- 1Department of Urology, Nanchong Central Hospital, The Second Clinical College, North Sichuan Medical University, Nanchong, Sichuan, China
- 2Department of Breast Surgery, Guizhou Provincial People’s Hospital, Guiyang, Guizhou, China
- 3Department of Nephrology, Blood Purification Center, Nanchong Central Hospital, The Second Clinical College, North Sichuan Medical University, Nanchong, Sichuan, China
- 4Department of Urology, Institute of Urology, West China Hospital, Sichuan University, Chengdu, Sichuan, China
- 5Department of Clinical Laboratory, The People’s Hospital of Gao County, Yibin, Sichuan, China
Background: Numerous clinical studies have reported an association between the pretreatment albumin to globulin ratio (AGR) and survival outcomes of urological cancers. However, these conclusions remain controversial. Therefore, we performed a meta-analysis to explore the prognostic value of the AGR in urinary system tumors.
Methods: We retrieved eligible studies published up to June 2022 through a comprehensive search of multiple databases. Pooled hazard ratios (HRs) with 95% confidence intervals (CI) for overall survival (OS), cancer-specific survival (CSS), recurrence-free survival (RFS), progression-free survival (PFS), and biochemical recurrence-free survival (BRFS) were used to evaluated the predictive effect of the AGR before treatment in urinary system tumors. Heterogeneity test, random-effects models, fixed-effects models and sensitivity tests were used for analyses.
Results: A total of 21 studies with 18,269 patients were enrolled in our meta-analysis. We found that patients with urinary system cancer with low AGR prior to treatment had poor OS [HR = 1.93, 95% CI (1.56–2.39), p < 0.001], CSS [HR = 2.22, 95% CI (1.67–2.96), p < 0.001], RFS [HR = 1.69, 95% CI (1.29–2.22), p < 0.001], and PFS [HR = 1.29, 95% CI (0.54–3.07), p < 0.001]. For prostate cancer (PCa), a low pretreatment AGR was associated with poor BRFS [HR = 1.46, 95% CI (1.28–1.67), p < 0.001]. Also, a subgroup analysis, stratified by ethnicity, cancer type, cutoff value, sample size and publication year, was conducted. The results showed that worse OS and CSS were significantly associated with these factors.
Conclusion: Our meta-analysis revealed that the AGR before treatment could be used as a non-invasive predictive biomarker to evaluate the prognosis of urological cancer patients in clinical practice.
Key messages
- Many studies have reported the association between the pretreatment albumin to globulin ratio (AGR) and prognosis of urological cancers, and these conclusions remain controversial.
- Meta-analysis was conducted for evaluating the prognostic value of pretreatment AGR for patients with urological cancer.
- AGR prior to treatment can be used to predict the prognosis of patients with urological cancer.
Introduction
According to cancer statistics, in 2022 approximately 1,918,030 cancer cases will be diagnosed worldwide; cancer is still one of the leading causes of death (1). Urinary system cancers (prostate cancer, renal cancer, bladder cancer and upper tract urothelial cancer), belonging to the ten leading cancer types of diagnosed malignances; the incidence and death rates of these four urological carcinomas are increasing each year, especially in both developing and developed countries (2, 3). Over the past decades, despite considerable advances in early detection and surgical techniques, and medical therapies (e.g., chemotherapy, radiotherapy, targeted therapy, and immunotherapy) used in urinary system cancers, the 5-year survival outcome of patients diagnosed with urinary tumors remains poor; and the risk of recurrence and progression of these tumors is high (4). Numerous clinical studies have evaluated the prognosis of patients with urological cancers and have assisted clinicians in making follow-up treatment protocols using TNM stage, grade, tumor size, symptoms, and paraneoplastic syndromes (5–7). However, using the above clinical prognostic indicators alone can not accurately assess the extent of disease extent or define prognosis (7, 8). Hence, reliable, non-invasive and cost-effective pretreatment prognostic biomarkers need to be identified to evaluate the prognosis of urinary cancers and guide clinical individualized clinical treatment.
In recent years, accumulating evidence has shown that several preoperative blood-based biomarkers, such as the De Ritis ratio, neutrophil-lymphocyte ratio (NLR), serum albumin, platelet–lymphocyte ratio (PLR), and lymphocyte–monocyte ratio (LMR) have been verified as an independent prognostic indicators for patients with urinary system tumors (9–12). Recent epidemiological studies have indicated that cancer-related inflammation and nutritional status are closely associated with tumorigenesis, tumor progression, and oncological outcomes (13). The major components of serum proteins, serum albumin and globulin are valuable predictors in cancer and play a crucial role in inflammation and immunity (14, 15). The albumin-to-globulin ratio (AGR) is calculated as the albumin level divided by the globulins level. Previous studies have reported that pretreatment AGR is inversely associated with poor survival in patients with genitourinary malignant tumors (16–20). However, according to the published articles, the use of pretreatment AGR as a prognostic biomarker remains controversial. Pradere et al. (16) failed to demonstrate that upper tract urothelial carcinoma (UTUC) patients with low pretreatment AGR were associated with overall survival (OS) and recurrence-free survival (RFS). Therefore, in this context, our meta-analysis aimed to use the related published studies to explore the prognostic value of pretreatment AGR in urological cancers, to make individualized clinical decisions, and to improve patients survival.
Materials and methods
Literature search and eligibility criteria
Eligible citations were retrieved from Web of Science, PubMed, Google Scholar, and Cochrane Library up to June of 2022. Additionally, we also conducted a manual search of the references of the relevant studies. All searches were limited to human studies and no language restrictions were applied.
On the basis of our research objectives, the following search terms were used: (“albumin to globulin ratio” OR “albumin/globulin ratio” OR “AGR”) and (“urinary malignancy” OR “urinary neoplasm” OR “urinary cancer” OR “urinary tumor” OR “renal malignancy” OR “renal neoplasm” OR “renal cancer” OR “renal tumor” OR “bladder malignancy” OR “bladder neoplasm” OR “bladder cancer” OR “bladder tumor” OR “upper tract urothelial malignancy” OR “upper tract urothelial neoplasm” OR “upper tract urothelial cancer” OR “upper tract urothelial tumor” OR “prostate malignancy” OR “prostate neoplasm” OR “prostate cancer” OR “prostate tumor” OR “testicular malignancy” OR “testicular neoplasm” OR “testicular cancer” OR “testicular tumor” OR “transitional cell malignancy”). Since we included published articles, there was no need for review by an ethics committee. Our meta-analysis was conducted according to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guideline (21).
Studies had to meet the following inclusion criteria: (1) patients were confirmed as urinary cancer by histopathology; (2) studies reported the correlation between pretreatment AGR and prognosis of patients with urinary cancer; (3) all the studies provided hazard ratios (HR) and related 95% confidence intervals (CIs) of survival outcomes, including overall survival (OS), cancer-specific survival (CSS), recurrence-free survival (RFS), progression-free survival (PFS), or biochemical recurrence-free survival (BRFS); and (4) studies with randomized controlled trials (RCTs), case-control studies, or cohort studies. The exclusion criteria were as follows: (1) duplicated studies; (2) conference abstracts, reviews, letters and case reports; (3) basic experiments and animal researches; and (4) studies with unavailable data.
Quality evaluation
In this study, the Newcastle-Ottawa Scale (NOS), which includes three domains (selection of the cohort, comparability of the groups, and quality of the outcomes), was used to assess the quality of the included studies (22). The NOS scale has nine stars, and a score of six or more was considered a high-quality study in our meta-analysis.
Data extraction
The primary basic information for each included study was as follows: first author’s name, country, study design, sample size, intervention, age, cancer type, cut-off value for AGR, analysis method, and follow-up time. In addition, the pooled HRs and corresponding 95% CIs of the survival outcomes (OS, CSS, RFS, PFS, and BRFS) were extracted. As a common index to evaluate the prognosis of cancer patients, OS is only concerned about whether the patient dies, not the specific cause of death and the follow-up time is long. The other tumor-specific prognostic indexes, such as CSS, RFS, PFS, or/and BRFS, are supplement to OS for better manage tumor patients. Therefore, in order to better evaluate the clinical efficacy of AGR in the prognosis of urinary cancers, we made a comprehensive analysis of these indexes. If univariate and multivariate analyses were conducted in a study, we extracted the multivariate analysis data for follow-up analysis.
The above steps independently performed by two authors, and a third author resolved any discrepancies.
Statistical analysis
All the statistical data were processed by Stata 16 (StataCorp LP, College station, TX, United States of America LP, University City, TX, USA). For studies that provided only the Kaplan-Meier curves, we used Engauge Digitizer 4.1 software to extract the relevant survival data. The HRs and corresponding 95% CIs of the included articles were extracted to assess the prognostic significance of the AGR in urological cancers. Cochrane’s Q test and Higgin’s I2 test were used to measure the heterogeneity among the included studies. According to the results of the heterogeneity test, the random-effects model was utilized with high heterogeneity (I2 ≥ 50% or p < 0.1). Otherwise, fixed-effects models were used for the analyses. Sensitivity analysis, involving the removal of each individual study, was also used to assess the reliability and stability of our survival outcomes. Additionally, Begg’s test was performed to identify potential publication bias across studies if ten or more articles were included in meta-analysis. A value of P less than 0.05 was considered as a statistical significance.
