
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Nutr., 18 January 2023
Sec. Clinical Nutrition
Volume 9 - 2022 | https://doi.org/10.3389/fnut.2022.1023997
Background: Curcumin (CUR) and quercetin (QUE), two natural polyphenols, possess diverse biological activities including broad-spectrum antiviral, antioxidant, and immunomodulatory effects. Both CUR and QUE have shown inhibition of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in in vitro assays.
Objective: In the present study we aimed to assess the possible treatment benefits of a combined curcumin and quercetin (CUR-QUE) oral supplement, alongside standard of care (SOC), in the early-stage COVID-19 infection.
Methods: This was an exploratory, pragmatic, open-label, randomized controlled clinical trial, conducted at the Department of Pathology, Liaquat University of Medical and Health Sciences, Jamshoro, PK. The study compared the treatment effect of an oral CUR-QUE supplement plus SOC vs. SOC alone, in the early-stage/mild to moderately symptomatic COVID-19 outpatients. Patients were randomized in a 1:1 ratio to CUR-QUE (n = 25) and control (n = 25) treatment groups. The CUR-QUE supplementation consisted of a daily intake of 168 mg curcumin and 260 mg quercetin, as two soft capsules, to be taken twice a day at home for 14 days.
Results: After one-week of treatment, most of the patients in the CUR-QUE group showed an expedited clearance of the viral infection i.e., 18 (72.0%) vs. 6 (24.0%) patients in the control group tested negative for SARS-CoV-2 in the nasal-oropharyngeal swab reverse transcription-polymerase chain reaction (RT-PCR) analysis (p = 0.0002). In addition, COVID-19-associated acute symptoms were also speedily resolved in the CUR-QUE treated patients, i.e., 10 (40.0%) vs. 4 (16.0%) patients in the control group (p = 0.061). The CUR-QUE supplementation therapy was well-tolerated by all 25 patients and no treatment-emergent effects or serious adverse events were reported.
Conclusion: The results revealed in this exploratory study suggest a possible therapeutic role of curcumin and quercetin in the early-stage of COVID-19. It is proposed that the two agents possibly acting in synergy, interfere the SARS-CoV-2 replication, and thus help a speedy recovery in the early-stage of COVID-19. Further research is highly encouraged.
Clinical trial registration: Clinicaltrials.gov, Identifier NCT04603690.
Coronavirus disease 2019 (COVID-19) caused by the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has resulted in the death of over 6 million people worldwide. The COVID-19 pandemic is ongoing, affecting the communities and poses an enormous challenge to the healthcare systems. The mass vaccination has significantly controlled the worldwide SARS-CoV-2 infection, transmission, rate of hospitalization and mortality. Mutation in the virus poses a potential risk to the effectiveness of the COVID-19 vaccines. Low-/middle-income countries are still suffering from COVID-19 impacts due to many challenges such as lack of vaccine, funding, and COVID-19 medications.
The clinical manifestations of COVID-19 is rather heterogenous ranging from no symptoms to mild cough, pneumonia, acute respiratory distress syndrome (ARDS) and multi organ failure, with 10–20% of the symptomatic patients likely to develop serious illness (1). Host’s immune response is believed to be closely related with the severity and outcomes in patients with COVID-19 (2–5). Older age, male sex and comorbidities have been associated with worst outcomes in patients with COVID-19 (6–8). The clinical course of COVID-19 is unpredictable, which can rapidly change in an irreversible outcome. Amongst the unique characteristics of COVID-19 is a predilection to elicit a maladaptive immune response leading to an excessive systemic inflammation (cytokine storm) and organ injury (9, 10). Inflammation is particularly severe in the lungs and vascular endothelium and is mostly associated with substantial alveolar damage and thrombosis of large and small lung vessels (11). The so-called cytokine storm is believed to be the major underlaying cause of mortality in COVID-19 patients (9).
For the management of mild to moderate symptomatic COVID-19 infection, commonly used analgesics such as paracetamol and ibuprofen are advised as first-line treatment for most people to lower the temperature and treat body aches and pain. Several new COVID-19 antivirals including molnupiravir (12), sotrovimab (13), casirivimab/imdevimab (14), and nirmatrelvir/ritonavir (15) have been recently developed, and claimed to be effective to prevent hospitalization in patients who are at risk of developing severe COVID-19. But these drugs are highly costly and available only in certain developed countries such as the UK, and USA. For severe COVID-19 condition, anti-inflammatory intervention such as corticosteroids (dexamethasone) (16), interleukin-6 (IL-6) receptor blockers (tocilizumab) (17), and baricitinib (18), a Janus kinase inhibitor, have shown to result in clinical improvement and reduce mortality. The treatment benefits of these anti-inflammatory therapies are yet to be conclusively proven, and their pros and cons remain unknown. Though, it is very unlikely that a single magic bullet drug will cure COVID-19, but rather a combination of early-stage antiviral and anti-inflammatory agents will be the most effective therapy for COVID-19. Supressing the SARS-CoV-2 viral replication in the early-stage and concomitantly modulating the host’s hyperinflammatory response is believed to be crucial for early recovery and prevention from progression to severe illness. There is thus an urgent need of safe, effective, cheap, and worldwide available early-stage COVID-19 medications for speedy early recovery and control of COVID-19 community transmission.
Diverse nutritional agents with demonstrated antimicrobial, antioxidant and immunomodulatory (anti-inflammatory) activities are believed to act as an adjuvant in the body and improve the immune and antioxidant defense systems against pathogenic infections. Amongst such agents, curcumin (CUR) (19–21), and quercetin (QUE) (22–24), (hereafter combinedly referred to as CUR-QUE), two most extensively studied polyphenols, have been proposed as possible adjuvants for early-stage COVID-19 due to their demonstrated antiviral, antioxidant, and immunomodulatory pharmacological effects.
Curcumin is the main curcuminoid present in the roots of turmeric (Curcuma longa) spice, has shown diverse pharmacological activities such as antioxidant, anticancer, antibacterial, antiviral, antidiabetic, and anti-inflammatory, both in in vitro and animal model studies (25). Over 300 clinical trials have reported the beneficial protective effects of curcumin against various diseases including respiratory disease such as chronic obstructive pulmonary disease (COPD), asthma, pulmonary fibrosis, acute lung injury, inflammatory diseases such as inflammatory bowel disease (IBD), rheumatoid arthritis, psoriasis, neurological diseases, cardiovascular diseases, metabolic diseases, liver diseases, and cancers (26–28). Curcumin reportedly is also a broad-spectrum antiviral, inhibiting a variety of viruses such as SARS-CoV-1 (29), human immunodeficiency virus (HIV)-1, HIV2, herpes simplex virus (HSV), human papillomavirus (HPV), human T-lymphotropic virus-1 (HTLV1), hepatitis B virus (HBV), hepatitis C virus (HCV), influenza A virus, Japanese encephalitis virus (JEV) (30), and SARS-CoV-2 (31–34).
