- 1Department of Nephrology, Sichuan Academy of Medical Sciences and Sichuan Provincial People’s Hospital, Chengdu, China
- 2Internal Medicine, School of Medicine, University of Electronic Science and Technology of China, Chengdu, China
Background: There is conflicting data on the effect of vitamin K supplementation against vascular calcification in chronic kidney disease (CKD). We aimed to summarize current evidence from randomized controlled trials (RCTs) to determine whether vitamin K supplementation in CKD could attenuate vascular calcification.
Methods: A systematic search was performed in MEDLINE, EMBASE, and Cochrane Central Library. RCTs assessing the effect of vitamin K supplementation on vascular calcification in CKD and reported measures relevant to vascular calcification were eligible for inclusion. Effect outcomes are changes of biochemical and imaging measures of vascular calcification, as well as vascular elasticity reflected by pulse wave velocity (PWV). Safety outcomes included any adverse event and death. The risk of bias was assessed according to Cochrane handbook guidelines. Mean differences or standardized mean differences (SMD) with 95% confidence intervals (CIs) of absolute and relative changes of each studied outcome between experimental and control groups were pooled using a random-effects model.
Results: In all, ten RCTs with 733 patients were included. Pooled results indicated a decrease in serum biomarkers relevant to vascular calcification to a certain extent, mild improvement in vascular elasticity reflected by PWV, yet, no significant change in calcification scores derived from radiology examinations. Half of the included studies had low risk of bias.
Conclusion: Therefore, there is not yet solid evidence to support protective effects of vitamin K supplementation against vascular calcification in CKD. The results of ongoing RCTs are needed to further elucidate the value of vitamin K in this field.
Systematic review registration: www.crd.york.ac.uk/prospero, identifier CRD42022343857.
Introduction
Vascular calcification is an important and challenging complication of chronic kidney disease (CKD) and has been proven as an independent risk factor for patients’ mortality and poor quality of life (1). Strategies that may attenuate or delay the progression of vascular calcification in CKD have gained great attention yet remain uncertain (2).
The theoretical effect of vitamin K to attenuate vascular calcification began with the observation that patients who had been treated by warfarin had more serious burden of vascular calcification than those who had not (3). Later studies revealed vitamin K deficiency induced dysfunction of gamma-carboxyglutamic acid (Gla) proteins that widely distribute in vessels and skeleton might cause impaired vascular metabolism, leading to accelerated vascular calcification (4). The prevalence of vitamin K deficiency in the hemodialysis (HD) population (5, 6) and close relationship between vitamin K deficiency and vascular calcification in HD patients (7) were further confirmed. Many studies have explored the effect of vitamin K supplementation to protect patients against vascular calcification, however, there is conflicting or insufficient data in CKD population (2).
Therefore, we conducted this systematic review and meta-analysis to summarize current evidence on the effects of vitamin K supplementation on vascular calcification in CKD, to determine whether vitamin K supplementation could attenuate vascular calcification and help further research and clinical practice on this topic.
Materials and methods
Data sources and searches
A systematic search according to the Preferred Reporting Items for Systematic Review and Meta-Analyses statement (8) was performed for eligible studies published up to 1st July, 2022 in the following data sources: MEDLINE via PubMed (from 1946 through July 2022), EMBASE (from 1980 through July 2022), and Cochrane Central Library (no date restriction). The search strategy used text words and medical subject headings relevant to vitamin K and CKDs (see Supplementary Table 1). The search was limited to publication in English. The study was registered on PROSPERO (Identifier# CRD42022343857).
Study selection
Randomized controlled trials (RCTs) that had assessed the effects of vitamin K supplementation on vascular calcification in CKDs and reported measures relevant to vascular calcification were considered eligible for inclusion. Vitamin K could be either vitamin K1 or K2.
Two reviewers (G.C.Y. and H.L.M.) independently conducted the review process following a standardized approach. Titles and abstracts of all returned records were carefully reviewed. Duplications, non-original studies (e.g., guidelines, reviews, opinions, and editorial commentaries), non-human studies, vitamin K studies in non-CKD populations, and vitamin K studies that had not reported vascular calcification indexes were excluded. Reference lists from full text reviewed articles were further manually scanned to identify any other relevant studies. Any discrepancy was adjudicated by a third reviewer (F.Y.L.).
Outcomes
Effect outcomes considered in this meta-analysis can be divided into three categories. The first category was absolute and relative changes of known biochemical measures relevant to vascular calcification, including osteocalcin (OC) and dephosphorylated uncarboxylated matrix Gla protein (dp-ucMGP). The second category was absolute and relative changes of imaging measures of vascular calcification, including Agaston scores of coronary artery, aortic artery, and valve as well as calcification volume scores of coronary artery and valve. The third category was vascular elasticity reflected by pulse wave velocity (PWV). In addition, information on safety outcomes was also collected, including any adverse event and death.
Data extraction and quality assessment
Two reviewers (G.C.Y. and H.L.M.) independently extracted data from eligible studies following a double-check procedure and compiled them into a shared sheet. Disagreements were resolved by the third reviewer (F.Y.L.). The data extracted included authors, year of publication, identifier number if registered, geographical origin, number of participants, details of vitamin K supplementation, details of control, studied outcomes, and adverse events. Information about potential sources of significant clinical heterogeneity, such as composition of participants, was also collected for potential sensitivity analysis.
Critical appraisal of included studies
Two reviewers (G.C.Y. and H.L.M.) independently assessed the risk of bias of the included studies based on the “Cochrane Handbook for Systematic Reviews of Interventions” imbedded in analysis software (9).
Data synthesis and analysis
RevMan software (Version 5.2; Cochrane, Oxford, UK) was used for data synthesis. In order to avoid data entry errors, forest plots were performed through double-checked process by two reviewers (F.Y.L. and G.C.Y.). The change of each individual outcome was calculated by subtracting the pre-intervention value from the post-intervention value, and mean differences (MD) or standardized mean differences (SMD) with 95% confidence intervals (CIs) of studied outcomes between experimental and control groups were pooled using a random-effects model. Prior to data synthesis, data which was reported instead of mean and standard deviation (e.g., in case of median and range) were transformed to mean and standard deviation using the methods reported by Luo et al. (10) and Wan et al. (11). Statistical heterogeneity was estimated using the I2 statistic (12). The pooled outcomes are deemed having low statistical heterogeneity if I2 < 25%, moderate statistical heterogeneity if I2 ranged from 26 to 75%, and high statistical heterogeneity if I2 > 75%. The statistical significance was set at p < 0.05. Sensitivity analyses and funnel plots analysis for publication bias were not applicable due to the limited number of studies included in the meta-analysis.
