
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Nutr., 27 September 2022
Sec. Sport and Exercise Nutrition
Volume 9 - 2022 | https://doi.org/10.3389/fnut.2022.1013310
This article is part of the Research TopicFunctional Foods, Supplements, and Dietary Approaches in Sports and Clinical NutritionView all 12 articles
 Xiang Li1†
Xiang Li1† Yunqi Luan2†
Yunqi Luan2† Yuejin Li3
Yuejin Li3 Shili Ye4
Shili Ye4 Guihui Wang5
Guihui Wang5 Xinlun Cai5
Xinlun Cai5 Yucai Liang6
Yucai Liang6 Hamed Kord Varkaneh6
Hamed Kord Varkaneh6 Yunpeng Luan1,7*
Yunpeng Luan1,7*High-fructose corn syrup (HFCS) has been speculated to have stronger negative metabolic effects than sucrose. However, given the current equivocality in the field, the aim of the present study was to determine the impact of HFCS use compared to sucrose on anthropometric and metabolic parameters. We searched PubMed, Scopus, Cochrane Central and web of sciences, from database inception to May 2022. A random effects model and the generic inverse variance method were applied to assess the overall effect size. Heterogeneity analysis was performed using the Cochran Q test and the I2 index. Four articles, with 9 arms, containing 767 participants were included in this meta-analysis. Average HFCS and sucrose usage equated to 19% of daily caloric intake. Combined data from three studies indicated that HFCS intake does not significantly change the weight (weighted mean difference (WMD): −0.29 kg, 95% CI: −1.34, 0.77, I2 = 0%) when compared to the sucrose group. Concordant results were found for waist circumstance, body mass index, fat mass, total cholesterol (TC), high-density lipoprotein (HDL), low-density lipoprotein (LDL), triglyceride (TG), systolic blood pressure (SBP), and diastolic blood pressure (DBP). Moreover, overall results from three studies indicated a significant increase in CRP levels (WMD: 0.27 mg/l, 95% CI: 0.02, 0.52, I2 = 23%) in the HFCS group compared to sucrose. In conclusion, analysis of data from the literature suggests that HFCS consumption was associated with a higher level of CRP compared to sucrose, whilst no significant changes between the two sweeteners were evident in other anthropometric and metabolic parameters.
Per capita consumption of sugar in the world increased sharply in the twentieth century. Although reports suggest that this consumption has now leveled off (1), it is of concern that high levels of added sugars have negative effects on human health (2). Sweeteners can be divided into two groups: nutritional and non-nutritional; where nutritional sweeteners contain monosaccharides (fructose and galactose), disaccharides (sucrose, maltose, and maize-based sweeteners) and polyols (sugar alcohol) that produce energy. On the other hand, non-nutritional sweeteners, such as acesulfame K, aspartame, neotame, saccharin and sucralose, do not contain sweet calories (3). Saccharose (GI 61-68) is a sweetener and well-used ingredient in the food industry, however, can cause harmful health problems (4). Glucose, fructose, and sucrose are natural nutrient sweeteners, where sucrose consists of a glucose molecule that is linked to a fructose molecule. High fructose corn syrup is a mixture of glucose and free fructose. The wide distribution of hexokinase enzymes, which are essential for the production of glucose-6-phosphate and glycolytic enzymes, means that glucose is metabolized by all cells of the organism. Fructose, on the other hand, is not so readily phosphorylated by the hexokinases and is metabolized exclusively in the intestine, liver and kidney. Glucose metabolism is regulated by insulin after a meal, whilst after consuming a fructose-only diet, the bulk enters the intestine and the liver, with a markedly longer transit time than glucose. Up to 20% of fructose may be stored as hepatic glycogen, and a large part is converted to LDL/VLDL (5). Furthermore, energy efficiency from fructose metabolism is lower than glucose; where at lower intake, fructose stimulates the metabolic pathway of hepatic de novo lipid production more than glucose does. However, this pathway plays only a minor role in the total excretion of fructose (6–8). Fructose is a natural monosaccharide (glycemic index, GI: 19-23), used as a sweetener in the food industry, whilst high fructose corn syrup (HFCS) is a liquid substitute for sucrose with 42 or 55% (dry) fructose, and is obtained from the hydrolysis of corn starch to glucose using glucoamylase and α-amylase, followed by glucose isomerization