- 1School of Systems Biology, George Mason University, Manassas, VA, United States
- 2Research Centre for Medical Genetics, Moscow, Russia
- 3Peking University Sixth Hospital/Institute of Mental Health, Beijing, China
- 4NHC Key Laboratory of Mental Health (Peking University), National Clinical Research Center for Mental Disorders (Peking University Sixth Hospital), Beijing, China
- 5PKU-IDG/McGovern Institute for Brain Research, Peking University, Beijing, China
- 6Chinese Institute for Brain Research, Beijing, China
- 7Institute of Neuropsychiatry, The Affiliated Brain Hospital of Nanjing Medical University, Nanjing, China
- 8Department of Psychiatry, The Affiliated Brain Hospital of Nanjing Medical University, Nanjing, China
Tea ingredients can effectively inhibit SARS-CoV-2 infection at adequate concentrations. It is not known whether tea intake could impact the susceptibility to COVID-19 or its severity. We aimed to evaluate the causal effects of tea intake on COVID-19 outcomes. We performed Mendelian randomization (MR) analyses to assess the causal associations between tea intake (N = 441,279) and three COVID-19 outcomes, including SARS-CoV-2 infection (122,616 cases and 2,475,240 controls), hospitalized COVID-19 (32,519 cases and 2,062,805 controls), and critical COVID-19 (13,769 cases and 1,072,442 controls). The MR analyses indicated that genetic propensity for tea consumption conferred a negative causal effect on the risk of SARS-CoV-2 infection (OR: 0.87, 95% confidence interval (CI): 0.78–0.97, P = 0.015). No causal effects on hospitalized COVID-19 (0.84, 0.64–1.10, P = 0.201) or critical COVID-19 (0.73, 0.51–1.03, P = 0.074) were detected. Our study revealed that tea intake could decrease the risk of SARS-CoV-2 infection, highlighting the potential preventive effect of tea consumption on COVID-19 transmission.
Introduction
COVID-19 has caused millions of deaths and is an ongoing threat to global public health. A collection of risk and protective factors have been identified to be associated with COVID-19 (1–10). Meanwhile, COVID-19 can give rise to a myriad of post-COVID-19 consequences (11–15). The continued effectiveness of the current generation of COVID-19 vaccines and the possible acquisition of resistance to antivirals remain a concern. Natural products, especially those present in the staple diet, may have the potential to provide a cost-efficient avenue to decrease the risk of COVID-19 transmission or symptomatic relief (16).
In ancient China, local outbreaks of infectious disease were common; hence, herbal or other natural medicines were developed to deal with illnesses. One of these herbal medicines was Camelia Sinensis, a tea. Now, drinking tea has become a habit worldwide, and its health benefits have been acknowledged. Through its anti-inflammatory, immunoregulatory, and antioxidant properties, tea protects against type 2 diabetes, obesity, cardiovascular conditions, cancer, and immune-related diseases (17–22). Epigallocatechin gallate (EGCG), a well-known catechin, is one of the most evaluated components of tea. EGCG inhibits the secretion of inflammatory cytokines, inhibits the main protease (MPro) of SARS-CoV-2 (23), and interferes with spike binding to ACE2 receptors (24, 25). Many in silico evaluations found that the oolongobobisflavan-A molecule had the potential to inhibit the bioactivity of Mpro of SARS-CoV-2 (26). Barrigenol, kaempferol, and myricetin had good docking scores in inhibiting non-structural protein 15 (Nsp15) of SARS-CoV-2 (27). Theaflavin could potentially inhibit non-structural protein 16 (Nsp16) of SARS-CoV-2 (28); epicatechin-3,5-di-O-gallate, epigallocatechin-3,5-di-O-gallate, and epigallocatechin-3,4-di-O-gallate had better inhibitory effects on the enzyme RNA-dependent RNA polymerase (RdRp) than some repurposed drug molecules (29).
