- 1Jiangsu Key Laboratory of Marine Bioresources and Environment/Jiangsu Key Laboratory of Marine Biotechnology, Jiangsu Ocean University, Lianyungang, China
- 2Jiangsu Key Laboratory of High-Tech Research and Development of Veterinary Biopharmaceuticals, Jiangsu Agri-Animal Husbandry Vocational College, Taizhou, China
- 3Co-Innovation Center of Jiangsu Marine Bio-industry Technology, Jiangsu Ocean University, Lianyungang, China
- 4Department of Chemical and Environmental Engineering, Faculty of Science and Engineering, University of Nottingham Malaysia, Semenyih, Malaysia
- 5College of Food Science and Technology, Nanjing Agricultural University, Nanjing, China
The demand for roasted seaweed sandwich (Porphyra yezoensis) product has risen in recent years. The product slicing process has created a huge number of scraps that are not utilized effectively. Three lactic acid bacteria (LAB) strains were used to ferment P. yezoensis sauces in this study, including Lactobacillus fermentum, Lactobacillus casei, Streptococcus thermophilus, and the mixed strains (1:1:1, v/v). The fermentation characteristics, antioxidant capacity in vitro, sensory properties, and flavoring substances of fermented P. yezoensis sauces were analyzed. After 21 days of fermentation, all LAB strains grew well in the P. yezoensis sauces, with protease activity increased to 6.6, 9.24, 5.06, and 5.5 U/mL, respectively. Also, the flavors of P. yezoensis sauces fermented with L. casei and L. fermentum were satisfactory. On this premise, gas chromatography-mass spectrometry (GC-MS) was used to investigate the changes in gustatory compounds in P. yezoensis sauces fermented with L. casei and L. fermentum. In general, 42 and 41 volatile flavor chemicals were identified after the fermentation of L. casei and L. fermentum. Furthermore, the fermented P. yezoensis sauce possessed greater DPPH scavenging activity and ferric-reducing ability power than the unfermented P. yezoensis. Overall, the flavor and taste of P. yezoensis sauce fermented by L. casei was superior.
Introduction
The East China Sea, South China Sea, Huanghai Sea, and other coastal regions produce Porphyra yezoensis. The protein level in dried P. yezoensis is 25–30%, while the carbohydrate amount is around 40% (1). It also contains vitamins like riboflavin and niacin (2). Moreover, P. yezoensis is rich in minerals that can assist youngsters and the elderly to absorb nutrition (3, 4).
Dietary P. yezoensis products are popular in Japan and Korea while Chinese consumers eat less P. yezoensis products. The local market of P. yezoensis products in China is underdeveloped with few product varieties and influential manufactors (5). One of the main reasons is that P. yezoensis processing are essential, whereas the products have a distinct flavor that some domestic consumers cannot tolerate. Currently, the primary domestic seaweed processing methods are drying and roasting. Thus, more processing methods of P. yezoensis are needed to boosting its economic value and increase the market of P. yezoensis products.
The slicing of roasted seaweed sandwich into small pieces generates a large number of wastes, which are rich in protein and maltose. The processing of sandwich seaweed filet to Porphyra sauce helps save waste. In addition, microbal fermentation, expecially lactic acid fermentation can preserve the nutritional value of Porphyra sauce, give it a unique taste and flavor, enrich its product form, and creat new opportunities for the Porphyra market. To our knowledge, the fermentation of P. yezoensis sauces has been poorly studied. Zhang et al. investigated the basic nutrient content of dried Porphyra, and fermented it with LAB and Aspergillus oryzae (6). Fan et al. utilized A. oryzae and Rhodozyme to co-ferment Porphyra and optimized the fermentation based on the protease activity and sensory index of Porphyra sauces (7).
LAB is a probiotic that may generate high quantities of amino acids in its fermentation P. yezoensis supernatant. It coud be a suitable species to ferment Porphyra sauces (8). This study chose L. fermentum, L. casei, and S. thermophilus to independently and co-ferment waste materials of roasted seaweed sandwich. The changes of fermentation characteristics and flavor profile were evaluated. Furthermore, the DPPH scavenging activity and ferric-reducing ability power (FRAP) of fermented P. yezoensis sauces were also investigated. The findings may help to produce probiotic fermented P. yezoensis sauces with excellent nutritional value and flavor.
Materials and Methods
Bacterial Strains and Culture Conditions
S. thermophilus FJAT-46738, L. fermentum FJAT-46744, and L. casei FJAT-7928 were obtained from the Fujian Academy of Agricultural Sciences, China. The single colony of the three LAB strains were innoculted to 10 mL MRS broth, cultivated for 36 h at 40°C except for L. fermentum (37°C). These strains were cultured with the AnaeroPack System C-32 (Mitsubishi Gas Chemical Company, Inc. Tokyo).
Fermentation of P. yezoensis Sauce
The waste materials of roasted seaweed (P. yezoensis) sandwich was obtained from a local plant (Lianyungang Wende Food Co., Ltd. Lianyungang) in Lianyungang, Jiangsu, China. The seaweed sandwich scraps were crushed to a specific value (0.01 mm) by a beater. The aperture of the machine sieve hole should be kept at around 0.01 mm to promote the release of protein from the tissue (9). Ten grams of seaweed sandwich scrap powders and 15 mL water were mixed with a ratio (m/v) of 1:1.5 in 100 mL glass bottles with blue lid. The bottles were then sterilized at 121°C for 30 min in a autoclave. This process both softened the tissue of P. yezoensis and inactivated the residue bacteria. After cooling to room tempreature, the activated L. fermentum, S. thermophilus, L. casei, and their mixtures (1:1:1 v/v) were, respectively, added into the bottles at a ratio of 3% (v/v). The blue lid bottles were then tightened and statically fermented at 40°C. The viable bacteria level, pH, protease vitality, and sensory quality of the fermented products were measured at 0, 3, 7, 14, and 21 days.
