- 1Department of Education, Taichung Veterans General Hospital, Taichung, Taiwan
- 2Department of Medical Research, Taichung Veterans General Hospital, Taichung, Taiwan
- 3Division of Endocrinology and Metabolism, Department of Internal Medicine, Taichung Veterans General Hospital, Taichung, Taiwan
- 4Department of Medicine, School of Medicine, National Yang Ming Chiao Tung University, Taipei, Taiwan
- 5Department of Post-baccalaureate Medicine, College of Medicine, National Chung Hsing University, Taichung, Taiwan
- 6Rong Hsing Research Center for Translational Medicine, Institute of Biomedical Science, National Chung Hsing University, Taichung, Taiwan
Background and aims: We investigated the association of adherence to the Dietary Approaches to Stop Hypertension (DASH) diet with all-cause mortality in patients with a history of heart failure.
Methods: We analyzed data from the National Health and Nutrition Examination Survey (NHANES). Dietary information was obtained from a 24-h dietary recall interview. Adherence to the DASH diet was assessed using the DASH score. The primary outcome was all-cause mortality which was confirmed by the end of 2011. Weighted Cox proportional hazards regression models were used to determine the hazard ratios and 95% CI for the association of the DASH score and all-cause mortality with multivariate adjustment.
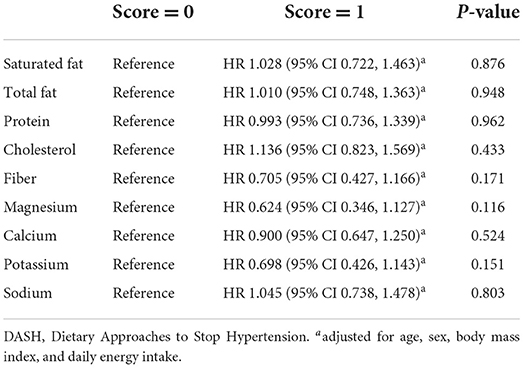
Results: The median DASH score was 2 among the 832 study participants. There were 319 participants who died after a median follow-up duration of 4.7 years. A higher DASH score (>2 vs. ≤2) was not associated with a decrease in the risk of all-cause mortality (adjusted HR 1.003, 95% CI 0.760–1.323, p = 0.983). With respect to the components of the DASH score, a lower sodium intake was not associated with a decreased risk of mortality (adjusted HR 1.045, 95% CI 0.738–1.478, p = 0.803).
Conclusion: A higher DASH score (>2 vs. ≤2) was not associated with all-cause mortality in patients with heart failure.
Introduction
The majority of deaths worldwide were attributed to non-communicable diseases (1). A healthy lifestyle, including dietary modifications, may help control risk factors and healthcare burdens of non-communicable diseases (2, 3). For instance, dietary salt reduction may lower blood pressure (4) and reduce long-term mortality risk non-communicable diseases (5). The Dietary Approaches to Stop Hypertension (DASH) diet has been recommended as a dietary modification that has effects on both blood pressure and blood cholesterol (6). The DASH diet is rich in fruits, vegetables, and low-fat dairy foods. These foods are high in potassium, magnesium, calcium, and fiber (7, 8). Combining the DASH dietary pattern with sodium reduction could reduce blood pressure and non-communicable diseases (6–9).
Among non-communicable diseases, the burden of heart failure is increasing (10, 11) and the quality of medical care for affected patients remains suboptimal (12). Guidelines recommend adopting a healthy diet as part of the management for heart failure; however, recommendations for specific dietary patterns are lacking (13). Dietary sodium restriction is frequently recommended to patients with heart failure (14) and is endorsed by most treatment guidelines (15, 16). Although the DASH diet has been associated with a reduced risk of mortality in the general population (17–19) and in patients with hypertension (20), its effect on patients' outcomes in heart failure is not yet clear. In this study, we examined the association between a DASH dietary pattern and all-cause mortality in patients with a history of heart failure using data from the National Health and Nutrition Examination Survey (NHANES).