Results
Search results and study characteristics
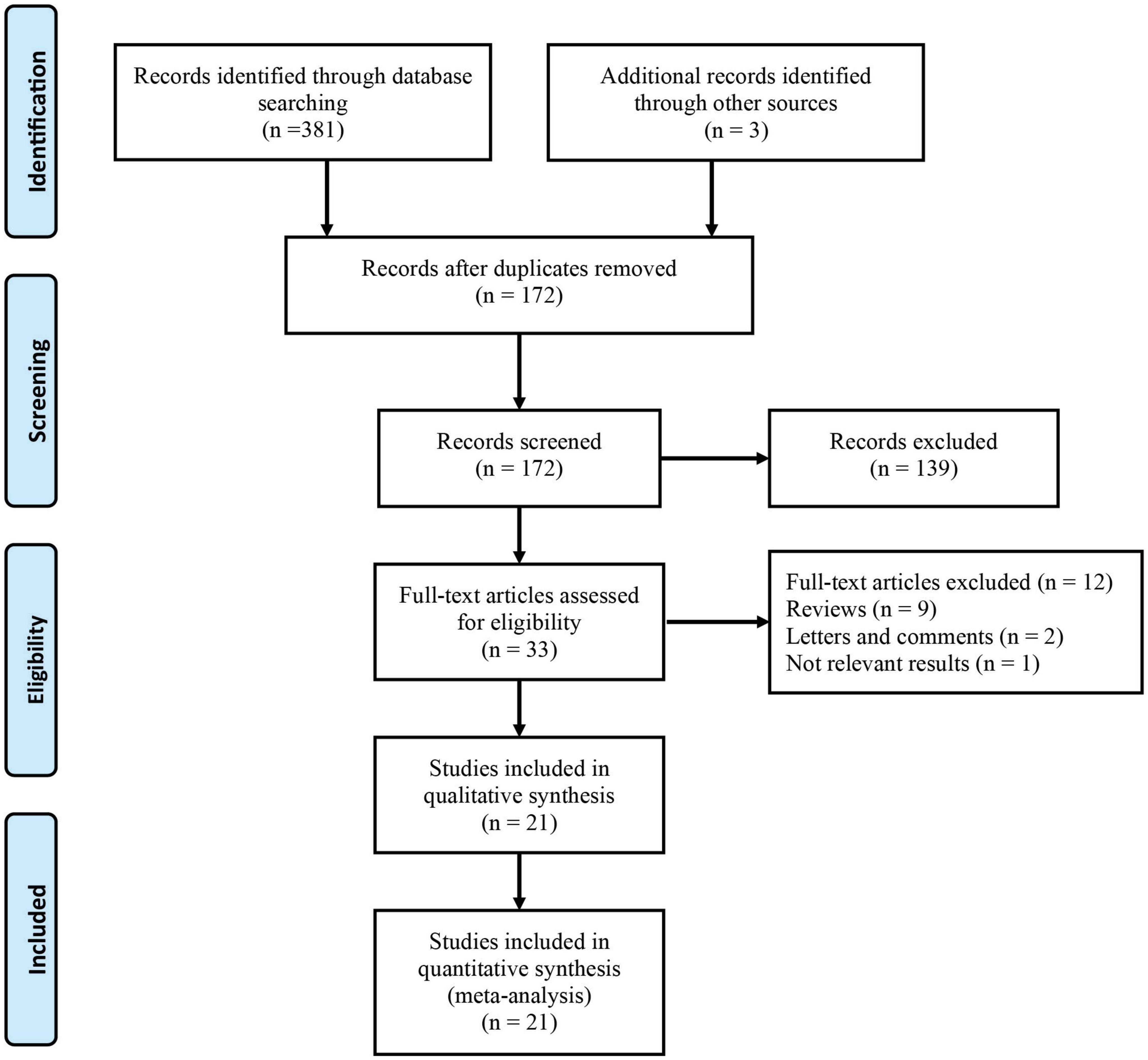
Figure 1 shows the detailed screening process used in this meta-analysis. Based on the search of electronic databases, 384 published articles were initially found, and 172 studies remained after removing 212 duplicates. After screening the titles and abstracts, 33 studies remained for further evaluation, and their full texts were read for eligibility. Twelve studies were excluded from the full-text analysis for the following reasons: nine reviews, two letters and comments, and one did not provide available data. Finally, there were 18,269 patients who were included in the 21 studies (16–19, 23–39)in this meta-analysis.
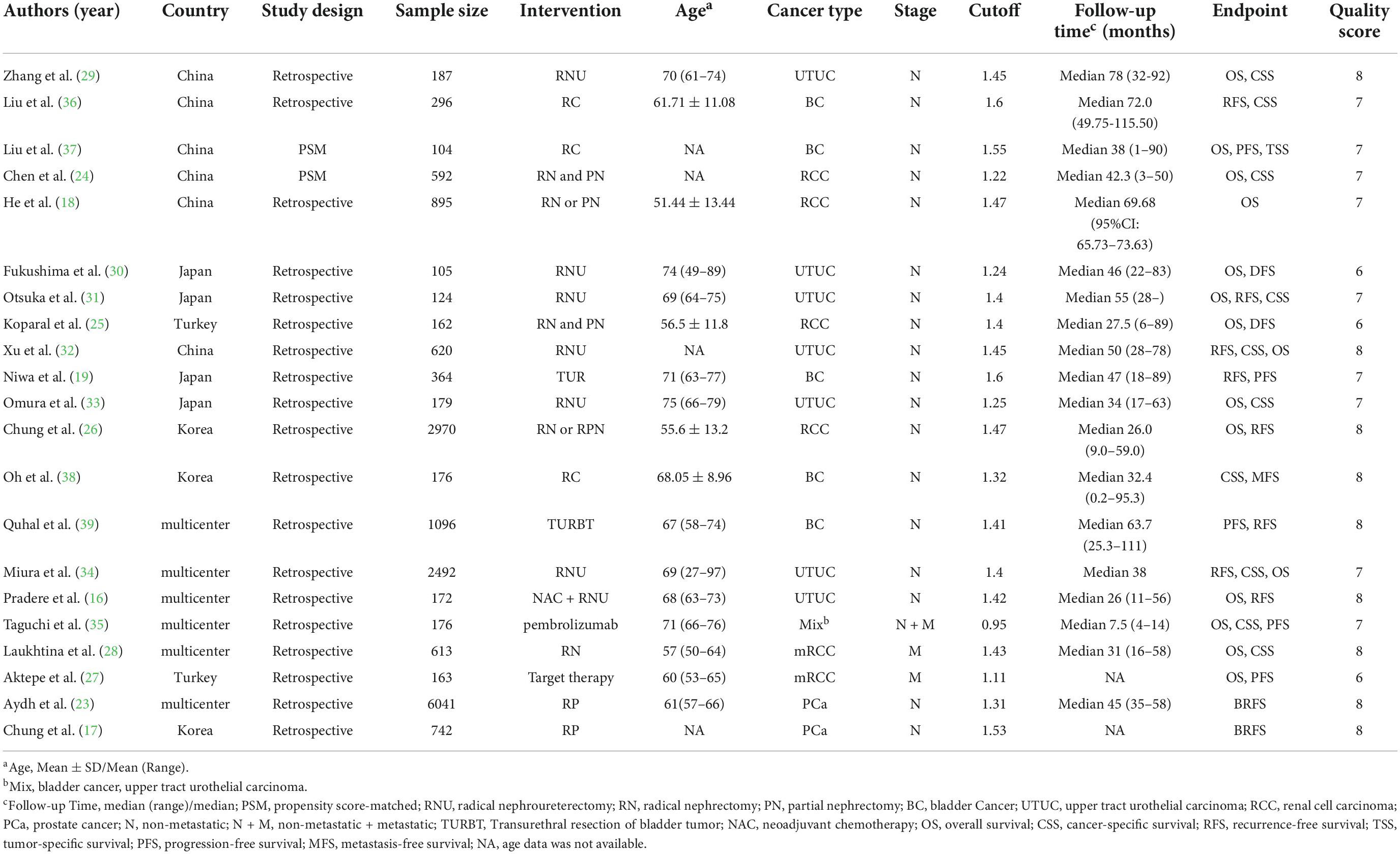
From 2017 to 2022, all the eligible studies were published, with a sample size between 104 and 6,041 participants and the cutoff values of AGR ranged from 1.11-1.6. Notably, all the included studies were only case-control, six of the 21 studies were conducted in a single center, and 15 studies were conducted in multiple centers. Of the included studies, five studies reported the correlation between pretreatment AGR and prognosis of bladder cancer (BC), seven articles explored upper tract urothelial carcinoma (UTUC), five investigated renal cell cancer (RCC), and two focused on prostate cancer (PC). Using the NOS score, eighteen articles scored 7-9 on the NOS and three studies scored 5 or 6 on the NOS, indicating high quality of the included articles. Table 1 summarizes the characteristics of the included articles.
