- 1“Carol Davila” University of Medicine and Pharmacy, Bucharest, Romania
- 2Center for Digestive Diseases and Liver Transplant, Fundeni Clinical Institute, Bucharest, Romania
- 3Center of Excellence in Translational Medicine, Fundeni Clinical Institute, Bucharest, Romania
- 4University Hospital Munster, Munster, Germany
Recurrent or de novo non-alcoholic fatty liver disease (NAFLD)/non-alcoholic steatohepatitis (NASH) following liver transplantation (LT) is a frequent event being increasingly recognized over the last decade, but the influence of recurrent NASH on graft and patient outcomes is not yet established. Taking into consideration the long term survival of liver transplanted patients and long term complications with associated morbidity and mortality, it is important to define and minimize risk factors for recurrent NAFLD/NASH. Metabolic syndrome, obesity, dyslipidemia, diabetes mellitus are life style risk factors that can be potentially modified by various interventions and thus, decrease the risk of recurrent NAFLD/NASH. On the other hand, genetic factors like recipient and/or donor PNPLA3, TM6SF2, GCKR, MBOAT7 or ADIPOQ gene polymorphisms proved to be risk factors for recurrent NASH. Personalized interventions to influence the different metabolic disorders occurring after LT in order to minimize the risks, as well as genetic screening of donors and recipients should be performed pre-LT in order to achieve diagnosis and treatment as early as possible.
Introduction
Non-alcoholic fatty liver disease (NAFLD) is a highly prevalent condition in Western Europe and USA, but has also an increasing trend in Southern and Eastern European countries and Asia as stated by the HEPAHEALTH Project (1). It is now the most frequent chronic liver disease worldwide (25% of all adults) and represents a major global public health challenge (2) and a cause of significant morbidity and mortality. NAFLD is a liver disease comprising different variants (3) from steatosis (non-alcoholic fatty liver, NAFL), in which plethoric hepatic fat is shown, and non-alcoholic steatohepatitis (NASH), a necroinflammatory form of the disorder manifested by histological inflammation and hepatocyte ballooning that conducts to severe liver fibrosis with end stage liver disease (>20% in NASH patients) and hepatocellular carcinoma requiring liver transplantation (LT). NAFLD/NASH can be present in the patient awaiting LT, but also in the donors because of increased risk of cardiovascular events, the major cause of death in people with NAFLD (4).
Transplant candidates with NASH commonly have certain metabolic comorbidities supplementary to the complexity of managing the complications of chronic liver disease. Obesity escalates the risk of decompensation while on the waiting list and can represent a surgical technical challenge (5). Sarcopenic obesity is multifactorial, affects up to 35% of patients awaiting LT and is associated with increased morbidity and mortality compared to either disease alone, as well as worse survival after LT (6, 7). The overall prevalence of NAFLD and NASH among patients with type 2 diabetes mellitus (T2DM) is 55.5% and, respectively, 37.3% (8), thus T2DM being another factor implicated in prognosis of patients with NASH related cirrhosis awaiting LT and following LT.
Recurrent or de novo NAFLD/NASH following LT is a frequent event being increasingly recognized over the past decade (9). The influence of recurrent NASH on graft and patient outcomes is not yet clearly stated. Several data suggest that it does not impact graft and patient survival (10–12), but there is a large variation in the diagnostic modalities, protocol liver biopsies or non-invasive evaluation of fibrosis and follow-up intervals. However, there are publications analyzing the factors that influence the occurrence of NAFLD/NASH after LT and demonstrate the association with adverse post-LT outcomes related to liver and non-liver related events (13–17).
Frequency, Progression and Significance of Post-transplant NAFLD
Recurrent and de novo NASH are increasingly being reported in post-LT NASH recipients, and quick diagnosis through non-invasive serological or imaging tests, followed by liver biopsy if needed, will help early intervention to avoid progression of NASH, and its related complications in the post-transplant period.
According to previous studies (14, 17, 18) there are variable prevalence of de novo NAFLD or recurrent NAFLD/NASH with different outcomes after LT. Recurrent NAFLD appears to be an earlier, more severe and with negative patient and graft outcomes. The recurrence of NAFLD has been reported to occur in 8.2–62.5% of recipients over variable follow-up periods ranging from <6 months to 10 years, and the rates for steatohepatitis have ranged from 4 to 33% over follow-up periods ranging from 6 weeks to 20 years. The rates of advanced fibrosis have ranged from 0 to 33% (short-term 6–12 months) or even 71.4% at 5 years after LT (14, 19, 20). One study even showed that almost 90% of patients developed recurrent NAFLD, but only 25% of them had advanced fibrosis following LT (21).
Taking into consideration the long term survival of liver transplanted patients and long term complications with associated morbidity and mortality, it is important to define and minimize risk factors for recurrent NAFLD/NASH.
