
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Nutr. , 22 November 2022
Sec. Food Chemistry
Volume 9 - 2022 | https://doi.org/10.3389/fnut.2022.1048520
Piper cubeba L.f. (Piperaceae), known as cubeb, is a popular traditional herbal medicine used for the treatment of many diseases, especially digestive and respiratory disorders. The plant is rich in essential oil, found mainly in fruits, and this makes it economically important. Many traditional utilizations have been also validated from the plant and its isolated compounds owing to their antioxidant, antibacterial, anti-inflammatory and anticancer effects. These biological activities are attributed to the phytochemicals (phenolic compounds, lignans and alkaloids) and the essential oil of the plant. The present work aims to provide an up-to-date review on the traditional uses, phytochemistry and pharmacology of the plant and discusses the future perspectives to promote its valorization for nutritional- and health-promoting effects.
Aromatic and medicinal plants (AMPs) have been used since antiquity as therapeutic and cosmeceutical agents (1). In addition, the vast ethnopharmacological applications of AMPs have inspired the current research to provide and discover new main drugs against various health disorders (2). The genus Piper belongs to the family Piperaceae which has more than 700 species. They are both erect and spreading herbs, shrubs, or rarely trees with great economic and medicinal importance (3). Piper cubeba is a native plant of Java and Borneo where the appellation of this plant is the Java pepper. It is one of the plants of the folk pepiraceae species that is used as a spice. The plant is cultivated for its berries, which are rich in essential oil (4). Economically, the plant is an important source of its dried berries as they have several applications in perfumes, cosmetics, and food preservatives (5). In Moroccan cuisine, cubeb is popular in savory dishes and pastries such as markouts and the famous Ras el hanout spice blend (a popular mixture of herbs and spices used throughout the Middle East and North Africa) (6). Cubeb is marketed by a Swiss company as a refreshing agent and used in various products such as chewing gums, alcoholic and soft drinks, sherbets, gelatin confectionery, and toothpaste (6). Cubeb is also used to flavor alcoholic and non-alcoholic drinks such as Bombay Sapphire gin and Pertsovka, a Russian pepper vodka which is prepared from a cubeb infusion (6). Traditionally, P. cubeba is used for the treatment of gonorrhea, dysentery, syphilis, abdominal pain, diarrhea, enteritis, and asthmatic diseases (5). The plant possesses also outstanding pharmacological activities. For instance, P. cubeba’s essential oil furnished antiparasitic, antimicrobial, and insecticidal activities (7). In addition, different extracts from the plant demonstrated antioxidant, antimicrobial, nephroprotetive, hepatoprotective, and anti-inflammatory activities (8). These biological activities are due to its chemical composition, especially, phenolic acids and flavonoids, that have been detected in P. cubeba extracts (9–11). The plant is also a rich source of lignans particularly cubebin, a bioactive compound with a wide range of biological activities such as antimicrobial, anticaner, and neuroprotective, among others (5, 12). Overall, the most reported modes of action by which P. cubeba extracts exert its biological activities involve many intracellular targets, among them the regulation of genes expression, inhibition of oxidative stress, induction of apoptosis and quorum sensing inhibition in pathogenic microbes.
The present review aims to collect and collate the available literature on the botany of plant and provide an insight about its chemical composition and diverse biological activities including antioxidant, anti-inflammatory, antibacterial, wound healing, antidiabetic, and renoprotective activities as well as its agricultural applications. It also highlights future perspectives to further maximize the exploitation of the plant in nutraceuticals, cosmeceuticals, and food applications.
The literature search was conducted through the Web of science, Scopus, PubMed, SciFinder, and other databases. The keywords used included “Piper cubeba,” “chemical composition,” “pharmacological properties,” and “biological activities.” Information has been collected from relevant textbook, reviews, and documents. Duplicated and irrelevant works were excluded as well as non-English documents, and those with unavailable full text (Figure 1). The species name was checked based on the online database.1 Various types of information regarding P. cubeba are discussed in corresponding parts of the paper.
P. cubeba is one of the most popular species of the Piperaceae family and the most widespread population of this species is generally found in Indonesia, India, medieval Europe, and North Africa (Figure 2) (5, 13). Cubeb is a woody climbing perennial that has stem and ashy-grey climbing branches. The length is 5–15 m high. The leaves are ovate with cordate or rounded base, glabrous with a thick pedicle, simple, smooth, and pointed at the apex, the lower surface is densely provided with tiny glands embedded. They are completely margined, tough and up to 15 cm long and 6 cm wide (Figure 3A) (5, 9). The flowers are small, dense unisexual that are glued to the peduncles, arranged in 4 cm long scaly spikes that have 2–3 stamens. The female tips have about 50 individual flowers with an ovary of 4 carpels fused with 4 sessile stigmas. Flowering takes place in winter (Figure 3B) (5). The fruits are globose from 6 to 8 mm in diameter. The upper part of the fruit has a diameter of 3–6 mm and covered by grayish brown, pericarp that extends at the base into a straight stem. They have a spicy, aromatic smell and a bitter taste. The fruit has a single dark brown sub-globose seed with a width of 3–4 mm (9) (Figure 3C). The plant has different vernacular names depending on its distribution (Table 1).
Piper species are characterized by the production of typical phytochemical compounds such as benzoic acids, amides, chromenos, terpenes, phenylpropanoids, lignans, alkaloids, fatty acids, and hydrocarbons (14). The alkaloid piperine and the two lignans cubebin and hinokinin are the most abundant compounds from the berries (15).
Altogether, 28 lignans were annotated from P. cubeba (leaves, berries, stalks) using GC, GC-MS, HPLC, and NMR. Out of which, 4 lignans were detected in all plant parts (leaves, berries, stalks), 9 lignans were found only in the leaves and berries, 2 lignans were solely characterized from the leaves and 13 lignans from the berries (Table 2) (5, 16, 17). Cubebininolide, hinokinin, yatein, and isoyatein, which represent the furanofuranic family, are the most known in Piper species and were identified in the berries, leaves, and stalks. Ashantin, clusin, cubebin, cubebinone, among others, were identified from the leaves and berries, while the hemiarensin was detected only in leaves (5). Yatein was the most predominant lignan in the berries, more than cubebin, while hinokinin was the most abundant lignan in the leaves and the stem from the Indonesia (5) (Figure 4). Noteworthy, the phytochemical profiling of the plant was mainly annotated from Indonesian flora and most of the studies targeted the extraction and identification of lignans.
Altogether, 91 volatile compounds were characterized in the essential oil, oleoresin, ethanol, and dichloromethane extracts from P. cubeba. Methyl eugenol (41.31%), eugenol (33.95%), beta-cubebene (18.3%), and alpha-cubebene (4.1%) dominated the essential oil while cubebol (26.1%) and beta-cubebene (12.3%) were the major compounds indentified from the oleoresin. As for ethanol extract, copaene was the most dominant compound that represented 13.47% of the extract followed by napthalene, 1,2,3,5,6,8a-hexahydro with 10.36%. Significant differences were detected between the extracts (Table 3). For instance, α-cubebene was found to be 4.1% in the essential oil content, 3.5% in the oleoresin, and 2.07% in the ethanolic extract; however, it was not detected at the dichloromethane extract. Copaene, another example, was detected only at the ethanol extract with 13.47% (18). Propylene glycol dominated the dichloromethane with 23.82%; however, it was not found in all other extracts (19). These differences might be attributed to the different extraction methods, detection techniques as well as geographic distribution and genetic chemotypes. Noteworthy, further experiments are required to annotate the non-volatile constituents of the extracts such as the ethanol extracts.
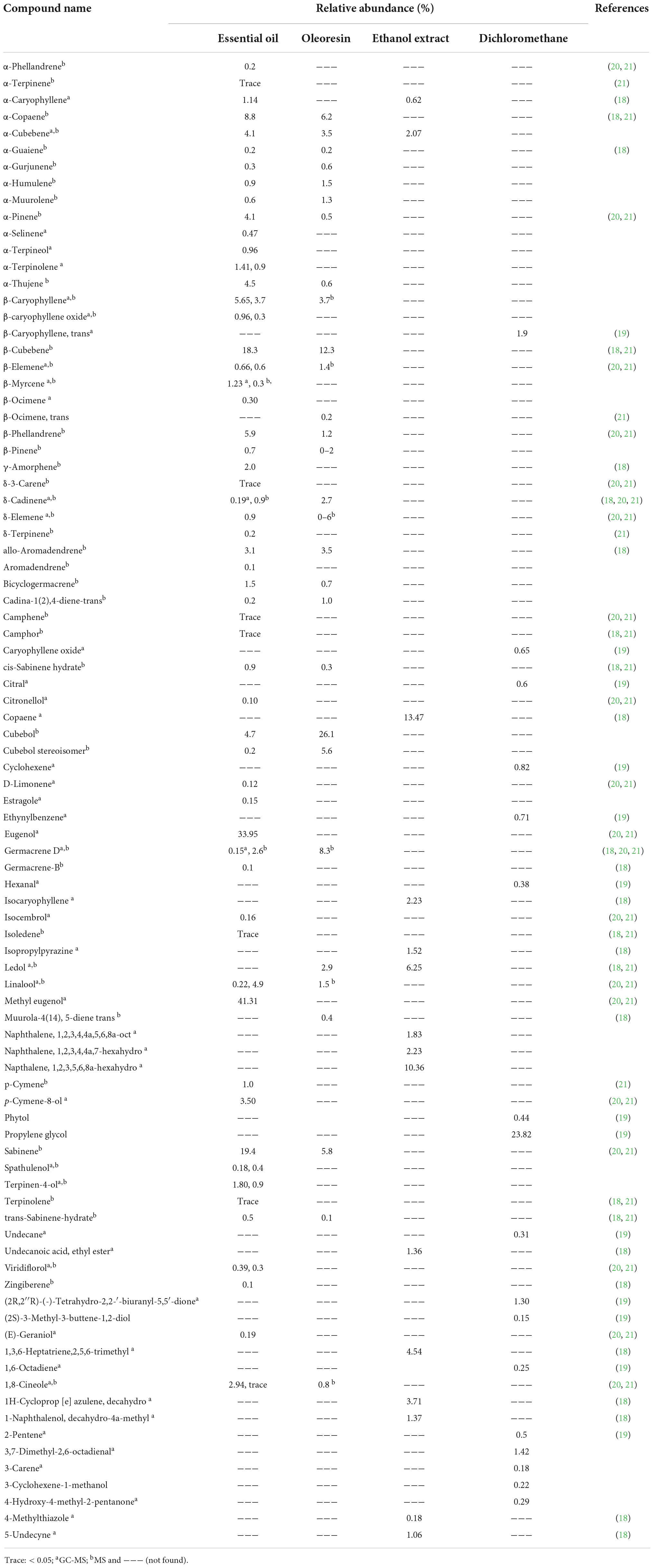
Table 3. Identified compounds from essential oil, oleoresin, ethanol and dichloromethane extracts of P. cubeba berries.
