- 1Department of Oral Implantology, Affiliated Stomatological Hospital of Southwest Medical University, Luzhou, Sichuan, China
- 2Department of VIP Dental Service, Affiliated Stomatological Hospital of Southwest Medical University, Luzhou, Sichuan, China
- 3Luzhou Key Laboratory of Oral and Maxillofacial Reconstruction and Regeneration, Affiliated Stomatological Hospital of Southwest Medical University, Luzhou, Sichuan, China
- 4Department of Nosocomial Infection Control, Affiliated Hospital of Southwest Medical University, Luzhou, Sichuan, China
- 5School of Stomatology, Southwest Medical University, Luzhou, Sichuan, China
Brick tea-type fluorosis (BTF) due to a high intake of brick tea is possible in Tibetan populations, and dental fluorosis (DF) and skeletal fluorosis (SF) are its primary manifestations. To determine the prevalence of DF and SF and their relationships with brick tea intake in Tibetan populations, a literature review was conducted for studies published between 1994 and 2021. The available evidence revealed that brick tea may be produced from older stems and leaves of the tea plant and that the fluoride content of brick tea exceeds the national standard. The harsh environment of the plateau has led to limited food sources for the local Tibetan people who form the habit of drinking tea leaves as a satiation solution to digest greasy food and replenish vitamins, and regular consumption of brick tea leads to excessive exposure of Tibetan residents to fluoride. Studies in Tibet showed that the prevalence of DF in children was 14.06–75.93% in different districts, and the overall pooled prevalence of DF was 26.08%. The prevalence of SF in adults was 19.90–74.77% in different Tibetan districts, and the overall pooled prevalence of SF was 33.84%. The analysis of risk factors showed that the prevalence of BTF may be related to high-altitude and different working and living conditions, and BTF in children may be associated with fluoride intake during mothers’ pregnancy and lactation. With the development of bioinformatics research, gene polymorphisms were suspected to be related to susceptibility to fluorosis in Tibetan populations. The study of BTF in Tibetan people needs to be further investigated and standardized, and additional studies evaluating the pathogenesis and preventive measures of BTF are warranted.
Introduction
Fluorosis is an endemic disease that is closely associated with the excessive intake of fluoride. Fluorine ranks 24th among all elements in universal abundance and 13th in terrestrial abundance, accounting for 0.08% of the Earth’s crust (1). An appropriate intake of fluoride is beneficial to health (2). Fluoride participates in the metabolism of calcium and phosphorus and improves the strength of teeth and bones (3, 4). However, fluoride overdose may cause detrimental health effects, and fluoride can be ingested through drinking water, fluoride supplementation, or fluoridated toothpaste. According to the World Health Organization, long-term fluoride intoxication is associated with damage to the teeth and bones as well as the endocrine, gastrointestinal, renal, neurological, and reproductive systems (5, 6). Dental fluorosis (DF) and skeletal fluorosis (SF) are the most common manifestations of excessive fluoride intake (7–9). Symptoms of DF include cloudy white or chalky lines, spots, or marks on teeth, yellow or brown discoloration, surface irregularities, and visible pits in tooth enamel. SF is a metabolic bone disease, and it is the most common and severe manifestation of fluorosis in adults. The main clinical presentations of SF include lumbar and leg joint pain, joint rigidity, bone deformation, and spinal cord compression.
Endemic fluorosis is prevalent in China because of the high-fluoride content of water sources and coal combustion in some areas (10). The fourth National Oral Health Survey conducted in mainland China in 2016 (11) revealed that DF had a prevalence of 40.3% in Tibet, which was the third highest after those of Guizhou Province and Tianjin City. The fluoride intake of Tibetan people is associated with certain local factors, and the excessive intake of brick tea may be one of them.
The tea plant is a perennial plant that can maintain a steady yield for up to 100 years. It prefers to grow in acidic soil (pH 3.5–5.6) where it can obtain the element fluorine. After the decomposition of the aluminum fluoride complex in the soil, fluoride in ionic form (F–) is absorbed by the roots, and excessive amounts of F– form complexes with Al3+ and are transferred to old leaves (12, 13). Tea beverages are popular in many regions worldwide, and the fluoride enrichment effect of the tea plant carries a risk of excessive fluoride intake for tea drinkers (14–19). The diversity of tea beverages, various production techniques, and soil conditions of tea-producing areas may result in varying differences in the fluoride content of tea (14, 16–18, 20–26).
Since ancient times, Chinese people have had the tradition of drinking tea. The types of tea and drinking habits differ across regions. Tibetans are primarily distributed in the Qinghai–Tibet Plateau, which has an average altitude of 3,000–5,000 m. Owing to the cold weather, high-altitude, and higher radiation exposure, the residents of this region are accustomed to eating high-fat and high-sugar foods to adapt to the harsh environment. Tea consumption is important for digesting greasy food and replenishing vitamins. However, Tibetan regions with high-altitude mountain landforms do not produce tea; thus, the supply of tea is primarily from low-altitude Han-inhabited areas (27). The ancient Tea Horse Road (28, 29), a famous passage for economic and cultural exchanges in ancient China that rose from the Tang Dynasty (approximately 8th century A.D.), was derived from the complementary trade of tea and horses between the Tibetan and Han people.
As tea plants selectively absorb fluoride from the soil, the concentration of fluoride increases with maturity (30, 31). The materials used to make brick tea include old leaves and stems from the tea plant, and as the name suggests, this tea is molded into a brick shape for better preservation (Figure 1). Therefore, drinking brick tea in large quantities may increase fluoride intake compared to consuming other drinks, potentially resulting in brick tea-type fluorosis (BTF).

Figure 1. A piece of brick tea produced in Yunnan province. Brick tea looks like a brick, and is a representative type of pressed tea, which is made of tea leaves, tea stems, and sometimes tea dust.
Existing studies (27, 32–36) have reported a relationship between brick tea consumption and fluorosis in Tibet. These studies were conducted in isolated cities, towns, or villages, and the information was relatively limited. To comprehensively compare fluoride intake from brick tea, prevent the occurrence of fluorosis, and confirm the relationship between brick tea consumption and fluorosis, the factors associated with excessive fluoride intake need further examination. A review is warranted to assess the prevalence of BTF and evaluate the effectiveness of the prevention and control of endemic BTF in recent years. In this study, the pooled prevalence rates of DF and SF and their existing risk factors were assessed to minimize bias and ensure objectivity, authenticity, and reliability of the research results, and a literature review was conducted to identify possible risk factors.
Materials and methods
Search strategy and identification criteria
Reasonable inclusion and exclusion criteria were established by evaluating the research content and scope of previous reports on BTF. Although many reports about brick tea are reported in Chinese, to ensure the objectivity, openness, and repeatability of this study, only articles written in English were included. The electronic search databases of PubMed and Cochrane Library were searched for studies published between January 1994 and September 2021. The search terms were “(“brick tea” OR “tea” OR “brick-tea”) AND (“fluorosis” OR “dental fluorosis” OR “skeletal fluorosis” OR “fluoride”).” The reference lists of the included articles were added to the screening list to identify eligible studies. Two authors independently screened the titles and abstracts of these studies and retrieved the full texts of potentially eligible articles if they met the inclusion criteria. Any conflicts regarding the inclusion or exclusion of a study were discussed, and a third researcher was invited to make the final judgment.
Inclusion and exclusion criteria
To meet the requirements of the analysis and reduce bias, studies were required to meet the following criteria: (1) The study was conducted in Tibetan residential areas, i.e., the Tibet Autonomous Region, the Qinghai Province, and portions of the Sichuan and Yunnan provinces; (2) the included participants were local individuals from the field sites rather than selected volunteers; (3) clear diagnostic criteria for fluorosis and accurate fluoride concentration measurement methods were reported; and (4) the time of the survey was reported. In cases of multiple reports of the same samples in the same area during the same period of time, the report with the most detailed information was included.
The exclusion criteria included duplicate articles; articles that discussed fluorosis of animals or laboratory research; articles focusing on fluoride intoxication that was not caused by brick tea; articles focusing on the association of fluoride with other health-related issues, such as cancer, intelligence, or physical development; studies not conducted on Tibetan individuals or in Tibetan-inhabited areas; and studies with unclear age, sex, and other demographic data of the surveyed participants or data that could not be obtained from the authors.
Literature screening, data extraction, and compilation
Relevant data were subsequently extracted and used to compile a database. The data from all included studies were clearly tabulated. Deviations were considered and identified during the quality assessment stage. The following data were collected from each study using specifically designed tables: Author; year of publication; time and place of investigation; altitude of the investigated place; sample size; demographic information; sex; occupation; volume of daily brick tea consumption; prevalence of DF and SF of the investigated participants; fluoride level of brick tea; and fluoride level of brick tea infusions. The altitude was based on the average height of the urban area where the study was located according to publicly available information, like Baidu Baike, if the data was not given in article.
The fluoride level of brick tea was the enumeration of the fluoride content of dry brick tea obtained from different studies, and the volume of daily brick tea consumption was based on the amount of brick tea consumed or the amount of liquid applied. Due to the different methods reported in various studies, the fluoride and tea consumption levels may use different units of measurement; this study enumerated them without changing the units. The prevalence of DF and SF were based on the ratio of the number of patients with fluorosis in the study to the total number of enrolled people in the study. The DF index was reported by the authors of the study if available. Based on these data, the overall pooled prevalence of DF and SF were calculated. Pearson’s correlation coefficients of the relationship between the prevalence of DF and SF and the altitudes of the investigated areas were also calculated.
Results
General database statistics
The initial electronic and manual searches yielded 297 articles. After screening the titles and abstracts, 274 articles were excluded because they were not human studies, were not conducted in Tibetan areas, did not focus on brick tea consumption, did not relate to DF and SF, or were not available in English. Then, 23 articles were read and reviewed in full. Among them, eight articles (37–44) were excluded because they had unclear demographic data or unclear SF or DF results, or the results were not consistent with the theme. Finally, 15 studies were included in the research: 6 studies (27, 35, 36, 45–47) that focused on BTF in Tibetan-inhabited areas were included in group 1; 4 studies (27, 45, 47, 48) that discussed the fluoride content of brick tea were included in group 2 (three articles were included in both group 1 and 2); and 8 articles (38, 43, 49–54) were included in group 3 because they primarily discussed the genetic susceptibility of Tibetans to fluorosis.
