
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Nutr., 08 December 2022
Sec. Clinical Nutrition
Volume 9 - 2022 | https://doi.org/10.3389/fnut.2022.1055517
This article is part of the Research TopicBiomarkers: Precision Nutrition in Chronic DiseasesView all 26 articles
 Mohammad Zamani1
Mohammad Zamani1 Mahtab Zarei2
Mahtab Zarei2 Mahlagha Nikbaf-Shandiz3
Mahlagha Nikbaf-Shandiz3 Fatemeh Gholami4
Fatemeh Gholami4 Amir Mehdi Hosseini5
Amir Mehdi Hosseini5 Maryam Nadery6
Maryam Nadery6 Farideh Shiraseb4*
Farideh Shiraseb4* Omid Asbaghi7,8*
Omid Asbaghi7,8*Introduction: Cardiovascular disease (CVD) is one of the leading causes of death and disability in the world and is estimated to involve more people in the next years. It is said that alternative remedies such as herbs can be used to manage the complications of this disease. For this reason, we aimed to conduct this meta-analysis to systematically assess and summarize the effects of saffron supplementation as an important herb on cardiovascular risk factors in adults.
Methods: A systematic search was done in PubMed, Scopus, and Web of Science to find eligible articles up to September 2022. Randomized controlled trials (RCTs) that evaluated the effects of saffron on lipid profiles, glycemic control, blood pressure, anthropometric measures, and inflammatory markers were included. In the meta-analysis, 32 studies were taken into account (n = 1674).
Results: Consumption of saffron significantly decreased triglyceride (TG) (WMD = −8.81 mg/dl, 95%CI: −14.33, −3.28; P = 0.002), total cholesterol (TC) (WMD = −6.87 mg/dl, 95%CI: −11.19, −2.56; P = 0.002), low density lipoprotein (LDL) (WMD = −6.71 mg/dl, 95%CI: −10.51, −2.91; P = 0.001), (P = 0.660), fasting blood glucose (FBG) level (WMD = −7.59 mg/dl, 95%CI: −11.88, −3.30; P = 0.001), HbA1c (WMD = −0.18%, 95%CI: −0.21, −0.07; P < 0.001), homeostasis model assessment-insulin resistance (HOMA-IR) (WMD = −0.49, 95%CI: −0.89, −0.09; P = 0.016), systolic blood pressure (SBP) (WMD = −3.42 mmHg, 95%CI: −5.80, −1.04; P = 0.005), tumor necrosis factor α (TNF-α) (WMD = −2.54 pg/ml, 95%CI: −4.43, −0.65; P = 0.008), waist circumference (WC) (WMD = −1.50 cm; 95%CI: −2.83, −0.18; P = 0.026), malondialdehyde (MDA) (WMD = −1.50 uM/L, 95%CI: −2.42, −0.57; P = 0.001), and alanine transferase (ALT) (WMD = −2.16 U/L, 95%CI: −4.10, −0.23; P = 0.028). Also, we observed that saffron had an increasing effect on total antioxidant capacity (TAC) (WMD = 0.07 mM/L, 95%CI: 0.01, 0.13; P = 0.032). There was linear regression between FBG and the duration of saffron intake. Additionally, the non-linear dose-response analysis has shown a significant association of saffron intervention with HDL (P = 0.049), HOMA-IR (P = 0.002), weight (P = 0.036), ALP (P = 0.016), FBG (P = 0.011), HbA1c (P = 0.002), and TNF-α (P = 0.042). A non-linear association between the length of the intervention and the level of HDL and DBP was also found.
Discussion: That seems saffron could effectively improve TG, TC, LDL, FBG, HbA1c, HOMA-IR, SBP, CRP, TNF-α, WC, MDA, TAC, and ALT.
Cardiovascular disease (CVD) is known as one of the main causes of morbidity and mortality in societies (1). This complication which includes ischemic heart disease, stroke, heart failure, peripheral arterial disease, and other conditions (2), reduces the quality of life and life expectancy among patients and also leads to high medical care expenses on health systems and governments in different countries around the world (3, 4). Numbers show that the global prevalence of CVD almost doubled from 271 million in 1990 to 523 million in 2019 besides reaching a mortality rate from 12.1 to 18.6 million which was a third of all death globally (5). It is estimated that CVD would be the cause of more than 23 million deaths in 2030 around the world (6). Many risk factors such as gender, family history, high blood pressure, dyslipidemia, obesity, glucose abnormalities, insulin resistance, lifestyle risk factors (7, 8), and inflammation (9) are involved in the development of this disease. Accordingly, lifestyle modification especially nutritional interventions and alternative remedies like herbs can be applied to manage and treat CVD and related diseases (10, 11).
Saffron with the scientific name of “Crocus sativus Linn” (12) and bioactive compounds of crocetin, crocin, picrocrocin, and safranal (13), is a plant with medical properties (14) and is mainly cultivated in Asian and European countries (15). It has been shown that saffron could have positive impacts on hyperglycemia, insulin resistance (16), and hyperlipidemia (17) due to increasing glucose uptake and enhancing insulin sensitivity in cells (18) besides mitochondrial-β-oxidation (19). Furthermore, it is shown that this herb has anti-inflammatory and anti-oxidative benefits (18) by raising the glutathione reductase levels (20) and lowering the levels of pro-inflammatory enzymes (21). A meta-analysis conducted in 2018 on 11 RCTs showed that saffron consumption has no significant effect on improving lipid profile, fasting insulin, systolic blood pressure (SBP), and body mass index (BMI) but in subgroup analysis, a significant reduction in fasting plasma glucose levels was seen. Inflammatory factors were not examined in this study (22). Also, another meta-analysis was done in 2018 on 9 RCTs that had been conducted on diabetes and metabolic syndrome. In this study, only waist circumferences (WC), HbA1c, and fasting plasma glucose (FPG) were examined and they concluded that saffron can improve WC as well as FPG levels in sub-group analysis when intervention durations were more than 12 weeks. There was no significant effect on HbA1c levels (23). In a recent meta-analysis on 25 RCTs evaluating the effects of saffron on cardiometabolic indices in overweight and obese patients, a significant reduction in FPG was seen in participants with metabolic syndrome but there was not any considerable effect on Hb1AC, weight, and BMI (24). Besides, Rahmani’s meta-analysis containing 9 RCTs showed FPG reduction in interventions longer than 12 weeks without affecting HbA1C levels (23). Regarding lipid profile, in 2019 another meta-analysis on six RCTs showed an improvement in serum concentration of total cholesterol (TC), triglyceride (TG), and high-density lipoprotein (HDL) following supplementation with saffron but no influence on serum FPG and low-density lipoprotein (LDL) concentrations was seen (25). In addition, a meta-analysis in 2019 demonstrated the positive impact of saffron on malondialdehyde (MDA) and total antioxidant capacity (TAC) in unhealthy patients (20). Based on a 2019 article, saffron supplementation did not affect inflammatory cytokines in adults (26).
Although some studies have been done in recent years, findings show contradictory impacts of saffron and its derivates on CVD risk factors. Due to this issue and because a comprehensive meta-analysis of all the risk factors related to CVD has not been performed on new findings since then, we conducted this meta-analysis on 32 RCTs and a wide range of related variables to systematically summarize the results and evaluate the effects of saffron supplementation on cardiovascular risk factors in adults.
This systematic review and meta-analysis was performed under the preferred reporting items for systematic reviews and meta-analyses (PRISMA) guidelines (27). This study is registered at PROSPERO (CRD42022358721).
To find relevant articles published up to September 2022, a systematic search was done in scientific databases including PubMed, Scopus, and Web of Science regardless of the length of studies and language. In addition, a manual search through the reference lists of relevant publications was performed to make sure we did not miss any potential studies. The PICO criteria (Participant, Intervention, Comparison/Control, Outcome) was used to search for items related to saffron supplementation and cardiovascular risk factors. (1) Participants: adults age≥18; (2) Intervention group (Saffron, Satiereal, Crocin); (3) Comparison/Control group (non-saffron supplementation), and (4) Outcome (all of the CVD risk factors that will be mentioned). The main terms and keywords we used to search the databases are as follow: (“Crocus sativus Linn” OR Safranal OR saffron OR crocin) AND (Intervention OR “Intervention Study” OR “Intervention Studies” OR “controlled trial” OR randomized OR randomized OR random OR randomly OR placebo OR “clinical trial” OR Trial OR “randomized controlled trial” OR “randomized clinical trial” OR RCT OR blinded OR “double blind” OR “double blinded” OR trial OR “clinical trial” OR trials OR “Pragmatic Clinical Trial” OR “Cross-Over Studies” OR “Cross-Over” OR “Cross-Over Study” OR parallel OR “parallel study” OR “parallel trial”).
Studies with the following criteria were included: (1) RCTs with either parallel or crossover design; (2) used oral supplementation of saffron; (3) investigated the effects of saffron on any of the cardiovascular risk factors and the desired variables such as triglyceride (TG), total cholesterol (TC), low-density lipoprotein (LDL), and high-density lipoprotein (HDL), fasting blood glucose (FBG), hemoglobin A1c (HbA1c), insulin, serum insulin, homeostasis model assessment-insulin resistance (HOMA-IR), systolic blood pressure (SBP), diastolic blood pressure (DBP), C-reactive protein (CRP), interleukin-6, (IL-6), tumor necrosis factor (TNF-α), total antioxidant capacity (TAC), weight, waist circumference (WC), body mass index (BMI), fat mass% (FM), aspartate transaminase (AST), alanine transaminase (ALT), malondialdehyde (MDA), alkaline phosphatase (ALP), (4) were performed on the adult population (≥ 18 years old); (5) had an intervention duration of at least four days (RCTs with two or more eligible arms were considered as separate studies); (6) provided means and standard deviations (SDs) for data, or any other effect sizes from which the calculation of mean and SD was possible; (7) human studies. Two authors (OA, MZ) independently screened the titles, abstracts, and full texts, Checked the results, and assessed the eligibility of the selected studies. Any disagreement was resolved by discussion. Exclusion criteria included animal and in vitro studies in addition to studies that examined the effect of another intervention along with saffron or done on children and adolescents. Moreover, studies with a non-RCT design, without a placebo group, unpublished documents, and gray literature like conference abstracts, editorial papers, and books were excluded.
The following required data were extracted from eligible studies by two independent authors (OA, MZ): The first author’s name, country, publication year, type of clinical trial, participant characteristics (mean age, BMI, sex), health condition of participants, randomization, blinding, sample size, the number of participants in the intervention and control groups, the form and dose of supplemented saffron, study duration, and the desired variables. Furthermore, for both parallel and cross-over trials, means ± Standard Deviation (SD) of variables at the beginning and end of the study were collected. If this data was not available, the mean difference was calculated by subtracting the mean value at baseline from the mean value at the end of the study. If there were insufficient data in articles with pre-determined methods contact authors via email.
To assess the quality of the studies, we benefited from the Cochrane Collaboration tool (28). All the studies were checked for the probability of bias. This included randomized sequence generation, allocation concealment, blindness (participants, staff, and outcome assessment), incomplete outcome data, selective outcome reporting, and other biases. Based on the recommendations of the Cochrane Handbook, three groups of high risk of bias, low risk of bias, and uncertain risk of bias were created. The quality of studies in which the number of high-risk biases was more than 2 was considered as bad and in the same way, those having 2 or less than 2 high-risk biases were considered fair and good, respectively. The quality of the work was checked by two authors (OA, MZ) and in case of any disagreement, the problem was resolved by discussion and consulting.
All statistical analyzes of eligible studies were performed using Stata software version 11.0 (Stata Corp, College Station, TX). All tests were two-tailed, and p < 0.05 were considered statistically significant. The pooled weighted mean difference (WMD) was calculated by a random-effects model to consider the existing heterogeneity (29) and also the Interstudy heterogeneity was performed using I-square (I2) test (30), with values greater than 40% indicating strong heterogeneity (31). The mean differences of required variables in both intervention and control groups at the beginning and end of the study were calculated and also the SD of these mean differences was computed using the following formula: SD = square root [(SD at baseline)2 + (SD at the end of study)2 − (2r × SD at baseline × SD at the end of study)] (32). All standard errors (SEs), 95 percent confidence intervals (CIs), and interquartile ranges (IQRs) to SDs which had been reported in studies, converted to SD using a method introduced by Hozo et al. and this formula: SD = SE × √n (n = the number of individuals in each group) (33). We applied a correlation coefficient of 0.8 for r (28). To define the source of heterogeneity, a subgroup analysis was done. Subgroups were selected based on the required minimum number of studies according to the established criteria, where there should be at least 6 to 10 studies for continuous and a minimum of 4 studies for categorical subgroup variables (34, 35). The analysis of baseline TG (<150 mg/dl, ≥150 mg/dl), TC (<200 mg/dl, ≥200 mg/dl), LDL (<100 mg/dl, ≥100 mg/dl), HDL (<40 mg/dl, ≥40 mg/dl), FBG (<100 mg/dl, ≥100 mg/dl), SBP (<120 mmHg, ≥120 mmHg), DBP (<80 mmHg, ≥80 mmHg), Intervention duration (≤12 weeks, >12 weeks), and dosage of saffron (<100 mg/day, ≥100 mg/day) were based on the median values of the included studies. Other subgroup analyses were performed according to health status (diabetic, non-diabetic), and baseline BMI [normal (18.5–24.9 kg/m2), overweight (25–29.9 kg/m2), and obese (≥30 kg/m2)].
