- College of Food Science and Technology, Hebei Agricultural University, Baoding, China
In this study, we investigated the structural features of the polysaccharide obtained from Craterellus cornucopioides (CCP2) by high-performance liquid chromatography, Fourier transform infrared spectroscopy and ion chromatography. The results showed that CCP2 was a catenarian pyranose that principally comprised of mannose, galactose, glucose, and xylose in the ratio of 1.86: 1.57: 1.00: 1.14, with a molecular weight of 8.28 × 104 Da. Moreover, the immunoregulation effect of CCP2 was evaluated both in vitro and in vivo. It displayed a remarkable immunological activity and activation in RAW264.7 cells by enhancing the phagocytosis of macrophages in a dose-dependent manner without showing cytotoxicity at the concentrations of 10–200 μg/mL in vitro. Additionally, Histopathological analysis indicated the protective function of CCP2 against immunosuppression induced by cyclophosphamide (Cy). Meanwhile, the intake of CCP2 had better immunoregulatory activity for immunosuppression BALB/c mice model. After prevention by CCP2, the spleen and thymus weight indexes of BALB/c mice model were significantly increased. The RT-qPCR and Western Blot results provided comprehensive evidence that the CCP2 could activate macrophages by enhancing the production of cytokines (IL-2, IL-6, and IL-8) and upregulating the protein expression of cell membrane receptor TLR4 and its downstream protein kinase (TRAF6, TRIF, and NF-κB p65) production of immunosuppressive mice through TLR4-NFκB p65 pathway. The results demonstrated that CCP2 could be a potential prebiotic and might provide meaningful information for further research on the immune mechanism.
Introduction
The immune system comprises a heterogeneous population of cells that are relatively quiescent in a steady-state, however, they respond to inflammation, infection, and other perturbations (1). In clinic settings, patients with compromised immunity may be particularly vulnerable to normal and opportunistic infections (2). The innate immune system comprises innate dendritic cells, natural killers cells (NK), macrophages, mast cells (MCs) and NKT cells as primary defense entity (3, 4) and protects against invading pathogens in non-specific way (4, 5). One of the most important non-specific immune actions is phagocytosis, which is performed by macrophages (6). Macrophages are involved in antiviral, anti-tumor activities, hypersensitivity reactions, autoimmune diseases, and immune regulation in adaptive and innate immune responses via the production of cytokines (such as interferon γ), interleukin-6 (IL-6), interleukin-10 (IL-10), and tumor necrosis factor α (TNF-α) (7). After regulating the activation and inhibition of receptors, the immune system activates the pathogen associated pattern-recognition receptors (PRRs) (8–10).
Polysaccharides, as metabolic products of plants, animals, and microorganisms, have attracted considerable attention due to their therapeutic effects, and are considered the immunological molecules of the innate immune system (11). It enhances the ability of macrophages to resist external stress and survive under various conditions by promoting the integrity and stability of the outer membrane (12). Several studies have been conducted to investigate the pharmacological activities and active components of edible and medicinal plants (13–16). It showed that fungal polysaccharides are efficacious in the treatment of diabetes, hypolipidemia, oxidative stress, and obesity, as well as in the activation of innate immune cells and stimulation of cytokines secretions (17, 18).
Yu, et al. (19) demonstrated that the porphyra-derived oligosaccharides possessed antigen-specific immune responses by regulating the levels of IgG1, IgG2a, and OVA-specific IgE, and producing IL-2, IFN-γ, IL-4, and IL17 in ovalbumin (OVA)-sensitized mice. Wusiman, et al. (20) verified that the Lagenaria siceraria (Molina) standl polysaccharide and sulfated modified LSP50 could induce long-lasting and high hemagglutination (HI) titers, antigen-specific lgG-NDV antibody, splenic lymphocyte proliferation, high immune organ index, which could be served as a novel and effective vaccine adjuvant in chicken to induce specific immune responses against infections and diseases.
Therefore, the activations of macrophages induced by fungal polysaccharides are essential for the innate immune system.
Craterellus cornucopioides is wild, edible fungus, that is widely distributed around the world (China, Japan, Korea, North America, and Europe). In our previous studies (21–23), a natural immune heteroglycan (average molecular weight of 1.97 × 106 Da) with the potential to activate RAW264.7 macrophages were obtained from C. cornucopioides (CCP) in vitro. This heteroglycan showed potent immunomodulatory properties and reversed immunosuppression by enhancing the development of the immune system and the activation of peritoneal macrophage phagocytosis via regulation of the TLR4-NFκB pathway in peritoneal macrophages of immunosuppressed mice, which shows excellent prospects for the commercial development of functional foods and medicines (21–24).
Similarly, polysaccharides obtained from C. cornucopioides (CCP2) also have strong immunoregulatory potential in the extrinsic pathway. However, to date, a comprehensive understanding of the immunomodulatory activity of CCP2 in vitro and its structural characteristics have not been reported. The structural and bioactivity diversities of CCP2 remain unclear. Generally, the comprehensive utilization of agricultural products has significant economic and social environmental benefits, and has thus gained growing interests in the development of agricultural products.
On this basis, the structure information and immunomodulatory activity of CCP2 were investigated by FTIR, and in terms of monosaccharide composition. The proliferation, phagocytosis, and morphology of RAW264.7 cells were applied to understand the relationship between structural properties and biological activities, which further expands the application and advantages of C. cornucopioides.
Materials and methods
Materials and reagents
The fruiting body of C. cornucopioides was collected at the Junzi mountain of Shizong in Yunnan Province, P.R. Different monosaccharide standards (L-rhamnose, D -glucose, D-mannose, D-galactose, D-arabinose, and D-xylose) and DEAE-52 column (1.6 cm × 100 cm) were provided by Solarbio Biological Technology Company (BJ, CHN). Neutral red and 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT) were provided by Sigma Company (St Louis, MO, USA). Phosphate buffered saline, dimethyl sulfoxide (DMSO), Dulbecco’s Modified Eagle Medium (DMEM), and fetal bovine serum (FBS) were purchased from Gibco BRL (NY, USA). All the other reagents were of analytical grade.
Extraction and purification of CCP2
The C. cornucopioides powder was extracted with distilled water at 85°C for 2.5 h (twice) after degreasing with acetone. The water extract was concentrated and deproteinized using the sevag reagent [Chloroform: n-butanol = 4:1 (V:V), 30 min, 10 times]. Finally, three volumes of ethanol were added to precipitate the crude polysaccharide (CCCP), which were collected after centrifugation at 3,000 rpm at 25°C for 10 min and freeze-dried under −80°C after redissolving in water. The yield was calculated using formula 1 as follows:
Where WCCCP and Wsample are the weights of CCCP and C. cornucopioides powder, respectively.
CCCP (80 mg) was dissolved in distilled water (2 mL), purified by the DEAE-A52 column (1.6 cm × 100 cm), and eluted at the flow rate of 0.6 mL/min. The eluent contained a macromolecule that was discovered by HPLC and named C. cornucopioides polysaccharide (CCP2).
Molecular weight of CCP2
The Mw of CCP2 was determined by high-performance gel permeation chromatography (Agilent-1200) with a Shodex OHpak gel SB-805HQ column (8.0 mm × 300 mm, 35°C) and a refractive index detector (30°C). The sample solution (20 μL, 5 mg/mL) was injected into the apparatus. Deionized water was used as the flowing phase at the flow rate of 0.6 mL/min. The standard curve was established using the T-series Dextran (T-2000, T-500, T-70, T-40, T-20, and T-10) (25, 26).
Determination of monosaccharide composition of CCP2
The ICS2500 chromatography system (Thermo) with the high-performance anion chromatography column Carbo Pac PA20 (150 mm × 3 mm) and a dual pulse current sensor was used to determine the monosaccharide composition of CCP2 (NaOH at 2.00 and 10.00 mM was used as the eluent, the flow rate was 0.45 mL/min, and the temperature was set at 30°C). In total, 5.00 mg of CCP2 was hydrolyzed with 2 M Trifluoroacetic Acid (TFA, 5 mL) for 3 h at 120°C. Followed, the samples were diluted with ionized water according to the gradient. One milliliter CCP2 solution was injected into the apparatus. D-mannose, D-xylose, D-arabinose, L-rhamnose, D-galactose, and D-glucose were derivatized as standards.
FT-IR analysis
The experimental methods were referred to the literature reports (27). Briefly, 1.00 mg of CCP2 and 150 mg of KBr were mixed evenly and pressed into flake. Pure KBr flake was used as the blank background, and then the polysaccharide sample was analyzed on an Fourier Transform Infrared Spectroscopy (FT-IR) spectrophotometer with a resolution of 4 cm–1 (range: 4000 –400 cm–1) (VECTOR 22, Bruker, Germany).
Cell culture
The RAW264.7 cells were cultured in DMEM supplemented with 10% (v/v) FBS streptomycin (100 units/mL), and penicillin (100 units/mL) at 37°C, and 5% CO2 in a humidified atmosphere. Cells were passaged every 48 h for reserve.