An in vitro study found that green tea in general and EGCGs in particular significantly inhibit the entry to cells and post-entry stages of the viral life cycle while suppressing the activity of SARS-CoV-2 protease (30, 31). However, a majority of published in vitro experiments assessed the viricidal properties of some components of tea leaf extracts at very high, supraphysiological concentrations (32, 33). Therefore, whether tea drinking could impact the susceptibility or severity of COVID-19 in virus-exposed human populations is not known. Exploring the link between tea intake and COVID-19 may lead to an improvement in the management of coronavirus infection. The Mendelian randomization (MR) framework may be used to infer a potential causative association between a phenotype (exposure) that can be genetically influenced and a disease outcome. To achieve this, genetic variants are utilized as instrumental variables (34). The MR framework has been widely used in recent studies to explore relationships between related phenotypes (35–39). This study used the MR approach to test whether habitual tea intake exerts any causal influences on contracting SARS-CoV-2 and severe outcomes of COVID-19.
Materials and methods
Study design and data sources
The study was based on publicly available genome-wide association study (GWAS) summary results. The summary statistics for the outcomes of COVID-19 were obtained from the COVID-19. Host Genetics Initiative (HGI) GWAS meta-analysis round 7, including SARS-CoV-2 infection (122,616 cases and 2,475,240 controls), hospitalized COVID-19 (32,519 cases and 2,062,805 controls), and critical COVID-19 (13,769 cases and 1,072,442 controls) (40). The SARS-CoV-2 infection dataset mainly reflects the overall susceptibility to the virus, whereas the hospitalized and critical COVID-19 datasets represent the severity of the disease. Therefore, we collectively called the latter two outcomes “severe COVID-19.” The tea intake GWAS dataset included 441,279 participants from the UK Biobank (UKB) (41), which was obtained from YangLab (42). All participants in the GWAS datasets were from the European population. Ethical approvals were obtained from the original studies.
MR analyses
The main analyses were performed by using the inverse-variance weighted (IVW) method, complemented with the weighted median and MR-Egger methods, and implemented in TwoSampleMR (43). The intercept from the MR-Egger regression was utilized to evaluate the average horizontal pleiotropy (44). The IVW model was used as the main statistical method.
Sensitivity analyses
The heterogeneity in the MR analysis was evaluated by Cochran's Q-test and I2 statistics (both P < 0.05 and I2 > 0.25) (45). The significant associations between tea intake and COVID-19 were determined by IVW-based P < 0.017 (0.05/3). Single-nucleotide polymorphisms (SNPs) with genome-wide significance (P < 5 × 10−8) in the tea intake dataset were selected as instrumental variables (IVs) and further pruned using a clumping r2 cutoff of 0.01 within a 10 Mb window. For each MR analysis, we removed SNPs not present in the outcome dataset and palindromic SNPs with intermediate allele frequencies. We harmonized each pair of exposure and outcome datasets by aligning the effect allele for exposure and outcome with the obtained variant effects and standard errors of each dataset.
We conducted the MR analyses in R (version 4.0.5) (46).
Results
MR analysis
In the MR analysis of the causal effects of tea intake on the COVID-19 outcomes, a total of 47 IVs were derived. Our MR-Egger analysis showed that genetic propensity for tea intake conferred a causal protective effect on susceptibility to SARS-CoV-2 infection [OR: 0.87, 95% confidence interval (CI): 0.78–0.97, P = 0.015]. However, no causal effects of tea intake on hospitalized COVID-19 (0.84, 0.64–1.10, P = 0.201) or critical COVID-19 (0.73, 0.51–1.03, P = 0.074) were detected (Table 1, Figure 1).