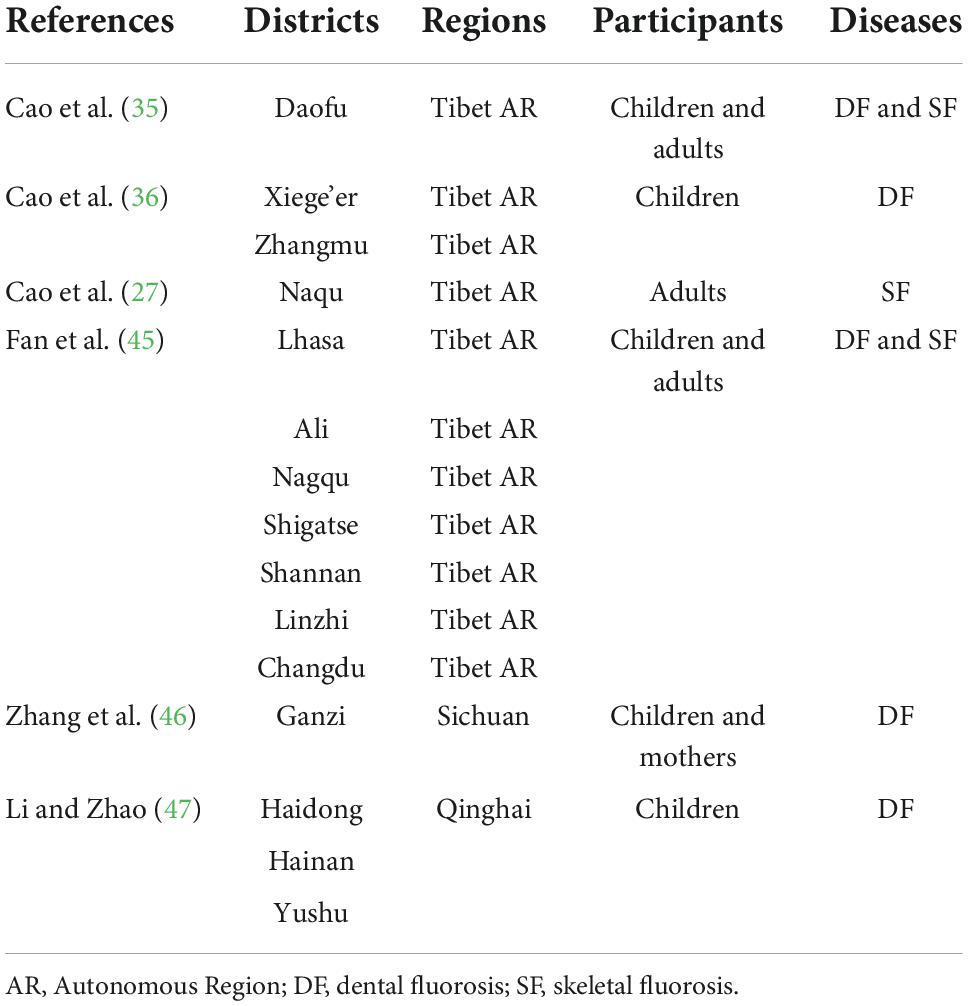
The PRISMA flowchart of the literature screening process was constructed (Supplementary material). Table 1 provides a summary of articles related to BTF prevalence. DF was investigated only in children in most included studies; however, the study by Fan et al. (45) studied DF in both children and their mothers. SF was only studied in adults in all included literature.
Fluoride level of brick tea
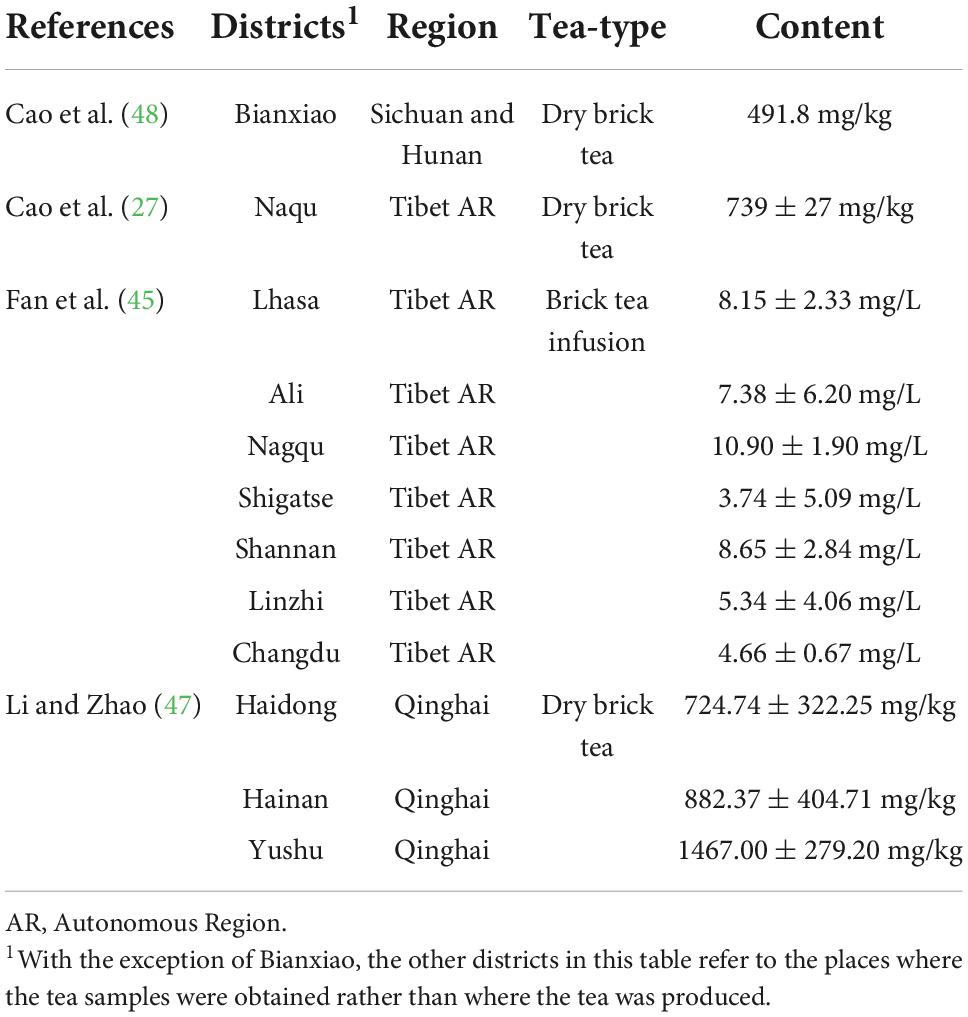
The Chinese national standard requires the fluoride concentration of dry tea to be within 300 mg/kg (GB 19,965-2005). Four articles disclosed the fluoride content of dry brick tea. Table 2 provides a summary of the fluoride content analysis. Three studies (27, 47, 48) compared the fluoride content of dry tea leaves in different sampling districts, whereas the researchers of one article (45) disclosed the fluoride content of tea infusions. Among the dry brick tea samples from Tibet, all samples had fluoride levels exceeding the national standard (i.e., 348.34–1085.70 mg/kg), except for one sample from Shigatse, which had a fluoride level of 96.53 mg/kg. The lowest reported fluoride content occurred in Shigatse (3.74 ± 5.09 mg/L), while the highest reported fluoride content occurred in Nagqu (10.90 ± 1.90 mg/L). Because the fluoride content of tea infusions is directly related to the method of mixing and there is no national standard for fluoride in beverages, it was impossible to conclude whether the fluoride content in tea beverage was significantly over the limit or not for studies that only evaluated tea solutions.
Prevalence of brick tea-type DF among children
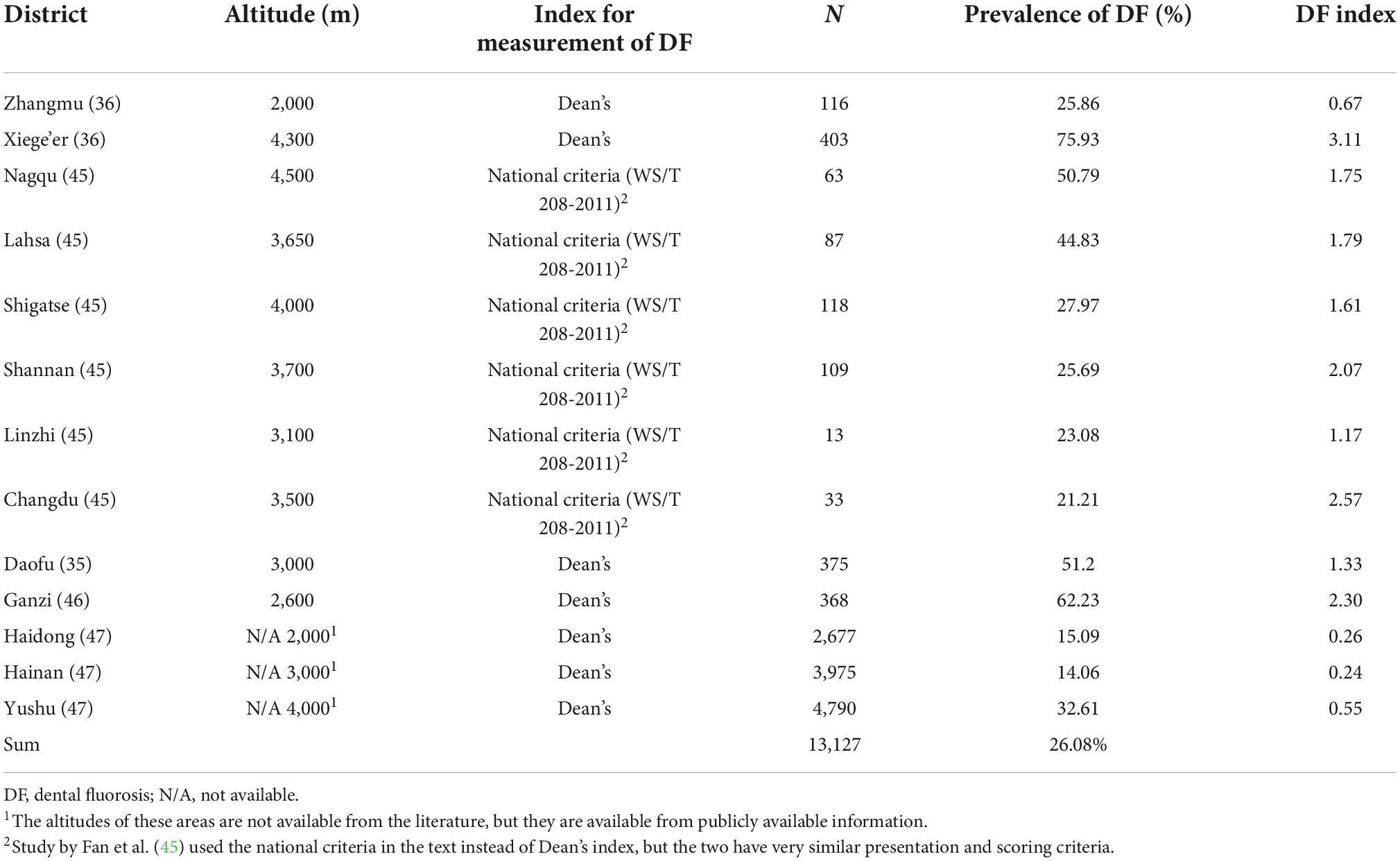
In the five studies that included the prevalence of DF, 13,127 children were examined and 2,404 children were thought to have developed some degree of DF. The reported prevalence rate of DF was 14.06–75.93%, and the pooled prevalence rate of DF was 26.08%. The areas with the highest DF prevalence rates were Xiege’er (75.93%) and Ganzi (62.23%). Dean’s DF index was used in most studies (36, 46, 47), and the Chinese national standard of DF was used in one study (45) (Table 3). According to our detailed examination of the statements of the two standards, the classification and statements of both are the same. The highest DF index was reported in Xiege’er (3.11), while the lowest index was reported in Hainan Autonomous Prefecture (0.24). However, it is important to note that differences may be due to the possible detection heterogeneity of different studies.
