
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Nutr. , 09 January 2023
Sec. Nutritional Epidemiology
Volume 9 - 2022 | https://doi.org/10.3389/fnut.2022.1023000
This article is part of the Research Topic Dietary and Nutritional Indices and Chronic Diseases View all 22 articles
 I-Hsin Lin1,2
I-Hsin Lin1,2 Te-Chih Wong3
Te-Chih Wong3 Tuyen Van Duong2
Tuyen Van Duong2 Shih-Wei Nien1
Shih-Wei Nien1 I-Hsin Tseng1
I-Hsin Tseng1 Hsu-Han Wang4,5
Hsu-Han Wang4,5 Yang-Jen Chiang4,5
Yang-Jen Chiang4,5 Shwu-Huey Yang2,6,7*
Shwu-Huey Yang2,6,7*Background: This study investigated the association between dietary quality indices and recurrent chronic kidney disease (rCKD) in Taiwanese post-renal transplant recipients (RTRs).
Methods: This prospective study recruited RTRs aged >18 years with a functioning allograft and without any acute rejection in the past 3 months from September 2016 to June 2018. Dietary quality indices included the Alternative Healthy Eating Index (AHEI) and AHEI-2010, and the Taiwanese version of the AHEI (AHEI-Taiwan) was calculated using 3-day dietary records, and calculated scores were divided into quartiles. Laboratory data were collected from medical records. rCKD was defined as an estimated glomerular filtration rate (eGFR) of <60 mL/min/1.73 m2. Logistic regression analysis was performed to analyze the associations.
Results: This study included 102 RTRs. The RTRs with higher AHEI, AHEI-Taiwan, and AHEI-2010 scores were older and had higher eGFRs and lower odds of rCKD. As compared with the lowest quartile, patients with the highest quartiles of the AHEI [odds ratio (OR), 0.10; 95% confidence interval (95% CI): 0.02, 0.49; p-trend = 0.004), AHEI-2010 (OR, 0.17; 95% CI: 0.04, 0.72; p-trend = 0.016], and AHEI-Taiwan (OR, 0.13; 95% CI: 0.03–0.59; p-trend = 0.008) had lower odds of rCKD, respectively. As compared with the lowest quartile, patients who consumed the highest quartiles of red and processed meat had 11.43 times higher odds of rCKD (OR, 11.43; 95% CI: 2.30–56.85; p for trend <0.01).
Conclusion: Higher dietary quality indices are associated with lower odds of rCKD in Taiwanese RTRs. Particularly, a positive association between a higher intake of red meat and processed meat and higher odds of rCKD remained exists after transplantation in Taiwanese RTRs. Further dietary guidelines and individualized dietary education were necessary for RTRs to prevent graft function deterioration.
Chronic kidney disease (CKD) was a major global public health problem, and its prevalence is 10–15% worldwide (1) and 11.3% in Taiwan (2). Among renal replacement therapies, renal transplantation was around 2,000 cases in Taiwan (2), which was more favorable compared with dialysis for patients with end-stage renal disease and those requiring dialysis because it had lower medical costs and results in better quality of life and higher survival rates (3). However, the elimination of dietary restrictions and conflict dietary recommendation and habits from dialysis to transplantation may also result in graft function deterioration and cause recurrent chronic kidney disease (rCKD) in renal transplant recipients (RTRs) (4).
Evidence indicates that lifestyle modifications including improved dietary quality can prevent metabolic abnormalities and reduce the risk of CKD (5, 6) and chronic diseases (7). In a previous study, we observed that RTRs had poor adherence to dietary recommendations and the intake of most nutrients was inadequate (8). The National Kidney Foundation (NKF) and National Health and Research Institutes in Taiwan (2, 9) published healthy guideline recommendations as a balance diet for RTRs includes foods from food guides, such as a variety of fresh fruits and vegetables, wholegrains, lean meats, low-fat dairy, and also low salt and high in fiber intake.
The Alternative Healthy Eating Index (AHEI) (10) includes food and nutrient components, such as trans fatty acid, the ratio of polyunsaturated fatty acid and saturated fatty acid (PSR), fruit, vegetables, wholegrains, the ratio of white and red meat, nut and soybean, and vitamin used and alcohol intake and is commonly used for the assessment of dietary quality. Both the AHEI and its updated version, AHEI-2010 (11), are based on the American Dietary Guidelines. AHEI-2010 was according to AHEI and was modified PSR to polyunsaturated fatty acid (PUFA) and n-3 PUFA, meanwhile focusing on red meat, sodium, and sugar intake. The previous study demonstrated that adherence to the AHEI and AHEI-2010 was associated with a lower risk of chronic diseases (12–14). However, Mccullough and Willett (15) reported that the dietary index can be modified according to the national dietary recommendations to be more approached to dietary culture in each country. The Taiwanese version of the AHEI-Taiwan (16) was composed as the original AHEI and modified the cutoff based on Taiwan’s dietary recommendations to adapt to Taiwanese dietary pattern.
In addition, several studies had reported that the adherence to the AHEI and AHEI-2010 was associated with decreased kidney function deterioration in CKD populations (6, 17, 18). However, the association between these indices, especially the AHEI-Taiwan, and graft function prevention had not been examined for Taiwanese RTRs. This study aimed to investigate the association between dietary quality indices and graft dysfunction in Taiwanese RTRs. We hypothesized that RTRs with higher AHEI, AHEI-Taiwan, and AHEI-2010 scores would have a lower risk of rCKD. Moreover, we explored the association between the dietary indices food component and the rCKD risk for further analysis.
This prospective cross-sectional study was conducted between September 2016 and June 2018 at Linkou Chang Gung Memorial Hospital. Inclusion criteria included that RTRs aged >18 years with a functioning allograft and without any acute rejection reaction in the past 3 months were recruited in this study. Excluded criteria included Patients with an estimated glomerular filtration rate (eGFR) variation of >25% in the past 3 months and other systemic inflammatory diseases.
A total of 106 eligible RTRs were enrolled and referred to qualified registered dietitians in the hospital for face-to-face interviews. Informed consent was obtained from each participant before the interview. The RTRs with considerably low-calorie or high-calorie intake (≤800 or ≥3,000 kcal) were excluded (n = 4). Hence, 102 RTRs were included in the final analysis. The study procedures complied with ethical standards for research with human participants, and the study protocol was reviewed and approved by the Institutional Review Board of Chang Gung Medical Foundation (IRB No. 201600954B0).
