- 1Department of Clinical Epidemiology, Shengjing Hospital of China Medical University, Shenyang, China
- 2Clinical Research Center, Shengjing Hospital of China Medical University, Shenyang, China
- 3Key Laboratory of Precision Medical Research on Major Chronic Disease, Shengjing Hospital of China Medical University, Shenyang, China
- 4Department of Obstetrics and Gynecology, Shengjing Hospital of China Medical University, Shenyang, China
Background: The impact of dietary trace elements intake on ovarian cancer (OC) severity is unknown.
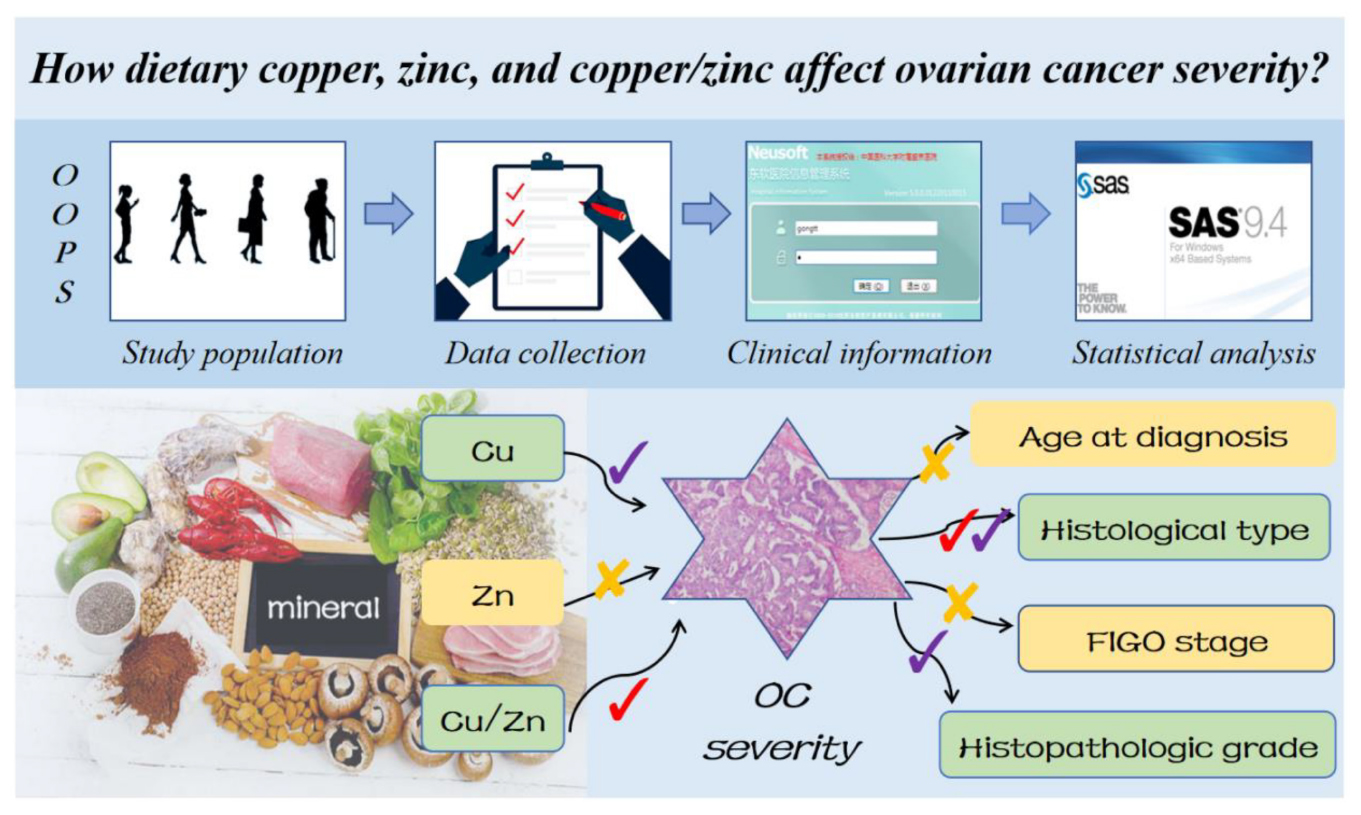
Objective: We firstly explore the relationship between dietary copper (Cu), zinc (Zn), and copper-to-zinc (Cu/Zn) ratio and severity of OC.
Methods: This cross-sectional study included 701 women from the OC follow-up study between 2015 and 2020. Dietary information was collected by a validated food frequency questionnaire (FFQ). The severity information of OC including age at diagnosis, histological type, International Federation of Gynecology and Obstetrics (FIGO) stage, and histopathologic grade was ascertained from medical records. Logistic regression model was used to estimate the odds ratios (ORs) and 95% confidence intervals (CIs) of aforementioned associations.
Results: Among 701 participants, the number of patients age at diagnosis older than 50 were 443 (63.2%). The number of patients diagnosed as serous, III–IV stage, and poorly differentiation OC were 477 (68.05%), 336 (47.93%), and 597 (85.16%), respectively. In addition, compared with the lowest tertile intake, higher possibility of non-serous OC was associated with the pre-diagnosis dietary Cu (OR = 2.39, 95% CI = 1.28–4.47, p trend < 0.05) and Cu/Zn ratio (OR = 2.06, 95% CI = 1.26–3.39, P trend < 0.05) in the highest tertile intake. The risk of poorly differentiation OC at diagnosis was significant inversely related to dietary Cu intake (OR = 0.40, 95% CI = 0.18–0.88, P trend < 0.05). Besides, the results of subgroup analyses were consistent with the main findings but not all of them showed statistical significance.
Conclusion: Pre-diagnostic dietary Cu and Cu/Zn ratio were contributed to reducing the severity of OC at diagnosis, especially for the risk of serous OC and poorly differentiation OC.
Introduction
Ovarian Cancer (OC) ranks as a leading barrier to increasing the life expectancy of women worldwide (1). According to the latest statistics in 2020, about 314 thousand women were diagnosed with OC and 207 thousand women died of this disease in 185 countries (1). In China, the increasing incidence and mortality rate make OC the third most common gynecological malignancy (2). Owing to its unfavorable anatomy and a lack of effective screening strategies, OC is often diagnosed at an advanced stage (3). Specifically, most serous OC cases were diagnosed at stage III or IV with a survival rate lower than 43%, while the survival rate of non-serous OC was above 66% (4). Similarly, women who died of OC were older on average, and more likely to be diagnosed with serous, stage III-IV tumors (5). Therefore, age at diagnosis, histological type, International Federation of Gynecology and Obstetrics (FIGO) stage, and histopathologic grade are important factors reflecting the severity and prognosis of OC (6–10).