Life Style Risk Factors for Recurrent NAFLD After LT and Potential Interventions
Metabolic syndrome (MS) has been described in 43–58% of LT recipients. Obesity, dyslipidaemia, diabetes or insulin resistance, as well as certain immunosuppressive agents after LT are frequent predictors of recurrence of NAFLD after transplantation (22).
Obesity
Obesity is encountered in more than one-third of all transplant recipients. Majority of the weight gain occurs during the first 1–3 years (23, 24), but persists to increase over the following years with an enlargement in abdominal girth and body fat content and corresponding low lean body mass. Obesity at 1-year post-transplantation shows a 2-fold increased mortality risk. Interventions to preclude the earliest weight gain might be more promising than later weight-loss endeavors.
Post-LT obesity management should comprise the same algorithm as in other obese persons: diet and exercise, pharmacologic therapy and surgical or endoscopic bariatric procedures. Weight loss is associated with improvement of recurrent NASH and better long term outcome. Weight loss medication can be used in this patient population, but the choice of medication should be individualized.
Orlistat, acting by directly stopping absorption of ~30% of dietary triglycerides, was evaluated in the post-LT setting and proved to be safe (25), but there are no data regarding its efficacy. Orlistat should be given at least 3 h before or after calcineurin inhibitors and levels should be monitored closely. There are no recorded interactions with antimetabolites or mammalian target of rapamycin (mTOR) inhibitors (26).
Liraglutide, a long-acting glucagon-like peptide-1 (GLP-1), appears to have no interactions with the immunosuppressive therapies and to have also cardio-protective effects in patients with known atherosclerotic disease or heart failure, making it an interesting option in these high risk patients. Following LT, liraglutide can be chosen in patients with diabetes mellitus, end stage renal disease or multiple drugs for different comorbidities, as well as in the early post-LT period to help avoid weight gain and possibly result in modest weight loss (27). Marked weight loss in patients with type 2 diabetes has also been noted in studies of semaglutide, a longer-acting GLP-1 analog, but there are no studies in LT recipients (28, 29).
Phentermine-topiramate suggests having the highest weight loss influence, by directly creating blockade of absorption of ~30% of dietary triglycerides. However, possible side effects are mentioned such as neuropsychiatric disorders, cardiovascular comorbidities, and drug-drug interactions that could limit their use. There are no known interactions with posttransplant immunosuppressants, but there are no data on the use of phentermine-topiramate following post-solid organ transplant setting (30).
Naltrexon-bupropion was authorized as a weight loss drug in 2014, leading also to improvement of fasting blood glucose and dyslipidemia. There are no data specific to the benefit of naltrexone-bupropion in the post-transplant setting, but there is no established interaction with post-transplant immunosuppressive medication. However, bupropion is a strong CYP2D6 inhibitor and can elevate the serum concentration of many drugs (26).
There is no specific immunosuppression strategy that has been shown to be useful in preventing weight gain after LT; however, immunosuppression should be tailored to diminish to minimum the risk of metabolic complications (30).
Bariatric surgery is also possible, may be safe and feasible after LT for weight loss, but may be more technically demanding, and is linked with elevated morbidity when compared with non-LT patients (17, 31). However, bariatric surgery should be taken into consideration for treatment of recurrent NAFLD because it ameliorates steatosis and steatohepatitis in most of the patients and improves or resolves liver fibrosis in 30% of patients (32).
Dyslipidemia occurs in 30–60% of LT recipients, being a major risk factor for allograft steatosis and posttransplant cardiovascular-related morbidity and mortality, and often continues despite dietary changes. A fasting lipid profile should be done every year in all LT recipients. mTOR inhibitors produce a stronger dyslipidemic effect compared to calcineurin inhibitors. Sirolimus proved to worsen hyperlipidemia in a dose-dependent manner (33).
Hypertriglyceridemia is the most common dyslipidemic change. Life-style changes should be realized when the low-density lipoprotein cholesterol (LDL-C) level is >100 mg/dL, although dietary modification alone is often inadequate, making pharmacotherapy necessary (24). Different circulating lipid components have varying effects. While circulating triglyceride (TG) levels are associated with the development of hepatic steatosis due to the imbalance between TG synthesis and breakdown process in hepatocytes, LDL-C is closely related to cardiovascular complications. Both lipid components should be addressed to reach different aspects of metabolic syndrome. The therapeutic goal for LDL-C should be below 100 mg/dL (even <70 mg/dL) in order to decrease the high cardiovascular risk in NASH patients after LT. Cholesterol is also a major lipotoxic molecule in NASH development. The gut microbiome represents an environmental factor contributing to the development of NAFLD and there are studies suggesting that dietary cholesterol caused advanced fibrosis by cholesterol-induced gut microbiota changes and metabolomic alterations (34). Thus, cholesterol inhibition and manipulation of the gut microbiota and its related metabolites might represent effective strategies in preventing NAFLD, but no studies are yet in the LT recipients.