The potential publication bias was reviewed by a funnel plot test (36, 37). Sensitivity analyses were conducted to explore the impact of each study on the pooled effect size. We used the trim-and-fill method to detect and adjust the publication bias’s impact (38). Meta-regression was performed to evaluate the potential effects of saffron (mg/d) dosage and duration on the variables. Furthermore, we used non-linear regression for dose-response analysis between saffron supplementation and our variables.
The overall quality of evidence in all studies was assessed and summarized using the GRADE (Grading of Recommendations Assessment, Development, and Evaluation) approach (39).
Initially, 2428 potentially eligible records were found in the literature using an electronic search [PubMed (973), ISI Web of Science (632), and Scopus (823)]. After duplicates were eliminated (n = 873) and title/abstract screening, 1500 articles were excluded because they had no relevance to the topic. As a result, 55 full-text papers were collected for a thorough evaluation. 23 of these studies had papers with no useful data (Figure 1). Finally, 32 trials (15, 17, 40–69), were considered eligible for the systematic review. The meta-analysis was conducted on 24 effect sizes for TG (15, 17, 40, 42, 44, 45, 47, 49, 51–55, 57, 58, 60, 61, 63, 67), 23 for TC (15, 17, 40, 42, 44, 45, 47, 49, 51–55, 57, 58, 60, 61, 63), 23 for LDL (15, 17, 40, 44, 45, 47, 49, 51–55, 57, 58, 60, 61, 63, 67), 23 for HDL (15, 17, 40, 44, 45, 47, 49, 51–55, 57, 58, 60, 61, 63, 67), 22 for FBG (15, 17, 42, 44, 45, 47, 49, 51–54, 57, 58, 60–62, 64, 66, 67), 7 for insulin (15, 44, 45, 57, 58, 64), 12 for HbA1c (15, 17, 44, 45, 54, 57, 60–62, 64), 7 for HOMA-IR (15, 45, 57, 58, 61, 64), 11 for SBP (40, 45, 48, 52, 53, 57, 63, 64, 66), 9 for DBP (40, 48, 52, 53, 57, 63, 64, 66), 10 for CRP (44, 46, 52, 57, 58, 62, 65, 68), 3 for IL-6 (53, 62, 66), 7 for TNF-α (53, 57, 59, 62, 65, 66, 68), 13 for weight (41, 44, 48–50, 52, 55, 57, 58, 60, 68, 69), 12 for BMI (44, 48–50, 52, 57, 58, 60, 63, 68, 69), 14 for WC (41, 44, 45, 48, 49, 52, 53, 55, 57, 60, 68, 69), 5 for FM% (41, 49, 57, 68), 7 for MDA (57–59, 62, 65, 68, 69), 7 for TAC (57–59, 62, 65, 68, 69), 11 for ALT (15, 42, 46, 54, 57, 61, 67, 68), 11 for AST (15, 42, 46, 54, 57, 61, 67, 68), and 5 for ALP (42, 46, 57, 61).
Table 1 lists the characteristics of the trials that included a total of 1674 participants who were enrolled in the studies, 842 of them were assigned to the intervention group and 832 to the control group. All publications that were included in the present systematic review were randomized controlled clinical trials in design, and a parallel research design was used in all studies (15, 17, 40–69). All of these investigations were conducted in France (41) and Iran (15, 17, 40, 42–69), and were published between 2008 and 2021. The participants’ average ages ranged from 27 to 57.95, while their average baseline BMIs ranged from 23.84 to 31.02 kg/m2. The follow-up period ranged from 1 to 12 weeks. Daily supplementation dosage of saffron varied between 5 (54) and 1000 (44, 48) mg/day in these studies. In two studies (40, 54), data were reported for two different doses, hence four effect sizes were calculated. Six effect sizes were estimated as a result of the three studies (45, 46, 49) data on two varieties of saffron being provided. Only one (46) of the included studies had a male-only population, two (41, 65) had a female-only population, and the remaining trials (15, 17, 40, 42–45, 47–64, 66–69) involved mixed-gender populations.
Subjects with a variety of health conditions were all included in the study: type 2 diabetes patients (15, 17, 44, 48, 56, 57, 61, 62, 64, 66), patients with schizophrenia (45, 46), patients with major depressive disorder (42), patients with coronary artery disease (49), patients with refractory diabetic maculopathy (54), subjects with mild to moderate generalized anxiety disorder (50), individuals with metabolic syndrome (47, 51–53, 55), healthy subjects (40, 43), mildly overweight healthy women (41), patients under methadone maintenance treatment (58), overweight/obese prediabetic patients (60), patients with mild and moderate persistent allergic asthma (63), multiple sclerosis patients (59), patients with non-alcoholic fatty liver disease (67, 68), patients with active rheumatoid arthritis (65), and ulcerative colitis patients (69). All research was done in English. Figure 2A (TG), 2B (TC), 2C (LDL), 2D (HDL), 2E (FBG), 2F (insulin), 2G (HbA1c), 2H (HOMA-IR), 2I (SBP), 2J (DBP), 2K (CRP), 2L (IL-6), 2M (TNF-α), 2N (weight), 2O (BMI), 2P (WC), 2Q (FM%), 2R (MDA), 2S (TAC), 2T (ALT), 2U (AST), and 2V (ALP) depict the WMD and 95% CI for outcomes forest plots.
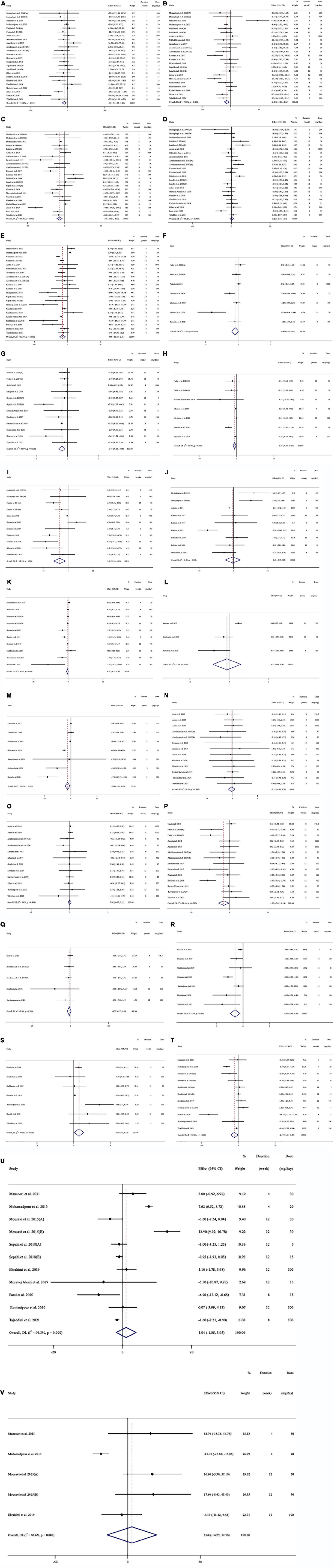
Figure 2. Forest plot detailing weighted mean difference and 95% confidence intervals (CIs) for the effect of saffron consumption on (A) TG (mg/dl); (B) TC (mg/dl); (C) LDL (mg/dl); (D) HDL (mg/dl); (E) FBG (mg/dl); (F) Insulin (miu/ml); (G) HbA1c (%); (H) HOMA-IR; (I) SBP (mmHg); (J) DBP (mmHg); (K) CRP (mg/l); (L); IL-6 (pg/ml); (M) TNF-α (pg/ml); (N) weight (kg); (O) BMI (kg/m2); (P) WC (cm); (Q) FM (%); (R) MDA (uM/L); (S) TAC (mM/L); (T) ALT (U/L); (U) AST (U/L) and (V) ALP (U/L). TG, triglyceride; TC, total cholesterol; LDL, low-density lipoprotein; HDL, high-density lipoprotein; FBG, fasting blood glucose; HOMA-IR, homeostasis model assessment for insulin resistance; HbA1C, hemoglobin A1C; CRP, C-reactive protein; IL-6, interleukin 6; TNF-α, tumor necrosis factor; TAC, total antioxidant capacity; BMI, body mass index; WC, waist circumference; FM, fat mass; ALT, alanine transaminase; AST, aspartate transaminase; ALP, alkaline phosphatase; MDA, malondialdehyde; SBP, systolic blood pressure; DBP, diastolic blood pressure; CI, confidence interval; WMD, weighted mean difference.
Most studies did not report specific side effects, but some side effects such as dry mouth, restlessness, anxiety, daily drowsiness, morning drowsiness (42), constipation, polydipsia, headache (17, 50), increased appetite, feet swelling, stomach ache, subconjunctival-hemorrhage, swelling, redness, and burning of the eyes (54), stomach pain (65), were reported in some studies.
Out of the 32 studies examined for this review, 11 trials (15, 17, 50, 56, 57, 60–63, 68, 69) were rated as having good quality, 12 trials (40, 41, 45, 47, 49, 51, 54, 58, 64–67) as having medium quality, and 9 trials (42–44, 46, 48, 52, 53, 59, 63) as having low quality. The details of the risk of bias in studies according to the domains used by the Cochrane collaboration’s tool are provided in Table 2.
In total, we pooled 24 effect sizes from 18 studies, with 1312 participants [intervention group (IG) = 655, control group (CG) = 657], to estimate the effect of saffron on plasma TG (15, 17, 40, 42, 44, 45, 47, 49, 51–55, 57, 58, 60, 61, 63, 67), and 23 effect sizes from 18 studies, for TC (15, 17, 40, 42, 44, 45, 47, 49, 51–55, 57, 58, 60, 61, 63) with 1208 participants (IG = 603, CG = 605), LDL (15, 17, 40, 44, 45, 47, 49, 51–55, 57, 58, 60, 61, 63, 67) with 1292 participants (IG = 645, CG = 647), and HDL (15, 17, 40, 44, 45, 47, 49, 51–55, 57, 58, 60, 61, 63, 67) levels with 1292 participants (IG = 645, CG = 647) (Table 3). According to the overall result of the meta-analysis, saffron significantly decreased serum TG (WMD = −8.81 mg/dl, 95%CI: −14.33, −3.28; P = 0.002; I2 = 55.1%, P = 0.001; Figure 2A), TC (WMD = −6.87 mg/dl, 95%CI: −11.19, −2.56; P = 0.002; I2 = 72.5%, P < 0.001; Figure 2B), and LDL (WMD = −6.71 mg/dl, 95%CI: −10.51, −2.91; P = 0.001; I2 = 81.3%, P < 0.001; Figure 2C). However, saffron on HDL showed no significant effect (WMD = 0.21 mg/dl, 95%CI: −0.73, 1.16; P = 0.660; I2 = 66.2%, P < 0.001; Figure 2D).
Different subgroup analyses were performed to determine the potential sources of heterogeneity among studies. The subgroup analysis revealed that saffron significantly decreased TG in studies with < 12 weeks of intervention (WMD = −11.18 mg/dl; 95%CI: −18.53, −3.84; P = 0.003), low (WMD = −7.80 mg/dl; 95%CI: −14.44, −1.16; P = 0.021) and high (WMD = −11.29 mg/dl; 95%CI: −20.69, −1.89; P = 0.019) doses of intervention, subjects with baseline TG < 150 (WMD = −4.65 mg/dl; 95%CI: −8.88, −0.43; P = 0.031), non-diabetic participants (WMD = −10.66 mg/dl; 95%CI: −17.70, −3.62; P = 0.003), and in studies that used saffron (WMD = −8.96 mg/dl; 95%CI: −16.01, −1.93; P = 0.013) as intervention, but when the baseline BMI was > 30 kg/m2, saffron significantly increased TG levels (WMD = 17.93 mg/dl; 95%CI: −35.31, −0.55; P = 0.043). Also, the reduction in TC and LDL levels was significant in some subgroups. In studies with ≥ 12 weeks intervention duration (WMD = −11.21 mg/dl; 95%CI: −19.74, −2.69; P = 0.010), intervention dose ≥ 100 mg/day (WMD = −10.76 mg/dl; 95%CI: −18.12, −3.41; P = 0.004), studies that used crocin (WMD = −7.15 mg/dl; 95%CI: −9.98, −4.33; P < 0.001)], obese (WMD = −19.59 mg/dl; 95%CI: −33.56, −5.61; P = 0.006) and normal weight (WMD = −7.43 mg/dl; 95%CI: −10.52, −4.34; P < 0.001) participants, non-diabetic individuals (WMD = −7.77 mg/dl; 95%CI: −13.03, −2.50; P = 0.004), and subjects with baseline TC < 200 (WMD = −7.39 mg/dl; 95%CI: −13.16, −1.62; P = 0.012), TC levels were reduced. The following subgroups showed a reduction in LDL: baseline LDL ≥ 100 (WMD = −8.10 mg/dl; 95%CI: −14.38, −1.82; P = 0.011), intervention dose < 100 mg/day (WMD = −5.10 mg/dl; 95%CI: −5.10, −1.23; P = 0.010), using crocin (WMD = −6.58 mg/dl; 95%CI: −10.91, −2.25; P = 0.003) as an intervention, obese (WMD = −13.36 mg/dl; 95%CI: −23.68, −3.03; P = 0.011) and non-diabetic (WMD = −9.41 mg/dl; 95%CI: −14.17, −4.65; P < 0.001) participants (Table 3).