Dietary, as a key modifiable risk factor, can be used to formulate recommendations for the prevention of cancers, including OC (11–13). High-quality diets were inversely associated with the risk and development of OC (14, 15). Therefore, exploring relevant nutritional factors and looking for effective interventions are crucial to reducing the severity of OC. A growing body of evidence indicated that copper (Cu) and zinc (Zn) played vital roles in a series of anticancer processes that proceed through various mechanisms (16, 17). Cu serves as a limiting factor for multiple aspects of tumor progression, including growth, angiogenesis, and metastasis (18, 19). Zn is required for the catalytic activity of more than 200 enzymes, which are essential in immune function, cell divisions, and anti-tumor actions (20, 21). Besides, Caglayan et al. revealed that Cu and Zn-superoxide dismutase might protect cells against the biological damage of oxidative stress induced by reactive oxygen species (22). To date, it has been shown that serum Cu, Zn level, and copper-to-zinc (Cu/Zn) ratio can be used as diagnosis and prognostic indicators for some cancers (23–25). Of these, a case-control study in northeast China suggested that the serum Cu level and the Cu/Zn ratio were effective predictive indicators of lung cancer and might help to evaluate the prognosis of patients with non-small cell lung cancer (23). In the development of colorectal cancer, Stepien et al. (24) suggested that Cu level in relation to Zn (Cu/Zn ratio) became imbalanced in a cohort study. Furthermore, another cohort proved that higher serum Cu and Cu/Zn ratio might be associated with worse hepatocellular carcinoma survival (25). Kazi et al. (26) showed that Cu isotopes and plasma trace elements might serve as suitable biomarkers of thyroid cancer diagnosis. Of note, one meta-analysis and Mendelian randomization study showed that lower circulating Zn might be causally associated with higher OC risk, while no causal effect of circulating Cu on OC risk was found (27).
Currently, no previous study has explored the associations between dietary Cu, Zn, and Cu/Zn ratio and OC severity. Therefore, to firstly elucidate this topic, we carried out this study based on the data from the ovarian cancer follow-up study (OOPS) in 701 OC patients.
Materials and methods
Study design and population
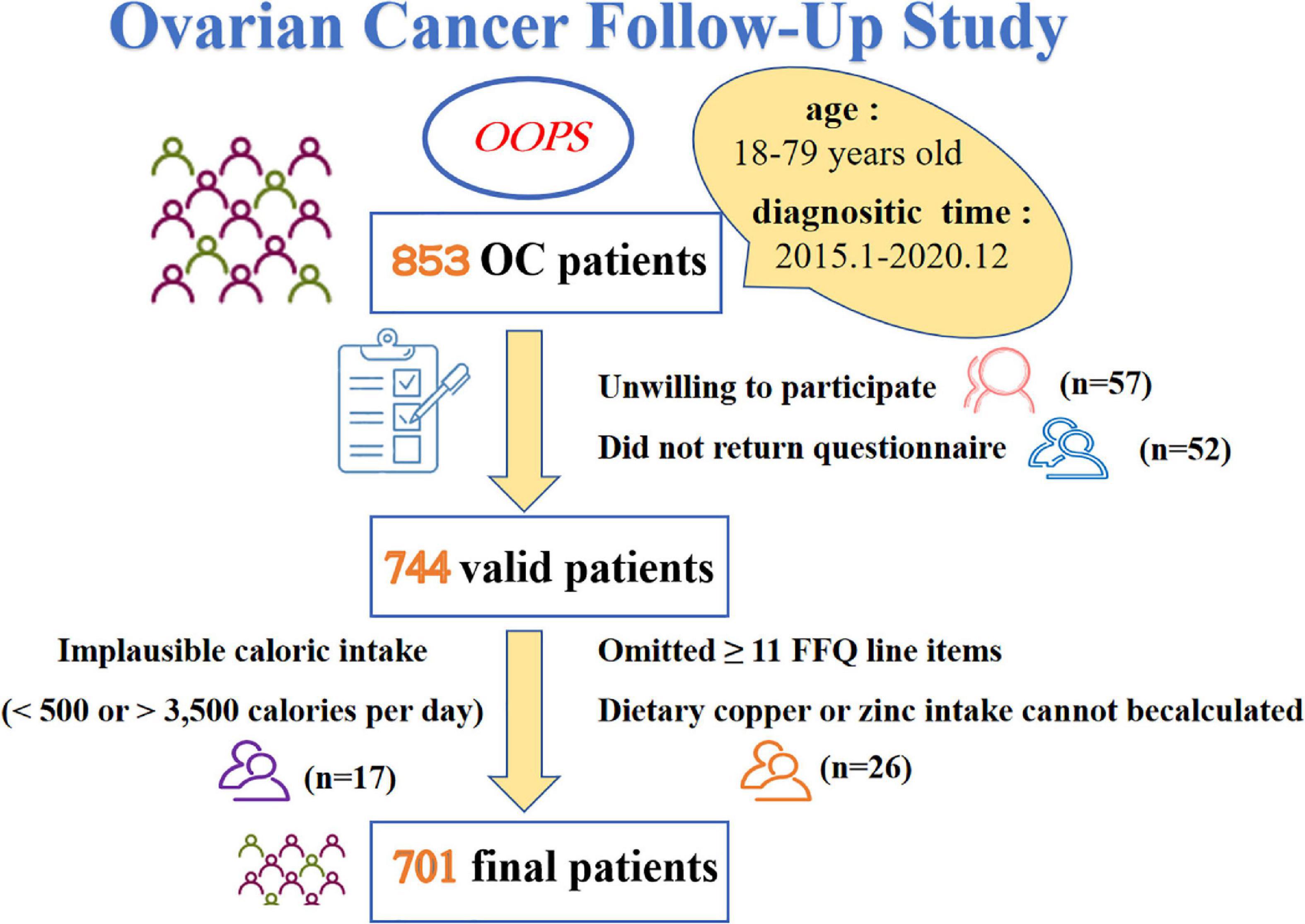
Patients included in this cross-sectional study were acquired from the OOPS (28), which is a prospective longitudinal cohort study of patients newly diagnosed with OC. Being approved by the Institutional Review Board of the Ethics Committee of Shengjing Hospital of China Medical University, Shenyang, China (2015PS38K), the OOPS was devoted to collecting clinical and lifestyle information and exploring their associations with cancer-related outcomes (29–34). Patients recruited during the baseline survey were histologically confirmed OC diagnosis, with age between 18 and 79 years old. We have diagnosed 853 patients with OC from January 2015 to December 2020, but only 796 women (93%) consented to participate and 744 (87%) women returned the completed study questionnaire. In addition, we excluded patients who were ineligible for analysis, such as implausible caloric intake was reported (< 500 or > 3,500 calories per day, n = 17) (35, 36), greater than or equal to 11 (10%) food frequency questionnaire (FFQ) line items were omitted (n = 24). Considering the necessity of Cu and Zn content for this study, we further excluded those whose dietary Cu or Zn intake cannot be calculated (n = 2). Finally, a total of 701 (82%) women contributed to the analyses (Figure 1).
