- 1College of Pharmacy, Al-Ain University, Abu Dhabi, United Arab Emirates
- 2Department of Food Science and Human Nutrition, University of Veterinary and Animal Sciences, Lahore, Pakistan
- 3Department of Public Health, Health Services Academy, Islamabad, Pakistan
- 4Department of Diet and Nutritional Sciences, IBADAT International University, Islamabad, Pakistan
- 5Department of Nutrition and Dietetics, National University of Medical Sciences, Rawalpindi, Pakistan
- 6The Kirby Institute, University of New South Wales, Sydney, NSW, Australia
- 7Department of Human Nutrition and Dietetics, University of Chakwal, Chakwal, Pakistan
Iron supplementation and fortification are the well-known approaches to treat iron deficiency anemia (IDA) in women of reproductive age. The objective of the current randomized controlled trial (RCT) was to evaluate the cumulative effects of prebiotics and iron fortification among women of reproductive age. For this purpose, a total of 75 iron deficient women of childbearing age were recruited and randomly divided into 5 groups (4 treatment groups and 1 control group). Four different types of fortified wheat flour were prepared using two iron fortificants (NaFeEDTA and FeSO4) and two prebiotics [inulin and galacto oligosaccharides (GOS)], while control group was treated with iron fortified flour without any prebiotics. Blood samples were collected from overnight fasted women on monthly basis up to 90 days. Hematological indices such as Hemoglobin (Hb), Hematocrit, Red Blood Cell (RBC) Count and Mean Corpuscular Volume (MCV), as well as iron biomarkers including serum iron, ferritin, transferrin, and Total Iron Binding Capacity (TIBC) were evaluated for analyses. The results showed a considerable positive improvement in all iron biomarkers as well as hematological indices among the treatment groups (P-value < 0.05), as compared to the control group. A maximum Hb (11.86 ± 0.24 mg/dL) and hematocrit value (35.06 ± 1.32%), was reported in group G3 which was treated with fortified wheat flour at a dose of 963 mg/kg GOS + 15 ppm FeSO4. On the other hand, highest mean values for RBC Count (4.73 ± 0.41 mil/mm3), MCV (81.41 ± 3.21 fL), serum iron (75.62 ± 2.79 μg/dL), serum transferrin (16.82 ± 0.30 mg/dL), and TIBC (403.68 ± 7.27 μg/dL) were observed in G4 group receiving the fortified wheat flour at a dose of 963 mg/kg GOS + 30 ppm FeSO4 level. The study concluded that prebiotic fortification along with iron salts helps to enhance iron absorption among iron deficiency anemic women of reproductive age.
Introduction
Iron, being an important constituent of certain proteins and several enzymes plays a major role in energy metabolism, oxygen transport, ATP synthesis, and biochemical transformation (1). However, iron deficiency is one amongst the major public health issues responsible to increase the global burden of disease, mainly affecting children and women (2). Such as, iron deficiency is a leading cause of infections, cognitive impairment, reduced work capacity, and high mortality risks in women of reproductive age (3). Besides, iron deficiency anemia during pregnancy has been reported to increase the risk of pregnancy complications and poor perinatal outcomes in low-and-middle income countries (4). Before conception, a woman should have adequate iron stores primarily required for the feto-placental development (∼360 mg Fe), expansion of red blood cells (450 mg Fe), and blood lost at delivery (∼150 mg), but unfortunately many women have insufficient preconception iron stores to meet these pregnancy needs (5). Recent estimates show that approximately 36.5% pregnant women and over half a billion women of reproductive age (15–49 years) are suffering from anemia (6). Considering these alarming figures, the World Health Organization (WHO) emphasizes on the target of 50% reduction in anemia by 2025 through improvement of dietary diversity, food fortification and iron supplementation (7).
Eating food processed from animal liver and animal sources during pregnancy is the safest and most effective method of iron supplementation. Food fortification with iron is the other well-known approach to treat anemia in women of reproductive age (8). Addition of vitamins and minerals through fortification helps to improve food quality and nutritional value. Equally, studies have shown that prebiotics such as galacto oligosaccharides and inulin might play an important role in enhancing iron absorption in anemic subjects (9). A recent study reported that co-administration of inulin and iron fortificants helps to improve iron biomarkers in female anemic rats (10). In concomitant, few studies suggested that Oligofructose helps to prevent iron deficiency anemia by increasing iron absorption in the gut (11, 12).
Prebiotics are known as functional food components that stimulate the growth and colonization of beneficial bacteria in the gut. These microorganisms scavenge iron from the environment though producing high/specific affinity iron-chelating compounds (siderophores system) (13). Furthermore, prebiotics stimulate the absorption of iron in proximal colon as well as duodenum through producing short chain fatty acids in the colon (14). Thus absorption of iron is enhanced in the presence of fermentable food involved in the growth of bacteria leading to the production of short-chain fatty acids such as propionic acid (13). However, scarcity of data and research on human subject warrants further studies in this regard considering high prevalence of IDA in low/middle income countries.
Pakistan being a low/middle income country is facing the major public health issue of iron deficiency in children and women of reproductive age. According to the latest estimates, approximately 42% women of reproductive age and more than half (56.6%) of adolescent girls are suffering with anemia in Pakistan (15). The current study therefore aimed to provide research-based evidence to target the preventive approach and minimizing the risk of IDA in the country. Thus, the objective of this randomized control trial (RCT) was to assess the cumulative effect of prebiotics and iron fortification on hematological indices and serum iron biomarkers among women of reproductive age group. Hemoglobin (Hb), Hematocrit, Red Blood Cell (RBC) Count and Mean Corpuscular Volume (MCV), as well as iron biomarkers including serum iron, ferritin, transferrin and Total Iron Binding Capacity (TIBC) were evaluated to observe the combined effects of prebiotics and iron fortification on iron deficiency anemic women.
Materials and methods
Study design
It was a double blind, randomized controlled trial which lasted for 3 months. For this purpose, the study population of university going iron deficient female adults (age 18–25 years) were screened based on the signs and symptoms associated with iron deficiency anemia. Later, these short-listed participants went for serum ferritin and complete blood count (CBC) tests to confirm diagnosis and to have baseline values. The participants were recruited from the three federal universities of Islamabad, Pakistan. An informed written consent was taken from all the study subjects prior to the start of RCT.
Study participants
A total of 113 women subjects were initially assessed for detection of iron deficiency (Figure 1). Out of these 113 shortlisted participants, 38 were excluded (26 did not meet the eligibility criteria whereas 12 participants refused to participate in the study). Remaining (75 participants) were finally recruited for this RCT. The flow chart of randomization has been presented in Figure 1. The selected participants underwent for a complete comprehensive medical examination to exclude the possibility of any confounders such as suffering from any chronic and GI disorders. Finally, 75 selected study subjects were divided into 5 groups comprising of 4 treatment groups and 1 control group (Table 1). The control group received only iron fortification without addition of any prebiotics. The current RCT continued from March 2019 to June 2019, while eligibility of the included participants started in January 2019 based on the convenience sampling technique.