to fructose, which results in the production of a mixture of glucose and fructose (9, 10). HFCS can provide flavor, color, texture, stability, and freshness in some food products, such as beverages, processed foods, baking products, ice cream and confectioneries (11). It has been demonstrated in a rat study that the addition of sugars, in general, and HFCS directly or indirectly contribute to obesity, as well as various types of metabolic disorders and diseases (12). Studies have shown that excess consumption of sugar can lead to weight gain, confers a greater risk of developing metabolic heart disease, and an increased risk of early mortality (2, 13–15). HFCS is a nutritional sweetener that is thought to be harmful for human health, partly attributed to preliminary research that shows consumption of large quantities of fructose (i.e. the main constituent of HFCS) can lead to deleterious metabolic consequences in the body (16–18). Sucrose is also comprised of glucose and fructose, which is absorbed in the digestive tract (19). Therefore, there is minimal difference between HFCS and sucrose, due to the ability of the human digestive system to absorb sucrose and fructose. Previous trials have shown that the use of HFCS in comparison with sucrose yields no significant difference in health-related indicators, such as glycemic index, calorie intake, lipid metabolism and inflammation (20–23). However, there is evidence to suggest that fructose consumption in comparison with sucrose has a significantly greater effect on indicators of health (24, 25). Thus, given the current equivocality in the field, the aim of the present study is to determine the impact of HFCS vs. sucrose on anthropometric and metabolic parameters.
This meta-analysis was performed and reported based on the ‘Preferred Reporting Items for Systematic Review and Meta-analysis’ (PRISMA) statement (26). The research questions were designed using the population, intervention, comparison, and outcomes (PICO) method.
We searched PubMed, Scopus, Cochrane Central and Clinicaltrials.gov and web of sciences, from database inception to May 2022. The search strategy combined Medical Subject Heading (MeSH) and non-MeSH terms: (“Clinical Trials as Topic”[Mesh] OR “Cross-Over Studies”[Mesh] OR “Double-Blind Method”[Mesh] OR “Single-Blind Method”[Mesh] OR “Random Allocation”[Mesh] OR RCT[Title/Abstract] OR “Intervention Studies”[Title/Abstract] OR “intervention”[Title/Abstract] OR “controlled trial”[Title/Abstract] OR “randomized”[Title/Abstract] OR “randomized”[Title/Abstract] OR “random”[Title/Abstract] OR “randomly”[Title/Abstract] OR “placebo”[Title/Abstract] OR “assignment”[Title/Abstract]) AND ((“High Fructose Corn Syrup 55%”[tiab] OR “HFCS-55”[tiab] OR “High Fructose Corn Syrup”[tiab] OR “High Fructose Corn Syrup”[Mesh]) AND ((((Sucrose[Title/Abstract] OR “Sucrose”[Mesh]) OR “Sugar”[tiab]) OR “Sugars”[Mesh]) OR “Sugars”[tiab])) (see Supplementary Table 1). Two of the authors (J.R and H.K) implemented independent searches to avoid missing any relevant articles.
The inclusion criteria were as follows: (1) RTs (2), HFCS use vs. sucrose (3), treatment for at least 48 weeks (4), reported sufficient information on BMI, WC, and body weight, lipid profile, CRP, blood pressure, and blood glucose both in High Fructose Corn Syrup group and Sucrose administration group (5), inclusion of patients ≥ 18 y old. Publications that did not provide outcome measures at study baseline and end of the intervention (or changes in outcome measures), Observational studies, case reports, case series, non-systematic reviews and trials published as abstracts were excluded.
Titles and abstracts were independently reviewed against eligibility criteria by two investigators (C.C and Y.Z). Any controversies or disagreements were resolved by the third author (H.K) via cooperative triangulation. Choice of the final eligible articles was according to the consensus between three investigators (C.C, Y.Z, and H.K). Moreover, with reference to the measures of outcome, the following data were extracted: (1) study's first author, (2) year of publication, (3) type of study population, (4) number of people in each groups, participants' mean age, participants' gender, study design, trial duration and results (means and standard deviations of outcomes at baseline, end of study and/or changes between baseline and end-intervention). To assess the quality of studies, we used the Cochrane Collaboration tool to assess risk of bias (27).