Prevalence of brick tea-type SF among adults
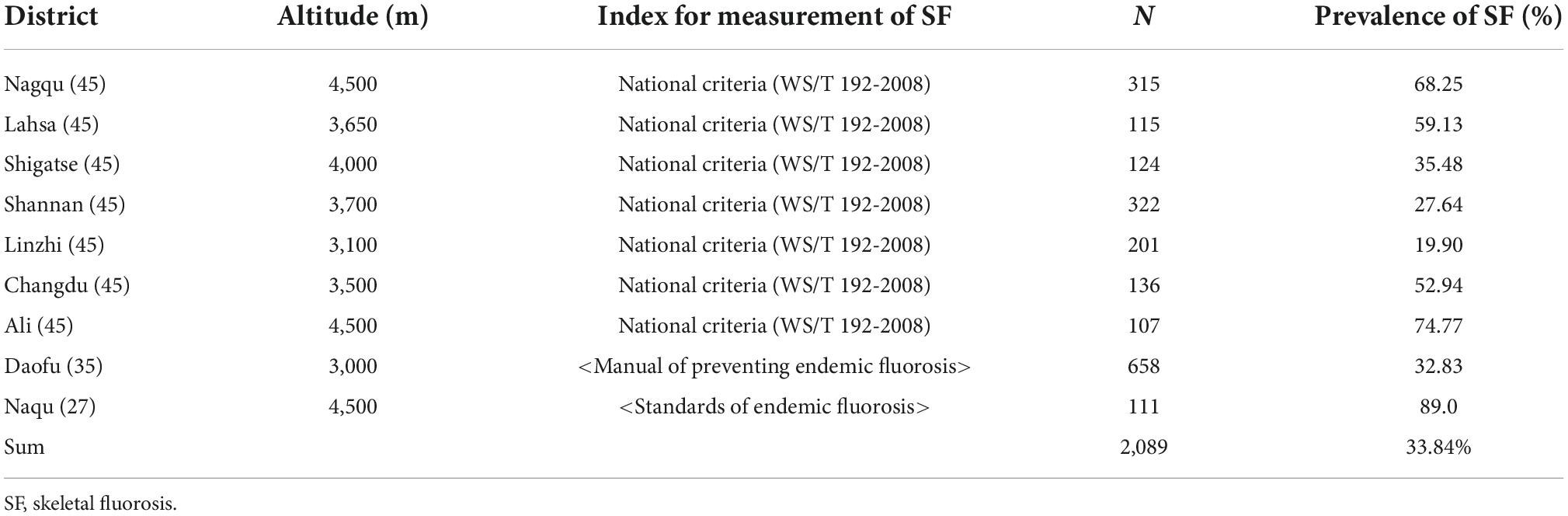
Three articles (27, 35, 45) discussed the prevalence rate of SF. A total of 2,089 adults were examined in these studies, and 923 adults were detected to have some degree of SF. The reported prevalence of SF was 19.90–74.77%, and the pooled prevalence rate of SF was 33.84%. The districts with the highest SF prevalence were Ali (74.77%) and Nagqu (68.25%). SF had no well-recognized index; therefore, the index of SF was not calculated (Table 4). Since there is no internationally recognized evaluation index for SF, no indexing score was given, but the authors classified them according to severity.
Fluoride intake per day for included participants
Fluoride intake among Tibetans may come from various sources, such as brick tea, zanba (a type of food made from cooked barley), butter tea, and water. Table 3 shows the average total daily fluoride intake per person from brick tea in different districts for the included participants. Fluoride intake differed significantly between districts and was much higher than the national standard in most areas (National standard GB 17,018-2011: The daily fluoride intake should be <3.5 mg per person). Among the four studies that reported daily fluoride intake, only Cao et al. (36) reported a fluoride intake of 2.905 mg/day in Zhangmu, which did not exceed the standard. Fan et al. (45) reported that the highest fluoride intakes were in Naqu (55.630 mg/day) and Ali (35.60 mg/day) in their study (Table 5).
Correlations of DF and SF with altitude
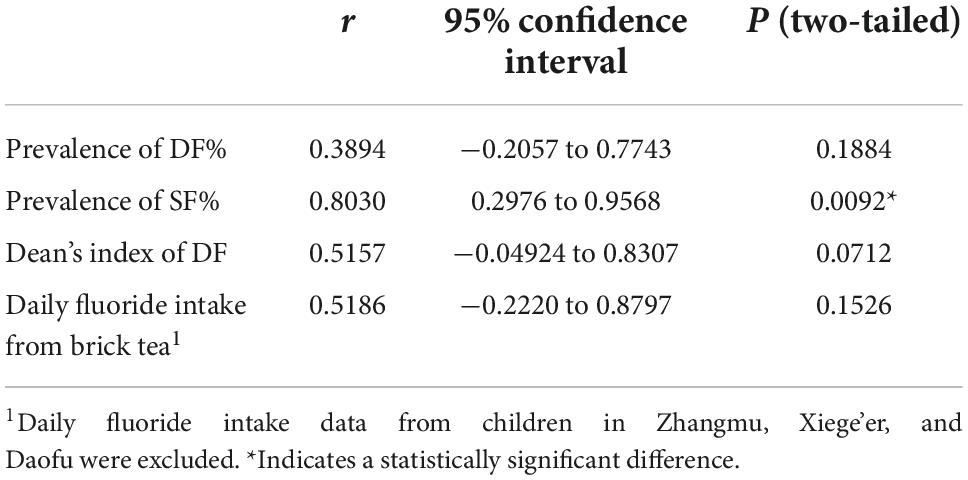
Pearson’s correlation coefficients of the relationship between the prevalence of DF and SF and the altitudes of the surveyed areas were 0.389 and 0.803, respectively. Moreover, Pearson’s correlation coefficient of the DF index with the altitude was 0.5157, and that of the daily fluoride intake from brick tea with the altitude was 0.5186. A significant difference in Pearson’s correlation coefficient was only detected between the prevalence of SF and the altitude (Table 6).
Relationship between fluorosis susceptibility and genotype
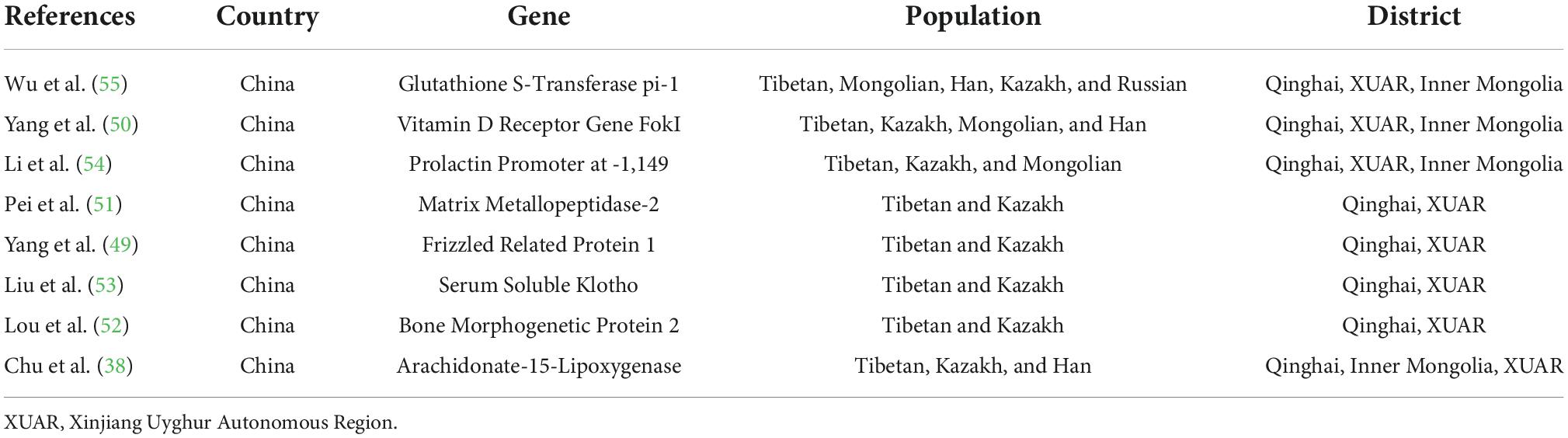
In this study, eight articles (38, 49–55) related to the genetic susceptibility to fluorosis were obtained (Table 7). Wu et al. (55) investigated the role of GSTP1 rs1695 polymorphisms in the susceptibility to fluorosis. Pei et al. (51) reported a significant correlation between the MMP2 rs2287074 genotype and SF severity, and the A allele of MMP2 rs2287074 was a protective factor in Tibetans. Yang et al. (50) indicated that Tibetans were more likely to develop moderate and severe brick tea-type SF, and CT/TT genotypes of vitamin D receptor-FokI may be a protective factor. Li et al. (54) indicated that a polymorphism in the extrapituitary prolactin promoter may decrease the risk of brick tea-induced SF in the Kazakh people. Chu et al. (38) suggested an association between the AlOX15 gene polymorphism and SF risk in Han participants. The CC/CC diplotype had a protective effect on SF risk in Han participants, whereas the CA/CC diplotype influenced SF risk in participants aged ≥65 years. Liu et al. (53) indicated that serum Klotho may be a potential mediator of SF in BTF-endemic areas. Yang et al. (49) suggested there might be differential genetic influence on SF risk in Kazakh and Tibetan participants and that this difference might be modified by tea fluoride intake. However, a study by Lou et al. (52) did not find an association between BMP2 single nucleotide polymorphisms and SF in their cross-sectional case-control study.
A schematic diagram (Figure 2) shows the fluoride accumulation effect of brick tea in old leaves and stems, the result of BTF in Tibetan populations, and possible influencing factors.
