
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Nutr., 14 October 2022
Sec. Nutrition and Food Science Technology
Volume 9 - 2022 | https://doi.org/10.3389/fnut.2022.1002147
This article is part of the Research TopicAdvances and Trends in Nutraceutical and Functional Plant-based FoodView all 12 articles
Syzygium aromaticum is an aromatic plant native to Indonesia, and introduced to tropical regions worldwide. As an ingredient in perfumes, lotions, and food preservation, it is widely used in the food and cosmetic industries. Also, it is used to treat toothache, ulcers, type 2 diabetes, etc. A variety of nutrients such as amino acids, proteins, fatty acids, and vitamins are found in S. aromaticum. In addition to eugenol, isoeugenol, eugenol acetate, β-caryophyllene and α-humulene are the main chemical constituents. The chemical constituents of S. aromaticum exhibit a wide range of bioactivities, such as antioxidant, antitumor, hypoglycemic, immunomodulatory, analgesic, neuroprotective, anti-obesity, antiulcer, etc. This review aims to comprehend the information on its taxonomy and botany, nutritional composition, chemical composition, bioactivities and their mechanisms, toxicity, and potential applications. This review will be a comprehensive scientific resource for those interested in pursuing further research to explore its value in food.
Spices are considered to be one of the earliest recorded functional foods, with international trade in spices dating back as far as 4500–1900 B.C. They are usually aromatic, dried plant parts obtained from seeds, fruits, leaves, roots, and bark (1). More than 100 species of plants are currently used worldwide as spices and flavorings that play an important role in cooking, health care, and preserving (2). In addition, spices serve as a rich reservoir of bioactive compounds and also have antioxidant, antibacterial, anti-inflammatory, antidiabetic, and anticancer properties that help fight various diseases in the human body (3).
Syzygium aromaticum L. (Myrtaceae), is also known as Eugenia caryophyllata Thunb. It is an evergreen tree native to the Maluku Islands in Indonesia (4). Its dried flower buds are referred to as “clove,” it is highly sought-after for medicinal and culinary purposes. In medieval Europe, it is cultivated for commercial purposes. Currently, it is produced mainly in Indonesia, West Indies, Madagascar, India, Tanzania, and Sri Lanka (5). especially Zanzibar and Pemba Island in Tanzania, which produce about 80% of the world’s production (6) (Figure 1). Clove has been used as a spice in ancient China for more than 2000 years and was included in the first list of medicinal food items in China in 2002. Recent studies have found that fruits, seeds and leaves of S. aromaticum also contain bioactive compounds. The Chinese Traditional Medicine Administration has listed the fruit as one of the 39 herbal species that should be given priority for further development. Despite having commercial and medicinal value, it is not been extensively reviewed on its nutritional composition and advances in its chemistry, pharmacology, toxicology, and applications. This review is the first time for the comprehensive analysis of the nutritional components of its various parts, which has never appeared in previous papers. Secondly, in terms of chemical composition, although some articles have introduced it, this paper summarizes the chemical compositions of each part for the first time and classifies them into volatile and non-volatile components. Thirdly, in terms of biological activity, although some articles introduced the biological activity of S. aromaticum, there was no detailed and clear mechanism diagram. In this paper, the biological activities of S. aromaticum were comprehensively summarized and the mechanism diagrams were drawn for the first time. Fourthly, in terms of application, most of the existing articles only introduced the fresh-keeping effect of clove. In this paper, the applications of S. aromaticum in medicine and food have been comprehensively analyzed for the first time, and the relevant popular contents of nanometer preparations have been added. Overall, this review will be a comprehensive scientific resource for those interested in pursuing further research to explore its value in medicine and food.
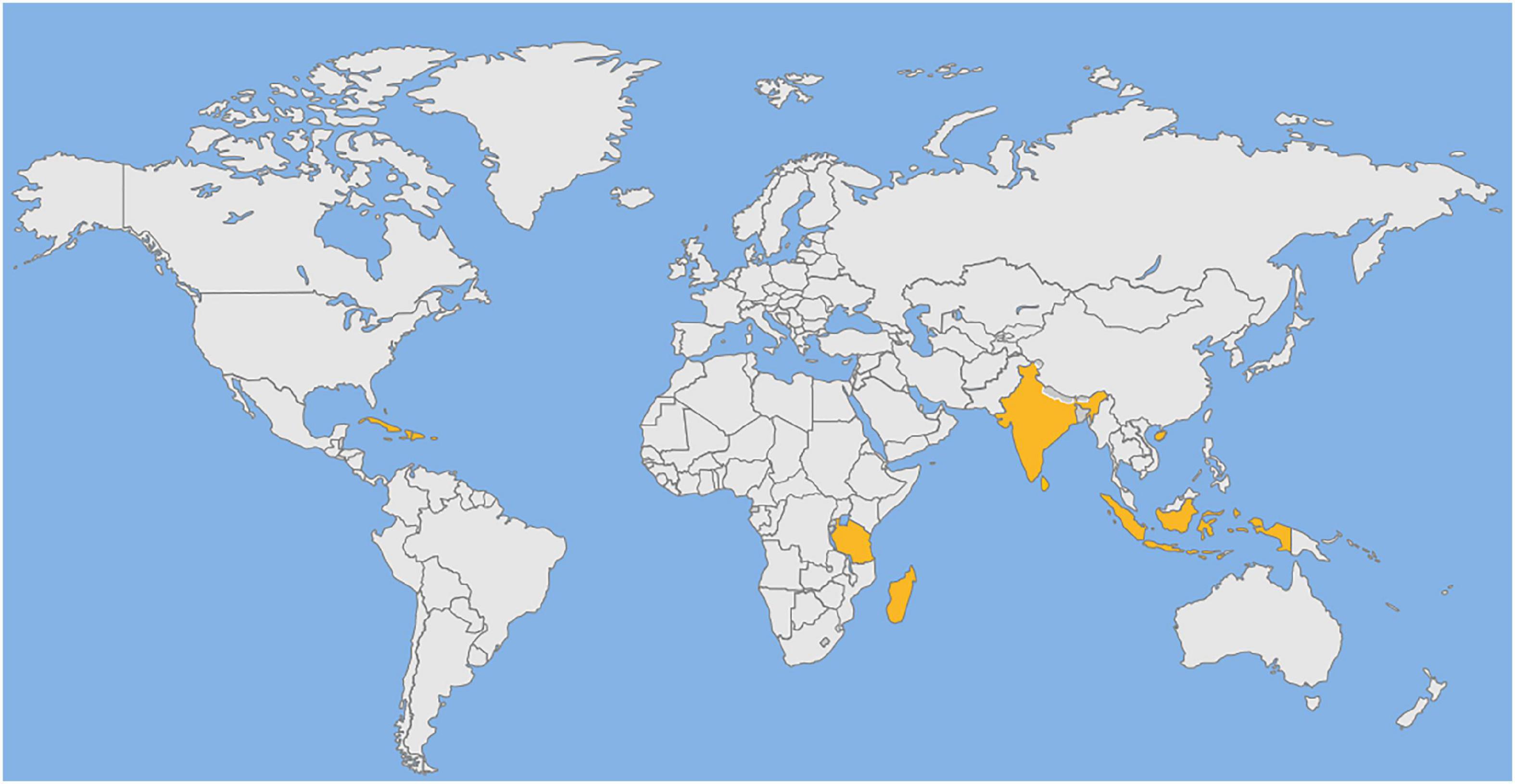
Figure 1. Distribution of S. aromaticum around the world. West Indies, Tanzania, Madagascar, India, Sri Lanka, Indonesia, Malaysia and Hainan province of China.
Syzygium aromaticum belongs to the Myrtales order, Myrtaceae family, and Syzygium genus. Myrtaceae family plants are mainly grown in tropical and subtropical regions of Australia and America (7). Syzygium is an important genus in the Myrtaceae family and contains more than 500 species, mainly distributed in tropical Asia and a few species in Oceania and Africa (8). S. aromaticum belongs to the plant kingdom, Angiospermae, Dicotyledoneae, and Archichlamydeae.
Syzygium aromaticum is an evergreen tree growing to about 10–20 m. The bark is gray and smooth. The leaves are large and opposite. The leaf blades are leathery, ovate-long elliptic, entire, and densely covered with oil glands. The petiole is conspicuous. The leaf buds are terminal, cymes or panicles. The flowers are red or pink, and 3 flowers are together in one group. The flowers contain 4 petals. The buds are white at the beginning and turn green and then red when the buds are 1.5 to 2cm long (9). The calyx is cylindrical, receptacle long, and 4-lobed at the tip. The lobes are triangular, bright red, with numerous stamens and an inferior ovary. The berries are oblong, red or dark purple, and contain one seed, which is oval. The fruit is called female or female S. aromaticum. The fruiting period is from June to July, and the flowering period is from January to February. The buds are called male or male S. aromaticum, and dried by removing the pedicel from the bright red buds (10). The different parts of S. aromaticum are shown in Figure 2.

Figure 2. Different parts of S. aromaticum. (A) Whole plant; (B) dried flower buds; (C) fruit; (D) leaf. Pictures are from https://baike.baidu.com/pic/%E4%B8%81%E5%AD%90%E9%A6%99/19435258.
The flower buds, fruits, branches, leaves, and seeds of S. aromaticum are rich in various nutrients, proteins, fatty acids, mineral elements, amino acids, vitamin, etc. The presence of these nutrients makes S. aromaticum as a plant with high economic value. The nutritional composition of each part of the plant is listed in Tables 1–4. Only a very few studies were conducted to identify the vitamins present in it, and the vitamins present in it are listed in Table 5.
The fruits contain the highest amount of total carbohydrate content. The buds contain the highest amount of crude fat. The leaves contain the highest protein content. The branches contain the highest amount of fiber. Table 1 lists the conventional nutrients present in various parts of S. aromaticum.
The fatty acid composition is similar in flower buds, fruits and branches. The leaves contain many fatty acids, and the number of fatty acids is 14. The contents palmitic, stearic, linoleic and linolenic acid were high in all parts. The higher proportion of polyunsaturated fatty acids in buds and seeds, is made up of α-linolenic acid and linoleic acid. α-Linolenic acid is one of the essential nutrients, with anti-inflammatory, anti-thrombotic, anti-arrhythmic, anti-cancer, lower blood pressure and other effects (17). Linoleic acid can be metabolized into arachidonic acid in the body, to play a pro-inflammatory or pro-thrombotic vasoconstriction (18). The highest proportion of saturated fatty acids present in branches amounts to 73.66%. The palmitic acid content in saturated fatty acids is about 47.69%. The palmitic acid can play an antitumor role by activating Saos-2 cell apoptosis through endoplasmic reticulum stress and autophagy (19). Thus S. aromaticum is rich in fatty acids and has broad prospects for preparing health supplements. The fatty acid composition in each part of S. aromaticum is shown in Table 2.
About 26 mineral elements have been detected in S. aromaticum. Fe is highest in the fruits, and Ca in the leaves. The total mineral element content in buds is higher than in other parts. The mineral element content of each part of S. aromaticum is shown in Table 3.
Amino acids are a combination of two organic substances, basic amino and acidic carboxyl groups, which give biochemical activity to protein molecules and are important components of proteins. The buds and fruits have similar percentages of essential amino acids, with total contents of 433.1–461.9 mg/kg and 406.2 mg/kg (32. 46%), respectively, which are higher than branches (113.9 mg/kg) and leaves (229.8 mg/kg). The details are shown in Table 4.