The changes in the mean values of body weight, body mass index, waist circumstance, fat mass, TC, HDL, LDL, TG, CRP, SBP, DBP was calculated for all included studies. The combined changes in outcomes were assessed as the difference (High Fructose Corn Syrup minus Sucrose) between its differences (post-trail values minus beginning trail) in the High Fructose Corn Syrup group and the Sucrose group. Standard deviations (SDs) of the mean difference were estimated using the following formula: SD = square root [(SDpre-intervention)2 C (SDpost- intervention)2 – (2R * SDpre-intervention*SDpost- intervention)], supposing a correlation coefficient (R) = 0.5 (28). A random-effects model and the generic inverse variance method were applied to assess overall effect size and the heterogeneity among studies. Heterogeneity analysis was performed using the Cochran Q test and the I2 index. Moreover, probable publication bias was determined visually by funnel plot and confirmed, statistically, through the Egger's and begg's tests. All statistical analyses were performed using Stata software, version 14 (Stata Corp. College Station, Texas, USA).
Figure 1 details the flow chart of our systematic search. In our comprehensive search of PubMed, Scopus, Web of Science, and Cochrane Library, and after removing duplicates, 141 articles were included in screening, and 102 articles were excluded because they did not meet the aforementioned inclusion criteria. In full-text screening, 35 articles were removed, and four articles, with 9 arms, containing 767 participants were included in this meta-analysis (20, 29–31).
Characteristics of the included studies are provided in Table 1. Mean age of participants was 41.51 years, ranging from 37 to 52 years and mean length of the interventions was 8.5 weeks, ranging from 2 to 12 weeks. All studies were conducted on both genders in the USA and articles were published between 2012 and 2016. Risk of bias of included studies was evaluated by the Cochrane's Handbook checklist, and most papers were confirmed as having good quality (Figure 2) (20, 29–31).
Three studies, with six arms, including 326 participants in intervention groups and 319 participants in control groups reported weight as an outcome measure (20, 29, 30), and combined results did not show any significant change in HFCS (WMD: −0.29 kg, 95% CI: −1.34, 0.77, I2 = 0%) compared to the sucrose group (Figure 3). There were comparable results for waist circumstance (WMD: −0.39 cm, 95% CI: −2.04, 1.25, I2 = 0%) (29, 30), Body mass index (WMD: −0.18 kg/m2, 95% CI: −0.46, 0.10, I2 = 0%) (20, 29–31), and fat mass (WMD: −0.24 %, 95% CI: −2.04, 1.55, I2 = 0%) (30).
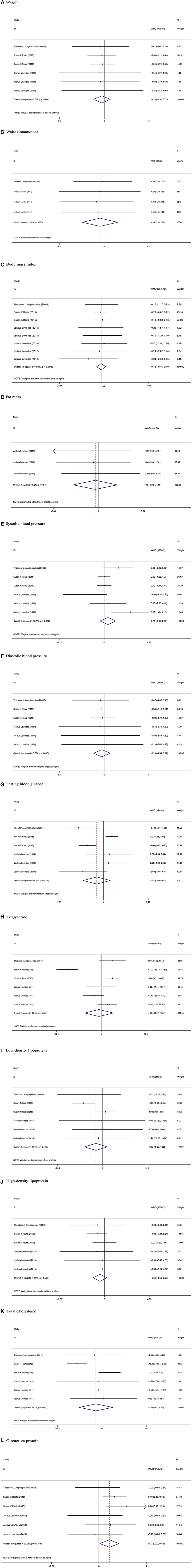
Figure 3. Meta-analysis of effect of High-fructose corn syrup on. (A) Weight. (B) Waist circumstance. (C) Body mass index. (D) Fat mass. (E) Systolic blood pressure. (F) Diastolic blood pressure. (G) Fasting blood glucose. (H) Triglyceride. (I) Low-density lipoprotein. (J) High-density lipoprotein. (K) Total Cholesterol. (L) C-reactive protein.
