
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Nutr., 07 November 2022
Sec. Food Chemistry
Volume 9 - 2022 | https://doi.org/10.3389/fnut.2022.1034751
This article is part of the Research TopicNatural Food Additives: Sustainable Development of Bio-Based MoleculesView all 4 articles
Artificial induction of polyploidy is an efficient technique for improving biological properties and developing new varieties of many plants. In this study, we analyzed and compared differences in characteristics (morphological and biological) of diploid and tetraploid Anoectochilus roxburghii plants. We found significant differences between tetraploid plants and their diploid counterparts. The tetraploid plants exhibited dwarfing and stockiness. They were also bigger and had more voluminous roots and larger stomata than the diploid plants. Moreover, the biochemical analyses showed that the contents of some amino acids and minerals elements were significantly higher in tetraploid plants. The chlorophyll content of the leaves exhibited no definitive changes, but the photosynthetic performance was higher in the tetraploid plants. In addition, contents of major bioactive compounds, such as kinsenoside and some flavonoids, were enhanced in tetraploids. This is the first detailed analysis of characteristics in diploid and tetraploid A. roxburghii plants. The results may facilitate breeding programs with the species.
Anoectochilus roxburghii (Wall.) Lindl, commonly known as Jinxianlian, belongs to the Orchidaceae family and is naturally distributed in many parts of a broad zone of the tropics, encompassing India, the Himalayas, southern China, large tracts of Southeast Asia and Hawaii (1). As a valuable element of Chinese herbal medicine, this species is frequently used in medical and health products in China and Asian countries (2–4). It is also often used as an indoor plant as it is highly ornamental (5). In recent years industrial-scale use of A. roxburghii has grown as a result of its wide application in many fields, such as medicine, healthcare and so on (6). Most raw material of the plant for industrial processing is obtained by artificial cultivation, as wild resources cannot meet the market demand (7–9).
Polyploid plants, either naturally occurring or generated with chromosome doubling techniques, are used in many breeding programs for medicinal plants to increase biomass and enhance desirable traits (10–12). Chromosome doubling is often accompanied by substantial changes in morphology and metabolic profiles (13, 14). It also has generally positive effects on stress resistance (15). Thus, polyploidy induction can be used to develop superior varieties. In this study, the morphology and biochemical characteristics of diploid and induced tetraploid A. roxburghii were compared. The ploidy level effects on the plant’s ability to resist high temperature stress were also assessed.
Flow cytometric peaks of the diploid mother and tetraploid offspring plants (at channels 13,000 and 6,500, respectively) confirmed their ploidy levels (Figure 1). We assessed various morphological characteristics of the diploid and tetraploid plants when they were 1 year old, and observed significant differences in some of them (Figure 2). Tetraploid plants had higher biomass (both fresh and dry weight), although the diploids were significantly taller, due to longer internodes. The tetraploid plants had thicker stems than the diploids, and shorter but broader and hypertrophic leaves. The tetraploid plants also produced more roots and had significantly higher total root length, root surface area, and root volume (Table 1).