Quercetin, a flavonoid polyphenol, is found in the highest concentrations in citrus fruits, apples, red onions, grapes, dark cherries, and dark berries such as blueberries, blackberries and bilberries, green tea, buckwheat, parsley, sage, and olive oil. Quercetin exhibits diverse pharmacological effects such as anti-infective, anti-inflammatory, antibacterial, antioxidant, antiapoptotic, anticancer, and antidiabetic (35, 36). Quercetin also inhibits a variety of viruses, including SARS-CoV-1 (37, 38), Influenza virus, Ebola virus, HCV, HSV, Respiratory Syncytial viruses (39) and SARS-CoV-2 (31, 40, 41). In SARS-CoV-2-infected hamsters and mice, quercetin treatment alongside dasatinib has shown senolytic activity, resulting in reduction in the inflammation and improvement in pulmonary injury (42).
The demonstrated pharmacological activities and readily availability of curcumin and quercetin nutritional supplements prompted us to explore the treatment benefits of a combined curcumin and quercetin (CUR-QUE) oral supplement as an add-on to the standard of care (SOC) in the management of early-stage/mild to moderately symptomatic COVID-19 outpatients.
The study compared the treatment effect of a CUR-QUE supplement plus SOC vs. SOC alone, in the early-stage/mild to moderately symptomatic COVID-19 outpatients. This was a pragmatic, open-label, randomized controlled clinical trial conducted at the Department of Pathology, Liaquat University of Medical and Health Sciences (LUMHS), Jamshoro, Pakistan (PK). The study was carried out in accordance with the Declaration of Helsinki Ethical Principles and Good Clinical Practices (GCP), and approved by the Research Ethics Committee (REC), LUMHS via Ref. No. LUMHS/REC/137. Informed written consent was obtained from participants before enrolling in the study. The study has been registered at clinicaltrials.gov with registration number NCT04603690.
Patients were enrolled at the medical outpatients’ clinics of Liaquat Medical University Hospital, from 21 September 2021 to 21 January 2022. Study inclusion criteria include: male or female aged ≥18 years; confirmed SARS-CoV-2 infection as shown by nasal-oropharyngeal swab reverse-transcription polymerase chain reaction (RT-PCR)-based positive analysis, associated with mild to moderate typical acute COVID-19 acute symptoms such as fever, cough, myalgia, pharyngitis, asthenia, dysgeusia, dyspnea (SpO2 ≥ 93%, and not needing supplementary oxygen) etc., and can be treated as outpatients (do not require hospitalization). Exclusion criteria include: history of self-reporting hypersensitivity or allergic reaction to curcumin or quercetin, end stage kidney or liver disease, severe thrombocytopenia, or any other condition or factor that, in the opinion of the treating physician, contraindicates the use of CUR-QUE supplementation or makes the subject at risk due to their participation in the study.
A total of 64 patients were enrolled and randomized in a 1:1 ratio to the CUR-QUE treatment arm (SOC plus CUR-QUE, n = 32) and control arm (SOC alone, n = 32). Randomization was performed by a trained healthcare professional who has no role in the study and carried out using a computer-generated random numbers code; patients assigned even numbers were allocated to the CUR-QUE treatment arm, and those assigned odd numbers were allocated to the control arm. Of the total 64 patients, 14 were lost in the follow-up, and the remaining 50 patients, consisting of 25 in each treatment arm (see CONSORT flow diagram in Figure 1), completed the study.
After treatment allocation, patient’s COVID-19-associated acute symptoms and serum levels of high-sensitivity C-reactive protein (hs-CRP), D-dimer, lactate dehydrogenase (LDH), and ferritin were evaluated and recorded by the outpatient’s physician, and treatment prescribed as per the randomization. Patients in both groups received the same SOC medications as per the hospital guidelines and include paracetamol 500 mg, oral azithromycin 500 mg, oral prednisolone 5 mg, with or without inj. ceftriaxone 1 g. The CUR-QUE supplementation to be taken at home, comprised of a daily intake of 168 mg curcumin, and 260 mg quercetin as two soft oral capsules, twice a day for 14 days. Each CUR-QUE supplement capsule (Nasafytol®, manufactured by Tilman SA, Belgium) contained 42 mg curcumin, and 65 mg quercetin, in a blend with 2.25 μg vitamin D3.
All patients were booked for day 7 follow-up in-person appointment with the physician at the medical outpatient’s clinic to test for the persistence of the SARS-CoV-2 infection (RT-PCR assay), assess COVID-19-associated acute symptoms, and evaluate laboratory biochemistry. If necessary, a further RT-PCR test was booked for day 14 of the treatment.
The proportion of the patients testing negative for SARS-CoV-2 in the RT-PCR test, and improvement in the COVID-19-associated acute symptoms at day 7 follow-up, were the primary outcomes of this study, while improvement in the laboratory biochemistry was the secondary outcome of this study. Other outcomes include CUR-QUE supplement safety and tolerability, hospitalization rate and length, length of supplementary oxygen support, rate of intensive care unit (ICU) transfer and mortality.
Welch two-sample t-test (normally distributed variables) was used to compare the continuous variables between the groups. Binary and categorical variables were described as counts (and%) and compared between groups using the Chi-squared test or, when counts were sparse, then with Fischer’s exact test. The t-tests and Chi-squared tests were performed using MedCalc for Windows, version 19.4 (MedCalc Software, Ostend, Belgium). Given the highly skewed distributions, non-parametric analysis of Covariance (ANCOVA) was used to test the effect of treatment on follow-up biomarker levels adjusted for their baseline values (43, 44) using the sm package in R 4.1.0 (45).
Patient’s demographics and baseline clinical characteristics are shown in Table 1. Overall, the median (IQR) age was 37.0 (48.0, 30.0) years and include 32 (64.0%) males. Of the total 50 patients, 17 (34.0%) had a history of underlying medical condition, including 6 (12.0%) patients had two or more conditions. The prevalent comorbidities were diabetes mellitus (DM), hypertension (HTN), and asthma in 9 (18.0%), 7 (14.0%), and 4 (8.0%) patients, respectively. At baseline, 26 (52.0%) patients presented five or more COVID-19-associated acute symptoms, while 24 (48.0%) patients had four or less symptoms. The most common symptoms were pyrexia [43 (86.0%)], cough [41 (82.0%)], myalgia [37 (74.0%)], pharyngitis [21 (42.0%)], flu [19 (38.0%)], and asthenia [27 (54.0%)]. Less common symptoms included dyspnea [17 (34.0%)], anosmia [18 (36.0%)], and dysgeusia [5 (10.0%)]. Most patients [48 (96.0%)] had received at least one dose of COVID-19 vaccination. Both the treatment groups were reasonably balanced with respect to the proportion of gender, age, COVID-19 vaccination status, and numbers of COVID-19-associated symptoms. The prevalence of myalgia and asthenia was higher in the control arm, while there were more patients with comorbidities in the CUR-QUE arm, incurred likely due to chance.