Saffron’s effects on FBG, insulin, HbA1c, and HOMA-IR were calculated in nineteen (22 effect sizes) (15, 17, 42, 44, 45, 47, 49, 51–54, 57, 58, 60–62, 64, 66, 67) with 1231 participants (IG = 616, CG = 615), six (7 effect sizes) (15, 44, 45, 57, 58, 64) with 422 participants (IG = 212, CG = 210), ten (12 effect sizes) with 761 participants (IG = 380, CG = 381) (15, 17, 44, 45, 54, 57, 60–62, 64) and six (7 effect sizes) (15, 45, 57, 58, 61, 64) trials with 405 participants (IG = 202, CG = 203), respectively. Pooled random-effects model analysis revealed significant decreasing effects of saffron on FBG level (WMD = −7.59 mg/dl, 95%CI: −11.88, −3.30; P = 0.001; I2 = 93.3%, P < 0.001; Figure 2E), HbA1c (WMD = −0.18%, 95%CI: −0.21, −0.07; P < 0.001; I2 = 56.9%, P = 0.008; Figure 2G), and HOMA-IR (WMD = −0.49, 95%CI: −0.89, −0.09; P = 0.016; I2 = 70.8%, P = 0.002; Figure 2H). However, the effects of saffron on serum insulin level (WMD = −0.46 miu/ml, 95%CI: −1.00, 0.06; P = 0.088; I2 = 75.6%, P < 0.001; Figure 2F) were not significant.
A subgroup analysis revealed that saffron at doses of less than 100 mg per day could considerably lower FBG levels (WMD = −10.05; 95%CI: −15.17, −4.92; P < 0.001), HbA1c (WMD = −0.21; 95%CI: −0.33, −0.09; P < 0.001) and HOMA-IR (WMD = −1.22; 95%CI: −2.42, −0.02; P = 0.045). The results also showed that saffron could significantly reduce FBG level and HbA1c, when the duration of intervention was ≥ 12 weeks (WMD FBG = −12.02 mg/dl; 95%CI: −18.28, −5.77; P < 0.001; WMD HbA1c = −0.27%; 95%CI: −0.45, −0.08; P = 0.004), and HOMA-IR, when the length of intervention was less than 12 weeks (WMD = −0.23; 95%CI: −0.41, −0.04; P = 0.013). Furthermore, both diabetic (WMD = −0.25%; 95%CI: −0.46, −0.03; P = 0.020) and non-diabetic (WMD = −0.17%; 95%CI: −0.22, −0.11; P < 0.001) participants who consumed saffron had significantly lower HbA1c levels. Saffron, however, only significantly affects FBG levels in diabetic patients (WMD = −14.08 mg/dl; 95%CI: −22.38, −5.78; P = 0.001). Additionally, the subgroup analysis showed that only the overweight patients’ serum insulin concentrations (WMD = −0.00 miu/ml; 95%CI: −0.33, 0.33; P = 0.002) could be considerably lowered by saffron.
Both saffron (WMD = −7.49 mg/dl; 95%CI: −13.98, −1.01; P = 0.023) and crocin (WMD = −8.13 mg/dl; 95%CI: −15.41, −0.86; P = 0.028) consumption resulted in significantly lower FBG levels, however, only saffron consumption resulted in significantly lower HbA1c (WMD = −0.15%; 95%CI: −0.22, 0.08; P < 0.001) values (Table 3).
In total, we pooled data from 9 (11 effect sizes) (40, 45, 48, 52, 53, 57, 63, 64, 66), six (7 effect sizes) with 564 participants (IG = 285, CG = 279), and 8 (9 effect sizes) with 476 participants (IG = 241, CG = 235), (40, 48, 52, 53, 57, 63, 64, 66) studies to evaluate the effect of saffron on SBP and DBP, respectively. The pooled effect demonstrated a significant reduction in SBP after consuming saffron (WMD = −3.42 mmHg, 95%CI: −5.80, −1.04; P = 0.005; I2 = 82.5%, P < 0.001; Figure 2I). Saffron had not significant effect on DPB (WMD = −0.19 mmHg, 95%CI: −2.42, 2.03; P = 0.862; I2 = 81.4%, P < 0.001; Figure 2J). A subgroup analysis revealed that saffron at doses of <100 mg/day (WMD = −4.97 mmHg; 95%CI: −8.06, −1.88; P = 0.002) for ≥ 12 weeks (WMD = −4.21 mmHg; 95%CI: −8.38, −0.05; P = 0.047) in patients with baseline SBP ≥ 120 (WMD = −4.24 mmHg; 95%CI: −8.22, −0.25; P = 0.037), and when crocin (WMD = −6.41 mmHg; 95%CI: −9.12, −3.69; P < 0.001) was used as an intervention, could significantly lower SBP. The results also showed that saffron could significantly reduce DBP in diabetic patients (WMD = −1.23 mmHg; 95%CI: −1.41, −1.05; P < 0.001) (Table 3).
Saffron’s effect on CRP, IL-6, and TNF-α was studied in 8 (10 effect sizes) (44, 46, 52, 57, 58, 62, 65, 68) with 596 participants (IG = 299, CG = 297), 3 (3 effect sizes) (53, 62, 66) with 165 participants (IG = 84, CG = 81), and 7 (7 effect sizes) studies with 427 participants (IG = 215, CG = 212), (53, 57, 59, 62, 65, 66, 68), respectively. A meta-analysis revealed that saffron significantly reduced TNF-α (WMD = −2.54 pg/ml, 95%CI: −4.43, −0.65; P = 0.008; I2 = 93.6%, P < 0.001; Figure 2M), and a subgroup analysis revealed that saffron had a significant influence on TNF-α in studies with < 12 weeks of intervention (WMD = −6.22 pg/ml; 95%CI: −10.31, −2.14; P = 0.003), and high dose interventions (≥ 100 mg/day) (WMD = −4.02 pg/ml; 95%CI: −7.94, −0.10; P = 0.044).
The variations in CRP (WMD = −0.20 mg/l, 95%CI: −0.46, 0.05; P = 0.127; I2 = 74.4%, P < 0.001; Figure 2K), and IL-6 (WMD = −0.12 pg/ml, 95%CI: −0.83, 0.59; P = 0.739; I2 = 87.4%, P < 0.001; Figure 2L) when compared to controls were not significant. Saffron consumption, on the other hand, resulted in significant decreases in CRP in high dose interventions (≥100 mg/day) (WMD = −0.72 mg/l; 95%CI: −1.30, −0.14; P = 0.014), non-diabetic subjects (WMD = −0.52 mg/l; 95%CI: −0.94, −0.10; P = 0.015) and when saffron (WMD = −0.57 mg/l; 95%CI: −1.12, −0.02; P = 0.040) used as intervention (Table 3).
Changes in body weight, BMI, WC, and FM% were assessed in 12 (13 effect sizes) (41, 44, 48–50, 52, 55, 57, 58, 60, 68, 69) with 841 participants (IG = 425, CG = 416), 11 (12 effect sizes) with 785 participants (IG = 396, CG = 389) (44, 48–50, 52, 57, 58, 60, 63, 68, 69), 15 (7 effect sizes) with 884 participants (IG = 447, CG = 437) (41, 44, 45, 48, 49, 52, 53, 55, 57, 60, 68, 69), and 4 (5 effect sizes) (41, 49, 57, 68) trials with 100 participants (IG = 50, CG = 50), respectively. Overall, we observed no significantly different change in weight (WMD = −0.12 kg, 95%CI: −0.82, 0.58; P = 0.732; I2 = 0.0%, P = 0.995; Figure 2N), BMI (WMD = 0.01 kg/m2, 95%CI: −0.17, 0.21; P = 0.853; I2 = 0.0%, P = 0.809; Figure 2O), and FM% (WMD = −0.57%, 95%CI: −1.57, 0.42; P = 0.262; I2 = 0.0%, P = 0.599; Figure 2Q) between the intervention and control groups. However, pooled effect sizes showed a substantial decrease in WC after saffron consumption (WMD = −1.50 cm; 95%CI: −2.83, −0.18; P = 0.026; I2 = 71.06%, P < 0.001; Figure 2P). A subgroup analysis revealed that saffron at doses of less than 100 mg per day (WMD = −2.68 cm; 95%CI: −4.88, −0.48; P = 0.017) could dramatically lower WC. Also, when crocin was used as an intervention, we saw a significant reduction in WC (WMD = −3.32 cm; 95%CI: −6.24, −0.40; P = 0.026) (Table 3).
For MDA and TAC, the study comprised and 455 subjects (IG: 230, CG: 225), and 454 subjects (IG:229, CG:225) from 7 trials (7 effect sizes) respectively (57–59, 62, 65, 68, 69), According to the meta-analysis, saffron had a decreasing effect on MDA (WMD = −1.50 uM/L, 95%CI: −2.42, −0.57; P = 0.001; I2 = 77.4%, P < 0.001; Figure 2R) and an enhancing effect on TAC (WMD = 0.07 mM/L, 95%CI: 0.01, 0.13; P = 0.032; I2 = 69.9%, P = 0.003; Figure 2S). The subgroup analysis revealed that MDA in both diabetic (WMD = −1.02 uM/L; 95%CI: −2.05, −0.01; P = 0.049) and non-diabetic (WMD = −1.52 uM/L; 95%CI: −2.48, −0.57; P = 0.002) patients decreased significantly after consuming saffron. Saffron also significantly raised TAC in non-diabetic subjects (WMD = 0.14 mM/L; 95%CI: 0.05, 0.23; P = 0.001), according to subgroup analysis. In studies which used saffron as an intervention (WMD MDA = −1.08 uM/L; 95%CI: −1.69, −0.46; P = 0.001; WMD TAC = 0.17 uM/L; 95%CI: 0.02, 0.31; P = 0.021), and studies with intervention doses of ≥100 (WMD MDA = −1.17 uM/L; 95%CI: −1.88, −0.47; P = 0.001; WMD TAC = 0.21 mM/L; 95%CI: 0.05, 0.37; P = 0.009) saffron significantly reduced MDA while increasing TAC. In studies with interventions lasting more than 12 weeks (WMD = −0.96 uM/L; 95%CI: −1.48, −0.43; P < 0.001), saffron dramatically decreased MDA, according to additional subgroup analyses (Table 3).
Saffron significantly affected ALT (WMD = −2.16 U/L, 95%CI: −4.10, −0.23; P = 0.028; I2 = 88.8%, P < 0.001; Figure 2T), according to the findings of a pooled analysis of 8 studies (11 effect sizes) (15, 42, 46, 54, 57, 61, 67, 68) with 637 participants (IG = 318, CG = 319). However, the results of a pooled analysis of 8 (12 effect sizes) (15, 42, 46, 54, 57, 61, 67, 68) with 637 participants (IG = 318, CG = 319) and 4 (5 effect sizes) (42, 46, 57, 61) trials with 296 participants (IG = 148, CG = 148), revealed no significant effect of saffron on AST (WMD = 1.03 U/L, 95%CI: −1.85, 3.92; P = 0.482; I2 = 96.3%, P < 0.001; Figure 2U) and ALP (WMD = 2.84 U/L, 95%CI: −14.29, 19.97; P = 0.745; I2 = 82.6%, P = 0.544; Figure 2V) respectively. The subgroup analysis revealed that saffron results in 5.58 (U/L) and 5.10 (U/L) reductions in ALT compared to controls in studies with a duration < 12 weeks (WMD = −5.58 U/L; 95%CI: −10.42, −0.75; P = 0.024) and non-diabetic patients (WMD = −5.10 U/L; 95%CI: −8.41, −1.78; P = 0.003), respectively. Crocin (WMD = −4.94 U/L; 95%CI: −9.38, −0.50; P = 0.029), when taken as an intervention, could dramatically lower AST. Additionally, after consuming saffron, the overweight individuals’ AST levels (WMD = −1.26 U/L; 95%CI: −1.85, −0.66; P < 0.001) significantly decreased (Table 3).
There was evidence of a non-linear relationship between saffron dosage and HDL (coefficients = 5.95, P = 0.049; Figure 4D), HOMA-IR (coefficients = 7.69, P = 0.002; Figure 4H), weight (coefficients = 0.06, P = 0.036; Figure 4N), and ALP (coefficients = 1.78, P = 0.016; Figure 4V). In addition, the non-linear dose-response analysis revealed a non-linear relationship between saffron dosage and FBG (coefficients = −0.67, P = 0.011; Figure 4E), HbA1c (coefficients = −0.02, P = 0.002; Figure 4G), and TNF-α (coefficients = −3.56, P = 0.042; Figure 4M).