We collected the following patient characteristics and laboratory data from medical records: age, sex, dialysis history, transplant history, years after dialysis or transplantation, body height (without shoes), body weight (two times, tenth of a point taken, no shoes, and wear light clothing), performance of handgrip (measure three times for maximum values), blood pressure (average of three times), fasting plasma glucose, homeostatic model assessment of insulin resistance (HOMA-IR), total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), triglyceride (TG), serum albumin, serum creatinine (Cr), estimated glomerular filtration rate, and high sensitive C-reactive protein. rCKD was defined as the deterioration of kidney function to end-stage renal disease (ESRD) after transplantation and was at risk for reverting to ESRD, which eGFR of <60 mL/min/1.73 m2 based on the Kidney Disease Outcomes Quality Initiative (KDOQI) guidelines (9).
Dietary intake was determined using self-reported 3-day dietary records (including 2 weekdays and 1 day on the weekend) and evaluated by the qualified registered dietitian one time during the latest clinical follow-up in the study period. Dietary food and nutrient intakes were calculated using nutrition analysis software (CofitPro version 1.0.0. Cofit HealthCare Inc., Taipei, Taiwan), according to Taiwan’s Ministry of Health and Welfare Food and Drug Administration database as described previously (19).
A total of three dietary indices based on food and nutrients were used to evaluate dietary quality: the AHEI (10), AHEI-2010 (11), and AHEI-Taiwan (16). The AHEI is based on the 2015–2020 Dietary Guidelines for Americans and includes nine components; the total AHEI score ranges from 0 (unhealthy eating quality) to 87.5 (healthy eating quality). Intermediate intake was proportionally calculated between the range of 0 and 10 points. The AHEI scores are based on the consumption of trans fat, the polyunsaturated-to-saturated fatty acid ratio (PSR) vegetables, fruits, nuts, and soybean, white and red meat, wholegrain fiber, daily multivitamins, and alcohol.
The AHEI-2010 is an updated version of the AHEI and includes 11 components; its total score ranges from 0 (unhealthy eating quality) to 110 (healthy eating quality). Compared with the AHEI, the AHEI-2010 considers the low consumption of sodium (10 points for the lowest decile) and sugar-rich beverages (10 points for <1 serving/day), the ratio of white meat to red/processed meat (10 points for 0 serving/day and 0 points for ≥1.5 servings/day), PUFA (10 points for ≥10% PUFA consumption), and n-3 PUFA (10 points for 250 mg). Moreover, in the AHEI-2010, the cutoff values were revised for the high consumption of wholegrain fiber (10 points for ≥90 g in men and ≥75 g in women) and the moderate consumption of alcohol (10 points for 0.5–3.5 equivalent in men and 0.5–2.5 equivalent in women).
The AHEI-Taiwan was developed from the AHEI according to Taiwan’s dietary recommendations for the convenience of a clinical study and better adaption to the Taiwanese population. Similar to the AHEI, the AHEI-Taiwan includes nine components, and its scores ranged from 0 (unhealthy eating quality) to 87.5 (unhealthy eating quality). In the AHEI-Taiwan, the consumption of trans fat is calculated in grams (10 points for ≥1 g and 0 points for ≤8 g); the measures for the high consumption of vegetables and fruits are revised from 5 and 4 servings/day to 3 and 2 servings/day, respectively; and the consumption of wholegrain cereal was calculated as the total recommended percent intake of cereals in Taiwan. These calculations differ from those in the AHEI.
The characteristics of the RTRs are summarized by the quartile of each dietary index score. Statistical analyses were performed using SAS (version 9.4, SAS Institute, Cary, NC, USA). Descriptive data are presented as the mean, standard deviation, interquartile range, and percentage as appropriate. Logistic regression analysis was performed to analyze associations between dietary quality and rCKD risk. The possible affecting factors of kidney function, such as age, sex, calorie intake, Charlson comorbidity index (CCI), body mass index, geriatric nutrition risk index, handgrip, transplantation time, and dialysis time, were adjusted based on KDOQI guidelines (9). Study data are presented as odds ratio (OR) with 95% confidence interval (95% CI). The significance was set at P < 0.05.
The mean scores of AHEI, AHEI-Taiwan, and AHEI-2010 were 43.6 ± 8.8, 44.6 ± 9.0, and 62.1 ± 10.2, respectively. The RTRs in the highest quartile of both the AHEI and AHEI-Taiwan were older (53.1 ± 14.3 vs. 40.8 ± 11.5, p < 0.001; 51.7 ± 14.6 vs. 42.1 ± 10.7, p < 0.05, respectively), had higher eGFRs (64.9 ± 10.3 vs. 48.7 ± 15.9, p < 0.01; 64.6 ± 19.7 vs. 48.5 ± 14.8, p < 0.01, respectively), and had lower body height (158.7 ± 7.6 vs. 165.5 ± 8.6, p < 0.01; 159.1 ± 8.1 vs. 166.7 ± 8.4, p < 0.01, respectively), TC (196.7 ± 42.0 vs. 221.2 ± 40.8, p < 0.05; 195.6 ± 41.4 vs. 217.5 ± 38.2, p < 0.05, respectively), LDL-C (111.3 ± 38.7 vs. 135.2 ± 34.3, p < 0.05; 108.8 ± 38.6 vs. 134.0 ± 32.9, p < 0.05, respectively), and Cr (1.1 ± 0.4 vs. 1.7 ± 1.0, p < 0.001; 1.2 ± 0.4 vs. 1.7 ± 1.0, p < 0.01, respectively) levels. A greater proportion of patients in the highest quartile of the AHEI-2010 were women and older (52.8 ± 13.7 vs. 41.0 ± 10.4, p < 0.001) and had higher eGFRs (61.4 ± 23.6 vs. 50.9 ± 18.4, p < 0.05), longer transplant time (10.4 ± 5.5 vs. 7.1 ± 4.4, p < 0.05), and lower body height (160.0 ± 8.6 vs. 166.4 ± 9.0, p < 0.05) and Cr (1.3 ± 0.7 vs. 1.8 ± 1.4, p < 0.01) levels. The albumin level was normal in both the lowest and highest quartiles of the AHEI-2010 group (4.2 ± 0.3 vs. 4.4 ± 0.3, p < 0.01), but the higher albumin level was significant higher in the highest quartiles of the AHEI-2010 (Table 1).
