bud of Panax notoginseng. Arab J Chem. (2021) 14:103409. doi: 10.1016/j.arabjc.2021.103409
52. Sun M, Ji Y, Li Z, Chen R, Zhou S, Liu C, et al. Ginsenoside Rb3 inhibits pro-inflammatory cytokines via MAPK/AKT/NF-κB pathways and attenuates rat alveolar bone resorption in response to porphyromonas gingivalis LPS. Molecules. (2020) 25:4815. doi: 10.3390/molecules25204815
53. Zhang Y, Ji H, Qiao O, Li Z, Pecoraro L, Zhang X, et al. Nanoparticle conjugation of ginsenoside Rb3 inhibits myocardial fibrosis by regulating PPAR-α pathway. Biomed Pharmacother. (2021) 139:111630. doi: 10.1016/j.biopha.2021.111630
54. Xing JJ, Hou JG, Ma ZN, Wang Z, Ren S, Wang YP, et al. Ginsenoside Rb3 provides protective effects against cisplatin-induced nephrotoxicity via regulation of AMPK-/MTOR-mediated autophagy and inhibition of apoptosis in vitro and in vivo. Cell Prolif. (2019) 52:e12627. doi: 10.1111/cpr.12627
55. Tan Y, Sun D, Chen J, Li R, Wang S. Ginsenoside Rb3 alleviates smoke-induced lung injury via the H19/mir-29B-3P/HGMB1/TLR4 signaling pathway. J Cell Mol Med. (2021) 25:2725–9. doi: 10.1111/jcmm.15844
56. Zhang H, Yi JK, Huang H, Park S, Kwon W, Kim E, et al. 20 (s)-ginsenoside Rh2 inhibits colorectal cancer cell growth by suppressing the AXL signaling pathway in vitro and in vivo. J Ginseng Res. (2022) 46:396–407. doi: 10.1016/j.jgr.2021.07.004
57. Song C, Yuan Y, Zhou J, He Z, Hu Y, Xie Y, et al. Network pharmacology-based prediction and verification of ginsenoside Rh2-induced apoptosis of A549 cells via the PI3K/AKT pathway. Front Pharmacol. (2022) 13:878937. doi: 10.3389/fphar.2022.878937
58. Peng K, Luo T, Li J, Huang J, Dong Z, Liu J, et al. Ginsenoside Rh2 inhibits breast cancer cell growth viaERβ-TNF-α pathway. Acta Biochim Biophys Sin. (2022) 54:647–56. doi: 10.3724/abbs.2022039
59. Su MX, Xu YL, Jiang XM, Huang MY, Zhang LL, Yuan LW, et al. c-MYC-mediated TRIB3/P62+ aggresomes accumulation triggers paraptosis upon the combination of everolimus and ginsenoside Rh2. Acta Pharm Sin B. (2022) 12:1240–53. doi: 10.1016/j.apsb.2021.09.014
60. Dai CY, Liu PF, Liao PR, Qu Y, Wang CX, Yang Y, et al. Optimization of flavonoids extraction process in Panax notoginseng stem leaf and a study of antioxidant activity and its effects on mouse melanoma B16 cells. Molecules. (2018) 23:2219. doi: 10.3390/molecules23092219
61. Elboughdiri N, Ghernaout D, Kriaa K, Jamoussi B. Enhancing the extraction of phenolic compounds from juniper berries using the Box-Behnken design. ACS Omega. (2020) 5:27990–8000. doi: 10.1021/acsomega.0c03396
Keywords: antioxidant, nutrients, Panax notoginseng, polyphenol, saponin
Citation: Liang Z, Liu K, Li R, Ma B, Zheng W, Yang S, Zhang G, Zhao Y, Chen J and Zhao M (2022) An instant beverage rich in nutrients and secondary metabolites manufactured from stems and leaves of Panax notoginseng. Front. Nutr. 9:1058639. doi: 10.3389/fnut.2022.1058639
Received: 30 September 2022; Accepted: 14 November 2022;
Published: 07 December 2022.
Edited by:
Bin Li, Shenyang Agricultural University, ChinaReviewed by:
Er Sheng Gong, Gannan Medical University, ChinaCopyright © 2022 Liang, Liu, Li, Ma, Zheng, Yang, Zhang, Zhao, Chen and Zhao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Junwen Chen, Y2p3MzE0MTJAMTYzLmNvbQ==; Ming Zhao, emhhb21pbmcwMjI5MjAwMkBhbGl5dW4uY29t
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.