Altogether, 19 fatty acids along with their esters were annotated from P. cubeba berries. They include dodecanoic acid (lauric acid, 24.05%), hexadecanoic acid (palmitic acid, 11.37%), 9-octadecenoic acid (10.00%), decanoic acid (capric acid, 2.62%), 9,12-octadecadienoic acid (Z,Z) (2.50%), octadecanoic acid (2.08%), methyl decanoate (capric acid methyl ester, 1.80%), tetradecanoic acid (myristic acid, 1.66%), ethyl-(R,E)-4-hydroxy-3-methylpent-2-enoate (1.12%), along with other compounds where their concentrations did not exceed a relative abundance of 1 like palmitic acid methyl ester (0.65%) and octanoic acid (caprylic acid) (0.18%) (19). The dichloromethane fraction gave the presence of two phenolic compounds which are 4-vinylphenol and 2,4-bis(1,1-dimethylethyl)-Phenol (19). Besides, several flavonoids and phenolic acids were isolated from the aqueous extract of P. cubeba fruits such as rutin, catechin, gallic acid, caffeic acid, syringic acid, ferulic acid (20, 21).
Determination of total phenolic contents from piper fruit was investigated using the Folin-Ciocalteu method. It amounted 123.1 and 185.65 μg of GAE/g extract from the ethanolic extract (20, 21), and 1,280 μg of GAE/g extract from the for the methanolic extract (20, 21). The quantification of flavonoids was carried out as well and amounted 65.83 μg QE/g extract from in ethanolic extract (20, 21). P. cubeba fruit aqueous extract contained zinc (Zn), selenium (Se), magnesium (Mg), phosphorus (P), iron (Fe), and manganese (Mn) (22).
P. cubeba is extensively used in several ways as powder, decoction, as an essential oil for numerous purposes. The fruit is frequently used to treat various diseases such as gastro-tonic and abdominal pain, anti-asthmatic, and sedative. In Morocco, the plant has been listed among the medicinal plants used in cancer treatment. Many of these traditional uses were supported by scientific evidence. These include antibacterial, nematocidal, analgesic, and anticancer activities. Moreover, P. cubeba fruits are widely exploited in spice market, and also used as food preservative, coloring aid and in cosmetics (9–11).
Several studies have shown that P. cubeba extracts, essential oils, and their constituents are endowed with many biological and pharmacological properties such as antioxidant, anti-inflammatory, antidiabetic, anticancer, reno-hepatoprotective, immunomodulatory, antidepressant, antimicrobial, anti-parasite, insecticidal, wound healing, and antidepressant activities. However, plant constituents can interact with biological components in the cells such as proteins and nucleic acids, toxicity studies are mandatory to ensure the beneficial and biosafety effects of the tested materials. In this section, we address the toxicity of P. cubeba, describe the biological and pharmacological activities of its extracts and/or compounds, underline some mechanisms of action, and discuss major findings.
Toxicity assessment of P. cubeba extracts were reported in many studies. For instance, it was shown that, using 3-(4,5-dimethylthiazol-2yl)-2,5-diphenyltetrazolium bromide (MTT) assay, P. cubeba extracts were not toxic to RAW 264.7 cells (monocyte/macrophage-like cells). In addition, normal oral fibroblasts treated with P. cubeba based compounds mainly methylcubebin, dihydrocubebin, and hinokinin showed neither cytotoxicity signs nor morphological changes (12). In vivo, it was found that the female Wister rats fed with the methanol extract of P. cubeba fruits were safe up to a maximum dose of 2,000 mg/kg body weight. It induced neither changes in behavioral patterns nor signs and symptoms of toxicity nor mortality (23). In another study, (−)-hinokinin, obtained by partial synthesis from (−)-cubebin isolated from the fruits of P. cubeba, was orally administered (1 mL/rat) to male Wistar rats daily for 1 week. This treatment did not cause any significant weight or water intake changes during the period of the experiment (24). Elsewhere, male Wistar rats subjected to treatment by P. cubeba essential oil ranging from 50 to 3,000 mg/kg showed no mortality nor overall behavioral alteration such as shaking, convulsion, writhing, chewing, pupil size, feeding behavior, and fecal output (25). The biosafety status was also monitored using other Piper species such as P. longum L. fruits which caused no significant acute (24 h) or chronic (90 days) mortality in mice (26). Likewise, the leaf extract from P. betle was nontoxic on the glyoxalase system of Swiss albino mice after 2 weeks of oral administration at 1.5 and 10 mg/kg (27).
Plant constituents are commonly known as the best source of antioxidants that neutralize reactive oxygen species (ROS) and free radicals (28). Consequently, an increasing attention is given to the antioxidant potential of plant-based molecules and their role in benefiting health and preventing aging and oxidation-related diseases (29). Like other plants, the antioxidant activities of P. cubeba extracts and essential oil were widely evaluated (Table 4). Many in vitro assays were used, mainly 2,2-diphenyl-1-picrylhydrazil (DPPH) radical scavenging, ferric-reducing antioxidant power (FRAP), β–carotene bleaching, thiobarbituric acid reactive substances (TBARS), phosphomolybdenum, CUPRAC (Cupric reducing antioxidant capacity), and total antioxidant capacity (TAC) assays (Table 4).
The antioxidant capacity of six extracts from P. cubeba fruits (petroleum ether, benzene, ethyl acetate, acetone, methanol, and ethanol) were evaluated in vitro (18). At 200 μg/mL, it was noticed that the ethanolic extract was the most potent in inhibiting DPPH, followed by acetone and ethyl acetate extracts at the highest concentration tested. Comparable pattern of antioxidant activities between extracts was observed using other methods such FRAP and CUPRAC assays. The antioxidant effect was also reported using P. cubeba essential oil. In fact, essential oil at 500 μg/mL elicited a good radical scavenging activity (84%) compared to ascorbic acid (92%), the reference antioxidant compound (25). Similarly, the radical scavenging activity induced by P. cubeba essential oil using DPPH and ABTS assays was up to 38.69% higher than the one that was exhibited by the essential oil of P. nigrum L. (7). Three other Piper species, namely P. guineense Schum and Thonn, P. nigrum L. and P. umbellatum L. showed also endowed scavenging activity (up to 89.9% inhibition) and metal chelating activity (up to 93.9% inhibition) (30). Consequently, Piper species can be considered as a great source of modulators of free radical induced disorders.
As several cancer chemotherapeutics are derived from plant-based molecules (31), multiple studies have explored the antitumor and cytotoxic activity of P. cubeba extracts against model cancer cells using several methods, mainly MTT test. For instance, the dichloromethane extract from P. cubeba fruits induced apoptosis on triple negative breast cancer cell lines (MCF-7 and MDA-MB-231), colon cancer cells (HT-29), cholangiocarcinoma cells (KKU-M213), with less cytotoxicity against normal fibroblast (L929). Sequential extraction showed that one fraction, named dichloromethane 15 (DE15), significantly enhanced multi-caspases activity in the breast cancer cell line MDA-MB-231 in a time-dependent fashion (19). Similarly, Graidist et al. (32) showed that the methanolic crude extract of P. cubeba fruits exhibited a better cytotoxic activity against MDA-MB-468 and MCF-7 breast cancer cell lines than the dichloromethane crude extract. From the six fractions, the most active fraction had an IC50 of > 4 μg/mL. DNA fragmentation assay demonstrated an apoptosis pattern in MCF-7, MDA-MB-468, MDA-MB-231, and L929 cancer lines, but not in MCF-12A normal cells (32).
Several compounds extracted from P. cubeba have been explored for their anticancer and cytotoxic potential, among them (–)-cubebin and its derivatives. Niwa et al. (33) studied the safety profile of (–)-cubebin by testing its cytotoxicity, mutagenicity, cell proliferation kinetics, and induction of apoptosis in human colon adenocarcinoma cells (HT29). MTT assay showed that (–)-cubebin was cytotoxic at 280 μM, whereas no cytotoxicity was demonstrated below 28 μM. In addition, micronucleus assay revealed that (–)-cubebin was not mutagenic, did not alter cell-growth kinetics over 4 days, and absence of induced apoptosis after 24 h (33). Moreover, the effect of P. cubeba extract and its major lignans (cubebin, dihydrocubebin, ethylcubebin, hinokinin, and methylcubebin) were evaluated on the larynx (Hep-2) and oral (SCC-25) squamous carcinoma cells and normal fibroblasts. They all decreased cell proliferation and migration with no change in cellular morphology and no genotoxic effects. This was attributed to the alteration of the expression of genes and proteins involved in the inflammatory process (12). This study concluded that cubebin and methylcubebin isolated from P. cubeba had a great effect on the proliferation, migration, and genotoxic profile of the head and neck cancer cells. Next to P. cubeba, the ethanolic extract P. nigrum L. was toxic to MCF-7 cells likely through calf thymus DNA (CT DNA) intercalation and damage (34). Additionally, compounds from Korean P. kadsura A549 such as kadsuketanone A, piperolactam A, and piperolactam B elicited a cytotoxic effect toward the SK-OV-3 (ovarian cancer cells), A549 (non-small cell lung adenocarcinoma), SK-MEL-2 (skin melanoma), and HCT-15 (colon cancer cells) cell lines (35). Many other Piper plants were reported to be used traditionally to treat cancer or cancer-like symptoms. These include P. aduncum L. for skin tumors (36), P. longum L. for breast cancer (37), P. nigrum L. for abdominal, respiratory, or gastric tumors/cancers (38–40). A plenty of extracts and compounds from the genus Piper were proved to be cytotoxic against cancer cells. For instance, amide alkaloids represent up to 53% of the most bioactive compounds. Outstandingly, piperlongumine showed excellent toxicity against dozens of cancer cell lines both in vitro and in vivo (41). Hence, conducting clinical anticancer studies on Piper plants, among them P. cubeba, and their bioactive principles seems to be worthwhile.
