
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Nutr. , 10 November 2022
Sec. Clinical Nutrition
Volume 9 - 2022 | https://doi.org/10.3389/fnut.2022.1038118
This article is part of the Research Topic Diet and Gastrointestinal Cancer: Advances in Clinical and Experimental Medicine View all 9 articles
Objective: Although the application of immunotherapy in gastric cancer has achieved satisfactory clinical effects, many patients have no response. The aim of this retrospective study is to investigate the predictive ability of the prognostic nutrition index (PNI) to the prognosis of patients with gastric cancer who received immune checkpoint inhibitors (ICIs).
Materials and methods: Participants were 146 gastric cancer patients with ICIs (PD-1/PD-L1 inhibitors) or chemotherapy. All patients were divided into a low PNI group and a high PNI group based on the cut-off evaluated by the receiver operating characteristic (ROC) curve. We contrasted the difference in progression-free survival (PFS) and overall survival (OS) in two groups while calculating the prognosis factors for PFS and OS by univariate and multivariate analyses. Moreover, the nomogram based on the results of the multivariate analysis was constructed to estimate the 1- and 3-year survival probabilities.
Results: There were 41 (28.1%) cases in the low PNI group and 105 (71.9%) cases in the high PNI group. The median survival time for PFS in the low PNI group and high PNI group was 12.30 months vs. 33.07 months, and 18.57 months vs. not reached in the two groups for OS. Patients in low PNI group were associated with shorter PFS and OS in all patients [Hazard ratio (HR) = 1.913, p = 0.013 and HR = 2.332, p = 0.001]. Additionally, in subgroup analysis, low PNI group cases also had poorer PFS and OS, especially in patients with ICIs. In addition, the multivariate analysis found that carbohydrate antigen 724 (CA724) and TNM stage were independent prognostic factors for PFS. At the same time, indirect bilirubin (IDBIL), CA724, PNI, and TNM stage were independent prognostic factors for OS.
Conclusion: Prognostic nutrition index was an accurate inflammatory and nutritional marker, which could predict the prognosis of patients with gastric cancer who received ICIs. PNI could be used as a biomarker for ICIs to identify patients with gastric cancer who might be sensitive to ICIs.
Because of the increase in population, although incidence rate and mortality have decreased, gastric cancer remains the fifth most diagnosed cancer, especially in East Asian countries such as China, Japan, and Korea. Gastric cancer is even the most common cancer in Korean and Japanese men (1). The treatment of patients with advanced gastric cancer is still a major challenge. Even after receiving radical resection and adjuvant therapy, patients’ 5-year survival rates remain low (2–5). Therefore, it is significantly important for patients with gastric cancer to find new treatments that can prolong survival time. Immune checkpoint inhibitors (ICIs) have been found to achieve favorable results in a variety of solid tumors, including triple-negative breast cancer, non-small cell lung cancer, and head and neck cancer (6–10). Therefore, people have focused on the application of ICIs in patients with gastric cancer. However, although the use of ICIs has brought benefits to patients with gastric cancer, some cases were not sensitive to ICIs, even if they were patients with PD-1/PD-L1 positive and microsatellite instability-high (MSI-H) (11, 12). At the same time, some studies have also shown that gastric cancer patients with PD-1/PD-L1 negative and microsatellite stable (MSS) might still benefit from ICIs (13, 14). Therefore, exploring a simple and accurate biomarker to predict patients with gastric cancer who can benefit from ICIs is necessary.
Many studies have shown that nutritional status was related to cancer prognosis (15–20). Due to the especially anatomical characteristics, nutritional status has a more obvious impact on patients with gastric cancer (21–24). Nutritional markers such as albumin, prealbumin, and body mass index have been found to be independent prognostic factors for gastric cancer (25). A systemic inflammatory state can lead to cancer metastasis and progression. Previous studies have shown that peripheral inflammatory markers such as lymphocytes, neutrophils, and C-reactive proteins were also related to the prognosis of patients with gastric cancer (26). In a word, gastric cancer patients with malnutrition and system inflammation status tended to have higher metastasis rates and shorter survival times. Numerous previous studies have shown that composite indexes based on serum markers such as the Controlling Nutritional Status (CONUT) score, Glasgow Prognostic Score (GPS), and neutrophil-lymphocyte ratio (NLR) could both predict the prognosis of patients with gastric cancer (27–29). In addition, their ability to predict the prognosis of patients with gastric cancer who received ICIs has also been further confirmed.
The prognostic nutrition index (PNI) is calculated by serum albumin and lymphocyte. Current studies have shown that PNI has high accuracy in predicting the prognosis of various cancers after treatment, especially gastric cancer (30, 31). The immune system function is an important cause of ICI’s works, and serum albumin and lymphocyte in the bloodstream play an important role in reflecting immune system function. However, there has been still no evidence to demonstrate the usefulness of PNI as a prognosis assessment tool for patients with gastric cancer who received ICIs.
In this study, we enrolled 146 patients with gastric cancer at our institution who received ICIs (PD-1/PD-L1 inhibitors) or chemotherapy, and primarily analyzed the predictive ability of PNI for the prognosis of patients with ICIs. At the same time, we also performed subgroup analysis and constructed a nomogram to further test and verify the effectiveness of PNI in predicting the prognosis of patients with gastric cancer.
There were 146 patients with gastric cancer treated with chemotherapy or ICIs at our institution from August 2016 to December 2020 enrolled in this study. All patients and their clinical information were analyzed based on the Helsinki Declaration as well as its amendments and included according to the following criteria: (1) all patients underwent invasive gastroscope examination and pathological diagnosis; (2) all patients had no chronic disease or other types of cancer; and (3) all patients were received ICIs or chemotherapy. Patients with incomplete clinical information and without a regular review after treatment or abandoned treatment were exclusion criteria. An electronic medical record system was used to collect clinical and pathological information. Informed consent was waived by the Ethics Committee of Harbin Medical University Cancer Hospital owing to the retrospective nature of this study.
Routine telephone follow-up was used for all enrolled patients. Clinical and pathological information was acquired by an electronic medical record system. Progression-free survival (PFS) and overall survival (OS) were obtained by follow-up. PFS was comprehended as the period from the first day of surgery, ICIs, or chemotherapy date to the date of disease progression. The evidence of progression was obtained by chest and abdomen X-ray or computed tomography. PFS was also defined at the date of death or last follow-up with no evidence of progression in patients. OS was described as the period from the first day of ICIs, surgery, or chemotherapy date to the date of death or the last follow-up. PNI was calculated as follows: PNI = albumin (g/L) + 5 × lymphocyte (109/L). The cut-off point was obtained by receiver operating characteristic (ROC), and patients were divided into low-value groups (PNI < 44.63) and high-value groups (PNI ≥ 44.63) according to the cut-off point.
All statistical analyses were completed through the SPSS software 25.0 (Chicago, IL, USA) and R 4.1.3 (Vienna, Austria). Two-sided P-values < 0.05 were considered as statistical differences. The Chi-square test or Fisher’s exact test was used to compare the discrepancies between the two groups, the Kaplan–Meier survival curve was used to compute the survival rate, and the Log-rank test was used to compare the difference in survival time. Relative risks were assessed by the hazard ratio (HR) and 95% confidence interval (CI). The independent prognostic factors were analyzed by the constructed Cox proportional hazards regression model. Finally, the nomogram based on independent prognostic factors was established to predict the survival probability of PFS and OS.