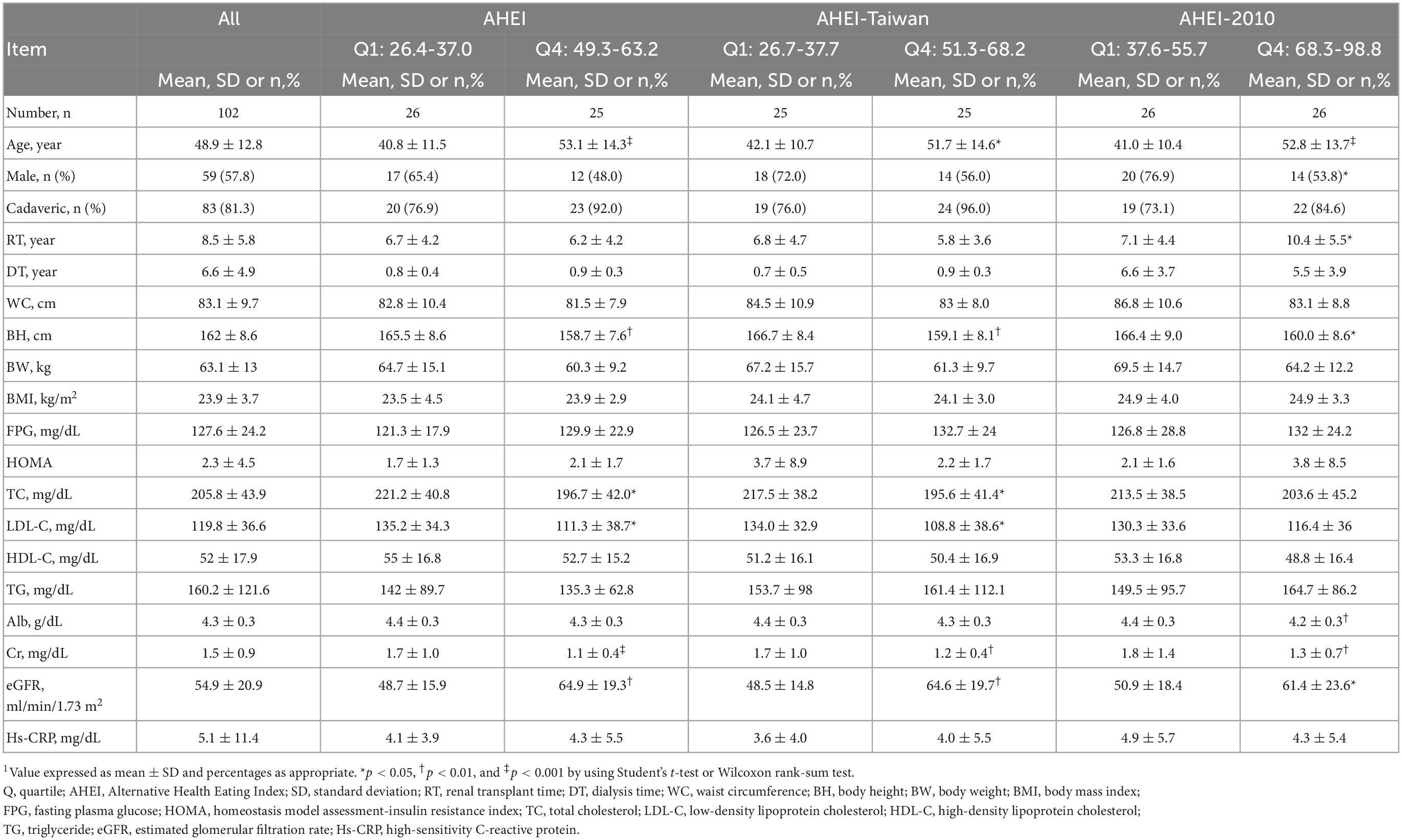
Table 1. Comparison of the components and scores of the AHEI and AHEI-Taiwan between the lowest and highest quartiles of dietary scores (n = 102)1.
The RTRs with the highest AHEI and AHEI-Taiwan scores had higher scores for the consumption of fruits (4.7 ± 2.7 vs. 1.1 ± 1.6; 7.9 ± 2.4 vs. 1.6 ± 3.0, p < 0.001, respectively), vegetables (6.4 ± 2.9 vs. 4.2 ± 1.7, p < 0.05; 8.9 ± 1.7 vs. 6.0 ± 2.1, p < 0.001, respectively), wholegrain (8.4 ± 3.6 vs. 0.8 ± 2.7, p < 0.001; 5.2 ± 5.1 vs. 0.5 ± 1.1, p < 0.001, respectively), white and red meat (4.8 ± 3.6 vs. 2.0 ± 1.7, p < 0.05; 4.9 ± 3.5 vs. 1.7 ± 1.6, p < 0.001, respectively), and nuts and soybean (9.1 ± 2.4 vs. 3.3 ± 4.2, p < 0.001; 7.8 ± 3.4 vs. 2.5 ± 3.5, p < 0.001, respectively) as well as higher total dietary scores (Table 2). The RTRs with the highest AHEI-2010 scores had higher scores for the consumption of fruits (4.9 ± 2.5 vs. 1.1 ± 1.6, p < 0.001), vegetables (6.3 ± 3.1 vs. 4.0 ± 1.7, p < 0.01), wholegrain (4.9 ± 4.2 vs. 0.6 ± 1.4, p < 0.001), and nuts and soybean (9.1 ± 2.4 vs. 4.0 ± 4.5, p < 0.01) and lower scores for the consumption of red or processed meat (2.4 ± 3.1 vs. 0.0 ± 0.0, p < 0.001), sodium (7.9 ± 2. vs. 3.7 ± 3.5, p < 0.001), and sugar (9.7 ± 0.4 vs. 9.4 ± 0.7, p < 0.05; Table 3).

Table 2. Comparison of the components and scores between the lowest and the highest quartiles of AHEI and AHEI-Taiwan dietary scores1.