Materials and methods
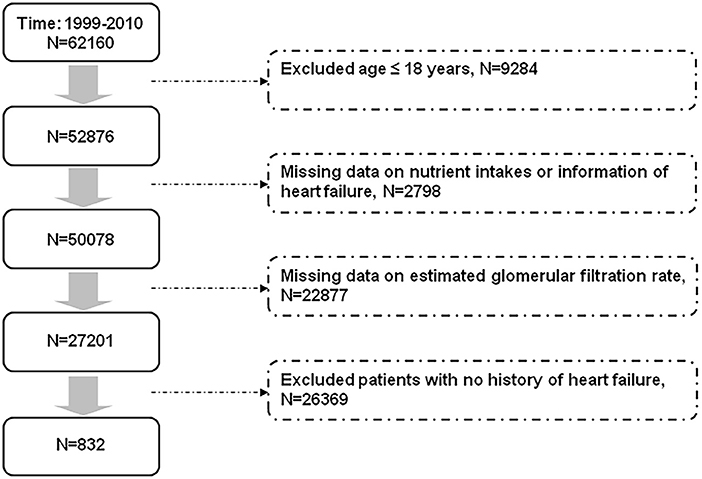
We selected our study population from the participants in the NHANES, which was conducted to assess the nutritional and health status of the US population. Briefly, data were collected through personal interviews, physical examinations, laboratory tests, and questionnaires (https://www.cdc.gov/nchs/surveys.htm). The selection of patients with heart failure is shown in Figure 1. We excluded participants aged ≤18 years, with missing information on nutrient intakes and renal function, with unknown history about heart failure, and those who reported no history of heart failure from the 62,160 participants [mean age 36.1 (95% CI 35.6–36.5) years] in the NHANES from 1999 to 2010. Finally, we included 832 participants with a history of heart failure. Survival status of the study population by the end of 2011 was confirmed according to information from the National Death Index.
The conduction of this study followed the Declaration of Helsinki. Our protocol was approved by the Institutional Review Board of Taichung Veterans General Hospital, Taichung, Taiwan (approval number: CE18312A). Informed consent was provided by all of the NHANES participants. Dietary information of the NHANES participants were obtained from a 24-h dietary recall interview, which was conducted in person by trained interviewers (https://www.cdc.gov/nchs/nhanes/measuring_guides_dri/measuringguides.htm). A computer-assisted software program, and measurement aids and visuals including charts and drawings were used to facilitate the 24-h dietary recall interview. Data from the 24-h dietary recall interview were translated for analyses according to the Food Patterns Equivalents Database (developed by the United States Department of Agriculture (https://www.ars.usda.gov/northeast-area/beltsville-md-bhnrc/beltsville-human-nutrition-research-center/food-surveys-research-group/docs/fped-databases/), which had been applied to the NHANES population (21–23).
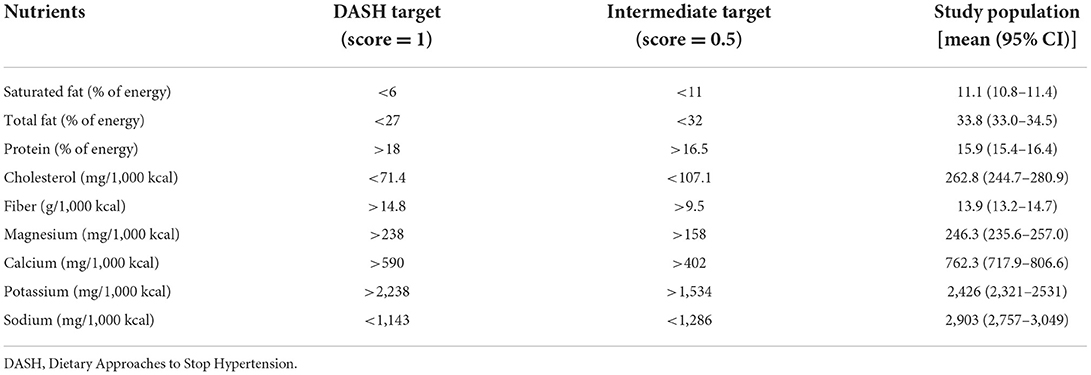
We used the DASH score (20) to assess the dietary concordance to the DASH diet. To determine the DASH score, data on 9 target nutrients (Table 1) (20) were obtained based on information from the 24-h dietary recall interview. We determined whether the participants' intake met the goal of each target nutrient. This method has been applied to the NHANES population (21–23). One point was assigned to each target nutrient if the study participants' intake met the goal (20, 23, 24), while half (0.5) a point was assigned if only the intermediate goal was met. The maximum DASH score is 9, and a higher score indicates better concordance with the DASH diet. We determined the study participants' renal function (estimated glomerular filtration rate, eGFR) using a well-documented method (25).