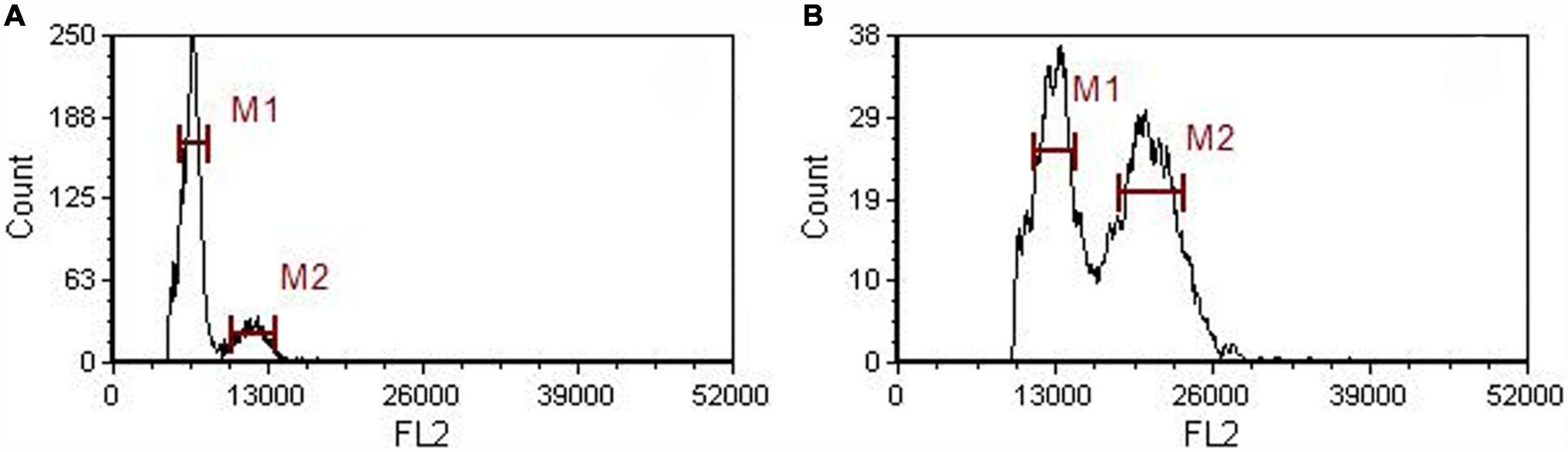
Figure 1. Histograms showing the results of flow cytometric analysis of diploid (A) and tetraploid (B) A. roxburghii plants.

Figure 2. Morphological characteristics of diploid and tetraploid A. roxburghii: whole plants (A–C), leaves (D) and stems (E). Diploids on the left and tetraploids on the right in each picture.
Anoectochilus roxburghii has traditionally been considered to be one of the most valuable medicinal plants because it is rich in amino acids, minerals and so on (8). The amino acids are necessary for metabolic processes, the transport and storage of all nutrients (16). Minerals play important roles in human health, not only affecting the enzyme activities, but also influencing the accumulation of metabolites (17). The types of free amino acids identified in diploids and tetraploids plants were identical, but the detected amounts differed (Table 2). Two of the most abundant amino acids in A. roxburghii were aspartate (Asp) and glutamate (Glu) and the average content was 2,254 μg/g and 1,067 μg/g in diploids plants, respectively. Contents of these two amino acids were greater in tetraploid than diploid plants. In addition to these two amino acids, there were some other amino acids that were more abundant in tetraploids plants, such as serine (Ser) and glycine (Gly). While there were some amino acids were more abundant in diploids, such as phenylalanine (Phe) and histidine (His). Tetraploid plants had higher concentrations of Ca, Mg, and Zn than diploids. The largest difference in concentration recorded between the plants was for Ca, which was about twice as high in tetraploids than diploids. Concentrations of Mn and Cr were lower in tetraploid plants (Table 3), but no significant differences in contents of the other measured elements were detected.
Kinsenoside and flavonoids, the main bioactive compounds in A. roxburghii, are often used as quality control markers (8). Narcissin, isorhamnetin, rutin, and quercetin are the characteristic flavonoids (8, 18). Thus, we examined contents of these five compounds in leaves, stems and roots of diploid and tetraploid plants (Table 4). They were mainly found in leaves. The kinsenoside concentration was non-significantly higher in tetraploid than diploid leaves, and the rutin level was slightly higher in tetraploid plants. Contents of the other three flavonoids were slightly higher in diploid plants. The results suggest that chromosome doubling had little impact on the main bioactive secondary metabolites.
Chlorophyll (Chl) is the most important pigment for capturing the light required for photosynthesis in higher plants and thus plays a key role in the conversion of light energy into chemical energy needed for plant growth. Chl content was not much affected by polyploidization. Chl a and Chl b levels were higher in diploid A. roxburghii plants than in tetraploids, but the differences were not significant (Table 5). However, tetraploids had significantly longer and wider stomata than diploids (Table 6).