Cell phagocytosis assays
The experimental method was according to the literature reports (28). For the neutral red uptake assay, the cell suspension (5 × 104 cell/mL) of the macrophages was added into 96-well plates at 37°C. After 4 h incubation, the supernates were removed and treated with different concentrations of CCP2 (0, 10, 25, 50, 100, 200, and 400 μg/mL) for 24, 36, and 48 h, respectively, and 100 μL of neutral red solutions were added and incubated for another 2 h. After staining, the cells were rinsed twice by Hank’s solution. Afterward, the cells were lysed with a lysis buffer [ethanol and 0.01% acetic acid at the ratio of 1:1 (100 μL per well) and detected at 540 nm].
General observation
During the experiment, all BALB/c mice were carefully monitored daily for signs of disease, the body weight and water intake of mice were recorded daily. The feeding environment was as follows: temperature: 22 ± 0.5°C, humidity: 50 ± 5%, light–dark cycle: 12:12 h. The mental state, stool consistency, diarrhea and rectal bleeding were observed and recorded. The mice were fasted for 24 h after gavage and sacrificed on the 17th day.
Histopathological observation
The experimental methods were referred to the literature reports (29).
Establishment of cy-induced immunosuppressive BALB/c mice model and treatments
Protective effects of CCP2 on immunosuppression mice were evaluated using a cyclophosphamide (Cy)-induced immunocompromised model recommended by China Food and Drug Administration (CFDA Publication No. 107, revised 2012). Briefly, 50 mice were randomly assigned into 5 groups (n = 10) according to the double-blind experiment after a week of adaptive feeding, including normal control group (NCG, 0.9% NaCl), immunocompromised model group (Cy-induced, CyMG), and three CCP2 as preventive treatment groups [CCP2 + Cy (L, M, H)]: 100, 200, and 400 mg kg–1 day–1. The details were as follows:
(1) NCG: Mice were intragastric administration once daily with 0.9% normal saline (0.2 mL) for 17 consecutive days, and intraperitoneally injected administration with normal saline (0.1 mL day–1) at 10th day for 3 days.
(2) CyG: Mice were intragastric administration once daily with 0.9% normal saline (0.2 mL) for 17 consecutive days and intraperitoneally injected administration with Cy (0.1 mL day–1, Mw 261.09 Da) at 10th day for 3 days.
(3) [CCP2 + Cy (L, M, H)]: Mice were intragastric administration with once daily with CCP2 at the doses of 100, 200, and 400 mg kg–1 day–1, respectively, for 17 consecutive days and intragastric administration with Cy at 10th day for 3 days.
On the 18th day after the various treatments, the BALB/c mice in each group were killed through the cervical dislocation method. The spleen and thymus were dissected and weighed.
The organ index was calculated as follows:
Western blot analysis
The experiment was conducted according to the method reported by Price et al. (30). Specifically, the total protein of peritoneal macrophage of BALB/c mice was extracted using radio immunoprecipitation assay lysis buffer according to the instruction of manufacture. After incubation of macrophages in 6-well plates (1 × 105 cells/mL) for 36 h, the macrophages were used for the protein extraction. All the primary antibodies were diluted with PBS for 1000 times (Cell Signaling Technology, Danvers, MA, USA).
In brief, cell lysates were subjected to 10% SDS-PAGE and transferred to nitrocellulose NC membranes, and then incubated overnight at 4°C with anti-TLR4, anti-TRIF, anti-TRAF6, anti-P-NF-kB p65, and anti-GAPHD monoclonal antibodies after a 1 h blocking on (5% (w/v) non-fat milk. The membranes were subsequently washed with Tris Buffered Saline Tween (TBST) and incubated for 1 h at room temperature with corresponding secondary anti-bodies. Immunoreactive bands were detected using enhanced chemiluminescence (ECL) kit (Millipore Co., Billerica, MA, USA), GAPHD was used as internal control.
Quantitative reverse transcription-polymerase chain reaction analysis
Quantitative reverse transcription Polymerase Chain Reaction (RT-qPCR) was conducted using SYBR RT-qPCR kit and Mx3000P™ RT-qPCR system (Stratagene, USA) in triplicate for each sample reaction according to previous report (31) to determine the mRNA expression of cytokines IL-2, IL-6, IL-8, and TNF-α. The total RNAs of peritoneal macrophage of BALB/c mice was extracted using Trizol reagent (Solarbio, Beijing, China) according to the instruction of manufacture and to synthesize cDNA by PrimeScript RT kit (Takara Biological Engineering Company, Dalian, China). The designed specific primers (Sangon Biotechnology company, Shanghai, China) were list in Table 1.
Data analysis
In this study, all statistical analyses were performed using SPSS 20.0 software (SPSS, Inc., IL, USA). Data were expressed as mean ± standard error (SE). One-way analysis of variance (ANOVA) and T-tests were used to test for statistical significance. P-values less than 0.05 were considered statistically significant.
Results and discussions
Extraction, purification and purity of CCP2
100 mg of CCCP was dissolved in distilled water (2 mL), purified by DEAE-A52 column (1.6 cm × 100 cm) and sequentially eluted with distilled water and 0.3 M NaCl at a flow rate of 0.6 mL/min. The eluant contained a macromolecule discovered by HPLC and named CCP1, which contained three fractions with similar polarity (Figure 1A). Further, the SephadexG-100 column (1.6 cm × 100 cm) was used to obtain CCP2. The single symmetrical peak (Figure 1B) at 15.592 min in HPLC indicates high purity. The UV absorption spectrum of thiirane revealed no obvious absorption peaks between 260 and 280 nm after full-wave scanning indicated little protein of CCP2. The average Mw of CCP2 was determined with a universal calibration curve using Dextran as a standard (32). Based on the calibration, the Mw of CCP2 was 8.28 × 104 Da.

Figure 1. Weight average distribution of (A) CCP1 (crude polysaccharides eluted with 0.3 M NaCl) and (B) CCP2 (purified polysaccharides extracted from C. cornucopioides).
The monosaccharide compositions and FTIR spectrum analysis of CCP2
The retention time of monosaccharide standard (Figure 2A) and CCP2 after degrading by TFA acid (Figure 2B) were shown in the Figure 2 and Table 2. After comparing the remain time and area between standard and CCP2, the results indicated that the CCP2 composed of D-Mannose, D-Galactose, D-Glucose, and D-Xylose with the molar ratio of 1.86: 1.57: 1.00:1.14, showing mannose might be the backbone of the CCP2 chain (33).
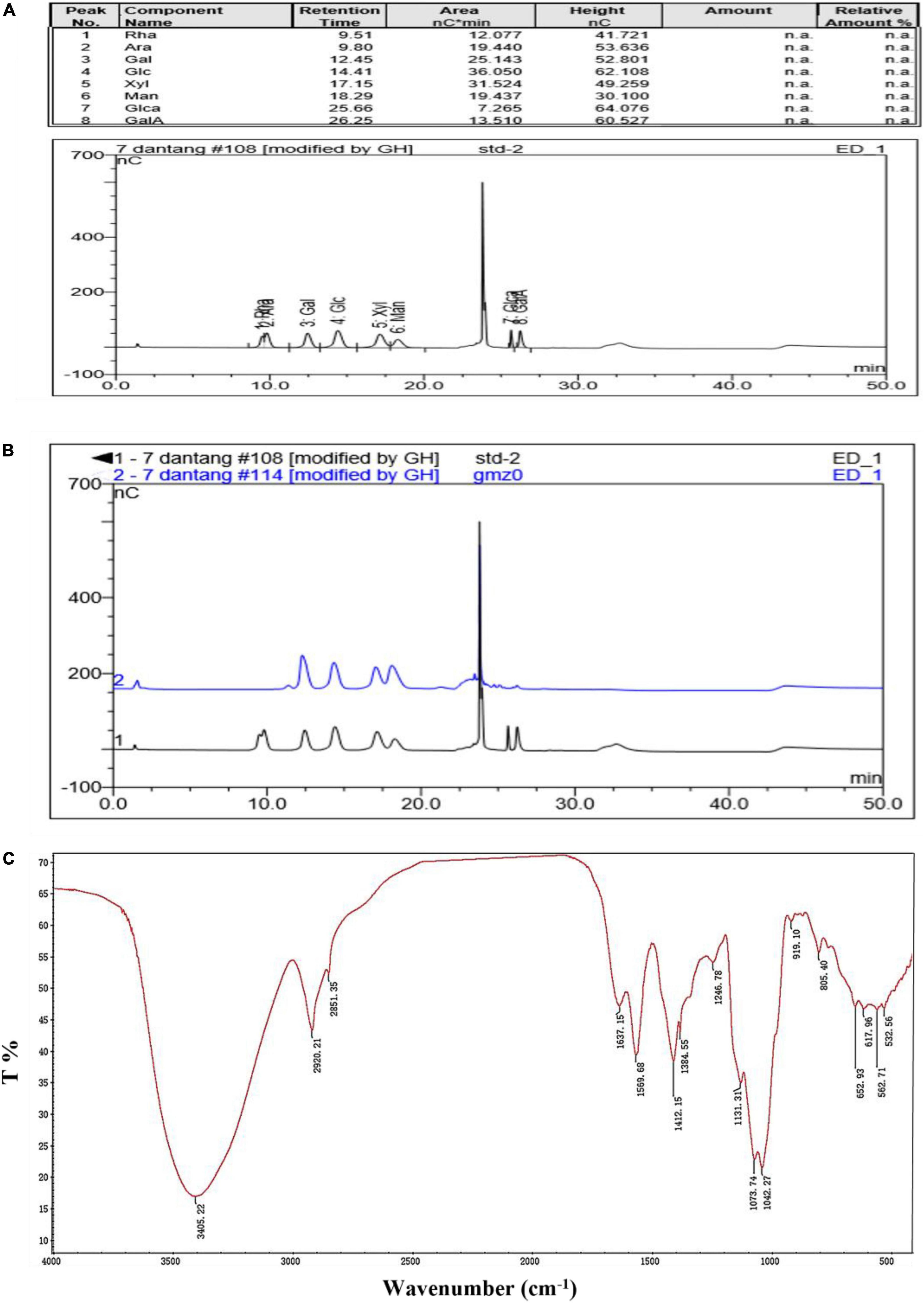
Figure 2. Monosaccharide composition [(A) Standards, (B) CCP2 (Blue line)] and the FTIR spectrum (C) of CCP2.