Among eligible studies, three trails, with six arms, containing a total of 645 participants (326 participants in HFCS group and 319 participants in sucrose group) reported SBP, DBP, fasting blood glucose (FBG), TG, LDL, HDL, and TC as an outcome measure (20, 29, 30). Pooled results from the random-effects model did not show any significant change between the two groups in SBP (WMD: 0.76 mmHg, 95% CI: −0.86, 2.38, I2 = 62%), DBP (WMD: −0.29 mmHg, 95% CI: −1.34, 0.77, I2 = 0%), FBG (WMD: −0.87 mg/dl, 95% CI: −2.56, 0.82, I2 = 84%), TG (WMD: −4.15 mg/dl, 95% CI: −30.37, 22.07, I2 = 91%), LDL (WMD: −2.02 mg/dl, 95% CI: −5.69, 1.65, I2 = 42%), HDL (WMD: −0.61 mg/dl, 95% CI: −1.55, 0.32, I2 = 0%), and TC (WMD: −2.45 mg/dl, 95% CI: −9.12, 4.22, I2 = 74%).
Overall results from three studies indicated a significant increase in CRP levels (WMD: 0.27 mg/l, 95% CI: 0.02, 0.52, I2 = 23%) in the HFCS group compared to the sucrose group (20, 29, 30).
Funnel plot assessment did not show any asymmetry in included studies (Supplemental Figure 1). Furthermore, The Egger's and Begg's tests did not show any significant publication bias for Weight (p = 0.09 and p = 0.57), WC (p = 0.25 and p = 0.99), BMI (p = 0.43 and p = 0.32), Fat mass (p = 0.62 and p = 0.60), SBP (p = 0.51 and p = 0.57), DBP (p = 0.60 and p = 0.57), FBS (p = 0.39 and p = 0.85), TG (p = 0.50 and p = 0.57), LDL (p = 0.84 and p = 0.57), HDL (p = 0.96 and p = 0.57), TC (p = 0.69 and p = 0.85), and CRP (p = 0.23 and p = 0.34), respectively.
Numerous empirical evidence has indicated that sugars, particularly HFCS and sucrose, can affect various anthropometric and metabolic parameters (6, 12). However, it remains debatable whether the effects of HFCS and sucrose are of equal magnitude. Some studies have demonstrated that consumption of HFCS and sucrose elicited comparable effects, while some other reports noted a marked difference between the two sugars (20, 22, 25). In this work, we performed a meta-analysis to determine whether the effect of HFCS and sucrose on anthropometric and metabolic parameters were concordant. We found that HFCS was significantly associated with an increased CRP level, compared to sucrose. However, we observed no difference between HFCS and sucrose in terms of their effects on weight, WC, BMI, fat mass, SBP, DBP, FBS, TG, LDL, HDL, and TC.
CRP is a biomarker for inflammation; and several previous investigations have shown that fructose-containing sweeteners, such as HFCS and sucrose, can induce the inflammatory process (32, 33). This is conceivably attributable to the unique metabolic process of fructose, which can cause oxidative stress to cells by elevating the intracellular levels of uric acid and reactive oxygen species (33, 34). To overcome the oxidative stress, cells release molecules such as monocyte chemotactic protein 1 (MCP-1), tumor necrosis factor (TNF) and interleukins, which are pro-inflammatory in nature and thus augment the inflammation process (33). HFCS and sucrose both contain the fructose moiety; however, the content of fructose in HFCS and sucrose is different, which conceivably explains why the two sugars induced inflammation differently. Despite this, in the present study, no significant difference was observed for any other anthropometric or metabolic parameter. The lack of significant difference could be attributed to several reasons. First, in some of the included studies, the duration of the experiment was short. For example, in the study by Raatz et al., the participants consumed the sugars for only 2 weeks (20); this duration is likely insufficient for significant weight changes to be manifest. In addition, compliance with the prescribed sugars was generally obtained through self-reported information from the participants, which could cause validity and bias issues (35, 36). Moreover, the studies generally delivered the sugars through milk or other foods, which contained other nutrients, such as vitamin D and calcium. These nutrients can affect several metabolic parameters, for example, vitamin D has been shown to alter cholesterol levels, especially LDL (37–39). On the other hand, calcium supplementation is known to decrease blood pressure (40). The presence of these nutrients in the foods used to deliver the sugars could act as confounders for our analyses; therefore, the findings should be interpreted with caution. Notwithstanding, the amount/concentration of the sugars consumed were different across the included studies; hence, it is conceivable that the effects elicited by the sugars was masked by studies which examined the lower concentration of the sugars.