Variations in structural and physiological elements may influence plants’ photosynthetic performance and we found significant differences in chl fluorescence traits between diploid and tetraploid plants (Table 7). The maximum photochemical efficiency of PSII photochemistry (Fv/Fm) was significantly higher in tetraploid plants. However, Y (I) and ETR (I) were higher in tetraploid than diploid plants and stronger than Y (II) and ETR (II). We also found that polyploidy resulted in a significant reduction in NPQ.
Multiple studies have shown that polyploid plants may differ morphologically, ecologically, physiologically, and cytologically from parental lines (10, 12–14). The variations have also been exploited in the development of superior varieties in breeding programs. Our results are consistent with findings that polyploidy is often associated with agronomic improvements, such as higher biomass, leaf thickness, stem diameter, and root development (12, 19). The mechanisms by which chromosome doubling produces superior traits in induced polyploid plants are not known. The increases in stem and leaf thickness may be due to increases in cell size, while genetic changes in the cells may enhance activities of key genes (19). It has also been suggested that changes in polyploid plants’ morphology may be associated with improvements in abiotic tolerance, genetic adaptability and tolerance of environmental stresses (20).
Although effects of polyploidy are not generally predictable and may be taxon-specific, doubling the chromosome number of plant species may enhance their overall nutrient contents and secondary metabolism (21, 22, 12). Accordingly, we found that concentrations of Asp (six times) and γ-ABA (more than twice) were higher in tetraploid A. roxburghii plants than in the diploids. Similar increases have been reported in artificial tetraploid rice (23). We also found much higher Ca contents, which may result in increased cell volumes (13), in tetraploid plants. The higher Ca levels in tetraploids may also be related to stress adaptation (24). The tetraploid leaves had significantly higher kinsenoside and rutin levels than diploid leaves, indicating that these phytochemical characteristics are strongly influenced by the plant ploidy level. Similar increases have been described in other artificial tetraploid plants, such as a higher yield of cichoric acid in tetraploid Echinacea purpurea (12) and a 47.7% increase in rubber concentration in tetraploid Taraxacum kok-saghyz (25) tetraploids.
Stomatal lengths and widths have often been used as morphological markers for identifying putative polyploid plants (26). We found that tetraploid A. roxburghii plants had significantly longer and wider stomata than the diploids, in accordance with previous findings that diploids Taraxacum kok-saghyz plants have lower stomatal length and width than counterparts with higher ploidy levels (25). Larger leaves and greater numbers of stomata may enhance photosynthesis, thereby indirectly increasing the plants’ environmental adaptability and improving their abiotic tolerance (13).
Chl is a key player in interactions with light during the entire life cycle of plants. We detected no clear ploidy level-associated variation in A. roxburghii leaves. No such variation had been detected in Urgenia indica either (27). However, chromosome doubling has reportedly increased chl contents of some species, e.g., Miscanthus× giganteus (13), and decreased those of Juncus effusus (28). Chl fluorescence measurements are mainstays of studies of photosynthetic regulation and plants’ environmental responses because of their sensitivity, convenience, and non-intrusive nature (29). Fv/Fm, indicating the amount of absorbed energy trapped in PSII reaction centers, is an excellent parameter for monitoring temperature stress (30). We found that tetraploid A. roxburghii plants had higher Fv/Fm values than diploids, indicating that tetraploid plants were more resistant to heat. NPQ reflects the amount of energy from photosynthetic electron transport that is not used but dissipated harmlessly as heat from PSII antennae, which is a key damage-avoidance mechanism in plants (29). We found that tetraploids had lower NPQ values than diploids, indicating higher ability to minimize heat damage and efficiently utilize absorbed light energy.
After thorough phenotypic and physiological evaluation, the tetraploids obtained exhibited obvious advantages in some traits. One the one hand, these plants can be directly applied. One the other hand, they can be used for further breeding, for example, to cross with diploids or tetraploids in efforts to obtain triploids or tetraploids with traits that provide higher economic value than the parents.
We cultivated A. roxburghii plants, vegetatively propagated in vitro, in the Chinese Medicinal Plants Garden on the South China Agricultural University campus. Tetraploid plants were obtained by colchicine treatment of diploid nodal explants, following the process described by Wang et al. (31). The ploidy level was estimated using a flow cytometer (CyFlow® Ploidy Analyzer, Sysmex, Japan) with a CyStain UV OxProtect kit (Jiyuan Biotech, China) according to Cai and Kang (32) and diploid A. roxburghii plants were used as controls.
