
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Nutr. , 10 October 2022
Sec. Clinical Nutrition
Volume 9 - 2022 | https://doi.org/10.3389/fnut.2022.1013449
The role of flavonoids in regulating the synthesis and function of skeletal muscles is increasingly recognized. However, randomized controlled trials have yielded inconsistent results on the influence of flavonoids on human muscular parameters. Therefore, we performed a meta-analysis to evaluate the possible effects of flavonoids on sarcopenia-related parameters in middle-aged and elderly people. Eligible literature and randomized controlled trials reports have been extensively searched from PubMed, Cochrane Library, Web of Science, and EMBASE databases until April 2022. A total of 20 articles involving 796 participants were available for the meta-analysis. There were significant benefits for participants in appendicular muscle mass gain (SMD = 0.29; 95% CI: 0.07, 0.52; P = 0.01) and 6-min walk distance (SMD = 0.37; 95% CI: 0.01, 0.73; P = 0.05). A subgroup analysis indicated that flavonoid significantly improves appendicular muscle mass (SMD = 0.50; 95% CI: 0.21, 0.80; P < 0.01) and Timed-Up and Go test (SMD = −0.47; 95% CI: −0.85, −0.09; P = 0.02) in Sarcopenia population. Our results provide insight into the effects of flavonoids on skeletal muscle mass and gait speed for those without exercise. However, there was no significant improvement in the subjects' muscle strength.
Systematic review registration: https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=334383, identifier: CRD42022334383.
Skeletal muscle is the most extensive mass system of the organ in the body, which plays a critical role in maintaining physical functioning and metabolic health (1). With aging, there is an unavoidable loss of muscle mass and strength (2). Muscle mass loss occurs at approximately 1–2% per year from middle age, and muscle strength decreases by 1.5% per year from 40 years of age and 3% per year thereafter (3). Such a trend is associated with increased adverse outcomes, including falls, functional decline, frailty, and mortality (4, 5), which translates into higher healthcare costs (6). In clinical practice, the EWGSOP2 (European Working Group on Sarcopenia in Older People) states that a person with low muscle mass and low muscle strength or quality will be diagnosed with Sarcopenia (7). Sarcopenia has been already recognized as an independent condition by an ICD-10-CM code in 2016 (8).
Flavonoids are polyphenolic phytochemicals distributed commonly in different fruits, vegetables, plants, and herbs, including anthocyanidins (such as cyandin, delphinidin, and malvidin, found in colored berries and red wine), flavan-3-ols (such as catechin, epicatechin, theaflavin, etc., found in cocoa, apples, and grapes), flavanones (such as eriodictyol, hesperidin, and naringenin exclusive of citrus fruits), flavones (such as apigenin and luteolin, commonly found in celery, parsley, and chamomile tea), flavonols (such as kaempferol, quercetin, and myricetin, found in tea, broccoli, and various fruits), and isoflavones (such as daidzein, genistein and glycitein, present mainly in soy and soy products) (9). Flavonoids are widely known for their anti-inflammatory and antioxidant properties. Current evidence indicates that several sub-classes of flavonoids and their primary food sources may regulate metabolism in skeletal muscle that acts as the prior site of glucose storage (10). Flavonoids could also preserve muscle structure and function directly through physiological mechanisms or indirectly through distinct molecular signal pathways (11, 12).
Although both in vitro and in vivo studies have shown the association of flavonoids with skeletal muscle mass, clinical findings have been inconsistent concerning the relationship between flavonoids and Sarcopenia (13, 14). Therefore, this systematic review aims to evaluate the current evidence of flavonoid intake/supplementation on skeletal muscle mass, strength, and physical performance in middle-aged and older adults with or without Sarcopenia, and draw research-based conclusions on the practical implications of the findings.
This systematic review followed the guidelines recommended by the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) (15). The protocol for this study was registered at PROSPERO (Registration Number: CRD42022334383).
We searched PubMed, Embase, Web of Science, and the Cochrane Library (Cochrane Central Register of Controlled Trials) in English, from the date of inception until 15 April 2022.
The following Medical Subject Heading (MeSH) terms and keywords were used to search the studies: “middle-aged,” “elderly,” “skeletal muscle,” “muscle performance,” “muscle strength,” “dynapenia,” “frailty,” “sarcopenia,” “flavonoids,” “flavonols,” “flavones,” “flavanones,” “isoflavones,” and “anthocyanidins” (Supplementary Table 1).
Two reviewers (YZL and YL) independently reviewed all relevant articles. Disagreements were resolved by group discussion.
Following the removal of duplicate articles, two reviewers (YZL and YL) independently screened titles and abstracts for eligibility using the inclusion and exclusion criteria discussed below. Disagreements were discussed by the reviewers and resolved through consensus.
Trials included in the meta-analysis must meet the following criteria: (1) The participants included adults in middle-age and elderly, with or without Sarcopenia. Middle age was defined as between 45 and 65 years old, with elderly age over 65 years, and Sarcopenia was diagnosed by EWGSOP or AWGS (7, 16); (2) The study design was a randomized control trial (RCT); (3) Experimental groups received flavonoids or flavonoid-rich foods as interventions; (4) Control groups received a placebo supplement, and subjects taking exercise training are also taken into account; (5) The study provided available data to calculate average differences between baseline and endpoints, including skeletal muscle mass indicators, muscle strength indicators, and physical performance indicators.
Studies were eliminated if (1) the trial was conducted in vitro or in an animal model or if (2) the trial had a Non-RCT design, such as a case report, case series, or a prospectively designed trial without a comparison group.
Relevant data were extracted including general characteristics of the study and population (authorship, year of publication, and type of study population), the number of cases and controls, type of intervention, intervention duration, and measured outcomes (skeletal muscle mass (SMM), skeletal muscle mass index (SMMI), appendicular skeletal muscle mass (ASM), appendicular skeletal muscle mass index (ASMI), lean body mass (LBM), gait speed, the Timed-Up and Go and 6-min walk distance) by 2 reviewers (YZL and YL) independently.
The quality of the included studies was assessed according to Cochrane Collaboration's tool for assessing the risk of bias, considering the following characteristics: (1) randomized sequence generation; (2) treatment allocation concealment; (3) blinding of participants; (4) completeness of the outcome data; (5) selective outcome reporting, and (6) other sources of bias (17). Within each domain, an independent judgment was made separately by the 2 reviewers (YZL and YL).
The quality Statistical analysis was performed by RevMan (Review Manager, version 5.3; London, UK). Standard mean difference (SMD) along with the corresponding 95% confidence intervals (95% CIs) were used to examine the differences between the flavonoids and placebo groups. Estimates of statistical heterogeneity between the studies were described using Cochran Q (Chi-square test) and I2 statistics, where values of 25–49% were considered low, 50–74% considered moderate, and 75–100% considered high heterogeneity. A random-effects model was used if there was significant heterogeneity (P ≤ 0.05), and a fixed-effects model was used otherwise (18). To investigate whether the changes in outcomes were affected by the subjects' characteristics, subgroup analyses were conducted on whether participants were Sarcopenia or if they were intervened with exercise. Publication bias was evaluated by generating funnel plots and the Egger bias test, and a two-sided p value ≤ 0.05 was considered statistically significant, which was performed using Stata MP, version 15 (StataCorp; 2015; Stat Statistical Software: Release15 College Station, TX, USA).
The quality of the evidence and the robustness of the recommendations were evaluated using the GRADE (Grade of Recommendations Assessment, Development, and Evaluation) method. The quality of evidence was categorized as high, moderate, low or very low depending on the risk judgments of bias, inconsistency, vagueness, indivisibility and publication bias (19).
The literature retrieval process is shown in Figure 1. A total of 2,426 articles were identified through the systematic search. After the removal of duplicates, 2,240 articles were screened for inclusion based on title and abstract, which resulted in 234 full-text articles considered for inclusion. After detailed reading, 214 articles were excluded for several reasons such as Non-RCTs, reporting irrelevant outcomes, including a population aged below 45 years, and the presence of insufficient data. In the end, 20 randomized placebo-controlled intervention studies were included in this systematic review, which enabled us to investigate the effects of flavonoids on skeletal muscle mass, strength, and physical performance (13, 20–38).
