
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Nutr. , 19 December 2022
Sec. Clinical Nutrition
Volume 9 - 2022 | https://doi.org/10.3389/fnut.2022.1026450
 Mohammad Nejadhosseinian1,2
Mohammad Nejadhosseinian1,2 Shirin Djalalinia3,4
Shirin Djalalinia3,4 Hoda Haerian2
Hoda Haerian2 Majid Alikhani2
Majid Alikhani2 Asieh Mansour5
Asieh Mansour5 Amir-Hossein Mousavian6,7
Amir-Hossein Mousavian6,7 Heydar Ali Mardani-Fard8
Heydar Ali Mardani-Fard8 Amir Kasaeian6,7,9*
Amir Kasaeian6,7,9* Seyedeh Tahereh Faezi2*
Seyedeh Tahereh Faezi2*Objective: Knee osteoarthritis (KOA) is one of the growing health problems with a considerable burden. With recent research on the possible effectiveness of antioxidants in the remission of KOA symptoms, a systematic review and meta-analysis was required to confirm this hypothesis.
Design: Literature studies were searched on the most comprehensive databases such as PubMed, International Scientific Indexing, and Scopus, with no language and time restrictions. On 17 July 2021, a search strategy was developed based on the roots of “osteoarthritis (OA)” and “antioxidants,” with no time or language limitations. As the primary outcome, pain was evaluated based on all indicators for evaluating pain [e.g., Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) pain scores, the visual analog scale (VAS), and the numerical rating scale (NRS)]. The symptoms and functions of KOA and quality of life (QOL) were also considered as secondary outcomes, each of which was measured and reported by the corresponding instrument in the studies. To measure the changes in pain, symptoms, and functions of participants, we included randomized controlled trials with a placebo control or other medical therapeutic interventions. Publication bias was assessed using Begg's funnel plot and Egger's regression test, which was deemed to be statistically significant at 0.1, and the results were checked by the trim-and-fill test.
Results: After refinement, data were extracted from 31 documents from 7,698 primary searched papers. Using the VAS as a reliable psychometric measuring instrument, the present study revealed that a significant difference in the characteristics of disease-related symptoms of patients with KOA was reached after antioxidant therapy (standardized mean difference (SMD): 0.467, 95% confidence interval (CI): 0.303–0.632, p < 0.0001). The results reported by WOMAC confirmed no significant difference in the combined score, difficulty score, pain score, and stiffness score.
Conclusion: As the first comprehensive systematic review of the association between antioxidant supplementation and KOA, this study showed that antioxidants can decrease disease-related symptoms in patients with KOA. The results can be useful for health policy decisions and future related studies.
Systematic review registration: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42022351060, identifier: CRD42022351060.
Knee osteoarthritis (KOA) is one of the most common causes of pain, loss of function, and disability, which progressively affects millions of people worldwide and makes their lives difficult (1, 2). This condition caused the protective cartilage that protects the ends of the bone from impact to break down. Evidence suggests a higher incidence of knee degeneration among the elderly and women (1, 3, 4).
According to recent estimates, as a consequence of aging and many other predisposing factors, the global burden of KOA becomes a major health problem. The increasing prevalence rate, economic burden, and adverse health outcomes on the quality of life (QOL) make osteoarthritis (OA) an urgent public health issue (2, 5). Considering the importance and priority of the problem, prevention approaches must be planned and followed as the first line of intervention. However, the successful management and control of the disease will depend on the recognition of early symptoms, accurate diagnosis, and appropriate treatment (5–7).
Much of the research is based on pharmacological, mechanical, and surgical interventions. Therapeutic approaches focus primarily on controlling pain and other symptoms, improving functional abilities, and reducing disease progression (3, 4, 8, 9).
Most treatments also include a combination of regular physical activities, weight control, joint protection, and medication. In severe cases where one of the abovementioned treatments is not successful, surgical methods are recommended (2, 10).
From pharmacological approaches, analgesics and anti-inflammatory compounds were used to reduce inflammation and pain. Antioxidants have been discussed as an alternative treatment in elderly people who are at increased risk of OA and tend to have a poor physical function (3–5). Recent research suggests that some therapeutic approaches have focused on the use of antioxidants to prevent the damage caused by OA to the cartilage. Conflicting evidence has been provided on the effects of antioxidants on disease mortality and morbidity, which still requires further study. Antioxidant supplements should be considered medicinal products and should be adequately evaluated before marketing (3, 6, 11). These studies have been carried out by changing the diet to increase the proportion of foods containing antioxidants or by prescribing antioxidant pharmaceutical forms and supplements (10). Several studies reported improvement and increase in muscle strength, the promotion of physical functions, and a decreased risk of disease progression. They may be rooted in the free radical theory of aging, which hypothesizes that oxygen-derived free radicals are responsible for age-related damages at the cellular and tissue levels (3, 12–14).
In light of this problem's importance, we need more evidence to assist policymakers and clinicians in designing effective interventions. To provide reliable high-level evidence, the present study was designed and conducted as a comprehensive systematic review to assess the effects of antioxidants on KOA.
With an aim to assess the association of antioxidants and OA symptoms, we followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (15). The review protocol was registered in the PROSPERO international prospective register of systematic reviews (CRD42022351060).
Using the Medical Subject Headings (MeSH) terms and Emtree, related published scientific papers and peer review documents were systemically searched on PubMed, ISI/WOS, and Scopus.
On 17 July 2021, a search strategy was developed based on the roots of “OA” and “antioxidants” with no time or language limitations. In case of encountering any non-English articles, we consulted with the Department of Foreign Languages at our university (Tehran University of Medical Sciences) and asked the help of relevant translation experts. Fortunately, no non-English articles entered the final stage of assessment. The results were limited to human subjects. Additional searches were considered for the reference list of studies (Supplementary Table S1).
The searched records were exported to the Endnote software. After three steps of relevance assessment based on titles, abstracts, and full texts, before data extraction, relevant papers were evaluated for their quality. Two studies independently followed all processes. A third reviewer resolved any disagreements between the reviewers.
In the present study, only randomized controlled trials (RCTs) were included.
The results were presented in a PRISMA flowchart according to the PRISMA guideline (16).
The specifically targeted points as inclusion criteria were as follows:
(1) Targeted patients with KOA.
(2) Conducted therapeutic intervention with any kind of antioxidant compared to the placebo or any other medical treatments.
(3) Addressed primary or secondary targeted outcomes.
All indicators for evaluating pain [e.g., Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) pain scores, the visual analog scale (VAS), and the numerical rating scale (NRS)].
Indicators for evaluating the symptoms and functions of KOA as a whole (e.g., indicators for WOMAC total scores and Lequesne's index) and patient QOL such as the Euro-QoL instrument (EQ5D) and the 36-Item Short-Form Health Survey (SF-36).
If there was more than one paper from the specific unique research, more complete data were considered. Papers with duplicate citations were deleted.
After three steps of relevance refinement through the Endnote software, including titles, abstracts, and full-text review, using the PRISMA 2020 checklist and Cochrane collaborators tools for assessing the risk of bias in randomized trials (Supplementary Table S2) (17). The quality assessment of the studies was followed by two independent researchers.
Using a predefined checklist, data were extracted from the qualified eligible papers for citation, publication year, study year, the place of study, the type of study, population, total sample size, mean age, the outcomes of interest in pain, symptoms and functions of participants, the type of measure, the results of measures, and other information.
Data extraction and quality assessment were carried out independently by two researchers (kappa statistic for agreement for quality assessment; 0.94). The probable discrepancy was resolved by referring to the opinion of a third expert.
We chose the standardized mean difference (SMD)/Cohen's d as the effect size, and eligible studies were meta-analyzed (18).
For the two groups (treatment and placebo), the SMD was calculated using the mean differences (endpoint from baseline) and standard deviations (SD) according to the following formula (19).