Results
Search findings
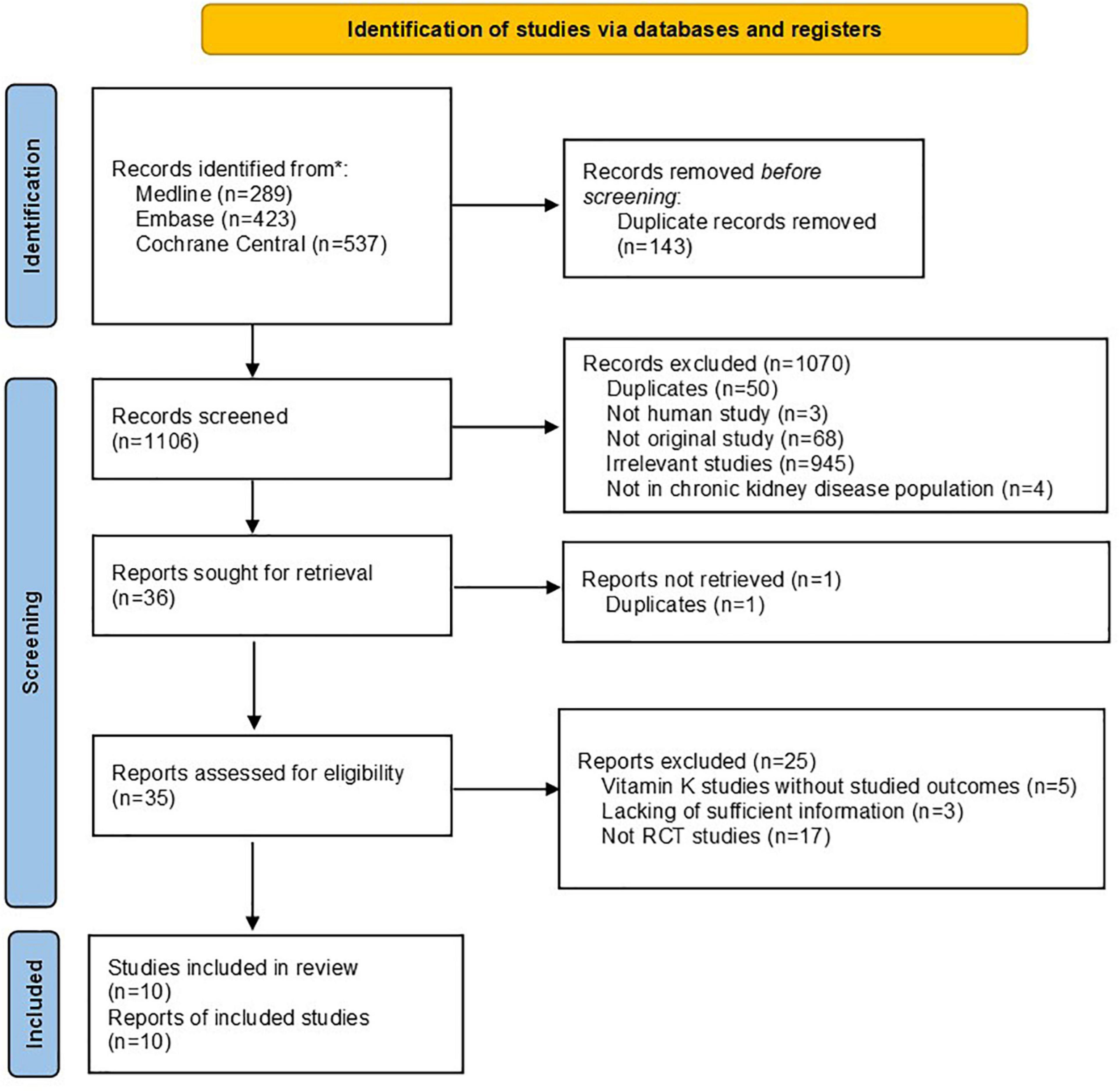
A total of 1,106 citations were identified from literature searching after removing duplications. A total of 35 citations were kept for full text review after abstract screening and full text retrieval, among which 25 citations were further excluded. Therefore, ten RCT studies were finally included in the systematic review (see Figure 1).
Study characteristics
In all, the ten RCTs involved 733 patients, including 365 in interventional group and 367 in control groups. Among these studies, six were conducted in hemodialysis patients (13–19), two were conducted in non-dialysis CKD patients (20, 21), and one was conducted in kidney transplantation recipients (22). All studies were conducted in adult population, except for one study which had been conducted in pediatric population. The percentages of male patients in individual studies ranged from 45.1 up to 71.6%. Two studies supplemented Vitamin K1, while the other eight studies supplemented Vitamin K2. Dosages of Vitamin K varied substantially from 90 μg/day up to 45 mg/day. Treatment durations also greatly varied from 3 months to 2 years. Characteristics of all included studies were shown in Table 1.
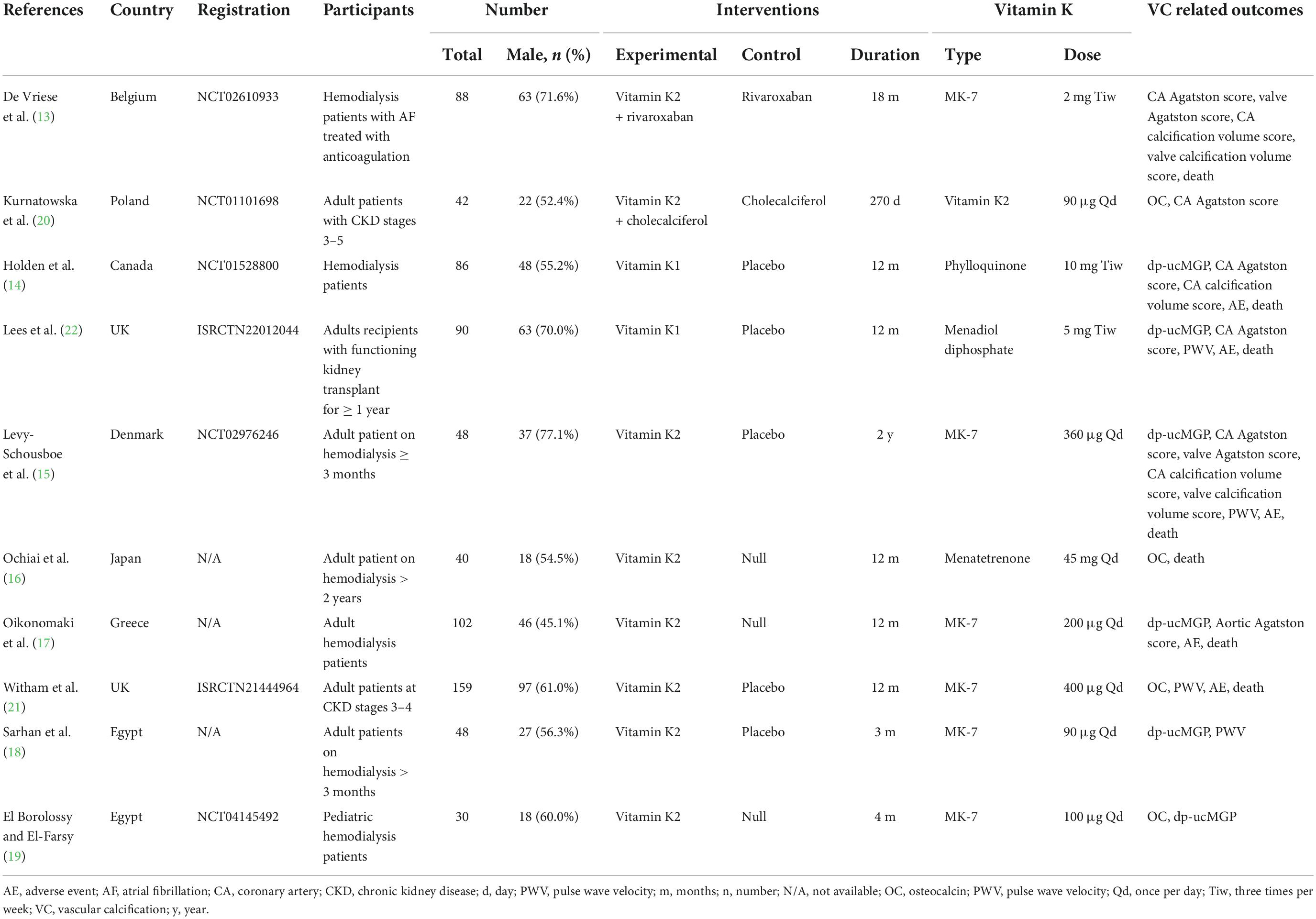
Table 1. Characteristics of included studies on the effect of vitamin K supplementation in chronic kidney disease.