Similar to non-transplant patients, statins are the drug of choice being usually well-accepted. Low doses of statins at beginning with slight increase as required and close follow-up should be taken into consideration. Pravastatin and fluvastatin are not metabolized by the CYP3A4 isoenzyme and should be first choice in post-LT recipients. Ezetimibe that acts through inhibition of enterohepatic recirculation of lipids, proved to effectively treat hypercholesterolemia with few side effects and to have no interaction with immunosuppressive agents. However, both pravastatin and fluvastatin are of low potency. Thus, combination therapy using ezetimibe will often be required to reach LDL-C targets. Alternatively, rosuvastatin is a substantially more potent option and is also not metabolized by cytochrome P450 (CYP) 3A4 (35).
Fibrates can be also safely used in patients with high triglyceridemia levels over 600 mg/dL, but caution is required when co-administrated with statins due to high risk of myotoxicity and renal dysfunction. For patients with hypertriglyceridemia, fish oil can be used with minimal side effects except potential increase of low-density lipoprotein level (18, 30).
Diabetes Mellitus
One-third of LT recipients have type 2 diabetes mellitus (post-transplant diabetes mellitus, PTDM), requiring long-term therapy and follow-up. There is lot of evidence that people with T2DM are at high risk of developing NASH, but also that NAFLD may precede and/or develop T2DM, hypertension and atherosclerosis (36). This complex link between NAFLD and T2DM can be extrapolated to post-LT recipients. Treatment of NAFLD/NASH patients could avoid T2DM occurrence and/or progression, but, also the other way around.
Recipients with PTDM are handled just the same as patients with type 2 diabetes mellitus in the general population and the aim is to normalize target values and re-establish metabolic control. Dietary and lifestyle modification are of great importance, but are usually unsatisfactory in this population, with most patients requiring pharmacological therapy with oral agents or insulin.
Metformin and thiazolidinediones, influencing insulin resistance, proved to have benefit on biochemical and metabolic features of NAFLD, but amelioration of patients' histological response or fibrosis was modest and studies were usually short-term, thus liver-related long-term outcomes could not be evaluated (37).
Sodium–glucose cotransporter 2 inhibitors (SGLT2i) and glucagon-like peptide-1 receptor agonists (GLP-1RAs) are now accepted as the best therapeutic option for patients with T2DM and cardiovascular disease, heart failure and/or chronic kidney disease. These two types of drugs determine weight loss, making them an attractive option for patients with associated obesity, and offer promising effects in reducing liver fat content (37, 38). However, there are no clinical studies performed in post-LT NAFLD/NASH patients with these two drug classes although this therapeutic approach would be completely justified.
Bariatric surgery has recently proved to be one of the most effective therapeutic options for T2DM through weight-dependent and weight-independent mechanisms (39). Factors associated with diabetes remission consists of duration of diabetes prior to surgery <4 years, higher C-peptide, younger age and use of oral agents or diet to control diabetes (40).
Due to the increased prevalence of NAFLD worldwide, along with a reduced organ pool donation in many countries, usage of donor grafts with steatosis is now rather common. Donor graft steatosis is also a significant risk factor for post-LT recurrence of NASH (41). Defatting strategies, like pharmacological agents (e.g., forskolin, peroxisome proliferator-activated receptor (PPAR) -alpha ligand, hypericin, scoparone, PPAR-delta ligand, visfatin, L-carnitine) and hypothermic or normothermic machine perfusion have been shown to decrease hepatocyte steatosis (42). To achieve significant defatting, the protocol of choice should shift the balance toward more efficient TG breakdown (lipolysis) and excretion of related byproducts, as well as minimizing TG synthesis. There is still much research to be done on how best to modulate this lipid metabolism using cocktails of agents or ex vivo machine perfusions in order to achieve rapid defatting without adversely affecting viability and other critical liver functions. Short term survival and functionality of steatotic livers for which TG content has been dramatically reduced is already proven (43), however long term prevention of post-LT complications is not yet established.
Genetic Risk Factors for Recurrent NAFLD After LT
There are few studies mentioning genetic influences on NAFLD recurrence post-LT. The range of recurrent NAFLD is wide and causes for this interindividual variability may be at least partially associated to differences in genetic background of both recipient and donor.