Prognostic significance of the albumin to globulin ratio for overall survival
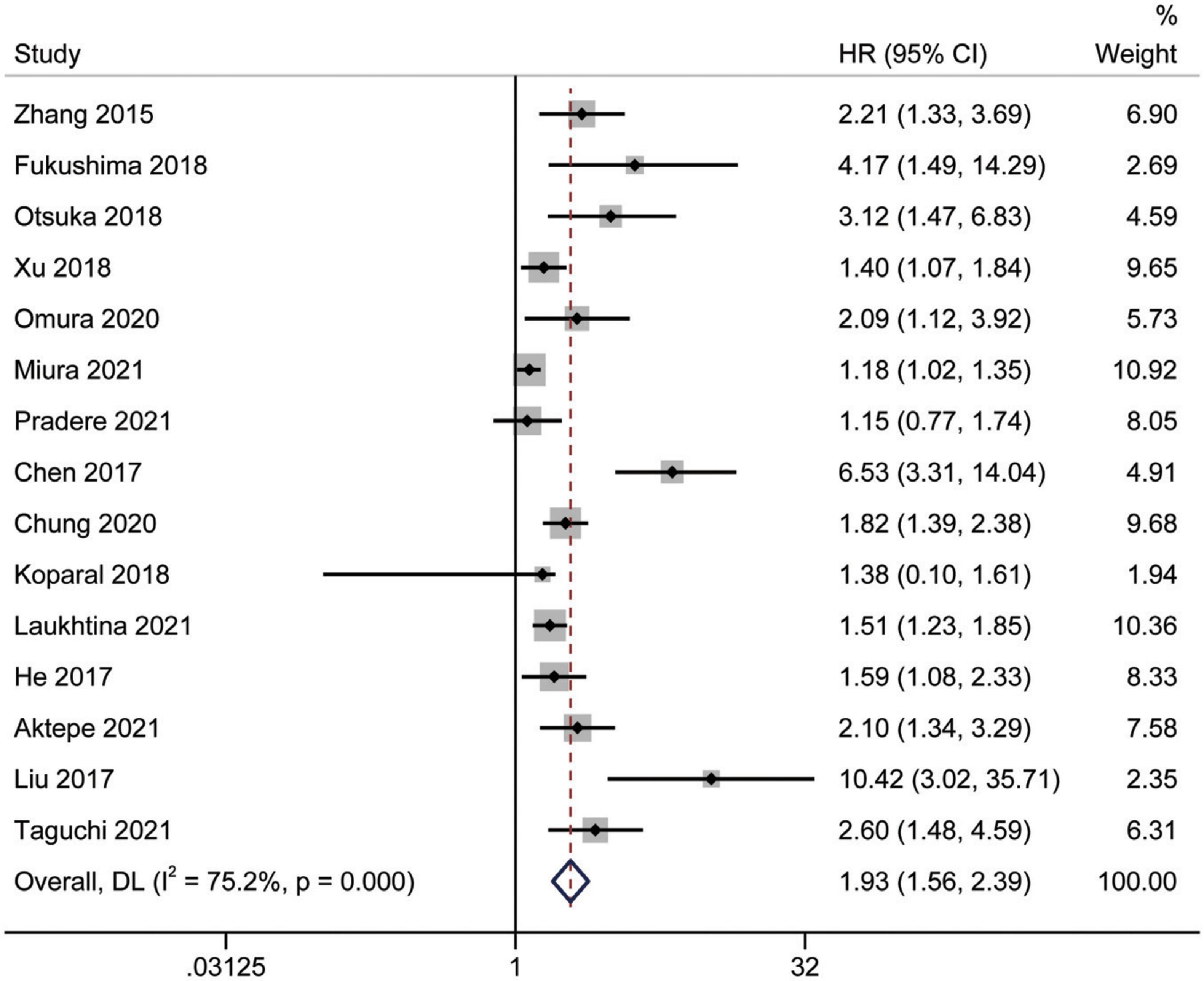
A total of 15 studies (16, 18, 24–35, 37) involving 9,554 patients revealed an association between AGR and OS in a multivariate analysis of urological cancers. According to Figure 2, patients with low pretreatment AGR had a worse survival outcomes than those with high AGR [HR = 1.93, 95% CI (1.56–2.39), p < 0.001]. Because of the high heterogeneity between the studies (I2 = 75.2%, p < 0.001), we used a random-effects model. To clarify the source of heterogeneity between studies, we performed subgroups analysis based on ethnicity, sample size, urothelial carcinoma (yes or no), AGR cut-off values and publication year. The results of the subgroups analysis also supported the fact that low AGR was related to poor OS and revealed that ethnicity, sample size, urothelial carcinoma (yes or no), AGR cut-off values, and publication year might be causes of high heterogeneity (Figure 3).
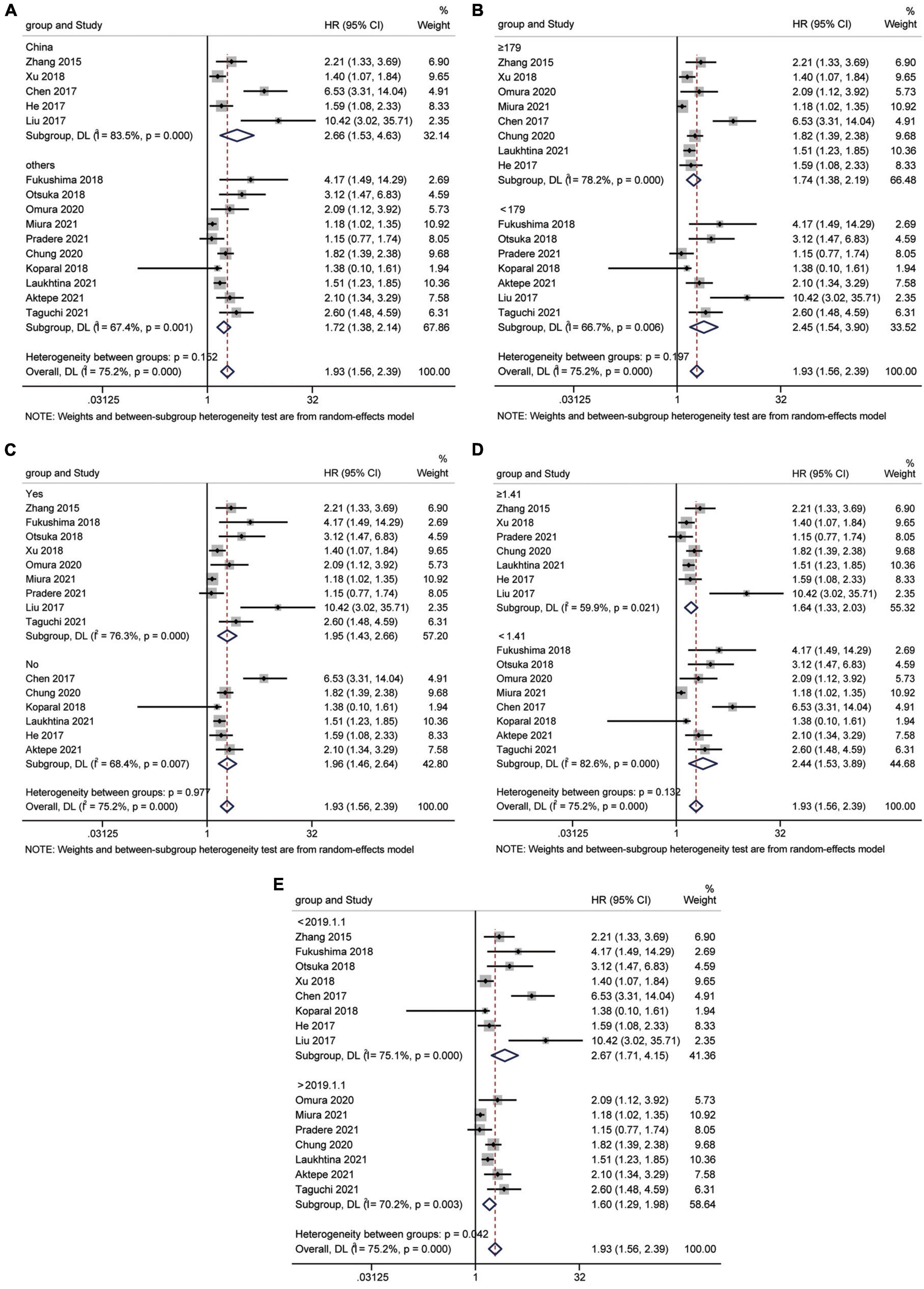
Figure 3. Forest plot for subgroup analysis of OS and AGR. (A) Subgroup analysis of OS and AGR according to ethnicity; (B) Subgroup analysis of OS and AGR according to sample size; (C) Subgroup analysis of OS and AGR according to urothelial carcinoma (yes or no); (D) Subgroup analysis of OS and AGR according to cut-off values; (E) Subgroup analysis of OS and AGR according to publication year.