Biochemical outcomes of vascular calcification
The pooled results on biochemical measures indicated serum proteins relevant to vascular calcification decreased after vitamin K supplementation, including both OC (standardized mean difference of absolute changes: −9.49 ng/mL, 95% CI: −14.21 to −4.76 ng/mL, I2 = 99%, p < 0.0001) and dp-ucMGP (standardized mean difference of absolute changes: −1.04 pmol/L, 95% CI: −1.70 to −0.39 pmol/L, I2 = 81%, p < 0.0001; standardized mean difference of relative changes: −1.31%, 95% CI: −2.85 to 0.23%, I2 = 95%, p < 0.0001) (Figure 2), although extremely high heterogeneities existed for both indexes. These results supported the findings of decreased osteogenesis biomarkers after Vitamin K intervention observed in previously published studies.
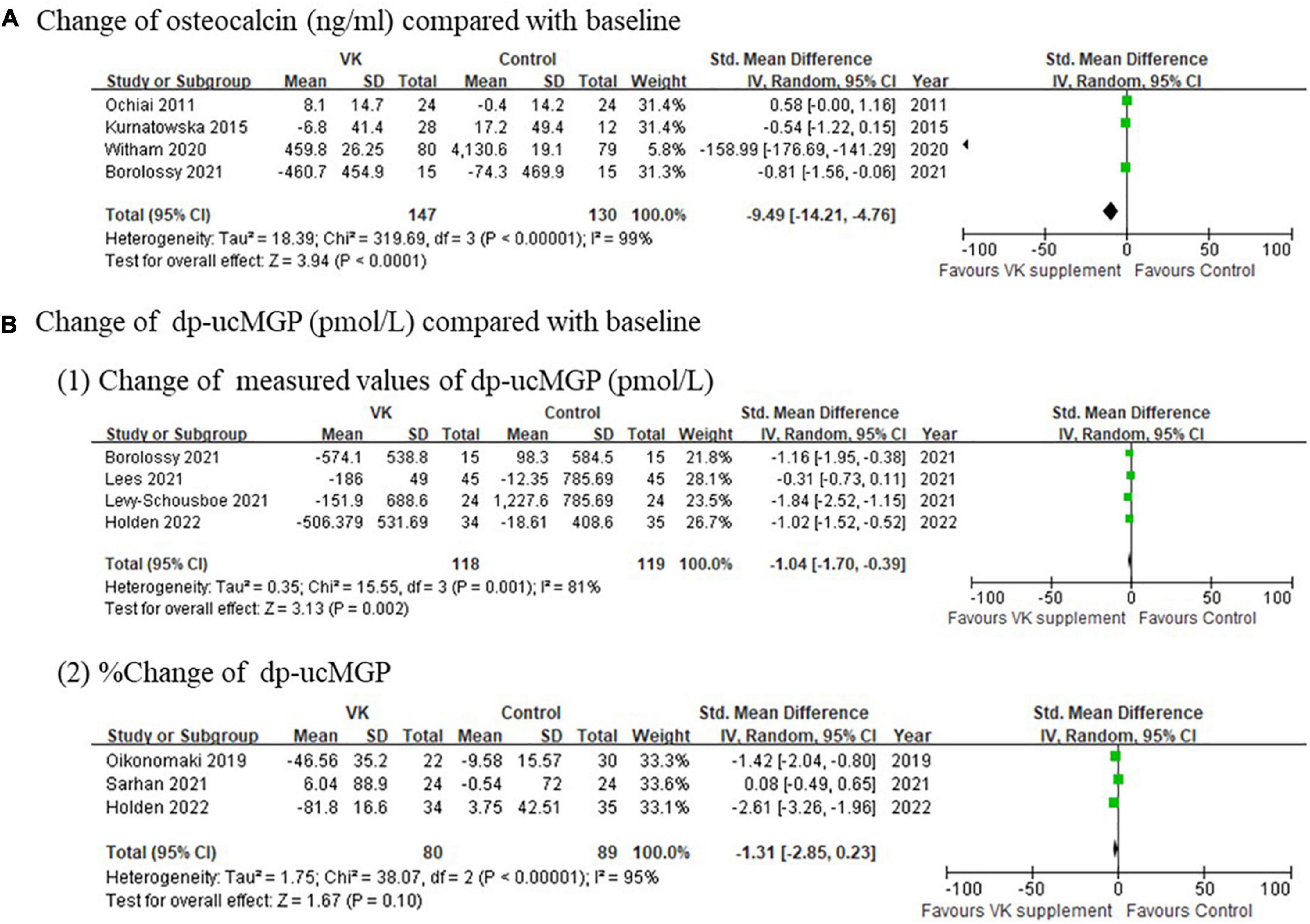
Figure 2. Pooled changes of (A) osteocalcin and (B) dp-ucMGP after Vitamin K supplementation compared with baseline.
Imaging outcomes of vascular calcification
Imaging outcomes of vascular calcification reported in individual studies exhibited inconsistent results. The changes of Agatston score for coronary artery (standardized mean difference of absolute changes: −0.03, 95% CI: −0.37 to 0.31, I2 = 40%, p = 0.17; mean difference of relative changes: 2.03%, 95% CI: −16.91 to 20.97%, I2 = 68%, p = 0.04), aortic artery (standardized mean difference of absolute changes: −0.03, 95% CI: −0.58 to 0.52), and valve (standardized mean difference of absolute changes: −0.33, 95% CI: −0.90 to 0.24; mean difference of relative changes:17.01%, 95% CI: −17.60 to 51.80%) showed no difference between Vitamin K supplementation and control groups (Figure 3), whereas the changes of calcification volume score of coronary artery (mean difference of absolute changes: 147.53, 95% CI: 63.77–231.29, I2 = 39%, p = 0.20; mean difference of relative changes: 9.65%, 95% CI: −0.93 to 20.23%, I2 = 44%, p = 0.18) and valve (standardized mean difference of absolute changes: −0.33, 95% CI: −0.90 to 0.241; mean difference of relative changes: 36.0%, 95% CI: 10.47–61.53%) partly favored the controls (Figure 4). However, this evidence was quite weak due to the limited number of studies included. Taken together, these findings failed to prove the effect of vitamin K to delay or prevent vascular calcification in CKD population from imaging perspective.