A random-effect model was applied if the level of significance of the Q-statistic for heterogeneity was set at 0.1 or (I2 < 50% considered as a fixed effect and I2 ≥ 50% considered as a random effect) (20).
In other cases, a fixed-effect model was used (21). All analyses were done using Stata version 16 (22). A p-value ≤ 0.05 was regarded as statistically significant.
The Cochrane Q test and I2 statistic were applied to measure the heterogeneity between the studies (23). The I2 statistic was used to quantify the degree of heterogeneity between the studies, with I2 values of 25, 50, and 75% being considered to correspond to low, medium, and high levels of heterogeneity, respectively. The result of the Q-test was considered to be statistically significant at 0.1. Publication bias was assessed using Begg's funnel plot and Egger's regression test, which was deemed statistically significant at 0.1, and the results were checked using the trim-and-fill test.
To assess the potential sources of heterogeneity in the results, the Galbraith plot and sensitivity analysis were carried out. Galbraith plot analysis was performed to detect the outliers as the potential sources of heterogeneity (24). In addition, sensitivity analysis, which eliminates any single study at a time, was used to assess the effect of each study on the overall results of a meta-analysis to show the stability and robustness of the results (25).
The present study was approved by the ethics committee of Tehran University of Medical Science (code: IR.TUMS.DDRI.REC.1400.034). All studies included in our review would be cited in all reports and all publications extracted from our study. For further information required, the corresponding authors were contacted.
Through a comprehensive search on the targeted databases, we found 7,698 papers. Of these, 1,033 papers were duplicated in different search sources and were excluded. The remaining 6,665 papers were screened according to the relevance of their titles and abstracts. After these refinement steps, 69 papers were left for a full-text review. After relevance assessment and quality control, data were extracted from 31 documents (43 comparison bands) (Figure 1). The kappa statistic for the agreement of processes, between the two independent research experts, from the search study development to data extraction and analysis was 0.94, which showed a good agreement.
We included 43 trial studies and pooled the results by including 6,605 participants in the data analysis. Of these, 10 studies were designed as multicenter RCTs.
Ten studies were designed as multi-center RCTs as the most significant number of studies performed; 10 articles were carried out in China, 6 in Iran, and 2 in Japan, Italy, France, the US, and Belgium. From other countries, only one paper was included in the study.
The first analytic studies were conducted in 1998, and the last paper was from 2020. Considering a variety of methodological approaches to the included studies, our systematic review yielded different measures of the association including hazard ratio (HR), odds ratio (OR), and relative risk (RR). Six papers did not mention the sex distribution of their participants, and two papers did not provide information on the age of participants. The characteristics of the included studies are presented in Table 1.
A random-effect meta-analysis of 27 trials and the pooled results obtained from 2,394 participants (1,193 cases and 1,201 controls) showed no significant difference in the VAS after antioxidant therapy (SMD: 0.045, 95% confidence interval (CI): (−0.78–0.87), p = 0.91) (Supplementary Figure S1). There was high heterogeneity between studies (p < 0.0001; I2 = 98.78%). Egger's test provided no evidence of publication bias (Egger's regression intercept: −3.13, 95%CI: (−10.90–4.64), p = 0.414). Begg's funnel plot of standard error (SE) vs. effect size (SMD) was asymmetric (Supplementary Figure S2). The Galbraith plot demonstrated that 15 studies were outside the 95% CI, which means they are outliers (Supplementary Figure S3).
After eliminating outlier studies, a fixed-effect meta-analysis of 12 trials and the pooled results obtained by including 910 participants (455 cases and 455 controls) showed no significant difference in the VAS after antioxidant therapy (SMD: 0.467, 95%CI: (0.303–0.632), p < 0.0001) (Figure 2). There was low heterogeneity between the studies (p = 0.161; I2 = 30.40%), which is not statistically significant. Egger's test provided no evidence of any publication bias (Egger's regression intercept: 0.802, 95%CI: (−2.25 3.85), p = 0.571). Begg's funnel plot of SE vs. effect size (SMD) was symmetrical, and the trim-and-fill correction also suggested no potentially missing studies on any side of the funnel plot (Supplementary Figure S4).
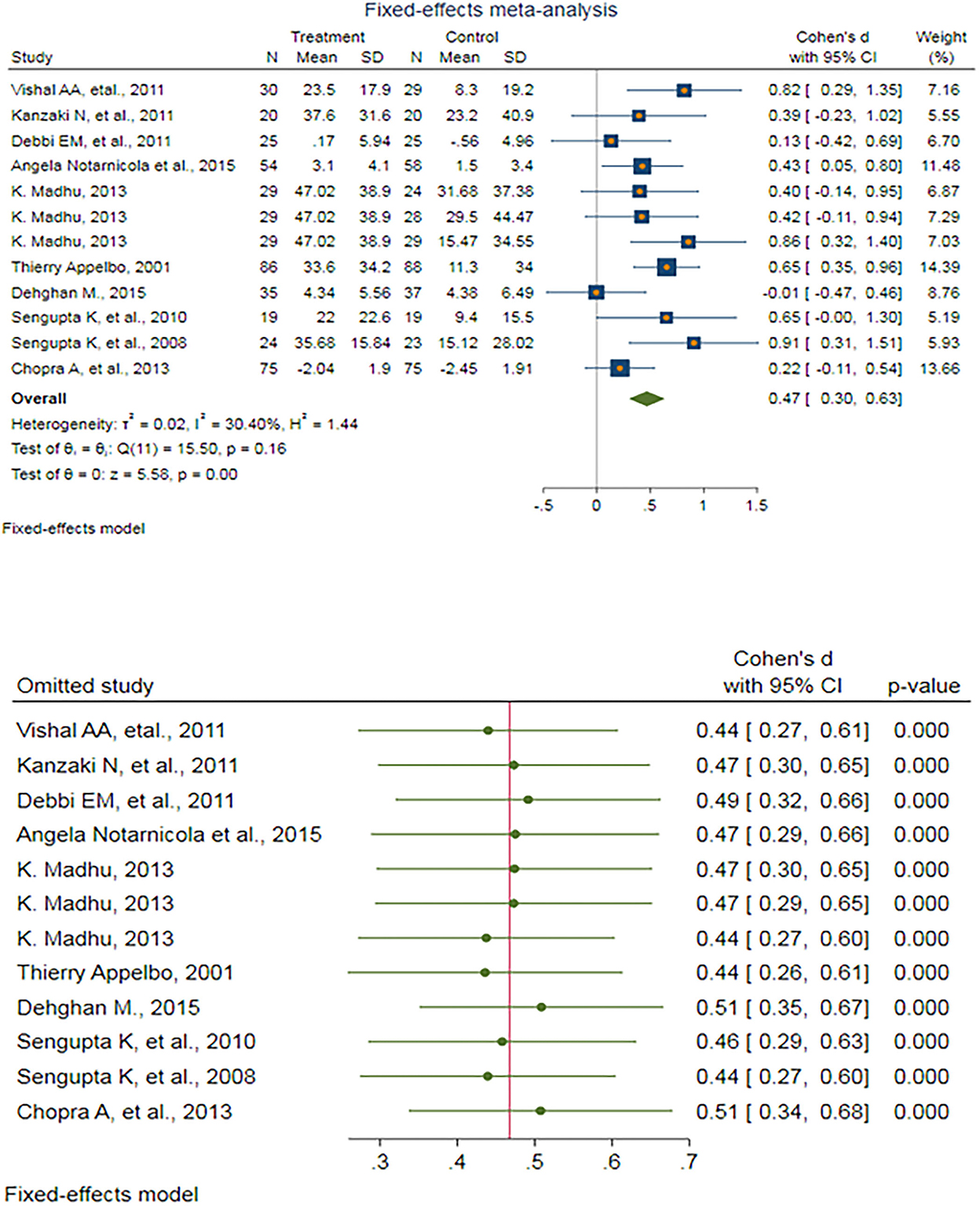
Figure 2. (UP) A forest plot for the association between antioxidants and the outcomes of interest in osteoarthritis (OA) based on the visual analog scale (VAS). (DOWN) Forest plot of sensitivity analysis for the association between antioxidants and the outcomes of interest in OA based on the VAS.