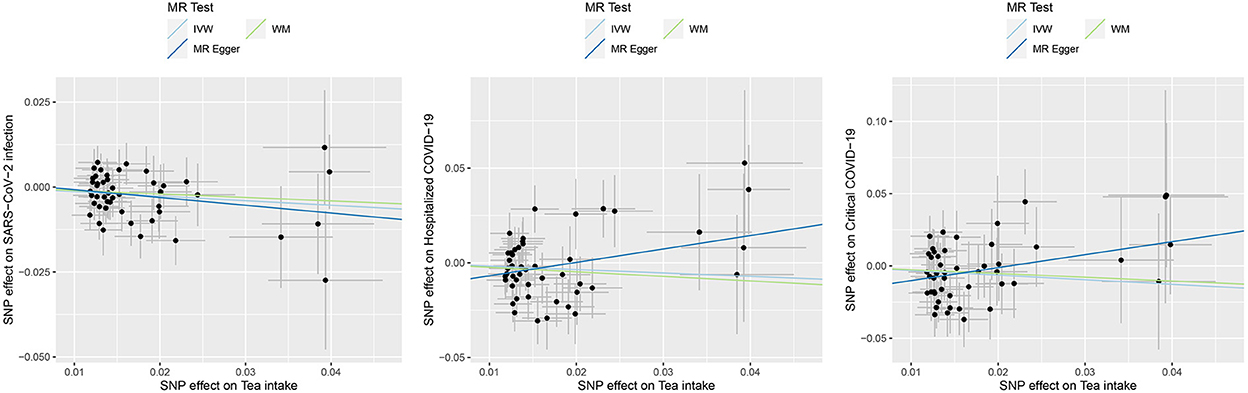
Figure 1. Causal effects of tea intake on the COVID-19 outcomes. Scatter plot for the SNP effect size estimate. Relationship between the SNP effect size estimate of tea intake (x-axis) and the corresponding effect size estimate of outcomes of COVID-19 (y-axis). The trait on the x-axis denotes exposure, the trait on the y-axis denotes outcome, and each cross point represents an instrumental variant. The dots and short lines through the dots denote the effect sizes (b) and the 95% CI of tea intake on the outcome of COVID-19. IVW, inverse variance weighted; WM, weighted median.
Sensitivity analysis
For the causal association between tea intake and SARS-CoV-2 infection, the directions of causal effect estimates across the three methods were largely the same. The effect sizes between tea intake and SARS-CoV-2 were 0.87 (0.78–0.97) for the IVW model, 0.90 (0.77–1.06) for the WM model, and 0.80 (0.53–1.19) for the MR-Egger model (Table 1, Figure 1). There was no evidence supporting the heterogeneity concerning the effects of habitual tea intake on SARS-CoV-2 infection critical COVID-19 (Cochran's P = 0.632, I2 < 0.25; Table 1, Figure 1).
The effect sizes between tea intake and hospitalized COVID-19 were 0.84 (0.64–1.10) for the IVW model, 0.79 (0.56–1.10) for the WM model, and 2.03 (0.75–5.51) for the MR-Egger model (Table 1, Figure 1). There was evidence suggesting heterogeneity of the causal association between tea intake and hospitalized COVID-19 (Cochran's P = 0.026, I2 = 0.308).
The effect sizes between tea intake and critical COVID-19 were 0.73 (0.51–1.03) for the IVW model, 0.77 (0.47–1.25) for the WM model, and 2.45 (0.70–8.60) for the MR-Egger model (Table 1, Figure 1). The heterogeneity analysis did not support the heterogeneity of the causal association (Cochran's P = 0.371, I2 = 0.052).
Notably, tests of MR-Egger regression did not support the directional pleiotropy of the genetic IVs for the MR analyses (MR-Egger intercept < 0.02, P > 0.05).
Discussion
Tea drinking is commonly regarded as a convenient and safe complementary therapy and healthy habit. Recent modeling studies have shown that various tea components are highly effective in blocking SARS-CoV-2 infection (24, 25, 47, 48). In addition, tea polyphenols might suppress the virus through their beneficial effects on intestinal microbiota (49). It has yet to be determined whether habitual tea consumption may exert an effect against COVID-19 in exposed populations.
Here, we provide convincing evidence for the potential influence of tea intake on the COVID-19 outcomes. In our study, a 1-SD increase in tea intake was associated with a 13% decreased risk for SARS-CoV-2 infection. The anti-COVID-19 effect of tea intake might be due to its inhibition of viral binding and/or enhancement of the human innate immune response (50). Green or black tea infusions are rich in bioactive nutrients: polyphenols, EGCG, theanine, theaflavin, alkaloids, caffeine, and its intermediates, theophylline, theobromine, and others, many of which have immunomodulatory or anti-inflammatory properties (51). Notably, the consumption of tea is safe for humans even at high levels, with adverse effects being rare (52). Our findings conclusively support the notion of possible protective effects of tea consumption against symptomatic COVID-19 (53, 54).
However, as our findings showed, routine tea intake was not associated with a decrease in the risks of COVID-19 hospitalization or critical COVID-19. These two severe outcomes are commonly due to a strong immunological response to the virus. It is necessary to explore whether the therapeutic effects of tea are related to its particular phytochemicals or a general habit of high tea intake. The full realization of the therapeutic potential of Camellia sinensis may require the development of advanced delivery systems for its molecular constituents.