Prognostic significance of the albumin to globulin ratio for cancer-specific survival
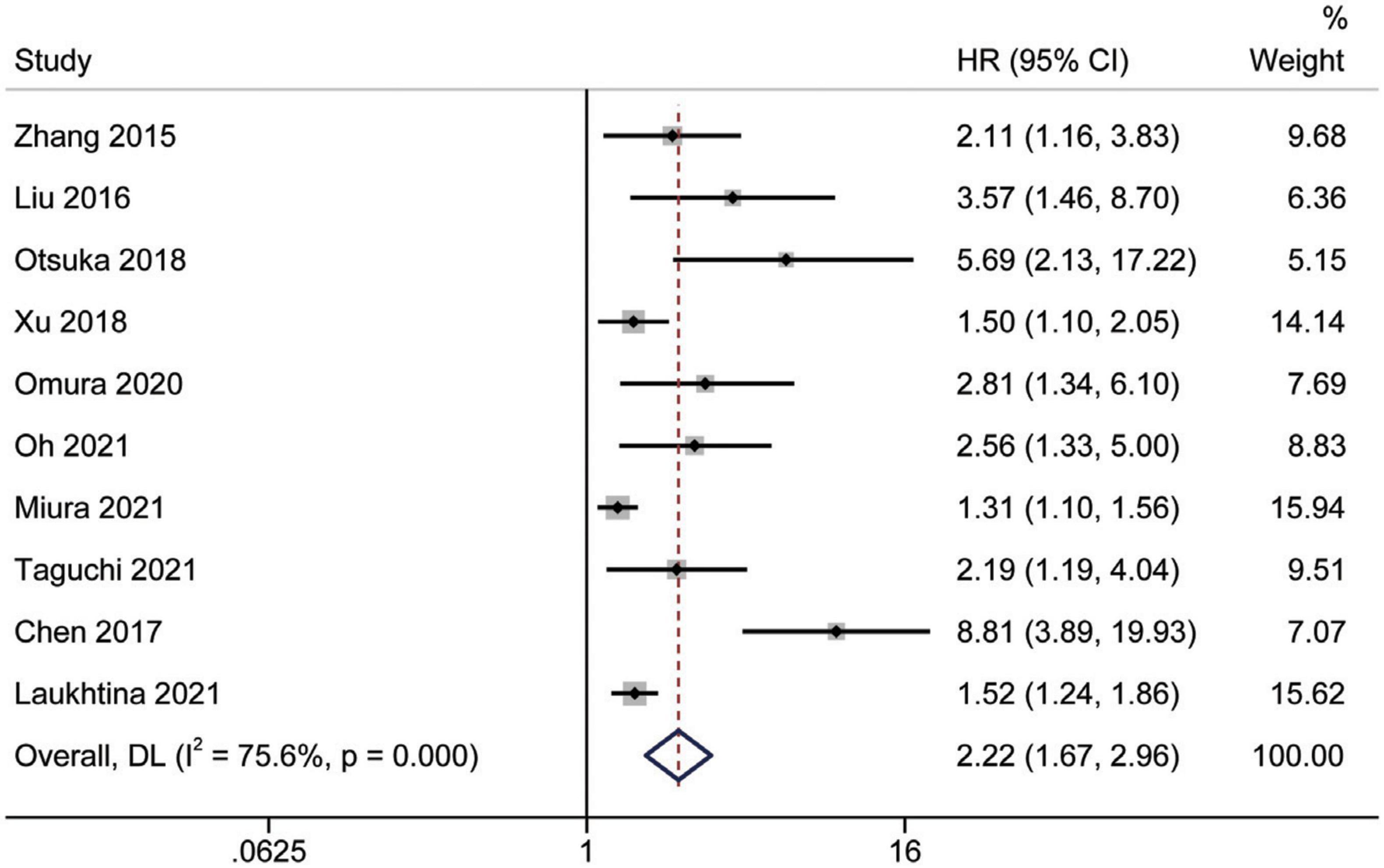
Study reports from 10 studies (24, 28, 29, 31–36, 38), with 5,455 patients enrolled, indicated the prognostic value of AGR before treatment in patients with urinary system cancers on CSS. Because of Cochrane’s Q test and I2 test revealed substantial heterogeneity among studies (I2 = 75.6%, p < 0.001), a random-effects model was used to combine the HR of each study. Pooled analysis showed that decreased pretreatment AGR was significantly associated with shorter CSS [HR = 2.22, 95% CI (1.67–2.96), p < 0.001] (Figure 4). Subgroups analysis stratified by ethnicity, sample size, urothelial carcinoma (yes or no), AGR cut-off values and publication year, and results showed that low pretreatment AGR was associated with poor CSS (Figure 5).
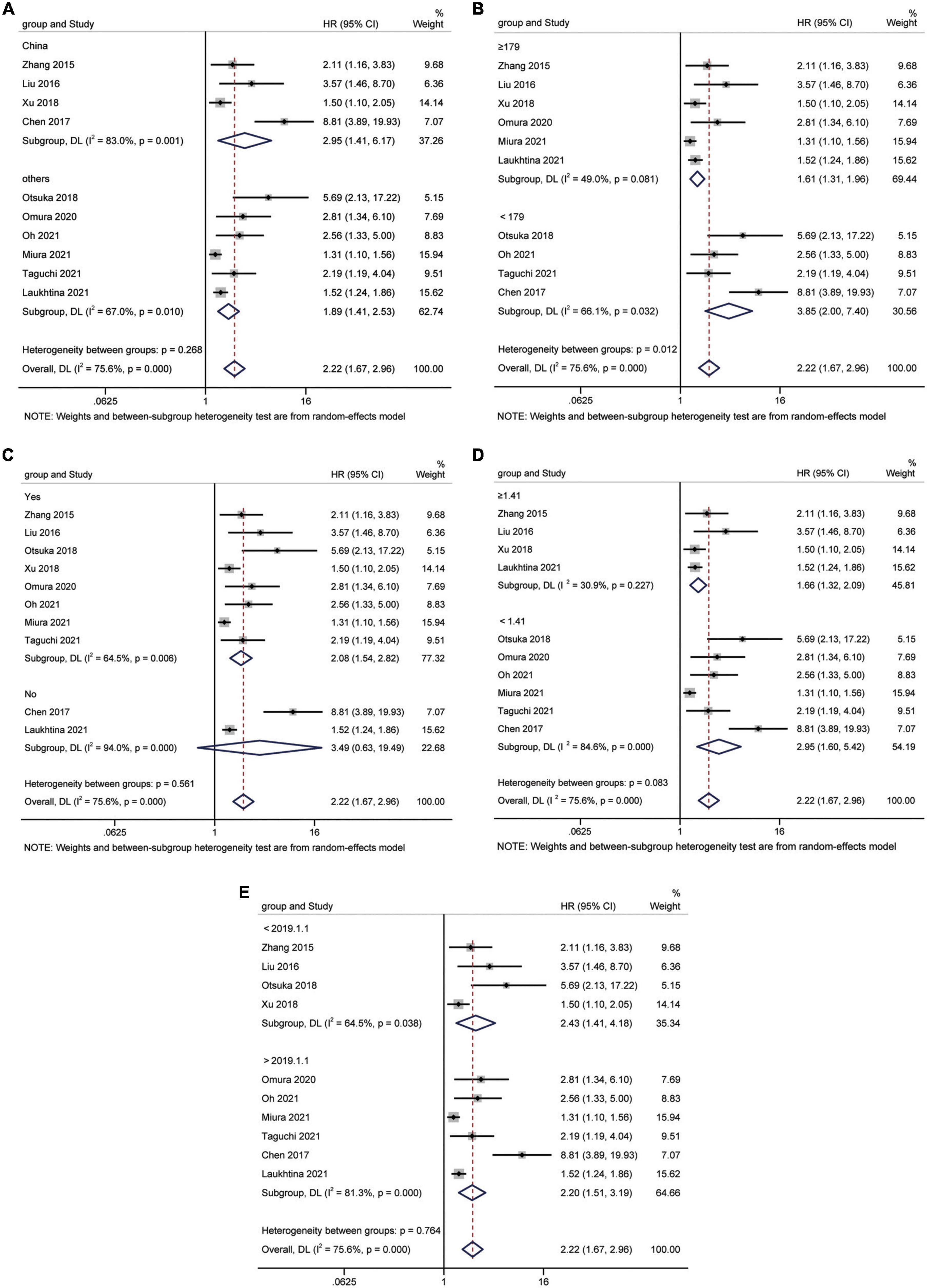
Figure 5. Forest plot for subgroup analysis of CSS and AGR. (A) Subgroup analysis of CSS and AGR according to ethnicity; (B) Subgroup analysis of CSS and AGR according to sample size; (C) Subgroup analysis of CSS and AGR according to urothelial carcinoma (yes or no); (D) Subgroup analysis of CSS and AGR according to cut-off values; (E) Subgroup analysis of CSS and AGR according to publication year.