The absorption band of CCP2 was performed (range: 4000–400 cm–1). The band at 3405.22 cm–1 and 2920.21–2851.35 cm–1 were ascribed to the -OH and C-H stretching vibrations, respectively (21). The characteristic absorption peak of crystal water bending vibration was observed at 1637.15 cm–1 and the band at 1412.15 cm–1 was ascribed to -CH2 deformation absorption (34). Bands around 1246.78 cm–1 reflected the deformation vibration the of C-H bond. Similarly, bands between 1042.27–1073.74 cm–1 reflected the C-O-C stretching in the pyranose ring. The absorption characteristic peak at 919.10 cm–1 indicated β-type glycosidic bond. The peaks at 1131.31 cm–1, 1073.74 cm–1, and 1042.27 cm–1 indicated the existence of pyranoid ring structure (12).
Effects of CCP2 on immunoregulation in vitro
CCP2 promoted phagocytosis activation of peritoneal macrophages
Recently, polysaccharides have been proven to participate in cell immune defense, proliferation, and differentiation (35). Several polysaccharides were used as immunotherapeutic agents in the treatment of cancer and were clinically applied in combination with chemotherapy. Phagocytosis is the most important index to evaluate the activation and function of macrophages (36–38). In this study, the neutral red uptake assay was used to evaluate the phagocytosis of macrophages in vitro.
Relative cell phagocytosis of macrophages in the presence of CCP2 was significantly increased in a dose-dependent manner (10–200 μg/mL) than that of the control group (P < 0.01), reached maximum value at 100 μg/mL (Figure 3). Meanwhile, CCP2 significantly enhanced the phagocytosis of macrophages in a time-dependent (24, 36, and 48 h) and reached maximum at 36 h. Generally, the phagocytosis of RAW264.7 was significantly increased, attaining a maximum value at 36 h and 100 μg/mL of CCP2 (P < 0.01). The above results indicated that CCP2 holded a strong potential to stimulate macrophages, which was the key participant in innate and adaptive immunity. Compared with previous reports, CCP2 showed stronger effect on the activation of the phagocytosis of macrophages (22, 34, 39).
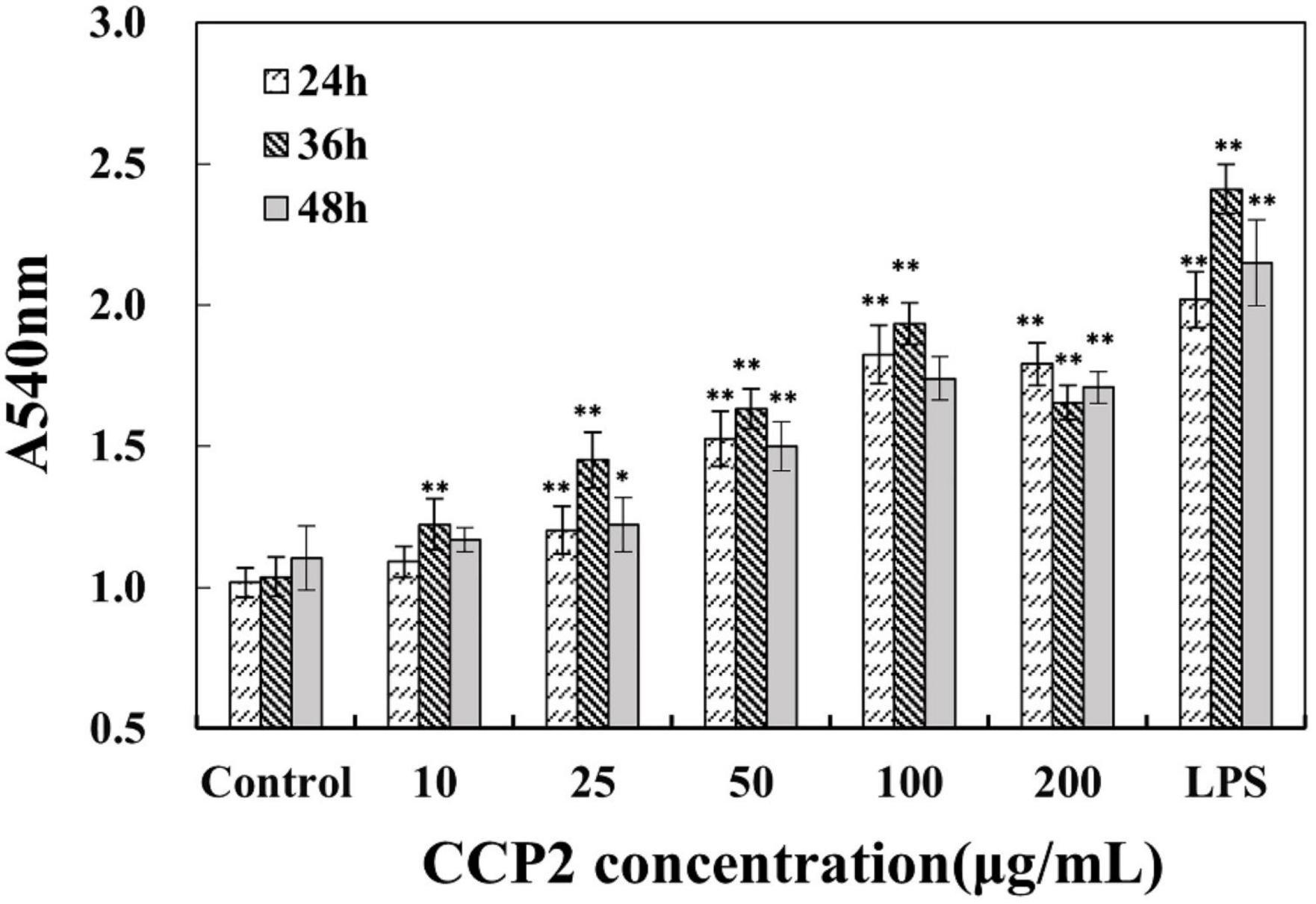
Figure 3. Effects of CCP2 on the phagocytic activity of macrophages. Cells were treated with CCP2 at various concentrations (10, 25, 50, 100, 200, and 400 μg/mL) for 24, 36, and 48 h, respectively. The control group was incubated with DMEM. *p < 0.05 and **p < 0.01 were significant when compared to the Control group. Data were expressed as means ± SD.
Effects of CCP2 on immunoregulation in vivo
The effect of CCP on thymus and spleen index of immunocompromised mice
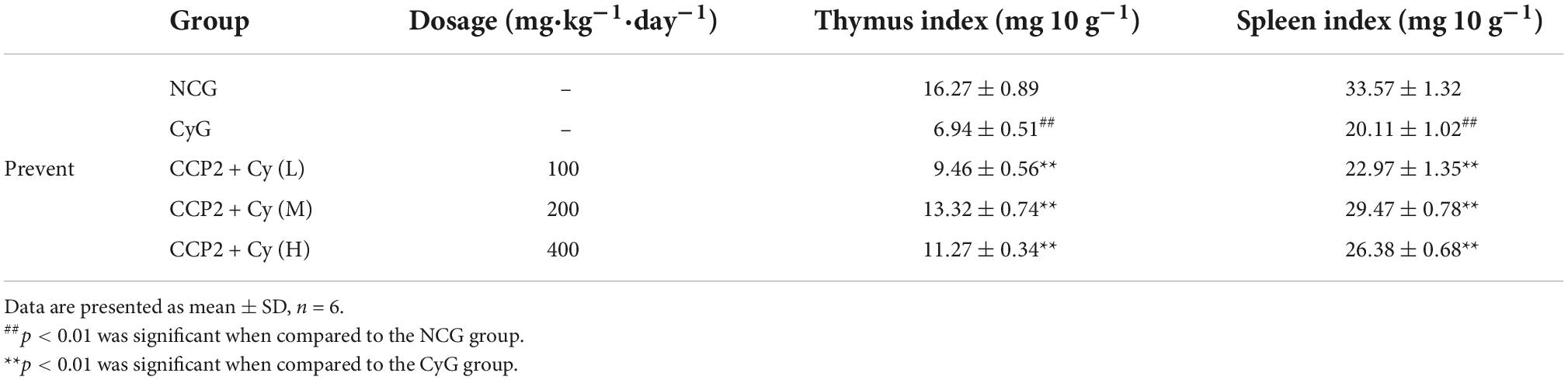
The spleen and thymus index of various treatment groups were dissected and weighed accurately on the 17th day. The relative thymus index and spleen index of CyG group decreased significantly (6.94 ± 0.51 vs. 16.27 ± 0.89 mg/10 g, 20.11 ± 1.02 vs. 33.57 ± 1.32 mg/10 g) compared with NCG group (Table 3). To compared with the CyG group, all the indexes in CCP2 treatment groups remarkable increased. The result implicated that the organic damage and immune function of immunocompromised mice might be recovered after CCP2 treatments.