Data collection
Self-administered questionnaires were applied for collecting all related characteristics of one year before diagnosis, such as basic personal data, dietary features, and sleep status. Anthropometrics including weight and height were measured at baseline, which were used to calculate body mass index (BMI). Besides, clinical information concerning the age at diagnosis, histological type, FIGO stage, histopathologic grade, and comorbidities was collected from the electronic medical records of the Shengjing hospital information system. After the patient finished the baseline survey, clinical specialists extracted patient medical data from the information system at Shengjing Hospital every 6 months.
Dietary exposure assessment
Our FFQ consisting of 111 items was used to assess dietary intake, which has been verified to have reasonable reliability and validity (29–31). The reproducibility coefficients (Spearman correlation coefficients and intraclass correlation coefficients) were above 0.5 for most food groups, and correlation coefficients (Spearman correlation coefficients) were between 0.3 and 0.7 for most food groups between the FFQ and weighed dietary records. Patients were required to recall their daily intake of these food items during the year before diagnosis. The dietary intake was assessed in times, including more than two times per day, one or two times per day, four to six times per week, two or three times per week, one time per week, two or three times per month, and almost never. To gather complete data on these items, face-to-face examinations on the self-administered questionnaires were carried out by well-trained researchers. Based on the Chinese Food Composition Tables (37), our nutrient intake was calculated by multiplying the frequency of consumption of each food by the nutrient content of the specified portions. Ultimately, we decided to select dietary Cu, Zn, and Cu/Zn ratio as our exposure to explore.
Outcome selection
Age at diagnosis, histological type, FIGO stage, and histopathologic grade are important factors reflecting the severity and prognosis of OC (6–10). Our primary outcome was the severity of OC, defined as a composite of some typical clinical characteristics containing age at diagnosis (< 50 or ≥ 50 years), histological type (serous or non-serous), FIGO stage (I – II or III – IV) serous or ical clintopathologic grade (well, moderately, and poorly differentiated) as our study outcome.
Statistical analysis
Baseline characteristics were described according to the tertiles of dietary Cu, Zn, and Cu/Zn ratio intake, where the lowest tertile served as the reference group. Descriptive statistics were performed to observe and distinguish individual features among groups. Continuous variables were described by the mean ± standard deviation, and categorical variables were exhibited using counts and percentages. Differences in general characteristics among groups were assessed with the one-way ANOVA or the Kruskal-Wallis test, except for categorical variables, which were assessed with the χ2-test. Logistic regression analyses were used to calculate the odds ratios (ORs) and 95% confidence intervals (CIs) for the association of dietary Cu, Zn, and Cu/Zn ratio intake with the severity of OC. The linear trend of cross-increasing tertiles was tested using the median value of each tertile as a continuous variable based on logistic regression.
The selection of covariates for the multivariable model was based on the degree of correlation with the exposure, clinical significance, and similar articles (25, 38, 39). Model 1 was adjusted for age at diagnosis (< 50 and ≥ 50 years), BMI (continuous, kg/m2), education (junior secondary or below, senior high school or technical secondary school, and junior college or university or above), income (< 5,000, 5,000– < 10,000, and ≥ 10,000), smoking status (yes or no), drinking status (yes or no), menarche age (≤ 16 and > 16), menopausal status (yes or no), physical activity (continuous, metabolic equivalent task/hours/days), and parity (≤ 1 and ≥ 2). We also applied a model adjustment for total energy intake (continuous, kcal), comorbidities (yes or no), diet change (yes or no), dietary protein intake (continuous, g/day), and dietary fiber intake (continuous, g/day) based on model 1. In the third model, dietary calcium intake (continuous, mg/day), and dietary iron intake (continuous, mg/day) were further adjusted based on model 2. Multivariate logistic regression collinearity diagnostic analysis was performed for adjustment models and no collinearity between variables was found.
In addition, we calculated adjusted OR and 95%CI in subgroup analyses and interaction analyses stratified by menopausal status (“no” compared to “yes”), BMI (< 25 compared to ≥ 25 kg/m2), and comorbidities status (“no” compared to “yes”). Finally, a restricted cubic spline model was applied to examine whether there was a non-linear relationship between exposures and outcomes. All statistical analyses were performed using SAS Version 9.4 (SAS Institute, Cary, NC, USA). All P-values were two-tailed and the difference was defined to be significant when P < 0.05.
Results
Table 1 presented the basic characteristics of the 701 OC patients by tertiles of dietary Cu and Zn intake. Among all patients, about 71.90% gave birth less than or equal to one time, while only 28.10% gave birth more than once. Besides, there were 59.92% patients had an average monthly income of lower than 5,000 yuan, 27.53% patients had an average monthly income of 5,000–10,000 yuan, and only 12.55% patients had an average monthly income of over than 10,000 yuan. The distribution of parity and income significantly differed by tertiles of dietary Cu and Zn, respectively (all P < 0.05). Moreover, socio-demographic and lifestyle status of 701 patients were described in Table 2, on the basic of tertiles of dietary Cu/Zn ratio. In summary, OC patients who had a higher intake of Cu, Zn, or Cu/Zn ratio tended to have higher total energy, dietary protein, fiber, calcium, and iron intake (all P < 0.05).