Determination of Viable Cell and pH
One gram of fermented P. yezoensis sauces were weighed and serially diluted with sterile 0.9% (w/v) NaCl solution. One hundred microlilter of each dilutions were, respectively, spread on MRS agar plates with two replicates and incubated in AnaeroPack System C-32 at 40°C for 36 h. The cell counts were expressed as colony numbers (Log 10 CFU per g). The P. yezoensis sauce without fermentation was used as control. An appropriate amount of fermented P. yezoensis sauces were sampled and the pH was measured by a digital pH meter (Instrument & Electricity Scientific Instruments Company, Shanghai).
Sensory Evaluations
Ten grams of each fermented P. yezoensis sauce were placed in plastic cups with cup and individually tasted by the sensory panelists. The sensory panel consisted of 7 persons (2 males and 5 females, aged 19–24) from School of Food Science and Engineering, Jiangsu Ocean University. All of the members were well-tranined before sensory evaluation. The sensory descriptors agreed by the team members include intensities (seaweed flavor, sauce flavor and no fishy flavor), preferences (aroma, sour flavor, bitter flavor and aftertaste), and overall quality. The intensity, preference, and overall quality scales ranged from 0 (weak) to 10 (strong), 0 (bad or dislike) to 10 (good or like), and 0 (dislike) to 10 (like), respectively (10).
About 10 g fermented P. yezoensis sauces at 25°C was randomly taken in the training and evaluation process. Water and white bread were provided to the assessors to wash the palate. The training and evaluation were organized in conformity to the International Organization for Standardization (ISO, 1993) and conducted in a sensory laboratory that complies with the American Society for Testing and Materials (ASTM) criteria.
Determination of Protease Activity
The protease produced during the fermentation could effectively enhanced biological activity of the products (11). The formaldehyde method was used to determine the protease activity of P. yezoensis sauce (12). Ten grams of fermented P. yezoensis sauce in a 250 mL tapered container was added with 80 mL 55°C ddH2O, and incubated in water bath at 55°C for 3 h. The samples were then boiled for 1 min to inactivate the inherent enzyme. The sample was cooled to room temperature and the volume was adjusted to 100 mL with ddH2O, and filtered through filter paper. Ten milliliter of the filtrate in a 150 mL tapered bottle was added with 50 mL ddH2O, 4–5 drops of phenolphthalein indicator, and titrated with 0.1 mol/L NaOH solution until the solution just turned red. The volume of consumed NaOH was recorded as V1. The sample in the tapered bottle was added with 10 mL formaldehyde, titrated with 0.1 mol/L NaOH to dark red as the endpoint. The volume of consumed NaOH was recorded as V2. The protease viability is then calculated as (Eq. 1):
where V1 is the volume of NaOH consumed by titration before adding formaldehyde, mL; V2 is the volume of NaOH consumed by titration after adding formaldehyde, mL; C is the concentration of standard NaOH, mol/L; W is the moisture content of fermented P. yezoensis sauces, mL.
GC-MS Analysis
Agilent 5977A series GC/MSD system (Agilent Technologies, CA) and Agilent HP-INNOWAX (30 m ×0.25 mm ×0.25 μm) were used to analyze the volatile compounds in the fermented P. yezoensis sauces. Two grams of sauce sample in a 20 mL headspace bottle was added with 3 mL of saturated sodium chloride solution (refrigerated at −20°C). The intake temperature was 250°C and the initial column temperature was 40°C. After incubation for 1 min, it was heated to 80°C at a rate of 4°C/min. This temperature was hold for 1 min, then heated to 160°C at a rate of 2°C/min, and finally heated to 220°C at a rate of 10°C/min. The temperature was then maintained for 10 min. Helium gas with high purity was used as the gas load and the flow rate was 1 mL/min. MS temperatures of the quaternary rod were 150°C. The ion source (230°C) was an electron-ion source with an electron energy of 70 eV and a scanning range of 35–350 m/z.
The semi-qualitative analysis was done with mass spectrometry in the computer spectral library (NIST/WILEY). The compound was identified when matched to a known volatile component with a score ≥80. The internal standard in this study was 2-octanol (concentration of 10 mg/L) (13, 14). The content of each volatile compound in the tested samples was calculated according to the following formula (Eq. 2).
where CX is the concentration of volatile compounds to be measured, MX is the peak area of the measured volatile compound, M intensor is the peak area of the internal standard, C intensor is the concentration of the internal standard.
The data of samples were sorted and drawn with Origin 2021 to analyze the changes in volatile profile (15).
Assay of DPPH Radical Scavenging and Ferric-Reducing Activity
Active ingredients of each P. yezoensis sauce (5 g) was extracted with 50 mL ddH2O. Ultrasound (Wuxi Gangzheng Technology Co., Ltd. Wuxi) was utilized to disrupt the cell wall and assist the extraction. The ultrasound power was 880 W the ultrasonic duration was 30 min, and the ultrasonic temperature was 50°C. The sample was then centrifuged at 8,000 g for 15 min, and the supernatant was collected (16, 17). P. yezoensis sauce extract (1 mL) was mixed with 1 mL of DPPH solution (0.1 mM), and incubated at 37°C in the dark for 30 min prior to measuring the absorbance at 517 nm (17). The scavenging activity of the DPPH radical was calculated as follows (Eq. 3).
A control was made by using absolute ethanol instead of DPPH ethanolic solution, and A blank was prepared by replacing the polysaccharide sample solution with ddH2O.
The Ferric-Reducing activity of fermented P. yezoensis sauces were determined by the FRAP assay with an commercial kits (Jiancheng Bioengineering Institute, Nanjing, China). The absorbance was recorded at 593 nm.
Statistical Analysis
All of the experiments were repeated triplicate. Data were expressed as the mean ± standard deviation. The data were analyzed by Excel, Origin 2021, and SPSS 20.0 software. Significance was defined at p < 0.05.