Sensitivity analyses were performed to test the robustness of the observed association. The elimination of any single study at a time from the meta-analysis ranged from 0.44 (95%CI: 0.26–0.61) to 0.51 (95%CI: 0.35–0.67), which indicated a high robustness of the pooled estimates of prevalence (Figure 2).
A random-effect meta-analysis of 22 trials and the pooled results obtained by including 2,378 participants (1,204 cases and 1,174 controls) showed no significant difference in the WOMAC combined pain score after antioxidant therapy (SMD: 0.117, 95%CI: (−0.319–0.554), p = 0.60) (Supplementary Figure S5). There was high heterogeneity between the studies (p < 0.0001; I2 = 96.18%). Egger's test did not provide any evidence of publication bias (Egger's regression intercept: −0.55, 95%CI: (−5.85–4.75), p = 0.831). Begg's funnel plot of SE vs. effect size (SMD) was asymmetrical (Supplementary Figure S6).
The Galbraith plot revealed that eight studies were outside the 95% CI, which means that they are outliers (Supplementary Figure S7).
After removing outlier studies, a fixed-effect meta-analysis of 14 trials and the pooled results obtained by including 1,762 participants (897 cases and 865 controls) showed no significant difference in the WOMAC combined pain score after antioxidant therapy (SMD: 0.061, 95%CI: −0.033–0.154) p = 0.203) (Figure 3). There was no heterogeneity between the studies (p = 0.43; I2 = 0.00%). Egger's test provided no evidence of publication bias (Egger's regression intercept: 0.81, 95%CI: (−1.08–2.70), p = 0.369). Begg's funnel plot of SE vs. effect size (SMD) was asymmetrical (Supplementary Figure S8). The trim-and-fill correction suggested two potentially missing studies on the left side of the funnel plot (Supplementary Figure S9). Imputation for these potentially missing studies yielded an effect size of 0.025 (95%CI: −0.080–0.130), which was not statistically significant.
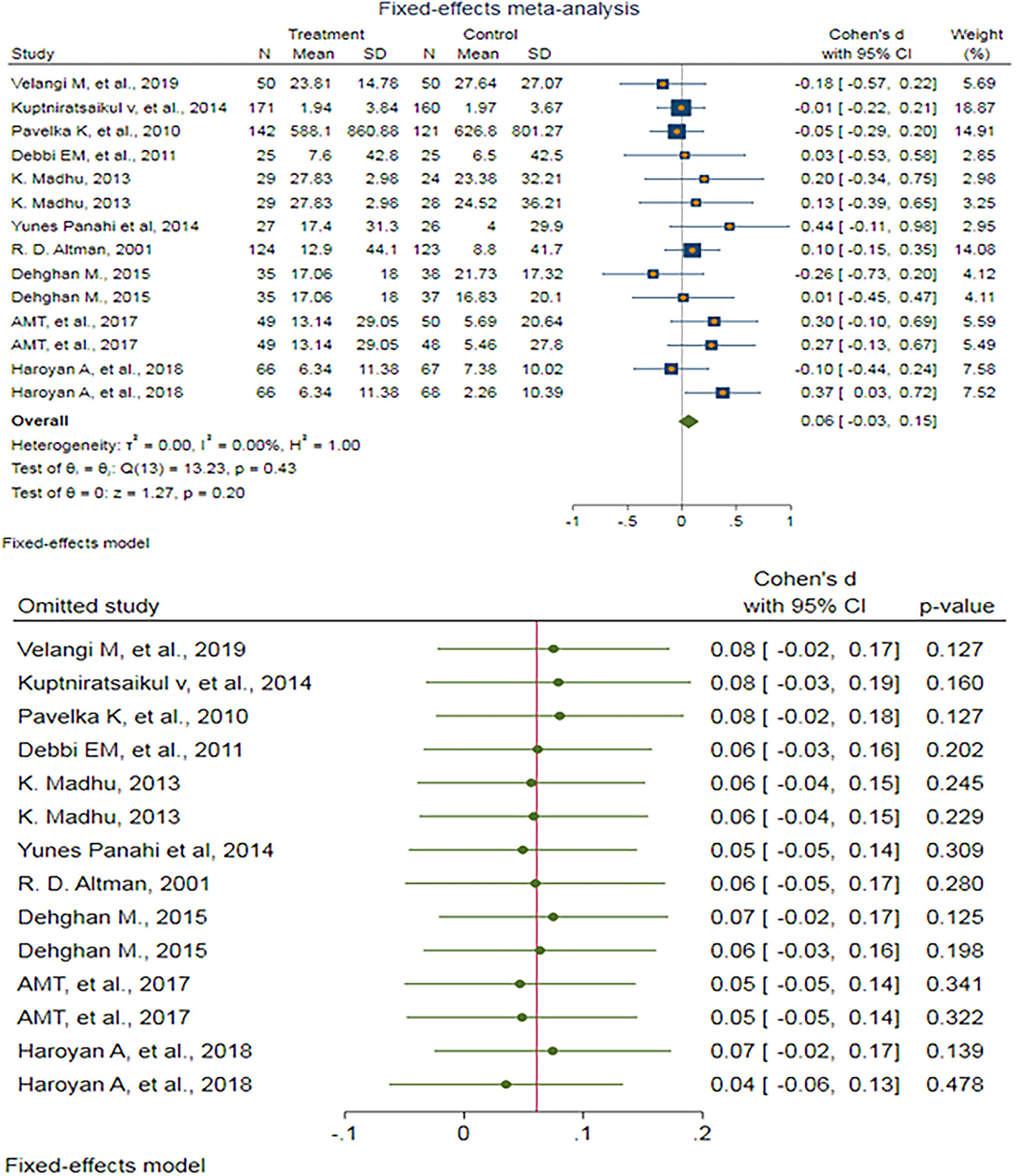
Figure 3. (UP) A forest plot for the association between antioxidants and the outcomes of interest in OA based on the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) combined pain score. (DOWN) Forest plot of sensitivity analysis for the association between antioxidants and the outcomes of interest in OA based on the WOMAC combined pain score.
Sensitivity analyses were done to test the robustness of the observed association. The elimination of any single study at a time from the meta-analysis ranged from 0.04 (95%CI: −0.06–0.13) to 0.08 (95%CI: −0.02–0.18), indicating high robustness of the pooled estimates of prevalence (Figure 3).
A random-effect meta-analysis of 25 trials and the pooled results obtained by including 2,547 participants (1,285 cases and 1,262 controls) showed no significant difference in the WOMAC difficulty pain score after antioxidant therapy (SMD: 0.296, 95%CI: −0.277–0.869), p = 0.31) (Supplementary Figure S10). There was non-ignorable heterogeneity between the studies (p < 0.0001; I2 = 97.94%). Egger's test provided no evidence of publication bias (Egger's regression intercept: 1.89, 95%CI: (−1.88–5.67), p = 0.310). Begg's funnel plot of SE vs. effect size (SMD) was asymmetrical (Supplementary Figure S11).
The Galbraith plot revealed that 11 studies were outside the 95%CI, which means they are outliers (Supplementary Figure S12).