Finkenstedt et al. (44) showed that recipient patatin-like phospholipase domain containing 3 (PNPLA3) rs738409 was correlated with graft steatosis according to the 5-year post-LT computed tomography imaging. Kim et al. (45) found that the presence of the rs738409-G risk allele in both donor and recipient was an important risk factor for 1 year post-LT histologically proven NAFLD. Other data by Trunecka et al. (46) proved donor PNPLA3 rs738409 is a powerful risk factor of graft steatosis based on histologic findings on liver biopsy. The actual insight into the role of the p.I148M mutated PNPLA3 protein in liver fat turnover should favor the hypothesis that donor, but not recipient PNPLA3 genotype is critical for fat aggregation in the liver graft (47).
The donor TM6SF2 (transmembrane 6 superfamily member 2) c.499A allele is an independent risk factor of liver graft steatosis following LT in addition to the effects of donor PNPLA3 c.444G allele (48). The TM6SF2 p.E167K (c.499G>A) variant is important in patients with NAFLD, being associated with more severe steatosis, necroinflammation and advanced fibrosis/cirrhosis. Variants in the genes encoding glucokinase regulator (GCKR) and membrane bound O-acyl transferase 7 (MBOAT7) also contribute to the risk of NAFLD, by increasing de novo lipogenesis and altering the remodeling of phospholipid.
The study by John et al. (49) newly indicated that recipient adiponectin (ADIPOQ) rs1501299 and rs17300539 polymorphisms are associated with de novo NAFLD among patients transplanted for hepatitis C. De novo diabetes mellitus, as risk factor for post-LT NAFLD was associated with the following SNPs: recipient angiotensinogen (AGT) rs699; recipient mTOR rs2295080 (only following everolimus use); recipient ADIPOQ rs1501299 and rs822396; donor and recipient small ubiquitin like modifier 4 (SUMO4) rs237025 (50).
Our group recently demonstrated that the allele 1993C of the SNP rs4794067 of gene TBX21 (T-box transcription factor 21), but not CYP3A5*3 genotype may predispose to the development of late significant fibrosis and severe steatosis of the liver graft (51). The functional polymorphism TBX21-1993T/C (rs4794067) increases the transcriptional activity of the TBX21 gene (essential for Th1 polarization) resulting in a preponderance of a Th-2 or Th17 response.
Whenever genetic screening of recipients and donors identifies high risk genotypes for NASH, it is of paramount importance to control the modifiable risk factors and to intensify screening after LT for early detection of NAFLD/NASH.
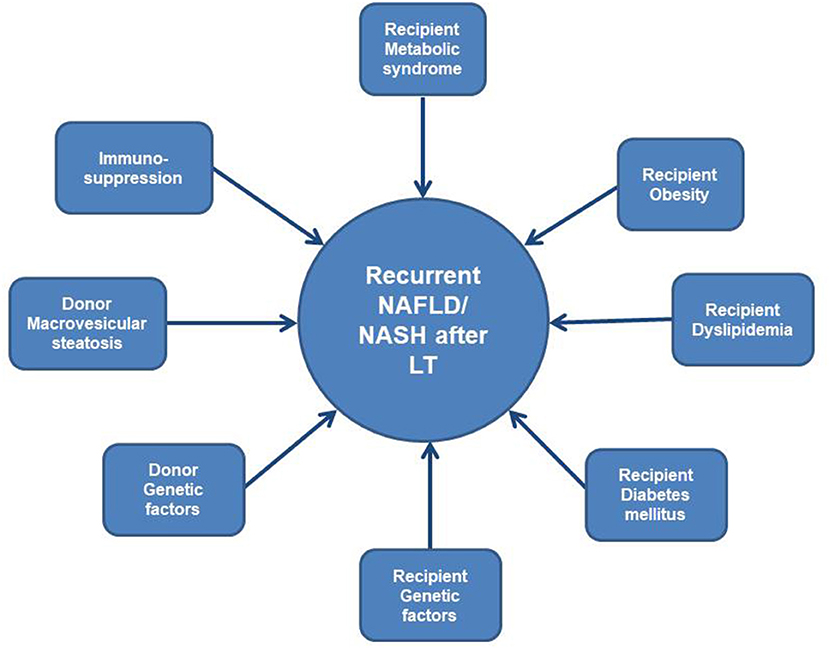
Screening for genetic risk factors before and after LT is very complex and interrelated (Figure 1). Multiple recipient and donor genetic factors are implicated in occurrence of all variants of NAFLD such as: risk factors for insulin resistance, for steatosis, for obesity and dyslipidemia, for metabolisation of immunosuppression, for gut microbiota, thus use of this data in clinical practice is still under investigation and constitutes one of the limitations of this review.
Discussion
NASH remains the fastest growing indication for LT worldwide and recurrent NAFLD is common. There remains a need for long-term studies in this patient population to specifically address approach to diagnosis of recurrent NASH, preventive measures, treatment and implications.