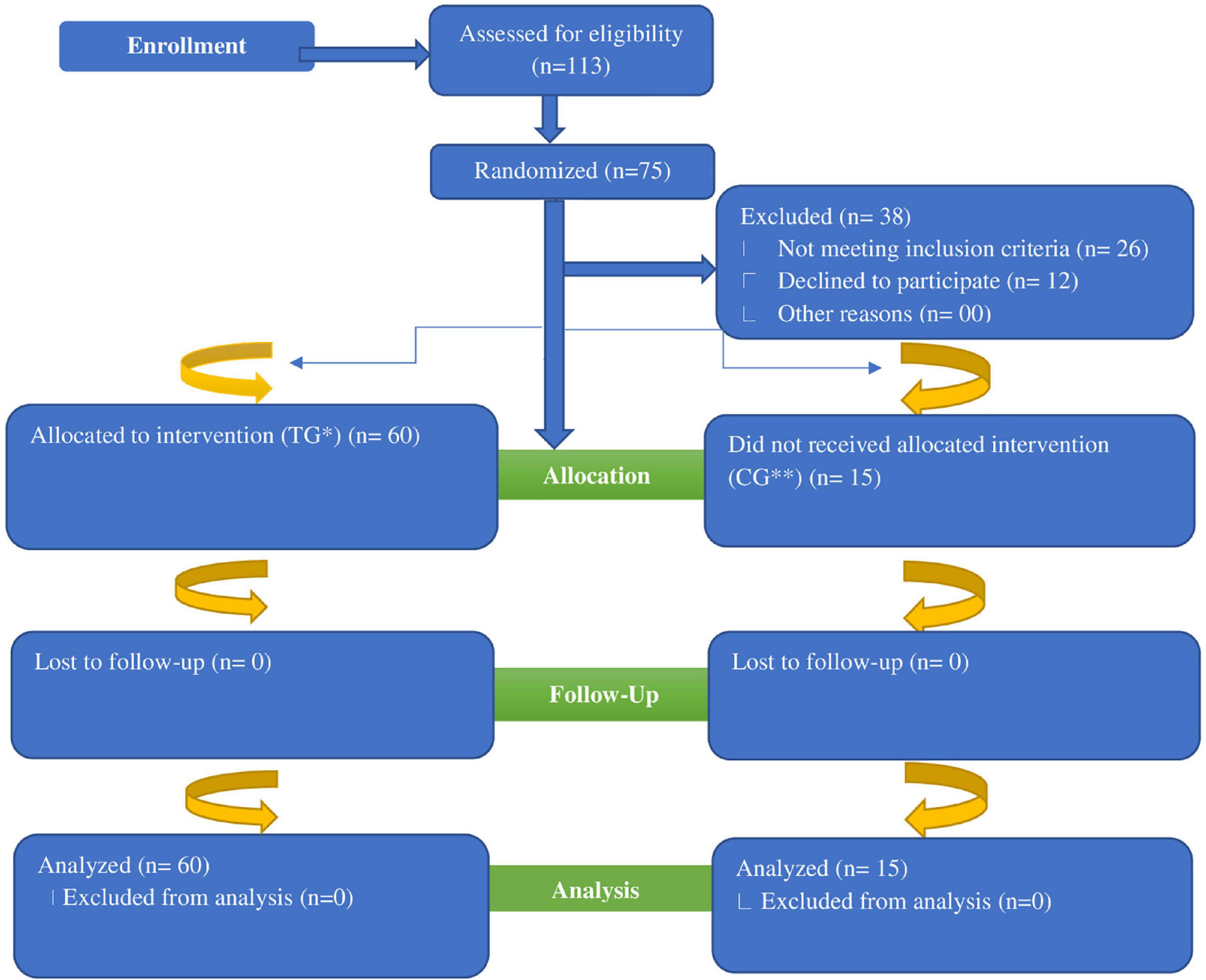
Figure 1. Flow diagram of women subjects through the randomized controlled trial. *TG, Treatment Group; **CG, Control Group.
Inclusion and exclusion criteria
All willing iron deficient female adults without any chronic diseases such as diabetes or hypertension were included in the study. On the other hand, women suffering from any chronic diseases, taking iron and/or prebiotic supplements, smokers and married or having children were excluded from the study.
Randomization and blinding
Simple randomization of study participants into groups was done via using computer generated random number tables. Since the study was double blinded, neither study participants nor data collectors in contact with the selected study subjects were aware about the type of fortified flour, allocation sequence of wheat flours, or method of preparing prebiotics and iron fortified flours used for this trial.
Diet plan
Prebiotics and iron fortificants required for this study were bought from the well reputed company of “Sigma Aldrich.” Proper storage was ensured as per the instructions on the label for each ingredient. In this study, two different types of iron fortificants “NaFeEDTA and FeSO4” were added at two different levels at 10 ppm and 20 ppm NaFeEDTA and 15 ppm and 30 ppm FeSO4. Similarly, two different prebiotics “Inulin and Galacto oligosaccharides” both at a level of 963 mg/kg body weight were used for current study.
The dosage for prebiotics was calculated using the human equivalent dose (HED) equation, that is,
Recommended dose of prebiotics for human consumption is 6–8 grams per day. Sample calculations for reference weight of 60 kgs human, 0.15 kg rat and 8 grams prebiotic consumption per day were performed as follows;
HED = 963 × (0.15/60)0.33
HED = 963 × (0.0025)0.33
HED = 963 × 0.1384
HED = 133.33 mg/kg/day
HED = 133.33 × 60
HED = 7999.8 ≈ 8 g per day
Exact dosage given to the study subjects was however calculated after weighing each study subject individually. Moreover, dosage was adjusted at the end of each week, according to the changes in body weight of each study participant.
Experimental protocol
For fortification, wheat flour was selected considering the major staple food consumed in Pakistan on daily basis by the wider regional/local community. Four different types of wheat flour were prepared based on the calculated dose of prebiotic as well as added iron fortificant (Table 1).
Wheat flours were prepared on weekly basis and participants were given the prepared flour for the subsequent week on each Sunday night. Participants were advised not to drink tea or coffee within 30 min after consumption of the fortified wheat flour.
Efficacy trials
For a period of 3 months, blood samples were collected from overnight fasted women on monthly basis. Four readings were taken overall, that is, at baseline, 30th day, 60th day, and 90th day.
Analytical procedures
Blood samples were collected from overnight fasted women and subjected to various analytical procedures.
Hematological indices
Four types of hematological indices Hb, Hematocrit, RBC Count and MCV were analyzed through automated cell counter. For determination of hemoglobin and MCV, automated hematology analyzer was used while hematocrit was determined as the ratio of the volume of packed red blood cells to the total blood volume. For RBC Count, flow cytometry technique was used (16).
Iron biomarkers
In addition to the hematological indices, four different iron biomarkers including serum iron, ferritin, transferrin, and TIBC were also evaluated on monthly basis up to 90 days of study duration.
Serum iron
Serum iron concentration was determined by Atomic Absorption Spectrophotometer. To determine serum iron, blood was centrifuged at 5,000 rpm followed by freezing of serum at –20°C. Serum iron was then measured using Atomic Absorption Spectrometry (17).