We used the Statistical Analysis System survey procedures (SAS version 9.4, 2013, Cary, NC, USA) to conduct all of the statistical analyses, which were adequately weighted (https://wwwn.cdc.gov/nchs/nhanes/analyticguidelines.aspx). We divided the study population into two groups according to their DASH score (>median vs. ≤ median). Between-group differences in categorical and continuous variables were examined using the Chi-square test and independent t test, respectively. The primary outcome was all-cause mortality. We conducted weighted Cox proportional hazards regression models to determine the hazard ratios (HR) and 95% CI for the association of DASH score (>median vs. ≤ median) and all-cause mortality, with multivariate adjustment. Adjusted variables included known factors related to risk of mortality (age, sex, race, body mass index, smoking, systolic blood pressure, diabetes, and eGFR) and dietary factor (daily energy intake). The association was also examined in subgroups of body mass index (≥30 vs. < 30 kg/m2), daily energy intake (≥25 vs. < 25 kcal/kg/day), glucose regulation status (diabetes vs. no diabetes), and renal function (eGFR ≥ 60 vs. < 60 ml/min/1.73 m2). We conducted Cubic spline of DASH score vs. all-cause mortality risk as a sensitivity test. Finally, the associations of the 9 parts of the DASH score (20) with all-cause mortality were determined. A two-sided P-value < 0.05 was considered statistically significant in all of the statistical analyses.
Results
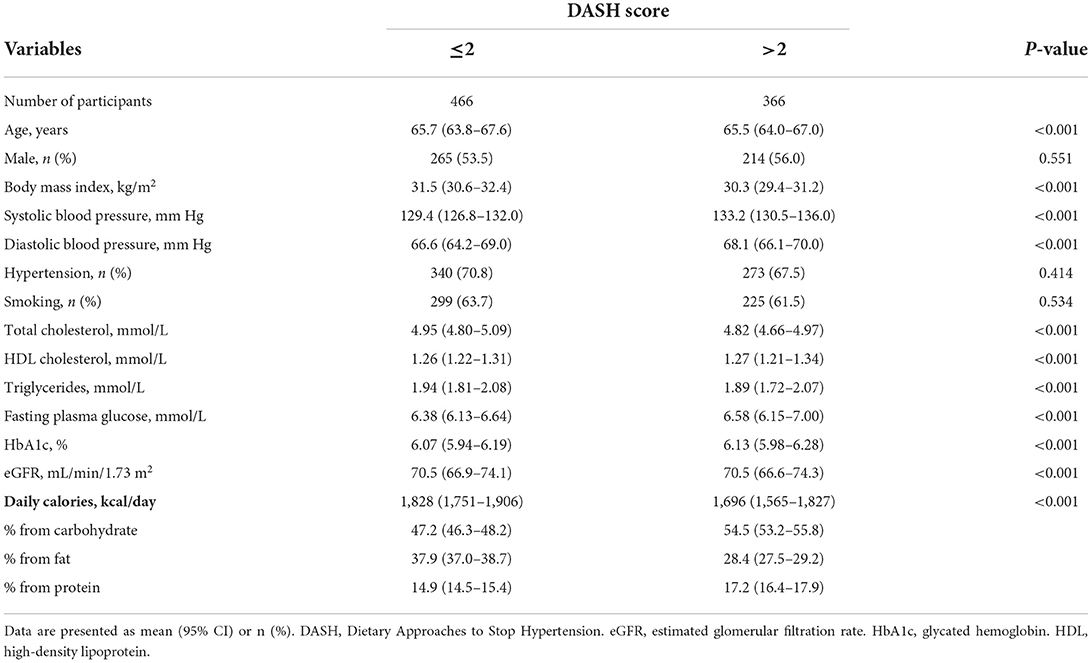
Table 1 shows the nutrient targets for the 9 parts of the DASH score, and the nutrient intakes of the study population. Overall, the study population had suboptimal concordance with the DASH diet (median DASH score = 2). Participants with a higher DASH score (>2 vs. ≤2, Table 2) had a lower body mass index, a higher systolic blood pressure, and a lower total cholesterol and triglyceride with a higher high-density lipoprotein cholesterol, compared with those who had a lower DASH score. The former group also had a lower intake of daily calories, which comprised a lower proportion from fat and higher proportions from carbohydrate and protein, compared with the latter group. There were only modest between-group differences for the other variables.