Several disorders and diseases are linked to the inflammatory responses including diabetes mellitus, rheumatoid arthritis, neurodegenerative diseases, and cancer (42). Many anti-inflammatory agents were isolated from plants such as curcumin, quercetin, capsaicin, resveratrol, and epigallocatechin-3-gallate (43). The anti-inflammatory activity of P. cubeba was studied in various studies both in vitro and in vivo. For instance, Mazlan et al. evaluated the anti-inflammatory effect of P. cubeba extracts and fractions by monitoring the nitric oxide (NO) production in lipopolysaccharide (LPS) RAW 264.7 cells. Compared to untreated cells, those treated with P. cubeba extracts and fractions showed a significant reduction in NO production up to 74.17%. The methanolic extract was the most potent in reducing NO production (44). Similarly, P. cubeba methanolic extract decreased NO production in macrophage RAW264.7 and HEK293T cells without any evidence of cell toxicity. In addition, it inhibited the expression level of proinflammatory cytokines such as iNOS and IL-6, downregulated NF-κB activation, and reduced the phosphorylation of IκBα, IKKα/β, Akt, p85, Src, and Syk (45). Interestingly, molecules such as 5-acetyl-2,3-dihydro-7-methyl-1H-pyrrolizine were identified in P. cubeba fruits aqueous extract and shown to reduce LPS-induced inflammation and inhibit LDL oxidation (46).
Some mechanisms underlying the inflammatory effect of P. cubeba, especially its essential oil, were studied using carrageenan induced pleurisy in rats (25). At 600 mg/kg, the essential oil substantially reduced the paw edema, the weight of cotton pellet granuloma, and the exudate volume. In addition, the level of polymorphonuclear cells was decreased as well as lung tissue myeloperoxidase, NO, and proinflammatory cytokines such as TNFα and IL-1β. The anti-inflammatory effects observed in vivo were attributed to the fact that P. cubeba essential oil contains sabinene, γ-terpinene, 4-terpineol, and α-thujene, known to be endowed with antioxidant and anti-inflammatory properties. Comparatively, the bioactive n-hexane and methanolic extracts from P. kadsura aerial parts were found to contain kadsuketanone A, ent-germacra-4(15),5,10(14)-trien-1β-ol, aristolactam A II, trans-2,3-diacetoxy-1-[(benzoy1oxy)methyl]-cyclohexa-4,6-diene, and piperolactam A and B. Both extracts induced a significant inhibition of both PGE2 and NO production in the LPS-activated microglia cells (35). Thus, expanding the research on the anti-inflammatory potential of Piper species can be a promising strategy to develop Piper-derived drugs or adjuvant medicines suitable for the treatment of inflammation-related disorders.
Diabetes is one of the most prevalent health problems worldwide (47). It has serious health consequences leading to increasing mortality. Synthetic anti-diabetic drugs have unavoidable side effects. Therefore, medicinal plants and their active components can act as alternative anti-diabetic medicines. Many plants are renowned for antidiabetic potential including P. cubeba (47–49). Noteworthy, the role of P.cubeba in the management of diabetes has been underexploited and is yet to receive sound scientific interest.
Muchandi et al. (50) demonstrated that the ethanolic extract of P. cubeba fruits administered to Albino rats protected them against D-galactose induced neuronal lipofuscinogenesis (51). In fact, using a dose of 400 mg/kg, p.o., of P. cubeba fruits significantly reduced lipofuscin fluorescence from the hippocampus region of animals comparatively to D-galactose treated rats. In addition, a decrease in the accumulation of lipofuscin granules in hippocampus of animals’ brains was observed in P. cubeba treated group. Observed effect was suggested to be due to the richness of the extract in lignans, mainly cubebin, hinokinin, yatein, and isoyatein, that are known as strong antioxidants.
As the intestinal enzymes α-amylase and α-glucosidase are key targets in the regulation of diabetes mellitus, P. cubeba extracts were reported as digestive enzymes inhibitors. It was found that the methanolic and aqueous extracts at 1 mg/mL were able to significantly inhibit α-glucosidase and α-amylase in vitro (48). Moreover, the anti-diabetes activity of both extracts has been suggested to be likely associated with their antioxidant properties. Next to P. cubeba, the root aqueous extract of P. longum administered by intraperitoneal route to streptozotocin induced diabetic male Wister albino rats at 200 mg/kg, b.w for 30 days, decreased, by 66.7%, the fasting blood glucose. These studies justify the traditional use of Piper species, including P. cubeba, and open up promising avenues for the management of diabetes and related complications.
Nowadays, the increasing prevalence of antibiotic resistance is one of the major challenges for public health worldwide. It has been attributed to the over- and misuse of antibiotics, as well as a declining trend in novel drug development by the pharmaceutical industry and challenging regulatory requirements (52, 53). As plants represent a great resource in drug discovery, for being mostly biocompatible, biodegradable, and less cytotoxic, their extracts and secondary metabolites are being widely explored to discover potential next antimicrobials (29, 54).
Extracts and compounds from P. cubeba parts, mainly fruits, were largely evaluated for antimicrobial activity (Table 5). Using four extracts (acetone, hexane, methanol, and ethanol) from P. cubeba fruits, Akshita et al. (55) found that all extracts showed high to moderate antibacterial activity against Klebsiella sp., Staphylococcus aureus, Escherichia coli, Enterococcus sp., Enterobacter sp., and Pseudomonas aeruginosa except hexane extract which exhibited no activity (Table 5). The best effects were observed toward Enterococcus sp. followed by E. coli and P. aeruginosa (55). Similar observation was reported that the hexane extract was not active in inhibiting different microbial strains (56). Noteworthy, P. cubeba extracts were more effective on Gram-positive than against Gram-negative bacteria. This is due to the single-layer cell wall in Gram-positive strains in contrast to the multilayered cell wall of Gram-negative bacteria that constitutes a barrier for the invasion of antimicrobial agents through the cell membrane.
Moreover, P. cubeba essential oil was also shown to be endowed with good antibacterial activity against methicillin-resistant S. aureus ATCC 43300 (Table 5). This was evaluated using atomic force microscopy and transmission electron microscopy. At 50 μg/mL, the essential oil severely damaged the bacterial cell walls while it was not active at microscopic levels at 25 μg/mL. However, at nanoscopic levels, it induced significant perturbation in the bacterial cell wall. These effects on the cell wall and plasma (cytoplasmic) membrane are likely to be the way by which this essential oil impaired bacterial activity (57).
Elsewhere, P. cubeba essential oil induced anti-Helicobacter pylori activity (MIC = 7.81 μg/mL) and thereby proposed as a therapeutic agent to protect and/or treat H. pylori infection (58). P. cubeba methanolic extract was also tested as a natural food preservative against microbial population in tofu using total plate counting (TPC) method. A decrease of upper to 3 Log10 CFU/g of TPC was observed against B. cereus, coliform and E. coli in tofu treated with 0.5% of the extract for 4 h. P. cubeba L. berries were suitable for use as a natural preservative to reduce the microbial load in raw food (59).
It was suggested that the essential oil targets the cell wall of bacterial cells, whereas the extracts attack and destroy the peptidoglycan causing cell collapse. P. cubeba essential oil injures the cell wall−anchored proteins that are involved in biofilm formation and adhesion. It could also injure the cytoplasmic membrane (57). Other Piper plants were reported for their huge antibacterial spectrum (60). For instance, Piper nigrum L. methanolic and chloroform extracts inhibited E. coli, S. aureus, S. typhi, and Proteus sp. except P. aeruginosa which was resistant to both extracts (61). Likewise, the leaf ethanolic extract of Piper betel L. exhibited pronounced antibacterial activities toward B. subtilis, S. aureus, E. coli, and moderate inhibition of P. aeruginosa. The aqueous extract was also tested and was active only against B. subitilis (62). These studies corroborate the traditional use of Piper plants, including P. cubeba, in managing infectious diseases. Therefore, it could serve as a source of novel therapeutic agents against human pathogenic bacteria and food borne pathogens. Alqadeeri et al. (63) isolated and identified, for the first time, two compounds, β-asarone, and asaronaldehyde, from the methanolic extract of P. cubeba and its fractions. Both compounds inhibited the growth of B. pumilus ATCC14884, B. cereus ATCC33019, B. megaterium ATCC14581, and B. subtilis ATCC6633 (MIC = 63.0–125.0 μg/mL) and inactivated more than 90.99% of the Bacillus spores 0.05%. More importantly, the compounds destroyed all the spores at 0.1% after 1 h of incubation. The antibacterial and anti-sporicidal activity of β-asarone and asaronaldehyde provide useful information about the antimicrobial effect of P. cubeba (63). However, in vivo studies would be of a great importance to support their development as antibacterial agents.
The antifungal activity of P. cubeba was fully explored in many studies by using several methods mainly agar disc diffusion, well diffusion method, microdilution, inverted petri plate, and poison food medium assays (Table 6). The antifungal potential of five extracts of P. cubeba berries against the opportunistic oral fungal pathogens Candida albicans and Saccharomyces cerevisiae was studied using the MIC assay (64). The acetone extract was the most potent against both species followed by the methanolic and ethanolic extracts. C. albicans was more sensitive than S. cerevisiae (64). Similarly, Salkar et al. (65) developed an oral gel from the essential oil (0.5%) of P. cubeba and tested its activity toward different strains of Candida. The gel elicited excellent activity against both normal (C. albicans ATCC 10231, C. glabrata H04FS fluconazole sensitive, C. krusei G06FS fluconazole sensitive) and resistant (C. albicans-fluconazole resistant, C. krusei G03FR fluconazole resistant, C. glabrata H05FR fluconazole resistant) Candida species. Interestingly, the developed oral gel was endowed with comparable inhibitory effect to marketed local-herbal gel samples (65). Hence, the crude extracts as well as essential oil from P. cubeba fruits were considered as promising treatments of oral fungal infections, especially C. albicans species.
Many oleoresins from P. cubeba fruits were tested against different food pathogenic fungi (66) (Table 6). Using inverted petri plate assay, the chloroform oleoresin at 6 μL was highly active against Penicillium purpurogenum. However, the petroleum benzene oleoresin was ineffective against Fusarium oxysporum at all doses. Other oleoresins elicited minimum to moderate activities. Using the food poison technique, the ethanol oleoresin at 6 μL was effective against Penicillium madriti. Many other fungal species were sensitive to P. cubeba essential oil such as Aspergillus fumigatus, A. flavus, and F. solani, among others. The antifungal activities of P. cubeba extracts and essential oils are presented in Table 6.
Comparatively, the crude methanolic extract and fractions (dichloromethane, hexane, and ethyl acetate) from Piper solmsianum, as well as four pure compounds namely eupomatenoid-5, eupomatenoid-3, conocarpan and orientin were all assessed against 12 pathogenic fungi (67). The methanolic extract and fractions elicited a good antifungal effect against all the dermatophytes strains (MIC, μg/mL = 20–60), a weak activity against the zigomycetes and were not active toward the hyaline hyphomycetes. Compounds eupomatenoid-5, conocarpan, and orientin exhibited pronounced activities against all the dermatophytes tested (MIC ≤ 1–9 μg/mL). Noteworthy, conocarpan showed a remarkable activity against all the yeasts. To sum up, the antifungal activity of P. cubeba and its relatives seems to be promising and is likely related to the presence of bioactive compounds belonging to neolignanes and flavonoids. However, the presence of other active compounds should be verified and evaluated.