Vitamins are essential nutrients for human health. The buds contain a variety of vitamins (Table 5), among which Vitamin A, B3, and Vitamin B6 are high. These vitamins promote bone development, protect eye vision, maintain normal skin function, maintain immunity, and promote blood red blood cell metabolism (20).
At present, it is believed that the chemical compositions of S. aromaticum are divided into two major parts: volatile and non-volatile components, mainly including aromatics, sesquiterpenoids, monoterpenoids, flavonoids, triterpenoids, organic acids, etc. These chemical components are mainly derived from flower buds, leaves, seeds, and other parts of S. aromaticum.
The volatile components of S. aromaticum have a unique and attractive fragrance. They can be used as a topical application to relieve pain and promote healing. They are also used in perfumey and flavor industries. They possess significant antioxidant and antibacterial effects. Studies have confirmed the presence of volatile components in buds, seeds and leaves, with the majority in buds. More than 110 compounds are present in the S. aromaticum volatile oil and are listed in Supplementary Table 1. The major compounds in volatile oil are eugenol, eugenyl acetate, caryophyllene and α-humulene.
Aromatic compounds (eugenol, isoeugenol and eugenyl acetate) are the main components in S. aromaticum. Eugenol, is a unique and widely studied volatile component of S. aromaticum, accounting for more than 50% of the volatile oil, with a variety of pharmacological activities (31). Eugenyl acetate, is another bioactive component of the volatile components. Forty-two aromatic compounds, including methyl eugenol, ethyl benzoate, and cinnamic aldehyde, were identified in the essential oil of clove by GC-MS.
The terpenoids in S. aromaticum include sesquiterpenes, monoterpenes, and diterpenes, which are the material basis for the clinical efficacy and an important chemical source for further activity screening. Terpenoids are an important class of natural compounds widely used in the cosmetics and food industries and have a large market potential (32). Twenty-six sesquiterpenes and eighteen monoterpenes were reported in the volatile oil of S. aromaticum. Only one diterpene, menthyl chavicol, was reported from S. aromaticum. Caryophyllene and α-humulene account for about 7-16% of the volatile oil.
S. aromaticum volatile oil also contains aliphatic compounds, such as alkanes, ketones, alcohols, esters and ethers. The most abundant aliphatic compounds are tritetracontane, 2-heptanone, 2-non-anone, ethyl caproate and menthyl octanoate. The percentage of aliphatic compounds is lower than aromatic compounds in the volatile oil.
A total of 73 non-volatile components were reported from S. aromaticum, including 36 flavonoids, 4 chromones, 26 tannins, 3 triterpenoids, 1 coumarin and 1 phenolic ester, and 2 other components, as shown in Table 6.
Studies have shown that flavonoids are one of the important components of S. aromaticum to exert strong antioxidative activity, anti-inflammatory, and immunomodulatory activities (42). Ryu et al. (33) reported the presence of luteolin, quercetin, rhamnocitrin, kumatakenin, and pachypodol in S. aromaticum. These flavonoids have shown anticancer activity on human ovarian cancer cells (A2780). In addition, flavonoids readily form O - and C - glycosides, with O - glycosides being more common and better absorbed. Ryu et al. (33) reported nine known flavonoid glycosides and four flavonoids from the buds.
The chromones in S. aromaticum are mainly eugenin, 8-C-glucosylnoreugenin, biflorin, isobiflorin, etc. Biflorin and isobiflorin were found to be isolated from S. aromaticum buds by Lee et al. (38) and showed an anti-inflammatory effect on Lipopolysaccharide (LPS) induced inflammation in macrophages through STAT1 inactivation. In addition, it was demonstrated that biflorin increased the activation of protein kinase C-ζ and its downstream signaling molecules in the hippocampus. These compounds improved the cognitive dysfunction in mice and reduced the viability of melanoma cell lines through DNA interactions (43). These findings provide a scientific basis for the neuroprotective and antitumor effects of S. aromaticum.
Tannins are a group of water-soluble polyphenolic compounds with complex structures present in plants. Based on the chemical structures, these can be classified into two major groups, hydrolyzable and condensed tannins. The tannins exhibit various pharmacological effects such as antibacterial, antioxidant, antitumor, antiviral, and hypoglycemic (44). Kim et al. (39) isolated four tannins from S. aromaticum, eugeniin, casuarictin, 1,3-di-O-galloyl-4,6-(S)-hexahydroxydiphenoyl-β-D-glucopyranose, and tellimagrandin I. The tellimagrandin was found to have significant inhibitory activity on syncytium formation. Ali et al. (35) analyzed the polyphenol and tannin content in 12 spices (allspice, black cardamom, black cumin, black pepper, cardamom, cinnamon, clove, cumin, fennel, nutmeg, star-anise, and turmeric) using LC-ESI-QTOF-MS and evaluated their antioxidant activity. Cloves contain the highest total polyphenol and total tannin content. The antioxidant activity is positively correlated with the total phenolic content. S. aromaticum is the most active antioxidant (45).
Triterpenoids (asiatic acid, arjunolic acid and oleanolic acid), coumarins (scopoletin), and phenolic acid esters (salvianolic acid C) were reported from S. aromaticum. Oleanolic acid, has shown significant analgesic effects. The oleanolic acid activates the bile acid receptor (TGR5), which plays a key role in treating metabolic diseases. The acetyl derivatives of oleanolic acid have shown better anti-inflammatory activity than oleanolic acid (46).
Different parts of S. aromaticum and its essential oil have various pharmacological activities, including antioxidant, hypoglycemic, antitumor, antibacterial antiviral, etc. The majority of the pharmacological investigations were mainly focused on the flower buds (clove). The details were discussed in each of the following paragraphs, and are recapitulative summary was presented in Table 7.
Clove essential oil (CEO) has shown powerful antioxidant activity with an EC50 of 0.36 μL/mL and it is the most potent volatile oil compared to eucalyptus, fennel and lavender (63). Phenols and flavonoids were attributed to antioxidant effects. The antioxidant activity is mediated via scavenging the free radicals, ferric reducing capacity, increasing the activity of antioxidant enzymes, and antagonizing lipid peroxidation (LPO).
Baghshahi et al. (64) showed that the antioxidant activity of clove extract (CE) was more than 10 times higher than that of vitamin E in the DPPH free radical scavenging capacity test. Reactive oxygen species (ROS) are oxygen-containing intermediate metabolites that modulate the host’s immune response in vivo and positively affect the clearance of dead cells and the inactivation of microorganisms, but excessive amounts of ROS in vivo can damage the organism at the cellular level. Neutrophils are the most abundant leukocytes in humans, and their hyperactivation can activate the NADPH oxidase to generate large amounts of ROS. Eugenol inhibits fMLF or PMA-induced superoxide anion production in neutrophils by inhibiting the Raf/MEK/ERK1/2/p47phox phosphorylation pathway, avoiding ROS accumulation (47). CEO can increase the activity of antioxidant enzymes by inducing the expression of SOD-3 or GST-4 to reduce ROS accumulation in vivo, exerting antioxidant effects (65). Eugenol also has Fe3+ reducing ability and electron donor properties, which can neutralize free radicals by forming stable products to exert antioxidant effects (66).
One of the common oxidative reactions, LPO, is associated with many diseases. DNA damage caused by ROS is a key factor in tumorigenesis and development. Eugenol can effectively block hydroxyl radical-induced DNA oxidation and LPO and has a significantly higher inhibitory effect on hydrogen peroxide than other reactive oxygen species (67). CEO also inhibits LPO in erythrocyte membranes, thereby increasing membrane resistance to spontaneous hemolysis, reducing membrane microviscosity, maintaining its structural integrity and functional activity, and significantly decreasing the intensity of LPO in mouse liver and brain, scavenging excess ROS and other free radicals from lipid chains, increasing the antioxidant capacity of liver and brain lipids and the activity of antioxidant enzymes in the liver (68). CE also significantly prevented oxidation-induced protein damage by reducing the formation of protein carbonyl groups and preventing the loss of protein sulfhydryl groups (48).
In conclusion, S. aromaticum can reduce free radical accumulation in vivo, decrease oxidative cellular damage, and increase antioxidant capacity (Figure 3). Thus, it has very high potential to be used as a natural antioxidant and anti-aging supplement.
Abnormal glucose metabolism leads to complications of various metabolic diseases, especially the prevalence of diabetes is increasing worldwide, but commonly used oral hypoglycemic drugs often cause serious side effects. So herbs and spices have been used in folk medicine for centuries to control the complications of diabetes. S. aromaticum has been widely studied for its beneficial and toxic effects. CE has shown comparable hypoglycemic effects to that of insulin in animal models and did not show toxic effects (69).
Abdulrazak et al. (51) found a significant increase in insulin and leptin levels in type 2 diabetic rabbits treated with 12.5% clove for 6 weeks suggesting its potential to use for patients with diabetes. Some studies have shown that CE and its compound nigricin enhance proximal insulin signaling by decreasing serine phosphorylation of insulin receptor substrate-1 (IRS-1), increasing tyrosine phosphorylation, modifying distal insulin signaling by enhancing protein kinase B (PKB) and glycogen synthase kinase-3-β (GSK-3β) phosphorylation in muscle cells signaling. Thus, they decrease free fatty acid-mediated insulin resistance in mouse myogenic cells, increase glucose uptake, and promote insulin secretion in muscle cells (70).
Glycogen metabolism has been one of the main causes of elevated blood glucose levels. Glycogen phosphorylase b (PYGB), a key enzyme in glycogen degradation, catalyzes the breakdown of glycogen to glucose-1-phosphate in the liver and skeletal muscle. Therefore, inhibition of hepatic glycogen phosphorylase is an effective therapeutic strategy to reduce hyperglycemia in type 2 diabetes. The clove’s 50% aqueous ethanol extract showed strong dose-dependent inhibitory activity on glycogen phosphorylase b and glucagon-stimulated gluconeogenesis in primary rat hepatocytes, significantly suppressed blood glucose and glycated hemoglobin (HbA1c) levels in db/db mice. Thus, it caused a significant reduction in plasma triglyceride and non-esterified fatty acid levels, and improved glucose and lipid metabolism (50). In addition, the dehydrodienol and dehydrodienol B compounds of clove ethanol extracts (ECE) have potent human peroxisome proliferator-activated receptor (PPAR)-γ ligand binding activity, which can stimulate 3T3-L1 preadipocyte differentiation through PPAR-γ activation and significantly inhibit the increase in blood glucose levels in type 2 diabetic KK-Ay mice (71). Studies on the effects of C2C12 cardiomyocyte metabolism revealed that CE increased the phosphorylation of AMP-activated protein kinase (AMPK) and its substrate acetyl coenzyme A carboxylase (ACC), and also transcriptionally regulated genes involved in metabolism, including sirtuin1 (SIRT1) and PPARγ coactivator 1α (PGC1α), as a way to increase muscle glycolysis and mitochondrial function (69).
Overall, S. aromaticum has the potential to exert type 2 diabetes through phosphorylation pathways that modify insulin signaling, activation of receptor PPAR-γ ligand binding activity, and activation of AMPK and SIRT1 pathways (Figure 4).