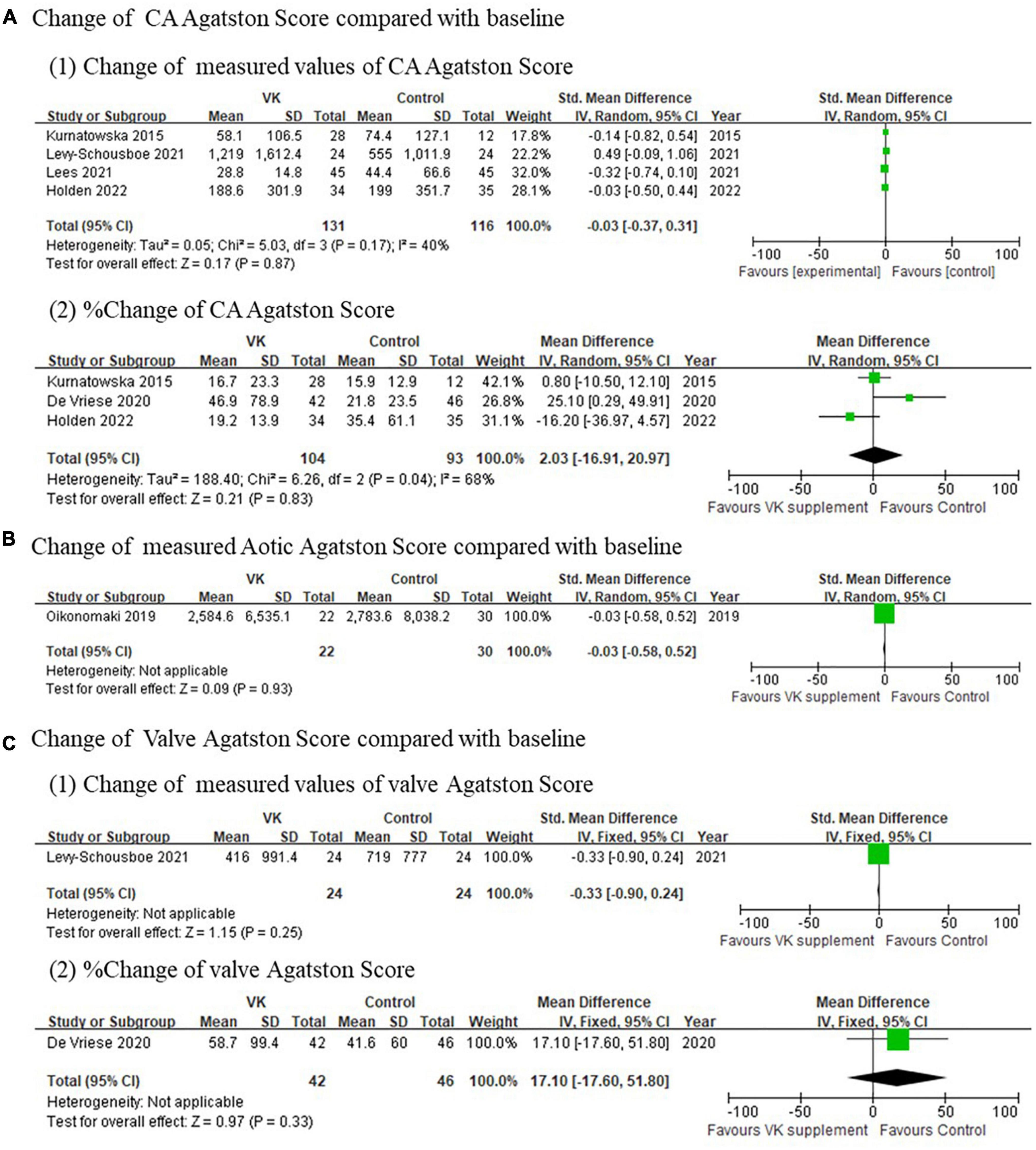
Figure 3. Pooled changes Agatston scores of coronary artery (A), aortic artery (B), and valve (C) after Vitamin K supplementation compared with baseline.
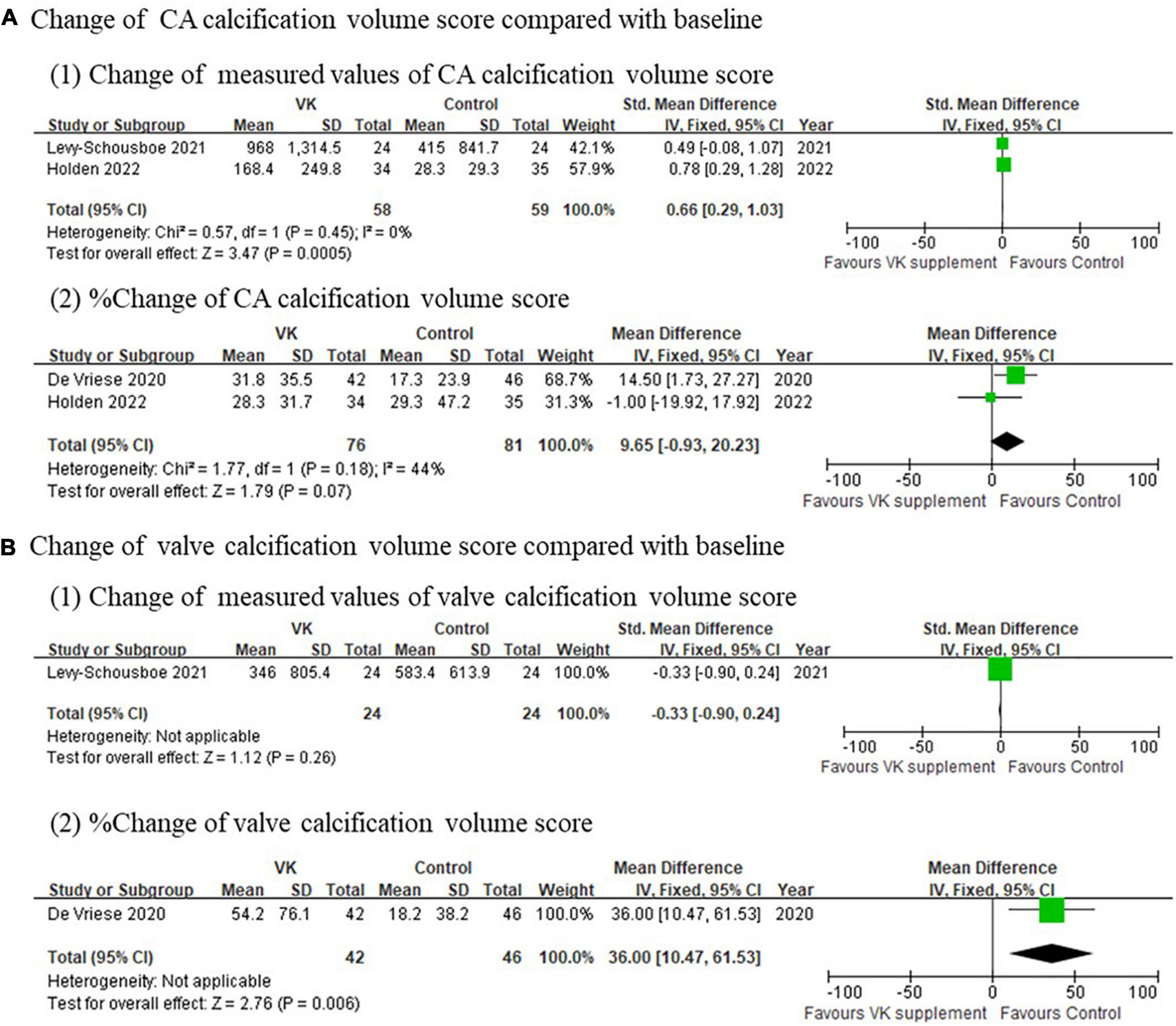
Figure 4. Pooled changes of calcification volume score of coronary artery and valve after Vitamin K supplementation compared with baseline. (A) Change of calcification volume score of coronary arteries and (B) change of calcification volume score of valves after Vitamin K supplementation compared with baseline.
Vascular elasticity
The pooled relative changes of PWV favored Vitamin K supplementation, whereas the pooled absolute changes did not support the differences (mean difference of absolute changes: 0.08 m/s, 95% CI: −0.41 to 0.57 m/s, I2 = 80%, p = 0.006; mean difference of relative changes: −11.01%, 95% CI: −13.84 to −8.18%, I2 = 90%, p = 0.001) (Figure 5). It should be noted that this finding was not solid due to high heterogeneities and limited number of studies included.