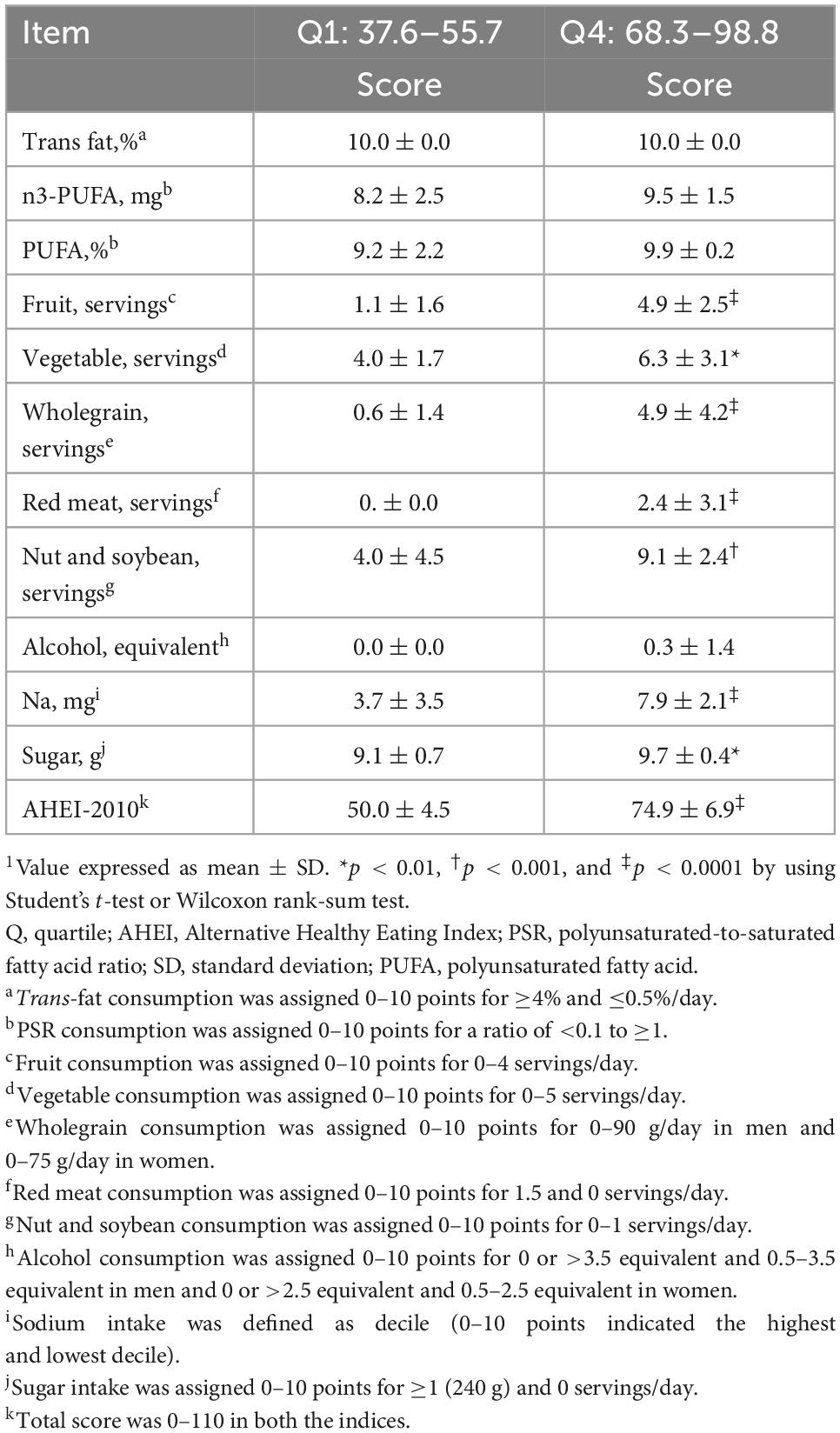
Table 3. Comparison of components and scores between the lowest and the highest quartiles of AHEI-2010 dietary scores1.
A total of 65 RTRs (64%) were diagnosed as having rCKD. All the dietary quality scores were associated with lower odds of rCKD. Compared with the lowest quartile, the highest quartile of the AHEI, AHEI-Taiwan, and AHEI-2010 had 88% (OR, 0.12; 95% CI: 0.03–0.46; p-trend <0.01), 87% (OR, 0.13; 95% CI: 0.03–0.48; p-trend <0.01), and 82% (OR, 0.18; 95% CI: 0.05–0.61; p-trend <0.01) lower odds of rCKD, respectively, in the crude model. In model 1, after adjustment for age and sex, the highest quartile of the AHEI, AHEI-Taiwan, and AHEI-2010 had 90% (OR, 0.09; 95% CI: 0.02–0.40; p-trend <0.01), 89% (OR, 0.11; 95% CI: 0.03–0.45; p-trend <0.01), and 85% (OR, 0.15; 95% CI: 0.04–0.59; p-trend <0.01) lower odds of rCKD, respectively. After additional adjustment for calorie intake and CCI, the RTRs in the highest quartile of the AHEI, AHEI-Taiwan, and AHEI-2010 had 90% (OR, 0.09; 95% CI: 0.02–0.39; p-trend <0.01), 90% (OR, 0.10; 95% CI: 0.03–0.43; p-trend <0.01), and 85% (OR, 0.15; 95% CI: 0.04–0.57; p-trend <0.05) lower odds of rCKD, respectively. In model 3, after further adjustment for body mass index (BMI), geriatric nutrition risk index (GNRI), handgrip, transplant time, and dialysis time, the RTRs in the highest quartile of the AHEI, AHEI-Taiwan, and AHEI-2010 had 91% (OR, 0.09; 95% CI: 0.02–0.46; p-trend <0.01), 88% (OR, 0.12; 95% CI: 0.03–0.59; p-trend <0.01), and 83% (OR, 0.17; 95% CI: 0.04–0.73; p-trend = 0.02) lower odds of rCKD, respectively (Table 4). Further analysis revealed that RTRs who consumed high amounts of red/processed meat had 11.43 times higher odds of rCKD (OR, 11.43; 95% CI: 2.30–56.85; p-trend <0.01; Table 5).