Results
Growth of LAB and pH Changes
The changes of viable cell counts and pH value of P. yezoensis sauces fermented with S. thermophilus, L. casei, L. fermentum, and the mixed strains during 21 days of fermentation are shown in Figure 1. All the three LAB strains were able to grow in the rehydrated roasted seaweed sandwich scraps. The initial viable bacteria counts of L. casei, L. fermentum, S. thermophilus and the mixed fermentation group were 7.38 ± 0.02, 7.15 ± 0.01, 7.19 ± 0.01 and 7.47 ± 0.03 log CFU/mL, respectively. After 21 days of fermentation, the viable cell counts in P. yezoensis sauce fermented with L. casei, L. fermentum, S. thermophilus and the mixed strains were 7.1 ± 0.01, 7.4 ± 0.01, 6.9 ± 0.015 and 6.5 ± 0.01 CFU/mL, indicating that the L. casei and L. fermentum strains were able to adapt to the fermentation environment. The number of viable bacteria of P. yezoensis sauce fermented with the mixed strains was considerably low at the end of fermentation. This was possibly due to the low pH (2.7 ± 0.29), which was one of the important factors affecting the number of viable bacteria during lactic acid fermentation (18). After 3 days of fermentation, the viable cell count frist rose and then decreased in the fermented P. yezoensis sauces. In contrast, no viable bacteria were found in the unfermented P. yezoensis sauce that was previously sterilized.
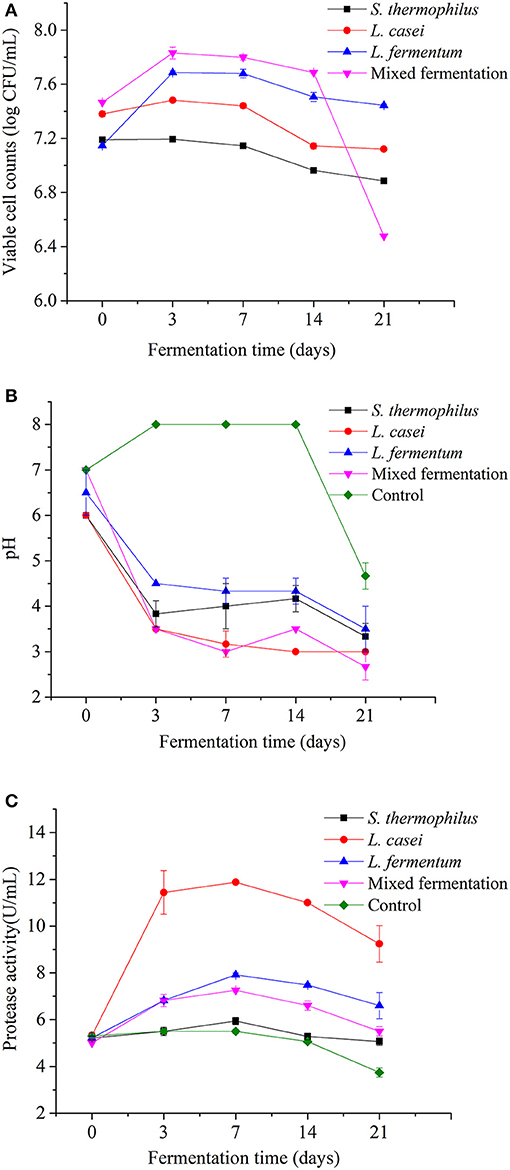
Figure 1. Evolutions of viable cell counts (A), pH value (B) and protease activity (C) of Porphyra yezoensis sauces during 21 days of LAB strains fermentation.
The pH value of the P. yezoensis sauce decreased during fermentation with all the LAB strains (Figure 1B). The pH values of the three strains have generally decreased. From 0 to Day 3, the pH value of fermented P. yezoensis sauces decreased the fastest. The pH value of each strain steadily dropped over a period of 3–21 days. When compared to the other two bacteria, L. casei has always had the lowest pH value. A low pH would efficiently inhibit the multiplication of pathogenic bacteria in samples and extend the shelf life (19).
Determination of Protease Activity
Microbial fermentation might enhance the protease activity of the product (20). Figure 1C revealed that the protease activity of P. yezoensis sauces was initially around 5.20 U/mL and increased during all the fermentation processes. The protease activity increased to the peak at Day 7 and then decreased in the following fermentation period. Among all the groups, the protease activity of P. yezoensis sauces fermented by L. casei were the greatest (11.88 U/mL at day 7) at the same stage, which was partly consistent with the low pH value. After 21 days of fermentation, the protease activities of P. yezoensis sauces fermented by L. fermentum, L. casei, S. thermophilus, and the mixed strains were increased to 6.60, 9.24, 5.06, and 5.50 U/mL, respectively. Furthermore, the protease activity of all fermented P. yezoensis sauces were significantly increased (p < 0.05) than the control.
Sensory Evaluation
The intensities of all the sensory attributes of fermented P. yezoensis sauces showed an increasing trend within the first week and then decreased gradually (Figure 2). The sensory quality of P. yezoensis sauces fermented by L. casei was the best among all the samples at the same fermentation period, followed by L. fermentum, S. thermophilus and the mixed strains. The fishy flavor, bitter flavor, and sour tastes in all samples got intensified with time, and the scent of seaweed and sauce flavor were more apparent in the L. casei fermented P. yezoensis sauce. Moreover, the sensory properties of the P. yezoensis sauce fermented by L. fermentum were relatively stable, and no significant changes in the flavors werwe observed. The aroma value increased the maximum at Day 7, and then declined significantly. Besides, the sensory quality of P. yezoensis sauces fermented by S. thermophilus were relatively low, among which the bitter and fishy smells were stonger than the aroma and sauce flavors. Taking all the sensory results into account, L. casei was more suitable for the P. yezoensis sauce fermentation.