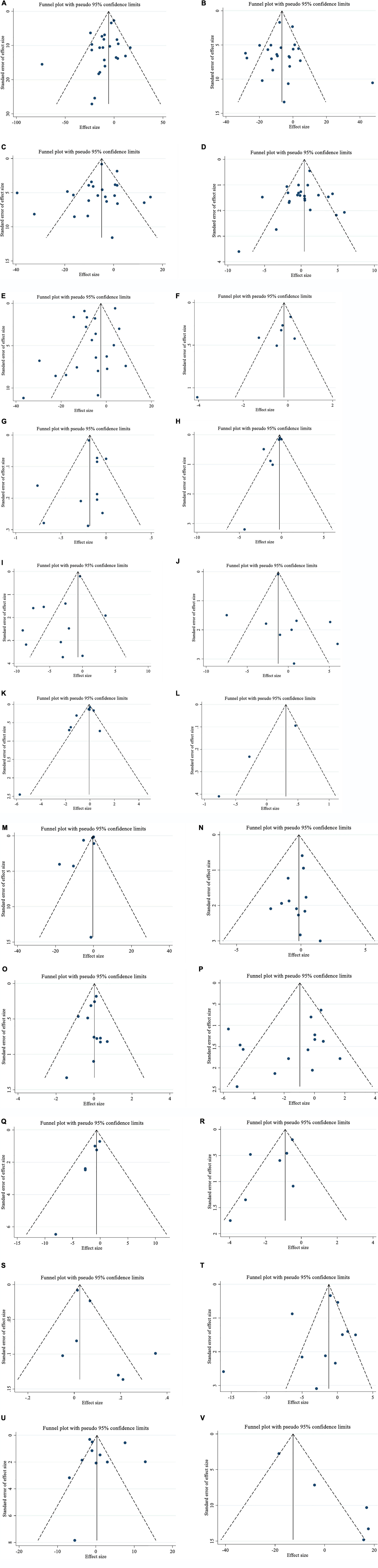
Figure 3. Funnel plots for the effect of saffron consumption on (A) TG (mg/dl); (B) TC (mg/dl); (C) LDL (mg/dl); (D) HDL (mg/dl); (E) FBG (mg/dl); (F) Insulin (miu/ml); (G) HbA1c (%); (H) HOMA-IR; (I) SBP (mmHg); (J) DBP (mmHg); (K) CRP (mg/l); (L); IL-6 (pg/ml); (M) TNF-α (pg/ml); (N) weight (kg); (O) BMI (kg/m2); (P) WC (cm); (Q) FM (%); (R) MDA (uM/L); (S) TAC (mM/L); (T) ALT (U/L); (U) AST (U/L) and (V) ALP (U/L). TG, triglyceride; TC, total cholesterol; LDL, low-density lipoprotein; HDL, high-density lipoprotein; FBG, fasting blood glucose; HOMA-IR, homeostasis model assessment for insulin resistance; HbA1c, hemoglobin A1c; CRP, C-reactive protein; IL-6, interleukin 6; TNF-α, tumor necrosis factor; TAC, total antioxidant capacity; BMI, body mass index; WC, waist circumference; FM, fat mass; ALT, alanine transaminase; AST, aspartate transaminase; ALP, alkaline phosphatase; MDA, malondialdehyde; SBP, systolic blood pressure; DBP, diastolic blood pressure; CI, confidence interval; WMD, weighted mean difference.
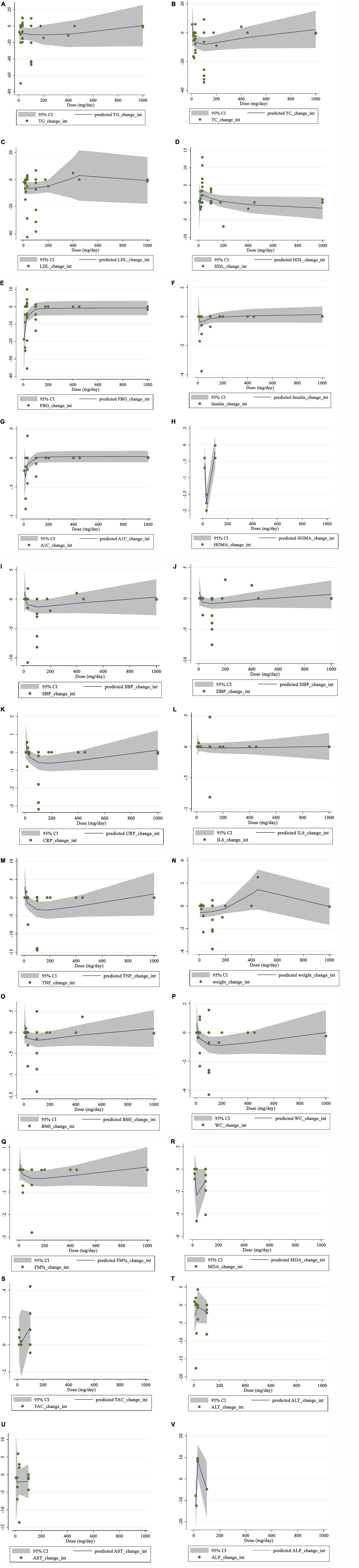
Figure 4. Non-linear dose-response relations between saffron consumption and absolute mean differences. Dose-response relations between dose (mg/day) and absolute mean differences (A) TG (mg/dl); (B) TC (mg/dl); (C) LDL (mg/dl); (D) HDL (mg/dl); (E) FBG (mg/dl); (F) Insulin (miu/ml); (G) HbA1c (%); (H) HOMA-IR; (I) SBP (mmHg); (J) DBP (mmHg); (K) CRP (mg/l); (L); IL-6 (pg/ml); (M) TNF-α (pg/ml); (N) weight (kg); (O) BMI (kg/m2); (P) WC (cm); (Q) FM (%); (R) MDA (uM/L); (S) TAC (mM/L); (T) ALT (U/L); (U) AST (U/L) and (V) ALP (U/L). TG, triglyceride; TC, total cholesterol; LDL, low-density lipoprotein; HDL, high-density lipoprotein; FBG, fasting blood glucose; HOMA-IR, homeostasis model assessment for insulin resistance; HbA1c, hemoglobin A1c; CRP, C-reactive protein; IL-6, interleukin 6; TNF-α, tumor necrosis factor; TAC, total antioxidant capacity; BMI, body mass index; WC, waist circumference; FM, fat mass; ALT, alanine transaminase; AST, aspartate transaminase; ALP, alkaline phosphatase; MDA, malondialdehyde; SBP, systolic blood pressure; DBP, diastolic blood pressure; CI, confidence interval; WMD, weighted mean difference.
Moreover, there was a non-linear relationship between the length of the intervention and HDL (coefficients = 3.20, P = 0.007; Figure 5D) and DBP (coefficients = −1.85, P = 0.033; Figure 5J). However, there was no evidence of a non-linear association between the duration of the intervention and other outcomes.
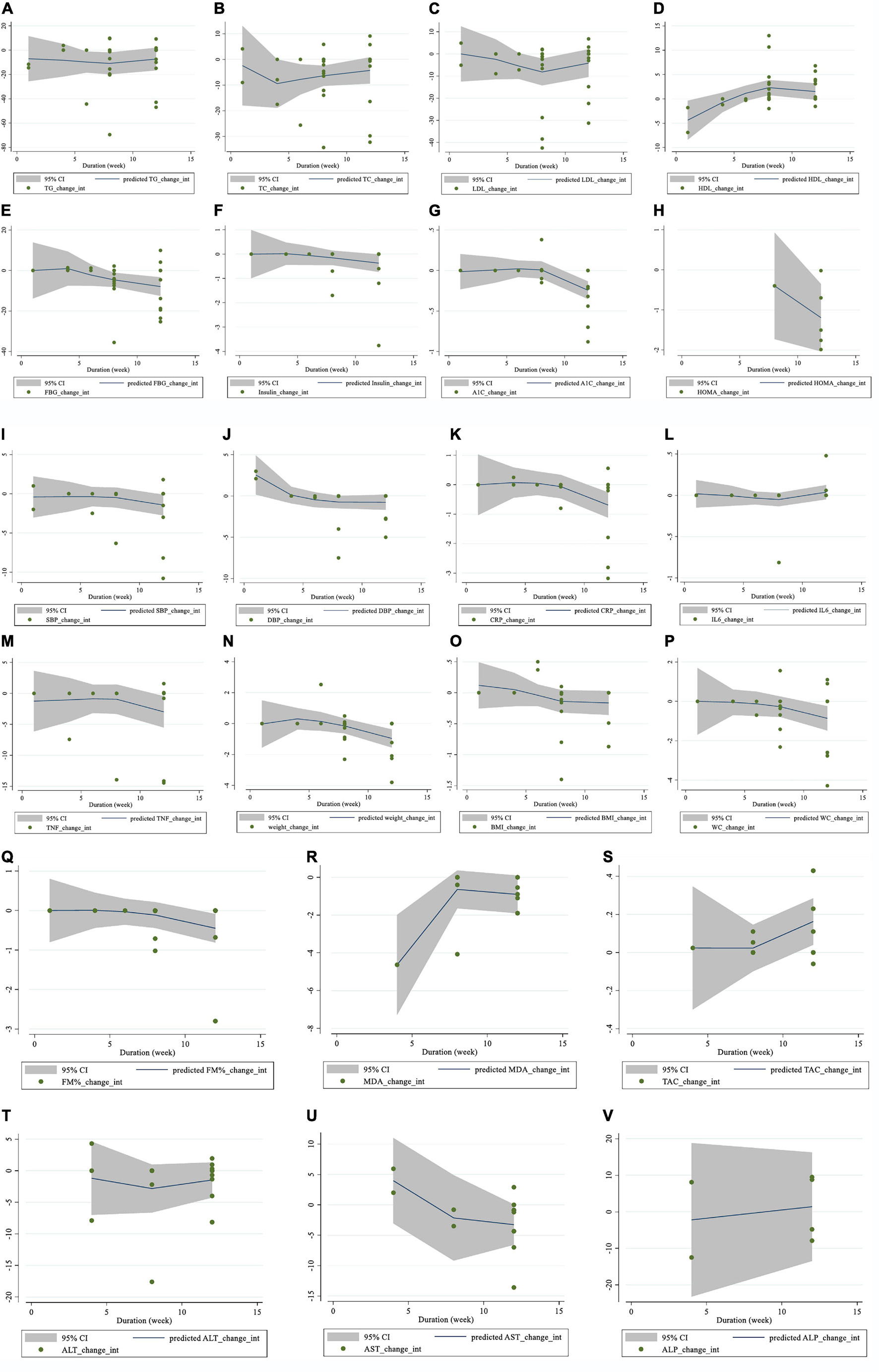
Figure 5. Non-linear dose-response relations between saffron consumption and absolute mean differences. Dose-response relations between duration of intervention (week) and absolute mean differences on (A) TG (mg/dl); (B) TC (mg/dl); (C) LDL (mg/dl); (D) HDL (mg/dl); (E) FBG (mg/dl); (F) Insulin (miu/ml); (G) HbA1c (%); (H) HOMA-IR; (I) SBP (mmHg); (J) DBP (mmHg); (K) CRP (mg/l); (L); IL-6 (pg/ml); (M) TNF-α (pg/ml); (N) weight (kg); (O) BMI (kg/m2); (P) WC (cm); (Q) FM (%); (R) MDA (uM/L); (S) TAC (mM/L); (T) ALT (U/L); (U) AST (U/L) and (V) ALP (U/L). TG, triglyceride; TC, total cholesterol; LDL, low-density lipoprotein; HDL, high-density lipoprotein; FBG, fasting blood glucose; HOMA-IR, homeostasis model assessment for insulin resistance; HbA1C, hemoglobin A1C; CRP, C-reactive protein; IL-6, interleukin 6; TNF-α, tumor necrosis factor; TAC, total antioxidant capacity; BMI, body mass index; WC, waist circumference; FM, fat mass; ALT, alanine transaminase; AST, aspartate transaminase; ALP, alkaline phosphatase; MDA, malondialdehyde; SBP, systolic blood pressure; DBP, diastolic blood pressure; CI, confidence interval; WMD, weighted mean difference.
Meta-regression analysis was used to assess how the dosage of saffron and the length of the intervention altered lipid profiles, glycemic profiles, blood pressure, inflammatory markers, anthropometric parameters, the immune system, and liver enzymes. Linear association was found between FBG and duration of intervention (coefficients = −0.29, P = 0.003; Figure 7E). There was no statistically significant linear association between the length and dosage of the intervention and changes in other outcomes (Figures 6 A–V, 7 A–D, F–V).