There are a number of strengths and limitations of the present study. Firstly, through the amalgamation of studies, we yielded an increased statistical power of analysis compared to the individual studies (41). In addition, most studies included in the present meta-analysis had high methodological quality and employed a randomized trial design. A randomized trial minimizes various types of biases and confounders and produces strong empirical evidence of the effects of an intervention (42). It is also noteworthy that publication bias was not evident in our meta-analysis, which suggests that the results obtained are likely not exaggerated (43). Apart from that, the sugars consumed by the participants were incorporated into their normal diets, which allowed a more accurate evaluation of the real-world effects of the added sugar compared to a totally controlled trial (20). However, one of the limitations of this meta-analysis was that subgroup analyses were not performed due to the lack of incumbent data in the included studies, which was out of the operational control of the study. For instance, the effects of HFCS and sucrose on male and female participants were not evaluated separately in the included studies. Importantly, many human and animal studies have demonstrated that males and females have different responses to fructose (44–48); for example, triglyceride levels in young women are less sensitive to modifications by fructose compared to men (44, 45, 48), and therefore represents a pragmatic avenue for further research. Besides gender, subgroup analysis by people of different age groups was not performed, although animal studies have indicated that fructose can induce metabolic changes differently in young and adult rats (49). Another limitation of this study was that, as mentioned above, compliance with the assigned sugar intake in the included studies was generally dependent on the self-reported information from the participants. This can cause misleading results if the participants did not adhere to the prescribed diet (35, 36). Finally, in this meta-analysis, we were unable to correct for some potential confounding factors, such as the co-incorporation of added vitamin D and calcium in the food used to deliver the sugars.
In conclusion, analysis of data from the literature suggests that HFCS is associated with a higher level of CRP compared to sucrose, and that little differences exist in other anthropometric and metabolic parameters. Nonetheless, considering the limitations of the study, careful interpretation of the results is warranted. Future studies are needed to address the limitations above, particularly related to gender-mediated differences. However, the present work successfully provided a reliable estimate of the effect and difference of HFCS and sucrose in a number of anthropometric and metabolic parameters.
The original contributions presented in the study are included in the article/Supplementary materials, further inquiries can be directed to the corresponding author.
XL, YunqL, YuejL, YunpL, and HK designed the study. The literature search, screening data, and data extraction were done by SY, GW, XC, and YucaL. Quality assessment was carried out by YunpL and HK. XL and YunqL analyzed and interpreted data. XL, YunqL, YuejL, YunpL, and HK wrote and edited the manuscript. All authors read and approved the final manuscript.
The present study was funded by National Natural Science Foundation of China (NSFC) (32160223), Young academic and technical leaders of Yunnan Province (202105AC160047), Joint Specific Project of basic Research of TCM Application of Yunnan Province (30370103808), Yunnan Provincial Science and Technology Department-Applied Basic Research Joint Special Funds of Chinese Medicine (202101AZ070001-274), Scientific Research Foundation of the Education Department of Yunnan Province, China (2021J0404), Yunnan Medical Reserve Talents Project (H-2018063), the Opening project of Clinical Medical Center of The First People's Hospital of Yunnan Province (2021LCZXXF-XG02), and doctoral Research Fund of The First People's Hospital of Yunnan Province (20206023).