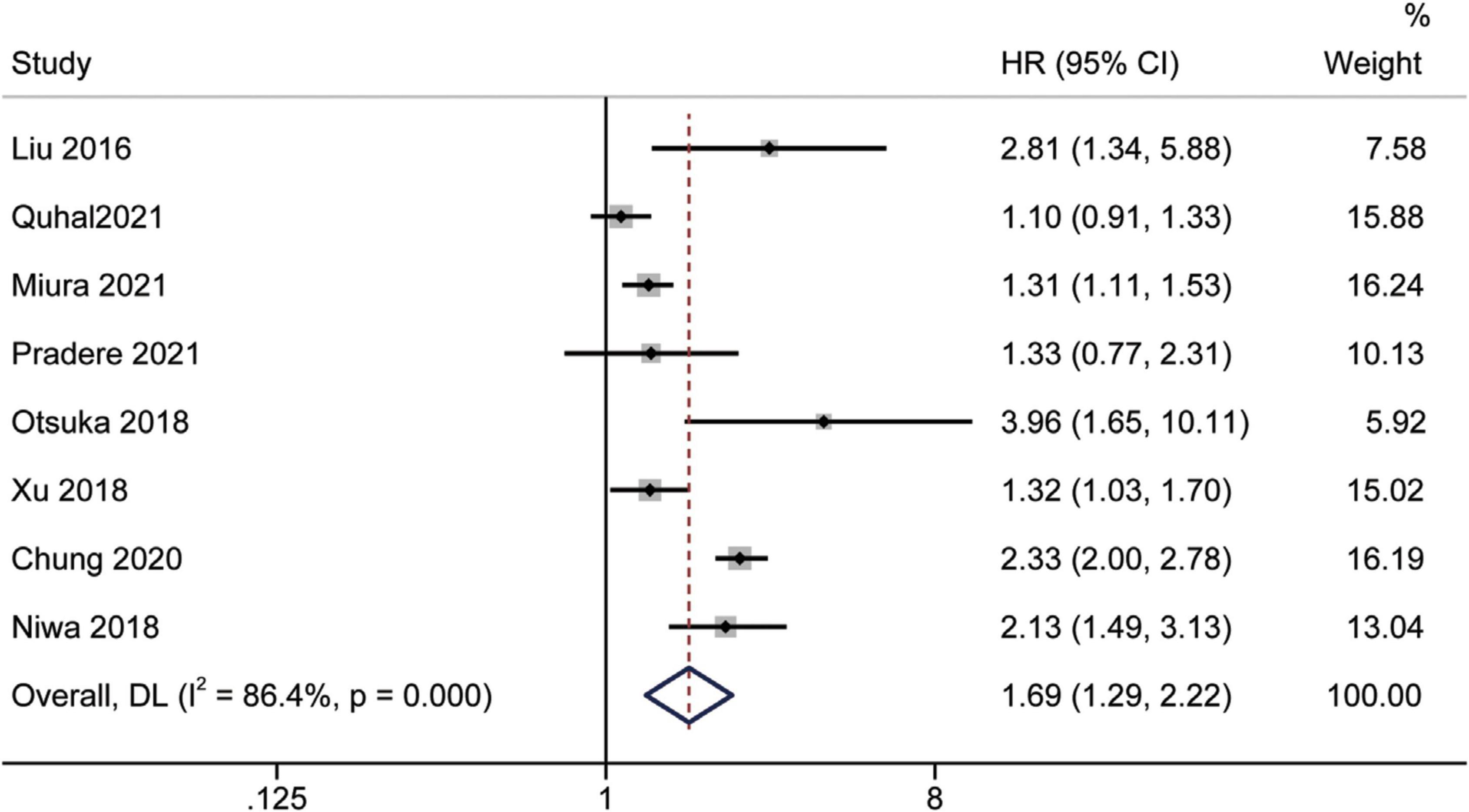
Prognostic significance of the albumin to globulin ratio for recurrence-free survival
Eight studies (16, 19, 26, 31, 32, 34, 36, 39) assessed the prognostic impact of AGR before treatment on RFS using multivariate analysis. Since the statistics showed considerable inter-study heterogeneity (I2 = 86.4%, p < 0.001), a random-effects model was used. The pooled meta-analysis results showed that low pretreatment AGR could predict inferior RFS independently [HR = 1.69, 95% CI (1.29–2.22), p < 0.001] (Figure 6).
Prognostic significance of the albumin to globulin ratio for progression-free survival and biochemical recurrence-free survival
Limited related data from four studies (19, 35, 37, 39) and two studies (17, 23) were suitable for PFS and BRFS analyses, respectively. Analysis of datasets revealed an association between low AGR and worse PFS [HR = 1.29, 95% CI (0.54–3.07), p < 0.001] (Figure 7A), and poor BRFS [HR = 1.46, 95% CI (1.28–1.67), p < 0.001] (Figure 7B).
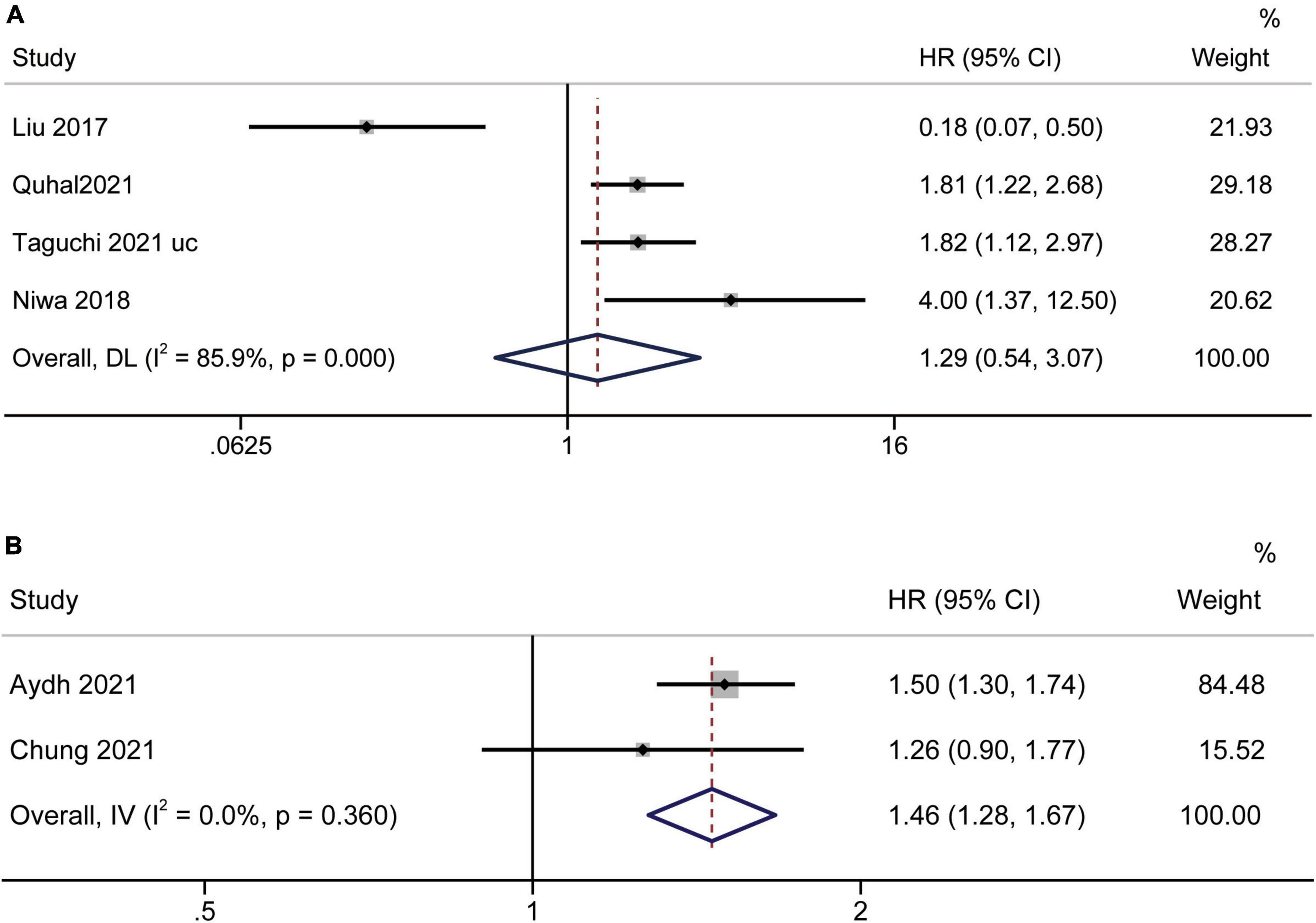
Figure 7. Forest plot reflects the association between AGR and PFS/BRFS for urological cancers. (A) AGR and PFS; (B) AGR and BRFS.
Sensitivity analysis
In this meta-analysis, we conducted a sensitivity analysis of OS and CSS outcomes to ascertain the strength of our results. The pooled HRs of OS and CSS were not significantly affected when the study was removed. Therefore, we believe that our results are reliable (Figure 8).
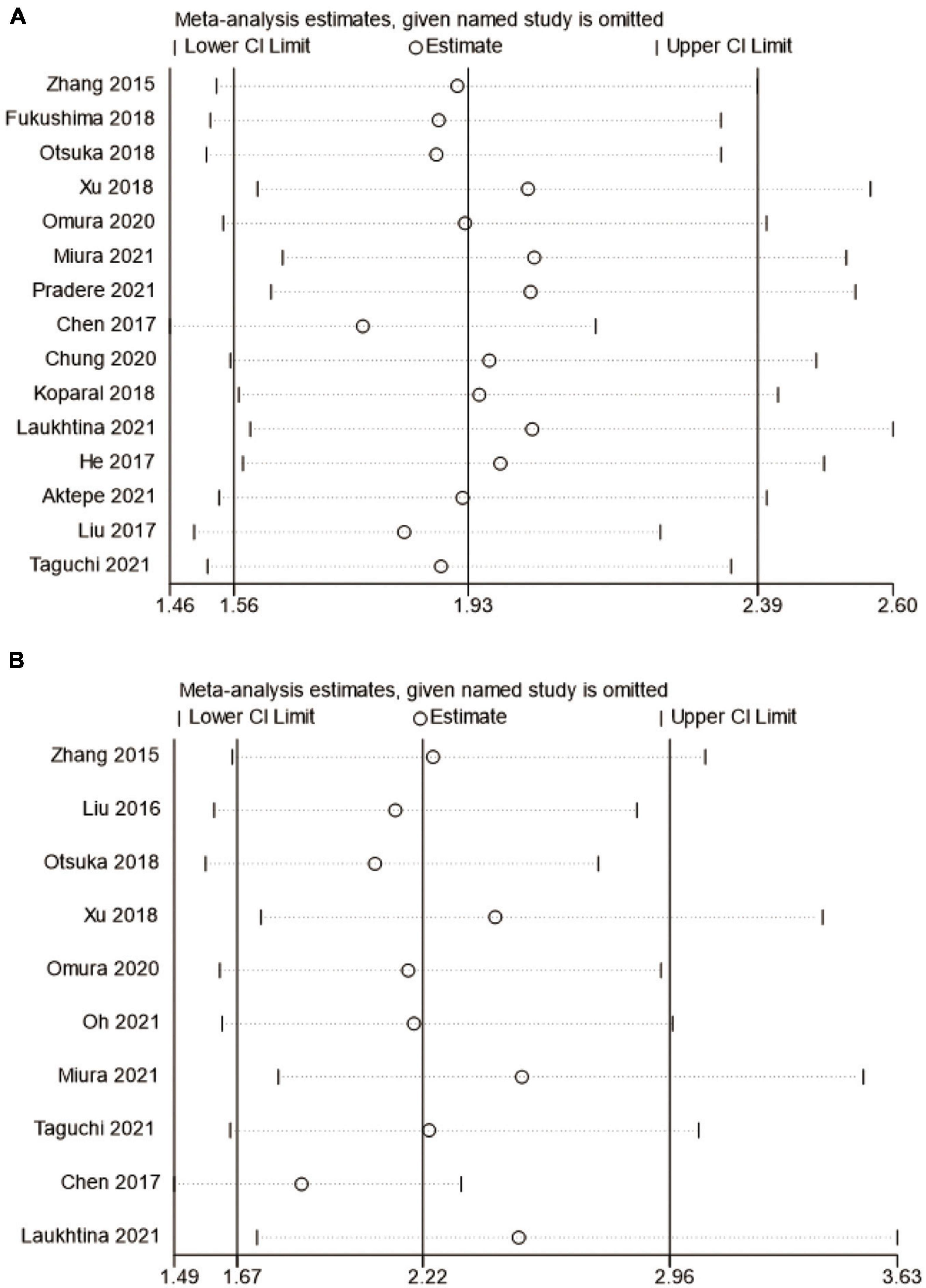
Figure 8. Forest plot for sensitivity analysis. (A) overall survival and (B) cancer-specific survival.