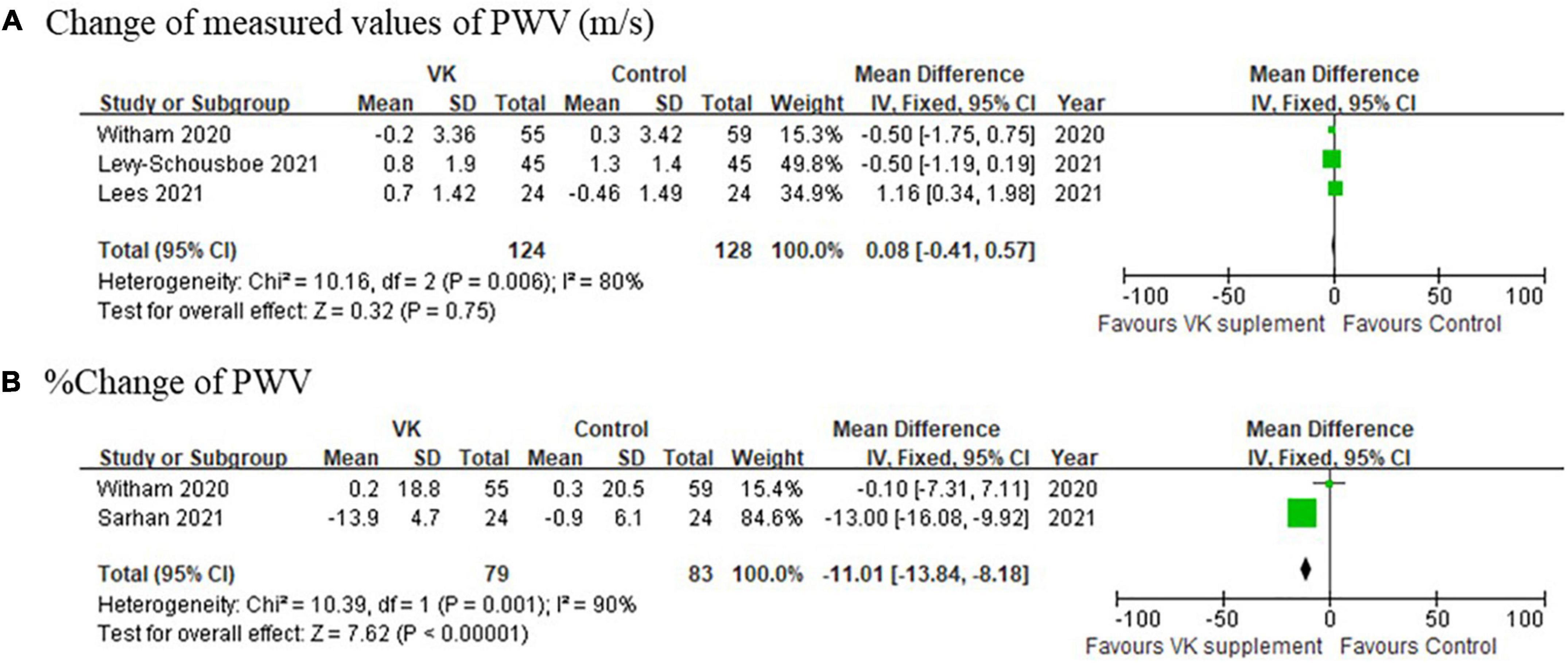
Figure 5. Pooled changes of pulse wave velocity after Vitamin K supplementation compared with baseline. (A) Absolute and (B) relative change of PWV after Vitamin K supplementation compared with baseline.
Adverse events
Pooled analysis of adverse events among the included studies indicated there was no significant difference in the incidence of adverse event between Vitamin K intervention and control groups (any adverse events: RR = 0.98, 95% CI: 0.82–1.18, p = 0.10, I2 = 48%, death: RR = 0.94, 95% CI: 0.57–1.54, p = 0.60, I2 = 0%) (Figure 6). These results consistently supported the safety of vitamin K supplementation in CKD population.
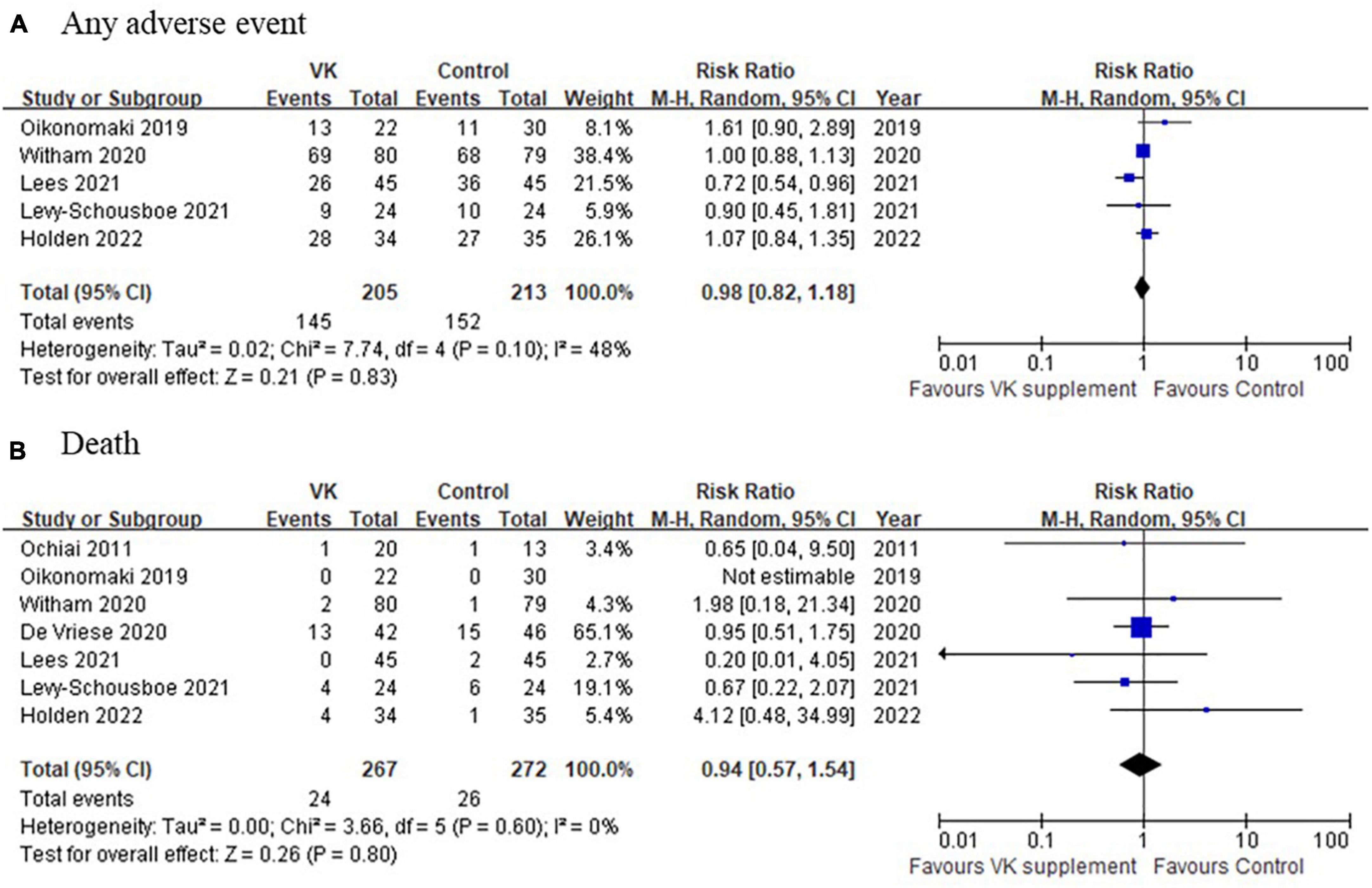
Figure 6. A comparison of adverse events between Vitamin K supplementation and control groups. (A) Any adverse event and (B) death between Vitamin K supplementation and control groups.