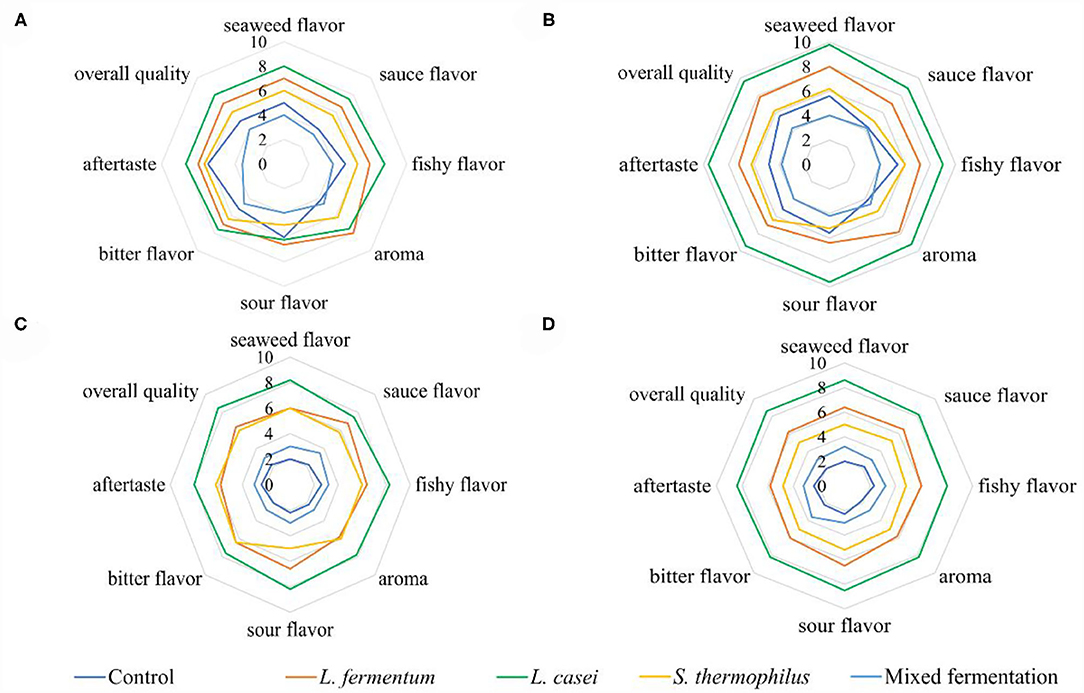
Figure 2. Radiation map of sensory profile for fermented P. yezoensis sauces. (A) 3 days of fermentation, (B) 7 days of fermentation, (C) 14 days of fermentation, (D) 21 days of fermentation.
Analysis of Volatile Composition
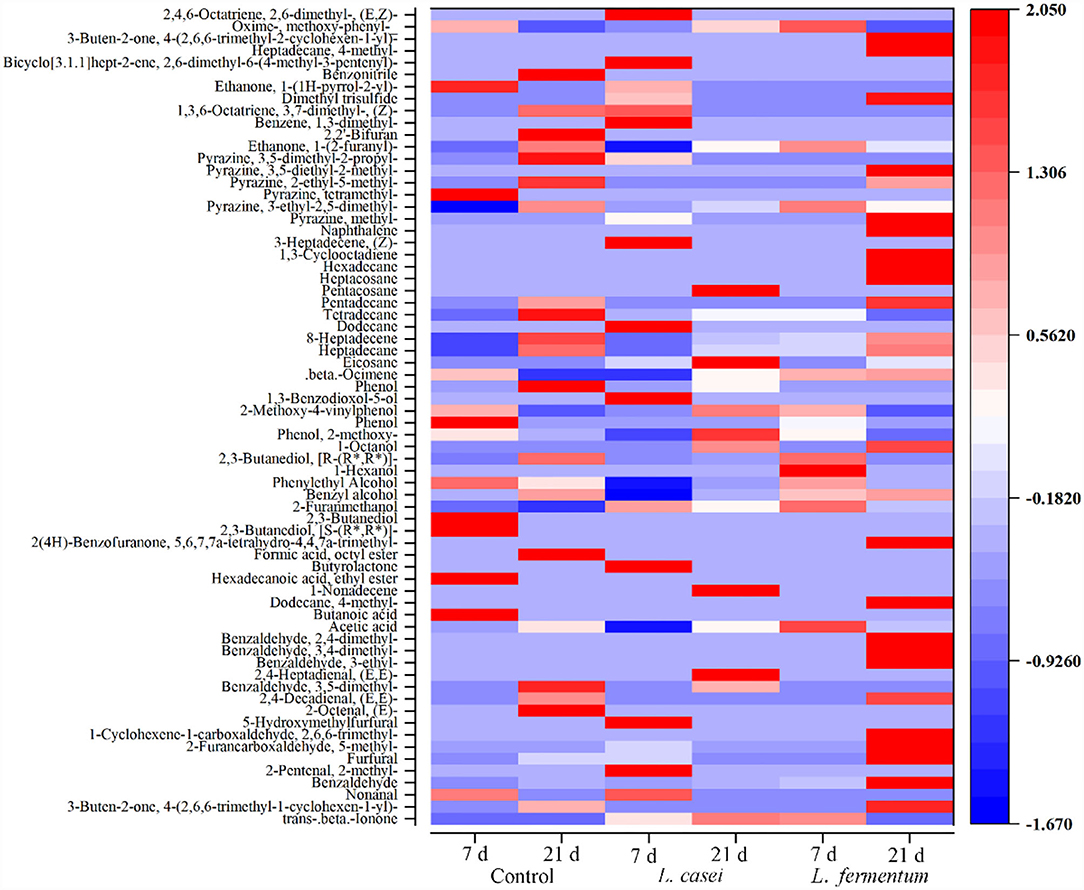
According to the above results, L. casei and L. fermentum strains were preferred for fermenting P. yezoensis sauce. Thus, GC-MS was employed to investigate flavor alterations in P. yezoensis sauce fermented by L. casei and L. fermentum. Figure 3 shows that during fermentation at 7 and 21 day, a total of 67 volatile compounds were detected from the P. yezoensis sauces. Specifically, 35, 41, and 42 volatile compounds were identified in the control group, L. fermentum group and L. casei group during fermentation, respectively. These volatile compounds mainly included aldehydes, alcohols, hydrocarbons, acids, phenols, ketones, esters, pyrazines, furans and benzodiazepines and others (Supplementary Table 1). At a given fermentation, the volatile profile changed with time due to the catabolic response of flavor precursor material (21). Alcohols presented the highest amount in all the 6 samples, accounting for 65.62%. Aldehydes and hydrocarbons accounted for 46.45 and 27.29% of the total volatile compounds, respectively. Alcohols and esters were mainly produced in the middle stage of fermentation, while hydrocarbons were produced in the later stage (22).