Serum ferritin
Commercially available kit of Ciba-Corning’s Automated Chemiluminescence System’s (ACS 180) was used to evaluate the levels of serum ferritin among anemic subjects. Serum ferritin levels were determined using the technique of Electrochemiluminescence immunoassay (ECLIA) (18).
Serum transferrin
Serum Transferrin was determined in anemic women as per the protocol described by Al-Buhairan and Oluboyede. For analysis of serum transferrin, Cobas Mira Plus Analyzer was used. The specific antiserum formed by Human transferrin, was measured at 340 nm turbidimetrically (19).
Total iron binding capacity
Total Iron Binding Capacity was calculated using the standard formula, that is, TIBC = Transferrin × 24.
Statistical analysis
Factorial design was employed to determine the level of significance using SPSS version 23.0. Data was considered significant at P-value < 0.05 (20). All the results were presented as Means and Standard Deviations.
Results
Effect of prebiotic and iron fortified diet on hematological indices and iron biomarkers
Mean square values for hemoglobin, hematocrit, RBC Count, MCV, serum iron, serum ferritin, Serum transferrin, and TIBC showed significant positive variations for the effect of groups, study intervals as well as their interaction (Table 2).
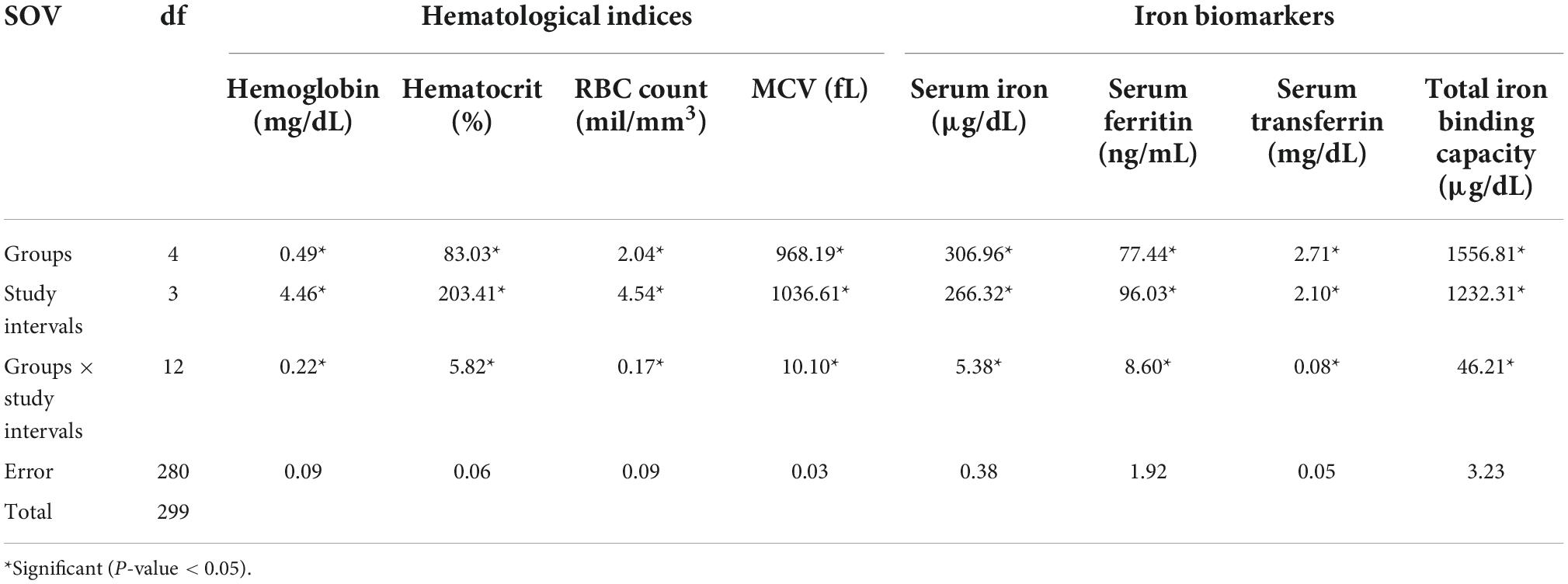
Table 2. Mean squares regarding hematological indices and iron biomarkers for anemic women fed with prebiotic and iron fortified diet.
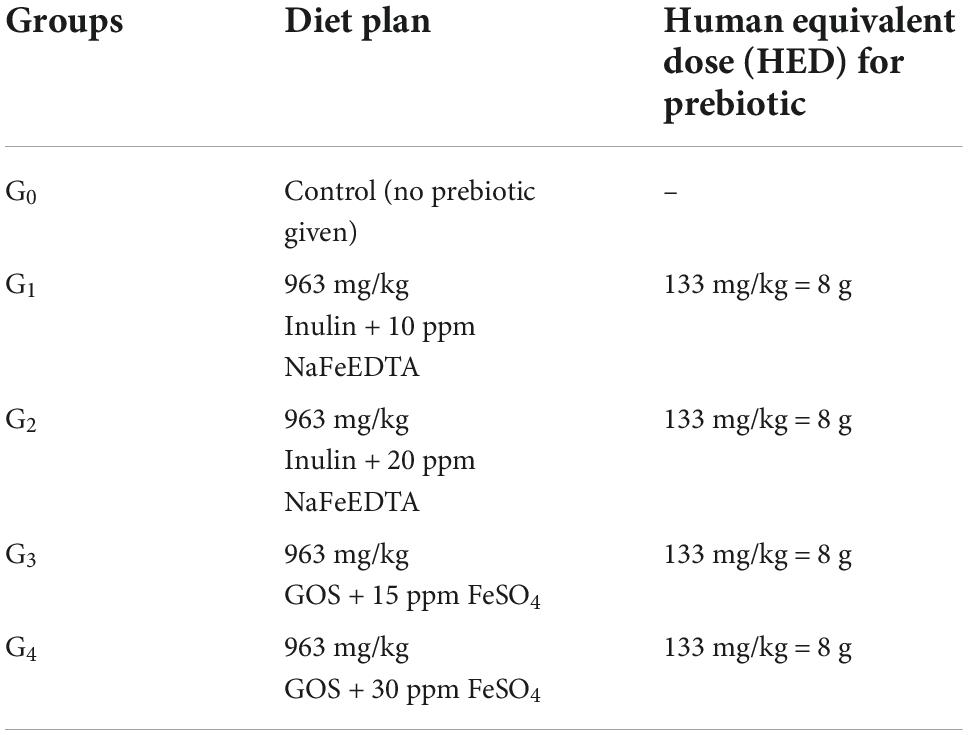
Hemoglobin
Mean values for hemoglobin levels along with their respective standard deviations have been shown in Table 3.
The results showed the maximum Hb value of 11.86 ± 0.24 mg/dL in group G3 which was given the fortified wheat dose at 963 mg/kg GOS + 15 ppm FeSO4, followed by the Hb value of G1 group at 11.79 ± 0.20 mg/dL, G4 at 11.74 ± 0.45 mg/dL and G2 at 11.68 ± 0.14 mg/dL. The control group G0 reported with the mean value for hemoglobin at 11.62 ± 0.21 mg/dL.