According to the cut-off value of PNI, 41 (28.1%) patients in this study entered the low PNI group and 105 (71.9%) patients were in the high PNI group. The median age of patients was 59 years, and there were 44 women (30.1%) and 102 men (69.9%) in all two groups’ cases. In this study, 86 (58.9%) patients received surgery plus adjuvant therapy and 60 (41.1%) patients received only adjuvant therapy. Chi-square test shown that PNI was related to age (p = 0.011), electrocardiogram (p = 0.006), pathology (p = 0.008), and PD-L1 (p = 0.046). The detailed clinical characteristics of all 146 cases grouped by PNI are shown in Table 1.
In this study, we analyzed the blood examination results of patients before treatment and studied their relationship to PNI by Chi-square test or Fisher’s exact test. We grouped patients according to the medians of total protein (TP), albumin (ALB), globulin (GLOB), prealbumin (PALB), total bilirubin (TBIL), direct bilirubin (DBIL), indirect bilirubin (IDBIL), lymphocyte (L), hemoglobin (Hb), lymphocyte carcinoembryonic antigen (CEA), carbohydrate antigen 199 (CA199), carbohydrate antigen 724 (CA724), and carbohydrate antigen 125II (CA125II). Their medians and detailed blood parameters are shown in Table 2. We found that PNI was related to TP (p < 0.001), PALB (p = 0.001), and Hb (p < 0.001). At the same time, patients in the low PNI group were found to have lower ALB (p < 0.001) and L (p < 0.001) than the high PNI group by Fisher’s exact test.
According to univariate analysis, the prognosis factors of patients in this study for PFS and OS were both IDBIL (p = 0.046 vs. p = 0.032), PALB (p = 0.013 vs. p = 0.006), PNI (p = 0.013 vs. p = 0.001), CEA (p = 0.037 vs. p = 0.004), CA199 (p = 0.032 vs. p = 0.009), CA724 (p = 0.003 vs. p = 0.001), radical resection (p = 0.001 vs. p < 0.001), surgery (p = 0.005 vs. p = 0.006), Borrmann type (p = 0.040 vs. p = 0.040), TNM stage (p = 0.004 vs. p = 0.001), Lauren type (p = 0.003 vs. p = 0.002), and treatment (p = 0.001 vs. p < 0.001). In addition, TBIL (p = 0.042) was also found to be the prognosis factor for PFS by univariate analysis. The multivariate analysis indicated that CA724 (p = 0.028 vs. p = 0.019) and TNM stage (p = 0.019 vs. p = 0.010) were both independent prognostic factors for PFS and OS. In addition, PNI (p = 0.030) and IDBIL (p = 0.032) were also the independent prognostic factors for OS. The detailed information is shown in Table 3.
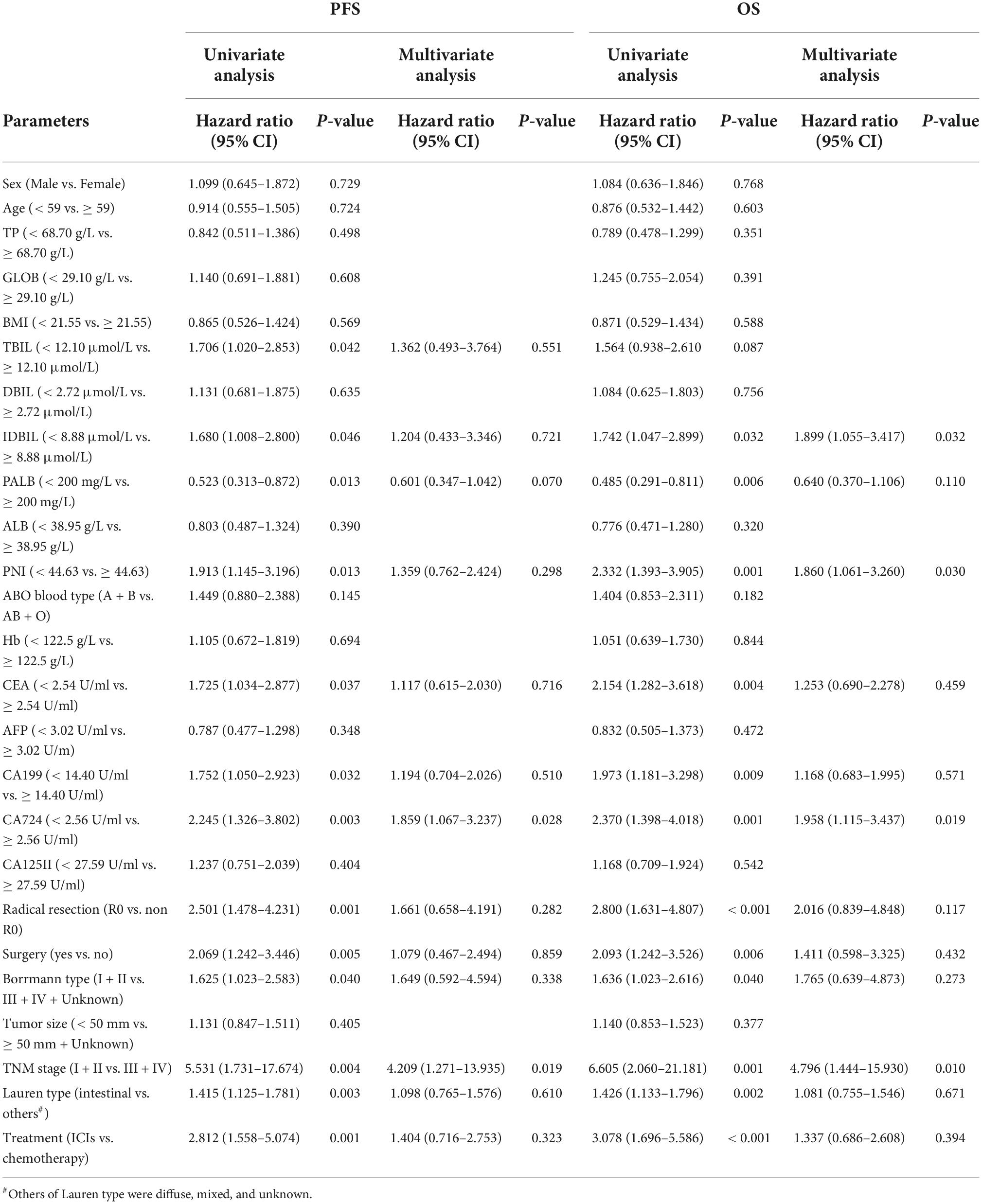
Table 3. Univariate and multivariate analyses for progression-free survival (PFS) and overall survival (OS).
The low PNI group’s median survival time (MST) for PFS and OS was 12.30 months and 18.57 months, while the MST for PFS and OS in the high PNI group was 33.07 months and not reached. Low PNI group’s patients had shorter PFS and OS than patients in the high PNI group (HR = 1.913, 95% CI: 1.145–3.196, p = 0.013 and HR = 2.332, 95% CI: 1.393–3.905, p = 0.001) (Figures 1A,B).