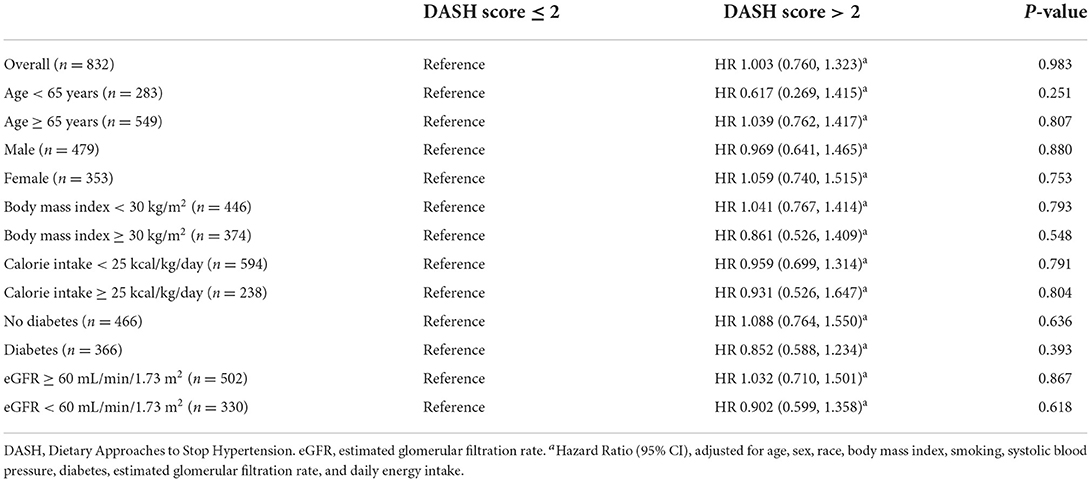
There were 319 participants who died after a median follow-up duration of 4.7 years (mortality rate 69.5 per 1,000 person-years). Table 3 shows a null association between a higher DASH score (>2 vs. ≤2) and all-cause mortality (adjusted HR 1.003, 95% CI 0.760 to 1.323, p = 0.983). The findings were similar in the subgroups of age (≥65 vs. < 65 years), sex (male vs. female), body mass index (≥30 vs. < 30 kg/m2), daily energy intake (≥25 vs. < 25 kcal/kg/day), glucose regulation status (diabetes vs. no diabetes), and renal function (eGFR ≥ 60 vs. < 60 ml/min/1.73 m2). Figure 2 shows the Cubic spline of DASH score vs. risk of all-cause mortality. There was a modest decrease (but not significant, p = 0.423) in all-cause mortality risk with an increase in DASH score.
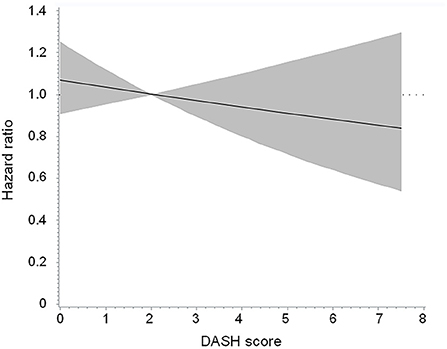
Figure 2. Cubic spline of DASH score vs. risk of all-cause mortality in the overall population. The p-value was 0.423 for testing for linear relation. DASH, Dietary Approaches to Stop Hypertension.
Table 4 shows the associations of the 9 target nutrients with all-cause mortality. We observed that concordance with each component of the DASH score was not associated with a significant difference in all-cause mortality risk (all p > 0.1). Notably, a lower sodium intake (< 1,143 mg/1,000 kcal) was not associated with a decrease in mortality risk in the study population (adjusted HR 1.045, 95% CI 0.738 to 1.478, p = 0.803).
Discussion
We demonstrated that a higher DASH score (>2 vs. ≤2) was not associated with risk of all-cause mortality (adjusted HR 1.003, 95% CI 0.760 to 1.323, p = 0.983) in patients with a history of heart failure during a median follow-up duration of 4.7 years. The findings were consistent across various subgroups (age, sex, body mass index, calorie intake, diabetes, and chronic kidney disease). Our results suggest that more evidence is needed for recommendation of the DASH diet for patients with heart failure to improve their outcomes.
Adopting a DASH dietary pattern has been associated with a lower all-cause mortality rate in the general population (17–19) and in patients with hypertension (20). Assessment of sodium intake is one of the components of the DASH score (20). Furthermore, dietary sodium restriction to prevent fluid overload has been endorsed as the cornerstone of therapy for patients with heart failure since the early 1950s (26). Nevertheless, it is not yet clear whether a dietary pattern concordance with the DASH diet helps reduce the mortality rate in patients with heart failure. In a cohort study of women who had experienced a hospitalization for heart failure (27), a higher DASH score was associated with a modest reduction in all-cause mortality (adjusted HR 0.84, 95% CI 0.70 to 1.00, p = 0.010). However, findings regarding the association between concordance with the DASH diet and incident heart failure were not consistent (28–33). We did not observe an association between the DASH score and all-cause mortality risk in patients with a history of heart failure (Table 3; Figure 2). Even if we selected participants who had a DASH score >4.5 and compared them with those who had a DASH score ≤2, there was no significant between-group difference in the risk of all-cause mortality (HR 0.838, 95% CI 0.383, 1.835, p = 0.656). In this context, the current evidence is insufficient to recommend the DASH diet for patients with heart failure to improve their outcomes.