Patients’ follow-up day 7 RT-PCR SARS-CoV-2 test results are shown in Figure 2. The results revealed that most of the patients in the CUR-QUE arm cleared the viral infection faster as compared to the control arm i.e., 18 (72%) of patients [including 9 (36%) patients with a history of underlying health condition] tested negative for SARS-CoV-2 vs. only 6 (24.0%) patients in the control arm; showing a statistically significant difference, p = 0.0002. Two patients in the CUR-QUE arm abstained COVID-19 test at day 7 follow-up appointment. Of the remaining 5 CUR-QUE and 19 control arm patients which were still SARS-CoV-2 positive on day 7, all of the CUR-QUE arm patients and 14 of the control arm patients tested negative at day 14, implying a delayed viral clearance in the control arm.
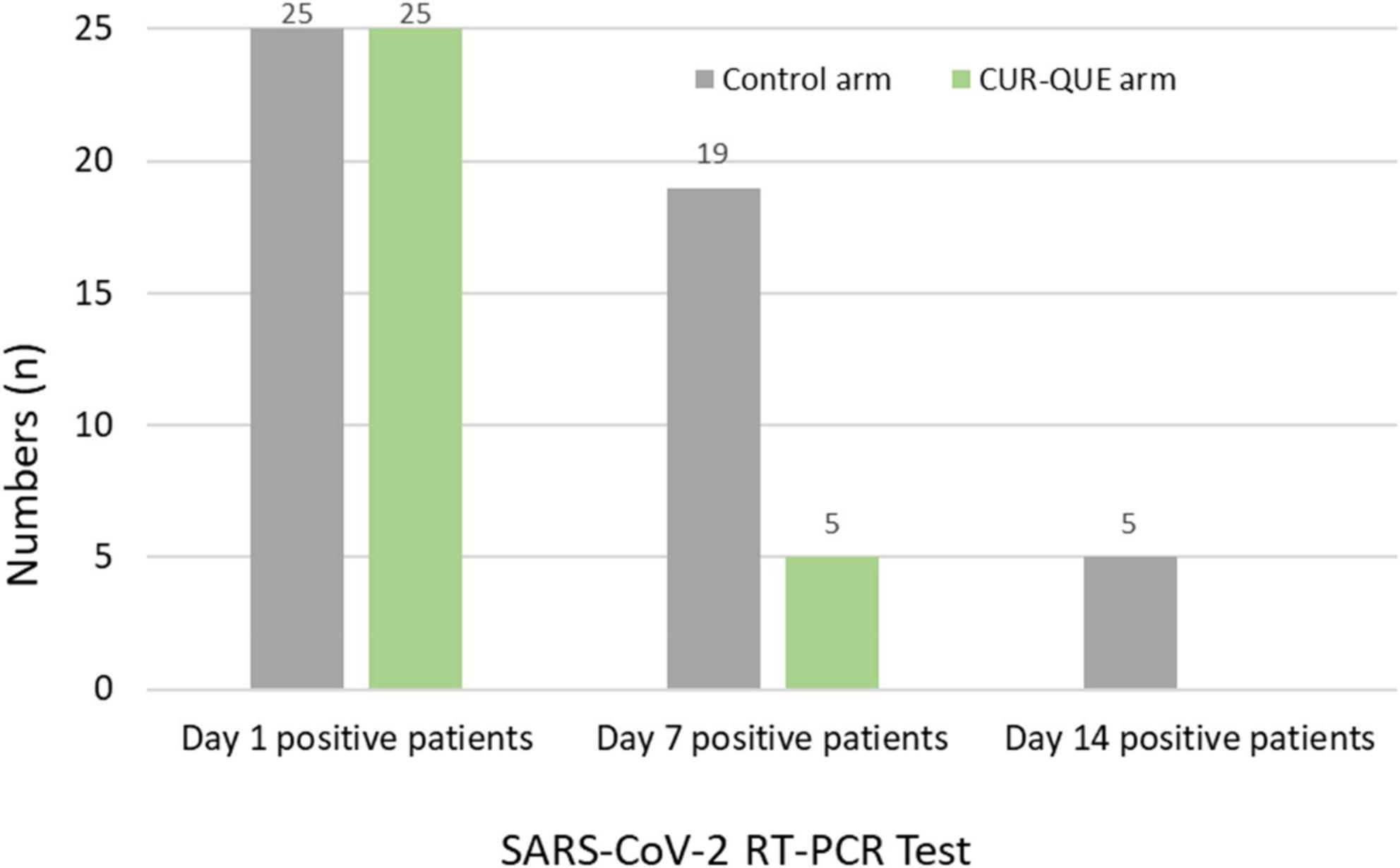
Figure 2. Patient’s RT-PCR COVID-19 test at day 1 and follow-up 7 and day 14 in the two treatment arms. Two patients in the CUR-QUE arm abstained the day 7 test.
In evaluating COVID-19-associated acute symptoms at follow-up day 7 (Figure 3), the number of patients showed improvement in the symptomatology was higher in CUR-QUE arm, i.e., 10 (40.0%) patients showed complete symptoms resolution, while 3 (12.0%) patient’s symptoms had reduced to three, 3 (12.0%) patients to two, and 9 (36.0%) patients symptoms reduced to one. In comparison, in the control arm, symptoms had resolved in only 4 (16.0%) patients, while 3 (12.0%) patients were left with four symptoms, 10 (40.0%) with three-symptoms, and 8 (32.0%) patients with two-symptoms. These results demonstrate a speedy recovery from COVID-19-associated acute symptoms in CUR-QUE arm as compared to control arm, which showed only partial symptoms improvement.