Critical appraisal
Five of the ten included studies were assessed as low risk of bias according to Cochrane systematic review guidelines, whereas the other five were assessed as high risk of bias (Figure 7). The domain with highest proportion of high risk was performance bias (4/10, 40%), i.e., blinding of participants and personnel, since these four studies utilized an open-label design.
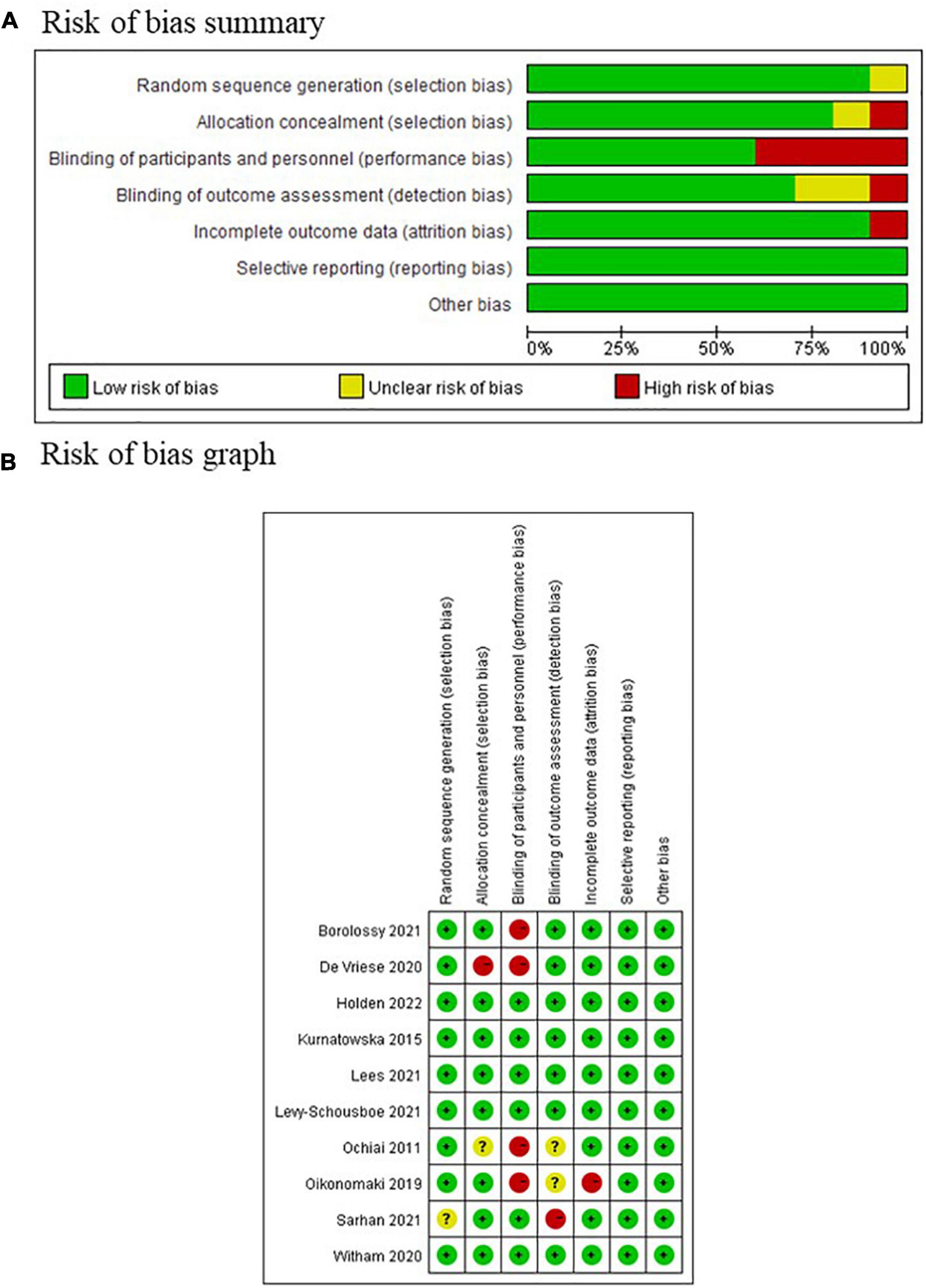
Figure 7. Risk of bias assessment of included study according to the Cochrane systematic review guidelines. (A) Risk of bias summary graph; (B) traffic light graph of risk of bias.
Discussion
The findings of this systematic review and meta-analysis indicated vitamin K supplementation helped to decrease serum biomarkers relevant to vascular calcification to a certain extent, might improve vascular elasticity reflected by PWV, however, failed to decrease calcification scores derived from radiology examinations. Half of the included studies had low risk of bias.
Despite of in-depth studies on potential mechanisms through which vitamin K supplementation protects against vascular calcification (23) and current and previous findings on the decrease of serum biomarkers relevant to vascular calcification after vitamin K intervention (24), there is insufficient data to support improvement of vascular calcification evidenced by radiology imaging, such as Agatston score and calcification volume score in our systematic review. Several possible reasons might explain this phenomenon. First, the current durations of vitamin K supplementation might be too short to observe obvious morphological changes, since vascular calcification is a long-term process of continuous progress. Second, there are many factors involved in the development and progression of vascular calcification in CKD, to name a few, serum phosphorus, serum calcium, serum parathyroid hormone, and dialysate calcium, and these factors closely relate to each other via complex crossover interactions. It is hardly possible to control or balance these factors throughout the study process. Therefore, theoretical improvement radiological outcomes, if existing, might be masked by effects from other confounders. Third, vitamin K supplementation might not be able reverse existing calcification lesions.
To the best of our acknowledgment, this is the first systematic review and meta-analysis that specifically addressed the value of vitamin K supplementation in CKD population and summarized the effects of vitamin K from biochemistry, imaging, and adverse effects perspectives. There are a number of ongoing trials on the effect of Vitamin K supplementation to delay or even stop vascular calcification in hemodialysis population (25–30) and pre-dialysis CKD population (31) (Table 2). Future results of these studies will help us better understand the value of vitamin K supplementation in the management of vascular calcification in CKD patients. It should be noted that vitamin K might have other clinical benefit other than preventing vascular calcification. It was reported vitamin K supplementation could reduce occurrence of muscle cramp in HD patients (32), possibly by improving vascular elasticity, as reflected in this systematic review. In addition, different doses of vitamin K supplementation were all very well tolerated in CKD patients. The net clinical benefits await future cost-effective research.