We further investigated the associations of the DASH score components with all-cause mortality in our study population. It is worth noting that sodium intake < 1,143 mg/1,000 kcal (score = 1) was not associated with a lower risk of all-cause mortality (Table 4). In a previous study which revealed a risk reduction for incident heart failure with an increase in DASH score (29), the mean sodium intakes were similar (~2,500 mg/day) across the DASH score quartiles. Whether a restriction in dietary sodium reduces the risk of cardiovascular diseases and mortality remains a subject of debate (34). A urinary sodium excretion rate of < 3.0 g/day (vs. 4.0–6.0 g/day) was associated with an increased risk of cardiovascular diseases or death in a large cohort study (35). Similar findings were noted regarding the associations of urinary sodium excretion and dietary sodium restriction with adverse heart failure outcomes (36–38). Our findings were in line with these reports, and were further supported by a recent randomized controlled trial (39) in which a low sodium diet (< 1,500 mg/day vs. usual care) was not associated with a risk reduction in clinical outcomes in patients with chronic heart failure. These results may be partly explained by some detrimental effects associated with low sodium intake, such as decreased cardiac output and renal perfusion, and activation of the renin–angiotensin–aldosterone system (40). Hence, whether or not to recommend a low sodium diet for patients with heart failure deserves careful consideration.
In contrast, an increase in urinary potassium excretion attenuated the cardiovascular risk associated with high urinary sodium excretion (41). We observed a modest, but non-significant, reduction in all-cause mortality risk (adjusted HR 0.698, 95% CI 0.426 to 1.143, p = 0.151, Table 4) associated with a higher potassium intake (potassium score = 1, >2,238 mg/1,000 kcal) in our study population. The use of potassium-enriched salt instead of regular salt has been shown to decrease cardiovascular mortality in an elderly population (42). An increase in potassium intake may improve blood pressure control (43) and decrease cardiovascular disease risk (44). In an animal model of non-ischemic heart failure (45), a potassium-supplemented diet alleviated cardiorespiratory dysfunction compared with a normal diet. The possible benefit of potassium-enriched salt in patients with heart failure awaits further studies. Likewise, more research is needed to explore the potential benefits of magnesium (46, 47) and fiber (48) intakes on all-cause mortality in heart failure.
This study has several limitations. First, this was an observational study. Our findings need to be confirmed in interventional studies. Second, information on nutritional intakes was obtained from a 24-h dietary recall interview. There might be some variations between the information from the 24-h recall and long-term dietary patterns. Third, we did not have information on left ventricular ejection fraction and N-terminal pro-brain natriuretic peptide, as well as medical treatment for some chronic diseases, such as hypertension and heart failure. This should be taken into account when interpreting our results. Last, the median DASH score was only two in the study population. This indicates that the degree of concordance with the DASH diet was low, which might help explain the null findings in this study. More evidence is required for specific recommendations of dietary components to improve outcomes in patients with heart failure. In this context, our results may have implications for future investigations.
In conclusion, a higher DASH score (>2 vs. ≤2) was not associated with all-cause mortality in patients with heart failure. Notably, a decrease in sodium intake (sodium score = 1) was not associated with a significant difference in the risk of mortality. More studies are needed to elucidate the effect of the DASH diet on outcomes in patients with a history of heart failure.
Data availability statement
Publicly available datasets were analyzed in this study. This data can be found here: https://www.cdc.gov/nchs/nhanes/index.htm.
Ethics statement
The studies involving human participants were reviewed and approved by the Institutional Review Board of Taichung Veterans General Hospital, Taichung, Taiwan. The patients/participants provided their written informed consent to participate in this study.
Author contributions
T-YC and J-SW designed and conducted the research and wrote the first draft of the manuscript. W-JL and C-LL analyzed data and revised the manuscript critically for important intellectual content. All authors approved the final draft of the manuscript.