In addition to the antimicrobial activities, P. cubeba essential oil was also active against Schistosoma mansoni, the trypomastigote and amastigote forms of Trypanosoma cruzi, and the promastigote forms of Leishmania amazonensis (68). The in vitro inhibitory effect against T. cruzi was dose dependent. In contrast, essential oil was inactive toward L. amazonensis.
In vivo, a recent study showed that intraperitoneal treatment of male BALB/c mice by encapsulated and unencapsulated (−)-cubebin isolated from P. cubeba showed up to 61.3% reduction in the number of the trypomastigotes of a strain of T. cruzi. Animals treated with encapsulated (−)-cubebin survived longer compared to those treated with Benznidazole used as standard antiparasitic drug (69). These findings open a promising application of encapsulated (−)-cubebin as antiparasitic agent.
Other Piper species were also reported for antiparasitic purposes. For instance, P. dennisii was shown to exhibit anti-plasmodial activity in vitro (70). Moreover, benzoic acid derivatives isolated from P. acutifolia and P. glabratum were effective toward both T. cruzi and Plasmodium falciparum (71). In vitro evaluation of extracts from different Piper plants such as P. barbatum, P. aduncum, P. acutifolium, and P. dilatatum showed that they are potent in inhibiting T. cruzi (72). These findings demonstrate that the antiparasitic potential of Piper plants, including P. cubeba, is worth exploring in drug discovery.
Antileishmanial activity of P. cubeba extracts was evaluated in vitro toward Leishmania donovani promastigotes (73). All tested extracts (n-hexane, ethyl acetate, methanol, and acetone) elicited a significant activity at 100 μg/mL with more than 90% inhibition. In the case of n-hexane extract, two lignans namely cubebin and hinokinin, were identified and isolated. Cubebin exhibited a significant in vitro antileishmanial activity at 100 μM. In vivo experiment carried out in golden hamsters against L. donovani amastigotes showed that cubebin slightly reduced parasitic burden and spleen weight (73). Comparatively, the antileishmanial activity was also demonstrated by of P. aduncum extracts (74). Moreover, the essential oils from P. angustifolium were effective against Leishmania infantum (75). Also P. cubeba exhibited anthelmintic activity against earthworms and tapeworms in vitro (76).
Medicinal plants are the major source of wound healing products with more than 70% while the remaining sources are mineral and animal-based pharma products (77–79). Several plants are known to accelerate wound healing (80). However, only few studies have explored this activity from P. cubeba. Shakeel et al. (81) assessed the wound healing effect of P. cubeba essential oil using self-nanoemulsifying drug delivery system (SNEDDS). Prepared formulation was evaluated for wound healing, collagen determination, and histo-morphological examination in female Wistar rats. Upon oral administration, it was found that EO-SNEDDS formula significantly accelerated wound healing and enhanced collagen content in tested animals in comparison with pure essential oil. Noteworthy, histopathological evaluation of the formula-treated animals showed no signs of inflammatory cells indicating that it is safe to female rats (81).
More recently, essential oil from P. cubeba fruits (PCEO) was tested for in vivo wound healing potential (20). Tested PCEO induced a powerful antibacterial activity especially against Listeria monocytogenes and S. aureus, known to be involved in wound infections. Interestingly, the application of PCEO as topical cream accelerated the wound healing process, increased the SOD level, and reduced the malondialdehyde (MDA) level. In addition, histopathological examination demonstrated that the derma was restored and arranged properly. The observed activities were attributed to the synergy between the antioxidants and antimicrobials present in PCEO.
Phytochemicals seem to elicit wound healing activity by targeting several factors mainly those known to be responsible for delaying and/or reducing the wound healing process such as infections, deficiency in blood supply, diabetes mellitus, necrotic tissue, and lymphatic blockage (82). Within Piper genus, the aqueous leaf extract of P. betle applied to wounds in vivo induced a significant contraction and complete epithelization of the wounds after 10 and 14 days of treatment, respectively (83). Elsewhere, topical application of the ointments prepared from the leaves, stems, and roots of P. hayneanum significantly improved the healing of rats’ wounds and reduced the infections by two wound pathogens: S. aureus and C. albicans (84). In conclusion, it is apparent that Piper plants contain active principles with great potential to be used as topical ointments to enhance wound healing and prevent the establishment of wound-related infections.
Phytochemicals, such as terpenoids, polysaccharides, glucosides, flavonoids, and alkaloids, are widely reported as immunomodulators to some extent (85). As for P. cubeba, the immunomodulatory activity of its protein extracts was evaluated on the proliferation of immune cells using MTT assay on the splenocytes. This was tested in presence and absence of the mitogenic agent, concanavalin-A (Con-A) (86). The protein extracts exhibited a more significant immunosuppressive activity compared to the total extract. In addition, Ikawati et al. (87) demonstrated that the hexane and ethanolic extracts of P. cubeba fruits caused lysis of 2H3 cells leading to the release of high level of histamine (87). This effect was comparable to that induced by the standard drug, Thapsigargin. These results suggest the potential of P. cubeba extracts to face allergic diseases. However, in vivo experiments would provide further useful information to identify the bioactive molecules and explain the underpinning mechanisms. Using another Piper member, the administration of the methanolic extract of P. longum and its major principle piperine, induced a significant increase in the total white blood cell (WBC) count, enhanced the bone marrow cellularity, and increased circulating antibody titer, α-esterase positive and plaque forming cells in Balb/c mice (88).
Several other phytochemicals have been evaluated for immunomodulatory purposes and some mechanisms of action have been uncovered. For instance, epigallocatechin-3-gallate (EGCG) was able to inhibit NF-kB activation and down-regulate the production of NO in macrophages as well as the expression of monocyte chemoattractant protein-1 (MCP-1). Moreover, resveratrol, the highest renowned active molecule in grapevine, acted via inhibiting TNF-α and/or (LPS)-mediated macrophages, NF-kB, dendritic cells, and myeloid (89).
As the need for anti-hepatitis C virus (HCV) agents is growing, the search for new candidates that can serve as drugs or as core-entities to design an effective HCV inhibitor and its enzymes is promising. P. cubeba aqueous extract inhibited HCV-PR activity in vitro with an IC50 of 18.0 μg/mL (45). When compared to other plants tested in this study (45), P. cubeba aqueous and methanol extracts were among the most active by inducing 94.2 ± 2.1 and 84.7 ± 1.8% inhibition at 100 mg/mL, respectively. In an attempt to study the renoprotective potential of P. cubeba, a 47 years old male patient diagnosed with hypertension induced chronic kidney disease (CKD) and altered serum creatinine level which was unable to revert to normal levels using the conventional medication, was orally given two capsules of P. cubeba at 4 g/day for 6 weeks (90). This resulted in a significant improvement in subjective symptoms (anorexia and fatigue) as well as the objective parameters of the disorder (blood urea, serum creatinine and urine routine and microscopy). In addition, no adverse effects were observed during and after the study. It was concluded that P. cubeba boost the effectiveness in reducing serum creatinine level and in increasing estimated glomerular filtration rate (eGFR) and may help reduce further complications related to renal parenchymal damage.
In another study, the antilithiatic activity of the hydroalcoholic extract of P. cubeba fruits was investigated in male Sprague Dawley rats (91). Animals having received the extract showed a significant decrease in crystals level in urine. Moreover, a reduction in serum creatinine and urea was also observed. Interestingly, magnesium in animals’ urine was increased while sodium, calcium, phosphorus, and chloride were significantly decreased. Likewise, histopathological examination showed a clear improvement in kidney tissue in treated rats with P. cubeba extract following induction of urolithiasis by ethylene glycol and ammonium chloride. This study strongly suggests that P. cubeba could be of significant utility in inhibiting calcium oxalate urolithiasis. Comparatively, streptozotocin induced diabetic Wister albino rats treated by the root aqueous extract of P. longum maintained the normal activities of hepatic [serum glutamic oxaloacetic transaminase (SGOT), serum glutamic pyruvic transaminase (SGPT), alkaline phosphatase (ALP)] and renal (serum creatinine and urea) functional markers. This showcases the protective and biosafety roles of P. longum extract against diabetes induced liver and kidney damages. In addition, the extract elicited an antihyperlipidemic activity demonstrated by a significant decrease in the total cholesterol (TC), very low density lipoprotein (VLDL), triglycerides (TG), low density lipoprotein (LDL), and an increase in the high density lipoprotein (HDL) (92). Besides, P. cubeba essential oil was also investigated for antihyperuricemic activity and showed strong effect against xanthine oxidase (IC50 = 54.87 μg/mL) compared to P. nigrum EO (IC50 = 77.11 μg/mL) (7).
The hydroethanolic extract of P. cubeba fruits was evaluated for melanogenesis stimulation activity using cultured murine B16 melanoma cells. At 10 mg/ml, the extract enhanced both intracellular and extracellular melanin contents comparatively to the negative control. In contrast, no significant effect was observed on cell proliferation rate. This stimulatory effect on melanin was attributable to the presence of cubebin, a known constituent of P. cubeba fruits (93). Comparatively, the extract of P. methysticum and P. nigrum also showed a strong stimulatory activity on melanogenesis. Following up these findings, guided bioassay allowed the isolation of two kavalactones yangonin and 7,8-epoxyyangonin from P. methysticum. When tested, both kavalactones significantly stimulated the melanogenesis in B16 melanoma cells (93). Nevertheless, more in deep studies are recommended to uncover the molecular targets and underpin the mechanisms of action.
The antidepressant potential of P. cubeba EO was investigated in vivo using Albino mice and Fluoxetine, a selective serotonin reuptake inhibitor, as antidepressant standard drug (94). Using forced swimming method, animals treated with essential oil gained weight, exhibited more mobility, and showed less immobility comparedatively to the mice treated with Fluoxetine. This reduction in passive behavior in animals highlighted the antidepressant-like effect of P. cubeba essential oil. Interestingly, piperine from P. nigrum was studied for its antidepressant-like effect using corticosterone-induced model of depression in mice for 3 weeks. Relative to the control animals, those treated with piperine showed a significant decrease in sucrose utilization and an increase in immobility time. In addition, it maintained the levels of brain-derived neurotrophic factor protein and mRNA (95). This demonstrates the antidepressant-like effect of piperine. In another recent study, seven compounds often found in P. nigrum (paprazine, pellitorine, piperine, sylvamide, cepharadione A, piperolactam D, and 10-tricosanone) were docked against two receptors namely the potassium channel and human serotonin transporter to assess their action on the anxiolytic and antidepressant activities observed in vivo. Results showed that tested compounds interact with these target proteins with docking scores ranging from −1.0 to −7.9 kcal/mol indicating that they are likely responsible for the antidepressant activity (96). Nevertheless, as the antidepressant activity is still poorly explored, further experiments with different Piper species, compounds, methods, and in vivo models are needed.