Assay of DPPH Radical Scavenging and Ferric-Reducing Activity
Polysaccharides, polyphenols, proteins, and porphyrins were the physiologically active compounds found in P. yezoensis, with anticancer, antioxidant, hypolipidemic, and anti-inflammatory properties (23, 24). Fermentation with LAB can increase the biological activity of food by bio-transforming active molecules (25). The antioxidant capacities of P. yezoensis sauces extract before and after fermentation with L. casei and L. fermentum are shown in Figure 4. The free radical scavenging capability and ferric reducing antioxidant capacity of unfermented P. yezoensis sauce were only 20.4% and 0.23, respectively. The antioxidant activity of all the tested extract from fermentated P. yezoensis sauces steadly increased during the 21 days of fermentation. For the P. yezoensis sauce fermented by L. casei for 21 days, the free radical scavenging capability and ferric reducing antioxidant capacity increased to 78% and 0.73, respectively. Similarly, L. fermentum fermented samples increased to 67.8% and 0.58, respectively.
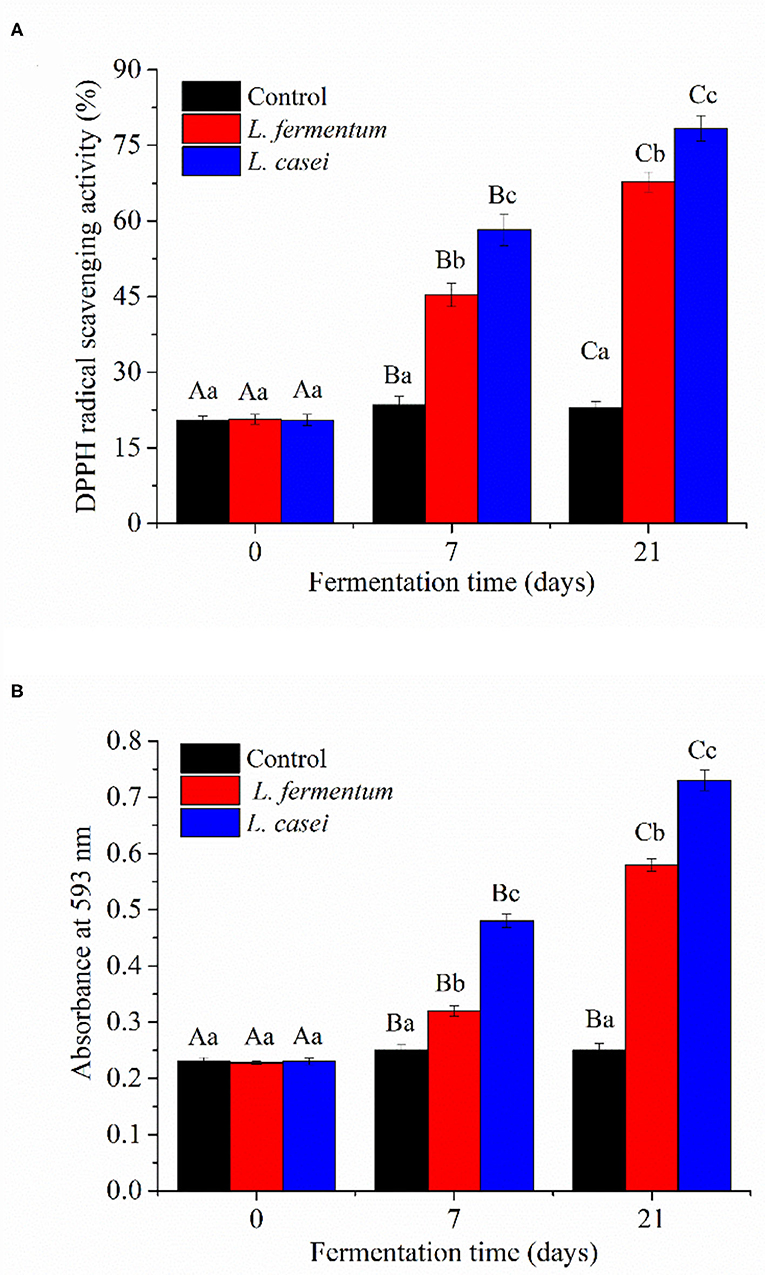
Figure 4. DPPH radical scavenging activity (A) and FRAP (B) of P. yezoensis sauces during fermentation. Values with different letters within each samples indicated a significant difference in content (p < 0.05).
Discussion
As more people become aware of the health benefits of laver, the laver industrial development has become more diverse and extended (26). The waste from the manufacturing of roasted seaweed sandwich were used to prepare the fermented P. yezoensis sauce. The obtained results revealed that L. casei was capable to grow in the the P. yezoensis sauce. The resulting seaweed sauce was featured by high enzyme vitality, and pronounced taste and flavor. In addition, compared with the control group and L. fermentum fermentation, L. casei fermentation improved the antioxidant activity of the sauce significantly, which was in line with the finding reported by Zubaidah et al., who fermented cabbage by L. casei (27).