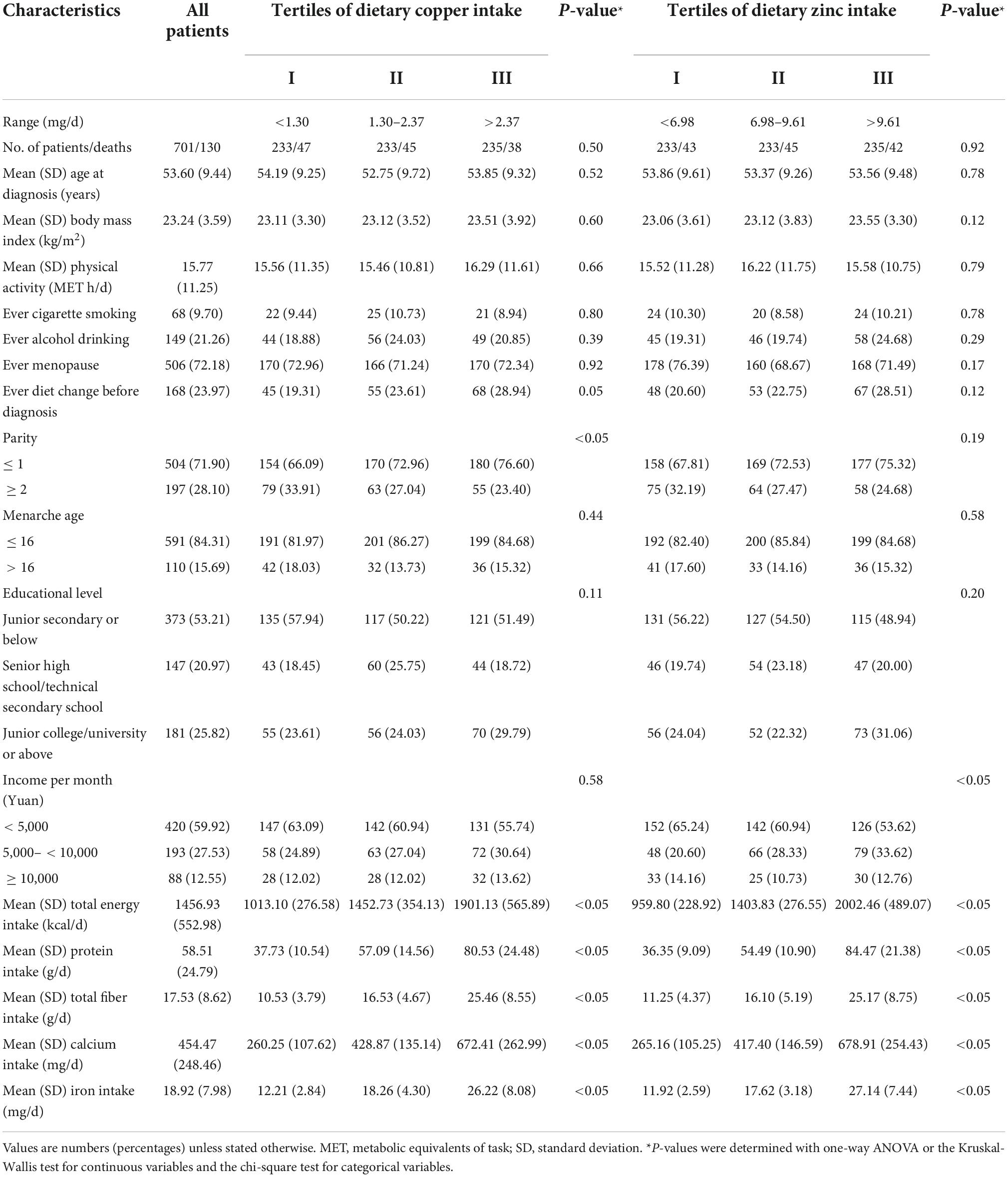
Table 1. Baseline characteristics of ovarian cancer patients by tertiles of dietary copper and zinc intake (N = 701).
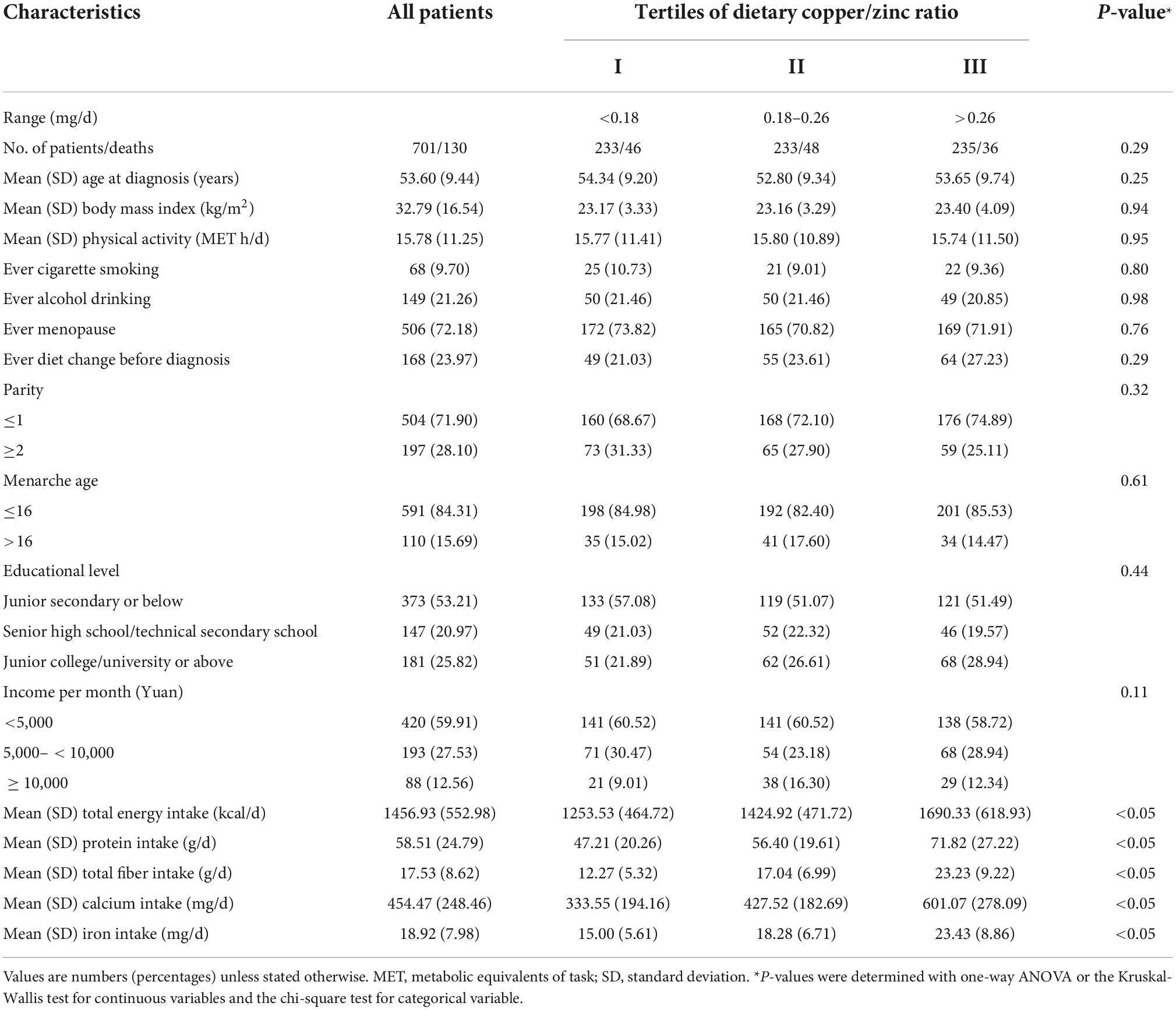
Table 2. Baseline characteristics of ovarian cancer patients by tertiles of dietary copper/zinc ratio (N = 701).