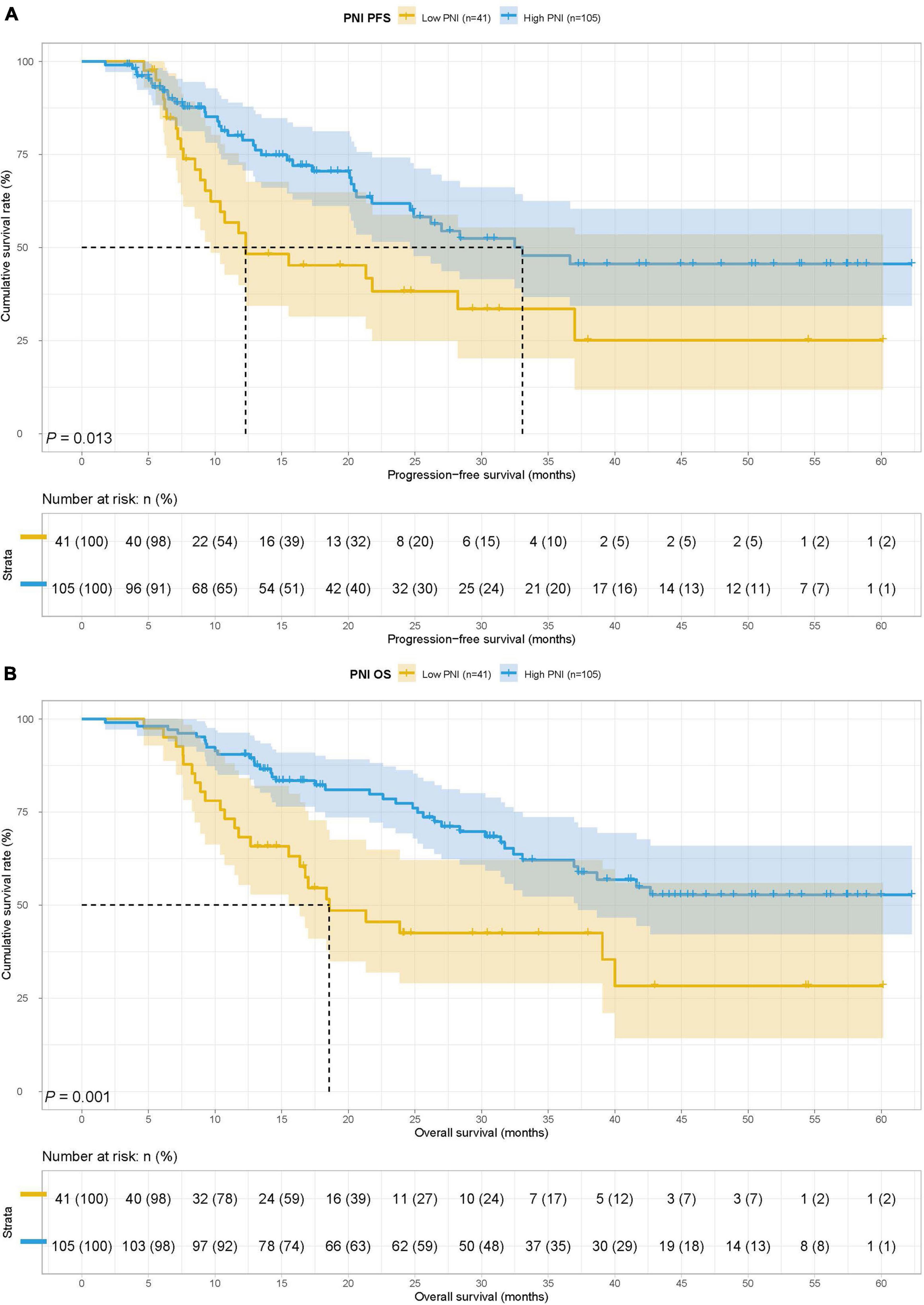
Figure 1. Prognostic nutrition index (PNI) related survival curve of (A) progression-free survival (PFS) and (B) overall survival (OS) in all patients.
To study the predictive ability of PNI for the prognosis of gastric cancer patients with ICIs, we divided the 146 cases into the ICIs group (89 patients) and the chemotherapy group (57 patients). Treatment was related to surgery (p < 0.001), tumor size (p = 0.046), TNM stage (p = 0.002), and Lauren type (p < 0.001). At the same time, Fisher’s exact test found that treatment was also related to Borrmann type (p < 0.001), PD-1 (p < 0.001), and PD-L1 (p < 0.001) (Table 4). The MST for PFS and OS in the ICIs group was 20.60 months and 30.27 months, and the MST for PFS and OS in the chemotherapy group was both not reached. Patients received chemotherapy had longer PFS (HR = 0.356, 95% CI: 0.197–0.642, P = 0.001) and OS (HR = 0.325, 95% CI: 0.179–0.590, p < 0.001) than those received ICIs (Figures 2A,B).
In the ICIs group, there were 27 cases in the low PNI group and 62 cases in the high PNI group, The MST for PFS in the low PNI group and high PNI group was 12.30 months vs. 21.77 months, while the MST for OS in the two groups was 18.37 vs. 32.40 months. Low PNI group had poorer PFS (HR = 1.583, 95% CI: 0.877–2.857, p = 0.124) and OS (HR = 2.210, 95% CI: 1.209–4.040, p = 0.008) than high PNI group in patients with ICIs (Figures 3A,B).
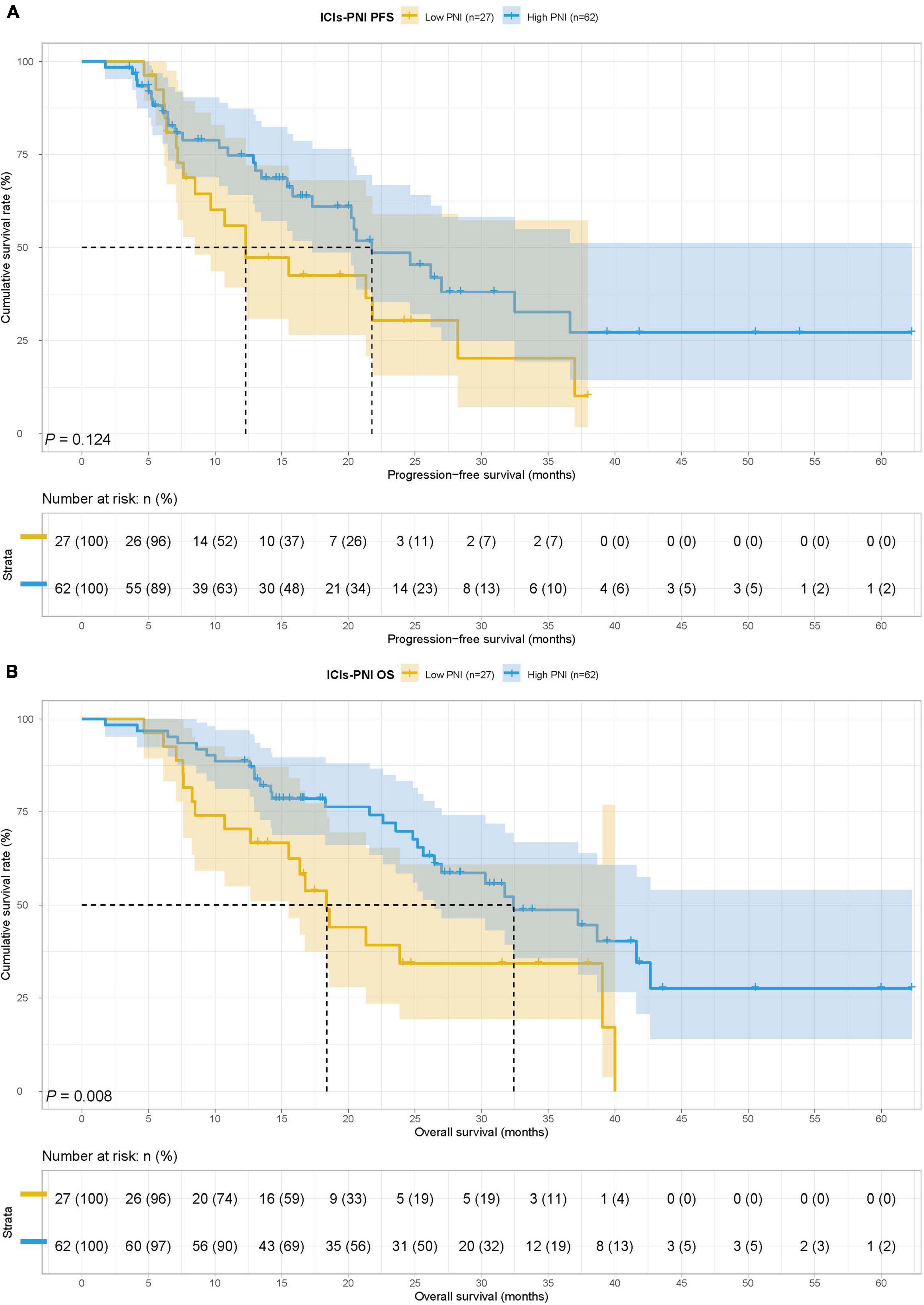
Figure 3. Prognostic nutrition index related survival curve of (A) PFS and (B) OS in the ICIs group.