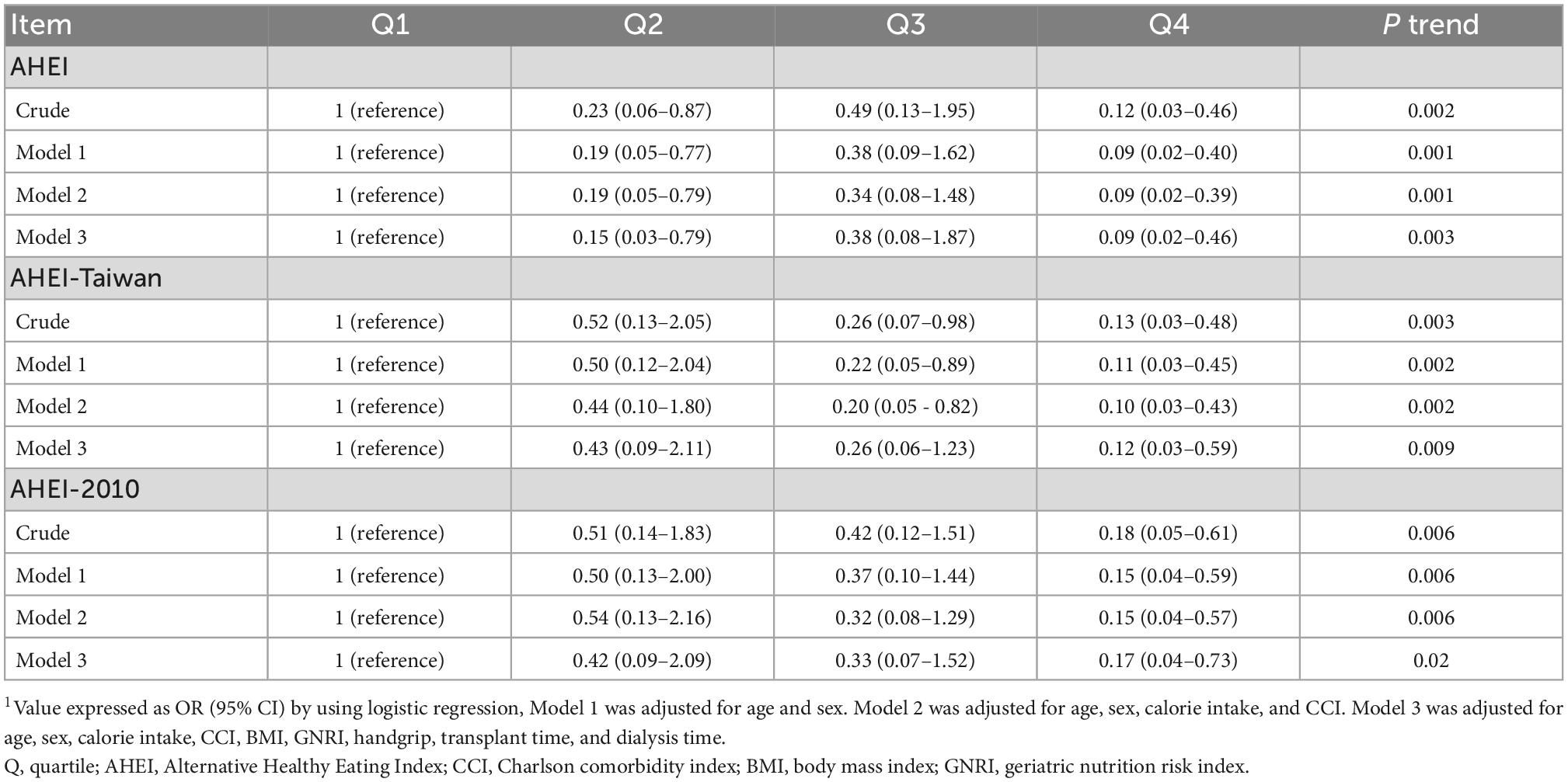
Table 4. Risk of incident chronic kidney disease by the AHEI, AHEI-Taiwan, and AHEI-2010 in the renal transplant recipients1.
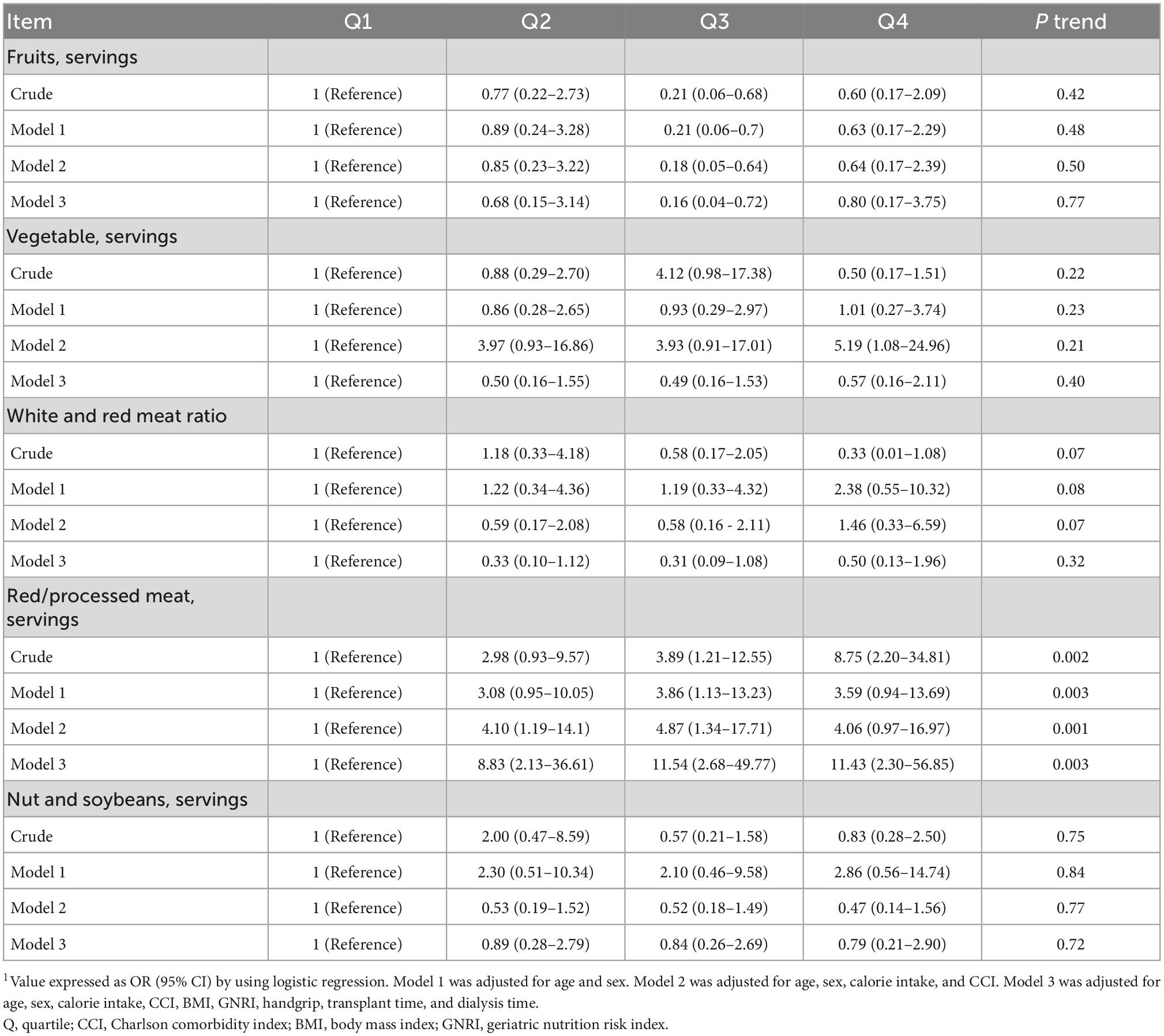
Table 5. Risk of incident chronic kidney disease by dietary indices in the renal transplant recipients.