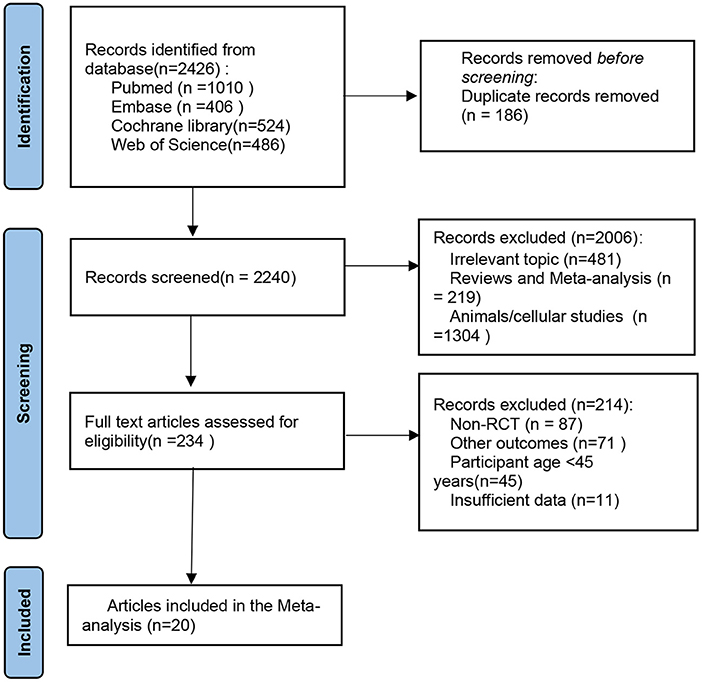
Figure 1. PRISMA flow diagram of the selection of the studies. PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
The included articles contain basic characteristics as shown in Table 1. Among them, six RCTs were conducted in Japan, four in Canada, four in Brazil, two in the USA, and one each in China, Mexico, Australia, and Iran. Fifteen studies used isoflavones or isoflavone-rich supplementations as intervention, two used catechin or epicatechin, and one used curcumin and rutin. The duration of the interventions spanned 8 to 24 weeks. Twelve studies conducted exercise training plans during supplement of flavonoids.
The studies' risks of bias are shown in Figure 2. They were typically at low risk of bias for most domains including completeness of the outcome (100%), selective reporting (100%), and random sequence generation (75%) and at an unclear risk of bias for the other bias (100%). The allocation concealment was at low risk in eleven studies (55%), unclear in eight studies (40%), and at high risk in one study (5%). The blinding of the participants and personnel was at low risk in ten studies (50%), unclear in eight studies (40%), and at high risk in two studies (10%). The blinding of the outcome assessment was at low risk in ten studies (50%), unclear in nine studies (45%), and high risk in one study (5%).
Of the eighteen studies that determined the effects of flavonoids on muscle mass outcomes, Results on muscle mass outcomes were clustered based on the following domains evaluated in the studies, (i) SMM or SMMI, (ii) ASM or ASMI, (iii) LBM.
A total of 10 trials with 399 participants reported the effects of flavonoids on SMM or SMMI. The pooled effect showed that supplementation with flavonoids did not exert muscle mass change (SMD = 0.17; 95% CI: −0.03, 0.36; P = 0.10), with an insignificant between-study heterogeneity (I2 = 3%, P = 0.41). A subgroup analysis was performed to explore differences in muscular change according to the combination with exercise intervention. We found that flavonoids for participants without taking exercise had a significant increase in SMM or SMMI compared to the control group (SMD = 0.48; 95% CI: 0.10, 0.86; P = 0.01) (Figure 3).
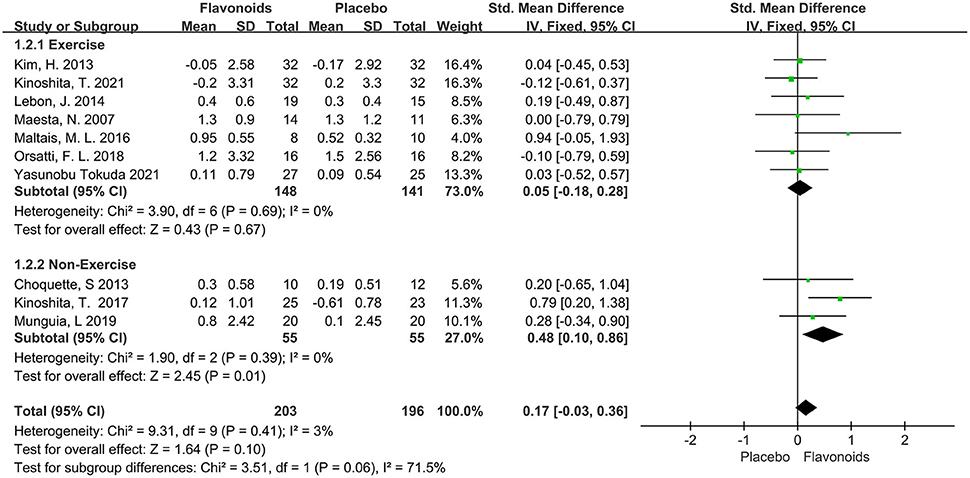
Figure 3. Forest plots of the included studies assessing effects of flavonoid supplementation on skeletal muscle mass categorized by exercise training intervention. IV, inverse-variance method; Fixed, fixed effect.
Seven studies including a total of 308 participants reported ASM or ASMI as an outcome measure. The combined results showed marginal significant benefits on the ASM or ASMI following flavonoid consumption (SMD = 0.29; 95% CI: 0.07, 0.52; P = 0.01), with low heterogeneity among the studies (I2 = 47%, P = 0.08). When the subgroup analysis was based on Sarcopenia, we found that studies for Sarcopenia showed significant improvements in the ASM or ASMI of subjects who received flavonoids (SMD = 0.50; 95% CI: 0.21, 0.80; P < 0.01), while those without Sarcopenia failed to show significant differences in the ASM or ASMI (Figure 4).
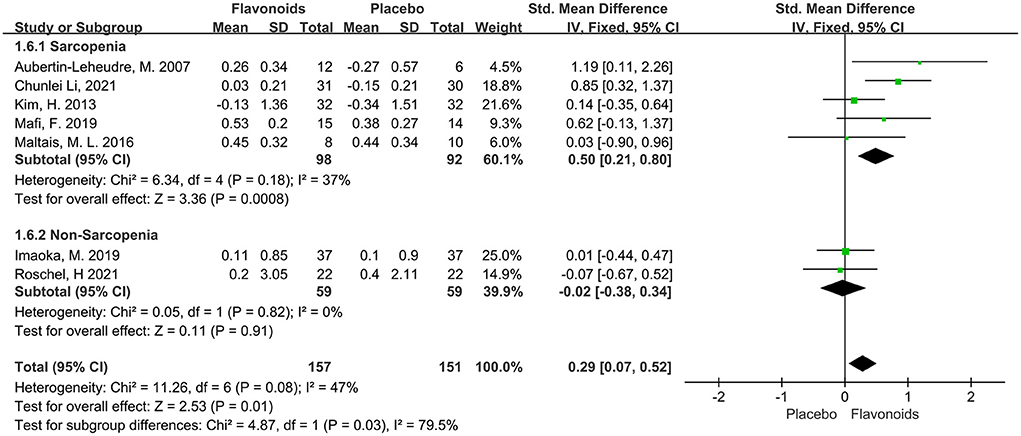
Figure 4. Forest plots of the included studies assessing effects of flavonoid supplementation on appendicular muscle mass categorized by Sarcopenia. IV, inverse-variance method; Fixed, fixed effect.
A total of 7 trials with 212 participants provided available data on the LBM, and the summary estimate showed that flavonoids supplementation did not exert significant effect on LBM (SMD = 0.07; 95% CI: −0.20, 0.35; P = 0.60) with no heterogeneity across the studies (I2 = 0.0%, P = 0.55) (Figure 5).
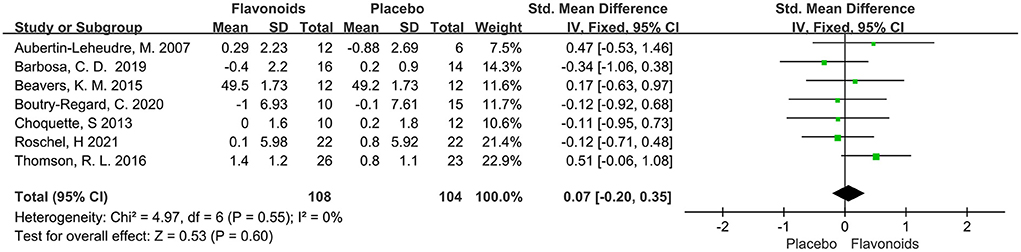
Figure 5. Forest plots of the included studies assessing effects of flavonoid supplementation on lean body mass. IV, inverse-variance method; Fixed, fixed effect.
This systematic analysis makes grip strength a representative parameter of muscle strength. A total of 10 studies with 494 participants reported the measures of grip strength, no difference was observed in the effect of flavonoids supplementation on grip strength compared with the control condition (SMD = −0.01; 95% CI: −0.19, 0.16; P = 0.87) with no intra-studies heterogeneity (I2 = 0%, P = 0.99). When the subjects were stratified in the combination with Sarcopenia, the results showed no difference in grip strength (SMD = 0.07; 95% CI: −0.28, 0.43; P = 0.68), with no intra-subgroup heterogeneity (I2 = 0.0%, P = 0.56) (Figure 6).