Individual phytochemicals of C. sinensis should be studied as possible dietary supplements suitable for the time of pandemics. As the effectiveness of EGCG and other polyphenols in tea is limited due to their low oral bioavailability, further studies on the advanced delivery of individual tea constituents are warranted.
The main strength of the study was that MR analysis is less affected by the causality pitfalls, namely, the presence of confounding factors and reverse causation, which is commonly seen in traditionally designed observational studies. Here, the largest available GWAS summary datasets were utilized for tracing the causative association between COVID-19 and tea intake. Our study had several limitations. In particular, we assessed only genetic effects with no regard to the effects of the environment, which are critical for both tea intake and COVID-19. MR analyses might be biased due to pleiotropy, especially in non-homogenous datasets. To mitigate the latter, we tested the MR assumptions using various models.
Conclusion
In summary, our MR-based study revealed a causal protective effect of habitual tea intake on symptomatic SARS-CoV-2 infections but no protective effect against severe COVID-19. Our study clarifies that the protection provided by tea intake is related to building the resilience of the human body rather than to direct therapeutic correction of entrenched pathology. The uncovered protective effects should be dissected further to determine the influences of particular tea leaf constituents, including EGCG, and the behavioral or general health-related traits associated with tea consumption. Encouraging tea consumption might be a cost-effective natural measure for the prevention of COVID-19 transmission in humans.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
FZ conceived the project, supervised the study, and analyzed the data. FZ, WY, HC, YS, and AB wrote the manuscript. All authors read and approved the final manuscript.
Acknowledgments
The authors thank all investigators and participants from the COVID-19 Host Genetics Initiative and UKB for sharing these data.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Docherty AB, Harrison EM, Green CA, Hardwick HE, Pius R, Norman L, et al. Features of 20 133 UK patients in hospital with covid-19 using the ISARIC WHO clinical characterisation protocol: prospective observational cohort study. BMJ. (2020) 369:m1985. doi: 10.1136/bmj.m1985
2. Rao S, Baranova A, Cao H, Chen J, Zhang X, Zhang F. Genetic mechanisms of COVID-19 and its association with smoking and alcohol consumption. Brief Bioinform. (2021) 22:bbab284. doi: 10.1093/bib/bbab284
3. Cao H, Baranova A, Wei X, Wang C, Zhang F. Bidirectional causal associations between type 2 diabetes and COVID-19. J Med Virol. (2022). doi: 10.1002/jmv.28100. [Epub ahead of print].
4. Yang J, Tian C, Chen Y, Zhu C, Chi H, Li J. Obesity aggravates COVID-19: an updated systematic review and meta-analysis. J Med Virol. (2021) 93:2662–74. doi: 10.1002/jmv.26677
5. Baranova A, Song Y, Cao H, Zhang F. Causal associations between basal metabolic rate and COVID-19. Diabetes. (2022) 10:db220610. doi: 10.2337/db22-0610
6. Baranova A, Xu Y, Cao H, Zhang F. Associations between pulse rate and COVID-19. J Med Virol. (2022). doi: 10.1002/jmv.28194. [Epub ahead of print].
7. Baranova A, Cao H, Chen J, Zhang F. Causal association and shared genetics between asthma and COVID-19. Front Immunol. (2022) 13:705379. doi: 10.3389/fimmu.2022.705379
8. Zhu G, Zhou S, Xu Y, Gao R, Li H, Su W, et al. Mendelian randomization study on the causal effects of COVID-19 on childhood intelligence. J Med Virol. (2022) 94:3233–9. doi: 10.1002/jmv.27736
9. Zhang F, Baranova A. Smoking quantitatively increases risk for COVID-19. Eur Respir J. (2021) 2101273. doi: 10.1183/13993003.01273-2021. [Epub ahead of print].