As the study intervals progressed, a significant improvement was seen in the mean hemoglobin levels of anemic women over the period of 90 days. Maximum Hb improvement could be seen in group G4 ranged from 11.28 ± 0.04 mg/dL at the start of the study to 12.33 ± 0.03 mg/dL at the end of the study. This was followed by group G3 in which the variation of the levels reported from 11.61 ± 0.02 mg/dL to 12.17 ± 0.04 mg/dL. The subsequent improvement was observed in groups G0, G1 and G2 where the hemoglobin values varied from 11.44 ± 0.04 mg/dL, 11.58 ± 0.02 mg/dL and 11.54 ± 0.01 mg/dL at 0 day to 11.91 ± 0.02 mg/dL, 12.04 ± 0.03 mg/dL and 11.85 ± 0.04 mg/dL at 90th day, respectively.
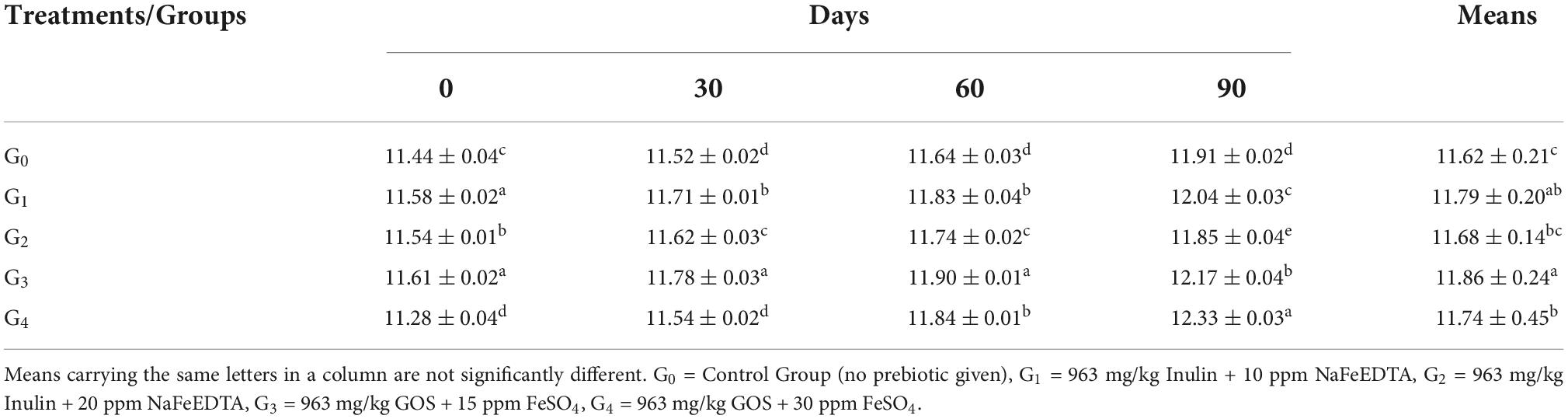
Hematocrit
According to the mean values of Hematocrit, the maximum value of 35.06 ± 1.32% was seen in group G3, followed by group G1 (35.04 ± 1.10%), group G4 (34.60 ± 2.66%), respectively (Table 4). On the other hand, the control group G0 showed a mean value of 32.22 ± 1.73%.
With the progression of study duration, maximum improvement was observed in group G4, followed by group G2, G3 and G1. For group G4, the hematocrit levels improved from 31.50 ± 0.06 at 0 day to 37.86 ± 0.26% at 90th day while for group G2, the improvement in hematocrit levels was 32.77 ± 0.07–36.17 ± 0.07%. In group G3, the hematocrit level enhanced from 33.50 ± 0.06 to 36.56 ± 0.06%, while Group G1 had a value of 33.76 ± 0.19% at the start and improved to 36.43 ± 0.07% at the end of 90 days.
Red blood cell count
Mean values for RBC Count (shown in Figures 2A,B) demonstrated a maximum value in group G4, followed by groups G1, G2, and G3. The recorded values for groups G4, G1, G2, and G3 were 4.73 ± 0.41 mil/mm3, 4.36 ± 0.19 mil/mm3, 4.34 ± 0.22 mil/mm3 and 4.29 ± 0.28 mil/mm3, respectively.
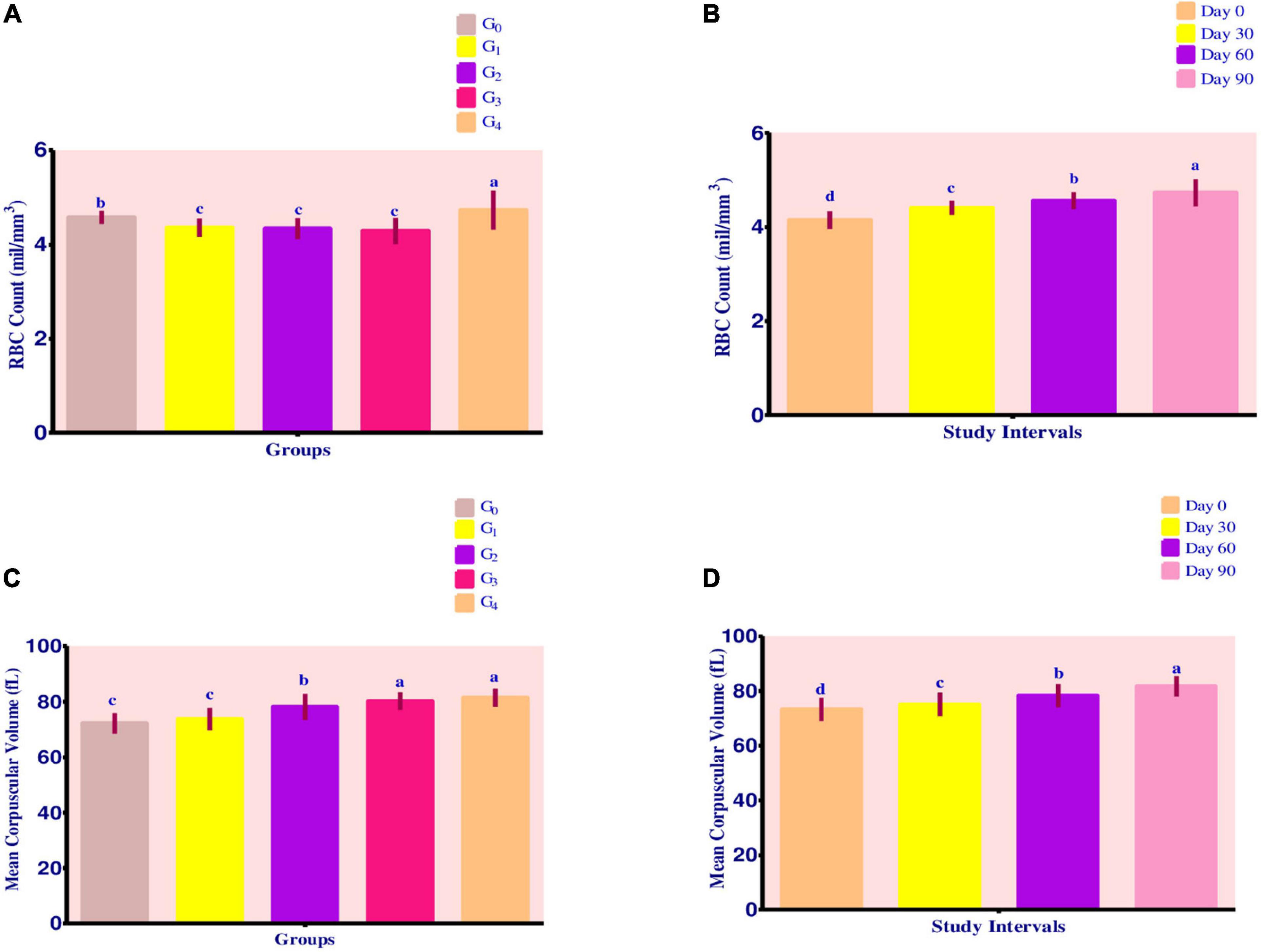
Figure 2. (A–D) Effect of fortified diets on RBC count and MCV among anemic women data are presented as Means ± SD. P-value < 0.05 is considered to be significant. Means carrying the same letters are not significantly different.