After controlling for potential confounders, we displayed the associations between dietary Cu, Zn, and Cu/Zn ratio and selected clinical outcomes in Table 3. Higher intake of dietary Cu was associated with a higher possibility to be diagnosed as non-serous OC, a linear trend was also evident (OR = 2.39, 95%CI: 1.28–4.47, P trend < 0.05). Besides, each 1-SD increment in dietary Cu was significantly associated with an increased non-serous OC incidence (OR = 1.33, 95%CI: 1.00–1.76). In addition, we observed that the association between dietary Cu/Zn ratio intake and non-serous OC were positive among different groups by tertiles (OR = 2.06, 95%CI: 1.26–3.39, P trend < 0.05). However, compared with the lowest tertile of dietary Cu, the risk of poorly differentiated OC was reduced in the patients with the highest tertile intake (OR = 0.40, 95%CI: 0.18–0.88, P trend < 0.05). Though we observe dietary Zn produced a borderline effect on histopathologic grade (OR = 0.39, 95%CI: 0.15–1.01, P trend = 0.05), each 1-SD increment in dietary Zn was significant inversely associated with the risk of poorly differentiated OC (OR = 0.44, 95%CI: 0.21–0.91). Furthermore, we did not find significant associations between dietary Cu, Zn, and Cu/Zn ratio levels and age at diagnosis or FIGO stage.
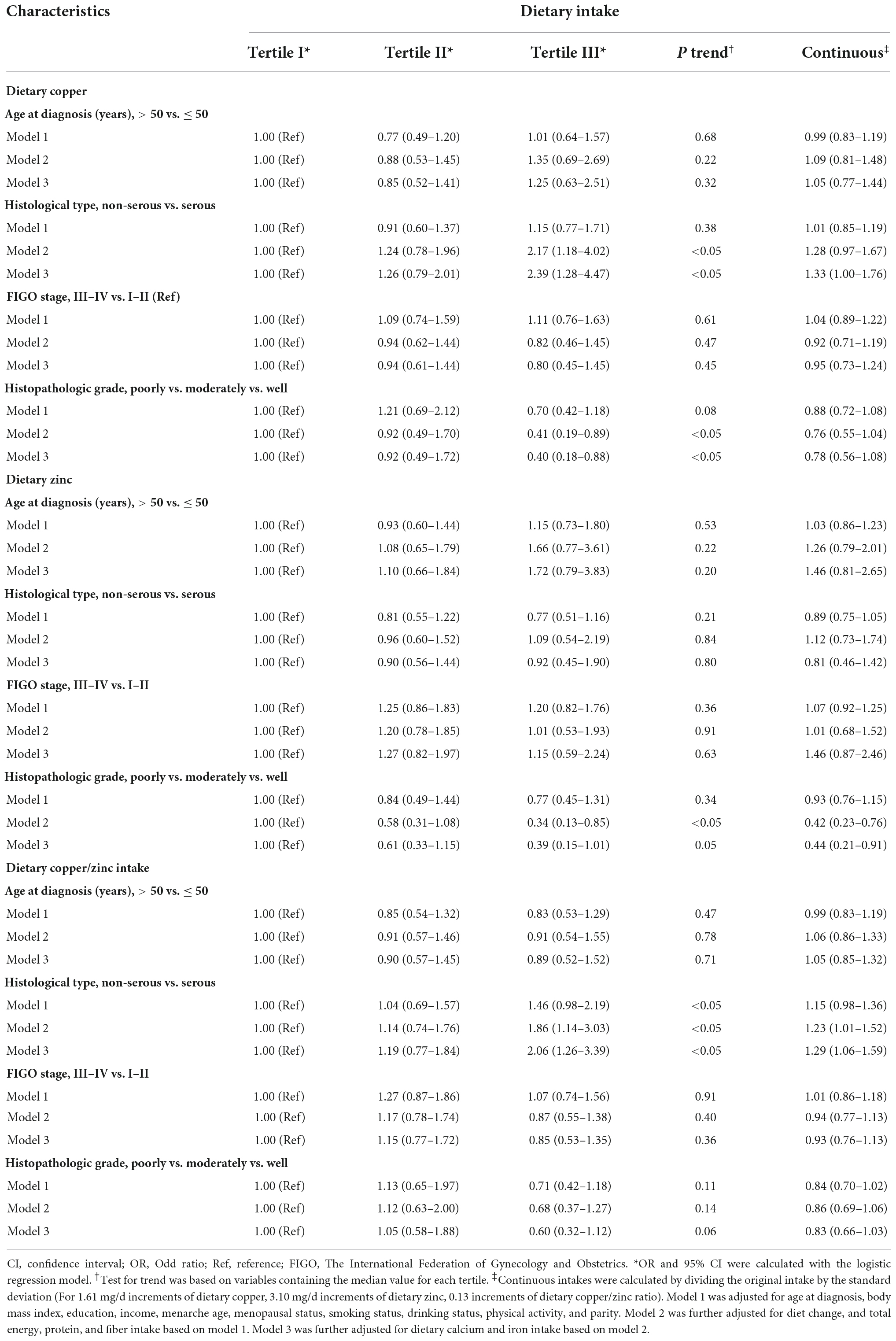
Table 3. Odds ratios (ORs) and 95% confidence intervals (CIs) for the association between dietary copper, zinc, and copper/zinc ratio intake and selected clinical characteristics among 701 ovarian cancer patients.
The relationships between dietary Cu, Zn, and Cu/Zn ratio intake and the severity of OC were evaluated across potential effect modifying variables. No significant interactions were found in the subgroup analyses stratified by menopausal status, BMI, and comorbidity (Supplementary Tables 1–4). The direction of these results was mainly consistent with the main findings but not all of them showed statistical significance. Of note, in menopausal or no comorbidity patients, the lowest serous OC risk was associated with the highest dietary Cu or Cu/Zn ratio intake (Supplementary Table 1). Additionally, OC patients with a higher intake of dietary Cu or Zn were more likely to be diagnosed as well-differentiated OC in the subgroup of BMI < 25 kg/m2 and comorbidities (Supplementary Table 2). Besides, we did not find the non-linear relationships between dietary Cu, Zn, and Cu/Zn ratio intake and the severity of OC.
Discussion
The present study with 701 OC patients reports, to our knowledge, the first one to study pre-diagnostic dietary Cu, Zn, and Cu/Zn and severity of OC to date. Our findings showed that higher dietary Cu and Cu/Zn ratio intake were related to patients diagnosed as non-serous OC, which means more diverse treatment options and longer survival time. This situation is more obvious in menopausal or no comorbidity patients. Moreover, in the women of BMI < 25 kg/m2 and comorbidities, high dietary Cu and Zn intake were accompanied by the emergence of well-differentiated OC with a lower severity. These findings were consistent with some studies (24, 26, 38, 40, 41) but different from others (23, 39, 42, 43).