10. Baranova A, Cao H, Teng S, Zhang F. A phenome-wide investigation of risk factors for severe COVID-19. J Med Virol. (2022) 95:e28264. doi: 10.1002/jmv.28264
11. Islam MK, Molla MMA, Hasan P, Sharif MM, Hossain FS, Amin MR, et al. Persistence of sleep disturbance among post-COVID patients: findings from a 2-month follow-up study in a Bangladeshi cohort. J Med Virol. (2022) 94:971–8. doi: 10.1002/jmv.27397
12. Azevedo MN, Rodrigues EDS, Passos E, Filho MAB, Barreto APA, Lima MCC, et al. Multimorbidity associated with anxiety symptomatology in post-COVID patients. Psychiatry Res. (2022) 309:114427. doi: 10.1016/j.psychres.2022.114427
13. Arjun MC, Singh AK, Roy P, Ravichandran M, Mandal S, Pal D, et al. Long COVID following omicron wave in eastern India-A retrospective cohort study. J Med Virol. (2022). doi: 10.1002/jmv.28214. [Epub ahead of print].
14. Chen JI, Hickok A, O'Neill AC, Niederhausen M, Laliberte AZ, Govier DJ, et al. Psychiatric disorders newly diagnosed among veterans subsequent to hospitalization for COVID-19. Psychiatry Res. (2022) 312:114570. doi: 10.1016/j.psychres.2022.114570
15. Asadi-Pooya AA, Akbari A, Emami A, Lotfi M, Rostamihosseinkhani M, Nemati H, et al. Long COVID syndrome-associated brain fog. J Med Virol. (2022) 94:979–84. doi: 10.1002/jmv.27404
16. Saied EM, El-Maradny YA, Osman AA, Darwish AMG, Abo Nahas HH, Niedbała G, et al. A comprehensive review about the molecular structure of severe acute respiratory syndrome Coronavirus 2 (SARS-CoV-2): insights into natural products against COVID-19. Pharmaceutics. (2021) 13:1759. doi: 10.3390/pharmaceutics13111759
17. Tang GY, Meng X, Gan RY, Zhao CN, Liu Q, Feng YB, et al. Health functions and related molecular mechanisms of tea components: an update review. Int J Mol Sci. (2019) 20:6196. doi: 10.3390/ijms20246196
18. Pan SY, Nie Q, Tai HC, Song XL, Tong YF, Zhang LJ, et al. Tea and tea drinking: China's outstanding contributions to the mankind. Chin Med. (2022) 17:27. doi: 10.1186/s13020-022-00571-1
19. Alam M, Ali S, Ashraf GM, Bilgrami AL, Yadav DK, Hassan MI. Epigallocatechin 3-gallate: from green tea to cancer therapeutics. Food Chem. (2022) 379:132135. doi: 10.1016/j.foodchem.2022.132135
20. Zhang Y, Yang H, Li S, Li WD, Wang Y. Consumption of coffee and tea and risk of developing stroke, dementia, and poststroke dementia: a cohort study in the UK Biobank. PLoS Med. (2021) 18:e1003830. doi: 10.1371/journal.pmed.1003830
21. Xu XY, Zhao CN, Li BY, Tang GY, Shang A, Gan RY, et al. Effects and mechanisms of tea on obesity. Crit Rev Food Sci Nutr. (2021) 1–18. doi: 10.1080/10408398.2021.1992748. [Epub ahead of print].