Tumor pathogenesis is complex, and impaired apoptosis mechanism is a major cause. So selective induction of apoptosis in tumor cells is one of the effective ways to treat tumors. S. aromaticum has anticancer and antitumor activity on the human colon, breast, liver, cervical, and gastric cancers. Its inhibitory effects are time- and dose-dependent. Arung et al. (72) found that CEO inhibited melanin in B16 melanoma cells up to 50% and 80% at 100 and 200 μg/mL, respectively. Ethyl acetate extract of clove and its active ingredient oleanolic acid both selectively increased the protein expression of p21(WAF1/Cip1) and γ-H2AX and downregulated the expression of cell cycle regulatory proteins, promoted G0/G1 cell cycle arrest and induced apoptosis in a dose-dependent manner. Its activity on colon tumor xenografts is superior to the chemotherapeutic drug 5-fluorouracil (52). PI3K/Akt/mTOR signaling pathway is an important pathway that regulates apoptosis and can be aberrantly activated by malignant tumor cells to exert apoptosis-inhibiting and proliferation-promoting effects in tumor cells. The active ingredients of clove inhibit PI3K/Akt/mTOR signaling pathway and activate the caspase-mediated cascade response to induce apoptosis of HCT116 cells in a dose-dependent manner (73).
Bcl-2 family proteins play a key role in apoptosis and play an important role in mitochondrial-mediated apoptosis by regulating mitochondrial outer membrane permeability. The study has shown that CE can induce endogenous caspase-dependent cell death by increasing oxidative stress mediated via oxygen and nitrogen radicals. It can mediate the release of Bcl-2 family protein pro-apoptotic factors, signaling oxidative stress-mediated DNA damage by modulating the cellular antioxidant SOD system and the activity of the Akt, p38mitogen-activated protein kinases (p38MAPK), c-Jun N-terminal kinase (JNK) and extracellular signal-regulated kinases (Erk1/2) pathways to induce apoptosis in human breast cancer MCF-7 cells (74). The aqueous extract of clove (ACE) upregulated the expression of pro-apoptotic proteins p53 and Bax in benzo[a]pyrene (BP)-induced lung carcinogenesis in mice. It downregulated the expression of anti-apoptotic protein Bcl-2 in the pre-cancerous stage. In the early stage of carcinogenesis (week 8), clove significantly activated the expression of caspase-3. In addition, clove downregulated the expression of COX-2, cMyc, HRA and other growth-promoting proteins expression. All these together promote a significant decrease in proliferating cells and an increase in apoptotic cells, exerting a chemopreventive potential (75). In addition, eugenol can also exert its chemotherapeutic potential by blocking the nuclear translocation of β-catenin, thus promoting its cytoplasmic degradation through N-terminal phosphorylation of Ser37. Thus, it effectively reduces cancer complications and prolongs and improves patient life (76).
In conclusion, the main pathway of S. aromaticum to induce apoptosis in cancer cells by upregulating the expression of intracellular pro-apoptotic proteins such as Bax, Caspase-3, p53, etc., downregulating the expression of intracellular anti-apoptotic proteins such as Bcl-2, and downregulating the expression of pro-growth proteins such as COX-2, cMyc, and HRA (Figure 5).
In recent years, the irrational application of broad-spectrum antibacterial drugs, immunosuppressive drugs, antitumor drugs and the widespread use of other surgical interventions such as organ transplantation have led to an increasingly serious crisis of bacterial and fungal drug resistance, which threatens the life and health of humans. Clove has strong antibacterial effects on Staphylococcus aureus, Candida albicans, Escherichia coli and Listeria monocytogenes.
It was found that ACE and ECE showed inhibitory activity against three foodborne pathogens, gram + ve Staphylococcus aureus, gram -ve Escherichia coli and Pseudomonas aeruginosa. S. aureus is the most sensitive to ACE (inhibition zone, 30.5 mm), P. aeruginosa is the most sensitive to ECE (inhibition zone, 38 mm). ACE inhibited the growth of S. aureus and E. coli at ≥500 μg/ml, P. aeruginosa at ≥700 μg/ml, respectively. ECE inhibited the growth of all three bacteria at ≥500 μg/ml (77). Similarly, Ajiboye et al. (55) found that aqueous extracts of clove seeds could enhance the membrane permeability of Escherichia coli, Pseudomonas aeruginosa, and Staphylococcus aureus, exerting antibacterial effects. In addition, four different CE (methanol, ethyl acetate, n-hexane and ether) showed inhibitory activity against Candida albicans, Candida glabrata and Candida tropicalis, with the ethyl acetate extract being the most active. CEO also antagonized Candida biofilm formation and effectively prevented the growth of Candida on abiotic surfaces (24). In addition, Zhang et al. (54) found that the eugenol in S. aromaticum leaf essential oil exhibited good antibacterial activity against Porphyromonas gingivalis at a concentration of 31.25 μm.
Xu et al. (78) showed that the antibacterial activity of S. aromaticum is via disrupting the cell wall and membrane, inhibiting the normal synthesis of bacterial DNA and proteins, eugenol is the main component in the antibacterial activity. Quorum sensing (QS) is a communication system associated with the virulence of pathogenic bacteria such as Pseudomonas aeruginosa. The molecular modeling studies have shown that eugenol binds to the QS receptor overcoming the antibiotic resistance through hydrophobic interactions and hydrogen bonding to Arg61 and Tyr41 of the LasR receptor (79). Elastase A, elastase B, proteinase IV and alkaline proteases activate host matrix metalloproteinases (MMPs) to establish infection by converting pre-MMP-2 to active MMP-2. S. aromaticum leads to a significant reduction of signaling molecules (Acyl-homoserine lactones) involved in population-sensing regulation, which can inhibit the activity of four proteases from establishing anti-infective effects, in addition to inducing a dose-dependent production of neutrophil extracellular traps (NETs) to destroy bacterial pathogens (80). CEO interferes with the expression of virulence-related genes involved in the flagellum, PEB1, PEB4, LPS and serine protease, with significant antibacterial and potentially virulence-modulating effects on Campylobacter jejuni (81). eEF1A protein interacted with several viral proteins, leading to enhanced viral replication, reduced apoptosis and increased cellular transformation; therefore, Wang et al. (82) suggested that downregulation of eEF1A protein expression may be another important mechanism for exerting its antifungal activity.
In conclusion, the permeability of phenolic substances such as eugenol to cell membranes and the irreversible disruption of plasma membrane integrity make S. aromaticum a potential source of natural antibacterial drugs with broad-spectrum antibacterial effects (Figure 6).
Clove essential oil (CEO) regulates the immune response and reduces inflammatory symptoms by enhancing humoral immunity and reducing the release of lymphokines to reduce cellular immunity. As a major component of S. aromaticum, eugenol is thought to regulate cellular inflammatory cascades in LPS induced inflammation in macrophages via (1) nuclear factor-κB (NF-κB) and ERK/MAPK pathways, (2) nitric oxide (NO) production, (3) pro-inflammatory interleukin release and (4) endogenous antioxidant defenses mechanisms (83). Secondly, CEO significantly inhibited the levels of several pro-inflammatory biomarkers such as (1) vascular cell adhesion molecule-1 (VCAM-1), (2) interferon γ-induced protein 10 (IP-10), (3) interferon-inducible T-cell α chemoattractant (I-TAC), and (4) monokine induced by γ interferon (MIG)-induced monokines (Figure 7). It also significantly inhibited (1) tissue remodeling protein molecules (collagen-I, collagen-III), (2) macrophage colony-stimulating factor (M-CSF), and (3) tissue inhibitor of metalloproteinase 2 (TIMP-2). It regulated the global gene expression and altered key signaling pathways of inflammation, tissue remodeling and cancer signaling processes (57). The water-soluble fraction of the hydroalcoholic extract of clove inhibited the production of pro-inflammatory cytokines (IL-1β and IL-6) by macrophages in BALB/c mice, thus exerting an anti-inflammatory effect (84). In addition, in an immunosuppressed mouse model, CEO (400 mg/kg) administration for one week significantly increased the total white blood cell (WBC) count. It and enhanced the delayed type hypersensitivity (DTH) in mice, restoring the cellular and humoral immune responses in a dose-dependent manner in cyclophosphamide immunosuppressed mice (85).
Ferland, Beaudry, and Vachon (86) showed eugenol (40 mg/kg) reduced substance P and CGRP, and increased the dynorphin level in a rat model of osteoarthritis. These results confirmed the therapeutic potential of eugenol in osteoarthritis. CEO also reduced the torsional movements in mice with acetic acid-induced abdominal contractions and significantly increased the latency of response to pain after 60 min by 82.3%. The CEO also inhibited the foot swelling in mice caused by carrageenan by 50.6%. It has significantly attenuated yeast-induced t hyperthermia by 2.7°C at ΔT-max (87).
Alzheimer’s disease (AD) is a common neurodegenerative disease characterized by progressive cognitive dysfunction and memory loss, which has been increasing in recent years, seriously affecting patients’ lives and quality of life. Oxidative stress plays a key role in AD, and CEO can reverse scopolamine-induced short- and long-term memory deficits by reducing oxidative stress (88). By administering CEO to intracerebroventricular (icv)-colchicine-induced cognitive dysfunction in rats, it was found that in addition to reducing oxidative stress damage, CEO exerted neuroprotective effects and improved cognitive dysfunction by improving mitochondrial dysfunction and inhibiting microglial activation (89). There are many links between SIRT1 and diseases such as AD. Studies have shown that ECE activates and increases SIRT1 level, inhibits NF-kB signaling in microglia, attenuates Aβ25-35-induced neuronal cell neurotoxicity, and also downregulates γ-secretase level, scavenges ROS and increases the percentage of antioxidant enzymes, which together delay the progression of neurodegenerative diseases and exert neuroprotective effects (90).
Glutamate is considered an excitatory neurotransmitter, but it causes oxidative stress at high concentrations and promotes apoptotic signaling cascades, leading to neurodegeneration. CE fermented with Trametes Versicolor (LTV), contains a higher content of dehydroeugenol. It inhibits apoptotic signaling such as Ca2+ inward flow, the excessive production of intracellular reactive oxygen species and LPO. It also has good protective properties against glutamate-induced toxicity in HT22 cells (91).
Rai et al. (92) reported that Sestrin2 (Sesn2) is a potential serum marker in Parkinson’s disease (PD). The Sesn2 concentrations were significantly elevated in the serum of PD patients. ECE caused a dose-dependent downregulation of p53, Sesn2 and phosphorylated AMPK in cells, accompanied by the increased phosphorylated p70S6K, alleviating SH-SY5Y cell damage and exhibiting neuroprotective effects in PD. In addition, cloves exhibit anticholinesterase activity and are protective against brain damage induced by CeCl3 and other substances (93).
In conclusion, S. aromaticum and its extracts exert neuroprotective effects by reducing acetylcholinesterase activity, restoring oxidative status, inhibiting microglia signaling and preventing histopathological changes (Figure 7).