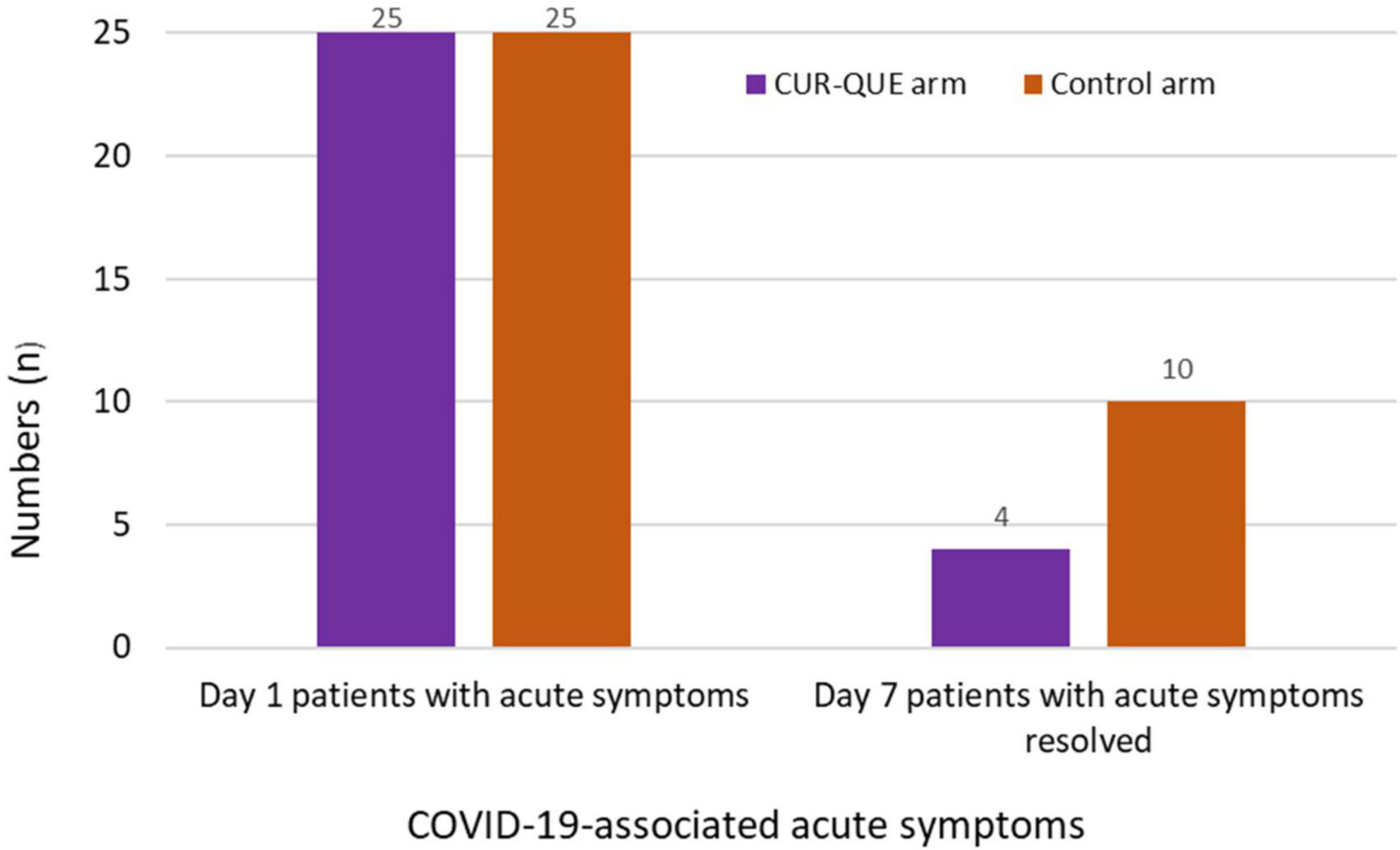
Figure 3. Patient’s COVID-19-associated symptoms at day 1 and follow-up day 7 in the two treatment arms.
Table 2 shows laboratory biochemistry (hs-CRP, D-dimer, LDH, ferritin) at baseline and day 7 follow-up by treatment group. Baseline serum levels of inflammatory biomarkers were comparable in the two groups. Both groups showed small change in D-dimer, LDH and ferritin levels between baseline and day 7 follow-up. Levels of hs-CRP fell in both groups, and there was weak evidence for a slightly greater fall in the CUR-QUE group, p = 0.068.
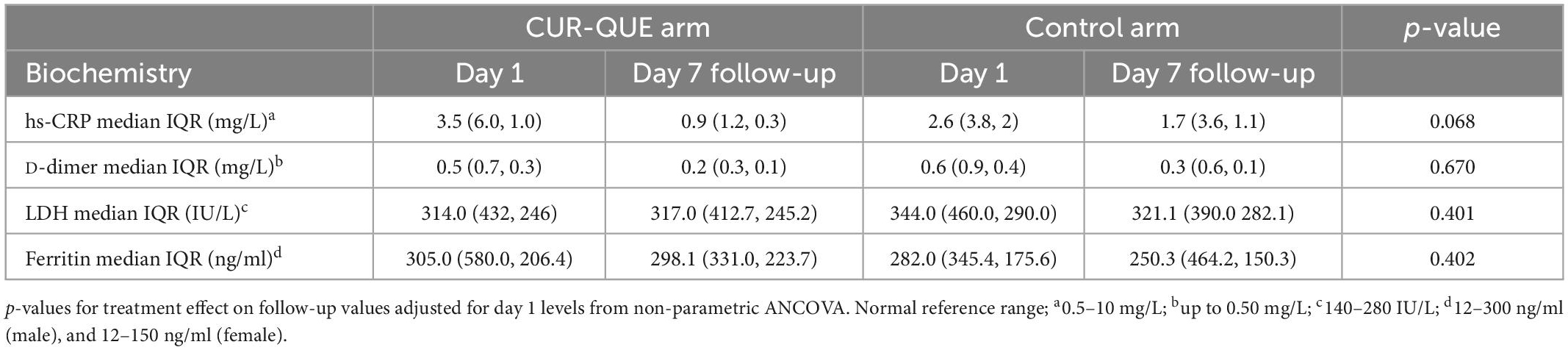
Table 2. Patients’ laboratory biochemistry at day 1 and follow-up day 7 in the two treatment groups.
Overall, in both the treatment groups patients showed recovery from COVID-19 and there was no case of hospitalization. The CUR-QUE supplementation was well-tolerated by all 25 patients, and no side-effects, or serious adverse events were reported.