Comparison with other studies
Up to now, no prior study aimed to explore the association between pre-diagnosis intake of dietary Cu, Zn, and Cu/Zn ratio and the severity of OC. Additionally, epidemiological evidence of other tumors has been limited and inconsistent (23, 24, 26, 38–43). On the one hand, our findings on the Cu and Cu/Zn ratio were consistent with the results from Atakul et al. (38) but contrary to others (23, 39, 42, 43). For example, a cross-sectional study suggested that endometrial cancer patients exhibited lower serum Cu, Zn, and Cu/Zn ratio when compared with controls (38). However, a case-control study with 146 patients and 146 controls suggested that increased serum Cu level and Cu/Zn ratio might improve the prognosis of patients with non-small cell lung cancer (23). The findings from a cohort study in the US proved that serum Cu level and Cu/Zn ratio had negative effects on all-cause death risk in lung cancer patients (39). Furthermore, a case-control study indicated that higher serum Cu levels and elevated Cu/Zn ratio might serve as biomarkers for the increased severity of viral hepatic damage, which may progress into more serious pathological outcomes and hepatocellular carcinoma eventually (42). Besides, Li et al. (43) revealed that each 1-SD increase in ln-transformed plasma copper were significantly associated with increased cancer incidence. The reasons for inconsistency may be the diversity in sample size, study population, especially for study outcomes. On the other hand, our findings of Zn from subgroup analysis are in line with previous studies (24, 26, 40, 41). For example, two multicenter prospective cohort studies from Europe presented that a higher circulating concentration of Zn was inversely related to the risk of hepatocellular carcinoma and colorectal cancer (24, 40). Kazi et al. (26) showed that plasma concentrations of Zn significantly lower in thyroid cancer patients (n = 46) as compared to healthy controls (n = 50). A cross-sectional study in Nigeria revealed that low plasma Zn status was associated with severe grade and advanced prostate cancer (41).
Potential biological mechanisms
Oxidative stress and trace elements have been established to be associated with cancer development (19, 21, 44). The role of Cu or Zn superoxide dismutase, whose functions are dependent on the adequate presence of both Cu and Zn, is to catalyze reactive oxygen species degradation originating from metabolic pathways to less reactive compounds, which are then removed by other antioxidant enzymes such as reduced glutathione peroxidase, thus reducing oxidative stress (22). In addition, cuproplasia is proved to be linked to a diverse array of cellular processes, including antioxidant defense, redox signaling, and autophagy (45, 46). Besides, Cu and Zn may control cancer development by maintaining normal cell cycle progression, inhibiting proliferation, tumor invasion, and inflammation (47). For example, an increased Cu requirement by prostate cancer cells because the presence of a mutant Cu-transporting Atp7b protein may change Cu-integration in serum and cause a remarkable reduction in prostate cancer burden and disease severity, abrogating adenocarcinoma development (48). Several studies showed that dysregulation of Zn transporters was implicated in the progression of cancer (49, 50). Furthermore, some researchers indicated Zn deficiency resulted in oxidative DNA damage, which means an inverse association between Zn levels and the risk of developing breast cancer (51). Consequently, the positive associations of dietary Cu, Zn, and Cu/Zn and the severity of OC are biologically plausible.
Strengthens and limitations
Our study had strengths that are worth mentioning. The innovation is the principal strength because this is the first study to explore the association between pre-diagnostic dietary Cu, Zn, and Cu/Zn ratio intake on the severity of OC. In addition, as a cross-sectional study with a face-to-face examination of the self-administered questionnaire by well-trained researchers, the integrity and authenticity of the data are well guaranteed. Furthermore, we strictly selected and controlled the confounding factors to reduce the confounding bias.
Nevertheless, several potential limitations also deserve our attention. First, recall bias is inevitable because diet information was self-reported at baseline. However, well-trained investigators, as well as a validated FFQ, were utilized to reduce the deviation. Second, dietary Cu and Zn intake were calculated by multiplying the frequency of consumption of each food by the nutrient content of the specified portions while their dietary supplements intake of them was not included. Data from the 2010 to 2012 China Nutrition and Health Surveillance (52), a nationally representative cross-sectional study covering all 31 provinces, autonomous regions, and municipalities in China, showed that only 0.11% of the Chinese population reported using Cu supplements. About Zn, the content in dietary supplements before diagnosis had no effect on the risk and development of OC (32). Therefore, we speculated that our results might not be materially altered by the dietary supplements used. Third, we focused on the influence of diet status 1 year before diagnosis on the OC severity, but we ignored the impact of diet on disease might be a long-term process. Therefore, we adjusted the dietary change during the analysis to reduce the bias. In addition, our cross-sectional study was carried out based on the OOPS from the Shengjing Hospital of China Medical University, which is a representative hospital in the northeast region, However, the current study is a hospital-based and single-center study, selection bias may be introduced and thus generalizability of the results is largely compromised. Furthermore, residual confounding is inevitable for any observational study. Even though generally disease-related confounding factors were comprehensively adjusted in our analyses, the effect of unmeasured or unknown confounders might not be ruled out. Eventually, it is impossible to infer causality due to the cross-sectional study design.
Conclusion
In conclusion, higher possibility of non-serous OC was associated with the higher pre-diagnosis dietary Cu and Cu/Zn ratio. The risk of poorly differentiation OC at diagnosis was significant inversely related to dietary Cu intake. Our present study provides evidence suggesting that higher pre-diagnosis dietary Cu and Cu/Zn ratio intake are conducive to reducing the severity of OC at diagnosis. However, further large prospective cohort studies are warranted to confirm these associations.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by the Institutional Review Board of the Ethics Committee of Shengjing Hospital of China Medical University, Shenyang, China (2015PS38K). The patients/participants provided their written informed consent to participate in this study.
Author contributions
SG, Y-HZ, and LW contributed to the study design. SG, LW, SY, and XQ collected the data. J-LY and Z-YW analyzed the data. J-LY, TT, Z-YW, RW, M-HS, CG, Y-JC, SG, and LW wrote the first draft of the manuscript and edited the manuscript. All authors read and approved the final manuscript and accept responsibility for the integrity of the data analyzed.
Funding
This work was supported by Clinical Research Cultivation Project of Shengjing Hospital (SG).
Acknowledgments
We thank the research team for their daily efforts in material collection and manuscript writing. Besides, we thank Qi-juWu and Ting-Ting Gong for their guidance and help in the process of research design and manuscript writing.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2022.1003675/full#supplementary-material
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
2. Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, et al. Cancer statistics in China, 2015. CA Cancer J Clin. (2016) 66:115–32. doi: 10.3322/caac.21338
3. Lheureux S, Gourley C, Vergote I, Oza AM. Epithelial ovarian cancer. Lancet. (2019) 393:1240–53. doi: 10.1016/S0140-6736(18)32552-2
4. Torre LA, Trabert B, DeSantis CE, Miller KD, Samimi G, Runowicz CD, et al. Ovarian cancer statistics, 2018. CA Cancer J Clin. (2018) 68:284–96. doi: 10.3322/caac.21456
5. Kim SJ, Rosen B, Fan I, Ivanova A, McLaughlin JR, Risch H, et al. Epidemiologic factors that predict long-term survival following a diagnosis of epithelial ovarian cancer. Br J Cancer. (2017) 116:964–71.