Authors YucaL and HK were employed by Lairui Biotechnology (Yunnan) Co., Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2022.1013310/full#supplementary-material
1. Newens K, Walton J. A review of sugar consumption from nationally representative dietary surveys across the world. J Hum Nutr Diet. (2016) 29:225–40. doi: 10.1111/jhn.12338
2. Ruxton C, Gardner E, McNulty H. Is sugar consumption detrimental to health? A review of the evidence 1995-−2006. Crit Rev Food Sci Nutr. (2009) 50:1–19. doi: 10.1080/10408390802248569
3. Liauchonak I, Qorri B, Dawoud F, Riat Y, Szewczuk MR. Non-Nutritive Sweeteners and Their Implications on the Development of Metabolic Syndrome. Nutrients. (2019) 11:644. doi: 10.3390/nu11030644
4. Stricker S, Rudloff S, Geier A, Steveling A, Roeb E, Zimmer KP. Fructose consumption-free sugars and their health effects. Dtsch Arztebl Int. (2021) 118:71–8. doi: 10.3238/arztebl.m2021.0010
5. Sun SZ, Empie MW. Fructose metabolism in humans - what isotopic tracer studies tell us. Nutr Metab (Lond). (2012) 9:89. doi: 10.1186/1743-7075-9-89
6. Stanhope KL, Havel PJ. Endocrine and metabolic effects of consuming beverages sweetened with fructose, glucose, sucrose, or high-fructose corn syrup. Am J Clin Nutr. (2008) 88:1733S−7S. doi: 10.3945/ajcn.2008.25825D
7. Mayes PA. Intermediary metabolism of fructose. Am J Clin Nutr. (1993) 58:754S−65S. doi: 10.1093/ajcn/58.5.754S
8. Bar-On H, Stein Y. Effect of glucose and fructose administration on lipid metabolism in the rat. J Nutr. (1968) 94:95–105. doi: 10.1093/jn/94.1.95
9. Scaman C. Corn Sweeteners: Enzyme Use, in Encyclopedia of Biotechnology in Agriculture and Food. Boka Raton: CRC Press. (2010). p. 178–82.
10. Nabors L, Gelardi R. Alternative sweeteners: an overview. Altern Sweeteners. (2001) 2:1–10. doi: 10.1201/b11242-2
11. Moeller S, Fryhofer S, Osbahr AJ 3rd, Robinowitz CB. The effects of high fructose syrup. Am J Clin Nutr. (2009) 28:619–26. doi: 10.1080/07315724.2009.10719794
12. Bocarsly ME, Powell ES, Avena NM, Hoebel BG. High-fructose corn syrup causes characteristics of obesity in rats: increased body weight, body fat and triglyceride levels. Pharmacol Biochem Behav. (2010) 97:101–6. doi: 10.1016/j.pbb.2010.02.012
13. Bray GA. Potential health risks from beverages containing fructose found in sugar or high-fructose corn syrup. Diabetes Care. (2013) 36:11–2. doi: 10.2337/dc12-1631
14. Bray GA, Nielsen SJ, Popkin BM. Consumption of high-fructose corn syrup in beverages may play a role in the epidemic of obesity. Am J Clin Nutr. (2004) 79:537–43. doi: 10.1093/ajcn/79.4.537
15. Elliott SS, Keim NL, Stern JS, Teff K, Havel PJ. Fructose, weight gain, and the insulin resistance syndrome. Am J Clin Nutr. (2002) 76:911–22. doi: 10.1093/ajcn/76.5.911