After excluding outlier studies, a fixed-effect meta-analysis of 14 trials and the pooled results obtained by including 1,934 participants (976 cases and 958 controls) showed no significant difference in the WOMAC difficulty pain score after antioxidant therapy (SMD: 0.079, 95%CI: −0.012–0.170), p = 0.09) (Figure 4). There was ignorable heterogeneity between the studies (p = 0.197; I2 = 2.13%). Egger's test provided no evidence of publication bias (Egger's regression intercept: 1.16 (95%CI: −0.79–3.12), p = 0.219). Begg's funnel plot of SE vs. effect size (SMD) was a bit asymmetric (Supplementary Figure S13). The trim-and-fill correction suggested one potentially missing study on the left side of the funnel plot (Supplementary Figure S14). Imputation for this potentially missing study yielded an effect size of 0.068 (95%CI: −0.024–0.160), which was not statistically significant.
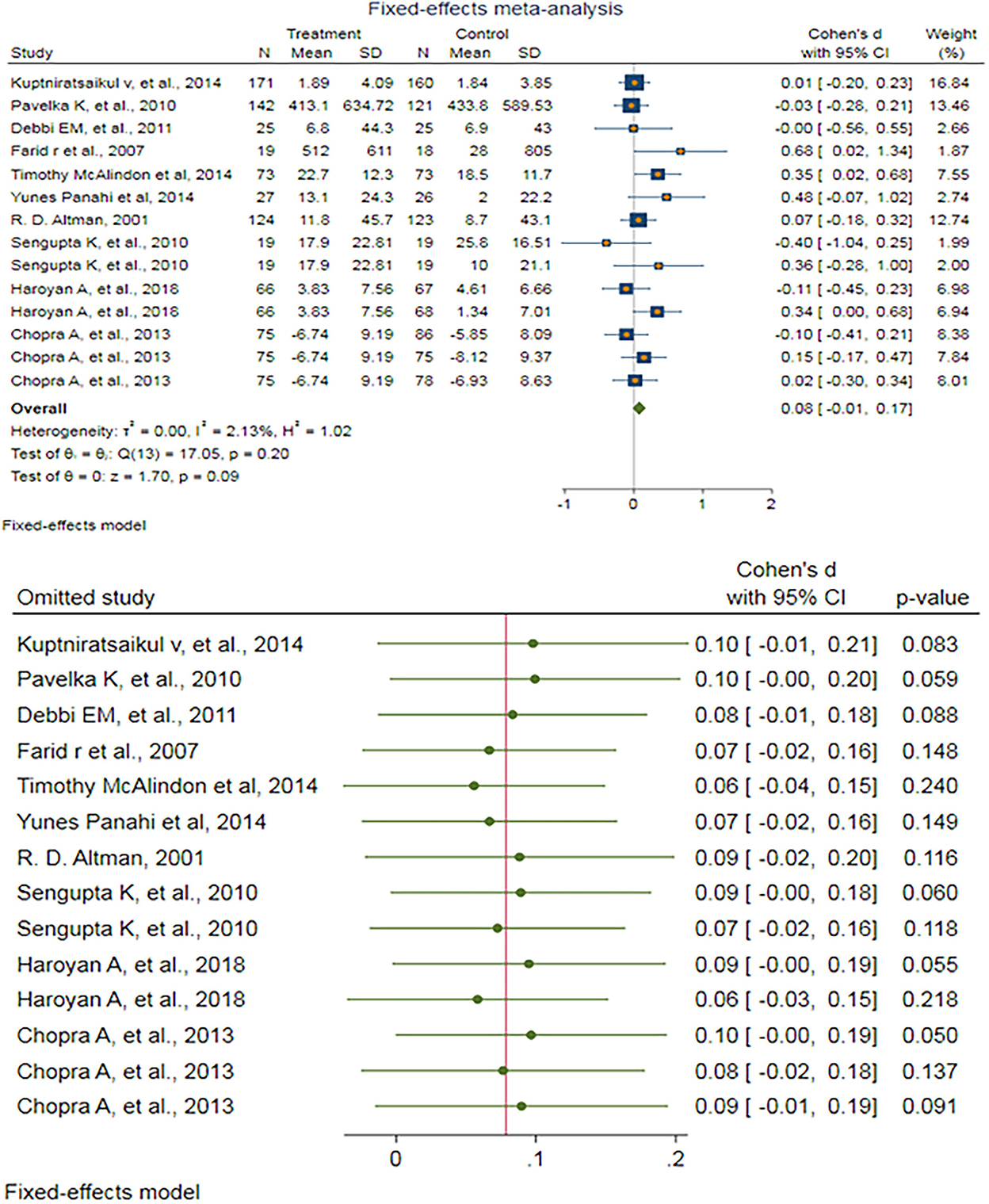
Figure 4. (UP) A forest plot for the association between antioxidants and the outcomes of interest in OA based on the WOMAC difficulty pain score. (DOWN) Forest plot of sensitivity analysis for the association between antioxidants and the outcomes of interest in OA based on the WOMAC difficulty pain score.
Sensitivity analyses were done to test the robustness of the observed association. The elimination of any single study at a time from the meta-analysis ranged from 0.06 (95%CI: −0.04–0.15) to 0.10 (95%CI: −0.01–0.21), indicating high robustness of the pooled estimates of prevalence (Figure 4).
A random-effect meta-analysis of 28 trials and the pooled results obtained by including 2,840 participants (1,427 cases and 1,413 controls) showed no significant difference in the WOMAC pain score after antioxidant therapy ((SMD: 0.297, 95%CI: −0.125–0.718), p = 0.17) (Supplementary Figure S15). There was high heterogeneity between the studies (p < 0.0001; I2 = 96.61%). Egger's test provided no evidence of publication bias (Egger's regression intercept: 2.09 (95%CI: −1.44–5.63), p = 0.235). Begg's funnel plot of SE vs. effect size (SMD) was asymmetrical (Supplementary Figure S16).
The Galbraith plot revealed that 10 studies were outside the 95%CI, which means they are outliers (Supplementary Figure S17).
A fixed-effect meta-analysis of 18 trials and the pooled results obtained by including 2,147 participants (1,081 cases and 1,066 controls) showed no significant difference in the WOMAC pain score after antioxidant therapy (SMD: 0.084 (95%CI: −0.024–0.192), p = 0.127) (Figure 5). There was low heterogeneity between the studies (p = 0.067; I2 =30.19%). Egger's test provided no evidence of publication bias (Egger's regression intercept: 1.55 (95%CI:−0.08–3.18), p = 0.061). Begg's funnel plot of SE vs. effect size (SMD) was asymmetrical (Supplementary Figure S18). The trim-and-fill correction suggested three potentially missing studies on the left side of the funnel plot (Supplementary Figure S19). Imputation for these potentially missing studies yielded an effect size of 0.044 (95%CI: −0.067–0.155), which was not statistically significant.