Effects of CCP2 on the spleen investigated via histological examinations in the BALB/c mice
The destruction of the immune system, lead to autoimmune diseases and inflammatory diseases, always accompanied the organic damage (40). As an vital extrinsic diagnostic technology, HE image is easily available to assess the immunosuppressive status of organism (41, 42).
In this study, the HE stained was performed to evaluate the effect of CCP2 on colon tissues ultrastructure. As shows in Figure 4, histological analysis showed that the ultrastructure of spleen cells in the NCG were dense and arrange regularly. To compared with NCG group, CyG group (B) showed unclear red and white pulp structure and obvious intercellular spaces dilatation. However, the administration of CCP2 could significantly decreased the extent of macroscopic and microscopic intestinal irregular arrangement of cells induced by Cy. As the ultrastructure of spleen cells in CCP2 + Cy (M) (200 mg kg–1 day–1) and CCP2 + Cy (H) (400 mg kg–1 day–1) groups were dense, arrange regularly with clear nuclei, red and white pulp structure, which were similar to the case of NCG group. Likewise, the microscopic structure of CCP2 + Cy (L) (100 mg kg–1 day–1) group also recovered slightly. The histopathological analysis showed that CCP2 could attenuate the immune lesions of spleen in immunosuppression mice after Cy intervention.
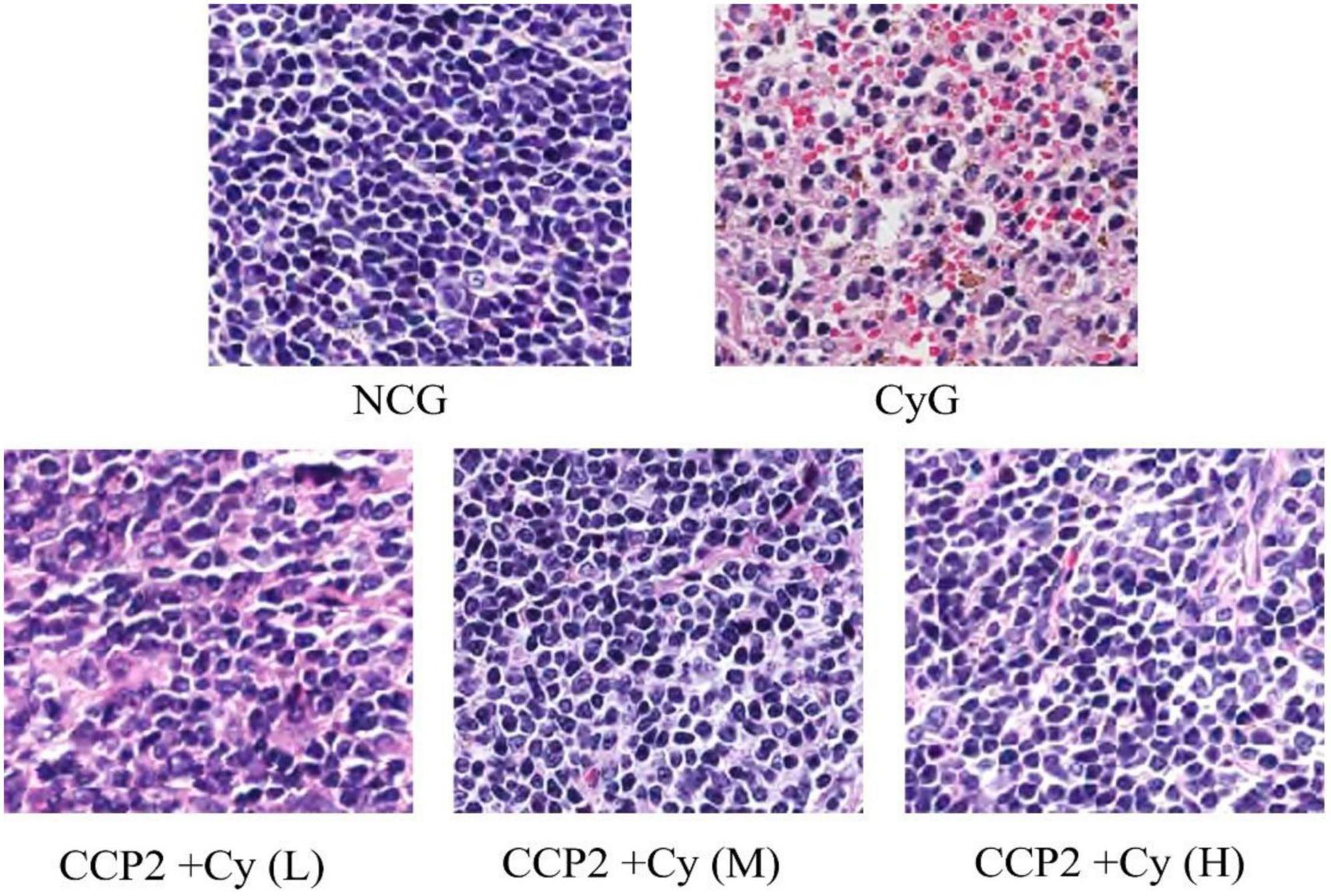
Figure 4. Effects of CCP2 on the spleen tissues showed in HE-stained histopathological images (scale bar = 100 μm, objective: 20×). NCG, normal control group; CyG, cyclophosphamide treatment group; CCP2 + Cy(L), Cy + CCP2 treatment group (100 mg kg–1 day–1); CCP2 + Cy(M): Cy + CCP2 treatment group (200 mg kg–1 day–1), CCP2 + Cy(H): Cy + CCP2 treatment group (400 mg kg–1 day–1).
Effects of CCP2 on the secretion of cytokines of the immunosuppressive BALB/c mice
Cytokines are synthesized and secreted by immune cells (macrophages, monocytes, B and T cells, DCs and neutrophils, etc.) and non-immune cells (endothelial cells, epidermal and fibroblasts, etc.) after stimulation by immunogen, inflammatory factors, and exogenous stimulant with the biological activities on regulating inflammatory, innate or adaptive immune response (43). Such as TNF-α and IL-1β were released robustly by monocytes and macrophages after treated with LPS or Tripalmitoyl-S-glyceryl-cysteine (Pam3Cys, a lipopeptide). ECs granulocyte-monocyte-colony stimulating factor (GM-CSF), secreted granulocyte-colony stimulating factor (G-CSF), IL-6, IL-10, and IL-1α as major cytokines upon TLR stimulation (44, 45).
To evaluate the immunosuppressive regulation capacity of CCP2, the mRNA expressions of immunological cytokines (IL-2, IL-6, TNF-α, and IL-8) in peritoneal macrophage of various treatment groups were analyzed (Figure 5). To compare with the NCG group, the expressions of IL-2, IL-6, IL-8, and TNF-α were suppressed significantly after Cy intervention (p < 0.01). As the comparison, CCP2 alleviated Cy-induced immunosuppression at a molecular level by promoting the production of IL-2, IL-6, and IL-8, but to different degrees in a dose-dependent manner. The CCP2 + Cy (M) and CCP2 + Cy (H) (200 and 400 mg kg–1 day–1) groups significantly enhanced the mRNA expression of above cytokines to compare with the CyG groups (p < 0.01) except TNF-α. Current data suggested that CCP2 capable of reversing the down-regulation of mRNA expressions to relieve the immunosuppressive.