Publication bias
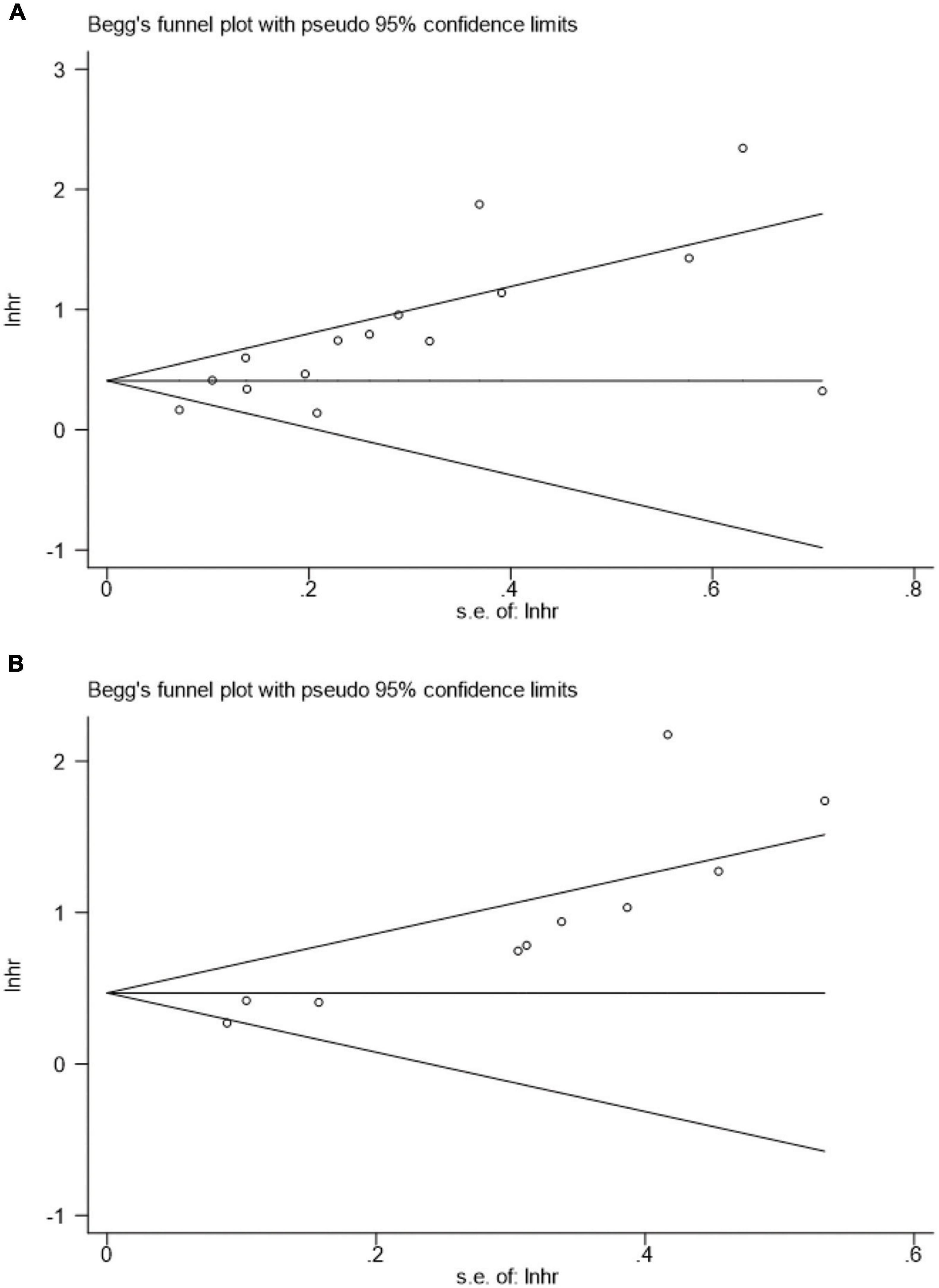
Figure 9 presents the funnel plots for OS and CSS, and asymmetry was observed by visual inspection of the Begg’s funnel plots. Therefore, a potential publication bias may have was existed for the association of pretreatment AGR, OS, and CSS based on funnel plots. Our Begg’s statistical tests aslo indicated that there was a significant publication bias (OS, p = 0.018; CSS, p < 0.001).
Discussion
Recurrence and metastasis of urinary tumors are common and seriously affect both the prognosis and quality of life of patients. Approximately 75% of high-grade non- muscle-invasive bladder cancer (NMIBC) cases would relapsed, progressed or died within 5 years (40). For urothelial cancer, even if the patients undergo radical surgery, the 5-year survival rate is less than 50% (41). Furthermore, 20% to 30% of patients with RCC undergoing curative resection have distant metastasis during follow-up, and the 5-year survival rate is very low (5-10%) (42). Therefore, it is of great significance to identify the predictive indicators that affect the prognosis and treatment decision-making for urinary cancer. To our knowledge, this is the first and most comprehensive meta-analysis to evaluate the prognostic impact of AGR on UC.
As a promising blood marker, some studies have reported a relationship between pretreatment AGR and urological cancers up to now. Owing to the inconsistent results of these studies, we iperformed a meta-analysis of twenty-one published studies involving 18,269 patients to further evaluate the potential value of AGR for predicting the survival of patients with urinary cancer. Based on the pooled data, our results are in line with the most of the related published literatures, which demonstrated that low pretreatment AGR is an independent predictor of survival outcomes. With a decrease in AGR, patients with urinary system cancer (BC, UTUC, RCC, and PCa) had worse OS, CSS, RFS, PFS, and BRFS outcomes. We then performed a subgroups analysis of OS and CSS based on ethnicity, cancer type, cutoff value, sample size or publication year. The results of the subgroup analysis were consistent with meta-regression analyses. Finally, sensitivity analyses also demonstrated the robustness of our outcomes. Albumin and globulins are the two most abundant proteins in human blood plasma, and can be easily and cost-effectively measured. Thus, AGR can be used as a competent prognostic biomarker in patients with urological cancer.
Growing evidence suggests that inflammation in the tumor microenvironment plays a critical role in tumor growth, progression and metastasis (43). Tumor growth, necrosis and hypoxia trigger the production of a series of inflammatory factors, such as tumor necrosis factor (TNF), interleukin-1 and interleukin-6, which increase vascular permeability by damaging vascular endothelial cells (44). In 2014, Duran and his colleagues first demonstrated that the AGR was a strong prognostic indicator of poor survival outcomes in lung adenocarcinoma patients (45). Although the specific mechanism is not clear, it is at least related to these two indicators. Albumin is the main component of total serum protein, and its level either reflects the body’s nutritional status or represents the systemic inflammation (18). Cytokines and chemokines produced by tumor cells can suppress albumin production and lead to malnutrition, which accelerates disease progression (46). Moreover, studies have reported that albumin plays an important role in delivering chemotherapy drugs to cancer patients, and this mechanism affects the survival outcomes (47). Furthermore, globulins, including complementary components, C-reactive protein (CRP), and immunoglobulin, is also involved in the inflammatory responses and immunosuppression against cancer cells in the human body (48). Chronic inflammation can affect not only the tumor growth but also angiogenesis and cancer migration (49). Studies have shown that some inflammatory markers, CRP and NLR, were closely related to the prognosis of cancer patients (50, 51). Equally, the AGR is also a inflammatory biomarker and decrease of AGR reflects the malnutrition and inflammatory response. Because of the AGR incorporates the advantages of albumin and globulin, and it is more likely to predict the poor survival in cancer patients.