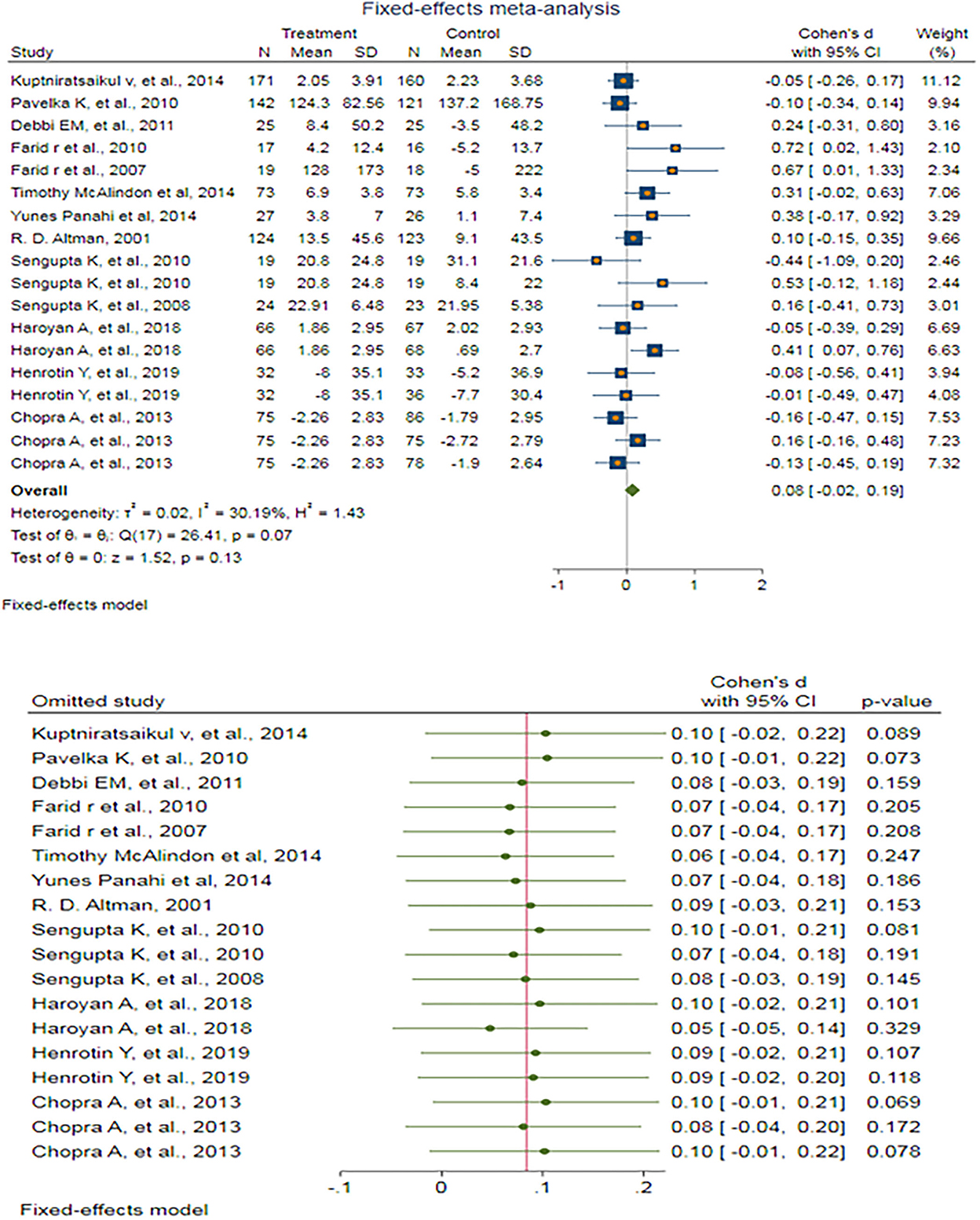
Figure 5. (UP) A forest plot for the association between antioxidants and the outcomes of interest in OA based on the WOMAC pain score. (DOWN) Forest plot of sensitivity analysis for the association between antioxidants and the outcomes of interest of OA based on the WOMAC pain score.
Sensitivity analyses were done to test the robustness of the observed association. The elimination of any single study at a time from the meta-analysis ranged from 0.05 (95%CI: −0.05–0.14) to 0.10 (95%CI: −0.02–0.22), indicating high robustness of the pooled estimates of prevalence (Figure 5).
A random-effect meta-analysis of 25 trials and the pooled results obtained by including 2,388 participants (1,208 cases and 1,180 controls) showed no significant decrease in the WOMAC stiffness score after antioxidant therapy (SMD: 0.128 (95%CI: −0.168–0.424), p = 0.40) (Supplementary Figure S20). There was high heterogeneity between the studies (p < 0.0001; I2 = 91.79%). Egger's test provided no evidence of publication bias (Egger's regression intercept: 0.64 (95%CI: −2.58–3.87), p = 0.685). Begg's funnel plot of SE vs. effect size (SMD) was symmetrical to some extent (Supplementary Figure S21).
The Galbraith plot (Supplementary Figure S22) revealed that seven studies were outside the 95%CI, which means that they are outliers.
A fixed-effect meta-analysis of 18 trials and the pooled results obtained by including 1,894 participants (962 cases and 932 controls) showed no significant decrease in the WOMAC stiffness score after antioxidant therapy (SMD: 0.074 (95%CI: −0.024–0.172), p = 0.140) (Figure 6). There was very low and ignorable heterogeneity between the studies (p = 0.332; I2 = 9.55%). Egger's test provided no evidence of publication bias (Egger's regression intercept: 1.27 (95%CI: −0.06–2.60), p = 0.06). Begg's funnel plot of SE vs. effect size (SMD) was asymmetrical (Supplementary Figure S23). The trim-and-fill correction suggested five potentially missing studies on any side of the funnel plot (Supplementary Figure S24). Imputation of these potentially missing studies yielded an effect size of −0.001 (95%CI: −0.116–0.113), which was also not statistically significant.
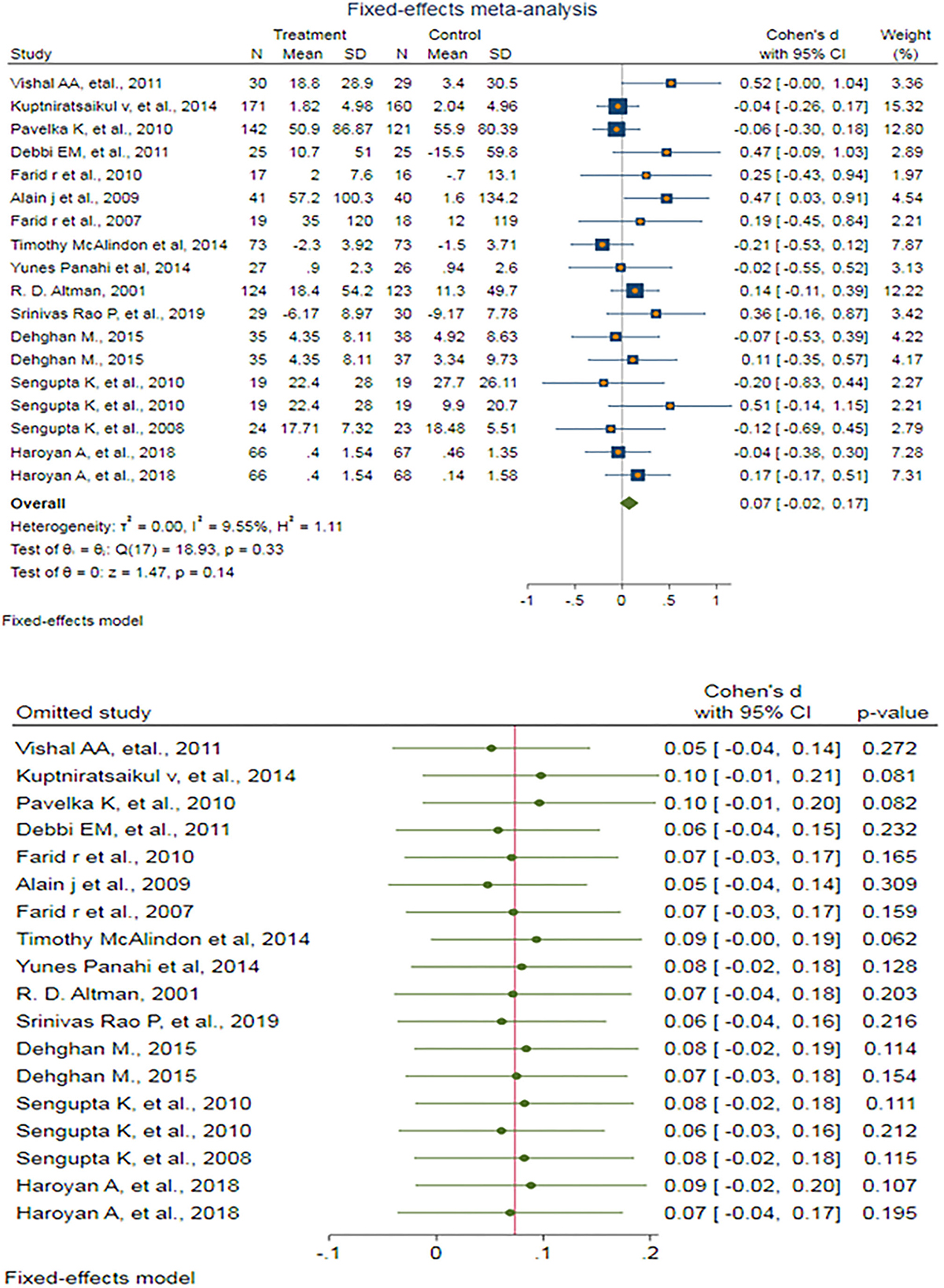
Figure 6. (UP) A forest plot for the association between antioxidants and the outcomes of interest in OA based on the WOMAC stiffness score. (DOWN) Forest plot of sensitivity analysis for the association between antioxidants and the outcomes of interest in OA based on the WOMAC stiffness score.