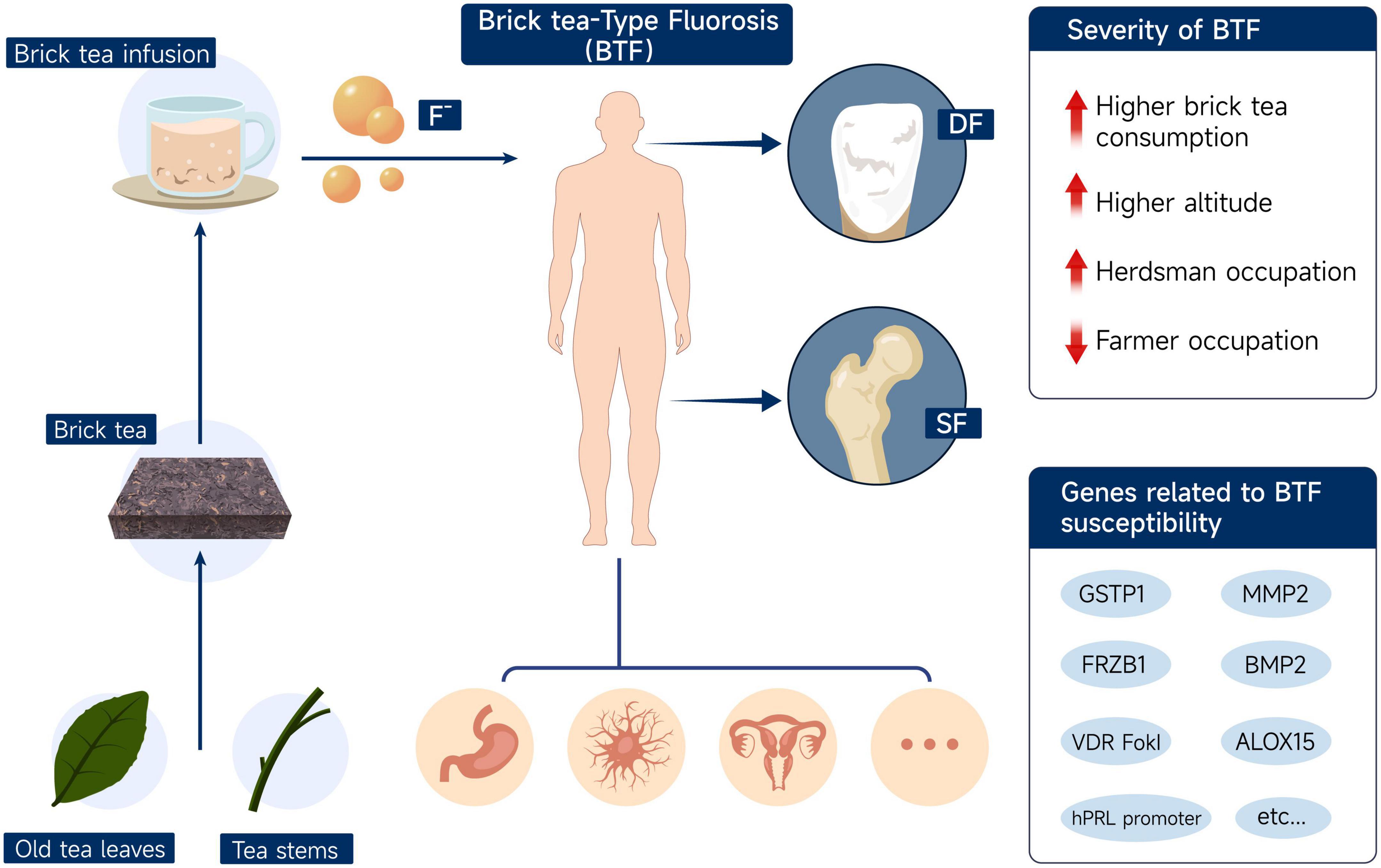
Figure 2. Schematic diagram of fluoride accumulation in brick tea, the manifestations of brick tea-type fluorosis, and possible influencing factors. DF, dental fluorosis; SF, skeletal fluorosis; BTF, brick tea-type fluorosis.
Discussion
Relationship between brick tea and fluoride
Because of the accumulation effect of fluoride in tea plants, drinking tea has become a source of excessive fluoride in the human body. The influences of different tea-producing areas, production methods, drinking habits, and dosages on the development of fluorosis should be noted.
Chandrajith et al. (16) reported that in Sri Lanka, the traditional habits of locally blended black tea consumption can cause an additional intake of fluoride and promote adverse health conditions that may also be related to chronic kidney disease. Whyte et al. (17) reported that the popular instant tea powder contributes to approximately 80% of F– exposure, and SF from the habitual consumption of large volumes of extra-strength instant tea calls for recognition. Apart from these, several studies (14, 56–60) have also raised health concerns regarding the excessive intake of fluoride from tea in certain regions.
Brick tea is relatively low-end tea, especially that sold in remote mountainous areas. Because of the long distances traveled, tea needed to be preserved for a long time and easily transported, so it was often pressed into the brick form that gave it its name. The ethnic people in the mountainous areas consume more meat and milk and less vegetables, and drinking tea not only helps digest greasy food, but it also supplements essential vitamins and trace elements. Therefore, it is said that “it is better to go without food for a day than without tea for a day.” Brick tea has become a necessity of Tibetan life.
Previous studies have reported that the fluoride content of brick tea was higher than that of ordinary tea (44, 48, 61). The Chinese national standard requires that the fluoride concentration of dry tea should be within 300 mg/kg. Studies (27, 47, 48) on BTF in Tibet have reported that the fluoride content of dry brick tea samples greatly exceeded the national standard, some of which reached three to five times the limit. In addition, animal experiments (47, 48) have also shown that rats exposed to brick tea infusions present with DF. Therefore, the association between high-fluoride brick tea consumption and endemic fluorosis in Tibet can be verified.
To distinguish the link between brick tea and fluorosis, we also need to know if the source of fluoride comes from other substances used in tea brewing, such as water and fuel. However, the fluoride content of drinking water in nearly all Tibetan residential areas is within the national drinking water range (≤1.2 mg/L, GB 5,749-2006), and the possibility of water-initiated fluorosis is low (27, 36, 39, 45). Among the four studies that reported fluoride concentrations in local drinking water samples, only the concentration of one water sample exceeded the national standard (in Ali, 2.01 mg/L). The fluoride concentrations of other water samples were lower than the national standard (0.03–0.96 mg/L). Moreover, the use of coal is often a cause of fluorosis, but Tibetan-inhabited areas are not coal-producing areas and do not have the tradition of using coal. Tibetan individuals generally use wood and cow dung as fuel in the countryside. Therefore, the possibilities of fluoride intake caused by coal pollution and drinking water can be eliminated.
Relationship between DF and brick tea
Dental fluorosis is characterized by chalky-white to brown patches on the enamel and, in severe cases, defects of the enamel. Fluorosis can be divided into three types based on severity: chalky type (mild), discoloration type (moderate), and defect type (severe) (62). DF is more common in permanent teeth, and affected teeth are less resistant to friction but more resistant to acid etching (63, 64). Excessive fluoride intake can delay the mineralization of the enamel and increase its surface roughness, thus promoting plaque accumulation (65). Excessive fluoride binds more enamel amelogenin and increases enamel organic substances in the secretory phase of enamel formation, thereby leading to hypomineralization and DF (66, 67). The typical histological feature of DF is the low mineralization rate of the enamel, which becomes fragile, easily eroded, and exfoliated.
Dental fluorosis is rare and mild in deciduous teeth due to their development in the embryonic and lactation stages, and the placenta has a certain barrier effect on fluoride. However, if fluoride intake exceeds the limit of its screening function, it can also result in irregularities on the deciduous teeth (68).
In the literature included in this study, most studies only examined children for DF. The reported prevalence of DF was quite different between different studies, indicating that the heterogeneity of these studies may be strong. Although the prevalence of DF varied, all included studies (35, 36, 45–47) identified DF caused by brick tea as severe and of great concern.
Relationship between SF and brick tea
Skeletal fluorosis is one of the most common and severe manifestations of fluorosis in adults, and it is also an important index to evaluate the severity of endemic fluorosis. Radiographic examination of SF may show increased bone matrix density and fibrous ossification, tendon attachment calcification, joint degeneration, degenerative hyperplasia, and ossification changes in the fibrous tissues (27, 69, 70). Such patients may also have DF, whereas those exposed to excess fluoride after 12 years of age may not have DF since the development of ameloblasts in the dental germ is finished by that age.
Cao et al. (27) indicated that the detection of SF positively correlated with long-term fluoride exposure from brick tea, and SF was increasing in individuals aged >50 years, while its severity was positively correlated with age.
Skeletal fluorosis needs to be differentiated from diseases such as osteoblastic metastatic carcinoma and renal bone disease. Patients with SF who experience pain should be given an appropriate dose of non-steroidal analgesics. Those with skeletal deformities should have local fixed or orthopedic surgery to prevent deformity exacerbation.
Relationship between fluorosis and altitude
Previous studies (36, 45, 46) have shown that high-altitude is a risk factor for fluorosis. Animal experiments have shown that under low oxygen conditions, mice ingested more fluoride, and their enamel formation was affected. Higher altitudes result in a lower oxygen concentration of the air and lower pH levels of the urine, and they affect the metabolism of fluoride and calcium and increase the reabsorption of fluoride by the kidneys (71–73). Changes in acid–base balance caused by high-altitude hypoxia may increase fluoride levels in organisms’ internal environments by increasing the reabsorption of fluoride and reducing fluoride excretion in the urine.
The effects of altitude on the prevalence and severity of fluorosis were first reported (73) in Kenya. Rwenyonyi et al. (74) indicated that altitude was a risk indicator of fluorosis after controlling for confounding variables, such as water source and vegetarianism. An animal study (75) also indicated that rats in the high-altitude group had more severe cartilage damage, coagulative kidney necrosis, and hydropic liver degeneration than those in the low-altitude group.
Zhang et al. (46) reported that a higher altitude was a risk factor for DF, and the prevalence of DF at higher altitudes (2,560–3,300 m) was nearly three times higher than that at lower altitudes (1,400 m). When a study included districts with varying altitudes, the prevalence of DF in low-altitude areas tended to be lower than that in high-altitude areas (Table 3 and Figure 3). Similarly, this trend also existed for SF (Table 4 and Figure 4). However, these trends may not be obvious in the surveys of small samples, and they may be affected by various factors, such as the economic and health statuses of individuals living in the surveyed areas. In low-altitude areas, residents may have a more diverse diet, relatively better economic and hygiene conditions, and easier access to clean water sources. These confounding factors should be noted in future studies. Thus, large data with less heterogeneous results are needed to illustrate this issue.