In the chemotherapy group, there were 14 patients in the low PNI group and 43 patients in the high PNI group. The MST for PFS and OS in the low PNI group and high PNI group was both not reached. Low PNI group was also associated with shorter PFS and OS than high PNI group (HR = 2.489, 95% CI: 0.882–7.028, p = 0.075 and HR = 2.778, 95% CI: 0.981–7.869, p = 0.045) (Figures 4A,B).
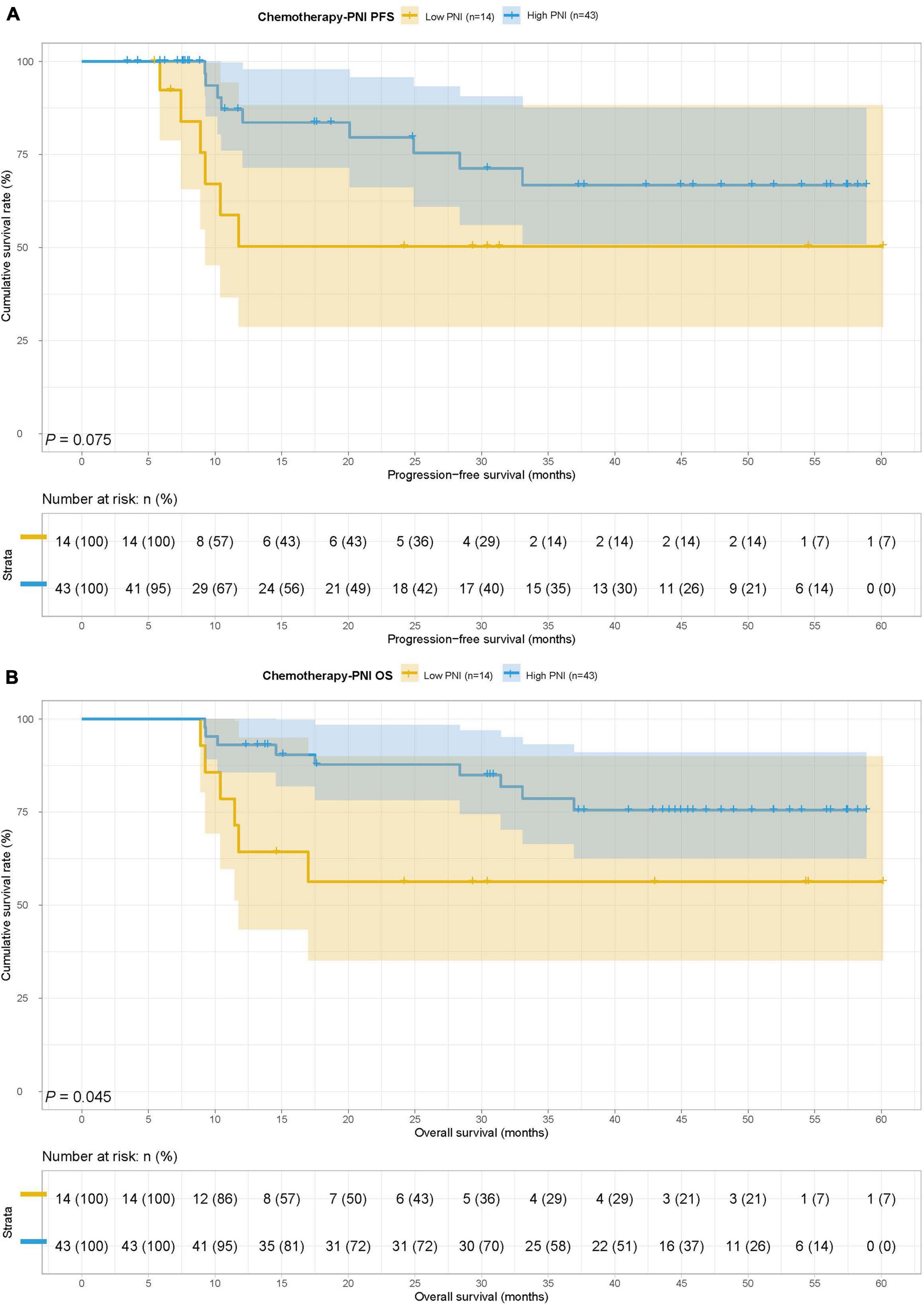
Figure 4. Prognostic nutrition index related survival curve of (A) PFS and (B) OS in the chemotherapy group.
In this study, we found that PALB was both PFS and OS’s independent prognostic factor by multivariate analysis. To further checked the prognostic prediction ability of PNI, we extra analyzed the application of PNI in gastric cancer patients with different PALB states. According to the median PALB value, patients in this study were enrolled in the low PALB group (PALB < 200) and high PALB group (PALB ≥ 200). The MST for PFS in the low PALB group and high PALB group was 20.10 months vs. 36.63 months, respectively, while the MST for OS in the two groups was 32.40 months vs. not reached. Patients with the low PALB group had shorter PFS and OS than high PALB group in this study (HR = 0.523, 95% CI: 0.313–0.872, p = 0.012 and HR = 0.485, 95% CI: 0.291–0.811, p = 0.005) (Figures 5A,B).
In the low PALB group, there were 29 cases in the low PNI group and 43 cases in the high PNI group. The MST for PFS in the low PNI group and high PNI group was 10.40 months vs. 33.07 months, respectively, while the MST for OS in the two groups was 17.00 months vs. 41.63 months. Low PNI group’s patients had poorer PFS and OS than patients in the high PNI group in this study (HR = 2.191, 95% CI: 1.150–4.175, p = 0.015 and HR = 2.429, 95% CI: 1.268–4.653, p = 0.006) (Figures 6A,B).