Sensitivity analyses were done to test the robustness of the observed association. The elimination of any single study at a time from the meta-analysis ranged from 0.05 (95% CI: −0.04–0.14) to 0.10 (95%CI: −0.01–0.21), indicating high robustness of the pooled estimates of prevalence (Figure 6).
Our systematic review, as the first comprehensive study of the effects of antioxidants on KOA, provides several major findings. We included 43 trial studies and the pooled results obtained by including 6,605 participants in the data analysis. Of these, 10 studies were designed as multicenter RCTs. A number of substances have been studied in several studies to treat or relieve the signs and symptoms of KOA. Some substances, such as glucosamine, coconut oil, vitamin D, ginger, Boswellia, and vitamin E, were prescribed in the form of compounds or as supplements along with other treatments.
Our findings are in accordance with those of other published RCTs or systematic reviews assessing the role of nutritional compounds or supplements for the prevention and reduction of the symptoms of KOA (52, 53). To compare the intervention effects of different types of antioxidants on the outcomes of interest in OA, we detected significant heterogeneity in the qualified included trials. According to the results detected using VASs as reliable psychometric measuring instruments, the present study revealed a significant difference in the characteristics of disease-related symptoms of patients with KOA who were achieved after antioxidant therapy (p < 0.0001). It is worth mentioning that the results reported by the WOMAC confirm no significant difference in the combined score, difficulty score, pain score, and stiffness score. Overall, according to the analysis of the results of standard indicators that measure the clinical symptoms of patients with KOA, it seems that the effectiveness of these compounds mainly prevents, reduces, and relieves symptoms, and studies have shown little success in the treatment and complete relief of clinical symptoms.
In addition to our experience, previous attempts have studied the difficulties and limitations of the problem. Lack of standards for many prescribed compounds, the possibility of the interference of many known and unknown factors on the type and severity of patients' symptoms, scattering of measuring instruments, self-reported baseline data and intervention effects, poorly conducted studies, lack of uniformity in the definition and diagnosis of diseases, and the deficiencies of some papers in the detail of the studies have been mentioned as some determining criteria in the promotion of scientific evidence used in making policies and clinical decisions (1, 3, 4, 32).
The results obtained from related studies are inconsistent on the effectiveness of antioxidants in the control and management of the clinical symptoms of patients with KOA. Much related research has shown that the different components of antioxidants are effective in reducing pain, stiffness, swelling, and improving physical function compared to both placebo or other non-antioxidant interventions (12, 46, 48, 54). The antioxidant supplements with the most evidence of benefit for pain management and function in patients with KOA were based on curcumin, avocado, soya bean, and vitamins D and E (3, 4, 36, 40, 42, 55). Boswellia and some herbs used in Ayurvedic and Chinese medicine may also be useful (14, 26, 46).
In the studies that have addressed this problem, different results have been reported, from the lack of a statistically significant effect of some antioxidant compounds to the registration of some significant indicators in the results of valid measurement tests such as VAS and WOMAC (3, 12–14).
According to the available evidence, the differences in the results obtained from the studies, in addition to what was mentioned above about the designs, implementations, and reporting of results, may be rooted in the complex mechanisms of action of these compounds and various influencing factors (2, 8, 9).
As a possible mechanism of action, antioxidants act against reactive oxygen species, which are produced by cells within the joints and slow down or stop the oxidative damage to various macromolecules and immunomodulatory effects on T- and B-cell functions. These mechanisms have been confirmed through previous in vivo and investor studies for vitamin C, vitamin E, and carotenoids (3, 4, 8, 9).
Considering previous attempts, the present study benefits from several achievements. This report presents scientific evidence to depict an association between antioxidants and KOA outcomes. All available sources of related data search use the most comprehensive database of related and most effective systematic search approaches. Despite data dispersion and serious differences in the design and implementation of studies, an attempt was made to present the best practical format of findings using appropriate updated analysis methods based on subgroup and comparative analyses.
Throughout this research, we faced several limitations. As the main limitation, the validity and applicability of the data included in a systematic review depend on the quality of the primary studies. Moreover, the widespread heterogeneity of the searched results limits the generalization of our findings. There was high heterogeneity between the studies that limited the possibility of aggregate analysis and required the elaboration and application of special statistical and analytical techniques. Also, the study design and research interventions were widely dispersed, which were considered in the final analysis. We must select the best approach to address differences in study design and the population that may play a major source of controversy in the analysis. However, the complex and multifactorial nature of antioxidants and possibly unknown interactive mechanisms should be considered in the exploitation of results.
As a practical implication, it could be recommended to increase the intake of antioxidant vitamins in the general diet of OA at-risk groups, especially in susceptible ethnic studies. Meanwhile, the possible effects of high doses of antioxidant vitamins as dietary supplements need to be evaluated against side effects. Many subjects at high risk for KOA and its complications may benefit from a combination of antioxidant and anti-inflammatory compounds. It is also recommended to study the beneficial effects of certain combination protocols for an intervention or for reducing specific symptoms of the disease through well-defined RCTs.
To our knowledge, this is the first comprehensive systematic review of the association between antioxidant supplementation and OA outcomes. Although the results were not statistically significant, in terms of the reported changes in clinical symptoms and the simplicity and cost-effectiveness of the interventions, it could be recommended to increase the intake of antioxidant vitamins in the general diet of OA at-risk groups, especially in susceptible ethnic studies. The antioxidant supplements with the most evidence of benefit for pain relief and function in KOA were based on curcumin. The benefits of diets containing appropriate antioxidants should be assessed because they may be more economical and easier to incorporate into the lifestyle. The results could be useful for better health policy decisions and future studies in this field. They can also be used for complementary analyses in the future.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
MN, SD, and AK wrote this manuscript. SD, AK, and STF contributed to the study design and the interpretation of the results. A-HM and MN were responsible for literature review and data extraction. AK performed the whole statistical analysis. All authors read and approved the final manuscript.