The GC-MS analysis revealed that 42, 41, and 35 volatile flavor compounds were detected in the P. yezoensis sauces fermentated by L. casei, L. fermentum and the control group, respectively. These volatile compounds were responsible for P. yezoensis sauce flavor. Aldehydes are generated by fat oxidation, providing a fat scent (28). In the L. casei fermentation group one aldehyde was detected at Day 7, and 8 aldehydes were found at Day 21. The portion of aldehyde in the total volatile components increased from 2.71% at Day 7 to 46.45% at Day 21. However, in the L. fermentum group, the relative amount of aldehydes dropped from 25.53 to 6.64% and the number of compounds dropped from 6 to 3. In the control group, the ratio of aldehyde species increased from 2.49 to 8.47%, and the number of aldehyde species increased from 2 to 5. The unsaturated aldehydes have attractive aromas (29). For example, benzaldehyde possesses pleasant fruity flavor, and furfural refers to thew sweet and almond scent. These components can contribute to the enhancement of the sauce flavor (30).
Alcohols are produced by the microbial metabolism including the breakdown of unsaturated fatty acids and the reduction of base chemicals, which impart a clove scent (31). A total of 8 alcoholic compounds were detected from the six groups, including 2,3-Butanediol, [S-(R*,R*)], 2,3-butanediol, 2-furanmethanol, benzyl alcohol, phenylethyl alcohol and 2,3-Butanediol, [R-(R*,R*)], etc. The relative content of 2,3-Butanediol, [R-(R*,R*)] increased up to 40.91% at Day 7 of L. casei fermentation, and then decreased to 1.71% at Day 21 of fermentation, thus providing little contribution to the overall flavor.
Hydrocarbon compounds were identified in P. yezoensis sauces, including β-ocimene, eicosane, heptadecane, 8-heptadecene, tetradecane and others. Although alkyene compounds were regarded as odorless due to the cleavage of the alkoxygen radical, some olefins generated aldehydes and ketones under specific circumstances still can contribute to the overall flavor of P. yezoensis sauces (32). Several alkanes, when degraded under certain circumstances, generated a fishy odor (33). After 21 days of fermentation with L casei, the number of hydrocarbon species increased from 4 to 9, with the relative content of 8-heptadecene increased from 7.15 to 19.15%. During fermentation, the relative content of 8-heptadecene in unfermented group increased from 2.21 to 16.48%, but the relative content of 8-heptadecene during L. fermentum fermentation was no significant difference. It was widely established that 8-heptadecene tadecene was a distinctive flavor compound found in a wide variety of macroalgae (34).
P. yezoensis sauces contained a total of four acid compounds, namely acetic acid, butanoic acid, dodecane, 4-methyl, and 1-non-adecene. Among them, acetic acid is primarily an enzymatic response in the lipogenesis enzyme pathway, which has an oily fishy smell. Also, acetic acid may create ester bonds with sugar to enhance the flavor (35). The L. casei fermentation reduced the concentration of acetic acid, consequently atteunating the pungent smell of P. yezoensis sauces (36). At the same time, the release of acetic acid indicates that L. casei has the ability to metabolize lactic acid (37).
Esters are formed by esterifying carboxylic acid and alcoho, which are usually related with fruit or floral scents, and contributing to the flavor of food products (38, 39). Ketones with a unique fruit smell, can be generated by heat oxidation or breakdown of amino acids (40). Pyrazines are nitrogen-containing heterocyclic compounds that are an intermediate in the Millard reaction (41). They often have a barbeque and nut flavor, an excellent dispersed fragrance, and an extreme deficient concentration. These volatile compounds were detected in P. yezoensis sauce fermented by L. casei. Therefore, it was determined that fermenting P. yezoensis sauce with L. casei produces the finest flavor.
Conclusion
In this study, three LAB strains fermented sandwich seaweed processing waste to prepare P. yezoensis sauce. The detection and analysis of the three LAB strains fermented P. yezoensis sauces revealed that the L. casei was able to adapt to the fermentation environment and had high protease vitality. Sensory evaluation results showed that fermentation of L. casei could enhanced the acceptability of P. yezoensis sauce. Following GC-MS detection, P. yezoensis sauce generated 42 volatile flavor compounds by fermentation of L. casei. These volatile chemicals are essential for P. yezoensis sauce taste. The antioxidant activity of P. yezoensis sauces fermented by L. casei was also higher. Overall, L. casei fermented P. yezoensis sauce had the best flavor and was appropriate for producing P. yezoensis sauce meals. The fermented P. yezoensis sauces has a distinctive taste and excellent nutritional value for the elderly, children and babies. This study can provide guidance for the development of LAB fermented P. yezoensis sauces in future studies.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Author Contributions