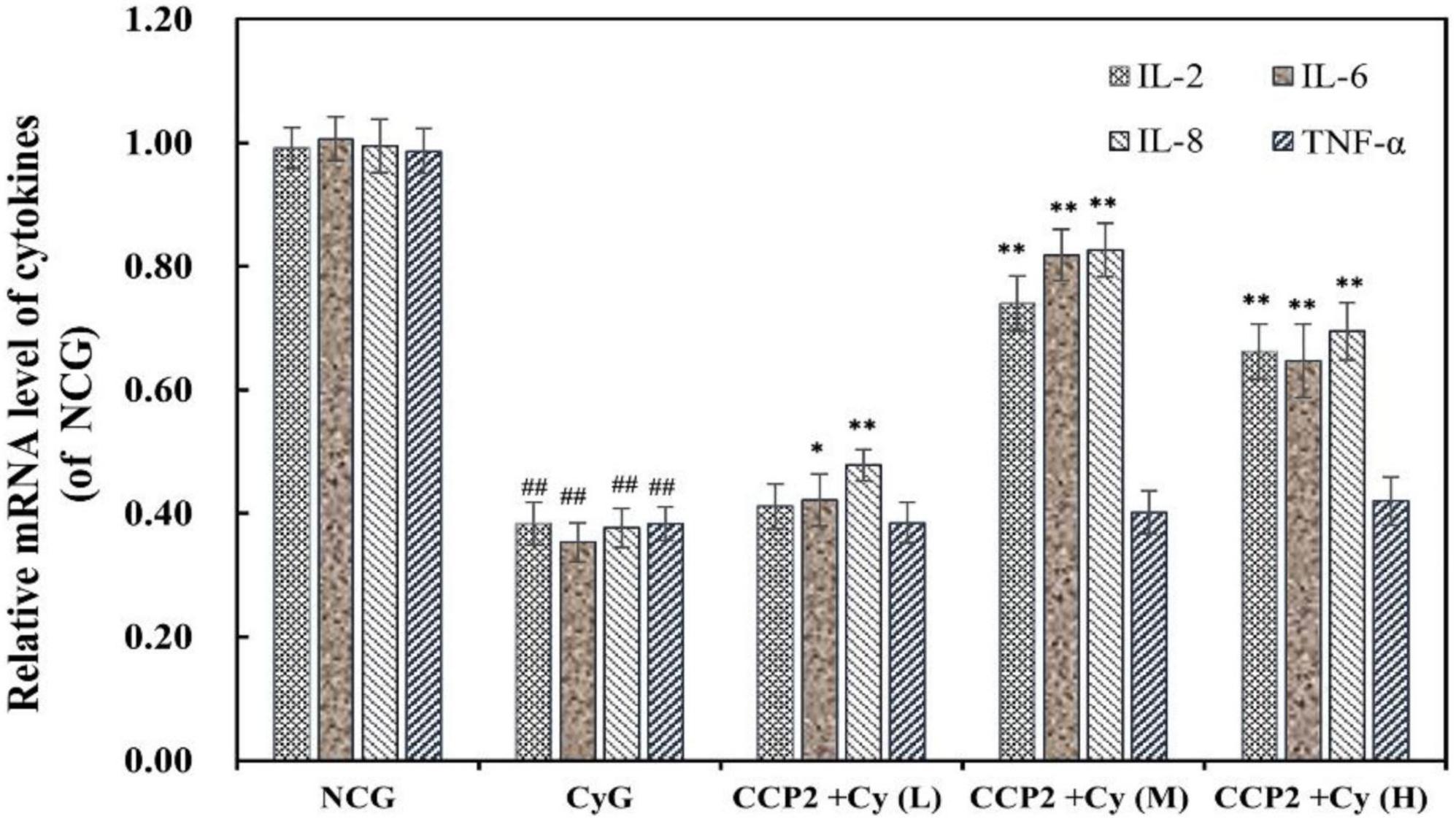
Figure 5. Effects of CCP2 on the secretion of cytokines (IL-2, IL-6, IL-8, and TNF-α) in the peritoneal macrophage of immunosuppressive mice induced by Cy. RT-qPCR analysis was relative to that of the reference gene (GAPHD). NCG: Normal control group, CyG: Cyclophosphamide treatment group, CCP2 + Cy (L): Cy + CCP2 treatment group (100 mg kg–1 day–1), CCP2 + Cy (M): Cy + CCP2 treatment group (200 mg kg–1 day–1), CCP2 + Cy (H): Cy + CCP2 treatment group (400 mg kg–1 day–1), ##p < 0.01 vs. the NCG group, *p < 0.05, **p < 0.01 vs. the CyG group. Data were expressed as mean ± SD, n = 6.
Effects of Craterellus cornucopioides on protein expressions in abdominal macrophages in BALB/c mice
Reportedly, TLRs and NF-κBs were involved in the stimulation of gene expression [such as Inducible Nitric Oxide Synthase (iNOS), IL-6, TNF-α mRNA] and cytokine secretion (such as NO, IL-6 and TNF-α) in immune responses. In view of the immunosuppression of Cy-induced injury as mentioned above, we elucidated an underlying mechanism of CCP2 effect via the TLR4-NF-κBp65 signal pathways, which were commonly involved in immune signaling cascades. The level of TLR4, TRIF, and TRAF6, and the phosphorylation of P- NFkB p65 were determined. As a result of Cy administration, the level of TLR4, TRIF, TRAF6, and P-NFκB p65 declined significantly, compared with those of mice in the NCG group. After administrating with CCP2, the phenomena (decrease of TLR4, TRIF, and TRAF6) were all alleviated significantly in a dose dependent manner, especially at the dose of 200 mg kg–1 day–1. Next, we evaluated the effect of CCP2 on the phosphorylation of p65. As shown in Figure 6, treatment with CCP2 increased the phosphorylation of p65 in a concentration-dependent manner. The CCP2 + Cy (M) and CCP2 + Cy (H) (200 and 400 mg kg–1 day–1) groups significantly enhanced the protein expression compared with the CyG groups (p < 0.01). Results suggested that CCP2 activated NF-κB signaling pathway which was implicated in transcriptional activation.
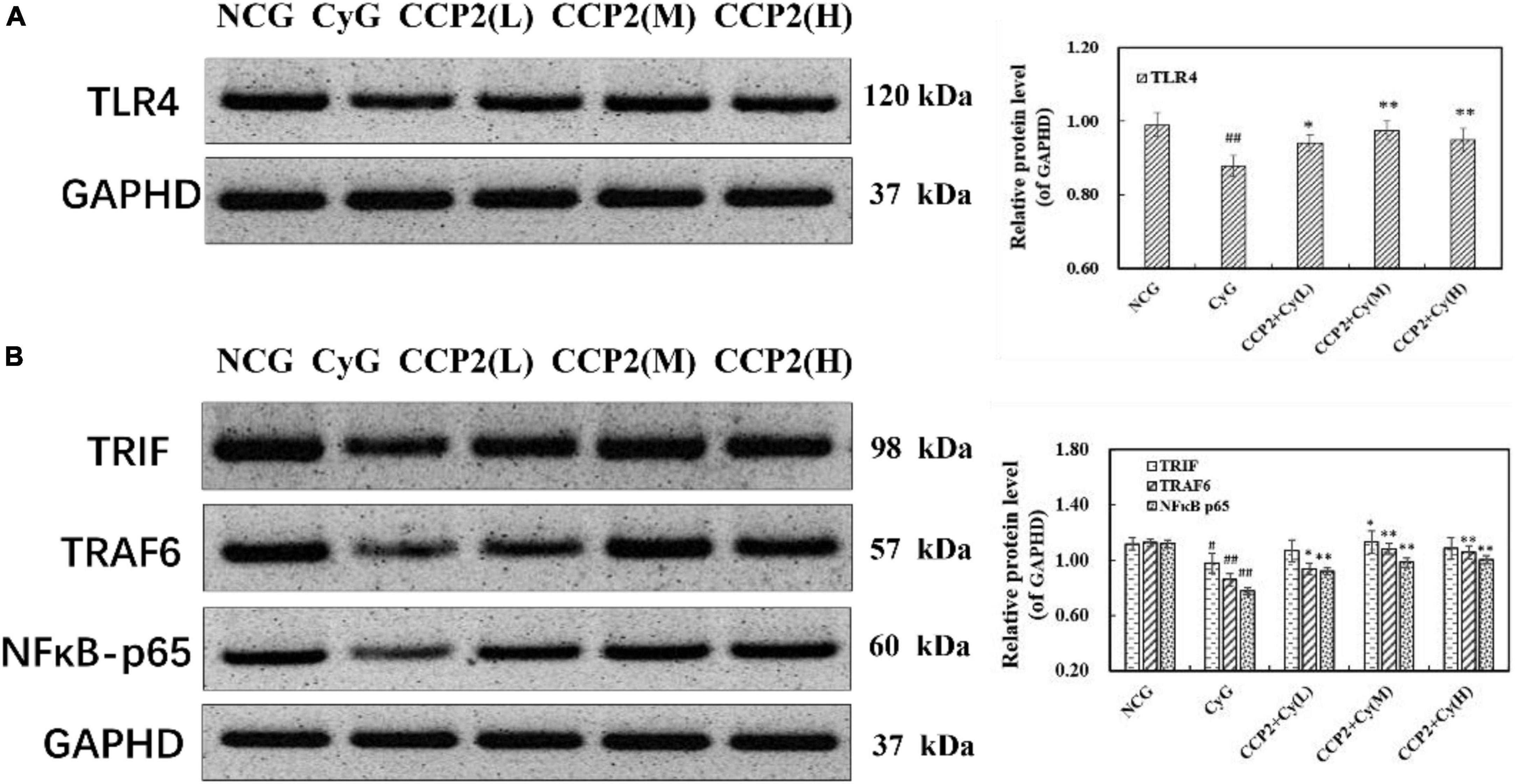
Figure 6. Detection of the protein expressions of TLR4, TRIF, TRAF6, and phosphor-NFκB-p65 by western blotting. (A) Effect of CCP on the expression of TLR4 in BALB/c mouse. (B) Effect of CCP on the expression of TRIF, TRAF6 and phosphor-NFκB-p65 in BALB/c mouse. GAPHD was used as an equal loading control. #p < 0.05, ##p < 0.01 vs. the NCG group, *p < 0.05, *p < 0.01 vs. the CyG group. Data were expressed as means ± SD, n = 10.