We would like to thank all of our research team for their participation in and support of the project.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2022.1026450/full#supplementary-material
1. Litwic A, Edwards M, Dennison E, Cooper C. Epidemiology and burden of osteoarthritis. Br Med Bull. (2013) 105:185. doi: 10.1093/bmb/lds038
2. Canter P, Wider B, Ernst E. The antioxidant vitamins A, C, E and selenium in the treatment of arthritis: a systematic review of randomized clinical trials. Rheumatology. (2007) 46:1223–33. doi: 10.1093/rheumatology/kem116
3. Gallagher B, Tjoumakaris FP, Harwood MI, Good RP, Ciccotti MG, Freedman KB. Chondroprotection and the prevention of osteoarthritis progression of the knee: a systematic review of treatment agents. Am J Sports Med. (2015) 43:734–44. doi: 10.1177/0363546514533777
4. Grover AK, Samson SE. Benefits of antioxidant supplements for knee osteoarthritis: rationale and reality. Nutr J. (2016) 15:1. doi: 10.1186/s12937-015-0115-z
5. Hsiao AF, Lien YC, Tzeng IS, Liu CT, Chou SH, Horng YS. The efficacy of high-and low-dose curcumin in knee osteoarthritis: a systematic review and meta-analysis. Complement Ther Med. (2021) 63:102775. doi: 10.1016/j.ctim.2021.102775
6. Suantawee T, Tantavisut S, Adisakwattana S, Tanavalee A, Yuktanandana P, Anomasiri W, et al. Oxidative stress, vitamin e, and antioxidant capacity in knee osteoarthritis. J Clin Diag Res JCDR. (2013) 7:1855. doi: 10.7860/JCDR/2013/5802.3333
7. Sengupta K, Alluri KV, Satish AR, Mishra S, Golakoti T, Sarma KV, et al. A double blind, randomized, placebo controlled study of the efficacy and safety of 5-Loxin for treatment of osteoarthritis of the knee. Arthritis Res Ther. (2008) 10:R85. doi: 10.1186/ar2461
8. Atabaki M, Shariati-Sarabi Z, Tavakkol-Afshari J, Mohammadi M. Significant immunomodulatory properties of curcumin in patients with osteoarthritis) a successful clinical trial in Iran. Int Immunopharmacol. (2020) 85:106607. doi: 10.1016/j.intimp.2020.106607
9. Setti T, Arab MGL, Santos GS, Alkass N, Andrade MAP, Lana JF. The protective role of glutathione in osteoarthritis. J Clin Orthopaed Trauma. (2021) 15:145. doi: 10.1016/j.jcot.2020.09.006
10. Santos JB, Mangili LR, Mangili MR, Mangili IR, Ribas Filho D. Nutritional approach in the prevention and treatment of knee osteoarthritis: a systematic review. Int J Nutrol. (2021) 14:. doi: 10.54448/mdnt21402
11. Rahimnia AR, Panahi Y, Alishiri G, Sharafi M, Sahebkar A. Impact of supplementation with curcuminoids on systemic inflammation in patients with knee osteoarthritis: findings from a randomized double-blind placebo-controlled trial. Drug Res. (2015) 65:521–5. doi: 10.1055/s-0034-1384536
12. Kulkarni PD, Damle ND, Singh S, Yadav KS, Ghante MR, Bhaskar VH, et al. Double-blind trial of solid lipid Boswellia serrata particles (SLBSP) vs. standardized Boswellia serrata gum extract (BSE) for osteoarthritis of knee. Drug Metab Pers Ther. (2020) 35:104. doi: 10.1515/dmpt-2020-0104
13. Jacquet A, Girodet P, Pariente A, Forest K, Mallet L, Moore N. Phytalgic®, a food supplement, vs placebo in patients with osteoarthritis of the knee or hip: a randomised double-blind placebo-controlled clinical trial. Arthritis Research and Therapy. (2009) 11:2891. doi: 10.1186/ar2891
14. Italiano G, Raimondo M, Giannetti G, Gargiulo A. Benefits of a Food supplement containing “yes” boswellia serrata and bromelain for improving the quality of life in patients with osteoarthritis: a pilot study. J Alt Complement Med. (2020) 26:123–9. doi: 10.1089/acm.2019.0258
15. Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA Statement. Open Medicine. (2009) 3:e123. doi: 10.1371/journal.pmed.1000097
16. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. doi: 10.1136/bmj.n71
17. Higgins JPT, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomized trials. BMJ. (2011) 343:d5928. doi: 10.1136/bmj.d5928
19. Higgins JP, Li T, Deeks JJ. Choosing effect measures and computing estimates of effect. Cochrane Handbook Sys Rev Intervent. (2019) 3:143–76. doi: 10.1002/9781119536604.ch6
20. DerSimonian R, Laird N. Meta-analysis in clinical trials. Cont Clin Trials. (1986) 7:177–88. doi: 10.1016/0197-2456(86)90046-2
21. Whitehead A, Whitehead J. A general parametric approach to the meta-analysis of randomized clinical trials. Stat Med. (1991) 10:1665–77. doi: 10.1002/sim.4780101105
23. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. Bmj. (2003) 327:557–60. doi: 10.1136/bmj.327.7414.557
24. Galbraith R. A note on graphical presentation of estimated odds ratios from several clinical trials. Stat Med. (1988) 7:889–94. doi: 10.1002/sim.4780070807
25. Tobias A. Assessing the influence of a single study in the meta-analysis estimate. Stata Tech Bull. (1999) 47:15–7.
26. Bolognesi G, Belcaro G, Feragalli B, Cornelli U, Cotellese R, Hu S, et al. Movardol® (N-acetylglucosamine, Boswellia serrata, ginger) supplementation in the management of knee osteoarthritis: preliminary results from a 6-month registry study. Eur Rev Med Pharmacol Sci. (2016) 20:5198–204.
27. Srivastava S, Saksena AK, Khattri S, Kumar S, Dagur RS. Curcuma longa extract reduces inflammatory and oxidative stress biomarkers in osteoarthritis of knee: a four-month, double-blind, randomized, placebo-controlled trial. Inflammopharmacology. (2016) 24:377–88. doi: 10.1007/s10787-016-0289-9
28. Vishal AA, Mishra A, Raychaudhuri SP. A double blind, randomized, placebo controlled clinical study evaluates the early efficacy of Aflapin®in subjects with osteoarthritis of knee. Int J Med Sci. (2011) 8:615. doi: 10.7150/ijms.8.615
29. Kanzaki N, Saito K, Maeda A, Kitagawa Y, Kiso Y, Watanabe K, et al. Effect of a dietary supplement containing glucosamine hydrochloride, chondroitin sulfate and quercetin glycosides on symptomatic knee osteoarthritis: a randomized, double-blind, placebo-controlled study. J Sci Food Agricult. (2012) 92:862–9. doi: 10.1002/jsfa.4660
30. Velangi M, Mandalika S, Shukla S, Pradhan V. Effect of vitamin D3 and virgin coconut oil on cartilage degeneration, inflammation and functional abilities in early knee osteoarthritis. Funct Foods Health Dis. (2019) 9:662–77. doi: 10.31989/ffhd.v9i10.653
31. Kuptniratsaikul V, Dajpratham P, Taechaarpornkul W, Buntragulpoontawee M, Lukkanapichonchut P, Chootip C, et al. Efficacy and safety of Curcuma domestica extracts compared with ibuprofen in patients with knee osteoarthritis: a multicenter study. Clin Intervent Aging. (2014) 9:451. doi: 10.2147/CIA.S58535
32. Pavelka K, Coste P, Géher P, Krejci G. Efficacy and safety of piascledine 300 versus chondroitin sulfate in a 6 months treatment plus 2 months observation in patients with osteoarthritis of the knee. Clin Rheumatol. (2010) 29:659–70. doi: 10.1007/s10067-010-1384-8