6. Costello AM, Elizondo-Riojas MA, Li X, Volk DE, Pillai AK, Wang H. Selection and characterization of vimentin-binding aptamer motifs for ovarian cancer. Molecules. (2021) 26:6525. doi: 10.3390/molecules26216525
7. Hand R, Fremgen A, Chmiel JS, Recant W, Berk R, Sylvester J, et al. Staging procedures, clinical management, and survival outcome for ovarian carcinoma. JAMA. (1993) 269:1119–22. doi: 10.3322/caac.21559
8. Yoshikawa K, Fukuda T, Uemura R, Matsubara H, Wada T, Kawanishi M, et al. Age-related differences in prognosis and prognostic factors among patients with epithelial ovarian cancer. Mol Clin Oncol. (2018) 9:329–34.
9. Chiang YC, Chen CA, Chiang CJ, Hsu TH, Lin MC, You SL, et al. Trends in incidence and survival outcome of epithelial ovarian cancer: 30-year national population-based registry in Taiwan. J Gynecol Oncol. (2013) 24:342–51. doi: 10.3802/jgo.2013.24.4.342
10. Cho KR, Shih I. Ovarian cancer. Annu Rev Pathol. (2009) 4:287–313. doi: 10.1146/annurev.pathol.4.110807.092246
11. Sun H, Gong TT, Xia Y, Wen ZY, Zhao LG, Zhao YH, et al. Diet and ovarian cancer risk: an umbrella review of systematic reviews and meta-analyses of cohort studies. Clin Nutr. (2021) 40:1682–90. doi: 10.1016/j.clnu.2020.11.032
12. GBD 2017 Diet Collaborators. Health effects of dietary risks in 195 countries, 1990-2017: a systematic analysis for the global burden of disease study 2017. Lancet. (2019) 393:1958–72.
13. Papadimitriou N, Markozannes G, Kanellopoulou A, Critselis E, Alhardan S, Karafousia V, et al. An umbrella review of the evidence associating diet and cancer risk at 11 anatomical sites. Nat Commun. (2021) 12:4579. doi: 10.1038/s41467-021-24861-8
14. Al Ramadhani RM, Nagle CM, Ibiebele TI, Grant P, Friedlander M, DeFazio A, et al. Pre- and post-diagnosis diet quality and ovarian cancer survival. Cancer Epidemiol Biomarkers Prev. (2021) 30:229–32. doi: 10.1158/1055-9965.EPI-20-1036
15. Karavasiloglou N, Pestoni G, Faeh D, Rohrmann S. Post-diagnostic diet quality and mortality in females with self-reported history of breast or gynecological cancers: results from the third national health and nutrition examination survey (NHANES III). Nutrients. (2019) 11:2558. doi: 10.3390/nu11112558
16. Chen J, Wang Y, Fang Y, Jiang Z, Wang A, Xue J. Improved photodynamic anticancer activity and mechanisms of a promising zinc (II) phthalocyanine-quinoline conjugate photosensitizer in vitro and in vivo. Biomed Opt Express. (2020) 11:3900–12. doi: 10.1364/BOE.394186
17. Khan MH, Cai M, Deng J, Yu P, Liang H, Yang F. Anticancer function and ROS-mediated multi-targeting anticancer mechanisms of copper (II) 2-hydroxy-1-naphthaldehyde complexes. Molecules. (2019) 24:2544. doi: 10.3390/molecules24142544
18. Hu J, Liao C, Mao R, Zhang J, Zhao J, Gu Z. DNA interactions and in vitro anticancer evaluations of pyridine-benzimidazole-based Cu complexes. Med Chem Comm. (2017) 9:337–43. doi: 10.1039/c7md00462a
19. Denoyer D, Masaldan S, La Fontaine S, Cater MA. Targeting copper in cancer therapy: ‘copper that cancer’. Metallomics. (2015) 7:1459–76. doi: 10.1039/c5mt00149h
20. Nojiri H, Saita Y, Morikawa D, Kobayashi K, Tsuda C, Miyazaki T, et al. Cytoplasmic superoxide causes bone fragility owing to low-turnover osteoporosis and impaired collagen cross-linking. J Bone Miner Res. (2011) 26:2682–94. doi: 10.1002/jbmr.489
21. Franklin RB, Costello LC. Zinc as an anti-tumor agent in prostate cancer and in other cancers. Arch Biochem Biophys. (2007) 463:211–7. doi: 10.1016/j.abb.2007.02.033
22. Caglayan A, Katlan DC, Tuncer ZS, Yüce K. Evaluation of trace elements associated with antioxidant enzymes in blood of primary epithelial ovarian cancer patients. J Trace Elem Med Biol. (2019) 52:254–62. doi: 10.1016/j.jtemb.2019.01.010
23. Wang W, Wang X, Luo J, Chen X, Ma K, He H, et al. Serum copper level and the copper-to-Zinc ratio could be useful in the prediction of lung cancer and its prognosis: a case-control study in northeast China. Nutr Cancer. (2021) 73:1908–15. doi: 10.1080/01635581.2020.1817957
24. Stepien M, Jenab M, Freisling H, Becker NP, Czuban M, Tjønneland A, et al. Pre-diagnostic copper and zinc biomarkers and colorectal cancer risk in the European prospective investigation into cancer and nutrition cohort. Carcinogenesis. (2017) 38:699–707. doi: 10.1093/carcin/bgx051
25. Fang AP, Chen PY, Wang XY, Liu ZY, Zhang DM, Luo Y, et al. Serum copper and zinc levels at diagnosis and hepatocellular carcinoma survival in the guangdong liver cancer cohort. Int J Cancer. (2019) 144:2823–32. doi: 10.1002/ijc.31991
26. Kazi Tani LS, Gourlan AT, Dennouni-Medjati N, Telouk P, Dali-Sahi M, Harek Y, et al. Copper isotopes and copper to zinc ratio as possible biomarkers for thyroid cancer. Front Med. (2021) 8:698167. doi: 10.3389/fmed.2021.698167
27. Lin S, Yang H. Ovarian cancer risk according to circulating zinc and copper concentrations: a meta-analysis and mendelian randomization study. Clin Nutr. (2021) 40:2464–8. doi: 10.1016/j.clnu.2020.10.011
28. Gong TT, Liu FH, Liu YS, Yan S, Xu HL, He XH, et al. A follow-up study of ovarian cancer (OOPS): a study protocol. Front Nutr. (2022) 9:872773. doi: 10.3389/fnut.2022.872773
29. Wen ZY, Liu C, Liu FH, Wei YF, Xu HL, Wang R, et al. Association between pre-diagnostic dietary pattern and survival of ovarian cancer: evidence from a prospective cohort study. Clin Nutr. (2022) 41:452–9. doi: 10.1016/j.clnu.2021.12.033
30. Wei YF, Hao YY, Gao S, Li XQ, Liu FH, Wen ZY, et al. Pre-diagnosis cruciferous vegetables and isothiocyanates intake and ovarian cancer survival: a prospective cohort study. Front Nutr. (2021) 8:778031. doi: 10.3389/fnut.2021.778031
31. Jiang L, Gong TT, Gao S, Li XQ, Liu FH, Wen ZY, et al. Pre-diagnosis dairy product intake and ovarian cancer mortality: results from the ovarian cancer follow-up study (OOPS). Front Nutr. (2021) 8:750801. doi: 10.3389/fnut.2021.750801
32. Gu JH, Gong TT, Wu QJ, Liu FH, Wen ZY, Gao C, et al. Association between pre-diagnostic dietary supplements intake and ovarian cancer survival: findings from a prospective cohort study in Chinese women. Front Nutr. (2021) 8:758178. doi: 10.3389/fnut.2021.758178
33. Liu FH, Du ZD, Li XY, Wei YF, Wen ZY, Yan S, et al. Pre-diagnosis fiber: carbohydrate intake ratio and mortality of ovarian cancer: results from a prospective cohort study. Food Funct. (2022) 13:10046–54. doi: 10.1039/d2fo01379g
34. Zhao JQ, Hao YY, Gong TT, Wei YF, Zheng G, Du ZD, et al. Phytosterol intake and overall survival in newly diagnosed ovarian cancer patients: an ambispective cohort study. Front Nutr. (2022) 9:974367. doi: 10.3389/fnut.2022.974367
36. Banna JC, McCrory MA, Fialkowski MK, Boushey C. Examining plausibility of self-reported energy intake data: considerations for method selection. Front Nutr. (2017) 4:45. doi: 10.3389/fnut.2017.00045