22. Gao N, Ni M, Song J, Kong M, Wei D, Dong A. Causal relationship between tea intake and cardiovascular diseases: a Mendelian randomization study. Front Nutr. (2022) 9:938201. doi: 10.3389/fnut.2022.938201
23. Chourasia M, Koppula PR, Battu A, Ouseph MM, Singh AK. EGCG, a green tea catechin, as a potential therapeutic agent for symptomatic and asymptomatic SARS-CoV-2 infection. Molecules. (2021) 26:1200. doi: 10.3390/molecules26051200
24. Liu J, Bodnar BH, Meng F, Khan AI, Wang X, Saribas S, et al. Epigallocatechin gallate from green tea effectively blocks infection of SARS-CoV-2 and new variants by inhibiting spike binding to ACE2 receptor. Cell Biosci. (2021) 11:168. doi: 10.1186/s13578-021-00680-8
25. Park R, Jang M, Park YI, Park Y, Jung W, Park J, et al. Epigallocatechin gallate (EGCG), a green tea polyphenol, reduces coronavirus replication in a mouse model. Viruses. (2021) 13:2533. doi: 10.3390/v13122533
26. Bhardwaj VK, Singh R, Sharma J, Rajendran V, Purohit R, Kumar S. Identification of bioactive molecules from tea plant as SARS-CoV-2 main protease inhibitors. J Biomol Struct Dyn. (2021) 39:3449–58. doi: 10.1080/07391102.2020.1766572
27. Sharma J, Kumar Bhardwaj V, Singh R, Rajendran V, Purohit R, Kumar S. An in-silico evaluation of different bioactive molecules of tea for their inhibition potency against non structural protein-15 of SARS-CoV-2. Food Chem. (2021) 346:128933. doi: 10.1016/j.foodchem.2020.128933
28. Singh R, Bhardwaj VK, Sharma J, Purohit R, Kumar S. In-silico evaluation of bioactive compounds from tea as potential SARS-CoV-2 nonstructural protein 16 inhibitors. J Tradit Complement Med. (2022) 12:35–43. doi: 10.1016/j.jtcme.2021.05.005
29. Bhardwaj VK, Singh R, Sharma J, Rajendran V, Purohit R, Kumar S. Bioactive molecules of tea as potential inhibitors for RNA-dependent RNA polymerase of SARS-CoV-2. Front Med. (2021) 8:684020. doi: 10.3389/fmed.2021.684020
30. Ngwe Tun MM, Luvai E, Nwe KM, Toume K, Mizukami S, Hirayama K, et al. Anti-SARS-CoV-2 activity of various PET-bottled Japanese green teas and tea compounds in vitro. Arch Virol. (2022) 167:1547–57. doi: 10.1007/s00705-022-05483-x
31. Henss L, Auste A, Schürmann C, Schmidt C, von Rhein C, Mühlebach MD, et al. The green tea catechin epigallocatechin gallate inhibits SARS-CoV-2 infection. J Gen Virol. (2021) 102:001574. doi: 10.1099/jgv.0.001574
32. Mokra D, Adamcakova J, Mokry J. Green tea polyphenol (-)-epigallocatechin-3-gallate (EGCG): a time for a new player in the treatment of respiratory diseases? Antioxidants. (2022) 11:1566. doi: 10.3390/antiox11081566
33. Dekant W, Fujii K, Shibata E, Morita O, Shimotoyodome A. Safety assessment of green tea based beverages and dried green tea extracts as nutritional supplements. Toxicol Lett. (2017) 277:104–8. doi: 10.1016/j.toxlet.2017.06.008
34. Lawlor DA, Harbord RM, Sterne JA, Timpson N, Davey Smith G. Mendelian randomization: using genes as instruments for making causal inferences in epidemiology. Stat Med. (2008) 27:1133–63. doi: 10.1002/sim.3034
35. Zhang F, Rao S, Cao H, Zhang X, Wang Q, Xu Y, et al. Genetic evidence suggests posttraumatic stress disorder as a subtype of major depressive disorder. J Clin Invest. (2022) 132:e145942. doi: 10.1172/JCI145942
36. Rao S, Baranova A, Yao Y, Wang J, Zhang F. Genetic relationships between attention-deficit/hyperactivity disorder, autism spectrum disorder, and intelligence. Neuropsychobiology. (2022) 1–13. doi: 10.1159/000525411. [Epub ahead of print].