The main experiments contents and the sketch map was showed in Figure 7. In the light of the analysis conducted, we concluded that the receptor TLR4 plays a key role in the CCP2-modulated immunoregulation in immunosuppression mice model. Moreover, we showed that TLR4 in the pathogenesis of CCP2 modulated NF-κB pathways.
Discussion
Macrofungus (mushroom) has been extensively applied as traditional oriental medicine and food component for centuries (46, 47). Several reports revealed the importance of Split gill mushroom [Schizophyllum commune (Fr.:Fr.)], Lingzhi (in China) [Ganoderma lucidum (W.Curt.:Fr.) P. Karst.], Shiitake mushrooms [Lentinus edodes (Berk.) Sing], among other (48, 49). Nowadays, mushrooms are used as natural product-based pharmaceuticals with higher treatment potential and lower toxic effects in different pathological processes.
Polysaccharides are made up of identical or different monosaccharides together with glycosidic linkages to be linear or branching structure, which have been produced as the first biopolymer on earth (50, 51). The macrofungus polysaccharides and polysaccharide complexes components attract considerable attention due to their bioactive [such as efficient immunomodulatory, anti-cancer (52) and anti-inflammatory effects (53, 54)]. However, the structural information of different functional polysaccharides needs to be analyzed to supply and to expand the application of macro-fungus polysaccharides. Thus, in this study, we obtained a polysaccharide that principally comprised of mannose, galactose, glucose, and xylose in the ratio of 1.86:1.57:1.00:1.14, obtained from C. cornucopioides (CCP2) that widely distributed around the world (China, Japan, Korea, North America and Europe).
There was a clear correlation between allowed conformations and linking pattern (55). As confirmed by reports, polysaccharides extracted from MAE showed excellent biological properties owing to their complete structure, functional glycosidic linkages with a higher Mw and uronic acid content. In this study, The CCP2 was a catenarian pyranose with the Mw of 8.28 × 104 Da. The high structural diversity reflects the functional diversity of these molecules (55, 56). The number of structural factors such as monosaccharide composition, uronic acids content, molecular weight (Mw), glycosidic bond type in the backbone chain, and the esterification degree are profoundly affected on the antiradical, antioxidant, and antimicrobial activities of polysaccharides extracted from biological sources (5, 57).
Recently, the structure of numbers of different heteropolysaccharides had been precisely defined. It indicated that the heteropolysaccharides in mushrooms revealed prominently biological activities, in which β-D-glucan was mainly relative to immunomodulatory and anti-tumor activity. The most conversant polysaccharide in medicinal mushroom is β-glucan due to their ability on stimulating cytokine secretion ability of T cells, NK cells, and macrophage (proliferation and differentiation) (47, 58, 59). The water soluble β-glucan isolated from edible mushroom Entoloma lividoalbum, contains (1→3,6)-β-D-Glcp, (1→3)-β-D-Glcp, (1→6)-β-D-Glcp, and terminal β-D-Glcp glucosides, showed antioxidant and immune-stimulate activities on thymocyte, splenocyte, and macrophage (44). Based on the result of monosaccharide composition, CCP2 composed of mannose, glucose and galactose showed potential utilization in hypoimmunity population, might be a potential immunomodulatory.
In our previous study, we obtained a polysaccharide (CCP) with a molecular weight of 1.97 × 103 kDa from edible C. cornucopioides fruiting bodies (21, 23). It was a heteroglycan with (1→3)-linked-β-D-Manp-(1→6)-linked-α-D-Galp backbone distributed by (1→4)-linked-α-D-Xylp-t-α-D-Manp and t-β-D-Glup units at O-6 and composed of mannose (48.73%), galactose (17.37%), glucose (15.97%), and xylose (17.93%), and stimulated macrophage function, rising phagocytosis, and activated cell morphology of RAW264.7 cells by TLR4-NFκB pathway. In the present paper, we investigated the chemical structure and biological activity of another C. cornucopioides polysaccharide. This study reported the isolation, structure analysis, and immunoregulatory activity of CCP2. Similarly, the immunomodulatory capacity was also found in CCP2. indicating the efficacy. Further studies including clinical trials need to be carried out to ascertain the safety of these compounds as adequate alternatives to conventional medicine. Our results showed that CCP2 could promote the phagocytosis of RAW264.7 cells in a concentration-dependent potency manner.
Cy has wide-spreading side-effects, such as hepatotoxicity and nephrotoxicity (60, 61). According to our results of thymus and spleen index (Table 2), and histological examination on the spleen of immunocompromised mice. We hypothesized that the immunosuppression in this group probably attributed to Cy side-effects on organic damage.
The stimulating factors affected adaptive immune cells (Th1, Th2, Th17, Tgd17, and CD8 T cells) to secrete IL-4, IL-5, IL-15, TNF-α, and chemokine CXCL8 (IL-8), which influenced neutrophils, macrophages (M1 and M2), and other granulocytes to fight against extracellular bacteria, tumors, viruses or extracellular parasites involved in immunologic processes of infection resistance, autoimmunity and allergic disease. It has previously been described that Cy polarizes the immune response from Th1 to Th2 (62). In the current study, mRNA and protein levels of all targeted elements were severely decreased in three CCP2-treatment immunosuppressed mice groups.
Some polysaccharides, characterized from plants, animals, fungi, etc., with various pharmacological properties by inducing cytokine secretion in immune cells, causing its segments similar to the cell membrane which were predominantly composed of various polysaccharides with species-specific monosaccharides or structures. The present data demonstrated that CCP2 significantly stimulated the mRNA expression of IL-2, IL-8, IL-6 to modulate immune response.
The western blot was used to explain the phenomenon. As an integral membrane protein in cytoplasmic domain, the toll protein is the first identified in D. melanogaster as potent classes of PRRs (63). It is an essential factor involve in the survival and development of the embryo along with patterning, and characterize in recognizing the polysaccharide structures of cell walls. Among TLRs, TLR4 is known to induce production of TNF-α and IL-6. In this study, the expression of cell surface receptor TLR4 was elevated significantly. Moreover, the production of TRIF, TRAF6, and phosphorylation of NF-κBp65 were detected after administration of CCP2, indicating the activation of TLR4- NF-kBp65 signaling pathway.
These results indicated the impact of Cy in suppressing immune system through diminishing immune cells production, circulation and infiltration. The chemotherapy might cause an overall depletion of adaptive immune system cells.
Nevertheless, the administration of CCP2 reversed the immunosuppression side-effects that caused by Cy, which provided us a better understanding of the molecular mechanisms of the activation of immune system. Further understanding of the signaling pathways might provide novel insights into the mechanisms of immunomodulation and new opportunities on rational application of CCP2.
Conclusion
Polysaccharides obtained from fungi have attracted considerable attention due to their unique biological activities. In the present study, we investigated the chemical structure and immunoregulatory activity of CCP2 for the first time. Available data indicated CCP2 possessed immune-enhancing effect in vivo and in vitro to alleviate immunosuppression, which could be considered as a functional component of C. cornucopioides and an immunological modulator in the food nutrition industry.
Data availability statement
The original contributions presented in this study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Ethics statement
This animal study was reviewed and approved by the Animal Ethical and Welfare Committee (AEWC).
Author contributions
CZ designed and conceived the experiments. MG performed the experiments and wrote the manuscript. YS and YL contributed the reagents, materials, and analysis tools and analyzed the data. All authors have read and approved the final manuscript.
Funding
This study was supported by the Science and Technology Research Project of University in Hebei Province (QN2021072), Introduced Talent Research Project of Hebei Agricultural University (YJ2021003), and Key Research and Development projects of Hebei Province (22327310D).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Pearce EL, Pearce EJ. Metabolic pathways in immune cell activation and quiescence. Immunity. (2013) 38:633–43.
2. Goulopoulou S, McCarthy CG, Webb RC. Toll-like receptors in the vascular system: Sensing the dangers within. Pharmacol Rev. (2016) 68:142–67.
3. van Dijk RA, Rijs K, Wezel A, Hamming JF, Kolodgie FD, Virmani R, et al. Systematic evaluation of the cellular innate immune response during the process of human Atherosclerosis. J Am Heart Assoc. (2016) 5:e002860. doi: 10.1161/JAHA.115.002860
4. Ohta T, Kusano K, Ido A, Miura C, Miura T. Silkrose: A novel acidic polysaccharide from the silkmoth that can stimulate the innate immune response. Carbohydr Polym. (2016) 136:995–1001.