33. Debbi EM, Agar G, Fichman G, Ziv YB, Kardosh R, Halperin N, et al. Efficacy of methylsulfonylmethane supplementation on osteoarthritis of the knee: a randomized controlled study. BMC Complement Alternat Med. (2011) 11:1–9. doi: 10.1186/1472-6882-11-50
34. Kanzaki N, Ono Y, Shibata H, Moritani T. Glucosamine-containing supplement improves locomotor functions in subjects with knee pain: a randomized, double-blind, placebo-controlled study. Clin Intervent Aging. (2015) 10:1743. doi: 10.2147/CIA.S93077
35. Notarnicola A, Maccagnano G, Moretti L, Pesce V, Tafuri S, Fiore A, et al. Methylsulfonylmethane and boswellic acids versus glucosamine sulfate in the treatment of knee arthritis: Randomized trial. Int. J Immunopathol Pharmacol. (2016) 29:140–6. doi: 10.1177/0394632015622
36. McAlindon T, LaValley M, Schneider E, Nuite M, Lee JY, Price LL, et al. Effect of vitamin d supplementation on progression of knee pain and cartilage volume loss in patients with symptomatic osteoarthritis. A randomized controlled trial JAMA. J Am Med Assoc. (2013) 309:155. doi: 10.1001/jama.2012.164487
37. Farid R, Rezaieyazdi Z, Mirfeiz Z, Hatef MR, Mirheidari M, Mansouri H, et al. Oral intake of purple passion fruit peel extract reduces pain and stiffness and improves physical function in adult patients with knee osteoarthritis. Nutri Res. (2010) 30:601–6. doi: 10.1016/j.nutres.2010.08.010
38. Farid R, Mirfeiz Z, Mirheidari M, Rezaieyazdi Z, Mansouri H, Esmaelli H, et al. Pycnogenol supplementation reduces pain and stiffness and improves physical function in adults with knee osteoarthritis. Nutri Res. (2007) 27:692–7. doi: 10.1016/j.nutres.2007.09.007
39. kumar Panda S, Nirvanashetty S, Parachur VA, Mohanty N, Swain T. A randomized, double blind, placebo controlled, parallel-group study to evaluate the safety and efficacy of curene®versus placebo in reducing symptoms of knee OA. Biomed Res Int. (2018). doi: 10.1155/2018/5291945
40. Henrotin Y, Malaise M, Wittoek R, de Vlam K, Brasseur JP, Luyten FP, et al. Bio-optimized Curcuma longa extract is efficient on knee osteoarthritis pain: a double-blind multicenter randomized placebo controlled three-arm study. Arthritis Res Ther. (2019) 21:179. doi: 10.1186/s13075-019-1960-5
41. Maheu E, Mazières B, Valat J-P, Loyau G, Loët XL, Bourgeois P, et al. Symptomatic efficacy of avocado/soybean unsaponifiables in the treatment of osteoarthritis of the knee and hip: a prospective, randomized, double-blind, placebo-controlled, multicenter clinical trial with a six-month treatment period and a two-month followup demonstrating a persistent effect. Arthritis Rheumat. (1998) 41:81–91. doi: 10.1002/1529-0131(199801)41:1<81::AID-ART11>3.0.CO;2-9
42. Madhu K, Chanda K, Saji MJ. Safety and efficacy of Curcuma longa extract in the treatment of painful knee osteoarthritis: a randomized placebo-controlled trial. Inflammopharmacology. (2013) 21:129–36. doi: 10.1007/s10787-012-0163-3
43. Panahi Y, Rahimnia AR, Sharafi M, Alishiri G, Saburi A, Sahebkar A. Curcuminoid treatment for knee osteoarthritis: A randomized double-blind placebo-controlled trial. Phytother Res. (2014) 28:1625–31. doi: 10.1002/ptr.5174
44. Altman RD, Marcussen KC. Effects of a ginger extract on knee pain in patients with osteoarthritis. Arthritis Rheumat. (2001) 44:2531–8. doi: 10.1002/1529-0131(200111)44:11<2531::AID-ART433>3.0.CO;2-J
45. Appelboom T, Schuermans J, Verbruggen G, Henrotin Y, Reginster JY. Symptoms modifying effect of avocado/soybean unsaponifiables (ASU) in knee osteoarthritis. Scand J Rheumatol. (2001) 30:242–7.
46. Kimmatkar N, Thawani V, Hingorani L, Khiyani R. Efficacy and tolerability of Boswellia serrata extract in treatment of osteoarthritis of knee–a randomized double blind placebo controlled trial. Phytomedicine. (2003 J) 10:3–7. doi: 10.1078/094471103321648593
47. Dehghan M. Comparative effectiveness of B and E vitamins with diclofenac in reducing pain due to osteoarthritis of the knee. Med Arch. (2015) 69:103–6. doi: 10.5455/medarh.2015.69.103-106
48. Rao PS, Ramanjaneyulu YS, Prisk VR, Schurgers LJ, A. Combination of tamarindus indica seeds and curcuma longa rhizome extracts improves knee joint function and alleviates pain in non-arthritic adults following physical activity. Int J Med Sci. (2019) 16:845–53. doi: 10.7150/ijms.32505
49. Lubis AMT, Siagian C, Wonggokusuma E, Marsetyo AF, Setyohadi B. Comparison of Glucosamine-Chondroitin Sulfate with and without Methylsulfonylmethane in Grade I-II Knee Osteoarthritis: A Double Blind Randomized Controlled Trial. Acta Med Indones. (2017) 49:105–11.
50. Haroyan A, Mukuchyan V, Mkrtchyan N, Minasyan N, Gasparyan S, Sargsyan A, et al. Efficacy and safety of curcumin and its combination with boswellic acid in osteoarthritis: a comparative, randomized, double-blind, placebo-controlled study. BMC Complement Altern Med. (2018) 18:7. doi: 10.1186/s12906-017-2062-z
51. Chopra A, Saluja M, Tillu G, Sarmukkaddam S, Venugopalan A, Narsimulu G, et al. Ayurvedic medicine offers a good alternative to glucosamine and celecoxib in the treatment of symptomatic knee osteoarthritis: a randomized, double-blind, controlled equivalence drug trial. Rheumatology (Oxford). (2013) 52:1408–17. doi: 10.1093/rheumatology/kes414
52. Brien S, Prescott P, Bashir N, Lewith H, Lewith G. Systematic review of the nutritional supplements dimethyl sulfoxide (DMSO) and methylsulfonylmethane (MSM) in the treatment of osteoarthritis. Osteoarth Cartil. (2008) 16:1277–88. doi: 10.1016/j.joca.2008.03.002
53. Dai W, Yan W, Leng X, Chen J, Hu X, Ao Y. Effectiveness of Curcuma longa extract versus placebo for the treatment of knee osteoarthritis: a systematic review and meta-analysis of randomized controlled trials. Phytother Res. (2021) 35:5921–35. doi: 10.1002/ptr.7204
54. Sengupta K, Krishnaraju AV, Vishal AA, Mishra A, Trimurtulu G, Sarma KV, et al. Comparative efficacy and tolerability of 5-Loxin and AflapinAgainst osteoarthritis of the knee: a double blind, randomized, placebo controlled clinical study. Int J Med Sci. (2010) 7:366–77. doi: 10.7150/ijms.7.366
Keywords: osteoarthritis, antioxidant, systematic reviews, meta-analysis, cartilage damage, knee osteoarthritis (KOA)
Citation: Nejadhosseinian M, Djalalinia S, Haerian H, Alikhani M, Mansour A, Mousavian A-H, Mardani-Fard HA, Kasaeian A and Faezi ST (2022) The effects of antioxidants on knee osteoarthritis: A systematic review and meta-analysis. Front. Nutr. 9:1026450. doi: 10.3389/fnut.2022.1026450
Received: 23 August 2022; Accepted: 10 November 2022;
Published: 19 December 2022.
Edited by:
Vijaya Juturu, Lonza Capsules and Health Ingredients, United StatesReviewed by:
Sook Yee Lim, UCSI University, MalaysiaCopyright © 2022 Nejadhosseinian, Djalalinia, Haerian, Alikhani, Mansour, Mousavian, Mardani-Fard, Kasaeian and Faezi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Amir Kasaeian, YW1pcl9rYXNhZWlhbkB5YWhvby5jb20=; Seyedeh Tahereh Faezi, dGZhZXppQHNpbmEudHVtcy5hYy5pcg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.