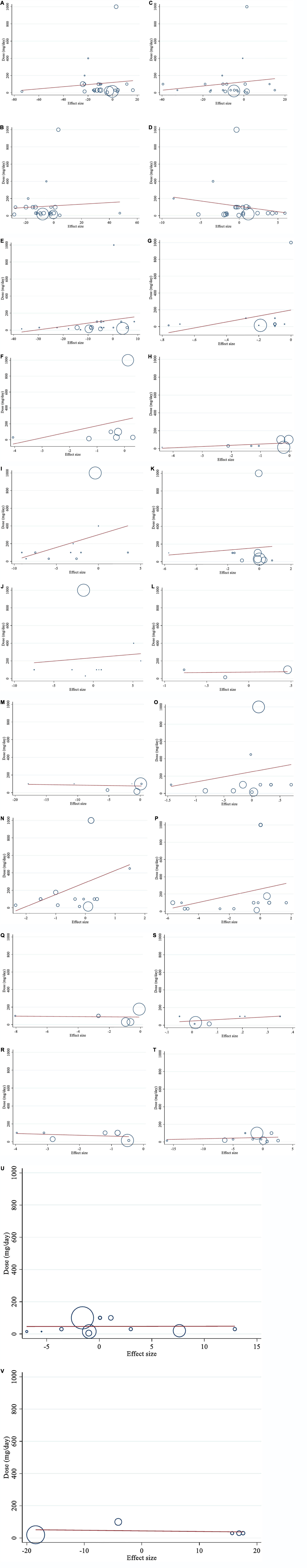
Figure 6. Linear dose-response relations between saffron consumption and absolute mean differences. Dose-response relations between dose (g/day) and absolute mean differences (A) TG (mg/dl); (B) TC (mg/dl); (C) LDL (mg/dl); (D) HDL (mg/dl); (E) FBG (mg/dl); (F) Insulin (miu/ml); (G) HbA1c (%); (H) HOMA-IR; (I) SBP (mmHg); (J) DBP (mmHg); (K) CRP (mg/l); (L); IL-6 (pg/ml); (M) TNF-α (pg/ml); (N) weight (kg); (O) BMI (kg/m2); (P) WC (cm); (Q) FM (%); (R) MDA (uM/L); (S) TAC (mM/L); (T) ALT (U/L); (U) AST (U/L) and (V) ALP (U/L). TG, triglyceride; TC, total cholesterol; LDL, low-density lipoprotein; HDL, high-density lipoprotein; FBG, fasting blood glucose; HOMA-IR, homeostasis model assessment for insulin resistance; HbA1C, hemoglobin A1C; CRP, C-reactive protein; IL-6, interleukin 6; TNF-α, tumor necrosis factor; TAC, total antioxidant capacity; BMI, body mass index; WC, waist circumference; FM, fat mass; ALT, alanine transaminase; AST, aspartate transaminase; ALP, alkaline phosphatase; MDA, malondialdehyde; SBP, systolic blood pressure; DBP, diastolic blood pressure; CI, confidence interval; WMD, weighted mean difference.
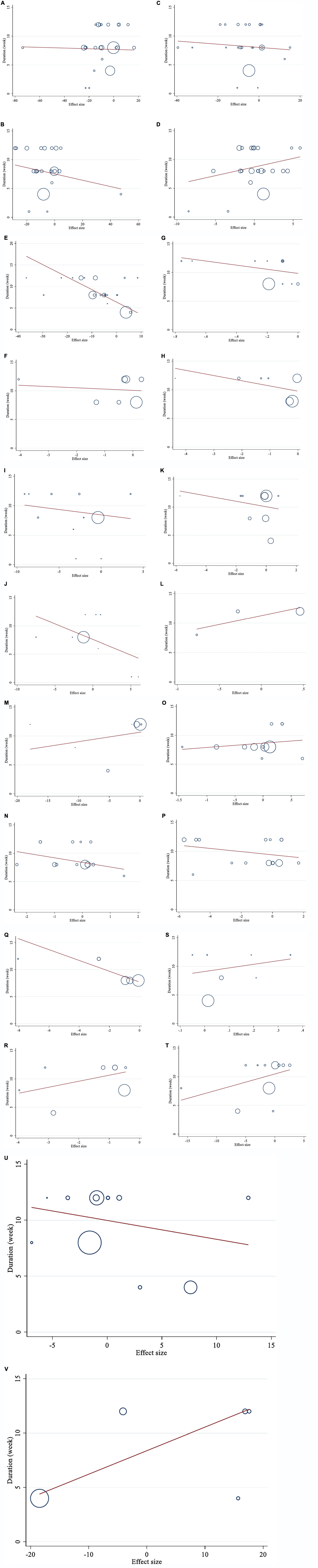
Figure 7. Linear dose-response relations between saffron consumption and absolute mean differences. Dose-response relations between duration of intervention (week) and absolute mean differences (A) TG (mg/dl); (B) TC (mg/dl); (C) LDL (mg/dl); (D) HDL (mg/dl); (E) FBG (mg/dl); (F) Insulin (miu/ml); (G) HbA1c (%); (H) HOMA-IR; (I) SBP (mmHg); (J) DBP (mmHg); (K) CRP (mg/l); (L); IL-6 (pg/ml); (M) TNF-α (pg/ml); (N) weight (kg); (O) BMI (kg/m2); (P) WC (cm); (Q) FM (%); (R) MDA (uM/L); (S) TAC (mM/L); (T) ALT (U/L); (U) AST (U/L) and (V) ALP (U/L). TG, triglyceride; TC, total cholesterol; LDL, low-density lipoprotein; HDL, high-density lipoprotein; FBG, fasting blood glucose; HOMA-IR, homeostasis model assessment for insulin resistance; HbA1C, hemoglobin A1C; CRP, C-reactive protein; IL-6, interleukin 6; TNF-α, tumor necrosis factor; TAC, total antioxidant capacity; BMI, body mass index; WC, waist circumference; FM, fat mass; ALT, alanine transaminase; AST, aspartate transaminase; ALP, alkaline phosphatase; MDA, malondialdehyde; SBP, systolic blood pressure; DBP, diastolic blood pressure; CI, confidence interval; WMD, weighted mean difference.
Findings regarding saffron consumption and lipid profiles, blood pressure, FBG, HbA1c, HOMA-IR, IL-6, weight, BMI, FM%, MDA, TAC, AST, and ALP remained robust in the sensitivity analysis. However, the significant effect of saffron on TNF-α, WC, and ALT disappeared when excluding the studies done by Ghiasian et al. (59) (WMD = −0.86, 95%CI: −2.19, 0.46) and Hamidi et al. (65) (WMD = −1.72, 95%CI: −3.45, 0.01) for TNF-α; Fadai et al. (A) (45) (WMD = −1.11, 95%CI: −2.36, 0.13), Fadai et al. (B) (45) (WMD = −1.08, 95%CI: −2.31, 0.14), Abedimanesh et al. (B) (49) (WMD = −1.29, CI 95%: −2.58, 0.01), Kermani et al. (52) (WMD = −1.18, 95%CI: −2.44, 0.07), and Ebrahimi et al. (57) (WMD = −0.87, 95%CI: −1.90, 0.16) for WC; Mohamadpour et al. (43) (WMD = −1.51, 95%CI: −3.20, 0.16), Parsi et al. (67) (WMD = −1.24, 95%CI: −2.83, 0.35), and Tajaddini et al. (15) (WMD = −2.52, 95%CI: −5.11, 0.06) for ALP. Sensitivity analysis indicated that exclusion of the articles done by Mohamadpour et al. (43) (WMD = −0.36, 95%CI: −0.65, −0.06), Azimi et al. (44) (WMD = −0.33, 95%CI: −0.66, −0.01), Mousavi et al. (A) (46) (WMD = −0.38, 95%CI: −0.74, −0.02), Mousavi et al. (B) (46) (WMD = −0.33, 95%CI: −0.66, −0.00), Ebrahimi et al. (57) (WMD = −0.33, 95%CI: −0.66, −0.00), and Shahbazian et al. (62) (WMD = −0.29, 95%CI: −0.57, −0.02) altered the overall effect of saffron on CRP concentration to a significant value. Additionally, the total effect of saffron on insulin was significantly changed by excluding the study by Fadai et al. (A) (45) (WMD = −0.61, 95%CI: -1.21, -0.01).
The GRADE system is used to grade the quality of the evidence by the outcome in Table 4. For TG, TC, LDL, FBG, HbA1c, HOMA-IR, SBP, weight, WC, MDA, TAC, and ALT, the quality of the evidence was very low. Additionally, the HDL, DBP, CRP, IL-6, TNF-, BMI, FM%, and AST evidence quality was low. Only for insulin and ALP were the evidence quality levels moderate and high, respectively.
There was no evidence of publication bias among the included articles assessing the effect of saffron on TG (PEgger = 0.077, PBegg = 0.413; Figure 3A), TC (PEgger = 0.950, PBegg = 0.916; Figure 3B), LDL (PEgger = 0.410, PBegg = 0.958; Figure 3C), HDL (PEgger = 0.352, PBegg = 0.958; Figure 3D), FBG (PEgger = 0.0.074, PBegg = 1.00; Figure 3E), HbA1c (PEgger = 0.866, PBegg = 0.273; Figure 3G), HOMA-IR (PEgger = 0.059, PBegg = 0.133; Figure 3H), DBP (PEgger = 0.529, PBegg = 0.348; Figure 3J), IL-6 (PEgger = 0.108, PBegg = 1.00; Figure 3L), TNF-α (PEgger = 0.130, PBegg = 1.00; Figure 3M), weight (PEgger = 0.183, PBegg = 0.702; Figure 3N), BMI (PEgger = 0.382, PBegg = 0.542; Figure 3O), WC (PEgger = 0.238, PBegg = 0.216; Figure 3P), MDA (PEgger = 0.105, PBegg = 0.138; Figure 3R), TAC (PEgger = 0.050, PBegg = 0.621; Figure 3S), ALT (PEgger = 0.403, PBegg = 0.131; Figure 3T), and AST (PEgger = 0.829, PBegg = 784; Figure 3U) levels, using Begg’s test and Egger’s tests. But among articles evaluating the impact of saffron on insulin (PEgger = 0.041, PBegg = 0.072; Figure 3F), SBP (PEgger = 0.042, PBegg = 1.00; Figure 3I), CRP (PEgger = 0.023, PBegg = 0.697; Figure 3K) FM% (PEgger = 0.001, PBegg = 0.060; Figure 3Q), and ALP (PEgger = 0.004, PBegg = 0.462; Figure 3V), publication biases were found.
The present study is a comprehensive systematic review and dose-response meta-analysis of the effects of saffron on all CVD risk factors. The results of 32 RCT with 1674 individuals showed that saffron intake can reduce TG, TC, LDL, FBG, HbA1c, HOMA-IR, SBP, CRP, TNF-α, WC, MDA, and ALT, and can elevate TAC levels. According to the subgroup analysis TG, TC, and LDL were reduced significantly in individuals with obesity, and FBG was reduced in overweight individuals. Moreover, participants with diabetes showed a significant reduction in FBG, HBA1c, and MDA levels by saffron supplementation. Saffron supplementation reduced LDL and SBP in individuals with abnormal baseline levels (LDL ≥ 100 mg/dl and SBP ≥ 120 mmHg), and reduced TG and TC in the categories of lower levels (TG < 150 mg/dl and TC < 200 mg/dl). This supplementation also reduced FBG in both categories of baseline higher and lower than 100 mg/dl. In the non-linear dose-response analysis, between dose for saffron intake and HDL, HOMA-IR, ALP, HbA1c, TNF-α, FBG, and weight was a significant association, and a significant linear association was seen between FBG and duration of saffron supplementation.
Saffron (crocus sativus) is a nutraceutical containing three phytochemical compounds including carotenoids (crocin and crocetin) that are responsible for saffron color, volatile oil component (safranal) that produces odor, and glycoside (picrocrocin) that is the bitter precursor for safranal (70–72). These different subtypes have different tastes, odors, absorption ways, and bioavailability (21). When the hydrophobic crocetin is esterified with two water-soluble sugars (gentiobioses), crocin will be produced which is water soluble and has a high bioavailability. The included studies in this meta-analysis have used two types of substances (saffron or crocin) for supplementation. According to the subgroup analysis, TG, CRP, MDA, and TAC were reduced only in the saffron group while LDL SBP, WC, and ALT were reduced in the crocin group. Both of these compounds could effectively reduce TC, FBG, and HbA1c.
This study revealed reductions in CRP and TNF-α but no changes in IL-6 were seen following the saffron intervention. A meta-analysis of 8 RCTs in 2021 by Asbaghi et al. did not reveal any significant impacts of saffron on CRP, TNF-α, and IL-6. However, significant reductions occurred in subgroups with higher baseline measures (CRP ≥ 3 mg/l and TNF-α ≥ 15 pg/ml), lower supplementation dosages (≤30 mg/day), and some other subgroups (26). The controversy can be due to the larger sample size of the present study. The limited number of included trials evaluating the effect of saffron on IL-6 (only three studies) hindered the implementation of subgroup analyses on IL-6. In the present study, the subgroups of non-diabetic individuals and intervention dosages of more than 100 mg/d showed significantly lower CRP and TNF-α levels. Moreover, there was a non-linear association between dose with TNF-α.
Saffron can inhibit serum levels of inflammatory markers such as nuclear factor kappa B (NF-kB), TNF-α, Interferon-gamma (IFN-γ), and some interleukins while acting as the agonist of peroxisome proliferator-activated receptor γ (PPARγ) (73). This medical spice can also downregulate key pro-inflammatory enzymes such as myeloperoxidase (MPO), inducible nitric oxide synthase (iNOS), cyclooxygenase-2 (COX-2), phospholipase A2, and prostanoids (73).