Previous meta-analyses have explored the correlation between AGR and different organs and systems. A meta-analysis conducted by Li et al. (52) discovered that a decreased AGR suffer from worse OS and DFS in patients with lung cancer. In colorectal cancer, Ma et al. (53) provided evidence that low pretreatment AGR was related to poor OS (HR = 2.07, P < 0.01) and DFS/PFS (HR = 2.10, P = 0.01), and advanced clinicopathological features, including age, tumor size, node metastasis stage, and tumor depth. Additionally, many recent studies have explored the association between the AGR and genitourinary cancers. Omura and his colleagues performed a retrospective study involving 179 patients with UTUC who underwent radical nephroureterectomy and revealed that a low AGR could predict the prognosis of patients with non-metastatic UTUC (33). A multicenter research team found that the UTUC patients with a low pretreatment AGR had a markedly shorter OS and RFS than those with a high AGR patients (16). In Korea, Chung’s study proved that the association between the preoperative serum AGR and the poor prognosis in patients with RCC in a large cohort (26). For non-metastatic PCa patients who received radical prostatectomy (RP), the findings of Aydh et al. and Chung et al. indicated that the pretreatment AGR can be a useful serological marker for predicting the BRFS and adverse pathology (17, 23). In 2021, Zhang et al. (48) verified that the AGR combined with other indices [C-reactive protein/albumin ratio (CAR), neutrophil-lymphocyte ratio (NLR), and other clinicopathological features] could predict OS and PFS in BC patients after radical cystectomy. This is the first meta-analysis to assess the prognostic value of the AGR in urinary system cancers. Our results further showed that a low pretreatment AGR indicates poor prognosis, which is in line with previous studies.
Although this meta-analysis provides evidence regarding the prognostic value of AGR in patients with urological cancer, several limitations cannot be avoided. First, most of the included studies were single-center and retrospective studies; therefore, the heterogeneity between studies is inevitable. Second, because of different treatment strategies for various cancers may introduce bias in the results. Third, the cutoff threshold of AGR selected by different studies was different, making it difficult to determine the optimal cutoff value. Fourth, only common urological tumors were included in this study, but the correlation between AGR and the prognosis of other genitourinary tumors is still unclear. Finally, considering the limitation of many factors affecting the prognosis of urinary cancers, the evaluation effectiveness of this index needs to be verified. Therefore, further studies need to be conducted.
Conclusion
In summary, our study proved that a low AGR before treatment is associated with inferior OS, CSS, RFS, PFS, and BRFS outcomes in urinary system cancers. As a non-invasive, effective, and cost-effective indicator, the AGR can be used to predict the prognosis of patients with urological cancer. However, further large-scale prospective studies with larger sample sizes are needed.
Data availability statement
The original contributions presented in this study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Author contributions
LT and JW conceived and designed the experiments. ZX, XF, JL, and XY analyzed the data. JL, JS, and HW contributed the reagents, materials, and analysis. ZX and XF wrote the manuscript. All authors contributed to the article and approved the submitted version.
Acknowledgments
The authors thank Editage (www.editage.cn) for English language editing.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. (2022) 72:7–33. doi: 10.3322/caac.21708
2. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
3. Li X, Gu L, Chen Y, Chong Y, Wang X, Guo P, et al. Systemic immune-inflammation index is a promising non-invasive biomarker for predicting the survival of urinary system cancers: a systematic review and meta-analysis. Ann Med. (2021) 53:1827–38. doi: 10.1080/07853890.2021.1991591
4. Peyton CC, Tang D, Reich RR, Azizi M, Chipollini J, Pow-Sang JM, et al. Downstaging and survival outcomes associated with neoadjuvant chemotherapy regimens among patients treated with cystectomy for muscle-invasive bladder cancer. JAMA Oncol. (2018) 4:1535–42. doi: 10.1001/jamaoncol.2018.3542
5. Mattesen TB, Rasmussen MH, Sandoval J, Ongen H, Árnadóttir SS, Gladov J, et al. MethCORR modelling of methylomes from formalin-fixed paraffin-embedded tissue enables characterization and prognostication of colorectal cancer. Nat Commun. (2020) 11:2025. doi: 10.1038/s41467-020-16538-5
6. Petitprez F, Ayadi M, de Reyniès A, Fridman WH, Sautès-Fridman C, Job S. Review of prognostic expression markers for clear cell renal cell carcinoma. Front Oncol. (2021) 11:643065. doi: 10.3389/fonc.2021.643065
7. Mbeutcha A, Mathieu R, Rouprêt M, Gust KM, Briganti A, Karakiewicz PI. Predictive models and prognostic factors for upper tract urothelial carcinoma: a comprehensive review of the literature. Transl Androl Urol. (2016) 5:720–34. doi: 10.21037/tau.2016.09.07
8. Moch H, Gasser T, Amin MB, Torhorst J, Sauter G, Mihatsch MJ. Prognostic utility of the recently recommended histologic classification and revised TNM staging system of renal cell carcinoma: a Swiss experience with 588 tumors. Cancer. (2000) 89:604–14. doi: 10.1002/1097-0142(20000801)89:3<604::AID-CNCR16>3.0.CO;2-Q
9. Mori K, Janisch F, Mostafaei H, Lysenko I, Kimura S, Egawa S, et al. Prognostic value of preoperative blood-based biomarkers in upper tract urothelial carcinoma treated with nephroureterectomy: a systematic review and meta-analysis. Urol Oncol. (2020) 38:315–33. doi: 10.1016/j.urolonc.2020.01.015
10. Li J, Cao D, Peng L, Meng C, Xia Z, Li Y, et al. Potential clinical value of pretreatment de ritis ratio as a prognostic biomarker for renal cell carcinoma. Front Oncol. (2021) 11:780906. doi: 10.3389/fonc.2021.780906
11. Dai J, Tang K, Xiao W, Yu G, Zeng J, Li W, et al. Prognostic significance of C-reactive protein in urological cancers: a systematic review and meta-analysis. Asian Pac J Cancer Prev. (2014) 15:3369–75. doi: 10.7314/APJCP.2014.15.8.3369
12. Ma JY, Hu G, Liu Q. Prognostic significance of the lymphocyte-to-monocyte ratio in bladder cancer undergoing radical cystectomy: a meta-analysis of 5638 individuals. Dis Markers. (2019) 2019:7593560. doi: 10.1155/2019/7593560
13. Wang Z, Wang X, Zou H, Dai Z, Feng S, Zhang M, et al. The basic characteristics of the pentraxin family and their functions in tumor progression. Front Immunol. (2020) 11:1757. doi: 10.3389/fimmu.2020.01757
14. Liu J, Chen S, Geng Q, Liu X, Kong P, Zhan Y, et al. Prognostic value of pretreatment albumin-globulin ratio in predicting long-term mortality in gastric cancer patients who underwent D2 resection. Onco Targets Ther. (2017) 10:2155–62. doi: 10.2147/OTT.S99282
15. Zhang X, Zhao W, Chen X, Zhao M, Qi X, Li G, et al. Combining the fibrinogen-to-pre-albumin ratio and prognostic nutritional index (FPR-PNI) predicts the survival in elderly gastric cancer patients after gastrectomy. Onco Targets Ther. (2020) 13:8845–59. doi: 10.2147/OTT.S264199
16. Pradere B, D’Andrea D, Schuettfort VM, Foerster B, Quhal F, Mori K, et al. Pre-therapy serum albumin-to-globulin ratio in patients treated with neoadjuvant chemotherapy and radical nephroureterectomy for upper tract urothelial carcinoma. World J Urol. (2021) 39:2567–77.
17. Chung JW, Ha YS, Kim SW, Park SC, Kang TW, Jeong YB, et al. The prognostic value of the pretreatment serum albumin to globulin ratio for predicting adverse pathology in patients undergoing radical prostatectomy for prostate cancer. Investig Clin Urol. (2021) 62:545–52. doi: 10.4111/icu.20210105
18. He X, Guo S, Chen D, Yang G, Chen X, Zhang Y, et al. Preoperative albumin to globulin ratio (AGR) as prognostic factor in renal cell carcinoma. J Cancer. (2017) 8:258–65. doi: 10.7150/jca.16525
19. Niwa N, Matsumoto K, Ide H, Nagata H, Oya M. Prognostic value of pretreatment albumin-to-globulin ratio in patients with non-muscle-invasive bladder cancer. Clin Genitourin Cancer. (2018) 16:e655–61. doi: 10.1016/j.clgc.2017.12.013
20. Guner E, Seker KG. The role of preoperative albumin to globulin ratio in predicting prognosis in testicular cancer patients. Actas Urol Esp. (2020) 44:469–76. doi: 10.1016/j.acuroe.2020.08.001
21. Li Y, He J, Hu Y. Comparison of the efficiency and safety of total ankle replacement and ankle arthrodesis in the treatment of osteoarthritis: an updated systematic review and meta-analysis. Orthop Surg. (2020) 12:372–7. doi: 10.1111/os.12635
22. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. (2010) 25:603–5. doi: 10.1007/s10654-010-9491-z
23. Aydh A, Mori K, D’Andrea D, Motlagh RS, Abufaraj M, Pradere B, et al. Prognostic value of the pre-operative serum albumin to globulin ratio in patients with non-metastatic prostate cancer undergoing radical prostatectomy. Int J Clin Oncol. (2021) 26:1729–35. doi: 10.1007/s10147-021-01952-6
24. Chen Z, Shao Y, Yao H, Zhuang Q, Wang K, Xing Z, et al. Preoperative albumin to globulin ratio predicts survival in clear cell renal cell carcinoma patients. Oncotarget. (2017) 8:48291–302. doi: 10.18632/oncotarget.15162
25. Koparal MY, Polat F, Çetin S, Bulut EC, Sözen TS. Prognostic role of preoperative albumin to globulin ratio in predicting survival of clear cell renal cell carcinoma. Int Braz J Urol. (2018) 44:933–46. doi: 10.1590/s1677-5538.ibju.2018.0012
26. Chung JW, Park DJ, Chun SY, Choi SH, Lee JN, Kim BS, et al. The prognostic role of preoperative serum albumin/globulin ratio in patients with non-metastatic renal cell carcinoma undergoing partial or radical nephrectomy. Sci Rep. (2020) 10:11999. doi: 10.1038/s41598-020-68975-3
27. Aktepe OH, Güner G, Güven DC, Taban H, Yıldırım HÇ, Şahin TK, et al. Impact of albumin to globulin ratio on survival outcomes of patients with metastatic renal cell carcinoma. Turk J Urol. (2021) 47:113–9.