5. Ferreira SS, Passos CP, Madureira P, Vilanova M, Coimbra MA. Structure-function relationships of immunostimulatory polysaccharides: A review. Carbohydr Polym. (2015) 132:378–96.
6. Jin M, Zhao K, Huang Q, Shang P. Structural features and biological activities of the polysaccharides from Astragalus membranaceus. Int J Biol Macromol. (2014) 64:257–66. doi: 10.1016/j.ijbiomac.2013.12.002
7. Cao J, Tang D, Wang Y, Li X, Hong L, Sun C. Characteristics and immune-enhancing activity of pectic polysaccharides from sweet cherry (Prunus avium). Food Chem. (2018) 254:47–54. doi: 10.1016/j.foodchem.2018.01.145
8. Kumar V. Corrigendum to ‘Toll-like receptors in immunity and inflammatory diseases: Past, present, and future’ [International Immunopharmacology 59 (2018) 391-412]. Int Immunopharmacol. (2018) 62:338. doi: 10.1016/j.intimp.2018.06.044
9. Bernareggi D, Pouyanfard S, Kaufman DS. Development of innate immune cells from human pluripotent stem cells. Exp Hematol. (2019) 71:13–23.
10. Gao L, Han Y, Deng H, Hu W, Zhen H, Li N, et al. The role of a novel C-type lectin-like protein from planarian in innate immunity and regeneration. Dev Comp Immunol. (2017) 67:413–26. doi: 10.1016/j.dci.2016.08.010
11. Zdorovenko EL, Shashkov AS, Kadykova AA, Kiseleva EP, Savich VV, Novik GI, et al. Structural analysis of the O-polysaccharide from the lipopolysaccharide of Pseudomonas putida BIM B-1100. Carbohydr Res. (2018) 457:8–13. doi: 10.1016/j.carres.2017.12.009
12. Zeng YJ, Yang HR, Wang HF, Zong MH, Lou WY. Immune enhancement activity of a novel polysaccharide produced by Dendrobium officinale endophytic fungus Fusarium solani DO7. J Funct Foods. (2019) 53:266–75.
13. Liu Y, Duan X, Zhang M, Li C, Zhang Z, Liu A, et al. Cooking methods effect on the nutrients, bioaccessibility and antioxidant activity of Craterellus cornucopioides. Lwt. (2020) 131:109768.
14. Liu Y, Duan X, Zhang M, Li C, Zhang Z, Hu B, et al. Extraction, structure characterization, carboxymethylation and antioxidant activity of acidic polysaccharides from Craterellus cornucopioides. Ind Crops Prod. (2021) 159:113079.
15. Guo H, Diao QP, Hou DY, Li ZH, Zhou ZY, Feng T, et al. Sesquiterpenoids from cultures of the edible mushroom Craterellus cornucopioides. Phytochem Lett. (2017) 21:114–7.
16. Yang WW, Wang LM, Gong LL, Lu YM, Pan WJ, Wang Y, et al. Structural characterization and antioxidant activities of a novel polysaccharide fraction from the fruiting bodies of Craterellus cornucopioides. I Int J Biol Macromol. (2018) 117:473–82. doi: 10.1016/j.ijbiomac.2018.05.212
17. Yi Y, Lamikanra O, Sun J, Wang LM, Min T, Wang HX. Activity diversity structure-activity relationship of polysaccharides from lotus root varieties. Carbohydr Polym. (2018) 190:67–76. doi: 10.1016/j.carbpol.2017.11.090
18. Zhang S, Pang G, Chen C, Qin J, Yu H, Liu Y, et al. Effective cancer immunotherapy by Ganoderma lucidum polysaccharide-gold nanocomposites through dendritic cell activation and memory T cell response. Carbohydr Polym. (2019) 205:192–202. doi: 10.1016/j.carbpol.2018.10.028
19. Wei YJ, Fang RE, Ou JY, Pan CL, Huang CH. Modulatory effects of Porphyra-derived polysaccharides, oligosaccharides and their mixture on antigen-specific immune responses in ovalbumin-sensitized mice. J Funct Foods. (2022) 96:105209.
20. Wusiman A, Rexiati S, Aziz M, Cheng X, Mai Z, Abulaiti A, et al. Preparation and sulfate modified of Lagenaria siceraria (Molina) Standl polysaccharide and its immune-enhancing adjuvant activity. Poult Sci. (2022) 101:102112. doi: 10.1016/j.psj.2022.102112
21. Guo MZ, Meng M, Duan SQ, Feng CC, Wang CL. Structure characterization, physicochemical property and immunomodulatory activity on RAW264.7 cells of a novel triple-helix polysaccharide from Craterellus cornucopioides. Int J Biol Macromol. (2019) 126:796–804. doi: 10.1016/j.ijbiomac.2018.12.246
22. Guo M, Meng M, Zhao J, Wang X, Wang C. Immunomodulatory effects of the polysaccharide from Craterellus cornucopioides via activating the TLR4-NFkappaB signaling pathway in peritoneal macrophages of BALB/c mice. Int J Biol Macromol. (2020) 160:871–9. doi: 10.1016/j.ijbiomac.2020.05.270
23. Guo MZ, Meng M, Feng CC, Wang X, Wang CL. A novel polysaccharide obtained from Craterellus cornucopioides enhances immunomodulatory activity in immunosuppressive mice models via regulation of the TLR4-NF-kappaB pathway. Food Funct. (2019) 10:4792–801. doi: 10.1039/c9fo00201d
24. Guo M, Yu H, Meng M, Wang C. Research on the structural characteristics of a novel Chinese Iron Yam polysaccharide and its gastroprotection mechanism against ethanol-induced gastric mucosal lesion in a BALB/c mouse model. Food Funct. (2020) 11:6054–65. doi: 10.1039/c9fo02642h
25. Zhu Z.-y, Liu R.-q, Si C.-l, Zhou F, Wang Y.-x, Ding L.-n, et al. Structural analysis and anti-tumor activity comparison of polysaccharides from Astragalus. Carbohydre Polym. (2011) 85:895–902.
26. Zhu Z, Dong F, Liu X, Lv Q, Yang Y, Liu F, et al. Effects of extraction methods on the yield, chemical structure and anti-tumor activity of polysaccharides from Cordyceps gunnii mycelia. Carbohydr Polym. (2016) 140:461–71. doi: 10.1016/j.carbpol.2015.12.053
27. Yan JK, Li L, Wang ZM, Wu JY. Structural elucidation of an exopolysaccharide from mycelial fermentation of a Tolypocladium sp. fungus isolated from wild Cordyceps sinensis. Carbohydr Polym. (2010) 79:125–30.
28. He LX, Ren JW, Liu R, Chen QH, Zhao J, Wu X, et al. Ginseng (Panax ginseng Meyer) oligopeptides regulate innate and adaptive immune responses in mice via increased macrophage phagocytosis capacity, NK cell activity and Th cells secretion. Food Funct. (2017) 8:3523–32. doi: 10.1039/c7fo00957g
29. Yang Z, Wang J, Li J, Xiong L, Chen H, Liu X, et al. Antihyperlipidemic and hepatoprotective activities of polysaccharide fraction from Cyclocarya paliurus in high-fat emulsion-induced hyperlipidaemic mice. Carbohydr Polym. (2018) 183:11. doi: 10.1016/j.carbpol.2017.11.033
30. Price SJ, Pangloli P, Dia VP. Pepsin-pancreatin hydrolysis reduced the ability of lunasin-enriched material to inhibit activation of the inflammasomes in THP-1 human macrophages. Food Funct. (2017) 8:4449–58. doi: 10.1039/c7fo00992e
31. Ren D, Lin D, Alim A, Zheng Q, Yang X. Chemical characterization of a novel polysaccharide ASKP-1 from Artemisia sphaerocephala Krasch seed and its macrophage activation via MAPK, PI3k/Akt and NF-kappaB signaling pathways in RAW264.7 cells. Food Funct. (2017) 8:1299–312. doi: 10.1039/c6fo01699e
32. Xie J, Wang Z, Shen M, Nie S, Gong B, Li H, et al. Sulfated modification, characterization and antioxidant activities of polysaccharide from Cyclocarya paliurus. Food Hydrocoll. (2016) 53:7–15.
33. Hu WB, Zhao J, Chen H, Xiong L, Wang WJ. Polysaccharides from Cyclocarya paliurus : Chemical composition and lipid-lowering effect on rats challenged with high-fat diet. J Funct Foods. (2017) 36:262–73.