In this exploratory pragmatic randomized clinical trial involving early-stage/mild to moderately symptomatic COVID-19 outpatients, those who received the CUR-QUE supplementation alongside SOC, showed a speedy recovery from COVID-19 as compared to patients who received the SOC alone. By treatment day 7, most of the patients in the CUR-QUE arm (i.e., 18 patients out of 25, 72.0%), including 9 (36.0%) patients with a history underlying health condition, cleared the SARS-CoV-2 infection earlier as compared to the control arm (i.e., 6 patients out of 25, 24.0%), showing a statistically significant difference (p = 0.0008). In addition, patients in CUR-QUE arm showed a speedy improvement in the COVID-19-associated acute symptoms as compared to the control arm i.e., 10 patients in the CUR-QUE arm showed complete symptoms resolution vs. 4 patients in the control arm (40.0% vs. 16.0%), p = 0.061. The results of this exploratory study suggest the possible adjuvant therapeutic effect of CUR-QUE nutritional supplement for early-stage/mild to moderate symptoms of COVID-19. It is speculated that the observed treatment benefits of CUR-QUE supplementation is possibly due to synergistic pharmacological effects of curcumin and quercetin. The speedy SARS-CoV-2 viral clearance in patients who received the CUR-QUE as shown in the RT-PCR analysis, is likely due to the interference in the replication of main protease (Mpro) of the SARS-CoV-2 by curcumin or quercetin or both as reported in the in vitro studies (31–34, 37, 40, 41). Mpro is a crucial enzyme involved in the replication of SARS-CoV-2 and is currently the inhibition target for the development of COVID-19 antiviral therapies. Other antiviral mechanisms shown by curcumin (19, 21) and quercetin (22, 24, 46, 47) including direct binding to the SARS-CoV-2 Spike protein (S-protein) RBD or disrupting the interaction of SARS-CoV-2 S-protein with its human specific receptor ACE2, which can prevent the viral entry to the host cells, are also possible.
The possible adjuvant effect of CUR-QUE supplementation alongside SOC in patients with COVID-19 have been investigated in several clinical trials. In the management of mild to moderately symptomatic COVID-19 patients, several trials have reported the possible adjuvant effect of oral curcumin supplementation, including speedy clearance of the SARS-CoV-2 in the RT-PCR test, early resolution of the COVID-19-associated acute symptoms, increase in oxygen saturation level, reduction in serum CRP levels, increase in lymphocyte count, reduction in duration of supplemental oxygen and hospitalization period (48–53). Several other clinical trials in hospitalized COVID-19 patients have also revealed the immunomodulatory (anti-inflammatory) effects of curcumin therapy, including reduction in the serum levels of IL-1β, IL-6, INF-γ, TNF-α, IL-17, IL-10, IL-35, TGF-β, PCT, FoxP3, IL-10, IL-35, TGF-β; reduction in the expression levels of IL-1β, IL-6, TBX21, and FOXP3, FoxP3, IL-10, IL-35, and TGF-β as well as upregulation of the frequency of Treg cells, reduction in the number of Th17 cells, Th17 cell-related cytokines levels, and downregulation of Th17 cell-related factors (54–59).
Similarly, the possible adjuvant effect of quercetin has also been investigated in several clinical trials in mild to moderately symptomatic COVID-19 patients, and have revealed beneficial effects including speedy viral clearance, early resolution of COVID-19-associated acute symptoms, and improvement in the serum levels of inflammatory biomarkers such as CRP, LDH, and alkaline phosphatase (ALP) (60–65). In study by Zupanets et al. in hospitalized COVID-19 patients associated with pneumonia, intravenous administration of quercetin/polyvinylpyrrolidone, followed by oral therapy of quercetin/pectin, showed accelerated restoration of the lung function (oxygen saturation, cough) and stabilization of the D-dimer level (66).
Moreover, the results observed in the present study are also in agreement and confirm the results of our previous collaborative study involving the same CUR-QUE supplement as an add-on to the SOC, in the management of mild to moderately symptomatic COVID-19 outpatients (67). Both studies showed a speedy viral clearance as shown in the RT-PCR test, and early resolution of the COVID-19-associated acute symptoms as compared to the SOC alone.
Both curcumin and quercetin supplementation have excellent safety profile, tolerability, and doses up to up to 8 g/day (68) and 1 g/day (69) respectively, for 3-months have failed to produce serious adverse effects. Both curcumin and quercetin have achieved the FDA GRASS (Generally Recognised As Safe) status for human use as nutritional supplements. The reported broad-spectrum antiviral, immunomodulatory, and antioxidant effects of curcumin and quercetin, and the results revealed in COVID-19 studies, supports the adjuvant therapeutic effect of these two polyphenols in the early-stage/mild to moderate symptoms of COVID-19. Supplementation of CUR-QUE in COVID-19 is suggested to have a greater impact when used early in the disease (i.e., outpatients) potentially preventing the spread of infection, hospitalization, and subsequent deaths, while reducing the healthcare system pressure, particularly in low-/middle income countries.
Limitations of our study include small sample-size and not been a double-blinded and placebo-controlled trial. Nevertheless, we have carried out a pragmatic randomized clinical trial that evaluated the treatment benefits of CUR-QUE supplement in patients with early-stage/mild to moderate symptoms of COVID-19, including in patients with underlying health conditions. Curcumin and quercetin supplements are safe, cheap, and worldwide available. The results revealed in this study are easily applicable in community-based patients and could possibly help in the management of mild to moderately symptomatic COVID-19 outpatients.
According to the results of this study, a daily oral co-supplementation of 168 mg of curcumin and 260 mg of quercetin by mild to moderately symptomatic COVID-19 outpatients for 14 days alongside SOC could possibly help in the early clearance of the viral infection, and resolution of the COVID-19-associated acute symptoms. Further research is highly encouraged.
The original contributions presented in this study are included in the article, further inquiries can be directed to the corresponding author.
The study was approved by the Research Ethics Committee (REC), Liaquat University of Medical and Health Sciences (LUMHS), Jamshoro, Pakistan via Ref. No. LUMHS/REC/-137. The patients/participants provided their written informed consent to participate in this study.
IU: study design, data collection, data interpretation, supervision, and critical revision of the manuscript. SK: study design, data interpretation, and manuscript revision. RN: data collection, supervision, and manuscript revision. HA: study administration, data collection, and manuscript revision. SA: study design, data interpretation, and critical revision of the manuscript. AK: study design, data interpretation, and writing the original draft and critical revision. All authors contributed to the article and approved the submitted version.