We measured the height, stem diameter, internode length, leaf area and thickness, stomata, root system, and whole weight of sampled plants. We also determined their fresh weight using an electronic balance (Sartorius, China), and their dry weight after drying to a constant weight at 65°C. Leaf thickness was analyzed using a digital caliper (Meinaite, China). We measure the size of mature leaves with a CL-203 laser area meter (CID, USA). The root system, plant height, and stem diameter were scanned using a WinRhizo Pro LA2400 system (Regent, Canada). Stomatal areas were measured in fresh fully developed and healthy adult leaf portions and observed under a microscope (Nikon Eclipse Ni-U, Japan).
Chlorophyll fluorescence parameters of the second and third leaves from the top of selected plants were measured with a dual PAM-100 fluorometer (Heinz-Walz, German), using red actinic light and a red measuring beam for fluorescence. After 30 min dark adaptation, minimal fluorescence (F0), the maximum fluorescence (Fm), and maximal level of P700 signal (Pm) were determined according to standard Dual-PAM-100 protocols. We calculated the maximum efficiency of PSII photochemistry (Fv/Fm), quantum yield of PSI Y(I), effective photochemical quantum yield of PSII Y(II), electron transport rates of PSI and PSII (ETR I and II, respectively), non-photochemical quantum yields of PSI due to acceptor-side limitation Y(NA) and donor-side limitation Y(ND), and non-photochemical quenching (NPQ) following Hikosaka (33).
Second leaves from the top of sampled plants were collected for determination of photosynthetic pigments. The fresh leaves were ground with 80% (Vacetone/Vwater) acetone at room temperature then centrifuged (5,000 g, 5 min). The supernatant’s absorbance was measured with a Varioskan LUX spectrometer (Thermo Scientific, Finland) at 663.2 and 646.8 nm. Chl a and Chl b concentrations were then calculated using equations of Porra (34).
Free amino acids were determined following Liu et al. (35) using a L-8900 automatic amino acid analyzer (Hitachi, China). The concentrations of macro nutrients (K, Ca, Mg, Fe, Mn, Zn, and Cu) were quantified by a 220FS flame atomic absorption spectrometer (Varian, USA), following Li et al. (36). Micro nutrients (Cr, Co, and Ni) were determined using a Zeenit 650P graphite furnace atomic absorption spectrometer (Analytik Jena, Germany) according to the method described by Manjusha et al. (37).
Kinsenoside, narcissin, rutin, quercetin, and isorhamnetin were extracted and analyzed. For this, samples were oven-dried and then ground to 100-mesh size. A sample of 10 mg powder was weighed and soaked in 1.2 mL of 70% (Vmethanol/Vwater) methanol. After ultrasonic extraction for 30 min, each resulting suspension was centrifuged (10,000 g, 10 min), then filtered with a 0.22 μm syringe filter, placed in a sample vial and stored at 4°C before measurement. The samples were analyzed using a HPLC-ESI-MS/MS system (Agilent 1290–6470, USA) equipped with a 2.1 × 50 mm, 1.8 μm C18 reverse phase column. The mobile phase consisted of water with 0.2% formic acid (solvent A) and acetonitrile (solvent B) at a flow rate of 0.4 mL/min, starting with a 1 min hold at 90% A followed by a linear 3-min gradient from 90 to 10% A, then a 1 min hold at 10% A, a 1 min linear rise back to 90% A and 4 min hold at 90% A for re-equilibration before the next injection. The column temperature was 40°C and injection volume 2 μL. The MS system was a 6470 Triple Quadrupole mass spectrometer, with the following settings: dry temperature 300°C, gas flow rate 6 L/min; nebulizer pressure 45 psi, sheath temperature 350°C, sheath gas flow rate 10 L/min, and capillary voltage 4 kV. Contents of analytes in samples were determined using the external standard method, with peak height as the quantitative parameter.