Patients with histologically established posttransplant NASH have elevated risk of poor outcome as one third of them die within 5 years of the diagnosis and 26% develop a cardiovascular event. Almost one third of patients with recurrent NASH may develop bridging fibrosis/cirrhosis at 5 years after LT (17, 18).
Transient elastography (TE) is an ideal, non-invasive and accessible method for diagnosing the stage of hepatic fibrosis post-LT in both viral [hepatitis C virus (HCV) vs. non-HCV] patients (52, 53). Our group proved that LT recipients can very well be evaluated for steatosis and fibrosis by TE with CAP (controlled attenuation parameter) (54). Screening of NASH via TE and CAP should notify the clinicians and patients to this additional comorbidity and the greater possibility for complications related to insulin resistance. Patients who are at high risk of developing MS after LT should receive personalized interventions in order to minimize the risks, and should undergo routine surveillance in order to achieve an earlier diagnosis and treatment. The influence of immunosuppression on the development of MS and NAFLD after LT was extensively discussed in other papers (55, 56) and will not be in the focus of this review. Weight loss through diet, lifestyle modifications, pharmacological agents or bariatric surgery is linked with resolution of NASH and improvement in liver fibrosis, and should be implemented in overweight LT recipients, with an objective of 7–10% decrease in body weight (30). An early diagnosis of MS will restraint associated comorbidities, thereby reducing the risk of cardiovascular events. Strength of our review consists in establishing patients at risk of recurrence of NAFLD through genotypic and phenotypic characterization at transplant that will help to interfere by targeted strategies to prevent recurrence of NAFLD/NASH.
Author Contributions
SI, SB, and LG: conceptualization. SI and RI: writing-original draft. CG, VC, SB, and IP: writing-review and editing. IP, SB, and LG: visualization. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Pimpin L, Cortez-Pinto H, Negro F, Corbould E, Lazarus JV, Webber L, et al. Burden of liver disease in Europe: epidemiology and analysis of risk factors to identify prevention policies. J Hepatol. (2018) 69:718–35. doi: 10.1016/j.jhep.2018.05.011
2. Hardy T, Wonders K, Younes R, Aithal GP, Aller R, Allison M, et al. The European NAFLD registry: a real-world longitudinal cohort study of nonalcoholic fatty liver disease. Contemp Clin Trials. (2020) 98:106175. doi: 10.1016/j.cct.2020.106175
3. Anstee QM, Reeves HL, Kotsiliti E, Govaere O, Heikenwalder M. From NASH to HCC: current concepts and future challenges. Nat Rev Gastroenterol Hepatol. (2019) 16:411–28. doi: 10.1038/s41575-019-0145-7
4. Targher G, Byrne CD, Tilg H. NAFLD and increased risk of cardiovascular disease: clinical associations, pathophysiological mechanisms and pharmacological implications. Gut. (2020) 69:1691–705. doi: 10.1136/gutjnl-2020-320622
5. Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, et al. The diagnosis and management of non-alcoholic fatty liver disease: practice guideline by the American association for the study of liver diseases, American college of gastroenterology, and the American gastroenterological association. Hepatology. (2012) 55:2005–23. doi: 10.1002/hep.25762
6. Kamo N, Kaido T, Hamaguchi Y, Okumura S, Kobayashi A, Shirai H, et al. Impact of sarcopenic obesity on outcomes in patients undergoing living donor liver transplantation. Clin Nutr. (2019) 38:2202–9. doi: 10.1016/j.clnu.2018.09.019
7. Eslamparast T, Montano-Loza AJ, Raman M, Tandon P. Sarcopenic obesity in cirrhosis – the confluence of two prognostic titans. Liver Int. (2018) 38:1706–17. doi: 10.1111/liv.13876
8. Younossi ZM, Golabi P, de Avila L, Paik JM, Srishord M, Fukui N, et al. The global epidemiology of NAFLD and NASH in patients with type 2 diabetes: a systematic review and meta-analysis. J Hepatol. (2019) 71:793–801. doi: 10.1016/j.jhep.2019.06.021