We would like to thank the patients who participated in this clinical trial, and the clinicians, nursing staff, laboratory staff, and other admin support of LUMHS who helped together in the collection of data recorded in the study. We would also like to thank Shona Livingstone, School of Medicine, University of Dundee, UK, for statistical support. Finally, we are grateful to Tilman SA, Belgium, for their help in donating the CUR-QUE supplement.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Tsai P, Lai W, Lin Y, Luo Y, Lin Y, Chen H, et al. Clinical manifestation and disease progression in COVID-19 infection. J Chin Med Assoc. (2021) 84:3–8. doi: 10.1097/jcma.0000000000000463
2. Lucas C, Wong P, Klein J, Castro T, Silva J, Sundaram M, et al. Longitudinal analyses reveal immunological misfiring in severe COVID-19. Nature. (2020) 584:463–9. doi: 10.1038/s41586-020-2588-y
3. Rydyznski Moderbacher C, Ramirez S, Dan J, Grifoni A, Hastie K, Weiskopf D, et al. Antigen-specific adaptive immunity to SARS-CoV-2 in Acute COVID-19 and associations with age and disease severity. Cell. (2020) 183:996–1012.e19. doi: 10.1016/j.cell.2020.09.038
4. Schultze J, Aschenbrenner A. COVID-19 and the human innate immune system. Cell. (2021) 184:1671–92. doi: 10.1016/j.cell.2021.02.029
5. Sette A, Crotty S. Adaptive immunity to SARS-CoV-2 and COVID-19. Cell. (2021) 184:861–80. doi: 10.1016/j.cell.2021.01.007
6. Docherty A, Harrison E, Green C, Hardwick H, Pius R, Norman L, et al. Features of 20 133 UK patients in hospital with covid-19 using the ISARIC WHO clinical characterisation protocol: prospective observational cohort study. BMJ. (2020) 369:m1985. doi: 10.1136/bmj.m1985
7. Grasselli G, Zangrillo A, Zanella A, Antonelli M, Cabrini L, Castelli A, et al. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the lombardy region, Italy. JAMA. (2020) 323:1574–81. doi: 10.1001/jama.2020.5394
8. Richardson S, Hirsch J, Narasimhan M, Crawford J, McGinn T, Davidson K, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York city area. JAMA. (2020) 323:2052–9. doi: 10.1001/jama.2020.6775
9. Hu B, Huang S, Yin L. The cytokine storm and COVID-19. J Med Virol. (2021) 93:250–6. doi: 10.1002/jmv.26232
10. Yang L, Xie X, Tu Z, Fu J, Xu D, Zhou Y. The signal pathways and treatment of cytokine storm in COVID-19. Signal Trans Target Ther. (2021) 6:255. doi: 10.1038/s41392-021-00679-0
11. Dorward D, Russell C, Um I, Elshani M, Armstrong S, Penrice-Randal R, et al. Tissue-specific immunopathology in fatal COVID-19. Am J Respir Crit Care Med. (2021) 203:192–201. doi: 10.1164/rccm.202008-3265OC
12. Jayk Bernal A, Gomes da Silva M, Musungaie D, Kovalchuk E, Gonzalez A, Delos Reyes V, et al. Molnupiravir for oral treatment of covid-19 in nonhospitalized patients. N Engl J Med. (2021) 386:509–20. doi: 10.1056/NEJMoa2116044
13. Gupta A, Gonzalez-Rojas Y, Juarez E, Crespo Casal M, Moya J, Falci D, et al. Early treatment for covid-19 with SARS-CoV-2 neutralizing antibody sotrovimab. N Engl J Med. (2021) 385:1941–50. doi: 10.1056/NEJMoa2107934
14. Weinreich D, Sivapalasingam S, Norton T, Ali S, Gao H, Bhore R, et al. REGN-COV2, a neutralizing antibody cocktail, in outpatients with covid-19. N Engl J Med. (2020) 384:238–51. doi: 10.1056/NEJMoa2035002
15. Hammond J, Leister-Tebbe H, Gardner A, Abreu P, Bao W, Wisemandle W, et al. Oral nirmatrelvir for high-risk, nonhospitalized adults with covid-19. N Engl J Med. (2022) 386:1397–408. doi: 10.1056/NEJMoa2118542
16. Collaborative Group R. Dexamethasone in hospitalized patients with covid-19. N Engl J Med. (2021) 384:693–704. doi: 10.1056/NEJMoa2021436
17. Collaborative Group R. Tocilizumab in patients admitted to hospital with COVID-19: a randomised, controlled, open-label, platform trial. Lancet. (2021) 397:1637–45. doi: 10.1016/S0140-673600676-0
18. Kalil A, Patterson T, Mehta A, Tomashek K, Wolfe C, Ghazaryan V, et al. Baricitinib plus remdesivir for hospitalized adults with covid-19. N Engl J Med. (2020) 384:795–807. doi: 10.1056/NEJMoa2031994
19. Zahedipour F, Hosseini S, Sathyapalan T, Majeed M, Jamialahmadi T, Al-Rasadi K, et al. Potential effects of curcumin in the treatment of COVID-19 infection. Phytother Res. (2020) 34:2911–20. doi: 10.1002/ptr.6738
20. Rattis B, Ramos S, Celes M. Curcumin as a potential treatment for COVID-19. Front Pharmacol. (2021) 12:675287. doi: 10.3389/fphar.2021.675287
21. Thimmulappa R, Mudnakudu-Nagaraju K, Shivamallu C, Subramaniam K, Radhakrishnan A, Bhojraj S, et al. Antiviral and immunomodulatory activity of curcumin: a case for prophylactic therapy for COVID-19. Heliyon. (2021) 7:e06350. doi: 10.1016/j.heliyon.2021.e06350
22. Colunga Biancatelli R, Berrill M, Catravas J, Marik P. Quercetin and vitamin C: an experimental, synergistic therapy for the prevention and treatment of SARS-CoV-2 related disease (COVID-19). Front Immunol. (2020) 11:1451. doi: 10.3389/fimmu.2020.01451
23. Derosa G, Maffioli P, D’Angelo A, Di Pierro F. A role for quercetin in coronavirus disease 2019 (COVID-19). Phytother Res. (2021) 35:1230–6. doi: 10.1002/ptr.6887
24. Manjunath S, Thimmulappa R. Antiviral, immunomodulatory, and anticoagulant effects of quercetin and its derivatives: potential role in prevention and management of COVID-19. J Pharm Analy. (2022) 12:29–34. doi: 10.1016/j.jpha.2021.09.009
25. Babaei F, Nassiri-Asl M, Hosseinzadeh H. Curcumin (a constituent of turmeric): new treatment option against COVID-19. Food Sci Nutr. (2020) 8:5215–27. doi: 10.1002/fsn3.1858
26. Aggarwal B, Harikumar K. Potential therapeutic effects of curcumin, the anti-inflammatory agent, against neurodegenerative, cardiovascular, pulmonary, metabolic, autoimmune and neoplastic diseases. Int J Biochem Cell Biol. (2009) 41:40–59. doi: 10.1016/j.biocel.2008.06.010