Studies have shown that ACE exhibits antiviral activity similar to pure eugenol on feline calicivirus (94). The plaque reduction assay demonstrated that ECE has anti-herpes simplex virus (HSV) properties, showing direct inactivation of standard HSV particles, as well as significant inhibition of HSV-1, HSV-2, and a decrease in total HSV virus yield 30 h after treatment with the extract (61). The methanol and aqueous extracts of clove also showed inhibition of HCV protease. Eugenol inhibited influenza A virus (IAV), presumably by a mechanism attributed to the inhibition of oxidative stress and activation of ERK1/2, p38MAPK, and IKK/NF-κB pathways. Eugenol inhibits Beclin1-Bcl2 heterodimer dissociation and autophagy, ultimately impairing IAV replication (95). In addition, eugenol showed significant inhibitory of the West African Ebola virus (EBOV) (60). It is also used for the prevention and control of SARS-CoV-2-associated diseases. Flavonoids of S. aromaticum can inhibit SARS-CoV-2 virus expression, while bicornin and biflorin in clove have a high affinity for Mpro and exhibit potential viral inhibitory activity (96).
In summary, S. aromaticum exerts antiviral effects, mainly by improving oxidative stress, reducing viral replication, and inhibiting autophagic gene expression.
Fatty acid synthase (FAS), a key enzyme in adipogenesis, has been considered a potential therapeutic target for cancer and obesity. ECE as a FASN inhibitor can inhibit S-phase DNA replication in HepG2 cells. Adipocyte differentiation in OP9 cells, regulates the content of total triglyceride and low-density lipoprotein cholesterol, limits the development of high-fat diet-induced obesity, reduces body weight and abdominal adipose tissue weight, and reduces lipid accumulation in liver and epididymal adipose tissue, making it a potential therapeutic agent for obesity (97, 98). In addition, ECE also inhibited lipid accumulation in mice by downregulating the mRNA levels of transcription factors such as Srebp and Pparg and suppressing the expression of lipid metabolism-related proteins such as SREBP-1, FAS, CD36 and PPARγ in liver and white adipose tissue (62).
In addition to the above effects, S. aromaticum also affects reproductive function, promoting transdermal absorption, and alleviating gastric injury. CE has a bidirectional effect on reproductive function in mice, with lower doses (15 mg/kg⋅BW) of clove increasing serum testosterone levels and testicular hydroxysteroid dehydrogenase activity, and improving sperm motility, sperm morphology, epididymal and seminal vesicle secretory activity, and the number of litters per female, but higher doses (30 and 60 mg/kg⋅BW) inhibiting testicular activity (99). Choi D et al. (100) hypothesized that the mechanism affects the reproductive endocrine system by acting on GnRH neuronal cells. ACE can treat ethanol-induced gastric mucosal injury in rats. The mechanism may involve antioxidant activity, promoting PGE2 production, and inhibiting gastric mucosal inflammatory cell infiltration and epithelial cell loss (101). CEO also has significant permeation-enhancing effects, and studies on the effects of CEO on the in vivo and in vitro transdermal administration of ibuprofen in rabbits revealed that clove significantly enhanced ibuprofen absorption in vitro and permeation in vivo (102). CEO can also be used for fish anesthesia, speculating that it may act through glutamate receptors.
The U.S. Food and Drug Administration (FDA) has confirmed the safety of clove buds, CEO, eugenol, and oleoresins as food additives. The World Health Organization (WHO) has established an acceptable daily intake of 2.5 mg/kg of CEO (6).
Some literature has reported cytotoxicity of eugenol, but it can rapidly reach peak plasma concentrations upon oral administration in rats and humans, with mean half-lives of 14.0 and 18.3 h, respectively, and is excreted in the urine in bound form within 24 h. Its genotoxicity and carcinogenicity are low (103). Acute toxicity studies in mice showed that LD50 of CEO is 4500 mg/kg upon oral administration for 24 h, which is a much higher dose than the doses usually used for infusions by humans (3 g/60 kg person in clinical therapy, equal to 9.35 mg/kg CEO). The long-term repeated toxicity studies (100, 200, and 400 mg/kg, orally) showed that only 400 mg/kg resulted in a significant decrease in body weight and no significant changes in relative organ weights and histopathological analysis were observed at all doses of CEO (104). Toxicity studies with clove buds polyphenol extract (Clovinol) in Wistar rats revealed no significant toxicity under acute (5 g/kg b.wt. for 14 days) and subchronic (0.25, 0.5 and 1 g/kg b.wt. for 90 days) conditions. The mutagenicity studies using Salmonella typhimurium strains revealed that Clovinol did not cause genetic mutations or shifts in the genome and showed significant mutagenic resistance against known mutagens, sodium azide, NPD, tobacco and 2-acetamidoflourene (105). In 2015, a panel of experts from the Association of Flavor and Extract Manufacturers initiated a re-evaluation of the safety of more than 250 natural flavor compounds (NFCs) and reported concluded that NFCs of clove was recognized as “Generally Recognized as Safe (GRAS)” under the conditions of their intended use as flavor ingredients (106). An acute oral toxicity study was conducted in normal rats according to the Organization for Economic Cooperation and Development (OECD) guidelines to assess the potential toxicity of S. aromaticum extracts after oral administration. Three animals per group were used in each step. Standard doses were given gradually, from 500 mg/kg b.w. continued up to 2500 mg/kg b.w. Mortality was recorded after 24 h. According to various acute and chronic toxicity studies of S. aromaticum, the oral LD50 of CEO in the food industry was 3597.5 mg/kg, with no adverse effects in subchronic toxicity tests and NAOEL levels of 900-2000 mg/kg/day. Oral LD50 of eugenol was reported as 2650–3000 mg/kg b.w. (105), all of which demonstrated the safety of S. aromaticum for human use at low doses.
As one of the traditional spices and herbs, clove has long been used to treat stomach disorders, abdominal pain, vomiting, etc. Many scientific studies have confirmed that clove possesses significant free radical scavenging and anti-inflammatory activities and has potential roles in alleviating and preventing diseases like cancer, type II diabetes and epilepsy. CEO also has a stomachic effect and can significantly improve the symptoms of loss of appetite (107). In addition, clove has long been used as a respiratory adjuvant in treating respiratory diseases such as cough, asthma, and bronchitis. It also plays an important role in preventing and early treatment of COVID-19 (108).
Because of its analgesic, anesthetic and anti-inflammatory properties, CEO is often used to treat dental diseases. The traditional treatment of toothache with CEO was first documented in 1640 in the French “practice of medicine.” Modern studies have shown that brushing with herbal toothpaste containing clove resulted in a significant reduction in salivary lactate dehydrogenase (LDH), improved cellular integrity, and reduced plaque and gingivitis (109). That hexane extract of S. aromaticum seeds exhibits preferential growth inhibitory activity against cariogenic pathogens in dental caries and may be used to treat dental caries (110). In addition, CE may also present new natural therapeutic potential by inhibiting dentin erosion (111).
In recent years, nanotechnology has rapidly evolved. A wide range of lipid nanostructures such as liposomes and solid lipid nanoparticles, metals, nanocrystals and polymeric particles have been tested in several drug delivery systems in different animal models. In addition, many nano-drug delivery systems containing S. aromaticum are being developed.
Metallic nanoparticles (NPs) have been widely used in cosmetics and medicine due to their unique antibacterial and antitumor properties. CE can be used as a reducing and stabilizing agent. CE-silver nanoparticles, synthesized by biosynthesis, showed good inhibitory activity against marine bacterial communities and Nitzschia closterium diatoms activity (112). Carboxymethyl cellulose structured silver-based nanocomposite (CMC-AgNPs) containing CE has shown antibacterial, in vivo anti-inflammatory, antileishmanial and antioxidant activities with low cytotoxicity (113). In addition, the synthesis of Au/Ag bimetallic nanoparticles by a single-step green route with CE significantly enhanced antioxidant and catalytic activity compared to individual monometallic nanoparticles (114).
Nano-encapsulation technology has been widely used in recent years. A study by Radün et al. (115) showed that the nano-encapsulated CEO had a strong antibacterial inhibitory capacity. CEO nanofibers formed by encapsulation in chitosan and polyethylene oxide polymers showed good antibacterial activity against Staphylococcus aureus and Escherichia coli, no cytotoxicity against humans fibroblast cell lines, and exhibited good wound healing potential (116). The retention of CEO in chitosan nanoparticles (ChNPs) was as high as 55.8-73.4%, and its antioxidant activity was significantly higher. Ashjazadeh et al. (117) also demonstrated that CE nanofibers showed the best granulation tissue by producing collagen and outperformed nanofibers such as nano zinc oxide in promoting wound healing in rats. Shetty et al. (118) found that ethosomal gel of the CEO was more effective in the treatment of cutaneous candidiasis than the pure CEO. In the croton oil-induced skin inflammation model in mice, it was found that nanofibers and nanoemulsions were more effective in the topical treatment of inflammation, and the efficacy of nanofibers was relatively higher than that of medicinal nanoemulsions (119). Plant essential oil is lipophilic, can easily cross cell membranes, and has greater anticancer efficacy potential. The CEO-based nanoemulsion system can increase drug retention time and improve bioavailability, making it a good candidate for cancer drug delivery systems. In addition, CEO nanoemulsions have greater potential in the food and agriculture industries. CEO-based nanoemulsions prepared by ultrasound using Tween 80 can be used as a natural delivery system to extend the shelf life of food products (120). The use of CEO-based nanoemulsions as green nanocarriers can significantly improve the solubility, bioavailability, and release of the pesticide Atrazine (ATZ). ATZ nanoemulsions also exhibited excellent herbicidal activity at low concentrations compared to commercial ATZ analogs (121).
Syzygium aromaticum showed beneficial advantages in antibacterial and antifungal activity, aromaticity and safety, especially against a wide range of food-borne microorganisms, making it a potential and valuable preservative in the food industry. The antifungal activity of clove is superior to that of lemongrass and thyme essential oils to prevent the natural preservation growth of fungi on dried apricots (122). Ju et al. (123) confirmed by accelerated storage tests that the CEO can extend the shelf life of food bakery products by 2–4 days under normal packaging. Hasheminejad et al. (124) found that encapsulation of CEO by ChNPs was more effective in extending the shelf life of food than CEO alone, and in a pomegranate shelf life and quality study, CEO-ChNPs improved the antifungal effect of CEO, effectively extending the shelf life by 54 days. Both CEO and CEO-based nanoemulsions inoculated into Talaga cheese were found to have significant inhibitory activity against foodborne pathogens such as Listeria monocytogenes and Shigella flexneri. Compared to CEO, CEO-based nanoemulsions significantly reduced the counts of inoculated pathogens from 8.2 to 1.5 log10cfu/g, showing greater antimicrobial effect (125). In addition, the addition of CEO to the structure of electrospun zein can better inhibit the growth of Listeria monocytogenes and Escherichia coli, effectively extending the shelf life of Iranian white cheese (126). The addition of clove powder to kimchi paste inhibited the growth of total aerobic and lactic acid bacteria, delayed the changes in O2 and CO2 concentrations and sugar and organic acid contents, and slowed down the decrease in pH, thus extending its shelf life (127). In addition, Eugenol-lean clove extracts can be used as a substitute for mustard flavoring in mayonnaise. They can improve its associated physical properties, giving it higher antioxidant activity and reducing capacity (128). Mixing the active ingredients in S. aromaticum into feeds has potential uses for improving meat quality, for example, adding S. aromaticum seeds to broiler chicken diets can significantly improve water retention, cooking loss percentage and tenderness of meat (129).