Three typical individuals of each ploidy and three biological replicates, were used in all the experiments. Data acquired in the experiments were sorted using Microsoft Office Excel (Microsoft Corp., Redmond, WA, USA), then statistical analyses were performed with SPSS software (Version 26.0; SPSS Inc., Chicago, IL, USA). Student’s t-tests were applied to assess the significance of differences between parameters of diploid and tetraploid plants. P-values ≤ 0.05 were considered statistically significant.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
YY and JZ conceived and designed research. XH, KO, YL, and GX conducted the experiments, collected, and analyzed the data. XH and JZ wrote the manuscript. YY obtained the fundings. All authors contributed to the article and approved the submitted version.
This work was funded by the Science and Technology Planning Project (grant no. 2021A090201) and awarded by the Science and Technology Bureau of Yunfu.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Shao QS, Deng YM, Liu HB, Zhang AL, Huang YQ, Xu GZ, et al. Essential oils extraction from Anoectochilus roxburghii using supercritical carbon dioxide and their antioxidant activity. Ind Crop Prod. (2014) 60:104–12. doi: 10.1016/j.indcrop.2014.06.009
2. Gao HS, Ding LL, Liu R, Zheng XH, Xia XC, Wang FA, et al. Characterization of Anoectochilus roxburghii polysaccharide and its therapeutic effect on type 2 diabetic mice. Int J Biol Macromol. (2021) 179:259–69. doi: 10.1016/j.ijbiomac.2021.02.217
3. Ye SY, Shao QS, Xu MJ, Li SL, Wu M, Tan X, et al. Effects of light quality on morphology, enzyme activities, and bioactive compound contents in Anoectochilus roxburghii. Front Plant Sci. (2017) 8:857. doi: 10.3389/fpls.2017.00857
4. Zeng BY, Su MH, Chen QX, Chang Q, Wang W, Li HH. Anoectochilus roxburghii polysaccharide prevents carbon tetrachloride-induced liver injury in mice by metabolomic analysis. J Chromatogr B. (2020) 1152:122202. doi: 10.1016/j.jchromb.2020.122202
5. Zhang AL, Wang HZ, Shao QS, Xu MJ, Zhang WS, Li MY. Large scale in vitro propagation of Anoectochilus roxburghii for commercial application: pharmaceutically important and ornamental plant. Ind Crop Prod. (2015) 70:158–62. doi: 10.1016/j.indcrop.2015.03.032
6. Wei M, Zhang M, Huang G, Yuan Y, Fu C, Yu L, et al. Coculture with two Bacillus velezensis strains enhances the growth of Anoectochilus plants via promoting nutrient assimilation and regulating rhizosphere microbial community. Ind Crop Prod. (2020) 154:112697. doi: 10.1016/j.indcrop.2020.112697
7. Wang W, Su MH, Li HH, Zeng BY, Chang Q, Lai ZX. Effects of supplemental lighting with different light qualities on growth and secondary metabolite content of Anoectochilus roxburghii. PeerJ. (2018) 6:e5274. doi: 10.7717/peerj.5274
8. Ye SY, Shao QS, Zhang AL. Anoectochilus roxburghii: a review of its phytochemistry, pharmacology, and clinical applications. J Ethnopharmacol. (2017) 209:184–202. doi: 10.1016/j.jep.2017.07.032
9. Zhang Y, Li YY, Guo SX. Effects of the mycorrhizal fungus Ceratobasidium sp. AR2 on growth and flavonoid accumulation in Anoectochilus roxburghii. PeerJ. (2020) 8:e346. doi: 10.7717/peerj.8346
10. Hannweg K, Visser G, De-Jager K, Bertling I. In vitro-induced polyploidy and its effect on horticultural characteristics, essential oil composition and bioactivity of Tetradenia riparia. S Afr J Bot. (2016) 106:186–91. doi: 10.1016/j.sajb.2016.07.013
11. Omidbaigi R, Mirzaee M, Hassani ME, Moghadam MS. Induction and identification of polyploidy in basil (Ocimum basilicum L.) medicinal plant by colchicine treatment. Int J Plant Prod. (2010) 4:87–98.