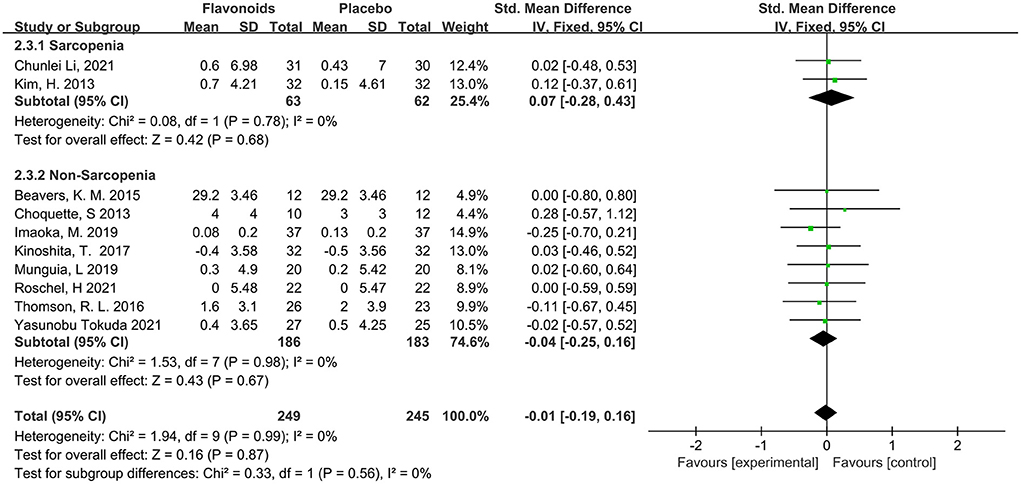
Figure 6. Forest plots of the included studies assessing effects of flavonoid supplementation on grip strength categorized by Sarcopenia. IV, inverse-variance method. Fixed, fixed effect.
Results on physical performance outcomes were clustered based on the following domains evaluated in the studies, (i) Gait speed, (ii) Timed-Up and Go test, and (iii) 6-min walk distance.
Six studies including a total of 294 participants reported gait speed as an outcome measure. Combined results from the fixed-effects model showed no significant impact on the gait speed following flavonoid consumption (SMD = 0.07; 95% CI: −0.17, 0.30; P = 0.58), with moderate heterogeneity among the studies (I2 = 53%, P = 0.06). Stratified analysis conducted according to combination with exercise intervention showed that flavonoids had a significant beneficial effect on gait speed for the non-exercise group (SMD = 0.64; 95% CI: 0.20, 1.08; P < 0.01) (Figure 7).
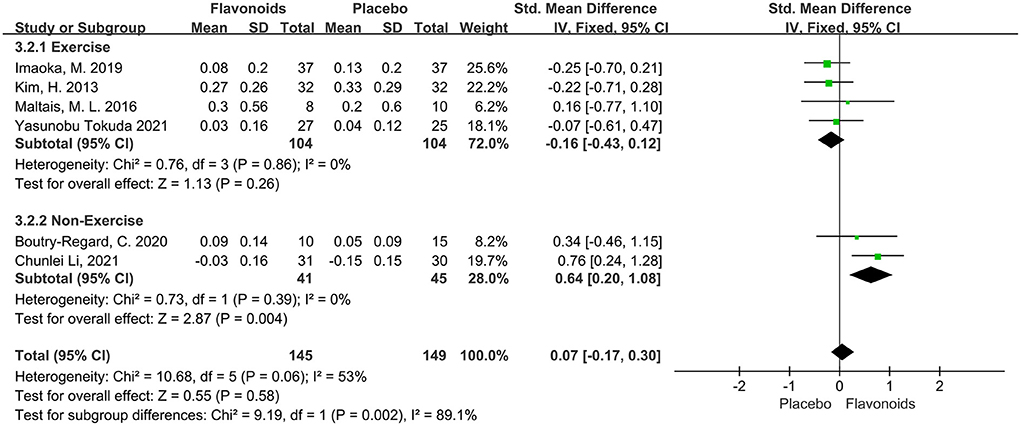
Figure 7. Forest plots of the included studies assessing effects of flavonoid supplementation on gait speed categorized by exercise intervention. IV, inverse-variance method; Fixed, fixed effect.
The variation of Timed-Up and Go test was reported in 5 trials of total 197 participants, and no significant effect was observed (SMD = −0.25; 95% CI: −0.53, 0.04; P = 0.09), with low heterogeneity (I2 = 34%, P = 0.19). The trials stratified by Sarcopenia indicated that the pooled effect of flavonoid supplements significantly reduced the Timed-Up and Go for the Sarcopenia population (SMD = −0.47; 95% CI: −0.85, −0.09; P = 0.02) (Figure 8).
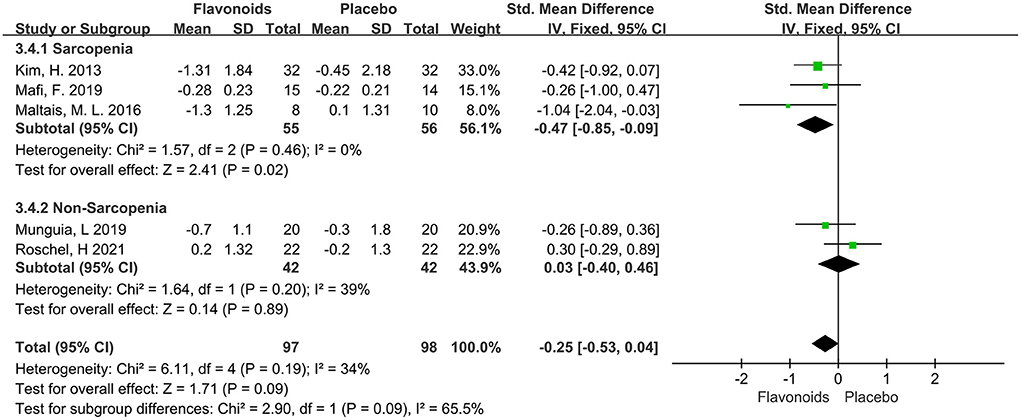
Figure 8. Forest plots of the included studies assessing effects of flavonoid supplementation on Timed-Up and Go test categorized by Sarcopenia. IV, inverse-variance method. Fixed, fixed effect.
Three trials of total 122 participants reported the effect of flavonoids supplementation on 6-min walk distance. The pooled effect showed that flavonoids supplementation has significant benefits effect on 6-min walk distance (SMD = 0.37; 95% CI: 0.01, 0.73; P = 0.05), with no heterogeneity across studies (I2 = 0%, P = 0.43) (Figure 9).
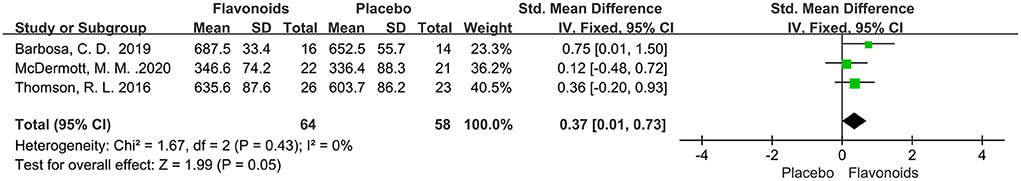
Figure 9. Forest plots of the included studies assessing effects of flavonoid supplementation on 6-min walk distance. IV, inverse-variance method; Fixed, fixed effect.
Sensitivity analysis to appraise the stability were carried out using the leave-one-out approach (Figure 10). It revealed that the effect of flavonoids on SMM, ASM, LBM, Grip strength, Gait speed, Time-Up and Go test, and 6-min walk distance was not substantially changed by excluding a particular study. Taken together, these results indicated that this meta-analysis showed good reliability.
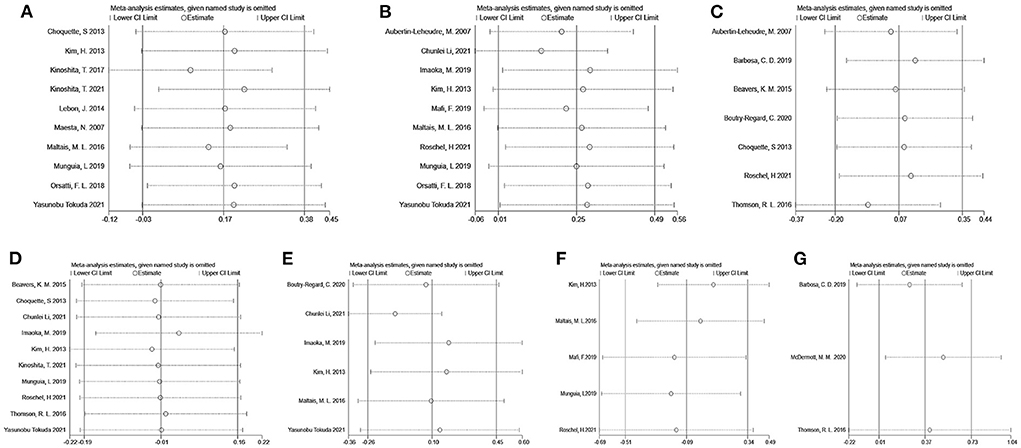
Figure 10. Sensitivity analyses by skeletal muscle mass (A), appendicular muscle mass (B), lean body mass (C), grip strength (D), gait speed (E), Timed-Up and Go test (F), and 6-min walk distance (G).