27. Jäger R, Lowery R, Calvanese A, Joy J, Purpura M, Wilson J. Comparative absorption of curcumin formulations. Nutr J. (2014) 13:11. doi: 10.1186/1475-2891-13-11
28. Lelli D, Sahebkar A, Johnston T, Pedone C. Curcumin use in pulmonary diseases: state of the art and future perspectives. Pharmacol Res. (2017) 115:133–48. doi: 10.1016/j.phrs.2016.11.017
29. Wen C, Kuo Y, Jan J, Liang P, Wang S, Liu H, et al. Specific plant terpenoids and lignoids possess potent antiviral activities against severe acute respiratory syndrome coronavirus. J Med Chem. (2007) 50:4087–95. doi: 10.1021/jm070295s
30. Zorofchian Moghadamtousi S, Abdul Kadir H, Hassandarvish P, Tajik H, Abubakar S, Zandi KA. Review on antibacterial, antiviral, and antifungal activity of curcumin. BioMed Res Int. (2014) 2014:186864. doi: 10.1155/2014/186864
31. Bahun M, Jukic M, Oblak D, Kranjc L, Bajc G, Butala M, et al. Inhibition of the SARS-CoV-2 3CL(pro) main protease by plant polyphenols. Food Chem. (2022) 373:131594. doi: 10.1016/j.foodchem.2021.131594
32. Bormann M, Alt M, Schipper L, van de Sand L, Le-Trilling V, Rink L, et al. Turmeric root and its bioactive ingredient curcumin effectively neutralize SARS-CoV-2 in vitro. Viruses. (2021) 13:1914. doi: 10.3390/v13101914
33. Leka K, Hamann C, Desdemoustier P, Frédérich M, Garigliany M, Ledoux A. In vitro antiviral activity against SARS-CoV-2 of common herbal medicinal extracts and their bioactive compounds. Phytother Res. (2022) 36:3013–5. doi: 10.1002/ptr.7463
34. Marin-Palma D, Tabares-Guevara J, Zapata-Cardona M, Florez-Alvarez L, Yepes L, Rugeles M, et al. Curcumin inhibits in vitro SARS-CoV-2 infection in vero E6 cells through multiple antiviral mechanisms. Molecules. (2021) 26:6900. doi: 10.3390/molecules26226900
35. Khursheed R, Singh S, Wadhwa S, Gulati M, Awasthi A. Enhancing the potential preclinical and clinical benefits of quercetin through novel drug delivery systems. Drug Discovery Today. (2020) 25:209–22. doi: 10.1016/j.drudis.2019.11.001
36. Yang D, Wang T, Long M, Li P. Quercetin: its main pharmacological activity and potential application in clinical medicine. Oxid Med Cell Longev. (2020) 2020:8825387. doi: 10.1155/2020/8825387
37. Chen L, Li J, Luo C, Liu H, Xu W, Chen G, et al. Binding interaction of quercetin-3-beta-galactoside and its synthetic derivatives with SARS-CoV 3CL(pro): structure-activity relationship studies reveal salient pharmacophore features. Bioorg Med Chem. (2006) 14:8295–306. doi: 10.1016/j.bmc.2006.09.014
38. Nguyen T, Woo H, Kang H, Nguyen V, Kim Y, Kim D, et al. Flavonoid-mediated inhibition of SARS coronavirus 3C-like protease expressed in Pichia pastoris. Biotechnol Lett. (2012) 34:831–8. doi: 10.1007/s10529-011-0845-8
39. Di Petrillo A, Orrù G, Fais A, Fantini M. Quercetin and its derivates as antiviral potentials: a comprehensive review. Phytother Res. (2022) 36:266–78. doi: 10.1002/ptr.7309
40. Abian O, Ortega-Alarcon D, Jimenez-Alesanco A, Ceballos-Laita L, Vega S, Reyburn H, et al. Structural stability of SARS-CoV-2 3CLpro and identification of quercetin as an inhibitor by experimental screening. Int J Biol Macromol. (2020) 164:1693–703. doi: 10.1016/j.ijbiomac.2020.07.235
41. Rizzuti B, Grande F, Conforti F, Jimenez-Alesanco A, Ceballos-Laita L, Ortega-Alarcon D, et al. Rutin is a low micromolar inhibitor of SARS-CoV-2 main protease 3CLpro: implications for drug design of quercetin analogs. Biomedicines. (2021) 9:375. doi: 10.3390/biomedicines9040375
42. Lee S, Yu Y, Trimpert J, Benthani F, Mairhofer M, Richter-Pechanska P, et al. Virus-induced senescence is a driver and therapeutic target in COVID-19. Nature. (2021) 599:283–9. doi: 10.1038/s41586-021-03995-1
43. Bowman A, Young S. Graphical comparison of nonparametric curves. Appl Statist. (1996) 45:83–98. doi: 10.2307/2986225
44. Young S, Bowman A. Non-parametric analysis of covariance. Biometrics. (1995) 51:920–31. doi: 10.2307/2532993
45. R Core Team. R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing (2015).
46. Munafò F, Donati E, Brindani N, Ottonello G, Armirotti A, De Vivo M. Quercetin and luteolin are single-digit micromolar inhibitors of the SARS-CoV-2 RNA-dependent RNA polymerase. Sci Rep. (2022) 12:10571. doi: 10.1038/s41598-022-14664-2
47. Pan B, Fang S, Zhang J, Pan Y, Liu H, Wang Y, et al. Chinese herbal compounds against SARS-CoV-2: puerarin and quercetin impair the binding of viral S-protein to ACE2 receptor. Comput Struct Biotechnol J. (2020) 18:3518–27. doi: 10.1016/j.csbj.2020.11.010
48. Ahmadi R, Salari S, Sharifi M, Reihani H, Rostamiani M, Behmadi M, et al. Oral nano-curcumin formulation efficacy in the management of mild to moderate outpatient COVID-19: a randomized triple-blind placebo-controlled clinical trial. Food Sci Nutr. (2021) 9:4068–75. doi: 10.1002/fsn3.2226
49. Chabot A, Huntwork M. Turmeric as a possible treatment for COVID-19-induced anosmia and ageusia. Cureus. (2021) 13:e17829. doi: 10.7759/cureus.17829
50. Honarkar Shafie E, Taheri F, Alijani N, Okhovvat A, Goudarzi R, Borumandnia N, et al. Effect of nanocurcumin supplementation on the severity of symptoms and length of hospital stay in patients with COVID-19: a randomized double-blind placebo-controlled trial. Phytother Res. (2022) 36:1013–22. doi: 10.1002/ptr.7374
51. Majeed M, Nagabhushanam K, Shah K, Mundkur L. A randomized, double-blind, placebo-controlled study to assess the efficacy and safety of a nutritional supplement (ImmuActive(TM)) for COVID-19 patients. Evid Based Complement Alternat Med. (2021) 2021:8447545. doi: 10.1155/2021/8447545
52. Pawar K, Mastud R, Pawar S, Pawar S, Bhoite R, Bhoite R, et al. Oral curcumin with piperine as adjuvant therapy for the treatment of COVID-19: a randomized clinical trial. Front Pharmacol. (2021) 12:669362. doi: 10.3389/fphar.2021.669362