Over the course of 90 days, RBC count levels steadily increased. Maximum improvement was recorded in groups G4, G3, G2, and G1, respectively. For group G4, change in RBC count levels was from 4.26 ± 0.02 mil/mm3 to 5.23 ± 0.02 mil/mm3. For group G3, the levels improved from 3.92 ± 0.03 mil/mm3 to 4.56 ± 0.02 mil mm3, whereas RBC count levels increased from 4.04 ± 0.02 mil/mm3 to 4.57 ± 0.01 mil/mm3 for group G2. Group G1 had a value of 4.13 ± 0.02 mil/mm3 improved to 4.57 ± 0.01 mil/mm3 at the end of 90-day trial period.
Mean corpuscular volume
Mean values for MCV showed a steady improvement (Figure 2C). Maximum value for MCV was attained by group G4 (81.41 ± 3.21 fL), followed by G3 80.21 ± 3.13 fL, G2 78.11 ± 4.71 fL, and G1 73.73 ± 3.99 fL. On the other hand, control group G0 showed a MCV value of 72.20 ± 3.68 fL.
Across the modeling trials, MCV levels improved steadily as shown by Figure 2D. With the progress in study intervals, maximum improvement occurred in group G2, followed by group G1, G0, G4, and G3. For group G2, the MCV levels ranged from 73.79 ± 0.19 fL at 0 day to 83.77 ± 0.13 fL at 90th day.
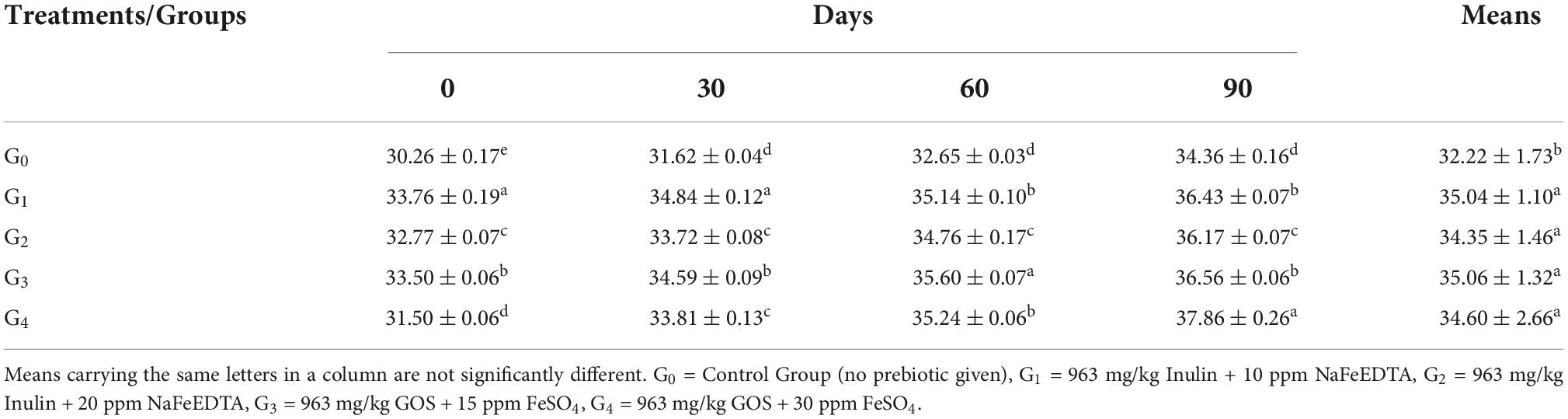
Serum iron
Table 5 shows that group G4 had a value of 75.62 ± 2.79 μg/dL while group G2 had a value of 74.70 ± 2.06 μg/dL. Group G3 recorded a value of 73.38 ± 2.04 μg/dL while group G1 was observed having a value of 73.07 ± 1.97 μg/dL. Group G0 showed a value of 69.69 ± 1.12 μg/dL.
Across the modeling trials, a significant improvement was witnessed from the time of initiation to the time of termination at 90th day. Maximum improvement in the trait was witnessed in group G4, with value ranging from 72.60 ± 0.33 μg/dL to 79.10 ± 0.95 μg/dL. In group G2, the value of serum iron ranged from 72.65 ± 0.50 μg/dL at 0 day to 77.42 ± 0.91 μg/dL. Values in group G3 and G1 ranged from 71.42 ± 0.60 μg/dL and 70.97 ± 0.70 μg/dL at initial level to 76.16 ± 1.09 μg/dL and 75.36 ± 1.23 μg/dL, respectively.