The results of this cohort study revealed that the RTRs in the highest quartiles of the AHEI, AHEI-Taiwan, and AHEI-2010 had 91, 88, and 83% lower odds of rCKD, respectively, compared with the RTRs in the lowest quartiles of these indices after adjustment for age, sex, calorie intake, CCI, BMI, GNRI, handgrip, transplant time, and dialysis time. In addition, higher consumption of red/processed meat was associated with 11.4 times higher odds of rCKD.
The results of the present study are consistent with those of a prospective cohort study (20) with a follow-up period of 14.3 years that recruited 4,848 participants and examined their dietary quality by using the Health Eating Index (HEI), AHEI-2010, Dietary Approaches to Stop Hypertension (DASH) diet, and alternate Mediterranean diet (aMED) and indicated that high dietary quality was associated with CKD prevention. Hu et al. (6) included 3,980 patients with CKD with a follow-up period of 24 years and indicated that high HEI, AHEI-2010, and aMED scores were associated with a 13–20% lower risk of incident CKD. Osté et al. (21) reported that the high scores of the DASH diet were associated with lower renal dysfunction and mortality in RTRs. In addition, some studies have demonstrated that the DASH diet and aMED were inversely associated with the risk of CKD and prevented a decrease in the eGFR and an increase in Cr and micro-albuminuria levels (22–25). These findings suggest that high dietary quality is associated with CKD prevention. Notably, the prevention of rCKD is more important for RTRs due to the elimination of dietary restrictions and incorrect dietary habits after transplantation (4). On the contrary, Song et al. (26) demonstrated that a revised version, the DASH-Japan Ube Modified diet Program (DASH-JUMP) and Korean DASH diet (K-DASH) were modified according to Japanese and Korean dietary recommendation, which is consistent with the present study of AHEI-Taiwan to adapt Taiwanese dietary recommendations.
Regarding the component of dietary indices, vegetable and fruit consumption were not associated with preserving the eGFR in the present study; this finding is in agreement with that of a previous study that enrolled Dutch (27) and American (28) participants. However, Jhee et al. (29) demonstrated that the high consumption of vegetables and fruits was associated with decreased albuminuria and kidney injury. A reason for this finding is that the consumption of vegetables and fruits rich in potassium is associated with lower blood pressure, which possibly prevents kidney function deterioration (30). Another reason for the positive association between vegetable and fruit consumption and lower risk of CKD may be the effect of decreased acid load. The high dietary acid load may increase metabolic acidosis and lead to kidney injury through an increase in the levels of endothelin-1, which stimulates aldosterone production by activating the renin–angiotensin–aldosterone system pathway, increasing the ammonium concentration, and leading to kidney tubular injury, endothelial dysfunction, and inflammation (31–33). Future studies should investigate the effect of vegetable and fruit consumption on rCKD in RTRs.
Previous studies (34) have examined the association between different protein sources such as red/processed meat, nuts, and soybean, and CKD prevalence. Red/processed meat can lead to inflammation, increase sodium load and iron’s pro-oxidative effects, and cause DNA damage, thus directly or indirectly affecting kidney function. In addition, animal protein sources increase the acid load, whereas plant protein sources increase alkalosis load; the association between acid load and CKD progression has also been demonstrated (35). No associations between white-to-red meat ratio, nut and soybean consumption, and rCKD were noted. However, O’Keefe et al. (36) demonstrated that the high consumption of soybean was associated with decreased phosphate intake and urinary protein excretion, thus preventing CKD progression. Haring et al. (37) and Mirmiran et al. (38) have reported that replacing one serving of total red/processed meat with one serving of legumes, nuts, wholegrain cereal, low-fat dairy, and fish and seafood was associated with 18–31% and 16–21% lower risk of CKD, respectively. Future studies should evaluate the association between replacing protein sources and rCKD risk in RTRs.
This study has some strengths and limitations. To date, this is the first study to investigate the association between dietary quality and rCKD in Taiwanese RTRs. However, the causality could not be interpreted because of the cross-sectional design of this study. Although the findings of the current study limit the evidence of randomized controlled trials, the results were obtained using 3-day dietary records including 2 weekdays and 1 day on the weekend, and 24-h recall was used to determine dietary quality based on food composition data provided by Taiwan’s Ministry of Health and Welfare. Furthermore, a composite definition was used to define rCKD. These methods helped us assess the dietary intake of the RTRs, evaluate nutrition-related problems, and enhance awareness regarding dietary quality and the rCKD in Taiwanese RTRs. The small sample size of this study may reduce the statistical power (β = 0.7) to detect significant associations. Finally, although many potential confounders were adjusted, the possibility of imperfectly measured or unknown confounders (such as non-immunological and immunological factors) was not excluded in this observational study.