16. Organization WH. Guideline: Sugars Intake for Adults and Children. World Health Organization. (2015).
17. Havel PJ. Dietary fructose: implications for dysregulation of energy homeostasis and lipid/carbohydrate metabolism. Nutr Rev. (2005) 63:133–57. doi: 10.1111/j.1753-4887.2005.tb00132.x
18. Trumbo P, Schlicker S, Yates AA, Poos M. Dietary reference intakes for energy, carbohdrate, fiber, fat, fatty acids, cholesterol, protein and amino acids. J Acad Nutr Diet. (2002) 102:1621. doi: 10.1016/S0002-8223(02)90346-9
19. Dahlqvist A, Thomson D. The digestion and absorption of sucrose by the intact rat. J Physiol. (1963) 167:193–209. doi: 10.1113/jphysiol.1963.sp007141
20. Raatz SK, Johnson LK, Picklo MJ. Consumption of honey, sucrose, and high-fructose corn syrup produces similar metabolic effects in glucose-tolerant and-intolerant individuals. J Nutr. (2015) 145:2265–72. doi: 10.3945/jn.115.218016
21. Kuzma JN, Cromer G, Hagman DK, Breymeyer KL, Roth CL, Foster-Schubert KE, et al. No difference in ad libitum energy intake in healthy men and women consuming beverages sweetened with fructose, glucose, or high-fructose corn syrup: a randomized trial. Am J Clin Nutr. (2015) 102:1373–80. doi: 10.3945/ajcn.115.116368
22. Yu Z, Lowndes J, Rippe J. High-fructose corn syrup and sucrose have equivalent effects on energy-regulating hormones at normal human consumption levels. Nutr Res Rev. (2013) 33:1043–52. doi: 10.1016/j.nutres.2013.07.020
23. Melanson KJ, Zukley L, Lowndes J, Nguyen V, Angelopoulos TJ, Rippe JM. Effects of high-fructose corn syrup and sucrose consumption on circulating glucose, insulin, leptin, and ghrelin and on appetite in normal-weight women. Nutrition. (2007) 23:103–12. doi: 10.1016/j.nut.2006.11.001
24. Teff KL, Elliott SS, Tschop M, Kieffer TJ, Rader DM, Heiman, et al. Dietary fructose reduces circulating insulin and leptin, attenuates postprandial suppression of ghrelin, and increases triglycerides in women. J Clin Endocrinol Metab. (2004) 89:2963–72. doi: 10.1210/jc.2003-031855
25. Anderson GH, Catherine NL, Woodend DM, Wolever TM. Inverse association between the effect of carbohydrates on blood glucose and subsequent short-term food intake in young men. Am J Clin Nutr. (2002) 76:1023–30. doi: 10.1093/ajcn/76.5.1023
26. Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. (2015) 4:1. doi: 10.1186/2046-4053-4-1
27. Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. (2011) 343:d5928. doi: 10.1136/bmj.d5928
28. Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. (2014) 14:135. doi: 10.1186/1471-2288-14-135
29. Angelopoulos TJ, Lowndes J, Sinnett S, Rippe JM. Fructose containing sugars at normal levels of consumption do not effect adversely components of the metabolic syndrome and risk factors for cardiovascular disease. Nutrients. (2016) 8:179. doi: 10.3390/nu8040179
30. Lowndes J, Sinnett S, Yu Z, Rippe J. The effects of fructose-containing sugars on weight, body composition and cardiometabolic risk factors when consumed at up to the 90th percentile population consumption level for fructose. Nutrients. (2014) 6:3153–68. doi: 10.3390/nu6083153
31. Lowndes J, Kawiecki D, Pardo S, Nguyen V, Melanson KJ, Yu Z, et al. The effects of four hypocaloric diets containing different levels of sucrose or high fructose corn syrup on weight loss and related parameters. Nutr J. (2012) 11:55. doi: 10.1186/1475-2891-11-55
32. Jameel F, Phang M, Wood LG, Garg ML. Acute effects of feeding fructose, glucose and sucrose on blood lipid levels and systemic inflammation. Lipids Health Dis. (2014) 13:195. doi: 10.1186/1476-511X-13-195
33. Cirillo P, Gersch MS, Mu W, Scherer PM, Kim KM, Gesualdo L, et al. Ketohexokinase-dependent metabolism of fructose induces proinflammatory mediators in proximal tubular cells. J Am Soc Nephrol. (2009) 20:545–53. doi: 10.1681/ASN.2008060576
34. Delbosc S, Paizanis E, Magous R, Araiz C, Dimo T, Cristol JP, et al. Involvement of oxidative stress and NADPH oxidase activation in the development of cardiovascular complications in a model of insulin resistance, the fructose-fed rat. Atherosclerosis. (2005) 179:43–9. doi: 10.1016/j.atherosclerosis.2004.10.018