JY performed the experiments, analyzed the data, and wrote the manuscript. TG and FG analyzed the data and wrote the manuscript. HS, ZC, and ZW analyzed and discussed the data. SW, PS, and YT provided samples and discussed the data. WW designed the research content, analyzed the data, and modified the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the Project funded by Opening Foundation of Key Laboratory of high-tech veterinary biopharmaceutical research, Jiangsu province (No. NSFK201803), National Natural Science Foundation of China (Nos. 32100037 and 32172284), Natural Science Research General Project of Jiangsu Higher Education Institutions (No. 20KJB550008), China Postdoctoral Science Foundation (No. 2019M661767), Natural Science Foundation of Jiangsu Province (No. BK20201028), Jiangsu Planned Projects for Postdoctoral Research Funds (No. 2021K316C), Jiangsu Ocean University Research Funds (No. KQ17028), and Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2021.810460/full#supplementary-material
References
1. Jin C, Wang J, Wang S, Xu X. Porphyra species: a mini-review of its pharmacological and nutritional properties. J Med Food. (2016) 19:111–9. doi: 10.1089/jmf.2015.3426
2. Watanabe F, Takenaka S, Katsura H, Miyamoto E, Nakano Y. Characterization of a vitamin B12 compound in the edible purple laver. porphyra yezoensis. J Agric Chem Soc Japan. (2000) 64:2712–5. doi: 10.1271/bbb.64.2712
3. Venkatraman KL, Syed AA, Indumathi P, Mehta A. Vitpor ai, a coagulation factor xiia inhibitor from porphyra yezoensis: In vivo mode of action and assessment of platelet function analysis. Prot Pept Lett. (2020) 27:243–50. doi: 10.2174/0929866526666191026111056
4. Qian L, Zhou Y, Ma JX. Hypolipidemic effect of the polysaccharides from porphyra yezoensis. Int J Biol Macromol. (2014) 68:48–9. doi: 10.1016/j.ijbiomac.2014.04.004
5. Tian Y, Jiang Y, Guo Y, Zhao Y, Li N, Yao L, et al. Research progress on nutritional quality and edible value of Porphyra. J Food Saf Qual. (2021) 12:4929–36. doi: 10.19812/j.cnki.jfsq11-5956/ts.2021.12.033
6. Zhang X, Luo J, Zhuo D, He Y, Zhou H, Ren D, et al. Fermentation technology of special Porphyra haitanensis sauce. J Food Saf Qual. (2020) 11:2609–16. doi: 10.19812/j.cnki.jfsq11-5956/ts.2020.08.047
7. Fan L, Shi X. Technology of fermentative Porphyra sauce. Food Sci Technol. (2015) 40:269–72. doi: 10.13684/j.cnki.spkj.2015.03.064
8. Uchida M, Miyoshi T, Yoshida G, Niwa K, Mori M, Wakabayashi H. Isolation and characterization of halophilic lactic acid bacteria acting as a starter culture for sauce fermentation of the red alga nori (porphyra yezoensis). J Appl Microbiol. (2014) 116:1506–20. doi: 10.1111/jam.12466
9. Ryu S. Study on manufactute of porphyran jam and eppiciency extraction method of porphyran from Porphyra yezoensis. J Korean Appl Sci Technol. (2013) 30:504–17. doi: 10.12925/jkocs.2013.30.3.504
10. Yuasa M, Shimada A, Matsuzaki A, Eguchi A, Tominaga M. Chemical composition and sensory properties of fermented citrus juice using probiotic lactic acid bacteria. Food Biosci. (2021) 39:100810. doi: 10.1016/j.fbio.2020.100810
11. Chen Y, Wang Y, Chen J, Tang H, Wang C, Li Z, et al. Bioprocessing of soybeans (Glycine max L.) by solid-state fermentation with Eurotium cristatum YL-1 improves total phenolic content, isoflavone aglycones, and antioxidant activity. RSC Adv. (2020) 10:16928–41. doi: 10.1039/C9RA10344A
12. Zhang S. Determination of the relationship between protease enzyme activity and number of psychrophilic bacteria in fresh milk in Harbin. China Dairy Ind. (2012) 40:41–9. doi: 10.3969/j.issn.1001-2230.2012.07.012
13. Apa B, Yha B, Rui M, Khe A, Lpt C, Mc A. Combination of solid phase microextraction and low energy electron ionization gas chromatography-quadrupole time-of-flight mass spectrometry to meet the challenges of flavour analysis. Talanta. (2021) 235:122793. doi: 10.1016/j.talanta.2021.122793
14. Augustini A, Sielemann S, Telgheder U. Strategy for the identification of flavor compounds in e-liquids by correlating the analysis of GCxIMS and GC-MS. Talanta. (2021) 230:122318. doi: 10.1016/j.talanta.2021.122318
15. Yang J, Lu J, Zhu Q, Tao Y, Zhu Q, Guo C, et al. Isolation and characterization of a novel Lactobacillus plantarum MMB-07 from traditional suanyu for Acanthogobius hasta fermentation. J Biosci Bioeng. (2021) 132:161–6. doi: 10.1016/j.jbiosc.2020.12.016
16. Tang WZ, Wang B, Wang MM, Wang MM. Ultrasound-assisted extraction of Osmanthus fragrans fruit oil and evaluation of its fatty acid composition, physicochemical properties and antioxidant activity. J Appl Res Med Aroma. (2021) 25:100331. doi: 10.1016/j.jarmap.2021.100331
17. Xu J, Xu L, Zhou Q, Hao S, Zhou T, Xie H. Enhanced in vitro antioxidant activity of polysaccharides from Enteromorpha prolifera by enzymatic degradation. J Food Biochem. (2016) 40:275–83. doi: 10.1111/jfbc.12218
18. Muhialdin BJ, Hussin A, K Adum H, Hamid AA, Jaafar AH. Metabolomic changes and biological activities during the lacto-fermentation of jackfruit juice using lactobacillus casei atcc334. LWT-Food Sci Technol. (2021) 141:110940. doi: 10.1016/j.lwt.2021.110940
19. Wang Y, Tao Y, Zhang X, Shao S, Han Y, Chu D, et al. Metabolic profile of ginkgo kernel juice fermented with lactic acid bacteria: a potential way to degrade ginkgolic acids and enrich terpene lactones and phenolics. Process Biochem. (2018) 76:25–33. doi: 10.1016/j.procbio.2018.11.006
20. Li W, Wang T. Effect of solid-state fermentation with Bacillus subtilis lwo on the proteolysis and the antioxidative properties of chickpeas. Int J Food Microbiol. (2020) 338:108988. doi: 10.1016/j.ijfoodmicro.2020.108988