Funding
This work was supported by Taichung Veterans General Hospital, Taichung, Taiwan (Grant Numbers: TCVGH-1083505C, 2019; TCVGH-1093504C, 2020; TCVGH-1103502C; TCVGH-1103504C; and TCVGH-1107305D, 2021).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. NCD Countdown 2030 collaborators. NCD Countdown 2030: worldwide trends in non-communicable disease mortality and progress towards sustainable development goal target 3.4. Lancet. (2018) 392:1072–88. doi: 10.1016/S0140-6736(18)31992-5
2. Beaglehole R, Bonita R, Horton R, Adams C, Alleyne G, Asaria P, et al. Priority actions for the non-communicable disease crisis. Lancet. (2011) 377:1438–47. doi: 10.1016/S0140-6736(11)60393-0
3. Arena R, Guazzi M, Lianov L, Whitsel L, Berra K, Lavie CJ, et al. Healthy lifestyle interventions to combat noncommunicable disease-a novel nonhierarchical connectivity model for key stakeholders: a policy statement from the American heart association, European society of cardiology, European association for cardiovascular prevention and rehabilitation, and American college of preventive medicine. Eur Heart J. (2015) 36:2097–109. doi: 10.1093/eurheartj/ehv207
4. He FJ, Li J, Macgregor GA. Effect of longer term modest salt reduction on blood pressure: cochrane systematic review and meta-analysis of randomised trials. BMJ. (2013) 346:f1325. doi: 10.1136/bmj.f1325
5. Kontis V, Mathers CD, Rehm J, Stevens GA, Shield KD, Bonita R, et al. Contribution of six risk factors to achieving the 25 × 25 non-communicable disease mortality reduction target: a modelling study. Lancet. (2014) 384:427–37. doi: 10.1016/S0140-6736(14)60616-4
6. Barone Gibbs B, Hivert MF, Jerome GJ, Kraus WE, Rosenkranz SK, Schorr EN, et al. Physical activity as a critical component of first-line treatment for elevated blood pressure or cholesterol: who, what, and how?: a scientific statement from the American heart association. Hypertension. (2021) 78:e26–37. doi: 10.1161/HYP.0000000000000196
7. Appel LJ, Moore TJ, Obarzanek E, Vollmer WM, Svetkey LP, Sacks FM, et al. A clinical trial of the effects of dietary patterns on blood pressure. DASH collaborative research group. N Engl J Med. (1997) 336:1117–24. doi: 10.1056/NEJM199704173361601
8. Sacks FM, Svetkey LP, Vollmer WM, Appel LJ, Bray GA, Harsha D, et al. Effects on blood pressure of reduced dietary sodium and the dietary approaches to stop hypertension (DASH) diet. DASH-sodium collaborative research group. N Engl J Med. (2001) 344:3–10. doi: 10.1056/NEJM200101043440101
9. Vollmer WM, Sacks FM, Ard J, Appel LJ, Bray GA, Simons-Morton DG, et al. Effects of diet and sodium intake on blood pressure: subgroup analysis of the DASH-sodium trial. Ann Intern Med. (2001) 135:1019–28. doi: 10.7326/0003-4819-135-12-200112180-00005
10. Callender T, Woodward M, Roth G, Farzadfar F, Lemarie JC, Gicquel S, et al. Heart failure care in low- and middle-income countries: a systematic review and meta-analysis. PLoS Med. (2014) 11:e1001699. doi: 10.1371/journal.pmed.1001699
11. Conrad N, Judge A, Tran J, Mohseni H, Hedgecott D, Crespillo AP, et al. Temporal trends and patterns in heart failure incidence: a population-based study of 4 million individuals. Lancet. (2018) 391:572–80. doi: 10.1016/S0140-6736(17)32520-5
12. Conrad N, Judge A, Canoy D, Tran J, O'Donnell J, Nazarzadeh M, et al. Diagnostic tests, drug prescriptions, and follow-up patterns after incident heart failure: a cohort study of 93,000 UK patients. PLoS Med. (2019) 16:e1002805. doi: 10.1371/journal.pmed.1002805
13. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. (2021) 42:3599–726. doi: 10.1093/eurheartj/ehab368
14. Riegel B, Moser DK, Powell M, Rector TS, Havranek EP. Nonpharmacologic care by heart failure experts. J Card Fail. (2006) 12:149–53. doi: 10.1016/j.cardfail.2005.10.004
15. Malcom J, Arnold O, Howlett JG, Ducharme A, Ezekowitz JA, Gardner M, et al. Canadian cardiovascular society consensus conference guidelines on heart failure−2008 update: best practices for the transition of care of heart failure patients, and the recognition, investigation and treatment of cardiomyopathies. Can J Cardiol. (2008) 24:21–40. doi: 10.1016/S0828-282X(08)70545-2