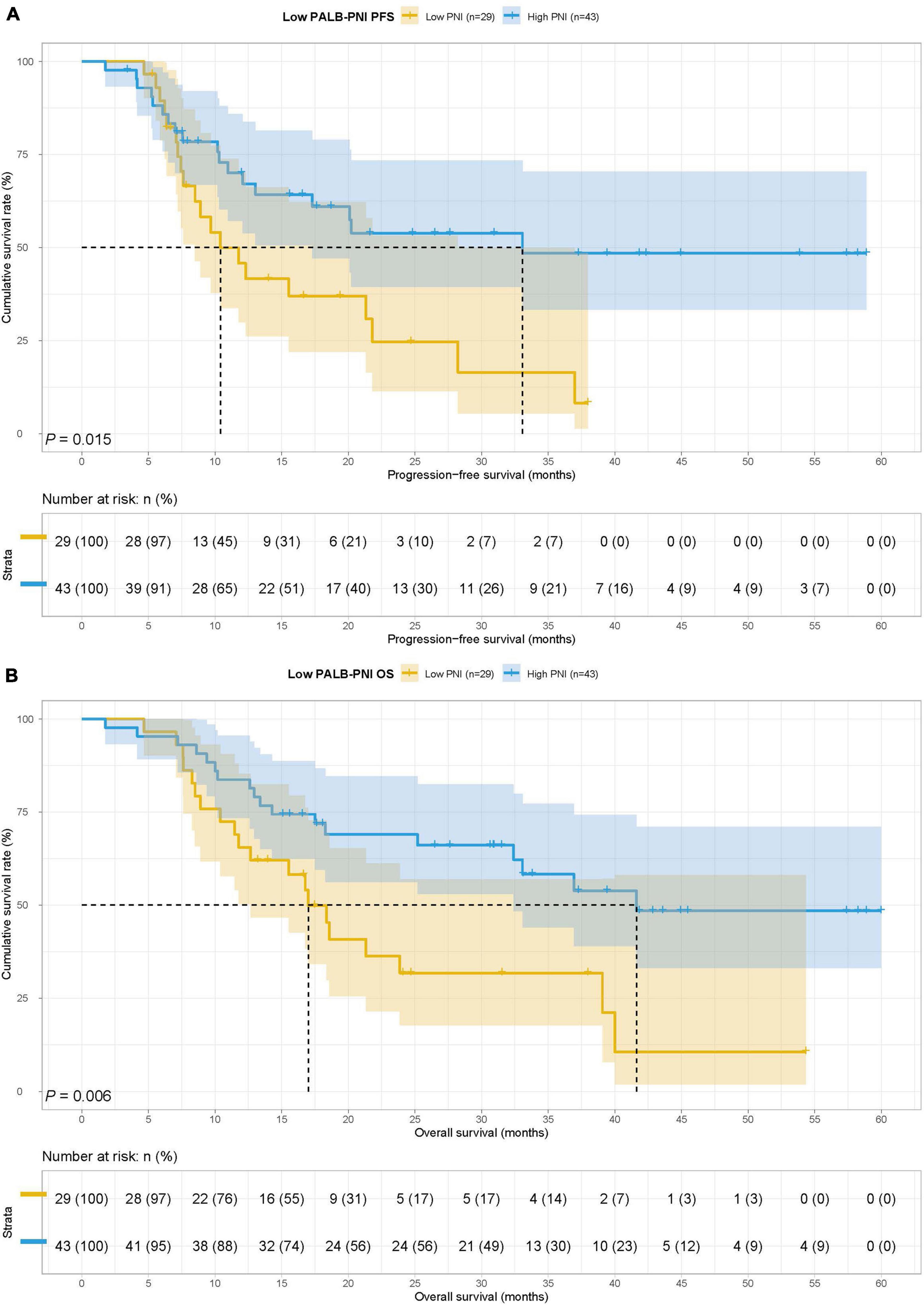
Figure 6. Prognostic nutrition index related survival curve of (A) PFS and (B) OS in the low PALB group.
In the high PALB group, there were 12 cases in the low PNI group and 62 cases in the high PNI group. The MST for PFS in the low PNI group and high PNI group was not reached vs. 28.37 months, while the MST for OS in the two groups was both not reached. There was no significant difference for PFS and OS in two groups (HR = 1.170, 95% CI: 0.399–3.433, p = 0.774 and HR = 0.841, 95% CI: 0.287–2.463, p = 0.753) (Figures 7A,B).
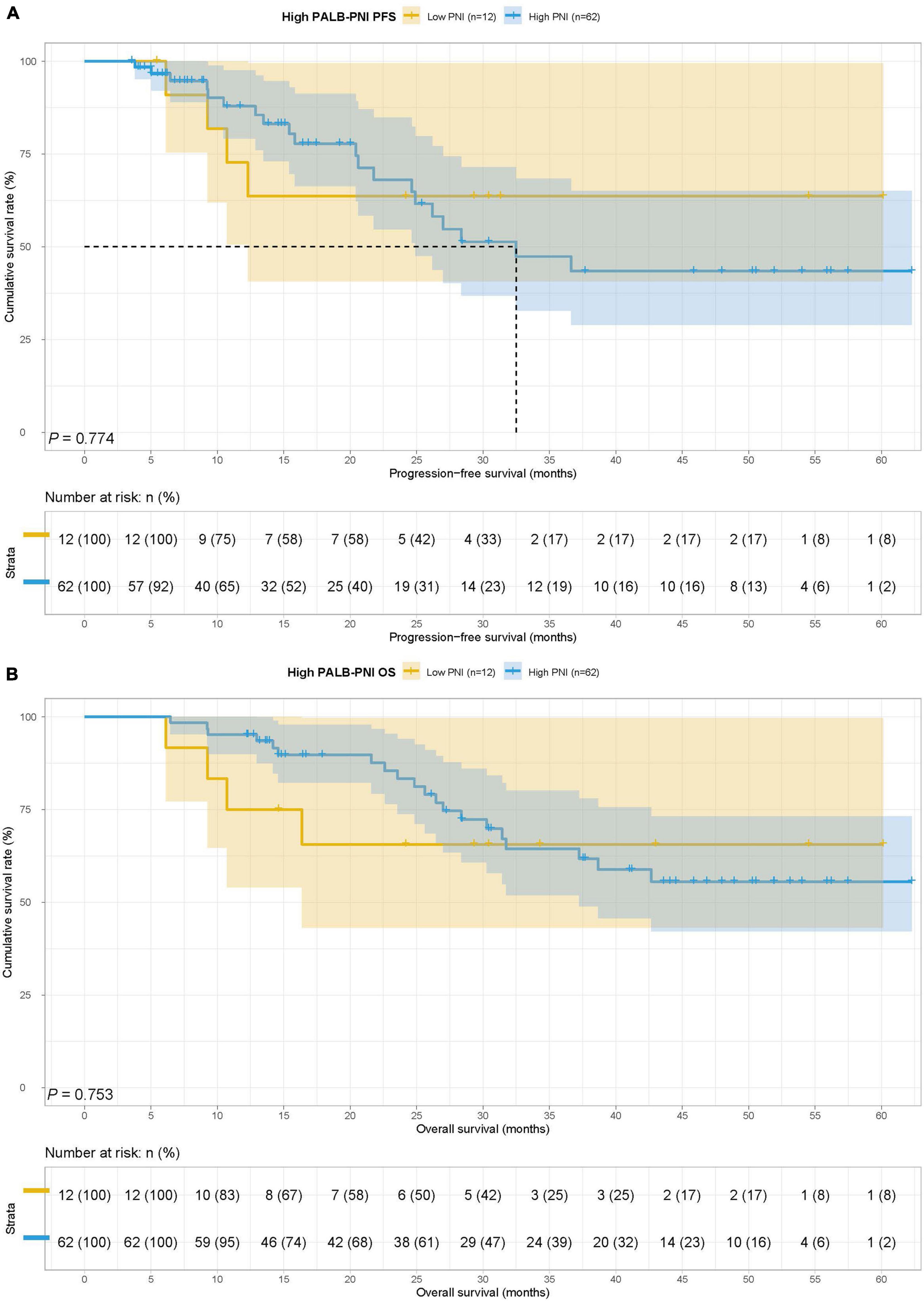
Figure 7. Prognostic nutrition index related survival curve of (A) PFS and (B) OS in the high PALB group.