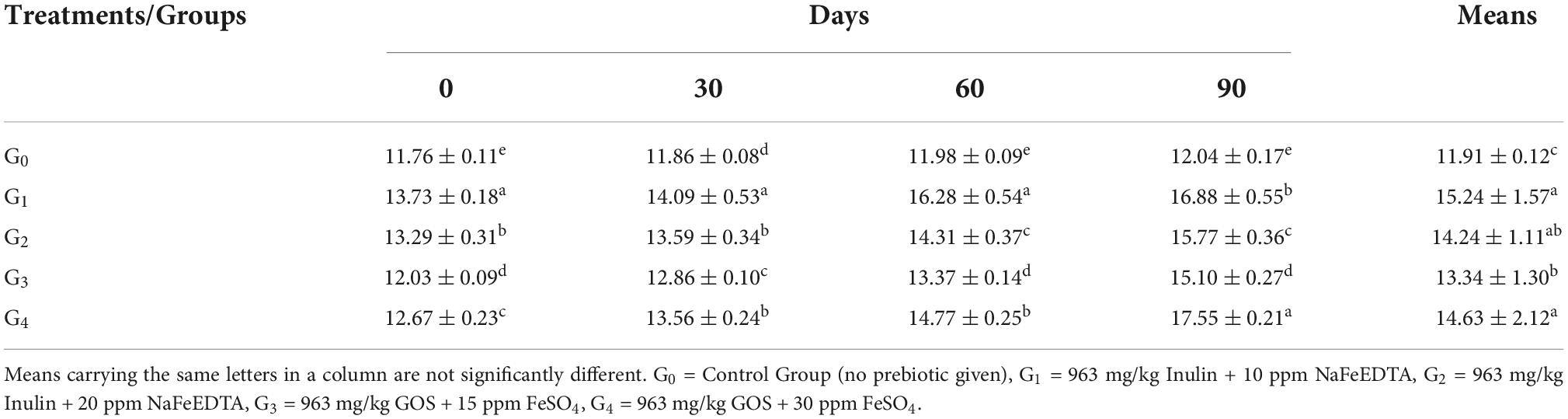
Serum ferritin
Maximum value for Serum Ferritin was observed in group G1 at 15.24 ± 1.57 ng/mL. Following the group G4 (14.63 ± 2.12 ng/mL), G2 (14.24 ± 1.11 ng/mL), G3 (13.34 ± 1.30 ng/mL), and G0 (11.91 ± 0.12 ng/mL), as shown by Table 6.
In terms of progression of study intervals, maximum improvement was seen in group G4, followed by G1, G3 and G2. Group G4 had an initial mean value of 12.67 ± 0.23 ng/mL improved to 17.55 ± 0.21 ng/mL at the end of trials. For group G1, the initial value of 13.73 ± 0.18 ng/mL increased to 16.88 ± 0.55 ng/mL at 90th day. Improvement in values of groups G3 and G2 were from 12.03 ± 0.09 ng/mL and 13.29 ± 0.31 ng/mL to 15.10 ± 0.27 ng/mL and 15.77 ± 0.36 ng/mL, respectively.
Serum transferrin
From Figure 3A, maximum value for Serum Transferrin was reported in group G4 which was 16.82 ± 0.30 mg/dL while the minimum value was shown by group G2 at 16.25 ± 0.12 mg/dL. Group G1 had a value of 16.58 ± 0.14 mg/dL while group G3 recorded at 16.63 ± 0.14 mg/dL. The control group G0 was observed having a mean value of 16.64 ± 0.18 mg/dL.
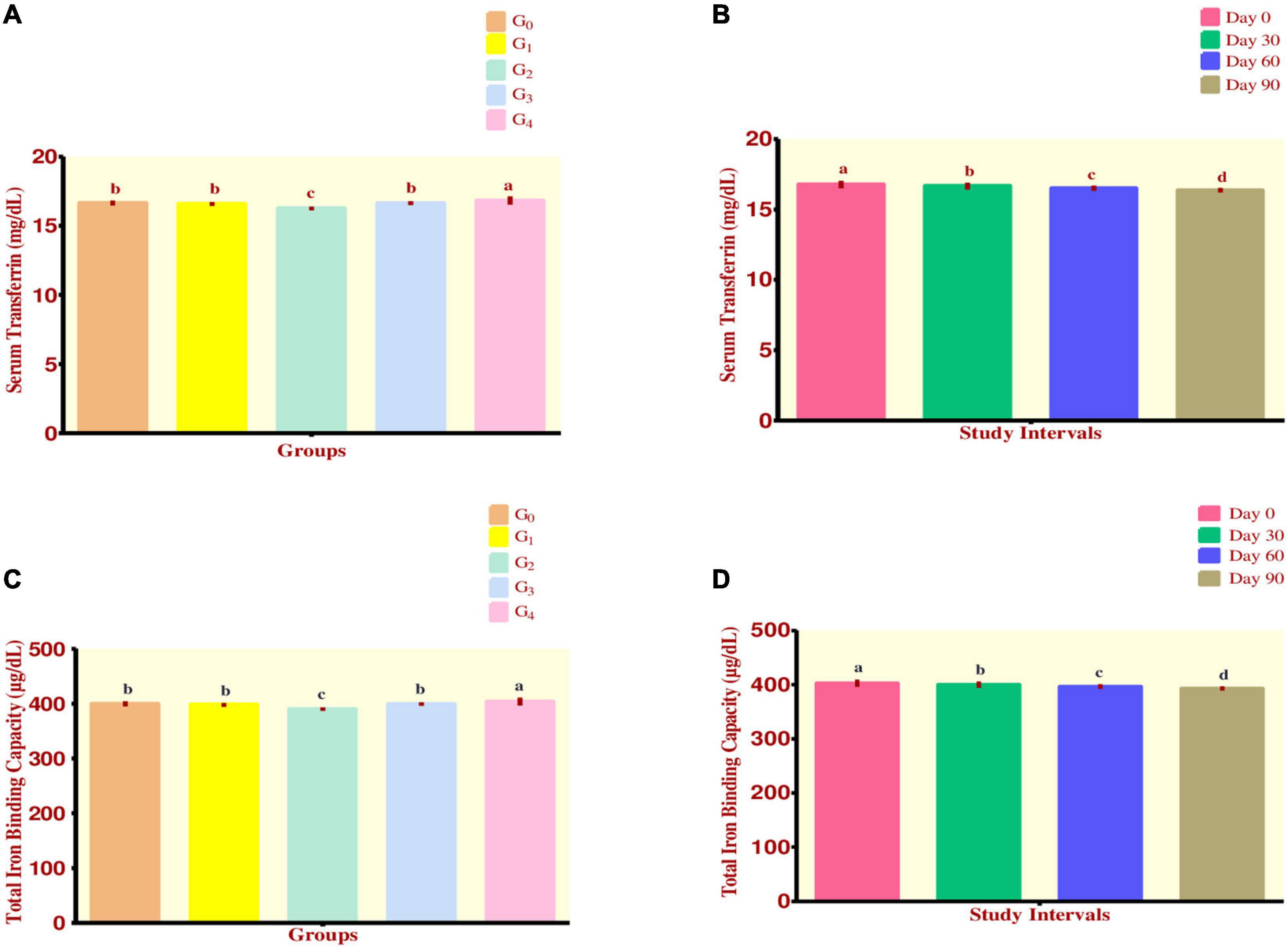
Figure 3. (A–D) Effect of fortified diets on serum transferrin and TIBC among anemic women data are presented as Means ± SD. P-value < 0.05 is considered to be significant. Means carrying the same letters are not significantly different.
As the study intervals progressed, it was observed that a steadily declining trend existed for the trait. Maximum decline occurred in group G4 which ranged from 17.15 ± 0.06 to 16.47 ± 0.12 mg/dL while minimum decline was observed in group G2 ranging from 16.38 ± 0.08 to 16.09 ± 0.10 mg/dL. During the efficacy trials, a significant decline in mean serum transferrin levels was observed (Figure 3B).