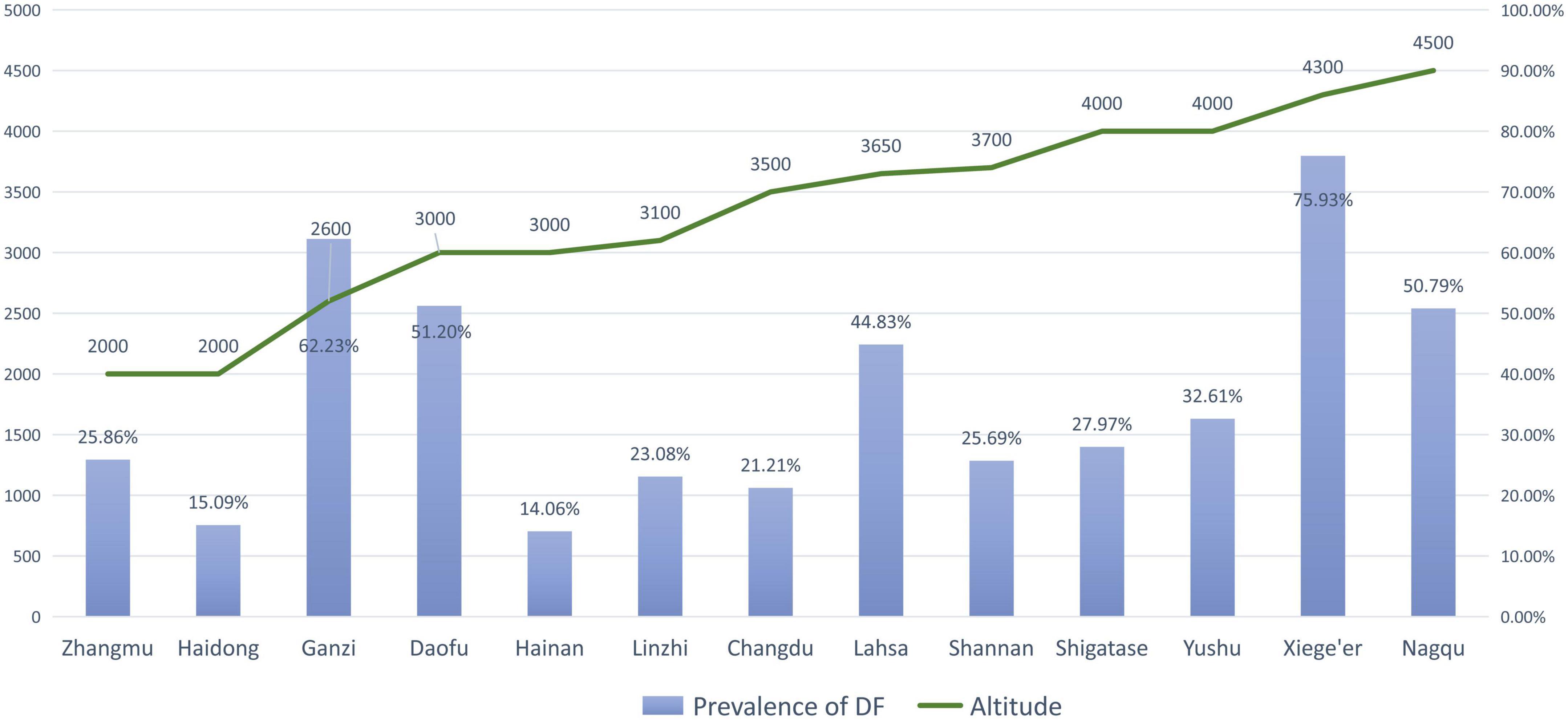
Figure 3. The prevalence of dental fluorosis (DF) and the altitude of surveyed areas (The line graph shows the altitude of the research site, and the bar graph shows the reported prevalence of DF at the research site).
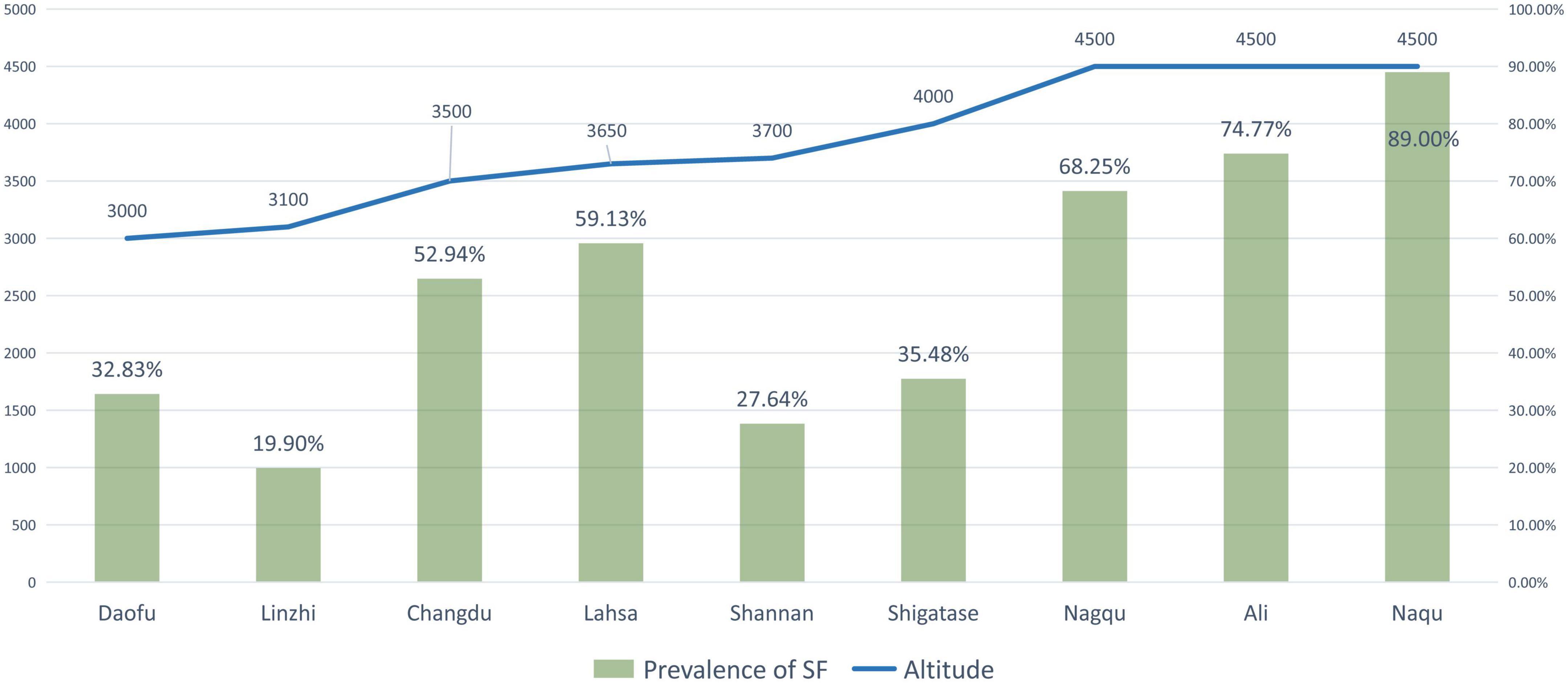
Figure 4. The prevalence of skeletal fluorosis (SF) and the altitude of surveyed areas (The line graph shows the altitude of the research site, and the bar graph shows the reported prevalence of SF at the research site).
Relationship between BTF and occupation
Some researchers have proposed the possibility of correlations between BTF and residents’ occupation, economic status, and health. Fan et al. (45) analyzed the prevalence of fluorosis across different occupational populations in Tibet. For the rural population, occupations can be briefly divided into agriculture and animal husbandry. Interestingly, they indicated that the likelihood of BTF was significantly associated with occupational factors; it was higher in villages where animal husbandry was the main occupation, whereas it was lower in agricultural villages (45). They explained that, owing to harsh living conditions, herdsmen consumed less vegetables but more brick tea for vitamins and minerals, which might have led to a higher prevalence of fluorosis among them.
Similarly, the correlation between BTF and occupation may be associated with living conditions and economic factors. The correlation between BTF and occupation, like the correlation between BTF and altitude, may be merely an external manifestation of the correlations between BTF and socioeconomic and living conditions. More detailed studies need to be conducted to identify confounding factors for this issue.
Considering the influence of occupation on diet and ultimately the likelihood of fluorosis, the large number of part-time or full-time religious workers in Tibet cannot be ignored. Tibetan Buddhist religious people may choose to become a monk or nun for life or a period of time, and the food sources for them are limited owing to their religious beliefs. Zanba, butter tea, and brick tea constitute a high proportion of their diet; however, to date, no data on the prevalence of fluorosis exist for this group of people.
Fluorosis and maternal brick tea consumption
During pregnancy and lactation, although the transfer of fluoride from mother to fetus is regulated by the placenta and the transfer of plasma to breast milk is inhibited (76), fluoride concentrations in breast milk and amniotic fluid increase significantly after the intake of fluorinated foods (77–81). Excessive fluoride exposure results in increased organic material in the secretory stage of enamel formation. Later, in the maturation period, the regulation of ameloblast modulation is disrupted by superfluous fluoride, and the mineralization of the enamel is disrupted, leading to hypomineralization and DF (66, 82, 83). Zhang et al. (46) reported an association between regular excessive maternal consumption of brick tea and DF in children. They indicated that the excessive consumption of brick tea by mothers during pregnancy and lactation could disrupt the development of children’s tooth germs.
In addition to the effect of fluoride levels on children’s teeth, existing research also suggests that higher levels of fluoride exposure in women during pregnancy may be associated with diseases or adverse reactions in their children. A report on fluoride intake in pregnant women indicated that those with a high intake produced offspring with lower IQs, and the effect was greater in boys (84). More attention should be paid to fluoride exposure in pregnant women.
Relationship between genetic polymorphisms and BTF
Recent advancements in bioinformatics have revealed many diseases to be associated with a genetic predisposition (85). Fluorosis may result from excessive fluoride intake as well as from prolonged exposure and the body’s response, but epidemiological and animal studies (86–88) have also indicated individual differences in the severity of fluorosis.
It is speculated that fluorosis may be caused by the complex interaction between candidate genes of fluorosis and specific environmental exposure, including dietary fluoride intake, among others. The severity of fluorosis does not always depend on the consumption of fluoride. Genetic polymorphisms may lead to individual differences in sensitivity or resistance to fluoride exposure.