In this study, we found that CA724 and TNM stages were the independent prognostic factors for PFS and PNI, IDBIL, CA724, and TNM stages were the independent prognostic factors for OS by the constructed Cox proportional hazards regression model. According to the results of multivariate analysis, the nomograms to predict the 1- and 3-year survival probabilities for PFS and OS were established (Figures 8A,B). The C-index and 95% CI for predicting the survival probability of PFS and OS were 0.649 (0.588–0.710) and 0.737 (0.674–0.799).
With the wide application of ICIs in gastric cancer, people began to seek accurate biomarkers to predict the sensitivity of patients with gastric cancer to ICIs. However, current biomarkers include PD-1/PD-L1 expression levels, microsatellite instability (MSI), tumor mutation burden (TMB), and Epstein–Barr virus (EBV) infection status were still some limitations (11, 12, 32). The function of the immune system, which is affected by nutrition and inflammation, is one of the important factors for ICIs (33, 34). Therefore, we attempted to find a biomarker based on nutritional and inflammatory status to predict the clinical outcomes of patients with gastric cancer who received ICIs.
The PNI is a biomarker to evaluate the nutritional and inflammatory status of patients. In 1984, Onodera et al. first created it to evaluate the nutritional status of surgical patients, predict the surgical risk, and judge the prognosis (35). In subsequent studies, the predictive role of PNI in various tumors was gradually discovered. Okadome et al. analyzed 337 patients with esophageal cancer and found that patients with low PNI values were significantly correlated with clinical outcomes. Low PNI group patients had shorter OS in their study (36). The studies of Bozkaya et al. and Li et al. on 280 patients with prostate cancer and 333 patients with metastatic non-small cell lung cancer obtained similar results. The low PNI group had poorer clinical outcomes (37, 38). At the same time, people found that PNI was more accurate in predicting the prognosis of patients with gastric cancer. In a retrospective study of 245 gastric cancer patients with total gastrectomy, Xishan et al. found that patients with low PNI status were closely correlated with poor prognosis by univariate analysis. Multivariate analysis also found that PNI was an independent prognostic factor for patients with gastric cancer (39). Park et al. studied the correlation between PNI and BMI change before/after surgery and prognosis in patients with gastric cancer. They collected 1,868 patients with gastric cancer treated with gastrectomy and found that preoperative low BMI and PNI status as well as decreased BMI and PNI before/after surgery were all associated with poor prognosis. At the same time, BMI and PNI were both gastric cancer patients’ independent prognostic factors (30). Hirahara et al. retrospectively recruited 368 gastric cancer patients with normal serum CEA levels and analyzed the predictive ability of PNI for these subjects. The results showed that both univariate and multivariate analyses found that PNI was significantly correlated with the prognosis of gastric cancer patients with normal serum CEA levels, and patients with low PNI values had poorer cancer-specific survival (CSS) (40). To sum up, PNI has a strong predictive ability for prognosis in various cancers, especially gastric cancer.
This study mainly analyzed the use of PNI in gastric cancer patients with ICIs or chemotherapy. We also further explored the predictive ability of PNI on the prognosis of patients with gastric cancer in different PALB states. The results showed that patients in the low PNI group had shorter PFS and OS in all subjects. Similar results were also obtained in the ICIs group and chemotherapy group in subgroup analysis, especially the ICIs group. In the multivariate analysis, we found that ALB, PALB, PNI, CA724, and TNM stage were significant independent prognostic factors for both PFS and OS. Similarly, further analysis revealed that low PNI value patients had shorter survival time in the low PALB group. However, restricted by the number of patients, we did not get meaningful results in the analysis of the high PALB group.
In this study, we analyzed the effects of ICIs and chemotherapy on the clinical outcomes of patients with gastric cancer. The results showed that patients who received ICIs had poorer PFS and OS. This is because ICIs are still not routinely administered to patients with gastric cancer at our institution. In most cases, ICIs are only considered for patients with progressive gastric cancer whose conventional therapy has failed. The results of correlation analysis also reflect this phenomenon, and the use of ICIs was significantly related to non-surgery and high TNM stages, which were both significant independent prognostic factors for patients with gastric cancer according to previous studies.
The mechanism that caused this observation remains unclear. PNI contains albumin and lymphocyte, which reflect the nutrition and immune status of the body to a certain extent. On the one hand, low serum albumin represents malnutrition. On the other hand, previous studies have shown that albumin was also a biomarker for systemic inflammation (41). Some inflammatory factors can inhibit the synthesis of albumin, and oxidative stress can lead to the denaturation of albumin, all these factors lead to the rapid decrease of serum albumin levels in patients with the inflammation state (42, 43). The state of malnutrition and systemic inflammation were important factors leading to tumor progression (44–47). The lymphocyte is part of the immune system and has strong anti-tumor activity (48). Studies have shown that low serum lymphocyte is significantly associated with poor prognosis in patients with gastric cancer (49–51). ICIs against tumors by relieving the inhibition of tumor cells on the immune system, so nutrition and immune status are important factors for its working (52). Therefore, PNI combined with serum albumin and lymphocyte can effectively predict the efficacy of ICIs.
Finally, our study inevitably had some limitations. First, this was a single-center retrospective study, a prospective randomized controlled trial was an important method to overcome potential information bias. Second, the types of ICIs were not strictly distinguished; patients used different ICIs in this study. Third, the cut-off value of PNI was often calculated by the ROC curve, and there was still no recognized standard. Forth, as a systemic inflammation and nutrition evaluation index, PNI only included albumin and lymphocyte, which could be combined with more markers such as the Geriatric Nutritional Risk Index (GNRI) which reflect the changes in body weight. The result of this study also needs to be further verified by a study with larger sample sizes and better design.
The PNI was an accurate prognostic tool for patients with gastric cancer. In patients with gastric cancer who received ICIs, it displayed a better ability to predict the prognosis. Patients with low PNI had poorer clinical outcomes. Therefore, PNI can be used as a biomarker for ICIs to identify patients with gastric cancer who are sensitive to ICIs.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by the Ethics Committee of Harbin Medical University Cancer Hospital. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
HSu, RH, and LC: writing—original draft, review, and editing. RZ and HP: data curation and investigation. YZ: methodology and supervision. YX and HSo: resources, funding acquisition, and project administration. All authors contributed to the article and approved the submitted version.