The visual inspection of funnel plots revealed an asymmetric distribution in SMM, ASM, and Gait speed, indicating potential publication bias (Figure 11). Therefore, we further performed the Egger bias test for quantitative detection. The Egger's test showed no significant publication bias regarding SMM (t = 1.30, P = 0.230), ASM (t = 0.97, P = 0.378), LBM (t = −0.26, P = 0.805), Grip strength (t = 1.46, P = 0.184), Gait speed (t = 0.64, P = 0.555), Time-Up and Go test (t = 0.06, P = 0.952) and 6-min walk distance (t = 1.32, P = 0.413). The evidence certainty for measured outcomes was rated from moderate to very low according to GRADE, with a summary of the effects of the flavonoids on muscle mass, strength, and physical performance summarized in Table 2.
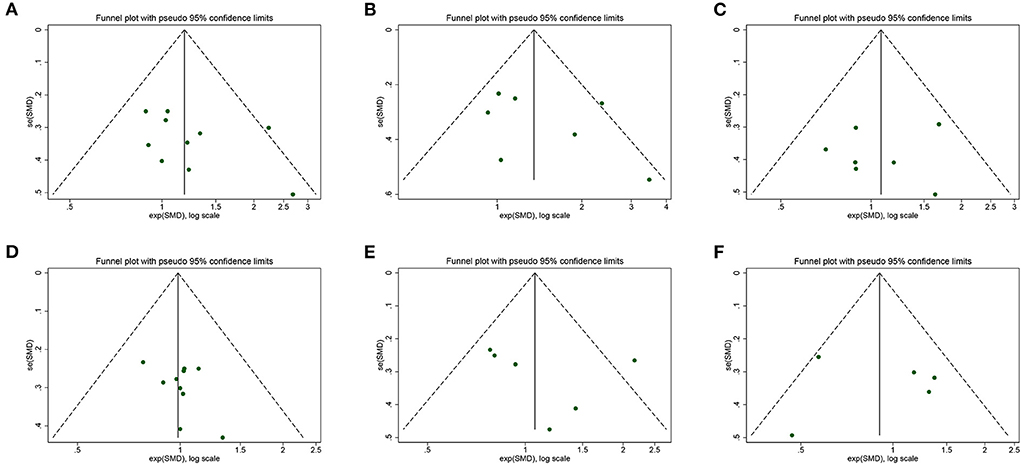
Figure 11. Funnel plot of meta-analysis result: (A) skeletal muscle mass, (B) appendicular muscle mass, (C) lean body mass, (D) grip strength, (E) gait speed, (F) Timed-Up and Go test.
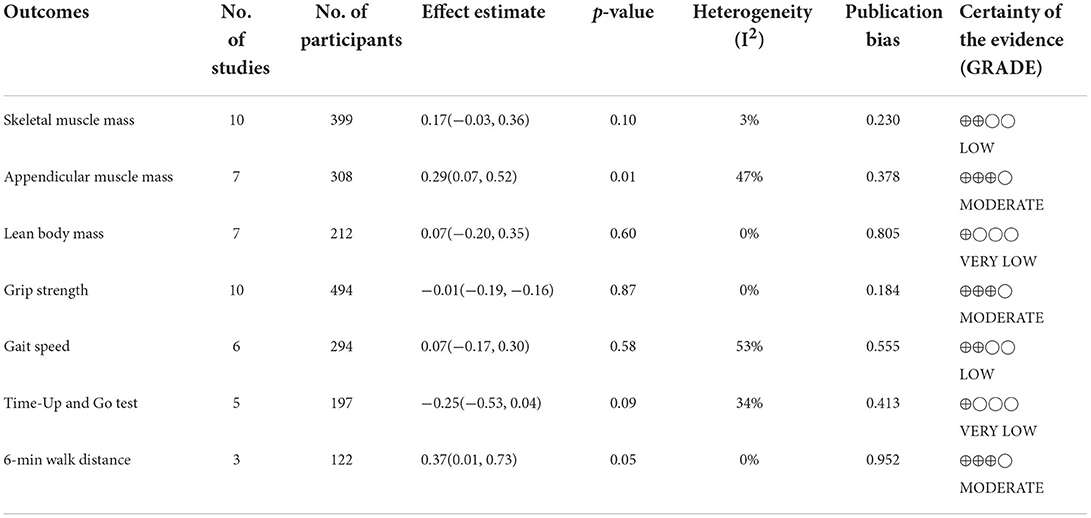
Table 2. Summary effects of flavonoids on the outcomes of interest among the included studies, publication bias, and quality evidence of the Grading of Recommendations Assessment, Development and Evaluation (GRADE).
This meta-analysis with 20 RCTs involving 796 elderly participants showed that flavonoid supplementation was associated with an increase in appendicular muscle mass by 0.29 kg for the middle-aged and elderly, especially for the Sarcopenia population. In terms of muscle strength, we found that flavonoid supplementation did not elicit greater handgrip strength. For muscle performance, flavonoid administration enhanced performance in the 6-min walk distance and facilitated sarcopenia up-and-go time.
Our result suggests that flavonoid supplementation has a beneficial effect on appendicular muscle mass which is recognized to be responsible for locomotion and physical independence, similar to a recently published systematic review and meta-analysis indicating that flavonoid improved muscle cross-sectional area and muscle mass (39). Muscle fiber loss and muscle fiber atrophy are the main mechanisms of muscle deterioration with aging. The former is primarily due to imbalances between muscle protein synthesis (MPS) and muscle protein breakdown (MPB). It has been observed that the blunting of MPS in combination with the suppressed inhibition of MPB exacerbates muscle fiber loss in the elderly (40). Multiple pathways participate in such skeletal muscle protein turnover at the molecular level. There is evidence that flavonol glycoside has skeletal muscle growth and differentiation effects by promoting the PI3K/Akt/mTOR pathways, which is the key molecule to promote protein synthesis and block protein degradation, and it can also down-regulate Smad pathways, which are negative regulators of skeletal muscle growth (41). Another study reported that licorice flavonoid oil promoted the phosphorylation of mTOR and p70S6K, which are involved in muscle synthesis pathways (42). Meador et al. (43) found that the green tea polyphenol epigallocatechin-3-gallate reduced the atrogin-1 and MuRF-1 in aged rats, the biomarker of muscle atrophy. Munguia et al. (44) found that epicatechin attenuated muscle damage by reducing the expression of Foxo1 in aged mice, a transcription factor of ubiquitin-proteasome for proteolytic activation. Chang and his colleagues also found that epigallocatechin gallate attenuated muscle atrophy and protein degradation upregulated miR-486-5p in aged mice, a microRNA modulates skeletal muscle epigenetic expression (45). Meanwhile, insulin, with a similar structure to IGF-1, potentially activates the anabolic mTOR pathway and inhibits the catabolic ubiquitin-proteasome pathway, contributing to maintaining skeletal muscle protein balance (46). Such balance can be broken because of the resistance to insulin in age-related Sarcopenia. Guevara et al. observed that genistein, a subclass of isoflavones, could improve insulin resistance and increase skeletal muscle fatty acid oxidation through changing gut microbiota (47). Bowser et al. (48) investigated the insulin-enhancing effects and insulin-mimetic activities of cocoa procyanidins in human skeletal muscle metabolism, which may lead to inducting MPS and contribute to the inhibition of MPB. Apart from MPS and MPB, recent studies demonstrated that anabolic resistance is the major driver of age-related muscle loss (40). Anabolic resistance is a situation where the skeletal muscle is unable to respond appropriately to these anabolic stimuli (e.g., nutrition and exercise) by stimulating protein synthesis (49–51). The main reason for this blunted response is fat infiltration into skeletal muscle and muscle ectopic fat deposition (52). Ohmae et al. (53) found that quercetin attenuated adipogenesis and fibrosis in human skeletal muscle. Xi et al. (54) found that baicalin attenuates insulin resistance and ectopic fat storage in skeletal muscle. A previous systemic review has shown that flavonoid-enriched diets may benefit lipid metabolism (55), indicating that flavonoids may improve anabolic resistance by improving lipid metabolism and ectopic deposition in muscle. On the other hand, anabolic resistance means that a more intense exercise is required to allow elderly subjects to gain muscle mass and enhance muscle strength. However, with weak sports ability and without the supervision of professionals, there are certain risks in sports reinforcement for the elderly. Therefore, exercise strengthening is not suitable for all the elderly. In our subgroup analysis, we found that non-exercise people can have better muscle mass benefits after supplementing flavonoids. Isoflavones have been usually used as a surrogate for hormone replacement therapy (HRT) for its plant estrogens property. A 12-month clinical trial combining exercise and HRT showed that it was of benefit on body composition only to non-exercisers (56), which is consistent with our present result. LBM consists of muscles, organs, and bones. Although a previous systematic review and meta-analysis has shown that soy isoflavones have the effect of increasing bone mineral density (57), our results did not observe beneficial effects on LBM. The sample size and intervention time might have contributed to the inconsistency in the findings between studies.