This prospective study examined food and nutrient intake, which reflect dietary quality in patients receiving renal transplantation. Overall, higher AHEI, AHEI-Taiwan, and AHEI-2010 scores were associated with lower odds of rCKD in Taiwanese RTRs. Notably, AHEI-Taiwan is based on Taiwan’s dietary recommendation, which may be more adaptive to Taiwanese populations. Moreover, further analysis for the dietary component as red/processed meat was positively associated with rCKD. Additional longitudinal and randomized controlled studies are required to verify the association between dietary quality and rCKD.
The original contributions presented in this study are included in this article/supplementary material, further inquiries can be directed to the corresponding author.
The studies involving human participants were reviewed and approved by the Institutional Review Board of Chang Gung Medical Foundation (IRB No. 201600954B0). The patients/participants provided their written informed consent to participate in this study.
I-HL, TD, T-CW, and S-HY: conceptualization. I-HL and TD: methodology and analysis and interpretation of data. I-HL, S-WN, and I-HT: software. TD, T-CW, and S-HY: validation and supervision. I-HL, S-WN, H-HW, and Y-JC: investigation. H-HW and Y-JC: resources. I-HL, TD, S-WN, and I-HT: data curation. I-HL: visualization and writing—original draft. I-HL, TD, and S-HY: writing—reviewing and editing. I-HL, S-WN, I-HT, H-HW, and Y-JC: project administration. All authors contributed to the article and approved the submitted version.
This research was funded by the Chang Gung Memorial Hospital, grant number CMRPG3F2001-2.
We express the appreciation to all medical staff and patients who participated in this study from the Chang Gung Memorial Hospital for helping with study conduction and data collections. The project was supported by the Chang Gung Memorial Hospitality (CMRPG3F2001-2). The manuscript was edited by the Wallace Academic Editing and also complies with the authorship and publishing of ethical guidelines.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Jha V, Garcia-Garcia G, Iseki K, Li Z, Naicker S, Plattner B, et al. Chronic kidney disease: global dimension and perspectives. Lancet. (2013) 382:260–72. doi: 10.1016/S0140-6736(13)60687-X
2. National Health and Research Institutes. Taiwan Chronic Kidney Disease Clinical Guidelines. Zhunan: National Health and Research Institutes (2015).
3. Reimer J, Franke G, Lütkes P, Kohnle M, Gerken G, Philipp T, et al. Quality of life in patients before and after kidney transplantation. Psychother Psychosom Med Psychol. (2002) 52:16–23. doi: 10.1055/s-2002-19662
4. Chan W, Bosch J, Jones D, McTernan P, Phillips A, Borrows R. Obesity in kidney transplantation. J Ren Nutr. (2014) 24:1–12. doi: 10.1053/j.jrn.2013.09.002
5. Snelson M, Clarke R, Coughlan M. Stirring the pot: can dietary modification alleviate the burden of CKD? Nutrients. (2017) 9:265–93. doi: 10.3390/nu9030265
6. Hu E, Steffen L, Grams M, Crews D, Coresh J, Appel L, et al. Dietary patterns and risk of incident chronic kidney disease: the atherosclerosis risk in communities study. Am J Clin Nutr. (2019) 110:713–21. doi: 10.1093/ajcn/nqz146
7. Dubois L, Girard M, Bergeron N. The choice of a diet quality indicator to evaluate the nutritional health of populations. Public Health Nutr. (2000) 3:357–65. doi: 10.1017/S1368980000000409
8. Lin IH, Wong TC, Nien SW, Chou YT, Chiang YJ, Wang HH, et al. Dietary compliance among renal transplant recipients: a single-center study in Taiwan. Transplant Proc. (2019) 51:1325–30. doi: 10.1016/j.transproceed.2019.02.026
9. Ikizler T, Burrowes J, Byham-Gray L, Campbell K, Carrero J, Chan W, et al. KDOQI clinical practice guideline for nutrition in CKD: 2020 update. Am J Kidney Dis. (2020) 76:S1–107. doi: 10.1053/j.ajkd.2020.05.006
10. McCullough M, Feskanich D, Stampfer M, Giovannucci E, Rimm E, Hu F, et al. Diet quality and major chronic disease risk in men and women: moving toward improved dietary guidance. Am J Clin Nutr. (2002) 76:1261–71. doi: 10.1093/ajcn/76.6.1261
11. Chiuve S, Fung T, Rimm E, Hu F, McCullough M, Wang M, et al. Alternative dietary indices both strongly predict risk of chronic disease. J Nutr. (2012) 142:1009–18. doi: 10.3945/jn.111.157222
12. Ma Y, Li W, Olendzki B, Pagoto S, Merriam P, Chiriboga D, et al. Dietary quality 1 year after diagnosis of coronary heart disease. J Am Diet Assoc. (2008) 108:240–6. doi: 10.1016/j.jada.2007.10.047
13. Schwingshackl L, Hoffmann G. Diet quality as assessed by the healthy eating index, the alternate healthy eating index, the dietary approaches to stop hypertension score, and health outcomes: a systematic review and meta-analysis of cohort studies. J Acad Nutr Diet. (2015) 115:780–800.
14. Turner-McGrievy G, Barnard N, Cohen J, Jenkins D, Gloede L, Green A. Changes in nutrient intake and dietary quality among participants with type 2 diabetes following a low-fat vegan diet or a conventional diabetes diet for 22 weeks. J Am Diet Assoc. (2008) 108:1636–45. doi: 10.1016/j.jand.2014.12.009
15. McCullough M, Willett W. Evaluating adherence to recommended diets in adults: the alternate healthy eating index. Public Health Nutr. (2006) 9:152–7. doi: 10.1079/PHN2005938
16. Lei WS. Association Between Alternate Healthy Eating Index for Taiwan (AHEI-T) and Anthropometry, Blood Sugar, Blood Pressure and Serum Lipid Profile Among Type 2 Diabetes Mellitus Patient. Ph.D Thesis. Taipei: School of Nutrition and Health Sciences Taipei Medical University (2010).