12. Xu CG, Tang TX, Chen R, Liang CH, Liu XY, Wu CL, et al. A comparative study of bioactive secondary metabolite production in diploid and tetraploid Echinacea purpurea (L.) Moench. Plant Cell Tissue Organ Cult. (2014) 116:323–32. doi: 10.1007/s11240-013-0406-z
13. Ghimire BK, Seong ES, Nguyen TX, Yoo JH, Yu CY, Kim SH, et al. Assessment of morphological and phytochemical attributes in triploid and hexaploid plants of the bioenergy crop Miscanthus x giganteus. Ind Crop Prod. (2016) 89:231–43. doi: 10.1016/j.indcrop.2016.04.051
14. Iannicelli J, Elechosa MA, Juárez MA, Martinez A, Bugallo V, Bandoni AL, et al. Effect of polyploidization in the production of essential oils in Lippia integrifolia. Ind Crop Prod. (2016) 81:20–9. doi: 10.1016/j.indcrop.2015.11.053
15. Chen HL, Lu ZW, Wang J, Chen T, Gao JM, Zheng JL, et al. Induction of new tetraploid genotypes and heat tolerance assessment in Asparagus officinalis L. Sci Hortic. (2020) 264:109168. doi: 10.1016/j.scienta.2019.109168
16. Veronika Z, Daniela P, František H, Milan P. Arsenic toxicity-induced physiological and metabolic changes in the shoots of Pteris cretica and Spinacia oleracea. Plants. (2021) 10:2009. doi: 10.3390/plants10102009
17. Zhang H, Mo X, Tang D, Ma Y, Xie Y, Yang H, et al. Comparative analysis of volatile and carotenoid metabolites and mineral elements in the flesh of 17 kiwifruit. J Food Sci. (2021) 86:3023–32. doi: 10.1111/1750-3841.15796
18. Chen M, Zeng X, Liu Y, Zhang H, Hu Q. An orthogonal design of light factors to optimize growth, photosynthetic capability and metabolite accumulation of Anoectochilus roxburghii (Wall.) Lindl. Sci Horticult. (2021) 288:110272. doi: 10.1016/j.scienta.2021.110272
19. Xue H, Zhang B, Tian JR, Chen MM, Zhang YY, Zhang ZH, et al. Comparison of the morphology, growth and development of diploid and autotetraploid ‘Hanfu’ apple trees. Sci Hortic. (2017) 225:277–85. doi: 10.1016/j.scienta.2017.06.059
20. Jiang JL, Yang N, Li L, Qin GW, Ren KX, Wang HT, et al. Tetraploidy in Citrus wilsonii enhances drought tolerance via synergistic regulation of photosynthesis, phosphorylation, and hormonal changes. Front Plant Sci. (2022) 13:875011. doi: 10.3389/fpls.2022.875011
21. Chung HH, Shi SK, Huang B, Chen JT. Enhanced agronomic traits and medicinal constituents of autotetraploids in Anoectochilus formosanus Hayata, a top-grade medicinal orchid. Molecules. (2017) 22:1907. doi: 10.3390/molecules22111907
22. Tavan M, Mirjalili MH, Karimzadeh G. In vitro polyploidy induction: changes in morphological, anatomical and phytochemical characteristics of Thymus persicus (Lamiaceae). Plant Cell Tissue Organ Cult. (2015) 122:573–83. doi: 10.1007/s11240-015-0789-0
23. Zhang XH. Polyploidization increases the lipid content and improves the nutritional quality of rice. Plants Basel. (2022) 11:132. doi: 10.3390/plants11010132
24. Elkelish AA, Alnusaire TS, Soliman MH, Gowayed S, Senousy HH, Fahad S. Calcium availability regulates antioxidant system, physio-biochemical activities and alleviates salinity stress mediated oxidative damage in soybean seedlings. J Appl Bot Food Qual. (2019) 92:258–66.