P. cubeba, especially its EO, was evaluated for its plant-based insecticides activity (97). It was proved that at 0.003125%, the EO significantly repelled Sitophilus oryzae adults. This effect was more potent than those induced by pure compounds α-pinene and β-caryophyllene. Following fumigation, the EO was the most potent in causing lethality of S. oryzae adults (LC50 = 1.07 mL cm–3 air). Comparatively, the EO and α-pinene exhibited more toxic effect compared to Zingiber officinale EO and β-caryophyllene. The noticed insecticidal activity was attributed to the ability of the EO to inhibit acetylcholinesterase enzyme (AchE) in fumigated rice weevil (S. oryzae). Moreover, the oviposition of Callosobruchus sp. was significantly reduced after fumigation with P. cubeba EO. Similarly, a combination of 4-methyl-3-heptanol and P. cubeba EO was revealed to be more effective as a bait for Scolytus scolytus than multilure traps. This was due to the synergetic action between α-cubebene and 4-methyl-3-heptanol (98). Many other Scolytinae species including Xyleborini and Corthylini tribes were presented to be sensitive to P. cubeba based compounds such as α-copaene, α-cubebene, α-humulene, and calamenene. Similarly, myristicin (4-methoxy-6[2-propenyl]-1,3-benzodioxole) isolated from the hexane fraction of P. mullesua D. Don fruits induced significant toxicity against the 4th instar larvae of Spilarctia obliqua after 24 h of topical application (LD 50 = 104 μg/larva) (99). Additionally, it was showed that in Piper genus, piperamides are the major compounds with the strongest insecticidal activity. Many extracts from P. nigrum, P. guineense, and P. tuberculatum were shown to be active against insect pests (100). In conclusion, Piper plants and compounds constitute an innovative source of biopesticide agents for controlling insects out-breaks.
Moreover, P. cubeba largely inhibited the germination and growth of tow weeds (Bidens pilosa and Echinochloa crus-galli). Noteworthy, P. cubeba EO reduced photosynthesis in the two weeds while lipid peroxidation electrolyte leakages were increased at 1.93 mg/mL (7).
Nature is the storehouse of many active compounds that we are currently using as pharmaceuticals. P. cubeba synthesizes many secondary metabolites, among them hinokinin, cubebin and cubebin derivatives that are reported to be the most pharmacologically active compounds. These compounds exhibited many biological activities mainly antimicrobial, anticancer, antimutagenic, antiparasitic, ovicidal, and anticholinesterase (Table 7). In fact, lignans from P. cubeba were shown to alter the expression of PTGS2 and MMP2 proteins in head and neck cancer cells (12). Additionally, (−)-cubebin derivatives, (−)-hinokinin, and (−)-O-benzyl cubebin (OBZ) at 40 mg/kg inhibited the inflammation in vivo induced by injection of either PGE2 or dextran into the paw of animals in comparison to indomethacin, the reference standard (101). Besides these activities, (−)-cubebin was found to exert a vasorelaxant effect mediated by the NO/cGMP signaling pathway without prostacyclin participation (102). In another study, sixteen compounds were isolated from P. cubeba extracts based on their antioxidant potential to scavenge free radicals, hydroxyl radical, superoxide anion radical, and DPPH (103). It was mainly found that crotepoxide was the most active against 5,5-dimethyl-1-pyrroline-N-oxide-OH with up to 57% inhibition. In contrast, less inhibitory activity was noticed using other compounds such as 1′-acetoxychavicol acetate, deoxypipoxide and 3-(3′,2′,5′ -trimethoxyphenyl) pyrrolidine. Moreover, several compounds including 5,6-dehydrokawain, benzyl benzoate, 1′-acetoxychavicol acetate, deoxypipoxide, and 5,7,3′,4′-tetrametoxyflavone exhibited superoxide dismutase (SOD)-like activity through their ability to deliver protons (103).
The present review comprehensively summarized the available literature on the uses of P. cubeba and its phytochemicals to promote health conditions and manage diseases-related issues. It also critically addressed the opportunity of using the plant as a source of natural drugs in clinical trials. P. cubeba has edible fruits and condiments with various medicinal properties. The most dominant phytochemicals characterized in P. cubeba were polyphenolics and flavonoids (rutin, catechin, gallic acid, caffeic acid, ferulic acid, etc.), lignans (Cubebininolide, hinokinin, yatein, and isoyatein, etc.), fatty acids (lauric acid, hexadecanoic acid, palmitic acid, 9-octadecenoic acid, etc.), and volatile compounds (eugenol, β-cubebene, α-cubebene). Owing to this phytochemical richness, the plant has proved a large spectrum of biological and pharmacological activities that corroborate the traditional uses. Moreover, P. cubeba volatiles and aromatic characteristics are used in cosmetics for deodorants production and in food industry as culinary flavor (9).
Regarding the medical applications, cubeb’s different extracts and compounds have demonstrated biosafety status both in vitro and in vivo. In addition, due to the presence of high amounts of polyphenols, P. cubeba extracts/compounds exhibited substantial antioxidant/scavenging and anti-inflammatory activities. This is mainly targeted by downregulating the expression of proinflammatory transcriptional factors and cytokines while augmenting the enzymatic and non-enzymatic antioxidants (45). These latter are in turn involved in maintaining hepatic and renal functional parameters. Interestingly, many research investigations were devoted to the anticancer potential of the plant. The toxicity of different phytochemicals against tumor cell lines is mainly due their capacity to suppress many pro-oncogenic pathways and genes and to stimulate tumor suppressor-like pathways (125). Moreover, elicitation of proapoptotic proteins and impairment of mitochondrial membrane potential could also be targeted by plant compounds (78). The plant phytochemicals were also corroborated to be useful in managing diabetes because of their inhibitory effect on diabetic intestinal enzymes such as α-amylase and α-glucosidase (48) as well as in fighting microbial infections by inhibiting pathogens’ growth and quorum sensing (57). Thanks to the antimicrobial, antioxidant, and immunomodulatory activities it induces, P. cubeba was also capable of accelerating wound healing process by enhancing blood supply, synthesizing collagen and inhibiting wound infections (20, 82). Overall, C. cubeba’s activities, like those elicited by most plants, occur through the modification of the metabolism or gene expression modification.
The present work has systematically and comprehensively reviewed the botany, traditional uses, phytochemistry and pharmacology of P. cubeba extracts and constituents. In recent years, an increasing interest was given to this plant as it is used in traditional medicine in many countries. Most of these traditional uses have been validated by pharmacological studies. Nevertheless, there is no yet systemic data regarding the pharmacokinetics and clinical research of P. cubeba. Therefore, there is not enough evidence to interpret the specific mechanisms for the observed biological activities. Also, there are a few studies to date on other parts of P. cubeba than the fruits. To ensure full utilization of the plant, it is necessary to investigate the chemical constituents of each part and tissue. According to current investigations, lignans are the main active constituents of the plant, in which cubebin is the most abundant. This lignan is known to possess several activities like anti-inflammatory, anticancer, analgesic, and antimicrobial. Next to it, other cubebin derivatives were also isolated from the fruits of the plant. Thus, it will be more interesting to investigate the biological activities of the isolated compounds from each part of the plant. This review also highlighted the need to study the bioavailability of cubebin, as it is the major compound, in terms of water solubility and bioavailability in vivo following different doses and modes of administration. In addition, more toxicity studies as well as preclinical trials are required using cell-based and animal models. Active extracts warrant establishing guided bioassay experiments to translate the beneficial effects into solid scientific data that can lead to molecules and/or formulas with a targeted therapeutic potential. Another therapeutic strategy could be combining cubeba’s major compounds to standard drugs as adjuvants. Finally, despite the continued progress on various aspects of P. cubeba, the elaboration and discovery of new drugs from it will need more advanced trials in preclinical and clinical phases.
BD, IM, MY, and WB reviewed the literature and wrote the manuscript. LB and MS revised the manuscript, designed, and conceived the work. All authors approved the final version.
APC was funded by the UM6P.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Harvey AL. Natural products in drug discovery. Drug Dis Today. (2008) 13:894–901. doi: 10.1016/j.drudis.2008.07.004
2. Koparde AA, Doijad RC, Magdum CS. Natural Products in Drug Discovery, in: Pharmacognosy-Medicinal Plants. London: IntechOpen (2019).
3. Parmar VS, Jain SC, Bisht KS, Jain R, Taneja P, Jha A, et al. Phytochemistry of the genus piper. Phytochemistry. (1997) 46:597–673. doi: 10.1016/S0031-9422(97)00328-2
4. Nahak G, Sahu RK. Phytochemical evaluation and antioxidant activity of Piper cubeba and Piper nigrum. J Appl Pharmaceut Sci. (2011) 1:153.
5. Ruslan K, Batterman S, Bos R, Kayser O, Woerdenbag HJ, Quax WJ. Lignan profile of Piper cubeba, an indonesian medicinal plant. Biochem Syst Ecol. (2007) 35:397–402. doi: 10.1016/j.bse.2007.01.003
6. Lim T. Piper cubeba, in: edible medicinal and non-medicinal plants. Berlin: Springer (2012). p. 311–21. doi: 10.1007/978-94-007-4053-2_40
7. Andriana Y, Xuan TD, Quy TN, Tran H-D, Le Q-T. Biological activities and chemical constituents of essential oils from Piper cubeba bojer and Piper nigrum L. Molecules. (2019) 24:1876. doi: 10.3390/molecules24101876
8. Mothana R, Alsaid M, Khaled JM, Alharbi NS, Alatar A, Raish M, et al. Assessment of antinociceptive, antipyretic and antimicrobial activity of Piper cubeba L. Essential oil in animal models. Pakistan J Pharmaceut Sci. (2016) 29:671–7.
9. Abdul-Jalil TZ, Nasser ZA. Piper cubeba: phytochemical and pharmacological review of a routinely used spices. Int J Pharmaceut Res. (2020) 1:761–8.