9. Andrade A, Cotrim HP, Bittencourt PL, Almeida CG, Sorte NC. Nonalcoholic steatohepatitis in posttransplantation liver: review article. Rev Assoc Med Bras 1992. (2018) 64:187–94. doi: 10.1590/1806-9282.64.02.187
10. Sharma P, Arora A. Approach to prevention of non-alcoholic fatty liver disease after liver transplantation. Transl Gastroenterol Hepatol. (2020) 5:51. doi: 10.21037/tgh.2020.03.02
11. Shaked O, Demetris J, Levitsky J, Feng S, Loza BL, Punch J, et al. Impact of donor and recipient clinical characteristics and hepatic histology on steatosis/fibrosis following liver transplantation. Transplantation. (2022) 106:106–16. doi: 10.1097/TP.0000000000003681
12. Galvin Z, Rajakumar R, Chen E, Adeyi O, Selzner M, Grant D, et al. Predictors of de novo nonalcoholic fatty liver disease after liver transplantation and associated fibrosis. Liver Transpl. (2019) 25:56–67. doi: 10.1002/lt.25338
13. Gitto S, de Maria N, di Benedetto F, Tarantino G, Serra V, Maroni L, et al. De-novo nonalcoholic steatohepatitis is associated with long-term increased mortality in liver transplant recipients. Eur J Gastroenterol Hepatol. (2018) 30:766–73. doi: 10.1097/MEG.0000000000001105
14. Vallin M, Guillaud O, Boillot O, Hervieu V, Scoazec JY, Dumortier J. Recurrent or de novo nonalcoholic fatty liver disease after liver transplantation: natural history based on liver biopsy analysis. Liver Transpl. (2014) 20:1064–71. doi: 10.1002/lt.23936
15. Younossi ZM, Stepanova M, Saab S, Kalwaney S, Clement S, Henry L, et al. The impact of type 2 diabetes and obesity on the long-term outcomes of more than 85 000 liver transplant recipients in the US. Aliment Pharmacol Ther. (2014) 40:686–94. doi: 10.1111/apt.12881
16. Gitto S, Marra F, De Maria N, Bihl F, Villa E, Andreone P, et al. Nonalcoholic steatohepatitis before and after liver transplant: keeping up with the times. Expert Rev Gastroenterol Hepatol. (2019) 13:173–8. doi: 10.1080/17474124.2019.1551132
17. van Son J, Stam SP, Gomes-Neto AW, Osté MCJ, Blokzijl H, van den Berg AP, et al. Post-transplant obesity impacts long-term survival after liver transplantation. Metabolism. (2020) 106:154204. doi: 10.1016/j.metabol.2020.154204
18. Spiritos Z, Abdelmalek MF. Metabolic syndrome following liver transplantation in nonalcoholic steatohepatitis. Transl Gastroenterol Hepatol. (2021) 6:13. doi: 10.21037/tgh.2020.02.07
19. Patil DT, Yerian LM. Evolution of nonalcoholic fatty liver disease recurrence after liver transplantation. Liver Transpl. (2012) 18:1147–53. doi: 10.1002/lt.23499
20. Unger LW, Herac M, Staufer K, Salat A, Silberhumer G, Hofmann M, et al. The post-transplant course of patients undergoing liver transplantation for nonalcoholic steatohepatitis versus cryptogenic cirrhosis: a retrospective case-control study. Eur J Gastroenterol Hepatol. (2017) 29:309–16. doi: 10.1097/MEG.0000000000000794
21. Bhati C, Idowu MO, Sanyal AJ, Rivera M, Driscoll C, Stravitz RT, et al. Long-term outcomes in patients undergoing liver transplantation for nonalcoholic steatohepatitis-related cirrhosis. Transplantation. (2017) 101:1867–74. doi: 10.1097/TP.0000000000001709
22. Sprinzl MF, Weinmann A, Lohse N, Tönissen H, Koch S, Schattenberg J, et al. Metabolic syndrome and its association with fatty liver disease after orthotopic liver transplantation. Transpl Int. (2013) 26:67–74. doi: 10.1111/j.1432-2277.2012.01576.x
23. Fussner LA, Heimbach JK, Fan C, Dierkhising R, Coss E, Leise MD, et al. Cardiovascular disease after liver transplantation. When, what and who is at risk. Liver Transpl. (2015) 21:889–96. doi: 10.1002/lt.24137
24. Watt KD. Keys to long-term care of the liver transplant recipient. Nat Rev Gastroenterol Hepatol. (2015) 12:639–48. doi: 10.1038/nrgastro.2015.172
25. Cassiman D, Roelants M, Vandenplas G, Van der Merwe SW, Mertens A, Libbrecht L, et al. Orlistat treatment is safe in overweight and obese liver transplant recipients: a prospective, open label trial. Transpl Int. (2006) 19:1000–5. doi: 10.1111/j.1432-2277.2006.00379.x