Plant-based biopesticides have been proposed to be the best pest control tools compared to conventional synthetic molecules. Many plants’ essential oils exhibit broad-spectrum insecticidal and repellent properties, are relatively non-toxic to mammals and fish and have gradually developed become potential alternatives to synthetic insecticides. S. aromaticum has been widely studied for its excellent insecticidal biological potential and higher safety for the environment and humans (130). The insecticidal potential of clove seed powder was evaluated by red palm weevil. It was found that S. aromaticum seeds powder at a dosage of 7 mg resulted in 100% mortality within three days (30). Terpenoids in S. aromaticum can reduce the respiratory rate of Sitophilus granarius L. exhibit a tropism effect, and prevent or delay the development of insecticide resistance (131). Viteri et al. (132) found that CEO had similar insecticidal activity to the synthetic pyrethroid insecticide deltamethrin, significantly affecting the oviposition of sublethally exposed Callosobruchus maculatus females and affecting their population growth. In addition, S. aromaticum showed high levels of repellency against fleas, aphids, nymphal instars, mites, mosquito species (Aedes aegypti, Anopheles gambiae, Culex quinquefasciatus etc.), termite and red imported fire ants etc. (133, 134). Toxicity assessment of clove powder, eugenol, eugenol acetate, and β-caryophyllene against red imported fire ants Solenopsis invicta Buren revealed that application of clove powder at 3 and 12 mg/cm2 provided 100% ant mortality within 6 h and repelled 99% within 3 h. Compared to eugenol acetate, β-caryophyllene and CEO, eugenol was the compound with the fastest action against red imported fire ants. And with increasing application rates, the LT50 values of the chemical compounds inclined exponentially (135). The findings suggest that S. aromaticum has potential as a natural source of insecticides.
While acetaldehyde is the main cytotoxin formed by alcohol metabolism and causes liver damage, extracellular matrix changes, inflammation, and hangover in heavy drinkers, clove polyphenol extract can accelerate the elimination of acetaldehyde from human blood, reduces hangover, and alleviates alcohol-related side effects (136). Clove has also been used worldwide as an anesthetic for various fish species, including several Amazon fish, Far Eastern catfish, small-sized tropical fish, and Pacific hagfish. Due to its better antioxidant effects, clove has also been used in recent years in studies on the role of skin barrier repair, where it activates the nuclear erythroid 2-related factor/antioxidant response element (Nrf2/ARE) signaling pathway to increase antioxidant activity. It significantly increases type I procollagen and elastin levels through TGF/Smad signaling and effectively ameliorates UVB-induced photoaging (137).
This paper review the nutritional composition, phytochemistry, pharmacological effects and application prospects of S. aromaticum by combining traditional literature with modern evidence. Eugenol is the main phytoconstituent of S. aromaticum, which is involved in almost all the pharmacological activities of S. aromaticum and has been extensively studied in recent years. S. aromaticum also has a variety of nutritional and other phytoconstituents, such as β-Caryophyllene, α-Humulene, sesquiterpene, flavonoids, etc. These compounds are responsible for the powerful antioxidant and antibacterial properties of S. aromaticum. Respectively, no in-depth studies were carried out on individual substances to explore their pharmacological effects and mechanisms.
The current research on S. aromaticum mainly focuses on the active ingredients of flower buds (clove). A large amount of literature has proved that the CE has various pharmacological activities, such as antioxidant, antibacterial, anti-inflammatory, antiviral, analgesic, neuroprotective, hypoglycemic, anticancer, etc. Clove has been widely used in food, medicine, and cosmetics. The other parts of S. aromaticum (seeds, leaves, etc.) also have similar active ingredients as those in flower buds. However, no extensive studies were carried out on other parts of S. aromaticum. Thus, more research on other parts of the S. aromaticum and the compounds present is worth it.
In addition, although there is a large amount of pharmacological evidence in the literature that can lay the foundation for clinical studies on S. aromaticum, a search through the ClinicalTrials.gov and Chinese Clinical Trials Registry shows that there are still less than 30 registered clinical studies on S. aromaticum. These studies mainly focus on the effects of clove oil on pain and dental caries, as well as the clinical efficacy observation of Chinese medicine such as Dingxiang Shidi Decoction, Dingxiang Kaiwei Paste combined with other therapies. The results of the only clinical studies on S. aromaticum have proved that its biological activity is consistent with the results of preclinical studies. However, due to the small number and scope of clinical studies, it still cannot fully reflect the real clinical situation, and the clinical value of S. aromaticum has not been fully explored. Therefore, it is recommended that controlled clinical studies be conducted to understand more about the pharmacology, molecular mechanisms, and safety of S. aromaticum. In-depth studies on the bioactivity of the constituents, their structure-activity relationships and potential interactions (either synergistic or antagonistic) should be carried out.
In conclusion, S. aromaticum is an edible plant with various bioactive components and biological activities. It has a lot of opportunities for further development to improve its value and use in the food and pharmaceutical industries.
XL coordinated technical support and funding. PG performed data collection and coordinated technical support. QX and ZX performed the study and drafted the manuscript. SW and ZC revised the manuscript. All authors read and approved the final manuscript.
This work was financially supported by Department of Science & Technology of Shandong Province (China) (Nos. YDZX2021083 and 2019JZZY020906), and Shandong University of Traditional Chinese Medicine (China) (No. 2021-0033).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2022.1002147/full#supplementary-material
CEO, clove essential oil; CE, clove extract; ECE, clove ethanol extract; ACE, the aqueous extract of clove; ChNPs, chitosan nanoparticles.
1. Gooderham NJ, Cohen SM, Eisenbrand G, Fukushima S, Guengerich FP, Hecht SS, et al. The safety evaluation of food flavoring substances: the role of genotoxicity studies. Crit Rev Toxicol. (2020) 50:1–27. doi: 10.1080/10408444.2020.1712589
2. Mathot AG, Postollec F, Leguerinel I. Bacterial spores in spices and dried herbs: the risks for processed food. Comprehens Rev Food Sci Food Safe. (2020) 20:840–62. doi: 10.1111/1541-4337.12690
3. Guldiken B, Ozkan G, Catalkaya G, Ceylan FD, Ekin Yalcinkaya I, Capanoglu E. Phytochemicals of herbs and spices: health versus toxicological effects. Food Chem Toxicol. (2018) 119:37–49. doi: 10.1016/j.fct.2018.05.050
4. Batiha GES, Beshbishy AM, Tayebwa DS, Shaheen HM, Yokoyama N, Igarashi I. Inhibitory effects of Syzygium aromaticum and Camellia sinensis methanolic extracts on the growth of Babesia and Theileria parasites. Ticks Tick-Borne Dis. (2019) 10:949–58. doi: 10.1016/j.ttbdis.2019.04.016
5. Kamatou GP, Vermaak I, Viljoen AM. Eugenol—from the remote maluku islands to the international market place: a review of a remarkable and versatile molecule. Molecules. (2012) 17:6953–81. doi: 10.3390/molecules17066953
6. Cortés-Rojas DF, de Souza CRF, Oliveira WP. Clove (Syzygium aromaticum): a precious spice. Asian Pacific J Trop Biomed. (2014) 4:90–6. doi: 10.1016/s2221-1691(14)60215-x
7. Golmakani MT, Zare M, Razzaghi S. Eugenol enrichment of clove bud essential oil using different microwave-assisted distillation methods. Food Sci Technol Res. (2017) 23:385–94. doi: 10.3136/fstr.23.385
8. Tunç MT, Koca L. Ohmic heating assisted hydrodistillation of clove essential oil. Indus Crops Prod. (2019) 141:111763. doi: 10.1016/j.indcrop.2019.111763
9. Alfikri FN, Pujiarti R, Wibisono MG, Hardiyanto EB. Yield, quality, and antioxidant activity of clove (Syzygium aromaticumL.) bud oil at the different phenological stages in young and mature trees. Scientifica. (2020) 2020:9701701. doi: 10.1155/2020/9701701
10. Wei MC, Xiao J, Yang YC. Extraction of α-humulene-enriched oil from clove using ultrasound-assisted supercritical carbon dioxide extraction and studies of its fictitious solubility. Food Chem. (2016) 210:172–81. doi: 10.1016/j.foodchem.2016.04.076
11. Liu JG, Liu HP. Total nutrient analysis of various parts of Eugenia caryophyllata. Biotic Res. (2021) 43:357–62. doi: 10.14188/j.ajsh.2021.04.005
12. Ma SS. Food safety standards and development of health food of clove. Ph.D.thesis. China: Hainan University (2018).
14. Al-Jasass FM, Al-Jasser MS. Chemical composition and fatty acid content of some spices and herbs under Saudi Arabia conditions. Sci World J. (2012) 2012:859892. doi: 10.1100/2012/859892
15. Li YL, Liu ZS. Optimal extraction and fatty acid analysis of oil from syringa linn leaves. Food Indus. (2020) 41:80–2.