Saffron could reduce MDA levels, and enhance TAC according to our analysis. MDA and TAC reduced significantly in the subgroups of intervention dose of ≥100 mg/d and MDA reduced in the subgroup of trial duration ≥12 months. Oxidative stress, which means the loss of balance between oxidants and antioxidants in favor of oxidants, occurs when the environmental stressors become overwhelming or in case of not enough antioxidant capacity in the body (74). A meta-analysis by Morvaridzadeh et al in 2021 showed the beneficial effect of saffron on TAC and MDA in unhealthy patients (20).
The mechanism by which saffron can affect oxidative stress can be attributed to increasing the levels of glutathione reductase (75). Safranal is suggested to act against the aging process due to its antioxidant properties and can act as a remedy for hepatic ischemia-reperfusion (IR) injury via the prevention of high intracellular reactive oxygen species (ROS) concentration and restoring the content of antioxidant enzymes (76). Existing research shows that saffron can enhance some antioxidant enzymes such as catalase and superoxide dismutase (SOD) (77). Moreover, animal studies revealed the anti-toxicity effects of saffron in different tissues against natural or chemical toxins (78).
The finding of this study shows significant reductions in TG, TC, and LDL after saffron supplementation. However, no significant changes in HDL levels were seen. A meta-analysis of six RCTs by Asbaghi et al. in 2019 demonstrated similar results in the reduction of TG and TC but showed a significant increase in HDL and no changes in LDL levels which are in contrast with this study (25). Another dose-response meta-analysis of 14 RCTs in 2019 by Rahmani et al. showed results similar to the Asbaghi et al. study in TC and TG reduction, no changes in LDL levels, and an increase in HDL levels after long-term consumption of saffron according to the meta-regression analysis (79). The optimum dose of saffron supplementation was 400 mg/d for TG reduction in this study, while dose-response analysis in the present study was not significant for TG (79). The controversy between these two studies in 2019 and the present study in 2022 can be related to the higher sample size of the present study owing to the recently published RCTs (15, 67). Moreover, another meta-analysis of ten studies also published in 2019 by Pourmasoumi et al. showed no effect of saffron on lipid profile (22). According to the subgroup analysis, the reduction in TC, TG, and LDL was significant in individuals with BMI ≥ 30 (obese). This can be explained by the anti-inflammatory properties of saffron (75) since inflammatory markers are higher in individuals with obesity compared to normal weight (80). A non-linear association has been seen between HDL levels with both the dose and duration of saffron intervention.
An animal study on the effect of saffron (crocetin) against alcoholic fatty liver showed that this substance can enhance mitochondrial-β-oxidation, decline fatty sediment, and prevent lipid peroxidation (19). Moreover, saffron and crocin could effectively reduce hyperlipidemia parameters in rats (81) and humans (79). Saffron also reduces cholesteryl ester transfer protein (CETP) which is involved in the regulation of serum lipid profile (51).
In this study saffron significantly impacted FBG, HbA1c, and HOMA-IR while not affecting insulin levels. A meta-analysis of ten studies in 2019 by Pourmasoumi et al. showed a significant reduction in fasting plasma glucose (FPG) following saffron supplementation and no changes in fasting insulin level (22). Another meta-analysis by Rahmani et al. in 2020 also showed a reduction in FPG especially when the intervention duration exceeds 12 weeks, but could not show a reduction in HbA1c (23). In contrast, another meta-analysis of six RCTs by Asbaghi et al. in 2019 revealed no significant changes in FBG after the supplementation (25). This is in line with the meta-analysis by Roshanravan et al. in 2022 that could not reveal any impact of saffron on blood glucose (82). The existing controversy can be due to different sample sizes, different participant morbidities, or different types of supplementations. According to the subgroup analysis FBG and HbA1c reduced significantly in individuals with diabetes after the saffron intervention. This can be justified by the anti-inflammatory properties of saffron in individuals with diabetes (83). There was shown a non-linear association between FBG, HbA1c, and HOMA-IR with a dose of saffron supplementation.
Regarding the effect of saffron on blood glucose profile, this agent enhances glucose uptake and insulin sensitivity in muscle cells via the phosphorylation of AMPK (AMP-activated protein kinase), ACC (acetyl-CoA carboxylase), and MAPKs (mitogen-activated protein kinase) (18).
Our results showed a significant reduction in SBP and a non-significant reduction in DBP. In contrast, a meta-analysis of ten publications by Pourmasoumi et al. in 2019 showed a significant reduction in DBP and no changes in SBP following saffron supplementation. Another dose-response meta-analysis by Setayesh et al. in 2021 showed the effective impact of saffron on both SBP and DBP and mentioned that the impact of the supplementation on DBP is dependent on the duration of the intervention and the effect would be more in case of longer durations (84). Subgroup analysis of the present study showed that SBP reduces in individuals with baseline SBP ≥ 120, intervention duration ≥ 12, and intervention dose of <100. DBP significantly decreased in the subgroup of diabetic patients. The dose-response analysis revealed a significant association in DBP in the optimum duration of 2 weeks.
The effect of saffron on endothelial nitric oxide (NO) synthases can lead to the elevation of NO production and the lowering of blood pressure (85). Moreover, Crocetin can down-regulate intracellular adhesion molecule-1 (ICAM-1) protein expression (86). This effect can affect the renin-angiotensin system and lead to hypertension suppression (87).
According to this meta-analysis, saffron intake can significantly reduce WC but has no significant effect on weight, BMI, and FM. However, the non-linear dose-response analysis showed that the optimum dose of 450 mg/d of the intervention can reduce weight. A dose-response meta-analysis of 14 studies by Rahmani et al. in 2019 could not show any significant effect of this intervention on weight (79). This result can be interpreted as the intervention dose being lower than optimum in this study. The mean dose of saffron administered in the meta-analysis by Rahmani et al. was 160 mg/d (79). An animal study by Mashmoul et al. in 2014 compared the anti-obesity effect of crocin and saffron. After inducing obesity in rats with a high-fat diet for 12 weeks, the supplementation showed a beneficial effect of saffron on prospective food consumption and LDL/HDL ratio while crocin had a beneficial effect on lipid profile (TG, and TC), and lowered the rate of body weight gain (88). This is in line with the present study in which WC was reduced only in the crocin subgroup but not in the saffron group. The justification can be related to the higher antioxidant properties of crocin compared to the same weight of saffron since crocin is the carotenoid component responsible for the color of saffron (72).
The medical herb of saffron can regulate the expression of leptin and adiponectin in adipose tissue (89) and inhibit the secretion of pancreatic and gastric lipase that regulates fat absorption (90). This effect can reduce central adipose tissue accumulation and decrease blood circulating leptin levels, leading to higher satiety perception (90).
The liver enzymes ALT, AST, and ALP were assessed. Only ALT reduces after saffron supplementation according to this meta-analysis. However, the dose-response analysis showed that at the optimum dose of 20 mg/d ALP can be reduced. A meta-analysis of 12 RCTs by Karimi et al in 2021 showed no beneficial effect of saffron on the mentioned three liver function markers (91). Another meta-analysis of nine RCTs in 2022 by Mousavi et al. showed results similar to this analysis on AST, ALT, and ALP. However, the dose-response analysis did not show any relationships (92). The existing controversy can be due to different sample sizes or different supplementation types (crocin or saffron) in these studies.
Liver enzymes may rise above normal levels in healthy individuals or stay normal in liver diseases (93). Regarding this unstable nature of liver enzymes, the results of this study on the effect of saffron on ALT, ASP, and ALP should be interpreted carefully. Moreover, existing diseases can affect liver enzyme levels differently (93) and the participants of this meta-analysis had different morbidities.
This study is the first comprehensive systematic review and dose-response meta-analysis on the effect of saffron on all cardiovascular risk factors. The strengths of this study are the use of the risk of bias assessment, GRADE classification of quality of evidence, non-linear dose-response analysis, subgroup analysis, sensitivity analysis, and meta-regression analysis that enhance the accuracy of the results. Moreover, the adverse effects reported in the study were summarized. The studies were included based on inclusion criteria with a variety of participants which provided the possibility of subgroup analysis and also can make the results eligible to be generalized. The randomized placebo-controlled design of included studies and the double- or triple-blind design of most of them are other strengths. However, some limitations also exist. The contrasting findings may be due to different supplementation types of saffron (crocin, crocetin, safranal, and picrocrocin). Although all studies used randomization, information on allocation concealment, randomization efficiency, and withdrawal was not consistently reported. The included studies were significantly heterogeneous. Some of the current meta-analysis outcomes were secondary outcomes in RCTs. Moreover, regarding the considerable number of the included studies, the types of measurements for outcomes could be different. The intra-assay coefficient of variation and inter-assay variability for biochemical kits in different studies might lead to heterogeneous results. Most of the studies were conducted in Iran due to the use of this plant as a spice in cooked foods, and therefore it seems that it cannot be generalized to other countries. Similarly, the anthropometric indices were measured by different scales and differently trained persons in the included studies. In addition, the blood pressure had been taken in different positions (seated or standing posture, supine position) which is another limitation. It is suggested that combining saffron with starchy food can enhance its bioavailability (21). Therefore, different timing of supplementation in the included RCTs, whether it was consumed simultaneously with food or not, could lead to different results. Another point to be mentioned is the high risk of bias in some of the included trials, highlighting the need for more high-quality clinical trials in the future.
This systematic review and dose-response meta-analysis revealed the beneficial effects of saffron on cardiovascular risk factors including TG, TC, LDL, FBG, HbA1c, HOMA-IR, SBP, CRP, TNF-α, WC, MDA, TAC, and ALT. The non-linear dose-response analysis showed a significant association between dose for saffron intake with HDL, HOMA-IR, ALP, HbA1c, TNF-α, FBG, weight, and showed between the supplementation duration and HDL level, and DBP. Given the significant beneficial results, saffron seems to be an appropriate supplement and adjunct therapy along with other conventional medicine used for preventing or alleviating CVD risk factors.
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author/s.
MoZ designed the study. MoZ and OA developed the search strategy. MaZ, MN, and MN-S extracted the data and conducted the analyses. MoZ, FG, and AH drafted the manuscript. MoZ, MN-S, and OA assessed the risk of bias of the meta-analyses. FS and OA interpreted the results and revised the manuscript. All authors read and approved the final manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
CVD, cardiovascular disease; TG, triglyceride; TC, total cholesterol; LDL, low-density lipoprotein; HDL, high-density lipoprotein; FBG, fasting blood glucose; HOMA-IR, homeostasis model assessment for insulin resistance; HbA1c, hemoglobin A1c; CRP, C-reactive protein; IL-6, interleukin 6; TNF-α, tumor necrosis factor; TAC, total antioxidant capacity; BMI, body mass index; WC, waist circumference; FM, fat mass; ALT, alanine transaminase; AST, aspartate transaminase; ALP, alkaline phosphatase; MDA, malondialdehyde; SBP, systolic blood pressure; DBP, diastolic blood pressure; CI, confidence interval; WMD, weighted mean difference; MPO, myeloperoxidase; iNOS, inducible nitric oxide synthase; COX-2, cyclooxygenase-2; NF-kB, nuclear factor kappa B; IFN-γ, Interferon gamma; PPARγ, peroxisome proliferator-activated receptorγ; IR, ischemia-reperfusion; ROS, reactive oxygen species; SOD, super oxide dismutase; CETP, cholesteryl ester transfer protein; FPG, fasting plasma glucose; AMPK, AMP-activated protein kinase; ACC, acetyl-CoA carboxylase; MAPKs, mitogen-activated protein kinase; NO, nitric oxide; ICAM-1, intracellular adhesion molecule-1; GRADE, grading of recommendations assessment, development, and evaluation.
1. Eckel RH, Jakicic JM, Ard JD, De Jesus JM, Miller NH, Hubbard VS, et al. 2013 AHA/ACC Guideline on Lifestyle Management to Reduce Cardiovascular Risk. Circulation. (2014) 129(25_suppl_2):S76–99. doi: 10.1161/01.cir.0000437740.48606.d1
2. Roth GA, Abate D, Abate KH, Abay SM, Abbafati C, Abbasi N, et al. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. (2018) 392:1736–88.
3. Amini M, Zayeri F, Salehi M. Trend analysis of cardiovascular disease mortality, incidence, and mortality-to-incidence ratio: results from global burden of disease study 2017. BMC Public Health. (2021) 21:401. doi: 10.1186/s12889-021-10429-0
4. Barrios V, Castellanos M, Campuzano Ruiz R, Gómez Cerezo JF, Egocheaga Cabello I, Gámez JM, et al. Treatment patterns and use of healthcare resources of patients with atherosclerotic cardiovascular disease and hypercholesterolemia and patients with familial hypercholesterolemia in Spain: Protocol of the Reality study. Front Cardiovasc Med. (2022) 9:966049. doi: 10.3389/fcvm.2022.966049
5. Roth GA, Mensah GA, Johnson CO, Addolorato G, Ammirati E, Baddour LM, et al. Global burden of cardiovascular diseases and risk factors, 1990-2019: Update From the GBD 2019 Study. J Am Coll Cardiol. (2020) 76:2982–3021.