17. Geng T, Jafar T, Neelakantan N, Yuan J, van Dam R, Koh W. Healthful dietary patterns and risk of end-stage kidney disease: the Singapore Chinese health study. Am J Clin Nutr. (2021) 113:675–83. doi: 10.1093/ajcn/nqaa348
18. Hu E, Coresh J, Anderson C, Appel L, Grams M, Crews D, et al. Dietary patterns and risk of chronic kidney disease progression and all-cause mortality: findings from the CRIC study. Curr Dev Nutr. (2020) 4:1415.
19. Lin I, Duong T, Wong T, Nien S, Tseng I, Chiang Y, et al. Dietary nutrients and cardiovascular risk factors among renal transplant recipients. Int J Environ Res Public Health. (2021) 18:8448–59. doi: 10.3390/ijerph18168448
20. Smyth A, Griffin M, Yusuf S, Mann J, Reddan D, Canavan M, et al. Diet and major renal outcomes: a prospective cohort study. The NIH-AARP diet and health study. J Ren Nutr. (2016) 26:288–98. doi: 10.1053/j.jrn.2016.01.016
21. Osté M, Gomes-Neto A, Corpeleijn E, Gans R, de Borst M, van den Berg E, et al. Dietary approach to stop hypertension (DASH) diet and risk of renal function decline and all-cause mortality in renal transplant recipients. Am J Transplant. (2018) 18:2523–33. doi: 10.1111/ajt.14707
22. Lin J, Fung T, Hu F, Curhan G. Association of dietary patterns with albuminuria and kidney function decline in older white women: a subgroup analysis from the nurses’ health study. Am J Kidney Dis. (2011) 57:245–54. doi: 10.1053/j.ajkd.2010.09.027
23. Chang A, Van Horn L, Jacobs D Jr, Liu K, Muntner P, Newsome B, et al. Lifestyle-related factors, obesity, and incident microalbuminuria: the CARDIA (coronary artery risk development in young adults) study. Am J Kidney Dis. (2013) 62:267–75. doi: 10.1053/j.ajkd.2013.02.363
24. Rebholz C, Crews D, Grams M, Steffen L, Levey A, Miller IE, et al. DASH (Dietary Approaches to Stop Hypertension) diet and risk of subsequent kidney disease. Am J Kidney Dis. (2016) 68:853–61. doi: 10.1053/j.ajkd.2016.05.019
25. Asghari G, Yuzbashian E, Mirmiran P, Azizi F. The association between dietary approaches to stop hypertension and incidence of chronic kidney disease in adults: the Tehran lipid and glucose study. Nephrol Dial Transplant. (2017) 32:224–30.
26. Song Y, Lobene A, Wang Y, Hill Gallant K. The DASH diet and cardiometabolic health and chronic kidney disease: a narrative review of the evidence in East Asian countries. Nutrients. (2021) 13:984–99. doi: 10.3390/nu13030984
27. Herber-Gast G, Boersma M, Verschuren W, Stehouwer C, Gansevoort R, Bakker S, et al. Consumption of whole grains, fruit and vegetables is not associated with indices of renal function in the population-based longitudinal doetinchem study. Br J Nutr. (2017) 118:375–82.
28. Foster M, Hwang S, Massaro J, Jacques P, Fox C, Chu A. Lifestyle factors and indices of kidney function in the Framingham heart study. Am J Nephrol. (2015) 41:267–74.
29. Jhee J, Kee Y, Park J, Chang T, Kang E, Yoo T, et al. A diet rich in vegetables and fruit and incident CKD: a community-based prospective cohort study. Am J Kidney Dis. (2019) 74:491–500. doi: 10.1053/j.ajkd.2019.02.023
30. Aburto N, Hanson S, Gutierrez H, Hooper L, Elliott P, Cappuccio F. Effect of increased potassium intake on cardiovascular risk factors and disease: systematic review and meta-analyses. BMJ. (2013) 346:f1378.
31. Khanna A, Simoni J, Hacker C, Duran M, Wesson D. Increased endothelin activity mediates augmented distal nephron acidification induced by dietary protein. J Am Soc Nephrol. (2004) 15:2266–75.
32. Wesson D, Simoni J, Broglio K, Sheather S. Acid retention accompanies reduced GFR in humans and increases plasma levels of endothelin and aldosterone. Am J Physiol Renal Physiol. (2011) 300:F830–7. doi: 10.1152/ajprenal.00587.2010
33. Scialla J. The balance of the evidence on acid–base homeostasis and progression of chronic kidney disease. Kidney Int. (2015) 88:9–11.
34. Mafra D, Borges N, de Franca Cardozo L, Anjos J, Black A, Moraes C, et al. Red meat intake in chronic kidney disease patients: two sides of the coin. Nutrition. (2018) 46:26–32. doi: 10.1016/j.nut.2017.08.015
35. Gaggl M, Sliber C, Sunder-Plassmann G. Effect of oral alkali supplementation on progression of chronic kidney disease. Curr Hypertens Rev. (2014) 10:112–20.
37. Haring B, Selvin E, Liang M, Coresh J, Grams M, Petruski-Ivleva N, et al. Dietary protein sources and risk for incident chronic kidney disease: results from the atherosclerosis risk in communities (ARIC) study. J Ren Nutr. (2017) 27:233–42. doi: 10.1053/j.jrn.2016.11.004
Keywords: dietary quality, kidney function, chronic kidney disease, renal transplant recipients, Taiwan
Citation: Lin I-H, Wong T-C, Duong TV, Nien S-W, Tseng I-H, Wang H-H, Chiang Y-J and Yang S-H (2023) Dietary quality indices and recurrent chronic kidney disease in Taiwanese post-renal transplant recipients. Front. Nutr. 9:1023000. doi: 10.3389/fnut.2022.1023000
Received: 19 August 2022; Accepted: 19 December 2022;
Published: 09 January 2023.
Edited by:
Sorayya Kheirouri, Tabriz University of Medical Sciences, IranReviewed by:
Jeanette Mary Andrade, University of Florida, United StatesCopyright © 2023 Lin, Wong, Duong, Nien, Tseng, Wang, Chiang and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shwu-Huey Yang,  c2hlcnJ5QHRtdS5lZHUudHc=
c2hlcnJ5QHRtdS5lZHUudHc=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.