16. Brima EI, Siddeeg SM. Pilot study of trace elements in the infusion of medicinal plants used for diabetes treatment. Int J Anal Chem. (2022) 2022:3021396. doi: 10.1155/2022/3021396
17. Stark AH, Reifen R, Crawford MA. Past and present insights on alpha-linolenic acid and the omega-3 fatty acid family. Crit Rev Food Sci Nutr. (2016) 56:2261–7. doi: 10.1080/10408398.2013.828678
18. Marangoni F, Agostoni C, Borghi C, Catapano AL, Cena H, Ghiselli A, et al. Dietary linoleic acid and human health: focus on cardiovascular and cardiometabolic effects. Atherosclerosis. (2020) 292:90–8. doi: 10.1016/j.atherosclerosis.2019.11.018
19. Yang L, Guan G, Lei L, Lv Q, Liu S, Zhan X, et al. Palmitic acid induces human osteoblast-like Saos-2 cell apoptosis via endoplasmic reticulum stress and autophagy. Cell Stress Chaperones. (2018) 23:1283–94. doi: 10.1007/s12192-018-0936-8
20. Diyya ASM, Thomas NV. Multiple micronutrient supplementation: as a supportive therapy in the treatment of covid-19. Biomed Res Int. (2022) 2022:3323825. doi: 10.1155/2022/3323825
21. Chaieb K, Hajlaoui H, Zmantar T, Kahla-Nakbi AB, Rouabhia M, Mahdouani K, et al. The chemical composition and biological activity of clove essential oil,Eugenia caryophyllata (Syzigium aromaticum L. Myrtaceae): a short review. Phytother Res. (2007) 21:501–6. doi: 10.1002/ptr.2124
22. Al-Hashimi AG, Ammar AB, G L, Cacciola F, Lakhssassi N. Development of a millet starch edible film containing clove essential oil. Foods. (2020) 9:184. doi: 10.3390/foods9020184
23. Achar PN, Quyen P, Adukwu EC, Sharma A, Msimanga HZ, Nagaraja H, et al. Investigation of the antifungal and anti-aflatoxigenic potential of plant-based essential oils against aspergillus flavus in peanuts. J Fungi. (2020) 6:383. doi: 10.3390/jof6040383
24. Yassin MT, Mostafa AAF, Al-Askar AA. In vitro anticandidal potency of Syzygium aromaticum (clove) extracts against vaginal candidiasis. BMC Complement Med Ther. (2020) 20:25. doi: 10.1186/s12906-020-2818-8
25. Hemalatha R, Nivetha P, Mohanapriya C, Sharmila G, Muthukumaran C, Gopinath M. Phytochemical composition, GC-MS analysis, in vitro antioxidant and antibacterial potential of clove flower bud (Eugenia caryophyllus) methanolic extract. J Food Sci Technol. (2015) 53:1189–98. doi: 10.1007/s13197-015-2108-5
26. Muñoz Castellanos L, Amaya Olivas N, Ayala-Soto J, de la O Contreras CM, Zermeño Ortega M, Sandoval Salas F, et al. In vitro and in vivo antifungal activity of clove (Eugenia caryophyllata) and pepper (Piper nigrum L.) essential oils and functional extracts against Fusarium oxysporum and Aspergillus niger in tomato (Solanum lycopersicum L.). Int J Microbiol. (2020) 2020:1702037. doi: 10.1155/2020/1702037
27. El-Garawani IM, El-Nabi SH, Dawoud GT, Esmail SM, Abdel Moneim AE. Triggering of apoptosis and cell cycle arrest by fennel and clove oils in Caco-2 cells: the role of combination. Toxicol Mechan Methods. (2019) 29:710–22. doi: 10.1080/15376516.2019.1650149
28. Oboh G, Akinbola IA, Ademosun AO, Sanni DM, Odubanjo OV, Olasehinde TA, et al. Essential oil from clove bud (Eugenia aromatica Kuntze) inhibit key enzymes relevant to the management of type-2 diabetes and some pro-oxidant induced lipid peroxidation in rats pancreas in vitro. J Oleo Sci. (2015) 64:775–82. doi: 10.5650/jos.ess14274
29. Jirovetz L, Buchbauer G, Stoilova I, Stoyanova A, Krastanov A, Schmidt E. Chemical composition and antioxidant properties of clove leaf essential oil. J Agric Food Chem. (2006) 54:6303–7. doi: 10.1021/jf060608c
30. Al Dawsari Mona M. Insecticidal potential of cardamom and clove extracts on adult red palm weevil Rhynchophorus ferrugineus. Saudi J Biol Sci. (2020) 27:195–201. doi: 10.1016/j.sjbs.2019.07.009
31. Barboza JN, da Silva Maia Bezerra Filho C, Silva RO, Medeiros JVR, de Sousa DP. An overview on the anti-inflammatory potential and antioxidant profile of eugenol. Oxid Med Cell Longev. (2018) 2018:3957262. doi: 10.1155/2018/3957262
32. Tholl D. Biosynthesis and biological functions of terpenoids in plants. Biotechnol Isopre. (2015) 148:63–106. doi: 10.1007/10_2014_295
33. Ryu B, Kim HM, Lee JS, Lee CK, Sezirahiga J, Woo JH, et al. New flavonol glucuronides from the flower buds of Syzygium aromaticum (Clove). J Agric Food Chem. (2016) 64:3048–53. doi: 10.1021/acs.jafc.6b00337
34. Cai L, Wu CD. Compounds from Syzygium aromaticum possessing growth inhibitory activity against oral pathogens. J Nat Prod. (1996) 59:987–90. doi: 10.1021/np960451q
35. Ali A, Wu H, Ponnampalam EN, Cottrell JJ, Dunshea FR, Suleria HAR. Comprehensive profiling of most widely used spices for their phenolic compounds through LC-ESI-QTOF-MS2 and their antioxidant potential. Antioxidants. (2021) 10:721. doi: 10.3390/antiox10050721
36. Sara, Ali SN, Begum S, Siddiqui BS. Chemical constituents of Syzygium aromaticum. Chem Nat Comp. (2018) 54:1192–3. doi: 10.1007/s10600-018-2593-7
37. Han AR. Identification and PEP inhibitory activity of acetophenone glucosides from the clove buds (Syzygium aromaticum). J Korean Soc Appl Biol Chem. (2010) 53:847–51. doi: 10.3839/jksabc.2010.129
38. Lee HH, Shin JS, Lee WS, Ryu B, Jang DS, Lee KT. Biflorin, isolated from the flower buds of Syzygium aromaticum L., suppresses LPS-induced inflammatory mediators via STAT1 inactivation in macrophages and protects mice from endotoxin shock. J Nat Prod. (2016) 79:711–20. doi: 10.1021/acs.jnatprod.5b00609
39. Kim HJ, Lee JS, Woo ER, Kim MK, Yang BS, Yu YG, et al. Isolation of virus-cell fusion inhibitory components from Eugenia caryophyllata. Planta Med. (2001) 67:277–9. doi: 10.1055/s-2001-11993
40. Hatano T, Eerdunbayaer, Nozaki A, Takahashi E, Okamoto K, Ito H, et al. Hydrolysable tannins isolated from Syzygium aromaticum: structure of a new c-glucosidic ellagitannin and spectral features of tannins with a tergalloyl group. Heterocycles. (2012) 85:365. doi: 10.3987/com-11-12392
41. Nassar MI. Flavonoid triglycosides from the seeds of Syzygium aromaticum. Carbohyd Res. (2006) 341:160–3. doi: 10.1016/j.carres.2005.10.012
42. Zakaryan H, Arabyan E, Oo A, Zandi K. Flavonoids: promising natural compounds against viral infections. Arch Virol. (2017) 162:2539–51. doi: 10.1007/s00705-017-3417-y
43. Jeon SJ, Kim B, Ryu B, Kim E, Lee S, Jang DS, et al. Biflorin ameliorates memory impairments induced by cholinergic blockade in mice. Biomol Ther. (2017) 25:249–58. doi: 10.4062/biomolther.2016.058
44. Javed A, Hussain MB, Tahir A, Waheed M, Anwar A, Shariati MA, et al. Pharmacological applications of phlorotannins: a comprehensive review. Curr Drug Disc Technol. (2021) 18:282–92. doi: 10.2174/1570163817666200206110243
45. Jiang TA. Health benefits of culinary herbs and spices. J AOAC Int. (2019) 102:395–411. doi: 10.5740/jaoacint.18-0418
46. Ladurner A, Zehl M, Grienke U, Hofstadler C, Faur N, Pereira FC, et al. Allspice and clove as source of triterpene acids activating the G protein-coupled bile acid receptor TGR5. Front Pharmacol. (2017) 8:468. doi: 10.3389/fphar.2017.00468
47. Chniguir A, Pintard C, Liu D, Dang PMC, El-Benna J, Bachoual R. Eugenol prevents fMLF-induced superoxide anion production in human neutrophils by inhibiting ERK1/2 signaling pathway and p47phox phosphorylation. Sci Rep. (2019) 9:18540. doi: 10.1038/s41598-019-55043-8
48. Suantawee T, Wesarachanon K, Anantsuphasak K, Daenphetploy T, Thien-Ngern S, Thilavech T, et al. Protein glycation inhibitory activity and antioxidant capacity of clove extract. J Food Sci Technol. (2015) 52:3843–50. doi: 10.1007/s13197-014-1452-1
49. Fauzya AF, Astuti RI, Mubarik NR. Effect of ethanol-derived clove leaf extract on the oxidative stress response in yeast Schizosaccharomyces pombe. Int J Microbiol. (2019) 2019:2145378. doi: 10.1155/2019/2145378
50. Sanae F, Kamiyama O, Ikeda-Obatake K, Higashi Y, Asano N, Adachi I, et al. Effects of eugenol-reduced clove extract on glycogen phosphorylase b and the development of diabetes in db/db mice. Food Funct. (2014) 5:214–9. doi: 10.1039/c3fo60514k
51. Abdulrazak A, Tanko Y, Mohammed A, Mohammed KA, Sada NM, Dikko AA. Effects of clove and fermented ginger on blood glucose, leptin, insulin and insulin receptor levels in high fat dietinduced type 2 diabetic rabbits. Nigerian J Physiol Sci. (2018) 33:89–93.
52. Liu H, Schmitz JC, Wei J, Cao S, Beumer JH, Strychor S, et al. Clove extract inhibits tumor growth and promotes cell cycle arrest and apoptosis. Oncol Res Featur Preclin Clin Cancer Ther. (2014) 21:247–59. doi: 10.3727/096504014x13946388748910
53. Sara, Begum S, Ali SN, Farooq AD, Siddiqui F, Siddiqui BS, et al. Volatile constituents and in vitro activity of Syzygium aromaticum flower buds (clove) against human cancer cell lines. Pak J Pharm Sci. (2020) 33:2659–65.