10. Kumar P. A review on medicinal plant Piper cubeba L. and its pharmaceutical properties. Int J Sci Food Agric. (2021) 5:174–9. doi: 10.26855/ijfsa.2021.03.022
11. Salehi B, Zakaria ZA, Gyawali R, Ibrahim SA, Rajkovic J, Shinwari ZK, et al. Piper species: a comprehensive review on their phytochemistry, biological activities and applications. Molecules. (2019) 24:1364. doi: 10.3390/molecules24071364
12. Gusson-Zanetoni JP, da Silva JSGM, Picão TB, Cardin LT, Prates J, Sousa SO, et al. Effect of Piper cubeba total extract and isolated lignans on head and neck cancer cell lines and normal fibroblasts. J Pharmacol Sci. (2022) 148:93–102. doi: 10.1016/j.jphs.2021.09.002
13. Usia T, Watabe T, Kadota S, Tezuka Y. Potent CYP3A4 inhibitory constituents of Piper cubeba. J Nat Prod. (2005) 68:64–8. doi: 10.1021/np0401765
14. Jensen S, Hansen J, Boll PM. Lignans and neolignans from piperaceae. Phytochemistry. (1993) 33:523–30. doi: 10.1016/0031-9422(93)85442-T
15. Yaffe PB, Doucette CD, Walsh M, Hoskin DW. Piperine impairs cell cycle progression and causes reactive oxygen species-dependent apoptosis in rectal cancer cells. Exp Mol Pathol. (2013) 94:109–14. doi: 10.1016/j.yexmp.2012.10.008
16. Arruda C, Mejía JAA, Pena Ribeiro V, Costa Oliveira L, Silva MLA, Bastos JK. Development of a validated high-performance liquid chromatography method and optimization of the extraction of lignans from Piper cubeba. J Agric Food Chem. (2018) 67:753–9. doi: 10.1021/acs.jafc.8b05359
17. Usia T, Watabe T, Kadota S, Tezuka Y. Metabolite-cytochrome P450 complex formation by methylenedioxyphenyl lignans of Piper cubeba: mechanism-based inhibition. Life Sci. (2005) 76:2381–91. doi: 10.1016/j.lfs.2004.12.005
18. Zahin M, Khan MS, Abul Qais F, Abulreesh HH, Ahmad I. Antioxidant properties and anti-mutagenic potential of Piper cubeba fruit extract and molecular docking of certain bioactive compounds. Drug Chem Toxicol. (2018) 41:358–67. doi: 10.1080/01480545.2018.1429459
19. Maungchanburi S, Rattanaburee T, Sukpondma Y, Tedasen A, Tipmanee V, Graidist P. Anticancer activity of Piper cubeba L. Extract on triple negative breast cancer MDA-MB-231. J Pharmacy Pharmacogn Res. (2022) 10:39–51. doi: 10.56499/jppres21.1160_10.1.39
20. Alminderej F, Bakari S, Almundarij TI, Snoussi M, Aouadi K, Kadri A. Antimicrobial and wound healing potential of a new chemotype from Piper cubeba L. Essential oil and in silico study on S. aureus tyrosyl-tRNA synthetase protein. Plants. (2021) 10:205. doi: 10.3390/plants10020205
21. Singh G, Marimuthu P, de Heluani CS, Catalan CA. Chemical constituents, antioxidative and antimicrobial activities of essential oil and oleoresin of tailed pepper (Piper cubeba L). Int J Food Eng. (2007) 3:1154. doi: 10.2202/1556-3758.1154
22. Mustafa RA, Hameed S. Nutritional, phytochemical, phenolic compound analysis of Piper cubeba extract as a food fortified. J Garmian Univ. (2017) 4:441–52. doi: 10.24271/garmian.153
23. Mouid MG, Kavimani S. Effect of methanolic extract of Piper cubeba linn. Fruits on the pharmacokinetics of pioglitazone in rats. World J Pharmaceut Sci. (2016) 4:104–9.
24. Barbosa LC, Furtado RA, Bertanha HCC, Tomazella IM, Costa ES, Bastos JK, et al. Chemopreventive effects of (−)-hinokinin against 1,2-dimethylhydrazine-induced genotoxicity and preneoplastic lesions in rat colon. J Nat Prod. (2014) 77:2312–5. doi: 10.1021/np500093u
25. Khaled M, Alharbi NS, Alatar A, Ahmad A. Chemical composition, anti-inflammatory and antioxidant activities of the essential oil of Piper cubeba L. Romanian Biotechnol Lett. (2017) 22:12366.
26. Shah AH, Al-Shareef AH, Ageel AM, Qureshi S. Toxicity studies in mice of common spices, Cinnamomum zeylanicum bark and Piper longum fruits. Plant Foods Hum Nutr. (1998) 52:231–9. doi: 10.1023/A:1008088323164
27. Choudhary D, Kale RK. Antioxidant and non-toxic properties of Piper betle leaf extract: in vitro and in vivo studies. Phytother Res. (2002) 16:461–6. doi: 10.1002/ptr.1015
28. Ochieng MA, Ben Bakrim W, Bitchagno GTM, Mahmoud MF, Sobeh M. Syzygium jambos L. alston: an insight into its phytochemistry, traditional uses, and pharmacological properties. Front Pharmacol. (2022) 13:786712. doi: 10.3389/fphar.2022.786712
29. Mahdi I, Bakrim WB, Bitchagno GTM, Annaz H, Mahmoud MF, Sobeh M. Unraveling the phytochemistry, traditional uses, and biological and pharmacological activities of thymus Algeriensis boiss. & reut. Oxid Med Cell Long. (2022) 2022:6487430. doi: 10.1155/2022/6487430
30. Agbor GA, Vinson JA, Oben JE, Ngogang JY. In vitro antioxidant activity of three piper species. Null. (2008) 7:49–64. doi: 10.1080/J157v07n02_04
31. Solowey E, Lichtenstein M, Sallon S, Paavilainen H, Solowey E, Lorberboum-Galski H. Evaluating medicinal plants for anticancer activity. Sci World J. (2014) 2014:721402. doi: 10.1155/2014/721402
32. Graidist P, Martla M, Sukpondma Y. Cytotoxic activity of Piper cubeba extract in breast cancer cell lines. Nutrients. (2015) 7:707. doi: 10.3390/nu7042707
33. Niwa AM, de Paula NA, Vesenick DC, Sartori D, Maistro EL, Ribeiro LR, et al. Evaluation of lignan (–)-cubebin extracted from Piper cubeba on human colon adenocarcinoma cells (HT29). Null. (2016) 79:92–100. doi: 10.1080/15287394.2015.1110067
34. Grinevicius VMAS, Andrade KS, Ourique F, Micke GA, Ferreira SRS, Pedrosa RC. Antitumor activity of conventional and supercritical extracts from Piper nigrum L. Cultivar bragantina through cell cycle arrest and apoptosis induction. J Super Fluids. (2017) 128:94–101. doi: 10.1016/j.supflu.2017.05.009
35. Kim KH, Choi JW, Choi SU, Ha SK, Kim SY, Park H-J, et al. The chemical constituents of Piper kadsura and their cytotoxic and anti-neuroinflammtaory activities. Null. (2011) 26:254–60. doi: 10.3109/14756366.2010.496363
36. Alonso-Castro AJ, Villarreal ML, Salazar-Olivo LA, Gomez-Sanchez M, Dominguez F, Garcia-Carranca A. Mexican medicinal plants used for cancer treatment: pharmacological, phytochemical and ethnobotanical studies. J Ethnopharmacol. (2011) 133:945–72. doi: 10.1016/j.jep.2010.11.055
37. Holdsworth DK. Traditional medicinal plants of rarotonga, cook islands. Part II. Null. (1991) 29:71–9. doi: 10.3109/13880209109082853
38. Chaveerach A, Mokkamul P, Sudmoon R, Tanee T. Ethnobotany of the genus Piper (Piperaceae) in Thailand. Khon Kaen (2006). doi: 10.17348/era.4.0.223-231
39. Chen T. Observation of the medicine made by oneself in treating with 97 cases with gastric diseases. J Pract Med Technol. (2008) 15:593–4.
40. Xin Y, Qi W, Han C. Traditional Chinese medicine for treating respiratory cancer. CN Patent. (2009) 2009:101455834.
41. Wang Y-H, Morris-Natschke SL, Yang J, Niu H-M, Long C-L, Lee K-H. Anticancer principles from medicinal piper (胡椒 hú jiāo) plants. J Tradit Complem Med. (2014) 4:8–16. doi: 10.4103/2225-4110.124811
42. Yassir M, Bakrim WB, Mahmoud MF, Drissi B, Kouisni L, Sobeh M. Watery rose apple: a comprehensive review of its traditional uses, nutritional value, phytochemistry, and therapeutic merits against inflammation-related disorders. Oxidat Med Cell Long. (2022) 2022:7502185. doi: 10.1155/2022/7502185
43. Fürst R, Zündorf I. Plant-derived anti-inflammatory compounds: hopes and disappointments regarding the translation of preclinical knowledge into clinical progress. Med Inflam. (2014) 2014:146832. doi: 10.1155/2014/146832
44. Raja Mazlan RN, Rukayadi Y, Maulidiani M, Ismail IS. Solvent extraction and identification of active anticariogenic metabolites in Piper cubeba L. Through 1H-NMR-based metabolomics approach. Molecules. (2018) 23:1730. doi: 10.3390/molecules23071730
45. Qomaladewi NP, Aziz N, Kim M-Y, Cho JY. Piper cubeba L. Methanol extract has anti-inflammatory activity targeting Src/Syk via NF-B inhibition. Evid Based Complem Alternat Med. (2019) 2019:1548125. doi: 10.1155/2019/1548125
46. Deme P, Aluganti Narasimhulu C, Parthasarathy S. Evaluation of anti-inflammatory properties of herbal aqueous extracts and their chemical characterization. J Med Food. (2019) 22:861–73. doi: 10.1089/jmf.2019.0009
47. Nurcahyanti ADR, Jap A, Lady J, Prismawan D, Sharopov F, Daoud R, et al. Function of selected natural antidiabetic compounds with potential against cancer via modulation of the PI3K/AKT/mTOR cascade. Biomed Pharmacother. (2021) 144:112138. doi: 10.1016/j.biopha.2021.112138
48. Ahmed AS, Ahmed QU, Saxena AK, Jamal P. Evaluation of in vitro antidiabetic and antioxidant characterizations of Elettaria cardamomum (L.) maton (zingiberaceae), Piper cubeba L. f.(piperaceae), and Plumeria rubra L.(apocynaceae). Pakistan J Pharmaceut Sci. (2017) 2017:30.