21. Li S, Jin Z, Hu D, Yang W, Chen X. Effect of solid-state fermentation with Lactobacillus casei on the nutritional value, isoflavones, phenolic acids and antioxidant activity of whole soybean flour. LWT Food Sci Technol. (2020) 125:109264. doi: 10.1016/j.lwt.2020.109264
22. Song J, Bi J, Chen Q. Assessment of sugar content, fatty acids, free amino acids, and volatile profiles in jujube fruits at different ripening stages. Food Chem. (2019) 270:344–52. doi: 10.1016/j.foodchem.2018.07.102
23. Isaka S, Cho K, Nakazono S, Abu R, Ueno M, Kim D, et al. Antioxidant and anti-inflammatory activities of porphyran isolated from discolored nori (porphyra yezoensis). Int J Biol Macromol. (2015) 74:68–75. doi: 10.1016/j.ijbiomac.2014.11.043
24. Wang F, Kong LM, Xie YY, Wang C, Zhou T. Purification, structural characterization, and biological activities of degraded polysaccharides from Porphyra yezoensis. J Food Biochem. (2021) 45:e13661. doi: 10.1111/jfbc.13661
25. Li S, Tao Y, Li D, Wen G, Zhou J, Manickam S, et al. Fermentation of blueberry juices using autochthonous lactic acid bacteria isolated from fruit environment: fermentation characteristics and evolution of phenolic profiles. Chemosphere. (2021) 276:130090. doi: 10.1016/j.chemosphere.2021.130090
26. Cho TJ, Min SR. Health functionality and quality control of laver (Porphyra, Pyropia): current issues and future perspectives as an edible seaweed. Marine Drugs. (2020) 18:14. doi: 10.3390/md18010014
27. Zubaidah E, Arum MS, Widyaningsih TD, Rahayu AP. Sauerkraut with the addition of lactobacillus casei: effects of salt and sugar concentrations on fermentation and antioxidant activity. Curr Nutr Food Sci. (2020) 16:1265–9. doi: 10.2174/1573401316666200217112642
28. Widjaja R, Craske JD, Wootton M. Comparative studies on volatile components of non-fragrant and fragrant rices. J Sci Food Agric. (2015) 70:151–61. doi: 10.1002/(SICI)1097-0010(199602)70:2<151::AID-JSFA478>3.0.CO;2-U
29. Jiye A, Trygg J, Gullberg J, Johansson AI, Jonsson P, Antti H, et al. Extraction and GC/MS analysis of the human blood plasma metabolome. Anal Chem. (2005) 77:8086–94. doi: 10.1021/ac051211v
30. Cao R, Hu M, Zhao L, Wang L, Liu Q. Flavor characteristics of different crops of laver (Porphyra yezoensis) during one harvest cycle. J Ocean U China. (2021) 20:213–20. doi: 10.1007/s11802-021-4447-3
31. Lee SM, Seo BC, Kim YS. Volatile compounds in fermented and acid-hydrolyzed soy sauces. J Food Sci. (2006) 71:C146–56. doi: 10.1111/j.1365-2621.2006.tb15610.x
32. Giannetti V, Mariani MB, Mannino P. Volatile fraction analysis by HS-SPME/GC-MS and hemometric modeling for traceability of apples cultivated in the Northeast Italy. Food Control. (2017) 78:215–21. doi: 10.1016/j.foodcont.2017.02.036
33. Chakraborty K, Joseph D. Production and characterization of refined oils obtained from Indian oil sardine (Sardinella longiceps). J Agric Food Chem. (2015) 63:998–1009. doi: 10.1021/jf505127e
34. Murathan ZT, Zarifikhosroshahi M, Kafkas NE. Determination of fatty acids and volatile compounds in fruits of rosehip (Rosa L.) species by HS-SPME/GC-MS and Im-SPME/GC-MS techniques. Turk J Agric For. (2016) 40:269–79. doi: 10.3906/tar-1506-50
35. Lalel H, Singh Z, Tan SC. Glycosidically-bound aroma volatile compounds in the skin and pulp of 'kensington pride' mango fruit at different stages of maturity. Postharvest Biol Tec. (2003) 29:205–18. doi: 10.1016/S0925-5214(02)00250-8
36. Nishino N, Touno E. Ensiling characteristics and aerobic stability of direct-cut and wilted grass silages inoculated with Lactobacillus casei or Lactobacillus buchneri. J Sci Food Agric. (2005) 85:1882–8. doi: 10.1002/jsfa.2189
37. Li S, Chen C, Ji Y, Lin J, Chen X, Qi B. Improvement of nutritional value, bioactivity and volatile constituents of quinoa seeds by fermentation with lactobacillus casei. J Cereal Sci. (2018) 84:83–9. doi: 10.1016/j.jcs.2018.10.008
38. Jabalpurwala F, Gurbuz O, Rouseff R. Analysis of grapefruit sulphur volatiles using SPME and pulsed flame photometric detection. Food Chem. (2010) 120:296–303. doi: 10.1016/j.foodchem.2009.09.079
39. Xiao Y, Huang Y, Chen Y, Fan Z, Chen R, He C, et al. Effects of solid-state fermentation with Eurotium cristatum YL-1 on the nutritional value, total phenolics, isoflavones, antioxidant activity, and volatile organic compounds of black soybeans. Agronomy. (2021) 11:1029. doi: 10.3390/agronomy11061029
40. Sarnoski PJ, O'Keefe SF, Jahncke ML, Mallikarjunan P, Flick GJ. Analysis of crab meat volatiles as possible spoilage indicators for blue crab (Callinectes sapidus) meat by gas chromatography-mass spectrometry. Food Chem. (2017) 122:930–5. doi: 10.1016/j.foodchem.2010.03.069
Keywords: Porphyra yezoensis sauce, lactic acid bacteria, volatile components, GC-MS, antioxidation
Citation: Yang J, Gao T, Ge F, Sun H, Cui Z, Wei Z, Wang S, Show PL, Tao Y and Wang W (2022) Porphyra yezoensis Sauces Fermented With Lactic Acid Bacteria: Fermentation Properties, Flavor Profile, and Evaluation of Antioxidant Capacity in vitro. Front. Nutr. 8:810460. doi: 10.3389/fnut.2021.810460
Received: 07 November 2021; Accepted: 20 December 2021;
Published: 13 January 2022.
Edited by:
Biao Yuan, China Pharmaceutical University, ChinaReviewed by:
Ronghai He, Jiangsu University, ChinaXu-Cong Lv, Fujian Agriculture and Forestry University, China
Copyright © 2022 Yang, Gao, Ge, Sun, Cui, Wei, Wang, Show, Tao and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wenbin Wang, d2VuYmluNjZAam91LmVkdS5jbg==
 Jie Yang
Jie Yang Tengqi Gao1
Tengqi Gao1 Yang Tao
Yang Tao Wenbin Wang
Wenbin Wang