Existing studies have been conducted with different laboratory mouse strains to identify possible genetic determinants of DF. Everett et al. (89) showed that different genotypes of mice with the same fluoride levels could produce different degrees of DF severity when controlling for variables such as sex, food, age, housing, and drinking water fluoride concentration. Mousny et al. (90) analyzed the role of genetic factors on the effects of fluoride on bone metabolism in an animal study. Their study evaluated the effect of increasing fluoride doses in three inbred strains of mice (susceptible, resistant, and intermediate). Significant changes in bone quality were observed in susceptible strains, moderate changes were seen in intermediate strains, and no changes were noted in resistant strains. Thus, the findings suggest that genetic factors may contribute to variations in bone quality.
Some human studies have also confirmed the role of genetic factors in fluorosis. Researchers found that genes related to signal transduction (G-protein, ERK, MEK1, and MEK2) (91–93), immune lymphokines (interleukin 6, interleukin 8) (94, 95), and estrogen receptors (96) may influence the occurrence and development of fluorosis. Case-control studies (97–99) carried out in fluorosis-endemic areas have analyzed the relationship between gene polymorphisms of candidate genes and fluorosis susceptibility.
We found that many studies in China have analyzed the genetic diversity of BTF in Tibetan populations and evaluated the susceptibility to BTF caused by different genes in Tibetan, Kazak, Mongolian, and Han populations. GSTP1, vitamin D receptor-FokI, prolactin promoter at -1,149, MMP2, FRZB1, serum soluble Klotho, BMP2, and ALOX15 and their related genes are thought to be involved (38, 49–55). However, the exact correlation between BTF and genetics needs further research.
Preventive measures and effects of brick tea consumption
The prevention and control of BTF have been underway since the discovery of a link between brick tea consumption and fluoride intake in Tibetan areas. To reduce the effect of BTF, the government and disease control authorities have acted to rectify the production of brick tea containing high-fluoride concentrations. The standard in China (GB 19,965-2005) stipulates that the fluoride content of brick tea should not exceed 300 mg/kg. According to the classification standard of endemic fluorosis (GB 17,018-2011), an endemic fluorosis area can be determined if the average daily tea fluoride intake of people over 16 years of age is more than 3.5 mg and there are patients with SF confirmed by X-ray examination.
Strengthening health education allows residents to understand the harm of high-fluoride brick tea and consciously develop healthier tea drinking habits, such as changing the single diet structure, balancing nutrition, advocating for the reduced dependence on brick tea, not drinking high-fluoride tea, and eating more fresh vegetables and fruits.
In addition, the tea preparation method should be changed; before adding water to boil, the tea should first be mashed and washed once with 80°C water. Strong tea, tea that has been boiled for a long time, and tea that has been soaked for too long should be avoided. Tea drinkers should avoid adding alkali when boiling brick tea since the dissolution efficiency of fluoride in tea is higher under alkaline conditions.
Jin et al. (39) reported their findings from a 3-year (2008–2011) observation study on the prevention of brick tea-induced intoxication. By reducing the availability of brick tea in school canteens and switching to low-fluoride brick tea, the total daily fluoride intake of children decreased to a relatively safe level, although it was still at the level of chronic intoxication. Though the short-term use of low-fluoride brick tea reduced urinary fluoride levels, this difference was not significant, and the serum fluoride levels remained unchanged. This finding may have been attributed to the maintenance of internal homeostasis, and the bones may have become fluoride buffers in the body. Fluoride accumulation in the body may take a long time to be eliminated, and the effect of preventive measures on brick tea needs to be verified by controlled experiments with a larger sample over a longer period of time.
Study limitations
To our knowledge, this is the first review of BTF in Tibetan residents, but limitations still exist. To date, the research on BTF is not comprehensive and has not been taken seriously enough. The literature that could be included in this study was limited, and most researches were conducted by the few limited scholars. In addition, the research methods are not unified and have a certain heterogeneity. The detection of fluoride in brick tea and the identification and rating of DF and SF should use widely recognized international standards. And no data are available on fluorosis among certain groups of Tibetans, especially Tibetan Buddhist monks and nuns. Lastly, the correlation between genotypes and the susceptibility to fluorosis caused by brick tea intake requires further investigation.
Conclusion
At present, the high-fluoride content of brick tea is an important pathogenic factor for fluorosis in Tibetan regions. To our knowledge, this is the first review on the issue of BTF. DF had prevalence rates of 14.06–75.93% and an overall pooled rate of 26.08% in children, whereas SF had prevalence rates of 19.90–74.77% and an overall pooled rate of 33.84% in adults. BTF susceptibility may be associated with occupation and the altitude at which individuals reside. Genetic polymorphisms were suggested risk factors. BTF among Tibetan residents needs more attention, and further studies evaluating its pathogenesis and preventive measures are warranted.
Author contributions
CW designed the study, applied for grant support, and conducted the literature search and screening, data collection, manuscript writing, and revision. QZ conducted the literature screening and data collection. FX participated in literature screening. JJ participated in data analysis. All authors read and approved the final manuscript.
Funding
This research was funded by grants: Sichuan Science and Technology Program Joint Innovation Project (No. 22ZDYF3789); Innovation and Entrepreneurship Training Program of SWMU (Nos. 2022074 and 2022045); Science and Technology Bureau of Aba Tibetan and Qiang Autonomous Prefecture (No. 21YYJSYJ0052); and Teaching Reform Project of Standardized training of residents from Affiliated Stomatological Hospital of SWMU (No. 2022GP04).
Acknowledgments
We would like to thank the Tibetan and Qiang people of Aba Autonomous Prefecture for their help in this study, especially Rong Jiang, Zhiqiang Zhang, Bo Lei, Yingquan Zhong, Guangmei Yang, Yuanzheng Yan, Cu Dong, and Cuo Ga from the Department of Dentistry of the People’s Hospital of Aba Tibetan and Qiang Autonomous Prefecture.
Cai Wen gives special gratitude and salute to a genius musician, Jay Chou, admires his unparalleled talent and inexhaustible creativity, and thanks him for the long-time companionship and encouragement by his works. One of his songs, “Grandpa’s Tea (Ye Ye Pao De Cha)”, which contains wonderful tributes to family affection and Chinese traditional culture, is the inspiration of this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2022.1030344/full#supplementary-material
References
2. Nielsen F. How should dietary guidance be given for mineral elements with beneficial actions or suspected of being essential? J Nutr. (1996) 126(Suppl. 9):2377S–85. doi: 10.1093/jn/126.suppl_9.2377S
3. Ciosek Z, Kot K, Kosik-Bogacka D, Lanocha-Arendarczyk N, Rotter I. The effects of calcium, magnesium, phosphorus, fluoride, and lead on bone tissue. Biomolecules. (2021) 11:506. doi: 10.3390/biom11040506
4. Gaffney-Stomberg E. The impact of trace minerals on bone metabolism. Biol Trace Element Res. (2019) 188:26–34. doi: 10.1007/s12011-018-1583-8
5. Akuno M, Nocella G, Milia E, Gutierrez L. Factors influencing the relationship between fluoride in drinking water and dental fluorosis: a ten-year systematic review and meta-analysis. J Water Health. (2019) 17:845–62. doi: 10.2166/wh.2019.300
6. Yadav K, Kumar S, Pham Q, Gupta N, Rezania S, Kamyab H, et al. Fluoride contamination, health problems and remediation methods in Asian groundwater: a comprehensive review. Ecotoxicol Environ Safety. (2019) 182:109362. doi: 10.1016/j.ecoenv.2019.06.045
7. Patil M, Lakhkar B, Patil S. Curse of fluorosis. Indian J Pediatr. (2018) 85:375–83. doi: 10.1007/s12098-017-2574-z
8. Everett E. Fluoride’s effects on the formation of teeth and bones, and the influence of genetics. J Dent Res. (2011) 90:552–60. doi: 10.1177/0022034510384626
9. Kebede A, Retta N, Abuye C, Whiting S, Kassaw M, Zeru T, et al. Dietary fluoride intake and associated skeletal and dental fluorosis in school age children in rural ethiopian rift valley. Int J Environ Res Public Health. (2016) 13:756. doi: 10.3390/ijerph13080756
10. Liu J, Yang S, Luo M, Chen T, Ma X, Tao N, et al. Association between dietary patterns and fluorosis in Guizhou, China. Front Nutr. (2019) 6:189. doi: 10.3389/fnut.2019.00189
11. Zhou Y, Chen D, Zhi Q, Tao Y, Wang X, Feng X, et al. The prevalence and associated risk indicators of dental fluorosis in china: findings from the 4th national oral health survey. Chin J Dent Res. (2018) 21:205–11. doi: 10.3290/j.cjdr.a41081
12. Kabata-Pendias A. Trace Elements in Soils and Plants. 4th ed. Boca Raton, FL: CRC Press (2010). doi: 10.1201/b10158
13. Gao H, Zhao Q, Zhang X, Wan X, Mao J. Localization of fluoride and aluminum in subcellular fractions of tea leaves and roots. J Agric Food Chem. (2014) 62:2313–9. doi: 10.1021/jf4038437
14. Chandrajith R, Abeypala U, Dissanayake C, Tobschall H. Fluoride in ceylon tea and its implications to dental health. Environ Geochem Health. (2007) 29:429–34. doi: 10.1007/s10653-007-9087-z
15. Kaczmarek U. [PH values and fluoride levels in some tea brands]. Ann Academ Med Stetin. (2004) 50(Suppl. 1):58–61.
16. Chandrajith R, Bhagya S, Diyabalanage S, Wimalasiri S, Ranatunga M, Barth J. Exposure assessment of fluoride intake through commercially available black tea (Camellia sinensis L.) from areas with high incidences of chronic kidney disease with undetermined origin (CKDU) in Sri Lanka. Biol Trace Element Res. (2022) 200:526–34. doi: 10.1007/s12011-021-02694-2
17. Whyte M, Totty W, Lim V, Whitford G. Skeletal fluorosis from instant tea. J Bone Mineral Res. (2008) 23:759–69. doi: 10.1359/jbmr.080101
18. Zhang R, Zhang H, Chen Q, Luo J, Chai Z, Shen J. Composition, distribution and risk of total fluorine, extractable organofluorine and perfluorinated compounds in Chinese teas. Food Chem. (2017) 219:496–502. doi: 10.1016/j.foodchem.2016.09.136
19. Karami M, Fakhri Y, Rezania S, Alinejad A, Mohammadi A, Yousefi M, et al. Non-carcinogenic health risk assessment due to fluoride exposure from tea consumption in iran using monte carlo simulation. Int J Environ Res Public Health. (2019) 16:4261. doi: 10.3390/ijerph16214261
20. Gupta P, Sandesh N. Estimation of fluoride concentration in tea infusions, prepared from different forms of tea, commercially available in Mathura city. J Int Soc Prevent Commun Dentist. (2012) 2:64–8. doi: 10.4103/2231-0762.109371
21. Kavanagh D, Renehan J. Fluoride in tea–its dental significance: a review. J Irish Dental Associat. (1998) 44:100–5.
22. Lung S, Cheng H, Fu C. Potential exposure and risk of fluoride intakes from tea drinks produced in Taiwan. J Exposure Sci Environ Epidemiol. (2008) 18:158–66. doi: 10.1038/sj.jes.7500574
23. Regelson S, Dehghan M, Tantbirojn D, Almoazen H. Evaluation of fluoride levels in commercially available tea in the United States. General Dentist. (2021) 69:17–20.