53. Saber-Moghaddam N, Salari S, Hejazi S, Amini M, Taherzadeh Z, Eslami S, et al. Oral nano-curcumin formulation efficacy in management of mild to moderate hospitalized coronavirus disease-19 patients: an open label nonrandomized clinical trial. Phytother Res. (2021) 35:2616–23. doi: 10.1002/ptr.7004
54. Asadirad A, Nashibi R, Khodadadi A, Ghadiri A, Sadeghi M, Aminian A, et al. Antiinflammatory potential of nano-curcumin as an alternative therapeutic agent for the treatment of mild-to-moderate hospitalized COVID-19 patients in a placebo-controlled clinical trial. Phytother Res. (2022) 36:1023–31. doi: 10.1002/ptr.7375
55. Hassaniazad M, Eftekhar E, Inchehsablagh B, Kamali H, Tousi A, Jaafari M, et al. A triple-blind, placebo-controlled, randomized clinical trial to evaluate the effect of curcumin-containing nanomicelles on cellular immune responses subtypes and clinical outcome in COVID-19 patients. Phytother Res. (2021) 35:6417–27. doi: 10.1002/ptr.7294
56. Karimi A, Mahmoodpoor A, Kooshki F, Niazkar H, Shoorei H, Tarighat-Esfanjani A. Effects of nanocurcumin on inflammatory factors and clinical outcomes in critically ill patients with sepsis: a pilot randomized clinical trial. Eur J Int Med. (2020) 36:101122. doi: 10.1016/j.eujim.2020.101122
57. Tahmasebi S, El-Esawi M, Mahmoud Z, Timoshin A, Valizadeh H, Roshangar L, et al. Immunomodulatory effects of nanocurcumin on Th17 cell responses in mild and severe COVID-19 patients. J Cell Physiol. (2021) 236:5325–38. doi: 10.1002/jcp.30233
58. Tahmasebi S, Saeed B, Temirgalieva E, Yumashev A, El-Esawi M, Navashenaq J, et al. Nanocurcumin improves Treg cell responses in patients with mild and severe SARS-CoV2. Life Sci. (2021) 276:119437. doi: 10.1016/j.lfs.2021.119437
59. Valizadeh H, Abdolmohammadi-Vahid S, Danshina S, Ziya Gencer M, Ammari A, Sadeghi A, et al. Nano-curcumin therapy, a promising method in modulating inflammatory cytokines in COVID-19 patients. Int Immunopharmacol. (2020) 89:107088. doi: 10.1016/j.intimp.2020.107088
60. Di Pierro F, Derosa G, Maffioli P, Bertuccioli A, Togni S, Riva A, et al. Possible therapeutic effects of adjuvant quercetin supplementation against early-stage COVID-19 infection: a prospective, randomized, controlled, and open-label study. Int J Gen Med. (2021) 14:2359–66. doi: 10.2147/IJGM.S318720
61. Di Pierro F, Iqtadar S, Khan A, Ullah Mumtaz S, Masud Chaudhry M, Bertuccioli A, et al. Potential clinical benefits of quercetin in the early stage of COVID-19: results of a second, pilot, randomized, controlled and open-label clinical trial. Int J Gen Med. (2021) 14:2807–16. doi: 10.2147/IJGM.S318949
62. Kamel A, Abdelseed H, Albalawi Y, Aslsalameen E, Almutairi Y, Alkattan A. Evaluation of the effect of zinc, quercetin, bromelain and vitamin C on COVID-19 patients. medRxiv [preprint] (2020). doi: 10.1101/2020.12.22.20245993
63. Onal H, Arslan B, Ucuncu Ergun N, Topuz S, Yilmaz Semerci S, Kurnaz M, et al. Treatment of COVID-19 patients with quercetin: a prospective, single center, randomized, controlled trial. Turk J Biol. (2021) 45:518–29. doi: 10.3906/biy-2104-16
64. Rondanelli M, Perna S, Gasparri C, Petrangolini G, Allegrini P, Cavioni A, et al. Promising effects of 3-month period of quercetin phytosome((R)) supplementation in the prevention of symptomatic COVID-19 disease in healthcare workers: a pilot study. Life. (2022) 12:10066. doi: 10.3390/life12010066
65. Shohan M, Nashibi R, Mahmoudian-Sani M, Abolnezhadian F, Ghafourian M, Alavi S, et al. The therapeutic efficacy of quercetin in combination with antiviral drugs in hospitalized COVID-19 patients: a randomized controlled trial. Eur J Pharmacol. (2022) 914:174615. doi: 10.1016/j.ejphar.2021.174615
66. Zupanets I, Golubovska O, Tarasenko O, Bezuhla N, Pasichnik M, Karabinyosh S, et al. Efficacy of quercetin in patients with pneumonia associated with coronavirus disease (COVID-19). Zaporizhia Med J. (2021) 23:636–43. doi: 10.14739/2310-1210.2021.5.231714
67. Khan A, Iqtadar S, Mumtaz S, Heinrich M, Pascual-Figal D, Livingstone S, et al. Oral co-supplementation of curcumin, quercetin, and vitamin D3 as an adjuvant therapy for mild to moderate symptoms of COVID-19-results from a pilot open-label, randomized controlled trial. Front Pharmacol. (2022) 13:898062. doi: 10.3389/fphar.2022.898062
68. Cheng A, Hsu C, Lin J, Hsu M, Ho Y, Shen T, et al. Phase I clinical trial of curcumin, a chemopreventive agent, in patients with high-risk or pre-malignant lesions. Anticancer Res. (2001) 21:2895–900.
69. Harwood M, Danielewska-Nikiel B, Borzelleca J, Flamm G, Williams G, Lines T. A critical review of the data related to the safety of quercetin and lack of evidence of in vivo toxicity, including lack of genotoxic/carcinogenic properties. Food Chem Toxicol. (2007) 45:2179–205. doi: 10.1016/j.fct.2007.05.015
Keywords: curcumin, quercetin, SARS-CoV-2, COVID-19, polyphenols
Citation: Ujjan ID, Khan S, Nigar R, Ahmed H, Ahmad S and Khan A (2023) The possible therapeutic role of curcumin and quercetin in the early-stage of COVID-19—Results from a pragmatic randomized clinical trial. Front. Nutr. 9:1023997. doi: 10.3389/fnut.2022.1023997
Received: 20 August 2022; Accepted: 29 December 2022;
Published: 18 January 2023.
Edited by:
Silvia Turroni, University of Bologna, ItalyReviewed by:
Renuka Suravajhala, Amrita Vishwa Vidyapeetham University, IndiaCopyright © 2023 Ujjan, Khan, Nigar, Ahmed, Ahmad and Khan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Amjad Khan,  YW1qYWRraGFuQGx1bWhzLmVkdS5waw==
YW1qYWRraGFuQGx1bWhzLmVkdS5waw==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.