49. Salehi B, Martorell M, Arbiser J, Sureda A, Martins N, Maurya PK. Sharifi-rad. Antidiabetic potential of medicinal plants and their active components. Biomolecules. (2019) 9:551–551. doi: 10.3390/biom9100551
50. Muchandi AA, Jadhav AS, Patil SB, Patil SA, Jadhav NB. Antioxidant and in vitro antidiabetic activity of methanol extract of Piper cubeba L. Int Res J Pharmacy Med Sci. (2018) 1:1–4.
51. Muchandi AA, Dhawale SC. Protective effects of ethanolic extract of Piper cubeba L. on D-galactose induced neuronal lipofuscinogenesis in albino rats. Sci Eng Health Stud. (2018) 2018:11–7.
52. Lokhasudhan G, Nasim I. Knowledge, attitude, and practice survey on usage of antibiotics among dental practitioners in southern region of India. J Adv Pharm Edu Res. (2017) 7:160–2.
53. Ventola CL. The antibiotic resistance crisis: part 1: causes and threats. P T. (2015) 40:277–83.
54. Okba MM, El-Shiekh RA, Abu-Elghait M, Sobeh M, Ashour RM. HPLC-PDA-ESI-MS/MS profiling and anti-biofilm potential of eucalyptus sideroxylon flowers. Antibiotics. (2021) 10:761. doi: 10.3390/antibiotics10070761
55. Akshita C, Vijay BV, Praveen D. Evaluation of phytochemical screening and antimicrobial efficacy of mesua ferrea and Piper cubeba fruit extracts against multidrug-resistant bacteria. Pharmacophore. (2020) 11:15–20.
57. Alharbi NS, Khaled JM, Alzaharni KE, Mothana RA, Alsaid MS, Alhoshan M, et al. Effects of Piper cubeba L. Essential oil on methicillin-resistant Staphylococcus aureus: an AFM and TEM study. J Mol Recogn. (2017) 30:e2564. doi: 10.1002/jmr.2564
58. Al-Sayed E, Gad HA, El-Kersh DM. Characterization of four piper essential oils (GC/MS and ATR-IR) coupled to chemometrics and their anti-Helicobacter pylori activity. ACS Omega. (2021) 6:25652–63. doi: 10.1021/acsomega.1c03777
59. Alqdeeri F, Abas F, Rukayadi Y. Tailed pepper (Piper cubeba) L. Berries extract reduced number of microbial population in tofu. Food Res. (2020) 4:738–45. doi: 10.26656/fr.2017.4(3).325
60. Abdallah EM, Abdalla WE. Black pepper fruit (Piper nigrum L.) as antibacterial agent: a mini-review. J Bacteriol Mycol Open Access. (2018) 6:141–5. doi: 10.15406/jbmoa.2018.06.00192
61. Ganesh P, Kumar RS, Saranraj P. Phytochemical analysis and antibacterial activity of pepper (Piper nigrum L.) against some human pathogens. Central Eur J Exp Biol. (2014) 3:36–41.
62. Kaveti B, Tan L, Sarnnia KT, Baig M. Antibacterial activity of piper betle leaves. Int J Pharmacy Teach Pract. (2011) 2:129–32.
63. Alqadeeri F, Rukayadi Y, Abbas F, Shaari K. Antibacterial and antispore activities of isolated compounds from Piper cubeba L. Molecules. (2019) 24:3095. doi: 10.3390/molecules24173095
64. Aneja KR, Joshi R, Sharma C, Aneja A. Antimicrobial efficacy of fruit extracts of two piper species against selected bacterial and oral fungal pathogens. Braz J Oral Sci. (2010) 9:421–6.
65. Salkar K, Suthar A, Chotalia C. Anti-candida activity of an oral gel developed using piper cubeba oil. J Pharmaceut Biol Sci. (2014) 2:44.
66. Singh G, Kiran S, Marimuthu P, de Lampasona MP, De Heluani CS, Catalan CAN. Chemistry, biocidal and antioxidant activities of essential oil and oleoresins from Piper cubeba (seed). Int J Essent Oil Ther. (2008) 2:50.
67. De Campos MP, Cechinel Filho V, Da Silva RZ, Yunes RA, Zacchino S, Juarez S, et al. Evaluation of antifungal activity of Piper solmsianum C. DC. var. solmsianum (piperaceae). Biol Pharmaceut Bull. (2005) 28:1527–30. doi: 10.1248/bpb.28.1527
68. Esperandim VR, da Silva Ferreira D, Rezende KCS, Magalhaes LG, Souza JM, Pauletti PM, et al. In vitro antiparasitic activity and chemical composition of the essential oil obtained from the fruits of Piper cubeba. Planta Med. (2013) 79:1653–5. doi: 10.1055/s-0033-1351022
69. Neres NBR, Montagnini D, Ferreira DS, Parreira RLT, Orenha RP, Lima TC, et al. In Vivo and in silico trypanocidal activity evaluation of (−)-cubebin encapsulated in PLGA microspheres as potential treatment in acute phase. Chem Biod. (2021) 18:e2100052. doi: 10.1002/cbdv.202100052
70. Céline V, Adriana P, Eric D, Joaquina A, Yannick E, Augusto LF, et al. Medicinal plants from the yanesha (peru): evaluation of the leishmanicidal and antimalarial activity of selected extracts. J Ethnopharmacol. (2009) 123:413–22. doi: 10.1016/j.jep.2009.03.041
71. Flores N, Jiménez IA, Giménez A, Ruiz G, Gutiérrez D, Bourdy G, et al. Benzoic acid derivatives from piper species and their antiparasitic activity. J Nat Prod. (2008) 71:1538–43. doi: 10.1021/np800104p
72. Calderón ÁI, Romero LI, Ortega-Barría E, Solís PN, Zacchino S, Gimenez A, et al. Screening of latin american plants for antiparasitic activities against malaria, chagas disease, and leishmaniasis. Null. (2010) 48:545–53. doi: 10.3109/13880200903193344
73. Bodiwala HS, Singh G, Singh R, Dey CS, Sharma SS, Bhutani KK, et al. Antileishmanial amides and lignans from Piper cubeba and Piper retrofractum. J Nat Med. (2007) 61:418–21. doi: 10.1007/s11418-007-0159-2
74. Braga FG, Bouzada MLM, Fabri RL, de O, Matos M, Moreira FO, et al. Antileishmanial and antifungal activity of plants used in traditional medicine in Brazil. J Ethnopharmacol. (2007) 111:396–402. doi: 10.1016/j.jep.2006.12.006
75. Bosquiroli LS, Demarque DP, Rizk YS, Cunha MC, Marques MCS, Matos M, et al. In vitro anti-Leishmania infantum activity of essential oil from Piper angustifolium. Rev Brasileira Farmacogn. (2015) 25:124–8. doi: 10.1016/j.bjp.2015.03.008
76. Khare CP. Indian herbal remedies: rational western therapy, ayurvedic, and other traditional usage, Botany. Berlin: Springer science & business media (2004). doi: 10.1007/978-3-642-18659-2
77. Bakrim WB, Nurcahyanti ADR, Dmirieh M, Mahdi I, Elgamal AM, El Raey MA, et al. Phytochemical profiling of the leaf extract of Ximenia americana var. Caffra and its antioxidant, antibacterial, and antiaging activities in vitro and in Caenorhabditis elegans: a cosmeceutical and dermatological approach. Oxidat Med Cell Long. (2022) 2022:3486257. doi: 10.1155/2022/3486257
78. Kumar B, Vijayakumar M, Govindarajan R, Pushpangadan P. Ethnopharmacological approaches to wound healing—exploring medicinal plants of India. J Ethnopharmacol. (2007) 114:103–13. doi: 10.1016/j.jep.2007.08.010
79. Lambebo MK, Kifle ZD, Gurji TB, Yesuf JS. Evaluation of wound healing activity of methanolic crude extract and solvent fractions of the leaves of Vernonia auriculifera hiern (asteraceae) in mice. J Exp Pharmacol. (2021) 13:677–92. doi: 10.2147/JEP.S308303
80. Kumarasamyraja D, Jeganathan NS, Manavalan R. A review on medicinal plants with potential wound healing activity. Int J Pharm Pharm Sci. (2012) 2:105–11. doi: 10.3329/icpj.v2i6.14869
81. Shakeel F, Alam P, Anwer MK, Alanazi SA, Alsarra IA, Alqarni MH. Wound healing evaluation of self-nanoemulsifying drug delivery system containing Piper cubeba essential oil. 3 Biotech. (2019) 9:82. doi: 10.1007/s13205-019-1630-y
82. Chithra P, Sajithlal G, Chandrakasan G. Influence of aloe vera on collagen turnover in healing of dermal wounds in rats. Indian J Exp Biol. (1998) 36:896–901.
83. Santhanam G, Nagarajan S. Wound healing activity of Curcuma aromatica and Piper betle. Fitoterapia. (1990) 61:458–9.
84. Bastos MLA, Houly RLS, Conserva LM, Andrade VS, Rocha EMM, Lyra Lemos R. Antimicrobial and wound healing activities of Piper hayneanum. J Chem Pharm Res. (2011) 3:213–22.
85. Alhazmi HA, Najmi A, Javed SA, Sultana S, Al Bratty M, Makeen HA, et al. Medicinal plants and isolated molecules demonstrating immunomodulation activity as potential alternative therapies for viral diseases including COVID-19. Front Immunol. (2021) 12:637553. doi: 10.3389/fimmu.2021.637553
86. Daoudi A, Aarab L, Abdel-Sattar E. Screening of immunomodulatory activity of total and protein extracts of some moroccan medicinal plants. Toxicol Ind Health. (2013) 29:245–53. doi: 10.1177/0748233711430972
87. Ikawati Z, Wahyuono S, Maeyama K. Screening of several indonesian medicinal plants for their inhibitory effect on histamine release from RBL-2H3 cells. J Ethnopharmacol. (2001) 75:249–56. doi: 10.1016/S0378-8741(01)00201-X
88. Sunila ES, Kuttan G. Immunomodulatory and antitumor activity of Piper longum linn. and piperine. J Ethnopharmacol. (2004) 90:339–46. doi: 10.1016/j.jep.2003.10.016
89. Jantan I, Ahmad W, Bukhari SNA. Plant-derived immunomodulators: an insight on their preclinical evaluation and clinical trials. Front Plant Sci. (2015) 6:655. doi: 10.3389/fpls.2015.00655
90. Jahan KI, Aafreen S, Quamri MA, Siddiqui MA. Efficacy of sufoof-e-kabab chini (Piper cubeba) in hypertension induced CHRONIC kidney disease. (2020).