24. Sofuoglu S, Kavcar P. An exposure and risk assessment for fluoride and trace metals in black tea. J Hazardous Materials. (2008) 158:392–400. doi: 10.1016/j.jhazmat.2008.01.086
25. Whyte M, Essmyer K, Gannon F, Reinus W. Skeletal fluorosis and instant tea. Am J Med. (2005) 118:78–82. doi: 10.1016/j.amjmed.2004.07.046
26. Waugh D, Potter W, Limeback H, Godfrey M. Risk assessment of fluoride intake from tea in the republic of ireland and its implications for public health and water fluoridation. Int J Environ Res Public Health. (2016) 13:259. doi: 10.3390/ijerph13030259
27. Cao J, Zhao Y, Liu J, Xirao R, Danzeng S, Daji D, et al. Brick tea fluoride as a main source of adult fluorosis. Food Chem Toxicol. (2003) 41:535–42. doi: 10.1016/s0278-6915(02)00285-5
28. Yang L, Chen J, Geng J, Fang Y, Yang W. Social resilience and its scale effects along the historical tea-horse road. Environ Res Lett. (2021) 16:045001. doi: 10.1088/1748-9326/abea35
29. Lancuo Z, Hou G, Xu C, Liu Y, Zhu Y, Wang W, et al. Simulating the route of the tang-tibet ancient road for one branch of the silk road across the Qinghai-Tibet plateau. PLoS One. (2019) 14:e0226970. doi: 10.1371/journal.pone.0226970
30. Huang C, Zhang H, Zeng W, Ma J, Zhao S, Jiang Y, et al. Enhanced fluoride adsorption of aluminum humate and its resistance on fluoride accumulation in tea leaves. Environ Technol. (2020) 41:329–38. doi: 10.1080/09593330.2018.1498135
31. Das S, de Oliveira L, da Silva E, Liu Y, Ma L. Fluoride concentrations in traditional and herbal teas: health risk assessment. Environ Pollut. (2017) 231(Pt. 1):779–84. doi: 10.1016/j.envpol.2017.08.083
32. Cao J, Zhao Y, Liu J. [Fluoride in the environment and brick-tea-type fluorosis in tibet]. Huan jing ke xue= Huanjing kexue. (2002) 23:97–100.
33. Cao J, Zhao Y, Liu J, Xirao R. [Brick-tea type adult bone fluorosis]. Wei sheng yan jiu = J Hygiene Res. (2003) 32:141–3.
34. Cao J, Zhao Y, Liu J, Xirao R, Danzeng S. [Environmental fluorine level in tibet]. Ying yong sheng tai xue bao = J Appl Ecol. (2000) 11:777–9.
35. Cao J, Bai X, Zhao Y, Liu J, Zhou D, Fang S, et al. The relationship of fluorosis and brick tea drinking in Chinese tibetans. Environ Health Perspect. (1996) 104:1340–3. doi: 10.1289/ehp.961041340
36. Cao J, Zhao Y, Liu J, Xirao R, Danzeng S. Varied ecological environment and fluorosis in tibetan children in the nature reserve of Mount Qomolangma. Ecotoxicol Environ Safety. (2001) 48:62–5. doi: 10.1006/eesa.2000.1988
37. Cao J, Zhao Y, Liu J, Bai X, Zhou D, Fang S, et al. Fluorine intake of a Tibetan population. Food Chem Toxicol. (1996) 34:755–7. doi: 10.1016/0278-6915(96)00041-5
38. Chu Y, Liu Y, Guo N, Lou Q, Wang L, Huang W, et al. Association between ALOX15 gene polymorphism and brick-tea type skeletal fluorosis in Tibetans, Kazaks and Han, China. Int J Environ Health Res. (2021) 31:421–32. doi: 10.1080/09603123.2019.1666972
39. Jin C, Yan Z, Jian-Wei L, Ruoden X, Sangbu D, Zeguo S, et al. Prevention and control of brick-tea type fluorosis—a 3-year observation in Dangxiong, Tibet. Ecotoxicol Environ Safety. (2003) 56:222–7. doi: 10.1016/s0147-6513(03)00065-4
40. Jin C, Yan Z, Jianwei L, Ruodeng X, Sangbu D. Environmental fluoride content in Tibet. Environ Res. (2000) 83:333–7. doi: 10.1006/enrs.2000.4066
41. Liu G, Ye Q, Chen W, Zhao Z, Li L, Lin P. Study of the relationship between the lifestyle of residents residing in fluorosis endemic areas and adult skeletal fluorosis. Environ Toxicol Pharmacol. (2015) 40:326–32. doi: 10.1016/j.etap.2015.06.022
42. Shu W, Zhang Z, Lan C, Wong M. Fluoride and aluminium concentrations of tea plants and tea products from Sichuan Province, PR China. Chemosphere. (2003) 52:1475–82. doi: 10.1016/s0045-6535(03)00485-5
43. Song J, Hou C, Guo J, Niu Q, Wang X, Ren Z, et al. Two new members of CsFEXs couple proton gradients to export fluoride and participate in reducing fluoride accumulation in low-fluoride tea cultivars. J Agric Food Chem. (2020) 68:8568–79. doi: 10.1021/acs.jafc.0c03444
44. Wong M, Fung K, Carr H. Aluminium and fluoride contents of tea, with emphasis on brick tea and their health implications. Toxicol Lett. (2003) 137:111–20. doi: 10.1016/s0378-4274(02)00385-5
45. Fan Z, Gao Y, Wang W, Gong H, Guo M, Zhao S, et al. Prevalence of brick tea-type fluorosis in the tibet autonomous region. J Epidemiol. (2016) 26:57–63. doi: 10.2188/jea.JE20150037
46. Zhang R, Cheng L, Zhang T, Xu T, Li M, Yin W, et al. Brick tea consumption is a risk factor for dental caries and dental fluorosis among 12-year-old Tibetan children in Ganzi. Environ Geochem Health. (2019) 41:1405–17. doi: 10.1007/s10653-018-0216-7
47. Li Q, Zhao Z. Prevalence of brick tea-type fluorosis in children aged 8-12 years in Qinghai Province, China. Biomed Environ Sci. (2021) 34:334–6. doi: 10.3967/bes2021.044
48. Cao J, Zhao Y, Liu J. Safety evaluation and fluorine concentration of Pu’er brick tea and Bianxiao brick tea. Food Chem Toxicol. (1998) 36:1061–3. doi: 10.1016/s0278-6915(98)00087-8
49. Yang Y, Zhao Q, Liu Y, Liu X, Chu Y, Yan H, et al. FRZB1 rs2242070 polymorphisms is associated with brick tea type skeletal fluorosis in Kazakhs, but not in Tibetans, China. Arch Toxicol. (2018) 92:2217–25. doi: 10.1007/s00204-018-2217-9
50. Yang D, Liu Y, Chu Y, Yang Q, Jiang W, Chen F, et al. Association between vitamin D receptor gene FokI polymorphism and skeletal fluorosis of the brick-tea type fluorosis: a cross sectional, case control study. BMJ Open. (2016) 6:e011980. doi: 10.1136/bmjopen-2016-011980
51. Pei J, Li B, Liu Y, Liu X, Li M, Chu Y, et al. Matrix metallopeptidase-2 Gene rs2287074 polymorphism is associated with brick tea skeletal fluorosis in tibetans and Kazaks, China. Sci Rep. (2017) 7:40086. doi: 10.1038/srep40086
52. Lou Q, Guo N, Huang W, Wu L, Su M, Liu Y, et al. Association between bone morphogenetic protein 2 gene polymorphisms and skeletal fluorosis of the brick-tea type fluorosis in tibetans and Kazakhs, China. Int J Environ Health Res. (2021) 32:1489–99. doi: 10.1080/09603123.2021.1892037
53. Liu Y, Yang Y, Wei Y, Liu X, Li B, Chu Y, et al. sKlotho is associated with the severity of brick tea-type skeletal fluorosis in China. Sci Total Environ. (2020) 744:140749. doi: 10.1016/j.scitotenv.2020.140749
54. Li B, Yang Y, Liu Y, Sun J, Ye Y, Liu X, et al. Prolactin rs1341239 T allele may have protective role against the brick tea type skeletal fluorosis. PloS One. (2017) 12:e0171011. doi: 10.1371/journal.pone.0171011
55. Wu J, Wang W, Liu Y, Sun J, Ye Y, Li B, et al. Modifying role of GSTP1 polymorphism on the association between tea fluoride exposure and the brick-tea type fluorosis. PloS One. (2015) 10:e0128280. doi: 10.1371/journal.pone.0128280
56. Izuora K, Twombly J, Whitford G, Demertzis J, Pacifici R, Whyte M. Skeletal fluorosis from brewed tea. J Clin Endocrinol Metab. (2011) 96:2318–24. doi: 10.1210/jc.2010-2891
57. Joshi S, Hlaing T, Whitford G, Compston J. Skeletal fluorosis due to excessive tea and toothpaste consumption. Osteoporos Int. (2011) 22:2557–60. doi: 10.1007/s00198-010-1428-6
58. Cai H, Zhu X, Peng C, Xu W, Li D, Wang Y, et al. Critical factors determining fluoride concentration in tea leaves produced from Anhui province, China. Ecotoxicol Environ Safe. (2016) 131:14–21. doi: 10.1016/j.ecoenv.2016.04.023
59. Lu Y, Guo W, Yang X. Fluoride content in tea and its relationship with tea quality. J Agric Food Chem. (2004) 52:4472–6. doi: 10.1021/jf0308354
60. Shomar B, Müller G, Yahya A, Askar S, Sansur R. Fluorides in groundwater, soil and infused black tea and the occurrence of dental fluorosis among school children of the Gaza strip. J Water Health. (2004) 2:23–35.