This study was funded by Clinical Research Foundation of Wu Jieping Medical Foundation (No: 320.6750.2022-07-13).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Ajani JA, D’Amico TA, Bentrem DJ, Chao J, Cooke D, Corvera C, et al. Gastric cancer, version 2.2022, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Ne. (2022) 20:167–92. doi: 10.6004/jnccn.2022.0008
2. Digklia A, Wagner AD. Advanced gastric cancer: current treatment landscape and future perspectives. World J Gastroentero. (2016) 22:2403–14. doi: 10.3748/wjg.v22.i8.2403
3. Johnston FM, Beckman M. Updates on management of gastric cancer. Curr Oncol Rep. (2019) 21:67. doi: 10.1007/s11912-019-0820-4
4. Chen QY, Zhong Q, Wang W, Chen S, Li P, Xie JW, et al. Prognosis of young survivors of gastric cancer in China and the U.S: determining long-term outcomes based on conditional survival. Oncologist. (2019) 24:e260–74. doi: 10.1634/theoncologist.2018-0220
5. Yu J, Huang C, Sun Y, Su X, Cao H, Hu J, et al. Effect of laparoscopic vs open distal gastrectomy on 3-year disease-free survival in patients with locally advanced gastric cancer: the class-01 randomized clinical trial. JAMA J Am Med Assoc. (2019) 321:1983–92. doi: 10.1001/jama.2019.5359
6. Keenan TE, Tolaney SM. Role of immunotherapy in triple-negative breast cancer. J Natl Compr Canc Ne. (2020) 18:479–89. doi: 10.6004/jnccn.2020.7554
7. Suresh K, Naidoo J, Lin CT, Danoff S. Immune checkpoint immunotherapy for non-small cell lung cancer: benefits and pulmonary toxicities. Chest. (2018) 154:1416–23. doi: 10.1016/j.chest.2018.08.1048
8. Zhou F, Qiao M, Zhou C. The cutting-edge progress of immune-checkpoint blockade in lung cancer. Cell Mol Immunol. (2021) 18:279–93. doi: 10.1038/s41423-020-00577-5
9. Rizzo A, Ricci AD, Brandi G. Recent advances of immunotherapy for biliary tract cancer. Expert Rev Gastroent. (2021) 15:527–36. doi: 10.1080/17474124.2021.1853527
10. Guidi A, Codecà C, Ferrari D. Chemotherapy and immunotherapy for recurrent and metastatic head and neck cancer: a systematic review. Med Oncol. (2018) 35:37. doi: 10.1007/s12032-018-1096-5
11. Takei S, Kawazoe A, Shitara K. The new era of immunotherapy in gastric cancer. Cancers. (2022) 14:1054. doi: 10.3390/cancers14041054
12. Li K, Zhang A, Li X, Zhang H, Zhao L. Advances in clinical immunotherapy for gastric cancer. BBA-Rev Cancer. (2021) 1876:188615. doi: 10.1016/j.bbcan.2021.188615
13. Formica V, Morelli C, Patrikidou A, Shiu KK, Nardecchia A, Lucchetti J, et al. A systematic review and meta-analysis of PD-1/PD-L1 inhibitors in specific patient subgroups with advanced gastro-oesophageal junction and gastric adenocarcinoma. Crit Rev Oncol Hemat. (2021) 157:103173. doi: 10.1016/j.critrevonc.2020.103173
14. Kim H, Hong JY, Lee J, Park SH, Park JO, Park YS, et al. Clinical sequencing to assess tumor mutational burden as a useful biomarker to immunotherapy in various solid tumors. Ther Adv Med Oncol. (2021) 13:1758835921992992. doi: 10.1177/1758835921992992
15. Muscaritoli M, Arends J, Bachmann P, Baracos V, Barthelemy N, Bertz H, et al. ESPEN practical guideline: clinical nutrition in cancer. Clin Nutr. (2021) 40:2898–913. doi: 10.1016/j.clnu.2021.02.005
16. Langius JA, Zandbergen MC, Eerenstein SE, van Tulder MW, Leemans CR, Kramer MH, et al. Effect of nutritional interventions on nutritional status, quality of life and mortality in patients with head and neck cancer receiving (chemo)radiotherapy: a systematic review. Clin Nutr. (2013) 32:671–8. doi: 10.1016/j.clnu.2013.06.012
17. Lewandowska A, Religioni U, Czerw A, Deptała A, Karakiewicz B, Partyka O, et al. Nutritional treatment of patients with colorectal cancer. Int J Environ Res Public Health. (2022) 19:6881. doi: 10.3390/ijerph19116881
18. Uster A, Ruehlin M, Mey S, Gisi D, Knols R, Imoberdorf R, et al. Effects of nutrition and physical exercise intervention in palliative cancer patients: a randomized controlled trial. Clin Nutr. (2018) 37:1202–9. doi: 10.1016/j.clnu.2017.05.027
19. Viana ECRM, Oliveira IDS, Rechinelli AB, Marques IL, Souza VF, Spexoto MCB, et al. Malnutrition and nutrition impact symptoms (NIS) in surgical patients with cancer. PLoS One. (2020) 15:e0241305. doi: 10.1371/journal.pone.0241305
20. Sadakane-Sakuramoto A, Hasegawa Y, Sugahara K, Horii N, Saito S, Nakao Y, et al. Change in nutritional status and dysphagia after resection of head and neck cancer. Nutrients. (2021) 13:2438. doi: 10.3390/nu13072438
21. Luo Z, Zhou L, Balde AI, Li Z, He L, ZhenWei C, et al. Prognostic impact of preoperative prognostic nutritional index in resected advanced gastric cancer: a multicenter propensity score analysis. EJSO Eur J Surg Onc. (2019) 45:425–31. doi: 10.1016/j.ejso.2018.09.004
22. Kim JH, Bae YJ, Jun KH, Chin HM. Long-term trends in hematological and nutritional status after gastrectomy for gastric cancer. J Gastrointest Surg. (2017) 21:1212–9. doi: 10.1007/s11605-017-3445-7
23. Kim YN, Choi YY, An JY, Choi MG, Lee JH, Sohn TS, et al. Comparison of postoperative nutritional status after distal gastrectomy for gastric cancer using three reconstructive methods: a multicenter study of over 1300 patients. J Gastrointest Surg. (2020) 24:1482–8. doi: 10.1007/s11605-019-04301-1
24. Mizukami T, Piao Y. Role of nutritional care and general guidance for patients with advanced or metastatic gastric cancer. Future Oncol. (2021) 17:3101–9. doi: 10.2217/fon-2021-0186
25. Liu ZJ, Ge XL, Ai SC, Wang HK, Sun F, Chen L, et al. Postoperative decrease of serum albumin predicts short-term complications in patients undergoing gastric cancer resection. World J Gastroentero. (2017) 23:4978–85. doi: 10.3748/wjg.v23.i27.4978
26. Lu J, Xu BB, Zheng ZF, Xie JW, Wang JB, Lin JX, et al. CRP/prealbumin, a novel inflammatory index for predicting recurrence after radical resection in gastric cancer patients: post hoc analysis of a randomized phase III trial. Gastric Cancer. (2019) 22:536–45. doi: 10.1007/s10120-018-0892-0
27. Chen L, Sun H, Zhao R, Huang R, Pan H, Zuo Y, et al. Controlling nutritional status (CONUT) predicts survival in gastric cancer patients with immune checkpoint inhibitor (PD-1/PD-L1) outcomes. Front Pharmacol. (2022) 13:836958. doi: 10.3389/fphar.2022.836958
28. Kurosaki T, Kawakami H, Mitani S, Kawabata R, Takahama T, Nonagase Y, et al. Glasgow prognostic score (GPS) and tumor response as biomarkers of nivolumab monotherapy in third- or later-line setting for advanced gastric cancer. In Vivo. (2020) 34:1921–9. doi: 10.21873/invivo.11989
29. Gou M, Qu T, Wang Z, Yan H, Si Y, Zhang Y, et al. Neutrophil-to-lymphocyte ratio (NLR) predicts pd-1 inhibitor survival in patients with metastatic gastric cancer. J Immunol Res. (2021) 2021:2549295. doi: 10.1155/2021/2549295
30. Park SH, Lee S, Song JH, Choi S, Cho M, Kwon IG, et al. Prognostic significance of body mass index and prognostic nutritional index in stage II/III gastric cancer. EJSO Eur J Surg Onc. (2020) 46(4 Pt A.):620–5. doi: 10.1016/j.ejso.2019.10.024
31. Liu JY, Dong HM, Wang WL, Wang G, Pan H, Chen WW, et al. The effect of the prognostic nutritional index on the toxic side effects of radiochemotherapy and prognosis after radical surgery for gastric cancer. Cancer Manag Res. (2021) 13:3385–92. doi: 10.2147/CMAR.S301140
32. Eso Y, Shimizu T, Takeda H, Takai A, Marusawa H. Microsatellite instability and immune checkpoint inhibitors: toward precision medicine against gastrointestinal and hepatobiliary cancers. J Gastroenterol. (2020) 55:15–26. doi: 10.1007/s00535-019-01620-7
33. Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. (2010) 140:883–99. doi: 10.1016/j.cell.2010.01.025
34. Suardi C, Cazzaniga E, Graci S, Dongo D, Palestini P. Link between viral infections, immune system, inflammation and diet. Int J Environ Res Public Health. (2021) 18:2455. doi: 10.3390/ijerph18052455
35. Onodera T, Goseki N, Kosaki G. [Prognostic nutritional index in gastrointestinal surgery of malnourished cancer patients]. Nihon Geka Gakkai Zasshi. (1984) 85:1001–5.