37. Baranova A, Wang J, Cao H, Chen JH, Chen J, Chen M, et al. Shared genetics between autism spectrum disorder and attention-deficit/hyperactivity disorder and their association with extraversion. Psychiatry Res. (2022) 314:114679. doi: 10.1016/j.psychres.2022.114679
38. Zhang F, Cao H, Baranova A. Genetic variation mediating neuroticism's influence on cardiovascular diseases. J Psychopathol Clin Sci. (2022) 131:278–86. doi: 10.1037/abn0000744
39. Zhang F, Rao S, Baranova A. Shared genetic liability between major depressive disorder and osteoarthritis. Bone Joint Res. (2022) 11:12–22. doi: 10.1302/2046-3758.111.BJR-2021-0277.R1
40. Initiative C-HG. The COVID-19 host genetics initiative, a global initiative to elucidate the role of host genetic factors in susceptibility and severity of the SARS-CoV-2 virus pandemic. Eur J Hum Genet. (2020) 28:715–8. doi: 10.1038/s41431-020-0636-6
41. Bycroft C, Freeman C, Petkova D, Band G, Elliott LT, Sharp K, et al. The UK Biobank resource with deep phenotyping and genomic data. Nature. (2018) 562:203–9. doi: 10.1038/s41586-018-0579-z
42. Jiang L, Zheng Z, Qi T, Kemper KE, Wray NR, Visscher PM, et al. A resource-efficient tool for mixed model association analysis of large-scale data. Nat Genet. (2019) 51:1749–55. doi: 10.1038/s41588-019-0530-8
43. Hemani G, Zheng J, Elsworth B, Wade KH, Haberland V, Baird D, et al. The MR-Base platform supports systematic causal inference across the human phenome. Elife. (2018) 7:e34408. doi: 10.7554/eLife.34408
44. Bowden J, Davey Smith G, Burgess S. Mendelian randomization with invalid instruments: effect estimation and bias detection through egger regression. Int J Epidemiol. (2015) 44:512–25. doi: 10.1093/ije/dyv080
45. Bowden J, Del Greco MF, Minelli C, Zhao Q, Lawlor DA, Sheehan NA, et al. Improving the accuracy of two-sample summary-data Mendelian randomization: moving beyond the NOME assumption. Int J Epidemiol. (2019) 48:728–42. doi: 10.1093/ije/dyy258
46. R Core Team. R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing (2020).
47. Wang L, Tao Q, Wang Z, Shi J, Yan W, Zhang L, et al. Tea ingredients have anti-coronavirus disease 2019 (COVID-19) targets based on bioinformatics analyses and pharmacological effects on LPS-stimulated macrophages. Front Nutr. (2022) 9:875765. doi: 10.3389/fnut.2022.875765
48. Ishimoto K, Hatanaka N, Otani S, Maeda S, Xu B, Yasugi M, et al. Tea crude extracts effectively inactivate severe acute respiratory syndrome coronavirus 2. Lett Appl Microbiol. (2022) 74:2–7. doi: 10.1111/lam.13591
49. Xiang Q, Cheng L, Zhang R, Liu Y, Wu Z, Zhang X. Tea polyphenols prevent and intervene in COVID-19 through intestinal microbiota. Foods. (2022) 11:506. doi: 10.3390/foods11040506
50. McKay DL, Blumberg JB. The role of tea in human health: an update. J Am Coll Nutr. (2002) 21:1–13. doi: 10.1080/07315724.2002.10719187
51. Chowdhury P, Barooah AK. Tea bioactive modulate innate immunity: in perception to COVID-19 pandemic. Front Immunol. (2020) 11:590716. doi: 10.3389/fimmu.2020.590716
52. Hu J, Webster D, Cao J, Shao A. The safety of green tea and green tea extract consumption in adults - results of a systematic review. Regul Toxicol Pharmacol. (2018) 95:412–33. doi: 10.1016/j.yrtph.2018.03.019
53. Zhong R, Zhang Q, Qiu Y, Chen L, Xie J, Chen Y, et al. Results of the adult COVID-19 lifestyle matching study. Int J Public Health. (2022) 67:1604329. doi: 10.3389/ijph.2022.1604329
Keywords: COVID-19, tea intake, Mendelian randomization, genetics, Camellia sinensis
Citation: Baranova A, Song Y, Cao H, Yue W and Zhang F (2023) Causal associations of tea intake with COVID-19 infection and severity. Front. Nutr. 9:1005466. doi: 10.3389/fnut.2022.1005466
Received: 28 July 2022; Accepted: 18 November 2022;
Published: 04 January 2023.
Edited by:
Agata Chmurzynska, Poznan University of Life Sciences, PolandReviewed by:
Rahul Singh, Academy of Scientific and Innovative Research (AcSIR), IndiaRituraj Purohit, Institute of Himalayan Bioresource Technology (CSIR), India
Copyright © 2023 Baranova, Song, Cao, Yue and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fuquan Zhang,  emhhbmdmcUBuam11LmVkdS5jbg==
emhhbmdmcUBuam11LmVkdS5jbg==
†These authors have contributed equally to this work and share first authorship
 Ancha Baranova
Ancha Baranova Yuqing Song3,4†
Yuqing Song3,4† Hongbao Cao
Hongbao Cao Weihua Yue
Weihua Yue Fuquan Zhang
Fuquan Zhang