61. Jin C, Yan Z, Jianwei L. Processing procedures of brick tea and their influence on fluorine content. Food Chem Toxicol. (2001) 39:959–62. doi: 10.1016/s0278-6915(01)00039-4
62. Dean H. Chronic endemic dentaln fluorosis: (mottled enamel). J Am Med Associat. (1936) 107:1269–73.
63. Bhagavatula P, Curtis A, Broffitt B, Weber-Gasparoni K, Warren J, Levy S. The relationships between fluoride intake levels and fluorosis of late-erupting permanent teeth. J Public Health Dent. (2018) 78:165–74. doi: 10.1111/jphd.12260
64. Cardenas Flores A, Flores Reyes H, Gordillo Moscoso A, Castanedo Cazares J, Pozos Guillen Ade J. Clinical efficacy of 5% sodium hypochlorite for removal of stains caused by dental fluorosis. J Clin Pediatr Dent. (2009) 33:187–91. doi: 10.17796/jcpd.33.3.c6282t1054584157
65. Revelo-Mejia I, Hardisson A, Rubio C, Gutierrez A, Paz S. Dental fluorosis: the risk of misdiagnosis-a review. Biol Trace Element Res. (2021) 199:1762–70. doi: 10.1007/s12011-020-02296-4
66. DenBesten P, Li W. Chronic fluoride toxicity: dental fluorosis. Monogr Oral Sci. (2011) 22:81–96. doi: 10.1159/000327028
67. Cao J, Yao Z, Yi J, Zhao Y, Zhong J, Yuan H. [Effect of broken black tea on the formation of dental enamel and the contents of twelve kinds of chemical elements]. Wei sheng yan jiu = J Hygiene Res. (2009) 38:725–9.
68. Ruan J, Wang Z, Yang Z, Bardsen A, Astrom A, Bjorvatn K. Dental fluorosis in primary teeth: a study in rural schoolchildren in Shaanxi Province. China. Int J Paediatric Dent. (2005) 15:412–9. doi: 10.1111/j.1365-263X.2005.00667.x
69. Yang C, Wang Y, Xu H. Treatment and prevention of skeletal fluorosis. Biomed Environ Sci. (2017) 30:147–9. doi: 10.3967/bes2017.020
70. Sellami M, Riahi H, Maatallah K, Ferjani H, Bouaziz M, Ladeb M. Skeletal fluorosis: don’t miss the diagnosis! Skeletal Radiol. (2020) 49:345–57. doi: 10.1007/s00256-019-03302-0
71. Buzalaf M, Whitford G. Fluoride metabolism. Monogr Oral Sci. (2011) 22:20–36. doi: 10.1159/000325107
72. Sah O, Maguire A, Zohoori F. Effect of altitude on urinary, plasma and nail fluoride levels in children and adults in Nepal. J Trace Elem Med Biol. (2020) 57:1–8. doi: 10.1016/j.jtemb.2019.09.003
73. Manji F, Baelum V, Fejerskov O. Fluoride, altitude and dental fluorosis. Caries Res. (1986) 20:473–80. doi: 10.1159/000260977
74. Rwenyonyi C, Bjorvatn K, Birkeland J, Haugejorden O. Altitude as a risk indicator of dental fluorosis in children residing in areas with 0.5 and 2.5 mg fluoride per litre in drinking water. Caries Res. (1999) 33:267–74. doi: 10.1159/000016528
75. Wang L-H, Liu L-Z, Shi Y-X, Gao Y-H, Liu Y-Q, Sun D-J. Investigation on histopathological damges of articular growth plate cartilage liver and kidney of rats with fluorosis induced by drinking brick-tea in the high altitude areas. Chinese J Endemiol. (2008) 27:5. doi: 10.3760/cma.j.issn.1000-4955.2008.01.008
76. Jha S, Mishra V, Sharma D, Damodaran T. Fluoride in the environment and its metabolism in humans. Rev Environ Contam Toxicol. (2011) 211:121–42. doi: 10.1007/978-1-4419-8011-3_4
77. Faraji H, Mohammadi A, Akbari B, Saatloo N, Lashkarboloki G, Mahvi A. Correlation between fluoride in drinking water and its levels in breast milk in Golestan Province, Northern Iran. Iranian J Public Health. (2014) 43:1664–8.
78. Hassunuma R, Zen Filho E, Ceolin D, Cestari T, Taga R, de Assis G. Ultrastructural and immunohistochemical study of the influence of fluoride excess on the development of rat incisor tooth buds. J Appl Oral Sci. (2007) 15:292–8. doi: 10.1590/s1678-77572007000400010
79. Abduweli Uyghurturk D, Goin D, Martinez-Mier E, Woodruff T, DenBesten P. Maternal and fetal exposures to fluoride during mid-gestation among pregnant women in northern California. Environ Health. (2020) 19:38. doi: 10.1186/s12940-020-00581-2
80. Campus G, Congiu G, Cocco F, Sale S, Cagetti M, Sanna G, et al. Fluoride content in breast milk after the use of fluoridated food supplement. A randomized clinical trial. Am J Dent. (2014) 27:199–202.
81. Cao J, Zhao Y, Liu J. [The relationship between dental fluorosis and dietary pattern among tibetan children in natural protective areas of Mount Qomolangma]. Zhonghua yu fang yi xue za zhi Chinese J Prevent Med. (2000) 34:297–9.
82. Bronckers A, Lyaruu D, DenBesten P. The impact of fluoride on ameloblasts and the mechanisms of enamel fluorosis. J Dent Res. (2009) 88:877–93. doi: 10.1177/0022034509343280
83. Lyaruu D, Bervoets T, Bronckers A. Short exposure to high levels of fluoride induces stage-dependent structural changes in ameloblasts and enamel mineralization. Eur J Oral Sci. (2006) 114(Suppl. 1):111–5. doi: 10.1111/j.1600-0722.2006.00346.x
84. Green R, Lanphear B, Hornung R, Flora D, Martinez-Mier E, Neufeld R, et al. Association between maternal fluoride exposure during pregnancy and IQ scores in offspring in Canada. JAMA Pediatr. (2019) 173:940–8. doi: 10.1001/jamapediatrics.2019.1729
85. Pramanik S, Saha D. The genetic influence in fluorosis. Environ Toxicol Pharmacol. (2017) 56:157–62. doi: 10.1016/j.etap.2017.09.008
86. Vieira A, Hanocock R, Eggertsson H, Everett E, Grynpas M. Tooth quality in dental fluorosis genetic and environmental factors. Calcif Tissue Int. (2005) 76:17–25. doi: 10.1007/s00223-004-0075-3
87. Dawson D. Preliminary evidence of an association between COL1A2 polymorphisms and dental fluorosis in a population with high fluoride exposure. J Evid Based Dent Pract. (2010) 10:96–8. doi: 10.1016/j.jebdp.2010.02.007
88. Escobar-Garcia D, Mejia-Saavedra J, Jarquin-Yanez L, Molina-Frechero N, Pozos-Guillen A. Collagenase 1A2 (COL1A2) gene A/C polymorphism in relation to severity of dental fluorosis. Commun Dent Oral Epidemiol. (2016) 44:162–8. doi: 10.1111/cdoe.12201
89. Everett E, McHenry M, Reynolds N, Eggertsson H, Sullivan J, Kantmann C, et al. Dental fluorosis: variability among different inbred mouse strains. J Dent Res. (2002) 81:794–8. doi: 10.1177/0810794
90. Mousny M, Banse X, Wise L, Everett E, Hancock R, Vieth R, et al. The genetic influence on bone susceptibility to fluoride. Bone. (2006) 39:1283–9. doi: 10.1016/j.bone.2006.06.006
91. Misra U, Gawdi G, Pizzo S. Beryllium fluoride-induced cell proliferation: a process requiring P21(ras)-dependent activated signal transduction and NF-kappaB-dependent gene regulation. J Leukoc Biol. (2002) 71:487–94. doi: 10.1189/jlb.71.3.487
92. Xu S, Khoo S, Dang A, Witt S, Do V, Zhen E, et al. Differential regulation of mitogen-activated protein/ERK kinase (MEK)1 and MEK2 and activation by a Ras-independent mechanism. Mol Endocrinol. (1997) 11:1618–25. doi: 10.1210/mend.11.11.0010
93. Matsuo S, Kiyomiya K, Kurebe M. Mechanism of toxic action of fluoride in dental fluorosis: whether trimeric G proteins participate in the disturbance of intracellular transport of secretory ameloblast exposed to fluoride. Arch Toxicol. (1998) 72:798–806. doi: 10.1007/s002040050576
94. Akashi M, Loussararian A, Adelman D, Saito M, Koeffler H. Role of lymphotoxin in expression of interleukin 6 in human fibroblasts. Stimulation and regulation. J Clin Invest. (1990) 85:121–9. doi: 10.1172/JCI114401
95. Hilger R, Koller M, Konig W. Inhibition of leukotriene formation and IL-8 release by the paf-receptor antagonist SM-12502. Inflammation. (1996) 20:57–70. doi: 10.1007/BF01487745
96. Ba Y, Zhang H, Wang G, Wen S, Yang Y, Zhu J, et al. Association of dental fluorosis with polymorphisms of estrogen receptor gene in Chinese children. Biol Trace Element Res. (2011) 143:87–96. doi: 10.1007/s12011-010-8848-1
97. Jiang M, Mu L, Wang Y, Yan W, Jiao Y. The relationship between alu I polymorphisms in the calcitonin receptor gene and fluorosis endemic to Chongqing, China. Med Princ Pract. (2015) 24:80–3. doi: 10.1159/000368435
98. Zhang S, Zhang X, Liu H, Qu W, Guan Z, Zeng Q, et al. Modifying effect of COMT gene polymorphism and a predictive role for proteomics analysis in children’s intelligence in endemic fluorosis area in Tianjin, China. Toxicol Sci. (2015) 144:238–45. doi: 10.1093/toxsci/kfu311
99. Zhang T, Shan K, Tu X, He Y, Pei J, Guan Z. Myeloperoxidase activity and its corresponding mrna expression as well as gene polymorphism in the population living in the coal-burning endemic fluorosis area in Guizhou of China. Biol Trace Element Res. (2013) 152:379–86. doi: 10.1007/s12011-013-9632-9
Keywords: brick tea-type fluorosis, brick tea, dental fluorosis, skeletal fluorosis, Tibetan, food security
Citation: Wen C, Zhang Q, Xie F and Jiang J (2022) Brick tea consumption and its relationship with fluorosis in Tibetan areas. Front. Nutr. 9:1030344. doi: 10.3389/fnut.2022.1030344
Received: 28 August 2022; Accepted: 28 November 2022;
Published: 13 December 2022.
Edited by:
Mainul Haque, National Defence University of Malaysia, MalaysiaReviewed by:
Racheal John, National Bureau of Plant Genetic Resources (ICAR), IndiaRogelio González-González, Juárez University of the State of Durango, Mexico
Copyright © 2022 Wen, Zhang, Xie and Jiang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Cai Wen, d2VuY2FpQHN3bXUuZWR1LmNu; orcid.org/0000-0002-3400-5382
 Cai Wen
Cai Wen Qing Zhang4
Qing Zhang4