25. Luo ZN, Iaffaldano BJ, Cornish K. Colchicine-induced polyploidy has the potential to improve rubber yield in Taraxacum kok-saghyz. Ind Crop Prod. (2018) 112:75–81. doi: 10.1016/j.indcrop.2017.11.010
26. Widoretno W. In vitro induction and characterization of tetraploid Patchouli (Pogostemon cablin Benth.) plant. Plant Cell Tissue Organ Cult. (2016) 125:261–7. doi: 10.1007/s11240-016-0946-0
27. Phulari SS. Polyploidy breeding in Urgenia indica-to study the effect of colchicines treatment on morphological character of Urgenia indica. Inidan Streams Res J. (2011) 1:207–10.
28. Xu L, Najeeb U, Naeem MS, Daud MK, Cao JS, Gong HJ, et al. Induction of tetraploidy in Juncus effusus by colchicine. Biol Plant. (2010) 54:659–63. doi: 10.1007/s10535-010-0117-9
29. Shao QS, Wang HZ, Guo HP, Zhou AC, Huang YQ, Sun YL, et al. Effects of shade treatments on photosynthetic characteristics, chloroplast ultrastructure, and physiology of Anoectochilus roxburghii. PLoS One. (2014) 9:e85996. doi: 10.1371/journal.pone.0085996
30. Jiang HY, Howell GS. Applying chlorophyll fluorescence technique to cold hardiness studies of grapevines. Am J Enol Vitic. (2002) 53:210–7. doi: 10.5344/ajev.2002.53.3.210
31. Wang LF, Huang J, Lin X. Polyploidy in Anoectochilus roxburghii induced by colchicine in vitro. Acta Agric Zhejiang. (2010) 22:760–3.
32. Cai X, Kang XY. In vitro tetraploid induction from leaf explants of Populus pseudo-simonii kitag. Plant Cell Rep. (2011) 30:1771–8. doi: 10.1007/s00299-011-1085-z
33. Hikosaka K. Photosynthesis, chlorophyll fluorescence and photochemical reflectance index in photoinhibited leaves. Funct Plant Biol. (2021) 48:815–26. doi: 10.1071/FP20365
34. Porra RJ. The chequered history of the development and use of simultaneous equations for the accurate determination of chlorophylls a and b. Photosynth Res. (2002) 73:149–56.
35. Liu WH, Pu XL, Sun JK, Shi XW, Cheng WD, Wang B. Effect of Lactobacillus plantarum on functional characteristics and flavor profile of fermented walnut milk. LWT Food Sci Technol. (2022) 160:113254. doi: 10.1016/j.lwt.2022.113254
36. Li LL, Guo SS, Sun Y, Li XD, Gao YF, Xu H, et al. Detoxification effect of single inoculation and co-inoculation of Oudemansiella radicata and Serratia marcescens on Pb and fluoranthene co-contaminated soil. J Soils Sediments. (2019) 19:3008–17. doi: 10.1007/s11368-019-02304-8
37. Manjusha R, Shekhar R, Kumar SJ. Ultrasound-assisted extraction of Pb, Cd, Cr, Mn, Fe, Cu, Zn from edible oils with tetramethylammonium hydroxide and EDTA followed by determination using graphite furnace atomic absorption spectrometer. Food Chem. (2019) 294:384–9. doi: 10.1016/j.foodchem.2019.04.104
Keywords: Anoectochilus roxburghii, tetraploid, kinsenoside, phenotype, biochemical
Citation: Huang X, Ouyang K, Luo Y, Xie G, Yang Y and Zhang J (2022) A comparative study of characteristics in diploid and tetraploid Anoectochilus roxburghii. Front. Nutr. 9:1034751. doi: 10.3389/fnut.2022.1034751
Received: 02 September 2022; Accepted: 24 October 2022;
Published: 07 November 2022.
Edited by:
Sandrina A. Heleno, Polytechnic Institute of Bragança (IPB), PortugalReviewed by:
Radjassegarin Arumugam, A.V.C. College, IndiaCopyright © 2022 Huang, Ouyang, Luo, Xie, Yang and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yuesheng Yang, eXN5YW5nQHNjYXUuZWR1LmNu; Junjie Zhang, emhhbmdqdW5qaWUzMTY4QDE2My5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.