With regard to muscle strength, the data analyzed do not allow us to draw any conclusions about the potential effect of flavonoids on handgrip in middle-aged adults and seniors. Our results are similar to previous systematic reviews that supplementation with dairy protein, well-established countermeasures against Sarcopenia, did not improve grip strength in older adults, with or without Sarcopenia (58). Given that muscle strength decreases at a faster rate compared with muscle mass and has better efficacy in predicting adverse outcomes, EWGSOP2 elevates low muscle strength to the forefront as a primary indicator of probable Sarcopenia (7). Actually, muscle strength does not depend solely on muscle mass, and the correlations between muscle strength and muscle mass are inconsistent and not linear (24). Dynapenia, defined as the loss of muscle strength and power, is specifically proposed to differentiate Sarcopenia (59). The evidence convincingly indicates that with age, muscle fibers undergo continued denervation and reinnervation owing to the accelerated loss of motor neurons in the spinal cord (60). Such a process is probably one of the main contributors to muscle atrophy resulting in the loss of muscle strength. Choi et al. found that dietary apigenin of flavone inhibits denervation-induced muscle atrophy by down-regulating the expression of inflammatory cytokines [tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6)] (61). Tabata et al. (62) also found that isoflavone aglycone could modulate muscle atrophy after denervation in mice based on the modulation of apoptosis-dependent signaling. It seems that the anti-denervation effect of flavonoids is theoretically inconsistent with our negative findings in muscle strength. However, age-related muscle fiber type conversion should be taken into account. Aging can cause remarkable shrinkage of explosive fast-twitch type II fibers and promote their transformation into slow-twitch type I fibers (63). Therefore, the loss in muscle strength associated with Sarcopenia might also be a consequence of the preferential atrophy of type II fibers, responsible for higher intensity (64). Chen et al. found that quercetin could promote skeletal fiber to switch from glycolytic type II to oxidative type I through Adiponectin signaling pathway (65). Xue et al. (66) also found that naringin induced skeletal muscle fiber type transformation from II to type I by activating the AMPK/PGC-1α signaling pathway, which supports the notion that flavonoids could not improve muscle strength due to their muscle fiber switch property. Although flavonoids decrease Type II fibers leading to a reduction in power bursts, they have been shown to improve the endurance of activity which we will discuss below.
In order to evaluate physic performance, the included studies evaluated the subjects' gait speed, Timed-Up and Go, and 6-min walk distance. Our research has found that flavonoid supplementation could increase gait speed and 6-min walk distance. Physical performance is a multidimensional concept that goes beyond skeletal muscle, and it also involves many other body organs and systems (cardiovascular aspects, peripheral nervous and vascular function, motivation, balance…) (67), leading to falls, fractures, and even death directly in aging. In 2018, EWGSOP2 has purposed physical performance to categorize the severity of Sarcopenia (7). Gait speed and 6-min walk distance are diagnostic indicators of Sarcopenia with limited mobility (68). The latter is a commonly used test for the objective assessment of cardiovascular disease, pulmonary disease, frailty, and cancer, which is also predictive of morbidity and mortality in patients with chronic obstructive pulmonary diseases or congestive heart failure (69). Our results show that flavonoids provide a statistically significant benefit in 6-min walk distance. One reason perhaps is their well-documented cardioprotective effects. Several studies confirmed that flavonoids may exert their cardiovascular protective properties through various signaling pathways, such as AMPK, PPAR-γ, PGC-1α, and NF-κB (70, 71). Besides the contribution of the cardiopulmonary function to physical performance, the peripheral vascular system in skeletal muscle is crucial to maintaining physical activity. Older subjects have impairments in the vascular structure and function, including reductions in leg blood flow (72), decrements in microvascular function (73), loss of endothelial cells, and a reduced muscle capillary density (74). These changes have the potential to further compromise physical performance by affecting the delivery of energy, nutrients, and oxygen. Sian et al. found that the cocoa favanols condition displayed significant increases in the lower limb muscles microvascular blood volume (MBV), thus enhancing nutrient and oxygen delivery (75). Roberts et al. (76) found that catechin-rich green tea promoted peripheral vascular function by flow-mediated vascular dilation of upper and lower limbs. Heiss et al. (77) found that cocoa flavanols increased circulating nitric oxide (NO) with enhanced vasodilation and improved muscle perfusion, which could be reversed by NO-synthase inhibitor L-NAME. Similarly, Ludovici et al. summarized the vascular protective effects of cocoa flavanols in their reviews (78). Accompanied by the deterioration of the vascular system, the oxygen delivery and utility are restricted, which are the core steps for aerobic capacity. Yeh et al. (79) found that pueraria isoflavone and soybean peptides were effective in promoting the utilization of free fatty acids and improving VO2max exhaustive cycling test performance in humans. In a previous Systematic Review and Meta-analysis, Kressler et al. reported that quercetin supplementation could improve human endurance exercise capacity (VO2max and endurance exercise performance) (80). In our subgroup analysis, it is found that a greater increase in gait speed is expected in patients who do not regularly exercise compared with those who do. Similar to these findings, it was also reported that the dietary flavonoid quercetin could increase VO2max and endurance capacity, especially for people without taking exercise training (81). Clinically, this apparent increase in fitness without physical training may have implications beyond enhancing health promotion and illness prevention performance. VO2max is not only limited to oxygen delivery by the cardiovascular system but is also influenced by muscle mitochondrial oxidative capacity. Skeletal muscle, especially type I muscle fibers, is abundant in mitochondria generating the energy and oxygen continuously required for physical activity. Recent evidence has proven that lower mitochondrial capacity and efficiency are associated with reduced physical performance in age-related Sarcopenia (82). Flavonoids could enhance muscle mitochondrial function through diverse signaling pathways. Davis et al. found that quercetin could induce mitochondrial biogenesis in skeletal muscle by overexpression of the transcriptional coactivators sirtuin 1 (SIRT1) and peroxisome proliferator-activated receptor-γ coactivator (PGC1α) (83). Moreno et al. (84) also found that epicatechin exhibited greater increases in mitochondrial protein expression and indices of mitochondrial content through NO-dependent and Nrf2 pathways. In addition, various flavonoids have been reported to promote the switch of mitochondrial-rich type I fibers in skeletal muscle as discussed above (65, 66), thus improving aerobic capacity. Our subgroup analysis conducted by complications with Sarcopenia showed that flavonoids effectively improved the Timed-Up and Go for Sarcopenia population. The underlying mechanism is probably that the muscle fibers reduction and transformation in severe Sarcopenia is revered by flavonoid supplementation.
Concerning the clinical practice, most of the supplementations in this study are isoflavones or isoflavone-rich foods, and the mean isoflavones intake ranges from about 50–135 mg/d for 8–24 weeks. Fan et al. in their review of dose-response analysis showed that an increment of 0.5 mg/d isoflavones, 5 mg/d flavones, 25 mg/d flavanols, 50 mg/d anthocyanins, 100 mg/d proanthocyanins, were associated with a 5 % reduction in coronary heart disease risk, respectively (85). However, evidence for flavonoids against Sarcopenia risk is quite scarce. The non-standardized flavonoids intervention and the uncategorizable outcomes made specific investigations into optimal composition, dosage and duration impossible in the current review.
There are several strengths of this review. To the best of our knowledge, this is the first systematic review based on RCTs to determine the impact of flavonoid intake on muscle mass, muscle strength, and physical performance separately in middle-aged to older adults with or without Sarcopenia. Furthermore, we provided a valuable quantitative analysis of the current research and performed subgroup analyses and found differential benefits in non-exercise and sarcopenic populations. It put forward some clinical implications. Thirdly, we also applied GRADE to qualify our level of evidence. Using GRADE, we show that, although statistically significant, some of our findings have been downgraded in terms of certainty, noting that study design issues preclude further conclusions. The main reasons for downgrading the certainty of evidence were increased risk of bias, mainly in the blinding domains, and for some subgroup analyses, the low number of subjects in each respective group.