28. Laukhtina E, Pradere B, D’Andrea D, Rosiello G, Luzzago S, Pecoraro A, et al. Prognostic effect of preoperative serum albumin to globulin ratio in patients treated with cytoreductive nephrectomy for metastatic renal cell carcinoma. Transl Androl Urol. (2021) 10:609–19. doi: 10.21037/tau-20-1101
29. Zhang B, Yu W, Zhou LQ, He ZS, Shen C, He Q, et al. Prognostic significance of preoperative albumin-globulin ratio in patients with upper tract urothelial carcinoma. PLoS One. (2015) 10:e0144961. doi: 10.1371/journal.pone.0144961
30. Fukushima H, Kobayashi M, Kawano K, Morimoto S. Prognostic value of albumin/globulin ratio in patients with upper tract urothelial carcinoma patients treated with radical nephroureterectomy. Anticancer Res. (2018) 38:2329–34. doi: 10.21873/anticanres.12478
31. Otsuka M, Kamasako T, Uemura T, Takeshita N, Shinozaki T, Kobayashi M, et al. Prognostic role of the preoperative serum albumin : globulin ratio after radical nephroureterectomy for upper tract urothelial carcinoma. Int J Urol. (2018) 25:871–8. doi: 10.1111/iju.13767
32. Xu H, Tan P, Ai J, Huang Y, Lin T, Yang L, et al. Prognostic impact of preoperative albumin-globulin ratio on oncologic outcomes in upper tract urothelial carcinoma treated with radical nephroureterectomy. Clin Genitourin Cancer. (2018) 16:e1059–68. doi: 10.1016/j.clgc.2018.06.003
33. Omura S, Taguchi S, Miyagawa S, Matsumoto R, Samejima M, Ninomiya N, et al. Prognostic significance of the albumin-to-globulin ratio for upper tract urothelial carcinoma. BMC Urol. (2020) 20:133. doi: 10.1186/s12894-020-00700-8
34. Miura N, Mori K, Laukhtina E, Schuettfort VM, Abufaraj M, Teoh JYC, et al. Prognostic value of the preoperative albumin-globulin ratio in patients with upper urinary tract urothelial carcinoma treated with radical nephroureterectomy: results from a large multicenter international collaboration. Jpn J Clin Oncol. (2021) 51:1149–57.
35. Taguchi S, Kawai T, Nakagawa T, Nakamura Y, Kamei J, Obinata T, et al. Prognostic significance of the albumin-to-globulin ratio for advanced urothelial carcinoma treated with pembrolizumab: a multicenter retrospective study. Sci Rep. (2021) 11:15623. doi: 10.1038/s41598-021-95061-z
36. Liu J, Dai Y, Zhou F, Long Z, Li Y, Liu B, et al. The prognostic role of preoperative serum albumin/globulin ratio in patients with bladder urothelial carcinoma undergoing radical cystectomy. Urol Oncol. (2016) 34:484.e1–8. doi: 10.1016/j.urolonc.2016.05.024
37. Liu Z, Huang H, Li S, Yu W, Li W, Jin J, et al. The prognostic value of preoperative serum albumin-globulin ratio for high-grade bladder urothelial carcinoma treated with radical cystectomy: a propensity score-matched analysis. J Cancer Res Ther. (2017) 13:837–43. doi: 10.4103/jcrt.JCRT_237_17
38. Oh JS, Park DJ, Byeon KH, Ha YS, Kim TH, Yoo ES, et al. Decrease of preoperative serum albumin-to-globulin ratio as a prognostic indicator after radical cystectomy in patients with urothelial bladder cancer. Urol J. (2021) 18:66–73.
39. Quhal F, Pradere B, Laukhtina E, Sari Motlagh R, Mostafaei H, Mori K, et al. Prognostic value of albumin to globulin ratio in non-muscle-invasive bladder cancer. World J Urol. (2021) 39:3345–52. doi: 10.1007/s00345-020-03586-1
40. Xiao M, Liu J, Xiang L, Zhao K, He D, Zeng Q, et al. MAFG-AS1 promotes tumor progression via regulation of the HuR/PTBP1 axis in bladder urothelial carcinoma. Clin Transl Med. (2020) 10:e241. doi: 10.1002/ctm2.241
41. Su X, Lu X, Bazai SK, Compérat E, Mouawad R, Yao H, et al. Comprehensive integrative profiling of upper tract urothelial carcinomas. Genome Biol. (2021) 22:7. doi: 10.1186/s13059-020-02230-w
42. Li Y, Gong Y, Ning X, Peng D, Liu L, He S, et al. Downregulation of CLDN7 due to promoter hypermethylation is associated with human clear cell renal cell carcinoma progression and poor prognosis. J Exp Clin Cancer Res. (2018) 37:276. doi: 10.1186/s13046-018-0924-y
43. Tao CJ, Chen YY, Jiang F, Feng XL, Jin QF, Jin T, et al. The C-reactive protein/albumin ratio is an independent prognostic factor for overall survival in patients with nasopharyngeal carcinoma receiving intensity-modulated radiotherapy. J Cancer. (2016) 7:2005–11. doi: 10.7150/jca.16210
44. Kinoshita A, Onoda H, Imai N, Iwaku A, Oishi M, Tanaka K, et al. The C-reactive protein/albumin ratio, a novel inflammation-based prognostic score, predicts outcomes in patients with hepatocellular carcinoma. Ann Surg Oncol. (2015) 22:803–10. doi: 10.1245/s10434-014-4048-0
45. Duran AO, Inanc M, Karaca H, Dogan I, Berk V, Bozkurt O, et al. Albumin-globulin ratio for prediction of long-term mortality in lung adenocarcinoma patients. Asian Pac J Cancer Prev. (2014) 15:6449–53. doi: 10.7314/APJCP.2014.15.15.6449
46. Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. (2008) 454:436–44. doi: 10.1038/nature07205
47. Matsumoto I, Tanaka M, Shirakawa S, Shinzeki M, Toyama H, Asari S, et al. Postoperative serum albumin level is a marker of incomplete adjuvant chemotherapy in patients with pancreatic ductal adenocarcinoma. Ann Surg Oncol. (2015) 22:2408–15. doi: 10.1245/s10434-014-4280-7
48. Zhang W, Yang F, Kadier A, Chen Y, Yu Y, Zhang J, et al. Development of nomograms related to inflammatory biomarkers to estimate the prognosis of bladder cancer after radical cystectomy. Ann Transl Med. (2021) 9:1440. doi: 10.21037/atm-21-4097
49. Wang J, Yang ZR, Dong WG, Zhang JX, Guo XF, Song J, et al. Cooperative inhibitory effect of sinomenine combined with 5-fluorouracil on esophageal carcinoma. World J Gastroenterol. (2013) 19:8292–300. doi: 10.3748/wjg.v19.i45.8292
50. Barak-Corren Y, Horovits Y, Erlichman M, Picard E. The prognostic value of C-reactive protein for children with pneumonia. Acta Paediatr. (2021) 110:970–6. doi: 10.1111/apa.15580
51. Wang J, Liu Y, Mi X, Shao M, Liu L. The prognostic value of prognostic nutritional index (PNI) and neutrophil to lymphocyte ratio (NLR) for advanced non-small cell lung cancer treated with platinum-based chemotherapeutics. Ann Palliat Med. (2020) 9:967–78. doi: 10.21037/apm.2020.04.31
52. Li J, Wang Y, Wu Y, Li J, Che G. Prognostic value of pretreatment albumin to globulin ratio in lung cancer: a meta-analysis. Nutr Cancer. (2021) 73:75–82. doi: 10.1080/01635581.2020.1737155
Keywords: urological cancers, albumin to globulin ratio, meta-analysis, prognosis, survival
Citation: Xia Z, Fu X, Yuan X, Li J, Wang H, Sun J, Wu J and Tang L (2022) Serum albumin to globulin ratio prior to treatment as a potential non-invasive prognostic indicator for urological cancers. Front. Nutr. 9:1012181. doi: 10.3389/fnut.2022.1012181
Received: 05 August 2022; Accepted: 12 October 2022;
Published: 26 October 2022.
Edited by:
Yulong Li, University of Nebraska Medical Center, United StatesReviewed by:
Utku Oflazoğlu, İzmir Kâtip Çelebi University, TurkeyMohammad Arjmand, Mashhad University of Medical Sciences, Iran
Copyright © 2022 Xia, Fu, Yuan, Li, Wang, Sun, Wu and Tang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ji Wu, d3VqaTIxNjhAMTYzLmNvbQ==; Lingtong Tang, MzY0Njg2MTQ5QHFxLmNvbQ==
†These authors have contributed equally to this work
 Zhongyou Xia
Zhongyou Xia Xueqin Fu
Xueqin Fu Xinzhu Yuan3†
Xinzhu Yuan3† Jinze Li
Jinze Li