37. Yang Y, Wang GY, Pan XC. China Food Composition (Standard Edition). Beijing: Peking University Medical Press (2018).
38. Atakul T, Altinkaya SO, Abas BI, Yenisey C. Serum copper and zinc levels in patients with endometrial cancer. Biol Trace Elem Res. (2020) 195:46–54. doi: 10.1007/s12011-019-01844-x
39. Zabłocka-Słowińska K, Prescha A, Płaczkowska S, Porȩbska I, Kosacka M, Pawełczyk K. Serum and whole blood Cu and Zn status in predicting mortality in lung cancer patients. Nutrients. (2020) 13:60. doi: 10.3390/nu13010060
40. Stepien M, Hughes DJ, Hybsier S, Bamia C, Tjønneland A, Overvad K, et al. Circulating copper and zinc levels and risk of hepatobiliary cancers in Europeans. Br J Cancer. (2017) 116:688–96. doi: 10.1038/bjc.2017.1
41. Wakwe VC, Odum EP, Amadi C. The impact of plasma zinc status on the severity of prostate cancer disease. Investig Clin Urol. (2019) 60:162–8. doi: 10.4111/icu.2019.60.3.162
42. Lin CC, Huang JF, Tsai LY, Huang YL. Selenium, iron, copper, and zinc levels and copper-to-zinc ratios in serum of patients at different stages of viral hepatic diseases. Biol Trace Elem Res. (2006) 109:15–24. doi: 10.1385/BTER:109:1:015
43. Li Z, Long T, Wang R, Feng Y, Hu H, Xu Y, et al. Plasma metals and cancer incidence in patients with type 2 diabetes. Sci Total Environ. (2021) 758:143616. doi: 10.1016/j.scitotenv.2020.143616
44. Samavarchi Tehrani S, Mahmoodzadeh Hosseini H, Yousefi T, Abolghasemi M, Qujeq D, Maniati M, et al. The crosstalk between trace elements with DNA damage response, repair, and oxidative stress in cancer. J Cell Biochem. (2018) Epub ahead of print. doi: 10.1002/jcb.27617
45. Ge EJ, Bush AI, Casini A, Cobine PA, Cross JR, DeNicola GM, et al. Connecting copper and cancer: from transition metal signalling to metalloplasia. Nat Rev Cancer. (2022) 22:102–13. doi: 10.1038/s41568-021-00417-2
46. Shanbhag VC, Gudekar N, Jasmer K, Papageorgiou C, Singh K, Petris MJ. Copper metabolism as a unique vulnerability in cancer. Biochim Biophys Acta Mol Cell Res. (2021) 1868:118893. doi: 10.1016/j.bbamcr.2020.118893
47. Theocharis SE, Margeli AP, Klijanienko JT, Kouraklis GP. Metallothionein expression in human neoplasia. Histopathology. (2004) 45:103–18. doi: 10.1111/j.1365-2559.2004.01922.x
48. Denoyer D, Pearson HB, Clatworthy SA, Smith ZM, Francis PS, Llanos RM, et al. Copper as a target for prostate cancer therapeutics: copper-ionophore pharmacology and altering systemic copper distribution. Oncotarget. (2016) 7:37064–80. doi: 10.18632/oncotarget.9245
49. Pan Z, Choi S, Ouadid-Ahidouch H, Yang JM, Beattie JH, Korichneva I. Zinc transporters and dysregulated channels in cancers. Front Biosci (Landmark Ed). (2017) 22:623–43. doi: 10.2741/4507
50. Bafaro E, Liu Y, Xu Y, Dempski RE. The emerging role of zinc transporters in cellular homeostasis and cancer. Signal Transduct Target Ther. (2017) 2:17029. doi: 10.1038/sigtrans.2017.29
51. Alam S, Kelleher SL. Cellular mechanisms of zinc dysregulation: a perspective on zinc homeostasis as an etiological factor in the development and progression of breast cancer. Nutrients. (2012) 4:875–903. doi: 10.3390/nu4080875
Keywords: ovarian cancer, severity, diet, copper, zinc, copper-to-zinc ratio
Citation: Yin J-L, Tao T, Wen Z-Y, Wang R, Sun M-H, Gao C, Chang Y-J, Yan S, Qin X, Zhao Y-H, Wang L and Gao S (2022) Association between pre-diagnostic dietary copper, zinc, and copper-to-zinc ratio and severity of ovarian cancer. Front. Nutr. 9:1003675. doi: 10.3389/fnut.2022.1003675
Received: 26 July 2022; Accepted: 19 October 2022;
Published: 15 November 2022.
Edited by:
Imre Lengyel, Queen’s University Belfast, United KingdomReviewed by:
Susmita Barman, University of Nebraska Medical Center, United StatesZhan Zhipeng, Jinzhou Medical University, China
Copyright © 2022 Yin, Tao, Wen, Wang, Sun, Gao, Chang, Yan, Qin, Zhao, Wang and Gao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lan Wang, d2FuZ2wzQHNqLWhvc3BpdGFsLm9yZw==; Song Gao, Z2Fvc0Bzai1ob3NwaXRhbC5vcmc=
†These authors have contributed equally to this work
 Jia-Li Yin1,2,3†
Jia-Li Yin1,2,3† Ran Wang
Ran Wang Ming-Hui Sun
Ming-Hui Sun Yu-Hong Zhao
Yu-Hong Zhao Song Gao
Song Gao