There are also notable limitations that should be recognized. Firstly, while a comprehensive search for four electronic databases has been undertaken, it is possible that some RCTs meeting the criteria of this review were overlooked, which may be present in gray literature, such as conference proceedings, or may have been published in another language. In addition, many of the RCTs discussed in this review utilized small sample sizes. Furthermore, the current meta-analysis failed to perform dose–response analysis hindering drawing a reliable conclusion on the optimal dose and duration. Meanwhile, we did not analyze the separate effects of different subclasses of flavonoids, which may introduce category errors. Therefore, the specific type, dosage, and duration of flavonoid supplementation to improve Sarcopenia-related parameters need to be further explored. Finally, although our quantitative test for publication bias was insignificant, careful consideration should be given when interpreting the results because some of the measured outcomes included relatively few studies with the potential risk of bias.
Our results suggest that significant improvements were primarily observed in appendicular skeletal muscle mass and 6-min walk distance after flavonoid supplementation. Results of subgroup analyses showed that flavonoids had more significant effects on appendicular muscle mass and Timed-Up and Go test in the Sarcopenia group, with a better impact on skeletal muscle mass and gait speed in the non-exercise group. However, no significant differences were found between the effects of the flavonoids on muscle strength.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Conceptualization: YunL. Design: YuL. Supervision, critical review, and funding acquisition: YanL and RT. Materials, data collection and processing, analysis and interpretation, literature search, and writing manuscript: YuL and YunL. All authors have read and agreed to the published version of the manuscript.
This research was funded by the Research Program of Sports Bureau of Guangdong Province (Grant Number GDSS2020M003), City-School Joint Program of Guangzhou Science and Technology Bureau (Grant Number 202201020033), and Research-oriented Hospital Program of Guangzhou (Grant Number 2022RHPG05). These funding sources had no role in the design, methods, data collection, analysis, or preparation of this manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2022.1013449/full#supplementary-material
1. Wolfe RR. The underappreciated role of muscle in health and disease. Am J Clin Nutr. (2006) 84:475–82. doi: 10.1093/ajcn/84.3.475
2. Kitamura A, Seino S, Abe T, Nofuji Y, Yokoyama Y, Amano H, et al. Sarcopenia: prevalence, associated factors, and the risk of mortality and disability in Japanese older adults. J Cachexia Sarcopenia Muscle. (2021) 12:30–8. doi: 10.1002/jcsm.12651
3. Keller K, Engelhardt M. Strength and muscle mass loss with aging process. Age and strength loss. Muscles Ligaments Tendons J. (2013) 3:346–50. doi: 10.32098/mltj.04.2013.17
4. Cruz-Jentoft AJ, Sayer AA. Sarcopenia. Lancet. (2019) 393:2636–46. doi: 10.1016/S0140-6736(19)31138-9
5. Li R, Xia J, Zhang XI, Gathirua-Mwangi WG, Guo J, Li Y, et al. Associations of muscle mass and strength with all-cause mortality among us older adults. Med Sci Sports Exerc. (2018) 50:458–67. doi: 10.1249/MSS.0000000000001448
6. Norman K, Otten L. Financial impact of Sarcopenia or low muscle mass - a short review. Clin Nutr. (2019) 38:1489–95. doi: 10.1016/j.clnu.2018.09.026
7. Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyère O, Cederholm T, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. (2019) 48:601. doi: 10.1093/ageing/afz046
8. Cao L, Morley JE. Sarcopenia is recognized as an independent condition by an International Classification of Disease, Tenth Revision, Clinical Modification (Icd-10-Cm) code. J Am Med Dir Assoc. (2016) 17:675–7. doi: 10.1016/j.jamda.2016.06.001
9. Pérez-Cano FJ, Castell M. Flavonoids, inflammation and immune system. Nutrients. (2016) 8:659. doi: 10.3390/nu8100659
10. Russo B, Picconi F, Malandrucco I, Frontoni S. Flavonoids and insulin-resistance: from molecular evidences to clinical trials. Int J Mol Sci. (2019) 20:2061. doi: 10.3390/ijms20092061
11. Li P, Zhang S, Song H, Traore SS, Li J, Raubenheimer D, et al. Naringin promotes skeletal muscle fiber remodeling by the Adipor1-Appl1-Ampk signaling pathway. J Agric Food Chem. (2021) 69:11890–9. doi: 10.1021/acs.jafc.1c04481
12. Daussin FN, Cuillerier A, Touron J, Bensaid S, Melo B, Al Rewashdy A, et al. Dietary cocoa flavanols enhance mitochondrial function in skeletal muscle and modify whole-body metabolism in healthy mice. Nutrients. (2021) 13:3466. doi: 10.3390/nu13103466
13. Boutry-Regard C, Vinyes-Parés G, Breuillé D, Moritani T. Supplementation with whey protein, omega-3 fatty acids and polyphenols combined with electrical muscle stimulation increases muscle strength in elderly adults with limited mobility: a randomized controlled trial. Nutrients. (2020) 12:1866. doi: 10.3390/nu12061866
14. Lynch HM, Buman MP, Dickinson JM, Ransdell LB, Johnston CS, Wharton CM. No significant differences in muscle growth and strength development when consuming soy and whey protein supplements matched for leucine following a 12 week resistance training program in men and women: a randomized trial. Int J Environ Res Public Health. (2020) 17:3871. doi: 10.3390/ijerph17113871
15. Page MJ, Moher D, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. Prisma 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ. (2021) 372:n160. doi: 10.1136/bmj.n160
16. Chen LK, Woo J, Assantachai P, Auyeung TW, Chou MY, Iijima K, et al. Asian working group for Sarcopenia: 2019 consensus update on Sarcopenia diagnosis and treatment. J Am Med Dir Assoc. (2020) 21:300–7.e2. doi: 10.1016/j.jamda.2019.12.012
17. Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The cochrane collaboration's tool for assessing risk of bias in randomised trials. BMJ. (2011) 343:d5928. doi: 10.1136/bmj.d5928
18. Cumpston M, Li T, Page MJ, Chandler J, Welch VA, Higgins JP, et al. Updated guidance for trusted systematic reviews: a new edition of the cochrane handbook for systematic reviews of interventions. Cochrane Database Syst Rev. (2019) 10:Ed000142. doi: 10.1002/14651858.ED000142
19. Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. Grade: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. (2008) 336:924–6. doi: 10.1136/bmj.39489.470347.AD
20. Maltais ML, Ladouceur JP, Dionne IJ. The effect of resistance training and different sources of postexercise protein supplementation on muscle mass and physical capacity in sarcopenic elderly men. J Strength Cond Res. (2016) 30:1680–7. doi: 10.1519/JSC.0000000000001255
21. Tokuda Y, Mori H. Effect of ingestion of essential amino acids and tea catechins after resistance exercise on the muscle mass, physical performance, and quality of life of healthy older people: a randomized controlled trial. Asia Pac J Clin Nutr. (2021) 30:213–33. doi: 10.6133/apjcn.202106_30(2).0005
22. Orsatti FL, Maestá N, de Oliveira EP, Nahas Neto J, Burini RC, Nunes PRP, et al. Adding soy protein to milk enhances the effect of resistance training on muscle strength in postmenopausal women. J Diet Suppl. (2018) 15:140–52. doi: 10.1080/19390211.2017.1330794
23. Munguia L, Rubio-Gayosso I, Ramirez-Sanchez I, Ortiz A, Hidalgo I, Gonzalez C, et al. High flavonoid cocoa supplement ameliorates plasma oxidative stress and inflammation levels while improving mobility and quality of life in older subjects: a double-blind randomized clinical trial. J Gerontol A Biol Sci Med Sci. (2019) 74:1620–7. doi: 10.1093/gerona/glz107