26. Brown S, Izzy M, Watt KD. Pharmacotherapy for weight loss in cirrhosis and liver transplantation: translating the data and underused potential. Hepatology. (2021) 73:2051–62. doi: 10.1002/hep.31595
27. Kukla A, Hill J, Merzkani M, Bentall A, Lorenz EC, Park WD, et al. The use of GLP1R. Agonists for the treatment of type 2 diabetes in kidney transplant recipients. Transplant Direct. (2020) 6:e524. doi: 10.1097/TXD.0000000000000971
28. Sorli C, Harashima SI, Tsoukas GM, Unger J, Karsbøl JD, Hansen T, et al. Efficacy and safety of once-weekly semaglutide monotherapy versus placebo in patients with type 2 diabetes (SUSTAIN 1): a double-blind, randomised, placebo-controlled, parallel-group, multinational, multicentre phase 3a trial. Lancet Diabetes Endocrinol. (2017) 5:251–60. doi: 10.1016/S2213-8587(17)30013-X
29. O'Neil P, Birkenfeld A, Barbara McGowan B, Mosenzon O, Pedersen SD, Wharton S, et al. Efficacy and safety of semaglutide compared with liraglutide and placebo for weight loss in patients with obesity: a randomised, double-blind, placebo and active controlled, dose-ranging, phase 2 trial. Lancet. (2018) 392:637–49. doi: 10.1016/S0140-6736(18)31773-2
30. Iacob S, Gheorghe L. Long term follow-up of liver transplant recipients: considerations for non-transplant specialists. J Gastrointestin Liver Dis. (2021) 30:283–90. doi: 10.15403/jgld-3616
31. Lee Y, Tian C, Lovrics O, Soon MS, Doumouras AG, Anvari M, et al. Bariatric surgery before, during, and after liver transplantation: a systematic review and meta-analysis. Surg Obes Relat Dis. (2020) 16:1336–47. doi: 10.1016/j.soard.2020.05.012
32. Fakhry TK, Mhaskar R, Schwitalla T, Muradova E, Gonzalvo JP, Murr MM. Bariatric surgery improves nonalcoholic fatty liver disease: a contemporary systematic review and meta-analysis. Surg Obes Relat Dis. (2019) 15:502–11. doi: 10.1016/j.soard.2018.12.002
33. Morrisett JD, Abdel-Fattah G, Hoogeveen R, Mitchell E, Ballantyne CM, Pownall HJ, et al. Effects of sirolimus on plasma lipids, lipoprotein levels, and fatty acid metabolism in renal transplant patients. J Lipid Res. (2002) 43:1170–80. doi: 10.1194/jlr.M100392-JLR200
34. Zhang X, Coker OO, Chu ES, Fu K, Lau HCH, Wang YX, et al. Dietary cholesterol drives fatty liver-associated liver cancer by modulating gut microbiota and metabolites. Gut. (2021) 70:761–74. doi: 10.1136/gutjnl-2019-319664
35. Hirota T, Fujita Y, Ieiri I. An updated review of pharmacokinetic drug interactions and pharmacogenetics of statins. Expert Opin Drug Metab Toxicol. (2020) 16:809–22. doi: 10.1080/17425255.2020.1801634
36. Lonardo A, Nascimbeni F, Mantovani A, Targher G. Hypertension, diabetes, atherosclerosis and NASH: cause or consequence? J Hepatol. (2018) 68:335–52. doi: 10.1016/j.jhep.2017.09.021
37. Berkovic MC, Virovic-Jukic L, Bilic-Curcic I, Mrzljak A. Post-transplant diabetes mellitus and preexisting liver disease - a bidirectional relationship affecting treatment and management. World J Gastroenterol. (2020) 26:2740–57. doi: 10.3748/wjg.v26.i21.2740
38. Scheen AJ. Effect of sodium-glucose cotransporter type 2 inhibitors on liver fat in patients with type 2 diabetes: hepatic beyond cardiovascular and renal protection? Ann Transl Med. (2018) 6:S68. doi: 10.21037/atm.2018.10.39
39. Affinati AH, Esfandiari NH, Oral EA, Kraftson AT. Bariatric surgery in the treatment of type 2 diabetes. Curr Diab Rep. (2019) 19:156. doi: 10.1007/s11892-019-1269-4
40. Panunzi S, Carlsson L, De Gaetano A, Peltonen M, Rice T, Sjöström L, et al. Determinants of diabetes remission and glycemic control after bariatric surgery. Diabetes Care. (2016) 39:166–74. doi: 10.2337/dc15-0575
41. Vinaixa C, Selzner N, Berenguer M. Fat and liver transplantation: clinical implications. Transpl Int. (2018) 31:828–37. doi: 10.1111/tri.13288
42. Mazilescu LI, Selzner M, Nazia Selzner N. Defatting strategies in the current era of liver steatosis. JHEP Rep. (2021) 3:100265. doi: 10.1016/j.jhepr.2021.100265