6. Mensah GA, Roth GA, Fuster V. The global burden of cardiovascular diseases and risk factors: 2020 and Beyond. J Am Coll Cardiol. (2019) 74:2529–32. doi: 10.1016/j.jacc.2019.10.009
7. Carnethon MR, Gidding SS, Nehgme R, Sidney S, Jacobs J, David R, et al. Cardiorespiratory fitness in young adulthood and the development of cardiovascular disease risk factors. JAMA. (2003) 290:3092–100. doi: 10.1001/jama.290.23.3092
8. Prasad DS, Kabir Z, Dash AK, Das BC. Cardiovascular risk factors in developing countries: A review of clinico-epidemiological evidence. CVD Prev Control. (2010) 5:115–23. doi: 10.1016/j.cvdpc.2010.09.001
9. Mozaffarian D, Wilson PW, Kannel WB. Beyond established and novel risk factors: lifestyle risk factors for cardiovascular disease. Circulation. (2008) 117:3031–8. doi: 10.1161/CIRCULATIONAHA.107.738732
10. Johnston TP, Korolenko TA, Pirro M, Sahebkar A. Preventing cardiovascular heart disease: Promising nutraceutical and non-nutraceutical treatments for cholesterol management. Pharmacol Res. (2017) 120:219–25. doi: 10.1016/j.phrs.2017.04.008
11. Rabito MJ, Kaye AD. Complementary and alternative medicine and cardiovascular disease: an evidence-based review. Evid Based Complement Alternat Med. (2013) 2013:672097. doi: 10.1155/2013/672097
12. Moshiri M, Vahabzadeh M, Hosseinzadeh H. Clinical applications of saffron (Crocus sativus) and its constituents: a review. Drug Res. (2015) 65:287–95. doi: 10.1055/s-0034-1375681
13. Arshad Husain R, Amjad Ali K, Yousef Homood A. Saffron (Crocus sativus) and its active ingredients: Role in the prevention and treatment of disease. Pharmacogn J. (2017) 9:873–9. doi: 10.5530/pj.2017.6.137
14. Elgazar AF, Rezq AA, Bukhari HM. Anti-hyperglycemic effect of saffron extract in alloxan-induced diabetic rats. Eur J Biol Sci. (2013) 5:14–22.
15. Tajaddini A, Roshanravan N, Mobasseri M, Aeinehchi A, Sefid-Mooye Azar P, Hadi A, et al. Saffron improves life and sleep quality, glycaemic status, lipid profile and liver function in diabetic patients: A double-blind, placebo-controlled, randomised clinical trial. Int J Clin Pract. (2021) 75:e14334. doi: 10.1111/ijcp.14334
16. Kianbakht S, Hajiaghaee R. Anti-hyperglycemic effects of saffron and its active constituents, crocin and safranal, in alloxan-induced diabetic rats. J Med Plants. (2011) 10:82–9.
17. Milajerdi A, Jazayeri S, Hashemzadeh N, Shirzadi E, Derakhshan Z, Djazayeri A, et al. The effect of saffron (Crocus sativus L.) hydroalcoholic extract on metabolic control in type 2 diabetes mellitus: A triple-blinded randomized clinical trial. J Res Med Sci. (2018) 23:16. doi: 10.4103/jrms.JRMS_286_17
18. Kang C, Lee H, Jung ES, Seyedian R, Jo M, Kim J, et al. Saffron (Crocus sativus L.) increases glucose uptake and insulin sensitivity in muscle cells via multipathway mechanisms. Food Chem. (2012) 135:2350–8. doi: 10.1016/j.foodchem.2012.06.092
19. Shi Y, Sheng L, Qian Z, Chen ZJ. Beneficial effects of crocetin on alcoholic fatty liver in rats and the mechanism. Chin J New Drugs. (2008) 17:2115–8.
20. Morvaridzadeh M, Agah S, Dulce Estêvão M, Hosseini AS, Heydari H, Toupchian O, et al. Effect of saffron supplementation on oxidative stress parameters: A systematic review and meta-analysis of randomized placebo-controlled trials. Food Sci Nutr. (2021) 9:5809–19. doi: 10.1002/fsn3.2463
21. Khorasany AR, Hosseinzadeh H. Therapeutic effects of saffron (Crocus sativus L.) in digestive disorders: a review. Iran J Basic Med Sci. (2016) 19:455–69.
22. Pourmasoumi M, Hadi A, Najafgholizadeh A, Kafeshani M, Sahebkar A. Clinical evidence on the effects of saffron (Crocus sativus L.) on cardiovascular risk factors: A systematic review meta-analysis. Pharmacol Res. (2019) 139:348–59. doi: 10.1016/j.phrs.2018.11.038
23. Rahmani J, Bazmi E, Clark C, Hashemi Nazari SS. The effect of Saffron supplementation on waist circumference, HA1C, and glucose metabolism: A systematic review and meta-analysis of randomized clinical trials. Complement Ther Med. (2020) 49:102298. doi: 10.1016/j.ctim.2020.102298
24. Tahmasbi F, Araj-Khodaei M, Mahmoodpoor A, Sanaie S. Effects of saffron (Crocus sativus L.) on anthropometric and cardiometabolic indices in overweight and obese patients: A systematic review and meta-analysis of randomized controlled trials. Phytother Res. (2022) 36:3394–414. doi: 10.1002/ptr.7530
25. Asbaghi O, Soltani S, Norouzi N, Milajerdi A, Choobkar S, Asemi Z. The effect of saffron supplementation on blood glucose and lipid profile: A systematic review and meta-analysis of randomized controlled trials. Complement Ther Med. (2019) 47:102158. doi: 10.1016/j.ctim.2019.07.017
26. Asbaghi O, Sadeghian M, Sadeghi O, Rigi S, Tan SC, Shokri A, et al. Effects of saffron (Crocus sativus L.) supplementation on inflammatory biomarkers: A systematic review and meta-analysis. Phytother Res. (2021) 35:20–32. doi: 10.1002/ptr.6748
27. Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. (2015) 4:1. doi: 10.1186/2046-4053-4-1
28. Green S, Higgins J, Alderson P, Clarke M, Mulrow C, Oxman A. Cochrane handbook for systematic reviews of interventions: Cochrane book series. Naunyn-Schmiedeberg’s Arch Exp Pathol Pharmakol. (2008) 5:S38.
29. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. (1986) 7:177–88. doi: 10.1016/0197-2456(86)90046-2
30. Namazi N, Larijani B, Azadbakht L. Low-Carbohydrate-Diet score and its association with the risk of diabetes: A systematic review and meta-analysis of cohort studies. Horm Metab Res. (2017) 49:565–71. doi: 10.1055/s-0043-112347
31. Brondani LA, Assmann TS, de Souza BM, Bouças AP, Canani LH, Crispim D. Meta-Analysis reveals the association of common variants in the uncoupling protein (UCP) 1–3 Genes with Body Mass Index Variability. PLoS One. (2014) 9:e96411. doi: 10.1371/journal.pone.0096411
32. Asbaghi O, Sadeghian M, Mozaffari-Khosravi H, Maleki V, Shokri A, Hajizadeh-Sharafabad F, et al. The effect of vitamin d-calcium co-supplementation on inflammatory biomarkers: A systematic review and meta-analysis of randomized controlled trials. Cytokine. (2020) 129:155050. doi: 10.1016/j.cyto.2020.155050
33. Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. (2005) 5:13. doi: 10.1186/1471-2288-5-13
34. Agency for Healthcare Research and Quality AHRQ. Methods for effective health care. methods guide for effectiveness and comparative effectiveness reviews. Rockville, MD: Agency for Healthcare Research and Quality (2008).
35. Fu R, Gartlehner G, Grant M, Shamliyan T, Sedrakyan A, Wilt TJ, et al. Conducting quantitative synthesis when comparing medical interventions: AHRQ and the Effective Health Care Program. J Clin Epidemiol. (2011) 64:1187–97. doi: 10.1016/j.jclinepi.2010.08.010
36. Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. (1994) 50:1088–101. doi: 10.2307/2533446
37. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. (1997) 315:629–34. doi: 10.1136/bmj.315.7109.629
38. Duval S. The trim and fill method. In: Rothstein HR, Sutton AJ, Borenstein M editors. Publication Bias in Meta-Analysis. Hoboken, NJ: John Wiley & Sons (2005). p. 127–44. doi: 10.1002/0470870168.ch8
39. Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. (2008) 336:924–6. doi: 10.1136/bmj.39489.470347.AD
40. Modaghegh MH, Shahabian M, Esmaeili HA, Rajbai O, Hosseinzadeh H. Safety evaluation of saffron (Crocus sativus) tablets in healthy volunteers. Phytomedicine. (2008) 15:1032–7. doi: 10.1016/j.phymed.2008.06.003
41. Gout B, Bourges C, Paineau-Dubreuil S. Satiereal, a Crocus sativus L extract, reduces snacking and increases satiety in a randomized placebo-controlled study of mildly overweight, healthy women. Nutr Res. (2010) 30:305–13. doi: 10.1016/j.nutres.2010.04.008
42. Mansoori P, Akhondzadeh S, Raisi F, Ghaeli P, Jamshidi AH, Nasehi AA, et al. A randomized, double-blind, placebo - controlled study of safety of the adjunctive saffron on sexual dysfunction induced by a selective serotonin reuptake inhibitor. J Med Plants. (2011) 10:121–30.
43. Mohamadpour AH, Ayati Z, Parizadeh MR, Rajbai O, Hosseinzadeh H. Safety evaluation of crocin (a constituent of saffron) tablets in healthy volunteers. Iran J Basic Med Sci. (2013) 16:39–46.
44. Azimi P, Ghiasvand R, Feizi A, Hariri M, Abbasi B. Effects of cinnamon, cardamom, saffron, and ginger consumption on markers of glycemic control, lipid profile, oxidative stress, and inflammation in Type 2 Diabetes Patients. Rev Diabetic Stud RDS. (2014) 11:258–66. doi: 10.1900/RDS.2014.11.258
45. Fadai F, Mousavi B, Ashtari Z, Ali beigi N, Farhang S, Hashempour S, et al. Saffron aqueous extract prevents metabolic syndrome in patients with schizophrenia on olanzapine treatment: a randomized triple blind placebo controlled study. Pharmacopsychiatry. (2014) 47:156–61. doi: 10.1055/s-0034-1382001
46. Mousavi B, Bathaie SZ, Fadai F, Ashtari Z, Ali Beigi N, Farhang S, et al. Safety evaluation of saffron stigma (Crocus sativus L.) aqueous extract and crocin in patients with schizophrenia. Avicenna J Phytomed. (2015) 5:413–9.
47. Nikbakht-Jam I, Khademi M, Nosrati M, Eslami S, Foroutan-Tanha M, Sahebkar A, et al. Effect of Crocin extracted from Saffron on Prooxidant-Antioxidant Balance in Subjects with Metabolic Syndrome: A randomized, placebo-controlled clinical trial. Eur J Integr Med. (2015) 8:307–12. doi: 10.1016/j.eujim.2015.12.008
48. Azimi P, Ghiasvand R, Feizi A, Hosseinzadeh J, Bahreynian M, Hariri M, et al. Effect of cinnamon, cardamom, saffron and ginger consumption on blood pressure and a marker of endothelial function in patients with type 2 diabetes mellitus: A randomized controlled clinical trial. Blood Pressure. (2016) 25:133–40. doi: 10.3109/08037051.2015.1111020
49. Abedimanesh N, Bathaie SZ, Abedimanesh S, Motlagh B, Separham A, Ostadrahimi A. Saffron and crocin improved appetite, dietary intakes and body composition in patients with coronary artery disease. J Cardiovasc Thorac Res. (2017) 9:200–8. doi: 10.15171/jcvtr.2017.35
50. Jafarnia N, Ghorbani Z, Nokhostin M, Manayi A, Nourimajd S, Razeghi S. Effect of Saffron (Crocus Satious L.) as an add-on therapy to sertraline in mild to moderate generalized anxiety disorder: A double blind randomized controlled trial. Arch Neurosci. (2017) 4:e14332. doi: 10.5812/archneurosci.14332
51. Javandoost A, Afshari A, Nikbakht-Jam I, Khademi M, Eslami S, Nosrati M, et al. Effect of crocin, a carotenoid from saffron, on plasma cholesteryl ester transfer protein and lipid profile in subjects with metabolic syndrome: A double blind randomized clinical trial. ARYA Atheroscler. (2017) 13:245–52.
52. Kermani T, Kazemi T, Molki S, Ilkhani K, Sharifzadeh G, Rajabi O. The efficacy of crocin of saffron (Crocus sativus L.) on the components of metabolic syndrome: A randomized controlled clinical trial. J Res Pharm Practice. (2017) 6:228–32. doi: 10.4103/jrpp.JRPP_17_26
53. Kermani T, Zebarjadi M, Mehrad-Majd H, Mirhafez SR, Shemshian M, Ghasemi F, et al. Anti-Inflammatory effect of Crocus sativus on serum cytokine levels in subjects with metabolic syndrome: A randomized, double-blind, placebo- controlled trial. Curr Clin Pharmacol. (2017) 12:122–6. doi: 10.2174/1574884712666170622082737
54. Sepahi S, Mohajeri SA, Hosseini SM, Khodaverdi E, Shoeibi N, Namdari M, et al. Effects of crocin on diabetic maculopathy: A placebo-controlled randomized clinical trial. Am J Ophthalmol. (2018) 190:89–98. doi: 10.1016/j.ajo.2018.03.007
55. Zilaee M, Soukhtanloo M, Ghayour-Mobarhan M, Shemshian M, Salehi M, Ferns GAA. Effect of saffron on serum leptin levels in patients with metabolic syndrome, a double-blind, randomized and placebo-controlled trial study. Prog Nutr. (2018) 20(1-S):140–4.