24. Kim H, Suzuki T, Saito K, Yoshida H, Kojima N, Kim M, et al. Effects of exercise and tea catechins on muscle mass, strength and walking ability in community-dwelling elderly japanese sarcopenic women: a randomized controlled trial. Geriatr Gerontol Int. (2013) 13:458–65. doi: 10.1111/j.1447-0594.2012.00923.x
25. Roschel H, Hayashi AP, Fernandes AL, Jambassi-Filho JC, Hevia-Larraín V, de Capitani M, et al. Supplement-based nutritional strategies to tackle frailty: a multifactorial, double-blind, randomized placebo-controlled trial. Clin Nutr. (2021) 40:4849–58. doi: 10.1016/j.clnu.2021.06.024
26. Kinoshita T, Matsumoto A, Yoshino K, Furukawa S. The effects of licorice flavonoid oil with respect to increasing muscle mass: a randomized, double-blind, placebo-controlled trial. J Sci Food Agric. (2017) 97:2339–45. doi: 10.1002/jsfa.8044
27. Barbosa CD, Costa JG, Giolo JS, Rossato LT, Nahas PC, Mariano IM, et al. Isoflavone supplementation plus combined aerobic and resistance exercise do not change phase angle values in postmenopausal women: a randomized placebo-controlled clinical trial. Exp Gerontol. (2019) 117:31–7. doi: 10.1016/j.exger.2018.08.007
28. Aubertin-Leheudre M, Lord C, Khalil A, Dionne IJ. Six months of isoflavone supplement increases fat-free mass in obese-sarcopenic postmenopausal women: a randomized double-blind controlled trial. Eur J Clin Nutr. (2007) 61:1442–4. doi: 10.1038/sj.ejcn.1602695
29. Maesta N, Nahas EA, Nahas-Neto J, Orsatti FL, Fernandes CE, Traiman P, et al. Effects of soy protein and resistance exercise on body composition and blood lipids in postmenopausal women. Maturitas. (2007) 56:350–8. doi: 10.1016/j.maturitas.2006.10.001
30. Lebon J, Riesco E, Tessier D, Dionne IJ. Additive effects of isoflavones and exercise training on inflammatory cytokines and body composition in overweight and obese postmenopausal women: a randomized controlled trial. Menopause. (2014) 21:869–75. doi: 10.1097/GME.0000000000000177
31. Mafi F, Biglari S, Ghardashi Afousi A, Gaeini AA. Improvement in skeletal muscle strength and plasma levels of follistatin and myostatin induced by an 8-week resistance training and epicatechin supplementation in sarcopenic older adults. J Aging Phys Act. (2019) 27:384–91. doi: 10.1123/japa.2017-0389
32. Kinoshita T, Maruyama K, Yamamoto N, Saito I. The effects of dietary licorice flavonoid oil supplementation on body balance control in healthy middle-aged and older Japanese women undergoing a physical exercise intervention: a randomized, double-blind, placebo-controlled trial. Aging Clin Exp Res. (2021) 33:3099–108. doi: 10.1007/s40520-020-01513-3
33. Li C, Meng H, Wu S, Fang A, Liao G, Tan X, et al. Daily supplementation with whey, soy, or whey-soy blended protein for 6 months maintained lean muscle mass and physical performance in older adults with low lean mass. J Acad Nutr Diet. (2021) 121:1035–48.e6. doi: 10.1016/j.jand.2021.01.006
34. Imaoka M, Nakao H, Nakamura M, Tazaki F, Maebuchi M, Ibuki M, et al. Effect of multicomponent exercise and nutrition support on the cognitive function of older adults: a randomized controlled trial. Clin Interv Aging. (2019) 14:2145–53. doi: 10.2147/CIA.S229034
35. Thomson RL, Brinkworth GD, Noakes M, Buckley JD. Muscle strength gains during resistance exercise training are attenuated with soy compared with dairy or usual protein intake in older adults: a randomized controlled trial. Clin Nutr. (2016) 35:27–33. doi: 10.1016/j.clnu.2015.01.018
36. Beavers KM, Gordon MM, Easter L, Beavers DP, Hairston KG, Nicklas BJ, et al. Effect of protein source during weight loss on body composition, cardiometabolic risk and physical performance in abdominally obese, older adults: a pilot feeding study. J Nutr Health Aging. (2015) 19:87–95. doi: 10.1007/s12603-015-0438-7
37. McDermott MM, Criqui MH, Domanchuk K, Ferrucci L, Guralnik JM, Kibbe MR, et al. Cocoa to improve walking performance in older people with peripheral artery disease: the cocoa-pad pilot randomized clinical trial. Circ Res. (2020) 126:589–99. doi: 10.1161/CIRCRESAHA.119.315600
38. Choquette S, Dion T, Brochu M, Dionne IJ. Soy isoflavones and exercise to improve physical capacity in postmenopausal women. Climacteric. (2013) 16:70–7. doi: 10.3109/13697137.2011.643515
39. Munguía L, Ortiz M, González C, Portilla A, Meaney E, Villarreal F, et al. Beneficial effects of flavonoids on skeletal muscle health: a systematic review and meta-analysis. J Med Food. (2022) 25:465–86. doi: 10.1089/jmf.2021.0054
40. Wilkinson DJ, Piasecki M, Atherton PJ. The age-related loss of skeletal muscle mass and function: measurement and physiology of muscle fibre atrophy and muscle fibre loss in humans. Ageing Res Rev. (2018) 47:123–32. doi: 10.1016/j.arr.2018.07.005
41. Oh M, Kim SY, Park S, Kim KN, Kim SH. Phytochemicals in Chinese Chive (Allium Tuberosum) induce the skeletal muscle cell proliferation via Pi3k/Akt/Mtor and smad pathways in C2c12 cells. Int J Mol Sci. (2021) 22:2296. doi: 10.3390/ijms22052296
42. Yoshioka Y, Yamashita Y, Kishida H, Nakagawa K, Ashida H. Licorice flavonoid oil enhances muscle mass in Kk-a(Y) mice. Life Sci. (2018) 205:91–6. doi: 10.1016/j.lfs.2018.05.024
43. Meador BM, Mirza KA, Tian M, Skelding MB, Reaves LA, Edens NK, et al. The green tea polyphenol Epigallocatechin-3-Gallate (Egcg) attenuates skeletal muscle atrophy in a rat model of Sarcopenia. J Frailty Aging. (2015) 4:209–15. doi: 10.14283/jfa.2015.58
44. Munguia L, Ramirez-Sanchez I, Meaney E, Villarreal F, Ceballos G, Najera N. Flavonoids from dark chocolate and (-)-epicatechin ameliorate high-fat diet-induced decreases in mobility and muscle damage in aging mice. Food Biosci. (2020) 37:100710. doi: 10.1016/j.fbio.2020.100710
45. Chang YC, Liu HW, Chan YC, Hu SH, Liu MY, Chang SJ. The green tea polyphenol epigallocatechin-3-gallate attenuates age-associated muscle loss via regulation of Mir-486-5p and myostatin. Arch Biochem Biophys. (2020) 692:108511. doi: 10.1016/j.abb.2020.108511
46. Tieland M, Trouwborst I, Clark BC. Skeletal muscle performance and ageing. J Cachexia Sarcopenia Muscle. (2018) 9:3–19. doi: 10.1002/jcsm.12238
47. Guevara-Cruz M, Godinez-Salas ET, Sanchez-Tapia M, Torres-Villalobos G, Pichardo-Ontiveros E, Guizar-Heredia R, et al. Genistein stimulates insulin sensitivity through gut microbiota reshaping and skeletal muscle ampk activation in obese subjects. BMJ Open Diabetes Res Care. (2020) 8:e000948. doi: 10.1136/bmjdrc-2019-000948
48. Bowser SM, Moore WT, McMillan RP, Dorenkott MR, Goodrich KM, Ye L, et al. High-molecular-weight cocoa procyanidins possess enhanced insulin-enhancing and insulin mimetic activities in human primary skeletal muscle cells compared to smaller procyanidins. J Nutr Biochem. (2017) 39:48–58. doi: 10.1016/j.jnutbio.2016.10.001
49. Wall BT, Gorissen SH, Pennings B, Koopman R, Groen BB, Verdijk LB, et al. Aging is accompanied by a blunted muscle protein synthetic response to protein ingestion. PLoS ONE. (2015) 10:e0140903. doi: 10.1371/journal.pone.0140903
50. Burd NA, Gorissen SH, van Loon LJ. Anabolic resistance of muscle protein synthesis with aging. Exerc Sport Sci Rev. (2013) 41:169–73. doi: 10.1097/JES.0b013e318292f3d5
51. Kumar V, Selby A, Rankin D, Patel R, Atherton P, Hildebrandt W, et al. Age-related differences in the dose-response relationship of muscle protein synthesis to resistance exercise in young and old men. J Physiol. (2009) 587:211–7. doi: 10.1113/jphysiol.2008.164483
52. Tardif N, Salles J, Guillet C, Tordjman J, Reggio S, Landrier JF, et al. Muscle ectopic fat deposition contributes to anabolic resistance in obese sarcopenic old rats through Eif2α activation. Aging Cell. (2014) 13:1001–11. doi: 10.1111/acel.12263