Total iron binding capacity
Regarding Total Iron Binding Capacity, it was observed that maximum value was attained by group G4 403.68 ± 7.27 μg/dL, followed by group G0 which had a value of 399.46 ± 4.35 μg/dL. Group G3 showed the value of 399.14 ± 3.34 μg/dL while group G1 had a value of 397.84 ± 3.38 μg/dL. Finally, group G2 was observed to be having a value of 389.80 ± 2.96 μg/dL (Figure 3C). Across the modeling trials, a significant drop in mean TIBC levels was witnessed (Figure 3D).
Discussion
Iron as a primary component of hemoglobin plays an import role in oxygen transport and energy metabolism (21). Moreover, it is essential for several other biological functions including respiration and proliferation of cells, DNA synthesis and repair. Besides, iron is a major constituent of almost 200 enzymes involved in various metabolic functions and biochemical reactions (22, 23). Additionally, iron is involved in normal functioning of the central nervous system (CNS) and engaged in the synthesis of neurotransmitters and myelination of nerves (24, 25).
In our study, we hypothesized that cumulative effect of prebiotics and iron fortification might enhance the bioavailability of iron, thus improving body iron reserves. The results of our RCT endorsed this hypothesis and showed a significant improvement in iron absorption among iron deficiency anemic women when fed iron fortificants (either NaFeEDTA or FeSO4) in conjugation to prebiotics (either Inulin or Galacto oligosaccharides) as compared to the control group. Though the absorption of iron was recorded in the control group (only treated with iron fortificant), the results were more pronounced in the treatments groups. The beneficial effects of prebiotics on iron absorption can be explained through the fermentation process by gut microbiota, which helps to (i) lower the pH of the luminal content, (ii) increase in the reduction of Fe + + + to Fe + +, and (iii) facilitate the expression of mineral transport proteins in epithelial cells (26).
Regarding hemoglobin and hematocrit, it was observed that group G3 treated with 963 mg/kg GOS + 15 ppm FeSO4 had maximum hemoglobin and hematocrit levels at the end of 90 days trial. It has been reported that bioavailability of ferric form is 3–4 times less compared to that of ferrous form due to poor solubility of ferric form in alkaline media (27). Therefore, maximum increase in Hb and hematocrit levels were seen in groups treated with FeSO4 which contains iron in ferrous form as compared to NaFeEDTA that contains iron in ferric form.
In agreement to the previous studies of Ahmad et al. and Gershoff et al. (10, 28), our study observed that serum iron levels were significantly improved in all the groups of anemic women when fed with iron fortificants and prebiotics, compared to the control group. Furthermore, maximum values of both RBC count and MCV levels were reported in group G4 fed with 963 mg/kg GOS and 30 ppm FeSO4 as compared to other treatment groups.
Hb levels are usually recommended for detection of iron deficiency anemia, despite that serum ferritin is a more reliable indicator used to detect iron deficiency anemia (29). Ferritin is considered to be the storage form of iron in the body and falls early in case of iron deficiency anemia (30). In our study, serum ferritin levels were considerably improved among anemic women at the end of the trial period, in accordance to a previous study conducted by Thuy et al. (31). Besides, studies have suggested that serum transferrin can be a useful indicator for diagnosis of iron deficiency anemia, highly sensitive and specific in nature (32).
In our trials, a significant decrease in serum transferrin levels was observed over a period of 90 days among all the treatment groups, with maximum decline occurring in group G2 (963 mg/kg Inulin + 20 ppm NaFeEDTA). The reason behind can be explained that serum transferrin has been known to increase in anemic individuals, indicating that more iron is needed by the body. On the other hand, as soon as anemia starts to improve, serum transferrin levels of an individual are declined (33). Similarly, TIBC is defined as the total amount of iron in the body which can be carried by transferrin. Transferrin is a protein found in the body which is responsible to carry iron throughout the body (30). TIBC levels are supposed to decline when anemia is being improved in patients as shown in our results. Lobo et al. on the similar lines conducted a study in 2011 and concluded that there was a steady decrease in TIBC levels over the course of trials, which indicated that iron reserves were being improved among anemic subjects (34).
Conclusion
In conclusion, our study revealed that cumulative diet containing prebiotics and iron fortificants improved levels of hemoglobin, hematocrit, RBC count, MCV, serum iron, serum ferritin, serum transferrin, and TIBC. These effects were more pronounced among treatment groups as compared to the control group (receiving only iron fortificants) highlighting that the addition of prebiotics appeared to increase absorption of iron among anemic women.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
This study was conducted according to the guidelines from the Declaration of Helsinki. All procedures involving human subjects/patients were approved by the Institutional Review Committee (IRC) for Biomedical Research of the University of Veterinary and Animal Sciences, Lahore (Reference No. 037/IRC/BMR). Moreover, informed written consent was taken from all the study subjects prior to recruiting them to the RCT. The trial was also registered at clinicaltrials.gov with ID number “NCT03894449” (https://clinicaltrials.gov/ct2/show/NCT03894449).
Author contributions
AA, WA, RA, and UF conceptualized the study. SZ, RA, and AA performed the data collection and analysis. WA, JA, HS, and AA wrote the initial manuscript. SA, MG, and JA proofread the manuscript. SI did the final editing. All authors contributed to the article and approved the submitted version.
Funding
This study was funded by the Higher Education Commission, Pakistan (Grant/Award Number: 315-7289-2BS3-087).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2022.1028956/full#supplementary-material
References
1. Lal A. Iron in health and disease: an update. Indian J Pediatr. (2020) 87:58–65. doi: 10.1007/s12098-019-03054-8
2. Pasricha S-R, Tye-Din J, Muckenthaler MU, Swinkels DW. Iron deficiency. Lancet. (2021) 397:233–48. doi: 10.1016/S0140-6736(20)32594-0
3. Sunuwar DR, Singh DR, Chaudhary NK, Pradhan PMS, Rai P, Tiwari K. Prevalence and factors associated with anemia among women of reproductive age in seven South and Southeast Asian countries: evidence from nationally representative surveys. PLoS One. (2020) 15:e0236449. doi: 10.1371/journal.pone.0236449
4. Watanabe A, Seki Y, Haruta H, Kikkawa E, Kasama K. Maternal impacts and perinatal outcomes after three types of bariatric surgery at a single institution. Arch Gynecol Obstet. (2019) 300:145–52. doi: 10.1007/s00404-019-05195-9
5. Fisher AL, Nemeth E. Iron homeostasis during pregnancy. Am J Clin Nutr. (2017) 106:1567S–74S. doi: 10.3945/ajcn.117.155812
6. Who Global Anaemia Estimates. Anaemia in women and children. (2021). Available online: https://www.who.int/data/gho/data/themes/topics/anaemia_in_women_and_children (accessed February 18, 2022).