56. Ebrahimi F, Aryaeian N, Pahlavani N, Abbasi D, Hosseini AF, Fallah S, et al. The effect of saffron (Crocus sativus L.) supplementation on blood pressure, and renal and liver function in patients with type 2 diabetes mellitus: A double-blinded, randomized clinical trial. Avicenna J Phytomed. (2019) 9:322–33.
57. Ebrahimi F, Sahebkar A, Aryaeian N, Pahlavani N, Fallah S, Moradi N, et al. Effects of saffron supplementation on inflammation and metabolic responses in type 2 diabetic patients: A randomized, double-blind, placebo-controlled trial. Diabetes Metab Syndr Obes. (2019) 12:2107–15. doi: 10.2147/DMSO.S216666
58. Ghaderi A, Rasouli-Azad M, Vahed N, Banafshe HR, Soleimani A, Omidi A, et al. Clinical and metabolic responses to crocin in patients under methadone maintenance treatment: A randomized clinical trial. Phytother Res. (2019) 33:2714–25. doi: 10.1002/ptr.6445
59. Ghiasian M, Khamisabadi F, Kheiripour N, Karami M, Haddadi R, Ghaleiha A, et al. Effects of crocin in reducing DNA damage, inflammation, and oxidative stress in multiple sclerosis patients: A double-blind, randomized, and placebo-controlled trial. J Biochem Mol Toxicol. (2019) 33:e22410. doi: 10.1002/jbt.22410
60. Karimi-Nazari E, Nadjarzadeh A, Masoumi R, Marzban A, Mohajeri SA, Ramezani-Jolfaie N, et al. Effect of saffron (Crocus sativus L.) on lipid profile, glycemic indices and antioxidant status among overweight/obese prediabetic individuals: A double-blinded, randomized controlled trial. Clin Nutr ESPEN. (2019) 34:130–6. doi: 10.1016/j.clnesp.2019.07.012
61. Moravej Aleali A, Amani R, Shahbazian H, Namjooyan F, Latifi SM, Cheraghian B. The effect of hydroalcoholic Saffron (Crocus sativus L.) extract on fasting plasma glucose, HbA1c, lipid profile, liver, and renal function tests in patients with type 2 diabetes mellitus: A randomized double-blind clinical trial. Phytother Res. (2019) 33:1648–57. doi: 10.1002/ptr.6351
62. Shahbazian H, Moravej Aleali A, Amani R, Namjooyan F, Cheraghian B, Latifi SM, et al. Effects of saffron on homocysteine, and antioxidant and inflammatory biomarkers levels in patients with type 2 diabetes mellitus: a randomized double-blind clinical trial. Avicenna J Phytomed. (2019) 9:436–45.
63. Zilaee M, Hosseini SA, Jafarirad S, Abolnezhadian F, Cheraghian B, Namjoyan F, et al. An evaluation of the effects of saffron supplementation on the asthma clinical symptoms and asthma severity in patients with mild and moderate persistent allergic asthma: a double-blind, randomized placebo-controlled trial. Respir Res. (2019) 20:39. doi: 10.1186/s12931-019-0998-x
64. Behrouz V, Dastkhosh A, Hedayati M, Sedaghat M, Sharafkhah M, Sohrab G. The effect of crocin supplementation on glycemic control, insulin resistance and active AMPK levels in patients with type 2 diabetes: a pilot study. Diabetol Metab Syndr. (2020) 12:59. doi: 10.1186/s13098-020-00568-6
65. Hamidi Z, Aryaeian N, Abolghasemi J, Shirani F, Hadidi M, Fallah S, et al. The effect of saffron supplement on clinical outcomes and metabolic profiles in patients with active rheumatoid arthritis: A randomized, double-blind, placebo-controlled clinical trial. Phytother Res. (2020) 34:1650–8. doi: 10.1002/ptr.6633
66. Mobasseri M, Ostadrahimi A, Tajaddini A, Asghari S, Barati M, Akbarzadeh M, et al. Effects of saffron supplementation on glycemia and inflammation in patients with type 2 diabetes mellitus: A randomized double-blind, placebo-controlled clinical trial study. Diabetes Metab Syndr. (2020) 14:527–34. doi: 10.1016/j.dsx.2020.04.031
67. Parsi A, Torkashvand M, Hajiani E, Rahimlou M, Sadeghi N. The effects of Crocus sativus extract on serum lipid profile and liver enzymes in patients with non-alcoholic fatty liver disease: A randomized placebo-controlled study. Obes Med. (2020) 17:100165. doi: 10.1016/j.obmed.2019.100165
68. Pour FK, Aryaeian N, Mokhtare M, Mirnasrollahi Parsa RS, Jannani L, Agah S, et al. The effect of saffron supplementation on some inflammatory and oxidative markers, leptin, adiponectin, and body composition in patients with nonalcoholic fatty liver disease: A double-blind randomized clinical trial. Phytother Res. (2020) 34:3367–78. doi: 10.1002/ptr.6791
69. Tahvilian N, Masoodi M, Faghihi Kashani A, Vafa M, Aryaeian N, Heydarian A, et al. Effects of saffron supplementation on oxidative/antioxidant status and severity of disease in ulcerative colitis patients: A randomized, double-blind, placebo-controlled study. Phytother Res. (2021) 35:946–53. doi: 10.1002/ptr.6848
70. Bhattacharjee B, Vijayasarathy S, Karunakar P, Chatterjee J. Comparative reverse screening approach to identify potential anti-neoplastic targets of saffron functional components and binding mode. Asian Pac J Cancer Prev APJCP. (2012) 13:5605–11. doi: 10.7314/APJCP.2012.13.11.5605
71. Tung NH, Shoyama Y. New minor glycoside components from saffron. J Nat Med. (2013) 67:672–6. doi: 10.1007/s11418-012-0721-4
72. Srivastava R, Ahmed H, Dixit RK, Dharamveer, Saraf SA. Crocus sativus L.: A comprehensive review. Pharmacogn Rev. (2010) 4:200–8. doi: 10.4103/0973-7847.70919
73. Zeinali M, Zirak MR, Rezaee SA, Karimi G, Hosseinzadeh H. Immunoregulatory and anti-inflammatory properties of Crocus sativus (Saffron) and its main active constituents: A review. Iran J Basic Med Sci. (2019) 22:334–44.
74. Ji LL, Yeo D. Oxidative stress: an evolving definition. Faculty Rev. (2021) 10:13. doi: 10.12703/r/10-13
75. Naghshineh A, Dadras A, Ghalandari B, Riazi GH, Modaresi SM, Afrasiabi A, et al. Safranal as a novel anti-tubulin binding agent with potential use in cancer therapy: An in vitro study. Chemico-Biol Interact. (2015) 238:151–60. doi: 10.1016/j.cbi.2015.06.023
76. Pan TL, Wu TH, Wang PW, Leu YL, Sintupisut N, Huang CH, et al. Functional proteomics reveals the protective effects of saffron ethanolic extract on hepatic ischemia-reperfusion injury. Proteomics. (2013) 13:2297–311. doi: 10.1002/pmic.201200551
77. El-Beshbishy HA, Hassan MH, Aly HA, Doghish AS, Alghaithy AA. Crocin “saffron” protects against beryllium chloride toxicity in rats through diminution of oxidative stress and enhancing gene expression of antioxidant enzymes. Ecotoxicol Environ Saf. (2012) 83:47–54. doi: 10.1016/j.ecoenv.2012.06.003
78. Razavi BM, Hosseinzadeh H. Saffron as an antidote or a protective agent against natural or chemical toxicities. Daru. (2015) 23:31. doi: 10.1186/s40199-015-0112-y
79. Rahmani J, Manzari N, Thompson J, Clark CCT, Villanueva G, Varkaneh HK, et al. The effect of saffron on weight and lipid profile: A systematic review, meta-analysis, and dose-response of randomized clinical trials. Phytother Res. (2019) 33:2244–55. doi: 10.1002/ptr.6420
80. Ellulu MS, Patimah I, Khaza’ai H, Rahmat A, Abed Y. Obesity and inflammation: the linking mechanism and the complications. Arch Med Sci AMS. (2017) 13:851–63. doi: 10.5114/aoms.2016.58928
81. Asdaq SM, Inamdar MN. Potential of Crocus sativus (saffron) and its constituent, crocin, as hypolipidemic and antioxidant in rats. Appl Biochem Biotechnol. (2010) 162:358–72. doi: 10.1007/s12010-009-8740-7
82. Roshanravan B, Samarghandian S, Ashrafizadeh M, Amirabadizadeh A, Saeedi F, Farkhondeh T. Metabolic impact of saffron and crocin: an updated systematic and meta-analysis of randomised clinical trials. Arch Physiol Biochem. (2022) 128:666–78. doi: 10.1080/13813455.2020.1716020
83. Nasimi Doost Azgomi R, Karimi A, Zarshenas MM, Moini Jazani A. The mechanisms of saffron (Crocus sativus’) on the inflammatory pathways of diabetes mellitus: A systematic review. Diabetes Metab Syndr. (2022) 16:102365. doi: 10.1016/j.dsx.2021.102365
84. Setayesh L, Ashtary-Larky D, Clark CCT, Rezaei Kelishadi M, Khalili P, Bagheri R, et al. The effect of saffron supplementation on blood pressure in adults: A systematic review and dose-response meta-analysis of randomized controlled trials. Nutrients. (2021) 13:2736. doi: 10.3390/nu13082736
85. Mousavi SH, Tayarani NZ, Parsaee H. Protective effect of saffron extract and crocin on reactive oxygen species-mediated high glucose-induced toxicity in PC12 cells. Cell Mol Neurobiol. (2010) 30:185–91. doi: 10.1007/s10571-009-9441-z
86. Xiang M, Qian ZY, Zhou CH, Liu J, Li WN. Crocetin inhibits leukocyte adherence to vascular endothelial cells induced by AGEs. J Ethnopharmacol. (2006) 107:25–31. doi: 10.1016/j.jep.2006.01.022
87. Cottone S, Mulè G, Nardi E, Vadalà A, Lorito MC, Guarneri M, et al. C-reactive protein and intercellular adhesion molecule-1 are stronger predictors of oxidant stress than blood pressure in established hypertension. J Hypertens. (2007) 25:423–8. doi: 10.1097/HJH.0b013e3280112d0e
88. Mashmoul M, Azlan A, Yusof BNM, Khaza’ai H, Mohtarrudin N, Boroushaki MT. Effects of saffron extract and crocin on anthropometrical, nutritional and lipid profile parameters of rats fed a high fat diet. J Funct Foods. (2014) 8:180–7. doi: 10.1016/j.jff.2014.03.017
89. Ghaffari S, Roshanravan N. Saffron; An updated review on biological properties with special focus on cardiovascular effects. Biomed Pharmacother. (2019) 109:21–7. doi: 10.1016/j.biopha.2018.10.031
90. Shafiee M, Aghili Moghaddam NS, Nosrati M, Tousi M, Avan A, Ryzhikov M, et al. Saffron against components of metabolic syndrome: Current status and prospective. J Agric Food Chem. (2017) 65:10837–43. doi: 10.1021/acs.jafc.7b03762
91. Karimi E, Farrokhzad A, Darand M, Arab A. The effect of saffron consumption on liver function: A systematic review and meta-analysis of randomized controlled clinical trials. Complement Med Res. (2021) 28:453–62. doi: 10.1159/000515003
92. Mousavi SM, Mokhtari P, Asbaghi O, Rigi S, Persad E, Jayedi A, et al. Does saffron supplementation have favorable effects on liver function indicators? A systematic review and meta-analysis of randomized controlled trials. Crit Rev Food Sci Nutr. (2022) 62:6315–27. doi: 10.1080/10408398.2021.1900059
Keywords: saffron, cardiovascular risk factors, systematic review, meta-analysis, adult
Citation: Zamani M, Zarei M, Nikbaf-Shandiz M, Gholami F, Hosseini AM, Nadery M, Shiraseb F and Asbaghi O (2022) The effects of saffron supplementation on cardiovascular risk factors in adults: A systematic review and dose-response meta-analysis. Front. Nutr. 9:1055517. doi: 10.3389/fnut.2022.1055517
Received: 27 September 2022; Accepted: 15 November 2022;
Published: 08 December 2022.
Edited by:
Shuang Song, Dalian Polytechnic University, ChinaReviewed by:
Elaheh Foroumandi, Sabzevar University of Medical Sciences, IranCopyright © 2022 Zamani, Zarei, Nikbaf-Shandiz, Gholami, Hosseini, Nadery, Shiraseb and Asbaghi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Farideh Shiraseb, ZmFyaWRlaF9zaGlyYXNlYkB5YWhvby5jb20=; Omid Asbaghi, b21pZC5hc2JhZ2hpQGdtYWlsLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.