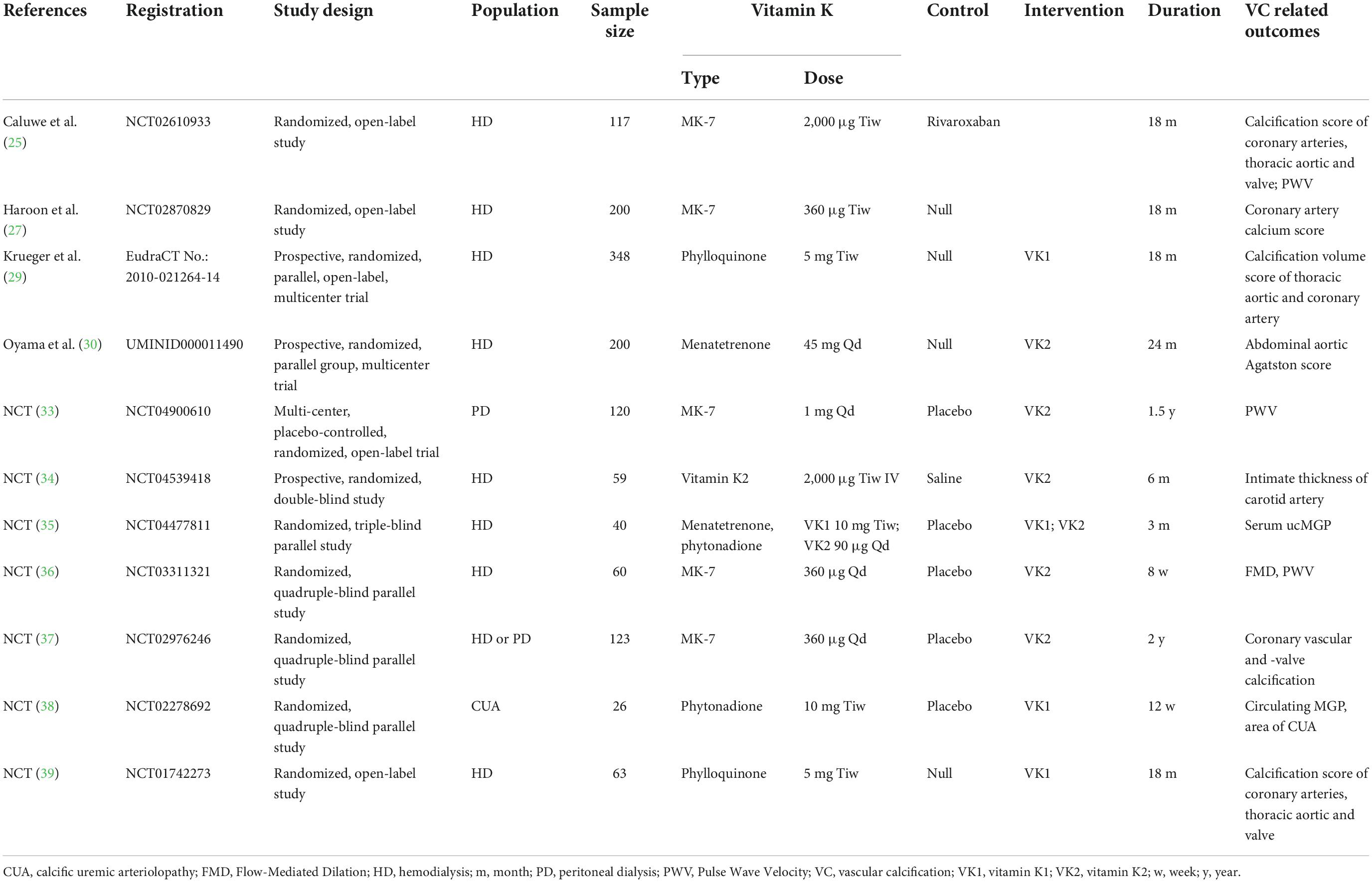
Table 2. A summary of ongoing clinical trials on the effect of Vitamin K supplementation to delay or even stop vascular calcification in CKD population.
There are a few limitations to be mentioned. First, the number of the included studies was too limited to allow sensitivity analysis or publication bias analysis. Second, the types of outcomes reported in the included bared great differences, weakening the power of horizontal comparisons. Future studies with standardized and consistent outcomes are needed to further clarify the value of vitamin K supplementation in this field.
Conclusion
Based on the findings of this systematic review and meta-analysis, there is not yet solid evidence from RCTs to support the protective effects against vascular calcification of vitamin K supplementation in CKD population, however, improvement in biochemical markers of vascular calcification and slight decrease of vascular elasticity were observed. Future results of ongoing RCTs are needed to further elucidate the value of vitamin K supplementation against vascular calcification in CKD.
Data availability statement
The original contributions presented in this study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
YF: conceptualization, project administration, and funding acquisition. YF and CG: methodology. CG and LH: software. CG, LH, and YF: data curation and formal analysis. YF, CG, LH, and LP: investigation. YF and LH: writing—original draft preparation. YF and LP: writing—review and editing. All authors have read and agreed to the published version of the manuscript, contributed to the article, and approved the submitted version.
Funding
This research was partly funded by Clinical research grant of Sichuan Provincial People’s Hospital (2020LY02).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2022.1001826/full#supplementary-material
References
1. Dusing P, Zietzer A, Goody PR, Hosen MR, Kurts C, Nickenig G, et al. Vascular pathologies in chronic kidney disease: Pathophysiological mechanisms and novel therapeutic approaches. J Mol Med. (2021) 99: 335–48.
2. Xu C, Smith ER, Tiong MK, Ruderman I, Toussaint ND. Interventions to attenuate vascular calcification progression in chronic kidney disease: A systematic review of clinical trials. J Am Soc Nephrol. (2022) 33: 1011–32.
3. Roumeliotis S, Roumeliotis A, Dounousi E, Eleftheriadis T, Liakopoulos V. Vitamin K for the treatment of cardiovascular disease in end-stage renal disease patients: Is there hope? Curr Vasc Pharmacol. (2020) 19:21–34. doi: 10.2174/1570161118666200320111745
4. Delanaye P, Liabeuf S, Bouquegneau A, Cavalier É, Massy ZA. [The matrix-gla protein awakening may lead to the demise of vascular calcification]. Nephrol Ther. (2015) 11:191–200.
5. Caluwe R, Verbeke F, De Vriese AS. Evaluation of vitamin K status and rationale for vitamin K supplementation in dialysis patients. Nephrol Dial Transplant. (2020) 35:23–33.
6. Feng Y, Ruan Y, He Q, Zhang W, Wang L. Suboptimal vitamin K status and its risk factors in a population of Chinese chronic haemodialysis patients. Nephrology. (2015) 20:625–31. doi: 10.1111/nep.12494
7. Roumeliotis S, Dounousi E, Eleftheriadis T, Liakopoulos V. Association of the inactive circulating matrix Gla protein with vitamin K intake, calcification, mortality, and cardiovascular disease: A review. Int J Mol Sci. (2019) 20:628.
8. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. BMJ. (2009) 339:b2700. doi: 10.1136/bmj.b2700
9. Higgins JPTTJCJ, Cumpston M, Li T, Page MJ, Welch VA editors. Cochrane handbook for systematic reviews of interventions version 6. 3th ed. London: Cochrane (2022).
10. Luo D, Wan X, Liu J, Tong T. Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Stat Methods Med Res. (2018) 27:1785–805.
11. Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. (2014) 14:135. doi: 10.1186/1471-2288-14-135
12. Ioannidis JP. Interpretation of tests of heterogeneity and bias in meta-analysis. J Eval Clin Pract. (2008) 14:951–7.
13. De Vriese AS, Caluwé R, Pyfferoen L, De Bacquer D, De Boeck K, Delanote J, et al. Multicenter randomized controlled trial of vitamin K antagonist replacement by rivaroxaban with or without vitamin K2 in hemodialysis patients with atrial fibrillation: The valkyrie study. J Am Soc Nephrol. (2020) 31:186–196.