54. Zhang Y, Wang Y, Zhu X, Cao P, Wei S, Lu Y. Antibacterial and antibiofilm activities of eugenol from essential oil of Syzygium aromaticum (L.) Merr. & L. M. Perry (clove) leaf against periodontal pathogen Porphyromonas gingivalis. Microb Pathog. (2017) 113:396–402. doi: 10.1016/j.micpath.2017.10.054
55. Ajiboye TO, Mohammed AO, Bello SA, Yusuf II, Ibitoye OB, Muritala HF, et al. Antibacterial activity of Syzygium aromaticum seed: studies on oxidative stress biomarkers and membrane permeability. Microb Pathog. (2016) 95:208–15. doi: 10.1016/j.micpath.2016.03.011
56. Ginting EV, Retnaningrum E, Widiasih DA. Antibacterial activity of clove (Syzygium aromaticum) and cinnamon (Cinnamomum burmannii) essential oil against extended-spectrum β-lactamase-producing bacteria. Vet World. (2021) 14:2206–11. doi: 10.14202/vetworld.2021.2206-2211
57. Han X, Parker TL. Anti-inflammatory activity of clove (Eugenia caryophyllata) essential oil in human dermal fibroblasts. Pharmac Biol. (2017) 55:1619–22. doi: 10.1080/13880209.2017.1314513
58. Dibazar SP, Fateh S, Daneshmandi S. Clove (Syzygium aromaticum) ingredients affect lymphocyte subtypes expansion and cytokine profile responses: an in vitro evaluation. J Food Drug Anal. (2014) 22:448–54. doi: 10.1016/j.jfda.2014.04.005
59. Akbar L, Juliandi B, Boediono A, Batubara I, Subangkit M. Effects of eugenol on memory performance, neurogenesis, and dendritic complexity of neurons in mice analyzed by behavioral tests and golgi staining of brain tissue. J Stem Cells Regen Med. (2021) 17:35–41. doi: 10.46582/jsrm.1701005
60. Lane T, Anantpadma M, Freundlich JS, Davey RA, Madrid PB, Ekins S. The natural product eugenol is an inhibitor of the ebola virus in vitro. Pharmac Res. (2019) 36:104. doi: 10.1007/s11095-019-2629-0
61. Tragoolpua Y, Jatisatienr A. Anti-herpes simplex virus activities of Eugenia caryophyllus (Spreng.) Bullock & S. G. Harrison and essential oil, eugenol. Phytother Res. (2007) 21:1153–8. doi: 10.1002/ptr.2226
62. Jung CH, Ahn J, Jeon TI, Kim TW, Ha TY. Syzygium aromaticum ethanol extract reduces high-fat diet-induced obesity in mice through downregulation of adipogenic and lipogenic gene expression. Exp Ther Med. (2012) 4:409–14. doi: 10.3892/etm.2012.609
63. Vella FM, Calandrelli R, Cautela D, Fiume I, Pocsfalvi G, Laratta B. Chemometric screening of fourteen essential oils for their composition and biological properties. Molecules. (2020) 25:5126. doi: 10.3390/molecules25215126
64. Baghshahi H, Riasi A, Mahdavi A, Shirazi A. Antioxidant effects of clove bud (Syzygium aromaticum) extract used with different extenders on ram spermatozoa during cryopreservation. Cryobiology. (2014) 69:482–7. doi: 10.1016/j.cryobiol.2014.10.009
65. Zhang L, Gu B, Wang Y. Clove essential oil confers antioxidant activity and lifespan extension in C. Elegans via the DAF-16/FOXO transcription factor. Comparat Biochem Physiol Part C. (2021) 242:108938. doi: 10.1016/j.cbpc.2020.108938
66. Gülçin L. Antioxidant activity of eugenol: a structure–activity relationship study. J Med Food. (2011) 14:975–85. doi: 10.1089/jmf.2010.0197
67. Nam H, Kim MM. Eugenol with antioxidant activity inhibits MMP-9 related to metastasis in human fibrosarcoma cells. Food Chem Toxicol. (2013) 55:106–12. doi: 10.1016/j.fct.2012.12.050
68. Misharina TA, Fatkullina LD, Alinkina ES, Kozachenko AI, Nagler LG, Medvedeva IB, et al. Effects of low doses of essential oil on the antioxidant state of the erythrocytes, liver, and the brains of mice. Прикладная Биохимия и Микробиология (2014) 50:101–7. doi: 10.7868/s0555109914010097
69. Tu Z, Moss-Pierce T, Ford P, Jiang TA. Syzygium aromaticumL. (Clove) extract regulates energy metabolism in myocytes. J Med Food. (2014) 17:1003–10. doi: 10.1089/jmf.2013.0175
70. Ghaffar S, Afridi SK, Aftab MF, Murtaza M, Hafizur RM, Sara S, et al. Clove and its active compound attenuate free fatty acid-mediated insulin resistance in skeletal muscle cells and in mice. J Med Food. (2017) 20:335–44. doi: 10.1089/jmf.2016.3835
71. Kuroda M, Mimaki Y, Ohtomo T, Yamada J, Nishiyama T, Mae T, et al. Hypoglycemic effects of clove (Syzygium aromaticum flower buds) on genetically diabetic KK-Ay mice and identification of the active ingredients. J Nat Med. (2012) 66:394–9. doi: 10.1007/s11418-011-0593-z
72. Arung ET, Matsubara E, Kusuma IW, Sukaton E, Shimizu K, Kondo R. Inhibitory components from the buds of clove (Syzygium aromaticum) on melanin formation in B16 melanoma cells. Fitoterapia. (2011) 82:198–202. doi: 10.1016/j.fitote.2010.09.008
73. Zhao G, Zhang D, Yang XH, Li XF, Liu MH. [Inhibition effect of active fraction from clove on PI3K/Akt/mTOR signaling pathway to induce apoptosis of human colon cancer HCT116 cells]. Zhongguo Zhong Yao Za Zhi. (2021) 46:1197–204. doi: 10.19540/j.cnki.cjcmm.20201027.401
74. Kello M, Takac P, Kubatka P, Kuruc T, Petrova K, Mojzis J. Oxidative stress-induced DNA damage and apoptosis in clove buds-treated MCF-7 cells. Biomolecules. (2020) 10:139. doi: 10.3390/biom10010139
75. Banerjee S. Clove (Syzygium aromaticum L.), a potential chemopreventive agent for lung cancer. Carcinogenesis. (2006) 27:1645–54. doi: 10.1093/carcin/bgi372
76. Choudhury P, Barua A, Roy A, Pattanayak R, Bhattacharyya M, Saha P. Eugenol restricts Cancer stem cell population by degradation of β-catenin via N-terminal Ser37 phosphorylation-an in vivo and in vitro experimental evaluation. Chemico-Biol Interact. (2020) 316:108938. doi: 10.1016/j.cbi.2020.108938
77. Mostaqim S, Saha SK, Hani U, Paul SK, Sharmin M, Basak S, et al. Antibacterial activities of clove (Syzygium aromaticum) extracts against three food borne pathogens: Staphylococcus aureus, Escherichia coli and Pseudomonas aeruginosa. Mymens Med J. (2019) 28:779–91.
78. Xu JG, Liu T, Hu QP, Cao XM. Chemical composition, antibacterial properties and mechanism of action of essential oil from clove buds against Staphylococcus aureus. Molecules. (2016) 21:1194. doi: 10.3390/molecules21091194
79. H J, Omanakuttan A, Pandurangan N, Vargis SV, Maneesh M, Nair GB, et al. Clove bud oil reduces kynurenine and inhibits pqs A gene expression in P. aeruginosa. Appl Microbiol Biotechnol. (2016) 100:3681–92. doi: 10.1007/s00253-016-7313-2
80. Haripriyan J, Omanakuttan A, Menon ND, Vanuopadath M, Nair SS, Corriden R, et al. Clove bud oil modulates pathogenicity phenotypes of the opportunistic human pathogen Pseudomonas aeruginosa. Sci Rep. (2018) 8:3437. doi: 10.1038/s41598-018-19771-7
81. Kovács JK, Felsõ P, Makszin L, Pápai Z, Horváth G, Ábrahám H, et al. Antimicrobial and virulence-modulating effects of clove essential oil on the foodborne pathogen campylobacter jejuni. Appl Environ Microbiol. (2016) 82:6158–66. doi: 10.1128/aem.01221-16
82. Wang Y, Ding Y, Wang S, Chen H, Zhang H, Chen W, et al. Extract of Syzygium aromaticum suppress eEF1A protein expression and fungal growth. J Appl Microbiol. (2017) 123:80–91. doi: 10.1111/jam.13478
83. Bahramsoltani R, Rahimi R. An evaluation of traditional persian medicine for the management of SARS-CoV-2. Front Pharmacol. (2020) 11:571434. doi: 10.3389/fphar.2020.571434
84. Rodrigues T, Fernandes A, Sousa J, Bastos J, Sforcin J. In vitro and in vivo effects of clove on pro-inflammatory cytokines production by macrophages. Nat Prod Res. (2009) 23:319–26. doi: 10.1080/14786410802242679
85. Carrasco FR, Schmidt G, Romero AL, Sartoretto JL, Caparroz-Assef SM, Bersani-Amado CA, et al. Immunomodulatory activity of Zingiber officinale Roscoe, Salvia officinalis L. and Syzygium aromaticum L. essential oils: evidence for humor- and cell-mediated responses. J Pharm Pharmacol. (2009) 61:961–7. doi: 10.1211/jpp/61.07.0017
86. Ferland CE, Beaudry F, Vachon P. Antinociceptive effects of eugenol evaluated in a monoiodoacetate-induced osteoarthritis rat model. Phytother Res. (2012) 26:1278–85. doi: 10.1002/ptr.3725
87. Taher YA, Samud AM, El-Taher FE, ben-Hussin G, Elmezogi JS, Al-Mehdawi BF, et al. Experimental evaluation of anti-inflammatory, antinociceptive and antipyretic activities of clove oil in mice. Libyan J Med. (2015) 10:28685. doi: 10.3402/ljm.v10.28685
88. Halder S, Mehta A, Kar R, Mustafa M, Mediratta P, Sharma K. Clove oil reverses learning and memory deficits in scopolamine-treated mice. Planta Med. (2011) 77:830–4. doi: 10.1055/s-0030-1250605
89. Kumar A, Aggrawal A, Pottabathini R, Singh A. Possible neuroprotective mechanisms of clove oil against icv-colchicine induced cognitive dysfunction. Pharmacol Rep. (2016) 68:764–72. doi: 10.1016/j.pharep.2016.03.005
90. Shekhar S, Yadav Y, Singh AP, Pradhan R, Desai GR, Dey A, et al. Neuroprotection by ethanolic extract of Syzygium aromaticum in Alzheimer’s disease like pathology via maintaining oxidative balance through SIRT1 pathway. Exp Gerontol. (2018) 110:277–83. doi: 10.1016/j.exger.2018.06.026
91. Lee HS, Yang EJ, Lee T, Song KS. Laccase fermentation of clove extract increases content of dehydrodieugenol, which has neuroprotective activity against glutamate toxicity in HT22 cells. J Microbiol Biotechnol. (2018) 28:246–54. doi: 10.4014/jmb.1709.09052
92. Rai N, Upadhyay AD, Goyal V, Dwivedi S, Dey AB, Dey S. Sestrin2 as serum protein marker and potential therapeutic target for Parkinson’s disease. J Gerontol A Biol Sci Med Sci. (2020) 75:690–5. doi: 10.1093/gerona/glz234
93. Kadri Y, Nciri R, Bardaa S, Brahmi N, Saber S, Harrath AH, et al. Syzygium Aromaticum alleviates cerium chloride-induced neurotoxic effect in the adult mice. Toxicol Mechan Methods. (2019) 29:26–34. doi: 10.1080/15376516.2018.1506849
94. Aboubakr HA, Nauertz A, Luong NT, Agrawal S, El-Sohaimy SAA, Youssef MM, et al. In vitro antiviral activity of clove and ginger aqueous extracts against feline calicivirus, a surrogate for human norovirus. J Food Protect. (2016) 79:1001–12. doi: 10.4315/0362-028x.jfp-15-593
95. Dai JP, Zhao XF, Zeng J, Wan QY, Yang JC, Li WZ, et al. Drug screening for autophagy inhibitors based on the dissociation of Beclin1-Bcl2 complex using BiFC technique and mechanism of eugenol on anti-influenza a virus activity. PLoS One. (2013) 8:e61026. doi: 10.1371/journal.pone.0061026
96. Rehman MT, AlAjmi MF, Hussain A. Natural compounds as inhibitors of SARS-CoV-2 main protease (3CLpro): a molecular docking and simulation approach to combat covid-19. Curr Pharmac Design. (2021) 27:3577–89. doi: 10.2174/1381612826999201116195851
97. Ding Y, Gu Z, Wang Y, Wang S, Chen H, Zhang H, et al. Clove extract functions as a natural fatty acid synthesis inhibitor and prevents obesity in a mouse model. Food Func. (2017) 8:2847–56. doi: 10.1039/c7fo00096k