7. World Health Organization. Global nutrition targets 2025: anaemia policy brief. Geneva: Google Scholar (2014).
8. Owais A, Merritt C, Lee C, Bhutta ZA. Anemia among women of reproductive age: an overview of global burden, trends, determinants, and drivers of progress in low-and middle-income countries. Nutrients. (2021) 13:2745. doi: 10.3390/nu13082745
9. Sundberg M. Iron bioavailability and pro-and prebiotics. Uppsala: SLU, Dept. of Food Science (2011).
10. Ahmad AMR, Farooq U, Anees M, Anis RA, Rashid S, Ahmed W. Co-administration of inulin and iron fortificants improves iron deficiency biomarkers in female sprague dawley rats. Food Sci Nutr. (2022) 10:2141. doi: 10.1002/fsn3.2337
11. Scholz-Ahrens KE, Schaafsma G, van den Heuvel EG, Schrezenmeir J. Effects of prebiotics on mineral metabolism. Am J Clin Nutr. (2001) 73:459s–64s. doi: 10.1093/ajcn/73.2.459s
12. Kobayashi Y, Ohbuchi T, Fukuda T, Wakasugi E, Yasui R, Hamada M, et al. Acidic xylooligosaccharide preserves hepatic iron storage level in adult female rats fed a low-iron diet. J Nutr Sci Vitaminol. (2011) 57:292–7. doi: 10.3177/jnsv.57.292
13. Rusu IG, Suharoschi R, Vodnar DC, Pop CR, Socaci SA, Vulturar R, et al. Iron supplementation influence on the gut microbiota and probiotic intake effect in iron deficiency—a literature-based review. Nutrients. (2020) 12:1993. doi: 10.3390/nu12071993
14. Bougle D, Vaghefi-Vaezzadeh N, Roland N, Bouvard G, Arhan P, Bureau F, et al. Influence of short-chain fatty acids on iron absorption by proximal colon. Scand J. Gastroenterol. (2002) 37:1008–11. doi: 10.1080/003655202320378176
15. Unicef. National nutrition survey 2018. Pakistan: Islamabad Government of Pakistan and UNICEF (2018).
16. Al Haj M, Kazzam E, Nagelkerke N, Nyberg F, Nicholls GM, Adem A. Effect of dehydration in the presence and absence of the angiotensin receptor blocker losartan on blood constituents in the camel. J Med Sci. (2011) 4:73–8. doi: 10.2174/1996327001104020073
17. Wojciak RW, Mojs E, Stanislawska-Kubiak M, Samborski W. The serum zinc, copper, iron, and chromium concentrations in epileptic children. Epilepsy Res. (2013) 104:40–4. doi: 10.1016/j.eplepsyres.2012.09.009
18. Kazuaki S, Yoshihiro N, Chiyo H, Hisahide N. Early diagnosis of health damage in arc welders based on their serum ferritin level. Open J Int Med. (2011) 2011:2162–5980.
19. Al-Buhairan AM, Oluboyede OA. Determination of serum iron, total iron-binding capacity and serum ferritin in healthy saudi adults. Ann Saudi Med. (2001) 21:100–3. doi: 10.5144/0256-4947.2001.100
20. Daniel WW, Cross CL. Biostatistics: a foundation for analysis in the health sciences. Hoboken, NJ: Wiley (2018).
21. Ferrara M, Coppola L, Coppola A, Capozzi L. Iron deficiency in childhood and adolescence: retrospective review. Hematology. (2006) 11:183–6. doi: 10.1080/10245330600775105
22. Hershko C, Skikne B. Pathogenesis and management of iron deficiency anemia: emerging role of celiac disease, helicobacter pylori, and autoimmune gastritis, seminars in hematology. Amsterdam: Elsevier (2009). p. 339–50. doi: 10.1053/j.seminhematol.2009.06.002
23. Banhidy F, Acs N, Puho EH, Czeizel AE. Iron deficiency anemia: pregnancy outcomes with or without iron supplementation. Nutrition. (2011) 27:65–72. doi: 10.1016/j.nut.2009.12.005
24. Monga M, Walia V, Gandhi A, Chandra J, Sharma S. Effect of iron deficiency anemia on visual evoked potential of growing children. Brain Dev. (2010) 32:213–6. doi: 10.1016/j.braindev.2009.02.009
25. Tettamanti M, Lucca U, Gandini F, Recchia A, Mosconi P, Apolone G, et al. Prevalence, incidence and types of mild anemia in the elderly: the “health and anemia” population-based study. Haematologica. (2010) 95:1849. doi: 10.3324/haematol.2010.023101
26. Ahmad AMR, Ahmed W, Iqbal S, Javed M, Rashid S. Prebiotics and iron bioavailability? Unveiling the hidden association-a review. Trends Food Sci Technol. (2021) 110:584–90. doi: 10.1016/j.tifs.2021.01.085
27. Santiago P. Ferrous versus ferric oral iron formulations for the treatment of iron deficiency: a clinical overview. Sci World J. (2012) 2012:846824. doi: 10.1100/2012/846824
28. Gershoff SN, Brusis OA, Nino HV, Huber AM. Studies of the elderly in boston. I. The effects of iron fortification on moderately anemic people. Am J Clin Nutr. (1977) 30:226–34. doi: 10.1093/ajcn/30.2.226
29. Goddard AF, James MW, McIntyre AS, Scott BB. Guidelines for the management of iron deficiency anaemia. Gut. (2011) 60:1309–16. doi: 10.1136/gut.2010.228874
31. Thuy PV, Berger J, Davidsson L, Khan NC, Lam NT, Cook JD, et al. Regular consumption of NaFeEDTA-fortified fish sauce improves iron status and reduces the prevalence of anemia in anemic vietnamese women. Am J Clin Nutr. (2003) 78:284–90. doi: 10.1093/ajcn/78.2.284
32. Zhu YI, Haas JD. Response of serum transferrin receptor to iron supplementation in iron-depleted, nonanemic women. Am J Clin Nutr. (1998) 67:271–5. doi: 10.1093/ajcn/67.2.271
33. Cable RG, Brambilla D, Glynn SA, Kleinman S, Mast AE, Spencer BR, et al. Effect of iron supplementation on iron stores and total body iron after whole blood donation. Transfusion. (2016) 56:2005–12. doi: 10.1111/trf.13659
Keywords: iron deficiency anemia, prebiotics, inulin, galacto oligosaccharides, iron fortification
Citation: Iqbal S, Ahmed W, Zafar S, Farooq U, Abid J, Shah HBU, Akram S, Ghazanfar M and Ahmad AMR (2022) Effect of inulin, galacto oligosaccharides and iron fortification on iron deficiency anemia among women of reproductive age; a randomized controlled trial. Front. Nutr. 9:1028956. doi: 10.3389/fnut.2022.1028956
Received: 26 August 2022; Accepted: 03 October 2022;
Published: 14 November 2022.
Edited by:
Haohao Wu, Ocean University of China, ChinaReviewed by:
Jin Sun, Qingdao University, ChinaNurpudji Astuti Taslim, Hasanuddin University, Indonesia
Copyright © 2022 Iqbal, Ahmed, Zafar, Farooq, Abid, Shah, Akram, Ghazanfar and Ahmad. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Abdul Momin Rizwan Ahmad, YWJkdWwubW9taW5AbnVtc3Bhay5lZHUucGs=
 Sehar Iqbal1
Sehar Iqbal1 Waqas Ahmed
Waqas Ahmed Abdul Momin Rizwan Ahmad
Abdul Momin Rizwan Ahmad