36. Okadome K, Baba Y, Yagi T, Kiyozumi Y, Ishimoto T, Iwatsuki M, et al. Prognostic nutritional index, tumor-infiltrating lymphocytes, and prognosis in patients with esophageal cancer. Ann Surg. (2020) 271:693–700. doi: 10.1097/SLA.0000000000002985
37. Bozkaya Y, Köstek O, Sakin A, Özyükseler DT, Şakalar T, Çil I. Is the prognostic nutritional index a prognostic and predictive factor in metastatic non-small cell lung cancer patients treated with first-line chemotherapy? Support Care Cancer. (2020) 28:2273–82. doi: 10.1007/s00520-019-05055-x
38. Li B, Lu Z, Wang S, Hou J, Xia G, Li H, et al. Pretreatment elevated prognostic nutritional index predicts a favorable prognosis in patients with prostate cancer. BMC Cancer. (2020) 20:361. doi: 10.1186/s12885-020-06879-1
39. Xishan Z, Ye Z, Feiyan M, Liang X, Shikai W. The role of prognostic nutritional index for clinical outcomes of gastric cancer after total gastrectomy. Sci Rep. (2020) 10:17373. doi: 10.1038/s41598-020-74525-8
40. Hirahara N, Tajima Y, Fujii Y, Kaji S, Yamamoto T, Hyakudomi R, et al. Prognostic nutritional index as a predictor of survival in resectable gastric cancer patients with normal preoperative serum carcinoembryonic antigen levels: a propensity score matching analysis. BMC Cancer. (2018) 18:285. doi: 10.1186/s12885-018-4201-4
41. Evans DC, Corkins MR, Malone A, Miller S, Mogensen KM, Guenter P, et al. The use of visceral proteins as nutrition markers: an ASPEN position paper. Nutr Clin Pract. (2021) 36:22–8. doi: 10.1002/ncp.10588
42. Coffelt SB, de Visser KE. Cancer: inflammation lights the way to metastasis. Nature. (2014) 507:48–9. doi: 10.1038/nature13062
43. Bito R, Hino S, Baba A, Tanaka M, Watabe H, Kawabata H. Degradation of oxidative stress-induced denatured albumin in rat liver endothelial cells. Am J Physiol-Cell Ph. (2005) 289:C531–42. doi: 10.1152/ajpcell.00431.2004
44. Anandavadivelan P, Lagergren P. Cachexia in patients with oesophageal cancer. Nat Rev Clin Oncol. (2016) 13:185–98. doi: 10.1038/nrclinonc.2015.200
45. Padoan A, Plebani M, Basso D. Inflammation and pancreatic cancer: focus on metabolism, cytokines, and immunity. Int J Mol Sci. (2019) 20:676. doi: 10.3390/ijms20030676
46. Tuomisto AE, Mäkinen MJ, Väyrynen JP. Systemic inflammation in colorectal cancer: underlying factors, effects, and prognostic significance. World J Gastroentero. (2019) 25:4383–404. doi: 10.3748/wjg.v25.i31.4383
47. Huang H, Liu Q, Zhu L, Zhang Y, Lu X, Wu Y, et al. Prognostic value of preoperative systemic immune-inflammation index in patients with cervical cancer. Sci Rep. (2019) 9:3284. doi: 10.1038/s41598-019-39150-0
48. Hirahara T, Arigami T, Yanagita S, Matsushita D, Uchikado Y, Kita Y, et al. Combined neutrophil-lymphocyte ratio and platelet-lymphocyte ratio predicts chemotherapy response and prognosis in patients with advanced gastric cancer. BMC Cancer. (2019) 19:672. doi: 10.1186/s12885-019-5903-y
49. Kim EY, Song KY. The preoperative and the postoperative neutrophil-to-lymphocyte ratios both predict prognosis in gastric cancer patients. World J Surg Oncol. (2020) 18:293. doi: 10.1186/s12957-020-02059-4
50. Mungan I, Dicle ÇB, Bektaş Ş, Sarı S, Yamanyar S, Çavuş M, et al. Does the preoperative platelet-to-lymphocyte ratio and neutrophil-to-lymphocyte ratio predict morbidity after gastrectomy for gastric cancer? Military Med Res. (2020) 7:9. doi: 10.1186/s40779-020-00234-y
51. Park SJ, Lee J, Kim H, Shin K, Lee M, Park JM, et al. Association between absolute lymphocyte count and overall mortality in patients with surgically resected gastric cancer. Korean J Intern Med. (2021) 36:679–88. doi: 10.3904/kjim.2019.358
Keywords: prognostic nutritional index, gastric cancer, immune checkpoint inhibitors, clinical outcomes, PD-1/PD-L1
Citation: Sun H, Chen L, Huang R, Pan H, Zuo Y, Zhao R, Xue Y and Song H (2022) Prognostic nutritional index for predicting the clinical outcomes of patients with gastric cancer who received immune checkpoint inhibitors. Front. Nutr. 9:1038118. doi: 10.3389/fnut.2022.1038118
Received: 06 September 2022; Accepted: 19 October 2022;
Published: 10 November 2022.
Edited by:
Massimo Marzorati, Ghent University, BelgiumReviewed by:
Yang Wo, Qingdao University, ChinaCopyright © 2022 Sun, Chen, Huang, Pan, Zuo, Zhao, Xue and Song. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hongjiang Song, aG9uZ2ppYW5nc29uZzIwMTZAMTYzLmNvbQ==
†These authors have contributed equally to this work
‡ORCID: Hao Sun, orcid.org/0000-0003-2001-7056
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.