16. Heart Heart Failure Society of America, Lindenfeld J, Albert NM, Boehmer JP, Collins SP, Ezekowitz JA, et al. HFSA 2010 comprehensive heart failure practice guideline. J Card Fail. (2010) 16: e1–194. doi: 10.1016/j.cardfail.2010.04.004
17. Sotos-Prieto M, Bhupathiraju SN, Mattei J, Fung TT, Li Y, Pan A, et al. Association of changes in diet quality with total and cause-specific mortality. N Engl J Med. (2017) 377:143–53. doi: 10.1056/NEJMoa1613502
18. Mokhtari Z, Sharafkhah M, Poustchi H, Sepanlou SG, Khoshnia M, Gharavi A, et al. Adherence to the dietary approaches to stop hypertension (DASH) diet and risk of total and cause-specific mortality: results from the golestan cohort study. Int J Epidemiol. (2019) 48:1824–38. doi: 10.1093/ije/dyz079
19. Yu D, Zhang X, Xiang YB, Yang G, Li H, Gao YT, et al. Adherence to dietary guidelines and mortality: a report from prospective cohort studies of 134,000 Chinese adults in urban Shanghai. Am J Clin Nutr. (2014) 100:693–700. doi: 10.3945/ajcn.113.079194
20. Mellen PB, Gao SK, Vitolins MZ, Goff DC Jr. Deteriorating dietary habits among adults with hypertension: DASH dietary accordance, NHANES 1988-1994 and 1999-2004. Arch Intern Med. (2008) 168:308–14. doi: 10.1001/archinternmed.2007.119
21. Ha K, Kim K, Sakaki JR, Chun OK. Relative validity of dietary total antioxidant capacity for predicting all-cause mortality in comparison to diet quality indexes in US adults. Nutrients. (2020) 12:1210. doi: 10.3390/nu12051210
22. Chang CY, Lee CL, Liu WJ, Wang JS. Association of adherence to the mediterranean diet with all-cause mortality in subjects with heart failure. Nutrients. (2022) 14:842. doi: 10.3390/nu14040842
23. Wang JS, Liu WJ, Lee CL. Associations of adherence to the DASH diet and the mediterranean diet with all-cause mortality in subjects with various glucose regulation states. Front Nutr. (2022) 9:828792. doi: 10.3389/fnut.2022.828792
24. Parikh A, Lipsitz SR, Natarajan S. Association between a DASH-like diet and mortality in adults with hypertension: findings from a population-based follow-up study. Am J Hypertens. (2009) 22:409–16. doi: 10.1038/ajh.2009.10
25. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF3rd, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. (2009) 150:604–12. doi: 10.7326/0003-4819-150-9-200905050-00006
26. Stock RJ, Mudge GH, Nurnberg MJ. Congestive heart failure; variations in electrolyte metabolism with salt restriction and mercurial diuretics. Circulation. (1951) 4:54–60. doi: 10.1161/01.CIR.4.1.54
27. Levitan EB, Lewis CE, Tinker LF, Eaton CB, Ahmed A, Manson JE, et al. Mediterranean and DASH diet scores and mortality in women with heart failure: the Women's Health Initiative. Circ Heart Fail. (2013) 6:1116–23. doi: 10.1161/CIRCHEARTFAILURE.113.000495
28. Levitan EB, Wolk A, Mittleman MA. Relation of consistency with the dietary approaches to stop hypertension diet and incidence of heart failure in men aged 45 to 79 years. Am J Cardiol. (2009) 104:1416–20. doi: 10.1016/j.amjcard.2009.06.061
29. Levitan EB, Wolk A, Mittleman MA. Consistency with the DASH diet and incidence of heart failure. Arch Intern Med. (2009) 169:851–7. doi: 10.1001/archinternmed.2009.56
30. Goyal P, Balkan L, Ringel JB, Hummel SL, Sterling MR, Kim S, et al. The dietary approaches to stop hypertension (DASH) diet pattern and incident heart failure. J Card Fail. (2021) 27:512–21. doi: 10.1016/j.cardfail.2021.01.011
31. Ibsen DB, Levitan EB, Åkesson A, Gigante B, Wolk A. The DASH diet is associated with a lower risk of heart failure: a cohort study. Eur J Prev Cardiol. (2022) 29:1114–23. doi: 10.1093/eurjpc/zwac003
32. Del Gobbo LC, Kalantarian S, Imamura F, Lemaitre R, Siscovick DS, Psaty BM, et al. Contribution of major lifestyle risk factors for incident heart failure in older adults: the cardiovascular health study. JACC Heart Fail. (2015) 3:520–8. doi: 10.1016/j.jchf.2015.02.009
33. Chang RS, Xu M, Brown SH, Cohen SS Yu D, Akwo EA, et al. Relation of the dietary approaches to stop hypertension dietary pattern to heart failure risk and socioeconomic status (from the southern community cohort study). Am J Cardiol. (2022) 169:71–7. doi: 10.1016/j.amjcard.2021.12.043