91. Bano H, Jahan N, Makbul SAA, Kumar BN, Husain S, Sayed A. Effect of Piper cubeba L. Fruit on ethylene glycol and ammonium chloride induced urolithiasis in male sprague dawley rats. Int Med Res. (2018) 7:358–65. doi: 10.1016/j.imr.2018.06.005
92. Nabi SA, Kasetti RB, Sirasanagandla S, Tilak TK, Kumar MVJ, Rao CA. Antidiabetic and antihyperlipidemic activity of Piper longum root aqueous extract in STZ induced diabetic rats. BMC Complem Alternat Med. (2013) 13:37. doi: 10.1186/1472-6882-13-37
93. Matsuda H, Hirata N, Kawaguchi Y, Naruto S, Takata T, Oyama M, et al. Melanogenesis stimulation in murine B16 melanoma cells by kava (Piper methysticum) rhizome extract and kavalactones. Biol Pharmaceut Bull. (2006) 29:834–7. doi: 10.1248/bpb.29.834
94. Khan M. Comparative physicochemical evaluation of fruits and anti depressant potential of volatile oils of fruits of local piper species. Oriental J Chem. (2015) 31:541–5. doi: 10.13005/ojc/310167
95. Damanhouri ZA, Ahmad A. A review on therapeutic potential of Piper nigrum L. black pepper): the king of spices. Med Aromat Plants. (2014) 3:161. doi: 10.4172/2167-0412.1000161
96. Emon NU, Alam S, Rudra S, Riya SR, Paul A, Hossen SMM, et al. Antidepressant, anxiolytic, antipyretic, and thrombolytic profiling of methanol extract of the aerial part of Piper nigrum: in vivo, in vitro, and in silico approaches. Food Sci Nutr. (2021) 9:833–46. doi: 10.1002/fsn3.2047
97. Chaubey MK. Fumigant toxicity of essential oils and pure compounds against Sitophilus oryzae L. (coleoptera: curculionidae). Null. (2012) 28:111–9. doi: 10.1080/01448765.2012.681352
98. Blight MM, King CJ, Wadhams LJ, Wenham MJ. Attraction of Scolytus scolytus (F.) to the components of multilure, the aggregation pheromone ofS. multistriatus (marsham) (coleoptera: scolytidae). Experientia. (1978) 34:1119–20. doi: 10.1007/BF01922902
99. Srivastava S, Gupta MM, Prajapati V, Tripathi AK, Kumar S. Insecticidal activity of myristicin from Piper mullesua. Null. (2001) 39:226–9. doi: 10.1076/phbi.39.3.226.5933
100. Scott IM, Jensen HR, Philogène BJR, Arnason JT. A review of piper spp. (piperaceae) phytochemistry, insecticidal activity and mode of action. Phytochem Rev. (2007) 7:65. doi: 10.1007/s11101-006-9058-5
101. Lima TC, Lucarini R, Volpe AC, de Andrade CQ, Souza AM, Pauletti PM, et al. In vivo and in silico anti-inflammatory mechanism of action of the semisynthetic (−)-cubebin derivatives (−)-hinokinin and (−)-O-benzylcubebin. Bioorg Med Chem Lett. (2017) 27:176–9. doi: 10.1016/j.bmcl.2016.11.081
102. Carvalho MTM, Rezende KCS, Evora PRB, Bastos JK, Cunha WR, Andrade Silva ML, et al. The lignan (−)−cubebin inhibits vascular contraction and induces relaxation via nitric oxide activation in isolated rat aorta. Phytother Res. (2013) 27:1784–9. doi: 10.1002/ptr.4932
103. Aboul-Enein HY, Kładna A, Kruk I. Radical scavenging ability of some compounds isolated from Piper cubeba towards free radicals. Luminescence. (2011) 26:202–7. doi: 10.1002/bio.1209
104. Alam MD, Shamim A, Husain S, Bano H, Ahmed Z, Azeez A. Kabab Chini (Piper cubeba) & its healing corollary in unani medicine: an overview. Am J Pharmacy Health Res. (2013) 1:2013.
105. Chitnis R, Abichandani M, Nigam P, Nahar L, Sarker SD. Actividad antibacteriana y antioxidante de los extractos de Piper cubeba (piperaceae). Ars Pharmaceutica. (2007) 48:343–50.
106. Alminderej F, Bakari S, Almundarij TI, Snoussi M, Aouadi K, Kadri A. Antioxidant activities of a new chemotype of Piper cubeba L. Fruit essential oil (methyleugenol/eugenol): in silico molecular docking and ADMET studies. Plants. (2020) 9:534. doi: 10.3390/plants9111534
107. Khalaf NA, Shakya AK, Al-Othman A, El-Agbar Z, Farah H. Antioxidant activity of some common plants. Turkish J Biol. (2008) 32:51–5.
108. Lin CW, Yu CW, Wu SC, Yih KH. DPPH free-radical scavenging activity, total phenolic contents and chemical composition analysis of forty-two kinds of essential oils. J Food Drug Analy. (2009) 17:386–95. doi: 10.38212/2224-6614.2594
109. Aqil F, Ahmad I, Mehmood Z. Antioxidant and free radical scavenging properties of twelve traditionally used Indian medicinal plants. Turkish J Biol. (2006) 30:177–83.
110. Ur Rehman J, Iqbal A, Mahmood A, Asif HM, Mohiuddin E, Akram M. Phytochemical analysis, antioxidant and antibacterial potential of some selected medicinal plants traditionally utilized for the management of urinary tract infection. Pakistan J Pharmaceut Sci. (2021) 34:1056–62.
111. Jain SR, Jain PR, Jain MR. Antibacterial evaluation of some indigenous volatile oils. Planta Med. (1974) 26:196–9. doi: 10.1055/s-0028-1097990
112. Kar A, Jain SR. Antibacterial evaluation of some indigenous medicinal volatile oils. Qualit Plant Mat Veget. (1971) 20:231–7. doi: 10.1007/BF01104967
113. Al-tememy TMK. Antibacterial activity of Piper cubeba linn. Fruit extracts against selected bacterial pathogens in Basrah city. Basrah J Vet Res. (2013) 12:142–51. doi: 10.33762/bvetr.2013.76184
114. Hanif MA, Bhatti HN, Jamil MS, Anjum RS, Jamil A, Khan MM. Antibacterial and antifungal activities of essential oils extracted from medicinal plants using CO2 supercritical fluid extraction technology. Asian J Chem. (2010) 22:7787–98.
115. Fazly Ann Z, Rukayadi Y. Antibacterial activity of ethanolic Piper cubeba L. Extract against Escherichia coli and its effect on microbiological quality of raw chicken meat during storage. Int Food Res J. (2019) 26:933–44.
116. Chudasama KS, Thaker VS. Biological control of phytopathogenic bacteria Pantoea agglomerans and Erwinia chrysanthemi using 100 essential oils. Arch Phytopathol Plant Protect. (2014) 47:2221–32. doi: 10.1080/03235408.2013.871435
117. Chudasama KS, Thaker VS. Screening of potential antimicrobial compounds against Xanthomonas campestris from 100 essential oils of aromatic plants used in India: an ecofriendly approach. Arch Phytopathol Plant Protect. (2012) 45:783–95. doi: 10.1080/03235408.2011.595967
118. Silva MLA, Coímbra H, dos S, Pereira AC, Almeida V, Lima T, et al. Evaluation of Piper cubeba extract,(−)−cubebin and its semi−synthetic derivatives against oral pathogens. Phytother Res Int J Dev Pharmacol Toxicol Eval Nat Prod Derivat. (2007) 21:420–2. doi: 10.1002/ptr.2088
119. Parreira RL, Costa ES, Heleno VC, Magalhães LG, Souza JM, Pauletti PM, et al. Evaluation of lignans from Piper cubeba against Schistosoma mansoni adult worms: a combined experimental and theoretical study. Chem Biod. (2019) 16:e1800305. doi: 10.1002/cbdv.201800305
120. Rajalekshmi DS, Kabeer FA, Madhusoodhanan AR, Bahulayan AK, Prathapan R, Prakasan N, et al. Anticancer activity studies of cubebin isolated from Piper cubeba and its synthetic derivatives. Bioorganic Med Chem Lett. (2016) 26:1767–71. doi: 10.1016/j.bmcl.2016.02.041
121. de Paula Carlis MS, Feboli A, de Laurentiz AC, da Silva Filardi R, de Oliveira AHP, Silva MLA, et al. In vitro anthelmintic activity of the crude hydroalcoholic extract of Piper cubeba fruits and isolated natural products against gastrointestinal nematodes in sheep. Vet Parasitol. (2019) 275:108932. doi: 10.1016/j.vetpar.2019.108932
122. Rezende K, Lucarini R, Símaro GV, Pauletti PM, Januário AH, Esperandim VR, et al. Antibacterial activity of (−)-cubebin isolated from Piper cubeba and its semisynthetic derivatives against microorganisms that cause endodontic infections. Rev Brasileira Farmacogn. (2016) 26:296–303. doi: 10.1016/j.bjp.2015.12.006
123. Medola JF, Cintra VP, Patrı É, de Andrade Royo V, Da Silva R, Saraiva J, et al. (−)-Hinokinin causes antigenotoxicity but not genotoxicity in peripheral blood of wistar rats. Food Chem Toxicol. (2007) 45:638–42. doi: 10.1016/j.fct.2006.10.012
124. Somani GS, Nahire MS, Parikh AD, Mulik MB, Ghumatkar PJ, Laddha KS, et al. Neuroprotective effect of cubebin: a dibenzylbutyrolactone lignan on scopolamine-induced amnesia in mice. Indian J Med Res. (2017) 146:255. doi: 10.4103/ijmr.IJMR_156_14
Keywords: cubeb, Piper cubeba, phytochemistry, traditional uses, pharmacological activities
Citation: Drissi B, Mahdi I, Yassir M, Ben Bakrim W, Bouissane L and Sobeh M (2022) Cubeb (Piper cubeba L.f.): A comprehensive review of its botany, phytochemistry, traditional uses, and pharmacological properties. Front. Nutr. 9:1048520. doi: 10.3389/fnut.2022.1048520
Received: 19 September 2022; Accepted: 01 November 2022;
Published: 22 November 2022.
Edited by:
Rajeev K. Singla, Sichuan University, ChinaReviewed by:
Lee Seong Wei, Universiti Malaysia Kelantan, MalaysiaCopyright © 2022 Drissi, Mahdi, Yassir, Ben Bakrim, Bouissane and Sobeh. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mansour Sobeh, bWFuc291ci5zb2JlaEB1bTZwLm1h
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.