43. Nativ NI, Maguire TJ, Yarmush G, Brasaemle DL, Henry SD, Guarrera JV, et al. Liver defatting: an alternative approach to enable steatotic liver transplantation. Am J Transplant. (2012) 12:3176–83. doi: 10.1111/j.1600-6143.2012.04288.x
44. Finkenstedt A, Auer C, Glodny B, Posch U, Steitzer H, Lanzer G, et al. Patatin-like phospholipase domain-containing protein 3 rs738409-G in recipients of liver transplants is a risk factor for graft steatosis. Clin GastroenterolHepatol. (2013) 11:1667–72. doi: 10.1016/j.cgh.2013.06.025
45. Kim H, Lee KW, Lee K, Seo S, Park MY, Ahn SW, et al. Effect of PNPLA3 I148M polymorphism on histologically proven non-alcoholic fatty liver disease in liver transplant recipients. Hepatol Res. (2018) 48:E162–71. doi: 10.1111/hepr.12940
46. Trunecka P, Mikova I, Dlouha D, Hubáček JA, Honsová E, Kolesár L, et al. Donor PNPLA3 rs738409 genotype is a risk factor for graft steatosis.A post-transplant biopsy-based study. Dig Liver Dis. (2018) 50:490–5. doi: 10.1016/j.dld.2017.12.030
47. Eslam M, Valenti L, Romeo S. Genetics and epigenetics of NAFLD and NASH: clinical impact. J Hepatol. (2018) 68:268–79. doi: 10.1016/j.jhep.2017.09.003
48. Míková I, Neroldová M, Hubáček JA, Dlouhá D, Jirsa M, Honsová E, et al. Donor PNPLA3 and TM6SF2 variant alleles confer additive risks for graft steatosis after liver transplantation. Transplantation. (2020) 104:526–34. doi: 10.1097/TP.0000000000002876
49. John BV, Aiken T, Garber A, Thomas D, Lopez R, Patil D, et al. Recipient but not donor adiponectin polymorphisms are associated with early posttransplant hepatic steatosis in patients transplanted for non-nonalcoholic fatty liver disease indications. Exp Clin Transpl. (2018) 16:439–45. doi: 10.6002/ect.2018.0070
50. Kelava T, Turcic P, Markotic A, Ostojic A, Sisl D, Mrzljak A. Importance of genetic polymorphisms in liver transplantation outcomes. World J Gastroenterol. (2020) 26:1273. doi: 10.3748/wjg.v26.i12.1273
51. Iacob S, Iacob R, Manea I, Uta M, Stoica L, Mandea M et al. TBX21 genotypes predict occurrence of non-alcoholic steatohepatitis following liver transplantation. JGLD. (2021) 30(Suppl. 1):86.
52. Beckebaum S, Iacob S, Klein CG, Dechêne A, Varghese J, Baba HA, et al. Assessment of allograft fibrosis by transient elastography and noninvasive biomarker scoring systems in liver transplant patients. Transplantation. (2010) 89:983–93. doi: 10.1097/TP.0b013e3181cc66ca
53. Barrault C, Roudot-Thoraval F, Tran Van Nhieu J, Atanasiu C, Kluger MD, Medkour F, et al. Non-invasive assessment of liver graft fibrosis by transient elastography after liver transplantation. Clin Res Hepatol Gastroenterol. (2013) 37:347–52. doi: 10.1016/j.clinre.2012.11.003
54. Iacob S, Onica M, Iacob R, Gheorghe C, Beckebaum S, Cicinnati V, et al. Impact of sustained virological response on metabolic profile and kidney function in cured HCV liver transplant recipients. Surg. Gastroenterol. Oncol. (2021) 26:104–10. doi: 10.21614/sgo-26-2-364
55. Azhie A, Sheth P, Hammad A, Woo M, Bhat M. Metabolic complications in liver transplantation recipients: how we can optimize long-term survival. Liver Transpl. (2021) 27:1468–78. doi: 10.1002/lt.26219
Keywords: genetic, liver, NAFLD, NASH, liver transplant
Citation: Iacob S, Beckebaum S, Iacob R, Gheorghe C, Cicinnati V, Popescu I and Gheorghe L (2022) Genetic and Life Style Risk Factors for Recurrent Non-alcoholic Fatty Liver Disease Following Liver Transplantation. Front. Nutr. 8:787430. doi: 10.3389/fnut.2021.787430
Received: 30 September 2021; Accepted: 22 December 2021;
Published: 14 January 2022.
Edited by:
Shira Zelber-Sagi, University of Haifa, IsraelReviewed by:
Matthias J. Bahr, Sana Kliniken Lübeck, GermanyHelena Katchman, Tel Aviv Sourasky Medical Center, Israel
Copyright © 2022 Iacob, Beckebaum, Iacob, Gheorghe, Cicinnati, Popescu and Gheorghe. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Speranta Iacob, bXNpYWNvYkBnbWFpbC5jb20=
 Speranta Iacob
Speranta Iacob Susanne Beckebaum
Susanne Beckebaum Razvan Iacob
Razvan Iacob Cristian Gheorghe1,2,3
Cristian Gheorghe1,2,3 Vito Cicinnati
Vito Cicinnati Irinel Popescu
Irinel Popescu Liana Gheorghe
Liana Gheorghe