53. Ohmae S, Akazawa S, Takahashi T, Izumo T, Rogi T, Nakai M. Quercetin attenuates adipogenesis and fibrosis in human skeletal muscle. Biochem Biophys Res Commun. (2022) 615:24–30. doi: 10.1016/j.bbrc.2022.05.033
54. Xi YL, Li HX, Chen C, Liu YQ, Lv HM, Dong SQ, et al. Baicalin attenuates high fat diet-induced insulin resistance and ectopic fat storage in skeletal muscle, through modulating the protein kinase B/Glycogen synthase kinase 3 beta pathway. Chin J Nat Med. (2016) 14:48–55. doi: 10.3724/SP.J.1009.2016.00048
55. Potì F, Santi D, Spaggiari G, Zimetti F, Zanotti I. Polyphenol health effects on cardiovascular and neurodegenerative disorders: a review and meta-analysis. Int J Mol Sci. (2019) 20:351. doi: 10.3390/ijms20020351
56. Teixeira PJ, Going SB, Houtkooper LB, Metcalfe LL, Blew RM, Flint-Wagner HG, et al. Resistance training in postmenopausal women with and without hormone therapy. Med Sci Sports Exerc. (2003) 35:555–62. doi: 10.1249/01.MSS.0000058437.17262.11
57. Akhlaghi M, Ghasemi Nasab M, Riasatian M, Sadeghi F. Soy isoflavones prevent bone resorption and loss, a systematic review and meta-analysis of randomized controlled trials. Crit Rev Food Sci Nutr. (2020) 60:2327–41. doi: 10.1080/10408398.2019.1635078
58. Hanach NI, McCullough F, Avery A. The impact of dairy protein intake on muscle mass, muscle strength, and physical performance in middle-aged to older adults with or without existing Sarcopenia: a systematic review and meta-analysis. Adv Nutr. (2019) 10:59–69. doi: 10.1093/advances/nmy065
59. Manini TM, Clark BC. Dynapenia and aging: an update. J Gerontol A Biol Sci Med Sci. (2012) 67:28–40. doi: 10.1093/gerona/glr010
60. Manini TM, Hong SL, Clark BC. Aging and muscle: a neuron's perspective. Curr Opin Clin Nutr Metab Care. (2013) 16:21–6. doi: 10.1097/MCO.0b013e32835b5880
61. Choi WH, Jang YJ, Son HJ, Ahn J, Jung CH, Ha TY. Apigenin inhibits sciatic nerve denervation-induced muscle atrophy. Muscle Nerve. (2018) 58:314–8. doi: 10.1002/mus.26133
62. Tabata S, Aizawa M, Kinoshita M, Ito Y, Kawamura Y, Takebe M, et al. The influence of isoflavone for denervation-induced muscle atrophy. Eur J Nutr. (2019) 58:291–300. doi: 10.1007/s00394-017-1593-x
63. Rogers MA, Evans WJ. Changes in skeletal muscle with aging: effects of exercise training. Exerc Sport Sci Rev. (1993) 21:65–102. doi: 10.1249/00003677-199301000-00003
64. Wiedmer P, Jung T, Castro JP, Pomatto LCD, Sun PY, Davies KJA, et al. Sarcopenia - molecular mechanisms and open questions. Ageing Res Rev. (2021) 65:101200. doi: 10.1016/j.arr.2020.101200
65. Chen X, Liang D, Huang Z, Jia G, Zhao H, Liu G. Quercetin regulates skeletal muscle fiber type switching via adiponectin signaling. Food Funct. (2021) 12:2693–702. doi: 10.1039/D1FO00031D
66. Xue Y, Huang Z, Chen X, Jia G, Zhao H, Liu G. Naringin induces skeletal muscle fiber type transformation via Ampk/Pgc-1α signaling pathway in mice and C2c12 myotubes. Nutr Res. (2021) 92:99–108. doi: 10.1016/j.nutres.2021.06.003
67. Beaudart C, Rolland Y, Cruz-Jentoft AJ, Bauer JM, Sieber C, Cooper C, et al. assessment of muscle function and physical performance in daily clinical practice : a position paper endorsed by the european society for clinical and economic aspects of osteoporosis, osteoarthritis and musculoskeletal diseases (Esceo). Calcif Tissue Int. (2019) 105:1–14. doi: 10.1007/s00223-019-00545-w
68. Morley JE, Abbatecola AM, Argiles JM, Baracos V, Bauer J, Bhasin S, et al. Sarcopenia with limited mobility: an international consensus. J Am Med Dir Assoc. (2011) 12:403–9. doi: 10.1016/j.jamda.2011.04.014
69. Agarwala P, Salzman SH. Six-minute walk test: clinical role, technique, coding, and reimbursement. Chest. (2020) 157:603–11. doi: 10.1016/j.chest.2019.10.014
70. Mozaffarian D, Wu JHY. Flavonoids, dairy foods, and cardiovascular and metabolic health: a review of emerging biologic pathways. Circ Res. (2018) 122:369–84. doi: 10.1161/CIRCRESAHA.117.309008
71. Zhang Q, Yue SJ. Editorial: flavonoids and cardiovascular metabolism. Front Nutr. (2022) 9:939798. doi: 10.3389/fnut.2022.939798
72. Donato AJ, Uberoi A, Wray DW, Nishiyama S, Lawrenson L, Richardson RS. Differential effects of aging on limb blood flow in humans. Am J Physiol Heart Circ Physiol. (2006) 290:H272–8. doi: 10.1152/ajpheart.00405.2005
73. Payne GW, Bearden SE. The microcirculation of skeletal muscle in aging. Microcirculation. (2006) 13:275–7. doi: 10.1080/10739680600618710
74. Bigler M, Koutsantonis D, Odriozola A, Halm S, Tschanz SA, Zakrzewicz A, et al. Morphometry of skeletal muscle capillaries: the relationship between capillary ultrastructure and ageing in humans. Acta Physiol. (2016) 218:98–111. doi: 10.1111/apha.12709
75. Sian TS, Din USU, Deane CS, Smith K, Gates A, Lund JN, et al. Cocoa flavanols adjuvant to an oral nutritional supplement acutely enhances nutritive flow in skeletal muscle without altering leg glucose uptake kinetics in older adults. Nutrients. (2021) 13:1646. doi: 10.3390/nu13051646
76. Roberts KA, Draijer R, Hopkins ND, de Graaf Y, Holder SM, Carter SE, et al. Impact of green tea on the deleterious cardiometabolic effects of 7-days unhealthy lifestyle in young healthy males. Physiol Rep. (2021) 9:e14720. doi: 10.14814/phy2.14720
77. Heiss C, Kleinbongard P, Dejam A, Perré S, Schroeter H, Sies H, et al. Acute consumption of flavanol-rich cocoa and the reversal of endothelial dysfunction in smokers. J Am Coll Cardiol. (2005) 46:1276–83. doi: 10.1016/j.jacc.2005.06.055
78. Ludovici V, Barthelmes J, Nägele MP, Enseleit F, Ferri C, Flammer AJ, et al. Cocoa, blood pressure, and vascular function. Front Nutr. (2017) 4:36. doi: 10.3389/fnut.2017.00036
79. Yeh TS, Chan KH, Hsu MC, Liu JF. Supplementation with soybean peptides, taurine, pueraria isoflavone, and ginseng saponin complex improves endurance exercise capacity in humans. J Med Food. (2011) 14:219–25. doi: 10.1089/jmf.2010.1096
80. Kressler J, Millard-Stafford M, Warren GL. Quercetin and endurance exercise capacity: a systematic review and meta-analysis. Med Sci Sports Exerc. (2011) 43:2396–404. doi: 10.1249/MSS.0b013e31822495a7
81. Davis JM, Carlstedt CJ, Chen S, Carmichael MD, Murphy EA. The dietary flavonoid quercetin increases Vo(2max) and endurance capacity. Int J Sport Nutr Exerc Metab. (2010) 20:56–62. doi: 10.1123/ijsnem.20.1.56
82. Ferri E, Marzetti E, Calvani R, Picca A, Cesari M, Arosio B. Role of age-related mitochondrial dysfunction in Sarcopenia. Int J Mol Sci. (2020) 21:5236. doi: 10.3390/ijms21155236
83. Davis JM, Murphy EA, Carmichael MD, Davis B. Quercetin increases brain and muscle mitochondrial biogenesis and exercise tolerance. Am J Physiol Regul Integr Comp Physiol. (2009) 296:R1071–7. doi: 10.1152/ajpregu.90925.2008
84. Moreno-Ulloa A, Cid A, Rubio-Gayosso I, Ceballos G, Villarreal F, Ramirez-Sanchez I. Effects of (-)-epicatechin and derivatives on nitric oxide mediated induction of mitochondrial proteins. Bioorg Med Chem Lett. (2013) 23:4441–6. doi: 10.1016/j.bmcl.2013.05.079
Keywords: middle-age, elderly, flavonoids, muscle mass, muscle strength, physical performance, Sarcopenia
Citation: Li Y, Liu Y, Tan R and Liu Y (2022) Effect of flavonoids on skeletal muscle mass, strength and physical performance in middle-aged and older adults with or without Sarcopenia: A meta-analysis of randomized controlled trials. Front. Nutr. 9:1013449. doi: 10.3389/fnut.2022.1013449
Received: 18 August 2022; Accepted: 20 September 2022;
Published: 10 October 2022.
Edited by:
Ming Yang, Sichuan University, ChinaReviewed by:
Jiling Liang, Wuhan Sports University, ChinaCopyright © 2022 Li, Liu, Tan and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yan Liu, cmFiYml0eWFuMTI3QDE2My5jb20=
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.