98. Pé,rez Gutiérrez RM, Arrioja MW. Rapid model to evaluate the anti-obesity potential of a combination of Syzygium aromaticum (Clove) and Cuminun cyminum (Cumin) on C57BL6/j mice fed high-fat diet. J Visual Exp. (2021) 173. doi: 10.3791/62087
99. Mishra RK, Singh SK. Biphasic effect of Syzygium aromaticum flower bud on reproductive physiology of male mice. Andrologia. (2016) 48:1011–20. doi: 10.1111/and.12533
100. Choi D, Roh HS, Kang DW, Lee JS. The potential regressive role of Syzygium aromaticum on the reproduction of male golden hamsters. Dev Reprod. (2014) 18:57–64. doi: 10.12717/dr.2014.18.1.057
101. Jin SE, Lee MY, Shin IS, Jeon WY, Ha H. Syzygium aromaticum water extract attenuates ethanol-induced gastric injury through antioxidant effects in rats. Mol Med Rep. (2016) 14:361–6. doi: 10.3892/mmr.2016.5269
102. Chen J, Jiang QD, Wu YM, Liu P, Yao JH, Lu Q, et al. Potential of essential oils as penetration enhancers for transdermal administration of ibuprofen to treat dysmenorrhoea. Molecules. (2015) 20:18219–36. doi: 10.3390/molecules201018219
103. Escobar-García M, Rodríguez-Contreras K, Ruiz-Rodríguez S, Pierdant-Pérez M, Cerda-Cristerna B, Pozos-Guillén A. Eugenol toxicity in human dental pulp fibroblasts of primary teeth. J Clin Pediatr Dentist. (2016) 40:312–8. doi: 10.17796/1053-4628-40.4.312
104. Liu BB, Luo L, Liu XL, Geng D, Li CF, Chen SM, et al. Essential oil of Syzygium aromaticum reverses the deficits of stress-induced behaviors and hippocampal p-ERK/p-CREB/brain-derived neurotrophic factor expression. Planta Med. (2015) 81:185–92. doi: 10.1055/s-0034-1396150
105. Vijayasteltar L, Nair GG, Maliakel B, Kuttan R, Krishnakumar IM. Safety assessment of a standardized polyphenolic extract of clove buds: subchronic toxicity and mutagenicity studies. Toxicol Rep. (2016) 3:439–49. doi: 10.1016/j.toxrep.2016.04.001
106. Gooderham NJ, Cohen SM, Eisenbrand G, Fukushima S, Guengerich FP, Hecht SS, et al. FEMA GRAS assessment of natural flavor complexes: clove, cinnamon leaf and West Indian bay leaf-derived flavoring ingredients. Food Chem Toxicol. (2020) 145:111585. doi: 10.1016/j.fct.2020.111585
107. Ogawa K, Honda M, Tanigawa A, Hatase A, Ito A, Higa Y, et al. Appetite-enhancing effects of inhaling cinnamon, clove, and fennel essential oils containing phenylpropanoid analogues. J Nat Med. (2020) 74:710–21. doi: 10.1007/s11418-020-01423-8
108. Vicidomini C, Roviello V, Roviello GN. Molecular basis of the therapeutical potential of clove (Syzygium aromaticum L.) and clues to its anti-covid-19 utility. Molecules. (2021) 26:1880. doi: 10.3390/molecules26071880
109. Sreenivasan PK, Kakarla VVP, Sharda S, Setty Y. The effects of a novel herbal toothpaste on salivary lactate dehydrogenase as a measure of cellular integrity. Clin Oral Invest. (2020) 25:3021–30. doi: 10.1007/s00784-020-03623-8
110. Uju DE, Obioma NP. Anticariogenic potentials of clove, tobacco and bitter kola. Asian Pacific J Trop Med. (2011) 4:814–8. doi: 10.1016/s1995-7645(11)60200-9
111. Sarialioglu Gungor A, Donmez N. Dentin erosion preventive effects of various plant extracts: an in vitro atomic force microscopy, scanning electron microscopy, and nanoindentation study. Micro Res Techniq. (2021) 84:1042–52. doi: 10.1002/jemt.23665
112. Lakhan MN, Chen R, Shar AH, Chand K, Shah AH, Ahmed M, et al. Eco-friendly green synthesis of clove buds extract functionalized silver nanoparticles and evaluation of antibacterial and antidiatom activity. J Microbiol Methods. (2020) 173:105934. doi: 10.1016/j.mimet.2020.105934
113. Asghar MA, Yousuf RI, Shoaib MH, Asghar MA, Zehravi M, Rehman AA, et al. Green synthesis and characterization of carboxymethyl cellulose fabricated silver-based nanocomposite for various therapeutic applications. Int J Nanomed. (2021) 16:5371–93. doi: 10.2147/ijn.s321419
114. Sharma C, Ansari S, Ansari MS, Satsangee SP, Srivastava M. Single-step green route synthesis of Au/Ag bimetallic nanoparticles using clove buds extract: enhancement in antioxidant bio-efficacy and catalytic activity. Material Sci Eng. (2020) 116:111153. doi: 10.1016/j.msec.2020.111153
115. Radünz M, da Trindade MLM, Camargo TM, Radünz AL, Borges CD, Gandra EA, et al. Antimicrobial and antioxidant activity of unencapsulated and encapsulated clove (Syzygium aromaticum, L.) essential oil. Food Chem. (2019) 276:180–6. doi: 10.1016/j.foodchem.2018.09.173
116. Hameed M, Rasul A, Waqas M, Saadullah M, Aslam N, Abbas G, et al. Formulation and evaluation of a clove oil-encapsulated nanofiber formulation for effective wound-healing. Molecules. (2021) 26:2491. doi: 10.3390/molecules26092491
117. Ashjazade MA, Jahandideh A, Abedi G, Akbarzadeh A, Hesaraki S. Histopathology and histomorphological study of wound healing using clove extract nanofibers (Eugenol) compared to zinc oxide nanofibers on the skin of rats. Arch Razi Institute. (2019) 74:267–77. doi: 10.22092/ari.2018.120170.1184
118. Shetty S, Jose J, Kumar L, Charyulu RN. Novel ethosomal gel of clove oil for the treatment of cutaneous candidiasis. J Cosmetic Dermatol. (2019) 18:862–9. doi: 10.1111/jocd.12765
119. Aman RM, Abu Hashim II, Meshali MM. Novel clove essential oil nanoemulgel tailored by taguchi’s model and scaffold-based nanofibers: phytopharmaceuticals with promising potential as cyclooxygenase-2 ors in external inflammation </p>. Int J Nanomed. (2020) 15:2171–95. doi: 10.2147/ijn.s246601
120. Singh, P, Kaur G, Singh A. Physical, morphological and storage stability of clove oil nanoemulsion based delivery system. Food Sci Technol Int. (2021) 108201322110694. doi: 10.1177/10820132211069470 [Epub ahead of print].
121. Kumar A, Kanwar R, Mehta SK. Development of phosphatidylcholine/tween 80 based biocompatible clove oil-in-water nanoemulsion as a green nanocarrier for controlled herbicide delivery. Environ Pollut. (2022) 293:118558. doi: 10.1016/j.envpol.2021.118558
122. Debonne E, Yilmaz MS, Sakiyan O, Eeckhout M. Comparison of antifungal activity of essential oils of clove, lemongrass and thyme for natural preservation of dried apricots. Food Sci Technol Int. (2021) 108201322110496. [Epub ahead of print]. doi: 10.1177/10820132211049603
123. Ju J, Xu X, Xie Y, Guo Y, Cheng Y, Qian H, et al. Inhibitory effects of cinnamon and clove essential oils on mold growth on baked foods. Food Chem. (2018) 240:850–5. doi: 10.1016/j.foodchem.2017.07.120
124. Hasheminejad N, Khodaiyan F. The effect of clove essential oil loaded chitosan nanoparticles on the shelf life and quality of pomegranate arils. Food Chem. (2020) 309:125520. doi: 10.1016/j.foodchem.2019.125520
125. Elsherif WM, Talaat Al Shrief LM. Effects of three essential oils and their nano-emulsions on Listeria monocytogenes and Shigella flexneri in Egyptian talaga cheese. Int J Food Microbiol. (2021) 355:109334. doi: 10.1016/j.ijfoodmicro.2021.109334
126. Tayebi-Moghaddam S, Khatibi R, Taklavi S, Hosseini-Isfahani M, Rezaeinia H. Sustained-release modeling of clove essential oil in brine to improve the shelf life of Iranian white cheese by bioactive electrospun zein. Int J Food Microbiol. (2021) 355:109337. doi: 10.1016/j.ijfoodmicro.2021.109337
127. Kang M, Park J, Yoo S. Effect of clove powder on quality characteristics and shelf life of kimchi paste. Food Sci Nutr. (2019) 7:537–46. doi: 10.1002/fsn3.833
128. Chatterjee D, Bhattacharjee P. Use of eugenol-lean clove extract as a flavoring agent and natural antioxidant in mayonnaise: product characterization and storage study. J Food Sci Technol. (2015) 52:4945–54. doi: 10.1007/s13197-014-1573-6
129. Suliman GM, Alowaimer AN, Al-Mufarrej SI, Hussein EO, Fazea EH, Naiel MA, et al. The effects of clove seed (Syzygium aromaticum) dietary administration on carcass characteristics, meat quality, and sensory attributes of broiler chickens. Poul Sci. (2021) 100:100904. doi: 10.1016/j.psj.2020.12.009
130. Toledo PF, Viteri Jumbo LO, Rezende SM, Haddi K, Silva BA, Mello TS, et al. Disentangling the ecotoxicological selectivity of clove essential oil against aphids and non-target ladybeetles. Sci Total Environ. (2020) 718:137328. doi: 10.1016/j.scitotenv.2020.137328
131. Plata-Rueda A, Campos JM, da Silva Rolim G, Martínez LC, dos Santos MH, Fernandes FL, et al. Terpenoid constituents of cinnamon and clove essential oils cause toxic effects and behavior repellency response on granary weevil, Sitophilus granarius. Ecotoxicol Environ Safe. (2018) 156:263–70. doi: 10.1016/j.ecoenv.2018.03.033
132. Viteri Jumbo LO, Haddi K, Faroni LRD, Heleno FF, Pinto FG, Oliveira EE. Toxicity to, oviposition and population growth impairments of Callosobruchus maculatus exposed to clove and cinnamon essential oils. PLoS One. (2018) 13:e0207618. doi: 10.1371/journal.pone.0207618
133. Wahed TB, Mondal M, Rahman MA, Hossen MS, Bhoumik NC, Saha S, et al. Protective role of syzygium cymosum leaf extract against carbofuran-induced hematological and hepatic toxicities. Chem Res Toxicol. (2019) 32:1619–29. doi: 10.1021/acs.chemrestox.9b00164
134. Nentwig G, Frohberger S, Sonneck R. Evaluation of clove oil, icaridin, and transfluthrin for spatial repellent effects in three tests systems against the aedes aegypti (Diptera: Culicidae). J Med Entomol. (2017) 54:150–8. doi: 10.1093/jme/tjw129
135. Kafle L, Shih CJ. Toxicity and repellency of compounds from clove (Syzygium aromaticum) to red imported fire ants Solenopsis invicta (Hymenoptera: Formicidae). J Econ Entomol. (2013) 106:131–5. doi: 10.1603/ec12230
136. Mammen RR, Natinga Mulakal J, Mohanan R, Maliakel B, Illathu Madhavamenon K. Clove bud polyphenols alleviate alterations in inflammation and oxidative stress markers associated with binge drinking: a randomized double-blinded placebo-controlled crossover Study. J Med Food. (2018) 21:1188–96. doi: 10.1089/jmf.2017.4177
Keywords: Syzygium aromaticum, medicinal food, nutritional composition, phytochemistry, bioactivities, applications
Citation: Xue Q, Xiang Z, Wang S, Cong Z, Gao P and Liu X (2022) Recent advances in nutritional composition, phytochemistry, bioactive, and potential applications of Syzygium aromaticum L. (Myrtaceae). Front. Nutr. 9:1002147. doi: 10.3389/fnut.2022.1002147
Received: 24 July 2022; Accepted: 13 September 2022;
Published: 14 October 2022.
Edited by:
Gengjun Chen, Kansas State University, United StatesReviewed by:
Sonia Malik, Université d’Orléans, FranceCopyright © 2022 Xue, Xiang, Wang, Cong, Gao and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaonan Liu, MTgzMDQ2MjUzMDRAMTYzLmNvbQ==; Peng Gao, Z2FvcGVuZ0BzZHV0Y20uZWR1LmNu
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.