34. Wang T, He H, Liu X, Liu C, Liang Y, Mei Y. Mycelial polysaccharides of Lentinus edodes (shiitake mushroom) in submerged culture exert immunoenhancing effect on macrophage cells via MAPK pathway. Int J Biol Macromol. (2019) 130:745–54. doi: 10.1016/j.ijbiomac.2019.03.023
35. Rong Y, Yang R, Yang Y, Wen Y, Liu S, Li C, et al. Structural characterization of an active polysaccharide of longan and evaluation of immunological activity. Carbohydr Polym. (2019) 213:247–56. doi: 10.1016/j.carbpol.2019.03.007
36. Deng Y, Xie J, Luo Z, Li SP, Zhao J. Synergistic immunomodulatory effect of complex polysaccharides from seven herbs and their major active fractions. Int J Biol Macromol. (2020) 165, (Pt A):530–41. doi: 10.1016/j.ijbiomac.2020.09.199
37. Perera N, Yang FL, Chiu HW, Hsieh CY, Li LH, Zhang YL, et al. Phagocytosis enhancement, endotoxin tolerance, and signal mechanisms of immunologically active glucuronoxylomannan from Auricularia auricula-judae. Int J Biol Macromol. (2020) 165, (Pt A):495–505. doi: 10.1016/j.ijbiomac.2020.09.171
38. Gan T, Feng C, Lan H, Yang R, Zhang J, Li C, et al. Comparison of the structure and immunomodulatory activity of polysaccharides from fresh and dried longan. J Funct Foods. (2021) 76:104323.
39. Wufuer R, Bai J, Liu Z, Zhou K, Taoerdahong H. Biological activity of Brassica rapa L. polysaccharides on RAW264.7 macrophages and on tumor cells. Bioorg Med Chem. (2020) 28:115330. doi: 10.1016/j.bmc.2020.115330
40. Li WJ, Zhang XY, Wu RT, Song YH, Xie MY. Ganoderma atrum polysaccharide improves doxorubicin-induced cardiotoxicity in mice by regulation of apoptotic pathway in mitochondria. Carbohydr Polym. (2018) 202:581–90. doi: 10.1016/j.carbpol.2018.08.144
41. Xu C, Ding C, Zhou N, Ruan X-M, Guo B-X. A polysaccharide from Aloe vera L. var. chinensis (Haw.) Berger prevents damage to human gastric epithelial cells in vitro and to rat gastric mucosa in vivo. J Funct Foods. (2016) 24:501–12.
42. Yun L, Wu T, Li Q, Zhang M. Dietary supplementation with purified wheat germ glycoprotein improve immunostimulatory activity in cyclophosphamide induced Balb/c mice. Int J Biol Macromol. (2018) 118, (Pt A):1267–75. doi: 10.1016/j.ijbiomac.2018.06.199
43. Dehghan P, Tolouie S, Baradaran B, Nami S, Morovati H. TLR-2, IL-10 and IL-17-mediated immunity in experimental chemotherapy murine model of systemic candidiasis, cyclophosphamides’ impact and roles. Microb Pathog. (2018) 119:183–92. doi: 10.1016/j.micpath.2018.04.026
44. Maity P, Sen IK, Maji PK, Paloi S, Devi K, Acharya K, et al. Structural, immunological, and antioxidant studies of β-glucan from edible mushroom Entoloma lividoalbum. Carbohydr Polym. (2015) 123:350–8. doi: 10.1016/j.carbpol.2015.01.051
45. Vijay K. Toll-like receptors in immunity and inflammatory diseases: Past, present, and future. Int Immunopharmacol. (2018) 59:391–412.
46. Vetvicka V, Gover O, Karpovsky M, Hayby H, Danay O, Ezov N, et al. Immune-modulating activities of glucans extracted from Pleurotus ostreatus and Pleurotus eryngii. J Funct Foods. (2019) 54:81–91.
47. Mirzadeh M, Arianejad MR, Khedmat L. Antioxidant, antiradical, and antimicrobial activities of polysaccharides obtained by microwave-assisted extraction method: A review. Carbohydr Polym. (2020) 229:115421. doi: 10.1016/j.carbpol.2019.115421
48. Yz A, Hk A, Yfa B, Kna C, Gopa B. Schizophyllan: A review on its structure, properties, bioactivities and recent developments. Bioact Carbohydr Diet Fibre. (2013) 1:53–71.
49. Chakraborty I, Sen IK, Mondal S, Rout D, Bhanja SK, Maity GN, et al. Bioactive polysaccharides from natural sources: A review on the antitumor and immunomodulating activities. Biocatal Agric Biotechnol. (2019) 22:101425.
50. Maity P, Nandi AK, Sen IK, Pattanayak M, Chattopadhyay S, Dash SK, et al. Heteroglycan of an edible mushroom Entoloma lividoalbum: Structural characterization and study of its protective role for human lymphocytes-ScienceDirect. Carbohydr Polym. (2014) 114:157–65. doi: 10.1016/j.carbpol.2014.07.080
51. Manna DK, Maity P, Nandi AK, Pattanayak M, Panda BC, Mandal AK, et al. Structural elucidation and immunostimulating property of a novel polysaccharide extracted from an edible mushroom Lentinus fusipes. Carbohydr Polym. (2017) 157:1657–65. doi: 10.1016/j.carbpol.2016.11.048
52. Han L, Suo Y, Yang Y, Jing M, Na H. Optimization, characterization, and biological activity of polysaccharides from Berberis dasystachya Maxim. Int J Biol Macromol. (2016) 85:655–66. doi: 10.1016/j.ijbiomac.2015.10.038
53. Tamiello CS, Nascimento G, Iacomini M, Cordeiro L. Arabinogalactan from edible jambo fruit induces different responses on cytokine secretion by THP-1 macrophages in the absence and presence of proinflammatory stimulus. Int J Biol Macromol. (2018) 107, (Pt A):35. doi: 10.1016/j.ijbiomac.2017.08.148
54. Weichao H, Zhao Y, Yang Y, Zhang H, Ding C. Microwave-assisted extraction, physicochemical characterization and bioactivity of polysaccharides from Camptotheca acuminata fruits. Int J Biol Macromol. (2019) 133:127–36. doi: 10.1016/j.ijbiomac.2019.04.086
55. Liu H, Xu J, Xu X, Yuan Z, Zhu D. Structure/function relationships of bean polysaccharides: A review. Crit Rev Food Sci Nutr. (2021) 13:1–15. doi: 10.1080/10408398.2021.1946480
56. Li S, Li M, Yue H, Zhou L, Huang L, Du Z, et al. Structural elucidation of a pectic polysaccharide from Fructus Mori and its bioactivity on intestinal bacteria strains. Carbohydr Polym. (2018) 186:168–75. doi: 10.1016/j.carbpol.2018.01.026
57. Chen X, Yang J, Shen M, Chen Y, Yu Q, Xie J. Structure, function and advance application of microwave-treated polysaccharide: A review. Trends Food Sci Technol. (2022) 123:198–209.
58. Song HN. Functional cordyceps coffee containing cordycepin and β-glucan. Prev Nutr Food Sci. (2020) 25:184–93. doi: 10.3746/pnf.2020.25.2.184
59. Zong S, Li J, Ye Z, Zhang X, Yang L, Chen X, et al. Lachnum polysaccharide suppresses S180 sarcoma by boosting anti-tumor immune responses and skewing tumor-associated macrophages toward M1 phenotype. Int J Biol Macromol. (2020) 144:1022–33. doi: 10.1016/j.ijbiomac.2019.09.179
60. Yu Y, Zhu H, Shen M, Yu Q, Chen Y, Xie J. Sulfation modification enhances the intestinal regulation of Cyclocarya paliurus polysaccharides in cyclophosphamide-treated mice via restoring intestinal mucosal barrier function and modulating gut microbiota. Food Funct. (2021) 12:12278–90. doi: 10.1039/d1fo03042f
61. Jeelani R, Khan SN, Shaeib F, Kohan-Ghadr HR, Aldhaheri SR, Najafi T, et al. Cyclophosphamide and acrolein induced oxidative stress leading to deterioration of metaphase II mouse oocyte quality. Free Radic Biol Med. (2017) 110:11–8. doi: 10.1016/j.freeradbiomed.2017.05.006
62. Viaud S, Flament C, Zoubir M, Pautier P, LeCesne A, Ribrag V, et al. Cyclophosphamide induces differentiation of Th17 cells in cancer patients. Cancer Res. (2011) 71:661–5. doi: 10.1158/0008-5472.CAN-10-1259
Keywords: Craterellus cornucopioides, polysaccharide, structural characterization, immunoregulation, pathway
Citation: Zhang C, Shu Y, Li Y and Guo M (2022) Extraction and immunomodulatory activity of the polysaccharide obtained from Craterellus cornucopioides. Front. Nutr. 9:1017431. doi: 10.3389/fnut.2022.1017431
Received: 12 August 2022; Accepted: 21 October 2022;
Published: 08 November 2022.
Edited by:
Chanchan Sun, Yantai University, ChinaReviewed by:
Fei Liu, Université de Strasbourg, FranceLiyuan Yun, Tianjin Agricultural University, China
Copyright © 2022 Zhang, Shu, Li and Guo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mingzhu Guo, Z216NTIzMjI1MzM0QGhlYmF1LmVkdS5jbg==
 Caixuan Zhang
Caixuan Zhang Mingzhu Guo
Mingzhu Guo