14. Holden RM, Booth SL, Zimmerman D, Moist L, Norman P, Day AG, et al. Inhibit progression of coronary artery calcification with vitamin k in hemodialysis patients (the iPACK-HD study): A randomized, placebo-controlled multi-centre, pilot trial. Nephrol Dial Transplant. (2022):gfac191. doi: 10.1093/ndt/gfac191
15. Levy-Schousboe K, Frimodt-Møller M, Hansen D, Peters CD, Kjærgaard KD, Jensen JD, et al. Vitamin K supplementation and arterial calcification in dialysis: Results of the double-blind, randomized, placebo-controlled RenaKvit trial. Clin Kidney J. (2021) 14:2114–23. doi: 10.1093/ckj/sfab017
16. Ochiai M, Nakashima A, Takasugi N, Kiribayashi K, Kawai T, Usui K, et al. Vitamin K2 alters bone metabolism markers in hemodialysis patients with a low serum parathyroid hormone level. Nephron Clin pract. (2011) 117:c15–19. doi: 10.1159/000319642
17. Oikonomaki T, Papasotiriou M, Ntrinias T, Kalogeropoulou C, Zabakis P, Kalavrizioti D, et al. The effect of vitamin K2 supplementation on vascular calcification in haemodialysis patients: A 1-year follow-up randomized trial. Int Urol Nephrol. (2019) 51:2037–2044. doi: 10.1007/s11255-019-02275-2
18. Sarhan II, Kamal OM, Said SA, Tawfik AM, Madbouly NN, Ismail A. Role of vitamin k 2 on matrix gla protein and vascular stiffness in hemodialysis patients. J Cardiovasc Dis Res. (2021) 12:1235–1245.
19. El Borolossy R, El-Farsy MS. The impact of vitamin K2 and native vitamin D supplementation on vascular calcification in pediatric patients on regular hemodialysis. A randomized controlled trial. Eur J Clin Nutr. (2022) 76:848–54. doi: 10.1038/s41430-021-01050-w
20. Kurnatowska I, Grzelak P, Masajtis-Zagajewska A, Kaczmarska M, Stefanczyk L, Vermeer C, et al. Effect of vitamin K2 on progression of atherosclerosis and vascular calcification in nondialyzed patients with chronic kidney disease stages 3-5. Pol Arch Med Wewn. (2015) 125:631–40. doi: 10.20452/pamw.3041
21. Witham MD, Lees JS, White M, Band M, Bell S, Chantler DJ, et al. Vitamin K supplementation to improve vascular stiffness in CKD: The K4Kidneys randomized controlled trial. J Am Soc Nephrol. (2020) 31:2434–2445. doi: 10.1681/ASN.2020020225
22. Lees JS, Rankin AJ, Gillis KA, Zhu LY, Mangion K, Rutherford E, et al. The ViKTORIES trial: A randomized, double-blind, placebo-controlled trial of vitamin K supplementation to improve vascular health in kidney transplant recipients. Am J Transplant. (2021) 21:3356–68. doi: 10.1111/ajt.16566
23. Bellone F, Cinquegrani M, Nicotera R, Carullo N, Casarella A, Presta P, et al. Role of vitamin K in chronic kidney disease: A focus on bone and cardiovascular health. Int J Mol Sci. (2022) 23:5282. doi: 10.3390/ijms23095282
24. Caluwé R, Verbeke F, De Vriese AS. Evaluation of vitamin K status and rationale for vitamin K supplementation in dialysis patients. Nephrol Dial Transplant. (2020) 35:23–33. doi: 10.1093/ndt/gfy373
25. Caluwe R, Pyfferoen L, De Boeck K, De Vriese AS. The effects of Vitamin K supplementation and Vitamin K antagonists on progression of vascular calcification: Ongoing randomized controlled trials. Clin Kidney J. (2016) 9:273–279. doi: 10.1093/ckj/sfv146
26. Euctr DE. Vitamin K1 to slow vascular calcification in hemodialysis patients. (2012). Available online at: https://trialsearch.who.int/ (Search for EUCTR2010-021264-14-DE) (accessed July 1, 2022).
27. Haroon SW, Tai BC, Ling LH, Teo L, Davenport A, Schurgers L, et al. Treatment to reduce vascular calcification in hemodialysis patients using vitamin K (Trevasc-HDK): A study protocol for a randomized controlled trial. Medicine. (2020) 99:e21906. doi: 10.1097/MD.0000000000021906
28. Jprn U. Evaluation of vascular calcification in relation to biomarker and vitminK in hemodialysis patients. (2013). Available online at: https://trialsearch.who.int/ (Search for JPRN-UMIN000011490) (accessed July 1, 2022).
29. Krueger T, Schlieper G, Schurgers L, Cornelis T, Cozzolino M, Jacobi J, et al. Vitamin K1 to slow vascular calcification in haemodialysis patients (VitaVasK trial): A rationale and study protocol. Nephrol Dial Transplant. (2014) 29:1633–1638. doi: 10.1093/ndt/gft459
30. Oyama S, Okamoto N, Koide S, Hayashi H, Nakai S, Takahashi K, et al. Vitamin K2 supplementation and the progression of abdominal aortic calcification in dialysis patients. Fujita Med J. (2021) 7:136–8.
31. ISRCTN. Vitamin K therapy to improve vascular health in patients with chronic kidney disease. (2015). Available online at: https://trialsearch.who.int/ (Search for ISRCTN21444964) (accessed July 1, 2022).
32. Xu D, Yang A, Ren R, Shan Z, Li YM, Tan J. Vitamin K2 as a potential therapeutic candidate for the prevention of muscle cramps in hemodialysis patients: A prospective multicenter, randomized, controlled, crossover pilot trial. Nutrition. (2022) 97:111608. doi: 10.1016/j.nut.2022.111608
33. NCT. The effect of vitamin K2 supplementation on arterial stifness and cardiovascular events in peritonial dialysis. (2021). Available online at: https://clinicaltrials.gov/show/NCT04900610 (accessed July 1, 2022).
34. NCT. Vitamin K2 supplementation and vascular calcification. (2020). Available online at: https://clinicaltrials.gov/show/NCT04539418 (accessed July 1, 2022).
35. NCT. Comparative study evaluating the effect of vitamin K1 versus vitamin K2 on vascular calcification in dialysis patients. (2020). Available online at: https://clinicaltrials.gov/show/NCT04477811 (accessed July 1, 2022).
36. NCT. Vitamin K to slow progression of cardiovascular disease risk in hemodialysis patients. (2017). Available online at: https://clinicaltrials.gov/show/NCT03311321 (accessed July 1, 2022).
37. NCT. Effect of vitamin K2 (MK7) on cardiovascular and bone disease in dialysis patients. (2016). Available online at: https://clinicaltrials.gov/show/NCT02976246 (accessed July 1, 2022).
38. NCT. Evaluation of vitamin K supplementation for calcific uremic arteriolopathy. (2014). Available online at: https://clinicaltrials.gov/show/NCT02278692 (accessed July 1, 2022).
39. NCT. Vitamin K1 to slow progression of vascular calcification in HD patients. (2012). Available online at: https://clinicaltrials.gov/show/NCT01742273 (accessed July 1, 2022).
Keywords: vitamin K, vascular calcification, chronic kidney disease, systematic review, meta-analysis
Citation: Geng C, Huang L, Pu L and Feng Y (2023) Effects of vitamin K supplementation on vascular calcification in chronic kidney disease: A systematic review and meta-analysis of randomized controlled trials. Front. Nutr. 9:1001826. doi: 10.3389/fnut.2022.1001826
Received: 24 July 2022; Accepted: 26 September 2022;
Published: 10 January 2023.
Edited by:
Elisabet Maria Rothenberg, Kristianstad University, SwedenReviewed by:
Gerd Faxén Irving, Karolinska Institutet (KI), SwedenSamantha Maurotti, Magna Græcia University, Italy
Copyright © 2023 Geng, Huang, Pu and Feng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yunlin Feng, ZmVuZ3l1bmxpbkBtZWQudWVzdGMuZWR1LmNu
†These authors have contributed equally to this work
 Chanyu Geng1,2†
Chanyu Geng1,2† Lei Pu
Lei Pu Yunlin Feng
Yunlin Feng