34. O'Donnell M, Mente A, Alderman MH, Brady AJB, Diaz R, Gupta R, et al. Salt and cardiovascular disease: insufficient evidence to recommend low sodium intake. Eur Heart J. (2020) 41:3363–73. doi: 10.1093/eurheartj/ehaa586
35. O'Donnell M, Mente A, Rangarajan S, McQueen MJ, Wang X, Liu L, et al. Urinary sodium and potassium excretion, mortality, and cardiovascular events. N Engl J Med. (2014) 371:612–23. doi: 10.1056/NEJMoa1311889
36. O'Donnell MJ, Yusuf S, Mente A, Gao P, Mann JF, Teo K, et al. Urinary sodium and potassium excretion and risk of cardiovascular events. JAMA. (2011) 306:2229–38. doi: 10.1001/jama.2011.1729
37. Pfister R, Michels G, Sharp SJ, Luben R, Wareham NJ, Khaw KT. Estimated urinary sodium excretion and risk of heart failure in men and women in the EPIC-Norfolk study. Eur J Heart Fail. (2014) 16:394–402. doi: 10.1002/ejhf.56
38. Doukky R, Avery E, Mangla A, Collado FM, Ibrahim Z, Poulin MF, et al. Impact of dietary sodium restriction on heart failure outcomes. JACC Heart Fail. (2016) 4:24–35. doi: 10.1016/j.jchf.2015.08.007
39. Ezekowitz JA, Colin-Ramirez E, Ross H, Escobedo J, Macdonald P, Troughton R, et al. Reduction of dietary sodium to less than 100 mmol in heart failure (SODIUM-HF): an international, open-label, randomised, controlled trial. Lancet. (2022) 399:1391–400. doi: 10.1016/S0140-6736(22)00369-5
40. Gupta D, Georgiopoulou VV, Kalogeropoulos AP, Dunbar SB, Reilly CM, Sands JM, et al. Dietary sodium intake in heart failure. Circulation. (2012) 126:479–85. doi: 10.1161/CIRCULATIONAHA.111.062430
41. O'Donnell M, Mente A, Rangarajan S, McQueen MJ, O'Leary N, Yin L, et al. Joint association of urinary sodium and potassium excretion with cardiovascular events and mortality: prospective cohort study. BMJ. (2019) 364:l772. doi: 10.1136/bmj.l772
42. Chang HY, Hu YW, Yue CS, Wen YW, Yeh WT, Hsu LS, et al. Effect of potassium-enriched salt on cardiovascular mortality and medical expenses of elderly men. Am J Clin Nutr. (2006) 83:1289–96. doi: 10.1093/ajcn/83.6.1289
43. Aburto NJ, Hanson S, Gutierrez H, Hooper L, Elliott P, Cappuccio FP. Effect of increased potassium intake on cardiovascular risk factors and disease: systematic review and meta-analyses. BMJ. (2013) 346:f1378. doi: 10.1136/bmj.f1378
44. Marklund M, Singh G, Greer R, Cudhea F, Matsushita K, Micha R, et al. Estimated population wide benefits and risks in China of lowering sodium through potassium enriched salt substitution: modelling study. BMJ. (2020) 369:m824. doi: 10.1136/bmj.m824
45. Schwarz KG, Pereyra KV, Toledo C, Andrade DC, Díaz HS, Díaz-Jara E, et al. Effects of enriched-potassium diet on cardiorespiratory outcomes in experimental non-ischemic chronic heart failure. Biol Res. (2021) 54:43. doi: 10.1186/s40659-021-00365-z
46. Douban S, Brodsky MA, Whang DD, Whang R. Significance of magnesium in congestive heart failure. Am Heart J. (1996) 132:664–71. doi: 10.1016/S0002-8703(96)90253-7
47. Ceremuzyński L, Gebalska J, Wolk R, Makowska E. Hypomagnesemia in heart failure with ventricular arrhythmias. beneficial effects of magnesium supplementation. J Intern Med. (2000) 247:78–86. doi: 10.1046/j.1365-2796.2000.00585.x
Keywords: DASH, heart failure, mortality, NHANES, sodium
Citation: Chou T-Y, Liu W-J, Lee C-L and Wang J-S (2022) Adherence to the dietary approaches to stop hypertension diet and all-cause mortality in patients with a history of heart failure. Front. Nutr. 9:1015290. doi: 10.3389/fnut.2022.1015290
Received: 09 August 2022; Accepted: 09 September 2022;
Published: 27 September 2022.
Edited by:
Lilia Castillo-Martinez, Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán (INCMNSZ), MexicoReviewed by:
Leyre Notario, Miguel Hernández University of Elche, SpainMidori Ogata Medel, Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán (INCMNSZ), Mexico
Copyright © 2022 Chou, Liu, Lee and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jun-Sing Wang, anN3YW5nQHZnaHRjLmdvdi50dw==; Chia-Lin Lee, dTUwMjEwN0B5YWhvby5jb20udHc=
†These authors have contributed equally to this work and share senior authorship
 Ting-Yu Chou1
Ting-Yu Chou1 Wei-Ju Liu
Wei-Ju Liu Chia-Lin Lee
Chia-Lin Lee Jun-Sing Wang
Jun-Sing Wang