35. Graziose MM. On the accuracy of self-report instruments for measuring food consumption in the school setting. Adv Nutr. (2017) 8:635–6. doi: 10.3945/an.117.015354
36. Rosenman R, Tennekoon V, Hill LG. Measuring bias in self-reported data. Int J Behav Health Res. (2011) 2:320. doi: 10.1504/IJBHR.2011.043414
37. Jastrzebski Z, Kortas J, Kaczor K, Antosiewicz J. Vitamin D supplementation causes a decrease in blood cholesterol in professional rowers. J Nutr Sci Vitaminol. (2016) 62:88–92. doi: 10.3177/jnsv.62.88
38. Schnatz PF, Jiang X, Vila-Wright S, Aragaki AK, Nudy M. O'sullivan DM, et al. Calcium/Vitamin D (CaD) Supplementation, Serum 25 (OH) Vitamin D Concentrations, and Cholesterol Profiles in the Women's Health Initiative CaD Randomized Trial. Menopause. (2014) 21:823. doi: 10.1097/GME.0000000000000188
39. Ponda MP, Dowd K, Finkielstein D, Holt PR, Breslow JL. The short-term effects of vitamin D repletion on cholesterol: a randomized, placebo-controlled trial. Arterioscler Thromb Vasc Biol. (2012) 32:2510–5. doi: 10.1161/ATVBAHA.112.254110
40. van Mierlo LA, Arends LR, Streppel MT, Zeegers M, Kok FJ, Grobbee DE, et al. Blood pressure response to calcium supplementation: a meta-analysis of randomized controlled trials. J Hum Hypertens. (2006) 20:571. doi: 10.1038/sj.jhh.1002038
41. Lee YH. An overview of meta-analysis for clinicians. Korean J Intern Med. (2018) 33:277. doi: 10.3904/kjim.2016.195
42. Bondemark L, Ruf S. Randomized controlled trial: the gold standard or an unobtainable fallacy? Eur J Orthod. (2015) 37:457–61. doi: 10.1093/ejo/cjv046
43. Murad MH, Chu H, Lin L, Wang Z. The effect of publication bias magnitude and direction on the certainty in evidence. BMJ Evid Based Med. (2018) 23:84–6. doi: 10.1136/bmjebm-2018-110891
44. Stanhope KL, Schwarz JM, Keim NL, Griffen SC, Bremer AA, Graham JL, et al. Consuming fructose-sweetened, not glucose-sweetened, beverages increases visceral adiposity and lipids and decreases insulin sensitivity in overweight/obese humans. J Clin Invest. (2009) 119:1322–34. doi: 10.1172/JCI37385
45. Swarbrick MM, Stanhope KL, Elliott SS, Graham JL, Krauss RM, Christiansen MP, et al. Consumption of fructose-sweetened beverages for 10 weeks increases postprandial triacylglycerol and apolipoprotein-B concentrations in overweight and obese women. Br J Nutr. (2008) 100:947–52. doi: 10.1017/S0007114508968252
46. Song D, Arikawa E, Galipeau D, Battell M, McNeill JH. Androgens are necessary for the development of fructose-induced hypertension. Hypertension. (2004) 43:667–72. doi: 10.1161/01.HYP.0000118018.77344.4e
47. Galipeau D, Verma S, McNeill JH. Female rats are protected against fructose-induced changes in metabolism and blood pressure. Am J Physiol Heart Circ Physiol. (2002) 283:H2478–84. doi: 10.1152/ajpheart.00243.2002
48. Hallfrisch J, Reiser S, Prather ES. Blood lipid distribution of hyperinsulinemic men consuming three levels of fructose. Am J Clin Nutr. (1983) 37:740–8. doi: 10.1093/ajcn/37.5.740
Keywords: high-fructose corn syrup, sucrose, fructose, weight, meta-analysis
Citation: Li X, Luan Y, Li Y, Ye S, Wang G, Cai X, Liang Y, Kord Varkaneh H and Luan Y (2022) The effect of high-fructose corn syrup vs. sucrose on anthropometric and metabolic parameters: A systematic review and meta-analysis. Front. Nutr. 9:1013310. doi: 10.3389/fnut.2022.1013310
Received: 06 August 2022; Accepted: 06 September 2022;
Published: 27 September 2022.
Edited by:
Mihnea-Alexandru Găman, Carol Davila University of Medicine and Pharmacy, RomaniaReviewed by:
Denisa Margina, Carol Davila University of Medicine and Pharmacy, RomaniaCopyright © 2022 Li, Luan, Li, Ye, Wang, Cai, Liang, Kord Varkaneh and Luan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yunpeng Luan, bHVhbnl1bnBlbmdfc2NpQG91dGxvb2suY29t
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.